Abstract
Objective:
Epstein-Barr virus (EBV)-associated gastric cancer (GC) has been proposed to be a distinct GC molecular subtype. The prognostic significance of EBV infection in GC remains unclear and needs further investigation. Our study aimed to analyze EBV-positive and EBV-negative GC patients regarding their personal and tumor-related characteristics, and compare their overall survival.
Methods:
GC patients consecutively treated at the Riga East University Hospital during 2009–2016 were identified retrospectively. Tumor EBV status was determined by in situ hybridization for EBV-encoded RNA (EBER). Information about clinicopathological characteristics was obtained from patient questionnaires and/or hospital records. Overall survival was ascertained through July 30, 2017. Cox proportional hazard regression models adjusted for personal and tumor-related covariates compared survival between EBV-positive and EBV-negative patients.
Results:
There were a total of 302 GC patients (61% males) with mean and standard deviation age 63.6 ± 11.5 years. EBER positivity was present in 8.6% of tumors. EBV-positive GC patients had better survival at 80 months (adjusted hazard ratio [HR] = 0.37, 95% confidence interval [CI] 0.19–0.72) compared to EBV-negative patients. Worse survival was observed for patients with stage III (HR = 2.76, CI 1.67–4.56) and stage IV (HR = 10.02, CI 5.72–17.57) compared to stage I GC, and overlapping and unspecified subsite (HR = 1.85; CI 1.14; 3.00) compared to distal tumors.
Conclusion:
Tumor EBV positivity is a favorable prognostic factor in gastric cancer.
Keywords: Epstein-Barr virus, EBER-in situ hybridization, gastric cancer, survival
Introduction
Epstein-Barr virus (EBV) associated gastric cancer (GC) has been proposed to be a distinct molecular GC subtype. Presence of the EBV genome in GCs tumors was first reported in 1990 by Burke et al. [1] In 2014, The Cancer Genome Atlas provided a molecular classification defining EBV-positive gastric cancer as a separate GC subtype. [2] In a systematic review of observational studies, the worldwide crude prevalence of EBV in gastric adenocarcinoma was 8.29%, with lower prevalence in Asia (7.99%), intermediate in Europe (8.75%) and higher in the Americas (11.9%). [3]
Recently, a number of studies have investigated the association between EBV positivity and the prognosis of GC with conflicting results. Two large meta-analyses of 8,336 cases across 24 studies (with EBV prevalence varying from 2.02 % to 33.3 % and overall EBV positivity 9.3 %)[4] and of 4,599 cases (overall EBV positivity 8.2%)[5] found EBV positivity associated with favorable prognosis[4,5], and two additional studies with 566 (EBV positivity 7.2%)[6] and 192 (EBV positivity 33.3%)[7]patients showed better survival with EBV-positive tumors[6,7]. However, other reports have shown no correlation between EBV positivity and survival[8] or even poorer survival in EBV-positive patients[9].
Standard GC treatment guidelines do not differentiate between EBV-positive and EBV-negative tumors[10,11]. Tumor EBV status has been recognized as an emerging potential biomarker for personalized treatment strategies in GC, but is not currently recommended for clinical care[10]. Nevertheless, specific treatments for EBV-positive GC patients have been proposed, including proteosome inhibitors, pan-histone deacetylase inhibitors, antiviral drugs, EBV vaccines and various targeted and immunotherapy agents directed at PIK3/Akt/mTOR, PD-1, PD-L1, CTLA-4 and JAK2 [10,12]. The therapeutic effectiveness of these approaches is yet to be established. Thus, the prognostic significance of tumor EBV positivity in GC merits further investigation. In this study, we analysed EBV-positive and EBV-negative Latvian GC patients concerning their personal and tumor-related factors, and to compare their overall survival.
Materials and Methods
Patient characteristics:
We retrospectively analysed data from consecutive GC patients treated at the Riga East University Hospital in 2009–2016 who were enrolled in the University of Latvia / Riga East University hospital biobank. At the time of enrolment all the participants have signed consent. Socio-demographic information was obtained by a standardized questionnaire. Personal characteristics obtained from the hospital database included sex, age, smoking status and body mass index (BMI). Age was dichotomized as ≤ 65 and > 65 years[13,14]. Smoking status was classified as never-smokers, current-smokers and former-smokers. BMI was classified as <18.5 kg/m2 (underweight); 18.5 – 24.9 kg/m2 (normal), and > 25 kg/m2 (overweight). Self-reported history of other cancers was obtained from questionnaires. Data on patient survival from the date of diagnosis until the end of follow-up (July 30, 2017) were obtained from the hospital records database and The Centre of Disease Prevention and Control of Latvia. Our study was approved by the Ethics Committee of Riga East University Hospital Support Foundation and Riga East University Hospital.
Tumor characteristics:
Tumors were classified by AJCC stages I – IV (American Joint Committee on Cancer 7th edition); proximal (C16.0-C16.2 and C16.5-C16.6), distal (C16.3-C16.4) or overlapping/unspecified (C16.8-C16.9) location based on International Statistical Classification of Diseases and Related Health Problems, 10th edition (ICD-10, 2017); and local recurrence as recorded in the hospital records database. All diagnoses of GC were confirmed histologically by an expert pathologist. We analyzed the following histopathological characteristics: Lauren’s classification (intestinal-type, diffuse-type, mixed, indeterminate), grade (G1, G2, G3), and peritumoral atrophy and intestinal metaplasia. We chose not to add figures showing standard histopathologic grades. A grade (1, 2 or 3) was assigned to each cancer tissue using standard histopathologic methods that reflect cytologic differentiation features of the malignant cells (following the protocol [15]). With low grade (1) signifying that the cells are well differentiated and are thus more likely to grow slowly and remain localized, and high grade (3) signifying the cells are less differentiated and thus predicted to proliferate or spread.
Addressing the loss of the patients’ data, we would like to note that none of the patients was lost during the follow up period. The focus of the study – survival in EBV positive and EBV negative gastric cancer patients had no missing data. This was achieved by gathering information from The Centre of Disease Prevention and Control of Latvia and strengthened the study’s integrity. We also had registered all data in following categories: sex, age, other cancer in personal history, local recurrence of the tumor, atrophy and intestinal metaplasia in the adjacent tissues.
Missing data:
smoking status: 3 in EBV positive and 26 EBV negative group;
BMI: 3 in EBV positive and 22 EBV negative group;
stage: 0 in EBV positive and 10 EBV negative group;
tumor anatomical location: 11 in EBV positive and 64 EBV negative group;
Lauren’s classification: 1 in EBV positive and 14 EBV negative group;
grade: 0 in EBV positive and 1 EBV negative group.
No participants that were excluded from the main analysis. Taking into account the bias missing data might cause we tried omitting categories which had missing values from the survival analysis.
Tissue cores were sampled from the paraffin embedded tumors and prepared as tissue microarrays (TMAs) in the Department of Pathology, University Medical Center Utrecht, Netherlands. TMAs were sent to the Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, North Carolina, USA for determination of EBV status by in situ hybridization of EBV-encoded small RNA (EBER) (Figure 1).
Figure 1.
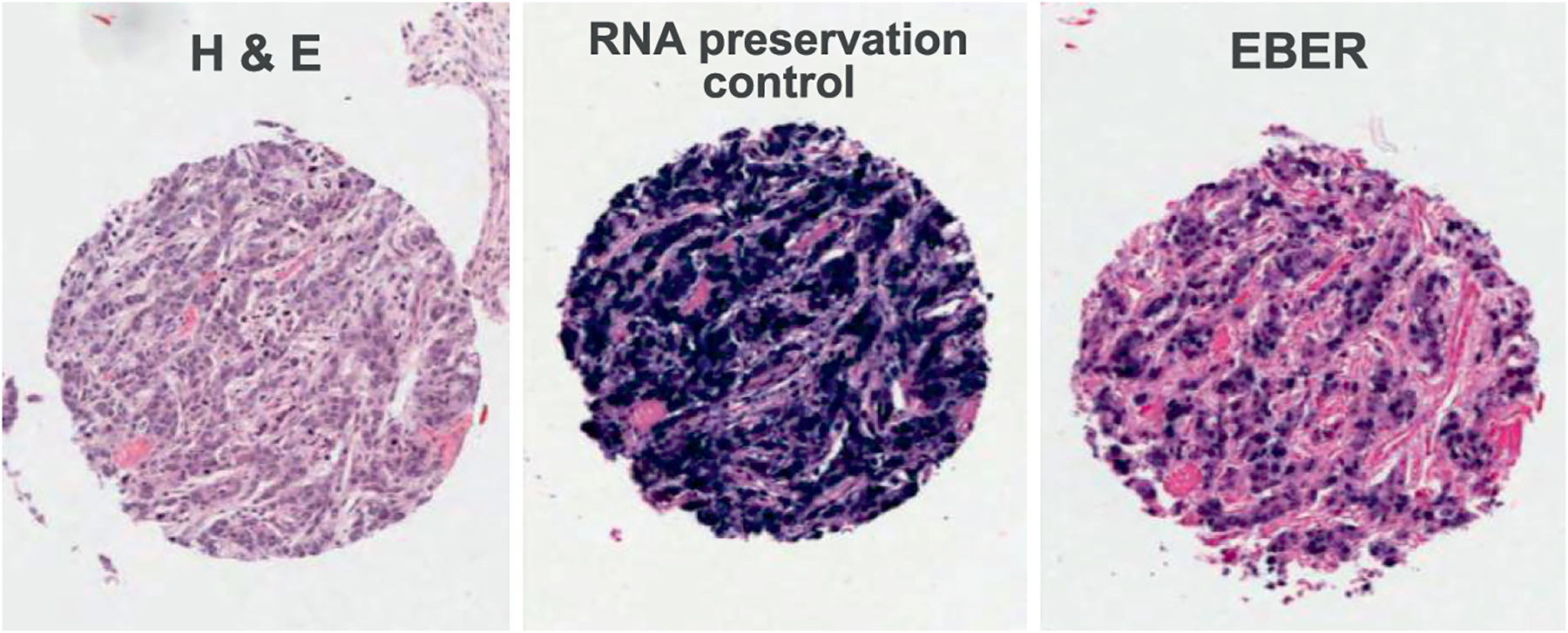
Representative photomicrographs of an EBV-positive gastric cancer tumor stained with hematoxylin and eosin (left panel), RNA preservation control (middle panel), and EBER-ISH (right panel)
Formalin-fixed, paraffin-embedded tissues from gastric cancer resections were retrieved from the University of Latvia / Riga East University hospital biobank. By an experienced pathologist, two representative tumor regions were marked on a hematoxylin and eosin (H&E)-stained section of each tumor, avoiding areas of necrosis. From these tumor regions, a tissue cylinder with a diameter of 1.0 mm was punched out of the corresponding paraffin block (‘donor block’) and placed into the TMA paraffin block using a manual tissue arrayer (MTA-I, Beecher Instruments, Sun Prairie, USA), which was guided by the MTABooster® (Alphelys, Plaisir, France). The distribution and position of the cores was determined in advance with the TMA-designer Software (Alphelys-TMA Designer®, Version 1.6.8, Plaisir, France). EBER in situ hybridization was performed by an automated method using fluorescein-labeled EBER and oligo(d)T control probes on the Ventana Benchmark in situ hybridization system (Ventana Medical Systems, Tuscon, AZ, USA) as previously described (Ryan et al., Lab Invest 2009 [16]). The oligo(d)T probe served as a control for RNA preservation in histological sections. A tumor was considered EBER-negative if EBER staining was undetected or was only expressed in benign-appearing lymphoid cells, and EBER-positive if the signal was localized to malignant epithelial cells.
Statistical analysis:
Chi-square tests were performed to compare EBV-positive and EBV-negative patients. Hazard ratios (HR) were derived from Cox proportional hazard regression models adjusted for personal (age, sex, BMI) and tumor-related (EBV status, tumor stage, topological localization) covariates. Cumulative survival curves were constructed by the Kaplan-Meier method. A two-sided p-value less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS 22 software (IBM Corp. Released 2013. IBM Statistics for Windows, Version 22.0, Armonk, NY: IBM Corp.).
Results
Patients’ characteristics:
There were 302 GC patients of which 61% were male. The mean age at diagnosis was 63.6 years (standard deviation [SD] 11.54; range 20 – 88). Slightly more patients were current or former smokers than never smokers. In almost half of the patients, BMI was within the normal range. There were 169 (56%) deaths during median follow-up of 34.3 months (range 0.27 – 156.2) (Table 1).
Table 1.
Socio-demographic and tumour-related characteristics of EBV- positive and EBV-negative GC patients
| Total % | EBV-positive gastric carcinomas (n=26) | EBV-negative gastric carcinomas (n=276) | p value (refers to all the group) | |
|---|---|---|---|---|
| N | N | |||
| female | 39.4 | 4 | 115 | |
| male | 60.6 | 22 | 161 | 0.01 |
| Mean ± SD | 63.6 ±11.54 | 63.8 ± 11.9 | 62.1 ± 11.5 | 0.86 |
| Age ≤ 65 years | 53.0 | 14 | 146 | |
| Age > 65 years | 47.0 | 12 | 130 | 0.54 |
| never | 43.4 | 8 | 123 | |
| current | 25.8 | 8 | 70 | |
| former | 21.2 | 7 | 57 | 0.60 |
| < 18.5 | 3.6 | 1 | 10 | |
| 18.5 – 24.9 | 45.7 | 12 | 126 | |
| ≥ 25 | 42.4 | 10 | 118 | 0.92 |
| no | 94.7 | 26 | 260 | |
| yes | 5.3 | 0 | 16 | 0.37 |
| 0 | 0.3 | 0 | 1 | |
| I | 22.2 | 4 | 63 | |
| II | 23.2 | 8 | 62 | |
| III | 36.1 | 7 | 102 | |
| IV | 14.9 | 7 | 38 | 0.33 |
| Proximal | 47.7 | 13 | 131 | |
| Distal | 18.9 | 2 | 55 | |
| Overlapping/unspecified | 33.4 | 11 | 90 | 0.27 |
| no | 95.4 | 25 | 263 | 0.84 |
| Intestinal | 49.0 | 14 | 134 | |
| Diffuse | 31.5 | 7 | 88 | |
| Mixed | 14.5 | 4 | 40 | 0.97 |
| G1 and G2 | 26.4 | 8 | 72 | |
| G3 | 73.2 | 18 | 203 | 0.51 |
| no | 74.2 | 19 | 205 | |
| yes | 25.8 | 7 | 71 | 0.52 |
| no | 42.7 | 11 | 118 | |
| yes | 57.3 | 15 | 158 | 0.56 |
EBV, Epstein-Barr virus.
smoking status: unknown – 9.6% of all patients, 3 patients in EBV positive and 26 EBV negative group;
BMI: unknown – 8.3% of all patients, 3 patients in EBV positive and 22 EBV negative group;
stage: unknown – 3.3% of all patients, 0 in EBV positive and 10 EBV negative group;
Lauren’s classification: indeterminate – 5% of all patients, 1 in EBV positive and 14 EBV negative group;
grade: unknown - 0.4% of all patients, 0 in EBV positive and 1 EBV negative group.
Tumor characteristics:
EBV positivity was present in 26 (8.6%) of the tumors. Male patients more often had EBV-positive GC (p = 0.01). A slight majority of all cancer cases (51%) were diagnosed at advanced stages, had histologically positive lymph nodes (51.3%, EBV-positive vs EBV-negative group p=0.15) and tumors located proximally (47.7%) in the stomach.
By Lauren classification, most cases were intestinal-type (49%), and poorly differentiated adenocarcinomas (73.2%). Patient and tumor characteristics (excluding sex) did not significantly differ between EBV-positive and EBV-negative GC patients (Table 1). The adjacent mucosa was atrophic for more than half of the tumors and exhibited intestinal metaplasia in one fourth, but these characteristics were not associated with tumor EBV status.
Statistical analysis:
In a multivariable Cox proportional hazard regression model adjusted for personal and tumor-related covariates, EBV-positive GC patients had better survival at 80 months (HR = 0.37; 95% confidence interval [CI] 0.19–0.72) compared to EBV-negative GC patients (Figure 2). Survival was not significantly associated with age, sex, BMI, stage II tumors and proximal tumor localization. Worse survival was observed for stages III and IV and for overlapping and unspecified tumor localisation (Table 2).
Figure 2.
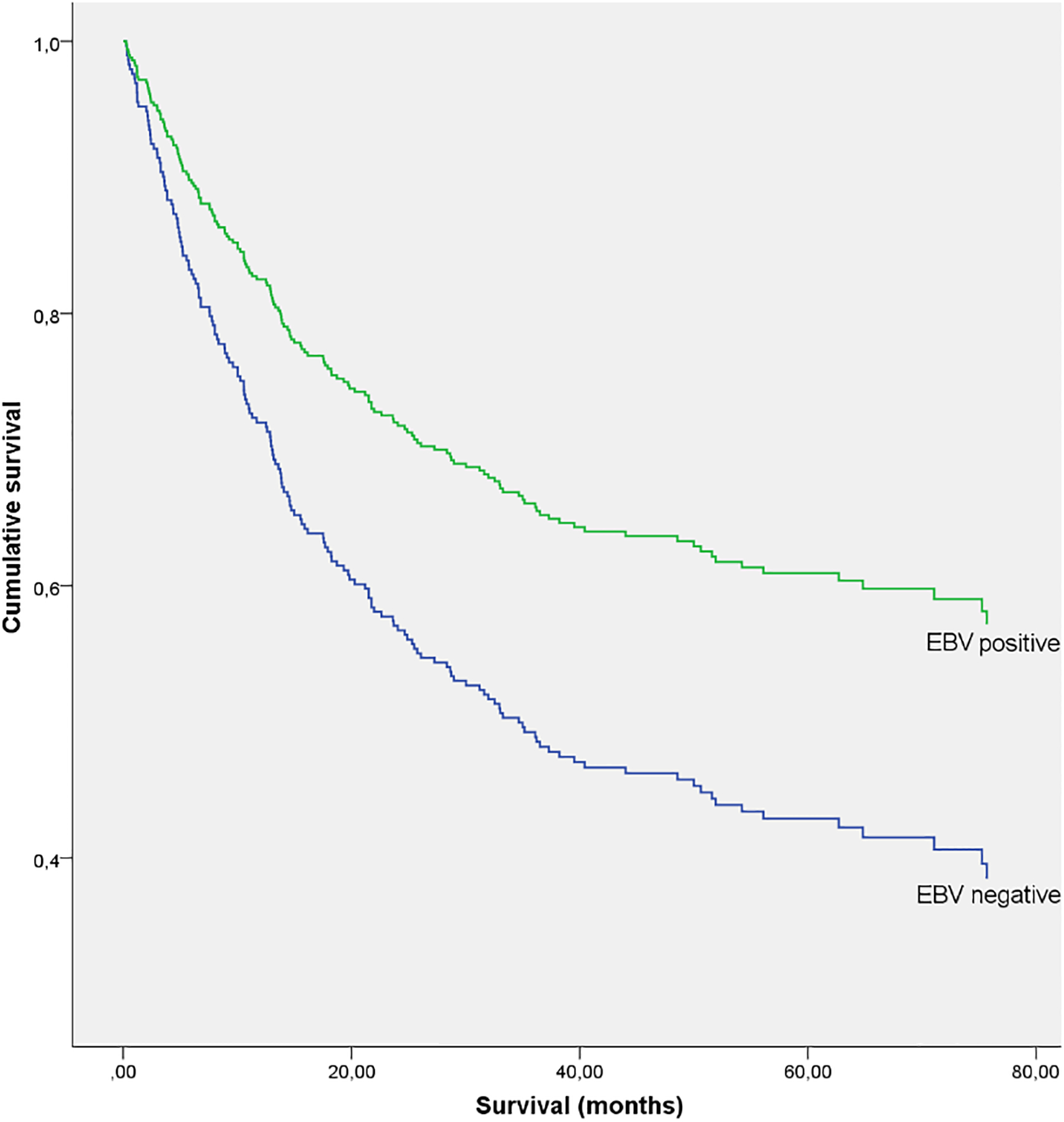
Kaplan-Meier curve of cumulative survival (all stages combined)
Table 2.
Associations of personal and tumour-related factors with survival
| Variable | Adjusted hazard ratio* | 95% CI | P value |
|---|---|---|---|
| EBV- positive status | 0.37 | 0.19; 0.72 | < 0.01 |
| Age > 65 years | 0.87 | 0.60; 1.12 | 0.21 |
| Female sex | 0.87 | 0.62; 1.22 | 0.42 |
| BMI – underweighta | 1.92 | 0.92; 4.02 | 0.08 |
| BMI – overweighta | 0.72 | 0.51; 1.01 | 0.06 |
| Tumour stage – IIb | 1.15 | 0.63; 2.12 | 0.64 |
| Tumour stage – IIIb | 2.76 | 1.67; 4.56 | < 0.01 |
| Tumour stage – IVb | 10.02 | 5.72; 17.57 | < 0.01 |
| Tumour anatomical location - proximal | 1.39 | 0.87; 2.21 | 0.17 |
| Tumour anatomical location – overlapping and unspecifiedb | 1.85 | 1.14; 3.00 | 0.01 |
CI, confidence interval; EBV, Epstein-Barr virus.
normal weight as referent
stage I as referent
distal location as referent
Discussion
In our series, EBV-positive GC patients had better survival compared to EBV-negative patients at median follow-up time of 34.3 months. These results are similar to several previous studies[4–7]. Reasons for this difference are uncertain, but may include enhanced cell-mediated cytotoxicity by tumor-infiltrating lymphocytes, more favorable mutation profile and/or greater sensitivity to chemotherapeutics[17].
We found tumor EBV positivity more frequently in male than female GC patients, similar to several other studies[3,5,6,18–21]. However, a few studies have found no difference in EBV frequency between sexes[22,23]. Males also have greater incidence of other EBV-associated malignancies, including nasopharyngeal carcinoma and Burkitt lymphoma, with somewhat less disparity post-menopause suggesting a potential protection by female sex hormones[24].
It has been reported that EBV-positive GC is more frequent in smokers[25,26]. Our study did not find any difference in smoking status between EBV-positive and EBV-negative patients. In the case-case comparison study of 2,648 patients (184 EBV-positive) by Camargo et al. (2014), the unadjusted OR of EBV-positivity with smoking was 2.2 (CI 1.6 – 3.2), which was attenuated to 1.5 [1.0 – 2.3] by adjustment for possible confounders[25]. A smaller study of 205 patients by C. Koriyama et al. (2005) found prevalence of smokers in EBV-positive GC cases higher than among EBV-negative GC cases, but the difference was not significant (p = 0.13)[26].
Similar to several others[19,21] our study found no distinct histological features in EBV-positive GC. Previous reports regarding histological data are inconclusive. Some studies described higher EBV positivity in diffuse-type GC[5,22] while other studies showed predominance of intestinal-type[6] and poorer differentiation[1,5,27,28]. Van Beek et al. (2004) in a study with 566 patients found that EBV-positivity associated with intestinal-type histology (p = 0.05)[6]. Regarding differentiation, Abdirad et al. (2007) reported Japanese classification for 273 GC cases; solid poorly differentiated adenocarcinoma (por1) and non-solid poorly differentiated adenocarcinoma (por2) were the predominant histologic types in EBV-positive GC, but low numbers of cases in each group precluded formal statistical comparison with EBV-negative GC[22].
EBV associations with anatomical localisation have been inconclusive. Some reports have described predilection for the cardia[5] or proximal stomach[6], while other studies found fundus or body favored[12] and still other reports[22] like our study did not find any relation to localisation. Several reports have described significantly lower tumor-node-metastasis system-stage[1],[5,6] and less lymph node involvement[1,5,6,29] for EBV-positive GC. In our study, we did not observe these differences. We also attempted to characterise the background mucosa adjacent to tumors because EBV-positivity has been described in association with severe atrophic gastritis and a paucity of intestinal metaplasia[30]. However, we found no difference regarding surrounding lesions of atrophy and intestinal metaplasia in EBV-positive and -negative GCs, in agreement with another report[9].
In our study, survival was not significantly associated with age, sex, BMI, stage II tumors and proximal tumor localization. Worse survival was observed for stages III and IV and for overlapping and unspecified tumor localisation.
Some of the hypothesis why EBV positive GC patients have better survival are:
Greater number of gene mutations and the production of neoantigenes:
Cancers with a greater number of gene mutations provoke a stronger antitumor immune response. The thinking behind this hypothesis relates to the production of neoantigens—fragments of proteins expressed on the surface of cancer cells that are encoded by mutated genes. Neoantigens are unique to cancer cells because they are derived from a mutant gene, which may encode a mutant protein that differs from that expressed by normal cells. Therefore, neoantigens have the potential to be recognized as foreign by the cells of the immune system that patrol the body. A greater number of neoantigens mean increased stimulation of immune cells and a stronger immune response [31] and correlate with patient response to both CTLA-4 and PD-1 inhibition. According to data 19% of gastric intestinal type adenocarcinomas have high mutation burden (defined as >20 mutations/Mb) [32].
The Cancer Genome Atlas classification distinguished EBV positive GC subtype based on molecular changes (some of them are: (1) higher prevalence of DNA hypermethylation, (2) strong predilection for PIK3CA mutation, (3) frequent ARID1A (55%) and BCOR (23%) mutations, recurrent JAK2 and ERBB2 amplifications and only rare TP53 mutations, (4) prominent pattern of nucleotide A to C transversions base changes and (5) highly transcribed EBV viral mRNAs and miRNAs). Mutation rates were below 11.4 mutations per megabase (Mb) [2].
High percentage of the tumor infiltrating lymphocytes:
EBV positive GC subtype is described to have high percentage of the tumor infiltrating lymphocytes [33] and the amount of lymphocytes is significantly associated with improved survival [34,35].
Morphological evidence of an activated cytotoxic T-cell infiltrate in EBV-positive gastric carcinoma preventing lymph node metastases:
Additionally, van Beek et al. have suggested that local triggering of cellular immune responses in EBV-positive GC prevents lymph node metastasis formation [36].
In summary, based on our findings which showed no substantial differences clinically (besides male sex in EBV positive group) and morphologically between EBV-positive and EBV-negative GC patients as well as evidence in the published papers, we suggest that reasons contributing to the survival difference could be: greater number of gene mutations and the production of neoantigenes, increased primary tumor inflammation and decreased secondary spread.
Our study is the first report presenting Northern European’ data regarding the association of EBV with GC and survival analysis of EBV-positive and -negative patients. We used standardized collection of patient data, biomaterial, and histological (including EBV status) analysis. We also included a range of covariates in the Cox regression model and our study is one of the largest single centre ones. Thus, the observed difference in survival between EBV-positive and -negative GC patients is meaningful. The novelty of the study is the analysis of the clinical and pathological characteristics in EBV positive and EBV negative gastric cancer groups in a high gastric cancer incidence country, with homogenous Caucasian population, similar diet patterns (all patients were carnivores) and evenly distributed characteristics (with exception of male sex) between both groups. As well as the multifactorial regression model used to analyse survival data.
Unfortunately, we did not have data on some important risk factors for developing gastric cancer, including salt intake and H. pylori infection. However, these characteristics would not be expected to confound an association between tumor EBV-status and mortality.
In conclusion, our study supports other data that EBV-positive GC has better survival. Tumor EBV status should be considered as a prognostic factor in design and analysis of clinical trials. Furthermore, EBV-positive GC may be amenable to targeted therapy and immunotherapy to improve patient outcomes in the future.
Source of funding:
Project No. LZP-2018/1-0135 ‘Research on implementation of a set of measures for prevention of gastric cancer mortality by eradication of H. pylori and timely recognition of precancerous lesions’ of the Latvian Council of Research. This study was supported in part by the Intramural Research Program, US National Cancer Institute.
Footnotes
Compliance with ethical standards
Consent to submit has been received explicitly from all co-authors, as well as from the responsible authorities.
At the time of enrolment all the participants have signed the informed consent. Our study was approved by the Ethics Committee of Riga East University Hospital Support Foundation and Riga East University Hospital.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Nishikawa J, Yoshiyama H, Iizasa H, Kanehiro Y, Nakamura M, Nishimura J, et al. Epstein-barr virus in gastric carcinoma. Cancers [Internet]. 2014. November 7;6(4):2259–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Network TCGAR, Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature [Internet]. 2014. July 23;513:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sousa H, Pinto-Correia A-L, Medeiros R, Dinis-Ribeiro M. Epstein-Barr virus is associated with gastric carcinoma: the question is what is the significance? World journal of gastroenterology [Internet]. 2008/07/21. 2008. July 21;14(27):4347–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Liu J, Qiu H, Kong P, Chen S, Li W, et al. Prognostic significance of Epstein-Barr virus infection in gastric cancer: a meta-analysis. BMC cancer [Internet]. 2015. October 24;15:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camargo MC, Kim W-H, Chiaravalli AM, Kim K-M, Corvalan AH, Matsuo K, et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut [Internet]. 2013/04/12. 2014. February;63(2):236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Beek J, zur Hausen A, Klein Kranenbarg E, van de Velde CJH, Middeldorp JM, van den Brule AJC, et al. EBV-Positive Gastric Adenocarcinomas: A Distinct Clinicopathologic Entity With a Low Frequency of Lymph Node Involvement. Journal of Clinical Oncology [Internet]. 2004. February 15;22(4):664–70. [DOI] [PubMed] [Google Scholar]
- 7.Koriyama C, Akiba S, Itoh T, Sueyoshi K, Minakami Y, Corvalan A, et al. E-cadherin and beta-catenin expression in Epstein-Barr virus-associated gastric carcinoma and their prognostic significance. World journal of gastroenterology [Internet]. 2007/08/07. 2007. August 7;13(29):3925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang MS, Kim WH. Epstein-Barr virus in human malignancy: a special reference to Epstein-Barr virus associated gastric carcinoma. Cancer research and treatment : official journal of Korean Cancer Association [Internet]. 2005/10/31. 2005. October;37(5):257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen H, Zhong M, Wang W, Liao P, Yin X, Rotroff D, et al. EBV infection and MSI status significantly influence the clinical outcomes of gastric cancer patients. Clinica Chimica Acta [Internet]. 2017;471:216–21. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Gastric Cancer, Version 2.2018 — May 22, 2018 [Internet] NCCN Guidelines. 2018. [cited 2018 Dec 12]. p. 1–115. Available from: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf [Google Scholar]
- 11.Smyth EC, Committee on behalf of the EG, Verheij M, Committee on behalf of the EG, Allum W, Committee on behalf of the EG, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Annals of Oncology [Internet]. 2016. September 1;27(suppl_5):v38–49. [DOI] [PubMed] [Google Scholar]
- 12.Riquelme I, Saavedra K, Espinoza JA, Weber H, García P, Nervi B, et al. Molecular classification of gastric cancer: Towards a pathway-driven targeted therapy. Oncotarget [Internet]. 2015. July 22;6(28):24750–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DePinho RA. The age of cancer. Nature [Internet]. 2000. November 9;408:248. [DOI] [PubMed] [Google Scholar]
- 14.Cancer Research UK. Stomach cancer incidence statistics [Internet]. 2018. [cited 2018 Dec 12]. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/stomach-cancer/incidence#heading-One
- 15.American, College of Pathologists, Shi Chanjuan, MD, PhD*; Berlin Jordan, MD; Branton Philip A., MD; Fitzgibbons Patrick L., MD; Frankel Wendy L., MD; Hofstetter Wayne L., MD; Kakar Sanjay, MD; Kelsen David, MD; Klepeis Veronica, MD, PhD; Talmadge Jason P L. Protocol for the Examination of Specimens From Patients With Carcinoma of the Stomach. Stomach 4000 [Internet]. 2017;Stomach 4.:1–16. Available from: https://cap.objects.frb.io/protocols/cp-stomach-17protocol-4000.pdf [Google Scholar]
- 16.Ryan JL, Morgan DR, Dominguez RL, Thorne LB, Elmore SH, Mino-Kenudson M, et al. High levels of Epstein-Barr virus DNA in latently infected gastric adenocarcinoma. Laboratory investigation; a journal of technical methods and pathology [Internet]. 2008/11/10. 2009. January;89(1):80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget [Internet]. 2016. April 28;7(22):32925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song H-J, Kim K-M. Pathology of epstein-barr virus-associated gastric carcinoma and its relationship to prognosis. Gut and liver [Internet]. 2011/06/24. 2011. June;5(2):143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogueira C, Mota M, Gradiz R, Cipriano MA, Caramelo F, Cruz H, et al. Prevalence and characteristics of Epstein-Barr virus-associated gastric carcinomas in Portugal. Infectious agents and cancer [Internet]. 2017. July 19;12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Truong CD, Feng W, Li W, Khoury T, Li Q, Alrawi S, et al. Characteristics of Epstein-Barr virus-associated gastric cancer: a study of 235 cases at a comprehensive cancer center in U.S.A. Journal of experimental & clinical cancer research : CR [Internet]. 2009. February 3;28(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. The American journal of pathology [Internet]. 1992. April;140(4):769–74. [PMC free article] [PubMed] [Google Scholar]
- 22.Abdirad A, Ghaderi-Sohi S, Shuyama K, Koriyama C, Nadimi-Barforoosh H, Emami S, et al. Epstein-Barr virus associated gastric carcinoma: a report from Iran in the last four decades. Diagnostic Pathology [Internet]. 2007;2(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu MS, Shun CT, Wu C, Hsu T, Lin M, Chang MC, et al. Epstein–Barr Virus—Associated Gastric Carcinomas: Relation to H. pylori Infection and Genetic Alterations. Gastroenterology. 2000;118(6):1031–1038. [DOI] [PubMed] [Google Scholar]
- 24.Xie S-H, Yu IT-S, Tse L-A, Mang OW, Yue L. Sex difference in the incidence of nasopharyngeal carcinoma in Hong Kong 1983–2008: Suggestion of a potential protective role of oestrogen. European Journal of Cancer [Internet]. 2013;49(1):150–5. [DOI] [PubMed] [Google Scholar]
- 25.Camargo MC, Koriyama C, Matsuo K, Kim W-H, Herrera-Goepfert R, Liao LM, et al. Case-case comparison of smoking and alcohol risk associations with Epstein-Barr virus-positive gastric cancer. International journal of cancer [Internet]. 2013/08/28. 2014. February 15;134(4):948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koriyama, Akiba S, Minakami Y E Y. Environmental factors related to Epstein-Barr virus-associated gastric cancer in Japan. Exp Clin Cancer Res [Internet]. 2005;24(4):547–53. [PubMed] [Google Scholar]
- 27.Lee J-H, Kim S-H, Han S-H, An J-S, Lee E-S, Kim Y-S. Clinicopathological and molecular characteristics of Epstein–Barr virus-associated gastric carcinoma: A meta-analysis. Journal of Gastroenterology and Hepatology [Internet]. 2009. March 1;24(3):354–65. [DOI] [PubMed] [Google Scholar]
- 28.Yoshiwara E, Koriyama C, Akiba S, Itoh T, Minakami Y, Chirinos JL, Watanabe J, Takano J, Miyagui J, Hidalgo H Chacon P, Linares V nEizuru Y. Epstein-Barr Virus-Associated Gastric Carcinoma in Lima, Peru. Exp Clin Cancer Res [Internet]. 2005;24(1):49–54. A [PubMed] [Google Scholar]
- 29.Ramos MFKP, Pereira MA, Dias AR, Faraj SF, Zilberstein B, Cecconello I, et al. Lymphoepithelioma-like gastric carcinoma: clinicopathological characteristics and infection status. Journal of Surgical Research [Internet]. 2017. April 1;210:159–68. [DOI] [PubMed] [Google Scholar]
- 30.Abe H, Kaneda A, Fukayama M. Epstein-Barr Virus-Associated Gastric Carcinoma: Use of Host Cell Machineries and Somatic Gene Mutations. Pathobiology [Internet]. 2015;82(5):212–23. [DOI] [PubMed] [Google Scholar]
- 31.De Lartigue J. Evidence Builds for Tumor Mutational Burden as Immunotherapy Biomarker. OncologyLive [Internet]. 2018;19(17). Available from: https://www.onclive.com/publications/oncology-live/2018/vol-19-no-17/evidence-builds-for-tumor-mutational-burden-as-immunotherapy-biomarker [Google Scholar]
- 32.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome medicine [Internet]. 2017. April 19;9(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Quadri S, Wolfgang CL, Zheng L. New Development of Biomarkers for Gastrointestinal Cancers: From Neoplastic Cells to Tumor Microenvironment. Biomedicines [Internet]. 2018;6(87):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng X, Song X, Shao Y, Xu B, Chen L, Zhou Q, et al. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis. Oncotarget [Internet]. 2017. May 22;8(34):57386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang BW, Kim JG, Lee IH, Bae HI, Seo AN. Clinical significance of tumor-infiltrating lymphocytes for gastric cancer in the era of immunology. World journal of gastrointestinal oncology [Internet]. 2017/07/15. 2017. July 15;9(7):293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Beek Josine, zur Hausen Axel, Snel Sander N., Berkhof Johannes, Kranenbarg Elma Klein van de V CJH, van den Brule Adriaan J. C. Chris JMM Meijer JLM and B E. Morphological Evidence of an Activated Cytotoxic T-Cell Infiltrate in EBV-Positive Gastric Carcinoma Preventing Lymph Node Metastases. Am J Surg Pathol [Internet]. 2006;Volume 30(Number 1):59–65. [DOI] [PubMed] [Google Scholar]


