Abstract
Objective
Coronavirus disease 2019 (COVID-19) was associated with a higher risk of arrhythmia in infected patients. However, there are no reports about the effect of the ongoing pandemic on arrhythmias in the non-infected population. We measured the arrhythmia burden in a non-infected population with cardiac implantable devices.
Methods
The arrhythmia burden during the COVID-19 pandemic was compared to a 6-month interval in the pre-COVID-19 period. The COVID-19 pandemic was divided into high-risk (17 January 2020 to 16 March 2020) and low-risk periods (17 March 2020 to 17 July 2020) according to whether there were locally infected patients. Arrhythmia burdens were compared among the pre-COVID-19, high-risk, and low-risk periods.
Results
A total of 219 patients with 1859 episodes were included. We observed a larger proportion of patients with atrial fibrillation (AF) during the COVID-19 pandemic (38.36% vs 26.03%, p = 0.006). There was not significantly more ventricular arrhythmia during the COVID period than the pre-COVID-19 period (p > 0.05). During the high-risk period, daily frequency of non-sustained ventricular tachycardia (NSVT) (0.0172, 0.0475 vs 0.0109, 0.0164, p < 0.05), atrial tachycardia (AT) (0.0345, 0.0518 vs 0.0164, 0.0219 p < 0.05) and AF (0.0345, 0.0432 vs 0.0164, 0.0186, p < 0.05) and daily duration of NSVT (0.1982, 0.2845 vs 0.0538, 0.1640 p < 0.05) were higher and longer than those in the pre-COVID-19 period. Regression modeling showed that the impact of COVID-19 pandemic lead to an increased onset of AF (odds ratio 2.465; p < 0.01). Patients with paroxysmal AF who had undergone a previous radiofrequency ablation had a lower burden of AF (incidence 21.43% vs 55.00%, P = 0.049, daily frequency 0.0000, 0.0027 vs 0.0000, 241.7978, P = 0.020) during the pandemic.
Conclusion
The COVID-19 pandemic contributed to a higher burden of arrhythmias in non-infected patients. Patients would experience a lower burden of AF following radiofrequency ablation treatment, and this effect persisted during the pandemic.
Keywords: COVID-19, arrhythmia, cardiac implantable device, non-infected population, radiofrequency ablation
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was a global pandemic that infected more than 200 million patients worldwide as of mid-September 2021. COVID-19 was associated with myocardial injury and a higher risk of arrhythmias in infected patients. Early reports from China suggested an overall cardiac arrhythmia incidence of 17% in hospitalized COVID-19 patients.1 A higher arrhythmia rate (44%) was observed in COVID-19 patients admitted to the intensive care unit.2 Another observational report from Italy described a concomitant increase in out-of-hospital cardiac arrests with the increased cumulative incidence of COVID-19.3
Evidence derived from various studies was inconsistent regarding the effect of COVID-19 on arrhythmias. In a large implantable cardiac defibrillator (ICD) population in the US, there was a 32% reduction in ventricular arrhythmia requiring ICD therapies following implementation of lockdown measures.4 Another report described an increase in ventricular arrhythmias in the 2 weeks before the lockdown order, and ventricular arrhythmia incidence decreased dramatically during the lockdown.5 However, a report from Italy declared that the complete nationwide lockdown did not affect the incidence of arrhythmias in an urban cohort of patients with ICDs.6 All these studies investigated the impact of COVID-19 on the incidence of ventricular arrhythmias in ICD recipients followed by remote monitoring. Nevertheless, remote monitoring does not allow the researcher to define the proportion of patients infected with the COVID-19. All these studies weighed the arrhythmia burden in the entire population without distinguishing infected from non-infected patients. As COVID infection itself is a well-recognized trigger for cardiac arrhythmias, therefore, it remains clear whether the variation in arrhythmia burden is directly correlated with the change of the proportion of infected people rather than the comprehensive effect of pandemic itself; none of these studies reflected the actual arrhythmia burden in most non-infected patients. Therefore, we measured the arrhythmia burden in the non-infected population during the COVID-19 pandemic using cardiac implantable device interrogation.
We had the following aims: 1) to compare the incidences of several arrhythmias between the pre-COVID-19 period and the COVID-19 period; 2) to compare the arrhythmia burden, defined by the combination of daily frequency and daily duration between the pre-COVID-19 period and the COVID-19 period; and 3) to identify potential factors giving rise to the increase of cardiac arrhythmias during the COVID-19 pandemic. A regression model was constructed to evaluate the association between the arrhythmia and each potential risk factor.
Methods
Study Design
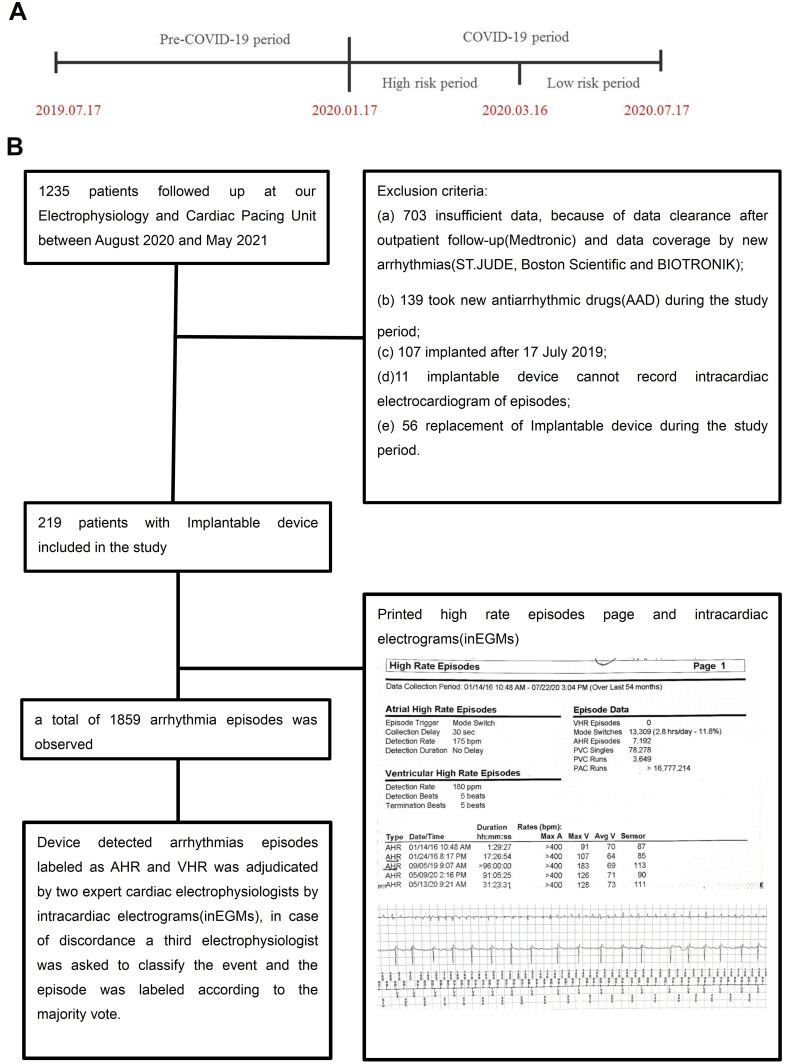
This was a cohort study conducted at the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China. The study period was selected based on the epidemiology of the COVID-19 pandemic in the area. Wenzhou, China, was the first area to experience an outbreak of COVID-19 outside Wuhan, where the first case was diagnosed on 17 January 2020, and the final case was recorded on 16 March 2020. We selected 17 January 2020 to 17 July 2020 as the COVID-19 period and 17 July 2019 to 16 January 2020 as the pre-COVID-19 period. To measure the influence of the COVID-19 pandemic locally, the COVID-19 pandemic period was divided into a high-risk period (17 January 2020 to 16 March 2020, 58 days) and a low-risk period (17 March 2020 to 17 July 2020, 125 days) according to whether there were infected patients locally. The details are shown in Figure 1A. The devices detected the arrhythmias were collected during the COVID-19 period and compared to those occurring during the pre-COVID-19 period. Comparisons were also made among pre-COVID-19, high-risk, and low-risk periods.
Figure 1.
(A) Timeline illustrating crucial periods of the study. The first case was diagnosed on 17 January 2020, and the final one recovered on 16 March 2020. We selected 17 January 2020 to 17 July 2020 as the COVID-19 period and 17 July 2019 to 16 January 2020 as the pre-COVID-19 period. To truly assess the influence of the COVID-19 pandemic locally, the COVID-19 pandemic period was divided into the high-risk period (17 January 2020 to 16 March 2020) and low-risk period (17 Mar 2020 to 17 July 2020) according to whether the existence of locally infected patients. (B) The diagram shows the study population’s selection and diagnosis process. The figure in diagram give an example of high rate episodes summary detected by Medtronic, which shows data collection period is 01/14/2016 10:48am-07/22/20 3:04pm (over last 54months), atrial high rate episodes(AHR) was triggered when the atrial rate exceeded 175bpm, the stored atrial inEGM is generated 30 seconds after the tachycardia meets AHR detection criteria. Ventricular high rate episodes(VHR) was triggered when the ventricular rate exceeded 180bpm and sustained for 5 beats. This figure depicts (top to bottom) 5 recorded episodes of AHR and provide the onset date and time along with the duration and stored intracardiac electrograms.
Study Population
Inclusion criteria included no SARS-CoV-2 infection as determined by polymerase chain reaction testing of a nasopharyngeal sample and having an implantable device including permanent pacemakers, ICD, cardiac resynchronization therapy (CRT), or a cardiac resynchronization therapy defibrillator (CRT-D). Between August 2020 and May 2021, 1235 patients followed up at our Electrophysiology and Cardiac Pacing Unit. The exclusion criteria and arrhythmia diagnosis process are shown in Figure 1B. (a) new antiarrhythmic drugs or interventional treatments during the study period; (b) incomplete implantable device-detected episodes because of data clearance after outpatient follow-up (Medtronic) or data coverage by new arrhythmias (St. Jude, Boston Scientific, and BIOTRONIK); (c) implantation after 17 July 2019; (d) the implantable device could not record intracardiac electrograms (inECGs) of episodes; and (e) replacement of implantable device during the study period. The final cohort consisted of 219 patients. Demographic, medical history, and echocardiographic data were collected from electronic medical records. The medical history was defined by eligible diagnosis codes or prescription fills.
Diagnostic Process
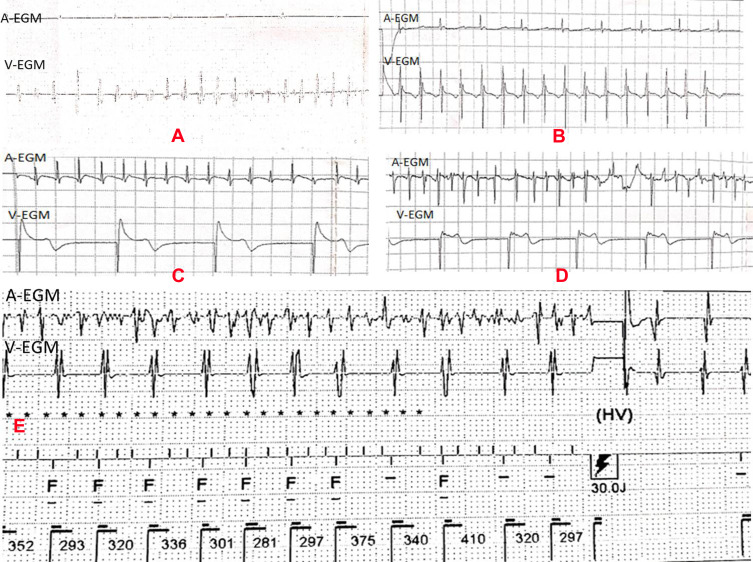
The high-rate episode detection algorithm, used by all devices in this study, was triggered when the atrial/ventricular rate exceeded the programmed atrial/ventricular rate for high-rate episode detection and when the atrial/ventricular tachyarrhythmia was sustained for the minimum programmed number of complexes. The recording of the detected episode was terminated when the atrial/ventricular rate decreased below the detection rate. We used the definition of the device’s manufacturer to classify the arrhythmia episodes and print inECGs.7,8 To reduce the selection bias arising from different algorithms across devices, device-detected arrhythmia episodes labeled as atrial or ventricular high-rate episodes were adjudicated by two expert cardiac electrophysiologists using inEGMs. In case of disagreement, a third electrophysiologist was asked to classify the event, and the episode was labeled according to the majority vote. The characteristics of the various arrhythmia inEGMs are shown in Figure 2.
Figure 2.
Intracardiac electrograms of arrhythmia. (A) Confirmed episode of SVT, A-A interval = 641–1289 ms, rate = 47–94 bpm; V–V interval = 172–273 ms, rate = 220–348 bpm; (B) Confirmed episode of NSVT, A-A interval = 590 ms (atrial pacing), rate = 47–94 bpm; V–V interval = 290–310 ms, rate = 194–207 bpm, duration = 4920 ms. (C) Confirmed episode of AT, regular A-A interval = 220 ms, rate = 273 bpm; V–V interval = 1000 ms (ventricular pacing), rate = 60 bpm; (D) confirmed episode of AF, A-A interval = 170–210 ms, rate = 286–352 bpm; V–V interval = 980 ms (ventricular pacing), 61 bpm. (E) False-positive example of electrograms (inEGMs) stored as VT/VF. The episode A-A interval= 80–240 ms, rate = 250–750 bpm; V–V interval = 281–410 ms, 146–213 bpm, according to intracavitary electrogram, according to inEGM, we diagnosed as AF with fast ventricular rate. Implantable device diagnosed this episode as VT/VF and given a 30J shock.
Abbreviations: A-ECG, atrial intracardiac electrogram; V-ECG, ventricular intracardiac electrogram; ms, millisecond; bpm, beats per minute.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation for data with normal distribution and median and inter-quartile range for data with skewed distributions. The Kolmogorov–Smirnov test was used to evaluate the normality of the distribution for continuous variables. The event rate in the pre-COVID-19 period was compared to the COVID-19 period or high-risk and low-risk period using the chi-squared test. Per-patient daily duration and daily frequency in the study period were calculated as the total occurrence times and duration divided by the number of days in the period (eg, during the high-risk period, 24 patients had NSVT, if someone had ten episodes of NSVT with a total duration of 40 seconds, and the high-risk period lasted 58 days; therefore the incidence of NSVT was 24/219, the daily patient duration was 40/58 seconds and daily frequency was 10/58 times). The daily frequency and daily duration across periods were compared using the Mann–Whitney U-test (between pre-COVID-19 and COVID-19 periods) and the Kruskal–Wallis H-test (for pre-COVID-19, high-risk, and low-risk periods).
A multivariate log-binomial regression model was established with adjustment for potential baseline confounders to evaluate the risk factors of arrhythmias further. We constructed a generalized estimation equation to evaluate the impacts of the COVID-19 pandemic on the risk of arrhythmias. Statistical analysis was performed using SPSS Statistics software, version 20.0 (IBM, Armonk, NY, USA). Two-sided p-values < 0.05 indicated statistical significance.
Follow-Up
This was a retrospective study after cardiac implantable device implantation in patients regularly followed up at our Electrophysiology and Cardiac Pacing Unit. All data on these episodes were obtained from a record review. We collected arrhythmia episodes, downloaded the intracardiac electrocardiogram of each patient, and asked each patient about antiarrhythmic drugs, interventional therapy, other treatments, and health status during the study period in detail. We exclude the patient from the study once treatment or other factors could influence the arrhythmia burden. In addition, when the severe arrhythmia episodes requiring intervention were monitored at device interrogation. The results were disseminated to patients truthfully, and they would obtain necessary treatment, including antiarrhythmic drugs or radiofrequency ablation.
Results
Patient Characteristics
In this 366-day observational study, a total of 219 patients were included (age, 68.86 ± 12.03 years; male, 60.7%). Etiologies leading to implantation were sick sinus syndrome in 85 (38.81%), atrial-ventricular block in 53 (24.20%), ejection fraction < 35% for primary prevention 46 (21.00%), and idiopathic ventricular arrhythmias in 35 (15.99%). A total of 138 (63.01%) patients had permanent pacemakers, 7 (3.20%) had undergone CRT, 40 (18.26%) had ICDs, and 34 (15.53%) had CRT defibrillators. The implantable cardiac device was from different manufacturers, 37 from BIOTRONIK, 31 from Boston Scientific, 54 from Medtronic, and 97 from St. Jude. Characteristics of the study population are provided in Table 1.
Table 1.
Baseline Characteristics of Study Population
| Characteristics | |
|---|---|
| Demographic and medical history | |
| Male, n (%) | 133(60.7) |
| Age (years), (mean ±SD) | 68.86±12.03 |
| BMI (kg/m2), (mean ± SD) | 23.77±3.06 |
| Habitual alcohol intake, n (%) | 41 (18.72) |
| Smoker, n (%) | 51 (23.3) |
| Heart failure, n (%) | 70(32.0) |
| Coronary heart disease, n (%) | 65(29.7) |
| Hypertension, n (%) | 138(63.0) |
| Diabetes, n (%) | 58(26.48) |
| DCM, n (%) | 15(6.8) |
| HCM, n (%) | 7(3.2) |
| Atrial fibrillation history, n (%) | 58(26.48) |
| Chronic kidney disease, n (%) | 24(10.96) |
| Peripheral artery disease, n (%) | 129(28.90) |
| COPD, n (%) | 10(4.57) |
| Hyperthyroidism, n (%) | 9(4.11) |
| Stroke history, n (%) | 17(7.76) |
| Echocardiographic parameters | |
| Left atrial diameter (mm) [median, range] | 40.53(28.76) |
| LVEDD (cm) [median, range] | 51.44(35.96) |
| LVESD (cm) [median, range] | 35.97(23.85) |
| LV systolic dysfunction, n (%) | |
| None | 156(71.23) |
| Mild | 19(8.68) |
| Moderate | 20(9.13) |
| Severe | 24(10.96) |
| LVEF (%)[median, range] | 57.53(17.75) |
| Indication for implanted devices, n(%) | |
| Sick sinus syndrome (SSS) | 85(38.81) |
| Atrial ventricular block (AVB) | 53(24.20) |
| LVEF<35% | 46(21.00) |
| IVT | 35(15.99) |
| Types of implanted devices, n(%) | |
| Permanent pacemaker | 138(63.01) |
| CRT | 7(3.20) |
| ICD | 40(18.26) |
| CRTD | 34(15.53) |
| Manufacturer, n(%) | |
| BIOTRONIK | 37(16.89) |
| Boston Scientific | 31(14.16) |
| Medtronic | 54(24.66) |
| St-jude | 97(41.55) |
Abbreviations: BMI, body mass index; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; COPD, chronic obstructive pulmonary disease; LVESD, left ventricular end-systolic diameter; LVESD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; IVT, idiopathic ventricular tachycardia; ICD, implantable cardiac defibrillator; CRT, cardiac resynchronization therapy; CRT-D, cardiac resynchronization therapy defibrillator.
Incidence of Various Types of Arrhythmias
During the pre-COVID-19 period, 5 (2.28%) patients had sustained ventricular tachycardia (SVT), 36 (16.44%) had Non-sustained ventricular tachycardia (NSVT), 55 (25.11%) had Atrial tachycardia (AT), and 57 (26.03%) had atrial fibrillation (AF). During the COVID-19 period, 9 (4.11%) had SVT, 44 (20.09%) had NSVT, 58 (26.48%) had AT, and 84 (38.36%) had AF. During the high-risk COVID-19 period, 5 (2.28%) had SVT, 24 (10.96%) had NSVT, 27 (12.33%) had AT, and 45 (20.55%) had AF. During the low-risk COVID-19 period, 7 (3.20%) patients had SVT, 34 (15.53%) had NSVT, 46 (21.00%) had AT, and 64 (29.22%) had AF.
There were 84 (38.36%) patients who suffered from AF during the COVID-19 period, more than the 57 (26.03%) who had AF in the pre-COVID-19 period (p = 0.006). There was no increase in ventricular arrhythmias and AT incidence during the COVID-19 period compared to the pre-COVID-19 period.
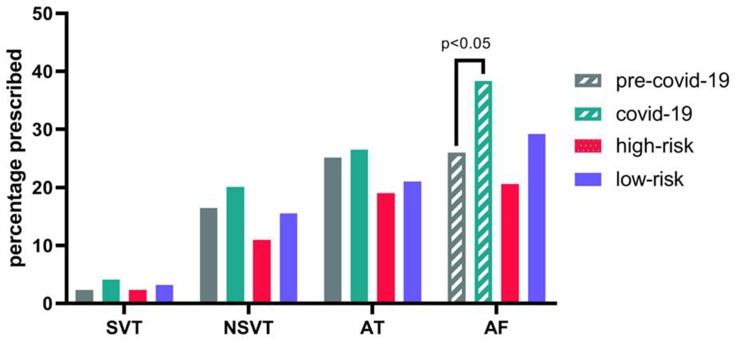
When the studied period was divided into pre-COVID-19, high-risk, and low-risk periods, there was no significant difference in SVT, NSVT, AT, or AF incidence among these three periods (all p > 0.05; Figure 3).
Figure 3.
The incidence of arrhythmias across periods. Patients suffering from AF in COVID-19 pandemic more than patients in the pre-COVID-19 period (P < 0.05), No significant changes were observed for the incidence of SVT, VT, AT compared among different periods.
Abbreviations: AT, atrial tachycardia; AF, atrial fibrillation; SVT, sustained ventricular tachycardia; NSVT, non-sustained ventricular tachycardia.
Comparison of Arrhythmia Burden
There was no evidence of increased daily frequency and duration of SVT, NSVT, AT, or AF when comparing the whole COVID-19 period to the pre-COVID-19 period. However, the daily NSVT frequency in the pre-COVID-19 period was less than it in the high-risk and low-risk periods (p < 0.05), and the daily NSVT duration in the high-risk period was significantly longer than during the pre-COVID-19 period (0.1982s Versus 0.0538, p = 0.023). The daily AT frequency in the high-risk period was more significant than the pre-COVID-19 period (0.0345 versus 0.0164 times, p = 0.034). The daily AF frequency was similar in the high-risk and low-risk periods, while it was relatively lower in the pre-COVID-19 period (P < 0.05) (Table 2).
Table 2.
Comparison of the Mean of Arrhythmia Burden Across Periods
| Pre-COVID-19 | COVID-19 | High-Risk | Low-Risk | F1/U1 | p1 | F2/χ2 | p2 | ||
|---|---|---|---|---|---|---|---|---|---|
| SVT | Frequency | 0.0186 ± 0.0126 | 0.0346 ± 0.0252 | 0.0759 ± 0.0707 | 0.0400 ± 0.0330 | 3.5 | 0.087 | 2.2 | 0.151 |
| Duration | 0.907, 2.1530 | 1.2022, 3.3688 | 2.5862, 10.8880 | 1.3920, 2.2960 | 18 | 0.606 | 1.9 | 0.380 | |
| NSVT | Frequency | 0.0109, 0.0164bc | 0.0164, 0.0314 | 0.0172, 0.0475a | 0.0240, 0.042a | 616 | 0.082 | 18 | 0.000 |
| Duration | 0.0538, 0.1640b | 0.0710, 0.1462 | 0.1982, 0.2845a | 0.6080, 0.3680 | 273 | 0.580 | 7.6 | 0.022 | |
| AT | Frequency | 0.0164, 0.0219b | 0.0219, 0.045 | 0.0345, 0.0518a | 0.0280, 0.0640 | 1565 | 0.861 | 7.3 | 0.025 |
| Duration | 0.8049, 3.5861 | 0.6448, 3.2869 | 2.0345, 8.1379 | 0.7480, 5.0100 | 1183 | 0.901 | 1.9 | 0.378 | |
| AF | Frequency | 0.0164, 0.0186bc | 0.0219, 0.0383 | 0.0345, 0.0432a | 0.0280, 0.0640a | 1967 | 0.070 | 19 | 0.000 |
| Duration | 11.1667, 503.6311 | 24.5792, 429.2486 | 40.7414, 631.0173 | 34.2560, 529.8260 | 1125 | 0.248 | 2.2 | 0.329 | |
Notes: F1/U1, p1 compare pre-COVID-19 period with COVID-19 period; F2/χ2, p2 compared with pre-COVID-19 period, high-risk period and Low-risk period. aCompared with the pre-COVID-19 group, and p < 0.05; bCompared with the high-risk group, and p < 0.05; cCompared with the low-risk group, and p<0.05.
Abbreviations: SVT, sustained ventricular tachycardia; NSVT, nonsustained ventricular tachycardia; AT, atrial tachycardia; AF, atrial fibrillation.
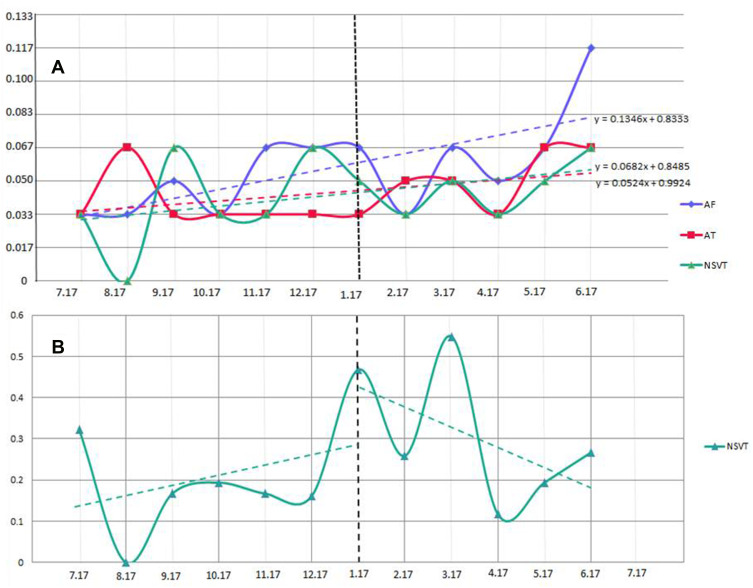
The per-patient daily arrhythmia frequency and duration among significantly different groups was illustrated to present the results more intuitively. We constructed a linear regression model for AT, AF, and NSVT in Figure 4A. During the COVID-19 period, the daily frequency of NSVT, AT, and AF increased month by month. In addition, we found that daily NSVT duration was correlated in parallel with the evolution of the COVID-19 pandemic. There was a sudden increase in daily NSVT duration after the first COVID-19 case in Wenzhou, China, was declared, and after the final case recovered on 16 March 2020, the per-patient daily NSVT duration dropped significantly (Figure 4B).
Figure 4.
Evolution of the burden of cardiac arrhythmias. (A) The mean of per-patient daily NSVT, AT, and AF occurrences per month. (B) The mean of per-patient NSVT duration per month. The dotted line: 1st COVID-19 case in Wenzhou.
Abbreviations: AT, atrial tachycardia; AF, atrial fibrillation; NSVT, non-sustained ventricular tachycardia.
Potential Risk Factors for Arrhythmia
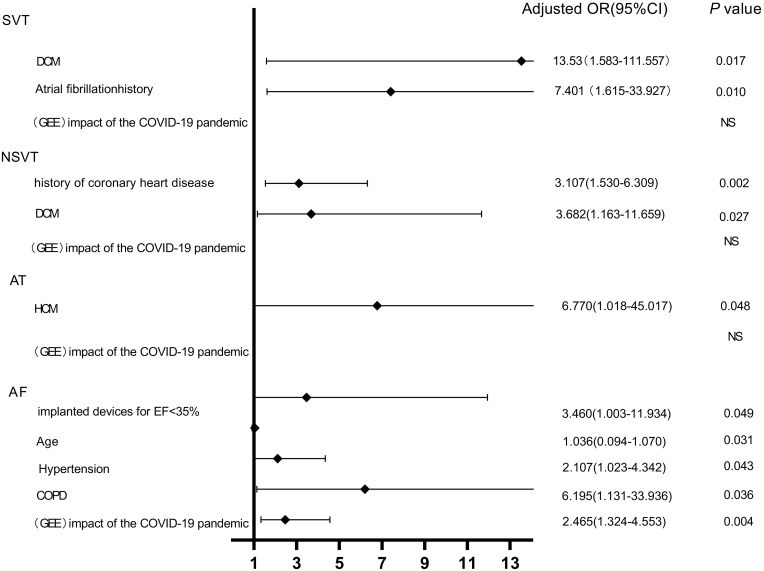
In our log-binomial regression and generalized estimation models, baseline variables that were considered clinically relevant and candidate variables with p-values < 0.2 on univariate analysis were included in the multivariable model to ensure parsimony of the final model.9 After adjusting for all confounding factors, we used a generalized estimation equation to evaluate the impact of the COVID-19 pandemic on the risk of any arrhythmias. We found the impact of the COVID-19 pandemic was a significant risk factor for occurrence AF in the non-infected population (odds ratio 2.465, 95% confidence interval, 1.324–4.553, p < 0.01). The COVID-19 pandemic had no impact on SVT, NSVT, or AT in our sample. The other potential risk factors for arrhythmia are shown in Figure 5.
Figure 5.
Logistic regression and generalized estimation equation analysis predicting the risk of arrhythmia during the COVID-19 epidemic.
Abbreviations: OR, odds ratio; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; COPD, chronic obstructive pulmonary disease; GEE, generalized estimation equation.
Previous RFCA Contributed to a Reduction of AF Burden During the COVID Period
AF incidence during the COVID-19 pandemic was higher, and the impact of pandemic was an independent risk factor to promote the onset of AF in non-infected patients. Therefore, during the pandemic, we primarily focused on identifying any method to reduce the AF burden. Interventional therapy might be an effective, long-lasting solution for these recurrent malignant arrhythmias and should be considered. Therefore, we retrospectively evaluated 34 patients with paroxysmal AF; 14 underwent previous RFCA to control heart rhythm, while the others still suffered from paroxysmal AF. We compared the incidence, per-patient daily duration, and daily frequency of the two groups across periods.
There were no significant differences in factors influencing the occurrence of AF, including age, the proportion of implanted devices for LVEF < 35%, hypertension, and chronic obstructive pulmonary disease (COPD) (Table 3). The incidence of AF in the COVID-19 period in the RFCA group was significantly lower than the non-RFCA group (21.43% versus 55.00% χ2 = 3.832, P = 0.049), So as the daily AF frequency (0.0000, 0.0027 versus 0.0000, 241.7978, P = 0.020); However, daily AF duration in RFCA group was similar with non-RFCA group (P > 0.05). As shown above, patients with AF could experience lower incidence and daily AF frequency following RFCA to control heart rhythm. This effect persisted during the pandemic; therefore, we recommend RFCA for patients with paroxysmal AF to reduce the arrhythmia burden in another unpredictable pandemic (Table 3).
Table 3.
Characteristics of Patients with Paroxysmal Atrial Fibrillation
| Characteristics | RFCA (n = 14) | Non-RFCA (n = 20) | χ2/U | p |
|---|---|---|---|---|
| Age(years) | 68.86 ± 9.28 | 71.40 ± 12.46 | 0.01 | 0.995 |
| Implanted devices for LVEF < 35%, n (%) | 3.00 (21.43) | 3.00 (15.00) | 0.234 | 0.672 |
| Hypertension, n (%) | 12.00 (85.71) | 12.00 (60.00) | 2.632 | 0.141 |
| COPD, n (%) | 0.00 (0.00) | 1.00 (5.00) | 0.721 | 1.000 |
| Incidence, n (%) | ||||
| Pre-COVID-19 period | 0.00 (0.00) | 7.00 (35.00) | 6.170 | 0.026 |
| COVID-19 period | 3.00 (21.43) | 11.00 (55.00) | 3.832 | 0.049 |
| Frequency | ||||
| Pre-COVID-19 period | 0.0000 | 0.0000, 8.4597 | 98 | 0.048 |
| COVID-19 period | 0.0000, 0.0027 | 0.0000, 241.7978 | 80.5 | 0.020 |
| High-risk period | 0.0000, 0.0345 | 0.0000, 8.8966 | 122.5 | 0.386 |
| Low-risk period | 0.0000, 0.0160 | 0.0000, 80.7320 | 96 | 0.056 |
| Duration | ||||
| Pre-COVID-19 period | 0.0000 | 0.0000, 0.0157 | 98 | 0.027 |
| COVID-19 period | 0.0000, 50.0068 | 0.0191, 0.0669 | 107.5 | 0.183 |
| High-risk period | 0.0000, 325.8276 | 0.0000, 0.0129 | 134 | 0.752 |
| Low-risk period | 0.0000, 141.6560 | 0.0000, 0.0440 | 112 | 0.207 |
Abbreviations: COPD, chronic obstructive pulmonary disease; RFCA, radiofrequency catheter ablation; LVEF, left ventricular ejection fraction.
Discussion
In our analysis of 219 out-of-hospital non-infected patients over 12 months, there were 1859 arrhythmia episodes recorded. The main results of this study are as follows: (a) there was a more significant proportion of patients with AF during the COVID-19 pandemic, and no increase was observed VAs; (b) the daily NSVT, AT, AF daily frequency, and NSVT duration were higher during the high-risk pandemic than the pre-COVID-19 period; (c) the COVID-19 pandemic appeared to promote the onset of AF in a non-infected population; However, we find that arrhythmias are the consequence of systemic illness, such as hypertension, dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), COPD, and a history of coronary heart disease; (d) controlling heart rhythm by RFCA may help reduce the incidence and daily AF frequency, this effect persisted during COVID-19 pandemic.
Environmental stress (such as earthquakes, war, or sporting events) may increase the risk of cardiovascular events.10,11 A growing body of evidence suggests that seasonal influenza pandemics trigger acute arrhythmias and coronary syndromes. Several studies reported that, during high activity influenza periods, patients were more likely to have ventricular arrhythmias treated with shock or anti-tachycardia pacing.12–14 Moreover, areas severely affected by the pandemic reported increased incidence of out-of-hospital cardiac arrest.3,15,16
In our study, the comprehensive effect of the COVID-19 pandemic on society was objectively explored by investigating the arrhythmias burden in majority non-infected patients opposing the minority infected patients. We did not find any increased incidence of ventricular arrhythmias during the COVID-19 pandemic in non-infected patients, consistent with previous studies on the topic.4–6,17 However, we found that, during the COVID-19 pandemic, the incidence of AF was higher than during the pre-COVID-19 period. Among the previous four studies on this topic, only one study from France mentioned atrial arrhythmia (the others only investigated ventricular arrhythmia burden); the authors demonstrated no changes regarding the evolution of atrial fibrillation/tachycardia episodes during the COVID-19 pandemic.17 The most likely explanation for the observed discrepancy was that the authors only studied the evolution of the incidence of atrial fibrillation/tachycardia episodes during the epidemic period and did not compare it with the pre-COVID-19 period. Additionally, we found a sudden increase in daily NSVT duration after the first COVID-19 case in Wenzhou, China, was declared and a significant drop after the final case recovered on 16 March 2020. We speculated that the COVID-19 pandemic could have triggered an integrated network of social factors, increasing the daily NSVT duration in the non-infected population. Similar results were shown in a study from France, where there was a sudden increase in the incidence of ventricular arrhythmia during the 2 weeks before the lockdown order. The two weeks before the lockdown order were associated explicitly with stress-inducing media coverage of the pandemic in France, including the first COVID-19 death in the country, the first televised presidential allocution on the topic, followed by a high number of COVID-19 cases and deaths.
One may hypothesize that this variation in AF incidence could be related to the stress generated by the outbreak. A study from Italy even revealed signs of psychological suffering after a protracted lockdown period.18 Other studies showed that, as the severity of the pandemic increased, the level of anxiety among the population also increased, bringing physical, mental, and psychological harm to people worldwide.19–22 These events contributed to the increase of adrenergic and dysfunction of the autonomic nervous system. Adrenergic inputs represent major neural triggers for atrial arrhythmias,23,24 especially in individuals with predisposing myocardial risk factors. Coumel et al reported that exercise and emotional stress could trigger adrenergic-mediated episodes of AF.25 Another study reported inpatient cardiac monitoring during the pandemic and found that of new arrhythmias detected, AF was the most common.26
RFCA could help to reduce the burden of AF, and this effect persisted during the pandemic. Patients with paroxysmal AF was found owning a reduction of incidence and daily AF frequency by previous RFCA. Therefore, we recommend RFCA for patients with paroxysmal AF in cases of frequent arrhythmia recurrence in another unpredictable pandemic. Especially in another national lockdowns where individuals are asked to remain isolated in their homes to stop the spread of the disease, electrophysiologists are redeployed to perform emergency work outside electrophysiology, elective work was canceled, and cases were performed only in emergencies,27 and protect patients from the severe complication of AF, such as embolism and stroke.
A recent study reported that all areas of cardiology service sustained significant reductions in general hospitals.28 It seems to contradict the result we observed that some arrhythmia burdens were increased during the high-risk period. Fersia et al demonstrated that urgent service did not decrease during COVID-19; the authors found that, despite a significant reduction in elective coronary angiography, there was no change in the number of urgent coronary angiography or primary percutaneous coronary interventions performed.28 We believe the reduction of cardiology service provision did not indicate a decrease in cardiovascular events. The reasons for these reductions are multifactorial. First, fear and trepidation regarding exposure to COVID-19 in clinical settings may compel many to avoid calling for help. Patients might have been reluctant to seek medical help during the pandemic apart from life-threatening events. The second reason is the restructuring and prioritization of health services, prioritizing urgent cases, and reducing access to primary care. This phenomenon also concerned us, as we observed that some arrhythmia burden increased during the pandemic. We were apprehensive that arrhythmias would not be treated sufficiently, and some arrhythmia-induced disability is unavoidable. We encourage exploring more flexible and available methods from medical services to help diagnose arrhythmias and prescribe appropriate treatment during pandemics for most non-infected patients.
Finally, we evaluated the association between arrhythmia and potential risk factors. As shown in Figure 4, arrhythmias are likely the consequence of systemic illness, and the impact of the pandemic was not related to the onset of life-threatening rhythms (SVT and NSVT), which may help reduce concerns experienced in the non-infected population during the pandemic. Furthermore, we could not ignore the non-infected people who had coronary heart disease, DCM, HCM, hypertension, COPD, or ejection fraction < 35% during the pandemic. These patients deserve special attention to ensure the early identification and intervention of arrhythmia.
Limitations
There were some limitations to the present study. First, this was a retrospective analysis, and several parameters could not be controlled for. We cannot claim with 100% certainty that the database contains accurately detected rhythms; despite device-detected arrhythmias, episodes were adjudicated by cardiac electrophysiologists to reduce potential bias. Second, this was a single-center study. We expect that sizable multi-center cohort studies will be conducted in the future to explore the long-term impact and the mechanism of the COVID-19 pandemic among the out-of-hospital non-infected population. Finally, in our sample, we did not systematically assess individual behavioral changes around physical activity, eating habits, and working from home arrangements; therefore, we could do no more than speculate regarding the association between the impact of the pandemic and arrhythmic events.
Conclusion
Based on our implantable device interrogation, we conclude that the COVID-19 pandemic contributed to a higher burden of arrhythmias in non-infected patients in a real-world setting. Patients with AF experienced a lower burden following RFCA to control heart rhythm, and this effect persisted during the pandemic; we recommend RFCA for patients with paroxysmal AF in cases of frequent arrhythmia recurrence in another unpredictable pandemic. We could not ignore the non-infected individuals who had coronary heart disease, DCM, HCM, hypertension, COPD, or ejection fraction < 35% during the pandemic. These patients deserve special attention to ensure the early identification and intervention of arrhythmia.
Funding Statement
This study was supported by Wenzhou Municipal Science and Technology Commission grant ZY2020018; and Department of Education of Zhejiang Province grant Y202044409. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations
COVID-19, coronavirus disease 2019; AT, atrial tachycardia; AF, atrial fibrillation; SVT, sustained ventricular tachycardia; NSVT, non-sustained ventricular tachycardia; RFCA, radiofrequency ablation; inECG, intracardiac electrogram; BMI, body mass index; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; COPD, chronic obstructive pulmonary disease; LVESD, left ventricular end-systolic diameter; LVESD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; IVT, idiopathic ventricular tachycardia; ICD, implantable cardiac defibrillator; CRT, cardiac resynchronization therapy; CRT-D, cardiac resynchronization therapy defibrillator.
Data Sharing Statement
Data are available on reasonable request to the corresponding author. (Email: linjiafeng_wzmcfey@163.com).
Ethics Approval and Informed Consent
This study was approved by the Institutional Review Board at The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (2021-K-36-01). Patient consent was waived as study a retrospective review of the medical files and anonymity was maintained all the way during data collection and manuscript writing. This study was conducted in accordance with the declaration of Helsinki.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. Erratum in: JAMA. 2021 Mar 16;325(11):1113. PMID: 32031570; PMCID: PMC7042881. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811–818. Erratum in: JAMA Cardiol. 2020 Jul 1;5(7):848. PMID: 32219356; PMCID: PMC7101506. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldi E, Sechi GM, Mare C, et al. Out-of-hospital cardiac arrest during the covid-19 outbreak in Italy. N Engl J Med. 2020;383(5):496–498. PMID: 32348640; PMCID: PMC7204428. doi: 10.1056/NEJMc2010418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Shea CJ, Thomas G, Middeldorp ME, et al. Ventricular arrhythmia burden during the coronavirus disease 2019 (COVID-19) pandemic. Eur Heart J. 2021;42(5):520–528. PMID: 33321517; PMCID: PMC7953962. doi: 10.1093/eurheartj/ehaa893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sassone B, Virzì S, Bertini M, et al. Impact of the COVID-19 lockdown on the arrhythmic burden of patients with implantable cardioverter-defibrillators. Pacing Clin Electrophysiol. 2021;44(6):1033–1038. PMID: 34022067; PMCID: PMC8207039. doi: 10.1111/pace.14280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malanchini G, Ferrari P, Leidi C, et al. Ventricular arrhythmias among patients with implantable cardioverter-defibrillator during the COVID-19 pandemic. J Arrhythm. 2021;37(2):407–413. PMID: 33821178; PMCID: PMC8014654. doi: 10.1002/joa3.12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowak B, Sperzel J, Rauscha F, et al. Diagnostic value of onset-recordings and marker annotations in dual chamber pacemaker stored electrograms. Europace. 2003;5(1):103–109. PMID: 12504649. doi: 10.1053/eupc.2002.0276 [DOI] [PubMed] [Google Scholar]
- 8.Pollak WM, Simmons JD, Interian A Jr, et al. Clinical utility of intraatrial pacemaker stored electrograms to diagnose atrial fibrillation and flutter. Pacing Clin Electrophysiol. 2001;24(4 Pt 1):424–429. PMID: 11341078. doi: 10.1046/j.1460-9592.2001.00424.x [DOI] [PubMed] [Google Scholar]
- 9.Kang SJ, Cho YR, Park GM, et al. Predictors for functionally significant in-stent restenosis: an integrated analysis using coronary angiography, IVUS, and myocardial perfusion imaging. JACC Cardiovasc Imaging. 2013;6(11):1183–1190. PMID: 24229771. doi: 10.1016/j.jcmg.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 10.Sato M, Fujita S, Saito A, et al. Increased incidence of transient left ventricular apical ballooning (so called ‘Takotsubo’ cardiomyopathy) after the mid-Niigata Prefecture earthquake. Circ J. 2006;70:947e953. doi: 10.1253/circj.70.947 [DOI] [PubMed] [Google Scholar]
- 11.Serra Grima R, Carreno MJ, Tomas Abadal L, Brossa V, Ligero C, Pons J. Acute coronary events among spectators in a soccer stadium. Rev Esp Cardiol. 2005;58:587–591. [PubMed] [Google Scholar]
- 12.Madjid M, Connolly AT, Nabutovsky Y, et al. Effect of high influenza activity on risk of ventricular arrhythmias requiring therapy in patients with implantable cardiac defibrillators and cardiac resynchronization therapy defibrillators. Am J Cardiol. 2019;124(1):44–50. PMID: 31047651. doi: 10.1016/j.amjcard.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 13.Russo V, Solimene F, Zanotto G, et al. Seasonal trend of ventricular arrhythmias in a nationwide remote monitoring database of implantable defibrillators and cardiac resynchronization devices. Int J Cardiol. 2019;275:104–106. PMID: 30327133. doi: 10.1016/j.ijcard.2018.10.025 [DOI] [PubMed] [Google Scholar]
- 14.Mamas MA, Fraser D, Neyses L. Cardiovascular manifestations associated with influenza virus infection. Int J Cardiol. 2008;130(3):304–309. PMID: 18625525. doi: 10.1016/j.ijcard.2008.04.044 [DOI] [PubMed] [Google Scholar]
- 15.Craen A, Logan G, Ganti L. Novel coronavirus disease 2019 and subarachnoid hemorrhage: a case report. Cureus. 2020;12(4):e7846. PMID: 32483497; PMCID: PMC7253081. doi: 10.7759/cureus.7846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan PS, Girotra S, Tang Y, et al. Outcomes for out-of-hospital cardiac arrest in the United States during the coronavirus disease 2019 pandemic. JAMA Cardiol. 2021;6(3):296–303. PMID: 33188678; PMCID: PMC7666759. doi: 10.1001/jamacardio.2020.6210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galand V, Hwang E, Gandjbakhch E, et al. Impact of COVID-19 on the incidence of cardiac arrhythmias in implantable cardioverter defibrillator recipients followed by remote monitoring. Arch Cardiovasc Dis. 2021;114(5):407–414. PMID: 34088625; PMCID: PMC8141722. doi: 10.1016/j.acvd.2021.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orlandi M, Rosselli M, Pellegrino A, et al. Gender differences in the impact on physical activity and lifestyle in Italy during the lockdown, due to the pandemic. Nutr Metab Cardiovasc Dis. 2021:S0939-4753(21)00133–2. PMID: 33975735. doi: 10.1016/j.numecd.2021.03.011 [DOI] [PubMed] [Google Scholar]
- 19.Wu T, Jia X, Shi H, et al. Prevalence of mental health problems during the COVID-19 pandemic: a systematic review and meta-analysis. J Affect Disord. 2021;281:91–98. PMID: 33310451; PMCID: PMC7710473. doi: 10.1016/j.jad.2020.11.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Wen X, Dong Y, et al. Psychological influence of Coronavirus Disease 2019 (COVID-19) pandemic on the general public, medical workers, and patients with mental disorders and its countermeasures. Psychosomatics. 2020;61(6):616–624. PMID: 32739051; PMCID: PMC7255244. doi: 10.1016/j.psym.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coelho CM, Suttiwan P, Arato N, et al. On the nature of fear and anxiety triggered by COVID-19. Front Psychol. 2020;11:581314. PMID: 33240172; PMCID: PMC7680724. doi: 10.3389/fpsyg.2020.581314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu J, Shen B, Zhao M, et al. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: implications and policy recommendations. Gen Psychiatry. 2020;33(2):e100213. Erratum in: Gen Psychiatr. 2020 Apr 27;33(2):e100213corr1. PMID: 32215365; PMCID: PMC7061893. doi: 10.1136/gpsych-2020-100213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan AY, Verrier RL. The role of the autonomic nervous system in cardiac arrhythmias. Handb Clin Neurol. 2013;117:135–145. PMID: 24095122. doi: 10.1016/B978-0-444-53491-0.00012-2 [DOI] [PubMed] [Google Scholar]
- 24.Franciosi S, Perry FKG, Roston TM, et al. The role of the autonomic nervous system in arrhythmias and sudden cardiac death. Auton Neurosci. 2017;205:1–11. PMID: 28392310. doi: 10.1016/j.autneu.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 25.Coumel P. Autonomic influences in atrial tachyarrhythmias. J Cardiovasc Electrophysiol. 1996;7(10):999–1007. PMID: 8894942. doi: 10.1111/j.1540-8167.1996.tb00474.x [DOI] [PubMed] [Google Scholar]
- 26.Braunstein ED, Reynbakh O, Krumerman A, et al. Inpatient cardiac monitoring using a patch-based mobile cardiac telemetry system during the COVID-19 pandemic. J Cardiovasc Electrophysiol. 2020;31(11):2803–2811. PMID: 32852868; PMCID: PMC7461402. doi: 10.1111/jce.14727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Mazzone P, Leung LWM, et al. Electrophysiology in the time of coronavirus: coping with the great wave. Europace. 2020;22(12):1841–1847. PMID: 32995866; PMCID: PMC7543596. doi: 10.1093/europace/euaa185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fersia O, Bryant S, Nicholson R, et al. The impact of the COVID-19 pandemic on cardiology services. Open Heart. 2020;7(2):e001359. PMID: 32855212; PMCID: PMC7454176. doi: 10.1136/openhrt-2020-001359 [DOI] [PMC free article] [PubMed] [Google Scholar]