Abstract
Coronavirus disease 2019 (COVID-19) continues to be a pandemic threat that is generating a constant state of alert in manifold countries. One of the strategies of defense against infectious diseases (e.g., COVID-19) is the vaccinations that decrease the numbers of infected individuals and deaths. In this context, the optimal level of vaccination for COVID-19 is a basic point to control this pandemic crisis in society. The study here,−using data of doses of vaccines administered per 100 inhabitants, confirmed cases and case fatality ratio of COVID-19 between countries (N=192) from March to May 2021,− clarifies the optimal levels of vaccination for reducing the number of infected individuals and, consequently, the numbers of deaths at global level. Findings reveal that the average level of administering about 80 doses of vaccines per 100 inhabitants between countries can sustain a reduction of confirmed cases and number of deaths. In addition, results suggest that an intensive vaccination campaign in the initial phase of pandemic wave leads to a lower optimal level of doses administered per 100 inhabitants (roughly 47 doses of vaccines administered) for reducing infected individuals; however, the growth of pandemic wave (in May, 2021) moves up the optimal level of vaccines to about 90 doses for reducing the numbers of COVID-19 related infected individuals. All these results here could aid policymakers to prepare optimal strategies directed to a rapid COVID-19 vaccination rollout, before the takeoff of pandemic wave, to lessen negative effects of pandemic crisis on environment and socioeconomic systems.
Keywords: COVID-19, Coronavirus Disease 2019; SARS‐CoV‐2, Severe acute respiratory syndrome coronavirus 2; CFRs, Case Fatality Ratios
Keywords: COVID-19 vaccine, Vaccination campaign, Public health, Herd immunity, Crisis management
1. Goals of the investigation
Coronavirus disease 2019 (COVID-19) is an infectious illness caused by the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which appeared in late 2019 (Anand et al., 2021; Bontempi et al., 2020, 2021; Bontempi and Coccia, 2021; Coccia, 2020, 2020a, 2021). COVID-19 is still circulating in 2021 with mutations of the novel coronavirus1 that generate continuous COVID-19 infections and deaths in manifold countries (Johns Hopkins Center for System Science and Engineering, 2021; Huang et al., 2021; National Academy of Medicine, 2021; Vicenti et al., 2021). Seligman et al. (2021) show some characteristics of people that are associated with COVID-19 mortality, such as: mean age of 71.6 years, nonwhite race/ethnicity, income below the median and less than a high school level of education. High numbers of COVID-19 related infected individuals and deaths worldwide have supported the development of different types of vaccines based on viral vector, protein subunit and nucleic acid-RNA (Abbasi, 2020; de Vlas and Coffeng, 2021; Jones and Helmreich, 2020). In vector vaccines, genetic material from the COVID-19 virus is placed in a modified version of a different virus (called, viral vector). When the viral vector gets into human cells, it delivers genetic material from the COVID-19 virus directed to instruct the cells to make copies of the Spike (S) protein (the main protein used as a target in COVID-19 vaccines). After that, human cells display the S proteins on their surfaces and immune system responds by creating antibodies and defensive white blood cells to fight the novel coronavirus (viral vector vaccines for COVID-19 are by Janssen/Johnson & Johnson and University of Oxford/AstraZeneca). Protein subunit vaccine includes only the parts of a virus that best stimulate immune system. This type of COVID-19 vaccine has harmless S proteins. The immune system recognizes S proteins and creates antibodies and defensive white blood cells to fight the viral agent ( Novavax, an American biotechnology company, has developed this type of protein subunit vaccine; cf., GAVI, 2021). Instead, the messenger Ribonucleic acid (mRNA) vaccines use genetically engineered mRNA to give to cells instructions on how to make the S protein found on the surface of the SARS-CoV-2, creating antibodies to fight this novel coronavirus (Mayo Clinic, 2021). The process of development of mRNA vaccines for COVID-19 is much faster than conventional vaccines to be redesigned and mass-produced (Cylus et al., 2021; Heaton, 2020; Jeyanathan et al., 2020; Komaroff, 2020). The first path-breaking mRNA vaccines for COVID-19 are due to premier biopharmaceutical companies: Pfizer-BioNTech and Moderna (cf., Coccia, 2015, 2017, 2017c, 2020c, 2021a). The investigation of vaccination plans between countries is a crucial aspect to determine how the novel infectious disease can be controlled and/or eradicated in the population (Aldila et al., 2021). Vaccination has the potential effect to reduce the diffusion of COVID-19, to relax non-pharmaceutical measures and maintain low basic reproduction number, but an important point is to clarify the levels of administering of vaccines between countries to timely reduce negative effects in society (Anser et al., 2020). Akamatsu et al. (2021) argue the vital role of governments to implement an efficient campaign of vaccination to substantially reduce infections in society, and avoid the collapse of healthcare system (cf., Coccia, 2021a, 2022). Aldila et al. (2021) maintain that higher levels of vaccination rate can eradicate COVID-19 in population by approaching herd immunity to protect vulnerable individuals (cf., Anderson et al., 2020; Randolph and Barreiro, 2020). Herd immunity indicates that only a share of population needs to be immune and therefore no longer susceptible to a viral agent (by overcoming natural infection or through vaccination) for controlling large outbreaks (Fontanet and Cauchemez, 2020). Scholars estimate the proportion of a population that needs to be vaccinated to support herd immunity, ceteris paribus (Redwan, 2021). The threshold level depends on basic reproduction number, R0— the number of average cases spawned by one infected individual in an otherwise fully susceptible (Coccia, 2020; Kwok et al., 2020). In particular, the formula for calculating the herd-immunity threshold is 1–1/R0 and it indicates that the more people who become infected by everyone who has the virus, the higher the proportion of the population that needs to be immune to reach herd immunity. The index R0 assumes that everyone is susceptible to the virus, but the level changes as the epidemic evolves, since it depends on changes in susceptibility of the population, mitigation and restriction policies, circulation of variants, etc. (Aschwanden, 2020, 2021; Coccia, 2021c, 2021d). In addition, the relax of mitigation and containment measures can move up herd-immunity threshold in specific situation (Buss et al., 2021; Dashtbali and Mirzaie, 2021). Kwok et al. (2021) argue that the estimates of effective reproduction number range from 1.06 to 6.64 and the minimum proportion (%) of total population required to confer COVID-19 immunity is 5.66 in Kuwait and 85 in Bahrain. Rosen et al. (2021) describe socioeconomic and organizational factors associated with the vaccination campaign in Israel, a virtuous country in the plan of vaccination for COVID-19 (cf., Prieto Cruriel et al., 2021).
In this context, a fundamental problem in COVID-19 pandemic crisis is the optimal level of vaccination that supports a drastic reduction of COVID-19 infected individuals and deaths. The present study confronts this problem here by developing a statistical analysis to explain, at global level, different optimal levels of vaccination, during the evolution of COVID-19 pandemic wave, that trigger a reduction of infected individuals in society. Results can suggest best practices for vaccination plans to guide effective and timely policy responses for constraining negative effects of COVID-19 pandemic crisis and future epidemics of similar infectious diseases in environment and socioeconomic systems. This study is part of a large research project directed to explain drivers of transmission dynamics of COVID-19 and design effective policy responses to cope with and/or to prevent pandemic threats in society (Coccia, 2020b, 2021b, 2021e, 2021h, 2021i, 2022a).
2. Materials and methods
2.1. Source and sample
The sample of this study is N = 192 countries worldwide. Period under study is from March to May 2021, using data of vaccines, confirmed cases and case fatality ratio of COVID-19. The list of countries under study is in Appendix A.
-
⁃Measures
-
−Doses of vaccines administered × 100 inhabitants on March 1, April 1 and May 1, 2021.
-
−
Doses of vaccines refer to the total number of vaccine doses, considering that an additional dose may be obtained from each vial (e.g., six doses for Pfizer BioNTech® Comirnaty), whereas number of doses administered refers to any individual receiving any dose of the vaccine (cf., Freed et al., 2021; Oliver et al., 2020). The data here considers all types of COVID-19 vaccines used in different countries, i.e., vaccines by Johnson & Johnson, Oxford/AstraZeneca, Pfizer/BioNTech, Sinopharm/Beijing, Sinovac, Sputnik V and Moderna (Ritchie et al., 2020). Of course, every country has been using a different combination of these COVID-19 vaccines to protect the population (CBC, 2021; CDC, 2021; Rossman et al., 2021). Source: Our World in Data (2021).
-
−
Number of COVID-19 infected individuals (%) is measured with confirmed cases of COVID-19 (on March 21, April 21 and May 21, 2021) divided by population of countries under study. This study considers a time lag of 21 days from doses of vaccines administered (1st day of the month) to confirmed cases of COVID-19 (21 day of the same month) to assure a certain level of protection in population that begins after a variable number of days (Faes et al., 2020; Zhang et al., 2020). Source of data: Johns Hopkins Center for System Science and Engineering (2021).
-
−
Number of COVID-19 deaths is measured with Case Fatality Ratio % (on March 21, April 21 and May 21, 2021). It indicates the severity of an infectious disease and evaluates the quality of health systems (Coccia, 2021e; Lau et al., 2021; WHO, 2020; Wilson et al., 2020). Case Fatality Ratio (CFR) estimates the proportion of deaths among identified confirmed cases of COVID-19:
Angelopoulos at al. (2020) maintain that Case Fatality Ratios (CFRs) between countries are critical measures of relative risk that guide policymakers to decide how to allocate medical resource to cope with COVID-19 pandemic crisis. This study also calculates the mortality rate per 100 000 people for a comparative analysis with CFRs to assess the effects of vaccination with two indexes. Source of data: Johns Hopkins Center for System Science and Engineering (2021).
2.2. Model and data analysis procedure
Firstly, data are analyzed with descriptive statistics of variables given by arithmetic mean and standard error of the mean. Data concerning doses of COVID-19 vaccines, confirmed cases and CFR in the dataset downloaded the days under study can refer to data of different days because of difficulties in countries associated with gather or transmission of information. Moreover, database here includes different COVID-19 vaccines having a different period of administering between the first and second dose to provide a certain level of protection that begins after a variable number of days (approximately after 10–15 days the first dose) and remains for some months (about six months). In the presence of this just mentioned issue, the study here considers a time lag of 21 days from date of doses of vaccines and date of confirmed cases. In addition, this study considers a large sample of N = 192 countries having a normal distribution that mitigates discrepancies among countries for significant statistical analyses.
We assume different scenarios considering the evolution of COVID-19 pandemic wave and vaccination over time and countries:
-
•
Scenario A. Initial stage of COVID-19 pandemic wave in March 2021. If countries of the sample have the level of vaccination at the time t (March 1, 2021) and confirmed cases at t+21 days (March 21, 2021), how would be the optimal level of doses of vaccines administered.
-
•
Scenario B. Maturity stage of COVID-19 pandemic wave in May 2021. If countries of the sample have the level of vaccination at the time t ' (May 1, 2021) and confirmed cases of t '+21 days (May 21, 2021), how would be the optimal level of doses of vaccines administered.
-
•
Scenario C. Overall evolution of COVID-19 pandemic wave from March to May to estimate the optimal level of doses of vaccines administered between countries.
Secondly, the analysis of simple regression applies quadratic models because they fit the scatter of data to detect nonlinear effects of relations understudy.
The specification of model for three scenarios just described is given by:
| [1] |
where:
-
o
xi, t-21 = Doses of vaccines administered × 100 inhabitants on 1st day of the month (Explanatory variable)
o y i,t = Number of confirmed cases of COVID-19 on 21st day of the month (Response or dependent variable)
o u i,t = Error term
o country i = 1, …, n; t = time
Remark 1
The square of the doses of vaccines administered × 100 inhabitants in model [1] is introduced to consider the possibility of non-linear effects in the relation under study.
Remark 2
Model [1] has a time lag effect between explanatory (t-21 days) and dependent variables (t) to reduce the endogeneity of explanatory variable in model and provide reliable (estimated) parameters.
Thirdly, the optimization of the estimated relationships [1] is performed with the perspective of maximization of equation [1] to find the optimal levels of doses of vaccines administered × 100 inhabitants (during the cycle of evolution of the COVID-19 pandemic) that support a consequential drastic reduction of confirmed cases of COVID-19 and negative effects in society. In particular, the estimated relationships [1] for three scenarios (A, B, and C) are objective functions of one (real) variable given by polynomial functions of second order. These estimated relations [1] are continuous and infinitely differentiable functions. The calculus applied on functional relation [1] provides the optimal levels of doses of vaccines administered × 100 inhabitants that reduce, subsequently, confirmed cases between countries in a specific stage of COVID-19 pandemic wave as described in scenarios A, B and C.
Finally, the optimal doses of vaccines administered, calculated as described in previous statistical and mathematical analyses, are used as cut-off points. In particular, average Case Fatality Ratios (%) and Mortality rates per 100 000 people are calculated for countries having lower or higher levels of doses of vaccines than cut-off points (i.e., optimal estimated values). This analysis also considers the population of countries in 2020 to assess if the size of countries plays a role in the policy responses to cope with COVID-19 pandemic crisis.
Statistical analyses are performed with the Statistics Software SPSS® version 26.
3. Results
Table 1 shows descriptive statistics of variables in March, April and May 2021.
□ Scenario A: Initial phase of COVID-19 pandemic wave in March 2021.
Table 1.
Descriptive statistics, N=192 countries worldwide.
| Variables | Mean | Std. Error |
|---|---|---|
| Doses vaccines per 100 inhabitants March 1, 2021 | 7.547 | 1.506 |
| Doses vaccines per 100 inhabitants April 1, 2021 | 12.750 | 1.573 |
| Doses vaccines per 100 inhabitants May 1, 2021 | 19.514 | 1.957 |
| Confirmed Cases/population % March 21, 2021 | 2.640 | 0.234 |
| Confirmed Cases/population % April 21, 2021 | 3.062 | 0.265 |
| Confirmed Cases/population % May 21, 2021 | 3.421 | 0.288 |
| Case Fatality Ratios % March 21, 2021 | 2.182 | 0.186 |
| Case Fatality Ratios % April 21, 2021 | 2.138 | 0.176 |
| Case Fatality Ratios % May 21, 2021 | 2.271 | 0.215 |
| Mortality rates per 100 000 people March 21, 2021 | 51.131 | 4.773 |
| Mortality rates per 100 000 people April 21, 2021 | 58.046 | 5.369 |
| Mortality rates per 100 000 people May 21, 2021 | 63.443 | 5.741 |
The estimated relationship, based on results of Table 2 , is:
Table 2.
Regression analyses of confirmed cases of March 21, 2021on doses of vaccines on March 1, 2021 based on quadratic model [1].
| Constant α (St. Err) | 3.25 *** (.47) |
|---|---|
| Coefficient β1 (St. Err.) | .187 ** (.07) |
| Coefficient β2 (St. Err.) | −.002* (.001) |
| R2 (St. Err. of Estimate) | .10 (3.24) |
| F | 4.79** |
| Total cases | 192 |
Note: Dependent variable is: Confirmed cases/population (%) of March 21, 2021. Explanatory variable is: doses of vaccines per 100 inhabitants on March 1, 2021.
Significance: *** p-value < 0.001,** p-value < 0.01, * p-value < 0.05.
The polynomial function is given by:
the necessary condition to maximize is:
The first derivative equal to 0 is:
h* = 46.75 doses of vaccine per 100 people; h* indicates the optimal level in the initial phase of pandemic wave (March 2021) between countries to trigger a sharply decrease of confirmed cases and diffusion of COVID-19 leading, whenever possible, to constraint negative effects of the pandemic crisis in society.
□ Scenario B. Maturity phase of COVID-19 pandemic wave in May 2021.
The estimated relationship, based on results of Table 3 , is:
Table 3.
Regression analyses of confirmed cases (%) of May 21, 2021 on doses of vaccines on May 1, 2021 based on quadratic model [1].
| Constant α (St. Err) | 1.45 *** (.40) |
|---|---|
| Coefficient β1 (St. Err.) | .180 *** (.03) |
| Coefficient β2 (St. Err.) | −.001*** (.000) |
| R2 (St. Err. of Estimate) | .33 (3.35) |
| F | 38.46*** |
| Total cases | 192 |
Note: Dependent variable is: Confirmed cases/population (%) of May 21, 2021. Explanatory variable is: doses of vaccines per 100 inhabitants on May 1, 2021.
Significance: *** p-value < 0.001.
The function is given by:
the necessary condition to maximize the function is:
| 0 |
The first derivative equal to 0 is:
x* = 90 doses of vaccines per 100 people; x* indicates the optimal level during the phase of maturity of the COVID-19 pandemic in May 2021 between countries to support, after this threshold, a sharply decrease of infections that reduces negative effects of COVID-19 pandemic in society.
□ Scenario C: Overall evolution of COVID-19 pandemic from March to May 2021 between countries
The estimated relationship, based on results of Table 4, Table 5 , is:
Table 4.
Regression analyses of confirmed cases of March 21, April 21, May21, 2021 on doses of vaccines on March 1, April 1 and May 1, 2021 based on quadratic model [1].
| Constant α (St. Err) | 2.33 *** (.24) |
|---|---|
| Coefficient β1 (St. Err.) | .161 *** (.020) |
| Coefficient β2 (St. Err.) | −.001*** (.000) |
| R2 (St. Err. of Estimate) | .21 (3.40) |
| F | 50.45*** |
Note: Dependent variable is: Confirmed cases/population March 21-Apri 21l-May 21, 2021. Explanatory variable is: Doses of vaccines per 100 inhabitants on March 1, April 1 and May 1, 2021.
Significance: ***p-value<0.001.
Table 5.
Negative effects of COVID-19 between countries having doses of vaccine below or above the optimal (estimated) level, used as cut-off point.
| Scenario A | Scenario B | Scenario C | ||
|---|---|---|---|---|
| Variables | Below or above the cut-off point = optimal level (estimated) | *Optimal level in March 2021 (Initial phase of pandemic) Doses of vaccines per 100 inhabitants = 46.8* Average value |
*Optimal level in May 2021 (Maturity phase of pandemic) Doses of vaccines per 100 inhabitants = 90* Average value |
*Optimal level over March–May 2021 (Evolution of COVID-19 pandemic) Doses of vaccines per 100 inhabitants = 80.5* Average value |
| Confirmed Cases/population % | Below | 4.15 | 3.88 | 3.82 |
| Above | 5.94 | 8.41 | 7.74 | |
| Case Fatality Ratios (CFR) % | Below | 1.94 | 1.84 | 1.86 |
| Above | 0.50 | 0.47 | 0.75 | |
| Mortality per 100 000 people | Below | 75.00 | 67.93 | 67.90 |
| Above | 32.74 | 41.11 | 63.20 | |
| Population | Below | 65 495 630.45 | 51 009 497.76 | 51 777 286.42 |
| Above | 6 401 920.67 | 6 401 920.67 | 6 642 840.00 |
The function is given by:
the necessary condition to maximize the function is:
The first derivative equal to 0 is:
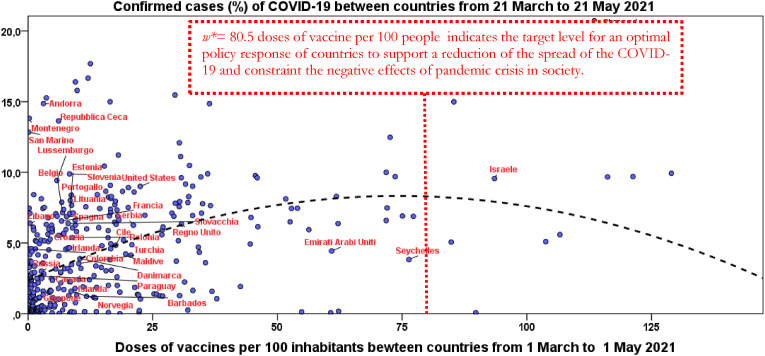
w* = 80.5 doses of vaccine per 100 people; w* indicates the optimal target between countries for a policy response to support a sharply decrease of confirmed cases of COVID-19 leading, whenever possible, to constraint the negative societal effects of pandemic crisis in society (Fig. 1 ).
Fig. 1.
Relation of confirmed cases/population (%) on doses of vaccines from March to May 2021 between countries based on quadratic model [1].
Fig. 1 shows the regression line and optimal level of doses of vaccines to trigger the reduction of confirmed cases. Some countries close to this optimal level are mainly small countries, such as Israel and United Arab Emirates.
-
□
Effects of vaccination in society measured with level of Case Fatality Ratios (CFRs) and mortality rates per 100 000 people of COVID-19
In general, these results suggest that countries with levels of vaccination above the optimal level of doses of vaccines have lower average values of CFR % and mortality rate, whereas the confirmed cases have a higher level likely because of pro-active testing activity across countries. Finally, results reveal that countries best performers in the administering of high levels of doses of vaccines per 100 inhabitants are mainly smaller countries, such as Israel (cf., Fig. 1).
4. Discussion and policy implications
The findings of the study here reveal that optimal level of vaccination is associated with the evolution of COVID-19 pandemic wave: a vaccination in the initial phase of pandemic wave has a lower optimal level of doses administered per 100 inhabitants to support the reduction of infected individuals, but the growth of pandemic wave moves up the optimal level of vaccines from 46.75 (in March 2021) to about 90 doses of vaccines in May 2021. This study also reveals that high levels of vaccination can reduce case fatality ratios and mortality rates of COVID-19 associated with other factors of health, environmental and economic system.
This study suggests that the optimal strategy and policy response to pandemic crisis are a rapid vaccination rollout to reduce timely numbers of infected individuals and deaths, before the takeoff of pandemic wave. These results may be interpreted through the lens of different studies that focus on many factors contributing to the success of implementing a rapid roll out of COVID-19 vaccination for reducing confirmed cases and negative social impact (Coccia, 2021a, 2021e, 2022). In fact, Sim et al. (2021) compare the COVID-19 pandemic in Israel and the UK, showing the importance of factors influencing the early days of the rollout of vaccination. Rosen et al. (2021) indicate three groups of facilitating factors the success of vaccination plan in Israel:
-
−
extrinsic factors to health care (small size in terms of both area and population), a relatively young population, warm weather in December 2020, a centralized national system of government, and well-developed infrastructure for implementing prompt responses to large-scale national emergencies
-
−
specific factors of the health-system, such as the organizational, IT and logistic capacities of community-based health care providers, the availability of well-trained cadre, a tradition of effective cooperation between government, health plans, hospitals, and emergency care providers, particularly during national emergencies; and effective tools and decision-making frameworks to support vaccination campaigns
-
−
finally, specific factors to the COVID-19 vaccination effort: the mobilization of special government funding for vaccine purchase and distribution, timely contracting for a large amount of vaccines for population, the use of simple, clear and easily criteria for determining who had priority for receiving vaccines in the early phases of the distribution process, demanding cold storage requirements of the Pfizer-BioNTech COVID-19 vaccines, and outreach efforts to encourage people to sign up for vaccinations and then show up to get vaccinated (cf., McKee and Rajan, 2021).
In general, optimal strategies to pandemic shocks should be based on a strong public governance driven by adequate and effective leadership that engages with the communities and adjusts to population needs (Williams et al., 2020). In fact, good governance can support the preparedness of nations for performing efficient campaigns of vaccination to reduce infections, mortality, morbidity, mental stress among the population and support economic recovery (Ardito et al., 2021; Coccia, 2017a, 2018a, 2019, 2021e, 2021f, 2021g, 2022; (Coccia, 2021l), Coccia and Bellitto, 2018; Coccia and Benati, 2018; Kluge et al., 2020). The public governance supporting efficient plans of vaccination is not limited to health system, but it involves other functions of public administration to work properly for strengthening health, economic and social systems (Sagan et al., 2020). As a matter of fact, effective crisis management of COVID-19 pandemic, supported by multi-level governance, should implement timely vaccine programmes to achieve, whenever possible, the goal of proposed scenarios (Abuza, 2020; Anttiroiko, 2021; Coccia, 2021a, 2021f; DeRoo et al., 2020; Frederiksen et al., 2020; Harrison and Wu, 2020; Ritchie et al., 2020, 2020a). Nevertheless, the optimal level of vaccination in the initial phase of pandemic has to face distribution and allocation hurdles, and in the presence of delays, governments have to cope with changes into the equation of herd-immunity with a strategy of vaccination directed to increase the thresholds of immunization in population to reduce infected individuals and negative effects in society (Callaway, 2021; Dooling et al., 2020; Vignesh et al., 2020). In fact, the study here clearly shows that a delay of effective vaccination plan from March to May 2021, considering the evolutionary growth of pandemic wave, it moves forward the optimal threshold between countries from about 47 to 90 doses of vaccines per 100 people to trigger the reduction of the transmission dynamics of COVID-19 (Buss et al., 2021; ECDC, 2021; Mallapaty, 2021; Whittaker et al., 2021). In short, the timely achievement of the optimal threshold of doses administered is a basic aspect of crisis management, because a quickly and thoroughly vaccination plan can constrain transmission dynamics and consequential socioeconomic issues (Akamatsu, 2021; Byun et al., 2021; Liu et al., 2021). Engelbrecht and Scholes (2021) argue that if effective strategies of vaccination have delayed, the progress of pandemic wave may generate additional health and socioeconomic issues. Overall, then, the policy response based on an optimal level of vaccination, according to suggested scenarios, is a significant challenge associated with manifold socio-cultural and political-administrative factors, and enormous public investments in health system of countries (Ethgen et al., 2018; Coccia, 2017b, 2019, 2021g, 2022). Hence, countries can adequately prepare to prevent, detect and respond to both epidemics and pandemics, over the next ten years, with a better governance, innovative partnerships, high public investments, and efficient utilization of economic resources in health and other sectors (U.S. Department of Health and Human Services, 2021).
5. Conclusions and prospects
COVID-19 and future epidemics of novel viruses pose, more and more, a serious threat to security and public health of nations. An influenza pandemic, similar to COVID-19, can occur at any time with little warning; any delay in detecting a novel influenza strain; sharing of influenza virus samples; and in developing, producing, distributing, or administering a therapeutic or vaccine could result in significant additional morbidity and mortality, and deterioration of socioeconomic systems (Coccia, 2021, 2021e; Huang et al., 2021).
This study suggests that efficient strategies of vaccination have to be rapid and responsive in the initial phase of COVID-19 pandemic wave for reducing the negative impact of novel viral agent in society. These results here can help policymakers to design satisfying goals to cope with current pandemic applying effective vaccination strategies to prevent and/or cope with future outbreaks of the COVID-19 and similar epidemics. The most important policy implications of findings here are that, though high vaccination efforts of most advanced countries worldwide, the theoretical threshold for vanquishing COVID-19 seems to be out of reach at global level (in the medium run) because of the inequality in the distribution of vaccines, the uncertainty if and how new vaccines prevent or not the transmission, the duration of immunity in vaccinated people, hesitancy of people to vaccination and new variants that modify the herd-immunity equation and transmission dynamics (cf., Aschwanden, 2020, 2021). In fact, on November 2021 about 52% of the world population has received at least one dose of a COVID-19 vaccine and in poor countries a mere 5% of people have received at least one dose (Our World in Data, 2021).
Although this study has provided interesting results, that are of course tentative, it has several limitations. First, a limitation of the study is the lack of data about doses administered and total vaccinations in manifold countries, mainly in the spring season of the year 2021, also for the difficulty of production and distribution of COVID-19 vaccines worldwide and transmission of data and information. Second, not all the possible confounding factors that affect the efficacy of vaccination are taken into consideration (such as, length of lockdown, mitigation measures, etc.) and in future these factors deserve to be controlled for supporting results here. Third, the lack of integration of data with age of vaccinated people (the priority given in many countries to elderly subjects, with a more compromised immune system) may have influenced the results of infected individuals and deaths across countries. In addition, structure of population and characteristics of patients (e.g., ethnicity, age, sex, and comorbidities) may vary between countries making comparative analysis in some cases a problematic approach (Angelopoulos et al., 2020; Coccia, 2018; WHO, 2020). Fourth, country-specific health norms may affect the gather and transmission of data, such that unreported confirmed cases and doses of vaccines in manifold countries may be present in database under study here. Antony et al. (2020) also argue that the similarity of symptoms between COVID-19 and influenza can have generated under-reported data across countries. Finally, the estimated relationships in this study focus on variables in specific months (based on recent data available) but an extension of period under study is needed in future development of the research here. Thus, generalizing the results of this research should be done with caution. Future research should consider new data, when available, and when possible, to examine also other variables between countries to explain dynamic relationships under study over time and space. Despite these limitations, the results presented here suggest the critical aspect of the relationship between the evolution of pandemic wave and timing of the vaccination rollout to achieve the goal of optimal threshold of vaccination at national (and global) level for reducing negative effects of pandemic crisis in society. However, there is need for much more detailed research in these topics and this study encourages further investigations for supporting optimal strategies of vaccination, using lessons learned of COVID-19, also considering the temporal interaction between the evolution of pandemic wave and vaccination plan. To conclude, different factors between countries that are not only parameters related to medicine but also to other sciences can improve the preparedness of countries for an effective and a rapid roll out of vaccination to control negative impact of pandemic crisis on public health, economy and society.
Author's contributions
Single author, Mario Coccia.
Declaration of competing interest
The author, Mario Coccia, declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
The World Health Organization (WHO) considers the following variants of concern: Beta, Gamma, Delta and Delta Plus; Variants of interest (Lambda and Mu) and manifold variants under monitoring (ECDC, 2021).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.112314.
Appendix A. List of countries N = 192
| Countries 1-46 | Countries 47-92 | Countries 93-138 | Countries 139-184 | Countries 185-192 |
|---|---|---|---|---|
| Afghanistan | Danimarca | Kuwait | Qatar | Uruguay |
| Albania | Diamond Princess | Laos | Regno Unito | Uzbekistan |
| Algeria | Dominica | Lesotho | Repubblica Ceca | Vanuatu |
| Andorra | Ecuador | Lettonia | Repubblica Centrafricana | Venezuela |
| Angola | Egitto | Libano | Repubblica democratica del Congo | Vietnam |
| Antigua e Barbuda | El Salvador | Liberia | Repubblica Dominicana | Yemen |
| Arabia Saudita | United Arab Emirates | Libia | Romania | Zambia |
| Argentina | Eritrea | Liechtenstein | Ruanda | Zimbabwe |
| Armenia | Estonia | Lituania | Russia | |
| Australia | Etiopia | Lussemburgo | Saint Kitts and Nevis | |
| Austria | Figi | Macedonia del Nord | Saint Lucia | TOTAL = 192 |
| Azerbaigian | Filippine | Madagascar | Saint Vincent e Grenadine | |
| Bahamas | Finlandia | Malawi | Samoa | |
| Bahrein | Francia | Maldive | San Marino | |
| Bangladesh | Gabon | Malesia | Sao Tome and Principe | |
| Barbados | Gambia | Mali | Senegal | |
| Belgio | Georgia | Malta | Serbia | |
| Belize | Germania | Marocco | Seychelles | |
| Benin | Ghana | Mauritania | Sierra Leone | |
| Bhutan | Giamaica | Mauritius | Singapore | |
| Bielorussia | Giappone | Messico | Siria | |
| Bolivia | Gibuti | Micronesia | Slovacchia | |
| Bosnia ed Erzegovina | Giordania | Moldavia | Slovenia | |
| Botswana | Grecia | Monaco | Somalia | |
| Brasile | Grenada | Mongolia | Spain | |
| Brunei | Guatemala | Montenegro | Sri Lanka | |
| Bulgaria | Guinea | Mozambico | United States of America | |
| Burkina Faso | Guinea Equatoriale | MS Zaandam | Sudafrica | |
| Burundi | Guinea-Bissau | Myanmar | Sudan | |
| Cambogia | Guyana | Namibia | Sudan del Sud | |
| Camerun | Haiti | Nepal | Suriname | |
| Canada | Honduras | Nicaragua | Sweden | |
| Capo Verde | India | Niger | Svizzera | |
| Ciad | Indonesia | Nigeria | Swaziland | |
| Cile | Iran | Norvegia | Tagikistan | |
| Cina | Iraq | Nuova Zelanda | Taiwan* | |
| Cipro | Irlanda | Oman | Tanzania | |
| Città del Vaticano | Islanda | Paesi Bassi | Thailandia | |
| Colombia | Isole Marshall | Pakistan | Timor Est | |
| Comore | Isole Salomone | Palestina | Togo | |
| Congo | Israel | Panama | Trinidad e Tobago | |
| Corea del Sud | Italy | Papua Nuova Guinea | Tunisia | |
| Costa d'Avorio | Kazakistan | Paraguay | Turchia | |
| Costa Rica | Kenya | Perù | Ucraina | |
| Croazia | Kirghizistan | Polonia | Uganda | |
| Cuba | Kosovo | Portugal | Ungheria |
Appendix. ASupplementary data
The following is the Supplementary data to this article:
References
- Abbasi J. COVID-19 and mRNA vaccines-first large test for a new approach. J. Am. Med. Assoc. 2020;324(12):1125–1127. doi: 10.1001/jama.2020.16866. [DOI] [PubMed] [Google Scholar]
- Abuza Z. The Diplomat; 2020. Explaining Successful (And Unsuccessful) COVID-19 Responses in Southeast Asia.https://thediplomat.com/2020/04/explaining-successful-and-unsuccessfulcovid-19-responses-in-southeast-asia/ Retrieved. [Google Scholar]
- Akamatsu T., Nagae T., Osawa M., Satsukawa K., Sakai T., Mizutani D. Model-based analysis on social acceptability and feasibility of a focused protection strategy against the COVID-19 pandemic. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-81630-9. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldila D., Samiadji B.M., Simorangkir G.M., Khosnaw S.H.A., Shahzad M. Impact of early detection and vaccination strategy in COVID-19 eradication program in Jakarta, Indonesia. BMC Res. Notes. 2021;14(1):132. doi: 10.1186/s13104-021-05540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U., Cabreros C., Mal J., Ballesteros F., Jr., Sillanpää M., Tripathi V., Bontempi E. Novel coronavirus disease 2019 (COVID-19) pandemic: from transmission to control with an interdisciplinary vision. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.M., Vegvari C., Truscott J., Collyer B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet (London, Engl.) 2020;396(10263):1614–1616. doi: 10.1016/S0140-6736(20)32318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelopoulos A.N., Pathak R., Varma R., Jordan M.I. On identifying and mitigating bias in the estimation of the COVID-19 case fatality rate. Harv. Data Sci. Revi. 2020 doi: 10.1162/99608f92.f01ee285. [DOI] [Google Scholar]
- Anser M.K., Yousaf Z., Khan M.A., Voo X.H., Nassani A.A., Alotaibi S.M., Abro M., Zaman K. Air Quality, Atmosphere, & Health. Advance online publication; 2020. The impacts of COVID-19 measures on global environment and fertility rate: double coincidence; pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony S.J., Almaghlouth N.K., Heydemann E.L. Are coinfections with COVID-19 and influenza low or underreported? An observational study examining current published literature including three new unpublished cases. J. Med. Virol. 2020;92(11):2489–2497. doi: 10.1002/jmv.26167. [DOI] [PubMed] [Google Scholar]
- Ari-Veikko Anttiroiko. Successful government responses to the pandemic: contextualizing national and urban responses to the COVID-19 outbreak in East and west. International Journal of E-Planning Research (IJEPR), IGI Global. 2021;10(2):1–17. [Google Scholar]
- Ardito L., Coccia M., Messeni Petruzzelli A. Technological exaptation and crisis management: evidence from COVID-19 outbreaks. R D Manag. 2021;51(4):381–392. doi: 10.1111/radm.12455. Special Issue: Providing solutions in emergencies: R&D and innovation management during Covid‐19 Part‐2, September 2021. [DOI] [Google Scholar]
- Aschwanden C. The false promise of herd immunity for COVID-19. Nature. Nov. 2020;587(7832):26–28. doi: 10.1038/d41586-020-02948-4.PMID:33087872. [DOI] [PubMed] [Google Scholar]
- Aschwanden C. Five reasons why COVID herd immunity is probably impossible. Nature. 2021;591(7851):520–522. doi: 10.1038/d41586-021-00728-2. [DOI] [PubMed] [Google Scholar]
- Bontempi E., Coccia M. International trade as critical parameter of COVID-19 spread that outclasses demographic, economic, environmental, and pollution factors. Environ. Res. 2021;201 doi: 10.1016/j.envres.2021.111514. Article number 111514, PII S0013-9351(21)00808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Coccia M., Vergalli S., Zanoletti A. Can commercial trade represent the main indicator of the COVID-19 diffusion due to human-to-human interactions? A comparative analysis between Italy, France, and Spain. Environ. Res. 2021;201 doi: 10.1016/j.envres.2021.111529. Article number 111529, PII S0013-9351(21)00823-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Vergalli S., Squazzoni F. Understanding COVID-19 diffusion requires an interdisciplinary, multi-dimensional approach. Environ. Res. 2020;188(2020) doi: 10.1016/j.envres.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss L.F., Prete C.A., Jr., Abrahim C., Mendrone A., Jr., Salomon T., de Almeida-Neto C., França R., Belotti M.C., Carvalho M., Costa A.G., Crispim M., Ferreira S.C., Fraiji N.A., Gurzenda S., Whittaker C., Kamaura L.T., Takecian P.L., da Silva Peixoto P., Oikawa M.K., Nishiya A.S., Sabino E.C. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science. 2021;371(6526):288–292. doi: 10.1126/science.abe9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun W.S., Heo S.W., Jo G., Kim J.W., Kim S., Lee S., Park H.E., Baek J.H. Is coronavirus disease (COVID-19) seasonal? A critical analysis of empirical and epidemiological studies at global and local scales. Environ. Res. 2021;196 doi: 10.1016/j.envres.2021.110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E. Fast-spreading COVID variant can elude immune responses. Nature. 2021;589(7843):500–501. doi: 10.1038/d41586-021-00121-z. [DOI] [PubMed] [Google Scholar]
- CBC . 2021. Politics, Stretch Interval between COVID-19 Vaccine Doses up to 4 Months, National Advisory Committee Recommends.https://www.cbc.ca/news/politics/naci-interval-advice-change-four-months-1.5934563#:∼:text=2048-,Canada's%20National%20Advisory%20Committee%20on%20Immunization%20(NACI)%20now%20says%20the,number%20of%20Canadians%20being%20vaccinated Accessed. [Google Scholar]
- CDC . 2021. Centers for Disease Control and Prevention, Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States.https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html Accessed. [Google Scholar]
- Coccia Mario. Spatial relation between geo-climate zones and technological outputs to explain the evolution of technology. Int. J. Transitions and Innovation Systems. 2015;4(1–2):5–21. doi: 10.1504/IJTIS.2015.074642. [DOI] [Google Scholar]
- Coccia M. Disruptive firms and industrial change. J. Econ. Soc. Thought. 2017;4(4):437–450. doi: 10.1453/jest.v4i4.1511. [DOI] [Google Scholar]
- Coccia M. Varieties of capitalism's theory of innovation and a conceptual integration with leadership-oriented executives: the relation between typologies of executive, technological and socioeconomic performances. Int. J. Public Sect. Perform. Manag. 2017;3(2):148–168. doi: 10.1504/IJPSPM.2017.084672. [DOI] [Google Scholar]
- Coccia Mario. New directions in measurement of economic growth, development and under development. J. Econ. Polit. Econ. 2017;4(4):382–395. doi: 10.1453/jepe.v4i4.1533. [DOI] [Google Scholar]
- Coccia Mario. Sources of disruptive technologies for industrial change. L’industria –rivista di economia e politica industriale. 2017;38(1):97–120. doi: 10.1430/87140. [DOI] [Google Scholar]
- Coccia M. An introduction to the methods of inquiry in social sciences. J. Soc. Adm. Sci. 2018;5(2):116–126. doi: 10.1453/jsas.v5i2.1651. [DOI] [Google Scholar]
- Coccia M. An introduction to the theories of institutional change. J. Econ. Librar. 2018;5(4):337–344. doi: 10.1453/jel.v5i4.1788. [DOI] [Google Scholar]
- Coccia M. Why do nations produce science advances and new technology? Technol. Soc. 2019;59:1–9. doi: 10.1016/j.techsoc.2019.03.007. November, 101124. [DOI] [Google Scholar]
- Coccia M. An index to quantify environmental risk of exposure to future epidemics of the COVID-19 and similar viral agents: theory and Practice. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729(138474) doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. How (Un)sustainable environments are related to the diffusion of COVID-19: the relation between coronavirus disease 2019, air pollution, wind resource and energy. Sustainability. 2020;12 doi: 10.3390/su12229709. [DOI] [Google Scholar]
- Coccia Mario. How does science advance? Theories of the evolution of science. J. Econ. Soc. Thought. 2020;7(3):153–180. doi: 10.1453/jest.v7i3.2111. [DOI] [Google Scholar]
- Coccia M. Effects of the spread of COVID-19 on public health of polluted cities: results of the first wave for explaining the dejà vu in the second wave of COVID-19 pandemic and epidemics of future vital agents. Environ. Sci. Pollut. Control Ser. 2021 doi: 10.1007/s11356-020-11662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Pandemic prevention: lessons from COVID-19. Encyclopedia. 2021;1:433–444. doi: 10.3390/encyclopedia1020036. https://www.mdpi.com/journal/encyclopedia MDPI, Basel, Switzerland, Encyclopedia of COVID-19 ISSN 2673-8392, open access journal. [DOI] [Google Scholar]
- Coccia M. How do low wind speeds and high levels of air pollution support the spread of COVID-19? Atmos. Pollut. Res. 2021;12(1):437–445. doi: 10.1016/j.apr.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. The relation between length of lockdown, numbers of infected people and deaths of COVID-19, and economic growth of countries: lessons learned to cope with future pandemics similar to Covid-19. Sci. Total Environ. 2021 doi: 10.1016/j.scitotenv.2021.145801. [DOI] [Google Scholar]
- Coccia M. The impact of first and second wave of the COVID-19 pandemic: comparative analysis to support control measures to cope with negative effects of future infectious diseases in society. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.111099. June, Article number. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. High health expenditures and low exposure of population to air pollution as critical factors that can reduce fatality rate in COVID-19 pandemic crisis: a global analysis. Environ. Res. 2021;199 doi: 10.1016/j.envres.2021.111339. Article number. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. In: Global Encyclopedia of Public Administration, Public Policy, and Governance. Farazmand A., editor. Springer Nature Switzerland AG, Springer; Cham: 2021. Comparative critical decisions in management. [DOI] [Google Scholar]
- Coccia M. In: Nezameddin Faghih, Ali Hussein Samadi., editors. Springer Nature Switzerland AG; 2021. How a good governance of institutions can reduce poverty and inequality in society? pp. 65–94. (Legal-Economic Institutions, Entrepreneurship, and Management, Perspectives on the Dynamics of Institutional Change from Emerging Markets). 978-3-030-60978-8_4. [DOI] [Google Scholar]
- Coccia Mario. Evolution and structure of research fields driven by crises and environmental threats: the COVID-19 research. Scientometrics. 2021 doi: 10.1007/s11192-021-04172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. The effects of atmospheric stability with low wind speed and of air pollution on the accelerated transmission dynamics of COVID-19. Int. J. Environ. Stud. 2021;78(1):1–27. doi: 10.1080/00207233.2020.1802937. [DOI] [Google Scholar]
- Coccia Mario. Effects of human progress driven by technological change on physical and mental health. Stud. Soc. 2021;(2):113–132. doi: 10.26350/000309_000116. [DOI] [Google Scholar]
- Coccia M. Preparedness of countries to face covid-19 pandemic crisis: strategic positioning and underlying structural factors to support strategies of prevention of pandemic threats. Environ. Res. 2022;203(111678) doi: 10.1016/j.envres.2021.111678. ISSN 0013-9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia Mario. Socioeconomic Dynamics of the COVID-19 Crisis, Global, Regional, and Local Perspectives. 1st. Springer International Publishing; 2022. The spread of the novel Coronavirus disease 2019 in polluted cities: Lessons learned from environmental and demographic factors for prevention of pandemic diseases.https://www.springer.com/gp/book/9783030899950 [DOI] [Google Scholar]
- Coccia M., Bellitto M. Human progress and its socioeconomic effects in society. J. Econ. Soc. Thought. 2018;5(2):160–178. doi: 10.1453/jest.v5i2.1649. [DOI] [Google Scholar]
- Coccia M., Benati I. Rewards in public administration: a proposed classification. J. Soc. Adm. Sci. 2018;5(2):68–80. doi: 10.1453/jsas.v5i2.1648. [DOI] [Google Scholar]
- Cylus J., Pantel D., van Ginneken E. Who should be vaccinated first? Comparing vaccine prioritization strategies in Israel and European countries using the Covid-19 Health System Response Monitor. Isr. J. Health Pol. Res. 2021;10(1):16. doi: 10.1186/s13584-021-00453-1. https://doi.org/10.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashtbali M., Mirzaie M. A compartmental model that predicts the effect of social distancing and vaccination on controlling COVID-19. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-86873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vlas S.J., Coffeng L.E. Achieving herd immunity against COVID-19 at the country level by the exit strategy of a phased lift of control. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-83492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRoo S., Pudalov N.J., Fu L.Y. Planning for a COVID-19 vaccination program. J. Am. Med. Assoc. 2020;323(24):2458–2459. doi: 10.1001/jama.2020.8711. [DOI] [PubMed] [Google Scholar]
- Dooling K., McClung N., Chamberland M., et al. The Advisory Committee on Immunization Practices' interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1857–1859. doi: 10.15585/mmwr.mm6949e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC . European Centre for Disease Prevention and Control; 2021. SARS-CoV-2 Variants of Concern as of 3 June 2021.https://www.ecdc.europa.eu/en/covid-19/variants-concern [Google Scholar]
- Engelbrecht F.A., Scholes R.J. vol. 12. One health; Amsterdam, Netherlands: 2021. (Test for Covid-19 Seasonality and the Risk of Second Waves). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethgen O., Rémy V., Wargo K. Vaccination budget in Europe: an update. Hum. Vaccines Immunother. 2018;14(12):2911–2915. doi: 10.1080/21645515.2018.1504528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faes C., Abrams S., Van Beckhoven D., Meyfroidt G., Vlieghe E., Hens N., Belgian Collaborative Group on COVID-19 Hospital Surveillance Time between symptom onset, hospitalisation and recovery or death: statistical analysis of Belgian COVID-19 patients. Int. J. Environ. Res. Publ. Health. 2020;17 doi: 10.3390/ijerph17207560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanet A., Cauchemez S. COVID-19 herd immunity: where are we? Nature reviews. Immunology. 2020;20(10):583–584. doi: 10.1038/s41577-020-00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen L., Zhang Y., Foged C., Thakur A. The long road toward COVID-19 herd immunity: vaccine platform technologies and mass immunization strategies. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed G.L. Actionable lessons for the US COVID vaccine program. Isr. J. Health Pol. Res. 2021;10(1):14. doi: 10.1186/s13584-021-00452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAVI . 2021. THE FOUR MAIN TYPES OF COVID-19 VACCINE.https://www.gavi.org/vaccineswork/there-are-four-types-covid-19-vaccines-heres-how-they-work accessed. [Google Scholar]
- Harrison E.A., Wu J.W. Vaccine confidence in the time of COVID-19. Eur. J. Epidemiol. 2020;35(4):325–330. doi: 10.1007/s10654-020-00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton P.M. The covid-19 vaccine-development multiverse. N. Engl. J. Med. 2020;383(20):1986–1988. doi: 10.1056/NEJMe2025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Liu Xiaoyue, Zhang Li, Zhao Yingjie, Wang Danfeng, Gao Jinfeng, Lian Xinbo, Liu Chuwei. The oscillation-outbreaks characteristic of the COVID-19 pandemic. Natl. Sci. Rev. 2021;8(n. 8) doi: 10.1093/nsr/nwab100. nwab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins Center for System Science and Engineering . 2021. Coronavirus COVID-19 Global Cases.https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 [Google Scholar]
- Jones D., Helmreich S. A history of herd immunity. Lancet (London, Engl.) 2020;396(10254):810–811. doi: 10.1016/S0140-6736(20)31924-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge H.H.P., Nitzan D., Azzopardi-Muscat N. COVID-19: reflecting on experience and anticipating the next steps. A perspective from the WHO Regional Office for Europe. Eurohealth. 2020;26(2) [Google Scholar]
- Komaroff A. Why are mRNA vaccines so exciting? Harvard Health Blog. 2020. https://www.health.harvard.edu/blog/why-are-mrna-vaccines-so-exciting-2020121021599 Posted December 10, 2020, 2:30 pm, Updated December 18, 2020, 7:52 pm.
- Kwok K.O., Lai F., Wei W.I., Wong S., Tang J. Herd immunity - estimating the level required to halt the COVID-19 epidemics in affected countries. J. Infect. 2020;80(6):e32–e33. doi: 10.1016/j.jinf.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau H., Khosrawipour T., Kocbach P., Ichii H., Bania J., Khosrawipour V. Evaluating the massive underreporting and undertesting of COVID-19 cases in multiple global epicenters. Pulmonology. 2021;27(2):110–115. doi: 10.1016/j.pulmoe.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Huang J., Li C., Zhao Y., Wang D., Huang Z., Yang K. The role of seasonality in the spread of COVID-19 pandemic. Environ. Res. 2021;195 doi: 10.1016/j.envres.2021.110874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. Are COVID vaccination programmes working? Scientists seek first clues. Nature. 2021;589(7843):504–505. doi: 10.1038/d41586-021-00140-w. [DOI] [PubMed] [Google Scholar]
- MAYO CLINIC Different types of COVID-19 vaccines: how they work. 2021. https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/different-types-of-covid-19-vaccines/art-20506465 accessed.
- McKee M., Rajan S. What can we learn from Israel's rapid roll out of COVID 19 vaccination? Isr. J. Health Pol. Res. 2021;10(1):5. doi: 10.1186/s13584-021-00441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academy of Medicine . Harnessing Lessons from the Efforts Mitigating the COVID-19 Pandemic. 2021. Advancing pandemic and seasonal influenza vaccine preparedness and response.https://nam.edu/programs/advancing-pandemic-and-seasonal-influenza-vaccine-preparedness-and-response-a-global-initiative/?utm_source=NASEM+News+and+Publications&utm_campaign=6c16b60cb9-What%27s_New_2021_03_08&utm_medium=email&utm_term=0_96101de015-6c16b60cb9-102245345&goal=0_96101de015-6c16b60cb9-102245345&mc_cid=6c16b60cb9&mc_eid=b005b4da57 accessed. [Google Scholar]
- Oliver S., Gargano J., Marin M., et al. The advisory committee on immunization practices' interim recommendation for use of pfizer-BioNTech COVID-19 vaccine — United States, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Our World in Data . 2021. Coronavirus (COVID-19) Vaccinations - Statistics and Research - Our World in Data.https://ourworldindata.org/covid-vaccinations Accessed. [Google Scholar]
- Prieto Curiel R., González Ramírez H. Vaccination strategies against COVID-19 and the diffusion of anti-vaccination views. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-85555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph H.E., Barreiro L.B. Herd immunity: understanding COVID-19. Immunity. 2020;52:737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwan E.M. COVID-19 pandemic and vaccination build herd immunity. Eur. Rev. Med. Pharmacol. Sci. 2021;25(2):577–579. doi: 10.26355/eurrev_202101_24613. [DOI] [PubMed] [Google Scholar]
- Ritchie H., Mathieu E., Rodés-Guirao L., Appel C., Giattino C., Ortiz-Ospina E., Hasell J., Macdonald B., Beltekian D., Roser M. Coronavirus pandemic (COVID-19) 2020. https://ourworldindata.org/coronavirus Published online at OurWorldInData.org. Retrieved from.
- Ritchie H., Ortiz-Ospina E., Beltekian D., Mathieu E., Hasel J., Macdonald B., Giattino C., Roser M. 2020. Policy Responses to the Coronavirus Pandemic. Our World in Data, Statistics and Research.https://ourworldindata.org/policy-responses-covid Retrieved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B., Waitzberg R., Israeli A. Israel's rapid rollout of vaccinations for COVID-19. Isr. J. Health Pol. Res. 2021;(1):6. doi: 10.1186/s13584-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman H., Shilo S., Meir T., et al. COVID-19 dynamics after a national immunization program in Israel. Nat. Med. 2021 doi: 10.1038/s41591-021-01337-2. 2021. [DOI] [PubMed] [Google Scholar]
- Sagan A., Thomas S., McKee M., Karanikolos M., Azzopardi-Muscat N., de la Mata I., Figueras J. COVID-19 and health systems resilience: lessons going forwards. Eurohealth. 2020;26(2) [Google Scholar]
- Seligman B., Ferranna M., Bloom D.E. Social determinants of mortality from COVID-19: a simulation study using NHANES. PLoS Med. 2021;18(1) doi: 10.1371/journal.pmed.1003490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim F. Early Covid-19 vaccination rollout: a commentary from England. Isr. J. Health Pol. Res. 2021;10(1):18. doi: 10.1186/s13584-021-00451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services . 2021. Public Health Emergency-Executive Summary.https://www.phe.gov/Preparedness/planning/nivms/Pages/executive-summary.aspx (accessed March 2021) [Google Scholar]
- Vignesh R., Shankar E.M., Velu V., Thyagarajan S.P. Is herd immunity against SARS-CoV-2 a silver lining? Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.586781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M., Filippini T., Rothman K.J., Di Federico S., Orsini N. SARS-CoV-2 infection incidence during the first and second COVID-19 waves in Italy. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker C., Kamaura L.T., Takecian P.L., da Silva Peixoto P., Oikawa M.K., Nishiya A.S., Sabino E.C. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science. 2021;371(6526):288–292. doi: 10.1126/science.abe9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Estimating Mortality from COVID-19, Scientific Brief.https://www.who.int/news-room/commentaries/detail/estimating-mortality-from-covid-19 Accessed. [Google Scholar]
- Williams G.A., Ulla Díez S.M., Figueras J., Lessof S. Translating evidence into policy during the covid-19 pandemic: bridging science and policy (and politics) Eurohealth. 2020;26(2) [Google Scholar]
- Wilson N., Kvalsvig A., Barnard L., et al. Case-fatality risk estimates for COVID-19 calculated by using a lag time for fatality. Emerg. Infect. Dis. 2020;26(6):1339–1441. doi: 10.3201/eid2606.200320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zhou X., Qiu Y., Song Y., Feng F., Feng J., et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.