Introduction
Hepatic sinusoidal obstruction syndrome (HSOS) is a hepatic vascular disease characterized by injury of the endothelial cells in the sinusoidal hepatic and interlobular veins, intra-hepatic congestion, liver dysfunction, and portal hypertension [1]. In Western countries, HSOS is often associated with myeloablative regimens used for hematopoietic stem-cell transplantation, while, in China, it is often associated with oral intake of Gynura segetum plants that contain pyrrolidine alkaloids [2]. In addition, new-onset HSOS after solid-organ transplantation has received increasing attention [3–8]. In this study, we report a case of HSOS caused by tacrolimus in post-liver-transplantation (LT) patients and present for the first time the dynamic course with complete clinical, radiological, and pathological information.
Case report
A 41-year-old Chinese male underwent LT on 23 December 2018 for hepatocellular carcinoma (single, 3 cm in diameter) in the setting of Wilson’s disease. No specific pathology was observed in the donated liver at the time of transplantation (Figure 1A). The transplant operation was performed successfully. The patient was discharged 14 days after LT and administered a tapering course of corticosteroids, mycophenolate mofetil (MMF), and tacrolimus (4 mg twice daily).
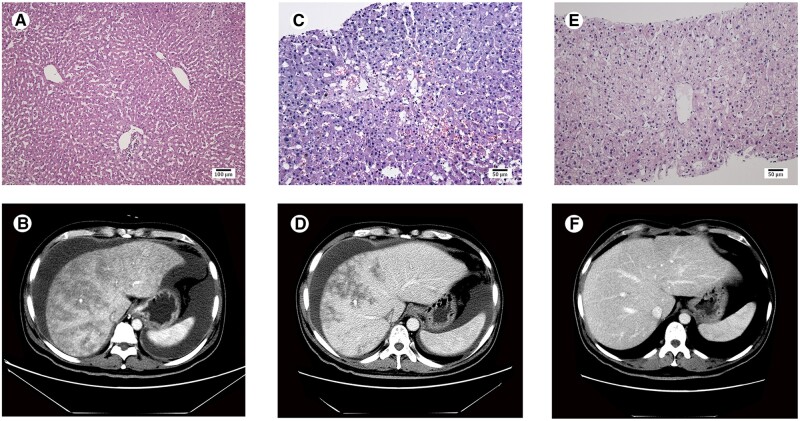
Figure 1.
Pathological manifestations and computed tomography imaging of the liver. Histological examination of the donated liver at the time of transplantation is normal (A). Liver biopsy shows significant dilation and congestion of the hepatic sinusoids in zone 3 of the hepatic acinus, regional hepatocellular necrosis, infiltration of the space of Disse by a few red blood cells, and deposition of collagenous fiber in the centrilobular veins on day 58 after transplantation (C) and recovered sinusoidal and centrilobular vein lesions on day 174 after transplantation (E). Abdominal computed tomography scan shows enlarged liver with patchy enhancement, massive ascites, and obscured hepatic veins on day 52 after transplantation (B), improved patchy enhancement, reduced ascites, and clear hepatic veins on day 90 after transplantation (D), and normal liver and hepatic veins without ascites on day 136 after transplantation (F). A, HE staining, ×100; C, HE staining, ×200; E, HE staining, ×200.
The patient was readmitted on day 36 after LT due to symptoms of abdominal distension and weight gain of 3 kg. Physical examination revealed shifting dullness. The results of laboratory examination including hepatic function, renal function, blood routine, and coagulation function were normal. The serum trough concentration of tacrolimus was 6.1 ng/mL. Ultrasonography demonstrated a moderate amount of ascites and enlarged liver without hepatic vascular problems. The immunosuppressive regimen at that time included tacrolimus (4 mg twice daily) combined with MMF (750 mg twice daily). Although diuretics were administered, the patient’s symptoms deteriorated with 9 kg weight gain on day 52 after transplantation. Abdominal computed tomography (CT) showed an enlarged liver with patchy enhancement, massive ascites, and obscured hepatic veins (Figure 1B). Serological testing demonstrated abnormal hepatic and renal function. The serum trough concentration of the tacrolimus was 9.2 ng/mL and the patient tested negative for hepatitis A, B, C, D, and E virus as well as cytomegalovirus and Epstein-Barr virus. Abdominal paracentesis showed no evidence of infection. A percutaneous liver biopsy was performed after complete drainage of the ascites on day 58 after transplantation. Sinusoidal congestion and deposition of collagenous fiber in the centrilobular veins were observed on the pathological slides (Figure 1C) in favor of the diagnosis of HSOS, excluding rejection, hepatotropic virus infection, chylous fistula, and obstruction of outflow. Based on this, we speculated that HSOS was secondary to tacrolimus due to its potential hepatotoxicity. Therefore, we switched the treatment from tacrolimus to cyclosporine A (CSA) 150 mg twice daily, with MMF being continued.
On day 90 after LT, the patient’s symptoms alleviated with 10 kg weight loss from the maximum weight. Abdomen CT scan showed improved patchy enhancement, mild ascites, and clear hepatic veins (Figure 1D). Hepatic and renal functions were normal. On day 136, CT scan showed normal liver and hepatic veins without ascites (Figure 1F) and hepatic and renal functions remained normal. On day 174, the second liver biopsy was performed and the pathology demonstrated no histologic features of HSOS (Figure 1E). The patient remained asymptomatic on CSA and MMF. Clinical characteristics during the disease course are shown in Supplementary Table 1.
Discussion
HSOS is a rare complication in LT recipients, with a reported incidence of ∼2% in the literature [9]. Despite its low incidence, HSOS can still cause graft failure. HSOS after LT is diagnosed after excluding other causative factors that can result in the obstruction of liver blood flow, such as rejection, hepatotropic virus infection, biliary complications, and vascular thrombosis or anastomosis stenosis. Extra-hepatic signs, such as ascites, hydrothorax, and splenomegaly, may be present. The typical CT findings include diffuse hepatomegaly, mottle-like heterogeneous hepatic parenchymal enhancement, and stenotic or obscured hepatic vein lumen [2]. Pathology is the golden diagnostic criterion characterized by sinusoid congestion, fibrosis and occlusion of hepatic lobular veins, and hepatic cell hemorrhagic necrosis.
Acute cellular rejection (ACR) is a major causative factor for HSOS in LT recipients [3, 9]. Azathioprine (AZA) therapy is another recognized predisposing factor for HSOS after organ transplantation [4], but has been rarely used in the current era due to its frequent vascular hepatotoxicity. With the application of new immunosuppressants, the occurrence of ACR- and AZA-related HSOS has decreased significantly. However, caution should be paid to the new immunosuppressants that are metabolized by the liver, such as tacrolimus. Tacrolimus is one of the most widely used calcineurin inhibitors and has been proven to be safe and effective in the prophylaxis and treatment of acute rejection among organ transplants. Newly diagnosed HSOS cases caused by tacrolimus have been reported in lung [5] and pancreas transplantations [6], as well as in LT recipients [7, 8]. The mechanism by which tacrolimus causes HSOS is unclear. A better comprehension of the genetic polymorphisms of cytochrome P450 and glutathione-S-transferase may be important in understanding the pathogenesis of HSOS [10]. Due to the lack of specific therapies for HSOS [1, 2], timely identification and removal of the initiating factor are crucial.
The patient in our study manifested abdominal distension, weight gain, and refractory ascites after LT, which were not relieved by routine diuretic therapy. HSOS was diagnosed according to the characteristic histological manifestations with congestion of the hepatic sinusoids. Considering that there was no evidence of rejection and AZA and other suspicious drugs were not applied, we speculated that tacrolimus was the main predisposing factor for HSOS. Clinical, radiological, and histological evidence of HSOS regression was observed after tacrolimus discontinuation, which further supported the inference that HSOS was triggered by tacrolimus. To the best of our knowledge, this is the first case reported with sequential liver biopsies, which provides direct evidence for tacrolimus-associated HSOS.
In conclusion, we describe a case of HSOS after LT, which was reversed following the withdrawal of tacrolimus. Tacrolimus should be considered a possible causative agent in LT recipients who present with HSOS on liver grafts.
Supplementary data
Supplementary data is available at Gastroenterology Report online.
Authors’ contributions
S.N.Z. and N.Z. drafted the manuscript; S.N.Z., D.N.F., H.L.L., and Z.W.L. treated the patient; S.N.Z., D.N.F., and X.Z. collected clinical data; Y.L.S. disposed of liver tissues and analysed the pathological data; Y.W.L. did radiological description and provided the computed tomography images; H.L.L. and Z.W.L. designed the study; N.Z. and J.D.Y. searched the literature and critically revised the manuscript. All authors read and confirmed the final version of this paper.
Funding
This work was supported by Beijing Municipal Science and Technology Commission Funding Project [Z161100000116058] and 302 Military Hospital Project [YNKT 2014006].
Supplementary Material
Acknowledgements
The study protocol was approved by the ethical committee of Fifth Medical Center of Chinese PLA General Hospital and written informed consent was obtained in accordance with the Declaration of Helsinki.
Conflicts of interest
None declared.
References
- 1.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: vascular diseases of the liver. J Hepatol 2016;64:179–202. [DOI] [PubMed] [Google Scholar]
- 2. Zhuge Y, Liu Y, Xie W, Chinese Society of Gastroenterology Committee of Hepatobiliary Disease et al. Expert consensus on the clinical management of pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome. J Gastroenterol Hepatol 2019;34:634–42. [DOI] [PubMed] [Google Scholar]
- 3. Sanei MH, Schiano TD, Sempoux C. et al. Acute cellular rejection resulting in sinusoidal obstruction syndrome and ascites postliver transplantation. Transplantation 2011;92:1152–8. [DOI] [PubMed] [Google Scholar]
- 4. Katzka DA, Saul SH, Jorkasky D. et al. Azathioprine and hepatic venocclusive disease in renal transplant patients. Gastroenterology 1986;90:446–54. [DOI] [PubMed] [Google Scholar]
- 5. Shah S, Budev M, Blazey H. et al. Hepatic veno-occlusive disease due to tacrolimus in a single-lung transplant patient. Eur Respir J 2006;27:1066–8. [DOI] [PubMed] [Google Scholar]
- 6. Wang SE, Shyr YM, Lee RC.. Hepatic veno-occlusive disease related to tacrolimus after pancreas transplantation. J Chin Med Assoc 2013;76:358–60. [DOI] [PubMed] [Google Scholar]
- 7. Shen T, Feng XW, Geng L. et al. Reversible sinusoidal obstruction syndrome associated with tacrolimus following liver transplantation. World J Gastroenterol 2015;21:6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hou Y, Tam NL, Xue Z. et al. Management of hepatic vein occlusive disease after liver transplantation: A case report with literature review. Medicine (Baltimore) 2018;97:e11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sebagh M, Azoulay D, Roche B. et al. Significance of isolated hepatic veno-occlusive disease/sinusoidal obstruction syndrome after liver transplantation. Liver Transpl 2011;17:798–808. [DOI] [PubMed] [Google Scholar]
- 10. Srivastava A, Poonkuzhali B, Shaji RV. et al. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood 2004;104:1574–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.