Abstract
The spinal cord contains an extraordinarily diverse population of interconnected neurons to process somatosensory information and execute movement. Studies of the embryonic spinal cord have elucidated basic principles underlying the specification of spinal cord neurons, while adult and postnatal studies have provided insight into cell type function and circuitry. However, the overarching principles that bridge molecularly defined subtypes with their connectivity, physiology, and function remain unclear. This review consolidates recent work in spinal neuron characterization, examining how molecular and spatial features of individual spinal neuron types relate to the reference points of connectivity and function. This review will focus on how spinal neuron subtypes are organized to control movement in the mouse.
Introduction
The spinal cord is a major site of output of the nervous system, where neural activity is converted into precise muscle contractions. It is also a place of integration, containing neural networks capable of processing a variety of sensory and descending inputs. Thus, the spinal cord is both a site of convergence and divergence, where multiple streams of information are synthesized and transformed into patterned activation of motor neurons.
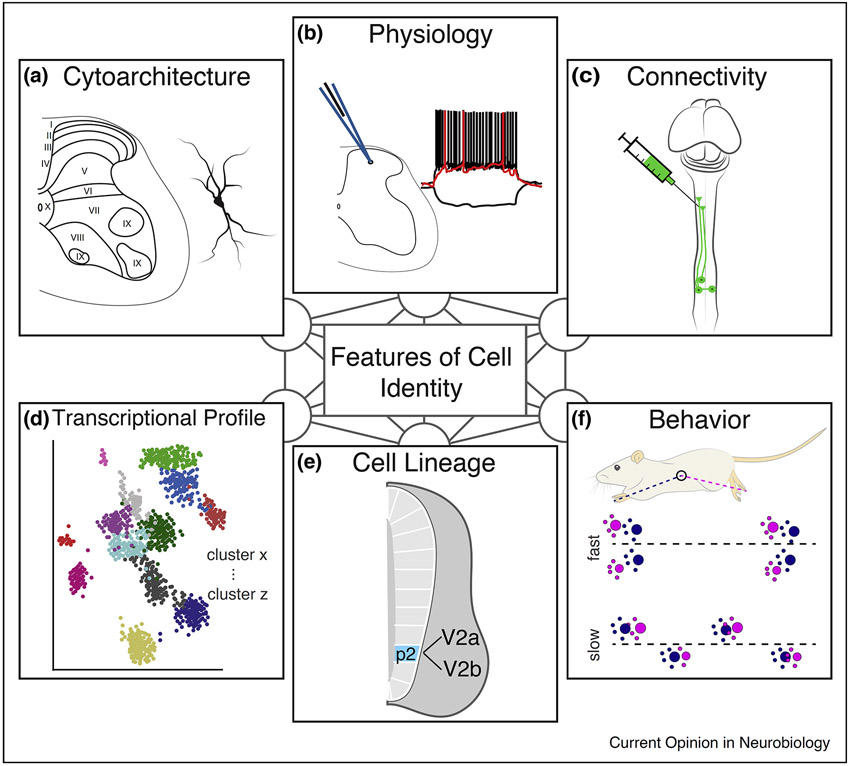
A heterogeneous network of spinal neurons underlies this sophisticated sensorimotor processing. Historically, investigators have identified spinal cord neuron types using one or two features at a time, through the lens of morphology, location, physiology, connectivity, molecular markers, developmental lineage, or behavior (Figure 1). These studies have provided a basic understanding of the major classes of spinal neurons and their most prominent features [1,2]. Experiments perturbing specific sets of interneurons point toward a modular organization of cell types and circuits within the spinal cord, where populations of neurons play somewhat independent roles [2-5].
Figure 1. Features encompassing neuronal identity in the spinal cord. Spinal neurons can be classified based on many individual features, but the relationships between these features are still unclear.
(a) Spinal neurons can be classified based on cell morphology and location. Laminar designation, originally described by Bror Rexed, is a common method to categorize spinal neurons.
(b) Spinal neurons possess a variety of intrinsic electrophysiological properties. Furthermore, cell recordings paired with stimulation (such as dorsal root stimulation) can reveal functional connections between neuronal populations.
(c) Various tracers and viral approaches have been used to examine connectivity between spinal neurons. Anterograde, retrograde, and trans-synaptic tracing have been used to identify neuronal populations with specific projection patterns.
(d) RNA sequencing, both at the population and single cell level, have identified many transcriptionally distinct populations within the spinal cord.
(e) Developmental studies have examined genes involved in the specification of spinal neurons. Overexpression and knockout studies have provided insight into gene function, while lineage tracing has allowed investigators to probe the anatomy and function of developmentally defined cell types.
(f) Spinal neurons can be classified based on their role in behaviors. Early activity gene markers such as c-fos have been used to identify cohorts of neurons active during specific behaviors. Activation, silencing, and ablation studies have allowed spinal neurons to be categorized by the behaviors they mediate.
Despite this division of function among cell types, recent studies using transcriptional profiling have uncovered an impressive array of molecular divisions within individual classes of spinal neurons, suggesting an even finer-grained level of cellular heterogeneity. The significance of many of these molecular subtypes are still obscure. In this review, we will examine how features of neuronal identity link to one another and attempt to understand what these relationships may suggest for spinal cord circuit assembly and function. We present evidence that interneuron molecular subtypes correspond to a complex mixture of neuronal features. Given this abstract relationship between molecular-identity and functional-identity, we suggest that mapping neuron subtypes into a multidimensional space of features will be necessary for uncovering the principles of spinal neuron diversity and motor control.
Spinal regions, their cell types, and segregated functions
The spinal cord can coarsely be divided into three regions: dorsal, intermediate, and ventral (Figure 2a). While these divisions are somewhat arbitrary anatomical, physiological, and functional studies provide evidence for distinct functions between these regions. The dorsal horn mediates exteroceptive sensory processing [4]. The ventral cord is critical for motor execution and the generation of rhythmic motor behaviors [3,5]. The intermediate spinal cord is thought to play a major role in integrating sensory and descending information to fine tune motor output [6]. Within each of these spinal regions, studies have characterized and manipulated molecularly defined cell populations (Figure 2b). The results of these studies point toward distinct molecular populations that make specific contributions to each region’s overall function.
Figure 2. Spinal cord regions and cell types.
(a) Spinal regions and corresponding Rexed laminae.
(b) Examples of molecularly defined cell types located in each region. In some cases, the gene of interest is also expressed in other spinal regions. Example dorsal cell types are RORα, GRP, and NPY expressing neurons [4,9,52-54]. Ventral cell types include En1, Chx10, and Hb9 expressing neurons [2,32••,34••]. Gad2 and Satb2 expressing neurons are intermediate spinal cord cell types [19,22•,26-28].
(c) Exteroceptive sensory fibers terminate in the dorsal cord in a topographic fashion [4,7,8]. Noxious sensory afferents primarily terminate within laminae I–II. Low threshold mechanoreceptors terminate within laminae II–IV of the spinal cord.
(d) Motor neurons are organized into columns and pools which reflect a musculotopic map [10-12]. Lumbar ventral interneurons form a network named the locomotor central pattern generator (CPG), which rhythmically excites motor neurons to generate locomotion [3,13].
(e) Many intermediate interneurons are monosynaptically connected to motor neurons [19,21]. Stimulation of intermediate premotor neurons evokes synergistic activity in multiple motor pools, thus these neurons are designated motor synergy encoders (MSE) [19]. The intermediate spinal cord receives a variety of sensory and descending inputs. Dorsal interneurons relay exteroceptive information onto intermediate spinal interneurons [20]. The corticospinal tract and proprioceptive afferents also densely terminate in the intermediate spinal cord [19,21]. Proprioceptive afferents also innervate motor neurons and subsets of ventral interneurons.
In the dorsal horn, cutaneous sensory neurons terminate in modality-specific patterns (Figure 2c) [4,7,8]. Noxious sensory afferents primarily terminate within laminae I–II, transmitting thermal or painful touch sensations. Innocuous touch is transmitted by a variety of low threshold mechanoreceptors (LTMRs) which terminate within laminae II–IV. These receptors transduce sensations such as skin indentation or hair vibration. Consequently, the ablation of dorsal spinal neuron subtypes results in selective deficits of sensory behaviors [4]. For example, ablation of spinal neurons expressing Retinoid-Related Orphan Receptor Alpha (RORα), which receive input from light touch LTMR subtypes, results in deficiencies in fine motor control and the detection of static and dynamic touch [9].
The ventral spinal cord contains motor neurons and a diverse network of interneurons that regulate motor neuron firing. Motor neuron subtypes are organized into pools that are arrayed in a musculotopic map [10-12]. In the lumbar spinal cord, ventral interneurons assemble into a network named the locomotor central pattern generator (CPG) (Figure 2d) [3,5]. The CPG drives rhythmic motor pool activity, alternating between left and right limbs, as well as between flexor and extensor muscles, producing a crude stepping pattern independent of sensory inputs [13]. The CPG comprises a variety of developmentally distinct classes of neurons identified through lineage tracing studies (Figure 3a-d) [3]. Manipulation of each ventral interneuron class has revealed a highly modular functional organization [3]. For example, V2a interneurons are excitatory, project ipsilaterally, and express the gene Chx10. Ablation of V2a interneurons does not stop or randomize the CPG, instead it disrupts high speed left–right alternation [14,15]. Many of the molecularly defined ventral classes of interneurons in the lumbar spinal cord are also present in other segments, where they support motor behaviors such as breathing and reaching [16-18].
Figure 3. Molecular organization of spinal neurons.
(a) During embryonic development, spinal interneurons are derived from 13 progenitor domains (including the late born dILa and dILb domains). Highlighted are the pMN, p1, and p2 domains. After neurogenesis, cells migrate to their final settling positions [2].
(b) The p1 domain generates V1 interneurons, which express En1. V1 interneurons settle throughout the ventral horn. The majority of V1 interneurons can be divided into 4 clades, expressing either Sp8, Pou6f2, FoxP2, or MafA. V1 clades can be further divided into many subtypes based on combinatorial expression of additional transcription factors. These additional transcriptional divisions often correspond to spatial divisions as well [32••].
(c) The p2 domain generates V2a–V2d interneurons [2,55]. V2a interneurons are divided into a medial and lateral column based on Nfib and Zfhx3 expression. Each column comprises multiple transcriptionally distinct subtypes; however, the spatial locations of these subtypes have yet to be examined in detail [34••].
(d) The pMN domain gives rise to motor neurons, which express Hb9 and settle in the ventral spinal cord. Motor neurons are organized into columns. While all motor neurons initially express Lhx3, only the MMC (Medial motor column) maintains its expression. The LMC (Lateral motor column) expresses FoxP1. Within each column, motor neurons can be further divided into motor pools, each of which is spatially clustered and has a unique muscle target [10-12].
(e–g) Categories of neurons based on their rostrocaudal distribution. Constant: a population of neurons which are present throughout the length of the spinal cord. Limb: neurons which reside in the cervical and lumbar cord. Thoracic: neurons which are located in the thoracic cord. Gradient: neurons that are arrayed in a gradient along the cord.
(e) V1 interneuron clades are preserved across the rostrocaudal axis. However, V1 subtypes specific to limb segments or thoracic segments can be identified based on the expression of two transcription factors [38••].
(f) V2a columns span the rostrocaudal axis. The composition of each column is a combination of Type I V2a interneurons (which maintain Chx10 expression) and Type II V2a interneurons (which downregulate Chx10). The ratio of the two types remains constant in the medial column. In the lateral column, the ratio is organized in a gradient manner which is dependent on spinal segment. Cervical segments are composed of primarily Type II V2a interneurons, while lumbar segments are primarily Type I V2a interneurons [34••].
(g) The MMC, HMC (Hypaxial motor column), and LMC synapse onto axial, intercostal, and limb muscles, respectively. The MMC spans the spinal cord, while the HMC and LMC are segment specific. Motor pools are segmentally organized as well [10-12]. Example LMC motor pools are shown (MMC and HMC pools are not shown). Motor pools generally span a few segments [10,12].
The intermediate spinal cord is a nodal point, receiving convergent input from proprioceptive, exteroceptive, and corticospinal neurons [19-21]. Subsets of intermediate neurons also project onto ventral CPG interneurons and directly onto motor neurons (Figure 2e) [19,22•]. Stimulation of intermediate premotor neurons, termed motor synergy encoders (MSE), evokes activation of multiple motor pools [19]. This may serve to coordinate the use of synergistic limb muscles for complex movements [19,23]. While the ventral spinal cord is pivotal for generating rhythmic motor patterns, the confluence of incoming sensory modalities allows the intermediate spinal cord to fine tune motor actions. Intermediate spinal neurons guide the movements necessary to withdraw the limb from sources of painful stimuli [20]. Recent studies have begun to identify and manipulate molecularly defined neuron populations enriched in the intermediate cord, such as Satb2, Gad2, and RORβ expressing interneurons [22•,24,25•,26-28]. Conditional deletion of Satb2 in the spinal cord results in a hyperflexion response during the limb withdrawal reflex. In general, it has been remarkable that targeted perturbations of spinal interneurons cause discrete functional phenotypes rather than an entire breakdown of motor control. One interpretation is that cell types are organized in modular systems within the spinal cord, rather than having broadly distributed functionality.
Hierarchical divisions of spinal neuron types
Developmental studies have identified 13 neural progenitor populations (i.e. lineages) that produce distinct cardinal classes of spinal neurons (Figure 3a) [2]. Analysis of these lineages has revealed that neurons arising from a common progenitor share particular features, often including neurotransmitter, settling location, and axonal projection pattern. While developmental origin offers a convenient way to organize spinal neurons into coherent groups, these lineage-defined classes are still rather broad cohorts of heterogeneous cells. The neural diversity arising from a single progenitor population is best understood for motor neurons, generated from pMN progenitors (Figure 3a,d) [12]. Motor neurons are organized hierarchically. As a first division, motor neurons are arranged into separate columns which each target a different peripheral structure such as limb muscle, axial muscle, or sympathetic ganglia. Within each column, somatic motor neurons form pools, each with a unique muscle target. Molecular studies have uncovered transcriptional codes that mark each motor column (e.g. LIM-Hd factors) and label individual motor pools (e.g. Hox and ETS factors) [11,12].
Extensive heterogeneity within interneuron classes has also been observed. A variety of molecular markers such as peptides and their receptors, calcium binding proteins, and transcription factors further divide interneuron classes into subtypes. In some cases, these molecular subtypes have been tied to specialized aspects of circuitry and function [1-4]. For example, Renshaw cells are a type of inhibitory neuron which receive input from motor neurons and in turn project on to motor neurons (Figure 4a) [29]. This circuit configuration allows Renshaw cells to provide feedback inhibition to motor neurons. Renshaw cells express Calbindin and are a subtype of V1 interneurons, a large ventral inhibitory class which express the gene En1 (Figure 3b) [30,31,32••].
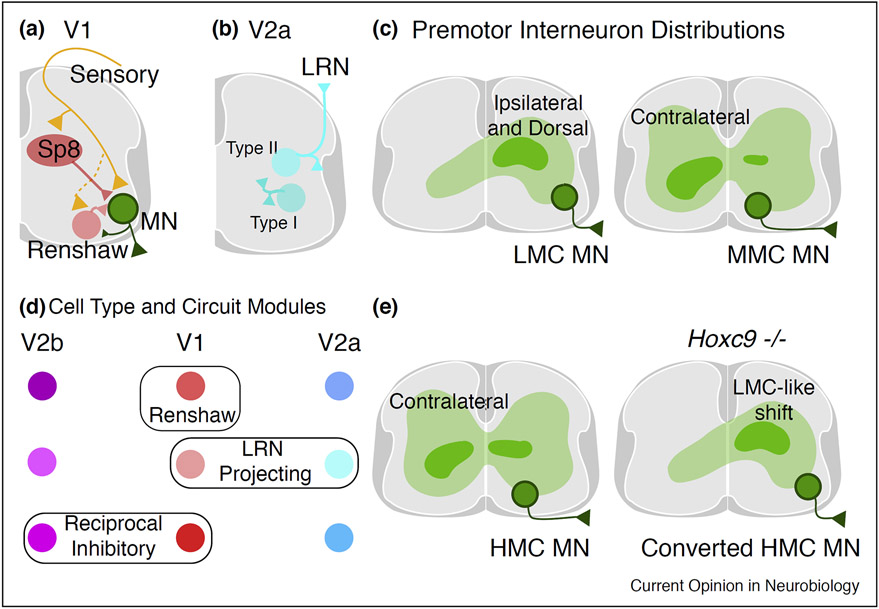
Figure 4. Specificity in spinal cord circuitry.
(a) Microcircuits in V1 interneurons for the Sp8 clade and Renshaw cells. Sp8 neurons receive proprioceptive input from Gluteus (GL) and Tibialis Anterior (TA) muscles while Renshaw cells only receive input from the GL. Renshaw cells receive input from GL and TA motor neurons while Sp8 neurons do not. Both Sp8 neurons and Renshaw cells synapse onto GL, TA, and intrinsic foot (IF) motor neurons [32••].
(b) Type II V2a interneurons, which downregulate Chx10 during development, are predominately located in cervical segments and project supraspinally to the lateral reticular nucleus (LRN). Type I V2a interneurons, which maintain Chx10 expression, project within the spinal cord [34••].
(c) Spatial distribution of premotor interneurons for different motor neuron column types. LMC (Lateral motor column) neurons predominately receive input from spinal interneurons located ipsilaterally and dorsally. MMC (Medial motor column) neurons receive a greater portion of input from contralaterally located spinal interneurons [19,21,42].
(d) Schematic depicting the relationship between spinal interneuron classes and circuit subtypes. Renshaw cells, which provide feedback inhibition to motor neurons, are a subtype exclusively found in V1 interneurons [49-51]. Subtypes of both V1 and V2a interneurons project to the brainstem lateral reticular nucleus (LRN) [39]. Reciprocal inhibitory neurons receive proprioceptive input from a limb muscle and inhibit the corresponding antagonist motor pool. Subtypes of V1 and V2b interneurons are reciprocal inhibitory neurons [51].
(e) Location of premotor HMC interneurons in the thoracic spinal cord. In Hoxc9 mutants, HMC neurons are converted into an LMC fate and their premotor interneuron distribution shifts accordingly [41••,42].
Recent studies have used transcriptional profiling to examine the fine-grained heterogeneity among ventral spinal interneuron classes [32••,34••,35•,36]. For V1 interneurons, transcriptional diversity assayed by 19 transcription factors correlated with differing position, electrophysiological properties, and input connectivity [32••]. The majority of V1 interneurons are organized into four non-overlapping clades defined by Sp8, MafA, FoxP2, and Pou6f2 expression [32••,33] (Figure 3b). Each clade can be further divided, resulting in ~50 subtypes of V1 interneurons based on their combinatorial expression of transcription factors. Likewise, considerable diversity was also demonstrated within V2a interneurons, with differentially expressed molecular markers corresponding to spatial location, projection targets, and timing of neurogenesis [34••]. Single cell RNA-sequencing revealed that V2a interneurons are divided into medial and lateral columns based on Nfib and Zfhx3 expression (Figure 3c). Additional molecular subtypes within each V2a columnar population were also found. A theme that has emerged for each interneuron population is the hierarchical organization of neuronal types into increasingly specialized cell groups, analogous to the way motor columns are divided into motor pools. While the function of each interneuron subtype remains poorly understood, elegant studies have begun to combine single cell sequencing and behavior to detect subsets of interneurons associated with specific sensorimotor responses [35•,37•]. Functionally targeting the discrete subtypes of V1 and V2a interneurons will help to define the role of these smaller neuronal units in motor control.
Rostrocaudal position as an additional axis of cellular diversity
Motor neuron columns are organized segmentally in registration with their peripheral muscle targets (Figure 3g). There is also increasing evidence for segmental specialization of interneurons. A recent study examined V1 molecular diversity in the thoracic spinal cord using the same set of 19 transcription factors initially identified in the lumbar cord. The study revealed V1 thoracic and limb-specific subtypes, as well as subtypes located throughout the spinal cord (Figure 3e) [38••]. Genetic manipulations demonstrated that segment-specific V1 interneuron subtypes were dependent on Hox genes and not dependent on extrinsic cues from segmentally organized motor neurons [38••].
Interestingly, RNA-sequencing of purified V2a interneurons revealed molecular distinctions which corresponded to V2a projection patterns [18,34••,39]. In more rostral segments, a portion of V2a interneurons possess an axon which bifurcates—one branch synapses locally and the other projects supraspinally, including to the lateral reticular nucleus (LRN) in the brainstem (Figure 4b). The ascending branch provides the brain with an efference copy of motor plans, allowing for predictions of the consequences of motor actions [18]. Transcriptional analysis revealed that the canonical V2a marker, Chx10, was downregulated in a subset of cells during embryonic development, resulting in Type I V2a neurons which maintain Chx10 expression and Type II V2a neurons which downregulate it. Type II V2a interneurons are primarily the brainstem-projecting population. Type I and Type II V2a neurons formed a counter-gradient along the spinal cord, with the majority of lumbar V2a interneurons being Type I and cervical V2a interneurons being a mix of the two types (Figure 3f). These observations have led to the speculative idea that V2a neurons participate in segment-specific activity using a Type I/Type II ratiometric computation.
These studies demonstrate that interneuron subtypes exhibit rostrocaudal spatial specificity, like their motor pool counterparts. Additionally, spinal cord segments are specialized through both qualitative differences in interneuron subtypes (e.g. V1 interneurons) and quantitative differences in the ratio of interneuron subtypes (e.g. V2a interneurons). The rostrocaudal specificity of interneuron subtypes likely relates to the functions of each spinal segment. Lumbar segments, which produce CPG activity, are essential for locomotion. Type I V2a neurons, which are enriched in lumbar segments, form extensive connections within the spinal cord (Figure 4b) [34••]. Stimulation of lumbar V2a interneurons drives motor neuron activity more quickly and reliably than stimulation of cervical V2a interneurons [34••]. Cervical segments play a smaller role in locomotion but are also vital for reaching behavior. Accordingly, the cervical spinal cord contains less Type I V2a interneurons but far more Type II V2a interneurons, whose supraspinal projection supports dexterity [18]. Interneuron subtypes present throughout the rostrocaudal axis presumably have broad-use functionality. An example is the V1 subtype Renshaw cells, which are prevalent throughout the spinal cord and provide feedback inhibition to multiple types of motor neurons (Figure 4a) [38••,40]. The identification of molecularly distinct interneuron subtypes with specific distributions in limb segments, thoracic segments, all segments, or in gradients suggests substantial circuit and physiological specialization of spinal interneurons across the spinal cord.
Molecular subtypes and their connectivity
Given the similarities in the hierarchical division of motor neurons and interneurons into motor pools and subtypes, it is intriguing to speculate that interneuron subtypes may be tailored to innervate single motor pools. However, at present there is not a clear indication that interneuron subtypes connect to motor pools in an exclusive relationship. For V1 interneurons, Sp8 clade neurons and Renshaw cells both form synapses onto the Gluteus (GL), Tibialis Anterior (TA), and intrinsic foot (IF) motor pools. Renshaw cells are a subtype of the MafA clade, which indicates that multiple clades can innervate a single motor pool and that the same clade can innervate multiple motor pools (Figure 4a) [32••].
While a variety of interneuron types connect to a single motor pool, the overall spatial distribution of spinal premotor interneurons has a stereotyped pattern [21,41••,42]. Interestingly, a major factor in premotor connectivity is the identity of the motor neuron itself [43]. Limb premotor neurons have distributions concentrated dorsally and ipsilaterally while axial and hypaxial premotor neurons have a tendency to be located contralaterally (Figure 4c,e) [41••,42]. Converting thoracic HMC (Hypaxial motor column) neurons into a LMC (Lateral motor column) identity resulted in a corresponding shift in premotor interneuron distribution (Figure 4e) [41••]. Perturbing motor neuron identity also alters the timing of motor neuron activity during CPG activation. Flexor-extensor motor timing is altered while rhythmicity and left–right alternation is maintained, indicating that motor neuron identity influences some, but not all components of the locomotor CPG [44,45]. Interneurons also play a role in establishing motor circuitry. Deletion of the midline guidance molecule EphA4 in dorsal interneurons results in aberrant contralateral premotor connections, indicating premotor connectivity is governed by an interplay of presynaptic and postsynaptic factors [46•].
Conclusion
With their unique muscle targets, motor pools have a pleasing simplicity across their features of development, genetics, connectivity, and function. As investigators begin to study spinal interneuron subtypes with equal precision and resolution, the correlations between neuronal features appear comparatively intricate and multidimensional. Differences between interneuron molecular subtypes may reflect a multitude of possible differences including inputs (sensory, descending, or other spinal interneurons), outputs (supraspinal, premotor, or other spinal interneurons), and physiology. The increasing division of neuron subtypes appears to apply to other regions of the central nervous system as well. Recent studies have demonstrated that the corticospinal tract can be divided into many parallel pathways based on cortical regions, which connect topographically onto different sets of spinal interneurons [47•,48].
Despite this complexity, it is likely that principles will emerge governing spinal interneuron identity as a variety of features of identity are cataloged for subtypes across spinal lineages. Developmental lineage often establishes certain neuronal features. For example, all V1 interneurons project ipsilaterally and express inhibitory neurotransmitters. However, additional layers of regulation help specify other aspects of spinal interneuron circuitry and physiology. Renshaw cells are a subtype that arise exclusively from the V1 interneuron lineage, thus other sets of transcription factors likely differentiate the Renshaw cell subtype from other V1 interneuron subtypes (Figure 4d) [30,32••,38••,49,50]. Neuron subtypes that are derived from separate lineages, but share aspects of circuitry or physiology, are potential entry points to uncovering overarching principles of subtype specification. In addition to V2a neurons, a subset of neurons from other lineages such as V1, V3, and dI3 also project supraspinally to the LRN [39]. This raises the possibility that Type I and Type II differences found in V2a interneurons extend to other populations [34••,39]. Comparing the transcriptional profiles of these interneuron subtypes which project to the LRN may reveal a common genetic signature for generating a brainstem-projecting subtype regardless of lineage (Figure 4d). Another example of a specialized circuit-defined interneuron subtype are reciprocal inhibitory neurons. Reciprocal inhibitory neurons receive proprioceptive input from a limb muscle and inhibit the corresponding antagonist motor pool [3]. They prevent unwanted co-contraction of flexor and extensor muscles [51]. Interestingly, populations of reciprocal inhibitory neurons include cells from both the V1 and V2b classes [51]. Thus, two separate lineages which generate inhibitory interneurons potentially share a genetic program for generating an interneuron input–output pattern (Figure 4d).
Beyond surveying and characterizing spinal interneuron diversity, it is critical to test the functional role of interneuron subtypes. Ablating and silencing spinal interneuron classes has proven to be an effective technique for examining how a circuit functions in the absence of a population of neurons. Additionally, studies which switch or perturb the genetic identity of cells without outright eliminating them are vital to understanding the function of neuronal diversity itself. Genetically converting the identity of one motor column into another type of motor column has revealed many insights into spinal neuron organization and function. Molecular studies in motor neurons initially identified mechanisms guiding motor neuron specification and their axon guidance [11,12]. Recently, motor neuron manipulations have also been used to study the role of motor neuron identity in establishing premotor circuitry and physiology [41••,44,45]. Thus, extending these types of studies to interneuron clades and subtypes will likely prove fruitful. While the role of molecular markers for establishing spinal interneuron lineages has been examined in some detail, less is known about the function of interneuron subtype markers [8]. Manipulating interneuron subtypes could potentially allow investigators to change subtype ratios or flatten subtype diversity altogether. Genetic manipulations may consequently alter spinal circuitry and physiology, providing causal insight into how molecular-identity relates to functional-identity and ultimately how subtypes shape behavior.
Acknowledgements
We would like to thank Ben Temple, Bianca Barriga, Gokhan Senturk, Kelsey Ladt, and Marito Hayashi for helpful comments on the manuscript. P.J.O was supported by the Christopher and Dana Reeve Foundation. S.L. P. is supported as a Howard Hughes Medical Institute Investigator and as a Benjamin H. Lewis chair in neuroscience. Research in the laboratory is supported by funding from the Howard Hughes Medical Institute and the Sol Goldman Charitable Trust.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Alaynick WA, Jessell TM, Pfaff SL: Snapshot: spinal cord development. Cell 2011, 146 178–178 e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu DC, Niu T, Alaynick WA: Molecular and cellular development of spinal cord locomotor circuitry. Front Mol Neurosci 2015, 8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiehn O: Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci 2016, 17:224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch SC, Acton D, Goulding M: Spinal circuits for touch, pain, and itch. Annu Rev Physiol 2018, 80:189–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gosgnach S, Bikoff JB, Dougherty KJ, El Manira A, Lanuza GM, Zhang Y: Delineating the diversity of spinal interneurons in locomotor circuits. J Neurosci 2017, 37:10835–10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granmo M, Petersson P, Schouenborg J: Action-based body maps in the spinal cord emerge from a transitory floating organization. J Neurosci 2008, 28:5494–5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abraira VE, Ginty DD: The sensory neurons of touch. Neuron 2013, 79:618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai HC, Seal RP, Johnson JE: Making sense out of spinal cord somatosensory development. Development 2016, 143:3434–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourane S, Grossmann KS, Britz O, Dalet A, Del Barrio MG, Stam FJ, Garcia-Campmany L, Koch S, Goulding M: Identification of a spinal circuit for light touch and fine motor control. Cell 2015, 160:503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romanes GJ: The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol 1951, 94:313–363. [DOI] [PubMed] [Google Scholar]
- 11.Bonanomi D, Pfaff SL: Motor axon pathfinding. Cold Spring Harb Perspect Biol 2010, 2:a001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasen JS, Jessell TM: Hox networks and the origins of motor neuron diversity. Curr Top Dev Biol 2009, 88:169–200. [DOI] [PubMed] [Google Scholar]
- 13.Kiehn O: Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 2006, 29:279–306. [DOI] [PubMed] [Google Scholar]
- 14.Crone SA, Quinlan KA, Zagoraiou L, Droho S, Restrepo CE, Lundfald L, Endo T, Setlak J, Jessell TM, Kiehn O et al. : Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron 2008, 60:70–83. [DOI] [PubMed] [Google Scholar]
- 15.Crone SA, Zhong G, Harris-Warrick R, Sharma K: In mice lacking V2a interneurons, gait depends on speed of locomotion. J Neurosci 2009, 29:7098–7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francius C, Harris A, Rucchin V, Hendricks TJ, Stam FJ, Barber M, Kurek D, Grosveld FG, Pierani A, Goulding M et al. : Identification of multiple subsets of ventral interneurons and differential distribution along the rostrocaudal axis of the developing spinal cord. PLoS One 2013, 8:e70325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romer SH, Seedle K, Turner SM, Li J, Baccei ML, Crone SA: Accessory respiratory muscles enhance ventilation in ALS model mice and are activated by excitatory V2a neurons. Exp Neurol 2017, 287:192–204. [DOI] [PubMed] [Google Scholar]
- 18.Azim E, Jiang J, Alstermark B, Jessell TM: Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature 2014, 508:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine AJ, Hinckley CA, Hilde KL, Driscoll SP, Poon TH, Montgomery JM, Pfaff SL: Identification of a cellular node for motor control pathways. Nat Neurosci 2014, 17:586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schouenborg J: Action-based sensory encoding in spinal sensorimotor circuits. Brain Res Rev 2008, 57:111–117. [DOI] [PubMed] [Google Scholar]
- 21.Tripodi M, Stepien AE, Arber S: Motor antagonism exposed by spatial segregation and timing of neurogenesis. Nature 2011, 479:61–66. [DOI] [PubMed] [Google Scholar]
- 22. •. Hilde KL, Levine AJ, Hinckley CA, Hayashi M, Montgomery JM, Gullo M, Driscoll SP, Grosschedl R, Kohwi Y, Kohwi-Shigematsu T et al. : Satb2 is required for the development of a spinal exteroceptive microcircuit that modulates limb position. Neuron 2016, 91:763–776. This study characterizes a population of inhibitory interneurons located in the intermediate spinal cord which express Satb2. Deletion of Satb2 from the interneurons causes the cells to be mispositioned in the spinal cord and form altered sensorimotor reflex circuitry.
- 23.Giszter SF, Mussa-Ivaldi FA, Bizzi E: Convergent force fields organized in the frog’s spinal cord. J Neurosci 1993, 13:467–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fink AJ, Croce KR, Huang ZJ, Abbott LF, Jessell TM, Azim E: Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 2014, 509:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. •. Koch SC, Del Barrio MG, Dalet A, Gatto G, Gunther T, Zhang J, Seidler B, Saur D, Schule R, Goulding M: RORbeta spinal interneurons gate sensory transmission during locomotion to secure a fluid walking gait. Neuron 2017, 96:1419–1431 e1415. The authors demonstrate inhibitory spinal interneurons expressing RORb play a key role in providing presynaptic inhibition to proprioceptive afferents. Ablation of RORβ interneurons results in a hyperflexion phenotype during locomotion.
- 26.Wildner H, Das Gupta R, Brohl D, Heppenstall PA, Zeilhofer HU, Birchmeier C: Genome-wide expression analysis of Ptf1a- and Ascl1-deficient mice reveals new markers for distinct dorsal horn interneuron populations contributing to nociceptive reflex plasticity. J Neurosci 2013, 33:7299–7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Weinrich JAP, Russ JB, Comer JD, Bommareddy PK, DiCasoli RJ, Wright CVE, Li Y, van Roessel PJ, Kaltschmidt JA: A role for dystonia-associated genes in spinal GABAergic interneuron circuitry. Cell Rep 2017, 21:666–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Betley JN, Wright CV, Kawaguchi Y, Erdelyi F, Szabo G, Jessell TM, Kaltschmidt JA: Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell 2009, 139:161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renshaw B: Influence of discharge of motoneurons upon excitation of neighboring motoneurons. J Neurophysiol 1941, 4:167–183. [Google Scholar]
- 30.Stam FJ, Hendricks TJ, Zhang J, Geiman EJ, Francius C, Labosky PA, Clotman F, Goulding M: Renshaw cell interneuron specialization is controlled by a temporally restricted transcription factor program. Development 2012, 139:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr PA, Alvarez FJ, Leman EA, Fyffe RE: Calbindin D28k expression in immunohistochemically identified Renshaw cells. Neuroreport 1998, 9:2657–2661. [DOI] [PubMed] [Google Scholar]
- 32. ••. Bikoff JB, Gabitto MI, Rivard AF, Drobac E, Machado TA, Miri A, Brenner-Morton S, Famojure E, Diaz C, Alvarez FJ et al. : Spinal inhibitory interneuron diversity delineates variant motor microcircuits. Cell 2016, 165:207–219. This study surveys subtype diversity in V1 interneurons. Subtypes, defined by differential expression of transcription factors, settle in distinct locations in the spinal cord and form specialized circuits.
- 33.Gabitto MI, Pakman A, Bikoff JB, Abbott LF, Jessell TM, Paninski L: Bayesian sparse regression analysis documents the diversity of spinal inhibitory interneurons. Cell 2016, 165:220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. ••. Hayashi M, Hinckley CA, Driscoll SP, Moore NJ, Levine AJ, Hilde KL, Sharma K, Pfaff SL: Graded arrays of spinal and supraspinal V2a interneuron subtypes underlie forelimb and hindlimb motor control. Neuron 2018, 97:869–884 e865. The authors explore how V2a interneurons are specialized across the rostrocaudal axis to support hindlimb and forelimb behaviors. They identify two subtypes of V2a interneurons, the first subtype is enriched in lumbar segments and supports locomotion, the second subtype is enriched in cervical segments and projects supraspinally to support dexterous movement.
- 35. •. Sathyamurthy A, Johnson KR, Matson KJE, Dobrott CI, Li L, Ryba AR, Bergman TB, Kelly MC, Kelley MW, Levine AJ: Massively parallel single nucleus transcriptional profiling defines spinal cord neurons and their activity during behavior. Cell Rep 2018, 22:2216–2225. In this study, the authors use single nucleus RNA-seq in the adult mouse spinal cord to identify transcriptionally distinct groups of neurons and assess their activity during different sensorimotor behaviors. During rotarod and formalin injection behavioral assays, different sets of neurons throughout the spinal cord are activated.
- 36.Delile J, Rayon T, Melchionda M, Edwards A, Briscoe J, Sagner A: Single cell transcriptomics reveals spatial and temporal dynamics of gene expression in the developing mouse spinal cord. Development 2019:146 10.1242/dev.173807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. •. Haring M, Zeisel A, Hochgerner H, Rinwa P, Jakobsson JET, Lonnerberg P, La Manno G, Sharma N, Borgius L, Kiehn O et al. : Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat Neurosci 2018, 21:869–880. This study characterizes cellular diversity in the dorsal horn using single cell RNA-seq. Excitatory and inhibitory neurons can be hierarchically organized, with subtypes demonstrating enrichment in specific dorsal laminae. Using the immediate early gene Arc, the authors identify neuronal subtypes that are activated during noxious heat or cold exposure.
- 38. ••. Sweeney LB, Bikoff JB, Gabitto MI, Brenner-Morton S, Baek M, Yang JH, Tabak EG, Dasen JS, Kintner CR, Jessell TM: Origin and segmental diversity of spinal inhibitory interneurons. Neuron 2018, 97:341–355 e343. This study examines V1 subtype diversity across the rostrocaudal axis. Limb and thoracic-specific subtypes were identified. The authors show development of these subtypes were not dependent on cues from motor neurons.
- 39.Pivetta C, Esposito MS, Sigrist M, Arber S: Motor-circuit communication matrix from spinal cord to brainstem neurons revealed by developmental origin. Cell 2014, 156:537–548. [DOI] [PubMed] [Google Scholar]
- 40.Saywell SA, Ford TW, Kirkwood PA: Axonal projections of Renshaw cells in the thoracic spinal cord. Physiol Rep 2013, 1: e00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. ••. Baek M, Pivetta C, Liu JP, Arber S, Dasen JS: Columnar-intrinsic cues shape premotor input specificity in locomotor circuits. Cell Rep 2017, 21:867–877. This study examines the role of motor neuron identity in shaping their sensory and interneuron inputs. Using Hoxc9 mutants to convert the columnar identity of HMC neurons to a LMC identity, the authors demonstrate that presynaptic input to motor neurons is converted as well.
- 42.Goetz C, Pivetta C, Arber S: Distinct limb and trunk premotor circuits establish laterality in the spinal cord. Neuron 2015, 85:131–144. [DOI] [PubMed] [Google Scholar]
- 43.Dasen JS: Master or servant? emerging roles for motor neuron subtypes in the construction and evolution of locomotor circuits. Curr Opin Neurobiol 2017, 42:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hinckley CA, Alaynick WA, Gallarda BW, Hayashi M, Hilde KL, Driscoll SP, Dekker JD, Tucker HO, Sharpee TO, Pfaff SL: Spinal locomotor circuits develop using hierarchical rules based on motorneuron position and identity. Neuron 2015, 87:1008–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machado TA, Pnevmatikakis E, Paninski L, Jessell TM, Miri A: Primacy of flexor locomotor pattern revealed by ancestral reversion of motor neuron identity. Cell 2015, 162:338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. •. Satoh D, Pudenz C, Arber S: Context-dependent gait choice elicited by EphA4 mutation in Lbx1 spinal interneurons. Neuron 2016, 89:1046–1058. By deleting the EphA4 axon guidance receptor from Lbx1 expressing interneurons, the authors demonstrate that dorsal premotor interneurons form bilateral premotor connections instead of ipsilateral connections. This ectopic bilateral wiring corresponds with an increase of left–right limb gait synchrony during swimming and airstepping.
- 47. •. Wang X, Liu Y, Li X, Zhang Z, Yang H, Zhang Y, Williams PR, Alwahab NSA, Kapur K, Yu B et al. : Deconstruction of corticospinal circuits for goal-directed motor skills. Cell 2017, 171:440–455 e414. Using viral tracing and genetically encoded calcium indicators, the authors examine the activity of corticospinal neurons during a food-pellet retrieval task. Populations of corticospinal neurons are activated sequentially throughout the task and project to distinct premotor populations within the spinal cord.
- 48.Ueno M, Nakamura Y, Li J, Gu Z, Niehaus J, Maezawa M, Crone SA, Goulding M, Baccei ML, Yoshida Y: Corticospinal circuits from the sensory and motor cortices differentially regulate skilled movements through distinct spinal interneurons. Cell Rep 2018, 23:1286–1300 e1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sapir T, Geiman EJ, Wang Z, Velasquez T, Mitsui S, Yoshihara Y, Frank E, Alvarez FJ, Goulding M: Pax6 and engrailed 1 regulate two distinct aspects of renshaw cell development. J Neurosci 2004, 24:1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benito-Gonzalez A, Alvarez FJ: Renshaw cells and Ia inhibitory interneurons are generated at different times from p1 progenitors and differentiate shortly after exiting the cell cycle. J Neurosci 2012, 32:1156–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Lanuza GM, Britz O, Wang Z, Siembab VC, Zhang Y, Velasquez T, Alvarez FJ, Frank E, Goulding M: V1 and v2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron 2014, 82:138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q et al. : Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 2015, 350:550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mishra SK, Hoon MA: The cells and circuitry for itch responses in mice. Science 2013, 340:968–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun S, Xu Q, Guo C, Guan Y, Liu Q, Dong X: Leaky gate model: intensity-dependent coding of pain and itch in the spinal cord. Neuron 2017, 93:840–853 e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dougherty KJ, Zagoraiou L, Satoh D, Rozani I, Doobar S, Arber S, Jessell TM, Kiehn O: Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. Neuron 2013, 80:920–933. [DOI] [PubMed] [Google Scholar]