Abstract
Our understanding of inherited retinal disease has benefited immensely from molecular genetic analysis over the past several decades. New technologies that allow for increasingly detailed examination of a patient’s DNA have expanded the catalog of genes and specific variants that cause retinal disease. In turn, the identification of pathogenic variants has allowed the development of gene therapies and low-cost, clinically focused genetic testing. Despite this progress, a relatively large fraction (at least 20%) of patients with clinical features suggestive of an inherited retinal disease still do not have a molecular diagnosis today. Variants that are not obviously disruptive to the codon sequence of exons can be difficult to distinguish from the background of benign human genetic variations. Some of these variants exert their pathogenic effect not by altering the primary amino acid sequence, but by modulating gene expression, isoform splicing, or other transcript-level mechanisms. While not discoverable by DNA sequencing methods alone, these variants are excellent targets for studies of the retinal transcriptome. In this review, we present an overview of the current state of pathogenic variant discovery in retinal disease and identify some of the remaining barriers. We also explore the utility of new technologies, specifically patient-derived induced pluripotent stem cell (iPSC)-based modeling, in further expanding the catalog of disease-causing variants using transcriptome-focused methods. Finally, we outline bioinformatic analysis techniques that will allow this new method of variant discovery in retinal disease. As the knowledge gleaned from previous technologies is informing targets for therapies today, we believe that integrating new technologies, such as iPSC-based modeling, into the molecular diagnosis pipeline will enable a new wave of variant discovery and expanded treatment of inherited retinal disease.
Keywords: Induced pluripotent stem cell, Inherited retinal disease, Retinal organoid, Transcriptome, Variant discovery, Rare Mendelian disease
1. The current landscape of variant identification in genetic retinal disease
1.1. The prevalence of genetically unsolved retinal disease
In recent years, the next frontier of retinal-disease-causing genetic variation has begun to come into clearer focus. Increasingly accessible and inexpensive DNA sequencing technology has enabled several large cohort studies of inherited retinal disease patients and their family members (Carrigan et al., 2016; Carss et al., 2017; Ellingford et al., 2016; Stone et al., 2017). These studies simultaneously revealed the efficiency and power of existing molecular methods and the unacceptably large diagnostic insensitivity that persists in the field. Specifically, the fraction of inherited retinal disease patients for whom a causative variant has not been identified ranges from 24% to 53% in the studies mentioned above. This large fraction of missing variants is not limited to retinal disease, it is a feature of the diagnostic pipelines that are common to all medical specialties (Hartley et al., 2020).
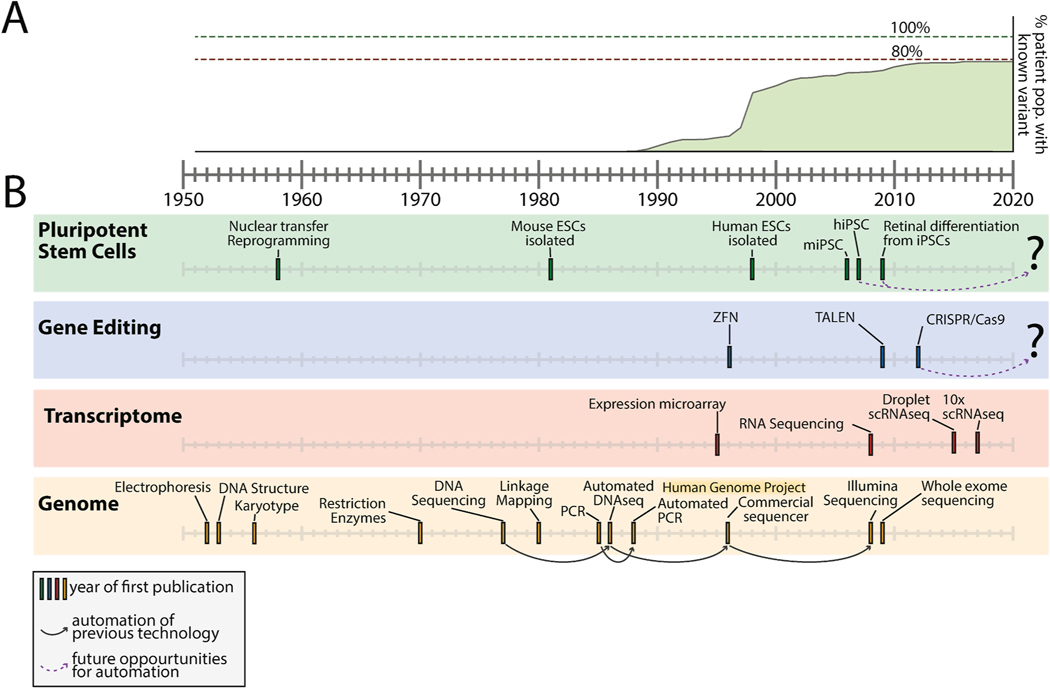
The consecutive nature of the large study by Stone et al. (2017) allows the fraction of disease caused by each gene to be accurately estimated. If one plots these gene specific fractions cumulatively according to the dates that the genes were first shown to cause disease, the overall cadence of disease gene discovery can be seen (Fig. 1A). The first 10% of this gene discovery function took 10 years (1987–1997) and resulted from the combined application of linkage analysis and candidate gene screening (the positional candidate approach) to study large families affected with dominant diseases. Forty percent of our current diagnostic sensitivity came from discoveries made in the two-year period from 1997 to 1999, which corresponds to the application of improved DNA sequencing technology fueled by the Human Genome Project to discover mutations in very large genes (e.g., ABCA4 and USH2A) causing autosomal recessive disease. After that, diagnostic sensitivity increased at the rate of about 2% per year for ten years (2000–2010), which resulted from continued improvements in DNA sequencing efficiency and the associated lower costs, which in turn allowed genes that cause a very small fraction of disease to be discovered. At that point, the rate of improvement in diagnostic sensitivity has fallen to about a tenth of a percent per year, asymptotically approaching 80%. The shape of this curve strongly suggests that very few new genes remain to be found that cause retinal disease in a high penetrance, Mendelian fashion.
Fig. 1. Timeline of technology development in the context on inherited retinal disease variant discovery.
A) The proportion of inherited retinal disease patients for whom the disease causing variant is known is plotted across time. Prevalence estimates are derived from a recent study of a large cohort of inherited retinal disease patients seen in consecutive order by a single inherited eye disease specialist (Stone et al., 2017). The technology and gene discovery timeline information is adapted from (Broadgate et al., 2017; Stuart and Satija, 2019; Wang et al., 2009). B) Breakthrough technologies in the fields of pluripotent stem cells (green, top), gene editing (blue, top middle), transcriptome analysis (red, bottom middle) and genome sequencing (yellow, bottom) are shown. Year of first publication of the method is indicated. Solid arrows connect technologies to subsequent related innovations in automation. Dashed arrows (found within top two boxes) indicate technologies that may be amenable to automation in the future.
What then accounts for the remaining 20% of patients that an experienced clinician finds indistinguishable from the other 80%? The immune system is definitely capable of injuring photoreceptor cells in a manner that is clinically similar to inherited eye disease (Stanwyck et al., 2019), as evidenced in our recent study of autoimmune retinopathy (Wiley et al., 2019). Such disease is often more rapid in time course and a bit less symmetric than Mendelian retinal disease, but it is likely that a significant fraction of the remaining 20% will have an immunological mechanism. Multigenic disease will also be responsible for some of the missing 20%. We know that there are some currently undefined elements in the “genetic background” that significantly modify Mendelian genotypes because virtually all autosomal dominant inherited retinal diseases manifest significant variable expressivity, often to the extreme of incomplete penetrance (Green et al., 2020; Llavona et al., 2017). These modifying alleles are much more common in the population than the high penetrance alleles that are currently counted among the almost 80% of solved cases. Rare multigenic combinations of these modifiers probably cause disease, but none of these alleles would be called “pathogenic” with current diagnostic algorithms. A few patients, especially those with unilateral disease, probably harbor somatic mutations in dominant disease genes similar to patients affected with dermatomal neurofibromatosis (Listernick et al., 2003).
Another very important group of patients that are currently undiagnosed are those with non-exomic mutations that cause disease by affecting the transcription and splicing of genes rather than their coding sequences. These variants would elude detection in whole exome sequencing (WES) and be difficult to functionally decipher in whole genome sequencing (WGS). Examples of such non-exomic mutations that are sufficiently far away from the normally assessed coding sequences that very focused experimental approaches were needed to find them include the splice variants in ABCA4 reported by Braun et al., in 2013 and the cluster of variants in a DNase I hypersensitivity site thousands of basepairs upstream from the PRDM13 transcription start site that cause North Carolina Macular Dystrophy (Small et al., 2016). Variants may also impact epigenetic regulation, a mechanism identified in a number of rare genetic disease cases (Hartley et al., 2020).
Although the true fraction of undiagnosed cases caused by non-exomic mutations will only be known once these patients are convincingly solved, most investigators in the field feel that the fraction is likely to be at least 50% of the currently unsolved families (i.e., 10% of all retinal disease-causing variations; Carrigan et al., 2016).
Creating a new inflection in the diagnostic sensitivity curve will require a fundamentally new approach. We believe that the best chance of discovering new disease variants of clinical significance will be to use transcriptomic analysis of patient-derived iPSCs to look for regulatory variants near genes known to be expressed in the retina. Kishore and coworkers recently published an example outside the retina of how this might be done. They used GATA6 mutant patient induced pluripotent stem cells (iPSCs) to discover a non-coding, regulatory single nucleotide polymorphism (SNP) that acts as a modifier for pancreatic development phenotype (Kishore et al., 2020).
1.2. The clinical usefulness of pathogenic variant discovery
Although the complete sequence of human DNA has been known for a number of years now, our knowledge of the transcription of this DNA and the specific functions of the tens of thousands of different proteins translated from these transcripts is in its infancy (Giacalone et al., 2016). Despite this, convincing disease-causing genotypes can already be identified in more than 75% of patients with an inherited retinal disease and this information will enable gene-specific therapies for these patients. In this section, we will briefly discuss the development of useful and accessible genetic testing and the role of the resulting information will play in the development of gene and cell-replacement therapies.
1.2.1. Gene therapy
Identification of disease-causing variants is central to the treatment of inherited retinal diseases. In addition to the prognostic and diagnostic benefits, knowledge of a patient’s disease-causing genotype allows gene-specific therapies like viral mediated gene replacement and CRISPR-corrected patient-derived stem cell transplants to be developed and delivered. Unlike drug treatments that usually address some cellular dysfunction that is mechanistically downstream from the genetic variants themselves, gene therapy relies on correct and precise identification of variants to be effective. In vitro confirmation of variant pathogenicity and the ability to demonstrate functional restoration after gene replacement are valuable components of an efficient gene therapy development pipeline (Tucker et al., 2014a).
Gene therapy has already shown promise for this class of diseases. The current state of gene therapy for retinal disease was recently reviewed in depth by Lee and colleagues (Lee et al., 2019). The importance of variant identification as a prerequisite for gene therapy is highlighted in this work. While technical challenges regarding transduction efficiency, packaging limitations, and other viral mechanics issues still remain to be solved, gene therapy logically cannot progress for any disease until the pathogenic gene is identified.
The utility of pathogenic variant identification also extends beyond the identification of gene therapy targets. Correcting a known pathogenic variant at the iPSC stage allows one to create isogenic control lines with the same genetic background as the patient but with the putative pathogenic variants corrected. Such model lines can be used to prototype gene therapy strategies, many of which are highly cell type specific (Burnight et al., 2014).
1.2.2. Cell replacement therapy
Cell replacement therapy is another therapeutic option for those patients whose inherited retinal disease has destroyed an essential population of cells. Without functional cells, gene replacement therapy is not an option and the photoreceptors must be replaced in order to restore vision. With the increasing refinement of in vitro differentiation strategies, the production of functional retinal cells in a laboratory is now a realistic option (Jayakody et al., 2015). Indeed, laboratory manufactured RPE sheets derived from iPSCs have already been transplanted into the subretinal space of age-related macular degeneration (AMD) patients (Araki et al., 2019; Jin et al., 2019; Mandai et al., 2017; Matsumoto et al., 2019), demonstrating the safety and potential efficacy of this approach. The topic of autologous versus off-the-shelf sources for stem cell therapy has been discussed at length previously (Doss and Sachinidis, 2019; Gagliardi et al., 2019; Jin et al., 2019) and while the development of the stem cell transplantation field is a major driving motivation for the creation of retinal in vitro differentiation techniques, the details of therapeutic approaches are not the focus of this review.
Nevertheless, autologous cell-based approach to therapy for inherited retina disease will still require precise knowledge of the genetic basis for disease. Our lab and others have observed that even relatively immature organoid models derived from patient cells show disease specific phenotypes (Burnight et al., 2014; Huang et al., 2019; Sharma et al., 2017; Tucker et al., 2013b, 2014b). In some cases, certain cell types will not develop in patient lines harboring inherited retinal disease-causing variants differentiated in vitro without mutation repair. This was shown to be the case by Lane, Jovanovic and colleagues, who recently demonstrated that iPSC-derived retinal organoids (iPSC-ROs) from RP2 patients undergo photoreceptor death during differentiation (Lane et al., 2020). This extreme example shows that, at least in some cases, autologous cell replacement therapy is not realistic without gene repair. It is uncertain whether or not replacing diseased retinal cells with iPSC-derived cells from the same patient without correcting the genetic aberration would yield long term disease reversal. As discussed later in this review, targeted editing of the genome will be valuable for determining the pathogenicity of non-coding variants. The same editing techniques will be required to “repair” autologous cells before they can be transplanted into a patient’s retina. This is just one of the many consideration that need to be included in the complex discussion required before choosing a starting material for transplantation (Jayakody et al., 2015; Wiley et al., 2014).
1.2.3. Genetic testing & clinical counseling
The most immediate utility of variant discovery is the development of genetic tests. Such testing, when performed in a targeted and logical manner informed by knowledge of variant-phenotype relationships, can be provided at low cost and high accuracy (Stone, 2003; Stone et al., 2012, 2017). As discussed by Stone and colleagues, genetic testing is not without hazards, particularly when a patient does not yet display symptoms (Stone et al., 2012). However, the benefits of targeted testing are also great, allowing for administration of preventative therapy, monitoring of disease progression, and reproductive planning on the part of adult patients (Stone et al., 2012). While the balance between hazard and benefit of genetic testing must be measured on an individual basis, adding curated variants to the catalog will steadily improve the clinical management of inherited retinal disease.
2. History of variant discovery in inherited retinal disease
In order to approach the yet unsolved inherited retinal diseases, one must first understand the relevant approaches taken to identify disease genes in the past. The history of inherited retinal disease gene discovery has been reviewed extensively by Broadgate and colleagues (Broadgate et al., 2017). In the current review, past approaches will be discussed only briefly in order to highlight key gaps and strategies that should inform the next generation of inherited retinal disease variant discovery.
The vast majority of inherited retinal disease genes were identified using DNA as the primary analysis substrate. DNA samples from whole blood was collected from members of families with a history of inherited retinal disease. Linkage analysis was then used to map variants across families, revealing the association of certain variants with disease phenotype by comparing presence in affected and unaffected family members. Candidate pathogenicity could then be assigned to such variants and tested using animal models (Fletcher et al., 2011; Kostic and Arsenijevic, 2016; Veleri et al., 2015). In some cases, naturally occurring retinal diseases in various animal species have suggested candidate genes for human disease. In Fig. 1B, various technologies are arranged by the date of their first publication. When one considers the progress in technologies that assay the DNA genome (Fig. 1, yellow box), it is evident that sequencing, PCR, and linkage mapping strategies correlate with the discovery of many inherited retinal disease genes. Automation of these techniques accelerated gene discovery (Fig. 1, solid arrows). The commercial availability of DNA sequencing facilitated studies of multiple individuals at a reasonable cost. Gene discovery resulting from the development and automation of new technologies will likely be paralleled in the stem cell and gene editing fields in the future (Fig. 1, green/blue boxes, dashed arrows). Even in 2008, at the emergence of next generation and RNA sequencing, Wold and Myers predicted the utility of RNA sequencing for the discovery of variants that functionally impact splicing instead of the resulting protein sequence (Wold and Myers, 2008). As noted above, such transcriptome-based approaches for variant discovery are among the most promising strategies for identifying the molecular cause of disease in patients who remain undiagnosed despite whole exome sequencing and these strategies will be a major focus of this review (see Section 5).
3. Limitations in variant discovery
3.1. Differentiation between rare benign variants and disease-causing variants
The pathogenicity of a variant that does not obviously disrupt the protein coding exons of a gene is difficult to assign in isolated individuals. Strategies to identify the pathogenicity of such variants rely on either population-level data and/or comparison to family members. Both of these comparisons inform pathogenicity through the same reasoning; if a variant is pathogenic, most individuals who harbor them should be clinically affected. Extension of this idea to the population scale requires that variants (or the genotypes they contribute to in the case of autosomal recessive or multigenic disease) be similarly prevalent to the disease in a given population in order to be considered pathogenic. The large and growing databases of genomic data from many populations (Karczewski et al., 2017, 2020) are making such frequency-based arguments much more reliable than they were in the past. A benefit of iSPC-based methods is the ability to empirically test pathogenicity using genome editing and in vitro phenotypic testing. These technologies make any approach that can narrow candidate SNPs to a handful, very valuable, since candidates can be rapidly tested in vitro. Without the ability to empirically test variants, a transcriptome-based strategy that yielded more than one candidate is unlikely to be clinically useful.
3.2. Determination of significance of variants in non-coding regions
Determining the pathogenicity of DNA variants that would not be expected to alter the protein structure directly can be challenging. An example of how transcriptome analysis can be used to do so is MAK, a recently described retinitis pigmentosa gene (Tucker et al., 2011b). MAK encodes a cilia-related kinase (Omori et al., 2010) and insertion of a repetitive element into exon 9 of this gene leads to gradual and progressive photoreceptor cell death. This insertion disrupts splicing, blocking the expression of the normal retina-specific isoform. This downstream effect following a variation in DNA was not inherently obvious from looking at the DNA code alone, and the pathogenic mechanism was only revealed after RNA analysis of retinal tissue (Tucker et al., 2011b). In this study, investigating expression of MAK was possible by generating iPSC-derived retinal tissue through a skin biopsy of living patients with this mutation.
Non-protein encoding variants often escape detection by methods that assay the DNA, as their effects will likely become evident only at the RNA transcript level. While RNA sequencing technology is now quite accessible, this technology has only recently been viewed through a diagnostic lens (Byron et al., 2016; Karczewski and Snyder, 2018; Lee et al., 2020). A major reason that RNA analysis lags behind DNA analysis in the gene discovery realm is that affected tissue is needed for analysis. While this is possible in subspecialties like dermatology and hematology where the affected tissue is abundant and accessible, it is not feasible for the diagnosis of retinal degenerations. While the DNA of virtually every cell in the body contains the same genetic code and can be considered equivalent for most traditional sequencing or PCR-based assays, the abundance and nucleotide sequence (due to splicing variants) of RNA for any particular gene varies greatly among different tissues (GTEx Consortium, 2017). For this reason, easily accessible tissues such as a skin biopsy or whole blood are not suitable for RNA analysis in the majority of Mendelian retinal diseases.
In designing studies that aim to discover pathogenic rare variants, the sequencing substrate must be carefully considered. The nuanced ways that heritable, Mendelian traits may be phenotypically expressed in a tissue-specific manner is thoroughly discussed by Hekselman and Yerger-Lotem in their recent review (Hekselman and Yeger-Lotem, 2020), and many such principles apply to inherited retinal disease. These authors found that while most Mendelian diseases display a disease phenotype in only one tissue, a large proportion of disease genes are in fact expressed at the RNA level in numerous (unaffected) tissues. This suggests that the cellular context in which a gene is expressed can help predict the impact of any potential variants. This context may be cell-autonomous or influenced by the cellular microenvironment. While discovery of these interactions is sure to be brought about by heterogeneous organoid studies, the details of such experiments are beyond the scope of this review.
To more generally apply the concept of tissue specific disease expressivity, consider a hypothetical gene consisting of 5 exons and 4 introns (Fig. 2). A patient has 3 rare variants associated with this gene: variant A is in an upstream regulatory region, variant B is in exon 1, and variant C is in an intronic region between exons 2 and 3. In the left half of the table below, two DNA sequencing methods are discussed: whole genome sequencing (WGS) and whole exome sequencing (WES). As the DNA sequence is identical in virtually all somatic cells, any tissue can be used as input for these studies. In the case of a WGS study of this gene, one would discover all three variants (A, B, and C). If B altered a codon or introduced a stop codon or frameshift, the protein-coding impact of B would also be known. The functional importance or pathogenicity of variants A and C, the retinal expression level, and the expected “normal” splice isoforms of this gene would remain unknown. In WES, DNA is enriched for protein-encoding exons prior to sequencing. In this example, only the existence of variant B would be discovered.
Fig. 2. Information gained regarding exonic and intronic variants from DNA and RNA sequencing modalities.
A) A hypothetical gene locus contains three potentially pathogenic variants (A, B, & C), indicated with red lines. Variant A is found in an upstream non-coding region, variant B is found within the first exon, and variant C is found within the second intron. The transcription and translation of this hypothetical gene are illustrated. B) Information gained about these variants varies between sequencing substrate (DNA, left versus RNA, right). Within each substrate, the experimental approach further defines what information can be gained. RNA sequencing of disease tissue provides the most information with regards to both coding and non-coding variants that may disrupt expression regulation or isoform splicing.
In this theoretical study, RNA sequencing is performed on two easily accessible tissues; whole blood and skin. Whole blood is the tissue typically used for WGS/WES studies. While RNA sequencing of blood does reveal the isoform level expression of the gene in blood, it does not reveal any information on isoform expression in the disease tissue of interest (i.e. retina). In this case, the isoform expressed in blood does not contain exon 1, thus the existence of all three variants remains unknown in this study.
Transcriptomic profiling of the disease tissue of interest provides an abundance of data relating to variant functionality. In this example, variant B is discovered due to inclusion of exon 1 in the retina isoforms. Allele-specific expression, relative isoform abundance, and retinal expression level of the gene are gleaned. While variants A and C may potentially possess pathogenic regulatory or splicing influences, the variants themselves are missed with RNA sequencing alone.
Transcriptomic analysis of the retina of living patients is not possible. However, stem cell culture and differentiation protocols developed over the past decades have made the in vitro derivation of retinal cells attainable. To the degree that these cells accurately recapitulate a patient’s retina, they can be used as a model system for transcriptome analysis. Combining existing cell culture methods and transcriptomic readouts with high throughput process design will bring RNA analysis into common use for inherited retinal disease variant discovery.
4. Stem cells and gene editing for retinal modeling
In rare cases, disease-causing mutations can be discovered, and their pathophysiologic mechanism characterized by the study of donated ocular tissue. Gene expression analyses can be performed on the retina of eyes donated post-mortem by individuals with inherited or acquired photoreceptor degeneration (Mullins et al., 2012; Voigt et al., 2020). However, this approach requires a tissue collection paradigm with sufficiently short death-to-preservation times to allow recovery of labile mRNA. In addition, since many photoreceptor degeneration genes are selectively expressed by photoreceptor cells, the loss of these cells that occurs in advanced degeneration also results in a loss in hundreds of transcripts associated with photoreceptor cell physiology.
Our group has focused on induced pluripotent stem cell (iPSC) technology as a surrogate source of retinal samples for many years. A typical patient-specific iPSC generation and differentiation workflow is depicted in Fig. 3. The benefits of the iPSC approach have been reviewed extensively for a number of organ systems and diseases (Blau and Daley, 2019; Rowe and Daley, 2019). In our own group, the development of in vitro retinal production schemes for therapeutic purposes has created a platform for gene therapy development and variant discovery (Tucker et al., 2011a, 2013a, 2013b; Wiley et al., 2016). In this section we have outlined the story of iPSC technology from the perspective of retinal disease and consider the next steps in using this platform for gene discovery.
Fig. 3. Reprogramming of patient somatic cells to an induced pluripotent state and subsequent differentiation towards retina.
Patient tissues such as skin or blood can be reprogrammed to iPSCs using a variety of non-integrating vectors to deliver the reprogramming factors (OSKM) (top). Once converted to iPSCs, the cells can be expanded indefinitely, allowing for quality control (gene expression, karyotype analysis, etc.) and genome editing (middle). Finally, these cells can be differentiated toward a retinal fate using a variety of protocols. The course of differentiation can be monitored using protein expression analysis of known markers as well as through global gene expression analysis and single cell-based trajectory analysis. Resulting retinal organoids can be used as a surrogate for patient retinal tissue in transcriptomic and other studies.
4.1. The development of in vitro pluripotent stem cells
All of the cells in an adult organism originated from a collection of undifferentiated, pluripotent cells. Such cells were first experimentally isolated from the embryonic mouse and grown in continuous culture in 1981 (Evans and Kaufman, 1981). In 1998, the same technique was applied to human blastocysts, and a number of pluripotent human embryonic stem cell (hESC) lines were derived (Thomson et al., 1998). As exciting as this development was, three limitations were quickly apparent. First, ethical questions were raised about the appropriateness of using human embryos for research; second, the embryonic lines were limited to a few genetic backgrounds; and third, if used for transplantation, the lines would usually not be a good immunological match for a given patient recipient. The technique of induced pluripotency solved the latter two problems via patient-specific pluripotent stem cell (PSC) lines; however, the ethical questions surrounding stem cell research remain incompletely addressed. This is especially true when scientists seek to create well-developed human brains or whole embryos, a topic we will elaborate upon later in this review.
Forty years prior to the derivation of ESCs, the evidence supporting pluripotency as an inducible state first emerged. In 1958, Gurdon and colleagues showed that the nucleus of an adult cell was programmable to pluripotency when transplanted into an enucleated oocyte (Gurdon et al., 1958). The factors present in the enucleated oocyte that provided the initial reprogramming trajectory would remain unknown for nearly 50 years until Takahashi and Yamanaka successfully demonstrated reprogramming of mouse somatic cells using defined transcription factors (Takahashi and Yamanaka, 2006). The first iPSCs were created by delivering transcription-factor-encoding sequences via retroviral vectors. The resulting cells were genetically identical to somatic cells from which they were derived except for the integrating reprogramming vectors. The latter have now been replaced with non-integrating vectors (Ban et al., 2011; Okita et al., 2013) such that the resulting cells are truly genetically identical to all other somatic cells in the donor animal’s body.
Takahashi and Yamanaka quickly followed up their mouse study using human fibroblasts, demonstrating that induced pluripotency was attainable in human somatic cells and outlining a set of factors that made this transition possible (Takahashi et al., 2007). The progression in iPSC technologies and techniques since 2007 has grown explosively. Following the demonstration that human cells were indeed reprogrammable, the cells of human patients were reprogrammed yielding the starting material for a new frontier of in vitro disease modeling (Park et al., 2008). More recently, it has been shown that iPSCs and ESCs share a common transcriptional profile (Choi et al., 2015). Fig. 4 illustrates various stages of patient-specific iPSC reprogramming in our laboratory. Fibroblasts are derived from a patient’s skin biopsy (Fig. 4A, B, C). After successful reprogramming, cells display typical pluripotent morphology (Fig. 4D), as well as expected gene expression profiles and protein markers (Fig. 4E–J).
Fig. 4. Reprogramming of donor dermal fibroblasts and quality control at the iPSC stage.
A-C) Patient dermal fibroblasts are expanded from a skin punch biopsy. D) Patient-specific iPSCs demonstrate expected pluripotent morphology under brightfield imaging, including prominent nucleoli (inset). E) hPSC Scorecard Analysis Software output demonstrates the expected pluripotent transcriptional profile; self-renewal without lineage-specific differentiation. F–H) Patient-specific iPSCs express known pluripotency markers SSEA3, TRA-1–81, SSEA4, and TRA-160, as demonstrated by immunofluorescence staining. I) Graph comparing the algorithm scores for expression of genes involved in self-renewal. Compared to the internal reference data set provided by the hPSC Scorecard Analysis Software, each patient line falls within the average. J). Data in this figure adapted from (Wiley et al., 2016), which is licensed under the Creative Commons Attribution 4.0 International License.
Currently, reprogramming to iPSCs is a relatively routine laboratory protocol that employs non-integrating vectors (Schlaeger, 2018; Schlaeger et al., 2015). For the purposes of this review, we will discuss iPSC reprogramming from the perspective of process optimization and scaling and consider the reprogramming process itself a solved problem for our purposes.
4.2. Pluripotent stem cells for generation of retinal tissue
Differentiation protocols suitable for generating retinal neurons from pluripotent embryonic stem cells have been under development for well over a decade (Lamba et al., 2006; Osakada et al., 2008; Pankratz et al., 2007). The primary goal of these early studies was to obtain cells suitable for transplantation and restoration of retinal function. With the advent of the human iPSC, and ability to generate pluripotent stem cells from patients with inherited disease, the field witnessed a rapid expansion in the number of labs interested in retinal differentiation. Stem cell culture and differentiation protocols quickly evolved and novel cell culture strategies, designed to produce mature tissue types, were developed. In this sub-section, we will discuss the development of various retinal differentiation strategies and their progression from two-to three-dimensional culture systems.
4.2.1. Early in vitro retinal models
As indicated above, the signals that would enable controllable in vitro differentiation to retinal lineages were already under investigation in 2006 when reprogramming to an induced pluripotent state was discovered by Takahashi and Yamanaka. Much of this work was conducted using human embryonic stem cells, originally isolated and cultured by Thomson and colleagues (Thomson et al., 1998). In 2006, Lamba et al., demonstrated that pluripotent stem cells could be pushed toward a retinal cell fate using embryoid-body based differentiation and supplementation with the developmental factors Noggin, DKK1, and IGF-1 (Lamba et al., 2006). When co-cultured with retinal tissue, enhanced expression of photoreceptor markers was achieved. Together, these experiments showed that exogenous factors, supplied either in media as recombinant proteins or by neighboring cells in co-culture, could lead pluripotent human cells down the neural retina differentiation pathway. Two years later, Osakada and coworkers used a systematic approach to screen various factors such as DAPT, aFGF, bFGF, Taurine, Shh, and retinoic acid to determine which combination of differentiation components would produce photoreceptor precursors from ESCs derived from mouse, monkey and human embryos (Osakada et al., 2008). This study revealed that retinal cell type sub-specification could be fine-tuned.
4.2.2. Early iPSC models of retina
In 2009, a series of papers published by groups in Kyoto, Japan and Wisconsin, USA described methods for retinal differentiation of iPSCs that are still widely used today. Meyer and colleagues (Meyer et al., 2009) adapted earlier strategies (Gamm et al., 2008; Pankratz et al., 2007) to generate retinal pigment epithelium (RPE) and neural retinal progenitor cells that passed through the states of normal development. This strategy is advantageous not only for the production of bona fide transplantable material, but also for modeling of genetic and congenital disease. Following the protocols of Osakada and Ikeda (Ikeda et al., 2005; Osakada et al., 2008), Hirami and coworkers utilized embryoid body differentiation with Wnt and Nodal inhibition followed by retinoic acid to produce photoreceptor precursors after 90 days in culture (Hirami et al., 2009). This same group later showed that this process could be completed with small molecules in lieu of recombinant factors, an important step toward clinical application (Osakada et al., 2009).
Also in 2009, Lamba, Reh, and colleagues continued development of their ESC protocol, showing that photoreceptor precursor cells could be successfully transplanted into the retina of a mouse model of retinal degeneration and that the transplant would restore some electro-retinal function (Lamba et al., 2009). The same was shown to be possible using iPSCs (Lamba et al., 2010). Collectively, these studies established pluripotent stem cells as a promising source of authentic retinal tissue for cellular therapy.
4.2.3. 3D retinal differentiation
The next series of seminal studies demonstrated differentiation via optic vesicle formation (Eiraku et al., 2011; Meyer et al., 2011; Nakano et al., 2012). Beyond expression of marker transcripts and proteins, these complex structures demonstrated the capacity of both mouse and human ESCs and human iPSCs to form laminated neural retinal tissue. Increasingly sophisticated protocols relying on a step-wise embryoid body-based approach with growth factor exposure or inhibition at appropriate junctures allowed more specific guidance toward a photoreceptor cell fate (Tucker et al., 2011a; Zhong et al., 2014). Unlike earlier planar culture systems, using this protocol intact three-dimensional retinal tissue could be developed (Tucker et al., 2011a). By maintaining cells in a 3D structure, photoreceptor precursor cells, which developed adjacent to retinal pigmented epithelial cells, extended primitive outer segments (Tucker et al., 2011a). Zhong and colleagues later showed that upon extended culture, 3D retinal organoids could yield more mature photoreceptors as evidenced by outer segment formation and phototransduction protein expression and functionality (Zhong et al., 2014). Subsequent studies went on to show that photoreceptor cell sub-types could be altered by making slight modifications to factors in the differentiation media. Specifically, Zhou et al. demonstrated that COCO and IGF-1 steered photoreceptor differentiation towards S-cones, while thyroid hormone added at the same stage favored more of a mixed population (Zhou et al., 2015).
At this point, the emerging differentiation techniques allowed for application of this technology in a number of ways. Following the optic cup-based differentiation protocol pioneered by Eiraku, Nakano, and Sasai, Gonzalez-Cordero and colleagues showed in 2013 that mouse ES cell-derived photoreceptors could integrate into a recipient mouse retina (Gonzalez-Cordero et al., 2013). As indicated above, our group (Tucker et al., 2011a) has previously expanded on the work of (Lamba et al., 2010) to show that similar integration was possible for mouse iPSC-derived photoreceptors. We also demonstrated electrical conduction indicative of successful graft integration (Tucker et al., 2011a).
Patient-specific iPSC studies also began during this era of PSC-based retina modeling (Phillips et al., 2014; Tucker et al., 2011b, 2013b). The fact that a disease phenotype could be observed in retina that was derived from patient iPSCs proved the usefulness of these cells for detection of novel variants and for therapeutic testing. The current state of iPSC-modeling of retinal disease was recently reviewed by Foltz and Clegg, whose work should be referred to for more detail (Foltz and Clegg, 2019). For the purposes of this review, the important takeaway is that iPSC models show abnormal phenotypes in patient derived samples. This fact is key to the strategy for pathogenic variant detection and confirmation with gene editing that is explored in more depth later in this work.
4.2.4. The current generation of in vitro retina differentiation
Since the introduction of complex, 3D-organoid-based retinal differentiation schemes, the techniques for generation and analysis of iPSC-based retinal cells have evolved. Differentiation schemes have been designed to suit distinct applications. Efficiency and scalability have become a focus for groups aiming to create retinal cells for transplantation (Reichman et al., 2014). In the same vein, clinically relevant cells must be manufactured under the strict quality-control guidelines of Good Manufacturing Practices (GMP) that govern drug creation. Towards this goal, our group previously created a protocol to manufacture patient-specific iPSCs and retinal cell types under GMP (Wiley et al., 2016). As depicted in Fig. 5, iPSC-derived retinal organoids (iPSC-ROs) generated in this manner express a variety of known developmental markers of neural differentiation and photoreceptor specification (Fig. 5A-T). Reichman and colleagues developed a similar protocol, additionally demonstrating how to cryopreserve the produced retinal tissues, an advance that allows such products to be more widely distributed for enhanced clinical accessibility (Reichman et al., 2017).
Fig. 5. Differentiation of retinal cell types from iPSCs using a cGMP compatible 3D retinal organoid protocol.
A-D) Early retinal organoids express expected neural and pro-proliferative markers. E-H) By 6–8 weeks of differentiation, organoids demonstrate multi-layered differentiation and the beginnings of cell type heterogeneity. I-L) Maturing organoids display expression of retinal precursor markers as well as decreased expression of early neural and proliferative markers. M-O) By 11–12 weeks, organoids express the known photoreceptor markers RCVRN and CRX. P-R) Neural rosettes are prominent in later stage organoids. Expression of photoreceptor specific factors are present. S) Healthy control iPSC-ROs display primarily NRL-expressing presumptive rod photoreceptors. T) iPSC-ROs derived from patient cells carrying a mutation in the transcription factor NR2E3 display differentiation biased toward blue cones as evidenced by SW opsin expression. Data in this figure adapted from (Wiley et al., 2016), which is licensed under the Creative Commons Attribution 4.0 International License.
Other contemporary differentiation techniques aim to more faithfully model the structure of the human retina. Long term culture and techniques then encourage proper morphological lamination and outer segment formation will enable in vitro modeling of diseases that disrupt these features (Capowski et al., 2019; Gonzalez-Cordero et al., 2017; Slembrouck-Brec et al., 2019; Wahlin et al., 2017).
4.2.5. The future of in vitro retina modeling
A major goal for the future of in vitro retina modeling using stem cells is closer recapitulation of the heterogenous retina. A downfall of many differentiation techniques across the stem cell field is the artificially pure cultures produced. While protocols that aim to drive differentiation toward a single cell type may be useful from a therapeutic manufacturing or downstream analysis perspective, these models do not truly emulate the complex interplay between many cell types and lineages found in normal retina. Achberger and colleagues recently addressed this problem by introducing vascularization and RPE interaction to contemporary 3D retinal organoid models (Achberger et al., 2019). Their “Retina-on-a-Chip” provides perfusion reminiscent of vascularization, as well as interaction of retinal cells with RPE and extracellular matrix. The better replication of natural retinal dynamics is expected to produce retinal cells that function more like their natural counterparts. For example, Achberger and coworkers showed that in their model, RPE cells carry out their normal function of photoreceptor outer segment phagocytosis, a function that is not possible either in a pure photoreceptor or RPE culture. Similarly, Akhtar and colleagues have recently published a study of iPSC retinal organoid differentiation in an RPE co-culture model (Akhtar et al., 2019).
The techniques used to examine these high-fidelity retinal models have also been refined and improved in recent years. Reichman et al. demonstrated how optical clearing of retinal organoids could be used to visualize the arrangement of cell types without the need for sectioning (Reichman et al., 2017). More recently, Cora and coworkers have developed a protocol to use hydrogel embedding of organoids to optically clear the structures, known as passive clarity technique (PACT). They show that large 3D retinal organoids can be labeled using immunofluorescent antibodies and visualized at extremely high resolution following this process (Cora et al., 2019). Aside from techniques to allow for the staining and visualization of fixed organoids, optical coherence tomography (OCT), a technique commonly used in clinical settings, has been adapted to the study of organoids and refined to allow for high resolution imaging of live organoids in recent years (Browne et al., 2017; Capowski et al., 2019; Scholler et al., 2020).
4.3. Current challenges in stem cell technology for retina modeling
Most protocols exploring retinal differentiation show efficacy in at most a handful of cell lines. To achieve usefulness in both variant discovery and autologous cell replacement therapy, differentiation and iPSC reprogramming techniques will have to be broadly reproducible and amenable to automated processes. Cell therapy and variant discovery applications require a relatively small amount of tissue to be produced from a very high number of individual donors. This is the reverse of typical manufacturing strategies that focus on large scale production of cell products from a single universal donor. While cells from a single individual may be therapeutically useful for a diverse patient population in some situations (Kamao et al., 2014; Papapetrou, 2016), this approach is of little value for genetic discovery studies.
4.3.1. Ethical concerns surrounding pluripotent stem cells and organoids
The ethical considerations surrounding stem cells in biomedical research have gradually evolved as the field has progressed. The initial human pluripotent stem cells were derived from embryos generated by an in vitro fertilization (IVF) clinic (Thomson et al., 1998). The original hESC lines were derived at a time of incredible legal scrutiny of stem cell research, and resulted from a strategic partnership between researchers in the United States and Israel (Itskovitz-Eldor, 2018). Shortly after the isolation of the original hESC lines around the turn of the millennium, the United States government imposed heavy restrictions on the use of embryonic stem cells in federally funded research. These restrictions effectively bottlenecked the field and led to a few hESC lines becoming the gold standard for pluripotency and stem cell research. These hESC lines (H1, H9, and others) remain popular today and have served as the starting material for many recent studies of the transcriptome of retinal organoids (Collin et al., 2019a, 2019b; Daniszewski et al., 2018; Kallman et al., 2020; Kim et al., 2019; Mao et al., 2019; Phillips et al., 2018; Sridhar et al., 2020). By the time the federal ban on funding for the derivation of embryonic stem cell lines was lifted, the field had shifted towards induced pluripotency for two reasons: to gain the advantage of patient specificity; and, to respond to the ethical focus on the source of stem cell lines as opposed to their potency.
The original hESC lines were derived from human embryos that were created to help hopeful couples to become parents. For opponents of hESC research, using these cells for research was tantamount to ending a human life, and was therefore inherently unethical. Before the possibility of induced pluripotency was realized, it seemed logical that most of the ethical weight associated with a cell line would be based on its origin and that some would feel that any cells derived from a human embryo would be unethical because an embryo could theoretically have become a human being. However, the creation of identically pluripotent cells from non-embryonic sources complicated this conversation.
Perhaps due to the long and intense focus on embryonic sources of cell lines, the ethics of induced pluripotent stem cell lines has been comparatively undiscussed. If we consider not only the source of a material, but also what that material has the potential to become, this stance may not be as obvious as has been assumed. That is, does the physical source of a cell line really matter if one can create a complete human embryo from induced pluripotent cells?
Bioethicists and scientists are now creating a framework of thought that is based more on what could be done with a cell line than where the line originated (Munsie et al., 2017; Rivron et al., 2018). This work is largely motivated by the increasingly sophisticated differentiation techniques, particularly in neural lineages and in blastocyst tissue development. To address the ethical considerations looming over the increasingly complex differentiation models, Hyun has proposed incorporating modern engineering ethics into the design of organoid experiments, essentially weighing benefits derived from increasing complexity with risks associated with the creation of human tissue (Hyun, 2017). In this approach, the goals of a research project must be set out from the beginning, and experiments tailored to these goals instead of just setting out to create the highest fidelity synthetic human tissue possible and dealing with the ethical consequences later. It is clear that at the time of this writing the ethics of stem cell research is continuing to evolve. iPSC technology, while revolutionary, did not displace embryonic cells, and as organoid technology continues to develop, one should expect increasingly complex ethical considerations. In the field of retinal development, and neural development more generally, investigators must remain acutely aware of the hazards that come with the high-fidelity in vitro tissue modeling that the stem cell field is energetically trying to create.
4.3.2. Quality control and variability in iPSCs
Human iPSC reprogramming and differentiation systems are notorious for their inefficient yields and unexplained variability. Inefficiency of reprogramming, while an active area of research, is not a large concern for retinal modeling studies. While contemporary reprogramming techniques typically result in only 0.1–1% of input somatic cells being successfully reprogrammed, as long as the primary culture is large enough, one can easily generate a sufficient number of monoclonal iPSC lines for most experiments (Schlaeger, 2018). Low efficiency of differentiation is similarly a minor concern in most applications. Highly scalable methods for rapid iPSC expansion have been developed to the point that iPSC availability should not prevent one from generating a sufficient number of differentiated cells for most applications.
Validation of in vitro differentiation models is necessary before one can use such models for investigation of normal biology. Comparison between in vitro derived tissues such as iPSC-derived retinal organoids (iPSC-ROs) and natural tissues (donor retina) is most often made through unbiased analysis such as RNA sequencing or by targeted analysis of marker genes (Wells and Choi, 2019).
Another consideration in the use of iPSCs for variant detection is the matching between control and patient samples. Even when individuals are of a similar ethnicity and a hypothesis is limited to the coding sequence of a few hundred genes, one will encounter dozens of potentially functional sequence differences between them. The use of genome editing and sophisticated bioinformatic analyses to address this problem will be discussed in more detail below.
4.3.3. Automation in iPSC manufacturing and differentiation
To be a useful tool for variant discovery and validation, iPSC reprogramming and the related differentiation and culture methods will need to be standardized and automated.
In the evolution of organoid-based differentiation strategies, a number of strides have recently been made in batch-to-batch standardization. Instead of using animal-derived media components and substrates such as serum and Matrigel, which have significant batch to batch variability, the use of small molecule synthetic materials dramatically improves reproducibility (Osakada et al., 2009; Wiley et al., 2016). The reduction of xenogeneic material in the production pipeline will also be important as iPSC-derived retinal cell products begin to move into clinical applications.
Aside from reducing batch-to-batch variability, the intrinsic differences in differentiation of iPSC lines must be addressed. This goal is again essential both to variant discovery and clinical cell therapy applications. In recent years, we and others have attempted to identify predictive markers of iPSC clones that are capable of reliable retinal differentiation. Scientists with extensive experience can recognize clones morphologically that are more likely to yield abundant relatively uniform organoids and it will be an important step forward to codify these morphological features sufficiently for image-based informatics to predict iPSC line yield. (Perestrelo et al., 2017; Schaub et al., 2020; Wakui et al., 2017).
Many automated pipelines for iPSC culture and differentiation have been proposed and prototyped (Archibald et al., 2016; Crombie et al., 2017; Kikuchi et al., 2018; Konagaya et al., 2015; Maddah et al., 2014; Paull et al., 2015). The crucial bottlenecks in the automated culture of a high number of cell lines involve quality control and passaging. Judgements of which iPSC colonies possess suitable morphology (i.e., lack of spontaneous differentiation) to warrant passaging are typically made by an experienced technician. In our lab, we have developed an automated iPSC culture system, which is designed around a custom multifunction robot manufactured by Cell X Technologies (Fig. 6A). This system has the ability to 1) image the entire surface of a culture dish allowing for real-time analysis of reprogramming kinetics, 2) pick select areas suitable for passage, 3) weed areas of spontaneous differentiation, and 4) rinse and replace culture media as needed (Fig. 6B and C). Automated cell culture, in addition to constant monitoring of reprogramming progress (Fig. 6D), will allow for the simultaneous production of many patient and control iPSC lines.
Fig. 6. Automation of iPSC reprogramming and routine culture.
A) Cell X robotic cell culture system housed in a cGMP-grade BioSpherix cell culture environment. B) Automated iPSC colony picking using disposable tips from a standard cell culture plate. C) Images of colonies acquired by the automated system prior to picking, selection of undifferentiated regions suitable for passage, and images of area after automated picking. D) Time course imaging of an entire well over the course of iPSC reprogramming allows for tracking of emerging colonies. Images were collected using the Cell X device over 26 days. Each column depicts a single time point captured during the time course and each row depicts a single field. Individual rows represent different magnifications.
When one takes the historical perspective of genetic discovery in retinal disease over the past century, a two-step pattern from invention to usefulness is evident (Fig. 1B). In the case of DNA and genome analysis tools, there was a decade long lag between the invention of DNA sequencing and the rapid discovery of pathogenic variants. The explanation for the eventual widespread use of this technology is the development and automation of Sanger sequencing from 1986 to 1996 (Gocayne et al., 1987; Smith et al., 1986). We anticipate that the automation of iPSC generation, editing, and differentiation will herald a similarly productive era of variant discovery and validation in retinal disease.
4.3.4. Pilot large scale iPSC studies
To date, there have only been a small number of studies that deploy iPSC technology in more than a handful of cell lines. These studies have largely been part of the National Heart, Lung, and Blood Institute’s (NHBLI) Next Generation Genetic Association Studies Consortium, an effort to use large cohorts of patient iPSCs to model heart, liver, lung, blood, and adipose tissue disorders (Panopoulos et al., 2017; Sweet, 2017; Warren et al., 2017). While not directly related to retinal modeling, the so-called NextGen consortium studies indicate that we are now in an era where population-level iPSC studies are possible. Classically, studies using hPSCs have relied on a small number of embryonic lines (such as the popular H1 and H9), a highly reliable “workhorse” iPSC line, and/or a relatively small cohort of patient iPSC lines. While experiments in such studies can be designed in such a way that mechanistic information is gleaned, small scale studies are inherently not well suited for genetic analysis. Advances in reprogramming techniques that increase efficiency and decrease variability have made iPSC technology attainable to a larger number of labs. Such advances have also increased the number of lines reprogrammable by a single staff member. Much research is now focused on similarly streamlining differentiation techniques. As discussed earlier, retinal differentiation is relatively reliable and is attractive for large scale, high throughput genetic studies of iPSCs.
4.4. Genome editing and in vitro phenotyping
While controlled population and family studies are essential first steps in detecting variants associated with disease, synthetic introduction of variants into wildtype cells is a powerful means of providing convincing evidence of a variant’s pathogenicity. Especially for non-coding variants whose function is not immediately evident, the ability to demonstrate that an artificially introduced variant causes sufficient cellular dysfunction to cause disease can be extremely helpful in supporting their pathogenicity. A similar approach with multiple variants will allow modifying genes and oligogenic diseases to be studied in ways that will be quicker, less expensive and more convincing than studying patients and family members alone.
In recent years, there has been an explosion of interest in CRISPR systems for targeted gene editing and modulation of gene expression. The history of gene editing and the applications of CRISPR systems in inherited retinal disease have been reviewed recently by our group (Burnight et al., 2018). The strategies employed for correction of variants for the purpose of translation are the same as those used for isogenic control generation and variant pathogenicity testing. The power of combining CRISPR genome editing with iPSC technology has been reviewed previously (Hockemeyer and Jaenisch, 2016).
Modified enzymes that target and change single DNA bases are another area of intense recent interest. So-called “base editors” use the specific targeting activity of Cas enzymes but instead of introducing double stranded breaks, make specific base modifications (Gaudelli et al., 2017; Komor et al., 2016). These methods provide more specific editing than traditional CRISPR-Cas9 and are sure to enable a new generation of gene editing applications. More recently, prime editing has been developed, which utilizes a single modified enzyme to both induce genomic nicks and generate the template for repair (Anzalone et al., 2019). A technological review of various genome editing strategies was recently published by Anzalone and colleagues (Anzalone et al., 2020) and should be referred to for further details.
Once variants are corrected using genome editing approaches, their pathogenicity can be confirmed using a range of in vitro assays designed to assess specific cellular functions (Fig. 7), a variety of which have been implicated in inherited retinal diseases. Although it is plausible that yet-to-be discovered variants will impact cellular functions other than those discussed here, the approach to in vitro variant testing using functional assays will remain essential too any future variant discovery workflow. To illustrate the usefulness of in vitro functional assays in determining variant pathogenicity, we will briefly discuss previous examples specific to inherited retinal disease.
Fig. 7. Photoreceptor cell functions measurable by in vitro assay known to be impacted in inherited retinal disease.
A) A mature rod photoreceptor is depicted, though cellular functions and related assays are relevant to cone photoreceptors as well. B) Transcription or transcript processing (e.g. cryptic splice variants, variants impacting gene expression regulation). C) Synaptic formation or function. D) Connecting cilia. E) Disc morphogenesis. F) Lysosomal storage. G) Protein folding or proteasomal degradation. H) Translation or ribosomal function. I) ER-stress. J) Mitochondrial function.
Variants in genes such as CEP290, USH2A, MAK, and ABCA4, which impact transcription or transcript processing within photoreceptor cells (Fig. 7B) have been shown to cause inherited retinal disease (Adato et al., 2005; Cremers et al., 1998; den Hollander et al., 2006; Khan et al., 2020b; Tucker et al., 2011b; van Wijk et al., 2004). While such variants can now be inferred from whole transcriptome profiling using bioinformatic techniques described later in this review, conventional PCR based strategies have been used to confirm abnormal transcript production and nonsense mediated decay caused by pathogenic variants.
Synapse formation and function is known to be impacted in retinal disease caused by variants in genes such as FLVCR1 and RS1 (Fig. 7C) (Rajadhyaksha et al., 2010; Sauer et al., 1997). Recently, assays performed on iPSC-based retinal models were shown to expose the abnormal cell-cell interactions caused by RS1 mutations, showing that variants causing such abnormalities may be confirmed in vitro (Huang et al., 2019). A variety of inherited retinal disease genes including IFT88, several of the BBS genes, and CEP290 are known to function at the level of the connecting cilia (Fig. 7D), in some cases perturbing formation and/or trafficking of proteins to the photoreceptor outer segment (Chekuri et al., 2018; den Hollander et al., 2006; Pazour et al., 2002; Scheidecker et al., 2014). Simple immunofluorescence-based assays have been shown to expose abnormal cilia formation caused by such variants (Chekuri et al., 2018).
Disc morphogenesis (Fig. 7E), a key process required for photoreceptor cell outer segment turnover and phototransduction, is known to be impacted by variants in genes such as ROM1, PRPH2, and C8ORF37, among others (Kajiwara et al., 1994; Loewen et al., 2001; Sharif et al., 2018). Mature retinal organoids that recapitulate key aspects of outer segment formation will allow for examination of proper disc formation and identification of variants that impact disc morphogenesis. Likewise, development of mature photoreceptor cells will be useful for analysis of novel variants in genes such as ABCA4 and PDE6β which are required for phototransduction and normal vision. Another cellular function that when disrupted is known to cause inherited retinal degeneration is lysosomal degradation (Fig. 7F), notably impacted in lysosomal storage disorders such as Batten disease, which is caused by variants in the gene CLN3 (Consortium, 1995). In vitro assays measuring arginine transport can be used to measure lysosome functionality and have been shown to be valuable in the study of such diseases (Ramirez-Montealegre and Pearce, 2005).
Defects in protein folding (Fig. 7G) have also been shown to cause retinal disease, as is the case in some Rho mutations (Illing et al., 2002), as reviewed by Tzekov et al. (2011). Measuring the expression levels of binding immunoglobulin protein (BiP) and C/EBP homologous protein (CHOP) has been shown to be an accurate measure of protein misfolding in retinal disease (Tzekov et al., 2011). Upstream of protein folding, defects in ribosome function and proper protein translation (Fig. 7H) are also functions disrupted in retinal degeneration caused by variants in genes such as SNRNP200 and TRNT1 (DeLuca et al., 2016; Zhao et al., 2009). In vitro assays that measure snRNA unwinding and autophagy or oxidative stress have been shown to elucidate the function of such variants (Sharma et al., 2017; Zhao et al., 2009). More generally, ER stress (Fig. 7I) is known to be disrupted by inherited retinal disease-causing variants such as those in USH2A and MYOC, among many others reviewed by Zhang et al. (Tucker et al., 2013b; Zhang et al., 2014). There exist a number of assays to examine ER stress in cultured cells (Oslowski and Urano, 2011) and such assays are known to be informative in the setting of retinal organoid models (Tucker et al., 2013b).
Finally, variants in mitochondrial genes such as MT-TL1 and MT-ND1 are known to cause retinal disease through abnormal mitochondrial function (Fig. 7J) (Kirby et al., 2004; Smith et al., 1999). As shown by Kirby et al., variants that impact the function of the electron transport chain can be tested using assays that measure complex I activity (Kirby et al., 2004). Additionally, there are many other cell culture-based assays developed to interrogate mitochondrial function in the cells of the eye (Jarrett et al., 2013).
Overall, the assays and cellular functions described in this section represent only a portion of those phenotypes known to be present in genetic retinal disease. Selection of functional assay to confirm pathogenicity of any discovered variant must be informed by clinical phenotype and prior knowledge of gene and cell function.
5. Transcriptomics’ place in inherited retinal disease variant discovery
5.1. Previous transcriptome-focused studies for the discovery of Mendelian disease variants
In recent years, the potential for RNA-sequencing-based transcriptome analysis as a diagnostic tool has been raised in various contexts (Byron et al., 2016). In the setting of unsolved Mendelian disease, multiple groups have conducted studies that utilize transcriptomic analysis to identify candidate pathogenic variants in patients for whom exome sequencing has failed to identify a convincing disease-causing genotype (Table 1) (Bronstein et al., 2020; Chandrasekharappa et al., 2013; Cummings et al., 2017; Evrony et al., 2017; Fresard et al., 2019; Gonorazky et al., 2019; Hamanaka et al., 2019; Kernohan et al., 2017; Kremer et al., 2017; Lee et al., 2020). On average, the diagnostic yield of such studies is approximately 29% (Table 1), ranging from 7.5% (Fresard et al., 2019) to 60% (Rentas et al., 2020).
Table 1.
Recent studies using transcriptomic methods to identify pathogenic variants in previously unsolved Mendelian disease.
| Citation | Year | Disease Phenotype | Sample Type | Diagnostic Yield | Variants Detected |
|---|---|---|---|---|---|
| Cummings et al. (2017) | 2017 | Muscular Dystrophy | Muscle Biopsy (63 patients) | 17/50 (34%) | Exon extension (2), Intronic splice gain (8), exonic splice gain (3), exon skipping (2), splice disruption (2) |
| Kremer et al. (2017) | 2017 | Mitochondriopathy | Dermal Fibroblasts (48 patients) | 5/48 (10.4%) | Monoallelic expression (2), aberrant splicing (2), aberrant gene expression (1) |
| Evrony et al. (2017) | 2017 | Microcephaly-Micromelia Syndrome | Whole Blood/Umbilical Cord (11 patients) | n/a | Intron retention (DONSON) |
| Kernohan et al. (2017) | 2017 | Sporadic Atypical Spinal Muscular Atrophy | Whole Blood (1 patient) | n/a | Splicing Outlier (1) |
| Gonorazky et al. (2019) | 2019 | Neuromuscular Disorder | iPSC-MT, Muscle Biopsy, Dermal Fibroblasts (70 samples, 29 families) | 9/25 (36%) | Splice site mutation (ASAH1) |
| Fresard et al. (2019) | 2019 | Various: Neurological, Musculoskeletal, Hematological, Ophthalmological | Whole Blood (143 patients) | 6/80 (7.5%) | Splicing Outlier (2), Under-expression Outlier (4) |
| Hamanaka et al. (2019) | 2019 | Nemaline Myopathy | Muscle Biopsy (6 patients) | 4/10 (40%) | Deep intronic, aberrant splicing (4) |
| Bronstein et al. (2020) | 2020 | Cone Dysfunction Syndrome | iPSC-RO (2 patients, 1 family) | n/a | Cryptic splice donor site (CNGB3) |
| Lee et al. (2020) | 2020 | Various: Neurological, Musculoskeletal, Orthopedic | Whole Blood, Dermal Fibroblasts, Muscle, Bone Marrow (113 patients) | 7/48 (14.6%) | Deep intronic (4), splice region (1), exonic (2) |
| Rentas et al. (2020) | 2020 | Cornelia de Lange Syndrome | B-lymphoblastoid cell line (5 patients) | 3/5 (60%) | Exon skipping (1), cryptic exon (1), exonic (1) |
| Avg. = 28.9% |
When reviewing this set of Mendelian transcriptomic studies, the categories of variants that were identified fall into the same groups discussed in Section 1. Novel variants were found to disrupt splicing, either through introduction or ablation of splice sites, or through retention of intronic regions (Table 1). These data, as well as previous estimates that 15–60% of genetic diseases are caused by disrupted splicing (Hartley et al., 2020), provide a general idea of what there is to find in the transcriptome of patients with inherited retina disease.
Unlike in DNA-based approaches, pathogenic variants that affect transcription will only be discoverable in tissues that at least express the affected gene. Further, alternative splicing is known to have tissue-specific patterns. As such, the ideal sample for such experiments would be tissue that is known to be affected by the disease and therefore most studies to date focus on diseases and patients from whom affected tissue can be easily sampled.
Cummings and colleagues published the first large study of the transcriptome of Mendelian disease in 2017 (Cummings et al., 2017) and sought to identify pathogenic variants in unsolved muscular disorders. They obtained and performed bulk RNA sequencing on muscle biopsy samples from 50 patients and discovered a number of splicing-related variants. More recently, Gonorazky and colleagues performed a similar study, this time including muscle biopsy samples and myocyte samples generated ex vivo from skin fibroblasts via transient expression of a single transcription factor, MyoD (Evrony et al., 2017; Gonorazky et al., 2019). Not only did this study uncover pathogenic variants in 9 of 25 previously unsolved families, but it also showed the suitability of transdifferentiated tissues as a source of patient material for transcriptome-based variant discovery pipelines. To validate the suitability of their induced myocytes, Gonorazky and co-workers compared expression and splice isoform abundance to patient biopsy material as well as to patient and control blood and skin fibroblasts. They found that while induced myocytes did not perfectly recapitulate the expression profile of biopsied muscle, they were much more suitable than other more easily accessible tissues like whole blood and skin.
Other transcriptome-based studies have focused on diseases that manifest in easy-to-access tissues themselves. In 2017, Kremer and colleagues used patient skin fibroblast cell lines to identify 5 causative variants in a cohort of 48 mitochondriopathy patients (Kremer et al., 2017). They reasoned that disrupted mitochondrial processes would be evident in all cell types due to the ubiquitous expression of mitochondrial genes. Similarly, when studying disorders that affect a number of different tissues, Lee, et al., and Evrony et al., both used patient whole blood for RNA sequencing and successfully identified previously undiscovered pathogenic variants (Evrony et al., 2017; Lee et al., 2020).
To date, the most notable venture away from testing easily biopsied tissues has been Bronstein and colleagues’ 2020 study of a single family with inherited cone dysfunction (Bronstein et al., 2020). The authors performed RNA sequencing on both accessible samples of blood and skin, but also on retinal organoids differentiated from iPSCs that were ultimately derived from patient fibroblasts. Vig and colleagues recently demonstrated the use of iPSC-derived retinal organoids, albeit not from an unsolved patient, in validating the pathogenicity of variants that affect retinal-specific transcription of DYNC2H1 (Vig et al., 2020). They demonstrated that organoids expressed a retinal-specific isoform starting at day 120 of differentiation. Khan et al. utilized a patient-derived iPSC line to generate photoreceptor precursor cells following a 30-day differentiation protocol, allowing them to investigate the effect on splicing of an ABCA4 intronic variant (Khan et al., 2020a). These works show that iPSC-derived tissues may be suitable for RNA-based discovery pipelines, a major opportunity for the retina field and others who study difficult-to-biopsy tissues. Additionally, these recent studies indicate that duration and conditions of culture may play an important and gene-specific role in the relevance of the organoid transcriptome to the disease state.
These studies are at the forefront of an emerging variant discovery modality. In our group, pursuing the genetic causes in the remaining unsolved inherited retinal disease patients will rely heavily on patient-specific iPSC-based modeling of the neural retina. While numerous protocols have been developed and characterized, the shift from isolated case studies to a diagnostic pipeline will require a significant increase in throughput, which as described above will likely be achieved by implementation of automated cell culture platforms.
5.2. Emerging bioinformatic techniques for the study of Mendelian disease
Techniques to analyze transcriptomic data with the goal of pathogenic variant discovery will need to address the experimental design limitations that are inherent to the study of rare diseases. While bioinformatic techniques for the analysis of next-generation sequencing has been reviewed previously in the context of retinal disease (Chaitankar et al., 2016), there are some tools specifically created for rare Mendelian disorders.
While sequencing and alignment strategies developed for routine RNAseq analysis will not likely need to be specially adapted for inherited retinal disease, the paucity of biological replicates (often only a single patient biopsy is available for iPSC reprogramming) and the variability between the transcriptome of iPSC-derived retinal cells and genuine human retinal tissue, create unique challenges. Strategies have been developed for identifying three specific aberrations in RNAseq data, including: 1) monoallelic expression; 2) aberrant splice isoform expression; and, 3) aberrant gene-level expression. These strategies are not in any way “tissue-specific” and are readily adaptable to retina.
Recently, Yepez and colleagues developed a bioinformatic package in Python and R to detect all three of the possible transcriptomic aberrations with a single pipeline (Yepez et al., 2020). This package, called “Detection of RNA Outliers Pipeline” or DROP, is composed of specially designed tools for aberrant splicing (Mertes et al., 2019) and gene expression detection (Brechtmann et al., 2018) in rare diseases, known as FRASER and OUTRIDER, respectively. DROP also includes built-in functionality for detection of mono-allelic expression. This tool, and others like it, will enable retinal and stem cell modeling laboratories to generate meaningful variant hypotheses from iPSC models of patient disease.
Another recent approach to differential splicing detection is an R and Python package known as LeafCutter (Li et al., 2018). A version of this package designed specifically for Mendelian disease in which there is only one patient sample and a larger number of controls was recently published by the same group and is known as LeafcutterMD (Jenkinson et al., 2020; Li et al., 2018). In demonstrating the benefit of LeafCutterMD, Jenkinson and colleagues showed that traditional splicing analysis tools are not very robust for the typical samples available in rare disease studies (Jenkinson et al., 2020). While they demonstrate this superiority of LeafCutterMD only against LeafCutter and not other splicing analysis packages such as FRASER, it is reasonable to infer that the older, traditional analysis tools are likely less suitable for inherited retinal disease iPSC-derived retinal organoid studies. The above approaches for splicing analysis were reviewed and benchmarked by Mehmood, and co-workers who nicely summarized the computational approaches, experimental design support, and performance of various tools (Mehmood et al., 2019). As bioinformatic packages such as DROP continue to emerge, a formal benchmarking study focused on rare Mendelian disease would be quite informative and aid the field in standardizing the analysis pipeline. Even with modern bioinformatic tools, the inherent limitations and inferences that drive these studies should be kept in mind, and traditional assays such as PCR for splicing isoform confirmation will remain important.
5.3. iPSCs enable transcriptomic analysis in the retina and other inaccessible tissues
5.3.1. Overview
As discussed in Section 4, techniques for the differentiation of retinal cells from iPSCs have become progressively refined in the past decade. The majority of such differentiation studies measure the success of differentiation by assaying expression of known cell type-specific genes, either at the mRNA (qPCR) or protein (immunohistochemistry) level. Functional assays are also common in these studies, because proper function implies proper gene expression and because clinical translation in the form of cell therapy is a major driving motivation in the organoid field. Common functional assays include electrical signal generation in response to light (ERG), calcium trafficking, and the testing of engraftment following transplantation of cells into an animal model of retinal degeneration (Lamba et al., 2006, 2009; Tucker et al., 2013b). While targeted assays to verify the identity of iPSC-derived cells are traditionally suitable for protocol development and modeling studies, they do not reveal global gene expression or its similarity or lack thereof to human retinal tissue. This global expression profile is of utmost importance when the goal of a study is to discover novel disease-causing variants. However, to date, only a handful of retinal differentiation studies using iPSCs have directly investigated global gene expression in an unbiased manner with RNA sequencing (Barabino et al., 2020; Gao et al., 2020; Kaya et al., 2019; Kim et al., 2019).
To examine the suitability of iPSC-derived retinal tissue and other common patient biopsy tissues for transcriptomic studies of the retina, we utilized published RNA sequencing datasets from whole blood and skin, retina, and iPSC-derived retinal organoids (Eldred et al., 2018; Li et al., 2014; Suntsova et al., 2019; Whitmore et al., 2014). Multidimensional scaling on the basis of global gene expression is shown in Fig. 8A. The similarity in transcriptomic profile between samples is represented as the distance between points. As expected, this plot corroborates that non-retinal somatic tissues, skin and whole blood, cluster at a great distance from the human retinal samples. iPSC-derived retinal organoids exhibit an increasingly similar expression profile to retina throughout a long-term differentiation protocol. Despite the drawbacks of comparing published data that was collected using a variety of different protocols and instruments, this re-analysis suggests that current retinal stem cell models may indeed be suitable for variant discovery using RNA sequencing. When we focus on splicing isoform abundance, as a function of exon inclusion as opposed to transcript-level expression, the same encouraging result emerges. Kim and co-workers show that retinal organoids and donor retina share a common splicing program (Kim et al., 2019). One example of the isoform-level similarity is shown in Fig. 8B. Here, REEP6 expression is shown across investigated samples in a Sashimi plot format. As iPSCs mature toward a photoreceptor cell fate, the sample increasingly displays inclusion of an exon known to be expressed in a photoreceptor-specific manner (Hao et al., 2014). Indeed, the role of aberrant splicing in retinal disease has been highlighted by Weisschuh and colleagues’, underscoring the fact that a good iPSC model should recapitulate not only retinal function but also splicing trends (Weisschuh et al., 2020). As demonstrated in our own group’s previous work, neither skin derived fibroblasts, whole blood nor iPSCs (prior to retinal differentiation) express MAK exon 12, which is a retinal specific isoform we found to be expressed in human iPSC derived photoreceptor precursor cells (Tucker et al., 2011b). In summary, patient specific iPSC-derived models are likely to be a suitable substrate for transcriptome-based approaches to inherited retinal disease variant discovery.
Fig. 8. iPSC-derived retinal organoids resemble human retina more closely than common biopsy tissue samples on a transcriptional level.
A) MDS plot based on transcript-level expression shows clustering of samples by tissue type. Whole blood and skin (Kremer et al., 2017; Suntsova et al., 2019), commonly biopsied patient samples, segregate away from iPSC-derived retinal organoids (Eldred et al., 2018) and human donor eye samples (Li et al., 2014; Whitmore et al., 2014). Over the course of a 250-day differentiation protocol, retinal organoid samples increasingly resemble the transcriptional profile of human retina. Retina and RPE/choroid/sclera samples show distinct transcriptional profiles. B) REEP6 is known to express a photoreceptor-specific isoform (ENST00000395479.9). This isoform consists of an exon that is not expressed in other tissues. The photoreceptor-specific isoform of REEP6 is detectable in macular retina as well as mature iPSC-derived retinal organoids. REEP6 is not well expressed in whole blood or skin.
5.3.2. Single cell analysis
Part of the reason that studying inherited retinal disease is challenging is because the retina has remarkable cellular diversity. If transcripts from a gene harboring a disease-causing variant are found only in a relatively rare retinal cell type, it may be difficult to investigate this gene at the RNA level due to the relatively higher contributions of transcripts from more abundant cell types. Trapnell has discussed this fundamental drawback of bulk analysis in heterogenous tissue known as Simpson’s Paradox (Trapnell, 2015). In bulk transcriptome profiling of a heterogenous tissue, effects such as the differential expression between disease and healthy samples are masked when cells are averaged. The same principle applies to perturbations or disease states that might plausibly affect either expression or cell type number. For example, if a patient has a mutation that results in loss of a certain cell type, genes expressed selectively by that cell type will appear down regulated in bulk analysis. In contrast, single cell sequencing will reveal the complete loss of a cell population.
Recent advances in single-cell RNA sequencing (scRNA-seq) have addressed these limitations and allow for RNA profiling within individual cells, which has many advantages for identifying disease variants. For an illustrative example, consider clarin-1 (CLRN1), a gene mutated in Usher Syndrome Type 3 (Adato et al., 2002). It took almost two decades for CLRN1 expression to be localized to Müller glial cells in the retina (Xu et al., 2020), because of its low relative level of expression. Indeed, CLRN1 was the 9,233rd most expressed retinal gene in an investigation using bulk RNA sequencing (Whitmore et al., 2014). However, in an scRNA-seq experiment conducted on the human retina, CLRN1 is the 115th most enriched gene in Müller glial cells, with 55% of Müller cells expressing this transcript (versus 1% expression in other retinal cell types) (Voigt et al., 2019b). Gene expression is highly correlated to cell function, and as a result, transcriptome analysis of rare diseases will benefit from stratifying the expression analysis across many different types of retinal cells.
Like the benefits of scRNA-seq in human postmortem retina, profiling the transcriptome of cell type subpopulations isolated from iPSC-derived retinal organoids offers many advantages for identifying disease causing variants. iPSC differentiation can recapitulate much of the cellular diversity of the retina, and retinal organoids contain all major classes of retinal neurons (Capowski et al., 2019; Wiley et al., 2016). Indeed, recent investigations have characterized single-cell level transcriptomes of organoid derived rod photoreceptor cells, cone photoreceptor cells, bipolar cells, retinal ganglion cells, amacrine cells, horizontal cells, as well as transitionary retinal progenitor cell populations (Collin et al., 2019a; Cowan et al., 2020; Kim et al., 2019; Lu et al., 2020; Mao et al., 2019; Xie et al., 2020). Retinal organoids express many of the genes previously implicated in inherited retinal diseases (Stone et al., 2017). For example, of all documented genes that cause photoreceptor degeneration (branch I disease genes (Stone et al., 2017), ), 113 genes are detectably expressed in at least one cell population from human postmortem retina (Voigt et al., 2019b) while 107 genes are expressed in retinal organoids (Sridhar et al., 2020) (Fig. 9A). Some (n = 22) of these genes are uniquely expressed in retinal organoids, including several genes that cause Leber Congenital Amaurosis or syndromic forms of retinal degeneration. This suggests that studying expression of these genes, many of which are important in development, is more appropriate in organoid models opposed to postmortem tissue. Thus scRNA-seq experiments with retinal organoids offers an opportunity to conduct transcriptome analysis on many known genes that cause retinal disease, including some genes that are not expressed in adult post-mortem tissues.
Fig. 9. Comparison of human postmortem versus retinal organoid expression of genes involved in inherited photoreceptor disease.
A) Venn diagram illustrating expression of genes that cause inherited retinal degeneration (branch I: photoreceptor disease (Stone et al., 2017)) in human post-mortem retina (Voigt et al., 2019b) and retinal organoids (Sridhar et al., 2020). Twenty-two unique genes to the retinal organoids are mutated in congenital and syndromic forms of retinal degeneration. B) Venn diagram illustrating which of the 107 organoid-expressed genes in (A) are expressed at different timepoints of differentiation. C) For both the human postmortem and retinal organoid study, the cell population with the highest expression of each of the 85 overlapping genes in (A) was identified. Blue dots represent genes that are expressed in the same cell type across both studies while red dots represent genes expressed by different cell types.
Because retinal organoids recapitulate retinal development, they allow one to study gene expression patterns that change dynamically over the course of differentiation. While the majority of known retinal disease-causing genes are expressed by more mature organoids, some disease-causing genes are transiently expressed during retinal maturation (Fig. 9B). A hallmark example is NR2E3, which encodes a retinal nuclear receptor transcription factor that suppresses the development of cone photoreceptors (Bohrer et al., 2019; Cheng et al., 2004). In a recent scRNA-seq investigation of retinal organoid differentiation, NR2E3 was only expressed in progenitor photoreceptor populations of day 90–110 organoids and remained absent from both day 60 and day 125 organoids (Sridhar et al., 2020). Genes such as NR2E3 that regulate differentiation and cell type specification are natural candidates for trajectory-based computational modeling. With this technique, cells can be ordered from immature to mature using individual transcriptomes, allowing expression analysis to be performed throughout development and at key timepoints such as lineage commitment, (Street et al., 2018; Trapnell et al., 2014). Such a trajectory inference experiment has been conducted on patient derived neural retinal leucine zipper (NRL) null organoids (Kallman et al., 2020). NRL activates the NR2E3 transcription factor, suppressing the expression of cone specific genes and promoting rod development (Cheng et al., 2004). Patients with NRL mutations can suffer from enhanced S-cone syndrome, which is characterized by an increased proportion of S-cones and a deficiency of rod photoreceptors, leading to night blindness (Littink et al., 2018). Similar to the human phenotype, retinal organoids lacking functional NRL were dominated by S-cone photoreceptors, while rod development was restored with wild type NRL correction. Cell fate trajectory reconstruction in this analysis facilitated the discovery of MEF2C, a novel transcription factor that may regulate cone versus rod commitment (Kallman et al., 2020).
Detecting aberrant expression patterns is an important outcome in the transcriptome analysis of rare diseases (Brechtmann et al., 2018; Bronstein et al., 2020; Cummings et al., 2017; Gonorazky et al., 2016; Kremer et al., 2017, 2018). We set out to determine if important genes in inherited retinal disease were expressed by the same cell types in human post-mortem eyes (Voigt et al., 2019b) and retinal organoids (Sridhar et al., 2020). For each of the 85 genes shared between these studies (Fig. 9A), we compared the cell population with the highest expression in the post-mortem versus organoid experiments (Fig. 9C). The majority (59%) of these inherited retinal disease genes were expressed in the same population between the two experimental models (Fig. 9C, blue dots). Unsurprisingly, 14 genes (16.5%) were most highly expressed in the multi-potent population of retinal progenitor cells, which are not found in the adult retina. A total of 12 genes (14%) were found to be most highly expressed in the cone photoreceptor subpopulation of retinal organoids while maximally expressed in primary adult rod photoreceptor cells, which is understandable as many inherited retinal disease genes affect both rod and cone photoreceptors. One example of this expression pattern (Fig. 9C, black arrow) is displayed by PROM1, known to be implicated in autosomal dominant Stargardt-like dominant macular dystrophy (Stone et al., 2017). Only 8 (9%) inherited retinal disease genes were misclassified in terms of cell type expression pattern in organoid versus primary tissue in a manner not involving rod/cone co-expression or progenitor expression. An example of such a misclassification is found in the case of CTSD (Fig. 9C, red arrow), which is most highly expressed in Müller glia in organoids, but in retinal ganglion cells in primary tissue. Collectively, these results suggest that retinal organoids largely recapitulate the cell-specific expression patterns of important retinal disease genes and would be amenable to detecting aberrant expression patterns in rare disease.
Aberrant expression analysis may also prove to be useful in the identification of variants causing multigenic disease. In such cases we would expect to discover two genes for which decreased expression of one or the other is not sufficient to cause disease, but decreased expression of both in the same individual does cause disease. This phenomenon is exemplified in the retina by the cooperativity in mutations of both peripherin and ROM1 genes leading to digenic retinitis pigmentosa (Kajiwara et al., 1994). Additional outcomes in transcriptome analysis of rare diseases are aberrant splicing and allele-specific expression (Karczewski and Snyder, 2018; Kremer et al., 2017). Detecting such changes using scRNA-seq can be challenging due to technical limitations of the sequencing technology. Different scRNA-sequencing platforms use different barcoding strategies that tag transcripts originating from the same cell. Many barcoding strategies utilize oligo-dT primers that hybridize to the poly(A) tail of processed mRNA transcripts, resulting in reverse transcription of only about 100 of the most 3′ nucleotides from each transcript (Macosko et al., 2015). While the resulting reads are of sufficient length to map to the reference genome, they are too short for analysis of splicing or allele-specific expression in most genes. Although lacking full gene coverage, such 3’ barcoding strategies are generally very high throughput and can generate expression profiles from thousands to tens of thousands of cells simultaneously. In contrast, barcoding strategies such as Smart-seq2 (Picelli et al., 2014) and Smart-seq3 (Hagemann-Jensen et al., 2020) perform “tagmentation” of barcoded reads, which allows for full length transcriptome coverage and subsequent analysis of splicing and allele specific expression. Allele specific expression analysis has been demonstrated in mouse embryos, human fibroblasts, and mouse ESCs (Borel et al., 2015; Chen et al., 2016; Deng et al., 2014). However, the additional sequence level information comes at a cost of throughput, as such experiments are conducted in individual wells of plates, generally capturing only hundreds or thousands of cells.
So far, these single cell approaches have not been used for variant discovery in Mendelian disease. Over the past few years, our group and others have begun assembling collections of single cell transcriptome data from human retina samples (Lukowski et al., 2019; Menon et al., 2019; Orozco et al., 2020; Sridhar et al., 2020; Voigt et al., 2019a, 2019b, 2020). Such studies are a necessary first step to understand where in the retina certain genes are expressed. When coupled with a clinical diagnosis that can often suggest specific cell types selectively affected by a disease, this cell-specific expression information will aid in narrowing the list of plausible candidate pathogenic variants observed in exome sequencing data from an individual patient.
6. Future directions and conclusions
6.1. Validating iPSC-models for transcriptomic studies
The first hurdle in transcriptome-based variant discovery in the retina will be validation of iPSC-derived retinal organoids. Beyond the protein-level analysis carried out in previous organoid studies, such validation will have to include isoform abundance and allele-specific expression. Importantly, the length of organoid culture required to reach a maturity relevant to disease is expected to vary from gene to gene (Khan et al., 2020a; Vig et al., 2020). This variability in experimental procedure will lead to highly variable costs in terms of personnel time and reagents for such strategies. For instance, evaluating the pathogenicity of a novel variant in the transcription factor VSX2, which is required for early retinal specification, will likely require much less differentiation time and associated reagents than a variant in ROM1, the expression of which will likely require long term culture to allow for development of mature rod photoreceptor cell outer segment like structures.
Once iPSC-based models are validated and deemed legitimate surrogates for the retinal transcriptome, we can expect to discover aberrant transcript events as discussed earlier. Coupled with genome sequencing, non-coding SNPs can be prioritized due to their physical proximity to the exons involved in such events.
6.2. What can we expect to find?
Mutations that lie outside of the protein-encoding exons are the most likely variants to be discovered by transcriptional studies. This trend has been seen in transcriptional studies in other rare genetic diseases (Table 1), and some examples of non-exomic variants in inherited retinal disease are already well-described (i.e., CEP290 (den Hollander et al., 2006), USH2A (van Wijk et al., 2004), ABCA4 (Albert et al., 2018), PRDM13 (Small et al., 2016)). In order to cause disease, non-exomic variants must in some way impact either the expression of RNA in magnitude or timing or the construction of the transcript itself through improper splicing. As discussed throughout this review, all of these downstream effects are detectable through RNA sequencing, as has been demonstrated in other studies of rare Mendelian disease. In some cases, the relationship between non-coding variants and the genes whose transcription they disrupt will be obvious. An example of such a case is an intronic variant in an alternatively spliced gene. Future work in these methods will focus on establishing causality between disrupted transcription and variants that are quite distant from an exon (Small et al., 2016).
6.3. Conclusion
There is no single approach that will easily identify the disease-causing genotypes in all of the families with inherited retinal disease whose mutations have not yet been found. However, many emerging technologies are now positioned to find a significant fraction of them. The fundamental shift from analysis of the genome to that of the transcriptome offers a new angle of discovery that has already gained traction in the field of rare unsolved genetic disease. The insights offered by a transcriptomic approach are highlighted in Fig. 10A. On the left and right of Fig. 10A in blue, the current information known about patients with unsolved retinal disease is shown. This information generally consists of clinical exam findings (right) and whole genome sequencing results (left). In the middle of Fig. 10A in red, the potential additional findings from a transcriptomic approach to the same patient are shown. Such insights may be used, in conjunction with current approaches, to narrow the list of candidate variants to a number amenable to empirical testing by gene editing strategies (Fig. 10B).
Fig. 10. Schematic of inherited retinal disease variant discovery pipeline utilizing transcriptomic analysis.
A) Currently, molecular diagnosis of inherited retinal disease consists of physical examination by an ophthalmologist and a targeted approach to molecular diagnosis. In patients with unsolved disease, whole genome sequencing may be employed to identify all potentially disease-causing variants present. These produce a list of pathogenic candidates (orange list) as well as a clinical phenotype and often an inferred affected cell type. iPSC-derived retinal organoids provide access to patient retinal cell types that could reveal defective expression/splicing regulation, affected cell type-specific gene expression, and defective functional pathways (red box). Together, these assays enable culling of the list of variants to a number of candidates that is amenable to CRISPR/Cas9-based screening of pathogenicity. B) Variant repair followed by transcriptomic and functional assays allows for variant pathogenicity confirmation in a single patient sample.
New bioinformatic techniques exist to help make sense of all of these new data. To gain access to the retinal transcriptome, iPSC technology will need to be employed for diagnostic and not just therapeutic purposes. Variant discovery will benefit from the tremendous work in retinal differentiation techniques developed in the past decade. Finally, phenotypic profiling coupled with gene editing in vitro will be required to functionally validate a new set of candidate variants.
There are two main lessons to be gleaned from the previous generation of inherited retinal disease variant discovery that may be applied in guiding future work. First, we can observe that technological breakthroughs must be coupled with automation and optimization in order to be useful for large scale discovery. In the case of DNA sequencing, it was not until sequencing technology was widely available and automated that it became a useful modality for discovery. iPSC generation and differentiation must be streamlined and automated to use these cells as viable patient sample surrogates. Second, molecular discovery assays must not occur in isolation from clinical observation. A great deal of information can be collected from a comprehensive retinal exam and patient interview. Even basic background information on patient phenotype has allowed genetic testing to be performed in a statistically powerful, targeted manner (Stone et al., 2017). This information will similarly be crucial to honing the list of candidate variants identified with emerging technologies.
Acknowledgements
We would like to thank the members of The University of Iowa Institute for Vision Research for their discussion and support. We would also like to thank the patients and families whose participation in research studies serves as the impetus for this work.
Funding
This work was supported by the National Institutes of Health (T32GM007337, T32GM008629, P30-EY025580, RO1-EY026008).
Abbreviations:
- CRISPR
clustered regularly interspersed palindromic repeats
- ESC
embryonic stem cell
- hESC
human embryonic stem cell
- iPSC-RO
iPSC-derived retinal organoid
- iPSC
induced pluripotent stem cell
- mESC
mouse embryonic stem cell
- PCR
polymerase chain reaction
- PSC
pluripotent stem cell
- RNA-seq
RNA sequencing
- SCNT
somatic cell nuclear transfer
- scRNA-seq
single cell RNA sequencing
- WES
whole exome sequencing
- WGS
whole genome sequencing
Footnotes
The authors declare no relevant conflicts of interest.
Author statement
Nathaniel K. Mullin: Conceptualization, Writing - Original Draft, Data analysis, Visualization. Andrew P. Voigt: Writing - Original Draft, Data analysis, Visualization. Jessica A. Cooke: Writing - Review & Editing. Laura R. Bohrer: Writing - Review & Editing. Erin R. Burnight: Writing - Review & Editing. Edwin M. Stone: Writing - Review & Editing, Supervision. Robert F. Mullins: Writing - Review & Editing, Supervision. Budd A. Tucker: Writing - Review & Editing, Supervision, Conceptualization.
References
- Achberger K, Probst C, Haderspeck J, Bolz S, Rogal J, Chuchuy J, Nikolova M, Cora V, Antkowiak L, Haq W, Shen N, Schenke-Layland K, Ueffing M, Liebau S, Loskill P, 2019. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adato A, Lefevre G, Delprat B, Michel V, Michalski N, Chardenoux S, Weil D, El-Amraoui A, Petit C, 2005. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum. Mol. Genet. 14, 3921–3932. [DOI] [PubMed] [Google Scholar]
- Adato A, Vreugde S, Joensuu T, Avidan N, Hamalainen R, Belenkiy O, Olender T, Bonne-Tamir B, Ben-Asher E, Espinos C, Millan JM, Lehesjoki AE, Flannery JG, Avraham KB, Pietrokovski S, Sankila EM, Beckmann JS, Lancet D, 2002. USH3A transcripts encode clarin-1, a four-transmembrane-domain protein with a possible role in sensory synapses. Eur. J. Hum. Genet. 10, 339–350. [DOI] [PubMed] [Google Scholar]
- Akhtar T, Xie H, Khan MI, Zhao H, Bao J, Zhang M, Xue T, 2019. Accelerated photoreceptor differentiation of hiPSC-derived retinal organoids by contact co- culture with retinal pigment epithelium. Stem Cell Res. 39, 101491. [DOI] [PubMed] [Google Scholar]
- Albert S, Garanto A, Sangermano R, Khan M, Bax NM, Hoyng CB, Zernant J, Lee W, Allikmets R, Collin RWJ, Cremers FPM, 2018. Identification and rescue of splice defects caused by two neighboring deep-intronic ABCA4 mutations underlying Stargardt disease. Am. J. Hum. Genet. 102, 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone AV, Koblan LW, Liu DR, 2020. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844. [DOI] [PubMed] [Google Scholar]
- Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, Chen PJ, Wilson C, Newby GA, Raguram A, Liu DR, 2019. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki H, Miura F, Watanabe A, Morinaga C, Kitaoka F, Kitano Y, Sakai N, Shibata Y, Terada M, Goto S, Yamanaka S, Takahashi M, Ito T, 2019. Base-resolution methylome of retinal pigment epithelial cells used in the first trial of human induced pluripotent stem cell-based autologous transplantation. Stem Cell Reports 13, 761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald PR, Chandra A, Thomas D, Chose O, Massourides E, Laabi Y, Williams DJ, 2016. Comparability of automated human induced pluripotent stem cell culture: a pilot study. Bioproc. Biosyst. Eng. 39, 1847–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban H, Nishishita N, Fusaki N, Tabata T, Saeki K, Shikamura M, Takada N, Inoue M, Hasegawa M, Kawamata S, Nishikawa S, 2011. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc. Natl. Acad. Sci. U. S. A. 108, 14234–14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabino A, Flamier A, Hanna R, Heon E, Freedman BS, Bernier G, 2020. Deregulation of neuro-developmental genes and primary cilium cytoskeleton anomalies in iPSC retinal sheets from human syndromic ciliopathies. Stem Cell Reports 14, 357–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM, Daley GQ, 2019. Stem cells in the treatment of disease. N. Engl. J. Med. 380, 1748–1760. [DOI] [PubMed] [Google Scholar]
- Bohrer LR, Wiley LA, Burnight ER, Cooke JA, Giacalone JC, Anfinson KR, Andorf JL, Mullins RF, Stone EM, Tucker BA, 2019. Correction of NR2E3 associated enhanced S-cone syndrome patient-specific iPSCs using CRISPR-cas9. Genes 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel C, Ferreira PG, Santoni F, Delaneau O, Fort A, Popadin KY, Garieri M, Falconnet E, Ribaux P, Guipponi M, Padioleau I, Carninci P, Dermitzakis ET, Antonarakis SE, 2015. Biased allelic expression in human primary fibroblast single cells. Am. J. Hum. Genet. 96, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechtmann F, Mertes C, Matuseviciute A, Yepez VA, Avsec Z, Herzog M, Bader DM, Prokisch H, Gagneur J, 2018. OUTRIDER: a statistical method for detecting aberrantly expressed genes in RNA sequencing data. Am. J. Hum. Genet. 103, 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadgate S, Yu J, Downes SM, Halford S, 2017. Unravelling the genetics of inherited retinal dystrophies: past, present and future. Prog. Retin. Eye Res. 59, 53–96. [DOI] [PubMed] [Google Scholar]
- Bronstein R, Capowski EE, Mehrotra S, Jansen AD, Navarro-Gomez D, Maher M, Place E, Sangermano R, Bujakowska KM, Gamm DM, Pierce EA, 2020. A combined RNA-seq and whole genome sequencing approach for identification of non-coding pathogenic variants in single families. Hum. Mol. Genet. 29, 967–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne AW, Arnesano C, Harutyunyan N, Khuu T, Martinez JC, Pollack HA, Koos DS, Lee TC, Fraser SE, Moats RA, Aparicio JG, Cobrinik D, 2017. Structural and functional characterization of human stem-cell-derived retinal organoids by live imaging. Invest. Ophthalmol. Vis. Sci. 58, 3311–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnight ER, Giacalone JC, Cooke JA, Thompson JR, Bohrer LR, Chirco KR, Drack AV, Fingert JH, Worthington KS, Wiley LA, Mullins RF, Stone EM, Tucker BA, 2018. CRISPR-Cas9 genome engineering: treating inherited retinal degeneration. Prog. Retin. Eye Res. 65, 28–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnight ER, Wiley LA, Drack AV, Braun TA, Anfinson KR, Kaalberg EE, Halder JA, Affatigato LM, Mullins RF, Stone EM, Tucker BA, 2014. CEP290 gene transfer rescues Leber congenital amaurosis cellular phenotype. Gene Ther. 21, 662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byron SA, Van Keuren-Jensen KR, Engelthaler DM, Carpten JD, Craig DW, 2016. Translating RNA sequencing into clinical diagnostics: opportunities and challenges. Nat. Rev. Genet. 17, 257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capowski EE, Samimi K, Mayerl SJ, Phillips MJ, Pinilla I, Howden SE, Saha J, Jansen AD, Edwards KL, Jager LD, Barlow K, Valiauga R, Erlichman Z, Hagstrom A, Sinha D, Sluch VM, Chamling X, Zack DJ, Skala MC, Gamm DM, 2019. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan M, Duignan E, Malone CP, Stephenson K, Saad T, McDermott C, Green A, Keegan D, Humphries P, Kenna PF, Farrar GJ, 2016. Panel-based population next-generation sequencing for inherited retinal degenerations. Sci. Rep. 6, 33248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carss KJ, Arno G, Erwood M, Stephens J, Sanchis-Juan A, Hull S, Megy K, Grozeva D, Dewhurst E, Malka S, Plagnol V, Penkett C, Stirrups K, Rizzo R, Wright G, Josifova D, Bitner-Glindzicz M, Scott RH, Clement E, Allen L, Armstrong R, Brady AF, Carmichael J, Chitre M, Henderson RHH, Hurst J, MacLaren RE, Murphy E, Paterson J, Rosser E, Thompson DA, Wakeling E, Ouwehand WH, Michaelides M, Moore AT, Consortium NI-BRD, Webster AR, Raymond FL, 2017. Comprehensive rare variant analysis via whole-genome sequencing to determine the molecular pathology of inherited retinal disease. Am. J. Hum. Genet. 100, 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaitankar V, Karakulah G, Ratnapriya R, Giuste FO, Brooks MJ, Swaroop A, 2016. Next generation sequencing technology and genomewide data analysis: perspectives for retinal research. Prog. Retin. Eye Res. 55, 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharappa SC, Lach FP, Kimble DC, Kamat A, Teer JK, Donovan FX, Flynn E, Sen SK, Thongthip S, Sanborn E, Smogorzewska A, Auerbach AD, Ostrander EA, Program NCS, 2013. Massively parallel sequencing, aCGH, and RNA-Seq technologies provide a comprehensive molecular diagnosis of Fanconi anemia. Blood 121, e138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekuri A, Guru AA, Biswas P, Branham K, Borooah S, Soto-Hermida A, Hicks M, Khan NW, Matsui H, Alapati A, Raghavendra PB, Roosing S, Sarangapani S, Mathavan S, Telenti A, Heckenlively JR, Riazuddin SA, Frazer KA, Sieving PA, Ayyagari R, 2018. IFT88 mutations identified in individuals with non-syndromic recessive retinal degeneration result in abnormal ciliogenesis. Hum. Genet. 137, 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Schell JP, Benitez JA, Petropoulos S, Yilmaz M, Reinius B, Alekseenko Z, Shi L, Hedlund E, Lanner F, Sandberg R, Deng Q, 2016. Single-cell analyses of X Chromosome inactivation dynamics and pluripotency during differentiation. Genome Res. 26, 1342–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Khanna H, Oh EC, Hicks D, Mitton KP, Swaroop A, 2004. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum. Mol. Genet. 13, 1563–1575. [DOI] [PubMed] [Google Scholar]
- Choi J, Lee S, Mallard W, Clement K, Tagliazucchi GM, Lim H, Choi IY, Ferrari F, Tsankov AM, Pop R, Lee G, Rinn JL, Meissner A, Park PJ, Hochedlinger K, 2015. A comparison of genetically matched cell lines reveals the equivalence of human iPSCs and ESCs. Nat. Biotechnol. 33, 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin J, Queen R, Zerti D, Dorgau B, Hussain R, Coxhead J, Cockell S, Lako M, 2019a. Deconstructing retinal organoids: single cell RNA-seq reveals the cellular components of human pluripotent stem cell-derived retina. Stem Cell. 37, 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin J, Zerti D, Queen R, Santos-Ferreira T, Bauer R, Coxhead J, Hussain R, Steel D, Mellough C, Ader M, Sernagor E, Armstrong L, Lako M, 2019b. CRX expression in pluripotent stem cell-derived photoreceptors marks a transplantable subpopulation of early cones. Stem Cell. 37, 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium TIBD, 1995. Isolation of a novel gene underlying batten disease, CLN3. The international batten disease consortium. Cell 82, 949–957. [DOI] [PubMed] [Google Scholar]
- Cora V, Haderspeck J, Antkowiak L, Mattheus U, Neckel PH, Mack AF, Bolz S, Ueffing M, Pashkovskaia N, Achberger K, Liebau S, 2019. A cleared view on retinal organoids. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CS, Renner M, De Gennaro M, Gross-Scherf B, Goldblum D, Hou Y, Munz M, Rodrigues TM, Krol J, Szikra T, Cuttat R, Waldt A, Papasaikas P, Diggelmann R, Patino-Alvarez CP, Galliker P, Spirig SE, Pavlinic D, Gerber-Hollbach N, Schuierer S, Srdanovic A, Balogh M, Panero R, Kusnyerik A, Szabo A, Stadler MB, Orgul S, Picelli S, Hasler PW, Hierlemann A, Scholl HPN, Roma G, Nigsch F, Roska B, 2020. Cell types of the human retina and its organoids at single-cell resolution. Cell 182, 1623–1640 e1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers FP, van de Pol DJ, van Driel M, den Hollander AI, van Haren FJ, Knoers NV, Tijmes N, Bergen AA, Rohrschneider K, Blankenagel A, Pinckers AJ, Deutman AF, Hoyng CB, 1998. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt’s disease gene ABCR. Hum. Mol. Genet. 7, 355–362. [DOI] [PubMed] [Google Scholar]
- Crombie DE, Daniszewski M, Liang HH, Kulkarni T, Li F, Lidgerwood GE, Conquest A, Hernandez D, Hung SS, Gill KP, De Smit E, Kearns LS, Clarke L, Sluch VM, Chamling X, Zack DJ, Wong RCB, Hewitt AW, Pebay A, 2017. Development of a modular automated system for maintenance and differentiation of adherent human pluripotent stem cells. SLAS Discov 22, 1016–1025. [DOI] [PubMed] [Google Scholar]
- Cummings BB, Marshall JL, Tukiainen T, Lek M, Donkervoort S, Foley AR, Bolduc V, Waddell LB, Sandaradura SA, O’Grady GL, Estrella E, Reddy HM, Zhao F, Weisburd B, Karczewski KJ, O’Donnell-Luria AH, Birnbaum D, Sarkozy A, Hu Y, Gonorazky H, Claeys K, Joshi H, Bournazos A, Oates EC, Ghaoui R, Davis MR, Laing NG, Topf A, Genotype-Tissue Expression C, Kang PB, Beggs AH, North KN, Straub V, Dowling JJ, Muntoni F, Clarke NF, Cooper ST, Bonnemann CG, MacArthur DG, 2017. Improving genetic diagnosis in Mendelian disease with transcriptome sequencing. Sci. Transl. Med. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniszewski M, Senabouth A, Nguyen QH, Crombie DE, Lukowski SW, Kulkarni T, Sluch VM, Jabbari JS, Chamling X, Zack DJ, Pebay A, Powell JE, Hewitt AW, 2018. Single cell RNA sequencing of stem cell-derived retinal ganglion cells. Sci. Data 5, 180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca AP, Whitmore SS, Barnes J, Sharma TP, Westfall TA, Scott CA, Weed MC, Wiley JS, Wiley LA, Johnston RM, Schnieders MJ, Lentz SR, Tucker BA, Mullins RF, Scheetz TE, Stone EM, Slusarski DC, 2016. Hypomorphic mutations in TRNT1 cause retinitis pigmentosa with erythrocytic microcytosis. Hum. Mol. Genet. 25, 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander AI, Koenekoop RK, Yzer S, Lopez I, Arends ML, Voesenek KE, Zonneveld MN, Strom TM, Meitinger T, Brunner HG, Hoyng CB, van den Born LI, Rohrschneider K, Cremers FP, 2006. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am. J. Hum. Genet. 79, 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Ramskold D, Reinius B, Sandberg R, 2014. Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science 343, 193–196. [DOI] [PubMed] [Google Scholar]
- Doss MX, Sachinidis A, 2019. Current challenges of iPSC-based disease modeling and therapeutic implications. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y, 2011. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56. [DOI] [PubMed] [Google Scholar]
- Eldred KC, Hadyniak SE, Hussey KA, Brenerman B, Zhang PW, Chamling X, Sluch VM, Welsbie DS, Hattar S, Taylor J, Wahlin K, Zack DJ, Johnston RJ Jr., 2018. Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingford JM, Barton S, Bhaskar S, O’Sullivan J, Williams SG, Lamb JA, Panda B, Sergouniotis PI, Gillespie RL, Daiger SP, Hall G, Gale T, Lloyd IC, Bishop PN, Ramsden SC, Black GCM, 2016. Molecular findings from 537 individuals with inherited retinal disease. J. Med. Genet. 53, 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH, 1981. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156. [DOI] [PubMed] [Google Scholar]
- Evrony GD, Cordero DR, Shen J, Partlow JN, Yu TW, Rodin RE, Hill RS, Coulter ME, Lam AN, Jayaraman D, Gerrelli D, Diaz DG, Santos C, Morrison V, Galli A, Tschulena U, Wiemann S, Martel MJ, Spooner B, Ryu SC, Elhosary PC, Richardson JM, Tierney D, Robinson CA, Chibbar R, Diudea D, Folkerth R, Wiebe S, Barkovich AJ, Mochida GH, Irvine J, Lemire EG, Blakley P, Walsh CA, 2017. Integrated genome and transcriptome sequencing identifies a noncoding mutation in the genome replication factor DONSON as the cause of microcephaly-micromelia syndrome. Genome Res. 27, 1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EL, Jobling AI, Vessey KA, Luu C, Guymer RH, Baird PN, 2011. Animal models of retinal disease. Prog. Mol. Biol. Transl. Sci. 100, 211–286. [DOI] [PubMed] [Google Scholar]
- Foltz LP, Clegg DO, 2019. Patient-derived induced pluripotent stem cells for modelling genetic retinal dystrophies. Prog. Retin. Eye Res. 68, 54–66. [DOI] [PubMed] [Google Scholar]
- Fresard L, Smail C, Ferraro NM, Teran NA, Li X, Smith KS, Bonner D, Kernohan KD, Marwaha S, Zappala Z, Balliu B, Davis JR, Liu B, Prybol CJ, Kohler JN, Zastrow DB, Reuter CM, Fisk DG, Grove ME, Davidson JM, Hartley T, Joshi R, Strober BJ, Utiramerur S, Undiagnosed Diseases N, Care4Rare Canada C, Lind L, Ingelsson E, Battle A, Bejerano G, Bernstein JA, Ashley EA, Boycott KM, Merker JD, Wheeler MT, Montgomery SB, 2019. Identification of rare-disease genes using blood transcriptome sequencing and large control cohorts. Nat. Med. 25, 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi G, Ben M’Barek K, Goureau O, 2019. Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: a pluripotent stem cell-based approach. Prog. Retin. Eye Res. 71, 1–25. [DOI] [PubMed] [Google Scholar]
- Gamm DM, Wright LS, Capowski EE, Shearer RL, Meyer JS, Kim HJ, Schneider BL, Melvan JN, Svendsen CN, 2008. Regulation of prenatal human retinal neurosphere growth and cell fate potential by retinal pigment epithelium and Mash1. Stem Cell. 26, 3182–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ML, Lei XL, Han F, He KW, Jin SQ, Zhang YY, Jin ZB, 2020. Patient-specific retinal organoids recapitulate disease features of late-onset retinitis pigmentosa. Front. Cell. Dev. Biol. 8, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, Liu DR, 2017. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature 551, 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacalone JC, Wiley LA, Burnight ER, Songstad AE, Mullins RF, Stone EM, Tucker BA, 2016. Concise review: patient-specific stem cells to interrogate inherited eye disease. Stem Cells Transl. Med. 5, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocayne J, Robinson DA, FitzGerald MG, Chung FZ, Kerlavage AR, Lentes KU, Lai J, Wang CD, Fraser CM, Venter JC, 1987. Primary structure of rat cardiac beta-adrenergic and muscarinic cholinergic receptors obtained by automated DNA sequence analysis: further evidence for a multigene family. Proc. Natl. Acad. Sci. U. S. A. 84, 8296–8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonorazky H, Liang M, Cummings B, Lek M, Micallef J, Hawkins C, Basran R, Cohn R, Wilson MD, MacArthur D, Marshall CR, Ray PN, Dowling JJ, 2016. RNAseq analysis for the diagnosis of muscular dystrophy. Ann. Clin. Transl. Neurol. 3, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonorazky HD, Naumenko S, Ramani AK, Nelakuditi V, Mashouri P, Wang P, Kao D, Ohri K, Viththiyapaskaran S, Tarnopolsky MA, Mathews KD, Moore SA, Osorio AN, Villanova D, Kemaladewi DU, Cohn RD, Brudno M, Dowling JJ, 2019. Expanding the boundaries of RNA sequencing as a diagnostic tool for rare mendelian disease. Am. J. Hum. Genet. 104, 466–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cordero A, Kruczek K, Naeem A, Fernando M, Kloc M, Ribeiro J, Goh D, Duran Y, Blackford SJI, Abelleira-Hervas L, Sampson RD, Shum IO, Branch MJ, Gardner PJ, Sowden JC, Bainbridge JWB, Smith AJ, West EL, Pearson RA, Ali RR, 2017. Recapitulation of human retinal development from human pluripotent stem cells generates transplantable populations of cone photoreceptors. Stem Cell Reports 9, 820–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cordero A, West EL, Pearson RA, Duran Y, Carvalho LS, Chu CJ, Naeem A, Blackford SJI, Georgiadis A, Lakowski J, Hubank M, Smith AJ, Bainbridge JWB, Sowden JC, Ali RR, 2013. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat. Biotechnol. 31, 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Sallah SR, Ellingford JM, Lovell SC, Sergouniotis PI, 2020. Variability in gene expression is associated with incomplete penetrance in inherited eye disorders. Genes 11. GTEx Consortium, 2017. Genetic effects on gene expression across human tissues. Nature 550, 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB, Elsdale TR, Fischberg M, 1958. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature 182, 64–65. [DOI] [PubMed] [Google Scholar]
- Hagemann-Jensen M, Ziegenhain C, Chen P, Ramsköld D, Hendriks G-J, Larsson AJM, Faridani OR, Sandberg R, 2020. Single-cell RNA counting at allele and isoform resolution using Smart-seq3. Nat. Biotechnol. 38, 708–714. [DOI] [PubMed] [Google Scholar]
- Hamanaka K, Miyatake S, Koshimizu E, Tsurusaki Y, Mitsuhashi S, Iwama K, Alkanaq AN, Fujita A, Imagawa E, Uchiyama Y, Tawara N, Ando Y, Misumi Y, Okubo M, Nakashima M, Mizuguchi T, Takata A, Miyake N, Saitsu H, Iida A, Nishino I, Matsumoto N, 2019. RNA sequencing solved the most common but unrecognized NEB pathogenic variant in Japanese nemaline myopathy. Genet. Med. 21, 1629–1638. [DOI] [PubMed] [Google Scholar]
- Hao H, Veleri S, Sun B, Kim DS, Keeley PW, Kim JW, Yang HJ, Yadav SP, Manjunath SH, Sood R, Liu P, Reese BE, Swaroop A, 2014. Regulation of a novel isoform of Receptor Expression Enhancing Protein REEP6 in rod photoreceptors by bZIP transcription factor NRL. Hum. Mol. Genet. 23, 4260–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Lemire G, Kernohan KD, Howley HE, Adams DR, Boycott KM, 2020. New diagnostic approaches for undiagnosed rare genetic diseases. Annu. Rev. Genom. Hum. Genet. 21. [DOI] [PubMed] [Google Scholar]
- Hekselman I, Yeger-Lotem E, 2020. Mechanisms of tissue and cell-type specificity in heritable traits and diseases. Nat. Rev. Genet. 21, 137–150. [DOI] [PubMed] [Google Scholar]
- Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, Yoshimura N, Takahashi M, 2009. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci. Lett. 458, 126–131. [DOI] [PubMed] [Google Scholar]
- Hockemeyer D, Jaenisch R, 2016. Induced pluripotent stem cells meet genome editing. Cell Stem Cell 18, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KC, Wang ML, Chen SJ, Kuo JC, Wang WJ, Nhi Nguyen PN, Wahlin KJ, Lu JF, Tran AA, Shi M, Chien Y, Yarmishyn AA, Tsai PH, Yang TC, Jane WN, Chang CC, Peng CH, Schlaeger TM, Chiou SH, 2019. Morphological and molecular defects in human three-dimensional retinal organoid model of X-linked juvenile retinoschisis. Stem Cell Rep. 13, 906–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun I, 2017. Engineering ethics and self-organizing models of human development: opportunities and challenges. Cell Stem Cell 21, 718–720. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Osakada F, Watanabe K, Mizuseki K, Haraguchi T, Miyoshi H, Kamiya D, Honda Y, Sasai N, Yoshimura N, Takahashi M, Sasai Y, 2005. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 102, 11331–11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illing ME, Rajan RS, Bence NF, Kopito RR, 2002. A rhodopsin mutant linked to autosomal dominant retinitis pigmentosa is prone to aggregate and interacts with the ubiquitin proteasome system. J. Biol. Chem. 277, 34150–34160. [DOI] [PubMed] [Google Scholar]
- Itskovitz-Eldor J, 2018. 20th anniversary of isolation of human embryonic stem cells: a personal perspective. Stem Cell Reports 10, 1439–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett SG, Rohrer B, Perron NR, Beeson C, Boulton ME, 2013. Assessment of mitochondrial damage in retinal cells and tissues using quantitative polymerase chain reaction for mitochondrial DNA damage and extracellular flux assay for mitochondrial respiration activity. In: Weber BHF, Langmann T (Eds.), Retinal Degeneration: Methods and Protocols. Humana Press, Totowa, NJ, pp. 227–243. [DOI] [PubMed] [Google Scholar]
- Jayakody SA, Gonzalez-Cordero A, Ali RR, Pearson RA, 2015. Cellular strategies for retinal repair by photoreceptor replacement. Prog. Retin. Eye Res. 46, 31–66. [DOI] [PubMed] [Google Scholar]
- Jenkinson G, Li YI, Basu S, Cousin MA, Oliver GR, Klee EW, 2020. LeafCutterMD: an algorithm for outlier splicing detection in rare diseases. Bioinformatics. 10.1093/bioinformatics/btaa259. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin ZB, Gao ML, Deng WL, Wu KC, Sugita S, Mandai M, Takahashi M, 2019. Stemming retinal regeneration with pluripotent stem cells. Prog. Retin. Eye Res. 69, 38–56. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Berson EL, Dryja TP, 1994. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science 264, 1604–1608. [DOI] [PubMed] [Google Scholar]
- Kallman A, Capowski EE, Wang J, Kaushik AM, Jansen AD, Edwards KL, Chen L, Berlinicke CA, Joseph Phillips M, Pierce EA, Qian J, Wang TH, Gamm DM, Zack DJ, 2020. Investigating cone photoreceptor development using patient-derived NRL null retinal organoids. Commun. Biol. 3, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, Kiryu J, Takahashi M, 2014. Characterization of human induced pluripotent stem cell- derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports 2, 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP, Gauthier LD, Brand H, Solomonson M, Watts NA, Rhodes D, Singer-Berk M, England EM, Seaby EG, Kosmicki JA, Walters RK, Tashman K, Farjoun Y, Banks E, Poterba T, Wang A, Seed C, Whiffin N, Chong JX, Samocha KE, Pierce-Hoffman E, Zappala Z, O’Donnell-Luria AH, Minikel EV, Weisburd B, Lek M, Ware JS, Vittal C, Armean IM, Bergelson L, Cibulskis K, Connolly KM, Covarrubias M, Donnelly S, Ferriera S, Gabriel S, Gentry J, Gupta N, Jeandet T, Kaplan D, Llanwarne C, Munshi R, Novod S, Petrillo N, Roazen D, Ruano-Rubio V, Saltzman A, Schleicher M, Soto J, Tibbetts K, Tolonen C, Wade G, Talkowski ME, Neale BM, Daly MJ, MacArthur DG, 2020. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski KJ, Snyder MP, 2018. Integrative omics for health and disease. Nat. Rev. Genet. 19, 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski KJ, Weisburd B, Thomas B, Solomonson M, Ruderfer DM, Kavanagh D, Hamamsy T, Lek M, Samocha KE, Cummings BB, Birnbaum D, The Exome Aggregation C, Daly MJ, MacArthur DG, 2017. The ExAC browser: displaying reference data information from over 60 000 exomes. Nucleic Acids Res. 45, D840–D845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya KD, Chen HY, Brooks MJ, Kelley RA, Shimada H, Nagashima K, de Val N, Drinnan CT, Gieser L, Kruczek K, Erceg S, Li T, Lukovic D, Adlakha YK, Welby E, Swaroop A, 2019. Transcriptome-based molecular staging of human stem cell-derived retinal organoids uncovers accelerated photoreceptor differentiation by 9-cis retinal. Mol. Vis. 25, 663–678. [PMC free article] [PubMed] [Google Scholar]
- Kernohan KD, Fresard L, Zappala Z, Hartley T, Smith KS, Wagner J, Xu H, McBride A, Bourque PR, Consortium CRC, Bennett SAL, Dyment DA, Boycott KM, Montgomery SB, Warman Chardon J, 2017. Whole-transcriptome sequencing in blood provides a diagnosis of spinal muscular atrophy with progressive myoclonic epilepsy. Hum. Mutat. 38, 611–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Arno G, Fakin A, Parfitt DA, Dhooge PPA, Albert S, Bax NM, Duijkers L, Niblock M, Hau KL, Bloch E, Schiff ER, Piccolo D, Hogden MC, Hoyng CB, Webster AR, Cremers FPM, Cheetham ME, Garanto A, Collin RWJ, 2020a. Detailed phenotyping and therapeutic strategies for intronic ABCA4 variants in Stargardt disease. Mol. Ther. Nucleic Acids 21, 412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Cornelis SS, Pozo-Valero MD, Whelan L, Runhart EH, Mishra K, Bults F, AlSwaiti Y, AlTalbishi A, De Baere E, Banfi S, Banin E, Bauwens M, Ben-Yosef T, Boon CJF, van den Born LI, Defoort S, Devos A, Dockery A, Dudakova L, Fakin A, Farrar GJ, Sallum JMF, Fujinami K, Gilissen C, Glavac D, Gorin MB, Greenberg J, Hayashi T, Hettinga YM, Hoischen A, Hoyng CB, Hufendiek K, Jagle H, Kamakari S, Karali M, Kellner U, Klaver CCW, Kousal B, Lamey TM, MacDonald IM, Matynia A, McLaren TL, Mena MD, Meunier I, Miller R, Newman H, Ntozini B, Oldak M, Pieterse M, Podhajcer OL, Puech B, Ramesar R, Ruther K, Salameh M, Salles MV, Sharon D, Simonelli F, Spital G, Steehouwer M, Szaflik JP, Thompson JA, Thuillier C, Tracewska AM, van Zweeden M, Vincent AL, Zanlonghi X, Liskova P, Stohr H, Roach JN, Ayuso C, Roberts L, Weber BHF, Dhaenens CM, Cremers FPM, 2020b. Resolving the dark matter of ABCA4 for 1054 Stargardt disease probands through integrated genomics and transcriptomics. Genet. Med. 22, 1235–1246. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Kino-Oka M, Wada M, Kobayashi T, Kato M, Takeda S, Kubo H, Ogawa T, Sunayama H, Tanimoto K, Mizutani M, Shimizu T, Okano T, 2018. A novel, flexible and automated manufacturing facility for cell-based health care products: tissue Factory. Regen. Ther. 9, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lowe A, Dharmat R, Lee S, Owen LA, Wang J, Shakoor A, Li Y, Morgan DJ, Hejazi AA, Cvekl A, DeAngelis MM, Zhou ZJ, Chen R, Liu W, 2019. Generation, transcriptome profiling, and functional validation of cone-rich human retinal organoids. Proc. Natl. Acad. Sci. U. S. A. 116, 10824–10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby DM, McFarland R, Ohtake A, Dunning C, Ryan MT, Wilson C, Ketteridge D, Turnbull DM, Thorburn DR, Taylor RW, 2004. Mutations of the mitochondrial ND1 gene as a cause of MELAS. J. Med. Genet. 41, 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore S, De Franco E, Cardenas-Diaz FL, Letourneau-Freiberg LR, Sanyoura M, Osorio-Quintero C, French DL, Greeley SAW, Hattersley AT, Gadue P, 2020. A non-coding disease modifier of pancreatic agenesis identified by genetic correction in a patient-derived iPSC line. Cell Stem Cell 27, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR, 2016. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konagaya S, Ando T, Yamauchi T, Suemori H, Iwata H, 2015. Long-term maintenance of human induced pluripotent stem cells by automated cell culture system. Sci. Rep. 5, 16647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic C, Arsenijevic Y, 2016. Animal modelling for inherited central vision loss. J. Pathol. 238, 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer LS, Bader DM, Mertes C, Kopajtich R, Pichler G, Iuso A, Haack TB, Graf E, Schwarzmayr T, Terrile C, Konarikova E, Repp B, Kastenmuller G, Adamski J, Lichtner P, Leonhardt C, Funalot B, Donati A, Tiranti V, Lombes A, Jardel C, Glaser D, Taylor RW, Ghezzi D, Mayr JA, Rotig A, Freisinger P, Distelmaier F, Strom TM, Meitinger T, Gagneur J, Prokisch H, 2017. Genetic diagnosis of Mendelian disorders via RNA sequencing. Nat. Commun. 8, 15824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer LS, Wortmann SB, Prokisch H, 2018. Transcriptomics”: molecular diagnosis of inborn errors of metabolism via RNA-sequencing. J. Inherit. Metab. Dis. 41, 525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, Gust J, Reh TA, 2009. Transplantation of human embryonic stem cell- derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell 4, 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Ware CB, Reh TA, 2006. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 103, 12769–12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA, 2010. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PloS One 5, e8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane A, Jovanovic K, Shortall C, Ottaviani D, Panes AB, Schwarz N, Guarascio R, Hayes MJ, Palfi A, Chadderton N, Farrar GJ, Hardcastle AJ, Cheetham ME, 2020. Modeling and rescue of RP2 retinitis pigmentosa using iPSC-derived retinal organoids. Stem Cell Reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Huang AY, Wang LK, Yoon AJ, Renteria G, Eskin A, Signer RH, Dorrani N, Nieves-Rodriguez S, Wan J, Douine ED, Woods JD, Dell’Angelica EC, Fogel BL, Martin MG, Butte MJ, Parker NH, Wang RT, Shieh PB, Wong DA, Gallant N, Singh KE, Tavyev Asher YJ, Sinsheimer JS, Krakow D, Loo SK, Allard P, Papp JC, Undiagnosed Diseases N, Palmer CGS, Martinez-Agosto JA, Nelson SF, 2020. Diagnostic utility of transcriptome sequencing for rare Mendelian diseases. Genet. Med. 22, 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Wang JH, Chen J, Li F, Edwards TL, Hewitt AW, Liu GS, 2019. Gene therapy for visual loss: opportunities and concerns. Prog. Retin. Eye Res. 68, 31–53. [DOI] [PubMed] [Google Scholar]
- Li M, Jia C, Kazmierkiewicz KL, Bowman AS, Tian L, Liu Y, Gupta NA, Gudiseva HV, Yee SS, Kim M, Dentchev T, Kimble JA, Parker JS, Messinger JD, Hakonarson H, Curcio CA, Stambolian D, 2014. Comprehensive analysis of gene expression in human retina and supporting tissues. Hum. Mol. Genet. 23, 4001–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YI, Knowles DA, Humphrey J, Barbeira AN, Dickinson SP, Im HK, Pritchard JK, 2018. Annotation-free quantification of RNA splicing using LeafCutter. Nat. Genet. 50, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listernick R, Mancini AJ, Charrow J, 2003. Segmental neurofibromatosis in childhood. Am. J. Med. Genet. 121A, 132–135. [DOI] [PubMed] [Google Scholar]
- Littink KW, Stappers PTY, Riemslag FCC, Talsma HE, van Genderen MM, Cremers FPM, Collin RWJ, van den Born LI, 2018. Autosomal recessive NRL mutations in patients with enhanced S-cone syndrome. Genes 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llavona P, Pinelli M, Mutarelli M, Marwah VS, Schimpf-Linzenbold S, Thaler S, Yoeruek E, Vetter J, Kohl S, Wissinger B, 2017. Allelic expression imbalance in the human retinal transcriptome and potential impact on inherited retinal diseases. Genes 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen CJ, Moritz OL, Molday RS, 2001. Molecular characterization of peripherin-2 and rom-1 mutants responsible for digenic retinitis pigmentosa. J. Biol. Chem. 276, 22388–22396. [DOI] [PubMed] [Google Scholar]
- Lu Y, Shiau F, Yi W, Lu S, Wu Q, Pearson JD, Kallman A, Zhong S, Hoang T, Zuo Z, Zhao F, Zhang M, Tsai N, Zhuo Y, He S, Zhang J, Stein-O’Brien GL, Sherman TD, Duan X, Fertig EJ, Goff LA, Zack DJ, Handa JT, Xue T, Bremner R, Blackshaw S, Wang X, Clark BS, 2020. Single-cell analysis of human retina identifies evolutionarily conserved and species-specific mechanisms controlling development. Dev. Cell 53, 473–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowski SW, Lo CY, Sharov AA, Nguyen Q, Fang L, Hung SS, Zhu L, Zhang T, Grunert U, Nguyen T, Senabouth A, Jabbari JS, Welby E, Sowden JC, Waugh HS, Mackey A, Pollock G, Lamb TD, Wang PY, Hewitt AW, Gillies MC, Powell JE, Wong RC, 2019. A single-cell transcriptome atlas of the adult human retina. EMBO J. 38, e100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, Trombetta JJ, Weitz DA, Sanes JR, Shalek AK, Regev A, McCarroll SA, 2015. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddah M, Shoukat-Mumtaz U, Nassirpour S, Loewke K, 2014. A system for automated, noninvasive, morphology-based evaluation of induced pluripotent stem cell cultures. J. Lab. Autom. 19, 454–460. [DOI] [PubMed] [Google Scholar]
- Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, Terada M, Nomiya Y, Tanishima S, Nakamura M, Kamao H, Sugita S, Onishi A, Ito T, Fujita K, Kawamata S, Go MJ, Shinohara C, Hata KI, Sawada M, Yamamoto M, Ohta S, Ohara Y, Yoshida K, Kuwahara J, Kitano Y, Amano N, Umekage M, Kitaoka F, Tanaka A, Okada C, Takasu N, Ogawa S, Yamanaka S, Takahashi M, 2017. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 376, 1038–1046. [DOI] [PubMed] [Google Scholar]
- Mao X, An Q, Xi H, Yang XJ, Zhang X, Yuan S, Wang J, Hu Y, Liu Q, Fan G, 2019. Single-cell RNA sequencing of hESC-derived 3D retinal organoids reveals novel genes regulating RPC commitment in early human retinogenesis. Stem Cell Reports 13, 747–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto E, Koide N, Hanzawa H, Kiyama M, Ohta M, Kuwabara J, Takeda S, Takahashi M, 2019. Fabricating retinal pigment epithelial cell sheets derived from human induced pluripotent stem cells in an automated closed culture system for regenerative medicine. PloS One 14, e0212369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmood A, Laiho A, Venalainen MS, McGlinchey AJ, Wang N, Elo LL, 2019. Systematic evaluation of differential splicing tools for RNA-seq studies. Brief Bioinform. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon M, Mohammadi S, Davila-Velderrain J, Goods BA, Cadwell TD, Xing Y, Stemmer-Rachamimov A, Shalek AK, Love JC, Kellis M, Hafler BP, 2019. Single-cell transcriptomic atlas of the human retina identifies cell types associated with age-related macular degeneration. Nat. Commun. 10, 4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertes C, Scheller I, Yépez VA, Çelik MH, Liang Y, Kremer LS, Gusic M, Prokisch H, Gagneur J, 2019. Detection of Aberrant Splicing Events in RNA-Seq Data with FRASER. biorXiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, Pinilla I, Martin JM, Tian S, Stewart R, Pattnaik B, Thomson JA, Gamm DM, 2011. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cell. 29, 1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, Zhang SC, Gamm DM, 2009. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc. Natl. Acad. Sci. U. S. A. 106, 16698–16703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Kuehn MH, Radu RA, Enriquez GS, East JS, Schindler EI, Travis GH, Stone EM, 2012. Autosomal recessive retinitis pigmentosa due to ABCA4 mutations: clinical, pathologic, and molecular characterization. Invest. Ophthalmol. Vis. Sci. 53, 1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsie M, Hyun I, Sugarman J, 2017. Ethical issues in human organoid and gastruloid research. Development 144, 942–945. [DOI] [PubMed] [Google Scholar]
- Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y, 2012. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785. [DOI] [PubMed] [Google Scholar]
- Okita K, Yamakawa T, Matsumura Y, Sato Y, Amano N, Watanabe A, Goshima N, Yamanaka S, 2013. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cell. 31, 458–466. [DOI] [PubMed] [Google Scholar]
- Omori Y, Chaya T, Katoh K, Kajimura N, Sato S, Muraoka K, Ueno S, Koyasu T, Kondo M, Furukawa T, 2010. Negative regulation of ciliary length by ciliary male germ cell-associated kinase (Mak) is required for retinal photoreceptor survival. Proc. Natl. Acad. Sci. U. S. A. 107, 22671–22676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco LD, Chen HH, Cox C, Katschke KJ Jr., Arceo R, Espiritu C, Caplazi P, Nghiem SS, Chen YJ, Modrusan Z, Dressen A, Goldstein LD, Clarke C, Bhangale T, Yaspan B, Jeanne M, Townsend MJ, van Lookeren Campagne M, Hackney JA, 2020. Integration of eQTL and a single-cell atlas in the human eye identifies causal genes for age-related macular degeneration. Cell Rep. 30, 1246–1259 e1246. [DOI] [PubMed] [Google Scholar]
- Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M, 2008. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat. Biotechnol. 26, 215–224. [DOI] [PubMed] [Google Scholar]
- Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, Sasai Y, Takahashi M, 2009. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J. Cell Sci. 122, 3169–3179. [DOI] [PubMed] [Google Scholar]
- Oslowski CM, Urano F, 2011. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 490, 71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratz MT, Li XJ, Lavaute TM, Lyons EA, Chen X, Zhang SC, 2007. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cell. 25, 1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panopoulos AD, Smith EN, Arias AD, Shepard PJ, Hishida Y, Modesto V, Diffenderfer KE, Conner C, Biggs W, Sandoval E, D’Antonio-Chronowska A, Berggren WT, Izpisua Belmonte JC, Frazer KA, 2017. Aberrant DNA methylation in human iPSCs associates with MYC-binding motifs in a clone-specific manner independent of genetics. Cell Stem Cell 20, 505–517 e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetrou EP, 2016. Induced pluripotent stem cells, past and future. Science 353, 991–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ, 2008. Disease-specific induced pluripotent stem cells. Cell 134, 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull D, Sevilla A, Zhou H, Hahn AK, Kim H, Napolitano C, Tsankov A, Shang L, Krumholz K, Jagadeesan P, Woodard CM, Sun B, Vilboux T, Zimmer M, Forero E, Moroziewicz DN, Martinez H, Malicdan MC, Weiss KA, Vensand LB, Dusenberry CR, Polus H, Sy KT, Kahler DJ, Gahl WA, Solomon SL, Chang S, Meissner A, Eggan K, Noggle SA, 2015. Automated, high-throughput derivation, characterization and differentiation of induced pluripotent stem cells. Nat. Methods 12, 885–892. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC, 2002. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J. Cell Biol. 157, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perestrelo T, Chen W, Correia M, Le C, Pereira S, Rodrigues AS, Sousa MI, Ramalho-Santos J, Wirtz D, 2017. Pluri-IQ: quantification of embryonic stem cell pluripotency through an image-based analysis software. Stem Cell Reports 9, 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MJ, Jiang P, Howden S, Barney P, Min J, York NW, Chu LF, Capowski EE, Cash A, Jain S, Barlow K, Tabassum T, Stewart R, Pattnaik BR, Thomson JA, Gamm DM, 2018. A novel approach to single cell RNA-sequence analysis facilitates in silico gene reporting of human pluripotent stem cell-derived retinal cell types. Stem Cell. 36, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MJ, Perez ET, Martin JM, Reshel ST, Wallace KA, Capowski EE, Singh R, Wright LS, Clark EM, Barney PM, Stewart R, Dickerson SJ, Miller MJ, Percin EF, Thomson JA, Gamm DM, 2014. Modeling human retinal development with patient-specific induced pluripotent stem cells reveals multiple roles for visual system homeobox 2. Stem Cell. 32, 1480–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picelli S, Faridani OR, Bjorklund AK, Winberg G, Sagasser S, Sandberg R, 2014. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha AM, Elemento O, Puffenberger EG, Schierberl KC, Xiang JZ, Putorti ML, Berciano J, Poulin C, Brais B, Michaelides M, Weleber RG, Higgins JJ, 2010. Mutations in FLVCR1 cause posterior column ataxia and retinitis pigmentosa. Am. J. Hum. Genet. 87, 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Montealegre D, Pearce DA, 2005. Defective lysosomal arginine transport in juvenile Batten disease. Hum. Mol. Genet. 14, 3759–3773. [DOI] [PubMed] [Google Scholar]
- Reichman S, Slembrouck A, Gagliardi G, Chaffiol A, Terray A, Nanteau C, Potey A, Belle M, Rabesandratana O, Duebel J, Orieux G, Nandrot EF, Sahel JA, Goureau O, 2017. Generation of storable retinal organoids and retinal pigmented epithelium from adherent human iPS cells in xeno-free and feeder-free conditions. Stem Cell. 35, 1176–1188. [DOI] [PubMed] [Google Scholar]
- Reichman S, Terray A, Slembrouck A, Nanteau C, Orieux G, Habeler W, Nandrot EF, Sahel JA, Monville C, Goureau O, 2014. From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc. Natl. Acad. Sci. U. S. A. 111, 8518–8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentas S, Rathi KS, Kaur M, Raman P, Krantz ID, Sarmady M, Tayoun AA, 2020. Diagnosing Cornelia de Lange syndrome and related neurodevelopmental disorders using RNA sequencing. Genet. Med. 22, 927–936. [DOI] [PubMed] [Google Scholar]
- Rivron N, Pera M, Rossant J, Martinez Arias A, Zernicka-Goetz M, Fu J, van den Brink S, Bredenoord A, Dondorp W, de Wert G, Hyun I, Munsie M, Isasi R, 2018. Debate ethics of embryo models from stem cells. Nature 564, 183–185. [DOI] [PubMed] [Google Scholar]
- Rowe RG, Daley GQ, 2019. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 20, 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer CG, Gehrig A, Warneke-Wittstock R, Marquardt A, Ewing CC, Gibson A, Lorenz B, Jurklies B, Weber BH, 1997. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat. Genet. 17, 164–170. [DOI] [PubMed] [Google Scholar]
- Schaub NJ, Hotaling NA, Manescu P, Padi S, Wan Q, Sharma R, George A, Chalfoun J, Simon M, Ouladi M, Simon CG Jr., Bajcsy P, Bharti K, 2020. Deep learning predicts function of live retinal pigment epithelium from quantitative microscopy. J. Clin. Invest. 130, 1010–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidecker S, Etard C, Pierce NW, Geoffroy V, Schaefer E, Muller J, Chennen K, Flori E, Pelletier V, Poch O, Marion V, Stoetzel C, Strahle U, Nachury MV, Dollfus H, 2014. Exome sequencing of Bardet-Biedl syndrome patient identifies a null mutation in the BBSome subunit BBIP1 (BBS18). J. Med. Genet. 51, 132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaeger TM, 2018. Nonintegrating human somatic cell reprogramming methods. Adv. Biochem. Eng. Biotechnol. 163, 1–21. [DOI] [PubMed] [Google Scholar]
- Schlaeger TM, Daheron L, Brickler TR, Entwisle S, Chan K, Cianci A, DeVine A, Ettenger A, Fitzgerald K, Godfrey M, Gupta D, McPherson J, Malwadkar P, Gupta M, Bell B, Doi A, Jung N, Li X, Lynes MS, Brookes E, Cherry AB, Demirbas D, Tsankov AM, Zon LI, Rubin LL, Feinberg AP, Meissner A, Cowan CA, Daley GQ, 2015. A comparison of non-integrating reprogramming methods. Nat. Biotechnol. 33, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholler J, Groux K, Goureau O, Sahel JA, Fink M, Reichman S, Boccara C, Grieve K, 2020. Dynamic full-field optical coherence tomography: 3D live-imaging of retinal organoids. Light Sci. Appl. 9, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif AS, Yu D, Loertscher S, Austin R, Nguyen K, Mathur PD, Clark AM, Zou J, Lobanova ES, Arshavsky VY, Yang J, 2018. C8ORF37 is required for photoreceptor outer segment disc morphogenesis by maintaining outer segment membrane protein homeostasis. J. Neurosci. 38, 3160–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma TP, Wiley LA, Whitmore SS, Anfinson KR, Cranston CM, Oppedal DJ, Daggett HT, Mullins RF, Tucker BA, Stone EM, 2017. Patient-specific induced pluripotent stem cells to evaluate the pathophysiology of TRNT1-associated Retinitis pigmentosa. Stem Cell Res. 21, 58–70. [DOI] [PubMed] [Google Scholar]
- Slembrouck-Brec A, Rodrigues A, Rabesandratana O, Gagliardi G, Nanteau C, Fouquet S, Thuret G, Reichman S, Orieux G, Goureau O, 2019. Reprogramming of adult retinal muller glial cells into human-induced pluripotent stem cells as an efficient source of retinal cells. Stem Cell. Int. 2019, 7858796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small KW, DeLuca AP, Whitmore SS, Rosenberg T, Silva-Garcia R, Udar N, Puech B, Garcia CA, Rice TA, Fishman GA, Heon E, Folk JC, Streb LM, Haas CM, Wiley LA, Scheetz TE, Fingert JH, Mullins RF, Tucker BA, Stone EM, 2016. North Carolina macular dystrophy is caused by dysregulation of the retinal transcription factor PRDM13. Ophthalmology 123, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, Sanders JZ, Kaiser RJ, Hughes P, Dodd C, Connell CR, Heiner C, Kent SB, Hood LE, 1986. Fluorescence detection in automated DNA sequence analysis. Nature 321, 674–679. [DOI] [PubMed] [Google Scholar]
- Smith PR, Bain SC, Good PA, Hattersley AT, Barnett AH, Gibson JM, Dodson PM, 1999. Pigmentary retinal dystrophy and the syndrome of maternally inherited diabetes and deafness caused by the mitochondrial DNA 3243 tRNALeu A to G mutation11The authors have no proprietary interest in the development or marketing of any device or medications mentioned in the article or any competing device. Ophthalmology 106, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Sridhar A, Hoshino A, Finkbeiner CR, Chitsazan A, Dai L, Haugan AK, Eschenbacher KM, Jackson DL, Trapnell C, Bermingham-McDonogh O, Glass I, Reh TA, 2020. Single-cell transcriptomic comparison of human fetal retina, hPSC-derived retinal organoids, and long-term retinal cultures. Cell Rep. 30, 1644–1659 e1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwyck LK, Place EM, Comander J, Huckfeldt RM, Sobrin L, 2019. Predictive value of genetic testing for inherited retinal diseases in patients with suspected atypical autoimmune retinopathy. Am. J. Ophthalmol. Case Rep. 15, 100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EM, 2003. Finding and interpreting genetic variations that are important to ophthalmologists. Trans. Am. Ophthalmol. Soc. 101, 437–484. [PMC free article] [PubMed] [Google Scholar]
- Stone EM, Aldave AJ, Drack AV, Maccumber MW, Sheffield VC, Traboulsi E, Weleber RG, 2012. Recommendations for genetic testing of inherited eye diseases: report of the American Academy of Ophthalmology task force on genetic testing. Ophthalmology 119, 2408–2410. [DOI] [PubMed] [Google Scholar]
- Stone EM, Andorf JL, Whitmore SS, DeLuca AP, Giacalone JC, Streb LM, Braun TA, Mullins RF, Scheetz TE, Sheffield VC, Tucker BA, 2017. Clinically focused molecular investigation of 1000 consecutive families with inherited retinal disease. Ophthalmology 124, 1314–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street K, Risso D, Fletcher RB, Das D, Ngai J, Yosef N, Purdom E, Dudoit S, 2018. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genom. 19, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart T, Satija R, 2019. Integrative single-cell analysis. Nat. Rev. Genet. 20, 257–272. [DOI] [PubMed] [Google Scholar]
- Suntsova M, Gaifullin N, Allina D, Reshetun A, Li X, Mendeleeva L, Surin V, Sergeeva A, Spirin P, Prassolov V, Morgan A, Garazha A, Sorokin M, Buzdin A, 2019. Atlas of RNA sequencing profiles for normal human tissues. Sci. Data 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DJ, 2017. iPSCs meet GWAS: the NextGen consortium. Cell Stem Cell 20, 417–418. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S, 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S, 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM, 1998. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- Trapnell C, 2015. Defining cell types and states with single-cell genomics. Genome Res. 25, 1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, Rinn JL, 2014. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Anfinson KR, Mullins RF, Stone EM, Young MJ, 2013a. Use of a synthetic xeno-free culture substrate for induced pluripotent stem cell induction and retinal differentiation. Stem Cells Transl. Med. 2, 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Mullins RF, Stone EM, 2014a. Stem cells for investigation and treatment of inherited retinal disease. Hum. Mol. Genet. 23, R9–R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Mullins RF, Streb LM, Anfinson K, Eyestone ME, Kaalberg E, Riker MJ, Drack AV, Braun TA, Stone EM, 2013b. Patient-specific iPSC- derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. Elife 2, e00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Park IH, Qi SD, Klassen HJ, Jiang C, Yao J, Redenti S, Daley GQ, Young MJ, 2011a. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PloS One 6, e18992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Scheetz TE, Mullins RF, DeLuca AP, Hoffmann JM, Johnston RM, Jacobson SG, Sheffield VC, Stone EM, 2011b. Exome sequencing and analysis of induced pluripotent stem cells identify the cilia-related gene male germ cell- associated kinase (MAK) as a cause of retinitis pigmentosa. Proc. Natl. Acad. Sci. U. S. A. 108, E569–E576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Solivan-Timpe F, Roos BR, Anfinson KR, Robin AL, Wiley LA, Mullins RF, Fingert JH, 2014b. Duplication of TBK1 stimulates autophagy in iPSC-derived retinal cells from a patient with normal tension glaucoma. J. Stem Cell Res. Ther. 3, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzekov R, Stein L, Kaushal S, 2011. Protein misfolding and retinal degeneration. Cold Spring Harb. Perspect Biol. 3, a007492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk E, Pennings RJ, te Brinke H, Claassen A, Yntema HG, Hoefsloot LH, Cremers FP, Cremers CW, Kremer H, 2004. Identification of 51 novel exons of the Usher syndrome type 2A (USH2A) gene that encode multiple conserved functional domains and that are mutated in patients with Usher syndrome type II. Am. J. Hum. Genet. 74, 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veleri S, Lazar CH, Chang B, Sieving PA, Banin E, Swaroop A, 2015. Biology and therapy of inherited retinal degenerative disease: insights from mouse models. Dis. Model. Mech. 8, 109–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig A, Poulter JA, Ottaviani D, Tavares E, Toropova K, Tracewska AM, Mollica A, Kang J, Kehelwathugoda O, Paton T, Maynes JT, Wheway G, Arno G, Genomics England Research C, Khan KN, McKibbin M, Toomes C, Ali M, Di Scipio M, Li S, Ellingford J, Black G, Webster A, Rydzanicz M, Stawinski P, Ploski R, Vincent A, Cheetham ME, Inglehearn CF, Roberts A, Heon E, 2020. DYNC2H1 hypomorphic or retina-predominant variants cause nonsyndromic retinal degeneration. Genet. Med. 10.1038/s41436-020-0915-1. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt AP, Binkley E, Flamme-Wiese MJ, Zeng S, DeLuca AP, Scheetz TE, Tucker BA, Mullins RF, Stone EM, 2020. Single-cell RNA sequencing in human retinal degeneration reveals distinct glial cell populations. Cells 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt AP, Mulfaul K, Mullin NK, Flamme-Wiese MJ, Giacalone JC, Stone EM, Tucker BA, Scheetz TE, Mullins RF, 2019a. Single-cell transcriptomics of the human retinal pigment epithelium and choroid in health and macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 116, 24100–24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt AP, Whitmore SS, Flamme-Wiese MJ, Riker MJ, Wiley LA, Tucker BA, Stone EM, Mullins RF, Scheetz TE, 2019b. Molecular characterization of foveal versus peripheral human retina by single-cell RNA sequencing. Exp. Eye Res. 184, 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlin KJ, Maruotti JA, Sripathi SR, Ball J, Angueyra JM, Kim C, Grebe R, Li W, Jones BW, Zack DJ, 2017. Photoreceptor outer segment-like structures in long-term 3D retinas from human pluripotent stem cells. Sci. Rep. 7, 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakui T, Matsumoto T, Matsubara K, Kawasaki T, Yamaguchi H, Akutsu H, 2017. Method for evaluation of human induced pluripotent stem cell quality using image analysis based on the biological morphology of cells. J. Med. Imaging 4, 044003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M, 2009. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CR, Jaquish CE, Cowan CA, 2017. The NextGen genetic association studies consortium: a foray into in vitro population genetics. Cell Stem Cell 20, 431–433. [DOI] [PubMed] [Google Scholar]
- Weisschuh N, Buena-Atienza E, Wissinger B, 2020. Splicing mutations in inherited retinal diseases. Prog. Retin. Eye Res. 100874. [DOI] [PubMed] [Google Scholar]
- Wells CA, Choi J, 2019. Transcriptional profiling of stem cells: moving from descriptive to predictive paradigms. Stem Cell Reports 13, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore SS, Wagner AH, DeLuca AP, Drack AV, Stone EM, Tucker BA, Zeng S, Braun TA, Mullins RF, Scheetz TE, 2014. Transcriptomic analysis across nasal, temporal, and macular regions of human neural retina and RPE/choroid by RNA-Seq. Exp. Eye Res. 129, 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley LA, Binkley EM, DeLuca AP, Workalemahu G, Tatro NJ, Luse MA, Kennedy EL, Folk JC, Scheetz TE, Ballas ZK, Tucker BA, Mullins RF, Han IC, Stone EM, 2019. Autoimmune retinopathy mimicking heritable retinal degeneration in a patient with common variable immune deficiency. Retin. Cases Brief Rep. 10.1097/ICB.0000000000000941. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley LA, Burnight ER, DeLuca AP, Anfinson KR, Cranston CM, Kaalberg EE, Penticoff JA, Affatigato LM, Mullins RF, Stone EM, Tucker BA, 2016. cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Sci. Rep. 6, 30742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley LA, Burnight ER, Mullins RF, Stone EM, Tucker BA, 2014. Stem cells as tools for studying the genetics of inherited retinal degenerations. Cold Spring Harb. Perspect Med. 5, a017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold B, Myers RM, 2008. Sequence census methods for functional genomics. Nat. Methods 5, 19–21. [DOI] [PubMed] [Google Scholar]
- Xie H, Zhang W, Zhang M, Akhtar T, Li Y, Yi W, Sun X, Zuo Z, Wei M, Fang X, Yao Z, Dong K, Zhong S, Liu Q, Shen Y, Wu Q, Wang X, Zhao H, Bao J, Qu K, Xue T, 2020. Chromatin accessibility analysis reveals regulatory dynamics of developing human retina and hiPSC-derived retinal organoids. Sci. Adv. 6, eaay5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Bolch SN, Santiago CP, Dyka FM, Akil O, Lobanova ES, Wang Y, Martemyanov KA, Hauswirth WW, Smith WC, Handa JT, Blackshaw S, Ash JD, Dinculescu A, 2020. Clarin-1 expression in adult mouse and human retina highlights a role of Muller glia in Usher syndrome. J. Pathol. 250, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepez VA, Mertes C, Muller MF, Andrade DS, Wachutka L, Fresard L, Gusic M, Scheller I, Goldberg PF, Prokisch H, Gagneur J, 2020. Detection of Aberrant Events in RNA Sequencing Data. Github. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Sanders E, Fliesler SJ, Wang JJ, 2014. Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration. Exp. Eye Res. 125, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Bellur DL, Lu S, Zhao F, Grassi MA, Bowne SJ, Sullivan LS, Daiger SP, Chen LJ, Pang CP, Zhao K, Staley JP, Larsson C, 2009. Autosomal- dominant retinitis pigmentosa caused by a mutation in SNRNP200, a gene required for unwinding of U4/U6 snRNAs. Am. J. Hum. Genet. 85, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, Gamm DM, Yau KW, Canto-Soler MV, 2014. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun. 5, 4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Flamier A, Abdouh M, Tetreault N, Barabino A, Wadhwa S, Bernier G, 2015. Differentiation of human embryonic stem cells into cone photoreceptors through simultaneous inhibition of BMP, TGFbeta and Wnt signaling. Development 142, 3294–3306. [DOI] [PubMed] [Google Scholar]