Abstract
Aims
This study investigates the effects of intra-articular injection of adipose-derived mesenchymal stem cells (AdMSCs) and platelet-rich plasma (PRP) on lameness, pain, and quality of life in osteoarthritic canine patients.
Methods
With informed owner consent, adipose tissue collected from adult dogs diagnosed with degenerative joint disease was enzymatically digested and cultured to passage 1. A small portion of cells (n = 4) surplus to clinical need were characterized using flow cytometry and tri-lineage differentiation. The impact and degree of osteoarthritis (OA) was assessed using the Liverpool Osteoarthritis in Dogs (LOAD) score, Modified Canine Osteoarthritis Staging Tool (mCOAST), kinetic gait analysis, and diagnostic imaging. Overall, 28 joints (25 dogs) were injected with autologous AdMSCs and PRP. The patients were followed up at two, four, eight, 12, and 24 weeks. Data were analyzed using two related-samples Wilcoxon signed-rank or Mann-Whitney U tests with statistical significance set at p < 0.05.
Results
AdMSCs demonstrated stem cell-like characteristics. LOAD scores were significantly lower at week 4 compared with preinjection (p = 0.021). The mCOAST improved significantly after three months (p = 0.001) and six months (p = 0.001). Asymmmetry indices decreased from four weeks post-injection and remained significantly lower at six months (p = 0.025).
Conclusion
These improvements in quality of life, reduction in pain on examination, and improved symmetry in dogs injected with AdMSCs and PRP support the effectiveness of this combined treatment for symptom modification in canine OA for six months.
Cite this article: Bone Joint Res 2021;10(10):650–658.
Keywords: Osteoarthritis, Stem cells, Platelet-rich plasma, adipose-derived mesenchymal stem cells, Intra-articular injection, Osteoarthritis (OA), clinical outcomes, platelet-rich plasma (PRP), stem cells, Mann-Whitney U tests, Adipose tissue, Flow cytometry, gait analysis
Article focus
Intra-articular injection of adipose-derived stem cells (AdMCs) and platelet-rich plasma (PRP).
The effect of stem cells and PRP injection on lameness and pain scores in osteoarthritic canine patients.
Key messages
Intra-articular injection of autologous AdMSCs and PRP can be used in the management of canine osteoarthritis (OA).
Improvement in OA symptoms in clinical canine patients with large variability in ages, breeds, and weights.
Strengths and limitations
A single intra-articular injection of AdMSCs and PRP was effective for six months.
This study is applicable in a clinical setting as patient progress was monitored noninvasively using gait analysis and pain scores.
Additional research needs to be performed in order to determine the optimal dose and timing for repeated injection of AdMSCs and PRP.
Introduction
Osteoarthritis (OA) is characterized by degeneration of the articular cartilage, with loss of matrix, fibrillation, and formation of fissures causing loss of the cartilage surface. Associated changes include osseous metaplasia of intra- and extracapsular structures, abnormal periarticular calcification, and joint mechanics. OA is the most common cause of chronic pain in dogs, with an estimated 20% of dogs presenting with clinical signs.1 It is a chronic degenerative condition commonly managed with analgesics and symptom-modifying medication, alone or in combination with supportive therapies such as exercise and physiotherapy. These management methods are, however, limited in their ability to attenuate disease progression, leaving arthroplasty as a common eventuality in OA patients.1
Research has focused on using stem cell therapy for the management of OA. Not only do stem cells have differentiation and self-renewal properties, which may directly contribute to cartilage repair, but they also have anti-inflammatory and immunosuppressive abilities and secrete a variety of bioactive factors that allow them to be attracted to the site of injury, reducing pain and inflammation.2-5 In the osteoarthritic joint, progressive cartilage degradation produces proinflammatory mediators which exacerbate the condition; consequently, the anti-inflammatory properties of stem cells may be beneficial in reducing the inflammatory processes associated with OA.6,7
Autologous adipose-derived mesenchymal stem cell (AdMSC) therapy requires isolating and expanding stem cells from the patient’s fat using tissue culture techniques. The cells can then be administered into the osteoarthritic joint through intra-articular injection. The use of AdMSCs in preference to BMSCs is becoming increasingly common as adipose tissue is easy to obtain; the procedure for isolating the cells from the fat is simple, and adipose tissue has been shown to yield approximately 500 times more stem cells than bone marrow.8-10 Studies reporting the application of MSCs in canine OA include autologous and allogeneic BMSCs and AdMSCs.1,8,11,12 Human clinical trials assessing the effect of AdMSC in OA demonstrated preservation and increased cartilage volume compared to control patients as assessed by MRI, clinical exam, and cartilage scoring.13-15
Platelet-rich plasma (PRP) is isolated from patient blood and consists of a mixture of growth factors such as hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor-β (TGF-β). Intra-articular injection of PRP is associated with reduced inflammation, pain relief, improved limb function, and possible cartilage regeneration in human OA patients,16-19 and has been shown to reduce lameness in dogs with OA.20 The aim of this study was therefore to investigate the effect of stem cells and PRP injection on lameness and pain scores in osteoarthritic canine patients.
Methods
We do not believe the ARRIVE guidelines apply to our study because the dogs in this study were injected under the guidance of Recognised Veterinary Practice (RVP). All patients were receiving treatment at a veterinary practice, treatment for which the owners were paying and have given informed consent.
Patient recruitment and radiological evaluation
A series of 25 skeletally mature dogs (28 joints) were recruited for this study (Table I). The mean age of the dogs included was 98.5 months (standard deviation (SD) 34.5); 18 to 138) and the mean weight was 32.8 kg (SD 13.0; 13 to 58.1). All patients included in this study were receiving their first stem cells and PRP injection as part of OA management.
Table I.
Patient characteristics.
| ID | Breed | Age, mths | Weight, kg | Sex | Joint injected | Other injected joints |
|---|---|---|---|---|---|---|
| 1 | Cane Corso | 49 | 53.7 | F | L stifle | None |
| 2 | Rottweiler | 67 | 58.1 | MN | L stifle | None |
| 3 | Doberman | 47 | 48.5 | MN | L stifle | None |
| 4 | Rhodesian Ridgeback | 127 | 30.0 | FN | R stifle | None |
| 5 | Doberman | 18 | 41.0 | M | L stifle | None |
| 6 | Labradoodle | 120 | 32.5 | MN | R hip | None |
| 7 | Crossbreed | 138 | 23.6 | M | L hip L elbow |
None |
| 8 | Labrador | 119 | 34.7 | MN | R hip | Bi elbows |
| 9 | Labrador | 100 | 26.3 | FN | L hock | Bi elbows |
| 10 | Collie crossbreed | 134 | 22.5 | M | L hock R elbow |
None |
| 11 | Great Dane crossbreed | 96 | 52.7 | MN | L hip L shoulder |
Bi hips Bi hips |
| 12 | Labrador | 134 | 27 | F | R hip | Bi elbows, LS spine |
| 13 | ESS | 116 | 26.3 | MN | R elbow | Bi elbows |
| 14 | Rhodesia Ridgeback crossbreed | 118 | 28.7 | M | R hip | Bi elbows |
| 15 | ESS | 110 | 17.7 | F | L elbow | None |
| 16 | SBT | 69 | 13.0 | M | L hock | Bi elbows |
| 17 | Hungarian Vizla | 114 | 28.0 | M | L hip | Bi elbows |
| 18 | Rottie X | 122 | 30.6 | M | L hip | Bi elbows |
Bi, bilateral; ESS, English Springer Spaniel; F, female; FN, female neutered; L, left; M, male; MN, male neutered; R, right; SBT, Staffordshire Bull Terrier.
Inclusion criteria included the presence of OA in one or more joints. Dogs were excluded if they had any other orthopaedic-related surgery to the injected and/or the contralateral limb. A complete clinical evaluation was performed including haematology and serum biochemistry. Radiographs were reviewed to determine the degree of OA in the affected joint(s). The International Elbow Working Group Scoring Scheme was used to assess osteophyte size to grade the severity of OA. The scoring system used for all the joints including shoulder, hip, and stifle was: grade 0 = no osteophytes, grade 1 = osteophytes < 2 mm, grade 2 = osteophytes 2 mm to 5 mm, grade 3 = osteophytes > 5 mm.21,22 Lameness and limb asymmetry were confirmed using pressure-sensitive walkway analysis (Strideway; Tekscan, USA) immediately prior to enrolment on the study.
Adipose tissue collection
Adipose tissue was collected from each dog. Dogs were pre-medicated using intravenous (IV) administration of acepromazine (0.02 mg/kg; ACP; Elanco, UK) and methadone (0.3 mg/kg; Comfortan; Dechra, UK). General anaesthesia was induced with propofol 1 to 4 mg/kg (PropoFl; Zoetis, UK), the dog intubated, and then anaesthesia maintained with inhaled 2% isoflurane in oxygen. Adipose tissue was harvested from subcutaneous tissue in the inguinal region or falciform ligament.23
Tissue and stem cell processing
The adipose tissue was washed with phosphate-buffered saline (PBS) (Thermo Fisher Scientific, UK) and 1% P/S solution. The fat was minced and digested with collagenase II enzyme (Sigma Aldrich, UK) for three hours at 37°C with agitation. Thereafter, the fat mixture was centrifuged at 644 ×g for five minutes. The resulting cell pellet was then re-suspended in growth media composed of Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma Aldrich) supplemented with 10% (v/v) foetal calf serum (FCS) (First Link, UK) and 1% P/S solution, and cultured in a flask. Cells were maintained in the growth media until passage 1, after which they were trypsinized, counted, and mixed with PRP for injection into the joints.
PRP collection
Using a syringe containing 2 ml of citrate anticoagulant (ACD-A, Anticoagulant Citrate Dextrose Solution, Zimmer Biomet, USA), 18 ml of blood was collected from the jugular vein. The blood was centrifuged in sediment PRP (sPRP) tubes (NTL Biologica, UK) at 1,900 ×g for three minutes, to separate the PRP from the blood. The leucocyte-rich platelets in the PRP fraction were then counted under a light microscope and mixed with the stem cells before injection. Platelets were added to AdMSCs at a ratio of ten platelets per AdMSC.
In vitro study for stem cell characterization: flow cytometry analysis of CD marker expression
Passage 1 cells surplus to clinical need were expanded further. Flow cytometry was used to investigate CD marker expression of cells at either passage 2 or 3 (n = 4). The cells were analyzed for expression of CD29, CD90 and CD105, CD34, and CD45.
Cells were fixed for 15 minutes with 4% formaldehyde solution and washed with 0.5% bovine serum albumin (BSA) solution. The cells were then incubated with the antibodies (Table II), and diluted as recommended by the company for one hour in the dark at room temperature. The excess antibody was washed off and the cells were analyzed using a flow cytometer (CytoFLEX; Beckman Coulter, USA). Analysis of CD105 required a fluorescent secondary antibody. After one-hour incubation in the primary antibody, the samples were incubated with the secondary antibody in the dark for 30 minutes. After the cells were washed in BSA, they were re-suspended in saline solution for flow cytometry analysis. The resultant data were analyzed with proprietary software (CytExpert; Beckman Coulter). Isotype antibody was used as the negative control.
Table II.
List of primary antibodies (conjugated and unconjugated) and their conjugated isotype controls.
| Primary antibodies | Isotype or secondary control |
|---|---|
| R-phycoerythrin conjugated anti-CD29 (eBioscience) MEM-101A | Mouse IgG1-K PE (eBioscience) P3.6.2.8.1 |
| PE conjugated anti-CD34 (eBioscience) -1 H6 | Mouse IgG1-K PE (eBioscience) P3.6.2.8.1 |
| Fluorescein isothiocyanate conjugated anti-CD45 (eBioscience) – YKIX716.13 | Rat IgG2b-K FITC (eBioscience) eB149/10H5 |
| Allophycocyanin conjugated anti-CD90 (eBioscience) KIX337.217 | Mouse IgG2b-K APC (eBioscience) – eBMG2b |
| Primary anti-CD105 (Abcam) – OTI8A1 | Goat anti-mouse secondary antibody (Abcam) – ab150113 |
Trilineage differentiation (performed on samples from four patients)
Osteogenic differentiation - 30,000 cells at passage 2 were seeded and treated with osteogenic media containing growth media, supplemented with 10 mM B-glycerophosphate (Sigma Aldrich), 200 µM L-ascorbic acid (Sigma Aldrich), and 100 nM dexamethasone (Sigma Aldrich). The samples were fixed at day 21 with 10% formaldehyde and stained for calcium phosphate deposition using Alizarin Red stain (Sigma Aldrich). The samples were imaged under inverted light microscopy (Primovert; Zeiss, Germany).
Adipogenic differentiation - 30,000 cells at passage 2 were seeded and cultured with adipogenic media consisting of growth media supplemented with 50 mM Indometacin, 0.1 nM dexamethasone, 0.45 mM 3-isobutyl-1-methylxanthine, and 10 mg/ml Insulin (all Sigma Aldrich). The samples were incubated at 37°C and 5% CO2. The samples were then stained for the presence of lipid droplets using Oil Red O stain at day 21. The cells were initially washed in PBS and fixed with 4% formaldehyde, after which the cells were washed with 60% isopropanol and stained with Oil Red O for 15 minutes. Images were taken under inverted light microscopy (Primovert; Zeiss).
Chondrogenic differentiation - 100,000 cells at passage 2 were seeded as a micro-mass and supplemented with chondrogenic media containing 2% FCS, 100 µM dexamethasone, 50 mM ascorbic acid, 0.05 M sodium pyruvate (Sigma Aldrich), 10 mg/ml transforming growth factor-β1 (Peprotech EC,UK), and insulin-transferrin-selenium premix (Thermo Fisher Scientific). The samples were incubated at 37°C and 5% CO2. After 21 days, the samples were washed with distilled water and fixed for three hours using 100% methanol (Sigma Aldrich) at -20°C. The samples were then stained overnight for glycosaminoglycan production using Alcian Blue stain (Sigma Aldrich).
Stem cell injection
Patients were sedated with an IV injection of medetomidine (12 µg/kg) plus methadone (0.02 mg/kg). The number of cells injected per joint was determined by the weight of the patient in approximately 1.5 ml of PRP (Table III). The site for intra-articular injection was clipped and cleaned using chlorohexidine followed by final alcohol prep. The injection was administered under aseptic conditions with a 5 ml syringe and one-inch 21-gauge needle. Elbows were injected by identifying the medial humeral condyle and olecranon and passing a needle caudomedial to craniolateral into the joint space. Coxofemoral joints were injected by identifying the great trochanter of the femur, externally rotating the femur, palpating a deficit between femoral head and pelvis, then passing a needle into the coxofemoral joint space. Stifles were injected by locating the distal aspect of the patella and tibial tuberosity. The needle was passed abaxial to the straight patella tendon angled underneath the patella. Joint fluid was aspirated to confirm intra-articular location. Post-procedural analgesia provided was by buprenorphine (0.01 mg/kg SC at six hours post-injection) and paracetamol (10 mg/kg by slow IV injection, then orally every 12 hours for the next 72 hours).
Table III.
The dosage of cells and platelets was determined by the weight of the dog. The cells and platelets were suspended in 1.5 ml of plasma for injection.
| Dog weight, kg | Cell number | Platelet number |
|---|---|---|
| 0 to 10 | 4 million | 40 million |
| 11 to 25 | 10 million | 100 million |
| 26 to 45 | 16 million | 160 million |
| 45+ | 30 million | 300 million |
Outcome measures
To assess mobility, a validated OA survey instrument, the Liverpool Osteoarthritis in Dogs (LOAD) questionnaire was given to owners at the time of study entry (18 dogs), two weeks (ten dogs), four weeks (13 dogs), eight weeks (13 dogs), 12 weeks (13 dogs), and 24 weeks (eight dogs) post-injection.24 A reduction in LOAD scores reflects an improvement in mobility.25
All Tekscan data were collected by the same person (LC) for this study. Tekscan data were collected on the day of injection to obtain the initial baseline data (25 dogs). Thereafter data were collected at two weeks (ten dogs), four weeks (15 dogs), eight weeks (18 dogs), 12 weeks (16 dogs), and 24 weeks (nine dogs) post-injection. Limb loading, corrected to percentage body weight (%BW) was used to calculate the asymmetry index, which is an indicator of weight distribution between the healthy and the osteoarthritic joint. Lameness was indicated by a high asymmetry value. When lameness occurs in a load-bearing limb, compensatory load adjustments are made in the contralateral limb, so a lower asymmetry index indicated equal weight distribution.26,27 The %BW was measured by walking the dogs in a straight line four times across a pressure mat, at a consistent walk, approximately 100 cm/s. Prior to measurements, all dogs were acclimatized to the room and the pressure mat. Asymmetry index between contralateral limbs was calculated by: ((Xr –Xl)/(Xr+ Xl))*100, whereby Xr and Xl are the %BW for the right and left limb, respectively.28
The Canine Osteoarthritis Staging Tool (COAST) is a standardized scoring system that uses observations from both the owner and veterinarian to monitor canine OA progression.29 A modified version of the COAST (mCOAST) scoring system was used in the current study to assess patients at baseline (16 dogs), 12 weeks (16 dogs), and 24 weeks (13 dogs). The system was modified by excluding values assigned to follow-up radiological assessment. In this study, the final score was determined by adding scores together from each category, rather than taking the highest value as per the original COAST scoring system, and a reduction in the score would reflect an improvement in the progression of OA symptoms.30
Statistical analysis
The data in this study were non-parametric, therefore statistical comparison was carried out using a Wilcoxon signed-rank or Mann-Whitney U test. All data were analyzed using SPSS version 25 (IBM, USA). Statistical significance was defined as p < 0.05.
Results
There were no adverse effects associated with either surgical harvest of fat tissue or after the injection of stem cells and PRP. Radiographs were available for 26 of 28 joints; three joints were assigned grade 0, three joints were assigned grade 1, eight joints were assigned grade 2, and 12 joints were assigned grade 3. Two joints were not radiographed. Of the 25 patients, three were injected in multiple joints. Altogether 28 joints were treated with AdMSCs and PRP (Table I).
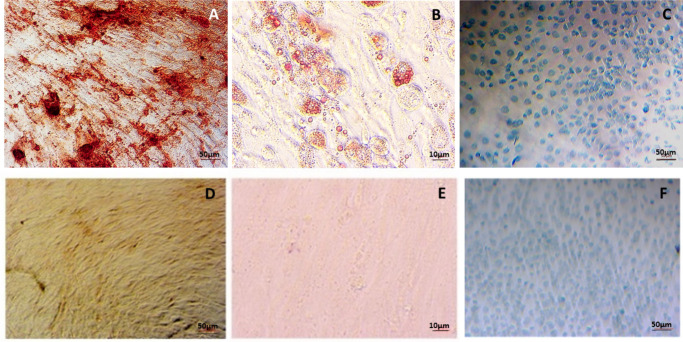
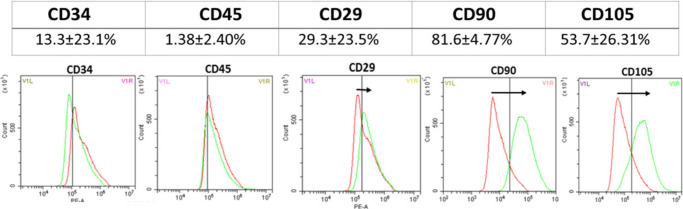
In accordance with the International Society for Stem Cell Research,31 AdMSCs differentiated to bone, fat, and chondrocytes (n = 4) after 21 days and stained positively for Alizarin Red, Oil Red O, and Alcian Blue stain, respectively (Figure 1). The cells (n = 4) were also analyzed using flow cytometry and showed limited expression of CD34 and CD45 with positive expression of CD29, CD90, and CD105 (Figure 2).
Fig. 1.
a) Alizarin Red, b) Oil Red O, and c) Alcian Blue stain for adipose-derived mesenchymal stem cells and their negative controls differentiated to d) bone, e) fat, and f) cartilage, respectively, after 21 days (n = 4).
Fig. 2.
Flow cytometry results for adipose-derived mesenchymal stem cells. The cells were negative for CD34 and CD45 and highly expressed CD90 (n = 4).
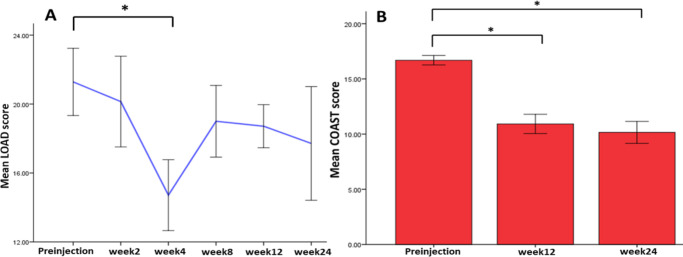
LOAD scores were significantly lower at week 4 (p = 0.021, Mann-Whitney U test) compared with preinjection. The mCOAST improved significantly after three months (p = 0.001, Mann-Whitney U test) and six months (p = 0.001, Mann-Whitney U test), implying that the injections made an improvement to the pain and mobility of the patients after four weeks, as assessed by the owner, and after three months (12 weeks) and six months (24 weeks), as assessed by the veterinarian (Figure 3).
Fig. 3.
Graphs showing: a) the mean Liverpool Osteoarthritis in Dogs (LOAD) score taken before injection (on the day of the injection) up to six months; and b) the mean Modified Canine Osteoarthritis Staging Tool (mCOAST) score assessed on the day of injection (preinjection), and three months and six months post-injection. The LOAD score was shown to decrease up to six months, demonstrating improved mobility and pain scores based on owner assessment. *p < 0.05, Mann-Whitney U test. For LOAD analysis: preinjection (n = 17), week 2 (n = 10), week 4 (n = 13), week 8 (n = 13), week 12 (n = 13), and week 24 (n = 8). For COAST analysis: preinjection (n = 16), week 12 (n = 16), and week 24 (n = 13).
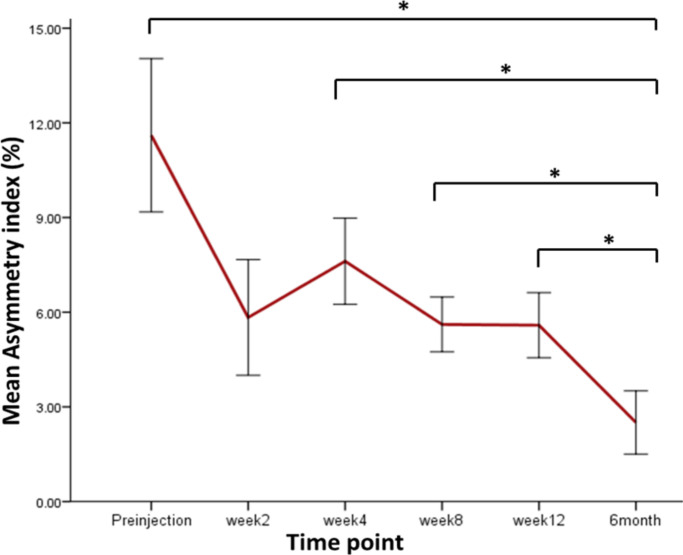
The asymmetry index decreased at week 2, possibly due to the physical effect of joint lavage from the injection procedure. The asymmetry index then increased at week 4 and then decreased consistently until 24 weeks (six months). Significant differences were recorded between weeks 8 and 24 (p = 0.021), weeks 4 and 24 (p = 0.008), weeks 12 and 24 (p = 0.011), and between weeks 24 and week 0/preinjection (p = 0.025, all Mann-Whitney U test) (Figure 4).
Fig. 4.
The asymmetry index calculated using the peak vertical force (PVF), calculated from the injected limb and the contralateral uninjected joint. Asymmetry index is a percentage, calculated by finding the difference in PVF between the treated and untreated limb and dividing by the sum of PVF for the same limbs. *p < 0.05, Mann-Whitney U test. Preinjection (n = 28), week 2 (n = 10), week 4 (n = 15), week 8 (n = 18), week 12 (n = 16), and six months (n = 9).
Discussion
Loss of articular cartilage with inflammation is a common feature of OA. It eventually results in impaired joint function.32 Results from this study demonstrate that AdMSC and PRP therapy improved pain and lameness scores over time, with statistical and clinical significance seen at 12 weeks and six months compared with preinjection. Clinically, this is an important finding because although these results did not reflect the asymmetry index from gait analysis, the AdMSCs and PRP injections appear to have enabled patients to experience a better quality of life with less pain, delaying the need for more invasive surgical techniques. Similar results were reported in a study in osteoarthritic human patients, in which 18 patients were injected with AdMSCs in the knee joint. This study showed significant improvement in Western Ontario and McMaster Universities Osteoarthritis (WOMAC) score and size of the cartilage defect, with no adverse events.33
Articular cartilage is susceptible to damage and has a poor potential for regeneration due to its lack of vascularity. As OA progresses, the load-bearing capacity and biomechanical properties of the thinning cartilage decreases and restoration of normal cartilage function is difficult to achieve. As well as the ability to differentiate into chondrocytes, stem cells also control the local microenvironment via anti-inflammatory and immunosuppressive factors, thereby protecting the cartilage from further tissue destruction and aiding regeneration of the remaining progenitor cells.34 The role that injected stem cells play in cartilage regeneration is therefore unclear and it is difficult to predict how long the cells’ stimulatory effect may last, and whether a reinjection of cells and PRP may be necessary.13 From the asymmetry measurements in our study, the treatment appears to remain effective for months.
The growth factors in PRP have an anti-inflammatory and analgesic effect.35,36 HGF has been shown to enhance the inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which decreases the production of the cytokine interleukin 6 (IL-6), leading to an increase in the anti-inflammatory cytokine IL-10.37,38 Indeed, PRP injection has been shown to be effective after three and six months of injections in human patients with initial stage of knee OA, compared with patients injected with hyaluronic acid (HA).39 Similar results have also been reported in canine patients injected with PRP, with an increase in PVF and improved pain and lameness scores at 12 weeks.12,40 Additionally, it has been reported that when MSCs were treated with PRP, proliferation and chondrogenic differentiation in vitro were enhanced.41,42 A study by Yun et al12 demonstrated superior disease modification when AdMSCs were combined with PRP, as compared to stem cells or PRP alone, in an experimental model of canine OA, whereby the dogs were injected every week for a month. However, unlike the study by Yun et al,12 dogs in our study had naturally occurring OA and have shown a statistically significantly long-term positive response to a single injection of cells and PRP injection, until six months. This is therefore more viable in a clinical setting in veterinary and human medicine, because all patients would demonstrate variability in ages and weight, and their progress would be monitored using gait analysis and pain scores.
Unlike MRI, which can be very costly, radiological analysis can be used in clinical practice for diagnosis of OA in dogs. However, it mainly provides information on osseous changes such as sclerosis and osteophyte formation, but provides limited data on soft tissues. Osteophyte measurements can be graded and used to diagnose OA, but it has been shown that osteophyte size can vary between different breeds of dogs.43 Therefore, radiological findings, taken in isolation, should be interpreted with caution, as they are not a reliable indicator of OA. The dogs in this study were therefore assessed clinically and using gait analysis.
The dogs in this study were injected under the guidance of RVP. Therefore, the limitations of this study were as follows. Firstly, the lack of a placebo or control group that would receive an intra-articular injection of saline solution or PRP in a prospective blinded randomized control manner to conclusively assess the effect of the treatment arm in an unbiased manner. The authors acknowledge that it would have been preferable to compare the results from this case series with those injected with only PRP or HA, but there are constraints on randomization in many veterinary practices. Secondly, the restriction to collect invasive outcome measures in a clinical setting following treatments such as AdMSCs. MRI and arthroscopy could have been used to assess cartilage volume and infill. This would have identified whether the improvement in lameness was due to cartilage regrowth or due to the anti-inflammatory effect of the cells. Determining the catabolic and anabolic biological markers in the synovial fluid would also have helped to establish the anti-inflammatory effect of the cells and PRP in OA. However, to subject these patients to additional anaesthesia for imaging and arthroscopy would have required an experimental licence. Finally, canine OA often affects joints bilaterally; it is difficult to report on the effects of stem cells in patients with asymmetrical OA. The effect of multiple joint OA may mask clinical outcomes following stem cell injection.
In conclusion, this study represents the first clinical series on the use of a combination of autologous culture-expanded AdMSCs and PRP in the management of canine OA. We report a significant improvement in pain and functional scores and lameness. Although these findings support the idea of using AdMSCs in the management of OA, additional research needs to be performed in order to determine the optimal dose and timing, and to confirm whether combination therapy is better than monotherapy.
Author contributions
A. Sanghani-Kerai: Investigation, Formal analysis, Writing – original draft, Writing – review & editing.
C. Black: Resources, Conceptualization, Writing – review & editing.
S. O. Cheng: Investigation, Formal analysis, Writing – review & editing.
L. Collins: Resources, Investigation, Writing – review & editing.
N. Schneider: Formal analysis, Writing – review & editing.
G. Blunn: Conceptualization, Supervision, Writing – review & editing.
F. Watson: Resources, Investigation, Writing – review & editing.
N. Fitzpatrick: Conceptualization, Resources, Investigation, Writing – review & editing.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
The authors declare no potential conflict of interest.
Open access funding
The authors confirm that the open access fees for this publication were self-funded.
Acknowledgements
Sarah Morgan, PhD, an employee of Fitzpatrick Referrals, provided medical writing assistance.
Contributor Information
Anita Sanghani-Kerai, Email: anitas@fitzpatrickreferrals.co.uk.
Cameron Black, Email: CBlack@fitzpatrickreferrals.co.uk.
Shuliang Oliver Cheng, Email: oliver.cheng.15@ucl.ac.uk.
Laura Collins, Email: imlaura7@hotmail.com.
Nadine Schneider, Email: NSchneider@fitzpatrickreferrals.co.uk.
G. Blunn, Email: gordon.blunn@port.ac.uk.
Fraje Watson, Email: frajew@fitzpatrickreferrals.co.uk.
Noel Fitzpatrick, Email: NoelF@fitzpatrickreferrals.co.uk.
References
- 1. Shah K, Drury T, Roic I. Outcome of allogeneic adult stem cell therapy in dogs suffering from osteoarthritis and other joint defects. Stem Cells Int. 2018;2018:7309201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–347. [DOI] [PubMed] [Google Scholar]
- 3. Abumaree M, Al Jumah M, Pace RA, Kalionis B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev Rep. 2012;8(2):375–392. [DOI] [PubMed] [Google Scholar]
- 4. Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28(8):1446–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kazemi D, Shams Asenjan K, Dehdilani N, Parsa H. Canine articular cartilage regeneration using mesenchymal stem cells seeded on platelet rich fibrin. Bone Joint Res. 2017;6(2):98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang R-K, Li G-W, Zeng C, et al. Mechanical stress contributes to osteoarthritis development through the activation of transforming growth factor beta 1 (TGF-β1). Bone Joint Res. 2018;7(11):587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Black LL, Gaynor J, Gahring D. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther. 2007;8(4):272–284. [PubMed] [Google Scholar]
- 9. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24(4):150–154. [DOI] [PubMed] [Google Scholar]
- 10. Cuervo B, Rubio M, Sopena J. Hip osteoarthritis in dogs: a randomized study using mesenchymal stem cells from adipose tissue and plasma rich in growth factors. Int J Mol Sci. 2014;15(8):13437–13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Black LL, Gaynor J, Adams C. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008;9(3):192–200. [PubMed] [Google Scholar]
- 12. Yun S, S-K K, Kwon Y-. S. Adipose-derived mesenchymal stem cells and platelet-rich plasma synergistically ameliorate the surgical-induced osteoarthritis in Beagle dogs. J Orthop Surg Res. 2016;11(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freitag J, Bates D, Wickham J, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen Med. 2019;14(3):213–230. [DOI] [PubMed] [Google Scholar]
- 14. Song Y, Du H, Dai C. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. 2018;13(3):295–307. [DOI] [PubMed] [Google Scholar]
- 15. Kim YS, Choi YJ, Lee SW, et al. Assessment of clinical and MRI outcomes after mesenchymal stem cell implantation in patients with knee osteoarthritis: a prospective study. Osteoarthritis Cartilage. 2016;24(2):237–245. [DOI] [PubMed] [Google Scholar]
- 16. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489–496. [DOI] [PubMed] [Google Scholar]
- 17. Sundman EA, Cole BJ, Karas V. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am J Sports Med. 2014;42(1):35–41. [DOI] [PubMed] [Google Scholar]
- 18. Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014;5(3):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berger DR, Centeno CJ, Steinmetz NJ. Platelet lysates from aged donors promote human tenocyte proliferation and migration in a concentration-dependent manner. Bone Joint Res. 2019;8(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Venator KP, Frye CW, Gamble L-J, Wakshlag JJ. Assessment of a single intra-articular stifle injection of pure platelet rich plasma on symmetry indices in dogs with unilateral or bilateral stifle osteoarthritis from long-term medically managed cranial cruciate ligament disease. Vet Med (Auckl). 2020;11:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lang J, Busato A, Baumgartner D, Flückiger M, Weber UT. Comparison of two classification protocols in the evaluation of elbow dysplasia in the dog. J Small Animal Practice. 1998;39(4):169–174. [DOI] [PubMed] [Google Scholar]
- 22. Szabo SD, Biery DN, Lawler DF. Evaluation of a circumferential femoral head osteophyte as an early indicator of osteoarthritis characteristic of canine hip dysplasia in dogs. J Am Vet Med Assoc. 2007;231(6):889–892. [DOI] [PubMed] [Google Scholar]
- 23. Jifcovici A, Solano MA, Fitzpatrick N, Findji L, Blunn G, Sanghani-Kerai A. Comparison of Fat Harvested from Flank and Falciform Regions for Stem Cell Therapy in Dogs. Vet Sci. 2021;8(2):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. No authors listed . Liverpool Osteoarthritis in Dogs (LOAD): Owner questionnaire for dogs with mobility problems. University of Liverpool. https://dspace.uevora.pt/rdpc/bitstream/10174/19611/2/liverpool%20OA%20in%20dogs%20-%20load.pdf (date last accessed 13 September 2021).
- 25. Hercock CA, Pinchbeck G, Giejda A, Clegg PD, Innes JF. Validation of a client-based clinical metrology instrument for the evaluation of canine elbow osteoarthritis. J Small Anim Pract. 2009;50(6):266–271. [DOI] [PubMed] [Google Scholar]
- 26. Fischer S, Anders A, Nolte I, Schilling N. Compensatory load redistribution in walking and trotting dogs with hind limb lameness. Vet J. 2013;197(3):746–752. [DOI] [PubMed] [Google Scholar]
- 27. Rumph PF, Kincaid SA, Visco DM, Baird DK, Kammermann JR, West MS. Redistribution of vertical ground reaction force in dogs with experimentally induced chronic hindlimb lameness. Vet Surg. 1995;24(5):384–389. [DOI] [PubMed] [Google Scholar]
- 28. Bockstahler BA, Skalicky M, Peham C, Müller M, Lorinson D. Reliability of ground reaction forces measured on a treadmill system in healthy dogs. Vet J. 2007;173(2):373–378. [DOI] [PubMed] [Google Scholar]
- 29. Cachon T, Frykman O, Innes JF, et al. Face validity of a proposed tool for staging canine osteoarthritis: Canine Osteoarthritis Staging Tool (COAST). Vet J. 2018;235:1–8:S1090-0233(18)30058-3. [DOI] [PubMed] [Google Scholar]
- 30. Cachon T, Frykman O, Innes J. Face validity of a proposed tool for staging canine osteoarthritis: Canine OsteoArthritis Staging Tool (COAST). Vet J. 2018;235:1–8. [DOI] [PubMed] [Google Scholar]
- 31. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 32. Krasnokutsky S, Attur M, Palmer G, Samuels J, Abramson SB. Current concepts in the pathogenesis of osteoarthritis. Osteoarthritis Cartilage. 2008;16(3):S1–S3. [DOI] [PubMed] [Google Scholar]
- 33. CH J, Lee YG, Shin WH. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–1266. [DOI] [PubMed] [Google Scholar]
- 34. Gupta PK, Das AK, Chullikana A, Majumdar AS. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther. 2012;3(4):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amable PR, Carias RBV, Teixeira MVT, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4(3):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamilton B, Tol JL, Knez W, Chalabi H. Exercise and the platelet activator calcium chloride both influence the growth factor content of platelet-rich plasma (PRP): overlooked biochemical factors that could influence PRP treatment. Br J Sports Med. 2015;49(14):957–960. [DOI] [PubMed] [Google Scholar]
- 37. Bendinelli P, Matteucci E, Dogliotti G. Molecular basis of anti‐inflammatory action of platelet‐rich plasma on human chondrocytes: Mechanisms of NF‐κB inhibition via HGF. J Cell Physiol. 2010;225(3):757–766. [DOI] [PubMed] [Google Scholar]
- 38. Coudriet GM, He J, Trucco M, Mars WM, Piganelli JD. Hepatocyte growth factor modulates interleukin-6 production in bone marrow derived macrophages: Implications for inflammatory mediated diseases. PLoS One. 2010;5(11):e15384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spaková T, Rosocha J, Lacko M, Harvanová D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411–417. [DOI] [PubMed] [Google Scholar]
- 40. Fahie MA, Ortolano GA, Guercio V. A randomized controlled trial of the efficacy of autologous platelet therapy for the treatment of osteoarthritis in dogs. J Am Vet Med Assoc. 2013;243(9):1291–1297. [DOI] [PubMed] [Google Scholar]
- 41. Mishra A, Tummala P, King A, et al. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Engineering Part C: Methods. 2009;15(3):431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Drengk A, Zapf A, Stürmer EK, Stürmer KM, Frosch K-H. Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells Tissues Organs. 2009;189(5):317–326. [DOI] [PubMed] [Google Scholar]
- 43. Pettitt RA, German AJ. Investigation and management of canine osteoarthritis. In pract. 2015;37(S1):1–8. [Google Scholar]