Abstract
Cancer remains one of the leading health burdens worldwide. One of the challenges hindering cancer therapy development is the substantial discrepancies between the existing cancer models and the tumor microenvironment (TME) of human patients. Constructing tumor organoids represents an emerging approach to recapitulate the pathophysiological features of the TME in vitro. Over the past decade, various approaches have been demonstrated to engineer tumor organoids as in vitro cancer models, such as incorporating multiple cellular populations, reconstructing biophysical and chemical traits, and even recapitulating structural features. In this review, we focus on engineering approaches for building tumor organoids, including biomaterial-based, microfabrication-assisted, and synthetic biology-facilitated strategies. Furthermore, we summarize the applications of engineered tumor organoids in basic cancer research, cancer drug discovery, and personalized medicine. We also discuss the challenges and future opportunities in using tumor organoids for broader applications.
Keywords: Tumor organoid, Biomaterial, 3D printing, Micropatterning, Microfluidic, Genome editing
1. Introduction
Cancer drug discovery and development aim to identify new anti-cancer drugs and determine the clinical safety and efficacy of these therapies. After the lead candidate is identified in various cancer models, phased evaluations are performed to understand the mechanisms of action, dose-related toxicity, response rate, and applicable patients [1]. Cancer drug development, however, is often associated with high failure rates, suboptimal efficacies, and long development timelines [2]. Successful approvals by the Food and Drug Administration (FDA) are historically lengthy (over 10 years) and less than 10% of cancer drug candidates flushing into the phase I trials eventually get approved by the FDA [3]. Discrepancies in efficacy, side effects, and varied response rates among patients contribute to the low FDA approval rate of new cancer drugs[4, 5]. Cancer modeling plays an indispensable role in anti-cancer drug development. Conventional cancer modeling strategies using two dimensional (2D) cancer cell lines, genetically engineered mouse models, and primary patient‐derived tumor xenografts (PDXs) have contributed to the success of some anti-cancer therapeutics [6]. Cancer cell lines are commonly derived from patient samples and require extensive genetic manipulations to be efficiently cultured in vitro. Although genetically engineered mouse models and PDXs have better tumor-mimicking features, they are time-consuming and cost-ineffective to generate and may show mouse-specific characteristics [7]. Furthermore, tumor formation is often accompanied by the progression of neoplastic cells, the remodeling of the extracellular matrix (ECM) and vasculature, and interactions with immune cells from initial outgrowth to eventual metastasis [8]. It is critical to treat tumors as organs instead of simple epithelial cell masses with genetic alternations [9]. The dynamically orchestrated recruitment of fibroblasts, remodeling of the ECM, establishment of vascular networks, and the specific immune populations all contribute to the complexity and heterogeneity of tumors, further shaping the differences in responding or resisting to therapeutics. These features contribute to the failure of many conventional cancer therapies among individual patients [10]. Therefore, additional cancer modeling strategies are needed to facilitate more efficient and accurate evaluations of cancer therapies. Personalized cancer models can also make it possible for personalized anti-cancer drug screening.
Three dimensional (3D) cell culture represents an advanced in vitro model that can create patient-like tumor microtissues. Spheroids and organoids are two main types of 3D tissue models. Spheroids are simple clusters of cells that are generally cultured as free-floating aggregates [11]. They may be generated from cell lines, primary cells, or tissues. Tumor spheroids can better recapitulate the behavior of tumors than 2D cell cultures. They have been applied in studying tumor growth, angiogenesis, and drug resistance [12]. However, tumor spheroids have limited complexity in cell composition and cell-cell interactions, which may hinder their clinical impact [13]. Organoids are microscopic 3D tissue models cultured in vitro to mimic in vivo organs. They may be generated from primary tissue samples, embryonic or somatic stem cells, or pluripotent stem cells [14]. Organoids may contain multiple organ-specific cell types with spatial architectures similar to their counterparts. In this way, they can better recapitulate the organ. Organoid culture protocols have been established for a variety of organs and their associated tumors, such as intestine [15], colon [16], liver [17], breast [18], lung [19], brain [20], and related tumors [21–25]. With physiologically more accurate cancer modeling, organoids could serve as advanced in vitro cancer models to closely recapitulate the tumor microenvironment (TME) and maintain diverse cell populations. The comparison of organoids to other cancer models is listed in Table 1. Different models are complementary to each other in terms of cancer research. Two dimensional cell cultures are the least expensive options for evaluating anti-cancer drug response. However, they are oversimplified and poor to recapitulate the physiological, structural, and transcriptional features of tumors [26]. For example, the 2D cultured hepatocytes tend to lose polarity due to structural distortion within the monolayers of cell culture [27]. The loss of polarity led to de-differentiation and death of the isolated hepatocytes [28]. PDX models are generated by transplanting patients’ tumor cells into immune-deficient animals so that PDX could recapitulate some aspects of the niche effects, such as the interactions between tumor cells, stromal cells, and the ECM. PDX models have been used to investigate drug resistance and develop novel therapeutics [14]. However, many tumors fail to be grafted in animals, such as non-metastatic colorectal cancer [29] and hormone-sensitive breast cancer [30], and this is the main limitation of PDX models. In addition, after being injected into an animal model, the patient-derived tumor cells need to adapt to the new environment, leading to genetic drift and making the model less accurate [31]. In contrast, 3D cultures can partially mimic the tumor microenvironment and help to evaluate chemoresistance in the presence of cell-ECM interactions [32, 33]. Furthermore, it does not need to adapt to a new host, avoiding the concern for genetic drift. It is a faster and cheaper alternative to the animal model, which can fill the gap between 2D cell culture and animal model [6]. In the meantime, organoids can be easily scaled up for high-throughput drug screening. This feature is generally absent in traditional cancer models, including xenografts and animal models.
Table 1.
Comparison of different cancer models.
| Features Cancer models | Mimic tumor microenvironment | Tumor cell-ECM interaction | Genetic and functional recapitulations | Mimic tumor heterogeneity | Biological stability | High-throughput drug screening | Low cost | Time consumption |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 2D cell lines | - | - | + | - | + | +++ | +++ | + |
| Organoids | +++ | +++ | +++ | +++ | ++ | +++ | ++ | ++ |
| Spheroid | ++ | ++ | ++ | ++ | ++ | +++ | ++ | ++ |
| Xenografts | +++ | +++ | +++ | +++ | + | + | + | +++ |
| Animal models | +++ | +++ | ++ | ++ | +++ | - | + | +++ |
Note: +++, Best; ++, good; +, possible; -, unsuitable.
Conventional strategies of generating cancer organoids rely on 3D culture of patient-derived tumor tissues or genetically modified stem cells with the supplement of various growth factors or scaffolds to support organoid growth. However, several disadvantages limit the applicability of this strategy, including the random configuration of tumor organoids, insufficient complexity in recapitulating microenvironmental cues during the organogenesis process, and low reproducibility [34]. To address the limitations of traditional strategies in organoid-based cancer modeling, various engineering approaches have been developed to enhance the fidelity of cancer organoids. Researchers have reconstructed the TME diversity by incorporating immune cells and stromal cells [35, 36], promoting vascularization [37], and establishing regional differences of biochemical (hypoxia, acidity, and the presence of growth factors) [38] and biophysical features (matrix stiffness) [39]. High fidelity of tumor organoids to native tumor tissues could potentially decrease the discrepancies in predicting therapeutic responses of patients [8]. Additionally, engineered tumor organoids could promote their reproducibility and efficiency in a high-throughput manner using well-defined biomaterials, microwells, or well-controlled bioprinting methods. Organoids engineered in high-throughput formats could serve as valuable platforms for screening anti-cancer compounds from large libraries of compounds. Deriving tumor organoids from patient samples could also contribute to personalized drug testing, which could help to guide medical advice. Even though many studies on developing engineering approaches for 3D tumor modeling used cell lines. It is expected that these approaches could be combined with primary tumor tissues to build more accurate in vitro cancer models.
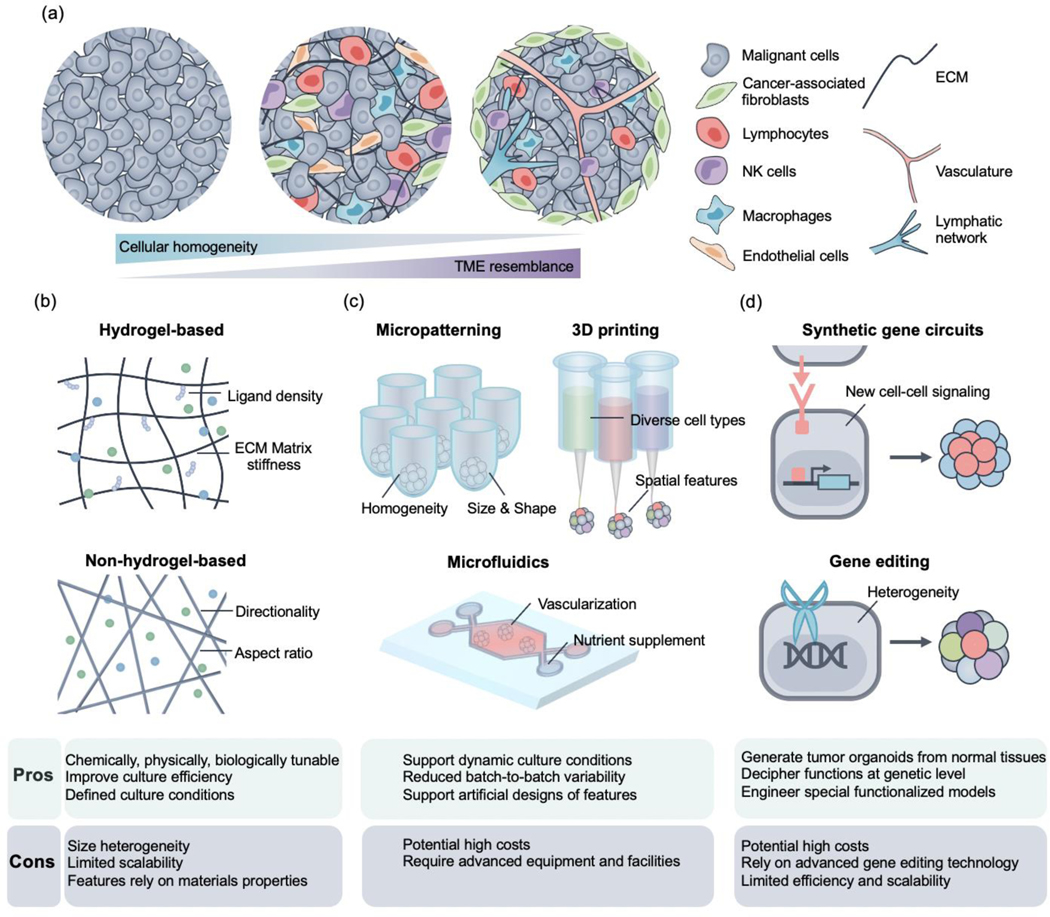
In this review, we first provide an overview of how engineering approaches could complement traditional methods in generating tumor organoids. Specifically, we categorize these approaches as biomaterial-based, microfabrication-assisted, and synthetic biology-facilitated strategies (as shown in Fig. 1). We then review how tumor organoids can be applied in basic cancer research, cancer drug discovery, and personalized medicine. Lastly, we discuss the potential opportunities and challenges in tumor organoid applications.
Fig. 1.
Schematic representation of reconstructing the tumor architecture into organoids via various engineering approaches. (a) From left to right: TME complexity increases with the incorporation of diverse cell types and ECM components. Physiologically relevant features could also be incorporated into tumor organoids by forming vascular and lymphatic networks. (b~d) Different engineering approaches enabled the generation of tumor organoids with corresponding pros and cons. (b) Biomaterial-based approaches could be generally divided into hydrogel- and scaffold-based ones, which provide physical support to the tumor organoids. The niche could be engineered by rationally tuning ligand density, changing matrix stiffness, and leveraging directionality and aspect ratios. (c) Microfabrication-assisted approaches can be summarized as micropatterning, 3D printing, and microfluidics-based ones. Micropatterning can facilitate the formation of homogenous organoids in specific shapes and sizes. 3D printing could organize diverse cell types with spatial specificity. Microfluidic platforms enable vascularization and provide biochemical cues in a physiologically relevant manner. (d) Synthetic biology-based approaches could engineer new cell-cell signaling for special tumor organoids formation and perform targeted gene editing to generate heterogeneous tumor organoids.
2. Approaches to generate organoids
2.1. Traditional approaches for organoid culture
Organoids, generally seen as “organ buds in a dish”, are 3D in vitro cell cultures that mimic the structure and function of organs. In early studies, they were formed from embryonic stem cells, induced pluripotent stem cells, or primary tissue either through self-organization or directed assembly with specific organogenesis cues [13, 40]. Based on the experience gained from culturing stem-cell-based organoids, several traditional methods have been developed to produce organoids, including tumor organoids. The simplest method uses a non-adhesive surface. In this method, the cells are grown on a Petri dish treated with bovine serum albumin, pluronic F-127, or the hydrophobic polymer to prevent cell adhesion. To generate compact organoids consisting of 10 to 20 cells, Bae et al. cultured a colon cancer cell line on a surface coated with non-adherent poly-2-hydroxyethyl methacrylate [41]. The non-adhesive method is easy to operate. However, it is difficult to control the size of organoids and track the growth of each organoid [42]. Spinner-flask is another approach to generate organoids. It holds the cells in suspension by stirring, which prevents the cells from adhering to the substrate and faciliates nutrient diffusion. Schneeberger et al. established an improved spinner-flask method for the expansion of large quantities of human liver organoids through improving the oxygenation system in the spinner flasks [43]. The organoids rapidly proliferated, and a 40-fold cell expansion was observed after 2 weeks. However, the spinner-flask method requires a high volume of cell culture media for cultivation. This drawback limits its usage for high-throughput drug screening or other multiplex experiments [44]. Hanging drop is another well-known method for generating organoids. Drops of cell suspensions are hung under the lid of a culture dish, the surface tension of the droplets induce the cells to form spherical structures. There are approximately 300 to 3000 cells in a droplet, and the volume of each droplet is usually 15 to 30 μL. Djomehri et al. used a scaffold-free 384-well hanging drop system to culture breast cancer organoids. Large (~1 mm diameter), scaffold-free, and highly-spherical organoids were obtained [45]. More importantly, the organoids retained the spindle cell morphology of the primary tumors.
By far, these traditional approaches have been used to generate various types of tumor organoids, such as pancreatic cancer [24], colon carcinoma [46], kidney cancer [47], gastrointestinal cancer [27], liver cancer [48], prostate cancer [49], uterine cancer [50], ovarian cancer [51], breast cancer [52], and bladder cancer [53]. The representative tumor organoid models are listed in Table 2. These organoids have been applied in basic cancer research, anti-cancer drug development, and personalized medicine that uses patient-specific tissue samples.
Table 2.
Typical tumor organoid models
| Types of tumor organoids | Aim of study | Source of organoids | Digestion condition | Culture condition | Reference |
|---|---|---|---|---|---|
|
| |||||
| Pancreatic cancer | To identify and interrogate pathways involved in pancreatic tumorigenesis | Neoplastic human pancreas tissues | Collagenase E, TrypLE | GFR Matrigel, human complete medium | [25] |
| Colorectal cancer | For high-throughput drug screening | Colorectal carcinoma patients | collagenase type IX, dispase type II | Human Intestinal Stem Cell medium minus Wnt Advanced DMEM/F12 added with | [54] |
| Kidney cancer | To provide a paediatric cancer organoid biobank | Children kidney tumor tissues | Collagenase | B27, R-spondin, EGF, N-acetylcysteine, fibroblast growth factor 10 (FGF-10), Y-27632 and A83–01 | [23] |
| Endometrial cancer | Drug screening | Human endometrial | Collagenase R TrypLE | Matrigel, DMEM/F12 supplement with Y-27632 | [55] |
| Prostate cancer | To establish the culture system of prostate epithelial and cancer tissue To establish a biobank | Circulating tumor cells and metastatic prostate cancer lesions Fresh | Collagenase type II supplemented with Y-27632, TrypLE | Matrigel, human prostate culture medium plus Y-27632 dihydrochloride | [56] |
| Gastric cancer | of patient-derived gastric cancer organoids for therapeutic screening | gastrectomy specimens from gastric tumor patients | Collagenase, hyaluronidase | Matrigel added with primocin, Y-27632 R-Spondin 1 conditioned medium, | [57] |
| Breast cancer | To establish a biobank of breast cancer organoids | Needle biopsies of metastatic breast cancer lesions | Collagenase | Neuregulin 1, FGF 7, FGF 10, EGF, Noggin, A83–01, Y-27632, SB202190, B27 supplement, N-Acetylcysteine, Nicotinamide, GlutaMax 100x, Hepes, Advanced DMEM/F12 | [18] |
| Bladder cancer | To establish a biobank of patient-derived bladder cancer organoids | Fresh human bladder tumor samples | Collagenase, hyaluronidase | Hepatocyte media with EGF, CS-FBS, Y-2763, and Glutamax supplemented with Primocin Advanced DMEM/F12 | [58] |
| Liver cancer | For disease modeling and drug screening | Patient-derived liver cancer specimens | / | supplemented with P/S, glutamax, HEPES, B27 without vitamin A, N2, N-acetyll-cysteine, Rspo-1, nicotinamide, (Leu15)-gastrin I, EGF, FGF10, HGF, forskolin and | [59] |
| A83–01 | |||||
| Cervical cancer | Establishment of a patient-derived cervical tumor organoid | Biopsy samples of a 23- year- old patient | dispase II, collagenase P and Accumax | Advanced DMEM/F12 supplemented with R-spondin1, Y27632, Noggin, EGF, Jagged-1, L-glutamine | [50] |
2.2. Engineering approaches for organoid culture
Conventional strategies in tumor organoid culture mainly focus on generating multicellular structures with either a single or multiple cell types, which are generally simple, disorganized, and limited in tissue-level features [60, 61]. Building tissue-level complexity into tumor organoids in a controlled manner can be facilitated by various state-of-art engineering approaches, such as utilization of biomaterials, microfabrication, microfluidic system, and synthetic biology approaches [62]. The overarching goals of next-generation tumor organoid engineering could be achieved by incorporating biological (such as involving key cell populations) and synthetic environmental controls (such as shear flow and stiffness of TME) [60]. Continuous understanding of the TME identified important cell types, such as stromal cells and immune cells, and other components, such as blood vessels, lymphatic vessels, and ECM components, which would increase the fidelity of tumor organoids. Engineering approaches can facilitate the self-organizing events and maximize the desired tissue-level organizations with spatiotemporal precisions [60]. In terms of biological environmental cues [63], the incorporation of various soluble factors and activation of selected signaling pathways through a time- and dose-specific strategy could trigger organoids’ growth in a specific pattern [16]. In addition, the ECM has an important role within the TME and it shapes the tumorigenesis processes [64]. Scaffolds derived from natural ECM, such as laminin-rich ECM (lrECM) [65] and collagen gel [66], are commonly used for tumor organoid culture [25, 67]. Other features of the ECM also affect tumor organoid growth and function, such as stiffness and adhesion specificity [68]. Bordeleau et al. investigated the effect of matrix stiffness on shaping tumor vasculature with collagen-based scaffold [69]. It was demonstrated that increased stiffness, regardless of matrix density, contributed to angiogenesis by enhancing vascular growth and integrity. Engineering synthetic or naturally derived ECM-like biomaterials with tunable biophysical and biochemical features could create organoids with higher fidelity to native tissues [70]. Furthermore, various synthetic environmental controls have been explored to facilitate in vitro organoid growth, such as air-liquid interface, on-gel surface, gel embedding, and roller ball cultures [61]. Microfabrication techniques generate better tumor organoids by controlling spatial architectures and incorporating various biological and synthetic environmental cues [62, 71, 72]. Synthetic biology approaches could program cell behaviors at genomic levels, which can be harnessed to further enhance the physiological fidelity of tumor organoids. In this section, we discuss engineering strategies for tumor organoids culture, including biomaterial-based, microfabrication-assisted, and synthetic biology-facilitated ones.
2.2.1. Biomaterial-based organoids culture
Advances in the engineering of synthetic or natural materials have been the foundation of tissue engineering. Tissue scaffold-based culture systems aim to mimic the native environment of the ECM by incorporating biological, physical, or biochemical features [73]. Tumor organoid culture could benefit from these tissue engineering tools [73, 74]. Traditionally used lrECM or collagen gels have undefined constituents that could cause batch-to-batch variabilities [75]. Therefore, synthetic or natural biomaterials that can be mass-produced with well-defined compositions can be alternatives to support 3D tumor organoids culture. In addition to their biochemical compositions, the stiffness of ECMs has a direct influence on the cellular behaviors, which are bidirectional interactions between cells and the surrounding ECM [76]. Cells could sense and respond differently to distinct mechanical environments, and ECM stiffness is dynamically modulated over the tumorigenesis processes, such as early (softer) versus late stages (stiffer) [77]. Therefore, engineering mechanically tunable biomaterials could potentially facilitate the optimization of organoid culture. To better organize the discussion, we classified the biomaterial-assisted approaches into hydrogel matrix- and non-hydrogel-based ones. Strategies involving cell-material interactions, different cell-adhesive ligands (such as Arg-Gly-Asp (RGD) and integrins), dynamic remodeling and degradation of ECM materials have been demonstrated.
2.2.1.1. Hydrogel matrix
The ECM plays important roles in regulating cell phenotypes through a series of biochemical and mechanical signals. Various ECM-derived or ECM-mimicking materials have been investigated to support organoid growth. Naturally derived polymers, such as hyaluronic acid (HA), gelatin, and chitosan, are commonly explored matrix materials for organoid culture [78–81]. These polymers are biocompatible and structurally similar to native ECM. To recapitulate in vivo invasiveness of glioma, Kievit et al. reported the use of chitosan-alginate (CA) hybrid hydrogel to mimic the TME of glioma and obtained more invasive glioma models [79]. The CA platform upregulated the secretion of matrix metalloproteinase 2 (MMP-2) and vascular endothelial growth factor (VEGF). It also increased the deposition of ECM proteins, including fibronectin and laminin, which provided the basis for more vascularized and malignant 3D culture. Another advantage of naturally derived polymers is that they can be chemically modified for tuning biophysical and biochemical properties, such as the conjugation of cross-linkable groups and cell adhesive peptides. Hao et al. engineered a hybrid hydrogel system based on thiolated HA (HA-SH) and an acrylated copolymer carrying repeated cell-adhesive peptide units (poly RGD-acrylate (AC)) (As shown in Fig. 2 a and b) [82]. Under physiological conditions, the hydrogel can be formed by simply mixing HA-SH and polyRGD-AC. Singular prostate cancer cells were dispersed within the hydrogel matrix, and multicellular spheroids (~95 μm in diameter) were formed by Day 28. The hybrid hydrogel enhanced cell-cell interactions through E-cadherin (E-CAD) and integrins but not polyRGD alone. A similar HA-SH-based platform was adopted by Fong et al. to investigate tumor-stroma interactions in the context of prostate cancer metastases to the bone [83]. HA was modified with an integrin-binding peptide and crosslinked with matrix metalloproteinase (MMP)-degradable peptides for co-culture of osteoblastic cell line (MC3T3-E1) and patient-derived prostate cancer cells. The osteoblastic cells were able to self-organize around prostate tumor organoids, closely mimicking the physiological architecture of prostate cancer metastases in bone. Ng et al. utilized enzymatically crosslinked gelatin and HA-based hydrogels for generating patient-derived colorectal cancer (CRC) organoid [78]. By conjugating phenol groups into the backbone of gelatin and HA, the addition of horseradish peroxidase (HRP) and H2O2 could catalyze the oxidative coupling among phenol groups. Thus, the mechanical strength and gelation rate of the hydrogel could be well-controlled. It was also demonstrated that phenol conjugated gelatin hydrogel could induce organoid growth and enhanced matrix stiffness could better support the growth of CRC organoids in vitro.
Fig. 2.

(a) Schematic of developing HA-PolyRGD hydrogels for the generation of prostate spheroids. (b) Confocal images of LNCaP prostate spheroid grown in HA-PolyRGD gels at days 1, 7, 14, and 28. Scale bar: 100 μm. Figures (a) and (b) were adapted with permission of ACS from [82]. (c) Schematic of the method for generating lymphoid organoids. (d) Confocal images of live/dead population of B cell lymphomas wrapped within various hydrogels. Figure (c and d) adapted with permission of Elsevier from [87].
Apart from naturally derived polymers, synthetic polymers are also widely investigated as a versatile and simple alternative strategy for tumor organoid culture. Both biological signals and mechanical cues within the synthetic material matrices can be defined and optimized for organoid growth. Chemically defined hydrogels with optimized post-processing steps could decrease batch-to-batch variability among organoid cultures. Polyethylene glycol (PEG) is one of the most commonly used synthetic materials in preparing hydrogels. Its unique chemical structure allows various modifications, enabling the control of its crosslinking degrees and biochemical activities [84]. Earlier work by Kloxin et al. developed synthetic PEG-based hydrogels. The physical and chemical properties of the hydrogels can be spatiotemporally tuned through photolytic reactions [85]. Both stiffness and the presence of adhesive peptides within the matrix network can be dynamically tuned via photodegradable crosslinkers. As a result, the morphology and behaviour of the encapsulated cells could be guided as desired [86].
The lack of effective ex vivo lymphoma models limits our capability to investigate lymphoma-associated drug resistance. To address this challenge Tian et al. utilized the PEG maleimide (MAL) click chemistry for hydrogel formation, which avoided the need for cytotoxic free-radicals or ultraviolet light (as shown in Fig. 2 c and d) [87]. By using a thiolated bio-adhesive peptide, the hydrogel can be functionalized with integrin-specific peptide in defined densities. The modularity of the hydrogel allows the recreation of a lymphoid neoplasm-like microenvironment. It has been demonstrated that T cell and B cell lymphomas have distinct integrin signaling demands for their survival. Furthermore, follicular dendritic cells were also included to function as a supporting stromal subtype, which demonstrated their critical roles in enhancing proliferation of lymphoma. In the case of drug screening, the engineered 3D microenvironment could induce drug resistance, including doxorubicin and histone deacetylase inhibitor panobinostat, which shows their potential to facilitate the screening and translation of various treatments in a faster and more rational way. Such versatility of incorporating different ligands at various densities in the hydrogel also enables the creation of personalized tumor models with patient-derived tissue samples. Recently, Mosquera et al. employed a similar PEG platform (PEG-4MAL) to develop patient-derived prostate tumor organoids [88]. With proteomics profiling and RNA sequencing, ECM cues involved in prostate tumor growth were identified, including fibronectin/vitronectin mimicking RGD, fibronectin mimicking Arg-Glu-Asp-Val (REDV), and collagen mimicking Gly-Phe-Hyp-Gly-Glu-Arg (GFOGER) peptides. This molecular information was further incorporated into the synthetic hydrogel to generate different prostate tumor organoids that exhibited morphologies, transcriptome profiles, and epigenetic hallmarks mimicking the original tumor biopsies. Compared to traditional ECM, different responses to small molecule inhibitors targeting epigenetic repressors were demonstrated in the synthetic tumor-specific ECM, revealing novel targets for prostate cancer therapeutics. Other commonly used matrices include thermo-responsive hydrogels and hybrids of natural and synthetic materials. Poly (N‐ isopropylacrylamide‐ co‐ acrylic acid) (PNIPAM-AA) [89] is a typical thermo-responsive hydrogel that can go through sol-gel transitions at specific temperatures for cell encapsulation. Exemplary hybrid materials include an aqueous two-phase system based on PEG and dextran (DEX) [90]. The immiscible two phases allow the confinement of tumor spheroids within the dextran phase after being immersed within the PEG phase. Selection of the two materials could be further optimized to achieve specialized tumor organoid cultures. Combined with automatic deposition, such as 3D printing, this strategy is simple and compatible with scaling up for high-throughput applications.
2.2.1.2. Non-hydrogel
In addition to hydrogel matrix-based platforms, various scaffolds have been developed for building 3D tumor models. One advantage of using scaffolds is tunable organoid architectures. The use of scaffolds with specific geometries could achieve the “guided self-organization” of organoids. For instance, recently, fiber-based scaffolds have been developed to produce elongated organoids by Lancaster et al. (as shown in Fig. 3 a~c) [91]. The authors utilized poly(lactide-co-glycolide) (PLGA) fibers as floating scaffolds to guide the self-organization of organoids, which recapitulated human forebrain tissue in terms of improved neuroectoderm formation and cortical development. For tumor organoids, Fischebach et al. engineered polymeric scaffolds based on PLGA for modelling oral squamous cell carcinoma [92]. Compared to Matrigel-based culture and 2D monolayer culture, the PLGA scaffold-based platform recreated features more filial to in vivo tissues, including growth profiles, hypoxia, and the pro-angiogenic factor expression (such as VEGF, bFGF, and IL-8). Fong et al. engineered 3D electrospun porous scaffolds based on poly(ε-caprolactone) (PCL) for generating sarcoma 3D model (as shown in Fig. 3 d and e) [93]. The fabrication was controlled to produce fibers with various diameters in a reproducible and straightforward manner. Efficient cell attachment was enabled on highly porous fibers with a large surface-area-to-volume ratio. The tumor organoid closely resembled those of the in vivo tumors in morphology, growth kinetics, and various biomarkers. Some natural polymers with particular architectures and high mechanical strength can also be used as scaffolds to support in vitro tumor organoid cultures, such as silk [94, 95]. Silk is mainly composed of fibroin, which has low degradability and high cell adhesiveness. Tan et al. fabricated a 3D porous silk sponge and seeded osteosarcoma cells in it [81]. The obtained osteosarcoma 3D scaffolds recapitulated several physiological features of in vivo osteosarcoma. The expression of various angiogenic and proliferative markers (bFGF, HIF-1α, IL-8, and VEGF-A) exhibited no statistical difference between in vitro silk scaffold-supported 3D tumor model and in vivo tumors.
Fig. 3.

(a) Schematic of engineering cerebral organoids (enCORs). (b) The PLGA microfilaments (left, arrowheads), fibers originated from sea sponge (middle), and cellulose fibers (right panel) serve as the scaffold to guide the organization of human pluripotent stem cells (hPSCs). Cellulose fibers fail to support the formation of elongated embryoids at day 3 using H9 cells. (c) Brightfield images of bioengineered embryoids formed from H9 cells at days 8 and 11. The embryoids display clearing along their edges and polarized neural ectoderm (arrows) during the neural induction. Figure adapted with permission from [91]. (d) Scanning electron microscopy images of electrospun 3D PCL scaffolds seeded with TC-71 cells with different magnifications (Upper: scale bar, 200 μm; Lower: scale bar, 50 μm). At Day 20, the cells closely resembled human Ewing sarcoma tumors with morphologically small and round structures in sheet-like clusters. (e) C-kit, IGF-1R, and pIGF-1R immunohistochemical staining of TC-71 human Ewing sarcoma cells cultured as 2D monolayer, in 3D PCL scaffolds, and as in vivo xenograft tumors. The expression levels of IGF-1R, pIGF-1R, and c-kit in 3D PCL scaffold is much more pronounced than 2D monolayer culture conditions. Expression levels of IGF-1R, pIGF-1R, and c-kit in the 3D culture shows similar results with in vivo model (the magnification: 400×). Figure adapted with permission from [93].
2.2.2. Microfabrication-assisted organoid culture
Advances in various microfabrication and 3D manufacturing techniques provide more tools in tumor organoid engineering [96, 97]. Despite the success of biomaterials in supporting the growth of organoids, organoids are commonly formed in varying shapes and sizes [98]. The batch-to-batch variability may have negative effects on biomedical applications, such as drug screening. Microfabrication-based approaches, such as microwells and 3D bioprinting, could conveniently produce arrays of organoids with consistent shape, size, and compositions. Micropatterning strategies and 3D bioprinting enable the generation of organoids with more complicated structures. Various stimuli have been incorporated in biomaterial-based approaches to support organoid growth, including the regulation of matrix stiffness and controlled release of growth factors. These aspects can be easily combined with microfabrication approaches. Traditional organoid cultures are generally closed systems without nutrient and waste transport, which are different from their in vivo tumor counterparts. These organoids typically lack vasculature and immune cell populations, limiting the size of organoids and failing to recapitulate in vivo tumor physiology [99]. 3D bioprinting can create vasculature within tissue constructs for exchanging nutrients, waste, and gases. Physiologically, the body is a dynamic system with various stimuli/nutrients supplemented and the waste removed. By using the microfluidic systems, additional external stimuli, such as various nutrients and flow conditions, could be easily added to the current organoid culture to better approximate the phenotypes of the corresponding tumor tissues [14].
2.2.2.1. Micropatterning
Advances in microfabrication techniques and understanding of the self-assembly of cells contributed to tumor organoid engineering by improving our control over organoids’ shapes and sizes. The most commonly used technique is the low-adhesive microwell array that can induce the formation of uniform tumor spheroid from cell aggregates at a predetermined cell density [100]. Brandenberg et al. developed an automated organoid culture platform based on micro-engineered hydrogel substrates (Fig. 4) [101]. The PEG hydrogel substrate was patterned with regularly spaced round-bottom microcavities to culture the cell suspensions. The confinement of the microcavities induced the formation of homogenous organoids. The platform was customizable in terms of cell seeding densities, stiffness of the hydrogel substrate, and the size of the microcavities. This strategy is also free of solid matrix, making it easy for downstream analysis. After preparing CRC tumor organoids, the authors tested 80 compounds (FDA-approved or currently under clinical trials) to select the most effective one. The microwell can be fabricated from micropatterned hydrogels and loaded with various cells. Yue et al. designed a stromal cell-laden hydrogel-based microwell array with tunable stiffness, mimicking normal and cancerous tissues [102]. The microwells can be further loaded with various cells to promote organoid formation. The authors demonstrated that the crosstalk between tumor organoids and stromal cells was significantly affected by the stiffness of ECM and the shape of tumor organoids. The microwell system has also been applied in cancer immunotherapy screening. Jiang et al. designed a high-throughput platform co-culturing tumor spheroids and immune cells for screening cancer immunotherapeutics (Fig. 5). 3D printing is utilized to create a conical microwell arrays for tumor spheroids formation. After the supplement of T cells and immune check point inhibitors, drug responses could be evaluated from viability of the tumor spheroid, cytokine release from the T cells, and T cell infiltration into the tumor organoids [103].
Fig. 4.

(a) Schematic of fabricating U-shaped microwell arrays with hydrogel. U-shaped microcavities were created on silicon substrates via standard Si Bosch and soft lithography approaches for subsequent polydimethylsiloxane (PDMS) molding. The microcavities were imprinted on the hydrogel surface (blue). (b) Schematic for generating intestinal organoids in U-shaped microcavities. Cell suspensions were seeded in the microwells. Once precipitated, the cells could form aggregates overnight with luminal stem cell (SC) colonies. After the expansion and differentiation of SC colonies for 6 days, the organoids are generated and post-processed for further use. (c) The fluorescence images of one screening replicate in a 96-well plate at different magnifications. The microtissues were stained with live (calcein acetoxymethyl ester, in green) and dead (ethidium homodimer-1, in red) dyes. The left column represents HCT116 microtissues and the right column represents the CRC organoids. Figure adapted with permission from [101].
Fig. 5.
(a). Schematic of the immunotherapeutic high-throughput observation chamber (iHOC). iHOC provided a platform to monitor the interaction among tumor spheroids, immune cells, and cancer immunotherapeutics in a high-throughput manner. B. Representative fluorescent microscopy images of LIVE/DEAD stained MDA-MB-231 tumor spheroids. The spheroids were cultured in 3D-printed microwell arrays for 48 h (Scale Bar = 100 μm). C. Representative fluorescent microscopy images of IL-2 detected by micropillar arrays. Groups S1 was seeded with MDA-MB-231 at a cell density of 0.5 × 106 cells mL−1. D. GFP-Jurkat T cells infiltration into MDA-MB-231 tumor spheroids with or without anti-PD-1 were observed by confocal microscopy after being cocultured for 2, 12, and 24 h. Tumor spheroids were stained by CellTracker CM-Dil Dye (Red). (*P < 0.05, **P < 0.01).
Other than device-based patterning, the utilization of material features or physical principles, including entropy-driven assemble, hydrophobic effect, and biphasic systems could guide pattern formation [104]. Du et al. presented a bottom-up approach to pattern the cell-laden microgels to construct a 3D tissue model [105]. The assembly process occurs through energy-favourable surface area minimization among different phases. Hydrophilic cell-containing microgels tend to assemble to minimize their surface energy in the presence of a hydrophobic oil phase. The cells can be loaded within PEG microgels and self-assembled by agitation. The modules, shapes, and encapsulants of the microgels can also be adapted for other applications. Tavana et al. utilized the aqueous biphasic systems (PEG and DEX) to pattern heterocellular constructs in desired patterns [106]. The authors demonstrated the patterning of stem cells in different sized colonies onto stromal cell monolayer. The differentiation fate of the stem cells is affected by the size of the colonies. A larger colony could enhance the differentiation due to the larger contact area with the stromal cell support. A similar strategy could be utilized for establishing stroma-tumor interactions or vascularizing tumor organoids through endothelial cell invasion. Atefi et al. employed a biphasic system for high-throughput generation of tumor spheroids by “patterning” cells contained in the DEX phase into the bath of PEG phase [90]. The resulting tumor spheroids are highly uniform, which are suitable for anticancer drug screening.
2.2.2.2. 3D bioprinting
3D bioprinting is a strategy to deposit bioinks with specified cell density, composition, and ECM components. It can control the volume of bioink to be deposited and the number and type of cells printed. 3D bioprinting can contribute to tumor organoid engineering by precisely controlling the shapes and sizes of organoids. It also allows the customization of various TME features, such as vasculature and immune cell infiltrations. Reid et al. optimized bioprinting about how many cells could be printed and the printing resolution. The authors demonstrated that as few as 10 cells could form 3D structures in a single printing [107]. By controlling the distance (up to 500 μm) between each printed dot, the singular 3D structure can gradually fuse together and form larger continuous organoids over time. By precisely controlling the number of cells and their coordinates, it generated organoids in different architectures, such as linear or luminal structures. Mollica et al. adapted the 3D bioprinting techniques for generating tumor organoids with human mammary-derived ECM hydrogels (as shown in Fig. 6 a~c) [108]. The native mammary ECM faithfully recapitulated the compositional features of the TME and preserved various signaling molecules for the growth of organoids. To be compatible with 3D bioprinting, the ECM should be able to gelate after the printing. The authors proposed slight enzymatic digestion with pepsin to promote spontaneous gelation. Furthermore, by printing ~50 cells at a time at 500 μm intervals, the authors generated MCF-7 cell-based solid structures, which have irregular borders similar to their tumorigenic phenotypes. In contrast, MCF-12A cells produced duct-like luminal morphologies (> 2 mm in length) within the human mammary-derived ECM hydrogels. Therefore, the combination of suitable substrate and 3D bioprinting can facilitate the recapitulation of morphological characteristics of tumor organoids. To create vasculature, various strategies have been demonstrated with 3D bioprinting. One common method is to print the vascular channels with a sacrificial bioink directly. Skylar-Scott et al. utilized embedded 3D printing to produce vascular networks in organoids (as shown in Fig. 6 d~f) [109]. The perfusable networks could facilitate the transport of nutrients to support organoid growth. Organoids made from human embryonic stem cells and induced pluripotent stem cells (iPSCs) could resemble their in vivo counterparts, which can be further differentiated under specific culture conditions [110]. Compacted organoid-based slurry was made by mixing organoids with collagen I and Matrigel under 4 °C. The slurry has self-healing capability to prevent defect formation while printing, which could serve as a matrix for direct writing of sacrificial gelatin ink within the organoid based slurry [111–113]. Followed by warming to 37 °C, the sacrificial ink would be melted and replaced with flowing oxygenated media. This strategy has inspired larger-scale in vitro tumor modeling by using tumor organoids as building blocks. A vascular network can be designed with features of tumor vasculatures, which are generally malformed and tortuous, to recreate hypoxic cores of solid tumors or mimic the transport of therapeutics within the abnormal vasculature [114–116].
Fig. 6.

(a~c) 3D bioprinted epithelial organoids and tumor organoids growing in rat and ECM hydrogels derived from human mammary tissues. (a) Fluorescent images of a linear pattern cellular arrays through bioprinting MCF-12A cells within Geltrex, rat-tail collagen (rtCOL), rat mammary ECM (rtMECM), and human mammary ECM (huMECM) (from left to right) at days 2 and 14. Large organoids are formed in all substrates and typically exceed 3 mm in length. (b) Fluorescent images of a linear pattern cellular arrays through bioprinting MCF-7 cells within Geltrex, rtCOL, rtMECM, and huMECM (from left to right) at days 2 and 14. Cells formed grape-like clusters in Geltrex while maintaining spherical shapes in other substrates by day 14. (c) Fluorescent images of a linear pattern cellular arrays through bioprinting MB-MDA-468 cells within Geltrex, rtCOL, rtMECM, and huMECM (from left to right) at days 2 and 14. Small clusters were formed in Geltrex, but single tumor organoids were generated in rtCOL and rtMECM. The cells did not grow well in huMECM. Scale bars 200 μm. Figure (a~c) adapted with permission of Elsevier from [108]. (d) Schematic representation of the sacrificial writing into functional tissue (SWIFT) process. (e) 3D printing of a vascular network with branched and interconnected channels within a compacted matrix made of embryoid bodies-based tissue. The connection between the fluid inlet and outlet from left to right represents the vascular network. Scale bar, 10 mm. (f) Image of a tissue construct with a perfusable vascular network with 12 hours of perfusion (top). Fluorescent images of cell viability (LIVE in green; DEAD in red) (bottom). Figure (d~f) adapted with permission from [109].
2.2.2.3. Microfluidics
The development of microfluidic systems and organ-on-a-chip technologies allows the manipulation of fluids in submillimeter channels. It enables precise control over fluid flow patterns, shear stress (mechanical cues), and biochemical or physical gradients [117]. Organoids are generally solid structures with a lack of tissue-tissue interfacing. Therefore, incorporating microfluidics or on-chip techniques could potentially generate more advanced tumor organoid models [71, 118]. Recently, Homan et al. explored the role of the ECM and fluidic shear stress (FSS) in enhancing in vitro vascularization and maturation of kidney organoids using a millifluidic chip-based platform (Fig. 7 a and b) [47]. The difference between millifluidic and microfluidic is that the cross-sectional size of millifluidic (> 1mm) is larger than that of microfluidic (< 500 μm) [119]. The bottom of the 3D-printed millifluidic chips were coated with gelatin-fibrin-based ECM to promote the adherence of embedded organoids. The fluid can flow within the perfusable fluidic chip with an optimized flow rate. Both ECM adherence and high FSS triggered an enhanced vascularization within organoids. Compared to strategies using in vivo transplantation to induce vascularization [120, 121], engineering approaches combining hydrogel substrates and mechanical cues provide versatile methods for producing vascularized tissues in vitro [122]. These approaches can also be adapted to vascularize tumor organoids and get scaled up for high-throughput applications [71, 123]. Shirure et al. designed a microfluidic device with three interconnected chambers to house perfusable 3D blood vessels and patient-derived breast cancer organoids under a culture medium flow that mimics physiological conditions (Fig. 7 c and d) [124]. The tumor organoids can be vascularized with endothelial cell migration from the vasculature chamber to the tumor organoid chamber, and the vascularized tumor organoids could be maintained for 22 days. Apart from using on-chip techniques for vascularization [125, 126], microfluidic systems could also help control the shapes and sizes of organoids. June et al. demonstrated a microfluidic-based platform for culturing lung cancer organoids under medium flow, mimicking physiological conditions [127]. The flow provided stable supplement of nutrients and oxygen to the organoid, generating monodispersed organoid arrays that can be used for high-throughput drug screening. Another advantage of microfluidic-based platforms over the traditional Matrigel droplet organoid culture is that it costs less time for organoids formation. Physiologically relevant flow can also enhance the phenotypical fidelity of tumor organoids to the original cancer tissues. The same microfluidic channels could be used to transport drugs and simulate drug treatment through diffusion. Less variability in drug responses could be achieved due to the monodispersed size and consistent spatial distributions of the organoids.
Fig. 7.
(a) A perfusable millifluidic chip was designed to culture renal organoids on an engineered ECM under controlled FSS (top). A timeline of the culturing conditions is shown (bottom). (b) Fluorescent microscopy images of organoids cultured on either adherent or nonadherent ECM stained with DAPI, MCAM, PECAM1, and PODXL. Scale bars, 100 μm. Figure adapted with permission from [47]. (c and d) The modeling of tumor-vasculature communication via an on-chip convection-diffusion-based system. (c) The device includes three parallel tissue chambers, each connected to two loading ports (LP) for tissues. A microporous wall (bottom insert) serves to separate the tissue chambers (PDMS is indicated in black, and empty chamber space is indicated in white). The two chambers on the side are used to for tumor and control tissues loading. Microvasculature is added to the central chamber. The central chamber is connected with two fluidic lines (FL1 and FL2) through their sources (So) and sinks (Si). Two side chambers are connected to a FL (white) to function as a sink to drain excess fluid. (d) Fluorescent microscopy images of the microvascular chamber showing endothelial cells (in green) and fibroblasts (in red) cultured at days 0 and 7. A vascular network is fully formed by day 7. Figure adapted with permission of RSC from [124].
2.2.3. Synthetic biology-assisted organoids culture
Cells, such as pluripotent stem cells, can proliferate, differentiate into distinct cell types, and spatially self-organize into complex architectures or patterns even with minimal external stimuli or instructions. Various cell behaviors and self-organized patterns are the results of genetically programmed cascades of events [61]. Synthetic biology has emerged as a powerful tool to program cells’ behaviors by building novel genetic pathways or networks [128, 129]. Various genetic engineering techniques, including RNA interference (RNAi), Cre-Lox-based recombination, and lentiviral small hairpin RNA (shRNA) have been applied in 3D cell culture, including organoids [130]. For example, Koo et al. presented a method for gene knockdown and overexpression in primary Lgr5 organoids via Cre recombinase-inducible retroviral vectors [131]. The method is broadly applicable to different organoid types and could inducibly knockdown or overexpress specific genes to modulate organoid growth. Morphological changes of organoids can also be achieved by engineering synthetic cell-cell signaling interactions [132]. Toda et al. programmed the self-assembly of multicellular systems by engineering a synthetic Notch (synNotch) system into the cells [133]. The synNotch system could drive the expression of a specified gene after recognizing cognate ligands on neighboring cells. The authors selected two different transcriptional outputs: expression of cadherin molecules or new synNotch ligands. The cells could self-organize into layered architectures as desired. The self-organized structures could either be spheroids or even asymmetric multicellular structures. These studies demonstrated the possibility of customizing self-organized organoids by programming genetic circuits. In terms of tumor organoids, Cheung et al. cultured breast cancer organoids and identified tumor cells with distinct expression of cytokeratin-14 (K14) and p63 could lead to the collective invasion [134]. By infecting the tumor organoids with lentivirus encoding shRNAs of K14 and p63, it was demonstrated that both K14 and p63 were indispensable to the invasive phenotype of breast cancer cells.
Multiple cancerous mutations can lead to the transformation of normal tissues into invasive cancers [135, 136]. Accumulated random alternations in the genome generated various genetic profiles, contributing to tumor heterogeneity. Advances in genome editing, such as the clustered regularly interspersed short palindromic repeats (CRISPR/Cas) systems, allow facile engineering of genomic mutations. When applied to tumor organoids, they could induce tumor organoid formation through genetically engineered tumorigenesis [137]. They can also help to elucidate the roles of different genetic mutations in tumorigenesis [138], and study tumor responses to various anti-cancer therapeutics [139]. CRISPR-based genome editing enables efficient introduction or correction of mutations in tumor organoids. Matano et al. introduced defined genetic alternations in human intestinal epithelial cells via CRISPR/Cas genome editing. Tumor suppressor genes adenomatous polyposis coli (APC), SMAD family member4 (SMAD4), and tumor protein p53 (TP53), and the oncogenes Kirsten rat sarcoma viral oncogene homolog (KRAS) and/or phosphatidylinositol 3-kinase catalytic subunit (PIK3CA) were engineered (Fig. 8 a~g) [137]. Previous reports on intestinal niche-specific culture and expansion of epithelial organoids can serve as an efficient selection strategy by supplementing specific culture conditions [16]. Only organoids with particular gene mutations can grow, facilitating the selection of driver gene mutations in the adenoma-carcinoma sequence. Organoids with all five genetic mutations can grow progressively in vitro without the need for niche factors and formed tumors after in vivo implantation. It was further demonstrated that invasive metastasis may require other molecular lesions, such as chromosomal instability, apart from the driver mutations. Takeda et al. further validated the driver genes of CRC in intestinal tumor organoids via CRISPR/Cas9-based gene disruptions (Fig. 8 h~j) [138]. They identified various tumor suppressors, including Acvr1b, Acvr2a, and Arid2. Mutations in genes encoding the activin receptor and transforming growth factor (TGF)-β promoted tumorigenesis synergistically.
Fig. 8.

(a~g) Incorporation of oncogenic driver mutations within human intestinal organoids via CRISPR/Cas9. Single cells from human intestinal organoids were treated with sgRNAs targeting APC, SMAD4, or TP53 via electroporation. (a) Control medium (supplemented with Wnt, EGF, noggin, R-spondin, and A83–01) was used for culturing control wild type (WT) organoids, and medium without Wnt and R-spondin was used to culture APC-targeted (A-) organoids. (b) Control medium was used for culturing control wild type (WT) organoids, and medium with TGF-β was used to culture SMAD4-targeted (S-) organoids. (c) Control medium was used for culturing control wild-type (WT) organoids, and medium with MDM inhibitor nutlin-3 was used to culture TP53-targeted (T-) organoids. (d) Control medium was used for culturing KRASWT A-organoids, and medium without EGF was used for culturing KRASG12V A-organoids. (e) Media without EGF was used for culturing KRASG12V and media without EGF but supplementing a MEK inhibitor (MEKi) was used for culturing KRASG12VPIK3CAE545K A-organoids. Scale bars, 1 mm (a~e). (f) Photographs of four representative isolated tumors after transplanting for 1 month (1M) or 2 months (2M). Organoids were transplanted under the kidney capsule with EGFP-labeling in NOG mice. AKST-organoids have four genetic mutations (APC, KRAS (G12V), SMAD4, and TP53) while AKSTP-organoids have an additional gene mutation in PIK3CA(E545K)). (g) LGR5+ stem cells (in brown) shown in xenografts formed from organoids via in situ mRNA hybridization. Figure (a~g) adapted with permission from [137]. (h~j) CRISPR-Cas9 mediated validation of the colorectal driver gene via mouse intestinal tumor organoids. Figure adapted with permission from [138]. (h) Schematic illustration of validation process: establishing mouse intestinal tumor organoids from ApcΔ716:Kras+/G12D:Villin-CreERT2 mice; the intestinal organoids were subjected to a Cas9 lentivirus with a GFP expression cassette; the GFP(+) organoids were subsequently treated with pools of gRNAs targeting 9 to 10 driver genes and then transplanted into mice for tumor formation monitoring. (i) Images of Cas9-expressing AK organoids. (j) Fluorescence images of GFP(+) organoids indicating the successful introduction of Cas9 into the organoids.
3. Applications of tumor organoids
With the development of 3D tissue culture technologies, organoids can now highly recapitulate the genomic characteristics, molecular profile, and tissue-level functions of a tumor. For example, Van de Wetering et al. compared the gene expression of CRC organoids with that of the original tumor. It showed that the heterogeneity of the original tumor was mostly retained. DNA sequencing and histological analysis verified resemblance in genetic mutational profiles and morphology between tumor organoids and parental tumor [54]. Weeber et al. also reported a 90% similarity of somatic mutations between the tumor organoid and matched patient tumor [98]. Moreover, Duarte et al. reported that mammary tumor organoids can better recapitulate the epithelial morphology and the drug response of the original tumor than 2D cell lines derived from the same tumor [140]. These properties enable tumor organoids to bridge the gap between 2D cell culture-based models and human patient-based clinical trials [141]. Consequently, tumor organoids have been widely used for fundamental cancer research, drug testing, and personalized therapy (Fig. 9) [142–146].
Fig. 9.
Engineered tumor organoids have been applied in anti-cancer drug development in a high-throughput manner. Tumor organoids could also be derived from individual patients for personalized medicine.
3.1. Tumor organoids for basic cancer research
Over the past decade, many new technologies have emerged for investigating cancer biology. Establishing reliable preclinical models is an essential step for basic cancer research and anti-cancer drug development. During the oncogenesis process, mechanical features and biochemical compositions in the TME are constantly changing [147]. TME is a complex environment that consists of stromal cells, tumor cells, immune cells, microvasculature, nutrients, growth factors, oxygen gradients, and other ECM components. Engineered 3D tumor organoids can faithfully recapitulate critical physiological features of TME [148]. They enabled the study of the communications among different cell types and the interactions between cells and ECM in vitro [149]. Therefore, tumor organoids can be a powerful platform for basic cancer research. For instance, the study of rare cancers is limited by the access to sufficient samples, such as signet-ring cell carcinoma (SRCC). Li et al. reported the first SRCC organoid line derived from a surgically resected tissue sample [150]. Chromosome analysis demonstrated that the SRCC organoids were aneuploid and the structure of chromosome was also aberrant. These features were consistent with the genetic characteristics of parent tumors. SRCC’s mucinous nature was verified by RNA sequencing of the organoids. Furthermore, the SRCC organoid line was used for screening drugable targets and effective drugs.The results showed that Janus kinase 2 was a potential target and AT9283 was an effective drug candidate for treating SRCC. The screening results were in line with the response of in vivo xenografts. Researchers have built various organoid biobanks with cancer tissues collected from individual patients, including breast cancer [18], kidney cancer [23], colorectal cancer [38, 54], gastric cancer [57], glioblastoma [21], bladder cancer [58], lung cancer [151], rectal cancer [152], ovarian cancer [153], neuroendocrine neoplasms [154] and prostate cancer [155]. These biobanks capture the heterogeneity of cancers and provide a representative collection of well-characterized models for basic cancer research [156]. Moreover, these biobanks conserve the genomic landscapes of tumors, which is crucial for genotype-phenotype correlation analyses and functional tests.
3.2. Tumor organoids for drug development
In the pharmaceutical industry, reliable preclinical models are essential for efficient anti-cancer drug discovery. They need to recapitulate human cancer biology faithfully [157]. Tumor organoids can be constructed from patient-derived cells, and they can be easily engineered on-the-bench. They have various advantages over animal models and 2D cell cultures (see Table 1) [158]. PDX was fabricated through injecting patient-derived tumor biopsies into immunodeficient or genetically modified animals. However, the use of animals is labor intense and time-consuming, making it unsuitable forhigh-throughput drug screening [142, 159]. Compared with PDX models, organoids allow the propagation and passage, can be expanded for long-term culture, and recapitulate the original cancer tissue both histologically and genetically [160]. More importantly, tumor organoids can faithfully show tumors’ responses to drugs in vitro, which are critical steps for drug development [161]. Therefore, tumor organoids have been a powerful tool for anti-drug development. As shown in Table 3, various tumor types of tumor organoids have been applied in drug development. Libraries of different therapeutic molecules, including small molecules and antibodies, have been tested on tumor organoid platforms.
Table 3.
Summary the studies of organoid cultures for drug screening
| Cancer Types | Source of organoids | Tested drug types | Total amount of tested drugs | Reference |
|---|---|---|---|---|
|
| ||||
| Breast cancer | 33 breast cancer patients | Target known inhibitors | 6 | [18] |
| Kidney cancer | 50 children patients | MEK and HDAC inhibitors | 150 | [23] |
| Colorectal cancer | 20 colorectal carcinoma patients | Chemo drugs and target known inhibitors | 83 | [54] |
| Bladder cancer | 18 bladder cancer patients | Target known inhibitors and chemo drugs | 50 | [58] |
| Liver cancer | Tumor cells from human PLC tissue | Anti-cancer compounds | 29 | [59] |
| Colon signet-ring cell carcinoma | A 43-year female patient | Janus kinase/signal transducers and activators, JAK2 inhibitors | 88 | [150] |
| Gastroesophageal cancer | 110 fresh biopsies from 71 patients | Drugs now in phase 1 to 3 clinical trials or in clinical practice | 55 | [161] |
| Pancreatic cancer | Organoid lines derived from CRC patients | Anti-cancer drugs and antibodies | 3 | [162] |
| Ovarian cancer | Patient-derived OC tissue | Platinum/taxane drugs | 10 | [163] |
| Colorectal cancer | Patient-derived organoids of CRC | FDA approved drugs and target known inhibitors | 2427 | [167] |
| Pancreatic cancer | hT1, hT1-CAF, hM1, and hM1-CAF cells | National Cancer Institute-approved drugs | 3300 | [168] |
| Colorectal cancer | Human colon | Existed antitumor agents and | 95 | [169] |
| carcinoma cell lines | molecular target drugs | |||
| HT29 | ||||
| Ductal pancreatic cancer | hESC- derived pancreatic progenitors | Gemcitabine, A366, UNC1999 | 3 | [170] |
| Liver cancer | Patient-derived organoids | Anti-cancer drugs | 129 | [171] |
| Colorectal cancer | Patient-derived organoids of CRC | Target known inhibitors | 71 | [172] |
| Ovarian cancer | Derived from ovarian cancer patients | Chemo drugs and target-known inhibitors | 22 | [173] |
| Colorectal cancer | Patient-derived organoids of CRC | Chemo drugs and target known inhibitors | 16 | [174] |
| Gastric cancer | Derived from 34 patients | Anti-cancer drugs | 37 | [57] |
The utilization of patient-derived organoid models for drug testing is a significant step in bridging the gap between animal models and human-based clinical trials. Steinhart et al. utilized CRC patient-derived organoids to screen novel biologics and verified the efficiency of anti-FZD5 antibodies as a potential candidate for targeted anti-tumor treatment [162]. Broutier et al. developed an organoid culture system mimicking physiological conditions using primary human healthy liver cells [59]. Organoids that retained liver tissue function and genetic stability were obtained, and they can self-expand for a long term. The PLC-derived organoids were used for drug-screening, and the researchers identified extracellular-signal-regulated kinase inhibitor SCH772984 as a potent candidate for treating primary liver cancer. Kopper et al. established a new organoid culture-based platform with 56 patient-derived ovarian cancer (OC) organoids which faithfully recapitulated the histological and genomic features of ovary lesions [163]. Ten chemotherapeutic drugs were tested on this platform, and OC organoids captured different tumor subtype responses to these drugs. For example, HGS-3.1 organoid line was highly sensitive to gemcitabine, adavosertib, carboplatin, and paclitaxel but resistant to drugs that target the PI3K/AKT/mTOR pathway. In contrast, HGS-23 organoid line demonstrated the opposite drug responses. Calandrini et al. built a pediatric kidney cancer-derived organoid biobank that preserved the heterogeneity of patient-derived tissues. A subset of Wilms tumor organoids was used for screening 150 compounds [23]. It was shown that the pediatric cancer organoid models were compatible with high-throughput screening and revealed patient-specific drug responses. Correlations between drug responses and gene mutations were established. Du et al. established a miniaturized human colon KRASG12D organoid culture platform for ultra-high-throughput screening in a 1536-well plate format [164]. A total of 10240 compounds was tested, and 43 of them passed the dose-response validation.
Microfabrication technologies enabled the rapid development of organoid-on-a-chip platforms for drug discovery. Microfluidic tumor organoid-on-a-chip platforms not only recapitulate the architecture of tumors but can also provide better nutrient and gas exchanges [99, 165]. More importantly, the organoid-on-a-chip platforms can be automated for continuous monitoring of organoid behaviors during drug testing. For example, Zhang et al. developed an automated microfluidic platform to measure soluble biomarkers and monitor organoid morphologies in an automated and continuous manner [166]. The designed platform could monitor the responses of liver cancer organoids when they are treated with drugs at different concentrations. Shirure et al. also described a patient-derived breast tumor organoid-on-a-chip system capable of analyzing the organoids’ responses to chemotherapy and anti-angiogenic therapy [124].
3.3. Tumor organoids for personalized medicine
Responses to anti-cancer therapies vary among individual patients. Different biomarkers have been investigated to predict treatment outcomes to maximize therapeutic efficacies and minimize side effects [14]. Even though cancer immunotherapy has revolutionized our management of cancer, their response rates are still unsatisfactory [175]. Apart from enhancing the general response rate of a specific therapy [176], it is also desirable to pursue personalized medicine, where medical advice could be given based on an individual patient’s response to a specific drug. Tumor organoid-based platforms provide an opportunity to achieve this goal by generating PDO and using it as an avatar of the patient to test drug responses [177]. PDOs could become personalized drug test models when they faithfully recapitulate individual patients’ tumor physiopathology. We can obtain valuable insight on selecting the treatment, optimizing efficacy, and reducing side effects for an individual patient. Notably, the tumor organoid strategy applies to different types of cancers and a wide range of therapeutics, making it a potentially universal strategy.
Ooft et al. derived PDOs from metastatic lesions of CRC patients. The authors aimed to identify patients who do not respond to standard chemotherapies, including combination therapies (5-fluorouracil with either oxaliplatin or irinotecan) or monotherapy (irinotecan) [178]. First-line oxaliplatin orsecond-line irinotecanwere evaluated in 22, 12, and 10 PDOs, respectively. Notably, 10 PDOs tested for irinotecan monotherapy displayed distinguishable GR50 and AUCDRC values (growth rate inhibition metrics (GR), dose-response curves (DRC)) between patients with progressive diseasesand stable disease. PDOs from stable disease patients exhibited higher sensitivity towards irinotecan monotherapy than PDOs derived from progressive disease patients. Similarly, PDO responses are predictive of irinotecan combination therapy but not oxaliplatin therapy. Sachs et al. recapitulated the responses of metastatic breast cancer to tamoxifen with patient-derived breast cancer organoids. Among 13 patients receiving tamoxifen as the standard-of-care, in vitro tests with organoids showed drug response or resistance matching clinical outcomes [18]. PDOs have also been used for distinguishing responders from non-responders to certain immunotherapies. 20 PDOs derived from responsive tumors were treated with anti‐ PD1. Assessment of interferon‐ gamma (IFN- γ ), granzyme B (GZMB), and perforin‐ 1 (PRF1) indicated T cell activation and tumor-killing capability, which was in agreement with clinical outcomes [179]. PDOs could also be applied for personalized cell therapy. Jacob et al. utilized patient‐ derived glioblastoma (GBM) organoids that could resemble parental tumors, including structural, cellular, genetic, and mutational features, for developing personalized CAR-T cell therapy. Antigen‐ specific killing was observed in cell therapy-responsive PDOs [21]. Established protocols for tumor organoid generation made it convenient for applying PDOs in personalized medicine. Different anti-cancer therapies, including chemotherapy and immunotherapy, and different drugs, such as small molecules, proteins, and cells, have been successfully tested on PDOs. The response of tumor organoids to drugs in vitro has the potential to mimic the patients’ response. However, success rates of culturing patient-derived organoids depend on sample quality, tumor types, and patients’ physiopathological conditions, which could complicate the screening process.
4. Challenges and Future Perspectives
Tumor organoid is a more faithful model of the human tumor tissue than the traditional cell culture. It can facilitate basic cancer research, drug development, and personalized medicine [180]. However, the engineered tumor organoids still have limitations to be addressed for broader applications. Even though many types of tumor organoids have been established, generating models for rare cancers is still needed to broaden its applicability. Different tumor types may need different culture conditions and have different success rates of forming organoids. The culture medium can be further optimized, and the time for successful organoid generation needs to be shortened. A more reliable and rapid organoid generation approach could facilitate tumor organoids’ clinical application as a personalized drug pre-screening platform. In preclinical cancer drug discovery, batch-to-batch variability among cultured organoids and compatibility with a high-throughput format need to be considered when designing organoids with increasing complexity and heterogeneity. High variability makes it difficult to interpret the drug responses. However, oversimplified organoid models may compromise their capability to recapitulate tumor biology. The balance between minimizing variability and maximizing the TME complexity should be considered in the design of engineering approaches [181].
Engineered tumor organoids have closely mimicked various aspects of the TME by incorporating immune cells and vasculature [182]. This increase in complexity also increases the genomic and epigenomic heterogeneity within these models. These features play important roles in tumor organoids’ responses to anti-cancer therapies. In addition, in vitro culture of these 3D models generally needs supplements different from physiological conditions. For instance, unnaturally elevated levels of growth factors supplemented in the media might lead to inaccurate growth profiles and abnormal drug responses in organoids [22]. Long-term maintenance of genetic stability during the culture without significant genomic mutations or epigenetic changes is desired for reliable tumor organoids in the clinic [160].
Organoid studies are gaining more complexity as researchers are investigating more fundamental mechanisms underlying stem cell differentiation. It requires concurrent evolutions of technologies relevant to 3D tumor. The single-cell technology can be applied to explore the genome, transcriptome, and epigenome at single-cell level, which facilitates the development of precision oncology [183]. Organoids combined with single-cell analysis have been applied to model various cancers and facilitated the study of tumor heterogeneity [18, 25, 58]. For example, Zhao et al. established the hepatobiliary PDOs and employed single-cell RNA sequencing to analyze the intertumoral and intratumoral heterogeneities [184]. The results revealed the inherent variability in their expression of transcriptional factors related to the cell cycle, hypoxia, and epithelial status. In recent years, artificial intelligence (AI) has also shown its potential in clinical decision-making. AI is a powerful tool in analyzing large datasets and identifying the relationship between variables. To evluate the fidelity of tumor organoid to the parent tumor tissue, tumor organoids are often subject to genomic analysis in comparison to the patients’ tumor. For instance, Chen et al. utilized machine learning and analyzed transcriptomics of tumor organoids to investigate the tumor heterogeneity. The researcher investigated if the subtypes/diversity are preserved in tumor organoids [185]. In addition, tumor organoids might be derived from large cohorts of patients and tested against many drugs. This could generate massive data sets that are unanalyzable manually, but AI can help to screen the results and decipher the correlations [177, 186]. Imaging has been essential to check the morphology of organoids, which is an important parameter to evaluate whether the organoids can faithfully recapitulate the tissue of origin. Light-sheet microscopy has been applied to image dynamic processes at the tissue level with limited light exposure and light toxicity [187]. Four-dimensional (4D) live imaging allowed the study of dynamic processes at high resolution. Combining 4D live imaging and engineered tumor organoid allowed a more precise determination of chromosome segregation defects and facilitated a better understanding of cancer progression. For example, Serra et al. characterized the development of intestinal organoids from single cells by using live-cell imaging and single-cell analysis [188]. They revealed single cells cultured in a growth-promoting environment have the intrinsic ability to generate complex multicellular asymmetric structures. The 4D live-cell imaging with light sheets makes it convenient to investigate organoids with higher complexity.
Even though many challenges exist, the area of engineering tumor organoids has been booming. It is expected that more and more advanced designs will generate more faithful organoid models. For instance, integrating bioprinting technology with on-chip platforms can minimize batch-to-batch variability and increase tissue-level complexity [118, 189]. When combined with the CRISPR/Cas system, organoids offer great opportunities for investigating mutation-induced tumorigenesis [190]. Gene editing can contribute to the building of more diverse tumor organoid biobanks. For the development of cancer immunotherapies, tumor organoids incorporating immune components could serve as effective drug screening models [191]. Also, tumor organoids are compatible with different therapeutics, such as small molecules, protein-, and cell-based drugs. They can also generate various readouts, such as cytotoxicity, biomarker secretion, infiltration levels of immune cells. Combination therapies based on radiology, such as radiotherapy and photothermal therapy, may also be screened on tumor organoids[192]. The 3D architecture of tumor organoids might be more suitable for screening these therapies in the solid tumor setting and give more accurate insight into the efficacy of these therapies.
Advances in tumor organoid engineering have pushed clinical evaluations forward, and the most recent recruiting clinical trials based on tumor organoids are summarized in Table 4 [57, 193]. Most trials aim to generate the PDOs from resectable primary tumor tissues or needle biopsies for drug screening or therapeutic response predictions. Combined with genomic analysis, biomarker monitoring, and immunochemical validations, the tumor organoid approach could help guide personalized medical advice and precision medicine development. Various organoid-related parameters have been evaluated for their correlations with drug responses, such as size, viability, and morphology [194]. Furthermore, tumor organoids can also be used to evaluate the dynamic clonal evolution of tumors [195], which might shed light on potential drug resistance or tumor relapse [196]. However, tumor organoids for drug response prediction are often used in proof-of-concept studies with limited patients. Apart from clinical applications, tumor organoids have also been applied in the pharmaceutical industry, aiming to establish large libraries of tumor organoids and matched healthy tissues. This approach could give insight into both drug efficacy and off-target toxicity. Large libraries can also cover better patient diversity, improving the accuracy of prediction. In the foreseeable future, more and more large scale clinical trials can be integrated with in vitro tumor models for more efficient and reliable tests [101].
Table 4.
Recruiting clinical trials involved with tumor organoids starting after 2021
| Cancer | Organoids | Treatment/ | Purpose | Estimated | Clinical trials |
|---|---|---|---|---|---|
|
| |||||
| types | culture | Intervention | enrollment | identifier | |
|
| |||||
| Colon cancer | Tumor biopsies | Cetuximab | Assess the consistency and accuracy of using colon cancer PDOs for prediction of clinical efficacy | 80 | NCT04906733 |
| Pancreatic cancer | Resectable cancer tissues | Adjuvant chemotherapy | Predict the treatment outcomes of individual patients by examining the relationship of the genomic mutations and reactivity to the anti-cancer drugs | 300 | NCT04736043 |
| Ovarian cancer | Primary cancer tissues after surgery | Standard regimens and treatments in clinical practice | Guide precision treatment by establishing organoids with genomic analysis and immunohistochemistry validations | 30 | NCT04768270 |
| HER2-nega tive breast cancer | Tumor biopsies | Standard regimens and treatments in clinical practice | Provide individualized genomic and drug reactivity information for patients before disease progression under standard treatment and examine how this will shape physicians’ choice of next-line therapy | 15 | NCT04450706 |
| Estrogen receptor positive breast cancer | Tumor biopsies | [18F] Fluoroestradiol positron emission tomography - computed tomography (FES PET/CT) | Establish the relationship between the FES-PET/CT results and drug screening results from PDOs | 6 | NCT04727632 |
| Breast cancer | Tumor biopsies | chemotherapy or endocrine therapy | Generate both PDX and tumor organoids from patients with residual disease after neoadjuvant therapy | 100 | NCT04703244 |
5. Conclusion
In summary, tumor organoids are models with high physiopathological fidelity to human cancer. They provide a powerful tool for investigating tumor biology and anti-cancer drug responses. Different engineering approaches have been demonstrated to improve tumor organoid generation, enhancing reproducibility and tissue-level functionality. Biomaterial-based, microfabrication-assisted, and synthetic biology-based strategies have been developed for organoid engineering. Tumor organoids could be further used in clinical screening for individual patients, which could help realize precision medicine goals. We believe tumor organoid models will revolutionize our cancer research approach and bring huge clinical benefits.
Acknowledgements
This work was supported by the National Institutes of Health (CA214411 and GM126571).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nass SJ, Rothenberg ML, Pentz R, Hricak H, Abernethy A, Anderson K, Gee AW, Harvey RD, Piantadosi S, Bertagnolli MM, Schrag D, Schilsky RL, Accelerating anticancer drug development - opportunities and trade-offs, Nat. Rev. Clin. Oncol, 15 (2018) 777–786. [DOI] [PubMed] [Google Scholar]
- [2].Hwang TJ, Carpenter D, Lauffenburger JC, Wang B, Franklin JM, Kesselheim AS, Failure of investigational drugs in late-stage clinical development and publication of trial results, JAMA internal medicine, 176 (2016) 1826–1833. [DOI] [PubMed] [Google Scholar]
- [3].Wong CH, Siah KW, Lo AW, Estimation of clinical trial success rates and related parameters, Biostatistics, 20 (2019) 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vineis P, Wild CP, Global cancer patterns: causes and prevention, Lancet, 383 (2014) 549–557. [DOI] [PubMed] [Google Scholar]
- [5].Imparato G, Urciuolo F, Netti PA, In vitro three-dimensional models in cancer research: a review, Int. Mater. Rev, 60 (2015) 297–311. [Google Scholar]
- [6].Tuveson D, Clevers H, Cancer modeling meets human organoid technology, Science, 364 (2019) 952–955. [DOI] [PubMed] [Google Scholar]
- [7].Drost J, Clevers H, Organoids in cancer research, Nat. Rev. Cancer, 18 (2018) 407–418. [DOI] [PubMed] [Google Scholar]
- [8].Junttila MR, de Sauvage FJ, Influence of tumour micro-environment heterogeneity on therapeutic response, Nature, 501 (2013) 346–354. [DOI] [PubMed] [Google Scholar]
- [9].Egeblad M, Nakasone ES, Werb Z, Tumors as organs: Complex tissues that interface with the entire organism, Dev. Cell, 18 (2010) 884–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fan H, Demirci U, Chen P, Emerging organoid models: leaping forward in cancer research, J. Hematol. Oncol, 12 (2019) 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Colella G, Fazioli F, Gallo M, De Chiara A, Apice G, Ruosi C, Cimmino A, de Nigris F, Sarcoma spheroids and organoids promising tools in the era of personalized medicine, Int J Mol Sci, 19 (2018) 615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gunti S, Hoke ATK, Vu KP, London NR Jr., Organoid and spheroid tumor models: Techniques and applications, Cancers (Basel), 13 (2021) 874–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thakuri PS, Liu C, Luker GD, Tavana H, Biomaterials-based approaches to tumor spheroid and organoid modeling, Adv. Healthc. Mater, 7 (2018) 1700980–1170100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Aboulkheyr Es H, Montazeri L, Aref AR, Vosough M, Baharvand H, Personalized cancer medicine: An organoid approach, Trends Biotechnol, 36 (2018) 358–371. [DOI] [PubMed] [Google Scholar]
- [15].Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H, Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche, Nature, 459 (2009) 262–266. [DOI] [PubMed] [Google Scholar]
- [16].Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H, Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium, Gastroenterology, 141 (2011) 1762–1772. [DOI] [PubMed] [Google Scholar]
- [17].Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, Ellis E, van Wenum M, Fuchs SA, de Ligt J, van de Wetering M, Sasaki N, Boers SJ, Kemperman H, de Jonge J, Ijzermans JN, Nieuwenhuis EE, Hoekstra R, Strom S, Vries RR, van der Laan LJ, Cuppen E, Clevers H, Long-term culture of genome-stable bipotent stem cells from adult human liver, Cell, 160 (2015) 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H, Korving J, van Boxtel R, Duarte AA, Lelieveld D, van Hoeck A, Ernst RF, Blokzijl F, Nijman IJ, Hoogstraat M, van de Ven M, Egan DA, Zinzalla V, Moll J, Boj SF, Voest EE, Wessels L, van Diest PJ, Rottenberg S, Vries RGJ, Cuppen E, Clevers H, A living biobank of breast cancer organoids captures disease heterogeneity, Cell, 172 (2018) 373–386. [DOI] [PubMed] [Google Scholar]
- [19].Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BLM, Basal cells as stem cells of the mouse trachea and human airway epithelium, P. Natl. Acad. Sci. USA, 106 (2009) 12771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Linkous A, Balamatsias D, Snuderl M, Edwards L, Miyaguchi K, Milner T, Reich B, Cohen-Gould L, Storaska A, Nakayama Y, Schenkein E, Singhania R, Cirigliano S, Magdeldin T, Lin Y, Nanjangud G, Chadalavada K, Pisapia D, Liston C, Fine HA, Modeling patient-derived glioblastoma with cerebral organoids, Cell Rep, 26 (2019) 3203–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jacob F, Salinas RD, Zhang DY, Nguyen PTT, Schnoll JG, Wong SZH, Thokala R, Sheikh S, Saxena D, Prokop S, Liu DA, Qian X, Petrov D, Lucas T, Chen HI, Dorsey JF, Christian KM, Binder ZA, Nasrallah M, Brem S, O’Rourke DM, Ming GL, Song H, A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity, Cell, 180 (2020) 188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gleave AM, Ci X, Lin D, Wang Y, A synopsis of prostate organoid methodologies, applications, and limitations, Prostate, 80 (2020) 518–526. [DOI] [PubMed] [Google Scholar]
- [23].Calandrini C, Schutgens F, Oka R, Margaritis T, Candelli T, Mathijsen L, Ammerlaan C, van Ineveld RL, Derakhshan S, de Haan S, Dolman E, Lijnzaad P, Custers L, Begthel H, Kerstens HHD, Visser LL, Rookmaaker M, Verhaar M, Tytgat GAM, Kemmeren P, de Krijger RR, Al-Saadi R, Pritchard-Jones K, Kool M, Rios AC, van den Heuvel-Eibrink MM, Molenaar JJ, van Boxtel R, Holstege FCP, Clevers H, Drost J, An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity, Nat. Commun, 11 (2020) 1310–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Saito Y, Establishment of an organoid bank of biliary tract and pancreatic cancers and its application for personalized therapy and future treatment, J. Gastroenterol. Hepatol, 34 (2019) 1906–1910. [DOI] [PubMed] [Google Scholar]
- [25].Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, Gracanin A, Oni T, Yu KH, van Boxtel R, Huch M, Rivera KD, Wilson JP, Feigin ME, Ohlund D, Handly-Santana A, Ardito-Abraham CM, Ludwig M, Elyada E, Alagesan B, Biffi G, Yordanov GN, Delcuze B, Creighton B, Wright K, Park Y, Morsink FH, Molenaar IQ, Borel Rinkes IH, Cuppen E, Hao Y, Jin Y, Nijman IJ, Iacobuzio-Donahue C, Leach SD, Pappin DJ, Hammell M, Klimstra DS, Basturk O, Hruban RH, Offerhaus GJ, Vries RG, Clevers H, Tuveson DA, Organoid models of human and mouse ductal pancreatic cancer, Cell, 160 (2015) 324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McMillin DW, Negri JM, Mitsiades CS, The role of tumour-stromal interactions in modifying drug response: challenges and opportunities, Nat Rev Drug Discov, 12 (2013) 217–228. [DOI] [PubMed] [Google Scholar]
- [27].Nguyen R, Bae SDW, Zhou G, Read SA, Ahlenstiel G, George J, Qiao L, Application of organoids in translational research of human diseases with a particular focus on gastrointestinal cancers, Biochim. Biophys. Acta Rev. Cancer, 1873 (2020) 188350–188361. [DOI] [PubMed] [Google Scholar]
- [28].Zeigerer A, Wuttke A, Marsico G, Seifert S, Kalaidzidis Y, Zerial M, Functional properties of hepatocytes in vitro are correlated with cell polarity maintenance, Exp Cell Res, 350 (2017) 242–252. [DOI] [PubMed] [Google Scholar]
- [29].Tetteh PW, Kretzschmar K, Begthel H, van den Born M, Korving J, Morsink F, Farin H, van Es JH, Offerhaus GJ, Clevers H, Generation of an inducible colon-specific Cre enzyme mouse line for colon cancer research, Proc Natl Acad Sci U S A, 113 (2016) 11859–11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, Factor R, Matsen C, Milash BA, Nelson E, Neumayer L, Randall RL, Stijleman IJ, Welm BE, Welm AL, Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes, Nat Med, 17 (2011) 1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gould SE, Junttila MR, de Sauvage FJ, Translational value of mouse models in oncology drug development, Nat Med, 21 (2015) 431–439. [DOI] [PubMed] [Google Scholar]
- [32].Weiswald LB, Bellet D, Dangles-Marie V, Spherical cancer models in tumor biology, Neoplasia, 17 (2015) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jager M, Blokzijl F, Sasselli V, Boymans S, Janssen R, Besselink N, Clevers H, van Boxtel R, Cuppen E, Measuring mutation accumulation in single human adult stem cells by whole-genome sequencing of organoid cultures, Nat Protoc, 13 (2018) 59–78. [DOI] [PubMed] [Google Scholar]
- [34].Sasai Y, Next-generation regenerative medicine: organogenesis from stem cells in 3D culture, Cell Stem Cell, 12 (2013) 520–530. [DOI] [PubMed] [Google Scholar]
- [35].Joyce JA, Fearon DT, T cell exclusion, immune privilege, and the tumor microenvironment, Science, 348 (2015) 74–80. [DOI] [PubMed] [Google Scholar]
- [36].Quail DF, Joyce JA, Microenvironmental regulation of tumor progression and metastasis, Nature medicine, 19 (2013) 1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dijkstra KK, Cattaneo CM, Weeber F, Chalabi M, van de Haar J, Fanchi LF, Slagter M, van der Velden DL, Kaing S, Kelderman S, Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids, Cell, 174 (2018) 1586–1598. e1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fujii M, Shimokawa M, Date S, Takano A, Matano M, Nanki K, Ohta Y, Toshimitsu K, Nakazato Y, Kawasaki K, A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis, Cell stem cell, 18 (2016) 827–838. [DOI] [PubMed] [Google Scholar]
- [39].Han YL, Pegoraro AF, Li H, Li K, Yuan Y, Xu G, Gu Z, Sun J, Hao Y, Gupta SK, Li Y, Tang W, Kang H, Teng L, Fredberg JJ, Guo M, Cell swelling, softening and invasion in a three-dimensional breast cancer model, Nature Physics, 16 (2020) 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Saglam-Metiner P, Gulce-Iz S, Biray-Avci C, Bioengineering-inspired three-dimensional culture systems: Organoids to create tumor microenvironment, Gene, 686 (2019) 203–212. [DOI] [PubMed] [Google Scholar]
- [41].Bae SI, Kang GH, Kim YI, Lee BL, Kim WH, Development of intracytoplasmic lumens in colon cancer cells cultured on non-adhesive surface, Exp. Toxicol. Pathol, 51 (1999) 21–26. [DOI] [PubMed] [Google Scholar]
- [42].Lee GH, Suh Y, Park JY, A paired bead and magnet array for molding microwells with variable concave geometries, J. Vis. Exp, (2018) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schneeberger K, Sanchez-Romero N, Ye S, van Steenbeek FG, Oosterhoff LA, Pla Palacin I, Chen C, van Wolferen ME, van Tienderen G, Lieshout R, Colemonts-Vroninks H, Schene I, Hoekstra R, Verstegen MMA, van der Laan LJW, Penning LC, Fuchs SA, Clevers H, De Kock J, Baptista PM, Spee B, Large-scale production of LGR5-positive bipotential human liver stem cells, Hepatology, 72 (2020) 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Goto-Silva L, Ayad NME, Herzog IL, Silva NP, Lamien B, Orlande HRB, da Costa Souza A, Ribeiro S, Martins M, Domont GB, Junqueira M, Tovar-Moll F, Rehen SK, Computational fluid dynamic analysis of physical forces playing a role in brain organoid cultures in two different multiplex platforms, BMC Dev. Biol, 19 (2019) 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Djomehri SI, Burman B, Gonzalez ME, Takayama S, Kleer CG, A reproducible scaffold-free 3D organoid model to study neoplastic progression in breast cancer, J. Cell Commun. Signal, 13 (2019) 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Aldebert E, Quaranta M, Sebert M, Bonnet D, Kirzin S, Portier G, Duffas JP, Chabot S, Lluel P, Allart S, Ferrand A, Alric L, Racaud-Sultan C, Mas E, Deraison C, Vergnolle N, Characterization of human colon organoids from Inflammatory Bowel Disease patients, Front Cell Dev. Biol, 8 (2020) 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R, Flow-enhanced vascularization and maturation of kidney organoids in vitro, Nat. Methods, 16 (2019) 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sun L, Yang H, Wang Y, Zhang X, Jin B, Xie F, Jin Y, Pang Y, Zhao H, Lu X, Sang X, Zhang H, Lin F, Sun W, Huang P, Mao Y, Application of a 3D bioprinted hepatocellular carcinoma cell model in antitumor drug research, Front. Oncol, 10 (2020) 878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Namekawa T, Ikeda K, Horie-Inoue K, Inoue S, Application of prostate cancer models for preclinical study: Advantages and limitations of cell lines, patient-derived xenografts, and three-dimensional culture of patient-derived cells, Cells, 8 (2019) 74–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Maru Y, Tanaka N, Ebisawa K, Odaka A, Sugiyama T, Itami M, Hippo Y, Establishment and characterization of patient-derived organoids from a young patient with cervical clear cell carcinoma, Cancer Sci, 110 (2019) 2992–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lohmussaar K, Kopper O, Korving J, Begthel H, Vreuls CPH, van Es JH, Clevers H, Assessing the origin of high-grade serous ovarian cancer using CRISPR-modification of mouse organoids, Nat. Commun, 11 (2020) 2660–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Roelofs C, Hollande F, Redvers R, Anderson RL, Merino D, Breast tumour organoids: promising models for the genomic and functional characterisation of breast cancer, Biochem. Soc. Trans, 47 (2019) 109–117. [DOI] [PubMed] [Google Scholar]
- [53].Abugomaa A, elbadawy M, Yamanaka M, Goto Y, Hayashi K, Mori T, Uchide T, Azakami D, fukushima R, Yoshida T, Shibutani M, Yamashita R, Kobayashi M, Yamawaki H, Shinohara Y, Kaneda M, Usui T, Sasaki K, Establishment of 2.5D organoid culture model using 3D bladder cancer organoid culture, Sci. Rep, 10 (2020) 9393–9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, Houdt W, Gorp J, Taylor-Weiner A, Kester L, McLaren-Douglas A, Blokker J, Jaksani S, Bartfeld S, Volckman R, Sluis P, Li VS, Seepo S, Sekhar Pedamallu C, Cibulskis K, Carter SL, McKenna A, Lawrence MS, Lichtenstein L, Stewart C, Koster J, Versteeg R, Oudenaarden A, Saez-Rodriguez J, Vries RG, Getz G, Wessels L, Stratton MR, McDermott U, Meyerson M, Garnett MJ, Clevers H, Prospective derivation of a living organoid biobank of colorectal cancer patients, Cell, 161 (2015) 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Boretto M, Maenhoudt N, Luo XL, Hennes A, Boeckx B, Bui B, Heremans R, Perneell L, Kobayashi H, Van Zundert I, Brems H, Cox B, Ferrante M, Uji-i H, Koh KP, D’Hooghe T, Vanhie A, Vergote I, Meuleman C, Tomassetti C, Lambrechts D, Vriens J, Timmerman D, Vankelecom H, Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening, Nat. Cell Biol, 21 (2019) 1041–1051. [DOI] [PubMed] [Google Scholar]
- [56].Drost J, Karthaus WR, Gao D, Driehuis E, Sawyers CL, Chen Y, Clevers H, Organoid culture systems for prostate epithelial and cancer tissue, Nat. Protoc, 11 (2016) 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yan HHN, Siu HC, Law S, Ho SL, Yue SSK, Tsui WY, Chan D, Chan AS, Ma S, Lam KO, Bartfeld S, Man AHY, Lee BCH, Chan ASY, Wong JWH, Cheng PSW, Chan AKW, Zhang J, Shi J, Fan X, Kwong DLW, Mak TW, Yuen ST, Clevers H, Leung SY, A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening, Cell Stem Cell, 23 (2018) 882–897. [DOI] [PubMed] [Google Scholar]
- [58].Lee SH, Hu W, Matulay JT, Silva MV, Owczarek TB, Kim K, Chua CW, Barlow LJ, Kandoth C, Williams AB, Bergren SK, Pietzak EJ, Anderson CB, Benson MC, Coleman JA, Taylor BS, Abate-Shen C, McKiernan JM, Al-Ahmadie H, Solit DB, Shen MM, Tumor evolution and drug response in patient-derived organoid models of bladder cancer, Cell, 173 (2018) 515–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Broutier L, Mastrogiovanni G, Verstegen MMA, Francies HE, Gavarró LM, Bradshaw CR, Allen GE, Arnes-Benito R, Sidorova O, Gaspersz MP, Georgakopoulos N, Koo B-K, Dietmann S, Davies SE, Praseedom RK, Lieshout R, Ijzermans JNM, Wigmore SJ, Saeb-Parsy K, Garnett MJ, van der Laan LJW, Huch M, Human primary liver cancer-derived organoid cultures for disease modeling and drug screening, Nat. Med, 23 (2017) 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Takebe T, Wells JM, Organoids by design, Science, 364 (2019) 956–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shamir ER, Ewald AJ, Three-dimensional organotypic culture: experimental models of mammalian biology and disease, Nat. Rev. Mol. Cell Biol, 15 (2014) 647–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Murrow LM, Weber RJ, Gartner ZJ, Dissecting the stem cell niche with organoid models: an engineering-based approach, Development, 144 (2017) 998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sato T, Clevers H, Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications, Science, 340 (2013) 1190–1194. [DOI] [PubMed] [Google Scholar]
- [64].Nallanthighal S, Heiserman JP, Cheon DJ, The role of the extracellular matrix in cancer stemness, Front. Cell Dev. Biol, 7 (2019) 86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kleinman HK, Martin GR, Matrigel: basement membrane matrix with biological activity, Semin. Cancer Biol, 15 (2005) 378–386. [DOI] [PubMed] [Google Scholar]
- [66].Sabeh F, Shimizu-Hirota R, Weiss SJ, Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited, J. Cell Biol, 185 (2009) 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chen JY, Zhao L, Peng HL, Dai SQ, Quan Y, Wang ML, Wang J, Bi ZY, Zheng Y, Zhou ST, Liu Y, Chen C, Na FF, An organoid-based drug screening identified a menin-MLL inhibitor for endometrial cancer through regulating the HIF pathway, Cancer Gene Ther, (2020) 1–14. [DOI] [PubMed] [Google Scholar]
- [68].Mason BN, Starchenko A, Williams RM, Bonassar LJ, Reinhart-King CA, Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior, Acta Biomater, 9 (2013) 4635–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bordeleau F, Mason BN, Lollis EM, Mazzola M, Zanotelli MR, Somasegar S, Califano JP, Montague C, LaValley DJ, Huynh J, Mencia-Trinchant N, Negron Abril YL, Hassane DC, Bonassar LJ, Butcher JT, Weiss RS, Reinhart-King CA, Matrix stiffening promotes a tumor vasculature phenotype, Proc. Natl. Acad. Sci. USA, 114 (2017) 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Henke E, Nandigama R, Ergun S, Extracellular matrix in the tumor microenvironment and its Impact on cancer therapy, Front. Mol. Biosci, 6 (2019) 160–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sontheimer-Phelps A, Hassell BA, Ingber DE, Modelling cancer in microfluidic human organs-on-chips, Nat. Rev. Cancer, 19 (2019) 65–81. [DOI] [PubMed] [Google Scholar]
- [72].Brusatin G, Panciera T, Gandin A, Citron A, Piccolo S, Biomaterials and engineered microenvironments to control YAP/TAZ-dependent cell behaviour, Nat. Mater, 17 (2018) 1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hutmacher DW, Biomaterials offer cancer research the third dimension, Nat. Mater, 9 (2010) 90–93. [DOI] [PubMed] [Google Scholar]
- [74].Abbott A, Biology’s new dimension, Nature, 424 (2003) 870–872. [DOI] [PubMed] [Google Scholar]
- [75].Lee GY, Kenny PA, Lee EH, Bissell MJ, Three-dimensional culture models of normal and malignant breast epithelial cells, Nat. Methods, 4 (2007) 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ingber DE, Can cancer be reversed by engineering the tumor microenvironment?, Semin. Cancer Biol, 18 (2008) 356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kaushik S, Pickup MW, Weaver VM, From transformation to metastasis: deconstructing the extracellular matrix in breast cancer, Cancer Metastasis Rev, 35 (2016) 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ng S, Tan WJ, Pek MMX, Tan MH, Kurisawa M, Mechanically and chemically defined hydrogel matrices for patient-derived colorectal tumor organoid culture, Biomaterials, 219 (2019) 119400–119412. [DOI] [PubMed] [Google Scholar]
- [79].Kievit FM, Florczyk SJ, Leung MC, Veiseh O, Park JO, Disis ML, Zhang MQ, Chitosan-alginate 3D scaffolds as a mimic of the glioma tumor microenvironment, Biomaterials, 31 (2010) 5903–5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Rao W, Zhao S, Yu J, Lu X, Zynger DL, He X, Enhanced enrichment of prostate cancer stem-like cells with miniaturized 3D culture in liquid core-hydrogel shell microcapsules, Biomaterials, 35 (2014) 7762–7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tan PH, Aung KZ, Toh SL, Goh JC, Nathan SS, Three-dimensional porous silk tumor constructs in the approximation of in vivo osteosarcoma physiology, Biomaterials, 32 (2011) 6131–6137. [DOI] [PubMed] [Google Scholar]
- [82].Hao Y, Zerdoum AB, Stuffer AJ, Rajasekaran AK, Jia X, Biomimetic hydrogels incorporating polymeric cell-adhesive peptide to promote the 3D assembly of tumoroids, Biomacromolecules, 17 (2016) 3750–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Fong EL, Wan X, Yang J, Morgado M, Mikos AG, Harrington DA, Navone NM, Farach-Carson MC, A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions, Biomaterials, 77 (2016) 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, Rizzi SC, Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells, Biomaterials, 31 (2010) 8494–8506. [DOI] [PubMed] [Google Scholar]
- [85].Kloxin AM, Kasko AM, Salinas CN, Anseth KS, Photodegradable hydrogels for dynamic tuning of physical and chemical properties, Science, 324 (2009) 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lutolf MP, Spotlight on hydrogels, Nat Mater, 8 (2009) 451–453. [DOI] [PubMed] [Google Scholar]
- [87].Tian YF, Ahn H, Schneider RS, Yang SN, Roman-Gonzalez L, Melnick AM, Cerchietti L, Singh A, Integrin-specific hydrogels as adaptable tumor organoids for malignant B and T cells, Biomaterials, 73 (2015) 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mosquera MJ, Bareja R, Bernheim JM, Asad M, Cheung C, Sigouros M, Prabhu V, Allen J, Martin ML, Puca L, Rubin M, Beltran H, Mosquera JM, Elemento O, Singh A, Extracellular microenvironment in patient-derived hydrogel organoids of prostate cancer regulates therapeutic response, medRxiv, 6 (2020) 160–183. [Google Scholar]
- [89].Wu YH, Zhao ZQ, Guan Y, Zhang YJ, Galactosylated reversible hydrogels as scaffold for HepG2 spheroid generation, Acta Biomater, 10 (2014) 1965–1974. [DOI] [PubMed] [Google Scholar]
- [90].Atefi E, Lemmo S, Fyffe D, Luker GD, Tavana H, High throughput, polymeric aqueous two-phase printing of tumor spheroids, Adv Funct Mater, 24 (2014) 6509–6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, Otani T, Livesey FJ, Knoblich JA, Guided self-organization and cortical plate formation in human brain organoids, Nat Biotechnol, 35 (2017) 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, Mooney DJ, Engineering tumors with 3D scaffolds, Nat Methods, 4 (2007) 855–860. [DOI] [PubMed] [Google Scholar]
- [93].Fong EL, Lamhamedi-Cherradi SE, Burdett E, Ramamoorthy V, Lazar AJ, Kasper FK, Farach-Carson MC, Vishwamitra D, Demicco EG, Menegaz BA, Amin HM, Mikos AG, Ludwig JA, Modeling Ewing sarcoma tumors in vitro with 3D scaffolds, Proc Natl Acad Sci USA, 110 (2013) 6500–6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Talukdar S, Kundu SC, A non-mulberry silk fibroin protein based 3D in vitro tumor model for evaluation of anticancer drug activity, Adv Funct Mater, 22 (2012) 4778–4788. [Google Scholar]
- [95].Talukdar S, Mandal M, Hutmacher DW, Russell PJ, Soekmadji C, Kundu SC, Engineered silk fibroin protein 3D matrices for in vitro tumor model, Biomaterials, 32 (2011) 2149–2159. [DOI] [PubMed] [Google Scholar]
- [96].Velasco V, Shariati SA, Esfandyarpour R, Microtechnology-based methods for organoid models, Microsyst Nanoeng, 6 (2020) 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Moroni L, Burdick JA, Highley C, Lee SJ, Morimoto Y, Takeuchi S, Yoo JJ, Biofabrication strategies for 3D in vitro models and regenerative medicine, Nat Rev Mater, 3 (2018) 21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Weeber F, van de Wetering M, Hoogstraat M, Dijkstra KK, Krijgsman O, Kuilman T, Gadellaa-van Hooijdonk CG, van der Velden DL, Peeper DS, Cuppen EP, Vries RG, Clevers H, Voest EE, Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases, Proc Natl Acad Sci USA, 112 (2015) 13308–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Yu F, Hunziker W, Choudhury D, Engineering microfluidic organoid-on-a-chip platforms, Micromachines, 10 (2019) 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bandaru P, Chu D, Sun W, Lasli S, Zhao C, Hou S, Zhang S, Ni J, Cefaloni G, Ahadian S, Dokmeci MR, Sengupta S, Lee J, Khademhosseini A, A microfabricated sandwiching assay for nanoliter and high-throughput biomarker screening, Small, 15 (2019) 1900300–1900309. [DOI] [PubMed] [Google Scholar]
- [101].Brandenberg N, Hoehnel S, Kuttler F, Homicsko K, Ceroni C, Ringel T, Gjorevski N, Schwank G, Coukos G, Turcatti G, Lutolf MP, High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays, Nat Biomed Eng, 4 (2020) 863–874. [DOI] [PubMed] [Google Scholar]
- [102].Yue XS, Nguyen TD, Zellmer V, Zhang SY, Zorlutuna P, Stromal cell-laden 3D hydrogel microwell arrays as tumor microenvironment model for studying stiffness dependent stromal cell-cancer interactions, Biomaterials, 170 (2018) 37–48. [DOI] [PubMed] [Google Scholar]
- [103].Jiang X, Ren L, Tebon P, Wang C, Zhou X, Qu M, Zhu J, Ling H, Zhang S, Xue Y, Wu Q, Bandaru P, Lee J, Kim HJ, Ahadian S, Ashammakhi N, Dokmeci MR, Wu J, Gu Z, Sun W, Khademhosseini A, Cancer-on-a-Chip for Modeling Immune Checkpoint Inhibitor and Tumor Interactions, Small, 17 (2021) 2004282–2004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Tavana H, Jovic A, Mosadegh B, Lee QY, Liu X, Luker KE, Luker GD, Weiss SJ, Takayama S, Nanolitre liquid patterning in aqueous environments for spatially defined reagent delivery to mammalian cells, Nat Mater, 8 (2009) 736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Du Y, Lo E, Ali S, Khademhosseini A, Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs, Proc Natl Acad Sci USA, 105 (2008) 9522–9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Tavana H, Mosadegh B, Takayama S, Polymeric aqueous biphasic systems for non-contact cell printing on cells: engineering heterocellular embryonic stem cell niches, Adv Mater, 22 (2010) 2628–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Reid JA, Mollica PA, Bruno RD, Sachs PC, Consistent and reproducible cultures of large-scale 3D mammary epithelial structures using an accessible bioprinting platform, Breast Cancer Res, 20 (2018) 122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mollica PA, Booth-Creech EN, Reid JA, Zamponi M, Sullivan SM, Palmer XL, Sachs PC, Bruno RD, 3D bioprinted mammary organoids and tumoroids in human mammary derived ECM hydrogels, Acta Biomater, 95 (2019) 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Skylar-Scott MA, Uzel SGM, Nam LL, Ahrens JH, Truby RL, Damaraju S, Lewis JA, Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels, Sci Adv, 5 (2019) 2459–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Yin X, Mead BE, Safaee H, Langer R, Karp JM, Levy O, Engineering stem cell organoids, Cell Stem Cell, 18 (2016) 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wu W, DeConinck A, Lewis JA, Omnidirectional printing of 3D microvascular networks, Advanced Healthcare Materials, 23 (2011) 178–183. [DOI] [PubMed] [Google Scholar]
- [112].Jia WT, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu WJ, Pi Q, Byambaa B, Dokmeci MR, Shin SR, Khademhosseini A, Direct 3D bioprinting of perfusable vascular constructs using a blend bioink, Biomaterials, 106 (2016) 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, Barabaschi G, Demarchi D, Dokmeci MR, Yang Y, Khademhosseini A, Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs, Lab Chip, 14 (2014) 2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK, Normalization of the vasculature for treatment of cancer and other diseases, Physiol Rev, 91 (2011) 1071–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Stylianopoulos T, Munn LL, Jain RK, Reengineering the tumor vasculature: improving drug delivery and efficacy, Trends Cancer, 4 (2018) 258–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Jain RK, Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy, Science, 307 (2005) 58–62. [DOI] [PubMed] [Google Scholar]
- [117].Bhatia SN, Ingber DE, Microfluidic organs-on-chips, Nat Biotechnol, 32 (2014) 760–772. [DOI] [PubMed] [Google Scholar]
- [118].Park SE, Georgescu A, Huh D, Organoids-on-a-chip, Science, 364 (2019) 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Beauchamp MJ, Nordin GP, Woolley AT, Moving from millifluidic to truly microfluidic sub-100-mum cross-section 3D printed devices, Anal Bioanal Chem, 409 (2017) 4311–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH, An in vivo model of functional and vascularized human brain organoids, Nature Biotechnology, 36 (2018) 432–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Berg CW, Ritsma L, Avramut MC, Wiersma LE, Berg BM, Leuning DG, Lievers E, Koning M, Vanslambrouck JM, Koster AJ, Howden SE, Takasato M, Little MH, Rabelink TJ, Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo, Stem Cell Reports, 10 (2018) 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA, 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs, Adv Mater, 26 (2014) 3124–3130. [DOI] [PubMed] [Google Scholar]
- [123].Bae H, Puranik AS, Gauvin R, Edalat F, Carrillo-Conde B, Peppas NA, Khademhosseini A, Building vascular networks, Sci Transl Med, 4 (2012) 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Shirure VS, Bi Y, Curtis MB, Lezia A, Goedegebuure MM, Goedegebuure SP, Aft R, Fields RC, George SC, Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids, Lab Chip, 18 (2018) 3687–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Meng F, Meyer CM, Joung D, Vallera DA, McAlpine MC, Panoskaltsis-Mortari A, 3D bioprinted in vitro metastatic models via reconstruction of tumor microenvironments, Adv Mater, 31 (2019) 1806899–1806908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Kim S, Lee H, Chung M, Jeon NL, Engineering of functional, perfusable 3D microvascular networks on a chip, Lab on a Chip, 13 (2013) 1489–1500. [DOI] [PubMed] [Google Scholar]
- [127].Jung DJ, Shin TH, Kim M, Sung CO, Jang SJ, Jeong GS, A one-stop microfluidic-based lung cancer organoid culture platform for testing drug sensitivity, Lab on a Chip, 19 (2019) 2854–2865. [DOI] [PubMed] [Google Scholar]
- [128].Khalil AS, Collins JJ, Synthetic biology: applications come of age, Nat Rev Genet, 11 (2010) 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Cheng AA, Lu TK, Synthetic biology: an emerging engineering discipline, Annu Rev Biomed Eng, 14 (2012) 155–178. [DOI] [PubMed] [Google Scholar]
- [130].Shamir ER, Pappalardo E, Jorgens DM, Coutinho K, Tsai WT, Aziz K, Auer M, Tran PT, Bader JS, Ewald AJ, Twist1-induced dissemination preserves epithelial identity and requires E-cadherin, J Cell Biol, 204 (2014) 839–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Koo BK, Stange DE, Sato T, Karthaus W, Farin HF, Huch M, van Es JH, Clevers H, Controlled gene expression in primary Lgr5 organoid cultures, Nat Methods, 9 (2011) 81–83. [DOI] [PubMed] [Google Scholar]
- [132].Morsut L, Roybal KT, Xiong X, Gordley RM, Coyle SM, Thomson M, Lim WA, Engineering customized cell sensing and response behaviors using synthetic notch receptors, Cell, 164 (2016) 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Toda S, Blauch LR, Tang SKY, Morsut L, Lim WA, Programming self-organizing multicellular structures with synthetic cell-cell signaling, Science, 361 (2018) 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Cheung KJ, Gabrielson E, Werb Z, Ewald AJ, Collective invasion in breast cancer requires a conserved basal epithelial program, Cell, 155 (2013) 1639–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., Kinzler KW, Cancer genome landscapes, Science, 339 (2013) 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL, Genetic alterations during colorectal-tumor development, N Engl J Med, 319 (1988) 525–532. [DOI] [PubMed] [Google Scholar]
- [137].Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, Watanabe T, Kanai T, Sato T, Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids, Nat Med, 21 (2015) 256–264. [DOI] [PubMed] [Google Scholar]
- [138].Takeda H, Kataoka S, Nakayama M, Ali MAE, Oshima H, Yamamoto D, Park JW, Takegami Y, An T, Jenkins NA, Copeland NG, Oshima M, CRISPR-Cas9-mediated gene knockout in intestinal tumor organoids provides functional validation for colorectal cancer driver genes, Proc Natl Acad Sci USA, 116 (2019) 15635–15644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EE, Beekman JM, Clevers H, Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients, Cell Stem Cell, 13 (2013) 653–658. [DOI] [PubMed] [Google Scholar]
- [140].Duarte AA, Gogola E, Sachs N, Barazas M, Annunziato S, R.d.R. J, Velds A, Blatter S, Houthuijzen JM, van de Ven M, Clevers H, Borst P, Jonkers J, Rottenberg S, BRCA-deficient mouse mammary tumor organoids to study cancer-drug resistance, Nat Methods, 15 (2018) 134–140. [DOI] [PubMed] [Google Scholar]
- [141].Weeber F, Ooft SN, Dijkstra KK, Voest EE, Tumor Organoids as a Pre-clinical Cancer Model for Drug Discovery, Cell Chem Biol, 24 (2017) 1092–1100. [DOI] [PubMed] [Google Scholar]
- [142].Xu H, Lyu X, Yi M, Zhao W, Song Y, Wu K, Organoid technology and applications in cancer research, J Hematol Oncol, 11 (2018) 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Yoshida GJ, Applications of patient-derived tumor xenograft models and tumor organoids, J Hematol Oncol, 13 (2020) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Xia X, Li F, He J, Aji R, Gao D, Organoid technology in cancer precision medicine, Cancer Lett, 457 (2019) 20–27. [DOI] [PubMed] [Google Scholar]
- [145].Praharaj PP, Bhutia SK, Nagrath S, Bitting RL, Deep G, Circulating tumor cell-derived organoids: Current challenges and promises in medical research and precision medicine, Biochim Biophys Acta Rev Cancer, 1869 (2018) 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Collins A, Miles GJ, Wood J, MacFarlane M, Pritchard C, Moss E, Patient-derived explants, xenografts and organoids: 3-dimensional patient-relevant pre-clinical models in endometrial cancer, Gynecol Oncol, 156 (2020) 251–259. [DOI] [PubMed] [Google Scholar]
- [147].Mandal K, Gong Z, Rylander A, Shenoy VB, Janmey PA, Opposite responses of normal hepatocytes and hepatocellular carcinoma cells to substrate viscoelasticity, Biomater Sci, 8 (2020) 1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Silva-Almeida C, Ewart MA, Wilde C, 3D gastrointestinal models and organoids to study metabolism in human colon cancer, Semin Cell Dev Biol, 98 (2020) 98–104. [DOI] [PubMed] [Google Scholar]
- [149].Astashkina A, Grainger DW, Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments, Adv Drug Deliv Rev, 69-70 (2014) 1–18. [DOI] [PubMed] [Google Scholar]
- [150].Li Y, Wang R, Huang D, Ma X, Mo S, Guo Q, Fu G, Li Y, Xu X, Hu X, Zhou Y, Deng Y, Zhang L, Chen H, Gao J, Zhang Z, Cai S, Hua G, Peng J, A novel human colon signet-ring cell carcinoma organoid line: establishment, characterization and application, Carcinogenesis, 41 (2020) 993–1004. [DOI] [PubMed] [Google Scholar]
- [151].Li Z, Qian Y, Li W, Liu L, Yu L, Liu X, Wu G, Wang Y, Luo W, Fang F, Liu Y, Song F, Cai Z, Chen W, Huang W, Human lung adenocarcinoma-derived organoid models for drug screening, iScience, 23 (2020) 101411–101441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L, Xia F, Fu G, Deng Y, Pan M, Guo Q, Gao X, Li Y, Rao X, Zhou Y, Liang L, Wang Y, Zhang J, Zhang H, Li G, Zhang L, Peng J, Cai S, Hu C, Gao J, Clevers H, Zhang Z, Hua G, Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer, Cell Stem Cell, 26 (2020) 17–26. [DOI] [PubMed] [Google Scholar]
- [153].Maenhoudt N, Defraye C, Boretto M, Jan Z, Heremans R, Boeckx B, Hermans F, Arijs I, Cox B, Van Nieuwenhuysen E, Vergote I, Van Rompuy AS, Lambrechts D, Timmerman D, Vankelecom H, Developing organoids from ovarian cancer as experimental and preclinical models, Stem Cell Reports, 14 (2020) 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Kawasaki K, Toshimitsu K, Matano M, Fujita M, Fujii M, Togasaki K, Ebisudani T, Shimokawa M, Takano A, Takahashi S, Ohta Y, Nanki K, Igarashi R, Ishimaru K, Ishida H, Sukawa Y, Sugimoto S, Saito Y, Maejima K, Sasagawa S, Lee H, Kim HG, Ha K, Hamamoto J, Fukunaga K, Maekawa A, Tanabe M, Ishihara S, Hamamoto Y, Yasuda H, Sekine S, Kudo A, Kitagawa Y, Kanai T, Nakagawa H, Sato T, An organoid biobank of neuroendocrine neoplasms enables genotype-phenotype mapping, Cell, 183 (2020) 1420–1435. [DOI] [PubMed] [Google Scholar]
- [155].Beshiri ML, Tice CM, Tran C, Nguyen HM, Sowalsky AG, Agarwal S, Jansson KH, Yang Q, McGowen KM, Yin J, Alilin AN, Karzai FH, Dahut WL, Corey E, Kelly K, A PDX/organoid biobank of advanced prostate cancers captures genomic and phenotypic heterogeneity for disease modeling and therapeutic screening, Clin Cancer Res, 24 (2018) 4332–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Artegiani B, Clevers H, Use and application of 3D-organoid technology, Hum Mol Genet, 27 (2018) R99–R107. [DOI] [PubMed] [Google Scholar]
- [157].Maru Y, Hippo Y, Current Status of Patient-Derived Ovarian Cancer Models, Cells, 8 (2019) 505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Devarasetty M, Mazzocchi AR, Skardal A, Applications of bioengineered 3D tissue and tumor organoids in drug development and precision medicine: Current and future, BioDrugs, 32 (2018) 53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Sachs N, Clevers H, Organoid cultures for the analysis of cancer phenotypes, Curr Opin Genet Dev, 24 (2014) 68–73. [DOI] [PubMed] [Google Scholar]
- [160].Liu C, Qin T, Huang Y, Li Y, Chen G, Sun C, Drug screening model meets cancer organoid technology, Transl Oncol, 13 (2020) 100840–100846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernandez-Mateos J, Khan K, Lampis A, Eason K, Huntingford I, Burke R, Rata M, Koh DM, Tunariu N, Collins D, Hulkki-Wilson S, Ragulan C, Spiteri I, Moorcraft SY, Chau I, Rao S, Watkins D, Fotiadis N, Bali M, Darvish-Damavandi M, Lote H, Eltahir Z, Smyth EC, Begum R, Clarke PA, Hahne JC, Dowsett M, de Bono J, Workman P, Sadanandam A, Fassan M, Sansom OJ, Eccles S, Starling N, Braconi C, Sottoriva A, Robinson SP, Cunningham D, Valeri N, Patient-derived organoids model treatment response of metastatic gastrointestinal cancers, Science, 359 (2018) 920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Steinhart Z, Pavlovic Z, Chandrashekhar M, Hart T, Wang X, Zhang X, Robitaille M, Brown KR, Jaksani S, Overmeer R, Boj SF, Adams J, Pan J, Clevers H, Sidhu S, Moffat J, Angers S, Genome-wide CRISPR screens reveal a Wnt-FZD5 signaling circuit as a druggable vulnerability of RNF43-mutant pancreatic tumors, Nat Med, 23 (2017) 60–68. [DOI] [PubMed] [Google Scholar]
- [163].Kopper O, de Witte CJ, Lohmussaar K, Valle-Inclan JE, Hami N, Kester L, Balgobind AV, Korving J, Proost N, Begthel H, van Wijk LM, Revilla SA, Theeuwsen R, van de Ven M, van Roosmalen MJ, Ponsioen B, Ho VWH, Neel BG, Bosse T, Gaarenstroom KN, Vrieling H, Vreeswijk MPG, van Diest PJ, Witteveen PO, Jonges T, Bos JL, van Oudenaarden A, Zweemer RP, Snippert HJG, Kloosterman WP, Clevers H, An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity, Nat Med, 25 (2019) 838–849. [DOI] [PubMed] [Google Scholar]
- [164].Du Y, Li X, Niu Q, Mo X, Qui M, Ma T, Kuo CJ, Fu H, Development of a miniaturized 3D organoid culture platform for ultra-high-throughput screening, J Mol Cell Biol, 12 (2020) 630–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Sun W, Luo Z, Lee J, Kim HJ, Lee K, Tebon P, Feng Y, Dokmeci MR, Sengupta S, Khademhosseini A, Organ-on-a-Chip for Cancer and Immune Organs Modeling, Adv Healthc Mater, 8 (2019) e1801363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Zhang YS, Aleman J, Shin SR, Kilic T, Kim D, Mousavi Shaegh SA, Massa S, Riahi R, Chae S, Hu N, Avci H, Zhang W, Silvestri A, Sanati Nezhad A, Manbohi A, De Ferrari F, Polini A, Calzone G, Shaikh N, Alerasool P, Budina E, Kang J, Bhise N, Ribas J, Pourmand A, Skardal A, Shupe T, Bishop CE, Dokmeci MR, Atala A, Khademhosseini A, Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors, Proc Natl Acad Sci U S A, 114 (2017) E2293–E2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Kondo J, Ekawa T, Endo H, Yamazaki K, Tanaka N, Kukita Y, Okuyama H, Okami J, Imamura F, Ohue M, Kato K, Nomura T, Kohara A, Mori S, Dan S, Inoue M, High‐throughput screening in colorectal cancer tissue‐originated spheroids, Cancer Sci, 110 (2019) 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Hou S, Tiriac H, Sridharan BP, Scampavia L, Madoux F, Seldin J, Souza GR, Watson D, Tuveson D, Spicer TP, Advanced Development of Primary Pancreatic Organoid Tumor Models for High-Throughput Phenotypic Drug Screening, SLAS Discov, 23 (2018) 574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Yoshii Y, Furukawa T, Waki A, Okuyama H, Inoue M, Itoh M, Zhang MR, Wakizaka H, Sogawa C, Kiyono Y, Yoshii H, Fujibayashi Y, Saga T, High-throughput screening with nanoimprinting 3D culture for efficient drug development by mimicking the tumor environment, Biomaterials, 51 (2015) 278–289. [DOI] [PubMed] [Google Scholar]
- [170].Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, Nostro C, Wang R, Muthuswamy LB, Crawford HC, Arrowsmith C, Kalloger SE, Renouf DJ, Connor AA, Cleary S, Schaeffer DF, Roehrl M, Tsao MS, Gallinger S, Keller G, Muthuswamy SK, Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids, Nat Med, 21 (2015) 1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Li L, Knutsdottir H, Hui K, Weiss MJ, He J, Philosophe B, Cameron AM, Wolfgang CL, Pawlik TM, Ghiaur G, Ewald AJ, Mezey E, Bader JS, Selaru FM, Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity, JCI Insight, 4 (2019) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Tashiro T, Okuyama H, Endo H, Kawada K, Ashida Y, Ohue M, Sakai Y, Inoue M, In vivo and ex vivo cetuximab sensitivity assay using three-dimensional primary culture system to stratify KRAS mutant colorectal cancer, Plos One, 12 (2017) e0174151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Jabs J, Zickgraf FM, Park J, Wagner S, Jiang X, Jechow K, Kleinheinz K, Toprak UH, Schneider MA, Meister M, Spaich S, Sutterlin M, Schlesner M, Trumpp A, Sprick M, Eils R, Conrad C, Screening drug effects in patient-derived cancer cells links organoid responses to genome alterations, Mol Syst Biol, 13 (2017) 955–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Schutte M, Risch T, Abdavi-Azar N, Boehnke K, Schumacher D, Keil M, Yildiriman R, Jandrasits C, Borodina T, Amstislavskiy V, Worth CL, Schweiger C, Liebs S, Lange M, Warnatz HJ, Butcher LM, Barrett JE, Sultan M, Wierling C, Golob-Schwarzl N, Lax S, Uranitsch S, Becker M, Welte Y, Regan JL, Silvestrov M, Kehler I, Fusi A, Kessler T, Herwig R, Landegren U, Wienke D, Nilsson M, Velasco JA, Garin-Chesa P, Reinhard C, Beck S, Schafer R, Regenbrecht CR, Henderson D, Lange B, Haybaeck J, Keilholz U, Hoffmann J, Lehrach H, Yaspo ML, Molecular dissection of colorectal cancer in pre-clinical models identifies biomarkers predicting sensitivity to EGFR inhibitors, Nat Commun, 8 (2017) 14262–14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Kvistborg P, Yewdell JW, Enhancing responses to cancer immunotherapy, Science, 359 (2018) 516–517. [DOI] [PubMed] [Google Scholar]
- [176].Riley RS, June CH, Langer R, Mitchell MJ, Delivery technologies for cancer immunotherapy, Nature reviews Drug discovery, 18 (2019) 175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Zhou XW, Qu MY, Tebon P, Jiang X, Wang CR, Xue YM, Zhu JX, Zhang SM, Oklu R, Sengupta S, Sun WJ, Khademhosseini A, Screening cancer immunotherapy: When engineering approaches meet artificial intelligence, Adv Sci, 7 (2020) 2001447–2001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Ooft SN, Weeber F, Dijkstra KK, McLean CM, Kaing S, van Werkhoven E, Schipper L, Hoes L, Vis DJ, van de Haar J, Prevoo W, Snaebjornsson P, van der Velden D, Klein M, Chalabi M, Boot H, van Leerdam M, Bloemendal HJ, Beerepoot LV, Wessels L, Cuppen E, Clevers H, Voest EE, Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients, Science Translational Medicine, 11 (2019) 2574–2582. [DOI] [PubMed] [Google Scholar]
- [179].Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, Liu IH, Chiou SH, Salahudeen AA, Smith AR, Deutsch BC, Liao L, Zemek AJ, Zhao F, Karlsson K, Schultz LM, Metzner TJ, Nadauld LD, Tseng YY, Alkhairy S, Oh C, Keskula P, Mendoza-Villanueva D, De La Vega FM, Kunz PL, Liao JC, Leppert JT, Sunwoo JB, Sabatti C, Boehm JS, Hahn WC, Zheng GXY, Davis MM, Kuo CJ, Organoid modeling of the tumor immune microenvironment, Cell, 175 (2018) 1972–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Liu Y, Chen YG, 2D- and 3D-based intestinal stem cell cultures for personalized medicine, Cells, 7 (2018) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Jin MZ, Han RR, Qiu GZ, Ju XC, Lou G, Jin WL, Organoids: An intermediate modeling platform in precision oncology, Cancer Lett, 414 (2018) 174–180. [DOI] [PubMed] [Google Scholar]
- [182].Merenda A, Fenderico N, Maurice MM, Wnt signaling in 3D: Recent advances in the applications of intestinal organoids, Trends Cell Biol, 30 (2020) 60–73. [DOI] [PubMed] [Google Scholar]
- [183].Tanay A, Regev A, Scaling single-cell genomics from phenomenology to mechanism, Nature, 541 (2017) 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Zhao Y, Li ZX, Zhu YJ, Fu J, Zhao XF, Zhang YN, Wang S, Wu JM, Wang KT, Wu R, Sui CJ, Shen SY, Wu X, Wang HY, Gao D, Chen L, Single-cell transcriptome analysis uncovers intratumoral heterogeneity and underlying mechanisms for drug resistance in hepatobiliary tumor organoids, Adv Sci, 1 (2021) 2003897–2003909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Chen KY, Srinivasan T, Lin C, Tung KL, Gao Z, Hsu DS, Lipkin SM, Shen X, Single-cell transcriptomics reveals heterogeneity and drug response of human colorectal cancer organoids, Annu Int Conf IEEE Eng Med Biol Soc, 2018 (2018) 2378–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Ma S, Zhao H, Galan EA, Integrating engineering, automation, and intelligence to catalyze the biomedical translation of organoids, Adv Biol, (2021) 2100535–2100541. [DOI] [PubMed] [Google Scholar]
- [187].Chen BC, Legant WR, Wang K, Shao L, Milkie DE, Davidson MW, Janetopoulos C, Wu XFS, Hammer JA, Liu Z, English BP, Mimori-Kiyosue Y, Romero DP, Ritter AT, Lippincott-Schwartz J, Fritz-Laylin L, Mullins RD, Mitchell DM, Bembenek JN, Reymann AC, Bohme R, Grill SW, Wang JT, Seydoux G, Tulu US, Kiehart DP, Betzig E, Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution, Science, 346 (2014) 1257998–1258025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Serra D, Mayr U, Boni A, Lukonin I, Rempfler M, Challet Meylan L, Stadler MB, Strnad P, Papasaikas P, Vischi D, Waldt A, Roma G, Liberali P, Self-organization and symmetry breaking in intestinal organoid development, Nature, 569 (2019) 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Adriani G, Pavesi A, Tan AT, Bertoletti A, Thiery JP, Kamm RD, Microfluidic models for adoptive cell-mediated cancer immunotherapies, Drug Discovery Today, 21 (2016) 1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Drost J, Boxtel R, Blokzijl F, Mizutani T, Sasaki N, Sasselli V, Ligt J, Behjati S, Grolleman JE, Wezel T, Nik-Zainal S, Kuiper RP, Cuppen E, Clevers H, Use of CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer, Science, 358 (2017) 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Jenkins RW, Aref AR, Lizotte PH, Ivanova E, Stinson S, Zhou CW, Bowden M, Deng J, Liu H, Miao D, Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids, Cancer Discovery, 8 (2018) 196–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Takahashi T, Organoids for drug discovery and personalized medicine, Annu Rev Pharmacol Toxicol, 59 (2019) 447–462. [DOI] [PubMed] [Google Scholar]
- [193].Liu L, Yu L, Li Z, Li W, Huang W, Patient-derived organoid (PDO) platforms to facilitate clinical decision making, J Transl Med, 19 (2021) 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Pasch CA, Favreau PF, Yueh AE, Babiarz CP, Gillette AA, Sharick JT, Karim MR, Nickel KP, DeZeeuw AK, Sprackling CM, Emmerich PB, DeStefanis RA, Pitera RT, Payne SN, Korkos DP, Clipson L, Walsh CM, Miller D, Carchman EH, Burkard ME, Lemmon KK, Matkowskyj KA, Newton MA, Ong IM, Bassetti MF, Kimple RJ, Skala MC, Deming DA, Patient-derived cancer organoid cultures to predict sensitivity to chemotherapy and radiation, Clin Cancer Res, 25 (2019) 5376–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Greaves M, Maley CC, Clonal evolution in cancer, Nature, 481 (2012) 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Wang J, Cazzato E, Ladewig E, Frattini V, Rosenbloom DIS, Zairis S, Abate F, Liu Z, Elliott O, Shin Y-J, Lee J-K, Lee I-H, Park W-Y, Eoli M, Blumberg AJ, Lasorella A, Nam D-H, Finocchiaro G, Iavarone A, Rabadan R, Clonal evolution of glioblastoma under therapy, Nature Genetics, 48 (2016) 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]