SUMMARY
Background
Impact of the Delta variant and vaccination on SARS-CoV-2 transmission remains unclear. In Singapore, quarantine of all close contacts, including entry and exit PCR testing, provided the opportunity to determine risk of infection by the Delta variant compared to other variants, vaccine efficacy against SARS-CoV-2 acquisition, symptomatic or severe COVID-19, and risk factors associated with SARS-CoV-2 acquisition and symptomatic disease.
Methods
This retrospective cohort study included all close contacts between September 1, 2020 and May 31, 2021. Regardless of symptoms, all were quarantined for 14 days with entry and exit PCR testing. Household contacts were defined as individuals who shared a residence with a Covid-19 index case. Secondary attack rates among household close contacts of Delta variant-infected indexes and other variant-infected indexes were derived from prevalence of diagnosed cases among contacts. Relative risk ratios and bootstrapping at the cluster level was used to determine risk of infection by the Delta variant compared to other variants and vaccine efficacy against SARS-CoV-2 acquisition, symptomatic or severe COVID-19. Logistic regression using generalized estimating equations was used to determine risk factors associated with SARS-CoV-2 acquisition and symptomatic disease
Findings
Of 1024 household contacts linked to 301 PCR-confirmed index cases, 753 (73.5%) were linked to Delta-infected indexes and 248 (24.2%) were exposed to indexes with other variants. Household secondary attack rate among unvaccinated Delta-exposed contacts was 25.8% (95% boostrap confidence interval [BCI] 20.6–31.5%) compared with 12.9% (95%BCI 7.0–20.0%) among other variant-exposed contacts. Unvaccinated Delta-exposed contacts were more likely to be infected than those exposed to other variants (Relative risk 2.01, 95%CI 1.24–3.84). Among Delta-exposed contacts, complete vaccination had a vaccine effectiveness of 56.4% (95%BCI 32.6–75.8%) against acquisition, 64.1% (95%BCI 37.8–85.4%) against symptomatic disease and 100% against severe disease. Among Delta-exposed contacts, vaccination status (adjusted odds ratio [aOR] 0.33, 95% robust confidence interval [RCI] 0.17–0.63) and older age of the index (aOR 1.20 per decade, 95%RCI 1.03–1.39) was associated with increased risk of SARS-CoV-2 acquisition by the contact. Vaccination status of the index was not associated with a statistically-significant difference for contact SARS-CoV-2 acquisition (aOR 0.73, 95%RCI 0.38–1.40).
Interpretation
Increased risk of SARS-CoV-2 Delta acquisition compared with other variants was reduced with vaccination. Close-contacts of vaccinated Delta-infected indexes did not have statistically significant reduced risk of acquisition compared with unvaccinated Delta-infected indexes.
1. INTRODUCTION
The SARS-CoV-2 Delta variant (B.1.617.2) has increased rapidly in many countries including the United Kingdom, United States of America, India and Singapore.1 Vaccination has been reported to prevent symptomatic disease2,3 and hospitalizations related to Delta variant infection. To reduce the spread of disease, it is important also to determine the impact of vaccination on preventing any infection, regardless of symptoms, and the relative risk of onward transmission of disease from vaccinated vs unvaccinated individuals, which remains unknown. As asymptomatic SARS-CoV-2 positive individuals are potential sources for onward transmission, especially in populations that do not have high levels of immunity, understanding vaccine efficacy against SARS-CoV-2 acquisition is essential for transmission risk assessment.4
Accurately determining secondary attack rates regardless of symptoms remains challenging as many settings rely on symptom-based SARS-CoV-2 testing. Symptom-based testing will not detect asymptomatic infections and mild non-medically consulted infection.5 This challenge can be addressed by studies of close contacts with routine SARS-CoV-2 testing regardless of symptoms to detect asymptomatic and mildly symptomatic cases. As household contacts of SARS-CoV-2-positive cases are likely to be highly exposed to the case and are known to be at high-risk of infection[5], [6], [7], they are an ideal group to provide estimates of the impact of variants and vaccination on SARS-CoV-2 attack rates.
We examined the impact of the Delta variant and vaccination among household close-contacts of all locally-acquired COVID-19 cases in Singapore, an island city-state in Southeast Asia with 5.8 million people. Since the identification of the first COVID-19 case on Jan 23, 2020, active contact tracing has been conducted to identify all close-contacts of every COVID-19 case. Close-contacts are quarantined for 14 days with routine PCRs conducted at entry to and exit from quarantine since May 2020. Whole genome sequencing is also performed on all local COVID-19 cases with a RT-PCR cycle threshold (Ct) value of less than 30. This cohort, where index case-contact pairs were known, provided a unique opportunity to determine secondary attack rates and risk factors for COVID-19 transmission.
2. METHODS
2.1. Case and close contact definitions
This retrospective cohort study included all close contacts of confirmed COVID-19 index cases in Singapore issued legally-binding quarantine orders between September 1, 2020 and May 31, 2021. Since January 2, 2020, surveillance for COVID-19 in Singapore has been conducted according to regularly-reviewed Ministry of Health (MOH) COVID-19 guidance. Since July 1, 2020, all persons aged 13 years old and above presenting with symptoms of febrile or non-febrile acute respiratory infection for any duration were actively offered to have a respiratory swab taken for RT-PCR testing. In addition, all close contacts of COVID-19 cases were also mandated to be tested by PCR upon entry and exit from quarantine, ensuring that additional cases are identified for further contact tracing. Physicians are required by law to report all COVID-19 cases to MOH. A confirmed COVID-19 case was defined as positive detection of SARS-CoV-2 nucleic acid by real-time RT-PCR of respiratory specimens.8
Contact-tracing was performed by MOH for every diagnosed COVID-19 case.9 Household close contacts were defined as persons who shared the same residential address as the index case, regardless of duration or proximity of contact. All identified close contacts were placed under legally-binding quarantine for 14 days, during which they were not allowed to leave their residence or assigned location. Persons under quarantine were monitored daily for development of symptoms, and symptomatic contacts were transferred to hospital via a dedicated transport service for COVID-19 testing and clinical evaluation. Regardless of symptoms, all quarantined close contacts were tested for COVID-19 by PCR, upon entry and exit from quarantine. The secondary attack rate was defined as the number of PCR-confirmed cases detected among all household close contacts of the index case.
All confirmed COVID-19 cases were isolated in hospital until MOH-specified discharge criteria were met. All cases of possible COVID-19 reinfection are independently adjudicated by an expert panel comprising specialists in infectious diseases and laboratory medicine.
2.2. Detection and identification of SARS-CoV-2 variants
The National Public Health Laboratory started whole genome sequencing (WGS)-based testing for SARS-CoV-2 variants routinely since December 2020. WGS was attempted for all SARS-CoV-2 positive cases with a RT-PCR Ct value of less than 30. Variant analysis was performed using Pangolin COVID-19 lineage assigner10 and CovSurver connected to GISAID11,12 to assign and verify lineages to each sequence.
2.3. National vaccination programme and vaccination status definitions
By end May 2021, only the Pfizer/BioNTech BNT162b2 and Moderna mRNA-1273 vaccines (authorised for use in Singapore on December 14, 2020 and February 3, 2021, respectively) had been offered to citizens and long-term residents on a voluntary basis under the national vaccination programme. The vaccination exercise was initiated with the Pfizer/BioNTech vaccine on December 30, 2020, prioritising healthcare and frontline workers13, and was expanded to the community-at-large, starting with seniors aged 70 and above from February 2021 and seniors aged 60 to 69 from end March 2021.14 As of May 30, 2021, approximately 2.3 million individuals (40% of the population) have received at least the first dose of the vaccine, of whom over 1.7 million individuals (30% of the population) have completed the full vaccination regimen. 73% of eligible seniors aged 60 and above, 72% of eligible persons aged 45 to 59 and 60% of those aged 40 to 44 have received the vaccine or scheduled a vaccination appointment.15
Both index cases and close contacts were considered to be partially vaccinated if they had received one vaccine dose before the day the quarantine order was issued, or were within 14 days of the second dose on the day the quarantine order was issued. If more than 14 days had elapsed after their second dose, they were taken to be fully vaccinated.
2.4. Epidemiological analysis
Data on the index cases and associated close contacts were obtained from MOH's contact tracing database. For both, demographic information, residential address and vaccination status (i.e. vaccine type, number of doses received and vaccination dates for received doses) were obtained. Additionally, unique national case number, date of COVID-19 diagnosis, WGS-confirmed variant assignment, date of symptom onset, date of hospital admission and reinfection status were obtained for index cases, while start date of quarantine, and if applicable, confirmed COVID-19 status, date of COVID-19 diagnosis and symptom onset, WGS-confirmed variant assignment, whether supplemental oxygen or intensive care were required, and reinfection status were obtained for close contacts. Symptom information was collected via interview by the MOH contact tracers soon after diagnosis while the requirement for supplemental oxygen or intensive care was determined throughout the duration of inpatient care.
Close contacts residing in foreign worker dormitories (as it is difficult to define a household in that setting), those of overseas or imported cases, and those lacking index case information were excluded. The remaining close contacts were linked to community-acquired cases (“community” close contacts), including household close contacts. If multiple linked index cases were listed for a close contact in the national records, the earliest-diagnosed index sharing the same residence as the contact was selected for analysis. It was assumed that all recorded cases in February 2021 and earlier with missing variant status information in the database were infected with variants other than the Delta variant of concern (“non-Delta variant”), as the first detection of the Delta variant in Singapore was in April 2021.
MOH actively investigates every new case for epidemiologically-linked transmission to prior cases and groups cases into transmission clusters which are reported.15 All cases with individually-assigned variant status in an epidemiologically-linked transmission cluster were found to have identical variant assignment. As such, cases with no individually-assigned variant status were assigned the transmission cluster variant if available.
2.5. Statistical analysis and modelling
Until the emergence of the Delta variant, the number of cases during the study period was low, and as a result, there were insufficient numbers of the other variants to analyze them separately. We therefore grouped all non-Delta variants together. Three outcomes were considered: infection, symptomatic disease, and severe disease (defined as either need for supplemental oxygen, and/or intensive care admission), though the small number of contacts with severe infection precluded some analyses of the final endpoint. We characterised contacts by their vaccine status and the variant they were exposed to, to account for potentially different protection against the Delta variant conferred by the vaccines. We adjusted for the age and gender of both index case and contact, the vaccine status of the index case, and the number of days of exposure from symptom onset or notification of the index to his or her isolation in a healthcare facility as these co-variates have prior data supporting possible effects on SARS-CoV-2 acquisition.[16], [17], [18], [19] To accommodate clustering we used generalized estimating equations in the logistic regression and bootstrap at the cluster level to derive relative risks, and hence vaccine effectiveness. In sensitivity analysis, we bootstrapped and adjusted for significant covariates through logistic regression. Statistical analysis was conducted in R.20 More details are available in the supplementary information.
2.6. Ethics
This work was performed as part of the outbreak investigation under the Infectious Diseases Act of Singapore.21
2.7. Role of the funding source
The sponsor(s) of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors were not precluded from accessing data in the study, and they accept responsibility to submit for publication.
3. RESULTS
3.1. Characteristics of COVID-19 cases and close contacts
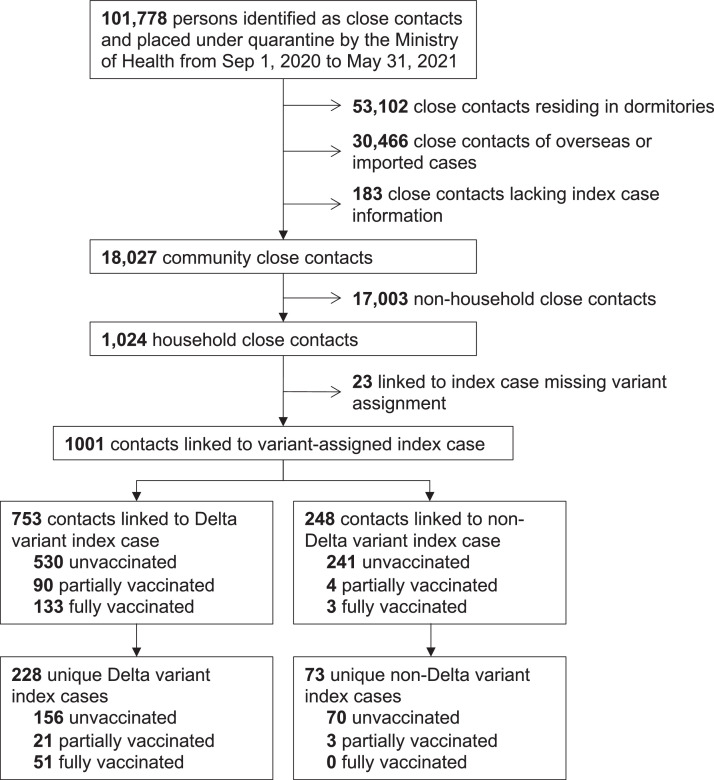
Between September 1, 2020 and May 31, 2021, 101,778 persons were identified as close contacts of COVID-19 cases and placed under quarantine. Close contacts residing in foreign worker dormitories, those linked to overseas or imported cases, and those lacking index case information were excluded, resulting in 18,027 close contacts associated with community cases, of whom 1,024 were classified as household close contacts, i.e. shared the same residence as the linked index case (Figure 1).
Figure 1.
Disposition of subjects identified by the Ministry of Health as close contacts of COVID-19 cases. 753 household close contacts linked to 228 unique Delta variant index cases was used for vaccine effectiveness estimation and risk factor analysis.
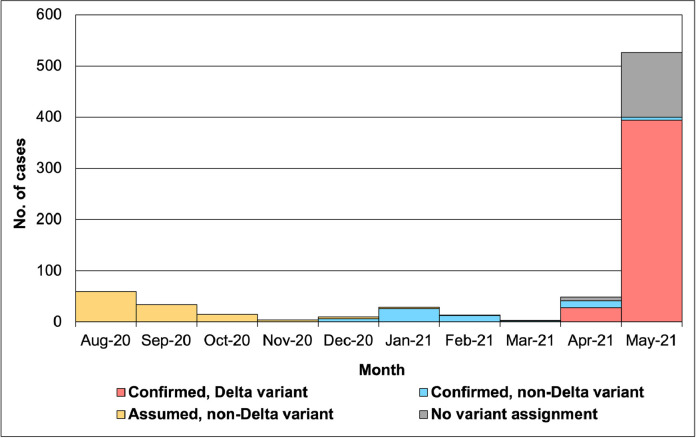
The SARS-CoV-2 Delta variant was first detected in Singapore on April 1, 2021, in an imported case. Among 743 community-acquired cases from August 1, 2020 to May 31, 2021, the first SARS-CoV-2 Delta variant infection was detected on April 27, 2021. By May 31, 2021, there were 422 confirmed community cases of the Delta variant, with 394 cases identified in May, i.e. 74.9% of all the community cases that month (Figure 2).
Figure 2.
Variant status of community cases in Singapore from Aug 1, 2020 to May 31, 2021. A total of 743 community cases were diagnosed from Aug 1, 2020 to May 31, 2021. The National Public Health Laboratory started whole genome sequencing (WGS)-based testing to identify SARS-CoV-2 variants from December 2020. It was assumed that all recorded cases in February 2021 and earlier with missing variant status information in the database were infected with variants other than the Delta variant of concern (“non-Delta variant”).
From February 1, 2021 to May 31, 2021, 23 (2.2%) household close contacts linked to nine index cases had missing data on the confirmed variant status of the index case in the household and were excluded from the analysis, of which all index cases had RT-PCR Ct value of greater than 30. Vaccinated index cases were not over-represented among the cases with no variant assigned as shown in Supplementary Table 5. Of the remaining 1,001 household close contacts, 753 were linked to 228 unique Delta variant index cases in the household (“Delta contact”), while 248 were linked to 73 unique non-Delta variant index cases (“non-Delta contact”) (Table 1). A breakdown of the PANGO lineage assignment for non-Delta variants are listed in Supplementary Table 1. None of the 301 index cases linked to the household close contacts nor any of the secondary cases identified in this dataset were re-infection cases. 72 (23.9%) Delta index cases received at least one dose of the COVID-19 vaccine before the contact quarantine start date, of which 51 (70.8%) of vaccinated individuals were fully vaccinated with both doses. In contrast, only 3 (4.1%) non-Delta index cases were partially-vaccinated.
Table 1.
Secondary attack rate among household close contacts (n=1,001)a of COVID-19 index cases, by variant status.
| Variant status of linked index case | ||
|---|---|---|
| Delta variant | non-Delta variant | |
| Number of contacts | 753 | 248 |
| Median age of contacts, in years (IQR) | 36 (25–51) |
35 (27–47) |
| Number of female contacts (%) | 354 (47.0) | 90 (36.3) |
| Number of unique index cases linked to all contacts | 228 | 73 |
| Median age of unique index cases linked to all contacts, in years (IQR) | 40b (28–53) |
36 (28–48) |
| Number of females among unique index cases linked to all contacts (%) | 91 (39.9) | 17 (23.3) |
| Unvaccinated indexes (%) | 156 (68.4) | 70 (95.9) |
| Vaccinated indexes (%) | 72 (31.6) | 3 (4.1) |
| Partially vaccinated (%) | 21 (9.2) | 3 (4.1) |
| Fully vaccinated (%) | 51 (22.4) | 0 |
| Number of unique contact groupsc,d,e | 228 | 73 |
| 1 contact | 45 | 22 |
| 2 contacts | 45 | 12 |
| 3 to 5 contacts | 112 | 31 |
| ≥ 6 contacts | 26 | 8 |
| Median number of contacts in each contact group (IQR)c | 3 (2–4) |
3 (1–5) |
| Mean number of contacts in each contact group (SD)c | 3 (2) | 3 (3) |
| Median days from symptom onset or notification of diagnosis (if asymptomatic) to hospital admission of index case (IQR) |
2 (1–3) |
2 (0–4) |
| Number of contact groups with no cases among contacts (%) | 135 (59.2) | 56 (76.7) |
| Number of contact groups with cases among contacts (%) | 93 (40.8) | 17 (23.3) |
| 1 case | 448 | 7 |
| 2 cases | 27 | 4 |
| 3 cases | 9 | 6 |
| ≥ 4 cases | 9 | 0 |
| Unvaccinated contacts (%) | 530 (70.4) | 241 (97.2) |
| Vaccinated contactsf (%) | 223 (29.6) | 7 (2.8) |
| Partially vaccinatedg (%) | 90 (12.0) | 4 (1.6) |
| Fully vaccinatedh (%) | 133 (17.7) | 3 (1.2) |
| Type of Vaccine (contacts) | ||
| Pfizer/BioNTech (% of vaccinated) | 185 (83.0) | 7 (100.0) |
| Moderna (% of vaccinated) | 38 (17.0) | 0 |
| Others | 0 | 0 |
| Number of cases among all contacts | 169 | 33 |
| Secondary attack rate (%) (95% BCI) | 22.4 (18.1–27.1) |
13.3 (7.3–20.6) |
| Number of cases among fully vaccinated contacts | 15 | 1 |
| Secondary attack rate (fully vaccinated) (%) (95%BCI) | 11.3 (6.1–17.3) |
33.3 (0–100)i |
| Number of cases among unvaccinated contacts | 137 | 31 |
| Secondary attack rate (unvaccinated) (%) (95% BCI) | 25.8 (20.6–31.5) |
12.9 (7.0–20.0) |
Abbreviations: BCI, bootstrap confidence interval; IQR, inter-quartile range; SD, standard deviation
Of 1024 household close contacts, 1001 (97.8%) contacts were linked to an index case with variant status data. 23 (2.2%) contacts did not have variant status data of the index case.
Based on 227 of 228 contacts, as data (age) was missing for one person
Contact group refers to a group consisting of an index case and their close contacts.
Refers to the number of contacts, excluding the linked index case(s).
Data and analysis for these endpoints for non-Delta variants were not available as linked severity data was not available prior to 1 Jan 2021.
Vaccination is defined as having received at least one dose of vaccine before the start date of quarantine.
Partially vaccinated is defined to mean having received one vaccine dose or to be within 14 days of the second vaccine dose on the start date of quarantine.
Fully vaccinated is defined to mean having received both doses of vaccine more than 14 days prior to the start date of quarantine.
Due to the small number of fully vaccinated non-Delta contacts, a usable estimate of the CI could not be obtained
Among Delta contacts, the median age was 36 (inter-quartile range [IQR] 25—51) and 354 (47.0%) were female, while non-Delta contacts had a median age of 35 (IQR 27—47) and 90 (36.3%) were female. The median age of Delta variant and non-Delta variant index cases was 40 (IQR 28—53) and 36 (IQR 28—48), respectively; 91 (39.9%) and 17 (23.3%) were female, respectively. 223 (29.6%) Delta contacts received at least one dose of the COVID-19 vaccine before their quarantine start date, of which 133 (59.6%) of vaccinated individuals were fully vaccinated with both doses. As vaccination only started from December 2020, in contrast, only seven (2.8%) non-Delta contacts were vaccinated, of whom three had received both doses.
The secondary attack rate, regardless of symptoms, among unvaccinated Delta contacts was 25.8% (95% bootstrap confidence interval [BCI] 20.6–31.5%) compared to 12.9% (95%BCI 7.0–20.0%) among unvaccinated non-Delta contacts. The secondary attack rates, regardless of symptoms, among fully vaccinated Delta contacts was 11.3% (95%BCI 6.1–17.3%) compared to 33.3% (there were very few fully vaccinated non-Delta contacts so a usable estimate of the CI cannot be obtained) among fully vaccinated non-Delta contacts (Table 1).
3.2. Overall risk by variant-vaccine combination
There was a significantly lower acquisition risk for fully vaccinated than unvaccinated contacts exposed to the Delta variant (Relative risk [RR] 0.44, 95%BCI 0.24–0.67; i.e. vaccine effectiveness against acquisition of 56.4% [95%BCI 32.6–75.8%]) (Table 2). The vaccine effectiveness was effectively unchanged when we repeated the bootstrapping adjusting for age of the index case (vaccine effectiveness [VE] 61.6, 95%CI 37.5–80.4). Complete vaccination was associated with reduced risk of symptomatic disease (VE 64.1%, 95%BCI 37.8–85.4%; or VE 67.9%, 95%BCI 41.3–87.8 after adjustment). No fully vaccinated contacts developed severe illness; 2.3% (95%BCI 1.1–3.6%) of those unvaccinated did (Table 2).
Table 2.
Vaccine effectiveness against SARS-CoV-2 acquistion, symptomatic COVID-19 disease and severe COVID-19 illness among household contacts exposed to the Delta variant.
| Unvaccinated contacts (N=530) | Unvaccinated attack rate (%) (95%BCI) | Fully vaccinated contacts (N=133) | Fully vaccinated attack rate (%) (95%BCI) | Unadjusted VE (95%BCI) | Adjusteda VE (95%BCI) | |
|---|---|---|---|---|---|---|
| SARS-CoV-2 acquisition | 137 | 25.8 (20.6–31.5) |
15 | 11.3 (6.1–17.3) |
56.4 (32.6–75.8) |
61.6 (37.5–80.4) |
| Symptomatic infection | 111 | 20.9 (15.9–26.3) |
10 | 7.5 (3.1–12.7) |
64.1 (37.8–85.4) |
67.9 (41.3–87.8) |
| Severe infection | 12 | 2.3 (1.1–3.6) |
0 | 0b | 100b | NAc |
Abbreviations: BCI, bootstrap confidence interval; VE, vaccine effectiveness; NA, not applicable
Adjusted for age and gender of the index and contact, vaccination status of the index case and time interval between symptom onset or notification (if asymptomatic) or notification (if asymptomatic) and hospitalization of the index case
The 95%CI could not be calculated because no vaccinated household member had a severe infection.
Adjusted vaccine effectiveness could not be calculated for the same reason.
There was a significantly higher risk of infection by the Delta variant compared to other variants, among unvaccinated contacts (RR 2.01, 95%BCI 1.24–3.84). The higher risk of infection remained unchanged when bootstrapping was repeated adjusting for age of the index case (RR 2.02, 95%BCI 1.22–3.91).
3.3. Analysis of risk factors by logistic regession for acquisition and symptomatic illness
After adjusting for age, gender and vaccination status of both the contact and index, as well as time exposure of the contact to the index case (i.e. time interval from symptom onset or notification (if asymptomatic) to hospitalization of index case due to COVID-19), vaccination status of the index was not associated with statistically significant difference for contact SARS-CoV-2 acquisition (adjusted odds ratio [aOR] 0.73, 95% robust confidence interval [RCI] 0.38–1.40). However, older index cases had more secondary infections (aOR 1.20 per decade, 95%CI 1.03–1.39) (Table 3). There was no evidence of other demographic effects on acquisition or symptomatic illness (Table 3 and Supplementary Table 3). Sensitivity analysis assuming all contacts with unassigned variants were exposed to the Delta variant did not alter the study inferences (Supplementary Table 6).
Table 3.
Multivariable logistic regression of risk factors associated with SARS-CoV-2 acquisition by household contacts of index cases infected with the Delta variant (N=753). Features of both contact and index are considered. Models are fit with generalized estimating equations to account for clustering at the household level.
| Variables | Case N=169 | Control N=584 | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|---|---|
| Person | Risk factor | OR (95%RCI) | P Value | aOR (95%RCI) | P Value | ||
| Contact | Unvaccinated | 137 | 393 | Ref | Ref | Ref | Ref |
| " | Partially vaccinateda | 17 | 73 | 0.67 (0.38–1.19) | 0.17 | 0.61 (0.33–1.12) | 0.11 |
| " | Fully vaccinatedb | 15 | 118 | 0.36 (0.20–0.66) | 0.0009 | 0.33 (0.17–0.63) | 0.0009 |
| " | Female | 83 | 271 | Ref | Ref | Ref | Ref |
| " | Male | 86 | 313 | 0.90 (0.62–1.29) | 0.56 | 0.92 (0.64–1.33) | 0.66 |
| " | Age (per decade) | — | — | 1.01 (0.90–1.12) | 0.90 | 1.07 (0.95–1.20) | 0.30 |
| " | Exposure to indexc (days) | — | — | 0.98 (0.91–1.05) | 0.59 | 0.97 (0.90–1.05) | 0.46 |
| Index | Unvaccinated | 127 | 425 | Ref | Ref | Ref | Ref |
| " | Partially vaccinateda | 13 | 50 | 0.87 (0.33–2.27) | 0.78 | 0.62 (0.22–1.69) | 0.35 |
| " | Fully vaccinatedb | 29 | 109 | 0.89 (0.49–1.61) | 0.70 | 0.73 (0.38–1.40) | 0.34 |
| " | Female | 74 | 218 | Ref | Ref | Ref | Ref |
| " | Male | 95 | 366 | 0.76 (0.47–1.25) | 0.29 | 0.86 (0.49–1.49) | 0.59 |
| " | Age (per decade) | — | — | 1.17 (1.02–1.33) | 0.021 | 1.20 (1.03–1.39) | 0.02 |
Abbreviations: aOR, adjusted odds ratio; RCI, robust confidence interval; Ref, reference level
Partially vaccinated is defined to mean having received one vaccine dose or to be within 14 days of the second vaccine dose on the start date of quarantine.
Fully vaccinated is defined to mean having received both doses of vaccine more than 14 days prior to the start date of quarantine.
Refers to the time interval between symptom onset or notification (if asymptomatic) and hospitalization of the index case
4. DISCUSSION
This study examined the impact of the Delta variant and vaccination among known index case-contact pairs in households to determine the secondary attack rates and risk factors for COVID-19 transmission. The household secondary attack rate among unvaccinated contacts exposed to index cases infected with the Delta variant was higher at 25.8% (95%BCI 20.6–31.5%) compared with 12.9% (95%CI 7.0–20.0%) among unvaccinated contacts exposed to other variants. There is a significantly higher risk of infection among unvaccinated close contacts by the Delta variant compared to other variants (RR 2.01, 95%BCI 1.24–3.84). This would explain the rapid and extensive epidemic in India, the first country that reported the Delta variant in late 20201; other South Asian countries with Delta variant-driven epidemics; and the difficulty in controlling the outbreaks caused by the Delta variant in countries and regions such as Singapore and Australia that have hitherto been successful in containing COVID-19 spread.1,22,23 This could be due to the higher viral load and prolonged viral shedding in persons infected with the Delta variant (Singapore unpublished data), and should be the focus of further studies on SARS-CoV-2 variants.
An interesting finding is that older primary cases had more secondary infections than younger cases did (aOR 1.20 per decade, 95%RCI 1.03–1.39), a finding corroborated by prior studies. A study from Korea of 4,048 household clusters determined that SARS-CoV-2 transmission was more common from adults to children than from children to adults.24 The increased transmission of SARS-CoV-2 from adults compared with children was also supported by studies from Spain and Germany.25,26 This is unlike many other respiratory diseases such as influenza, where children and young adults tend to be the conduits of transmission.27 More studies should be performed to determine the possible causes for this observation—whether older cases have more viral shedding, or increased interaction with household members that facilitate transmission.
Among contacts exposed to the Delta variant, there is a lower risk of being infected by SARS-CoV-2 for fully vaccinated compared to unvaccinated contacts (Relative risk [RR] 0.44, 95%BCI 0.24–0.67), and lower risk of symptomatic disease in both fully (RR 0.36 [95%BCI 0.15–0.62]) and partially (RR 0.37 [95%BCI 0.13–0.70]) vaccinated contacts. The vaccine effectiveness of 56.4% (95%BCI 32.6–75.8%) for PCR-confirmed infections, or 64.1% (95%BCI 37.8–85.4%) against symptoms, provide important information on the overall effectiveness of the mRNA vaccines used in Singapore. While the estimated vaccine effectiveness of 56.4% against Delta variant acquisition (regardless of symptoms) and 64.1% against symptomatic disease is lower than the 88% (95%CI 85.3–90.1) reported for the Pfizer/BioNTech BNT162b2 vaccine at preventing symptomatic infection by the Delta variant in England3, it is important to note that the household setting is one where vaccine effectiveness estimates are likely to be the lowest, given the context of prolonged close contact between members of the same household. Our estimates show that even for the Delta variant, the mRNA vaccines remain very effective at preventing all infections—both symptomatic and asymptomatic. Asymptomatic infections, if unprevented and undetected, can be the conduits of community transmission for SARS-CoV-2. In addition to preventing infections, vaccinated individuals also have significantly less severe outcomes if infected2, although in this study the absence of any severe outcomes in the vaccinated group prevented accurate estimation of the residual risk of severe Covid-19. The estimate of 100% vaccine effectiveness against this outcome should therefore be treated with caution.
Our analysis did not demonstrate a statistically-significant reduction in household contact SARS-CoV-2 acquisition comparing vaccinated index cases with unvaccinated index cases. Our point estimate suggested a possibility of reduced onward transmission and the ability to demonstrate a statistically-significant difference could be affected by the study sample size and index case misclassification. Primary cases who are vaccinated are less likely to experience symptomatic infection3, and thus less likely to be tested and diagnosed as index cases. As individuals with more symptoms are associated with increased infectiousness28, underdetection of asymptomatic or minimally symptomatic vaccinated index cases could result in underestimation of the preventive effect of vaccination on onward transmission to household contacts. In comparison, Harris and colleagues found that the likelihood of household transmission (pre-Delta) was lowered by approximately 40 to 50% in vaccinated index patients29, and another study (pre-print) from Guangdong, China, reported that unvaccinated Delta index cases were more likely to transmit infection to their contacts than those who had received two doses of vaccine.30 If infected cases who are vaccinated remain sources of onward infection, it is important to ensure that testing and isolating of cases, and public health measures to prevent the community spread of COVID-19, remain in place until high levels of immunity from vaccination and/or natural infection is present. Many countries are relaxing measures such as mask-wearing and testing for vaccinated indvidiuals.31,32 If these are not in place, infection among vaccinated individuals, especially if asymptomatic or mild, are likely to go undetected and may result in spread to other individuals who are not immune to SARS-CoV-2, resulting in further waves of infection.
This study has some limitations. The risk of household contacts acquiring infections from non-household sources cannot be excluded, but this would be minimal due to the low number of community cases in Singapore during the study period. The index case, which is the first diagnosed COVID-19 infected person in the household may in some cases not be the true primary case of infection. However this scenario would likely be minimized due to aggressive contact tracing and establishment of transmission chains in Singapore since the first diagnosed case, and where possible the assignment of the index case would be dependent on the earliest symptom onset or likely transmission source outside of the household.33,34 The older age of vaccinated indexes could have affected the estimates of the preventive effect of vaccination on onward transmission, a confounding which was adjusted for in the logistic regression (Table 3 and Supplementary Table 4).
Contacts’ symptom data was based on interview by MOH contact tracers soon after index case diagnosis and may have resulted in pre-symptomatic individuals (who were asymptomatic at time of interview) being classified as asymptomatic, a misclassification which could affect the estimates of vaccine effectiveness against symptomatic disease and not the esimates of vaccine effectiveness against acquisition or severe illness. Chronic disease data is not routinely captured in the MOH contact tracing database and hence we were not able to adjust for chronic disease in our risk factor analysis. Multiple null hypothesis significance tests were conducted, based on potential risk factors that were deemed epidemiologically important, and so some caution should be taken in interpreting the results as no correction for multiple testing was attempted. Bonferroni correction for the logistic regression would result in the age of vaccinated indexes not meeting threshold for statistical significance but would not alter the inference with regards to fully vaccinated individuals.
The majority of non-Delta household indexes occurred prior to March 2021 and all the household Delta index cases occurred after March 2021, and there were differences in public measures between these time periods. The main measure potentially affecting household exposures is the increased recommendation for telecommuting (Supplementary Table 2). If there was significantly increased exposure time within the household as a result, this may contribute to over-estimation of the increased transmissibility of the Delta variant but would not be expected to bias the estimates of vaccine effectiveness or determinants of Delta variant transmission.
5. CONCLUSION
Individuals exposed to the Delta variant have an increased risk of SARS-CoV-2 acquisition compared to those exposed to other variants. This risk is reduced by 55% among vaccinated individuals, which shows the importance of high vaccination coverage to increase population-level immunity. Our findings suggest that vaccinated cases remain at risk of onward transmission of SARS-CoV-2 Delta, and therefore it is important to continue to contain spread through testing and isolation, and public health measures to prevent widespread community transmission if significant numbers of at risk persons remain unvaccinated.
6. CONTRIBUTORS
OTN and KM conceived of and led the study. CJC, TMM, JKC, SSHO, YKL, KBT were involved in data collection. OTN, VK, NMT, ZF and AKJ have accessed and verified the data. OTN, VK, NMT, ARC and VLJM were involved in data analysis, data interpretation and writing of the manuscript in consultation with CJC, KM, TMM, JKC, SSHO, YKL, ZF, AKJ, MI-CC, SM-S, LC, RTPL, KBT, ARC and YSL.
7. DATA SHARING STATEMENT
Individual-level participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices), is regarded as sensitive and will not be shared. The study methods and statistical analyses are described in detail in the manuscript.
Declaration of Competing Interest
We declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by funds administered by the Singapore Ministry of Health's National Medical Research Council, namely, the NMRC COVID-19 Research Fund (MOH-000469 and MOH-000717), the NMRC Collaborative Grants: Collaborative Solutions Targeting Antimicrobial Resistance Threats in Health Systems (CoSTAR-HS) (NMRC CGAug16C005) and the Singapore Population Health Improvement Centre (NMRC/CG/C026/2017_NUHS), NMRC Clinician Scientist Award (MOH-000276) and NMRC Clinician Scientist Individual Research Grant (MOH-CIRG18Nov-0034). Additional support was provided by the German Federal Ministry of Health (BMG) COVID-19 Research and development funding to WHO (Award number 70826). Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not reflect the views of MOH/NMRC/WHO.
Footnotes
Funding: National Medical Research Council.
Contributor Information
Oon Tek Ng, Email: oon_tek_ng@ncid.sg.
Prof. Vernon JM Lee, Email: vernon_lee@moh.gov.sg.
REFERENCES
- 1.GISAID. GISAID: Tracking of Variants. https://www.gisaid.org/hcov19-variants/ (accessed June 22, 2021).
- 2.Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. The Lancet. 2021 doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez Bernal J, Andrews N, Gower C. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021 doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richterman A, Meyerowitz EA, Cevik M. Indirect Protection by Reducing Transmission: Ending the Pandemic with SARS-CoV-2 Vaccination. Open Forum Infectious Diseases. 2021 doi: 10.1093/ofid/ofab259. published online May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng OT, Marimuthu K, Koh V. SARS-CoV-2 seroprevalence and transmission risk factors among high-risk close contacts: a retrospective cohort study. The Lancet Infectious Diseases. 2021;21:333–343. doi: 10.1016/S1473-3099(20)30833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook AR, Dickens BL, Wilder-Smith A. Differential Household Attack Rates Mirror the Ability to Control Coronavirus Disease 2019 (COVID-19) Clinical Infectious Diseases. 2021;72:e1166–e1167. doi: 10.1093/cid/ciaa1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madewell ZJ, Yang Y, Longini IM, Halloran ME, Dean NE. Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Y, Koh V, Marimuthu K. Epidemiological and Clinical Predictors of COVID-19. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa322. published online March 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng Y, Li Z, Chua YX. Evaluation of the Effectiveness of Surveillance and Containment Measures for the First 100 Patients with COVID-19 in Singapore — January 2–February 29, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:307–311. doi: 10.15585/mmwr.mm6911e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rambaut A, Holmes EC, O'Toole Á. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5:1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shu Y, McCauley J.GISAID. Global initiative on sharing all influenza data - from vision to reality. Euro Surveill. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.A*STAR Bioinformatics Institute. CoVSurver. 2020 https://corona.bii.a-star.edu.sg.
- 13.Ministry of Health, Singapore. MOH News Highlight: Government Accepts Recommendations Of Expert Committee On Covid-19 Vaccination. 2020; published online Dec 27. https://www.moh.gov.sg/news-highlights/details/government-accepts-recommendations-of-expert-committee-on-covid-19-vaccination (accessed June 18, 2021).
- 14.Ministry of Health, Singapore. MOH News Highlight: Covid-19 Vaccination Brought Forward For All Seniors; Extended To Essential Services Personnel And Higher Risk Groups. 2021; published online March 8. https://www.moh.gov.sg/news-highlights/details/covid-19-vaccination-brought-forward-for-all-seniors-extended-to-essential-services-personnel-and-higher-risk-groups (accessed June 18, 2021).
- 15.Ministry of Health, Singapore. MOH News Highlight: Updates On Local Situation And Vaccination Programme. 2021; published online May 30. https://www.moh.gov.sg/news-highlights/details/updates-on-local-situation-and-vaccination-programme (accessed June 18, 2021).
- 16.Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 17.Dehingia N, Raj A. Sex differences in COVID-19 case fatality: do we know enough? Lancet Glob Health. 2021;9:e14–e15. doi: 10.1016/S2214-109X(20)30464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020:7. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Zhang M, Yang L. Prevalence and patterns of tobacco smoking among Chinese adult men and women: findings of the 2010 national smoking survey. J Epidemiol Community Health. 2017;71:154–161. doi: 10.1136/jech-2016-207805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Core Team R. R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A language and environment for statistical computing. https://www.R-project.org/ [Google Scholar]
- 21.Singapore Statutes Online. Infectious Diseases Act (Chapter 137). 2003; published online July 31. https://sso.agc.gov.sg/Act/IDA1976 (accessed Sept 17, 2020).
- 22.World Health Organization. COVID-19 situation report for the Western Pacific Region #57: 9 June 2021 - 15 June 2021. 2021; published online June 16. https://www.who.int/westernpacific/internal-publications-detail/covid-19-situation-report-for-the-western-pacific-region-57-9-june-2021-15-june-2021 (accessed June 22, 2021).
- 23.Campbell F, Archer B, Laurenson-Schafer H. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.24.2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi S, Kim Y-M, Choe YJ, Ahn S, Han S, Park Y-J. Geospatial Analysis of Age-specific SARS-CoV-2 Transmission Patterns in Households, Korea. J Korean Med Sci. 2021;36:e63. doi: 10.3346/jkms.2021.36.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soriano-Arandes A, Gatell A, Serrano P. Household SARS-CoV-2 transmission and children: a network prospective study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab228. published online March 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galow L, Haag L, Kahre E. Lower household transmission rates of SARS-CoV-2 from children compared to adults. Journal of Infection. 2021;83:e34–e36. doi: 10.1016/j.jinf.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worby CJ, Chaves SS, Wallinga J, Lipsitch M, Finelli L, Goldstein E. On the relative role of different age groups in influenza epidemics. Epidemics. 2015;13:10–16. doi: 10.1016/j.epidem.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu X, Nergiz AI, Maraolo AE, Bogoch II, Low N, Cevik M. The role of asymptomatic and pre-symptomatic infection in SARS-CoV-2 transmission—a living systematic review. Clinical Microbiology and Infection. 2021;27:511–519. doi: 10.1016/j.cmi.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris RJ, Hall JA, Zaidi A, Andrews NJ, Dunbar JK, Dabrera G. Effect of Vaccination on Household Transmission of SARS-CoV-2 in England. The New England Journal of Medicine. 2021:2. doi: 10.1056/NEJMc2107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang M, Xin H, Yuan J, et al. [Preprint] Transmission dynamics and epidemiological characteristics of Delta variant infections in China. medRxiv2021;: 2021.08.12.21261991.
- 31.Cabinet Office, United Kingdom. COVID-19 Response - Spring 2021 (Summary). 2021 https://www.gov.uk/government/publications/covid-19-response-spring-2021/covid-19-response-spring-2021-summary#step-4-not-before-21-june (accessed June 22, 2021).
- 32.Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. 2021 https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated-guidance.html (accessed June 22, 2021).
- 33.Pung R, Chiew CJ, Young BE. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. The Lancet. 2020;395:1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yong SEF, Anderson DE, Wei WE. Connecting clusters of COVID-19: an epidemiological and serological investigation. The Lancet Infectious Diseases. 2020 doi: 10.1016/S1473-3099(20)30273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]