Abstract
Brain degeneration and damage is difficult to cure due to the limited endogenous repair capability of the central nervous system. Furthermore, drug development for treatment of diseases of the central nervous system remains a major challenge. However, it now appears that using human pluripotent stem cell‐derived neural cells to replace degenerating cells provides a promising cell‐based medicine for rejuvenation of brain function. Accordingly, a large number of studies have carried out preclinical assessments, which have involved different neural cell types in several neurological diseases. Recent advances in animal models identify the transplantation of neural derivatives from pluripotent stem cells as a promising path toward the clinical application of cell therapies [Stem Cells Transl Med 2019;8:681‐693; Drug Discov Today 2019;24:992‐999; Nat Med 2019;25:1045‐1053]. Some groups are moving toward clinical testing in humans. However, the difficulty in selection of valuable critical quality criteria for cell products and the lack of functional assays that could indicate suitability for clinical effect continue to hinder neural cell‐based medicine development [Biologicals 2019;59:68‐71]. In this review, we summarize the current status of preclinical studies progress in this area and outline the biological characteristics of neural cells that have been used in new developing clinical studies. We also discuss the requirements for translation of stem cell‐derived neural cells in examples of stem cell‐based clinical therapy.
Keywords: cell replacement, cell therapy, neural cell, neurological disease, pluripotent stem cells, preclinical study
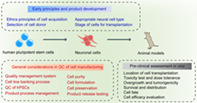
Significance statement.
Human pluripotent stem cell‐derived neural cells provide a promising cell‐based medicine for the treatment of neurological diseases. There are many challenges for cell derivatives preparation and quality evaluation, as well as for the establishment of critical quality attributes for cell‐based products. This review outlines the biological characteristics of neural cells that have been used in clinical studies and summarizes the requirements of neural cells in stem cell‐based clinical therapy for translation.
1. INTRODUCTION
The nervous system is the control center for various cognitive and motor functions of human beings, and its disruption can lead to severe neurological disorders. 1 , 2 , 3 , 4 In recent years, neurological disorders have become an increasingly significant cause of death and disability globally, such as neurodegenerative disease and neural damage, which affected 9 million and 276 million people, respectively, in 2016. 5 Because of the limited capacity of the central nervous system (CNS) to spontaneously repair itself, there is still a paucity of curative treatments for diseases of the CNS.
Cell‐based replacement therapy has long been a very promising approach in the treatment of neurological diseases. 6 Fetal brain‐derived neural stem cells (NSCs) and ventral midbrain (VM) tissue have shown positive effects in the treatment of stroke 7 and Parkinson's disease (PD), 3 respectively. However, the application of fetal tissue is often restricted due to the ethical restrictions and variation of cell quality. 8 , 9 Human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), provide a viable source of neuronal cells for transplantation because of their indefinite self‐renewal capacity and multi‐differentiation potential into desired ectodermal cell types. Until now, several hPSC‐derived neuronal cell types have been successfully tested in neurological diseases models where in vivo safety and efficacy have been proved. 10 , 11 First‐in‐human clinical studies have also been initiated in some countries. 8
There are still many challenges for the preparation and quality evaluation of cell derivatives, including the choice of the appropriate cell types and optimal differentiation stage for transplantation, preclinical animal models, quality control (QC), and quality standards for all stages in the manufacturing process, including cryopreservation, product formulation, transportation, and assessment in appropriate potency assays. Therefore, the evaluation and QC of stem cells in clinical use, as well as the establishment of critical quality attributes for cell‐based products, 4 have long been a major issue restricting the clinical application and future commercialization of stem cells.
Because of differing national requirements, many countries have developed local laws and regulations, leading to difference in regulatory policies between countries. In recent years, the International Stem Cell Banking Initiative (ISCBI) brought worldwide researchers together to publish a series of documents including white papers and meeting reports on ISCBI publications (https://www.iscbi.org/copy‐of‐expertise). These documents and ongoing ISCBI workshop discussions developed global consensus from expert centers for the clinical translation of hPSCs. These include cell QC principles, attention to the ethical requirements of donor selection, cell bank operation, cell safety evaluation, and raw materials selection. These aspects can help to promote the translation of stem cell research to therapies and ensure the quality and safety of the final cell product. 12 , 13 , 14 , 15 ISCBI has coordinated with other organizations to contribute to other international forums on standardization in cell‐based medicine manufacture including meetings of the International Alliance for Biological Standardization 16 (www.iabs.org). More specifically, in the area of stem cell‐based therapy for PD, international coordination has been provided by the GForce‐PD (a new global initiative around stem cell‐based therapies for PD, http://www.gforce-pd.com/). In this review, we summarize general principles for preclinical studies of stem cell therapy. The aim of this review is to provide guidance for early academic product developers in their efforts to promote clinical translation of stem cells to cell‐based medicine, but it should be used in the context of overarching national or local laws.
2. EARLY PRINCIPLES AND PRODUCT DEVELOPMENT
2.1. Ethics principles of cell acquisition
Both hESCs and hiPSCs cannot be produced without human donors, and donor cell acquisition should comply with internationally recognized principles (guidelines issued by ISSCR in 2016) and local laws and regulations. 17 , 18 Briefly, each cell line should have informed consent signed by donors/responsible doctor and ethical support files from the hospital. In addition, in view of the need for safety and traceability, donors should be informed of restrictions on privacy protection. For detailed discussion see ISCBI 2015. 12
2.2. Selection of cell donor
Embryos or cell donors should undergo careful risk assessment and screening for various potential human infectious pathogens. Typically, these include the serious blood‐borne viral diseases such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), human T‐cell lymphotropic virus (HTLV), Epstein‐Barr virus (EBV), cytomegalovirus (CMV), and syphilis. 12 However, other organisms may need to be considered based on information from the donor selection process. Genetic disease should also be considered for professional donor assessment. It is recommended to collect the donor information of ABO blood group, human leukocyte antigen (HLA) classes I and II genotype from a donor blood sample. These data may prove useful to check identity of the derived cell line but will also be important if there is a possibility to match cell line blood and tissue type to reduce patient immune response to therapeutic cells.
2.3. Appropriate neural cell type
Suitable cell differentiation condition establishes the cell fate along the anteroposterior axis and the dorsoventral axis in the embryonic neural tube, leading to the diversity of neuronal cells. 19 Each cell phenotype has distinct properties and performs unique functions in vivo. For example, PD is caused by the loss of midbrain dopaminergic neurons (mDA) in the substantia nigra, which projects to the dorsolateral striatum. 20 Therefore, the goal of PD cell therapy is to restore the lost DA in patients. Several studies have proved that only mDA cells can establish the correct connectivity within the host tissue to have suitable therapeutic outcomes in PD models, while forebrain/hindbrain neurons will not bring any recovery. 20 , 21 , 22 , 23 These results showed that in vivo efficacy of grafted neurons was largely determined by their intrinsic characteristics. Another example is amyotrophic lateral sclerosis (ALS), which is characterized by the loss of upper and lower motor neurons (MNs) mainly caused by toxicity of reactive astrocytes. Transplanted MNs may be vulnerable and susceptible. In this case, to improve the microenvironment by transplantation of healthy astrocytes may be a powerful treatment in addition to transplantation of MNs. 24 , 25
On the other hand, for the treatment of other nervous system damage, such as brain injury, stroke, and spinal cord injury (SCI), which involve loss of function of many nerve cell types and dramatic changes in local microenvironment, there are still challenges to restore the connectivity using a single type of neuronal cell. This would entail different considerations regarding the neural replacement. NSCs can not only differentiate into neuronal cells, but also secrete neurotrophic factors. 26 Therefore, NSCs are a promising therapeutic cell type worthy of consideration and would be expected to replace damaged cells and promote axonal regeneration. 27 , 28
2.4. Stage of cells for transplantation
Another important consideration of the hPSCs‐based neural replacement therapy is the suitable stage for transplantation. Early stage neural progenitors give rise to high yield of surviving neurons, but have the risk of uncontrolled proliferation, while mature neurons showed poor survival postgraft, 29 , 30 which is confirmed by several fetal brain tissue transplantation studies. 31 The survival of grafted cells and behavior improvement of animal models will provide some clues to appropriate cell stages for transplantation. It is essential, therefore, to find out the specific markers in different stages such as proliferative progenitors, postmitotic neuroblasts and mature neurons. Based on the research experience of cell therapy for PD, early postmitotic stage neurons are most widely used for transplantation. 31 For astrocytes, lineage‐restricted progenitors are also widely used for transplantation, 32 whereas further studies are still needed to determine the optimal cell phenotypes at each manufacturing stage.
3. GENERAL CONSIDERATIONS IN QC OF CELL MANUFACTURING
3.1. Quality management system
A well‐constructed QMS is crucial to help assure reliable output from cell bank operation and cell production. It is also recommended to follow good manufacturing practices (GMP) that are applicable for licensed products and will be required for final manufacture of the respective cell‐based medicine.
The QMS should be specific to the manufactured product, describing the manufacturers' responsibilities, standards adopted and a summary of all manufacturing procedures used. It should include all standard operating procedures used and higher level quality documents relevant to the manufacturing process which may include, but is not limited to, donor sample collection, raw material selection, facility validation/qualification, bioprocessing methods, QC, storage, product release, shipment and staff training procedures, and so on. The QMS should also ensure the traceability of all materials and procedures used from the original material to the final product. This is vital to demonstrate all procedures have been carried out as described and this is fundamental to GMP. It is typically described in a summary document sometimes called a quality manual which identifies the general procedures and associated standards and policies. For a review specific to hPSCs, refer to the ISCBI report. 12
3.2. Cell line banking process
To prepare the clinical cell product, the first step is to assure suitability of donors used to establish hPSCs, preservation of early stocks of production cells, and selection of appropriate scientifically selected QC and safety testing lines to meet GMP standards. 12 This will include the selection and qualification of reagents and materials (eg, “xeno‐free” and chemically defined media, medium supplements, cell detachment reagents, tissue culture‐ware) to applicable standards such as ISO13485. In order to reduce the variability of different batches of cells in the production process, a multilevel cell bank system is recommended as the starting point for supply of production cultures, including quality‐controlled master cell banks (MCB) and working cell banks (WCB). 12 , 14
3.3. QC of hPSCs
As a starting material, the quality of the hPSCs is important to help assure the quality and reproducibility of the final product. To ensure that the cell line retains effective and stable ability to differentiate into target cells under long‐term expansion, it is essential to implement appropriate QC assays for cell banked material, including tests of cell morphology, viability, DNA fingerprinting, karyotype, sterility, mycoplasma, viral, appropriate hPSCs markers expression and three germ layers formation ability. 12 , 14 , 33 , 34 , 35 , 36 , 37 A notable point is that batch‐to‐batch variation of hPSCs maybe a dominant source of process variation. Therefore, there is a need for development of a large number of hPSCs of the same batch.
3.4. Product process management
The quality of the cell product must be strictly controlled during the whole production process. The QMS should indicate specific QC tests, including cell marker and microbiological tests, at critical steps as these are a crucial element of quality assurance for cell production. These can ensure the supply of reproducible cell preparations with a specific passage level during a long preparation period. For hPSC‐derived products, the process mainly includes two parts: hPSC production and neuronal cell production. In the first part, hPSCs are expanded under in‐process testing to ensure hPSCs maintain intrinsic characteristics, for example, biomarkers, genetic integrity. Accordingly, MCB and WCB of the production hPSC line are established under QC check test as described in Section 3.3. In the second part, cell product is prepared with several QC tests performed at different time points during differentiation. The current generic published workflows for a neuronal cell production manufacturing process used by several groups are summarized in Figure 1. 34 , 36 , 38
FIGURE 1.
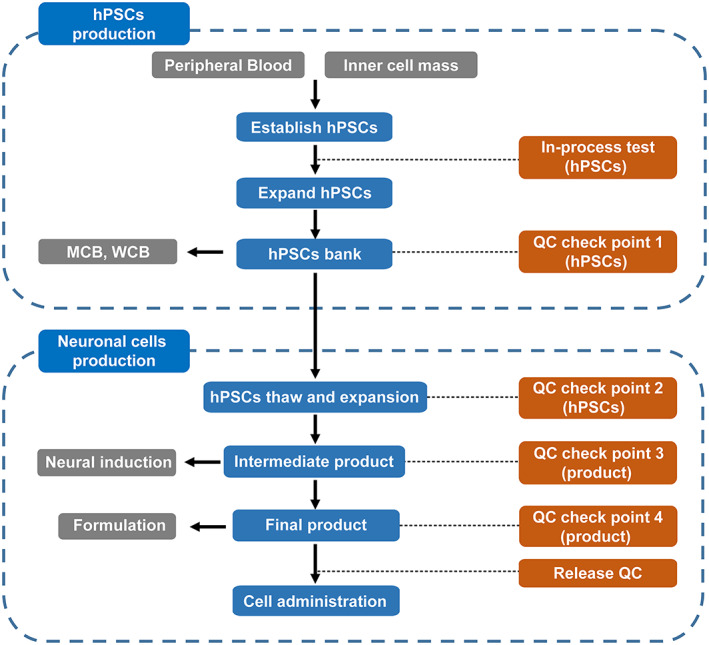
An overview of manufacturing workflow of clinical neuronal cells. In‐process test (hPSCs): morphology, karyotype, sterility, mycoplasma, viral. QC check point 1 (hPSCs): morphology, karyotype, sterility, mycoplasma, viral, cell number, viability, endotoxin, hPSCs marker expression, three germ layers formation ability. QC check point 2 (hPSCs): morphology, karyotype, sterility, mycoplasma, cell number, viability, endotoxin, hPSCs marker expression. QC check point 3 (intermediate product): morphology, neural marker expression, cell number, sterility, mycoplasma. QC check point 4 (final product, before cryopreservation): morphology, neural marker expression, residual undifferentiated hPSCs, cell number, viability, sterility, mycoplasma. Release test (final product, after cryopreservation): neural marker expression, cell number, viability, sterility, mycoplasma, endotoxin, maturation ability test. hPSCs, human pluripotent stem cells; MCB, master cell bank; QC, quality control; WCB, working cell bank
3.5. Purity of cells for transplantation
A major challenge of hPSCs‐based neural replacement therapy is to precisely obtain desired cell types with high homogeneity. Because the remaining undifferentiated cells or untargeted cells carry inherent safety risks, it is necessary to figure out the full composition of final cell product and eliminate the known residual stem cells before transplantation. Previous clinical evidence has suggested that the contaminating 5‐hydroxytryptamine (5‐HT) neurons may cause side effects called graft‐induced dyskinesia after transplantation in PD patients. 39 Furthermore, preclinical studies also showed that residual progenitor cells can excessively proliferate in the brain 40 or form cystic‐like structures in the spinal cord. 41 Some groups have developed strategies to improve cell purity, such as cell sorting using specific surface markers 42 , 43 and adding quercetin in the medium to eliminate undifferentiated cells. 44 Note: It should be confirmed by validation studies that no undifferentiated cells remain in the cell product.
3.6. Cell formulation and preservation
Unlike traditional small‐molecule chemicals, cells are “living drugs.” Biological processes such as metabolism, secretion, and death occur all the time and cells respond to changes in their environment. Therefore, it is important to establish an effective cell preservation regime to retain the cell preparation characteristics. In general, there are two main types of formulations: fresh nonfrozen cells 34 and cryopreserved cells. 45 Fresh cell preparations retain cell viability and characteristics very well, but this is typically short lived, thus, fresh live cells are not suitable for long‐term storage and full QC testing. In contrast, cryopreserved cells have a long shelf life, which makes the full QC and safety release testing possible prior to administration. Nevertheless, the freezing and thawing processes may cause cell damage, cell selection, and delay in recovery of function. 45 Recently, some studies have selected suitable cryopreservation solutions for neuronal cells. 46 , 47 These require appropriate cooling rates and thawing conditions validated for each neuronal cell product, suggesting that precisely controlled cooling and warming conditions will further improve cell viability. 48 Notably, the same study showed that the viability and recovery of cells at 24 hours post‐thawing could more accurately reflect the effect of cryopreservation and this is a principle now well established in cell cryobiology. 49 No matter what formulation is used, the stability of the cell product during storage (liquid nitrogen cryopreservation and temporary storage before cell implantation) and transportation should be studied as described in the International Council on Harmonization of Technical Requirements for Pharmaceuticals for Human Use guideline Q5C (https://www.ema.europa.eu/en/ich-q5c-stability-testing-biotechnologicalbiological-products).
3.7. Cell product release testing
The critical quality attributes of cell product are the prerequisite for its safety and efficacy in vivo. 4 It can be important to test a predetermined proportion of each product lot which may be taken from any appropriate part of the lot. The establishment of an evaluation system requires a series of in vitro tests, including the tests for biological contamination (microorganisms and endotoxin), cell viability, cell number, morphology, biomarkers, and ability to differentiate into mature neurons. 34 , 35 , 36 , 37 The related tolerances for release testing should also be developed based on a review of the data from multiple production batches. In addition, the appearance of genetic variants during culture processes is a major aspect of cell safety risk. The use of a genome integrity assay such as whole‐genome sequencing could be helpful, but it will be important to understand the relevance of any genetic changes observed during culture to patient safety. Although there is no unified standard for determining genome stability, several published studies provide valuable reference. 34 , 36 , 50 To avoid the tumorigenic hazards that may be associated with genetic variants, it may be helpful to choose cells with no detected large abnormalities or nonsynonymous mutation in cancer related genes. However, such detection methods can only provide a generic cancer risk assessment, not a certification of in vivo safety.
4. PRECLINICAL ASSESSMENT IN VIVO
4.1. Location of cell transplantation
Tissue replacement or local nutrition is the main purpose of neuronal cell transplantation and so it is desirable to deliver cells to the target sites. For example, cell transplantation in stroke has mainly focused on transplantation of cells in the ischemic penumbra to rescue the dying cells and rebuild the neural network, 51 whereas, for ALS, intrathecal injection is the best route for neuronal cell transplantation. 24 , 52 For PD treatment, current strategies involve grafting cells into the dorsal striatum 53 , 54 or substantia nigra. 21 , 55 On the other hand, transplantation at one to two sites can provide neural innervation in rodents. 21 , 56 In contrast, the primate brain is much bigger than rodent brain and to achieve sufficient innervation in this case, cell transplantation at multiple sites is generally required. In fact, three to eight injection tracts per hemisphere have been attempted in PD clinical studies. 36 , 57 , 58 , 59 Thus, the cell transplantation strategy for small animals is likely insufficient to model human therapy. Subsequently, nonhuman primate models have unique advantages in evaluation of grafting strategy, because the brain of nonhuman primate has similar size and structure to human brain.
4.2. Toxicity test and dose tolerance
Definition of the cell toxicity and dose tolerance are key questions for a new drug. Toxicity tests should follow the clinical route of cell transplantation and study the toxicity effects to target and nontarget organs (eg, liver, heart, kidney). In general, the high‐dose cell administration may produce a greater safety risk. It is generally considered best practice to set different dose groups in animal experiments and determine the maximum feasible dose, which will provide support to the clinical research. Dose determinations may also be more challenging where the cells are likely to proliferate in vivo. Typically, rats or mice usually receive a total of 50 000 to 400 000 cells in brain 30 , 35 , 45 or 100 000 to 2.4 million cells in spinal cord. 24 , 60 , 61
4.3. Overgrowth and tumorigenicity assay
Uncontrolled proliferation and tumor formation are two major considerations for the safety of hPSC‐derived cells. Especially for the nervous system, excessive cell growth will impact the host's cell networks and lead to dysfunction. It is therefore necessary to investigate the graft volumes and proliferation markers by histopathological and immunohistochemical analysis in sufficient numbers of animals. Previous work suggests that the graft volume might be primarily due to the nature of cells that are used for transplantation. For example, younger neural cells (such as SOX1 + PAX6 + KI67+) or less differentiated mDA neurons get extensive expansion in recipient brains, 62 , 63 while purified neuron preparations suffer from significant cell loss and graft size reduction compared with that of unpurified cells. 40
In addition, carcinogenicity or oncogenicity should also be taken seriously, which is distinct from cell tumorigenicity. Carcinogenicity/oncogenicity refers to tumor formation of the host's normal cells stimulated by agent, while tumorigenicity is associated with grafted cells. 12 To determine the contamination of undifferentiated hPSCs, some groups have performed spiking studies by mixing different proportions of hPSCs and cell products. 34 , 35
4.4. Survival and distribution in vivo
The purpose of neuronal cell graft is to achieve long‐term survival in the host and replace the lost cells; however, due to cell digestion, mechanical damage, changes in the microenvironment, and so on, a large number of grafted cells will die and lead to abrogation of function. Several reports have showed that the survival rate of transplanted DA is less than 10%. 31 Furthermore, studies also shown that events occurring within the first week after cell transplantation are critical to cell survival, 64 which makes it possible to predict the number of surviving cells at an early stage. Significant advances have been made to enhance the survival of transplanted cells via p53 inhibition, 65 polysialic acid expression 66 or GDNF injection. 30
Although neuronal cells show little evidence of long‐distance migration from the injection site, it is necessary to demonstrate cell distribution post infusion. For this purpose, noninvasive cell tracking methods will help researchers monitor animals over time, instead of sacrificing animals at multiple time points.
4.5. Cell fate in vivo
As mentioned above, neuronal cell transplantation is mainly composed of NSCs and immature progenitors, which requires further differentiation and maturation in vivo. Although the in vitro differentiation technology is reliable, the complex microenvironment in vivo may affect the fate of transplanted cells and cause cell heterogeneity. Indeed, single‐cell RNA sequencing data revealed that the grafted dopaminergic progenitors not only generated neurons, but also produced glial and vascular‐like cells, 67 as well as choroid plexus epithelia‐like cells. 34 In addition, several studies have revealed that NSCs will give rise to neurons, oligodendrocytes, and astrocytes in spinal cord, while different NSC lines may exhibit different ratios of the three cell types. 61 , 68 , 69 , 70 Hence, it is important to confirm whether the cell product has converted into the desired cell type in vivo, and whether there are dangerous cell types present. 71
4.6. Cell efficacy evaluation
Efficacy evaluation in animal models is a prerequisite for clinical trials/studies. An ideal animal model should reproduce the pathological lesion and behavioral symptoms seen in patients as closely as possible. Since rodents are readily available and low cost, using mouse and rat models is a common strategy in efficacy testing. On the other hand, compared with small animals, nonhuman primate models have more advantages in neurological diseases research, 72 since they are more likely to improve the predictive accuracy of cell dose effects and efficacy in humans. No matter which animal model is used, effective behavior evaluation methods and standards should be developed and used to prove the efficacy of the cell product. Functional endpoints may include the study of cell innervation, neural circuit reconstruction, pathological improvement, and behavior improvement and should inform understanding of the possible mechanism of action (eg, cell replacement, factor secretion, or others). In addition, researchers are encouraged to publish preclinical results in peer‐reviewed journal for rigor validation. Several clinical studies using hPSC‐derived neuronal cells are under way (Table 1).
TABLE 1.
Preclinical studies using hPSC‐derived neuronal cells
| Cell product | Disease | Registration no./program name | Reference | In vitro study | Animal | In vivo study |
|---|---|---|---|---|---|---|
| hiPSC‐derived dopaminergic progenitors | PD | R000038278 | Kikuchi et al 73 and Doi et al 34 | Marker expression; differentiation ability; electrophysiological analysis; dopamine release; genome analysis; single‐cell analysis; residual plasmids; residual pluripotent cells; mycoplasma; endotoxin; sterility | NOG mice; 6‐OHDA‐lesioned nude rat; MPTP‐lesioned monkey | Marker expression; toxicity; tumorigenicity; biodistribution; graft size; imaging (MRI, PET‐CT); efficacy |
| hiPSC‐derived neural stem/progenitor cells | SCI | jRCTa031190228 | Nakamura et al 74 and Nori et al 75 | Marker expression; differentiation ability; electrophysiological analysis | SCI in NOD/SCID mice | Marker expression; cell tracing; tumorigenicity; synapse connection; cell counting; efficacy |
| Human parthenogenetic ESC‐derived dopaminergic neurons | PD | NCT03119636 | Wang et al 37 | Marker expression; electrophysiological analysis; differentiation ability; residual pluripotent cells; mycoplasma; endotoxin; bacteria and fungi | SCID mice; MPTP‐lesioned monkey | Marker expression; cell counting; tumorigenicity; blood biochemistry; cell tracing; graft size; dopamine release; imaging (MRI); efficacy |
| Human parthenogenetic ESC‐derived neural stem cells | PD | NCT02452723 | Gonzalez et al 76 and Garitaonandia et al 38 | Marker expression; differentiation ability; bacteria and fungi; mycoplasma; karyotype; residual pluripotent cells; RNA‐seq | Nude rat; MPTP‐lesioned monkey | Marker expression; cell counting; tumorigenicity; biodistribution; efficacy |
| hESC‐derived astrocytes | ALS | NCT03482050 | Izrael et al 24 | Marker expression; differentiation ability; karyotype; pluripotent cells residuals; biological functionality | SOD1G93A transgenic mice and rats; immunodeficient NSG mice | Marker expression; cell counting; tumorigenicity; biodistribution; efficacy |
| hESC‐derived oligodendrocyte progenitors | SCI | NCT02302157 | Priest et al 41 | Marker expression; differentiation ability; sterility; mycoplasma; biological functionality | Thoracic SCI athymic nude rats; Shiverer/Rag2 mice; SCID/Bg mice | Marker expression; tumorigenicity; biodistribution; efficacy |
| Human autologous iPSC‐derived dopaminergic progenitors | PD | — | Song et al 44 | Marker expression; differentiation ability; karyotype; electrophysiological analysis; residual pluripotent cells; residual plasmids; dopamine release; genome analysis; mycoplasma | NOD/SCID mice; Athymic rats; 6‐OHDA‐lesioned athymic rats | Marker expression; cell counting; tumorigenicity; biodistribution; efficacy |
| hESC‐derived dopaminergic progenitors | PD | STEM‐PD trial | Kirkeby et al, 54 Nolbrant al, 45 Tiklova et al, 67 and Grealish et al 20 | Marker expression; differentiation ability; electrophysiological analysis; residual pluripotent cells; mycoplasma; endotoxin; sterility; single‐cell analysis | 6‐OHDA‐lesioned rats; athymic “nude” rats | Marker expression; cell counting; graft size; tumorigenicity; biodistribution; predictive biomarkers; imaging (MRI, PET‐CT); efficacy |
| hESC‐derived dopaminergic progenitors | PD | NCT04802733 | Kikuchi et al, 73 Nakamura et al, 74 Kriks et al, 77 and Ganat et al 29 | Marker expression; differentiation ability; residual pluripotent cells; mycoplasma; endotoxin; electrophysiological analysis; dopamine release; RNA‐seq; sterility | 6‐OHDA‐lesioned mice/rat; SCID mice; MPTP‐lesioned monkey | Marker expression; cell counting; graft size; biodistribution; toxicology; tumorigenicity; efficacy |
Abbreviations: ALS, amyotrophic lateral sclerosis; ESC, embryonic stem cells; hESC, human embryonic stem cells; hiPSC, human induced pluripotent stem cells; hPSC, human pluripotent stem cells; MRI, magnetic resonance imaging; MPTP, 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine; NOD, non‐obese diabetes; NSG, NOD‐SCID IL2rg‐/‐; PD, Parkinson's disease; PET‐CT, Positron Emission Tomography‐Computed Tomography; SCI, spinal cord injury; 6‐OHDA, 6‐hydroxydopamine.
5. CHALLENGES AND FUTURE PERSPECTIVES
5.1. A ready‐to‐use cryopreserved format
Striking advances in cell cryopreservation, particularly neuronal cell cryopreservation, pave the way for cell replacement therapy. As a kind of innovative treatment, one challenge for effective cell product is the issue of formulation development. As described earlier, cryopreserved cell product has advantages in QC and safety testing. Up until now, however, most frozen products need post‐thaw washing and concentration procedures to remove dimethyl sulfoxide (DMSO) and/or excipients prior to infusion. Such additional handling increases the safety risk due to changes during cryopreservation, thawing, and recovery of cell products and may compromise cell stability. 78 In the future, a ready‐to‐use neuronal cell product with fewer excipients, simpler cryopreservation solution (even DMSO‐free), no additional operation or simple dilution before administration will be a major research direction for formulation development. 49 Other cryoprotectants, such as glycerol, which is already a pharmaceutical excipient, 79 have long been considered as an alternative. A recent report showed that ground squirrel (a hibernating mammal) iPSC‐derived neurons have distinct pathways in response to cold, which are critical for cold tolerance. 80 Such a mechanism displays long‐term cold tolerance potential of mammal neuronal cells; furthermore, it will greatly promote the clinical application of neuronal cells.
5.2. Alternative markers for functional assay
Prediction of neural maturation and outcome in vivo is still a challenge for cell replacement. Since the maturation and integration of grafted neuronal cells requires several months, it takes a lot of time and animals for functional evaluation. The identification of alternative markers will greatly improve the cell quality and research efficiency and may provide the basis for product potency assays to use in release testing. Notably, the Parmar group defined the relationship between gene expression and in vivo outcomes of dopaminergic progenitors by RNA‐Seq. 56 The group identified several groups of markers that correlate with graft volume, neuron yield, and density, which has significantly promoted the progress of research in the field. It is expected that more biomarkers that can predict in vivo function will be discovered but importantly will need investment in validation as in the work of the Parmar group.
5.3. Hypoimmunogenic cell product
Immune rejection is one of the most important issues for cell replacement therapy as the vast majority of trials use allogenic cell products. Neuronal cell replacement therapies that work by reconstructing missing cells or enzymes require long‐term survival of the engrafted cells. Although the CNS is considered as a relatively immune privileged site, many nonhuman primates' studies revealed that immunological rejection of allogeneic cells in the brain still exists. 81 , 82 So far, the traditional method to overcome this issue is using immunosuppressive drugs. These immunosuppressive drugs can bring side effects, and increase the risk of infection and tumor formation, which may be life‐threatening, particularly for the elderly which are a key cohort for therapies for degenerative diseases.
The most feasible approach is to generate universally compatible cells. There are two ways to achieve this goal: One is HLA silencing of hPSCs by gene editing. Recent work has proved the reliability of this strategy in vitro and in vivo. 83 , 84 To our knowledge, there is no report on the application of such engineering universal neuronal cells yet. The other option is collection of hPSCs lines with various HLA types or haplotypes. This strategy has been validated by nonhuman primates and has been tried in clinical research. 31 However, both methods have their advantages and disadvantages. It is pivotal to collect strong evidence proving the safety and hypoimmunogenicity of cells prior to clinical applications. 85
In conclusion, despite the rapid advances in clinical studies of hPSC‐derived neuronal cell, many challenges still exist. It remains imperative to carefully consider the issues mentioned above. Our work indicates that further studies are required to improve the safety and efficacy of neuronal cell product and in particular potential tumorigenicity and functional potency assays.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Y.S., L.F., L.L., G.N.S., C.W.: manuscript writing; Y.W.: manuscript writing, conception and design, financial support, and final approval of the manuscript; B.H.: conception and design, financial support, and final approval of the manuscript.
ACKNOWLEDGMENTS
This research was supported by the National Key Research and Development Program of China (2018YFA0108001 to B.Y.H., 2019YFA0110800 to Y.K.W., 2018YFA0108402 to Y.S.), Beijing Science and Technology Major Project (Z181100001818002 to B.Y.H., Z181100001818009 to Y.K.W.), the National Natural Science Foundation of China (31771642 to B.Y.H., 31970821 to Y.K.W.), Chinese Academy of Sciences President's International Fellowship for Special Experts (2018FSB0009 to G.N.S.).
Sun Y, Feng L, Liang L, et al. Neuronal cell‐based medicines from pluripotent stem cells: Development, production, and preclinical assessment. STEM CELLS Transl Med. 2021;10(S2):S31–S40. 10.1002/sctm.20-0522
Yun Sun, Lin Feng, and Lingmin Liang contributed equally as first authors.
Funding information Chinese Academy of Sciences President's International Fellowship for Special Experts, Grant/Award Number: 2018FSB0009; National Natural Science Foundation of China, Grant/Award Numbers: 31970821, 31771642; Beijing Science and Technology Major Project, Grant/Award Numbers: Z181100001818009, Z181100001818002; National Key Research and Development Program of China, Grant/Award Numbers: 2018YFA0108402, 2019YFA0110800, 2018YFA0108001
Contributor Information
Yukai Wang, Email: wangyukai@ioz.ac.cn.
Baoyang Hu, Email: byhu@ioz.ac.cn.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Ramotowski C, Qu X, Villa‐Diaz LG. Progress in the use of induced pluripotent stem cell‐derived neural cells for traumatic spinal cord injuries in animal populations: meta‐analysis and review. Stem Cells Translational Medicine. 2019;8:681‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farkhondeh A, Li R, Gorshkov K, et al. Induced pluripotent stem cells for neural drug discovery. Drug Discov Today. 2019;24:992‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barker RA, TRANSEURO consortium . Designing stem‐cell‐based dopamine cell replacement trials for Parkinson's disease. Nat Med. 2019;25:1045‐1053. [DOI] [PubMed] [Google Scholar]
- 4. Creasey AA, Stacey G, Bharti K, Sato Y, Lubiniecki A. A strategic road map to filing a Biologics License Application for a pluripotent stem cell derived therapeutic product. Biologicals. 2019;59:68‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collaborators GBDN . Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Armstrong JPK, Keane TJ, Roques AC, et al. A blueprint for translational regenerative medicine. Sci Transl Med. 2020;12(572). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sinden JD, Hicks C, Stroemer P, Vishnubhatla I, Corteling R. Human neural stem cell therapy for chronic ischemic stroke: charting progress from laboratory to patients. Stem Cells Dev. 2017;26:933‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parmar M, Grealish S, Henchcliffe C. The future of stem cell therapies for Parkinson disease. Nat Rev Neurosci. 2020;21:103‐115. [DOI] [PubMed] [Google Scholar]
- 9. McCune JM, Weissman IL. The ban on US government funding research using human fetal tissues: how does this fit with the NIH mission to advance medical science for the benefit of the citizenry? Stem Cell Rep. 2019;13:777‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stoker TB, Barker RA. Recent developments in the treatment of Parkinson's disease. F1000Res. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han F, Lu P. Introduction for stem cell‐based therapy for neurodegenerative diseases. Adv Exp Med Biol. 2020;1266:1‐8. [DOI] [PubMed] [Google Scholar]
- 12. Andrews PW, Baker D, Benvinisty N, et al. Points to consider in the development of seed stocks of pluripotent stem cells for clinical applications: International Stem Cell Banking Initiative (ISCBI). Regen Med. 2015;10:1‐44. [DOI] [PubMed] [Google Scholar]
- 13. Kim JH, Kurtz A, Yuan BZ, et al. Report of the international stem cell banking initiative workshop activity: current hurdles and progress in seed‐stock banking of human pluripotent stem cells. Stem Cells Translational Medicine. 2017;6:1956‐1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sullivan S, Stacey GN, Akazawa C, et al. Quality control guidelines for clinical‐grade human induced pluripotent stem cell lines. Regen Med. 2018;13:859‐866. [DOI] [PubMed] [Google Scholar]
- 15. Stacey GN, Andrews PW, Barbaric I, et al. Stem cell culture conditions and stability: a joint workshop of the PluriMes Consortium and pluripotent stem cell platform. Regen Med. 2019;14:243‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abbot S, Agbanyo F, Ahlfors JE, et al. Report of the international conference on manufacturing and testing of pluripotent stem cells. Biologicals. 2018;56:67‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peng YJ, Huang X, Zhou Q. Ethical and policy considerations for human embryo and stem cell research in China. Cell Stem Cell. 2020;27:511‐514. [DOI] [PubMed] [Google Scholar]
- 18. Pandya SK. Guidelines for stem cell science and clinical translation. Indian J Med Ethics. 2016;1:160‐161. [DOI] [PubMed] [Google Scholar]
- 19. Lumsden A, Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109‐1115. [DOI] [PubMed] [Google Scholar]
- 20. Grealish S, Diguet E, Kirkeby A, et al. Human ESC‐derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson's disease. Cell Stem Cell. 2014;15:653‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiong M, Tao Y, Gao Q, et al. Human stem cell‐derived neurons repair circuits and restore neural function. Cell Stem Cell. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirkeby A, Grealish S, Wolf DA, et al. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703‐714. [DOI] [PubMed] [Google Scholar]
- 23. Adler AF, Cardoso T, Nolbrant S, et al. hESC‐derived dopaminergic transplants integrate into basal ganglia circuitry in a preclinical model of Parkinson's disease. Cell Rep. 2019;28:3462‐3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Izrael M, Slutsky SG, Admoni T, et al. Safety and efficacy of human embryonic stem cell‐derived astrocytes following intrathecal transplantation in SOD1(G93A) and NSG animal models. Stem Cell Res Ther. 2018;9:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kondo T, Funayama M, Tsukita K, et al. Focal transplantation of human iPSC‐derived glial‐rich neural progenitors improves lifespan of ALS mice. Stem Cell Rep. 2014;3:242‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu Z, Men Y, Dong P. Schwann cells promote the capability of neural stem cells to differentiate into neurons and secret neurotrophic factors. Exp Ther Med. 2017;13:2029‐2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang DJ, Oh SH, Lee N, et al. Contralaterally transplanted human embryonic stem cell‐derived neural precursor cells (ENStem‐A) migrate and improve brain functions in stroke‐damaged rats. Exp Mol Med. 2013;45:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vishwakarma SK, Bardia A, Tiwari SK, Paspala SA, Khan AA. Current concept in neural regeneration research: NSCs isolation, characterization and transplantation in various neurodegenerative diseases and stroke: a review. J Adv Res. 2014;5:277‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ganat YM, Calder EL, Kriks S, et al. Identification of embryonic stem cell‐derived midbrain dopaminergic neurons for engraftment. J Clin Invest. 2012;122:2928‐2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gantner CW, de Luzy IR, Kauhausen JA, et al. Viral delivery of GDNF promotes functional integration of human stem cell grafts in Parkinson's disease. Cell Stem Cell. 2020;26:511‐526. [DOI] [PubMed] [Google Scholar]
- 31. Kim TW, Koo SY, Studer L. Pluripotent stem cell therapies for Parkinson disease: present challenges and future opportunities. Front Cell Dev Biol. 2020;8:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicaise C, Mitrecic D, Falnikar A, Lepore AC. Transplantation of stem cell‐derived astrocytes for the treatment of amyotrophic lateral sclerosis and spinal cord injury. World J Stem Cells. 2015;7:380‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. International Stem Cell Initiative , Adewumi O, Aflatoonian B, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:803‐816. [DOI] [PubMed] [Google Scholar]
- 34. Doi D, Magotani H, Kikuchi T, et al. Pre‐clinical study of induced pluripotent stem cell‐derived dopaminergic progenitor cells for Parkinson's disease. Nat Commun. 2020;11:3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piao J, Zabierowski S, Dubose BN, et al. Preclinical efficacy and safety of a human embryonic stem cell‐derived midbrain dopamine progenitor product, MSK‐DA01. Cell Stem Cell. 2021;28:217‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schweitzer JS, Song B, Herrington TM, et al. Personalized iPSC‐derived dopamine progenitor cells for Parkinson's disease. N Engl J Med. 2020;382:1926‐1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang YK, Zhu WW, Wu MH, et al. Human clinical‐grade parthenogenetic ESC‐derived dopaminergic neurons recover locomotive defects of nonhuman primate models of Parkinson's disease. Stem Cell Rep. 2018;11:171‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garitaonandia I, Gonzalez R, Christiansen‐Weber T, et al. Neural stem cell Tumorigenicity and biodistribution assessment for phase I clinical trial in Parkinson's disease. Sci Rep. 2016;6:34478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Politis M, Wu K, Loane C, et al. Serotonergic neurons mediate dyskinesia side effects in Parkinson's patients with neural transplants. Sci Transl Med. 2010;2:38ra46. [DOI] [PubMed] [Google Scholar]
- 40. Samata B, Doi D, Nishimura K, et al. Purification of functional human ES and iPSC‐derived midbrain dopaminergic progenitors using LRTM1. Nat Commun. 2016;7:13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Priest CA, Manley NC, Denham J, Wirth ED 3rd, Lebkowski JS. Preclinical safety of human embryonic stem cell‐derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regen Med. 2015;10:939‐958. [DOI] [PubMed] [Google Scholar]
- 42. Doi D, Samata B, Katsukawa M, et al. Isolation of human induced pluripotent stem cell‐derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep. 2014;2:337‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lehnen D, Barral S, Cardoso T, et al. IAP‐based cell sorting results in homogeneous transplantable dopaminergic precursor cells derived from human pluripotent stem cells. Stem Cell Rep. 2017;9:1207‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Song B, Cha Y, Ko S, et al. Human autologous iPSC‐derived dopaminergic progenitors restore motor function in Parkinson's disease models. J Clin Invest. 2020;130:904‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nolbrant S, Heuer A, Parmar M, Kirkeby A. Generation of high‐purity human ventral midbrain dopaminergic progenitors for in vitro maturation and intracerebral transplantation. Nat Protocol. 2017;12:1962‐1979. [DOI] [PubMed] [Google Scholar]
- 46. Parker SS, Moutal A, Cai S, et al. High fidelity cryopreservation and recovery of primary rodent cortical neurons. eNeuro. 2018;5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wakeman DR, Hiller BM, Marmion DJ, et al. Cryopreservation maintains functionality of human iPSC dopamine neurons and rescues parkinsonian phenotypes in vivo. Stem Cell Rep. 2017;9:149‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Drummond NJ, Singh Dolt K, Canham MA, Kilbride P, Morris GJ, Kunath T. Cryopreservation of human midbrain dopaminergic neural progenitor cells poised for neuronal differentiation. Front Cell Dev Biol. 2020;8:578907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Awan M, Buriak I, Fleck R, et al. Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity? Regen Med. 2020;15:1463‐1491. [DOI] [PubMed] [Google Scholar]
- 50. Mandai M, Watanabe A, Kurimoto Y, et al. Autologous induced stem‐cell‐derived retinal cells for macular degeneration. N Engl J Med. 2017;376:1038‐1046. [DOI] [PubMed] [Google Scholar]
- 51. Kawabori M, Shichinohe H, Kuroda S, Houkin K. Clinical trials of stem cell therapy for cerebral ischemic stroke. Int J Mol Sci. 2020;21(19):7380‐7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mazzini L, Gelati M, Profico DC, et al. Results from phase I clinical trial with intraspinal injection of neural stem cells in amyotrophic lateral sclerosis: a long‐term outcome. Stem Cells Translational Medicine. 2019;8:887‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Studer L. Strategies for bringing stem cell‐derived dopamine neurons to the clinic—the NYSTEM trial. Prog Brain Res. 2017;230:191‐212. [DOI] [PubMed] [Google Scholar]
- 54. Kirkeby A, Parmar M, Barker RA. Strategies for bringing stem cell‐derived dopamine neurons to the clinic: a European approach (STEM‐PD). Prog Brain Res. 2017;230:165‐190. [DOI] [PubMed] [Google Scholar]
- 55. Grealish S, Heuer A, Cardoso T, et al. Monosynaptic tracing using modified rabies virus reveals early and extensive circuit integration of human embryonic stem cell‐derived neurons. Stem Cell Reports. 2015;4:975‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kirkeby A, Nolbrant S, Tiklova K, et al. Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC‐based therapy for Parkinson's disease. Cell Stem Cell. 2017;20:135‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li W, Englund E, Widner H, et al. Extensive graft‐derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating parkinsonian brain. Proc Natl Acad Sci USA. 2016;113:6544‐6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Olanow CW, Goetz CG, Kordower JH, et al. A double‐blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003;54:403‐414. [DOI] [PubMed] [Google Scholar]
- 59. Hagell P, Schrag A, Piccini P, et al. Sequential bilateral transplantation in Parkinson's disease: effects of the second graft. Brain. 1999;122(Pt 6):1121‐1132. [DOI] [PubMed] [Google Scholar]
- 60. Manley NC, Priest CA, Denham J, Wirth ED 3rd, Lebkowski JS. Human embryonic stem cell‐derived oligodendrocyte progenitor cells: preclinical efficacy and safety in cervical spinal cord injury. Stem Cells Translational Medicine. 2017;6:1917‐1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zalfa C, Rota Nodari L, Vacchi E, et al. Transplantation of clinical‐grade human neural stem cells reduces neuroinflammation, prolongs survival and delays disease progression in the SOD1 rats. Cell Death Dis. 2019;10:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Doi D, Morizane A, Kikuchi T, et al. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC‐derived neural cells in a primate model of Parkinson's disease. Stem Cells. 2012;30:935‐945. [DOI] [PubMed] [Google Scholar]
- 63. Katsukawa M, Nakajima Y, Fukumoto A, Doi D, Takahashi J. Fail‐safe therapy by gamma‐ray irradiation against tumor formation by human‐induced pluripotent stem cell‐derived neural progenitors. Stem Cells Dev. 2016;25:815‐825. [DOI] [PubMed] [Google Scholar]
- 64. Brundin P, Karlsson J, Emgard M, et al. Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell Transplant. 2000;9:179‐195. [DOI] [PubMed] [Google Scholar]
- 65. Chou J, Greig NH, Reiner D, Hoffer BJ, Wang Y. Enhanced survival of dopaminergic neuronal transplants in hemiparkinsonian rats by the p53 inactivator PFT‐alpha. Cell Transplant. 2011;20:1351‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Battista D, Ganat Y, El Maarouf A, Studer L, Rutishauser U. Enhancement of polysialic acid expression improves function of embryonic stem‐derived dopamine neuron grafts in Parkinsonian mice. Stem Cells Translational Medicine. 2014;3:108‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tiklova K, Nolbrant S, Fiorenzano A, et al. Single cell transcriptomics identifies stem cell‐derived graft composition in a model of Parkinson's disease. Nat Commun. 2020;11:2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Galiakberova AA, Dashinimaev EB. Neural stem cells and methods for their generation from induced pluripotent stem cells in vitro. Front Cell Dev Biol. 2020;8:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Abeysinghe HC, Bokhari L, Quigley A, et al. Pre‐differentiation of human neural stem cells into GABAergic neurons prior to transplant results in greater repopulation of the damaged brain and accelerates functional recovery after transient ischemic stroke. Stem Cell Res Ther. 2015;6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Oh SH, Jeong YW, Choi W, et al. Multimodal therapeutic effects of neural precursor cells derived from human‐induced pluripotent stem cells through episomal plasmid‐based reprogramming in a rodent model of ischemic stroke. Stem Cells Int. 2020;2020:4061516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Heslop JA, Hammond TG, Santeramo I, et al. Concise review: workshop review: understanding and assessing the risks of stem cell‐based therapies. Stem Cells Translational Medicine. 2015;4:389‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Potashkin JA, Blume SR, Runkle NK. Limitations of animal models of Parkinson's disease. Parkinsons Dis. 2010;2011:658083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kikuchi T, Morizane A, Doi D, et al. Human iPS cell‐derived dopaminergic neurons function in a primate Parkinson's disease model. Nature. 2017;548:592‐596. [DOI] [PubMed] [Google Scholar]
- 74. Nakamura M, Okano H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Res. 2013;23:70‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nori S, Okada Y, Yasuda A, et al. Grafted human‐induced pluripotent stem‐cell‐derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci USA. 2011;108:16825‐16830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gonzalez R, Garitaonandia I, Poustovoitov M, et al. Neural stem cells derived from human parthenogenetic stem cells engraft and promote recovery in a nonhuman primate model of Parkinson's disease. Cell Transplant. 2016;25:1945‐1966. [DOI] [PubMed] [Google Scholar]
- 77. Kriks S, Shim JW, Piao J, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hoogendoorn KH, Crommelin DJA, Jiskoot W. Formulation of cell‐based medicinal products: a question of life or death? J Pharm Sci. 2020;110(5):1885‐1894. [DOI] [PubMed] [Google Scholar]
- 79. Lewis DA, Young PM, Buttini F, et al. Towards the bioequivalence of pressurised metered dose inhalers 1: design and characterisation of aerodynamically equivalent beclomethasone dipropionate inhalers with and without glycerol as a non‐volatile excipient. Eur J Pharm Biopharm. 2014;86:31‐37. [DOI] [PubMed] [Google Scholar]
- 80. Ou J, Ball JM, Luan Y, et al. iPSCs from a hibernator provide a platform for studying cold adaptation and its potential medical applications. Cell. 2018;173:851‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Morizane A, Kikuchi T, Hayashi T, et al. MHC matching improves engraftment of iPSC‐derived neurons in non‐human primates. Nat Commun. 2017;8:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Aron Badin R, Bugi A, Williams S, et al. MHC matching fails to prevent long‐term rejection of iPSC‐derived neurons in non‐human primates. Nat Commun. 2019;10:4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xu H, Wang B, Ono M, et al. Targeted disruption of HLA genes via CRISPR‐Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell. 2019;24:566‐578. [DOI] [PubMed] [Google Scholar]
- 84. Suzuki D, Flahou C, Yoshikawa N, et al. iPSC‐derived platelets depleted of HLA class I are inert to anti‐HLA class I and natural killer cell immunity. Stem Cell Rep. 2020;14:49‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Benabdellah K, Sanchez‐Hernandez S, Aguilar‐Gonzalez A, et al. Genome‐edited adult stem cells: next‐generation advanced therapy medicinal products. Stem Cells Translational Medicine. 2020;9:674‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


