Supplemental digital content is available in the text.
Abstract
Background
Itch, the most bothersome symptom in atopic dermatitis, is largely mediated by pruritogenic cytokines via Janus kinase 1 signaling in cutaneous sensory neurons.
Objectives
The aims of the study were to assess the magnitude and rapidity of itch relief with the Janus kinase 1 selective inhibitor abrocitinib and to evaluate the extent to which the effect of abrocitinib on itch relief is independent of overall disease improvement.
Methods
Pooled data from 1 phase 2b (NCT02780167) and 2 phase 3 (NCT03349060, NCT03575871) double-blind, randomized, placebo-controlled monotherapy trials in moderate to severe atopic dermatitis (N = 942) were analyzed.
Results
Abrocitinib produced significant and clinically meaningful itch relief versus placebo from week 2 through week 12 (end of treatment) that was associated with marked sleep and quality-of-life improvements. Mean percentage reductions in itch scores 24 hours after the first dose were greater for both abrocitinib doses (200 and 100 mg) versus placebo. Itch improvement occurred regardless of baseline itch severity, sex, race, body mass index, or Investigator Global Assessment response, suggesting that abrocitinib-associated itch relief is at least partially independent of overall disease improvement.
Conclusions
Abrocitinib showed a rapid and profound antipruritic effect, partially independent of improvement in overall disease.
Atopic dermatitis (AD) is a common and heterogeneous chronic inflammatory skin disease. Patients with AD are more likely to be dissatisfied with life and have a poorer health rating than patients without AD, with the degree of dissatisfaction being proportional to disease severity.1 Itch is the most bothersome symptom for patients with AD.1,2 Chronic itch (ie, itch lasting >6 weeks) is a central feature of AD and has a profoundly negative impact on quality of life (QoL).1,3–5 Daily itch is common, and more pronounced in the evenings.6 Although oral antihistamines are broadly used to treat itch associated with AD, there is minimal evidence to support their efficacy, and they are likely used for their ability to promote somnolence, thereby overcoming nocturnal itch.7,8 In a recent systematic review and network meta-analysis, cyclosporine and dupilumab were associated with moderate (standardized mean difference, −0.8 for both) improvements in itch compared with placebo, whereas azathioprine and methotrexate were associated with smaller (standardized mean difference, −0.2 to −0.3) improvements.9
The maintenance of chronic itch in mammals relies on neuronal Janus kinase 1 (JAK1) signaling. Janus kinase 1 simultaneously promotes the neuronal signaling of T helper type 2 (TH2) inflammatory cytokines (ie, interleukin [IL] 4 and IL-13) and the responsiveness of these same pruriceptive skin sensory neurons to a variety of other itch-inducing factors (eg, IL-31).10 The anti-itch effects of JAK1 inhibition are mediated predominantly through direct neuronal JAK1 inhibition rather than through the suppression of skin inflammation.10 Keratinocyte-derived thymic stromal lymphopoietin (TSLP), which is also dependent on JAK1 for intracellular signaling, has been shown to directly activate cutaneous sensory neurons to promote itch.11 Thus, selective JAK1 inhibition represents a strategy to simultaneously suppress multiple cytokine-mediated itch circuits in AD and alleviate itch indirectly (ie, by decreasing inflammation) and directly (ie, through inhibition of neuronal signals).
Abrocitinib is an oral once-daily JAK1 selective inhibitor under investigation for the treatment of moderate to severe AD. In addition to inhibiting signaling of IL-4 and IL-13, abrocitinib inhibits signaling of other cytokines (eg, IL-31, IL-22, TSLP) important in AD pathogenesis and itch via shared reliance on JAK1 for intracellular signaling.12,13 This analysis was conducted to assess the efficacy and speed of action of abrocitinib on itch relief in AD and the degree to which these anti-itch effects are mediated independently of overall disease improvement.
METHODS
Study Designs
Data were pooled for patients who received abrocitinib 200 mg, abrocitinib 100 mg, or placebo in 3 abrocitinib monotherapy trials for the treatment of moderate to severe AD, including a phase 2b trial (NCT02780167) and 2 phase 3 trials (JADE MONO-1, NCT03349060; JADE MONO-2, NCT03575871). Patients were randomly assigned 1:1:1:1:1 in the phase 2b study to receive abrocitinib (200, 100, 30, or 10 mg) or placebo and 2:2:1 in the phase 3 studies to receive abrocitinib (200 or 100 mg) or placebo. The primary end point in the phase 2b study was the proportion of patients achieving Investigator Global Assessment (IGA) response (clear [0] or almost clear [1] with ≥2-grade improvement) at week 12; the coprimary end points in the phase 3 studies were the proportion of patients achieving IGA response and the proportion of patients achieving 75% or more improvement in Eczema Area and Severity Index (EASI14), both at week 12. The 3 trials had similar study designs,15 and complete details of the study designs can be found elsewhere.16–18
Study Participants
Study participants were patients aged 18 to 75 years (phase 2b) or 12 years or older (phase 3) with clinical diagnosis of moderate to severe AD (IGA ≥3, EASI ≥12 [phase 2b] or ≥16 [phase 3], percentage of body surface area involvement [%BSA] ≥10, Peak Pruritus Numerical Rating Scale [PP-NRS19; used with permission from Regeneron Pharmaceuticals, Inc, and Sanofi] ≥4 [phase 3 only]) for 1 or more years and recent (within 12 months in phase 2b; within 6 months in phase 3) history of inadequate response to topical medications (corticosteroids or calcineurin inhibitors) given for 4 or more weeks or an inability to receive topical treatment because it was medically inadvisable. Previous dupilumab use was permitted in all 3 studies if it had been discontinued for more than 6 weeks before study initiation. Patients who previously used JAK inhibitors within 12 weeks (phase 2b) or ever (phase 3) or oral immunosuppressant agents (ie, cyclosporine, azathioprine, methotrexate, mycophenolate mofetil, and systemic corticosteroids) within 4 weeks or 5 half-lives (whichever was longer) were excluded. Rescue medication, including topical corticosteroids, was prohibited during the study. Full inclusion and exclusion criteria are published elsewhere.16–18
The study protocol and informed consent documents were reviewed and approved by the institutional review board and/or independent ethics committee at each of the investigational sites. All patients provided written informed consent. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, the International Council for Harmonization Good Clinical Practice guidelines, and all local regulatory requirements.
Assessments and End Points
For phase 2b trial, Pruritus-NRS (self-report of itch in the last 24 hours) was assessed daily for the first 15 days, and for 2 phase 3 trials, PP-NRS (self-report of worst itch in the last 24 hours) scores were recorded daily by patients in an eDiary for the first 15 days. Afterward, for all 3 trials, Pruritus-NRS/PP-NRS (hereafter referred to as PP-NRS for simplicity) was assessed only on study visit days at weeks 4, 6 (phase 2b only), 8, and 12. Additional end points measured at these study visits were the IGA, EASI, SCORing of Atopic Dermatitis (SCORAD),20 and Dermatology Life Quality Index (DLQI,21 for patients aged ≥18 years). The SCORAD includes a sleep instrument surveying sleep quality over the preceding 3 nights rated using a patient-reported visual analog scale (VAS) from no sleeplessness (0) to worst imaginable sleeplessness (10).
The proportion of patients achieving 4-point or greater reduction in PP-NRS (PP-NRS4) at weeks 2, 4, 8, and 12 were measured in all trials; values at weeks 2, 4, and 12 were key secondary end points (multiplicity controlled) in the phase 3 trials. Subgroup multivariate analyses stratified by baseline characteristics (ie, itch severity, sex, race, and body mass index [BMI]) were performed. The proportions of patients achieving PP-NRS 0/1 (ie, near resolution of itch) and/or DLQI 0/1 (ie, no effect on QoL) at week 12; percentage change from baseline in PP-NRS scores at days 2–15 and at weeks 4 (day 29 ± 2), 8 (day 57 ± 3), and 12 (day 85 ± 3); times to PP-NRS4 response and 3-point or greater reduction in PP-NRS (PP-NRS3); and changes from baseline in mean SCORAD sleep VAS scores at week 12 were also calculated. A mediation analysis was performed to estimate the direct effect of abrocitinib on itch relief (ie, PP-NRS4 response) independent of overall disease improvement (ie, IGA response).
Statistical Analysis
The primary analysis population for efficacy was the full analysis set, which included patients who received 1 or more doses of randomized treatment with abrocitinib 200 mg, abrocitinib 100 mg, or placebo. Binary end points were analyzed using the Cochran-Mantel-Haenszel test, adjusted by randomization strata. Patients who permanently discontinued the study were defined as nonresponders at all visits after the last observation. Continuous end points were analyzed using a mixed-effects model with repeated measures based on all observed data. The model included factors for treatment group, randomization strata, visit, treatment-by-visit interaction, and relevant baseline value. No explicit imputations were made for missing data, and the mixed-effects model with repeated measures yielded valid inferences under assumption of missing data at random mechanism. Times to achieve PP-NRS3 and PP-NRS4 responses were analyzed using Kaplan-Meier methods based on observed data only (no imputations) with times to event censored at treatment discontinuation or last observation if no response was achieved. Subgroup analyses by baseline characteristics were performed for the week 12 PP-NRS4 response. A multivariate, linear regression analysis was performed to assess the factors associated with PP-NRS4 response at week 2. Factors included in this model were study treatment, age group, sex, race, region of participation, baseline %BSA group, baseline IGA, baseline EASI group, baseline PP-NRS4 group, baseline BMI, baseline comorbidities (yes/no), and previous use of AD medications (systemic/topical). A mediation analysis22 was performed to estimate the direct effect of abrocitinib on PP-NRS4 response and the indirect effect of abrocitinib on PP-NRS4 response mediated through IGA response (mediator). The percentage of direct effect of the total effect was reported based on this analysis.
RESULTS
Demographics and Baseline Disease Characteristics
Overall, 942 patients received abrocitinib 200 mg (n = 363), abrocitinib 100 mg (n = 369), or placebo (n = 210) and were included in this analysis. The median age was 31 years (range = 12–84 years), and 13.2% were adolescents. Most patients were male (56.2%) and White (66.3%). Of the patients, 62.7% had moderate disease (IGA 3), and 37.3% had severe disease (IGA 4). The mean (SD) baseline EASI score was 28.8 (12.7), and the median (range) %BSA was 43 (10–100). The median (range) DLQI was 14 (1–30), and 43.7% of the patients had used systemic treatment in the preceding year, of whom 4.8% had used dupilumab. The mean (SD) PP-NRS score was 7.0 (1.9). When stratified by baseline disease severity, the mean (SD) PP-NRS scores were 6.7 (1.9) and 7.5 (1.9) for moderate (IGA 3) and severe (IGA 4) disease, respectively.
The baseline characteristics and demographics of patients were similar among the 3 individual trials.15
Rapid and Clinically Meaningful Itch Improvement With Abrocitinib Versus Placebo
After 12 weeks of abrocitinib monotherapy, 57.3% (200 mg), 42.9% (100 mg), and 16.5% (placebo) of the patients experienced clinically meaningful itch improvement (PP-NRS4; Fig. 1). Regardless of baseline characteristics, higher PP-NRS4 response was observed in both abrocitinib groups compared with placebo at week 12 (Fig. 2). On multivariate analysis, the female patients (difference in response rate [95% confidence interval] = 3.1% [−2.6% to 8.8%]) and the patients with more severe baseline itch (PP-NRS = 8–10; 11.9% [6.2% to 17.7%]) had a higher PP-NRS4 response rate (Fig. S1, http://links.lww.com/DER/A73).
Figure 1.
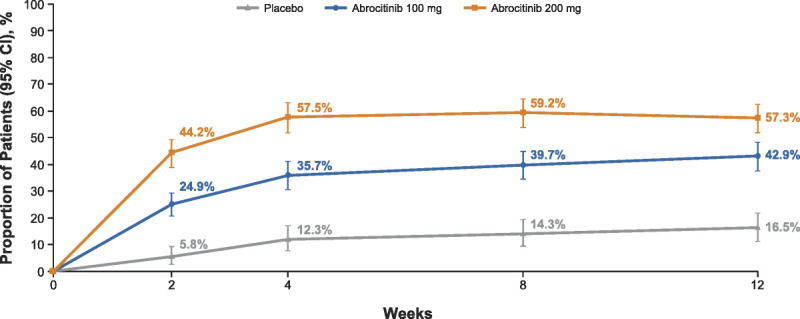
The PP-NRS4 response. Formal hypothesis testing was not performed for monotherapy pool. Formal hypothesis testing performed in phase 3 trials MONO-1 and MONO-2 for the key secondary end point (PP-NRS4 response at weeks 2, 4, and 12): abrocitinib 200 mg and 100 mg were superior to placebo at all time points (P < 0.05 for all). CI, confidence interval; PP-NRS4, 4-point or greater improvement in Peak Pruritus Numerical Rating Scale.
Figure 2.
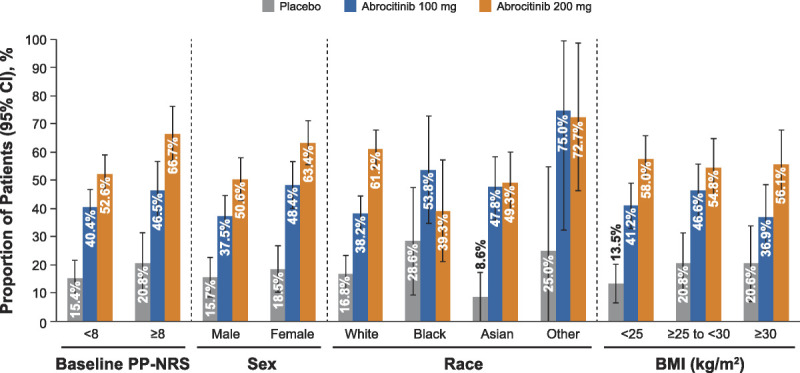
The PP-NRS4 response at week 12 subgroup analyses: baseline itch severity, sex, race, and BMI. BMI, body mass index; CI, confidence interval; PP-NRS, Peak Pruritus Numerical Rating Scale; PP-NRS4, 4-point or greater improvement in Peak Pruritus Numerical Rating Scale.
Twenty-four hours after receiving the first dose (day 2), the mean percentage reductions in PP-NRS scores were greater for both doses of abrocitinib versus placebo; this difference was maintained through week 12 (Fig. 3). Overall, the median time to achieving a PP-NRS3 response was 9, 13, and 59 days after initiating abrocitinib 200 mg, abrocitinib 100 mg, and placebo, respectively. For PP-NRS4 response, the median time to response in the 200- and 100-mg groups was 15 and 57 days, respectively; the median time to response in the placebo group was beyond the duration of the study (Fig. 4). A significant proportion of the patients also achieved itch-free or virtually itch-free status (ie, PP-NRS 0/1) at week 12 with abrocitinib 200 or 100 mg (36.6% and 23.4%, respectively) compared with placebo (5.3%).
Figure 3.
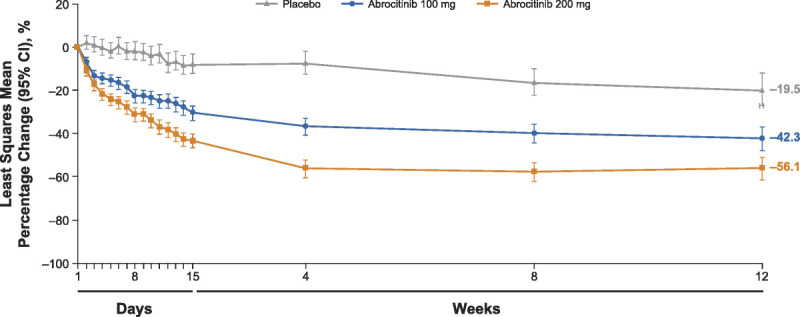
Least squares mean percentage change from baseline in PP-NRS. CI, confidence interval; PP-NRS, Peak Pruritus Numerical Rating Scale.
Figure 4.
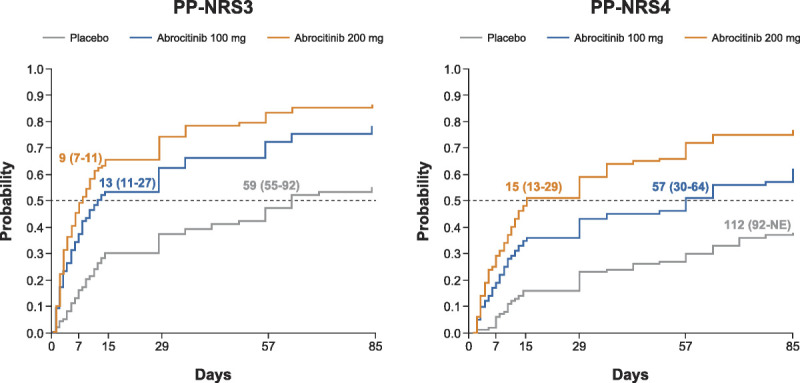
Time to achieve PP-NRS3 and PP-NRS4 response. Values represent median (95% CI). NE, not evaluable; PP-NRS3, 3-point or greater improvement in Peak Pruritus Numerical Rating Scale; PP-NRS4, ≥4-point improvement in Peak Pruritus Numerical Rating Scale.
Abrocitinib Itch Relief Is Associated With Marked Improvements in Disease-Related QoL and Sleep
Achievement of PP-NRS4 response was a strong driver for improvement in disease-related QoL. Among PP-NRS4 responders at week 12, similar proportions of the patients achieved DLQI 0/1 (ie, no effect on QoL) regardless of treatment (49.0%, 41.7%, and 37.9% for abrocitinib 200 mg, abrocitinib 100 mg, and placebo, respectively). Likewise, the PP-NRS4 nonresponders at week 12 were similarly unlikely to achieve DLQI 0/1 regardless of treatment (9.4%, 4.9%, and 4.8%, respectively). Among the patients receiving either dose of abrocitinib who achieved itch-free or virtually itch-free status at week 12, proportions of the patients achieving DLQI 0/1 were 86.8%, 71.0%, and 83.3% for abrocitinib 200 mg, abrocitinib 100 mg, and placebo, respectively. For these same patients, the median SCORAD sleep VAS scores improved from 5.4, 5.9, and 6.0 at baseline, respectively, to 0.0 for all groups at week 12.
Abrocitinib-Associated Itch Relief Occurs Partially Independent of Overall Disease Improvement
To explore whether the observed anti-itch effects of abrocitinib were independent of an anti-inflammatory effect, the proportion of the patients who experienced overall disease improvement (IGA response) was compared with the proportion of the patients who experienced clinically meaningful itch improvement (PP-NRS4 response) at weeks 2 and 12. Among the patients who achieved IGA response at week 2, 58.3% (200 mg), 38.6% (100 mg), and 15.4% (placebo) also achieved a week 2 PP-NRS4 response. However, 32.1% (200 mg), 19.9% (100 mg), and 5.3% (placebo) of the week 2 IGA nonresponders still achieved a PP-NRS4 response at this time (Table S1, http://links.lww.com/DER/A74). At week 12, 86.1% (200 mg), 81.3% (100 mg), and 61.5% (placebo) of the IGA responders achieved PP-NRS4 response, whereas 43.7% (200 mg), 34.3% (100 mg), and 19.5% (placebo) of the IGA nonresponders still achieved PP-NRS4 response (Table S1, http://links.lww.com/DER/A74). For either dose, approximately 62% of the total treatment effect on PP-NRS4 response at week 12 could be attributed to the treatment, whereas approximately 38% was attributable to an indirect effect mediated through overall disease improvement.
DISCUSSION
We demonstrated that abrocitinib induces rapid, profound, and clinically meaningful itch relief both associated with and independent of overall disease improvement as measured by IGA response. Itch relief was generally observed regardless of baseline itch severity, sex, race, or BMI. A considerable percentage of patients achieved itch-free or virtually itch-free status by week 12, which was also associated with a significant improvement in sleep and disease-related QoL. Itch relief seems to have been mediated by both a direct effect of abrocitinib (ie, neuronal JAK1 inhibition) and an indirect effect via overall disease improvement (ie, IGA response). This is supported by a recent report of marked itch relief in patients with no evidence of skin inflammation or primary dermatologic disorders treated with the JAK1/3 inhibitor tofacitinib.23 The dynamics of AD itch relief occurring independently of improvement in overall disease severity is further supported by recent work investigating how proinflammatory mediators promote itch.10,24,25 In a 2017 study, Oetjen et al10 demonstrated that the maintenance of chronic itch in mammalian skin sensory neurons relies on JAK1, which simultaneously promotes neuronal signaling of TH2 inflammatory cytokines (eg, IL-4, IL-13) and the neuronal responsiveness to other itch-inducing factors (eg, IL-31).10 As such, antipruritic effects of JAK1 inhibition are mediated predominantly through direct neuronal JAK1 inhibition rather than through the suppression of skin inflammation.10 Keratinocyte-derived TSLP (also dependent on JAK1 for intracellular signaling) directly activates cutaneous sensory neurons to promote itch.11
The emergence of dupilumab, an injectable monoclonal antibody against the common IL-4 and IL-13 receptor, has shown the importance of these TH2 inflammatory cytokines in AD pathophysiology and has enhanced treatment options available for patients with moderate to severe AD.26 Nevertheless, fewer than 40% of dupilumab-treated patients achieve IGA and/or PP-NRS4 responses at week 16.26 In addition, the majority of itch improvement (as measured by PP-NRS4) is observed after week 2, with much of the improvement happening after week 4.26 In contrast, the majority of itch improvement with abrocitinib occurred in the first 2 weeks. These cross-study findings indicate that targeting a broader set of pruritogenic cytokines may result in faster and more profound itch relief that is mediated through a combination of direct effects on cutaneous sensory neurons and indirect effects via improvement of skin inflammation and overall disease severity. However, additional research is necessary to confirm this.
Limitations of these analyses include the short duration of the trials (12 weeks) and that patients were not allowed to use topical medications, which may not reflect real-world clinical practice. In addition, there was a key limitation to accurately estimate the median time to PP-NRS4 response observed for abrocitinib 100 mg; assessments were daily for the first 15 days, but thereafter, the assessment was less frequent (days 29, 57, and 85). Therefore, the true median time to PP-NRS4 response for abrocitinib 100 mg may be between day 16 and day 56.
CONCLUSIONS
Abrocitinib rapidly and profoundly relieves AD itch, partially independent of overall disease severity, which is associated with marked improvements in sleep- and disease-related QoL. These findings support that the anti-itch effects of abrocitinib are likely mediated both directly through neuronal JAK1 inhibition and indirectly through suppression of skin inflammation and subsequent improvement of overall disease severity. This may be explained by the dependence of pruritogenic cytokines on JAK1 signaling in skin sensory neurons and the capacity of this signaling to also enhance the responsiveness of sensory neurons to a variety of pruritogenic stimuli. Whether broader targeting of such cytokines via selective JAK1 inhibition results in additional or more rapid anti-itch activity in AD will be addressed in forthcoming studies that directly compare dupilumab and abrocitinib in the treatment of moderate to severe AD.
Footnotes
This study was sponsored by Pfizer, Inc. Medical writing support under the guidance of the authors was provided by Irene Park, PhD, and Juan Sanchez-Cortes, PhD, of ApotheCom, San Francisco, CA, and was funded by Pfizer, Inc, New York, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–464).
B.S.K. is a consultant and advisor for Pfizer, Inc, AbbVie, Boehringer Ingelheim, Cara Therapeutics, Kiniksa, Menlo Therapeutics, Inc, and Sanofi-Regeneron; has received research grants from Cara Therapeutics, Celgene, and LEO Pharma and is a founder and stockholder in Nuogen Pharma, Inc. J.I.S. is an investigator for AbbVie, Celgene, Eli Lilly, GSK, Kiniksa, LEO Pharma, Menlo Therapeutics, Inc, Realm Therapeutics, Regeneron, Roche, and Sanofi; a consultant for Pfizer, Inc, AbbVie, Anacor, AnaptysBio, Arena Pharmaceuticals, Inc, Asana BioSciences, Dermira, Dermavant, Eli Lilly, Galderma, GSK, Glenmark, Incyte, Kiniksa, LEO Pharma, MedImmune, Menlo Therapeutics, Inc, Novartis, Realm Therapeutics, Regeneron, and Sanofi; a speaker for Regeneron and Sanofi; and served on advisory boards for Pfizer, Inc, Dermira, LEO Pharma, and Menlo Therapeutics, Inc. S.S. is an investigator for DERMASENCE, Galderma, Kiniksa, Menlo Therapeutics, Inc, Novartis, Sanofi, Trevi Therapeutics, Inc, and Vanda Pharmaceuticals, Inc, and is a consultant and/or a member of an advisory board for Pfizer, Inc, Almirall, Bayer, Beiersdorf, Bellus Health, Bionorica, Cara Therapeutics, Celgene, Clexio Biosciences, DS Biopharma, Galderma, Menlo Therapeutics, Inc, Novartis, Perrigo, and Trevi Therapeutics, Inc. G.Y. is a consultant and advisor for Pfizer, Inc, Bellus Health, Eli Lilly, Galderma, Kiniksa, Sanofi-Regeneron, Novartis, LEO Pharma, Kiniksa, and Trevi Therapeutics, Inc, and a principal investigator for Pfizer, Inc, Kiniksa, LEO Pharma, Novartis, Sun Pharmaceutical Industries, Ltd, and Sanofi-Regeneron. E.L.S. reports grants from Pfizer, Inc, Eli Lilly, Kyowa Kirin, LEO Pharma, Merck, and Regeneron and personal fees from Pfizer, Inc, Bausch Health (Valeant), Dermira, Eli Lilly, Galderma, LEO Pharma, Menlo Therapeutics, Inc, Novartis, Regeneron, and Sanofi Genzyme. M.D., U.K., S.A.F., P.B., H.V., and M.C.C. are employees and shareholders of Pfizer, Inc.
At the time of analysis, M.C.C. is affiliated with Pfizer, Inc, New York.
Clinical Trials registry and numbers: ClinicalTrials.gov: NCT02780167, NCT03349060, and NCT03575871.
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer, Inc, will provide access to individual deidentified participant data from Pfizer, Inc–sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer, Inc, will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer, Inc, trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions and, for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer, Inc.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.dermatitisjournal.com).
Contributor Information
Brian S. Kim, Email: briankim@wustl.edu.
Jonathan I. Silverberg, Email: jonathanisilverberg@gmail.com.
Gil Yosipovitch, Email: yosipog@gmail.com.
Eric L. Simpson, Email: simpsone@ohsu.edu.
Marco DiBonaventura, Email: Marco.DiBonaventura@pfizer.com.
Urs Kerkmann, Email: Urs.Kerkmann@pfizer.com.
Saleem A. Farooqui, Email: Saleem_Farooqui@pfizer.com.
Pinaki Biswas, Email: Pinaki.Biswas@pfizer.com.
Hernan Valdez, Email: Hernan.Valdez@pfizer.com.
Michael C. Cameron, Email: michael.cameron@pfizer.com.
REFERENCES
- 1.Silverberg JI Gelfand JM Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol 2018;121(3):340–347. [DOI] [PubMed] [Google Scholar]
- 2.Augustin M Langenbruch A Blome C, et al. Characterizing treatment-related patient needs in atopic eczema: insights for personalized goal orientation. J Eur Acad Dermatol Venereol 2020;34(1):142–152. [DOI] [PubMed] [Google Scholar]
- 3.Kini SP DeLong LK Veledar E, et al. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol 2011;147(10):1153–1156. [DOI] [PubMed] [Google Scholar]
- 4.Matterne U Apfelbacher CJ Loerbroks A, et al. Prevalence, correlates and characteristics of chronic pruritus: a population-based cross-sectional study. Acta Derm Venereol 2011;91(6):674–679. [DOI] [PubMed] [Google Scholar]
- 5.Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med 2013;368(17):1625–1634. [DOI] [PubMed] [Google Scholar]
- 6.Steinke S Zeidler C Riepe C, et al. Humanistic burden of chronic pruritus in patients with inflammatory dermatoses: results of the European Academy of Dermatology and Venereology Network on Assessment of Severity and Burden of Pruritus (PruNet) cross-sectional trial. J Am Acad Dermatol 2018;79(3):457–463.e5. [DOI] [PubMed] [Google Scholar]
- 7.Apfelbacher CJ van Zuuren EJ Fedorowicz Z, et al. Oral H1 antihistamines as monotherapy for eczema. Cochrane Database Syst Rev 2013;2013(2):CD007770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverberg NB, Duran-McKinster C. Special considerations for therapy of pediatric atopic dermatitis. Dermatol Clin 2017;35(3):351–363. [DOI] [PubMed] [Google Scholar]
- 9.Drucker AM Ellis AG Bohdanowicz M, et al. Systemic immunomodulatory treatments for patients with atopic dermatitis: a systematic review and network meta-analysis. JAMA Dermatol 2020;156(6):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oetjen LK Mack MR Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 2017;171(1):217–228.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson SR Thé L Batia LM, et al. The epithelial cell–derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013;155(2):285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev 2009;228(1):273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol 2016;51(3):263–292. [DOI] [PubMed] [Google Scholar]
- 14.Hanifin JM Thurston M Omoto M, et al. The Eczema Area and Severity Index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001;10(1):11–18. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg JI Thyssen JP Simpson EL, et al. Impact of oral abrocitinib monotherapy on patient-reported symptoms and quality of life in adolescents and adults with moderate-to-severe atopic dermatitis: a pooled analysis of patient-reported outcomes. Am J Clin Dermatol 2021. doi: 10.1007/s40257-021-00604-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gooderham MJ Forman SB Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol 2019;155(12):1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverberg JI Simpson EL Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis (AD): results from the phase 3 JADE MONO-2 study. J Am Acad Dermatol 2020;156(8):1–11. doi: 10.1001/jamadermatol.2020.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson EL Sinclair R Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2020;396(10246):255–266. [DOI] [PubMed] [Google Scholar]
- 19.Yosipovitch G Reaney M Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol 2019;181(4):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Severity scoring of atopic dermatitis: the SCORAD index. Consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1993;186(1):23–31. [DOI] [PubMed] [Google Scholar]
- 21.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19(3):210–216. [DOI] [PubMed] [Google Scholar]
- 22.MacKinnon DP. Introduction to Statistical Mediation Analysis. New York, NY: Taylor and Francis Group; 2008. [Google Scholar]
- 23.Wang F Morris C Bodet ND, et al. Treatment of refractory chronic pruritus of unknown origin with tofacitinib in patients with rheumatoid arthritis. JAMA Dermatol 2019;155(12):1426–1428. [DOI] [PubMed] [Google Scholar]
- 24.Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci 2014;17(2):175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yosipovitch G, Berger T, Fassett MS. Neuroimmune interactions in chronic itch of atopic dermatitis. J Eur Acad Dermatol Venereol 2020;34(2):239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson EL Bieber T Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016;375(24):2335–2348. [DOI] [PubMed] [Google Scholar]


