Abstract
Functional constipation (FC) is common, yet the etiology is not clear. Accumulating evidence suggests an association between FC and abnormal gut microbiota. The relationship between the gut microbiota and the gut transit is likely bidirectional. This review summarizes the current evidence regarding the impact of gut microbiota on the pathogenesis of FC. By modulating the colonic motility, secretion, and absorption, gut microbiota may contribute to the development of FC through microbial metabolic activities involving bile acids, short-chain fatty acids, 5-hydroxytryptamine, and methane. In support of the key roles of the gut microbiota in FC, treatment with probiotics, prebiotics, synbiotics, and traditional Chinese medicine often result in compositional and functional changes in the gut microbiota. Further studies on the pathogenesis of FC and the therapeutic mechanism of microecological agents will provide a knowledge base for better management of FC.
Keywords: gut microbiota, functional constipation, bile acids, SCFA, serotonin, traditional Chinese medicine
Introduction
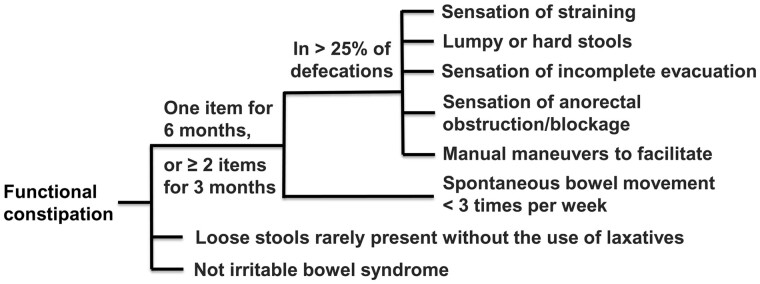
Functional constipation (FC) refers to constipation without an organic etiology [1, 2]. Patients with FC have symptoms of predominantly difficult, infrequent, or a feeling of incomplete defecation, which may be accompanied by abdominal pain and bloating. FC has a significant impact on patients’ quality of life. According to the Rome IV criteria, to diagnose FC [3] (Figure 1), the patient must have one of the following conditions for more than 6 months and have two or more of the following conditions within the last 3 months: (i) sensation of straining during >25% of defecations, (ii) lumpy or hard stools of >25% of defecations (Bristol stool type 1 and 2) [4], (iii) sensation of incomplete evacuation during >25% of defecations, (iv) sensation of anorectal obstruction/blockage during >25% of defecations, (v) manual maneuvers for >25% of defecations, and (vi) fewer than three spontaneous bowel movements per week. In addition, diagnosis of FC should meet the requirement that loose stools are rarely present without using laxatives and that irritable bowel syndrome (IBS) is not diagnosed at the same time.
Figure 1.
Diagnosis of functional constipation according to the Rome IV criteria.
A recent demographic survey of 6,300 cases from three countries showed that the prevalence of FC was 6.9% (95% confidence interval [CI], 5.8%–8.0%) in the USA, 7.9% (95% CI, 6.7%–9.1%) in Canada and 8.6% (95% CI, 7.4%–9.9%) in the UK, according to the Rome IV criteria [5]. Globally, the prevalence of FC from 1947 to 2010 was 14% (95% CI, 12.0%–17.0%) according to the Rome I, II, III criteria or another informal diagnostic standard. South Asia and East Asia had the lowest prevalence of 11% (95% CI, 7.0%–15.0%), while South America had the highest prevalence of 18% (95% CI, 15.0%–22.0%). Caution needs to be exercised in comparing the incidences of FC in different countries since the diagnosis criteria and the methods for data collection may differ among studies. FC is positively associated with age and more frequently occurs in people who are >60 years old [6]. The pathophysiology of FC remains unknown, but it is generally considered to be multifactorial. Recognized pathophysiological factors include genetic traits; lifestyle including diet, fluid intake, physical activity; colonic dismotility; psychological factors such as anxiety and depression; and the gut microbiota, which is the main focus of this review. Traditionally, three types of FC have been recognized: normal-transit constipation (NTC), slow-transit constipation (STC) and rectal-evacuation disorders [7]. The majority of FC patients have NTC (65%), followed by evacuation disorder (30%) and STC (5%) [8].
The first choices for FC treatment are nonpharmacological interventions including education on toileting posture and behavior, dietary recommendations, and regular physical activity [9]. Ohlsson and Manjer [10] showed that the lack of exercise and regular diet habit are independent risk factors for gastrointestinal symptoms in patients with functional gastrointestinal diseases. Traditional pharmacological treatments include osmotic laxatives and stimulant laxatives. Polyethylene glycol (PEG), the most frequently used osmotic laxative for FC, increases fecal volume and promotes intestinal peristalsis. Many studies have shown that PEG increases the frequency of defecation and has fewer side effects [11]. Therefore, PEG can be used as a long-term first-line treatment. In contrast, although stimulant laxatives can make a fast improvement in stool consistency and frequency, they should not be used for >4 weeks considering the possible adverse effects [12]. New therapeutic agents, including prosecretory agents (e.g. linaclotide), serotonergic agents (e.g. 5-hydroxytryptamine 4 agonist), cholinesterase inhibitors (e.g. pyridostigmine), and bile-acid (BA) regulators (e.g. elobixibat) etc. may improve FC symptoms by promoting colon secretion and enhancing gastrointestinal motility [9]. Other treatment options with evidence for efficacy include biofeedback therapy [13], transanal irrigation [14], surgical interventions [15, 16], and neuromodulation [14]. Despite all these intervention options, 40% of pediatric [17] and more than half of adult FC patients [18–20] are dissatisfied with the treatments due to the lack of efficacy and adverse effects. Therefore, new management strategies have been explored. One of the possible intervention targets is the gut microbiome, which was supported by the observation that interventions with probiotics, prebiotics, and fecal-microbiota transplantation improved colonic transit and defecation frequency [21–23]. Herein, we summarize the current knowledge on the potential contribution of the gut microbiota in the pathogenesis of FC.
Intestinal flora characteristics of patients with FC
Zoppi et al. [24] pioneered the study of the gut microbiota in FC using culture-based microbiological methods in 1998. They reported that constipation in children was associated with increased abundance in clostridia and bifidobacteria in the gut compared to healthy controls. Later, Khalif et al. [25] conducted a similar study with adult patients, still using culture-based microbiological methods, and reported that the abundances of Bifidobacterium and Lactobacillus were lower in constipated patients than in the controls. The opposite observations regarding the abundance of bifidobacteria may be explained by the pathophysiological differences between pediatric and adult patients. It is also important to note that culture-based methods tend to cause inaccurate observations in microbiota study because: (i) many species are not cultured, (ii) strict anaerobes die in an oxygenated environment and therefore tend to be underestimated, and (iii) in vitro culture changes the original structure of the microbiota.
In around 2015, Kim et al. [26] studied the microbiota of FC using a culture-independent method: the quantitative real-time polymerase chain reaction method. They reported that Bifidobacterium and Bacteroides species were decreased in the feces of FC. Although the methodology that Kim et al. [26] used is one large step more advanced than those reported in most studies on this topic, we have now progressed to the era of high-throughput sequencing and we conducted the first 16S rRNA sequencing study of the gut microbiota with adolescent FC patients [27]. Because we excluded those patients treated with antibiotics or proton-pump inhibitors, which are known to impact the gut microbiota, we were able to identify significant changes in the gut microbial composition of FC at every taxonomic level, with a relatively small sample size. At the genus level, the microbiota of FC exhibited a decreased abundance of Prevotella and increased abundance of Coprococcus, Ruminococcus, Blautia, Anaerotruncus, and Clostridium. Prevotella encodes a large set of enzymes for fiber metabolism [28] and is known for its association with dietary fibers [29]. Therefore, the decreased abundance of Prevotella in FC is consistent with the observation that FC patients usually have a low-fiber diet [30, 31]. In contrast to the findings of previous studies, conventional probiotic genera Lactobacillus and Bifidobacterium exhibited a trend for increased abundance in FC. At the community level, increased species richness was observed in the gut of FC, likely because of the prolonged incubation time of the gut microbiota in the presence of FC.
Recently, Mancabelli et al. [32] examined the gut microbial composition of adult FC patients using both the 16S rRNA sequencing and the whole-genome sequencing methods. Their 16S rRNA sequencing data indicated that the gut microbiota of FC patients was depleted of Bacteroides, Roseburia, and Coprococcus 3, which would predict a decreased level of short-chain fatty acid (SCFA) production. However, their whole-genome sequencing data did not validate this functional change.
Apparently, at this time, there is no consensus on the gut microbial structure characteristic of FC patients. Inconsistent observations may have been the consequences of the cultural and demographical differences of the study cohorts, different analysis techniques, and possible evolution of the disease over time. Table 1 summarizes several typical studies on the structural change in the gut microbiota in patients with FC.
Table 1.
Structural changes in gut microbiota in functional constipation
| Reference | Year | Methods | Inclusion criteria | Patients | Controls | Outcomes |
|---|---|---|---|---|---|---|
| Zoppi et al. [24] | 1998 | Microbial culture | Stool frequency less than one per 48 h and hard stool consistency | Children (n = 28, mean age 9.5 years) | Children (n = 14, mean age 7.9 years) | Bifidobacteria↑*Lactobacilli↑ Bacteroides↑ Clostridia↑* |
| Khalif et al. [25] | 2005 | Microbial culture | Rome II | Adults (n = 57, mean age 42.2 years) | Adults (n = 25) | Bifidobacterium↓*Lactobacillus↓ Bacteroides↓ Clostridium↓ |
| Kim et al. [26] | 2015 | qRT-PCR | Rome III | Adults (n = 30, mean age 35 years) | Adults (n = 30, mean age 32 years) | Bifidobacterium↓*Lactobacillus↓ Bacteroides↓*Clostridium↓ |
| Zhu et al. [27] | 2014 | 16S rRNA | Clinical practice guideline developed by the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition | Children (n = 8, mean age 11.8 years) | Children (n = 14, mean age 13.2 years) | Prevotella↓*Coprococcus↑*Ruminococcus↑*Blautia↑*Anaerotruncus↑*Clostridium*↑ |
| Mancabelli et al. [32] | 2017 | 16S rRNA and shotgun metagenomics | Rome III | Adults (n = 68, mean age 44 years) | Adults (n = 44, mean age 39 years) | Bacteroides↓*Roseburia↓*Coprococcus 3↓* Faecalibacterium↑* |
P < 0.05.
The relationship between the gut microbiota and the gut transit is likely bidirectional. Prolonged colonic transit in FC may facilitate the amplification and colonization of slow-growing species, leading to profound structural and functional alterations of the entire microecology. On the other hand, environmental factors may cause alterations in the gut microbiota, which, in turn, may contribute to the pathogenesis of FC through microbial metabolic activities.
The contribution of intestinal flora in the pathophysiological mechanism of FC
The current hypothesis is that the gut microbiota contributes to the pathogenesis of FC. This was supported by the observations that many risk factors of FC including age, diet, obesity, and stress have a large impact on the gut microbiota [33–35]. Thus, it is speculated that these risk factors may cause FC through mechanisms involving altered gut microbiota. The underlying mechanisms are the focus of the current review (Figure 2).
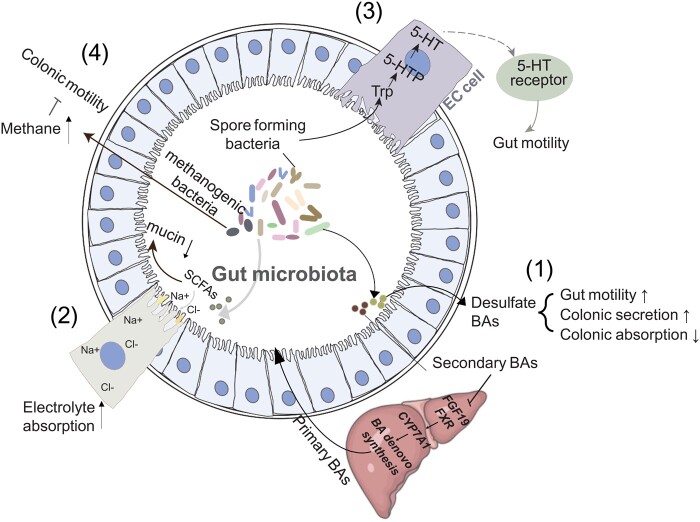
Figure 2.
Potential molecular mechanisms for the gut microbiota that contribute to the pathogenesis of functional constipation (FC). (1) Bile acids (BAs) stimulate bowel movement and colonic secretion and suppress colonic absorption. The gut microbiota impacts these processes by regulating the BA levels, as well as the sulfation of BAs, which abolishes the effects of BAs on colonic transit. (2) Elevated levels of short-chain fatty acids (SCFAs), the major group of microbial fermentation products, stimulate electrolyte absorption and suppress mucin secretion, thus contributing to the pathogenesis of FC. The effect of SCFA on colonic motility is a controversial topic. (3) 5-hydroxytryptamine (5-HT) stimulates colonic motility. The gut microbiota regulates the level of 5-HT through several mechanisms and thereby affects the pathogenesis of FC. (4) Methane produced by the gut microbiota reduces bowel movement. 5-HTP, 5-hydroxytryptophan.
Mechanisms involving BAs
Primary BAs are initially produced in the liver. Normally, most BAs are reabsorbed into the small intestine while ∼5% may arrive at the colon, where primary BAs are deconjugated and modified into secondary BAs by the gut microbiota [36, 37].
BAs may participate in the pathophysiology of FC through their effect on intestinal motility and colonic fluid transport. BAs are known to stimulate the release of 5-hydroxytryptamine (5-HT) and calcitonin gene-related peptide from enterochromaffin cells and intrinsic primary afferent neurons by activating the G protein-coupled BA receptor (TGR5), leading to the bowel peristaltic reflex [38, 39]. Several studies on FC patients treated with an ileal BA transporter inhibitor (elobixibat) demonstrated a causal relationship between elevated BA level and improved colonic transit [40–43].
There are direct and indirect mechanisms for BAs to stimulate fluid transport. BAs stimulate Cl− secretion and inhibit Na+ absorption from colonic epithelial cells through regulation of the ion pumps, exchangers, and transporters [44]. In addition, BAs may indirectly stimulate colonic secretion through their effect on local neurons [45] and immune cells [46].
Animal studies suggest that it is the deconjugated BAs that impact colonic transit. BAs are conjugated with glycine or taurine before being secreted from hepatocytes. After arriving at the colon, BAs are deconjugated by bacterial bile salt hydrolase before further modification. Microbial transplantation studies in mice indicated that altered microbiota affects gastrointestinal transit, through its impact on BA deconjugation [47, 48]. It is noteworthy that the microbial metabolisms of BAs are different between mice and humans, thus the mice studies remain to be validated in humans.
Several mechanisms have been proposed to explain how BAs mediate the microbial effects on FC. First, the gut microbiota may affect gastrointestinal transit through its regulation of BA synthesis. The gut microbiota is equipped with enzymes for the production of secondary BAs, which may suppress the FGF19- and FXR-mediated signaling, leading to the induction of CYP7A1 [49, 50], and consequently elevated de novo BA synthesis and improved colonic transit. To test this hypothesis, an integrated study on the colonic BA profiles, the abundances of BA metabolizing bacteria, and the cell biology of the colonic epithelium is required.
Another mechanism for the gut microbiota to influence FC is through its impact on BA sulfation, which abolishes the effect of the BAs on fluid transport [51, 52]. BA sulfation likely occurred in hepatocytes, while colonic bacterial bile salt sulfatase may desulfate BA, empowering its effect to stimulate colonic secretion and transit, and consequently improve colonic fluid transport [53]. Future study may characterize the abundances of sulfated BAs in the gut of patients with FC and its association with sulfatase-encoding bacteria.
Mechanisms involving SCFAs
SCFAs including acetate, propionate, and butyrate are the major fermentation products of the gut microbiota. Elevated levels of SCFAs are found in the stools of FC patients. Jalanka et al. [54] reported that the level of fecal acetate in FC patients was 2.2-fold higher than that in healthy controls, and that the levels of acetate, butyrate, and propionate were associated with the transit time in constipated patients. Iso-butyrate levels were also significantly higher in FC subjects than in healthy subjects [55].
SCFAs may contribute to the pathogenesis of FC by regulating colonic electrolyte absorption and secretion. SCFAs, especially butyrate, stimulate electrolyte absorption. The stimulation of Na+ and Cl− absorption by mucosal butyrate was greater than that by propionate and acetate [56]. The effect of butyrate on mucin secretion takes a biphasic mode: butyrate stimulation of mucin section peaks at 5 mM of butyrate. At concentrations of >5 mM, butyrate is inversely correlated with mucin section [57]. These results consistently support the roles of elevated SCFAs in increased electrolyte absorption and decreased mucin secretion. On the other hand, the effect of SCFAs on colonic motility is controversial. In some studies, SCFAs increased colonic motility in rats [58, 59], whereas other studies reported opposite observations [60, 61]. Several gaps and pitfalls are to be addressed to understand the role of SCFAs in FC. First, SCFAs measured in the published studies represent what were not absorbed, whereas the relevant SCFAs are those in contact with the SCFA receptors. Second, the working concentrations in different species likely vary and caution is needed when interpreting observations made in studies using SCFA concentrations that are far higher than physiological concentrations. Third, the long-term effects of SCFAs on the neural structure are important and need to be considered when interpreting the SCFA effects on colonic secretion, absorption, and motility [59].
Studies on the structure of the gut microbiota seem to support a role for SCFAs in the pathogenesis of FC. Butyrate-producing genera, Roseburia and Faecalibacterium, were increased in adolescent FC according to 16S rRNA sequencing studies [27, 62]. In a similar 16S rRNA sequencing study for adult FC patients, Mancabelli et al. [32] reported that Faecalibacterium is elevated in FC. However, their data indicated that other butyrate-producing bacteria Roseburia and Coprococcus 3 were depleted in FC. Shotgun metagenomic sequencing by Mancabelli et al. [32] does not support altered SCFA production in FC, which may be explained by very small sample size. Further study integrating microbiome survey, metabolome analysis, colonic absorption, secretion, and colonic motility is warranted to understand the role of the gut microbiota in promoting FC through altered SCFA production.
Mechanisms involving 5-HT
Produced by enterochromaffin cells, 5-HT is an abundant neurotransmitter in the enteric nervous system. Although it is a controversial subject, a significant amount of evidence suggests that 5-HT stimulates colonic motility through its receptors 5-HT3 and 5-HT4 [63]. For example, prucalopride, a highly selective agonist for serotonin 5-HT4 receptor, increases the number of bowel movements per week in adults with chronic FC [64]. Thus, the gut microbiota may impact the gut motility by regulating the production of 5-HT. Theoretically, the gut microbiota may regulate the production of 5-HT via several mechanisms. First, the gut microbiota influences the growth of colon enterochromaffin cells, suggested by the upregulation of 5-HT–positive enterochromaffin cells in germ-free rats [65]. In contrast to that of rats, the gut microbiota of mice and humans seem to have an opposite effect on 5-HT production. Mediated by microbial metabolites, indigenous spore-forming bacteria (Sp) from the mouse and human microbiota promote 5-HT biosynthesis from colonic ECs [66]. Independent studies have suggested that microbial metabolites BAs and SCFAs may induce the release of 5-HT from enterochromaffin cells [38, 39, 67].
A recent study suggests a different mechanism. Cao et al. [68] reported that the gut microbiota of FC patients upregulates the expression of serotonin transporter, which removes 5-HT from the gut. This causes decreased colonic transit and FC. It is noteworthy that the authors stated several times that they performed a 16S pyrosequencing study, but provided a description of illumina Miseq sequencing in the method section [68].
Finally, the gut microbiota may influence 5-HT production by regulating tryptophan metabolism in the gut [69]. For example, the gut microbiota may upregulate the production of indole and kynurenine from tryptophan, thereby reducing the substrate for the production of 5-HT and consequently leading to FC.
A fundamental gap in the study of 5-HT in FC is whether mucosal 5-HT is altered in FC patients. While some studies reported decreased 5-HT [64, 70], there were reports that the 5-HT level was not altered [71] or was increased in FC [72]. Vigorous studies are needed to validate the role of 5-HT in mediating the effect of gut microbiota on the pathogenesis of FC.
Mechanisms involving methane
It has long been proposed that methanogenic gut microbiota causes constipation by reducing bowel movements [73]. The hypothesis has been supported by the observations that FC patients carry gut microbiota enriched with methanogenic bacteria [74, 75]. In line with this, in patients with constipation-predominant IBS, treatment with antibiotics reduced the methanogenic bacteria in the gut microbiota and led to improved symptoms [76]. However, it is worth noting that no control was used in this retrospective study. On the other hand, one study reported that in patients with FC, methane production was associated with the gut microbial composition, but not with constipation or colonic transit [77]. Caution is also required to interpret these data as all of the 25 patients with constipation included 13 FC, 6 IBS with constipation, and 6 mixed IBS. Intervention studies with more strict inclusion criteria and a larger sample size are needed to clarify the role of methanogenic bacteria in FC.
FC treatments targeting the gut microbiota
In support of the roles of the gut microbiota in the pathogenesis of FC, various microbial interventions including probiotics, prebiotics, synbiotics, and traditional Chinese medicine (TCM) have shown beneficial effects on FC. In addition, some of the intervention studies support the mechanisms discussed above.
Probiotics
Probiotics, the most widely used microecologics, are effective in treating a wide variety of diseases by regulating the immune response, preventing the colonization of pathogens, improving gut barrier function, and reducing stress and anxiety, etc. [78]. Although individual studies have reported varied efficacies [79, 80], an earlier systemic review and meta-analysis of randomized–controlled trials indicated that probiotics may improve the whole-gut transit time, stool frequency, and stool consistency; Bifidobacterium lactis showed better efficacies than Lactobacillus casei Shirota [81]. A more recent systemic review and meta-analysis arrived at a similar conclusion that probiotics increase stool frequency and decrease intestinal transit time in FC patients [82]. Most recently, a meta-analysis of randomized–controlled trials of probiotics on FC concluded that probiotics consisting of multispecies, not single species such as B. lactis or B. longum alone, significantly reduced the whole-gut transit time, increased the defecation frequency, improved stool consistency, and decreased bloating [83]. It is worth noting that probiotics are likely to have a greater effect on the small bowel than on the colon, as the small bowel has far fewer competing bacteria. One study showed that probiotics reduced both the small-bowel transit time and the colonic transit time [84]. It is possible that the shortened small-bowel transit would increase the inflow to the colon and would consequently accelerate colonic transit.
However, similar studies with pediatric patients do not support the efficacy of probiotics on pediatric FC [85, 86]. The difference between adult and pediatric patients with FC in response to probiotics may be related to different microbial compositions in adult and pediatric subjects: while adult patients with FC exhibited a decreased abundance of Bifidobacterium [26], adolescent patients with FC exhibited a trend for elevated abundance of Bifidobacterium and Lactobacillus [27].
To explore the different effects of probiotic strains in FC, Wang et al. [87] treated constipated mice with B. longum, B. infantis, B. animalis, B. bifidum, B. adolescentis, and B. breve, respectively. They observed that B. longum, B. infantis, and B. bifidum were the most effective strains to relieve constipation. The improved symptoms were attributed to increased abundance of Lactobacillus and reduced levels of pathogenic bacteria (Alistipes, Odoribacter, and Clostridium). It is important to note that a randomized, double-blind, placebo-controlled probiotics treatment trial on FC is rare. In one of these trials, Ibarra et al. [88] reported no difference between probiotics and the placebo in primary analysis, but in a post hoc analysis, they reported that B. animalis subsp. lactis HN019 (HN019) increased the frequency of spontaneous defecations and reduced the degree of straining in FC patients.
Prebiotics
Prebiotics refers to non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon [89]. Recently, a randomized placebo-controlled trial of prebiotics for the treatment of FC was reported. UG1601, composed of inulin, lactitol, and aloe vera gel, was used to treat female patients with mild chronic FC [90]. Although UG1601 seemed to be more effective than placebo in improving abdominal and fecal symptoms, statistical significance was not achieved. Other interesting observations include reduced levels of serum cluster of differentiation 14 (CD14) and lipopolysaccharide (LPS), and increased abundance of Roseburia hominis, a butyrate-producing bacterium, upon UG1601 treatment.
D-tagatose is a monosaccharide often used as a food supplement. Liang et al. [91] found that high-dose d-tagatose restored the gastrointestinal transit rate of constipated mice induced by loperamide to a similar level of that of control mice, and improved the overall defecation condition including fecal weight, fecal number, and time to the first black-stool defecation. These therapeutic effects were attributed to the increased levels of excitatory neurotransmitters (Ach and SP) and the reduced level of inhibitory neurotransmitters (NO). The therapeutic mechanisms of d-tagatose may also involve the gut microbiota as the prebiotic therapy restored the composition of the intestinal flora.
Similarly, partially hydrolysed guar gum (HHGG), a fiber supplement, was shown to increase the fecal water content and enhance the small-intestinal transit of loperamide-induced constipated mice [92]. The therapeutic effects may be mediated by the gut microbiota as the prebiotics caused significant changes in the gut microbiota and elevated production of SCFAs.
Another popular prebiotics, β-glucan, is a polysaccharide widely found in yeast, fungus, and plants. Chen et al. [93] used the β-glucan extracted from bread yeast cells to treat loperamide-induced constipated mice and found enhanced intestinal motility. The pharmacological effect of β-glucan may be mediated by the enhanced expression of epithelial tight junction proteins (zonula occludens-1 and mucin-2) and neurotransmitters (acetylcholinesterase and serotonin). The gut microbiota was likely involved in the therapeutic effect of β-glucan as it restored the intestinal flora of the constipated mice toward a normal composition.
Although efficacies were shown with the loperamide-induced mice model, these prebiotics remain to be validated in randomized, double-blind, placebo-controlled trials.
Synbiotics
Synbiotics are combinations of probiotics and prebiotics, which may exhibit synergistic effects of both components [94]. In a pilot randomized, double-blind, controlled trial of a small sample size, synbiotic supplement Psyllogel Megafermenti improved defecation and decreased ITT [95]. However, in another randomized, double-blind, placebo-controlled trial with a larger sample size, no significant effect was found for a synbiotic composed of B. lactis BB12, L. plantarum LP01, and inulin-oligofructose [96]. A more recent trial using a combination of polydextrose and L. helveticus found beneficial effects on intestinal transit and fecal pH, but no significant advantage was found with this synbiotic compared with L. helveticus alone [97]. Perhaps different types of synbiotics have different therapeutic efficacies on FC. More clinical trials are needed to identify effective synbiotics and to confirm the therapeutic effects.
TCM
Several TCM herbs and formulations are effective for FC. The hemp seed soft capsule (HSSC) was developed from the ancient traditional prescription ‘hemp seed pill’, which consists of Semen Cannabis, Magnolia officinalis, Fructus Aurantii Immaturus, Radix Paeoniae Alba, Almond, and Rheum rhabarbarum. As a representative prescription of TCM in the treatment of constipation [98], the hemp seed pill has been known to improve colonic secretion and transit [99]. With loperamide-induced constipated rats, Lu et al. [100] showed that HSSC increased the fecal wet weight and water content, which was attributed to the combined actions of cAMP-dependent and Ca2+-dependent Cl− channels, NKCC, Na+-HCO3− co-transporter, or Cl−/HCO3− exchanger.
Recently, the gut microbiota has been often reported to participate in the therapeutic effects of these herbs and formulations. Invented in the Qing Dynasty ∼300 years ago, Zengye decoction (ZYD) has been used to cure ‘dryness’ by promoting the production of body fluids according to TCM theory. Liu et al. [101] examined the effect of ZYD on the gut microbiota of constipated rats. They found that ZYD restored the composition of the gut microbiota toward a normal state by reducing the abundance of Helicobacteraceae, Desulfovibrionaceae, Ruminococcaceae, Lactobacillaceae, Prevotellaceae, and Dorea, while increasing the abundance of Aeromonadaceae, Oxalobacteraceae, Veillonellaceae, Clostridiaceae, and Roseburia. Metabolomic analysis revealed that ZYD caused microbial changes in the metabolism of energy, amino acids, methane, and glutathione.
Records of mulberry fruit for the treatment of constipation and other digestive diseases date back to 200 BC. According to TCM theory, mulberry fruit can be used to treat ‘yin’ deficiency. Hu et al. [102] used the mulberry fruit to treat diphenoxylate-induced constipated mice and found that the treatment increased the fecal water content, shortened the first red-stool defecation time, and increased the gastric-intestinal transit rate. The mulberry-fruit treatment caused alterations in the gut microbiota, with increased abundance of Lactobacillus, Bifidobacterium, and Eubacterium, and decreased abundance of Helicobacter, Alloprevotella, and Prevotellaceae. The compositional change in the microbiota was accompanied by decreased expression of aquaporin genes (Aqp3, Aqp4, Aqp8, and AqP9), reduced levels of inhibitory neurotransmitters, and increased levels of excitatory neurotransmitters and SCFAs, suggesting a therapeutic mechanism whereby mulberry fruit causes a change in the microbiota, leading to changes in microbial metabolites, which, in turn, improves colonic motility and secretion.
Sennoside A, the main active constituent of Da-Huang-Gan-Cao-Tang (Daiokanzoto, DKT), is converted by microbial β-glucosidases to generate rheinanthrone, the molecule with laxative activity. Because of the close connection between sennoside A and the gut microbiota, it was hypothesized that the therapeutic effect of sennoside A depends on the composition and function of the gut microbiota, which was proved in mice carrying different types of gut microbiota. Takayama et al. [103] proposed that different types of gut microbiota represent different ‘patterns’ defined by TCM and therefore they established a model to investigate the biological mechanisms behind the personalized medicinal practices in TCM. In DKT, sennoside A is mainly found in its herbal component of rhubarb. In fact, many other TCM formulations for the treatment of FC have a component of rhubarb, which was shown to increase intestinal secretion and improve stool consistency [104].
TCM usually takes the form of a complex composition and is multi-targeting. Understanding the links between changes in the composition of the intestinal flora, the altered gene expression of the intestines, and the metabolites produced after TCM therapy requires further investigation.
Conclusions
Microecological imbalance is an important feature in FC, which may contribute to the pathogenesis via multiple mechanisms mediated by microbial metabolites including BAs, SCFAs, 5-HT, and methane. The therapeutic effects of probiotics, prebiotics, synbiotics, and TCM often involve compositional and functional changes in the gut microbiota. Further studies on the pathomechanisms of FC and the therapeutic mechanisms of microecological agents will provide a knowledge base for better management of FC patients. Given the very different diet and the gut microbiota of laboratory animals compared to those of humans, understanding the therapeutic efficacy and the mechanisms of microecological agents may require adequately powered mechanistic clinical trials with FC patients.
Authors’ Contributions
L.Q.Z. and L.X.Z. conceived of this work and designed the outlines of this review. S.S.Z. and L.X.Z. selected the references and prepared the first draft. All authors critically revised the manuscript and approved the final version for submission.
Funding
This work was supported by the Clinical Medicine Development Project of Beijing Municipal Administration of Hospitals [ZYLX201411], the Beijing Nova Program [Z201100006820119] from Beijing Municipal Science & Technology Commission, the Beijing Science and technology project [z181100001718218], the National Natural Science Foundation of China [81770571], the Guangdong Province ‘Pearl River Talent Plan’ Innovation and Entrepreneurship Team Project [2019ZT08Y464], and the National Key Clinical Discipline of China.
Acknowledgements
We thank Professor Guoxun Chen (University of Tennessee at Knoxville, Tennessee, USA) for critical review and advice during the preparation of this manuscript.
Conflict of Interest
None declared.
References
- 1. Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology 2016;150:1262–79.e2. [DOI] [PubMed] [Google Scholar]
- 2. Sperber AD, Bangdiwala SI, Drossman DA. et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology 2021;160:99–114.e3. [DOI] [PubMed] [Google Scholar]
- 3. Mearin F, Lacy BE, Chang L. et al. Bowel disorders. Gastroenterology 2016;150:1393–407.e5. [DOI] [PubMed] [Google Scholar]
- 4. Drossman DA. Functional gastrointestinal disorders: what's new for Rome IV? Lancet Gastroenterol Hepatol 2016;1:6–8. [DOI] [PubMed] [Google Scholar]
- 5. Palsson OS, Whitehead W, Törnblom H. et al. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020;158:1262–73.e3. [DOI] [PubMed] [Google Scholar]
- 6. Suares NC, Ford AC.. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol 2011;106:1582–91. quiz 1581, 1592. [DOI] [PubMed] [Google Scholar]
- 7. Hinton JM, Lennard-Jones JE.. Constipation: definition and classification. Postgrad Med J 1968;44:720–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nullens S, Nelsen T, Camilleri M. et al. Regional colon transit in patients with dys-synergic defaecation or slow transit in patients with constipation. Gut 2012;61:1132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vriesman MH, Koppen IJN, Camilleri M. et al. Management of functional constipation in children and adults. Nat Rev Gastroenterol Hepatol 2020;17:21–39. [DOI] [PubMed] [Google Scholar]
- 10. Ohlsson B, Manjer J.. Physical inactivity during leisure time and irregular meals are associated with functional gastrointestinal complaints in middle-aged and elder subjects. Scand J Gastroenterol 2016;51:1299–307. [DOI] [PubMed] [Google Scholar]
- 11. Paré P, Fedorak RN.. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol 2014;28:549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Noergaard M, Traerup Andersen J, Jimenez-Solem E. et al. Long term treatment with stimulant laxatives—clinical evidence for effectiveness and safety? Scand J Gastroenterol 2019;54:27–34. [DOI] [PubMed] [Google Scholar]
- 13. Skardoon GR, Khera AJ, Emmanuel AV. et al. Review article: dyssynergic defaecation and biofeedback therapy in the pathophysiology and management of functional constipation. Aliment Pharmacol Ther 2017;46:410–23. [DOI] [PubMed] [Google Scholar]
- 14. Mosiello G, Marshall D, Rolle U. et al. Consensus review of best practice of transanal irrigation in children. J Pediatr Gastroenterol Nutr 2017;64:343–52. [DOI] [PubMed] [Google Scholar]
- 15. Bharucha AE, Pemberton JH, Locke GR 3rd. American Gastroenterological Association technical review on constipation. Gastroenterology 2013;144:218–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xie XY, Sun KL, Chen WH. et al. Surgical outcomes of subtotal colectomy with antiperistaltic caecorectal anastomosis vs total colectomy with ileorectal anastomosis for intractable slow-transit constipation. Gastroenterol Rep (Oxf) 2019;7:449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pijpers MA, Bongers ME, Benninga MA. et al. Functional constipation in children: a systematic review on prognosis and predictive factors. J Pediatr Gastroenterol Nutr 2010;50:256–68. [DOI] [PubMed] [Google Scholar]
- 18. Johanson JF, Kralstein J.. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther 2007;25:599–608. [DOI] [PubMed] [Google Scholar]
- 19. Wald A, Scarpignato C, Mueller-Lissner S. et al. A multinational survey of prevalence and patterns of laxative use among adults with self-defined constipation. Aliment Pharmacol Ther 2008;28:917–30. [DOI] [PubMed] [Google Scholar]
- 20. Wald A, Mueller-Lissner S, Kamm MA. et al. Survey of laxative use by adults with self-defined constipation in South America and Asia: a comparison of six countries. Aliment Pharmacol Ther 2010;31:274–84. [DOI] [PubMed] [Google Scholar]
- 21. Choi CH, Chang SK.. Alteration of gut microbiota and efficacy of probiotics in functional constipation. J Neurogastroenterol Motil 2015;21:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Castillejo G, Bulló M, Anguera A. et al. A controlled, randomized, double-blind trial to evaluate the effect of a supplement of cocoa husk that is rich in dietary fiber on colonic transit in constipated pediatric patients. Pediatrics 2006;118:e641–8. [DOI] [PubMed] [Google Scholar]
- 23. Ding C, Fan W, Gu L. et al. Outcomes and prognostic factors of fecal microbiota transplantation in patients with slow transit constipation: results from a prospective study with long-term follow-up. Gastroenterol Rep (Oxf) 2018;6:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zoppi G, Cinquetti M, Luciano A. et al. The intestinal ecosystem in chronic functional constipation. Acta Paediatr 1998;87:836–41. [DOI] [PubMed] [Google Scholar]
- 25. Khalif IL, Quigley EM, Konovitch EA. et al. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig Liver Dis 2005;37:838–49. [DOI] [PubMed] [Google Scholar]
- 26. Kim SE, Choi SC, Park KS. et al. Change of fecal flora and effectiveness of the short-term VSL#3 probiotic treatment in patients with functional constipation. J Neurogastroenterol Motil 2015;21:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu L, Liu W, Alkhouri R. et al. Structural changes in the gut microbiome of constipated patients. Physiol Genomics 2014;46:679–86. [DOI] [PubMed] [Google Scholar]
- 28. Purushe J, Fouts DE, Morrison M. et al. ; North American Consortium for Rumen Bacteria. Comparative genome analysis of Prevotella ruminicola and Prevotella bryantii: insights into their environmental niche. Microb Ecol 2010;60:721–9. [DOI] [PubMed] [Google Scholar]
- 29. De Filippo C, Cavalieri D, Di Paola M. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 2010;107:14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dukas L, Willett WC, Giovannucci EL.. Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. Am J Gastroenterol 2003;98:1790–6. [DOI] [PubMed] [Google Scholar]
- 31. Nakaji S, Tokunaga S, Sakamoto J. et al. Relationship between lifestyle factors and defecation in a Japanese population. Eur J Nutr 2002;41:244–8. [DOI] [PubMed] [Google Scholar]
- 32. Mancabelli L, Milani C, Lugli GA. et al. Unveiling the gut microbiota composition and functionality associated with constipation through metagenomic analyses. Sci Rep 2017;7:9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moloney RD, Desbonnet L, Clarke G. et al. The microbiome: stress, health and disease. Mamm Genome 2014;25:49–74. [DOI] [PubMed] [Google Scholar]
- 34. Shi W, Xu X, Zhang Y. et al. Epidemiology and risk factors of functional constipation in pregnant women. PLoS One 2015;10:e0133521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rao SS, Rattanakovit K, Patcharatrakul T.. Diagnosis and management of chronic constipation in adults. Nat Rev Gastroenterol Hepatol 2016;13:295–305. [DOI] [PubMed] [Google Scholar]
- 36. Wahlström A, Sayin SI, Marschall HU. et al. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 2016;24:41–50. [DOI] [PubMed] [Google Scholar]
- 37. Appleby RN, Walters JR.. The role of bile acids in functional GI disorders. Neurogastroenterol Motil 2014;26:1057–69. [DOI] [PubMed] [Google Scholar]
- 38. Begley M, Hill C, Gahan CG.. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 2006;72:1729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bunnett NW. Neuro-humoral signalling by bile acids and the TGR5 receptor in the gastrointestinal tract. J Physiol 2014;592:2943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simrén M, Bajor A, Gillberg PG. et al. Randomised clinical trial: the ileal bile acid transporter inhibitor A3309 vs. placebo in patients with chronic idiopathic constipation--a double-blind study. Aliment Pharmacol Ther 2011;34:41–50. [DOI] [PubMed] [Google Scholar]
- 41. Wong BS, Camilleri M, McKinzie S. et al. Effects of A3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. Am J Gastroenterol 2011;106:2154–64. [DOI] [PubMed] [Google Scholar]
- 42. Nakajima A, Seki M, Taniguchi S. et al. Safety and efficacy of elobixibat for chronic constipation: results from a randomised, double-blind, placebo-controlled, phase 3 trial and an open-label, single-arm, phase 3 trial. Lancet Gastroenterol Hepatol 2018;3:537–47. [DOI] [PubMed] [Google Scholar]
- 43. Chey WD, Camilleri M, Chang L. et al. A randomized placebo-controlled phase IIb trial of a3309, a bile acid transporter inhibitor, for chronic idiopathic constipation. Am J Gastroenterol 2011;106:1803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Keely SJ, Walters JR.. The Farnesoid X Receptor: good for BAD. Cell Mol Gastroenterol Hepatol 2016;2:725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun Y, Fihn BM, Sjövall H. et al. Enteric neurones modulate the colonic permeability response to luminal bile acids in rat colon in vivo. Gut 2004;53:362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gelbmann CM, Schteingart CD, Thompson SM. et al. Mast cells and histamine contribute to bile acid-stimulated secretion in the mouse colon. J Clin Invest 1995;95:2831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dey N, Wagner VE, Blanton LV. et al. Regulators of gut motility revealed by a gnotobiotic model of diet-microbiome interactions related to travel. Cell 2015;163:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kashyap PC, Marcobal A, Ursell LK. et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 2013;144:967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jiao N, Baker SS, Chapa-Rodriguez A. et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018;67:1881–91. [DOI] [PubMed] [Google Scholar]
- 50. Parks DJ, Blanchard SG, Bledsoe RK. et al. Bile acids: natural ligands for an orphan nuclear receptor. Science 1999;284:1365–8. [DOI] [PubMed] [Google Scholar]
- 51. Hofmann AF, Loening-Baucke V, Lavine JE. et al. Altered bile acid metabolism in childhood functional constipation: inactivation of secretory bile acids by sulfation in a subset of patients. J Pediatr Gastroenterol Nutr 2008;47:598–606. [DOI] [PubMed] [Google Scholar]
- 52. Breuer NF, Rampton DS, Tammar A. et al. Effect of colonic perfusion with sulfated and nonsulfated bile acids on mucosal structure and function in the rat. Gastroenterology 1983;84:969–77. [PubMed] [Google Scholar]
- 53. Robben J, Caenepeel P, Van Eldere J. et al. Effects of intestinal microbial bile salt sulfatase activity on bile salt kinetics in gnotobiotic rats. Gastroenterology 1988;94:494–502. [DOI] [PubMed] [Google Scholar]
- 54. Jalanka J, Major G, Murray K. et al. The effect of psyllium husk on intestinal microbiota in constipated patients and healthy controls. Int J Mol Sci 2019;20:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kang DW, DiBaise JK, Ilhan ZE. et al. Gut microbial and short-chain fatty acid profiles in adults with chronic constipation before and after treatment with lubiprostone. Anaerobe 2015;33:33–41. [DOI] [PubMed] [Google Scholar]
- 56. Binder HJ, Mehta P.. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology 1989;96:989–96. [DOI] [PubMed] [Google Scholar]
- 57. Barcelo A, Claustre J, Moro F. et al. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut 2000;46:218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yajima T. Contractile effect of short-chain fatty acids on the isolated colon of the rat. J Physiol 1985;368:667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Soret R, Chevalier J, De Coppet P. et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 2010;138:1772–82. [DOI] [PubMed] [Google Scholar]
- 60. Cherbut C, Ferrier L, Rozé C. et al. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol 1998;275:G1415–22. [DOI] [PubMed] [Google Scholar]
- 61. Squires PE, Rumsey RD, Edwards CA. et al. Effect of short-chain fatty acids on contractile activity and fluid flow in rat colon in vitro. Am J Physiol 1992;262:G813–7. [DOI] [PubMed] [Google Scholar]
- 62. Pryde SE, Duncan SH, Hold GL. et al. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett 2002;217:133–9. [DOI] [PubMed] [Google Scholar]
- 63. Kendig DM, Grider JR.. Serotonin and colonic motility. Neurogastroenterol Motil 2015;27:899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sajid MS, Hebbar M, Baig MK. et al. Use of prucalopride for chronic constipation: a systematic review and meta-analysis of published randomized, controlled trials. J Neurogastroenterol Motil 2016;22:412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Uribe A, Alam M, Johansson O. et al. Microflora modulates endocrine cells in the gastrointestinal mucosa of the rat. Gastroenterology 1994;107:1259–69. [DOI] [PubMed] [Google Scholar]
- 66. Yano JM, Yu K, Donaldson GP. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fukumoto S, Tatewaki M, Yamada T. et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol 2003;284:R1269–76. [DOI] [PubMed] [Google Scholar]
- 68. Cao H, Liu X, An Y. et al. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci Rep 2017;7:10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Agus A, Planchais J, Sokol H.. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018;23:716–24. [DOI] [PubMed] [Google Scholar]
- 70. Coates MD, Mahoney CR, Linden DR. et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 2004;126:1657–64. [DOI] [PubMed] [Google Scholar]
- 71. Cremon C, Carini G, Wang B. et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol 2011;106:1290–8. [DOI] [PubMed] [Google Scholar]
- 72. Miwa J, Echizen H, Matsueda K. et al. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion 2001;63:188–94. [DOI] [PubMed] [Google Scholar]
- 73. Sahakian AB, Jee SR, Pimentel M.. Methane and the gastrointestinal tract. Dig Dis Sci 2010;55:2135–43. [DOI] [PubMed] [Google Scholar]
- 74. Lee KM, Paik CN, Chung WC. et al. Breath methane positivity is more common and higher in patients with objectively proven delayed transit constipation. Eur J Gastroenterol Hepatol 2013;25:726–32. [DOI] [PubMed] [Google Scholar]
- 75. Attaluri A, Jackson M, Valestin J. et al. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol 2010;105:1407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Low K, Hwang L, Hua J. et al. A combination of rifaximin and neomycin is most effective in treating irritable bowel syndrome patients with methane on lactulose breath test. J Clin Gastroenterol 2010;44:547–50. [DOI] [PubMed] [Google Scholar]
- 77. Parthasarathy G, Chen J, Chen X. et al. Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology 2016;150:367–79.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Suez J, Zmora N, Segal E. et al. The pros, cons, and many unknowns of probiotics. Nat Med 2019;25:716–29. [DOI] [PubMed] [Google Scholar]
- 79. Martoni CJ, Evans M, Chow CT. et al. Impact of a probiotic product on bowel habits and microbial profile in participants with functional constipation: a randomized controlled trial. J Dig Dis 2019;20:435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Airaksinen K, Yeung N, Lyra A. et al. The effect of a probiotic blend on gastrointestinal symptoms in constipated patients: a double blind, randomised, placebo controlled 2-week trial. Benef Microbes 2019;10:617–27. [DOI] [PubMed] [Google Scholar]
- 81. Dimidi E, Christodoulides S, Fragkos KC. et al. The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2014;100:1075–84. [DOI] [PubMed] [Google Scholar]
- 82. Miller LE, Ouwehand AC, Ibarra A.. Effects of probiotic-containing products on stool frequency and intestinal transit in constipated adults: systematic review and meta-analysis of randomized controlled trials. Ann Gastroenterol 2017;30:629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang C, Jiang J, Tian F. et al. Meta-analysis of randomized controlled trials of the effects of probiotics on functional constipation in adults. Clin Nutr 2020;39:2960–9. [DOI] [PubMed] [Google Scholar]
- 84. Agrawal A, Houghton LA, Morris J. et al. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2009;29:104–14. [DOI] [PubMed] [Google Scholar]
- 85. Korterink JJ, Ockeloen L, Benninga MA. et al. Probiotics for childhood functional gastrointestinal disorders: a systematic review and meta-analysis. Acta Paediatr 2014;103:365–72. [DOI] [PubMed] [Google Scholar]
- 86. Koppen IJ, Benninga MA, Tabbers MM.. Is there a role for pre-, pro- and synbiotics in the treatment of functional constipation in children? A systematic review. J Pediatr Gastroenterol Nutr 2016;63(Suppl 1):S27–35. [DOI] [PubMed] [Google Scholar]
- 87. Wang L, Hu L, Xu Q. et al. Bifidobacteria exert species-specific effects on constipation in BALB/c mice. Food Funct 2017;8:3587–600. [DOI] [PubMed] [Google Scholar]
- 88. Ibarra A, Latreille-Barbier M, Donazzolo Y. et al. Effects of 28-day Bifidobacterium animalis subsp. lactis HN019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: a double-blind, randomized, placebo-controlled, and dose-ranging trial. Gut Microbes 2018;9:236–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gibson GR, Roberfroid MB.. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 1995;125:1401–12. [DOI] [PubMed] [Google Scholar]
- 90. Chu JR, Kang SY, Kim SE. et al. Prebiotic UG1601 mitigates constipation-related events in association with gut microbiota: a randomized placebo-controlled intervention study. World J Gastroenterol 2019;25:6129–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Liang YX, Wen P, Wang Y. et al. The constipation-relieving property of d-tagatose by modulating the composition of gut microbiota. Int J Mol Sci 2019;20:5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fu X, Li R, Zhang T. et al. Study on the ability of partially hydrolyzed guar gum to modulate the gut microbiota and relieve constipation. J Food Biochem 2019;43:e12715. [DOI] [PubMed] [Google Scholar]
- 93. Chen Z, Lin S, Jiang Y. et al. Effects of bread yeast cell wall beta-glucans on mice with loperamide-induced constipation. J Med Food 2019;22:1009–21. [DOI] [PubMed] [Google Scholar]
- 94. Cencic A, Chingwaru W.. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010;2:611–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bazzocchi G, Giovannini T, Giussani C. et al. Effect of a new synbiotic supplement on symptoms, stool consistency, intestinal transit time and gut microbiota in patients with severe functional constipation: a pilot randomized double-blind, controlled trial. Tech Coloproctol 2014;18:945–53. [DOI] [PubMed] [Google Scholar]
- 96. Lim YJ, Jamaluddin R, Hazizi AS. et al. Effects of synbiotics among constipated adults in Serdang, Selangor, Malaysia-a randomised, double-blind, placebo-controlled trial. Nutrients 2018;10:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bahrudin MF, Abdul Rani R, Tamil AM. et al. Effectiveness of sterilized symbiotic drink containing Lactobacillus helveticus comparable to probiotic alone in patients with constipation-predominant irritable bowel syndrome. Dig Dis Sci 2020;65:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang SS, Shen H, Zhang L. et al. Expert consensus on TCM diagnosis and treatment of constipation. Zhong Yi Za Zhi 2017;58:1345–50. [in Chinese] [Google Scholar]
- 99. Yang J, Zhang SS.. Progress in traditional Chinese medicine treatment of chronic functional constipation. Bei Jing Zhong Yi 2007;26:61–3 (in Chinese). [Google Scholar]
- 100. Lu XF, Jia MD, Zhang SS. et al. Effects of Hemp seed soft capsule on colonic ion transport in rats. World J Gastroenterol 2017;23:7563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Liu D, Lin L, Lin Y. et al. Zengye decoction induces alterations to metabolically active gut microbiota in aged constipated rats. Biomed Pharmacother 2019;109:1361–71. [DOI] [PubMed] [Google Scholar]
- 102. Hu TG, Wen P, Fu HZ. et al. Protective effect of mulberry (Morus atropurpurea) fruit against diphenoxylate-induced constipation in mice through the modulation of gut microbiota. Food Funct 2019;10:1513–28. [DOI] [PubMed] [Google Scholar]
- 103. Takayama K, Takahara C, Tabuchi N. et al. Daiokanzoto (Da-Huang-Gan-Cao-Tang) is an effective laxative in gut microbiota associated with constipation. Sci Rep 2019;9:3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wilkinson-Smith VC, Major G, Ashleigh L. et al. ; Nottingham GI MRI Research Group. Insights into the different effects of food on intestinal secretion using magnetic resonance imaging. JPEN J Parenter Enteral Nutr 2018;42:1342–8. [DOI] [PubMed] [Google Scholar]