Abstract
Stem cells, which could be developed as starting or raw materials for cell therapy, hold tremendous promise for regenerative medicine. However, despite multiple fundamental and clinical studies, clinical translation of stem cells remains in the early stages. In contrast to traditional chemical drugs, cellular products are complex, and efficacy can be altered by culture conditions, suboptimal cell culture techniques, and prolonged passage such that translation of stem cells from bench to bedside involves not only scientific exploration but also normative issues. Establishing an integrated system of standards to support stem cell applications has great significance in efficient clinical translation. In recent years, regulators and the scientific community have recognized gaps in standardization and have begun to develop standards to support stem cell research and clinical translation. Here, we discuss the development of these standards, which support the translation of stem cell products into clinical therapy, and explore ongoing work to define current stem cell guidelines and standards. We also introduce general aspects of stem cell therapy and current international consensus on human pluripotent stem cells, discuss standardization of clinical‐grade stem cells, and propose a framework for establishing stem cell standards. Finally, we review ongoing development of international and Chinese standards supporting stem cell therapy.
Keywords: cell therapy, clinical translation, standard, stem cell
Stem cell standard system. According to the requirements of stem cell preparation and evaluation, the standard system for stem cell clinical applications could include standards that cover the process management, quality of stem cell products, and the analytical method used for evaluation.
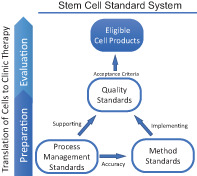
Significance statement.
Developments in stem cell research are advancing rapidly; nevertheless, the clinical translation of stem cell research remains quite limited. In contrast to translational chemical drugs, cellular products are complex in nature and can be altered by suboptimal cell culture techniques, prolonged passage, or changes in culture conditions. Therefore, the translation of stem cell research from bench to bedside involves not only scientific exploration, but also normative issues. In this review, the authors provide a discussion on the development of standards and propose establishing an integrated standard system for stem cell clinical applications that could include standards to cover the process management, the quality of stem cell products, and the analytical method used for evaluation.
1. INTRODUCTION
Stem cells, which are capable of self‐renewal and differentiating into one or more different types of specialized cells, could be developed as the starting or raw materials for cell therapy. Based on differentiation potential, stem cells can be classified as totipotent, pluripotent, multipotent, oligopotent, or unipotent. Totipotent stem cells (eg, zygotes) can differentiate into all possible cell types as well as extra‐embryonic cells (eg, placenta and yolk sac). Pluripotent stem cells, such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), can differentiate into all cells types in the body, whereas multipotent stem cells, such as hematopoietic stem cells and neural stem cells, can differentiate into only a limited number of cell types in a particular lineage. Oligopotent stem cells (eg, myeloid stem cells) have further restrictions in potency and differentiate into closely‐related cell types. Unipotent stem cells such as muscle stem cells, can self‐renew and differentiate into a single cell type. According to incomplete statistics, there are approved stem cell products on the market in the US, Europe, Japan, Korea, Canada, Australia, and India. Representative stem cell products are listed in Table 1.
TABLE 1.
Representative approved stem cell products on the market
| Region | Trade name | Cell type | Manufacturer | Indications |
|---|---|---|---|---|
| United States | ALLOCORD | HPC cord blood | SSM Cardinal Glennon Children's Medical Center | Hematopoietic and immunologic reconstitution |
| United States | Ducord | HPC cord blood | Duke University School of Medicine | Hematopoietic and immunologic reconstitution |
| United States | Clevecord | HPC cord blood | Cleveland Cord Blood Center | Hematopoietic and immunologic reconstitution |
| United States | Hemacord | HPC cord blood | New York Blood Center, Inc | Hematopoietic and immunologic reconstitution |
| Europe | Alofisel | Allogeneic MSC extracted from adipose tissue | Takeda | Complex anal fistulas in adults with Crohn's disease |
| Europe | Zynteglo | Genetically modified autologous CD34+ cell enriched population that contains HSC | Bluebird bio (Netherlands) B.V. | Beta‐thalassemia |
| Europe | Strimvelis | Autologous CD34+ enriched cell fraction contains genetically modified CD34+ cells | Orchard Therapeutics (Netherlands) BV | Severe combined immunodeficiency |
| Japan | Temcell | Allogeneic MSC from BM | JCR Pharmaceuticals Co. Ltd | Acute GVHD |
| Japan | Stemirac | Autologous MSC from BM | Nipro Corporation | Spinal cord injury |
| South Korea | NeuroNATA‐R | Autologous MSC from BM | CORESTEM | ALS |
| South Korea | Cupistem | Autologous adipose‐derived MSC | Anterogen | Crohn's disease complicated with anal fistul |
| South Korea | Cartistem | Allogeneic derived MSC from UCB | Medipost | Damaged cartilage |
| South Korea | Hearticellgram‐AMI | Autologous MSC from BM | Pharmicell | Aute myocardial infarction |
| Canada/New Zealand | Prochymal (Remestemcel‐L) | Human MSC | Osiris | Acute GVHD in pediatric patients |
| Australia | MPC | Mesenchymal precursor cell | Mesoblast | Repair and regeneration of damaged tissue |
| India | Stempeucel | Autologous MSC from BM | Stempeutics | CLI |
Abbreviations: ALS, amyotrophic lateral sclerosis; BM, bone marrow; CLI, critical limb ischemia; GVHD, graft‐vs‐host disease; HPC, hematopoietic progenitor cell; HSC, hematopoietic stem cells; MSC, mesenchymal stem cells; UCB, umbilical cord blood.
Using the search term “stem cells” returned 8544 clinical trials that were registered in the National Institutes of Health (NIH) clinical trial website through early June 2021 (ClinicalTrials.gov). These included 4167 and 1283 studies involving hematopoietic and mesenchymal stem cells, respectively. Another 69 studies focused on functional cells derived from ESCs, and 104 studies examined functional cells derived iPSCs.
The safety and efficacy of a new treatment are essential in clinical trials. Although some trials reported exciting positive results, 1 , 2 , 3 , 4 , 5 there remains a lot to be learned about how stem cells and their derivatives can be manufactured and applied safely in disease therapy and restore function. 6 Some clinical trials were unsuccessful due to safety concerns or inefficacy. For example, a 9‐year‐old boy was diagnosed with a glioneuronal neoplasm after undergoing transplantation with neural stem cells derived from two donors. 7 In another study, patients lost vision after intravitreal injection of autologous stem cells to treat age‐related macular degeneration. 8 In contrast to traditional chemical drugs, cellular products are complex and can be impacted by suboptimal handling or processing. 9 , 10 As such, translation of stem cells from bench to bedside involves not only scientific exploration but normative issues as well. Establishing an integrated system of standards will facilitate efficient clinical translation of stem cell‐based therapies. Standards represent a level of quality and attainment, and are typically defined by collaborative groups comprising individuals with subject matter expertise and knowledge of the needs of the organizations they represent, such as manufacturers, sellers, buyers, customers, trade associations, users, or regulators. Scientists, physicians, regulators, funders, entrepreneurs, and others who are involved in stem cell clinical translation are encouraged to collaborate on the timely development of standards for stem cell research and translation. To address issues associated with gaps in standardization, regulators and scientific societies have begun to convene experts from various fields related to stem cell therapy applications to develop standards for stem cell clinical translation. 11 , 12 In this review, we discuss the development of stem cell standards based on current consensus and recent progress in stem cell standardization.
2. INTERNATIONAL CONSENSUS ON HUMAN PLURIPOTENT STEM CELLS
The emerging demands of stem cell research and therapeutics necessitate the availability of abundant stem cell resources. Pluripotent stem cells could serve as an unlimited donor source for various types of cells and tissues that could be used for cell therapy for devastating and intractable diseases, such as spinal cord injury, macular degeneration, and neurodegenerative disorders, and hold enormous promise for regenerative medicine. When human ESC lines were first successfully cultured in the late 1990s, stem cell research raised both promise for regenerative medicine and pressing ethical and regulatory questions on how to advance such research in a responsible way. Countries around the world subsequently developed national laws, polices, and guidelines to guide human ESC research within country‐specific boundaries that are ethically and socially acceptable. 13 These laws, polices, and guidelines, unsurprisingly, varied among countries and created an international “patchwork” that in subsequent years was exploited by businesses offering direct‐to‐consumer stem cell interventions. 14
To address this challenge, the International Society for Stem Cell Research (ISSCR), the largest professional organization of stem cell researchers from around the world, issued Guidelines for the Conduct of Human Embryonic Stem Cell Research in 2006 15 and Guidelines for the Clinical Translation of Stem Cells in 2008. 16 These guidelines were reviewed and updated in 2016 according to the remarkable advances in the fundamental knowledge and clinical application of stem cell science. The 2016 Guidelines for Stem Cell Research and Clinical Translation 17 extended the scope to encompass institutional oversight, government regulation, and public communications surrounding basic and translational stem cell research. In May 2021, the ISSCR released and updated Guidelines for Stem Cell Research and Clinical Translation. This update reflects emerging advances including stem cell‐based embryo models, human embryo research, chimeras, organoids, and genome editing. 18 , 19 The ethical principles outlined in the guidelines include integrity of the research enterprise, primacy of patient/participant welfare, respect for patients and research subjects, transparency, and social and distributive justice. The guidelines recommend that research related to human embryos (eg, preimplantation stages of human development, in vitro human embryo culture, derivation of new embryo‐derived cells or cell lines, integrated stem cell‐based embryo models, and production of human gametes in vitro that are tested by fertilization or involve creation of embryos) shall be subject to a specialized oversight process to evaluate the unique aspects of the science and associated ethical issues. The guidelines outlined three categories to specify the types of research involving human embryos and related stem cell research that should be subject to review: Category 1: 1A, exempt from review and 1B, reportable to an oversight process, but normally exempt from review; Category 2: requires review; Category 3: 3A, research activities currently not permitted and 3B, prohibited research activities (see Section 2.2 of the 2021 guidelines for detailed information). The 2021 guidelines include an important update wherein the 14‐day rule stating: “In vitro culture of any intact human preimplantation embryo or organized embryo‐like cellular structure with human organismal potential, regardless of derivation method, beyond 14 days or formation of the primitive streak, whichever occurs first.”, was removed from Category 3, prohibited activities. Considering the advancing technology in human embryo culture, and the potential beneficial knowledge that promotes human health and well‐being, the 2021 guidelines softened the restriction and encouraged public discussion about allowing such research to be conducted.
3. MANUFACTURE OF CLINICAL GRADE CELLS
Through mid‐June 2021, 799 human ESC lines and 2744 human iPSC lines from 42 countries and regions were registered at the website http://hPSCreg.eu: the human pluripotent stem cell registry database funded by European Union. This registry includes 1662 cell lines from the United Kingdom, 943 cell lines from the US, 88 cell lines from Japan, and 565 cell lines from China. However, many of these cell lines are suitable only for research use due to the source of embryonic material, derivation process and subsequent handling procedures. 20 , 21 , 22 For example, most of the human ESC lines collected by the National Institutes of Health (NIH) were deemed ineligible for future use as therapeutic products because their derivation processes did not follow “Tissue Donor Guidance.” 22
According to current national and international regulation policies, most countries require rigorous donor screening and good manufacturing practice (GMP) for the generation of clinical grade human pluripotent stem cells. 23 , 24 GMP is the quality assurance that covers both manufacturing and final product testing to ensure that medicinal products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the product specification. According to GMP, general measures shall be defined to ensure that processes necessary for production and testing are clearly defined, validated, reviewed, and documented, and that the personnel, premises and materials are suitable for production of pharmaceuticals and biologicals.
Transfer of cell production to GMP standards involves three important aspects: (a) validated standard operating procedures (SOPs) for the entire process including cell establishment, culturing, passaging, freezing, storage, and transport; (b) release of criteria for the cells that are produced; and (c) description of quality control methodology.
In the past decade, scientists devised extensive standard operational procedures for establishing clinical‐grade human pluripotent stem cell lines. 23 , 25 , 26 , 27 , 28 According to these studies, these general recommendations specify that (a) donor eligibility screening is conducted and informed consent is obtained; (b) completely xenofree reagents are used; (c) pluripotency is characterized; and (d) stability and biosafety are tested.
Donor eligibility requires screening for risk factors associated with infection and communicable disease. For example, in the United States, the Food and Drug Administration (FDA) “Tissue Donor Guidance (2007)” requires the donor to be tested and demonstrated to be negative/nonreactive for the following infectious antigens/disease agents: HIV types 1 and 2; hepatitis B virus, hepatitis C virus; human transmissible spongiform encephalopathy (TSE), including Creutzfeldt‐Jakob Disease (CJD) (prion diseases); Treponema pallidum (syphilis); Human T‐lymphotropic virus (HTLV), types I and II; Chlamydia trachomatis; and Neisseria gonorrhoeae before donation.
Ancillary materials are materials that come into contact with the cellular therapeutic product during the manufacturing process, but are not intended to be in the final product. These materials can have a chemical or biological origin such as sera, media, growth factors, and monoclonal antibodies. Ancillary materials used in the culture process will greatly affect the safety and quality of the cells. Most countries advocate use of Xeno‐free culture media, feeder cells or a feeder‐free matrix in all cell operating processes to decrease the risk of infections by animal microbes and posttransplantation immune rejection due to immune responses to animal proteins. 29 , 30
Characterization of pluripotency is usually carried out by testing the following cell features: expression of pluripotent specific genes such as Oct4, Nanog, Sox2, Rex1 and the PSC surface antigens SSEA4, TRA‐1‐60, TRA‐1‐81; alkaline phosphatase (AP) activity; random or direct differentiation of cell types in the three germ layers in vitro and ability to form teratomas containing tissues of the three germ layers.
Genetic changes are reported to occur in cultured human pluripotent stem cells lines 31 , 32 , 33 that may cause deleterious effects including loss of functional characteristics and transformation into a tumorigenic state. 34 , 35 Karyological analyses are usually used to evaluate genome integrity. There a number of new techniques for these analyses including comparative genome hybridization (CGH) microarray and SNP microarrays. Whole genome sequencing can be used to characterize the genome and detect small genetic changes (eg, mutation of proto‐oncogenes such as p53, raf, mos, ras, src, abl, fes, sis, erbB, fms, myc, myn, fos) for assessment of tumorigenesis risk.
To further ensure the safety of cells used in clinical settings, testing to detect various pathogens such as bacteria, fungi, mycoplasma, virus and endotoxin are needed. 36 In addition, validation of the pluripotency and biosafety of clinical human pluripotent stem cells can help support clinical applications of the cells for regulatory documentation. 25
To provide cost‐effective treatments that are affordable for public health systems, storage of sufficient numbers of clinical‐grade human pluripotent stem cell lines in a stem cell bank for HLA matching is needed to meet the demands of cell therapies in the future. Biobanking involves acquisitioning and storage, together with activities related to collection, preparation, preservation, testing, analyzing, and distributing defined biological material as well as related information and data. The International Stem Cell Banking Initiative (ISCBI) aims to harmonize global stem cell banking and to facilitate best practices in stem cell research and clinical cell delivery. The ISCBI has published guidelines for the development of pluripotent stem cell seed stocks for clinical applications. These guidelines consider governance and ethical factors, provenance and selection of donor tissue and safety assessment, characterization, regulation and quality assurance. 37
4. ESTABLISHING AN INTEGRATED SYSTEM OF STANDARDS FOR STEM CELL CLINICAL APPLICATIONS
Before beginning a clinical trial, the therapeutic cell product should undergo critically processes for preparation and evaluation. The preparation process usually starts with the donation intention obtained from the donor and encompasses informed consent and ethics review, collection of biological source material, establishment of cell lines, expansion, characterization, quality control, and final cell preparations. The preclinical evaluation could include indications screening, animal disease model, in vivo kinetics and evaluation of safety and viability (Figure 1). Manufacturers can have their own SOPs, which can help reduce the lot‐to‐lot variation and could be updated flexibly for progress and innovation, when appropriate. The standards on the guidance and requirements on management of process, such as personnel, facilities and environmental conditions for handling the stem cells, donor screening and informed consent, ethical review, ancillary materials, could be developed to supporting the optimization and validation of the SOPs for different manufacturers and different cell products. According to the requirements of stem cell preparation and evaluation, the standard system for stem cell clinical applications could include standards that cover the process management, quality of stem cell products and the analytical method used for evaluation (Figure 2).
FIGURE 1.
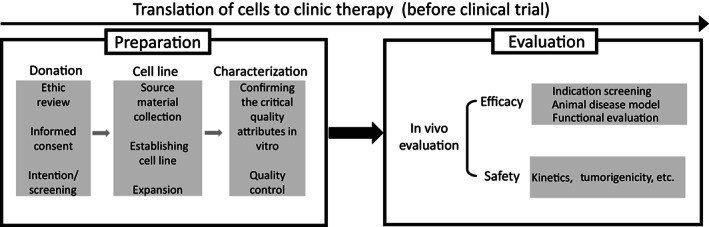
Preclinical processes for cell preparation and evaluation. The cell preparation process includes donation of biological source material, establishment of cell lines, and in vitro characterization of cellular preparations. Preclinical evaluation includes in vivo analysis of efficacy and safety
FIGURE 2.
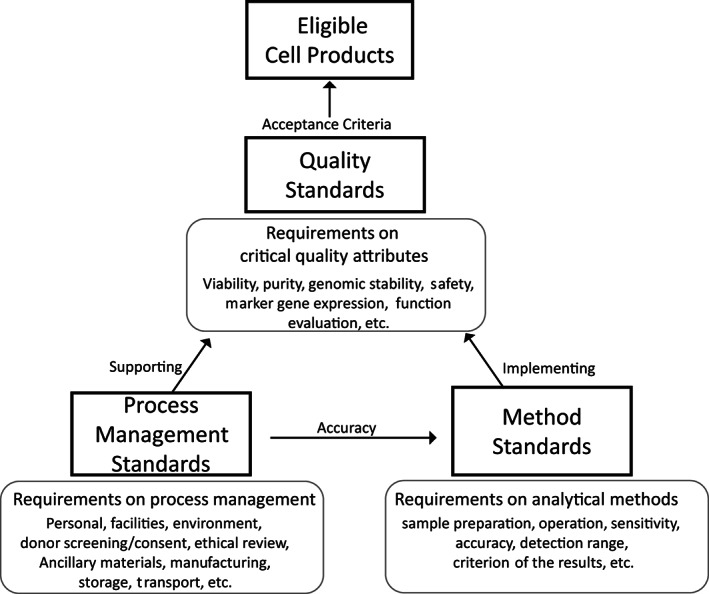
Stem cell standard systems. Quality standards specify the acceptance criteria of critical quality attributes of stem cell products, separating the acceptability from unacceptability. Method standards, which specify the requirements for evaluation of the critical quality attributes of cell products, facilitate implementation of quality standards. Process standards specify the management requirements supporting the whole operating process, thus ensuring the accuracy of testing results and quality of final cell products
The standards for quality should specify the requirements for every quality attribute that will influence effectiveness and safety during clinical therapy. These critical quality attributes could include, but are not limited to, cell viability, purity, genomic stability, sterility, expression of specific genes, functional evaluation in vitro and in vivo, and absence of infectious pathogens and tumorigenicity. Most importantly, the acceptance criteria that separate acceptability from unacceptability shall be established.
To implement quality standards, the analytical methods used to evaluate quality attributes should be developed. For the clinical translation of cell products, technology and testing method standards are needed that consider the requirements of a cell sample in addition to the sensitivity, accuracy, detection range and criterion of the results. 38
To ensure that the quality of final cell products meets eligibility standards and that testing results are accurate, standards covering the entire operating process should be established. These standards can include requirements for personnel, facilities and environmental conditions for handling the stem cells, donor screening and informed consent, ethical review, ancillary materials, storage and transport.
5. INTERNATIONAL STANDARDS SUPPORTING STEM CELL THERAPY
The International Organization for Standardization (ISO), founded in 1946, is an independent, nongovernmental organization made up of members from the national standards bodies of 165 countries. The ISO has developed nearly 24 000 international standards that cover almost all aspects of technology and manufacturing. A number of relative standards for therapeutic cells have been developed by the ISO and cover risk management, manufacturing equipment systems, ancillary materials, packaging, transportation, and analytical methods. The representative ISO standards regarding stem cell research and clinical translation are listed in Table 2.
TABLE 2.
Representative ISO standards regarding stem cell research and clinical translation
| Standards | Status |
|---|---|
| ISO 13022:2012 Medical Products Containing Viable Human Cells—Application of Risk Management and Requirements for Processing Practices | Published |
| ISO 20387:2018 Biotechnology‐Biobanking—General Requirements for Biobanking | Published |
| ISO 20391‐1:2018 Biotechnology—Cell Counting—Part 1: General Guidance on Cell Counting Methods | Published |
| ISO 20391‐2:2019 Biotechnology—Cell Counting—Part 2: Experimental Design and Statistical Analysis to Quantify Counting Method Performance | Published |
| ISO/TS 20399‐1:2018 Biotechnology—Ancillary Materials Present During the Production of Cellular Therapeutic Products—Part 1: General Requirements | Published |
| ISO/TS 20399–2:2018 Biotechnology—Ancillary Materials Present During the Production of Cellular Therapeutic Products—Part 2: Best Practice Guidance for Ancillary Material Suppliers | Published |
| ISO/TS 20399‐3:2018 Biotechnology—Ancillary Materials Present During the Production of Cellular Therapeutic Products—Part 3: Best Practice Guidance for Ancillary Material Users | Published |
| ISO 21709:2020 Biotechnology—Biobanking—Process and Quality Requirements for Establishment, Maintenance and Characterization of Mammalian Cell Lines | Published |
| ISO 21973:2020 Biotechnology—General Requirements for Transportation of Cells for Therapeutic Use | Published |
| ISO 21899:2020 Biotechnology—Biobanking—General Requirements for the Validation and Verification of Processing Methods for Biological Material in Biobanks | Published |
| ISO 23033:2021 Biotechnology—Analytical methods—General Requirements and Considerations for the Testing and Characterization of Cellular Therapeutic Products | Published |
| ISO/CD 20399 Biotechnology—Ancillary Materials Present During the Production of Cellular Therapeutic Products and Gene Therapy Products | Under development |
| ISO/CD 20404 Biotechnology—Bioprocessing—General Requirements for the Design of Packaging to Contain Cells for Therapeutic Use | Under development |
| ISO/DTS 22859 Biotechnology—Biobanking—Requirements for Human Mesenchymal Stromal Cells Derived from Umbilical Cord Tissue | Under development |
| ISO/CD 23511 Biotechnology—General Requirements for Cell Line Authentication | Under development |
| ISO/TS 23565 Biotechnology—Bioprocessing—General Requirements and Considerations for Equipment Systems Used in the Manufacturing of Cells for Therapeutic Use | Under development |
| ISO/DIS 24603 Biotechnology—Biobanking—Requirements for Human and Mouse Pluripotent Stem Cells | Under development |
| ISO/DIS 24651 Biotechnology—Biobanking—Requirements for Human Mesenchymal Stromal Cells Derived from Bone Marrow | Under development |
ISO 13022:2012 Medical Products Containing Viable Human Cells—Application of Risk Management and Requirements for Processing Practices, 39 which specifies requirements and guidance for processing practices and managing risk associated with viable cellular components of products to be regulated such as medicinal products, biologics, medical devices and active implantable medical devices, or combinations thereof.
In ISO/TS 20399:2018 Ancillary Materials Present During the Production of Cellular Therapeutic Products, 40 the standard is applicable to cellular therapeutic products, including gene therapy products in which cells form part of the final product. This standard has three parts. Part 1 specifies definitions and general requirements for ancillary materials used in processing of cellular therapeutic products. Part 2 provides guidance for ancillary material suppliers to maintain a high level of lot‐to‐lot consistency in terms of identity, purity, stability, biosafety and performance, as well as accompanying documentation. Part 3 provides guidance for ancillary material users.
ISO 21973:2020 Biotechnology—General Requirements for Transportation of Cells for Therapeutic Use 41 specifies general requirements and reviews points to consider for transportation of cells for therapeutic use, including development of a transportation plan that sets forth verification and validation procedures, communication between the client and the transportation service provider, and associated documentation.
International standards relative to analytical methods, as outlined in ISO 23033:2021 Biotechnology—Analytical Methods—General Guidelines for the Characterization and Testing of Cellular Therapeutic Products, ISO/CD 20404 Biotechnology—Bioprocessing—General Requirements for the Design of Packaging to Contain Cells for Therapeutic Use, ISO/TS 23565 Biotechnology—Bioprocessing—General Requirements and Considerations for Equipment Systems Used in Manufacturing of Cellular Therapeutic Products are presently under development in ISO/TC276 Biotechnology.
In addition to standards for cells having therapeutic use, the ISO also developed biobanking standards related to cells for research and development, including ISO 20387:2018 Biotechnology—Biobanking—General Requirements for Biobanking 42 ; ISO 21899:2020 Biotechnology—Biobanking—General Requirements for the Validation and Verification of Processing Methods for Biological Material in Biobanks 43 ; ISO 21709:2020 Biotechnology—Biobanking—Process and Quality Requirements for Establishment, Maintenance and Characterization of Mammalian Cell Lines 44 ; ISO/DIS 24603 Biotechnology—Biobanking—Requirements for Human and Mouse Pluripotent Stem Cells (under development); ISO/DTS 22859 Biotechnology—Biobanking—Requirements for Human Mesenchymal Stromal Cells Derived from Umbilical Cord Tissue (under development) and ISO/DIS 24651 Biotechnology—Biobanking—Requirements for Human Mesenchymal Stromal Cells Derived from Bone Marrow (under development).
Standards Coordinating Body (SCB) (https://www.standardscoordinatingbody.org/), a fully independent and functioning nonprofit organization, coordinates activities between the standards development organizations (SDOs) by serving as a liaison, recruiting subject matter experts to serve on working groups. To help inform the impact and urgency of developing new standards, SCB publishes stakeholder surveys regarding standard needs: Community Perspectives: Needed Standards in Regenerative Medicine Report in December 2020. For educating stakeholders on how standards are developed and used, SCB has assembled helpful introductory resources including Standards terminology, benefits of standards, case studies, organizations developing standards, guidance and regulations and community publications. SCB maintains an interactive database (https://portal.standardscoordinatingbody.org/) for searching current information on published and in‐development regenerative medicine standards, in addition to ISO standards, the representative of documents and standards developed by other SDOs are listed in Table 3.
TABLE 3.
The representative documents and standards developed by other standards development organizations in addition to ISO
| SDO | Documents/standards |
|---|---|
| ASTM International | ASTM F2997‐21 Standard Practice for Quantification of Calcium Deposits in Osteogenic Culture of Progenitor Cells Using Fluorescent Image Analysis |
| AABB | Standards for Cellular Therapy Services (10th Edition) |
| ASTM International | ASTM E3231‐19 Standard Guide for Cell Culture Growth Assessment of Single‐Use Material |
| ASTM International | ASTM F3163‐16 Standard Guide for Classification of Cellular and/or Tissue‐Based Products (CTPs) for Skin Wounds |
| ASTM International | ASTM F3368‐19 Standard Guide for Cell Potency Assays for Cell Therapy and Tissue Engineered Products |
| ATCC | ASN‐0002‐2011 Authentication of Human Cell Lines: Standardization of STR Profiling |
| BSI | PAS 93:2011 Characterization of Human Cells for Clinical Applications. Guide |
| EDQM | EP 2.7.23 Numeration of CD34/CD45 + Cells in Haematopoietic Products |
| EDQM | EP 2.7.29 Nucleated Cell Count and Viability |
| EDQM | EP 5.2.12 Raw Materials of Biological Origin for the Production of Cell‐Based and Gene Therapy Medicinal Products |
| FACT | FACT Common Standards for Cellular Therapies (Second Edition) |
| FACT | FACT Standards for Immune Effector Cells (First Edition, Version 1.1) |
| FACT | FACT‐JACIE International Standards for Hematopoietic Cellular Therapy Product Collection, Processing, & Administration (Eighth Edition) |
| ISCT | Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement |
| ISCT | Potency Assay Development for Cellular Therapy Products: An ISCT Review of the Requirements and Experiences in the Industry |
| ISCT | Stromal Cells from the Adipose Tissue‐Derived Stromal Vascular Fraction and Culture Expanded Adipose Tissue‐Derived Stromal/Stem Cells: A Joint Statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) |
| PDA | TR 81 Cell‐Based Therapy Control Strategy |
| PMDA | MHLW No. 1314 Guidelines on Ensuring the Quality and Safety of Products Derived from Processed Human Stem Cells |
| PMDA | MHLW No. 266 General Principles for the Handling and Use of Cellular/Tissue‐Based Products |
| PMDA | MHLW Notifications: Autologous No. 0208003, Allogenic No. 0912006 Guidelines on Ensuring Quality and Safety of Products Derived from Processed Cell/Tissue |
| USP | 41‐ NF 36 (1044) Cryopreservation of Cells |
| USP | USP <1043> Ancillary Materials for Cell, Gene, and Tissue‐Engineered Products |
| USP | USP <1046> Cell and Gene Therapies Products |
| USP | USP <127> Flow Cytometric Enumeration of CD34 + Cells |
Abbreviations: ATCC, American Type Culture Collection; BSI, British Standards Institution; EDQM, European Directorate for the Quality of Medicines; FACT, Foundation for the Accreditation of Cellular Therapy; ISCT, International Society for Cellular Therapy; PDA, Parenteral Drug Association; PMDA, Pharmaceuticals and Medical Devices Agency, Japan; USP, United States Pharmacopeia.
6. DEVELOPMENT OF REGULATIONS AND STANDARDS FOR STEM CELLS IN CHINA
In the past decades, health and drug regulatory agencies in China have developed a series of regulations and guidance documents on stem cell research. The earliest regulatory efforts were made in the early 1990s in response to the then latest advancements in cell and gene therapies. 45 Although most regulatory documents produced during that early stage are now outdated, the Ethical Guidelines for Human Embryo Cell Research 46 and Guidelines for the Research and Preparations Quality Control of Human Cell Therapy 47 remain in effect. Between 2009 and 2011, stem cell research and clinical practices in China were guided by Measures for the Administration of the Clinical Application of Medical Technologies. 48
Nonetheless, prior to 2012, without proper implementation and oversight, stem cell clinical research in China was sidetracked by commercial interests. Some rogue stem cell clinics ignored government regulations and warnings from the scientific community, and offered costly but largely ineffective treatments. 49 Other jurisdictions have also experienced similar problems and situations. 50 , 51 In this regard, there were growing doubts about the commitment of the Chinese research community and government to the development of safe and effective stem cell therapies for patients. In response to these concerns, the regulatory agencies in China made a considerable effort to develop effective regulations. In 2015, the National Health and Family Planning Commission (NHFPC) and the China Food and Drug Administration (CFDA) issued Management Methods for Clinical Research of Stem Cells (Trial) 52 and Guidelines for Quality Control of Stem Cell Preparation and Pre‐clinical Research (Trial). 53 In 2017, the CDFA issued Guideline for Research and Evaluation of Cell Therapy Products (Trial), 54 which normalized the research and evaluation of the cell therapy products in China and refined guidance for risk control as well as pharmaceutical, nonclinical and clinical research, and also supported the supervision and application of stem cell therapy.
To accompany governmental guidance and in response to the ISSCR proposal for developing standards, the stem cell research community and industry associations in China also developed specific standards to address quality control issues. The China Medicinal Biotechnology Association (CMBA) published Preparation of Stem Cell Preparation for Quality Management Discipline. 55 In 2016, the Chinese Society for Stem Cell Research (CSSCR) and the Chinese Society for Cell Biology (CSCB) founded the Stem Cell Standardization Working Group (now recognized as the Standard Committee of Chinese Society for Cell Biology), which aimed to develop standards for facilitating normalized practices in stem cell research and clinical translation and ensure that the cells have sufficient quality for research, development and clinical therapy. In 2017, the standards committee published the first stem cell standard in China, T/CSCB 0001 General Requirements for Stem Cell. 56 This document is applicable to stem cell research and production, and specifies stem cell classification, as well as requirements for ethical considerations, quality and quality control, detection control and waste disposal for stem cells. In 2019, the first specific standard for quality control of human ESCs, T/CSCB 0002 Human Embryonic Stem Cell, 57 was published. This standard specifies the technical requirements, test methods, test regulations, instructions for use, labeling requirements, packaging requirements, storage requirements and transportation requirements for human ESCs, which is applicable to the quality control for human ESCs. The English version of these two standards was published in 2020. 58 , 59 In 2021, the committee released another six specific standards for quality control of various cell types including T/CSCB 0003 Human Mesenchymal Stem Cell, 60 T/CSCB 0004 Human Hematopoietic Stem/Progenitor Cell, 61 T/CSCB 0005 Human Induced Pluripotent Stem Cell, 62 T/CSCB 0006 Human Retinal Pigment Epithelial Cell, 63 T/CSCB 0007 Human Cardiomyocytes 64 and T/CSCB 0008 Primary Human Hepatocyte. 65 These quality control standards specified criteria for critical quality attributes including cell morphology, chromosome karyotype, cell viability, cell authentication, cell markers, microorganisms and function. The English version of the six standards is going to be published. These standards developed by Committee of Chinese Society for Cell Biology are listed in Table 4. The committee continuously convenes proposals for standardizing stem cell research and clinical applications. A number of standards are currently under development with the committee and cover ethical guidance, analytical methods, and quality control standards.
TABLE 4.
Standards developed by Committee of Chinese Society for Cell Biology
| Published time | Standards | English version |
|---|---|---|
| 2017 | T/CSCB 0001 General Requirements for Stem Cell | Cell Proliferation, 2020 |
| 2019 | T/CSCB 0002 Human Embryonic Stem Cell | Cell Proliferation, 2021 |
| 2020 | T/CSCB 0003 Human Mesenchymal Stem Cell | To be published |
| 2020 | T/CSCB 0004 Human Hematopoietic Stem/Progenitor Cell | To be published |
| 2020 | T/CSCB 0005 Human Induced Pluripotent Stem Cell | To be published |
| 2020 | T/CSCB 0006 Human Retinal Pigment Epithelial Cell | To be published |
| 2020 | T/CSCB 0007 Human Cardiomyocytes | To be published |
| 2020 | T/CSCB 0008 Primary Human Hepatocyte | To be published |
7. CONCLUSION AND PERSPECTIVES
Clinical translation of stem cells requires collaborations among scientists, clinics, industry, regulators, and patients. Standardization can be used as a tool to create a level playing field that benefits everyone and supports efficient clinical translation in many ways. Standardized management for clinical cell procedures and quality control can help maintain sufficient quality and minimize lot‐to‐lot variations of stem cells products, which will assist in the scientific analysis of clinical trial outcomes, enable positive therapy effects reported in published studies to be reproducible in clinic, and protect the rights and interests of patients. The standards will also reduce the costs of uncertainty for private entities and facilitate independent review by regulators. 19 Stem cell research is advancing rapidly; standards should be set out and updated on time to keep pace with progress in regenerative medicine and biology.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
J.C., J.H., L.W.: conception and design, manuscript writing, and figure preparation; Y. Tan, Y. Tian, S.L.: data collection and figure preparation; A.M., B.F., J.D., P.Z., P.X., Y.Z.: critically read and revised the manuscript; T.C., Y.P., Q.Z., T.Z: conception and design, final approval of the manuscript.
ACKNOWLEDGMENTS
This work was supported by grants from the National Key R&D Program of China (2018YFA0108400, 2018YFE0204400) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16040501), the National Natural Science Foundation of China (81890990), and Chinese Academy of Medical Sciences (2021‐1‐I2M‐040).
Cao J, Hao J, Wang L, et al. Developing standards to support the clinical translation of stem cells. STEM CELLS Transl Med. 2021;10(S2):S85‐S95. 10.1002/sct3.13035
Jiani Cao, Jie Hao, and Lei Wang contributed equally to this study.
Funding information Strategic Priority Research Program of the Chinese Academy of Sciences, Grant/Award Number: XDA16040501; National Key R&D Program of China, Grant/Award Numbers: 2018YFE0204400, 2018YFA0108400
Contributor Information
Tao Cheng, Email: chengtao@ihcams.ac.cn.
Yaojin Peng, Email: yaojin.peng@ioz.ac.cn.
Qi Zhou, Email: qzhou@ioz.ac.cn.
Tongbiao Zhao, Email: tbzhao@ioz.ac.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell‐derived retinal pigment epithelium in patients with age‐related macular degeneration and Stargardt's macular dystrophy: follow‐up of two open‐label phase 1/2 studies. Lancet. 2015;385(9967):509‐516. [DOI] [PubMed] [Google Scholar]
- 2. Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379(9817):713‐720. [DOI] [PubMed] [Google Scholar]
- 3. Song WK, Park KM, Kim HJ, et al. Treatment of macular degeneration using embryonic stem cell‐derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Rep. 2015;4(5):860‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. da Cruz L, Fynes K, Georgiadis O, et al. Phase 1 clinical study of an embryonic stem cell‐derived retinal pigment epithelium patch in age‐related macular degeneration. Nat Biotechnol. 2018;36(4):328‐337. [DOI] [PubMed] [Google Scholar]
- 5. Kashani AH, Lebkowski JS, Rahhal FM, et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age‐related macular degeneration. Sci Transl Med. 2018;10(435):eaao4097. [DOI] [PubMed] [Google Scholar]
- 6. Daley GQ. Polar extremes in the clinical use of stem cells. New Engl J Med. 2017;376(11):1075‐1077. [DOI] [PubMed] [Google Scholar]
- 7. Amariglio N, Hirshberg A, Scheithauer BW, et al. Donor‐derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6(2):221‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuriyan AE, Albini TA, Townsend JH, et al. Vision loss after intravitreal injection of autologous “stem cells” for AMD. New Engl J Med. 2017;376(11):1047‐1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amps K, Andrews PW, Anyfantis G, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol. 2011;29(12):1132‐U1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kilpinen H, Goncalves A, Leha A, et al. Common genetic variation drives molecular heterogeneity in human iPSCs (vol 546, pg 370, 2017). Nature. 2017;546(7660):686‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarkar S, Lin‐Gibson S, Allocca CM, Henke D, Getz A. The critical role of standards in tissue engineering and regenerative medicine. In: Reis R, ed. Encyclopedia of Tissue Engineering and Regenerative Medicine. 1st ed. Cambridge, MA: Academic Press; 2019:1‐14. [Google Scholar]
- 12. Simon CGKL Jr. Role of standards for testing and performance requirements of biomaterials. In: Wagner W, Sakiyama‐Elbert S, Zhang G, Yaszemski M, eds. Biomaterials Science: An Introduction to Materials in Medicine. 4th ed. New York, NY: Elsevier; 2020:1475‐1483. [Google Scholar]
- 13. Isasi RM, Knoppers BM. Mind the gap: policy approaches to embryonic stem cell and cloning research in 50 countries. Eur J Health Law. 2006;13(1):9‐25. [DOI] [PubMed] [Google Scholar]
- 14. Caulfield T, Zarzeczny A, McCormick J, et al. The stem cell research environment: a patchwork of patchworks. Stem Cell Rev Rep. 2009;5(2):82‐88. [DOI] [PubMed] [Google Scholar]
- 15. ISSCR Guidelines for the conduct of human embryonic stem cell research. 2007. [DOI] [PubMed]
- 16. ISSCR Guidelines for the clinical translation of stem cells. 2008. [DOI] [PubMed]
- 17. ISSCR . Guidelines for Stem Cell Research and Clinical Translation ; 2016.
- 18. Skokie I. The ISSCR Releases Updated Guidelines for Stem Cell Research and Clinical Translation; 2021. https://www.isscr.org/news-publicationsss/isscr-news-articles/article-listing/2021/05/26/the-isscr-releases-updated-guidelines-for-stem-cell-research-and-clinical-translation. [DOI] [PMC free article] [PubMed]
- 19. ISSCR . ISSCR Guidelines for Stem Cell Research and Clinical Translation ; 2021. [DOI] [PMC free article] [PubMed]
- 20. Arabadjiev B, Petkova R, Chakarov S, Momchilova A, Pankov R. Do we need more human embryonic stem cell lines? Biotechnol Biotechnol Equip. 2010;24(3):1921‐1927. [Google Scholar]
- 21. Fraga AM, de Araujo ESS, Stabellini R, et al. A survey of parameters involved in the establishment of new lines of human embryonic stem cells. Stem Cell Rev Rep. 2011;7(4):775‐781. [DOI] [PubMed] [Google Scholar]
- 22. Jonlin EC. Differing standards for the NIH Stem Cell Registry and FDA approval render most federally funded hESC lines unsuitable for clinical use. Cell Stem Cell. 2014;14(2):139‐140. [DOI] [PubMed] [Google Scholar]
- 23. Unger C, Skottman H, Blomberg P, Sirac Dilber M, Hovatta O. Good manufacturing practice and clinical‐grade human embryonic stem cell lines. Hum Mol Genet. 2008;17(R1):R48‐R53. [DOI] [PubMed] [Google Scholar]
- 24. Andrews PW, Cavagnaro J, Deans R, et al. Harmonizing standards for producing clinical‐grade therapies from pluripotent stem cells (vol 32, pg 724, 2014). Nat Biotechnol. 2014;32(11):1166‐1166. [DOI] [PubMed] [Google Scholar]
- 25. Gu Q, Wang J, Wang L, et al. Accreditation of biosafe clinical‐grade human embryonic stem cells according to Chinese regulations. Stem Cell Rep. 2017;9(1):366‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ye J, Bates N, Soteriou D, et al. High quality clinical grade human embryonic stem cell lines derived from fresh discarded embryos. Stem Cell Res Ther. 2017;8(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sartipy P, Bjorquist P. Employment of the Triple Helix concept for development of regenerative medicine applications based on human pluripotent stem cells. Clin Transl Med. 2014;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carpenter MK, Rao MS. Concise review: making and using clinically compliant pluripotent stem cell lines. Stem Cell Transl Med. 2015;4(4):381‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11(2):228‐232. [DOI] [PubMed] [Google Scholar]
- 30. European Medicines Agency, CAT Secretariat & US Food and Drug Administration. Regen Med. 2011;6(6 suppl):90‐96. [DOI] [PubMed] [Google Scholar]
- 31. Martins‐Taylor K, Xu RH. Concise review: genomic stability of human induced pluripotent stem cells. Stem Cells. 2012;30(1):22‐27. [DOI] [PubMed] [Google Scholar]
- 32. Nguyen HT, Geens M, Spits C. Genetic and epigenetic instability in human pluripotent stem cells. Hum Reprod Update. 2013;19(2):187‐205. [DOI] [PubMed] [Google Scholar]
- 33. Ben‐David U, Arad G, Weissbein U, et al. Aneuploidy induces profound changes in gene expression, proliferation and tumorigenicity of human pluripotent stem cells. Nat Commun. 2014;5:ncomms5825. [DOI] [PubMed] [Google Scholar]
- 34. Sun Y, Yang YX, Zeng SC, Tan Y, Lu G, Lin G. Identification of proteins related to epigenetic regulation in the malignant transformation of aberrant Karyotypic human embryonic stem cells by quantitative proteomics. PLoS One. 2014;9(1):e85823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lund RJ, Emani MR, Barbaric I, et al. Karyotypically abnormal human ESCs are sensitive to HDAC inhibitors and show altered regulation of genes linked to cancers and neurological diseases [in English]. Stem Cell Res. 2013;11(3):1022‐1036. [DOI] [PubMed] [Google Scholar]
- 36. Devito L, Petrova A, Miere C, et al. Cost‐effective master cell bank validation of multiple clinical‐grade human pluripotent stem cell lines from a single donor. Stem Cells Translational Medicine. 2014;3(10):1116‐1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Andrews PW, Baker D, Benvinisty N, et al. Points to consider in the development of seed stocks of pluripotent stem cells for clinical applications: International Stem Cell Banking Initiative (ISCBI). Regen Med. 2015;10(2):1‐44. [DOI] [PubMed] [Google Scholar]
- 38. International Conference on Harmonisation . Validation of Analytical Procedures: Text and Methodology Q2(R1) . Current Step 4 Version; 2005.
- 39. ISO . 13022:2012 Medical Products Containing Viable Human Cells—Application of Risk Management and Requirements for Processing Practices ; 2012.
- 40. ISO . 20399:2018 Ancillary Materials Present During the Production of Cellular Therapeutic Products ; 2018.
- 41. ISO . 21973:2020 Biotechnology—General Requirements for Transportation of Cells for Therapeutic Use ; 2020.
- 42. ISO . 20387:2018 Biotechnology—Biobanking—General Requirements for Biobanking ; 2018.
- 43. ISO . 21899:2020 Biotechnology—Biobanking—General Requirements for the Validation and Verification of Processing Methods for Biological Material in Biobank ; 2020.
- 44. ISO . 21709:2020 Biotechnology—Biobanking—Process and Quality Requirements for Establishment, Maintenance and Characterization of Mammalian Cell Lines ; 2020.
- 45. Ministry of Health . Key Points of Quality Control in Clinical Research of Cell Therapy and Gene Therapy for Human [in Chinese]; 1993.
- 46. Ministry of Health, Ministry of Science and Technology . Ethical Guidelines for Human Embryonic Stem Cell Research [in Chinese]; 2003.
- 47. CFDA . Guidelines for the Research and Preparations Quality Control of Human Cell Therapy [in Chinese]; 2003.
- 48. Ministry of Health . Measures for the Administration of the Clinical Application of Medical Technologies [in Chinese]; 2009.
- 49. Cyranoski D. China's stem‐cell rules go unheeded. Nature. 2012;484(7393):149‐150. [DOI] [PubMed] [Google Scholar]
- 50. Cyranoski D. Strange lesions after stem‐cell therapy. Nature. 2010;465(7301):997‐997. [DOI] [PubMed] [Google Scholar]
- 51. Cyranoski D. Stem cells in Texas: cowboy culture. Nature. 2013;494(7436):166‐168. [DOI] [PubMed] [Google Scholar]
- 52. NHFPC, CFDA . Management Methods for Clinical Research of Stem Cells (Trial) [in Chinese]; 2015.
- 53. NHFPC, CFDA . Guidelines for Quality Control of Stem Cell Preparation and Pre‐Clinical Research (Trial) [in Chinese]; 2015.
- 54. CFDA . Guideline for Research and Evaluation of Cell Therapy Products (Trial) [in Chinese]; 2017.
- 55. CMBA . The Preparation of Stem Cell Preparation for Quality Management Discipline [in Chinese]; 2016.
- 56. CSCB . T/CSCB 0001 General Requirements for Stem Cell [in Chinese]; 2017.
- 57. CSCB . T/CSCB 0002 Human Embryonic Stem Cell [in Chinese]; 2019.
- 58. Hao J, Ma AJ, Wang L, et al. General requirements for stem cells. Cell Prolif. 2020;53(12):e12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hao J, Cao JN, Wang L, et al. Requirements for human embryonic stem cells. Cell Prolif. 2020;53(12):e12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. CSCB . T/CSCB 0003 Human Mesenchymal Stem Cell [in Chinese]; 2021.
- 61. CSCB . T/CSCB 0004 Human Hematopoietic Stem/Progenitor Cell [in Chinese]; 2021.
- 62. CSCB . T/CSCB 0005 Human Induced Pluripotent Stem Cell [in Chinese]; 2021.
- 63. CSCB . T/CSCB 0006 Human Retinal Pigment Epithelial Cell [in Chinese]; 2021.
- 64. CSCB . T/CSCB 0007 Human Cardiomyocytes [in Chinese]; 2021.
- 65. CSCB . T/CSCB 0008 Primary Human Hepatocyte [in Chinese]; 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


