Abstract
Umbilical cord blood transplantation (UCBT) has been performed in the clinic for over 30 years. The biological and immunological characteristics of umbilical cord blood (UCB) have been re‐recognized in recent years. UCB, previously considered medical waste, is rich in hematopoietic stem cells (HSCs), which are naïve and more energetic and more easily expanded than other stem cells. UCB has been identified as a reliable source of HSCs for allogeneic hematopoietic stem cell transplantation (allo‐HSCT). UCBT has several advantages over other methods, including no harm to mothers and donors, an off‐the‐shelf product for urgent use, less stringent HLA match, lower incidence and severity of chronic graft‐vs‐host disease (GVHD), and probably a stronger graft‐vs‐leukemia effect, especially for minimal residual disease‐positive patients before transplant. Recent studies have shown that the outcome of UCBT has been improved and is comparable to other types of allo‐HSCT. Currently, UCBT is widely used in malignant, nonmalignant, hematological, congenital and metabolic diseases. The number of UCB banks and transplantation procedures increased exponentially before 2013. However, the number of UCBTs increased steadily in Asia and China but decreased in the United States and Europe year‐on‐year from 2013 to 2019. In this review, we focus on the development of UCBT over the past 30 years, the challenges it faces and the strategies for future improvement, including increasing UCB numbers, cord blood unit selection, conditioning regimens and GVHD prophylaxis for UCBT, and management of complications of UCBT.
Keywords: graft‐vs‐host disease, graft‐vs‐leukemia, stem cell, transplantation, umbilical cord blood
Advantages and disadvantages of UCBT and improving strategies.

Significance statement.
This article focuses on the development of umbilical cord blood transplantation (UCBT) over the past 30 years, the challenges faced, and the strategies for future improvement. Optimal cord blood unit selection for transplantation is discussed. With the development of HLA matching, cord blood unit selection, modified conditioning regimens, and effective management of complications, UCBT has achieved comparable overall survival and better graft‐versus‐host disease‐free and relapse‐free survival than other allo‐HSCT types. Future directions should focus on innovative research on the basic biology of UCB stem cells, novel randomized controlled clinical trials, and perfect quality control of UCB banking, making UCBT more popular for more patients.
1. DEVELOPMENT OF UMBILICAL CORD BLOOD TRANSPLANTATION IN THE PAST 30 YEARS
Allogeneic hematopoietic stem cell transplantation (allo‐HSCT) remains an effective and curative therapy for malignant, nonmalignant, hematological, congenital, and metabolic diseases. 1 , 2 Unfortunately, fully matched related donors, which is preferred, are not always available for the majority (approximately 70%) of patients 3 and are even less available in China because of the once one‐child policy. Finding matched unrelated donors through the registry is also difficult and time consuming. Since the first successful umbilical cord blood transplantation (UCBT) was performed on a 5‐year‐old boy with Fanconi anemia in 1988 at Hospital Saint‐Louis in Paris, France, 4 umbilical cord blood (UCB) has become an available graft source of allo‐HSCT for over 30 years, with the advantages of rapid availability, no harm to mothers and donors, low immunogenicity, decreased chronic graft‐vs‐host disease (GVHD), and low relapse rate in minimal residual disease (MRD). 5 , 6
Unrelated donor cord blood transplantation (CBT) is an effective and reliable alternative to peripheral blood (PB) or bone marrow (BM) transplant and has emerged as a widely accepted treatment for a wide variety of hematologic diseases such as: acute lymphoblastic leukemia (ALL), 7 , 8 , 9 acute myeloid leukemia (AML), 10 , 11 , 12 myelodysplastic syndrome (MDS), 13 , 14 , 15 and aplastic anemia (AA) 16 , 17 , 18 (Table 1).
TABLE 1.
Umbilical cord blood transplantation for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), and aplastic anemia (AA)
| References | Diagnosis | Patients (n) | Graft" | Median age, years (range) | Neutrophil engraftment | Platelet engraftment (≥20 × 109/L) | Relapse | TRM | Grade II‐IV Acute GVHD | Chronic GVHD | Overall survival | Disease‐free survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matsumura et al 7 | ALL | 256 | Single unit | 40 (16‐74) | 78% at day 100 | 64% at day 100 | 43% at 2 years | 35% | 37% | 24% at 2 years | 42% at 2 years | 36% at 2 years |
| Page et al 8 | ALL | 640 | Single unit | 6.4 (0.5‐17.9) | / | / | 26% | / | 40.4% | 17% at 5 years | 51.3% at 5 years | 47.4% at 5 years |
| Piñana et al 9 | PH + ALL | 45 | Single unit | 31 (3–47) | 96% at 44 days | 73% at 183 days | 31% at 5 years | 31%(18%–45%) at 5 years | 31% | 53% (36‐70%) at 5‐years | 44% (28‐60%) at 5 years | 36% at 5 years |
| Sanz et al 10 | High‐risk AML | 49 | Single unit | 34 (16‐52) | 96% at 57 days | 73% at 250 days | 19% at 2 years | 2‐year NRM: 39% | 26% | 46% at 2 years | 37% at 4 years | |
| Baron et al 11 | AML | 1068 | Single unit: 567, double units: 501 | 45.5 (18‐73) | 77% | / | 32% at 2 years | 38% at 2 years | 31% | 42% at 2 years | 32% at 2 years | 30% at 2 years |
| Yanada et al 12 | AML | 1355 | Single unit | 52 (16‐85) | / | / | 18.2% at 3 years | 29.5% at 3 years | / | / | 55.1% at 3 years | / |
| Madureira et al 13 | MDS | 70 | Single unit | 6 (<1‐17) | 76% at day 60 | 57% at day 180 | 13 of 70 | 53% prior to 2001 (n = 30); 31% after 2001 (n = 40) | 30% | 23% at 3 years | 42% at 3 years | 39% at 3 years |
| Robin et al 14 | MDS | 129 | Single unit: 49, Double units: 80 | 57 (20‐72) | 78% | / | 30% | 42% at 2 years | 31% | 23% | 30% | 28% ± 4% |
| Gerds et al 15 | MDS | 176 | Single unit: 36, double units: 140 | 56 (18–73) | / | / | 32% at 3 years | 40% at 3 years | 38% at 3 years | 28% at 3 years | 31% at 3 years | 28% at 3 years |
| Kuwatsuka et al 16 | SAA | 69 | Single unit | 49 (17‐73) | 71% at day 42 | / | / | / | 32%, | 21% at 3 years | 69% at 3 years | / |
| de Latour et al 17 | SAA | 26 | Single unit: 16, double units: 10 | 16 (9‐23) | / | / | / | 11.5% at 1 year | 45.8% | 36% at 1 year | 88.5% at 1 year | / |
| Yoshimi et al 18 | SAA | 31 | Single unit | 27.9 (0.8‐72.7) | 54.8 | 72.2% | / | / | 17.1% | 19.7% | 41.1% at 2 years | / |
UCB, previously considered medical waste, was suggested as a potential source of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) by Hal Broxmeyer in a private meeting with the late Edward A. Boyse and Judith Bard in 1982. This conference led to the creation of a UCB company named Biocyte Corporation and a series of studies on the biology and cryopreservation of UCB cells. 19 , 20 These studies identified the possibility of using UCB as an available source of HSCs and HPCs, leading to the first HLA‐identical sibling UCBT 4 and subsequent UCBTs, including the first HLA‐identical sibling UCBT in a patient with juvenile chronic myelogenous leukemia (JCML) at Johns Hopkins University in 1992, 21 the first unrelated UCBTs in children reported by Joanne Kurtzberg et al in 1996 22 and the initial unrelated UCBT experience with adults in 1996. 23
The first public UCB bank was established at the New York Blood Center in 1993, 24 and the Eurocord Netcord network was created by Gluckman et al in 1997, 25 and there are currently more than 100 UCB banks in Asia, Europe, Oceania, North America, and South America. 26 According to the World Marrow Donor Association (WMDA), over 778 000 cord blood units are available worldwide to be used for any patient in need, and approximately 35 000 UCBTs have been performed up to the end of 2019. The number of UCB banks and transplantation increased exponentially before 2013. However, according to data from the WMDA and China Bone Marrow Transplantation Registry (CBMTR), with the widespread application of haploidentical transplants, the number of UCBTs decreased in the United States and Europe year‐on‐year from 2013 to 2019, although it increased steadily in Asia (from 1397 to 1645) and China (from 106 to 514) (Figure 1). Patients who undergo UCBT have had to face the challenges of delayed engraftment, risk of graft failure, increased transplant‐related mortality (TRM) and infection. Many strategies have been attempted to address these issues to further improve UCBT as a feasible and more attractive option for allo‐HSCT.
FIGURE 1.
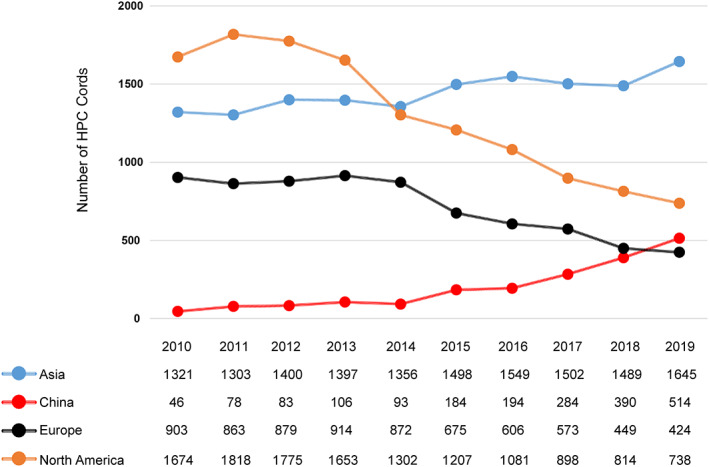
Shipments of HPC cords provided by the continents from WMDA and the number of UCBTs in China from CBMTR from 2010‐2019
2. ADVANTAGES OF UCBT
2.1. Biology of UCB
Research on the biology and cryopreservation of UCB cells showed that UCB from a single donor could be used as a source of autologous or major histocompatibility complex‐matched allogeneic transplantable hematopoietic repopulating cells. The process of cryopreservation of UCB cells should not require the need to discard any type of cells prior to freezing, and the cells should not be washed or otherwise handled after thawing, as all of these procedures would result in severe loss of HPCs. 19 The above‐mentioned process allows UCB units to be rapidly available for patients in urgent need of transplantation. In addition, a UCB unit could be collected at birth without any harm to the newborn or mother. These scientific findings paved the way for UCB as a potential source of transplantable HSCs/HPCs. Since then, our understanding of the biological characteristics of UCB has increased, emphasizing the advantages of UCBT.
UCB units usually contain one log less total nucleated cell (TNC) and CD34+ cells than a unit of bone marrow or peripheral blood, accompanied by delayed engraftment of neutrophils and platelets or higher incidence of graft failure. 27 , 28 Many studies have revealed the proportions of hematopoietic progenitor cells such as primitive HPCs and multipotent colony‐forming cells in UCB are significantly higher than those of BM CD34+ cells and peripheral blood stem cells. Moreover, UCB CD34+ progenitors have higher proliferation and multiple cell division potential. 29 , 30 , 31 Furthermore, the in vivo hematopoietic reconstitution capacity of UCB‐derived HSCs in a nonobese diabetic/severe combined immunodeficiency (NOD/SCID) repopulation assay is superior to that of BM CD34+ cells. 32 , 33 Several unique characteristics of UCB HSCs may lead to the above observations, including longer telomeres, a higher self‐renewal capacity due to overrepresentation of transcription factor such as NF‐kB, and autocrine production of certain cytokines such as granulocyte‐macrophage colony‐stimulating factor and IL‐3. 34 , 35
Furthermore, in vitro and in vivo studies showed that HSPCs derived from UCB have higher proliferation and expansion potential than their adult BM cells, which may be because UCB cells exit the G0/G1 phase of the cell cycle more rapidly than adult BM progenitors and have longer telomeres than BM cells. 36 It should be noted that in addition to being a rich source of HSCs and HPCs, UCB contains an abundance of B cells with immunoregulatory functions. In patients who underwent UCBT, the recovery frequencies and absolute numbers of IL‐10‐producing Bregs were higher than those of healthy donors or patients before transplant. The reconstituting Bregs showed a strong inhibitory effect against allogeneic CD4+ T cells in vitro but were deficient in patients with chronic GVHD. 37 IL‐10‐producing B cells may protect against chronic GVHD after UCBT. In addition, T cells from UCB mediated enhanced antitumor responses compared with peripheral blood (PB) T cells in a murine model of B‐cell lymphoma. The antitumor activity was correlated with increased tumor‐homing of CCR7high UCB CD8+ T cells and rapid gain of cytotoxic and T‐helper (Th) 1 function, 38 which may be related to the advantage of a lower relapse rate in MRD positive patients before UCBT.
As for Tregs, the expression of CD4+ CD25+ T cells and Foxp3 between UCB and APB remains controversial. 39 , 40 , 41 Many in vivo studies have demonstrated that donor or host Tregs are able to prevent GVHD in allogeneic transplantation mouse models. 42 , 43 , 44 The infusion of UCB Tregs in humans who received UCBT was safe and effective for reducing the incidence of GVHD. 45 , 46
2.2. Comparison of UCBT to other graft sources
A series of clinical cohort studies comparing UCBT to other graft sources have further confirmed the advantages of UCBT: a lower incidence of chronic GVHD and stronger graft‐vs‐leukemia (GVL) effects for MRD‐positive patients.
Chronic GVHD is usually accompanied by severe morbidity and impairment of quality of life (QoL). The Blood and Marrow Transplant Clinical Trials Network defined a novel composite GVHD‐free endpoint, namely, relapse‐free survival (GRFS), which represents a better QoL and ideal recovery after HCT. 47 UCBT has indicated comparable overall survival and a very low incidence of chronic GvHD with favorable GRFS vs matched related or unrelated transplantation. 48 , 49 , 50
In a retrospective study performed by our transplantation center, the First Affiliated Hospital of the University of Science and Technology of China (USTC), unrelated UCBT was compared with HLA‐matched sibling donor (MSD) transplants using a myeloablative regimen in AML patients. A total of 162 consecutive AML patients receiving a single unit of unrelated UCBT (n = 107) or MSD transplant (n = 55) were investigated. No differences were seen in grade II‐IV or III‐IV acute GVHD and TRM between the two transplant types. A lower incidence of chronic GVHD and extensive chronic GVHD, and a lower relapse rate and better GVHD‐free and relapse‐free survival (GRFS) were observed in the UCBT arm. 48 Another retrospective study reported by Sharma et al 49 compared outcomes among adult MSD transplants (n = 123) and adult patients undergoing double‐unit UCBT (dUCBT) (n = 190). Overall survival (OS) was comparable, and GRFS was significantly improved among UCBT patients (P = .0056), primarily because of decreased moderate to severe chronic GVHD following CBT (P < .0001).
When UCBT was compared with HLA‐matched or mismatched unrelated donor transplants in patients with acute leukemia or MDS using myeloablative conditioning, the relative risks of death and relapse appeared to vary according to the presence of MRD status before transplantation. Among patients with MRD, the probability of OS after UCBT was at least as favorable as that after an HLA‐matched unrelated donor transplant and was significantly higher than the probability of OS after an HLA‐mismatched unrelated donor transplant. Furthermore, the relapse rate was lower in the UCBT group than in the other groups. 5 In a multicenter retrospective study, 79 acute leukemia (AL) patients who underwent UCBT and 96 AL patients who underwent unrelated peripheral blood stem cell transplantation (UPBSCT) with myeloablative conditioning were compared. Acute GVHD, TRM, OS, and leukemia‐free survival (LFS) were similar between the two transplant types. Less chronic GVHD, less moderate and severe chronic GVHD and lower incidences of Epstein‐Barr virus viremia and posttransplantation lymphoproliferative disease were found in the UCBT group. UCBT recipients had higher Karnofsky performance scores for activity and 3‐year GRFS than the UPBSCT group. 50
Haploidentical donors are one of the three alternative donor options, whereas the other two options are UCB and mismatched unrelated donors. In 2014, the number of haploidentical transplants surpassed the total number of UCB transplants performed in the United States. This increasing trend has continued, with these transplants representing 21% of transplants, and the number of UCB transplants is nearing the number of MSD transplants, which represented 25% of allo‐HSCTs in the United States in 2018 (CIBMTR Summary Slides, 2019, available at https://www.cibmtr.org). A pilot study was conducted by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) to compare the results of two parallel multicenter phase 2 trials about reduced intensity conditioning (RIC) dUCBT (BMT CTN 0604) and HLA‐haploidentical‐related donor BM (haplo‐marrow) transplant (BMT CTN 0603) for patients with leukemia or lymphoma at 27 transplantation centers in the United States. 51 Lower TRM but a higher relapse rate was seen for haplo‐marrow transplant, which ultimately resulted in similar OS and progression‐free survival (PFS), 62% and 48%, respectively, after haplo‐marrow transplantation (n = 50), and 54% and 46%, respectively, after dUCBT (n = 50). These multicenter studies set the stage for the development of a multicenter randomized phase III clinical trial BMT CTN 1101. 52 No differences were seen in cumulative incidences of platelet recovery, grade II to IV and grade III to IV acute GVHD, chronic GVHD or the relapse rate. Although the results did not show a statistically significant difference in 2‐year PFS between the donor sources, delayed neutrophil recovery, increased TRM, and decreased OS were observed in the UCBT cohort. In a retrospective study from our transplant center and Peking University People's Hospital, the therapeutic effects of single UCBT and unmanipulated haplo‐HSCT in high‐risk ALL children were compared. 53 The incidences of grade II to IV and III to IV acute GVHD, chronic GVHD, and moderate and severe chronic GVHD were lower for UCBT patients with decreased DFS than patients who underwent haplo‐HSCT. Thus, UCBT is a reasonable option with these advantages, especially for those patients who do not have a donor available and are in urgent need of transplantation.
3. CURRENT CHALLENGES
Although UCBT is immediately available and associated with a lower incidence of chronic GVHD, limited numbers of total nucleated cells and CD34+ cell doses in UCB units are still the main deficiency, which result in delayed hematopoietic recovery and increased rates of graft failure, thus increasing the risks of infection and TRM. Moreover, antithymocyte globulin (ATG) is commonly used in UCB transplant recipients, especially in Europe. T‐cell depletion in vivo may reduce the risk of GVHD, which in turn increases graft failure and relapse of the primary disease. 6 Relapse remains the major cause of death after transplant. 26 To overcome these challenges, many investigators and clinicians have explored different ways to improve the efficacy of UCBT.
4. MAJOR STRATEGIES FOR IMPROVEMENT
4.1. Double‐unit UCBT
The first double‐unit UCBT (dUCBT) was performed in Europe in 1999. Both recipients had signs of donor engraftment but unfortunately died of relapse and hemorrhage 3 months post dUCBT (Eurocord, unpublished data, 2010). 54 In 2001, the first 2 units of UCB from male infant donors into a 53‐year‐old, 84‐kg woman with accelerated‐phase chronic myelogenous leukemia (CML) was performed by Barker et al, 55 and each unit contributed to hematopoiesis for at least 60 days after transplantation. Although this patient died of disseminated Aspergillus infection 68 days after transplantation, these findings prompted further investigation of UCBT from two partially HLA‐matched donors as a method of increasing cell dose, especially for adult recipients. Since then, dUCBT has become a strategy for patients with insufficient units. According to Eurocord, since 2005, the number of adult patients receiving dUCBT has exceeded that of adults with single‐unit UCBT (sUCBT). 56 Generally, only one unit can persist for a long time after dUCBT, which indicates that the 2 units might react against each other and have an impact on the efficacy of transplant. The effects of sUCBT and dUCBT were compared through several studies (Table 2). 57 , 58 , 59 , 60 , 61 , 62 An open‐label, phase 3, multicenter, randomized trial reported by Wagner et al 58 determined the effect of the graft composition (double‐unit vs single‐unit) on 1‐year survival among patients who received the same conditioning and GVHD prophylaxis regimen. The results showed that recipients of dUCBT had no engraftment or survival benefit compared with those receiving a sufficient dose of sUCBT. In addition, poorer platelet recovery and higher rates of grade III to IV acute and extensive chronic GVHD were observed after dUCBT. Michel et al 59 also found that the incidence of extensive chronic GVHD in dUCBT was higher than that in sUCBT. However, in MRD‐positive patients who had not received ATG during their conditioning regimen, the relapse rate was lower in the dUCBT group than that in the sUCBT group, leading to a higher 3‐year OS. We retrospectively analyzed 79 patients with hematological malignancies who received UCBT between November 2005 and December 2013 in our single transplant center. Patients who had dUCBT had a lower myeloid and platelet engraftment rate, higher TRM, and reduced OS, DFS and GRFS than patients who had sUCBT with a sufficient cell dose. 62
TABLE 2.
Comparison of transplant effects between single‐unit and double‐unit UCBT
| Ref. | Transplant type | Patient no., conditioning | Neutrophil engraftment rate | Median neutrophil engraftment time | Grade III to IV acute GVHD | Chronic GVHD | Relapse rate | TRM | OS | LFS |
|---|---|---|---|---|---|---|---|---|---|---|
| Kindwall‐Keller et al 57 | sUCBT | 27, RIC | 85% | 25 | 19.2% | 21.7% | 59.3% | 11.1% | 35.9% | 28.6% |
| dUCBT | 23, RIC | 91% | 23 | 17.4% | 26.3% | 30.4% | 17.4% | 39.1% | 39.1% | |
| P value | / | .99 | .99 | .87 | .73 | .045 | .48 | .86 | .71 | |
| Wagner et al 58 | sUCBT | 113, MAC | 89% | 21 | 13% | 9% a | 12% | 19% | 73% | 70% |
| dUCBT | 111, MAC | 88% | 23 | 23% | 15% a | 14% | 22% | 65% | 64% | |
| P value | / | .29 | >.05 | .02 | .05 a | .12 | .43 | .17 | .11 | |
| Michel et al 59 | sUCBT | 68, MAC | 92.6% | 24.8 | 25.0% | 14.7% a | 23.5% | 5.9% | 68.8% | 67.6% |
| dUCBT | 69, MAC | 94.2% | 23.5 | 18.8% | 31.9% a | 17.4% | 11.6% | 74.8% | 68.1% | |
| P value | / | >.05 | >.05 | .40 | .02 a | .31 | .25 | .56 | .74 | |
| Baron et al 60 | sUCBT | 172, RIC | 77% | 19 | 11% | 28% | 32% | 22% | 41% | 46% |
| dUCBT | 362, RIC | 83% | 24 | 13% | 36% | 35% | 29% | 51% | 36% | |
| P value | / | .40 | <.001 | .6 | .2 | .5 | .2 | .03 | .06 | |
| Balligand et al 61 | sUCBT | 56, MAC (MRD+) | N.R. | N.R. | N.R. | N.R. | 41.7% | 19% | 53.6% | 53% |
| dUCBT | 59, MAC (MRD+) | N.R. | N.R. | N.R. | N.R. | 10.5% | 22% | 82.6% | 82.6% | |
| P value | / | N.R. | N.R. | N.R. | N.R. | .025 | .43 | .031 | .028 | |
| Zheng et al 62 | sUCBT | 60, MAC | 96.7% | 19 | 12.1% | 24.4% | 11.7% | 33.3% | 56.7% | 55.0% |
| dUCBT | 37, MAC | 89.2% | 22 | 8.7% | 28.4% | 13.5% | 54.1% | 37.8% | 32.4% | |
| P value | / | .026 | .079 | .59 | .72 | .82 | .026 | .037 | .017 |
Extensive chronic GVHD.
Abbreviations: dUCBT, double‐unit UCBT; GVHD, graft‐vs‐host disease; LFS, leukemia free survival; MAC, myeloablative conditioning; MRD, minimal residual disease; N.R., not reported; OS, overall survival; RIC, reduced intensified conditioning; sUCBT, single‐unit UCBT; TRM, transplant‐related mortality; UCBT, umbilical cord blood transplantation.
4.2. Ex vivo expansion of UCB cells
Several investigators have explored a variety of approaches to expand functional UCB cells (HSCs and HPCs) in vitro, including recombinant hematopoietic cytokines, growth factors, stromal cells, and different small molecules. Recombinant hematopoietic cytokines were initially used to expand primitive hematopoietic cells from UCB, which was beneficial for self‐renewal. 63 , 64 Based on the favorable effect of cytokines on the ex vivo expansion of UCB, various growth factors, including FLT3 ligand, stem cell factor, erythropoietin, and thrombopoietin, were extensively tested. Although the number of HPCs increased significantly, no positive effects were observed in myeloid, erythroid, or platelet engraftment when UCB cells expanded with these growth factors were infused into patients. 65 , 66
Stromal cells were considered to be effective in the expansion of HSCs, since the maintenance of HSCs in vivo is closely related to special microenvironments, termed niches. 67 , 68 As a part of the hematopoietic microenvironment, mesenchymal stem cells (MSCs) can be isolated from a variety of fetal and adult tissues. 69 , 70 Research has shown that CB coculture with MSCs results in superior ex vivo expansion of total nucleated cells (TNCs) and HPCs. 71 A clinical trial to assess the safety and efficacy of transplantation of CB expanded with an MSC coculture strategy was conducted (Funded by the National Cancer Institute and others; ClinicalTrials.gov number, NCT00498316). The results demonstrated that this approach appeared to be safe and effective, significantly promoting neutrophil and platelet engraftment time from 24 and 49 days, respectively, in historical controls to 15 and 42 days, respectively, in the recipients of expanded CB (P < .001; P = .03). The 26‐day cumulative incidence of neutrophil engraftment was 88% with expansion vs 53% without expansion (P < .001); the 60‐day cumulative incidence of platelet engraftment was 71% and 31%, respectively (P < .001). 72
More recently, different small molecules, including but not limited to, diethylaminobenzaldehyde.
(DEAB), copper chelator (StemEx), Notch ligand, StemRegenin 1 (SR1), nicotinamide, and UM171, have been reported as agonists for experimental ex vivo expansion of human HSCs and HPCs. 73 , 74 , 75 , 76 , 77 , 78 Clinical trials of some small molecules have been reported and are summarized in Table 3. 72 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 Delaney et al 79 infused ex vivo expansion CB in the presence of Notch ligand Delta 1 in a clinical setting for stem cell transplantation, and the time to neutrophil recovery was substantially shortened to 16 days. The Nicord product, first used in the dUCBT setting with the expansion of a single CB unit before infusion, showed 13‐day neutrophil engraftment and 1‐year OS and PFS rates of 82% and 73%, respectively. 80 Then, a phase I/II clinical study of sUCBT expanded ex vivo in the presence of nicotinamide was performed, which shortening median neutrophil recovery to 11.5 days and median platelet recovery to 34 days. 81 This trial established the feasibility, safety, and efficacy of an ex vivo expanded UCB unit as a stand‐alone graft. SR1 and UM171 are effective amplification agents for HSCs and are also used as stand‐alone grafts. SR‐1 produced a 330‐fold increase in CB CD34+ cells and led to 100% engraftment at a median of 15 days for neutrophils and 49 days for platelets. 84 A recent phase I/II clinical study of single UM171‐expanded cord blood transplantation explored 100% engraftment, and was feasible, safe, and allowed for the use of small single cords without compromising engraftment. 85 Although these ex vivo expansion results are exciting, due to the limited sample size, there is still much work to be done in this area, and more mechanisms of HSC amplification need to be further explored.
TABLE 3.
Clinical trials for ex vivo manipulation of UCB to transplant
| Expansion approach | Clinical trial staging | Expanded TNC, Median (range), ×107/kg | Expanded CD34+, Median (range), ×105/kg | Neutrophil engraftment rate | Neutrophil engraftment time, median (range), days | Platelet engraftment time median (range), days | |
|---|---|---|---|---|---|---|---|
| Delaney et al 79 | Notch ligand | Phase I (n = 10) | 4.6 (0.6‐9.1) | 60 (9.3‐130) | N.R. | 16 (7‐34) | N.R. |
| Horwitz et al 80 | NiCord dUCBT | Phase I (n = 11) | 2.5 (1.7‐3.8) | 35 (9‐183) | 42d 90.9% | 13 (7‐26) | 33 (26‐49) |
| Horwitz et al 81 | NiCord sUCBT | Phase I/II (n = 36) | 4.9 (2.0‐13.3) | 63 (14‐149) | 42d 94% | 11.5 (9‐14) a | 34 (32‐42) a |
| de Lima et al 82 | Copper chelator (StemEx) | Phase I/II (n = 10) | 0.9 (0.1‐29.8) | 1.2 (0.16‐45.04) | 46d 90% | 30 (16‐46) | 48 (35‐105) |
| Stiff et al 83 | Copper chelator (StemEx) | N.R. (n = 101) | 2.2 (N.R.) | 9.26 (0.83‐117.56) | 91.9% | 21 (18.4‐23.5) a | 54 (43.3‐61.9) a |
| Wagner et al 84 | StemRegenin‐1 (SR1) | Phase I/II (n = 17) | 5 (1‐12) | 175 (14‐483) | 100% | 15 (6‐30) | 49 (28‐136) |
| Cohen et al 85 | UM171 | Phase I/II (n = 22) | N.R. | 28.75 (7.89‐54.58) | 100% | 18 (12.5‐20.0) b | 42 (35‐47) b |
| de Lima et al 72 | MSC | N.R. (n = 24) | 5.84 (0.03‐14.37) | 9.5 (16‐93.4) | 42d 96% | 15 (9‐42) | 42 (15‐62) |
| Mehta et al 86 | MPC | N.R. (n = 27) | 5.7 (1.35‐11.8) | 16 (0.4‐53) | 26d 78% | 12 (1‐28) | 31 (9‐52) |
95% confidence interval.
Interquartile range.
Abbreviations: dUCBT, double‐unit UCBT; MPC, mesenchymal precursor cell; MSC, mesenchymal stromal cell; N.R., not reported; sUCBT, single‐unit UCBT; TNC, total nucleated cell; UCB, umbilical cord blood.
4.3. Cord blood unit selection
Choosing the most suitable CB unit is the first step of successful UCBT. Optimal unit selection requires consideration of HLA match, unit quality and cell dose. Conventionally, HLA typing for UCBT relies on low‐resolution typing for HLA‐A and HLA‐B (antigen‐level) and allele‐level typing for DRB1 and does not consider HLA‐C locus matching. In 2011, Eapen et al 87 found that HLA‐C antigen matching should be included to minimize mortality risks for units that were matched at HLA‐A, HLA‐B, or HLA‐DRB1 or in the presence of a single locus mismatch at HLA‐A, HLA‐B, or HLA‐DRB1. Later reports support the importance of high‐resolution typing and the degree of HLA mismatch at HLA‐A, HLA‐B, HLA‐C, and HLA‐DRB1 when selecting UCB units for transplantation both for malignant and nonmalignant diseases. 88 , 89 Additionally, the role of donor‐specific anti‐HLA antibodies (DSA) and other immunogenetic factors, such as killer‐cell immunoglobulin‐like receptors (KIRs) and non‐inherited maternal antigen (NIMA) compatibility, are currently under investigation. 90 A retrospective analysis using the database of the Japan Society for Hematopoietic Cell Transplantation (JSHCT) showed that pretransplant DSA with a mean fluorescence intensity (MFI) ≥ 1000 was associated with an increased risk of graft failure (GF) in sUCBT. In an MD Anderson Cancer Center (MDACC) analysis of 110 sUCBT patients, patients homozygous for HLA‐C2 group alleles had a higher 1‐year relapse rate and worse survival than HLA‐C1/C1 or HLA‐C1/C2 (HLA‐C1/x) patients. 91 Patients lacking a KIR ligand of HLA group C1 or C2 had a better outcome after UCBT. 92 However, Tanaka et al 93 found no effects of KIR ligand incompatibility in the GVH direction on sUCBT outcomes for AL patients without ATG use. Different results indicate that the impact of KIR alloreactivity on UCBT outcomes may depend on the preconditioning regimen and GVHD prophylaxis. Some studies showed that HLA‐mismatched UCBT in which the mismatched antigen in the patient matched the NIMA of the UCB donor (NIMA‐matched transplantation) was associated with greater neutrophil recovery and better transplant outcomes. 94 , 95 However, since the frequency of NIMA matching is below 10%, whether this should be considered in CB selection needs to be further investigated.
In the setting of UCBT, non‐HLA factors are as critical as HLA matching in CB unit selection. Unit quality mainly depends on the qualification of UCB banks and is highly related to unit efficacy, including the viability and recovery of CD34+ cells after thawing. 96 Eurocord criteria recommend that cord blood units meet ≤2 HLA disparities and > 3 × 107 NC/kg or ≥ 2 × 105 CD34+ cells/kg before freezing in malignant diseases, and the cell dose should be increased to >3.5 × 107 NC/kg and ≤1 HLA disparities in nonmalignant diseases. 97 The UK consensus guidelines state different requirements for HLA matching and cell dose. 98 Less stringent criteria consisting of a TNC dose ≥2.0 × 107/kg and 4/6 or better matching for HLA‐A, HLA‐B, and HLA‐DR, all at the antigen level, are acceptable for the Japanese patient population. 12 Recent unrelated CB unit selection guidelines from the National Marrow Donor Program and the Center for International Blood and Marrow Transplant Research (NMDP/CIBMTR) recommend a minimum of 8 high‐resolution (HLA‐A, HLA‐B, HLA‐C, and HLA‐DRB1) for both patients and CB units, ≥ 4/6 HLA‐A and HLA‐B antigen, HLA‐DRB1 high‐resolution (traditional match), ≥ 4/8 high‐resolution match (some centers are investigating the use of 4/6 and 3/8 units if there is an adequate dose), TNC ≥2.5 × 107/kg, and CD34+ cells ≥1.5 × 105/kg (some centers recommend a higher CD34+ dose as minimum) in sUCBT. 99 Overall, suitable UCB unit selection should use the following principles: (a) optimal allele‐level HLA matching at HLA‐A, HLA‐B, HLA‐C, and HLA‐DRB1; DSA should be avoided; (b) adequate unit quality; and (c) minimum required TNC and CD34 cell doses.
4.4. Pitfalls of the ATG‐containing conditioning regimen in UCBT
ATG, with its well‐documented effect on T‐cell depletion, has been used to improve engraftment and reduce the risk of GVHD after allogeneic HSCT from related sibling, haplo‐identical and unrelated donors. However, the use of ATG as part of the conditioning regimen in UCBT is still under debate. Sanz et al 10 from Hospital Universitario La Fe in Spain routinely used ATG as part of the conditioning regimen prior to UCBT. This conditioning regimen consists of thiotepa, busulfan, cyclophosphamide and horse ATG (Lymphoglobuline, 15 mg/kg per day on days −5, −4, −3, and −2). The cumulative incidence of neutrophil and platelet engraftment was 96% and 73% at median times of 20 and 62 days, respectively. The cumulative incidences of grade II ~ IV and III ~ IV acute GVHD and extensive chronic GVHD were 26%, 15%, and 30%, respectively. LFS, NRM, and relapse at 2 years were 42%, 39%, and 19%, respectively. Patients transplanted in first complete remission (CR1) receiving TNC >2 × 107/kg had a 4‐year LFS of 75%.
Controversially, ATG has a long half‐life and contributes to viral reactivation and the development of lymphoproliferative disease. On the other hand, the CD8+ T‐cell dose influences neutrophil engraftment time when CD34+ cells are lower. In vivo depletion of donor graft‐facilitating CD8+ lymphocytes by ATG may affect CB engraftment in the setting of UCBT. Pascal et al 100 investigated the role of ATG in UCBT with RIC consisting of low‐dose total body irradiation (TBI), cyclophosphamide, and fludarabine (Cy/Flu/TBI 200). In multivariate analyses, the use of ATG was associated with a decreased incidence of acute GVHD (P < .0001), a higher incidence of NRM (P = .0009), and decreased OS (P = .003). Therefore, these results suggested that the use of ATG could be detrimental, especially if it was administered too close to the graft infusion in adults undergoing UCBT following the Cy/Flu/TBI 200 regimen. A retrospective analysis evaluated the effect of ATG on patient outcomes in 207 children with high‐risk or advanced hematological malignancies at our transplant center and the other seven child blood disease centers in China. The results demonstrated that patients who received conditioning that omitted ATG had a faster platelet recovery, a comparable GVHD and TRM, a significantly lower relapse risk, and improved long‐term survival compared with those patients who received ATG during conditioning. 101 A retrospective (development) and a prospective (validation) study in our single transplant center confirmed the superiority of modified myeloablative conditioning without ATG to myeloablative conditioning with ATG in UCBT for hematological malignancies. 102 The use of ATG in UCBT conditioning regimens remains controversial. A randomized study is required to determine whether omitting ATG confers a survival advantage for patients undergoing UCBT.
4.5. Pre‐engraftment syndrome
Pre‐engraftment syndrome (PES) was initially described as a pre‐engraftment immune reaction (PIR) in 2005 and was first proposed by Professor Young‐Ho Lee. 103 , 104 Although a uniform definition is lacking, PES has overlapping features with hyperacute GVHD and engraftment syndrome (ES), which are commonly characterized by noninfectious fever, erythematous rash, diarrhea, jaundice, and capillary leak syndrome (CLS), including noncardiogenic fluid retention or pulmonary manifestations such as tachypnea, hypoxemia, and pulmonary edema, before neutrophil engraftment. 103 , 104 , 105 The pathogenesis and severity classification are still not clear; PES may be caused by cytokine storms associated with toxicities of the conditioning regimen, GVHD prophylaxis drugs, DMSO, G‐CSF, or mismatched antigens by donor T cells. However, heterogeneity of the conditioning regimens and specific GVHD prophylaxis strategies may account for the different ranges (20‐78%) of the reported incidences of PES. 106 , 107 , 108 , 109
Generally, PES may lead to a higher incidence of grade II‐IV acute GVHD, 20 , 21 but data are conflicting as to whether PES may benefit cord blood engraftment and whether PES influences TRM, relapse and survival. 103 , 104 , 110 , 111 , 112 , 113 PES may be self‐limited and require no therapy in some mild patients, and most patients are responsive to methylprednisolone (MP). Tocilizumab‐targeted anticytokine therapy may be an effective adjuvant treatment in steroid‐resistant cases or in severe patients who have clinically significant manifestations of CLS, especially hypoxemia and pulmonary edema.
5. CYTOMEGALOVIRUS REACTIVATION
CMV reactivation is a significant complication in UCBT patients, associated with increased transplant‐related morbidity and mortality. 114 , 115 Because of the difference in condition regimens and GVHD prophylactics, the infection rates of CMV post UCBT varies substantially in many studies. Recipient CMV serostatus was the most important risk factor that predicted the reactivation of CMV viremia or disease, while CMV serologies of cord blood donor infants and their mothers may not improve the risk of CMV reactivation. 116 , 117 The association between CMV reactivation and the clinical outcome of cord blood transplantation is still controversial. In a recent large‐scale study, 3147 eligible UCBT patients older than 16 years showed a favorable effect of CMV reactivation on relapse and OS was observed in high‐risk AML and MDS. 118 While a Korean study revealed that CMV reactivation did not impact leukemia relapse or survival, and CMV disease can resulted in higher TRM and lower survival. 119 The results were not consistent in different studies, this might be due to discrepancies in patient characteristics such as age and the use of antithymocyte globulin. 118 , 119 , 120
6. CONCLUSION
UCB remains a viable donor option for allo‐HSCT. Rapid availability and easy transport, especially during the COVID‐19 pandemic, lower immunogenicity of UCB, lower incidence and severity of chronic GVHD, and a stronger GVL effect on recipients with MRD are advantages of UCBT. However, delayed engraftment and GF, increased infection risks and TRM remain important challenges for UCBT. To overcome these challenges, new strategies are constantly being explored, including dUCBT and ex vivo expansion of UCB cells, but no significant improvement in transplant outcomes has been demonstrated. With the development of HLA matching, CB unit selection, modified conditioning regimens and effective management of complications, UCBT has achieved comparable OS and better GRFS than other allo‐HSCT types. Future directions should focus on innovative research on the basic biology of UCB stem cells, novel randomized controlled clinical trials, and perfect quality control of UCB banking, making UCBT more popular for more patients.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
X.Z., B.T.: conception and design of the study as well as writing of the manuscript; Z.S.: conception and design of the study, final approval of the manuscript.
ACKNOWLEDGMENT
This work was supported by the National Natural Science Foundation of China (grant # 81670165), International Cooperation Projects in Anhui Province (grant # 1804b06020352), the Fundamental Research Funds for the Central Universities (grants # WK9110000001 and WK9110000060).
Zhu X, Tang B, Sun Z. Umbilical cord blood transplantation: Still growing and improving. STEM CELLS Transl Med. 2021;10(S2):S62‐S74. 10.1002/sctm.20-0495
Xiaoyu Zhu and Baolin Tang contributed equally to this work and should be considered as co‐first authors.
Contributor Information
Xiaoyu Zhu, Email: xiaoyuz@ustc.edu.cn.
Zimin Sun, Email: zmsun@ustc.edu.cn.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Passweg JR, Baldomero H, Bader P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant. 2016;51:786‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lv M, Huang XJ. Allogeneic hematopoietic stem cell transplantation in China: where we are and where to go. J Hematol Oncol. 2012;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem‐cell grafts in the U.S. registry. N Engl J Med. 2014;371:339‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical‐cord blood from an HLA‐identical sibling. N Engl J Med. 1989;321:1174‐1178. [DOI] [PubMed] [Google Scholar]
- 5. Milano F, Gooley T, Wood B, et al. Cord‐blood transplantation in patients with minimal residual disease. N Engl J Med. 2016;375:944‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kindwall‐Keller TL, Ballen KK. Umbilical cord blood: the promise and the uncertainty. Stem Cells Translational Medicine. 2020;9:1153‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsumura T, Kami M, Yamaguchi T, et al. Allogeneic cord blood transplantation for adult acute lymphoblastic leukemia: retrospective survey involving 256 patients in Japan. Leukemia. 2012;26:1482‐1486. [DOI] [PubMed] [Google Scholar]
- 8. Page KM, Labopin M, Ruggeri A, et al. Factors associated with long‐term risk of relapse after unrelated cord blood transplantation in children with acute lymphoblastic leukemia in remission. Biol Blood MarrowTransplant. 2017;23:1350‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piñana JL, Sanz J, Picardi A, et al. Umbilical cord blood transplantation from unrelated donors in patients with Philadelphia chromosome‐positive acute lymphoblastic leukemia. Haematologica. 2014;99:378‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanz J, Sanz MA, Saavedra S, et al. Cord blood transplantation from unrelated donors in adults with high‐risk acute myeloid leukemia. Biol Blood Marrow Transplant. 2010;16:86‐94. [DOI] [PubMed] [Google Scholar]
- 11. Baron F, Ruggeri A, Beohou E, et al. Occurrence of graft‐versus‐host disease increases mortality after umbilical cord blood transplantation for acute myeloid leukaemia: a report from Eurocord and the ALWP of the EBMT. J Intern Med. 2018;283:178‐189. [DOI] [PubMed] [Google Scholar]
- 12. Yanada M, Konuma T, Kuwatsuka Y, et al. Unit selection for umbilical cord blood transplantation for adults with acute myeloid leukemia in complete remission: a Japanese experience. Bone Marrow Transplant. 2019;54:1789‐1798. [DOI] [PubMed] [Google Scholar]
- 13. Madureira ABM, Eapen M, Locatelli F, et al. Analysis of risk factors inflfluencing outcome in children with myelodysplastic syndrome after unrelated cord blood transplantation. Leukemia. 2011;25:449‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robin M, Ruggeri A, Labopin M, et al. Comparison of unrelated cord blood and peripheral blood stem cell transplantation in adults with myelodysplastic syndrome after reduced‐intensity conditioning regimen: a collaborative study from Eurocord (Cord blood Committee of Cellular Therapy & Immunobiology Working Party of EBMT) and Chronic Malignancies Working Party. Biol Blood Marrow Transplant. 2015;21:489‐495. [DOI] [PubMed] [Google Scholar]
- 15. Gerds AT, Woo Ahn K, Hu ZH, et al. Outcomes after umbilical cord blood transplantation for myelodysplastic syndromes. Biol Blood Marrow Transplant. 2017;23:971‐979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuwatsuka Y, Kanda J, Yamazaki H, et al. A comparison of outcomes for cord blood transplantation and unrelated bone marrow transplantation in adult aplastic anemia. Biol Blood Marrow Transplant. 2016;22:1836‐1843. [DOI] [PubMed] [Google Scholar]
- 17. de Latour RP, Chevret S, Jubert C, et al. Unrelated cord blood transplantation in patients with idiopathic refractory severe aplastic anemia: a nationwide phase 2 study. Blood. 2018;132:750‐754. [DOI] [PubMed] [Google Scholar]
- 18. Yoshimi A, Kojima S, Taniguchi S, et al. Unrelated cord blood transplantation for severe aplastic anemia. Biol Blood Marrow Transplant. 2008;14:1057‐1063. [DOI] [PubMed] [Google Scholar]
- 19. Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:3828‐3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Broxmeyer HE, Kurtzberg J, Gluckman E, et al. Umbilical cord blood hematopoietic stem and repopulating cells in human clinical transplantation. Blood Cells. 1991;17:313‐329. [PubMed] [Google Scholar]
- 21. Wagner JE, Broxmeyer HE, Byrd RL, et al. Transplantation of umbilical cord blood after myeloablative therapy: analysis of engraftment. Blood. 1992;79:1874‐1881. [PubMed] [Google Scholar]
- 22. Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157‐166. [DOI] [PubMed] [Google Scholar]
- 23. Laporte JP, Gorin NC, Rubinstein P, et al. Cord blood transplantation from an unrelated donor in an adult with chronic myelogenous leukemia. New Engl J Med. 1996;335:167‐170. [DOI] [PubMed] [Google Scholar]
- 24. Rubinstein P, Rosenfield RD, Adamson JW, Stevens CE. Stored placental blood for unrelated bone marrow reconstitution. Blood. 1993;81:1679‐1690. [PubMed] [Google Scholar]
- 25. Gluckman E, Rocha V, Boyer Chammard A, et al. Outcome of cord blood transplantation from related and unrelated donors. New Engl J Med. 1997;337:373‐381. [DOI] [PubMed] [Google Scholar]
- 26. Ballen K. Umbilical cord blood transplantation: challenges and future directions. Stem Cells Translational Medicine. 2017;6:1312‐1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barker JN, Wagner JE. Umbilical cord blood transplantation: current practice and future innovations. Crit Rev Oncol Hematol. 2003;48:35‐43. [DOI] [PubMed] [Google Scholar]
- 28. Rocha V, Wagner JE Jr, Sobocinski KA, et al. Graft‐versus‐host disease in children who have received a cord‐blood or bone marrow transplant from an HLA‐identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342:1846‐1854. [DOI] [PubMed] [Google Scholar]
- 29. Brown JA, Boussiotis VA. Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution. Clin Immunol. 2008;127:286‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. da Silva CL, Gonçalves R, Porada CD, et al. Differences amid bone marrow and cord blood hematopoietic stem/progenitor cell division kinetics. J Cell Physiol. 2009;220:102‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Theunissen K, Verfaillie CM. A multifactorial analysis of umbilical cord blood, adult bone marrow and mobilized peripheral blood progenitors using the improved ML‐IC assay. Exp Hematol. 2005;33:165‐172. [DOI] [PubMed] [Google Scholar]
- 32. Bock TA, Orlic D, Dunbar CE, Broxmeyer HE, Bodine DM. Improved engraftment of human hematopoietic cells in severe combined immunodefificient (SCID) mice carrying human cytokine transgenes. J Exp Med. 1995;182:2037‐2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vormoor J, Lapidot T, Pflflumio F, et al. Immature human cord blood progenitors engraft and proliferate to high levels in severe combined immunodefificient mice. Blood. 1994;83:2489‐2497. [PubMed] [Google Scholar]
- 34. Mayani H, Wagner JE, Broxmeyer HE. Cord blood research, banking, and transplantation: achievements, challenges, and perspectives. Bone Marrow Transplant. 2020;55:48‐61. [DOI] [PubMed] [Google Scholar]
- 35. Mayani H. Biological differences between neonatal and adult human hematopoietic stem/progenitor cells. Stem Cells Dev. 2010;19:285‐298. [DOI] [PubMed] [Google Scholar]
- 36. Mayani H, Lansdorp PM. Biology of human umbilical cord blood‐derived hematopoietic stem/progenitor cells. Stem Cells. 1998;16:153‐165. [DOI] [PubMed] [Google Scholar]
- 37. Sarvaria A, Basar R, Mehta RS, et al. IL‐10+ regulatory B cells are enriched in cord blood and may protect against cGVHD after cord blood transplantation. Blood. 2016;128:1346‐1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hiwarkar P, Qasim W, Ricciardelli I, et al. Cord blood T cells mediate enhanced antitumor effects compared with adult peripheral blood T cells. Blood. 2015;126:2882‐2891. [DOI] [PubMed] [Google Scholar]
- 39. Wing K, Ekmark A, Karlsson H, Rudin A, Suri‐Payer E. Characterization of human CD25+CD4+ T cells in thymus,cord and adult blood. Immunology. 2002;106:190‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee C‐C, Lin S‐J, Cheng P‐J, Kuo M‐L. The regulatory function of umbilical cord blood CD4(+) CD25(+) T cells stimulated with anti‐CD3/anti‐CD28 and exogenous interleukin (IL)‐2 or IL‐15. Pediatr Allergy Immunol. 2009;20:624‐632. [DOI] [PubMed] [Google Scholar]
- 41. Wing K, Lindgren S, Kollberg G, et al. CD4 T cell activation by myelin oligodendrocyte glycoprotein is suppressed by adult but not cord blood CD25+ T cells. Eur J Immunol. 2003;33:579‐587. [DOI] [PubMed] [Google Scholar]
- 42. Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor‐type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft‐versus host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft‐versus‐host disease lethality. Blood. 2002;99:3493‐3499. [DOI] [PubMed] [Google Scholar]
- 44. Trenado A, Charlotte F, Fisson S, et al. Recipient type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft‐versus‐host disease while maintaining graft‐versus leukemia. J Clin Invest. 2003;112:1688‐1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brunstein CG, Miller JS, McKenna DH, et al. Umbilical cord blood‐derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016;127:1044‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profifile and detection kinetics. Blood. 2011;117:1061‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft‐versus‐host disease‐free, relapse‐free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zheng CC, Zhu XY, Tang BL, et al. Clinical separation of cGvHD and GvL and better GvHD free/relapse‐free survival (GRFS) after unrelated cord blood transplantation for AML. Bone Marrow Transplant. 2017;52:88‐94. [DOI] [PubMed] [Google Scholar]
- 49. Sharma P, Purev E, Haverkos B, et al. Adult cord blood transplant results in comparable overall survival and improved GRFS vs matched related transplant. Blood Adv. 2020;4:2227‐2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tong J, Xuan L, Sun Y, et al. Umbilical cord blood transplantation without antithymocyte globulin results in similar survival but better quality of life compared with unrelated peripheral blood stem cell transplantation for the treatment of acute leukemia‐a retrospective study in China. Biol Blood Marrow Transplant. 2017;23:1541‐1548. [DOI] [PubMed] [Google Scholar]
- 51. Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA‐mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fuchs EJ, O'Donnell PV, Eapen M, et al. Double unrelated umbilical cord blood versus HLA‐haploidentical bone marrow transplantation (BMT CTN 1101). Blood. 2021;137:420‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mo XD, Tang BL, Zhang XH, et al. Comparison of outcomes after umbilical cord blood and unmanipulated haploidentical hematopoietic stem cell transplantation in children with high‐risk acute lymphoblastic leukemia. Int J Cancer. 2016;139:2106‐2115. [DOI] [PubMed] [Google Scholar]
- 54. Rocha V, Crotta A, Ruggeri A, et al. Double cord blood transplantation: extending the use of unrelated umbilical cord blood cells for patients with hematological diseases. Best Pract Res Clin Haematol. 2010;23:223‐229. [DOI] [PubMed] [Google Scholar]
- 55. Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical‐cord blood from two partially matched unrelated donors. N Engl J Med. 2001;344:1870‐1871. [DOI] [PubMed] [Google Scholar]
- 56. Sideri A, Neokleous N, Brunet De La Grange P, et al. An overview of the progress on double umbilical cord blood transplantation. Haematologica. 2011;96:1213‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kindwall‐Keller TL, Hegerfeldt Y, Meyerson HJ, et al. Prospective study of one‐ vs two‐unit umbilical cord blood transplantation following reduced intensity conditioning in adults with hematological malignancies. Bone Marrow Transplant. 2012;47:924‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wagner JE Jr, Eapen M, Carter S, et al. One‐unit versus two‐unit cord‐blood transplantation for hematologic cancers. N Engl J Med. 2014;371:1685‐1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Michel G, Galambrun C, Sirvent A, et al. Single‐ vs double‐unit cord blood transplantation for children and young adults with acute leukemia or myelodysplastic syndrome. Blood. 2016;127:3450‐3457. [DOI] [PubMed] [Google Scholar]
- 60. Baron F, Ruggeri A, Beohou E, et al. Single‐ or double‐unit UCBT following RIC in adults with AL: a report from Eurocord, the ALWP and the CTIWP of the EBMT. J Hematol Oncol. 2017;10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Balligand L, Galambrun C, Sirvent A, et al. Single‐unit versus double‐unit umbilical cord blood transplantation in children and young adults with residual leukemic disease. Biol Blood Marrow Transplant. 2019;25:734‐742. [DOI] [PubMed] [Google Scholar]
- 62. Zheng CC, Zhu XY, Tang BL, et al. Double vs. single cord blood transplantation in adolescent and adult hematological malignancies with heavier body weight (≥50 kg). Hematology. 2018;23:96‐104. [DOI] [PubMed] [Google Scholar]
- 63. Mayani H, Dragowska W, Lansdorp PM. Cytokine‐induced selective expansion and maturation of erythroid versus myeloid progenitors from purified cord blood precursor cells. Blood. 1993;81:3252‐3258. [PubMed] [Google Scholar]
- 64. Cicuttini FM, Welch KL, Boyd AW. The effect of cytokines on CD34+ Rh‐123high and low progenitor cells from human umbilical cord blood. Exp Hematol. 1994;22:1244‐1251. [PubMed] [Google Scholar]
- 65. Shpall EJ, Quinones R, Giller R, et al. Transplantation of ex vivo expanded cord blood. Biol Bone Marrow Transpl. 2002;8:368‐376. [DOI] [PubMed] [Google Scholar]
- 66. Jaroscak J, Goltry K, Smith A, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo‐expanded UCB cells: results of a phase I trial using the AastromReplicell System. Blood. 2003;101:5061‐5067. [DOI] [PubMed] [Google Scholar]
- 67. Scadden DT. The stem cell niche as an entity of action. Nature. 2006;441:1075‐1079. [DOI] [PubMed] [Google Scholar]
- 68. Nagasawa T, Omatsu Y, Sugiyama T. Control of hematopoietic stem cells by the bone marrow stromal niche: the role of reticular cells. Trends Immunol. 2011;32:315‐320. [DOI] [PubMed] [Google Scholar]
- 69. Moreno R, Martínez‐González I, Rosal M, Farwati A, Gratacós E, Aran JM. Characterization of mesenchymal stem cells isolated from the rabbit fetal liver. Stem Cells Dev. 2010;19:1579‐1588. [DOI] [PubMed] [Google Scholar]
- 70. Goyal U, Sen A, Ta M. Isolation and molecular characterization of progenitor cells from human umbilical cord. Methods Mol Biol. 2019;2029:1‐13. [DOI] [PubMed] [Google Scholar]
- 71. Robinson SN, Ng J, Niu T, et al. Superior ex vivo cord blood expansion following co‐culture with bone marrow‐derived mesenchymal stem cell. Bone Marrow Transplant. 2006;37:359‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. de Lima M, McNiece I, Robinson SN, et al. Cord‐blood engraftment with ex vivo mesenchymal‐cell coculture. N Engl J Med. 2012;367:2305‐2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chute JP, Muramoto GG, Whitesides J, et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci USA. 2006;103:11707‐11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Peled T, Mandel J, Goudsmid RN, et al. Pre‐clinical development of cord blood‐derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy. 2004;6:244‐255. [DOI] [PubMed] [Google Scholar]
- 75. Figueroa E, Villanueva‐Toledo J, Garrido E, et al. In vitro effects of stromal cells expressing different levels of Jagged‐1 and Delta‐1 on the growth of primitive and intermediate CD34+ cell subsets from human cord blood. Blood Cells Mol Dis. 2011;47:205‐213. [DOI] [PubMed] [Google Scholar]
- 76. Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Peled T, Shoham H, Aschengrau D, et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol. 2012;40:342‐355. [DOI] [PubMed] [Google Scholar]
- 78. Fares I, Chagraoui J, Gareau Y, et al. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self‐renewal. Science. 2014;345:1509‐1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Delaney C, Heimfeld S, Brashem‐Stein C, Voorhies H, Manger RL, Bernstein ID. Notch‐mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Horwitz ME, Chao NJ, Rizzieri DA, et al. Umbilical cord blood expansion with nicotinamide provides long‐term multilineage engraftment. J Clin Invest. 2014;124:3121‐3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Horwitz ME, Wease S, Blackwell B, et al. Phase I/II study of stem‐cell transplantation using a single cord blood unit expanded ex vivo with nicotinamide. J Clin Oncol. 2019;37:367‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. de Lima M, McMannis J, Gee A, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant. 2008;41:771‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stiff PJ, Montesinos P, Peled T, et al. Cohort‐controlled comparison of umbilical cord blood transplantation using carlecortemcel‐L, a single progenitor‐enriched cord blood, to double cord blood unit transplantation. Biol Blood Marrow Transplant. 2018;24:1463‐1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wagner JE Jr, Brunstein CG, Boitano AE, et al. Phase I/II trial of stemregenin‐1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand‐alone graft. Cell Stem Cell. 2016;18:144‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cohen S, Roy J, Lachance S, et al. Hematopoietic stem cell transplantation using single UM171‐expanded cord blood: a single‐arm, phase 1‐2 safety and feasibility study. Lancet Haematol. 2020;7:e134‐e145. [DOI] [PubMed] [Google Scholar]
- 86. Mehta RS, Saliba RM, Cao K, et al. Ex vivo mesenchymal precursor cell‐expanded cord blood transplantation after reduced‐intensity conditioning regimens improves time to neutrophil recovery. Biol Blood Marrow Transplant. 2017;23:1359‐1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Eapen M, Klein JP, Sanz GF, et al. Effect of donor‐recipient HLA matching at HLA a, B, C, and DRB1 on outcomes after umbilical‐cord blood transplantation for leukaemia and myelodysplastic syndrome: a retrospective analysis. Lancet Oncol. 2011;12:1214‐1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Eapen M, Klein JP, Ruggeri A, et al. Impact of allele‐level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123:133‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Eapen M, Wang T, Veys PA, et al. Allele‐level HLA matching for umbilical cord blood transplantation for non‐malignant diseases in children: a retrospective analysis. Lancet Haematol. 2017;4:e325‐e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fuji S, Oshima K, Ohashi K, et al. Impact of pretransplant donor‐specific anti‐HLA antibodies on cord blood transplantation on behalf of the Transplant Complications Working Group of Japan Society for Hematopoietic Cell Transplantation. Bone Marrow Transplant. 2020;55:722‐728. [DOI] [PubMed] [Google Scholar]
- 91. Sekine T, Marin D, Cao K, et al. Specific combinations of donor and recipient KIR‐HLA genotypes predict for large differences in outcome after cord blood transplantation. Blood. 2016;128:297‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Martínez‐Losada C, Martín C, Gonzalez R, Manzanares B, García‐Torres E, Herrera C. Patients lacking a KIR‐ligand of HLA group C1 or C2 have a better outcome after umbilical cord blood transplantation. Front Immunol. 2017;8:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tanaka J, Morishima Y, Takahashi Y, et al. Effects of KIR ligand incompatibility on clinical outcomes of umbilical cord blood transplantation without ATG for acute leukemia in complete remission. Blood Cancer J. 2013;3(11):e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. van Rood JJ, Stevens CE, Smits J, Carrier C, Carpenter C, Scaradavou A. Reexpsoure of cord blood to noninherited maternal HLA antigens improves transplant outcome in hematological malignancies. Proc Natl Acad Sci USA. 2009;106:19952‐19957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rocha V, Spellman S, Zhang MJ, et al. Effect of HLA‐matching recipients to donor noninherited maternal antigens on outcomes after mismatched umbilical cord blood transplantation for hematologic malignancy. Biol Blood Marrow Transplant. 2012;18:1890‐1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Purtill D, Smith K, Devlin S, et al. Dominant unit CD34+ cell dose predicts engraftment after double‐unit cord blood transplantation and is influenced by bank practice. Blood. 2014;124:2905‐2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gluckman E, Ruggeri A, Volt F, Cunha R, Boudjedir K, Rocha V. Milestones in umbilical cord blood transplantation. Br J Haematol. 2011;154:441‐447. [DOI] [PubMed] [Google Scholar]
- 98. Hough R, Danby R, Russell N, et al. Recommendations for a standard UKapproach to incorporating umbilical cord blood into clinical transplantation practice: an update on cord blood unit selection, donor selection algorithms and conditioning protocols. Br J Haematol. 2016;172:360‐370. [DOI] [PubMed] [Google Scholar]
- 99. Dehn J, Spellman S, Hurley CK, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/CIBMTR. Blood. 2019;134:924‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pascal L, Tucunduva L, Ruggeri A, et al. Impact of ATG‐containing reduced‐intensity conditioning after single‐ or double‐unit allogeneic cord blood transplantation. Blood. 2015;126:1027‐1032. [DOI] [PubMed] [Google Scholar]
- 101. Zheng C, Luan Z, Fang J, et al. Comparison of conditioning regimens with or without antithymocyte globulin for unrelated cord blood transplantation in children with high‐risk or advanced hematological malignancies. Biol Blood Marrow Transplant. 2015;21:707‐712. [DOI] [PubMed] [Google Scholar]
- 102. Sun Z, Liu H, Luo C, et al. Better outcomes of modified myeloablative conditioning without antithymocyte globulin versus myeloablative conditioning in cord blood transplantation for hematological malignancies: a retrospective (development) and a prospective (validation) study. Int J Cancer. 2018;143:699‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lee YH, Lim YJ, Kim JY, Kim YD, Lee SW. Pre‐engraftment syndrome in hematopoietic stem cell transplantation. J Korean Med Sci. 2008;23:98‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kishi Y, Kami M, Miyakoshi S, et al. Early immune reaction after reduced‐intensity cord‐blood transplantation for adult patients. Transplantation. 2005;80:34‐40. [DOI] [PubMed] [Google Scholar]
- 105. Lee YH, Rah WJ. Pre‐engraftment syndrome: clinical significance and pathophysiology. Blood Res. 2016;51:152‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Konuma T, Kohara C, Watanabe E, et al. Cytokine profiles of pre‐engraftment syndrome after single‐unit cord blood transplantation for adult patients. Biol Blood Marrow Transplant. 2017;23:1932‐1938. [DOI] [PubMed] [Google Scholar]
- 107. Matsuno N, Yamamoto H, Watanabe N, et al. Rapid T‐cell chimerism switch and memory T‐cell expansion are associated with pre‐engraftment immune reaction early after cord blood transplantation. Br J Haematol. 2013;160:255‐258. [DOI] [PubMed] [Google Scholar]
- 108. Isobe M, Konuma T, Kato S, et al. Development of pre‐engraftment syndrome, but not acute graft‐versus‐host disease, reduces relapse rate of acute myelogenous leukemia after single cord blood transplantation. Biol Blood Marrow Transplant. 2019;25:1187‐1196. [DOI] [PubMed] [Google Scholar]
- 109. Morita‐Hoshi Y, Mori SI, Soeda A, et al. Identification of molecular markers for pre‐engraftment immune reactions after cord blood transplantation by SELDI‐TOF MS. Bone Marrow Transplant. 2010;45:1594‐1601. [DOI] [PubMed] [Google Scholar]
- 110. Park M, Lee SH, Lee YH, et al. Pre‐engraftment syndrome after unrelated cord blood transplantation: a predictor of engraftment and acute graft‐versus‐host disease. Biol Blood Marrow Transplant. 2013;19:640‐646. [DOI] [PubMed] [Google Scholar]
- 111. Frangoul H, Wang L, Harrell FE Jr, Ho R, Domm J. Preengraftment syndrome after unrelated cord blood transplant is a strong predictor of acute and chronic graft‐versus‐host disease. Biol Blood Marrow Transplant. 2009;15:1485‐1488. [DOI] [PubMed] [Google Scholar]
- 112. Kanda J, Kaynar L, Kanda Y, et al. Pre‐engraftment syndrome after myeloablative dual umbilical cord blood transplantation: risk factors and response to treatment. Bone Marrow Transplant. 2013;48:926‐931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hong KT, Kang HJ, Kim NH, et al. Peri‐engraftment syndrome in allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013;48:523‐528. [DOI] [PubMed] [Google Scholar]
- 114. Takami A, Mochizuki K, Asakura H, Yamazaki H, Okumura H, Nakao S. High incidence of cytomegalovirus reactivation in adult recipients of an unrelated cord blood transplant. Haematologica. 2005;90:1290‐1292. [PubMed] [Google Scholar]
- 115. Castillo N, García‐Cadenas I, Barba P, et al. Early and long‐term impaired T lymphocyte immune reconstitution after cord blood transplantation with antithymocyte globulin. Biol Blood Marrow Transplant. 2017;23:491‐497. [DOI] [PubMed] [Google Scholar]
- 116. Albano MS, Taylor P, Pass RF, et al. Umbilical cord blood transplantation and cytomegalovirus: posttransplantation infection and donor screening. Blood. 2006;108:4275‐4282. [DOI] [PubMed] [Google Scholar]
- 117. Theiler RN, Caliendo AM, Pargman S, et al. Umbilical cord blood screening for cytomegalovirus DNA by quantitative PCR. J Clin Virol. 2006;37:313‐316. [DOI] [PubMed] [Google Scholar]
- 118. Yokoyama H, Takenaka K, Nishid T, et al. Favorable effect of cytomegalovirus reactivation on outcomes in cord blood transplant and its differences among disease risk or type. Biol Blood Marrow Transplant. 2020;26:1363‐1370. [DOI] [PubMed] [Google Scholar]
- 119. Park M, Lee YH, Lee SH, et al. Cytomegalovirus infection in seropositive unrelated cord bloodrecipients: a study of 349 Korean patients. Ann Hematol. 2015;94:481‐489. [DOI] [PubMed] [Google Scholar]
- 120. Ramanathan M, Teira P, Battiwalla M, et al. Impact of early CMV reactivation in cord blood stem cell recipients in the current era. Bone Marrow Transplant. 2016;51:1113‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


