Abstract
Background
Atopic dermatitis (AD) is associated with considerable financial cost. However, the full burden of out-of-pocket (OOP) expenses is not well understood.
Objective
We sought to characterize the OOP health care expenses associated with AD management.
Methods
A 25-question voluntary online survey was administered to National Eczema Association members worldwide (n = 113,502). Inclusion criteria (US residents age ≥18 years who either self-reported had AD or were primary caregivers of individuals with AD) were met by 77.3% (1118/1447) of respondents.
Results
Respondents reported OOP expenses in 3 categories: (1) health care providers and prescriptions, including health care provider visit deductibles (68.7% [686]), prescription co-pays (64.3% [635]), and prescriptions not covered by insurance (48.6% [468]); (2) nonprescription health care products, including moisturizers (94.3% [934]), hygiene products (85.0% [824], allergy medications (75.1% [715]), itch relievers (68.25% [647]), dietary supplements (52.2% [491]), and sleep aids (37.0% [336]); and (3) complementary approaches, including cleaning products (74.7% [732]), clothing/bedding (44.8% [430]), alternative medications (19.0% [180]), and adjunctive therapies (15.9% [150]). The median annual AD OOP expense was US $600 (range, US $0–$200,000), with 41.9% (364) reporting expenditures US $1000 or greater.
Conclusions
Out-of-pocket expenses place a significant financial burden on individuals with AD. Additional studies are needed to better understand associations and impact of OOP costs.
Atopic dermatitis (AD) is a common chronic inflammatory skin disease that affects 13% of children and 7% of adults in the United States.1–3 Atopic dermatitis is associated with significant morbidity, including profound itch, skin pain, sleep disruption, and mental health disturbances.3–7 In addition, AD is associated with impaired health-related quality of life and a multidimensional burden consisting of physical, emotional, and psychosocial effects.7–13
Children and adults with AD have more outpatient office visits,9,14 emergency department visits,9,15 and hospital admissions with prolonged hospitalizations9,16,17 in the United States compared with those without AD. Increased health care resource utilization is associated with substantial direct costs and increasing costs over time even after adjusting for inflation.15,17 The multidimensional burden of AD is also associated with considerable indirect costs to society, including sick days and lost work productivity. The combined direct and indirect inflation-adjusted annual costs of AD were estimated to be US $5.3 billion in 2015; this amount may underestimate the actual disease-related financial burden.18
Out-of-pocket (OOP) expenses are particularly important in the day-to-day lives of AD patients and their families. Although US population-based surveys demonstrated multifactorial increases in overall OOP health expenses related to AD,12,19 OOP costs are not well characterized from an individual perspective. Few studies investigated the broader complement of health care expenses and OOP economic burden of AD on patients and their caregivers. Furthermore, management of AD and its comorbidities can be challenging. Currently available treatment options have variable long-term effectiveness and safety, which may warrant switching between therapies, use of combination therapy, and use of complementary or adjunctive health care approaches to achieve satisfactory disease control. We hypothesized that individuals with AD have a wide array of unappreciated OOP costs beyond major categories of health care expenses, such as health care provider (HCP) visits and prescription medications. In this study, we sought to characterize and quantify the OOP health care expenses and financial burden associated with AD management.
METHODS
Study Design
A 25-question voluntary online survey was administered between November 14, 2019, and December 21, 2019, to all National Eczema Association members, including 113,502 unique individuals with AD and nonaffected family members worldwide. To enhance reach, the survey was also advertised on a variety of social media platforms. Electronic informed consent was obtained prior to survey initiation, and respondents who reached the end of the survey were offered an optional opportunity to enter in a drawing to win 1 of 10 US $50 gift cards. Survey responses were not linked in any way to the gift cards. Study inclusion criteria included the following: US residents, adults (age ≥18 years) and either self-reportedly had AD or were primary caregivers of children with AD.
Survey Questions
Diagnosis of AD was determined by an affirmative answer to the question: “Have [you/the person with atopic dermatitis] been diagnosed with atopic dermatitis by a health care provider?” Demographics were collected, including age, sex, race/ethnicity, region of residence, geographical setting, household income, and insurance coverage. Current AD severity (clear, mild, moderate, and severe) and control (very well, moderately well, somewhat, minimally, and not controlled) were determined by patient global assessment, and the number of flare days in the last month (0/1–3/4–7/8–10/≥11) was also assessed. Comorbid diseases were assessed by asking about HCP-diagnosed asthma, allergic rhinitis, food allergy, frequent/persistent skin infections, and anxiety and/or depression.
Respondents were asked about specific topical/external (antimicrobials, corticosteroids, crisaborole, pimecrolimus, tacrolimus, phototherapy) and systemic (azathioprine, cyclosporine, methotrexate, tacrolimus, mycophenolate mofetil, dupilumab, oral corticosteroids, injectable corticosteroids) prescription therapies, total number of prescriptions in the past year (0/1/2/3/4/5/≥6), and number of HCP visits for AD in the past year (0/1/2/3/4/5/≥6). The OOP expenses related to evaluation or treatment of AD in the past 30 days (US $0/US $1–50/US $51–100/US $101–150/US $151–200/US $201–250/US $251–275/US $275–300/>US $300) were assessed for (1) current medical and therapeutic approaches (eg, HCP, individual prescription drugs, and care coordination expenses), (2) nonprescription over-the-counter (OTC) medications and other health-related personal products (eg, bandages and bathing/hygiene products), and (3) adjunctive approaches (eg, traditional Chinese medicine or similar, specialized clothing, bedding, or cleaning products). Respondents were also asked to compare OOP expenses in the past 30 days to average monthly OOP expenses (significantly more, somewhat more, same, somewhat less, significantly less) and estimate yearly OOP expenses for AD.
Data Analysis
Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC). χ2 Tests were used for comparisons of categorical variables, including sociodemographic and AD severity and control measures. Corrected P values of 0.05 or less were considered significant.
RESULTS
Respondent Characteristics
The survey was started by 1447 persons, of which 954 (65.9%) fully completed; 1118 (77.3%) met the inclusion criteria. The cohort included adults with AD (% prevalence [frequency]: 77.5% [866]) and parents and/or primary caregivers of children/teens (<18 years: 20.0% [224]) or young adults (18–25 years: 2.5% [28]) with AD (Table 1). The majority of respondents identified as female (76.5% [855]), White (72.38% [697]), and non-Hispanic (90.5% [871]). More than half of respondents had employer-sponsored insurance coverage (57.7% [550]) and a household income of US $50,000 or greater (61.7% [589]), with a median income of US $50,000 to US $74,999. Respondents were fairly evenly distributed across the United States, and most lived in a suburban location (56.6% [544]).
TABLE 1.
Respondent Characteristics
| Overall | No. Treatments | Step-up Therapy | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable—Freq (%) | 0 | 1–2 | ≥3 | P | No | Yes | P | |
| Age, y | ||||||||
| ≤2 | 42 (3.8%) | 5 (4.5%) | 17 (4.9%) | 18 (2.9%) | 0.20 | 31 (4.9%) | 9 (2.0%) | 0.16 |
| 3–5 | 69 (6.2%) | 8 (7.1%) | 21 (6.1%) | 39 (6.3%) | 49 (7.7%) | 18 (4.1%) | ||
| 6–11 | 68 (6.1%) | 6 (5.4%) | 18 (5.2%) | 41 (6.7%) | 41 (6.5%) | 23 (5.2%) | ||
| 12–17 | 49 (4.4%) | 4 (3.6%) | 14 (4.1%) | 28 (4.5%) | 21 (3.3%) | 25 (5.7%) | ||
| 18–25 | 139 (12.4%) | 15 (13.4%) | 31 (9.0%) | 83 (13.5%) | 70 (11.0%) | 60 (13.6%) | ||
| 26–35 | 130 (11.6%) | 16 (14.3%) | 43 (12.5%) | 67 (10.9%) | 76 (12.0%) | 51 (11.5%) | ||
| 36–50 | 173 (15.5%) | 24 (21.4%) | 51 (14.8%) | 96 (15.6%) | 103 (16.2%) | 70 (15.8%) | ||
| 51–64 | 247 (22.1%) | 18 (16.1%) | 74 (21.5%) | 148 (24.0%) | 135 (21.3%) | 103 (23.3%) | ||
| ≥65 | 201 (18.0%) | 16 (14.3%) | 75 (21.8%) | 97 (15.7%) | 109 (17.2%) | 83 (18.8%) | ||
| Sex | ||||||||
| Female | 855 (76.5%) | 97 (86.6%) | 261 (75.9%) | 473 (76.7%) | 0.10 | 496 (78.1%) | 335 (75.8%) | 0.76 |
| Male | 251 (22.5%) | 13 (11.6%) | 78 (22.7%) | 139 (22.5%) | 129 (20.3%) | 105 (23.8%) | ||
| Nonbinary/other | 4 (0.4%) | 0 (0.0%) | 2 (0.6%) | 2 (0.3%) | 3 (0.5%) | 1 (0.2%) | ||
| Prefer not to answer | 8 (0.7%) | 2 (1.8%) | 3 (0.9%) | 3 (0.5%) | 7 (1.1%) | 1 (0.2%) | ||
| Race | ||||||||
| White | 697 (72.4%) | 65 (69.9%) | 231 (74.3%) | 401 (71.7%) | <0.0001 | 412 (73.8%) | 281 (70.6%) | 0.50 |
| Black/African American | 102 (10.6%) | 10 (10.8%) | 29 (9.3%) | 63 (5.7%) | 53 (9.5%) | 48 (12.1%) | ||
| Asian | 58 (6.0%) | 1 (1.1%) | 25 (8.0%) | 32 (5.7%) | 37 (6.6%) | 21 (5.3%) | ||
| Native Hawaiian/Pacific Islander | 7 (0.7%) | 2 (2.2%) | 1 (0.3%) | 4 (0.7%) | 2 (0.4%) | 5 (1.3%) | ||
| American Indian or Alaskan Native | 8 (0.8%) | 2 (2.2%) | 0 (0.0%) | 6 (1.1%) | 5 (0.9%) | 2 (0.5%) | ||
| Multiracial | 63 (6.5%) | 9 (9.7%) | 20 (6.4%) | 34 (6.1%) | 36 (6.5%) | 26 (6.5%) | ||
| Other | 28 (2.9%) | 4 (4.3%) | 5 (1.6%) | 19 (3.4%) | 13 (2.3%) | 15 (3.8%) | ||
| Hispanic ethnicity | ||||||||
| No | 871 (90.5%) | 82 (88.2%) | 283 (91.0%) | 506 (90.5%) | 0.65 | 510 (91.4%) | 354 (88.9%) | 0.53 |
| Yes | 92 (9.6%) | 11 (11.8%) | 28 (9.0%) | 53 (9.5%) | 48 (8.6%) | 44 (11.1%) | ||
| Household income, US $ | ||||||||
| ≤24,999 | 175 (18.3%) | 25 (27.5%) | 63 (20.5%) | 87 (15.7%) | 0.04 | 101 (18.3%) | 73 (18.5%) | 0.80 |
| 25,000–49,999 | 190 (19.9%) | 23 (25.3%) | 57 (18.5%) | 110 (19.8%) | 115 (20.8%) | 74 (18.7%) | ||
| 50,000–74,999 | 192 (20.1%) | 20 (22.0%) | 61 (19.8%) | 111 (20.0%) | 117 (21.2%) | 73 (18.5%) | ||
| 75,000–99,999 | 122 (12.8%) | 10 (11.0%) | 45 (14.6%) | 67 (12.1%) | 69 (12.5%) | 53 (13.4%) | ||
| 100,000–124,999 | 103 (10.8%) | 4 (4.4%) | 30 (9.7%) | 69 (12.4%) | 52 (9.4%) | 50 (12.7%) | ||
| 125,000–149,999 | 61 (6.4%) | 1 (1.1%) | 23 (7.5%) | 37 (6.7%) | 36 (6.5%) | 25 (6.3%) | ||
| ≥150,000 | 111 (11.6%) | 8 (8.8%) | 29 (9.4%) | 74 (13.3%) | 63 (11.4%) | 47 (11.9%) | ||
| Insurance | ||||||||
| None | 41 (4.3%) | 8 (8.8%) | 15 (4.9%) | 18 (3.2%) | 0.08 | 27 (4.9%) | 14 (3.5%) | 0.56 |
| Employer-sponsored coverage | 550 (57.7%) | 46 (50.6%) | 168 (54.6%) | 336 (60.5%) | 310 (56.1%) | 235 (59.5%) | ||
| Medicaid or state assistance | 93 (9.8%) | 10 (11.0%) | 32 (10.4%) | 51 (9.2%) | 53 (9.6%) | 40 (10.1%) | ||
| Medicare | 160 (16.8%) | 11 (12.1%) | 58 (18.8%) | 91 (16.4%) | 93 (16.8%) | 66 (16.7%) | ||
| Policy purchased on state/federal health exchange | 37 (3.9%) | 4 (4.4%) | 16 (5.2%) | 17 (3.1%) | 22 (4.0%) | 15 (3.8%) | ||
| Policy purchased on the commercial market | 29 (3.0%) | 3 (3.3%) | 7 (2.3%) | 19 (3.4%) | 21 (3.8%) | 8 (2.0%) | ||
| Tricare or VA benefit | 22 (2.3%) | 3 (3.3%) | 7 (2.3%) | 12 (2.2%) | 10 (1.8%) | 12 (3.0%) | ||
| Unsure | 22 (2.3%) | 6 (6.6%) | 5 (1.6%) | 11 (2.0%) | 17 (3.1%) | 5 (1.3%) | ||
| Geographical setting | ||||||||
| Urban | 229 (23.8%) | 20 (21.5%) | 69 (22.3%) | 140 (25.1%) | 0.08 | 107 (19.2%) | 80 (20.2%) | 0.60 |
| Suburban | 544 (56.6%) | 47 (50.5%) | 191 (61.6%) | 306 (54.8%) | 310 (55.6%) | 230 (58.1%) | ||
| Rural | 188 (19.6%) | 26 (28.0%) | 50 (16.1%) | 112 (20.1%) | 141 (25.3%) | 86 (21.7%) | ||
| Region | ||||||||
| New England | 65 (6.8%) | 2 (2.2%) | 28 (9.0%) | 35 (6.3%) | 0.18 | 35 (6.3%) | 30 (7.5%) | 0.87 |
| Mid-Atlantic | 128 (13.3%) | 17 (18.3%) | 36 (11.6%) | 75 (13.4%) | 82 (14.7%) | 45 (11.3%) | ||
| East North Central | 145 (15.1%) | 14 (15.1%) | 41 (13.2%) | 90 (16.1%) | 87 (15.6%) | 57 (14.3%) | ||
| West North Central | 53 (5.5%) | 9 (9.7%) | 17 (5.5%) | 27 (4.8%) | 31 (5.6%) | 22 (5.5%) | ||
| South Atlantic | 185 (19.2%) | 17 (18.3%) | 55 (17.7%) | 113 (20.2%) | 103 (18.5%) | 81 (20.4%) | ||
| East South Central | 61 (6.3%) | 4 (4.3%) | 20 (6.4%) | 37 (6.6%) | 33 (5.9%) | 28 (7.0%) | ||
| West South Central | 90 (9.4%) | 5 (5.4%) | 36 (11.6%) | 49 (8.8%) | 51 (9.1%) | 37 (9.3%) | ||
| Mountain | 74 (7.7%) | 11 (11.8%) | 22 (7.1%) | 41 (7.3%) | 44 (7.9%) | 29 (7.3%) | ||
| Pacific | 162 (16.8%) | 14 (15.1%) | 56 (18.0%) | 92 (16.5%) | 92 (16.5%) | 69 (17.3%) | ||
Respondent Disease Burden and Comorbidities
Nearly three-quarters of respondents classified AD severity as either moderate (% prevalence [frequency]: 47.5% [531]) or severe (26.5% [296]) (Table 2). Most reported only minimally (23.2% [259]) or somewhat (40.1% [448]) controlled AD, and approximately half had 8 disease flare days or more (53.0% [588]) in the past month and 3 HCP visits or more (48.8% [523]) for evaluation or management of AD in the past year. Comorbidities among respondents included asthma (34.5% [382]), allergic rhinitis (50.4% [557]), food allergy (38.5% [426]), frequent/persistent skin infections (19.0% [210]), and anxiety and/or depression (36.5% [404]).
TABLE 2.
Respondent Disease Burden
| Overall | No. Treatments | Step-up Therapy | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable—Freq (%) | 0 | 1–2 | ≥3 | P | No | Yes | P | |
| Current atopic dermatitis severity | ||||||||
| Clear | 29 (2.6%) | 5 (4.5%) | 10 (2.9%) | 11 (1.8%) | <0.0001 | 18 (2.8%) | 10 (2.3%) | <0.0001 |
| Mild | 238 (21.3%) | 39 (34.8%) | 103 (29.9%) | 89 (14.4%) | 159 (25.0%) | 69 (15.6%) | ||
| Moderate | 531 (47.5%) | 53 (47.3%) | 150 (43.6%) | 305 (49.4%) | 318 (50.1%) | 192 (43.4%) | ||
| Severe | 296 (26.5%) | 12 (10.7%) | 73 (21.2%) | 200 (32.4%) | 126 (19.8%) | 163 (36.9%) | ||
| Current atopic dermatitis control | ||||||||
| Minimally controlled | 259 (23.2%) | 21 (18.8%) | 81 (23.6%) | 146 (23.7%) | 0.03 | 157 (24.7%) | 93 (21.0%) | 0.21 |
| Somewhat controlled | 448 (40.1%) | 41 (36.6%) | 126 (36.6%) | 264 (42.8%) | 256 (40.3%) | 179 (40.5%) | ||
| Moderately well controlled | 300 (26.8%) | 29 (25.9%) | 99 (28.8%) | 161 (26.1%) | 166 (26.1%) | 119 (26.9%) | ||
| Very well controlled | 102 (9.1%) | 19 (17.0%) | 34 (9.9%) | 43 (7.0%) | 49 (7.7%) | 49 (11.1%) | ||
| No. flare days in past 30 d | ||||||||
| 0 | 44 (4.0%) | 7 (6.3%) | 17 (4.9%) | 17 (2.8%) | 0.05 | 22 (3.5%) | 20 (4.5%) | 0.62 |
| 1–3 | 271 (24.4%) | 33 (29.5%) | 93 (27.0%) | 133 (21.6%) | 155 (24.4%) | 105 (23.8%) | ||
| 4–7 | 206 (18.6%) | 19 (17.0%) | 69 (20.1%) | 114 (18.5%) | 126 (19.8%) | 74 (16.8%) | ||
| 8–10 | 151 (13.6%) | 11 (9.8%) | 47 (13.7%) | 91 (14.8%) | 84 (13.2%) | 65 (14.7%) | ||
| ≥11 | 437 (39.4%) | 42 (37.5%) | 118 (34.3%) | 261 (42.4%) | 248 (39.1%) | 177 (40.1%) | ||
| Comorbidities | ||||||||
| Asthma | 382 (34.5%) | 32 (28.8%) | 105 (30.6%) | 234 (38.1%) | 0.02 | 203 (32.0%) | 171 (38.7%) | 0.03 |
| Allergic rhinitis | 557 (50.4%) | 48 (43.2%) | 158 (46.1%) | 335 (54.5%) | 0.03 | 305 (48.0%) | 242 (54.8%) | 0.04 |
| Food allergy | 426 (38.5%) | 30 (27.0%) | 104 (30.3%) | 278 (45.2%) | <0.0001 | 223 (35.1%) | 192 (43.4%) | 0.01 |
| Frequent/persistent skin infections | 210 (19.0%) | 10 (9.0%) | 31 (9.0%) | 164 (25.7%) | <0.0001 | 79 (12.4%) | 125 (28.3%) | <0.0001 |
| Anxiety and/or depression | 404 (36.5%) | 33 (29.7%) | 122 (35.6%) | 240 (39.0%) | 0.14 | 211 (33.2%) | 185 (41.9%) | 0.009 |
| HCP visits in past year | ||||||||
| 0 | 113 (10.6%) | 56 (50.0%) | 40 (11.7%) | 17 (2.8%) | <0.0001 | 100 (15.9%) | 12 (2.8%) | <0.0001 |
| 1–2 | 435 (40.6%) | 44 (39.3%) | 219 (63.9%) | 172 (27.9%) | 295 (46.9%) | 135 (31.2%) | ||
| 3–4 | 284 (26.5%) | 9 (8.0%) | 61 (17.8%) | 214 (34.7%) | 141 (22.4%) | 142 (32.8%) | ||
| ≥5 | 239 (22.4%) | 3 (2.7%) | 23 (6.7%) | 213 (34.6%) | 93 (14.8%) | 144 (33.3%) | ||
OOP Expenses
Current Medical and Therapeutic Approaches
When asked about OOP expenses for HCPs and prescriptions in the past 30 days (incurred on top of insurance premiums), 68.7% (frequency: 686) of the respondents reported OOP expenses for co-pays and/or deductibles for doctor or other HCP office visits (excluding mental health providers), with 31.2% (311) spending more than US $100 (Fig. 1A). The majority of respondents also reported OOP expenses for prescription medication co-pays covered by insurance (64.3% [635]), with 33.9% (335) spending greater than US $50 and nearly half (48.6% [468]) spending money on prescription medications not covered by insurance. Most respondents did not report OOP expense for emergency room/urgent care visits (86.7% [804]), hospitalization (97.5% [896]), outpatient phototherapy (95.4% [875]), outpatient laboratory testing (76.8% [716]), and mental health services or other behavioral counseling (85.6% [790]).
Figure 1.
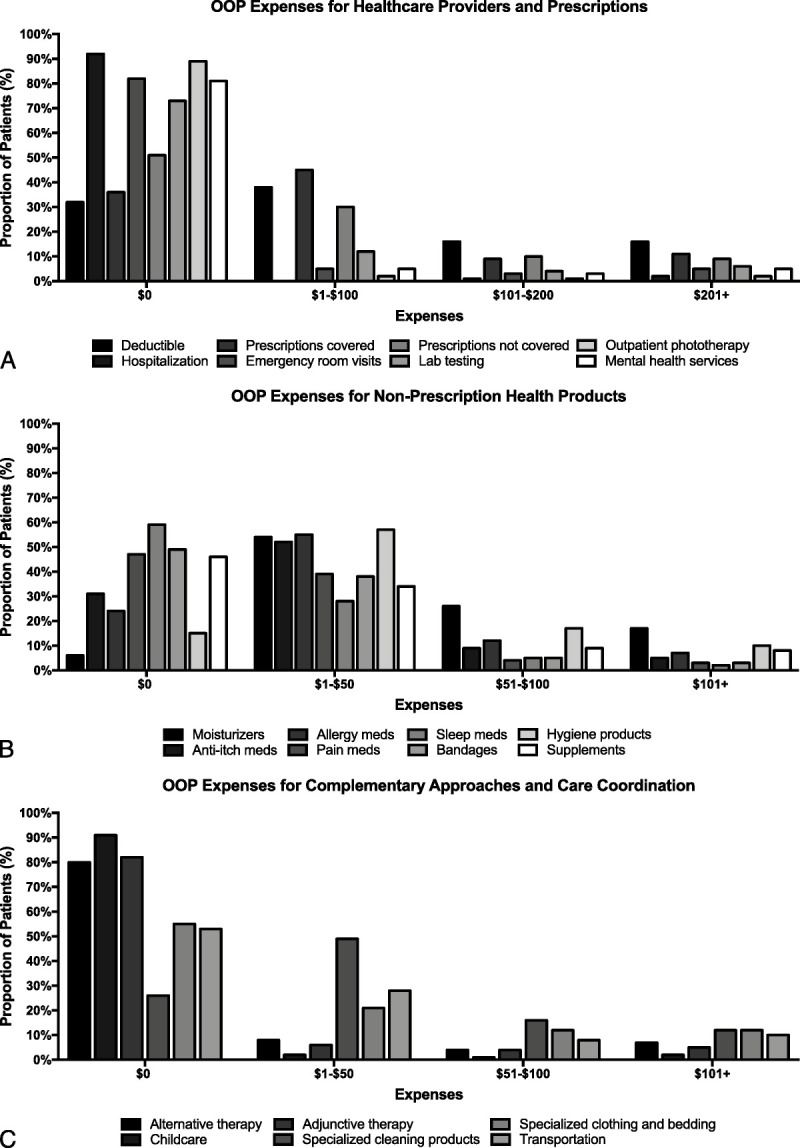
Breakdown of out-of-pocket (OOP) expenses. A, Out-of-pocket expenses for HCPs and prescriptions. B, Out-of-pocket expenses for nonprescription health products. C, Out-of-pocket expenses for complementary approaches and care coordination.
Nonprescription Health-Related Products
Nearly all respondents reported OOP expenses for nonprescription OTC moisturizers/emollients (94.3% [934]) in the past 30 days, with most spending up to US $50 (52.8% [523]) (Fig. 1B). Similarly, the majority of respondents spent up to US $50 on OTC hydrocortisone and other agents for itch relief (53.5% [507]), allergy medications (eg, antihistamines) (56.0% [533]), and hygiene/bathing products (eg, specific hair care products, soaps, and bath additives, such as bleach) (57.2% [555]). Similar numbers of respondents also reported OOP expenditures on pain relief (49.3% [449]), bandages or other dressings (48.4% [446]), dietary supplements (52.2% [491]), and sleep aids (excluding antihistamines) (37.0% [336]).
Complementary Approaches and Care Coordination
Approximately 1 in 5 respondents (19.0% [180]) reported expenditures for alternative OTC medicines (eg, traditional Chinese medicine, ayurvedic medicine, naturopathic medicine, and/or homeopathic medicine), and 15.9% (150) reported expenditures for adjunctive therapies (eg, acupuncture, yoga, or other relaxation approaches (Fig. 1C). Many respondents reported spending up to US $100 on specialized cleaning products (ie, laundry, household cleaners) (63.3% [620]) and specialized clothing (eg, pajamas, bedding) (32.6% [313]). Although nearly half of all respondents spent money on transportation/parking to obtain medical care or prescription medicine (46.8% [444]), most had no expenditures for childcare services while obtaining medical care (94.8% [872]).
Total Costs
A similar number of respondents reported that their OOP expenses over the past 30 days were either the same (42.5% [410]) or higher (40.7% [393]) than their average monthly OOP expenses (Fig. 2A). The median annual estimated OOP expense due to AD was US $600 (range, US $0–$200,000). Forty-two percent (364) of the respondents reported expenditures in excess of US $1000, and 8.5% (74) reported expenditures in excess of US $5000 (Fig. 2B).
Figure 2.
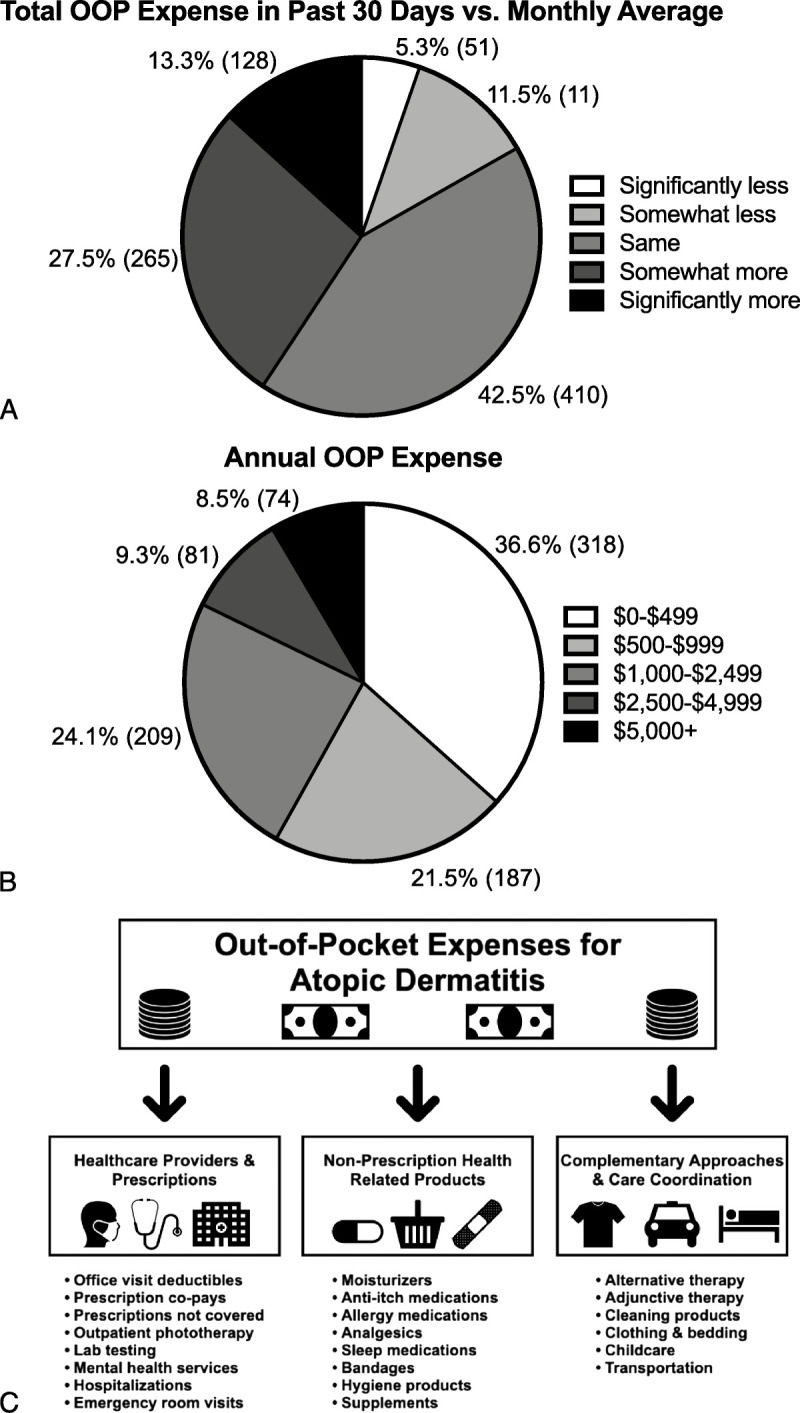
Total OOP expenses. A, Total OOP expense in past 30 days versus monthly average. B, Annual OOP expense. C, Summary of OOP expenses for AD. AD, atopic dermatitis; OOP, out-of-pocket.
Polypharmacy and Step-up Therapy
Most respondents reported currently using 3 or more prescription therapies (% prevalence [frequency], 57.5% [617]), with 32.1% (344) using 1 to 2 therapies and 10.4% (112) reporting no prescriptions. Those with more prescription therapies were more likely to be White or Asian and have increasing household income, increased disease severity, reduced control, increased number of flare days per month, allergic and infectious comorbidities, and increased number of HCP visits (P ≤ 0.05 for all) (Tables 1 and 2). Step-up therapy (ie, systemic therapy including injectable, oral, or phototherapy) was used in 41.0% (442) of the respondents. Those requiring step-up therapy were more likely to have increased disease severity; allergic, infectious, and mental health comorbidities; and increased number of HCP visits (P < 0.04 for all).
DISCUSSION
Using a survey-based approach to highlight the perspective of AD patients and caregivers in the United States, we found a wide array of previously unreported OOP expenses in medical, nonmedical, and supportive health care categories (Fig. 2C). Most respondents reported OOP expenses for co-pays and/or deductibles for office visits and prescription medication, moisturizers, OTC itch relievers, allergy medications, hygiene products, dietary supplements, and specialized cleaning supplies; nearly half reported OOP costs for prescription medications not covered by insurance, analgesics, dressings, and transportation/parking; many others reported OOP expenditures for sleep aids, alternative or adjunctive therapies, and specialized clothing. Most individuals had equivalent or higher OOP expenses in the past month when compared with average monthly OOP expenses for AD care, and many reported yearly OOP costs greater than US $1000. A majority of individuals had recently used or were concurrently using at least 3 prescription therapies, with almost half requiring step-up therapy. Together, these results show a significant OOP financial burden for AD, reflecting the real-life efforts of patients to better manage their disease.
The majority patients and caregivers reported an OOP expense for an HCP visit for AD in the past 30 days, and nearly all (89.4%) had at least 1 office visit in the last year. In contrast, OOP expenditures for emergency department visits and inpatient hospitalizations were relatively lower among respondents. This is in line with health care utilization findings from a recent US population-based study that showed that the median number of AD-related HCP visits for individuals with AD was approximately 1 per year, and the proportions of AD patients reporting at least 1 urgent care, emergency department visit, and inpatient hospitalization were 8.2%, 9.6%, and 6.7%, respectively.9 The OOP costs for mental health services were quite low among respondents, which was surprising given the strong association between AD and mental health disorders, including depression, anxiety, and psychological distress.7,20,21 The mental health burden of AD is underappreciated, and a substantial proportion of individuals with AD with mental health symptoms go undiagnosed.20 Thus, only a subset of AD patients with mental health comorbidities may seek mental health care. In addition, AD patients with mental health comorbidities may only seek out psychological or psychiatric care if it is well covered by their insurance. Thus, it is important that HCPs, who manage AD regularly, screen for mental health symptoms. Other contributing factors to assess in future studies might include stigma for seeking psychiatric care, impaired access because of lack of referral from the primary provider, and cost considerations (eg, unwilling to pay, too expensive).
The most common OOP expenses for OTC health products, and for any therapeutic approach in general, were emollients/moisturizers and hygiene/bathing products. This reflects the results of a similar survey-based study in France, which showed that these were the 2 most commonly used products associated with OOP cost among AD patients (74.4% and 65.2%, respectively).22 Neither of these categories is routinely reimbursed by health insurance, because they are seen as patient comfort rather than direct medical treatment. However, optimization of moisturization and bathing are universally seen as first-line nonpharmacologic approaches for managing AD.23 Emollients in particular are critical for increasing skin hydration and addressing the dysfunctional epidermal barrier underlying AD, thereby reducing pruritus, xerosis, erythema, lichenification, and fissuring. However, commonly used moisturizers vary significantly in cost, as well as potential allergenicity and irritancy, without a clear correlation to efficacy.24 More than 60% of the respondents also reported OOP expenditures for specialized laundry/household cleaning products and specialized clothing. Although some patients report better AD control when avoiding certain chemical and mechanical irritants, well-controlled studies to broadly support these types of costly environmental modification are lacking.25 Ultimately, given that personal preferences dictate the use of many of these products, HCPs should prioritize patient education, willingness for use, and adherence in the context of affordability to help control OOP cost.
These data highlight significant OOP expenses for health-related categories that are not routinely recommended or discussed during standard AD care encounters. The majority of respondents reported expenditures for OTC dietary supplements and a sizeable proportion of money spent on alternative, nonwestern medical care or adjunctive approaches. Although there is some evidence to suggest a positive role for certain types of complementary and alternative medicine (CAM) in improving the symptoms of AD, current data are largely insufficient to make widely applicable evidence-based recommendations.26,27 Previous studies showed that CAM use in AD patients is not uncommon and is associated with a longer disease duration, increased severity, and history of multiple previous conventional treatments.28 Our data highlight the real-life considerations of patients who struggle with controlling the symptoms of AD and use treatment approaches that may not be currently proven and/or recommended. Health care providers should make a renewed effort to understand the patient perspective (eg, motivations, expectations, benefits), review the most up-to-date evidence regarding CAM use, and assist in crafting a fiscally responsible care plan.
Reflecting the marked impairment in health-related quality of life, AD patients assume high OOP costs for disease management.29 However, studies examining the total burden of OOP expenses for AD patients in the United States are scarce. A survey-based study of a large managed care organization in 1997 estimated the total OOP cost per patient year to be US $314 (US $500 in 2019), with medications and household products being the top categories of expenditure.30 The total OOP cost for US AD patients was estimated to be US $371–$489 per person year in 2010 and 2012 (US $435–$573 in 2019) based on the results of the National Health Interview Survey, which did not include specific breakdown of OOP cost categories.12 Although not directly comparable because of differences in insurance structure and health care costs, recent surveys of large European countries showed similar OOP expenses when converted to US dollars, with moisturizers and hygiene products occupying the top categories of expenditure.22,31 Our data revealed a median annual cost of US $600, with the most common areas of expenses for moisturizers, hygiene and household products, and deductibles for outpatient care and medications. This figure is likely more accurate than previous estimates given that our study was the first to directly survey AD patients and caregivers across the United States.
Atopic dermatitis is a highly heterogenous disorder with variable severity, lesional distribution, symptoms, and disease course. Furthermore, AD patients also experience a chronic disease course punctuated by intermittent flares, which necessitate different treatment approaches. These features likely contribute to polypharmacy, complicated treatment regimens, and alternative treatments among patients with AD.32 Our data demonstrate considerable prescription polypharmacy among AD patients, especially for those with increased severity, poorer control, increased HCP visits, and private or Medicare insurance. Many respondents reported OOP expenses for prescription medications regardless of insurance coverage. Atopic dermatitis patients are likely to benefit from simplification of the treatment regimen by deprescribing ineffective or redundant treatments33 and transitioning to streamlined step-up therapy as warranted. Indeed, our data show much room for improvement in the use of step-up therapy among those with the highest disease burden (ie, increased severity, flares, and HCP visits). Additionally, most individuals also reported expenditures for OTC allergy, anti-itch, and pain medications. Although treatments in these categories may have a legitimate role in managing symptoms and comorbidities associated with AD, the use of certain therapies (eg, oral antihistamines for chronic treatment of AD) may not be aligned with evidence-based guidelines and are likely to further increase polypharmacy and contribute to excessive OOP financial burden. Although combination treatment may be warranted in some cases, HCPs should be sensitive to treatment efficacy, patient safety, evidence-based recommendations, and OOP costs when considering the treatment plan.
Strengths of this study include a large cohort of AD patients and caregivers distributed across the United States assessing real-world, OOP expenses for necessary AD care. The inclusion of 22 unique categories of OOP expenses, including HCP office visits, prescription therapies, nonprescription products, complementary approaches, and care coordination, allowed for a detailed understanding and accurate estimate of the OOP financial burden. Limitations include the cross-sectional nature of this study and inability to assess longitudinal changes in costs, treatments, and health care utilization. Selection bias is possible, because this was an Internet-based survey administered to members of the National Eczema Association and not the US population at large, although the respondent demographics indicate a variety of individuals with different insurance status, household income, and disease severity spread fairly evenly throughout the country. Self-reporting of costs and utilization may not be as accurate as a claims-based approach; however, this is offset by the ability to gather data directly from patients and caregivers about disease severity and comprehensive OOP costs. Diagnosis of AD was confirmed by self-reporting, which was previously validated in other types of studies.34,35 Future studies are necessary to confirm these findings and better understand associations of OOP costs and their impact on family finances. In addition, more studies incorporating patient-reported outcomes in cost analysis are needed to better understand predictors of OOP expenses.
In conclusion, OOP expenses for individuals with AD are broad and occupy a number of unique health care categories. Health care providers and patients should be cognizant of these costs and engage in shared decision making to create a treatment plan that minimizes financial burden.
Footnotes
W.S.B. and R.C. contributed to this work equally.
W.S.B. had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. W.S.B., I.J.T., and J.I.S. participated in the study concept and design. W.S.B. and I.J.T. participated in the acquisition of data. W.S.B., R.C., I.J.T., and J.I.S. participated in the analysis and interpretation of data. W.S.B., R.C., I.J.T., and J.I.S. participated in the drafting of the article. W.S.B., R.C., I.J.T., and J.I.S. participated in the critical revision of the manuscript for important intellectual content. W.S.B., R.C., I.J.T., and J.I.S. participated in the statistical analysis.
Funding support was received from National Eczema Association.
R.C. reports personal fees from Abbvie and RegeneronSanofi. J.S. reports personal fees from Abbvie, Anaptysbio, Asana, EliLilly, Galderma, GlaxoSmithKline, Kiniksa, Leo, Menlo, Pfizer, Realm, RegeneronSanofi, and Roivant, and grants from GlaxoSmithKline, RegeneronSanofi, and Galderma. The other authors have no conflicts of interest to declare.
Contributor Information
Raj Chovatiya, Email: raj.chovatiya@gmail.com.
Isabelle J. Thibau, Email: isabelle@nationaleczema.org.
Jonathan I. Silverberg, Email: jonathanisilverberg@gmail.com.
REFERENCES
- 1.Hua T, Silverberg JI. Atopic dermatitis in US adults: epidemiology, association with marital status, and atopy. Ann Allergy Asthma Immunol 2018;121(5):622–624. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis 2014;25(3):107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverberg JI Gelfand JM Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol 2018;121(3):340–347. [DOI] [PubMed] [Google Scholar]
- 4.Dawn A Papoiu AD Chan YH, et al. Itch characteristics in atopic dermatitis: results of a web-based questionnaire. Br J Dermatol 2009;160(3):642–644. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg JI Gelfand JM Margolis DJ, et al. Pain is a common and burdensome symptom of atopic dermatitis in United States adults. J Allergy Clin Immunol Pract 2019;7:2699–2706.e7. [DOI] [PubMed] [Google Scholar]
- 6.Li JC Fishbein A Singam V, et al. Sleep disturbance and sleep-related impairment in adults with atopic dermatitis: a cross-sectional study. Dermatitis 2018;29(5):270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng BT, Silverberg JI. Depression and psychological distress in US adults with atopic dermatitis. Ann Allergy Asthma Immunol 2019;123(2):179–185. [DOI] [PubMed] [Google Scholar]
- 8.Cheng BT, Silverberg JI. Association between atopic dermatitis and lower health utility scores in US adults. Ann Allergy Asthma Immunol 2020;124(1):88–89. [DOI] [PubMed] [Google Scholar]
- 9.Silverberg JI Gelfand JM Margolis DJ, et al. Atopic dermatitis in US adults: from population to health care utilization. J Allergy Clin Immunol Pract 2019;7(5):1524–1532.e2. [DOI] [PubMed] [Google Scholar]
- 10.Silverberg JI Margolis DJ Boguniewicz M, et al. Distribution of atopic dermatitis lesions in United States adults. J Eur Acad Dermatol Venereol 2019;33(7):1341–1348. [DOI] [PubMed] [Google Scholar]
- 11.Chiesa Fuxench ZC Block JK Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol 2019;139(3):583–590. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol 2015;151(7):743–752. [DOI] [PubMed] [Google Scholar]
- 13.McCleary KK. Understanding the lived experience of eczema: the “voice of the patient” report on the eczema patient-focused drug development meeting updated 3/20/2020. Available at: http://www.morethanskindeep-eczema.org/report.html. Accessed October 4, 2020.
- 14.Singh P, Silverberg JI. Outpatient utilization patterns for atopic dermatitis in the United States. J Am Acad Dermatol 2019. [DOI] [PubMed] [Google Scholar]
- 15.Kwa L, Silverberg JI. Financial burden of emergency department visits for atopic dermatitis in the United States. J Am Acad Dermatol 2018;79(3):443–447. [DOI] [PubMed] [Google Scholar]
- 16.Hua T, Silverberg JI. Atopic dermatitis is associated with increased hospitalization in US children. J Am Acad Dermatol 2019;81(3):862–865. [DOI] [PubMed] [Google Scholar]
- 17.Narla S Hsu DY Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis 2018;29(1):22–31. [DOI] [PubMed] [Google Scholar]
- 18.Drucker AM Wang AR Li W-Q, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol 2017;137(1):26–30. [DOI] [PubMed] [Google Scholar]
- 19.Whiteley J Emir B Seitzman R, et al. The burden of atopic dermatitis in US adults: results from the 2013 National Health and Wellness Survey. Curr Med Res Opin 2016;32(10):1645–1651. [DOI] [PubMed] [Google Scholar]
- 20.Silverberg JI Gelfand JM Margolis DJ, et al. Symptoms and diagnosis of anxiety and depression in atopic dermatitis in U.S. adults. Br J Dermatol 2019;119(548–552):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel KR Immaneni S Singam V, et al. Association between atopic dermatitis, depression, and suicidal ideation: a systematic review and meta-analysis. J Am Acad Dermatol 2019;80(2):402–410. [DOI] [PubMed] [Google Scholar]
- 22.Launois R Ezzedine K Cabout E, et al. Importance of out-of-pocket costs for adult patients with atopic dermatitis in France. J Eur Acad Dermatol Venereol 2019;33(10):1921–1927. [DOI] [PubMed] [Google Scholar]
- 23.Eichenfield LF Tom WL Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014;71(1):116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu S Kwa M Lohman ME, et al. Consumer preferences, product characteristics, and potentially allergenic ingredients in best-selling moisturizers. JAMA Dermatol 2017;153(11):1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidbury R Tom WL Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. Prevention of disease flares and use of adjunctive therapies and approaches. Consensus Development Conference presented at the Journal of the American Academy of Dermatology; December 2014. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0190962214018878. Accessed October 4, 2020. [DOI] [PMC free article] [PubMed]
- 26.Lu C-L Liu X-H Stub T, et al. Complementary and alternative medicine for treatment of atopic eczema in children under 14 years old: a systematic review and meta-analysis of randomized controlled trials. BMC Complement Altern Med 2018;18(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vieira BL Lim NR Lohman ME, et al. Complementary and alternative medicine for atopic dermatitis: an evidence-based review. Am J Clin Dermatol 2016;17(6):557–581. [DOI] [PubMed] [Google Scholar]
- 28.Holm JG Clausen M-L Agner T, et al. Use of complementary and alternative therapies in outpatients with atopic dermatitis from a dermatological university department. Dermatology 2019;235(3):189–195. [DOI] [PubMed] [Google Scholar]
- 29.Beikert FC Langenbruch AK Radtke MA, et al. Willingness to pay and quality of life in patients with atopic dermatitis. Arch Dermatol Res 2014;306(3):279–286. [DOI] [PubMed] [Google Scholar]
- 30.Fivenson D Arnold RJ Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm 2002;8(5):333–342. [DOI] [PubMed] [Google Scholar]
- 31.Zink AGS Arents B Fink-Wagner A, et al. Out-of-pocket costs for individuals with atopic eczema: a cross-sectional study in nine European countries. Acta Derm Venereol 2019;99(3):263–267. [DOI] [PubMed] [Google Scholar]
- 32.Singh P, Silverberg J. Real-world outpatient prescription patterns for atopic dermatitis in the United States. Dermatitis 2019;30(5):294–299. [DOI] [PubMed] [Google Scholar]
- 33.Scott IA Hilmer SN Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med 2015;175(5):827–834. [DOI] [PubMed] [Google Scholar]
- 34.Flohr C Weinmayr G Weiland SK, et al. How well do questionnaires perform compared with physical examination in detecting flexural eczema? Findings from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. Br J Dermatol 2009;161(4):846–853. [DOI] [PubMed] [Google Scholar]
- 35.Vissing NH, Jensen SM, Bisgaard H. Validity of information on atopic disease and other illness in young children reported by parents in a prospective birth cohort study. BMC Med Res Methodol 2012;12:160. [DOI] [PMC free article] [PubMed] [Google Scholar]


