Abstract
The effects of nitroglycerin (glyceryl trinitrate, GTN) on baroreflex sensitivity (BRS) are incompletely understood. Moreover, there are no reports evaluating the acute responses in both the sympathetic BRS (SBRS) and the cardiovagal BRS (CBRS) to the administration of sublingual GTN. We hypothesized that sublingual GTN modulates both CBRS and SBRS. In 10 healthy subjects, beat-to-beat heart rate (HR), blood pressure (BP), and muscle sympathetic nerve activity (MSNA) were recorded before and for 10 min after sublingual administration of GTN 0.4 mg. SBRS was evaluated from the relationship between spontaneous variations in diastolic BP and MSNA. CBRS was assessed with the sequence technique. These variables were assessed during baseline, during 3rd–6th min (post A), and 7th–10th min (post B) after GTN administration. Two min after GTN administration, MSNA increased significantly and remained significantly elevated during recording. Compared with baseline, CBRS decreased significantly (post A: 12.9 ± 1.6 to 7.1 ± 1.0 ms/mmHg, P < 0.05), whereas SBRS increased significantly (post A: 0.8 ± 0.2 to 1.5 ± 0.2 units·beat−1·mmHg−1, P < 0.05) with an upward shift of the operating point. There were no differences in these variables between posts A and B. A clinical dose of GTN increased MSNA rapidly through effects on both CBRS and SBRS. These effects should be kept in mind when nitrates are used to clinically treat chest pain and acute coronary syndromes and used as vasodilators in experimental settings.
Keywords: baroreflex sensitivity, muscle sympathetic nerve activity, nitrate
INTRODUCTION
Nitrates such as nitroglycerin (glyceryl trinitrate, GTN), isosorbide dinitrate and mononitrate, and nitroprusside are among the most commonly used vasodilator substances in clinical practice. These agents work by producing nitric oxide (NO), a regulator of vascular tone, via the l-arginine-NO synthetase (NOS) pathway, and NOS-independent pathways, that convert NO2− and NO3−, products of NO metabolism, back to NO (1). Acute favorable effects of nitrates on relaxing the vascular tone and reducing myocardial workload are well-established (2). Therefore, GTN and other nitrates are generally used to treat patients with ischemic heart disease and hypertension, especially when complicated by acute heart failure or stroke (3, 4). Nitroprusside is also used as a hypotensive drug in experimental studies to evaluate the autonomic nervous responses to acute changes in blood pressure (BP) (5, 6). However, the direct effect of nitrates on parasympathetic nerve activity (para-SNA) and sympathetic nerve activity (SNA) is still debated (7).
It was previously reported that muscle sympathetic nerve activity (MSNA) increased 90 min after the administration of isosorbide dinitrate (8). In that study, an arterial baroreceptor-mediated mechanism was speculated as the cause of SNA excitation; however, baroreflex function was not measured in detail. The arterial baroreflex is an important feedback mechanism that counteracts the change in BP (7), via regulating heart rate (HR) and sympathetic outflow to peripheral vascular beds. Generally, the sensitivity of baroreflex (BRS) is used to evaluate baroreflex control of HR (i.e., cardiovagal BRS, CBRS) and sympathetic activities (SBRS). The baroreflex plays a key role in maintaining homeostasis under various physiological conditions, such as exercise or orthostatic stress, and both SBRS and CBRS as well as the set points can be altered under these conditions (9–12). Impaired CBRS has been reported to contribute to adverse cardiovascular outcomes, including life-threatening arrhythmias and sudden death (13, 14). In addition, blunted SBRS was observed in many conditions characterized by augmented SNA, including drug-resistant hypertension (15) and chronic heart failure with reduced ejection fraction (HFrEF) (16). The degree of impairment in SBRS was associated with HFrEF severity (17). Importantly, the spontaneous BRS method was used in most of these studies (15, 17). Moreover, augmented SNA in cardiovascular diseases is significantly associated with poor prognosis and therefore is regarded as a therapeutic target (18, 19). Hence, understanding the effects of nitrates on SNA and BRS responses in the setting of cardiovascular diseases is of considerable clinical importance.
Sublingual GTN is a time-tested supplier of organic nitrate. Because of its fast-acting characteristics, it is commonly used to treat angina pectoris (2). Decreased CBRS after GTN administration was reported previously (20), but CBRS was evaluated 6 days after administration, and the acute effect of GTN on CBRS was not assessed in that study. In addition, neither SBRS nor MSNA were measured in the study. To our knowledge, there have been no reports so far evaluating both SBRS and CBRS responses to GTN, simultaneously. Moreover, the acute responses of SNA and BRS to the administration of GTN have never been investigated. Sublingual GTN is often used to quell chest pain acutely in patients with acute coronary syndromes including acute myocardial infarctions (MI). Interestingly over 50% of deaths occur within the first 60 min of MI (21). Fatal arrhythmias during the acute phase of MI are induced by sympathetic over activation (22). Accordingly, understanding the causes of sympathetic over activation during the acute phase of MI is an area of critical clinical importance. Therefore, the purpose of this study was to evaluate the acute effects of a clinical dose of sublingual GTN on SNA, SBRS, and CBRS in healthy subjects. We hypothesized that sublingual GTN would rapidly increase MSNA by altering both SBRS and CBRS.
METHODS
Subjects
Ten healthy subjects (9 male and 1 female) were included in this study. All subjects were free of any cardiovascular or other diseases and received no medications. The average age, height, and weight were 60 ± 2 yr, 175 ± 3 cm, and 87 ± 6 kg, respectively. Subjects refrained from alcohol, exercise, and caffeine for 24 h before measurement. The experimental protocol of this study was approved by the Institutional Review Board of the Milton S. Hershey Medical Center and conformed with the Declaration of Helsinki. Each subject had the purposes, the protocol, and the risks explained to them before written informed consent was obtained.
Measurements
Beat-to-beat HR was acquired via electrocardiogram (Cardicap 5, Datex-Ohmeda, GE, Healthcare). Beat-to-beat BP was recorded by finger photoelectric plethysmography (Finometer, Finapress Medical System, Amsterdam, The Netherlands) with resting values verified by the cuff pressure from the brachial artery (SureSigns, VS3, Philips, Philip Medical system). The cuff pressure was measured at least two times at rest. Respiratory frequency was monitored using piezoelectric pneumography, and subjects were instructed to avoid breath holding during the protocols. Postganglionic multiunit MSNA was recorded directly from a peroneal nerve using a tungsten microelectrode, as described previously (23). Briefly, the recording electrode was inserted percutaneously and adjusted until spontaneous pulse synchronous multiunit bursts of MSNA, which meet the established criteria (24), could be obtained. The nerve signal was amplified, band pass filtered (500–5,000 Hz), rectified, and integrated with a time constant of 0.1 s (Iowa Bioengineering, Iowa City, IA). The MSNA signal was monitored both audibly and visually throughout the study protocol. This was done to determine if the MSNA recording remained stable during the study.
Experimental Protocol
The subjects entered the laboratory after voiding, underwent anthropometric measurements, and were then placed in a supine position for instrumentation, including the placement of tungsten electrodes to obtain sympathetic nerve recordings (∼30 min). Subjects were asked to rest quietly during an acclimation period before the data collection period. The acclimation period was at least 5 min or until the hemodynamic variables and MSNA were stable. After a 5-min baseline data collection, sublingual GTN 0.4 mg was administered. Data collection was continued for 10 min after GTN administration.
Data Analysis
All signals were recorded at a sampling frequency of 200 Hz via a data-acquisition system (MacLab, AD Instruments, Castle Hill, Australia) (25). First, MSNA bursts in the integrated MSNA traces were identified by visual inspection of the data, together with the burst sound from the audio amplifier, then these bursts were further evaluated by software that identified bursts based upon fixed criteria, including an appropriate latency following the R-wave of the electrocardiogram (25, 26). MSNA bursts in the integrated MSNA traces were normalized by assigning a value of 100 to the mean amplitude of the top 10% largest bursts among all bursts during the baseline periods to reduce variability between subjects attributed to factors including needle position and signal amplification (25). Using the mean amplitude of the top 10% largest bursts for the normalization was to decrease the possible influence of background noise on the peaks of the largest bursts. MSNA was expressed as burst frequency (BF) (bursts/min), burst incidence (BI) (bursts/100 heartbeats), total MSNA (units/min), and burst area (units/burst). Total MSNA was calculated as the sum of the burst area of the integrated neurogram on a beat-to-beat basis. If no MSNA bursts were detected for a particular cardiac cycle, a zero value was assigned for the cardiac cycle. Beat-to-beat HR, RRI, SBP, DBP, and MSNA were recorded simultaneously. MSNA, BP, and HR were analyzed over the baseline (5 min) and every 2 min after GTN at first (from 1st to 5th period) (Fig. 1A). There were no significant changes from the baseline values during the first 2 min after GTN. Thus, the data of the first 2 min after GTN administration were excluded in the BRS analysis. In addition to absolute SBP and DBP value, a linear relationship between SBP or DBP and time (s) was also evaluated (Y: DBP or SBP, X: time s).
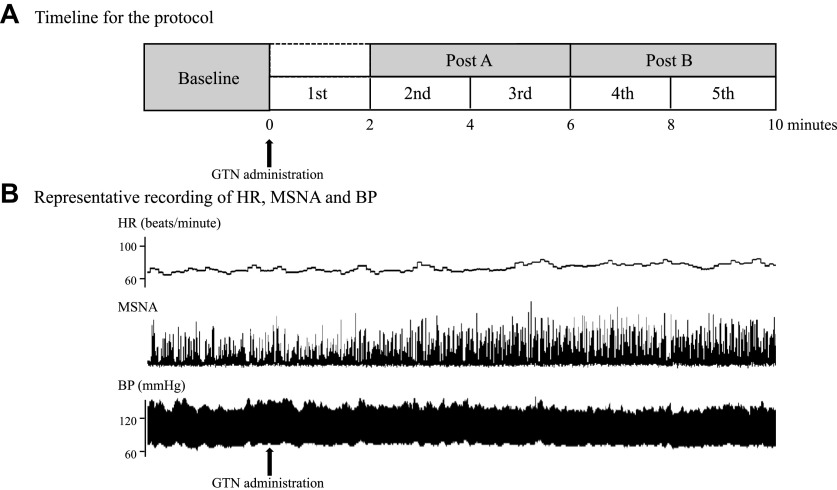
Figure 1.
A: block diagram of the timeline for the protocol. B: representative recordings of beat-to-beat heart rate (HR), integrated muscle sympathetic nerve activity (MSNA), and blood pressure (BP). MSNA clearly increased 2 min after nitroglycerin (GTN) administration.
The slope of the relationship between systolic BP (SBP) and cardiac RR interval (RRI) was used as an index of CBRS (7, 27), whereas the slope of the relationship between diastolic BP (DBP) and MSNA parameters (BI, total MSNA, and burst area) was used as an index of SBRS (SBRS-BI, SBRS-total MSNA, and SBRS-burst area) (28, 29). In this study, CBRS was assessed using the sequence method (27), and SBRS was assessed during spontaneous BP changes (28, 30). Our initial data analysis showed that the 2-min data segment was too short to reliably evaluate CBRS and SBRS, because with spontaneous BP changes, the sequence numbers were small in some data segments, and the variability in the CBRS was large. On the other hand, the correlation coefficient (R value) for SBRS was also low and unacceptable (R < 0.5) (31) in most subjects. Therefore, to analyze BRS, the timeline was divided into three periods (baseline; post A, from 2nd to 3rd period; post B, from 4th to 5th period) (Fig. 1A).
To determine reflex vagal control, the beat-to-beat time series of SBP and RR interval (RRI) were analyzed using Hemolab software (Harald Stauss Scientific, Iowa City, IA) as previously reported (32, 33). Briefly, sequences of three or more beats, during which SBP and the RRI of the following beats altered in parallel (i.e., positive correlation), were detected and divided into two groups (up sequences, rising SBP; down sequences, decreasing SBP). The mean value of the slope calculated by the relationship between the change of SBP and RRI (ms/mmHg) was regarded as CBRS. The up sequences and down sequences were calculated separately at first. Moreover, the mean slope of all sequences (i.e., the combination of both up and down sequences) was calculated as general CBRS. A least-square linear regression analysis was applied to each individual sequence, and only sequences in which R2 > 0.80 were accepted.
As described in prior reports (33–35), SBRS was assessed from the slope of the linear regression between DBP and MSNA with spontaneous BP changes. In brief, DBP for each cardiac cycle was grouped into 3 mmHg interval bins. “Burst incidence” for a given 3 mmHg DBP bins was determined and was expressed as the percentage of cardiac cycles in which a burst occurred for a given DBP bin. In each 3 mmHg DBP interval, the total burst area was calculated, and the total burst area was divided by the number of MSNA bursts occurring within the described DBP group. This value presented the mean “Burst area” for a given DBP bin. The total burst area within a DBP bin was divided by the number of cardiac cycles that occurred within that interval, which represented the “Total MSNA” for a given DBP bin. By using all binned data, we identified the slopes for the relationship between the MSNA (burst incidence, burst area, and total MSNA) and mean DBP, using linear regression analysis. For all linear regression analyses, the data were weighted for the number of cardiac cycles within each DBP bin. These slopes were used as indices for the SBRS. If the R value of the regression line ≥ 0.5, the slope of the line was regarded as an acceptable SBRS slope (31). The points corresponding to the averaged DBP of each subject on the regression lines relating MSNA (BI, total MSNA, and burst area) to DBP (not the points corresponding to the mean MSNA value) were determined as the operating points, an index of the MSNA corresponding to the BRS operating pressure for assessing the shift of the BRS curves (36–38). From the SBRS-BI slope, we calculated the DBP at which 50% of the cardiac cycles were related to a burst, which is known as the T50 value (28). The difference between T50 and average DBP (T50-DBP) in each subject was calculated as “error signal” (38).
To evaluate SBRS hysteresis, SBRS which used only DBP rising beats for x-axis (SBRSrise) and SBRS which used only DBP falling beats for x-axis (SBRSfall) were calculated separately by the same methods with previous studies (39, 40). DBP rising beats were defined as the cardiac cycles that were preceded by a cardiac cycle with a lower DBP, and thus a DBP rising sequence was defined as a collection of consecutive DBP rising beats (≥ 2 beats). DBP falling beats were defined as the cardiac cycles that were preceded by a cardiac cycle with a higher DBP, and a DBP falling sequence was defined as a collection of consecutive DBP falling beats (≥ 2 beats). Linear regression analyses between DBP and MSNA of rising or falling beats were performed and weighted for the number of beats and the slopes of their R value ≥ 0.5 were accepted as SBRS slope. Because the relationship between burst area and DBP was weak (R < 0.5 in 5 subjects at baseline), similar to a previous report (37), and it is expected that SBRS slope with acceptable R value is further decreased by dividing beats into rising or falling, we did not calculate the hysteresis of the SBS-burst area. To evaluate beat-to-beat DBP fluctuations in more detail, DBP falling beats/all beats (%) and DBP falling rate mmHg/s were calculated. In addition, with the DBP falling sequences in above SBRSfall analysis, DBP falling sequences number/min and DBP falling beats/sequence were calculated.
Statistical Analysis
We performed a sample size calculation by using G*Power 3.1 (41). The effect size for significant MSNA (BF) change was decided as 0.6 based on the previous report using MSNA change as the primary outcome (42). Using this effect size, an a priori power analysis indicated that eight subjects would be required to achieve statistical significance with a desired power of 0.8 and α-error of 5%. After data were collected, all statistical analyses were performed using SPSS software (SPSS Science v. 27.0, IBM). A linear mixed-effects model was performed to assess the effects of time on the HR, BP, and MSNA over each 2-min period. The measurements (SBRS, CBRS, T50, T50 error signal, HR, BP, and MSNA) over each 4-min period were also evaluated by linear mixed-effects models including fixed effects for time (baseline, post A, and post B). Age and body mass index (BMI) were applied as a covariate to all mixed-effect models. DBP and SBP were applied as a covariate to SBRS and CBRS analysis, respectively. When a significant interaction effect was observed, a post hoc for multiple comparison was conducted using a Holm-Sidak correction. All continuous variables are presented as means ± standard error of the mean (SEM). Values of P < 0.05 (two-sided) were considered statistically significant.
RESULTS
Responses of HR, BP, and MSNA to GTN Administration
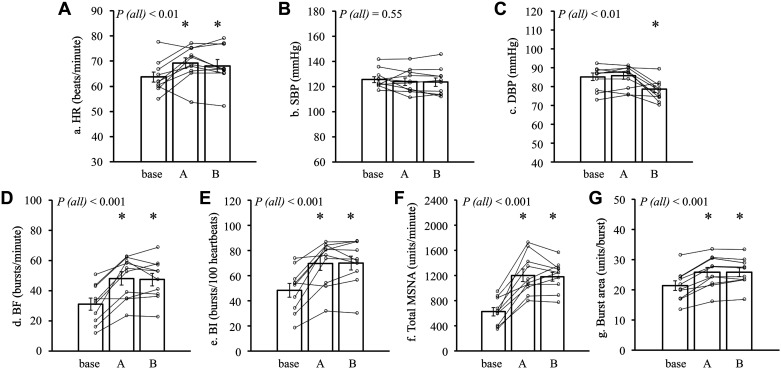
Representative recordings of beat-to-beat HR, integrated MSNA, and BP after GTN administration are shown in Fig. 1B. The recordings show that MSNA was clearly increased 2 min after GTN with minimal change in BP (Fig. 1B). In the whole group data analysis (n = 10), compared with baseline, SBP was statistically unchanged during 10 min after GTN administration. DBP during the 4–5th period was significantly lower than baseline values (both, P < 0.05) (Table 1). MSNA did not change significantly from baseline during the first 2 min after GTN. All MSNA parameters significantly (BF, BI, total MSNA, and burst area) rose after 2 min and remained elevated for 10 min after GTN (all, P < 0.05) (Table 1). The averaged HR, SBP, DBP, and MSNA over posts A and B are shown in Fig. 2. HR in posts A and B was significantly greater than baseline (both, P < 0.05). SBP in posts A and B was statistically unchanged compared with baseline (post A, P = 0.67; post B, P = 0.56). DBP in post A was statistically unchanged compared with baseline (P = 0.94); however, DBP in post B was significantly decreased compared with baseline (P < 0.05). All MSNA indices (BF, BI, total MSNA, and burst area) in posts A and B were significantly increased (all, P < 0.05) compared with baseline (Fig. 2).
Table 1.
Changes in HR, BP, and MSNA
| Period |
|||||||
|---|---|---|---|---|---|---|---|
| Baseline | 1st | 2nd | 3rd | 4th | 5th | P (all) | |
| HR, beats/min | 63.7 ± 2.0 | 63.1 ± 2.1 | 68.4 ± 2.1* | 70.0 ± 2.2* | 69.3 ± 2.6* | 66.9 ± 2.4 | <0.001 |
| SBP, mmHg | 125.5 ± 2.4 | 125.9 ± 2.5 | 124.9 ± 3.1 | 122.9 ± 2.9 | 123.5 ± 3.4 | 123.6 ± 3.3 | 0.391 |
| DBP, mmHg | 85.2 ± 2.0 | 86.0 ± 1.8 | 86.9 ± 2.0 | 84.6 ± 1.7 | 79.0 ± 1.7* | 78.1 ± 1.80* | <0.001 |
| MSNA BF, bursts/min | 31.1 ± 4.0 | 34.2 ± 4.1 | 46.5 ± 4.2* | 49.9 ± 4.7* | 48.8 ± 4.2* | 45.9 ± 4.2* | <0.001 |
| MSNA BI, bursts/100 heartbeats | 48.2 ± 5.5 | 53.8 ± 5.6 | 68.1 ± 5.6* | 71.3 ± 6.1* | 70.9 ± 5.9* | 68.7 ± 5.5* | <0.001 |
| Total MSNA, units/min | 625 ± 66.3 | 727 ± 70.7 | 1,139 ± 110* | 1,263 ± 96.8* | 1,210 ± 81.7* | 1,143 ± 68.6* | <0.001 |
| MSNA burst area, units/burst | 21.3 ± 1.6 | 22.7 ± 1.7 | 25.2 ± 1.8* | 26.3 ± 1.6* | 25.7 ± 1.4* | 25.9 ± 1.5* | <0.001 |
Data are presented as means ± SE. BF, burst frequency; BI, burst incidence; DBP, diastolic blood pressure; HR, heart rate; MSNA, muscle sympathetic nerve activity; SBP, systolic blood pressure; period (1st, 2nd, 3rd…), these periods are defined as shown in Fig. 1. P (all): P value for the effect of time on the whole trial by linear mixed effects model. *P < 0.05 compared with baseline by linear mixed effects model; n = 10 subjects.
Figure 2.
The average values in heart rate (HR; A), systolic blood pressure (SBP; B), diastolic blood pressure (DBP; C), burst frequency (BF; D), burst incidence (BI; E), total muscle sympathetic nerve activity (MSNA; F), and burst area (G) during each period. Small symbols represent individual data (subject number n = 10). Bars represent the average value of each period. P (all): P value for the effect of time on the whole trial by linear mixed-effects model (covariate; age and BMI). *P < 0.05 compared with baseline by linear mixed-effects model. BMI, body mass index.
Both SBP and DBP in most patients were loosely correlated with time after GTN (Table 2), but the correlation coefficient values were low (R < 0.5). The slope of the linear relationship between DBP (or SBP) and time was not significantly changed after GTN (Table 2).
Table 2.
Correlation between BP and time
| 0-10 Min after GTN | Baseline | Post A | Post B | P (all) | |
|---|---|---|---|---|---|
| Slope (DBP vs. time) |
−0.02 ± 0.004 (n = 10) |
−0.01 ± 0.01 (n = 7) |
−0.02 ± 0.003 (n = 9) |
−0.01 ± 0.004 (n = 8) |
0.29 |
|
R (DBP vs. time) |
−0.45 ± 0.12 (n = 10) |
−0.13 ± 0.18 (n = 7) |
−0.43 ± 0.06 (n = 9) |
−0.26 ± 0.09 (n = 8) |
0.40 |
| Slope (SBP vs. time) |
−0.01 ± 0.005 (n = 7) |
0.01 ± 0.02 (n = 8) |
−0.01 ± 0.01 (n = 10) |
0.001 ± 0.01 (n = 7) |
0.88 |
|
R (SBP vs. time) |
−0.21 ± 0.11 (n = 7) |
0.09 ± 0.15 (n = 8) |
−0.18 ± 0.13 (n = 10) |
0.004 ± 0.14 (n = 7) |
0.97 |
Data are presented as means ± SE. DBP, diastolic blood pressure; GTN, glyceryl trinitrate; n, number of subjects in who the correlation between SBP (or DBP) vs. time was significant; SBP, systolic blood pressure; time, time scale represented by seconds; P (all) indicates the P value for the effect of time on the whole trial by linear mixed-effects model (comparison between baseline, Post A and post B).
Responses of CBRS to GTN Administration
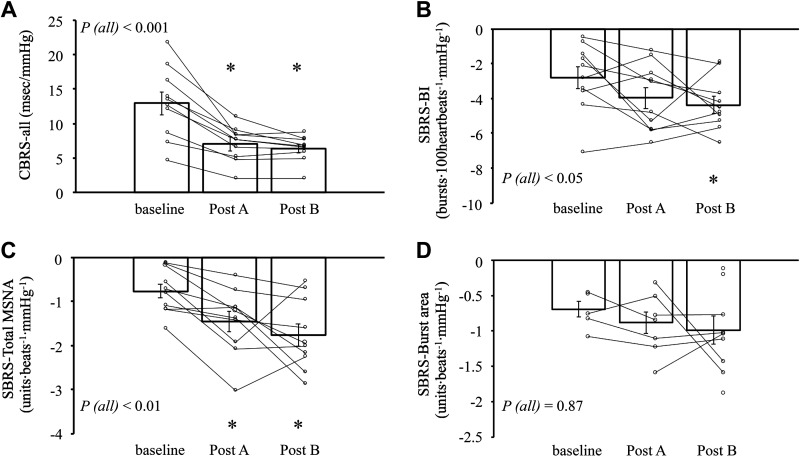
The group average CBRS of all sequences (i.e., the combination of both up and down sequences) and individual values are presented in Fig. 3. The CBRS slopes of up and down sequences are shown in Table 3. CBRS slopes in all directions (all, up, and down sequences) during posts A and B were significantly lower than baseline (all, P < 0.05; linear mixed model; covariate, SBP, BMI, and age).
Figure 3.
The slope of CBRS-all (A), SBRS-BI (B), SBRS-total MSNA (C), and SBRS-burst area (D). Small symbols represent individual data. Bars represent the average value of each period. n = 10 for CBRS, SBRS-BI, and SBRS-total MSNA analysis. In SBRS-burst area analysis, n = 5 for baseline, n = 7 for post A, and n = 9 for post B, others were excluded by their R value < 0.5. In CBRS analysis, age, BMI, and SBP were used as a covariate in linear mixed-effects model for the effect of time. In SBRS analysis, age, BMI, and DBP were used as a covariate. P (all): P value for the effect of time on the whole trial by linear mixed effects model. *P < 0.05 compared with baseline by linear mixed-effects model; n, number of subjects. BI, burst incidence; BMI, body mass index; CBRS, cardiovagal baroreflex sensitivity; DBP, diastolic blood pressure; MSNA, muscle sympathetic nerve activity; SBRS, sympathetic baroreflex sensitivity.
Table 3.
Variables in the BRS assessment
| Time, min | Baseline | Post A | Post B | P (all) |
|---|---|---|---|---|
| Cardiovagal BRS | ||||
| Slope-up, ms/mmHg | 12.3 ± 1.5 | 6.9 ± 0.8* | 6.1 ± 0.6* | <0.001 |
| Slope-down, ms/mmHg | 13.4 ± 2.0 | 7.2 ± 0.8* | 6.6 ± 0.7* | <0.001 |
| P (up vs. down) | 0.47 | 0.73 | 0.49 | |
| Sympathetic BRS | ||||
| R-BI | 0.8 ± 0.04 | 0.9 ± 0.02 | 0.9 ± 0.03 | 0.06 |
| R-total MSNA | 0.8 ± 0.05 | 0.9 ± 0.02 | 0.9 ± 0.03 | 0.06 |
| R-burst area | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.14 |
| T50, mmHg | 80.1 ± 7.6 | 89.3 ± 1.9 | 82.9 ± 2.8 | 0.26 |
| Error signal, mmHg | −5.1 ± 7.3 | 3.5 ± 2.7 | 4.4 ± 2.1 | 0.10 |
Data are presented as means ± SE; n = 10 subjects. BI, burst incidence; BMI, body mass index; BRS, baroreflex sensitivity; DBP, diastolic blood pressure; MSNA, muscle sympathetic nerve activity; R, correlation coefficient of the regression line; SBP, systolic blood pressure; T50, the midpoint pressure in the sympathetic BRS (SBRS) curve; error signal, the difference between SBRS midpoint (T50) and actual operating DBP. P (all): P value for the effect of time on the whole trial. *P < 0.05 compared with baseline by linear mixed-effects model (covariate for cardiovagal BRS; age, BMI, and SBP. Covariate for sympathetic BRS; age, BMI, and DBP).
Responses of SBRS to GTN Administration
The group average SBRS (BI, total MSNA, and burst area) slopes, as well as individual values over each period, are presented in Fig. 3. The correlation coefficient (R) of SBRS slopes over each period is shown in Table 3. The SBRS-total MSNA slope (Fig. 3) significantly increased after GTN (both in posts A and B) (P < 0.05, linear mixed model; covariate, DBP, age, and BMI). The SBRS-BI slope in post B was significantly greater than baseline (P < 0.05; covariate, DBP, age, and BMI). SBRS-burst area slopes through all periods were obtained completely from only three subjects and other data were excluded due to low R value (< 0.5). SBRS-burst area slopes did not change significantly during the measurement (P = 0.87, linear mixed model; covariate, DBP, age, and BMI) (Table 3).
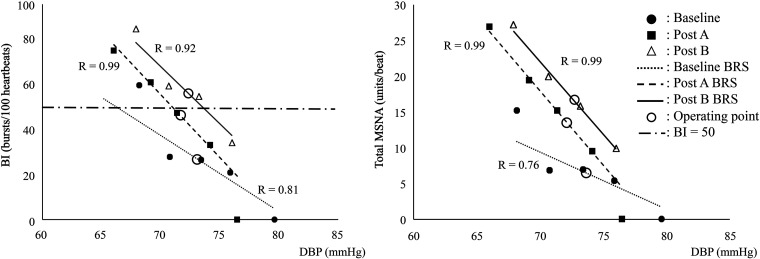
T50 and the error signal value were statistically unchanged during the measurements (Table 3). Representative SBRS curves with operating points are presented in Fig. 4. DBP at the intersection point between the SBRS-BI curve and the straight line of BI = 50 (bursts/100 heartbeats) indicates the T50 value. BI and DBP, as well as Total MSNA and DBP, showed linear relationships with high R values. In the representative data (Fig. 4), obvious increases of slope and upward shift of both BI and total MSNA SBRS in post A and post B were observed.
Figure 4.
Representative data of the relationship between DBP and BI (left) or DBP and total MSNA (right). Operating point, point on the regression line corresponding to the averaged diastolic pressure of the subject. BI, burst incidence; BRS, baroreflex sensitivity; DBP, diastolic blood pressure.
As shown in Fig. 2, averaged DBP did not significantly change in post A. However, averaged BI, total MSNA, and burst area at the operating point were significantly increased compared with baseline (P < 0.05), indicating upward shift of operating points (Table 4).
Table 4.
Comparison of MSNA values at the operating point among each period
| Baseline | Post A | Post B | P (all) | |
|---|---|---|---|---|
| MSNA BI, bursts/100 heartbeats | 49.6 ± 6.0 | 69.2 ± 5.9* | 69.5 ± 5.7* | <0.001 |
| Total MSNA, units/beat | 10.0 ± 1.1 | 17.0 ± 1.3* | 17.6 ± 1.2* | <0.001 |
| MSNA burst area, units/burst | 20.0 ± 1.7 | 25.0 ± 2.7* | 24.9 ± 1.9* | <0.001 |
Data are presented as means ± SE; n = 10 subjects. P (all): P value for the effect of time on the whole trial by linear mixed-effects model. *P < 0.05 compared with baseline by linear mixed-effects model (covariate; age and BMI). BI, burst incidence; BMI, body mass index; MSNA, muscle sympathetic nerve activity.
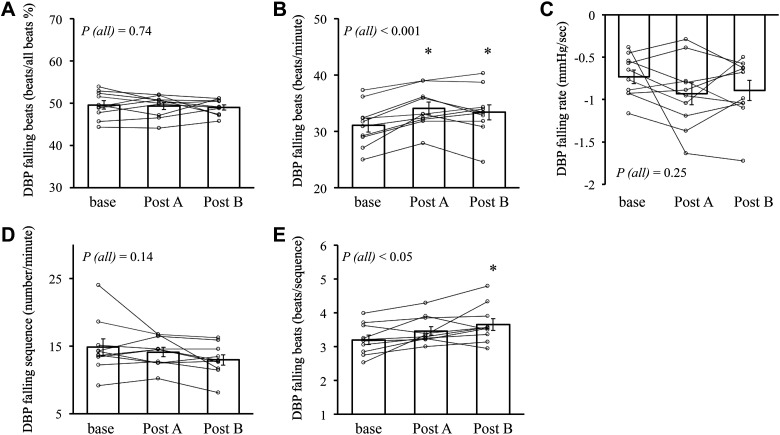
The results of SBRS hysteresis analysis and beat-to-beat blood pressure analysis are shown in Table 5. There were no differences between the SBRSrise slope and SBRSfall slope (both in SBRS-BI and SBRS-total MSNA, P > 0.05) (Table 5). SBRS-total MSNA slope of both rising and falling beats was significantly increased after GTN as with the result of SBRS slope of all beats. The ratio of falling beats per all beats was statistically unchanged during all periods, whereas falling beats/min was significantly increased after GTN (both in posts A and B) (Fig. 5). DBP falling rate (mmHg/s) and DBP falling sequences/min were unchanged after GTN; however, DBP falling beats/sequence in post B was significantly increased compared with baseline (Fig. 5).
Table 5.
Hysteresis of the SBRS and beat-to-beat blood pressure analysis
| Baseline | Post A | Post B | P (all) | |
|---|---|---|---|---|
| SBRS-slope | ||||
| SBRSrise-BI | −3.2 ± 0.6 | −2.9 ± 0.2 | −3.5 ± 0.7 | 0.70 |
| SBRSfall-BI | −2.9 ± 0.7 | −4.1 ± 0.7 | −3.8 ± 0.6 | 0.34 |
| P (rising vs. falling) | 0.56 | 0.15 | 0.68 | |
| SBRSrise-total MSNA | −0.8 ± 0.2 | −1.4 ± 0.1* | −1.6 ± 0.3* | <0.01 |
| SBRSfall-total MSNA | −0.7 ± 0.2 | −1.1 ± 0.2 | −1.4 ± 0.1* | <0.01 |
| P (rising vs. falling) | 0.16 | 0.26 | 0.41 |
Data are presented as means ± SE; n = 10 subjects. BI, burst incidence; BMI, body mass index; DBP, diastolic blood pressure; MSNA, muscle sympathetic nerve activity; SBRS, sympathetic baroreflex sensitivity; SBRSfall, SBRS comprised of DBP falling beats; SBRSrise, SBRS comprised of DBP rising beats; P (all): P value for the effect of time on whole trial by linear mixed effects model. *P < 0.05 compared with baseline by linear mixed-effects model (covariate; age, BMI, and DBP). P (rising vs. falling); P value between SBRSrise and SBRSfall was evaluated by paired t test.
Figure 5.
The proportion of DBP falling beats over all beats (%) in all sequences (A), number of DBP falling beats per minute (beats/min; B), DBP falling rate in DBP falling sequences (mmHg/s; C), number of DBP falling sequences per minute (number/min; D), and number of falling beats per DBP falling sequence (E); small symbols represent individual data. Bars represent the average value of each period. DBP, diastolic blood pressure; P (all): P value for the effect of time on the whole trial by linear mixed-effects model. *P < 0.05 compared with baseline by linear mixed-effects model (covariate; age, and BMI); n = 10 subjects.
DISCUSSION
The novel findings of this study are that 1) a typical clinical dose of GTN induced an acute increase in MSNA and 2) these responses in MSNA and hemodynamic variables after GTN were associated with an increase in SBRS slope and upward shift of the operating point and a decrease in CBRS.
Nitrate and Sympathetic Nerve Activity
Nitrates including GTN are widely used as vasodilator drugs. However, the development of tolerance that attenuates the vasodilator effect of nitrates during long-term use is often observed (43, 44). Long-term use of nitrates has been reported to induce lack and/or dysfunction of endogenous NO synthases (NOS), and abnormalities of NOS could cause reduction of endogenous nitrate production, resulting in the development of nitrate tolerance (45, 46). It has been suggested that nitrate tolerance is accompanied by the enhancement of SNA (47, 48). In an animal model, lack of NOS in the brain was reported to attenuate the central sympatho-inhibitory effect of nitrate (45). Although the relationship between nitrates and SNA in the chronic phase has been previously studied (45, 47, 48), the acute response of SNA to nitrates before the development of tolerance remains uncertain. In this study, GTN, a representative organic nitrate, increased all MSNA parameters (BF, BI, total MSNA, and burst area) within 10 min after administration in healthy subjects. DBP in our study tended to decrease after GTN and significantly decreased during the last 4 min of the measurements (post B). In addition, a loosely linear negative relationships between DBP and time after GTN were observed in most patients, but the correlations were weak (R < 0.5) and the slope was not significantly different compared with baseline (Table 2). Thus, the decrease in mean DBP was not large. Therefore, we speculate that with the increased SBRS slope and the upward shift set operating point, the BP decrease during the BP fluctuations would evoke greater MSNA responses, which would mainly contribute to the increase in mean MSNA after GTN. On the other hand, the role of the slight decrease in mean DBP on the MSNA activation cannot be excluded.
Effect of GTN on BRS
Previously, and in contrast to our results, one study using rabbits demonstrated that transdermal GTN treatment shifted the CBRS curve leftward without a change in slope (49). In that study, CBRS was calculated by manipulating BP using nitroprusside and phenylephrine, and BP was significantly decreased by transdermal GTN. It cannot be excluded that the use of the nitrate, nitroprusside, directly affected CBRS, and significant BP change by transdermal GTN might also have affected BRS. In our study, we evaluated CBRS with the spontaneous method, and averaged SBP in each period was unchanged by GTN. We believe these conditions made it possible to evaluate the direct effects of GTN on BRS in our study. To our knowledge, only one study has evaluated the effect of GTN on BRS in humans (20). In that study, 0.6 mg/h transdermal GTN decreased CBRS 6 days after administration had begun. The authors speculated that decreased CBRS might have contributed to the development of nitrate tolerance. This result is similar to our result where CBRS was decreased by GTN. However, in this study, a reduction in CBRS was observed within 10 min after administration of GTN. Our results indicate that decreased CBRS was not linked to nitrate tolerance, but rather that decreased CBRS can be induced rapidly after a single dose of GTN. In addition, increased SBRS, as well as an upward shift of the operating point, was confirmed in this study. These findings provide insights about the sympatho-excitatory effect of GTN unrelated to nitrate tolerance but caused by both altered SBSR and CBRS.
Total MSNA reflects both burst frequency and strength, and thus it is reported that total MSNA is a better parameter for quantifying the level of MSNA than evaluating BI and Burst area, separately (50, 51). It was demonstrated that total MSNA reflected the increase of plasma norepinephrine concentration, NE spillover rate, and the change of calf vascular resistance more closely than BI and BF (52, 53). Therefore, it is not surprising that the change of SBRS-Total MSNA was more sensitive compared with SBRS-BI (Fig. 3, Table 5). On the other hand, it is suggested that changes in the number of bursts (gating effect) and the strength of the bursts are regulated differently in the central nervous system (34). It cannot be excluded that the effect of GTN (directly or via other reflexes) was more dominant on the region of regulating the strength of bursts than on the region of regulating gating effect. At least, our results indicate that GTN affects both the frequency and strength of MSNA because both BF (BI) and burst area were significantly increased by GTN. In our study, SBRS-burst area through all periods could be obtained from only three subjects because of a weak linear relationship (R < 0.5). Thus, comparing the differences in SBRS-burst area among periods was difficult, which has also been reported previously (37, 50). Future studies with a larger number of subjects are needed to verify this issue.
In this study, SBRS was analyzed with the approach for spontaneous BP changes, whereas the mean DBP was gradually decreased. A prior report (10) suggested that SBRS assessed by this method could be affected by the gradual BP changes. However, in this study, the significant linear relationship between DBP and MSNA (BI and total MSNA) was not compromised in posts A and B (both average R = 0.9). This could be due to that the BP changes in this study were less than that study (10) and that the negative relationship between DBP and time was loose (i.e., low R value). Moreover, after adjusted with DBP data, the SBRS during post A and post B was still greater than the baseline values. Therefore, we believe it is unlikely that a small BP change in this study affected SBRS slope value.
It was suggested that DBP fluctuations could affect BRS (54). It was reported that the SBRS slope consisted of cardiac cycles preceded by a higher DBP cardiac cycle (DBP falling beat) was higher than the SBRS slope consisted of cardiac cycles preceded by a lower DBP cardiac cycle (DBP rising beat) in young healthy subjects (55). It was also suggested that a greater ratio of DBP falling beats caused a significant increase in SBRS slope despite a small absolute DBP change (54). Therefore, we performed a SBRS hysteresis analysis (Table 5) and calculated the ratio of DBP falling beats. There were no statistical differences between the SBRSrise slope and the SBRSfall slope. Thus, the SBRS increased during both DBP rising and falling phases in DBP fluctuations. As aforementioned, differences between the SBRSrise slope and SBRSfall slope were reported in young healthy people (54, 55). However, in elderly people, there were no differences between the SBRSrise slope and SBRSfall slope (39, 55). The average age of our subjects was relatively high (60 ± 2 yr) and baseline MSNA was as high as age-appropriate values. Our results are consistent with these prior observations in elderly individuals. The age difference between studies could cause the difference between our results and previous observations in young subjects.
The presented data show that the ratio of DBP falling beats/all beats was unchanged among all periods (Fig. 5). Thus, increased SBRS slope was not caused by the increased ratio of cardiac cycles with drops in DBP. Moreover, the DBP change rate (mmHg/s) during the DBP falling phase in the fluctuations was not altered after GTN. The BP fluctuations (indexed by DBP falling sequences/min) were unchanged after GTN. Thus, the increased SBRS or the MSNA activation after GTN should not be caused by these factors.
On the other hand, DBP falling beats/min and DBP falling beats/sequence were significantly increased after GTN. This could be caused by the increased HR when DBP falling sequences/min was unchanged after GTN. We could not exclude that more continuous pressure reduction beats in the sequence affected SBRS change. However, the increased DBP falling beats/min was very small, and thus the role of this factor could be limited.
Potential Pathways between GTN and SNA Aside from BRS
The vasodilator effect of nitrate could unload the cardiopulmonary baroreceptors by reducing preload. In our study, although SBP and DBP change were not statistically significant in post A, HR was significantly increased during post A and post B periods, which might be affected by decreased preload. Therefore, it is likely that the unloading of cardiopulmonary baroreceptors partly contributed to the increase in SNA. Mild lower body negative pressure (LBNP) (−10 and −20 mmHg) was reported to augment MSNA without a significant change in BP (56). However, the degree of MSNA increase after GTN in our study (e.g., baseline, 31.1 bursts/min; after GTN, 49.9 bursts/min) was larger than that in the previous study that examined the effect of mild LBNP (e.g., baseline, 23.6 bursts/min; during LBNP, 31.1 bursts/min) (56). It seems less likely that cardiopulmonary baroreceptor unloading with a small change of BP alone (especially in post A) could have contributed to the large MSNA increase we noted in our study. As for the effect of GTN on central command, NO, a product of nitrate, is thought to inhibit central SNA activation (45, 57). It was reported that inorganic nitrate reduced both BP and MSNA (58). In that study, the SBRS slope was unchanged despite a significant reduction of MSNA, and therefore it was concluded that the central SNA inhibitory effect of nitrates plays an important role in reducing SNA. In this study, T50 and error signal (T50-DBP) in SBRS-BI were measured. T50 can evaluate the threshold of MSNA occurrence (gating effect) which can be affected by baroreflex-independent factors (34). On the other hand, the error signal values may be related to the degree of baroreflex loading (or unloading) (e.g., negative error signal value means < 50% probability of MSNA occurrence, and a larger absolute value of negative/positive error signal indicates stronger BRS loading/unloading) (38). In our study, T50 value did not significantly change after GTN even though MSNA was significantly increased. The lack of a significant change may suggest that the baroreflex-dependent effect was dominant in altering SNA after GTN. However, the effects from other baroreflex-independent factors (e.g., nonneural hormones) cannot be fully excluded. It should be noted that the mean values of T50 error signal numerically changed from negative to positive. The positive error signal would suggest the high probability of MSNA occurrence. Future studies with a larger sample size are necessary to verify this issue.
Effects of GTN on BRS: Possible Mechanisms
There are several possible mechanisms by which GTN may affect BRS. First, altered peripheral arterial compliance and arterial baroreflex transduction were reported to be positively correlated with CBRS (59, 60). GTN may modulate peripheral artery compliance; however, GTN has not been reported to impair peripheral compliance before the establishment of tolerance. Rather NO, produced by the nitrate, is known to improve arterial distensibility (60). Therefore, it is not likely that GTN reduced the CBRS slope by reducing arterial compliance in our study.
Second, the interaction with other cardiovascular reflexes should be considered. Previously, phased LBNP (from −10 to −60 mmHg) was performed to evaluate the relationship between SBRS and cardiopulmonary baroreceptor unloading (37) and demonstrated an upward shift of SBRS without a change of the SBRS slope. In this study, to evaluate SBRS resetting, the operating points were determined. As a result, increased MSNA (BI and total MSNA) without significant DBP change at the operating point was observed in post A, indicating an upward shift of the SBRS operating point. Because we analyze spontaneous BRS and did not alter BP via interventions, clearly confirming the resetting of the full SBRS curve is difficult. However, before a significant increase in SBRS-BI slope, BF and BI were significantly increased in post A. Moreover, T50 was unchanged by GTN indicating that the SBRS curve was not shifted to the right (or left). From these results, upward resetting of the SBRS curve is most explainable for significant MSNA (BF and BI) increase in post A. As mentioned in the DISCUSSION section Potential Pathways between GTN and SNA Aside from BRS, it cannot be excluded that the cardiopulmonary baroreceptors might be unloaded after GTN and might contribute to resetting the operating points of SBRS in our study. To evaluate the effect of GTN on SBRS resetting in more detail, future studies using full BRS analysis (e.g., using neck suction device) are warranted.
Animal studies suggest that afferent nerve ending in peripheral veins senses peripheral blood volume changes and contributes to the blood pressure regulation via a reflex response (61–63). In humans, we reported that peripheral venous distention independently induced significant SNA excitation via a mechanism termed venous distention reflex (VDR) (42, 64). GTN might activate the VDR by its vasodilator effect. The interaction between VDR and BRS is still not understood. In addition, it can be assumed that the vasodilator effect of GTN extends more systemically compared with LBNP, which could have affected the different BRS response between our study and other work using phased LBNP (37).
In the previous studies evaluating both SBRS and CBRS, they both changed in the same direction (65, 66), or were in response to various interventions (e.g., orthostatic stress or mental stress), one changed and another remained unchanged (67, 68). Interestingly, in this study, CBRS and SBRS changed in opposite directions (SBRS, increase; CBRS, decrease). These opposite BRS responses might reflect two or more reflexes induced by GTN administration (e.g., improved arterial compliance and/or central effect of nitrate). In addition, as mentioned in the discussion for “Potential Pathways between GTN and SNA aside from BRS”, cardiopulmonary baroreceptor unloading could also affect the resetting of SBRS in this study. To evaluate these issues in more detail, future studies, evaluating both BRS and VDR, isolated from other reflexes (e.g., cardiopulmonary baroreceptor) are needed.
Limitation
This study has several limitations. First, we studied only healthy subjects. GTN is commonly used in patients with cardiovascular and in particular coronary artery diseases. It is uncertain that the results in our study would extend to these patients. In addition, many of these patients receive other drugs, such as calcium channel antagonists, that also could affect SNA (69), and might therefore make it difficult to clarify the relationship between SNA and GTN. Following our result of healthy subjects, studies including matched patients with coronary artery diseases are expected. Second, only one female was included in this study. Our results cannot be generalized to females. Third, we observed the responses of SNA and BRS to GTN until 10 min after the administration. We could not determine how long the effects will continue after GTN administration, which can be examined in further studies. Fourth, subjects were examined in the supine position; however, sublingual GTN could be used in different postures (e.g., upright). It is uncertain whether the same results with our study are confirmed in patients with different postures. Fifth, this study had no control and/or other drugs (e.g., calcium antagonist, inorganic nitrate) group. So, it is difficult to confirm the difference between GTN and them. Future studies comparing the effect of GTN and other vasodilators are expected. Sixth, the subject number was limited, although the sample size was estimated based on the statistical analysis. There is the possibility that BP data were underpowered given the low sample size.
Perspectives and Significance
In this study, we found that acutely, i.e., even before tolerance develops, nitrates increase MSNA and alter the baroreflex function. This finding is important, because in clinical practice, nitrates are used in patients with cardiovascular diseases, where elevated SNA is associated with a poor prognosis (18, 19). Moreover, sublingual GTN is often administered to patients who need immediate treatment for important diseases including the presentation of acute coronary syndromes. Impaired BRS was related to sudden cardiac and arrhythmic mortality after myocardial infarction (14) and sympathetic nerve stimulation during cardiac ischemia is a well-known risk factor for fatal arrhythmias (70). Sublingual GTN is effective in improving myocardial ischemia; however, considering the results of our study, it would be important for clinicians to be vigilant and consider the possibility that acute delivery of GTN to treat chest pain during acute coronary syndromes could evoke an excessive SNA response and raise the risk of serious arrhythmic complications. On the other hand, nitrates are often applied as a hypotensive drug in experiments to evaluate reflex function including BRS (5, 6). The direct effects of nitrates on SNA and BRS shown in our study should be considered when interpreting these experimental studies on reflex function.
Conclusion
In conclusion, our data showed that a typical clinical dose of sublingual GTN induced an increase in MSNA 2 min after the administration, which was associated with a decrease in CBRS, and an increase in SBRS with an upward shift of DBP-MSNA relationship. We speculate that altered baroreflex control of both HR and MSNA contributes to the sympathetic and hemodynamic responses to GTN. These results suggest that sublingual GTN can affect SNA via altering autonomic control.
GRANTS
This work was supported by National Institutes of Health Grant R01 HL144781 (to J. Cui and L. I. Sinoway) and UL1 TR002014 (L. I. Sinoway).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.I.S. and J.C. conceived and designed research; C.B., J.C.L., U.A.L., and J.C. performed experiments; T.H., C.B., and J.C. analyzed data; T.H., J.C.L., U.A.L., L.I.S., and J.C. interpreted results of experiments; T.H. prepared figures; T.H., J.C.L., U.A.L., L.I.S., and J.C. drafted manuscript; T.H., C.B., J.C.L., U.A.L., L.I.S., and J.C. edited and revised manuscript; T.H., C.B., J.L., U.A.L., L.I.S., and J.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We express appreciation to the subjects for their willingness to participate in this protocol. We thank Mr. Allen Kunselman and Dr. Jonathon Stavres for their technical support. We are grateful to Jennifer L. Stoner for secretarial help in preparing this manuscript.
REFERENCES
- 1.Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol 88: 767–773, 2000. doi: 10.1152/jappl.2000.88.2.767. [DOI] [PubMed] [Google Scholar]
- 2.Boden WE, Padala SK, Cabral KP, Buschmann IR, Sidhu MS. Role of short-acting nitroglycerin in the management of ischemic heart disease. Drug Des Devel Ther 9: 4793–4805, 2015. doi: 10.2147/DDDT.S79116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang K, Samai K. Role of high-dose intravenous nitrates in hypertensive acute heart failure. Am J Emerg Med 38: 132–137, 2020. doi: 10.1016/j.ajem.2019.06.046. [DOI] [PubMed] [Google Scholar]
- 4.Bath PM, Scutt P, Anderson CS, Appleton JP, Berge E, Cala L, et al. Prehospital transdermal glyceryl trinitrate in patients with ultra-acute presumed stroke (RIGHT-2): an ambulance-based, randomised, sham-controlled, blinded, phase 3 trial. Lancet 393: 1009–1020, 2019. doi: 10.1016/S0140-6736(19)30194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol Heart Circ Physiol 276: H1691–H1698, 1999. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- 6.Osculati G, Grassi G, Giannattasio C, Seravalle G, Valagussa F, Zanchetti A, Mancia G. Early alterations of the baroreceptor control of heart rate in patients with acute myocardial infarction. Circulation 81: 939–948, 1990. doi: 10.1161/01.CIR.81.3.939. [DOI] [PubMed] [Google Scholar]
- 7.La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol 13: 191–207, 2008. doi: 10.1111/j.1542-474X.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noll G, Wenzel RR, de Marchi S, Shaw S, Lüscher TF. Differential effects of captopril and nitrates on muscle sympathetic nerve activity in volunteers. Circulation 95: 2286–2292, 1997. doi: 10.1161/01.CIR.95.9.2286. [DOI] [PubMed] [Google Scholar]
- 9.Eckberg DL, Cooke WH, Diedrich A, Levine BD, Pawelczyk JA, Buckey JC Jr, Ertl AC, Biaggioni I, Cox JF, Robertson D, Baisch FJ, Blomqvist CG, Kuusela TA, Tahvanainen KU. Human baroreflex rhythms persist during handgrip and muscle ischaemia. Acta Physiol (Oxf) 209: 114–123, 2013. doi: 10.1111/apha.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Incognito AV, Duplea SG, Lee JB, Sussman J, Shepherd AD, Doherty CJ, Cacoilo JA, Notay K, Millar PJ. Arterial baroreflex regulation of muscle sympathetic nerve activity at rest and during stress. J Physiol 597: 4729–4741, 2019. doi: 10.1113/JP278376. [DOI] [PubMed] [Google Scholar]
- 11.Raven PB, Young BE, Fadel PJ. Arterial baroreflex resetting during exercise in humans: underlying signaling mechanisms. Exerc Sport Sci Rev 47: 129–141, 2019. doi: 10.1249/JES.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 12.O'Leary DD, Kimmerly DS, Cechetto AD, Shoemaker JK. Differential effect of head-up tilt on cardiovagal and sympathetic baroreflex sensitivity in humans. Exp Physiol 88: 769–774, 2003. doi: 10.1113/eph8802632. [DOI] [PubMed] [Google Scholar]
- 13.Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation 66: 874–880, 1982. doi: 10.1161/01.CIR.66.4.874. [DOI] [PubMed] [Google Scholar]
- 14.La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT Jr, Camm AJ, Schwartz PJ; Autonomic Tone and Reflexes After Myocardial Infarction (ATRAMI) Investigators. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation 103: 2072–2077, 2001. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 15.Dell’Oro R, Quarti-Trevano F, Seravalle G, Zanchettin F, Bertoli S, Airoldi F, Mancia G, Grassi G. Sympathetic nerve traffic and arterial baroreflex function in apparent drug-resistant hypertension. Hypertension 74: 903–909, 2019. doi: 10.1161/HYPERTENSIONAHA.119.13009. [DOI] [PubMed] [Google Scholar]
- 16.Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, Pozzi M, Mancia G. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation 92: 3206–3211, 1995. doi: 10.1161/01.CIR.92.11.3206. [DOI] [PubMed] [Google Scholar]
- 17.Seravalle G, Quarti-Trevano F, Dell’Oro R, Gronda E, Spaziani D, Facchetti R, Cuspidi C, Mancia G, Grassi G. Sympathetic and baroreflex alterations in congestive heart failure with preserved, midrange and reduced ejection fraction. J Hypertens 37: 443–448, 2019. doi: 10.1097/HJH.0000000000001856. [DOI] [PubMed] [Google Scholar]
- 18.Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrão CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 135: 302–307, 2009. doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 19.Gomes ME, Aengevaeren WR, Lenders JW, Verheugt FW, Smits P, Tack CJ. Improving myocardial perfusion by percutaneous coronary intervention reduces central sympathetic activity in stable angina. Clin Cardiol 33: E16–E21, 2010. doi: 10.1002/clc.20676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gori T, Floras JS, Parker JD. Effects of nitroglycerin treatment on baroreflex sensitivity and short-term heart rate variability in humans. J Am Coll Cardiol 40: 2000–2005, 2002. doi: 10.1016/S0735-1097(02)02532-9. [DOI] [PubMed] [Google Scholar]
- 21.Löwel H, Dobson A, Keil U, Herman B, Hobbs MS, Stewart A, Arstila M, Miettinen H, Mustaniemi H, Tuomilehto J. Coronary heart disease case fatality in four countries. A community study. The Acute Myocardial Infarction Register Teams of Auckland, Augsburg, Bremen, FINMONICA, Newcastle, and Perth. Circulation 88: 2524–2531, 1993. doi: 10.1161/01.cir.88.6.2524. [DOI] [PubMed] [Google Scholar]
- 22.Pantridge JF, Webb SW, Adgey AA. Arrhythmias in the first hours of acute myocardial infarction. Prog Cardiovasc Dis 23: 265–278, 1981. doi: 10.1016/0033-0620(81)90016-5. [DOI] [PubMed] [Google Scholar]
- 23.Cui J, Gao Z, Blaha C, Mast J, Herr M, Sinoway L. Distension of central great vein decreases sympathetic outflow in humans. Am J Physiol Heart Circ Physiol 305: H378–H385, 2013. doi: 10.1152/ajpheart.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallbo AB, Hagbarth KE, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 25.Cui J, Blaha C, Moradkhan M, Gray K, Sinoway L. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J Physiol 576: 625–634, 2006. doi: 10.1113/jphysiol.2006.116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui J, Wilson TE, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during cold pressor test in humans. Am J Physiol Heart Circ Physiol 282: H1717–H1723, 2002. doi: 10.1152/ajpheart.00899.2001. [DOI] [PubMed] [Google Scholar]
- 27.Parati G, Di Rienzo M, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, Pedotti A, Zanchetti A, Mancia G. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension 12: 214–222, 1988. doi: 10.1161/01.hyp.12.2.214. [DOI] [PubMed] [Google Scholar]
- 28.Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest: Relationship to blood pressure and age. J PhCysiol (London) 274: 621–637, 1978. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dutoit AP, Hart EC, Charkoudian N, Wallin BG, Curry TB, Joyner MJ. Cardiac baroreflex sensitivity is not correlated to sympathetic baroreflex sensitivity within healthy, young humans. Hypertension 56: 1118–1123, 2010. doi: 10.1161/HYPERTENSIONAHA.110.158329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson JT, Hedner JA, Sellgren J, Elam M, Wallin BG. Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am J Respir Crit Care Med 154: 1490–1496, 1996. doi: 10.1164/ajrccm.154.5.8912770. [DOI] [PubMed] [Google Scholar]
- 31.Hissen SL, Sayed KE, Macefield VG, Brown R, Taylor CE. The stability and repeatability of spontaneous sympathetic baroreflex sensitivity in healthy young individuals. Front Neurosci 12: 403, 2018. doi: 10.3389/fnins.2018.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iellamo F, Hughson RL, Castrucci F, Legramante JM, Raimondi G, Peruzzi G, Tallarida G. Evaluation of spontaneous baroreflex modulation of sinus node during isometric exercise in healthy humans. Am J Physiol Heart Circ Physiol 267: H994–H1001, 1994. doi: 10.1152/ajpheart.1994.267.3.H994. [DOI] [PubMed] [Google Scholar]
- 33.Cui J, Boehmer J, Blaha C, Sinoway LI. Muscle sympathetic nerve activity response to heat stress is attenuated in chronic heart failure patients. Am J Physiol Regul Integr Comp Physiol 312: R873–R882, 2017. doi: 10.1152/ajpregu.00355.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stauss HM, Moffitt JA, Chapleau MW, Abboud FM, Johnson AK. Baroreceptor reflex sensitivity estimated by the sequence technique is reliable in rats. Am J Physiol Heart Circ Physiol 291: H482–H483, 2006. doi: 10.1152/ajpheart.00228.2006. [DOI] [PubMed] [Google Scholar]
- 36.Monahan KD, Leuenberger UA, Ray CA. Effect of repetitive hypoxic apnoeas on baroreflex function in humans. J Physiol 574: 605–613, 2006. doi: 10.1113/jphysiol.2006.108977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ichinose M, Saito M, Fujii N, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during severe orthostatic stress. J Physiol 576: 947–958, 2006. doi: 10.1113/jphysiol.2006.117507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wehrwein EA, Joyner MJ, Hart EC, Wallin BG, Karlsson T, Charkoudian N. Blood pressure regulation in humans: calculation of an “error signal” in control of sympathetic nerve activity. Hypertension 55: 264–269, 2010. doi: 10.1161/HYPERTENSIONAHA.109.141739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hart EC, Wallin BG, Curry TB, Joyner MJ, Karlsson T, Charkoudian N. Hysteresis in the sympathetic baroreflex: role of baseline nerve activity. J Physiol 589: 3395–3404, 2011. doi: 10.1113/jphysiol.2011.208538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Incognito AV, Samora M, Shepherd AD, Cartafina RA, Guimaraes GMN, Daher M, Vianna LC, Millar PJ. Sympathetic arterial baroreflex hysteresis in humans: different patterns during low- and high-pressure levels. Am J Physiol Heart Circ Physiol 319: H787–H792, 2020. doi: 10.1152/ajpheart.00505.2020. [DOI] [PubMed] [Google Scholar]
- 41.Faul F, Erdfelder E, Lang AG, Buchner AG. *Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191, 2007. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 42.Cui J, McQuillan P, Blaha CA, Kunselman AR, Sinoway LI. Limb venous distension evokes sympathetic activation via stimulation of the limb afferents in humans. Am J Physiol Heart Circ Physiol 303: H457–H463, 2012. doi: 10.1152/ajpheart.00236.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bassenge E, Zanzinger J. Nitrates in different vascular beds, nitrate tolerance, and interactions with endothelial function. Am J Cardiol 70: 23B–29B, 1992. doi: 10.1016/0002-9149(92)90591-l. [DOI] [PubMed] [Google Scholar]
- 44.Harrison DG, Bates JN. The nitrovasodilators. New ideas about old drugs. Circulation 87: 1461–1467, 1993. doi: 10.1161/01.cir.87.5.1461. [DOI] [PubMed] [Google Scholar]
- 45.Zanzinger J, Czachurski J, Seller H. Impaired modulation of sympathetic excitability by nitric oxide after long-term administration of organic nitrates in pigs. Circulation 97: 2352–2358, 1998. doi: 10.1161/01.cir.97.23.2352. [DOI] [PubMed] [Google Scholar]
- 46.Gori T, Mak SS, Kelly S, Parker JD. Evidence supporting abnormalities in nitric oxide synthase function induced by nitroglycerin in humans. J Am Coll Cardiol 38: 1096–1101, 2001. doi: 10.1016/S0735-1097(01)01510-8. [DOI] [PubMed] [Google Scholar]
- 47.Parker JD, Farrell B, Fenton T, Cohanim M, Parker JO. Counter-regulatory responses to continuous and intermittent therapy with nitroglycerin. Circulation 84: 2336–2345, 1991. doi: 10.1161/01.cir.84.6.2336. [DOI] [PubMed] [Google Scholar]
- 48.Dupuis J, Lalonde G, Lemieux R, Rouleau JL. Tolerance to intravenous nitroglycerin in patients with congestive heart failure: role of increased intravascular volume, neurohumoral activation and lack of prevention with N-acetylcysteine. J Am Coll Cardiol 16: 923–931, 1990. doi: 10.1016/s0735-1097(10)80342-0. [DOI] [PubMed] [Google Scholar]
- 49.Serone AP, Angus JA, Wright CE. Baroreflex resetting but no vascular tolerance in response to transdermal glyceryl trinitrate in conscious rabbits. Br J Pharmacol 118: 93–104, 1996. doi: 10.1111/j.1476-5381.1996.tb15371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ichinose M, Saito M, Ogawa T, Hayashi K, Kondo N, Nishiyasu T. Modulation of control of muscle sympathetic nerve activity during orthostatic stress in humans. Am J Physiol Heart Circ Physiol 287: H2147–H2153, 2004. doi: 10.1152/ajpheart.00215.2004. [DOI] [PubMed] [Google Scholar]
- 51.Ichinose M, Saito M, Wada H, Kitano A, Kondo N, Nishiyasu T. Modulation of arterial baroreflex control of muscle sympathetic nerve activity by muscle metaboreflex in humans. Am J Physiol Heart Circ Physiol 286: H701–H707, 2004. doi: 10.1152/ajpheart.00618.2003. [DOI] [PubMed] [Google Scholar]
- 52.Hjemdahl P, Fagius J, Freyschuss U, Wallin BG, Daleskog M, Bohlin G, Perski A. Muscle sympathetic activity and norepinephrine release during mental challenge in humans. Am J Physiol Endocrinol Physiol 257: E654–E664, 1989. doi: 10.1152/ajpendo.1989.257.5.E654. [DOI] [PubMed] [Google Scholar]
- 53.Vissing SF, Scherrer U, Victor RG. Relation between sympathetic outflow and vascular resistance in the calf during perturbations in central venous pressure. Evidence for cardiopulmonary afferent regulation of calf vascular resistance in humans. Circ Res 65: 1710–1717, 1989. doi: 10.1161/01.res.65.6.1710. [DOI] [PubMed] [Google Scholar]
- 54.Nardone M, Teixeira AL, Incognito AV, Vermeulen TD, Shafer BM, Millar PJ, Foster GE. Within-breath sympathetic baroreflex sensitivity is modulated by lung volume but unaffected by acute intermittent hypercapnic hypoxia in men. Am J Physiol Heart Circ Physiol 319: H213–H221, 2020. doi: 10.1152/ajpheart.00296.2020. [DOI] [PubMed] [Google Scholar]
- 55.Studinger P, Goldstein R, Taylor JA. Age- and fitness-related alterations in vascular sympathetic control. J Physiol 587: 2049–2057, 2009. doi: 10.1113/jphysiol.2009.170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rea RF, Wallin BG. Sympathetic nerve activity in arm and leg muscles during lower body negative pressure in humans. J Appl Physiol (1985) 66: 2778–2781, 1989. doi: 10.1152/jappl.1989.66.6.2778. [DOI] [PubMed] [Google Scholar]
- 57.Ye S, Nosrati S, Campese VM. Nitric oxide (NO) modulates the neurogenic control of blood pressure in rats with chronic renal failure (CRF). J Clin Invest 99: 540–548, 1997. doi: 10.1172/JCI119191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Notay K, Incognito AV, Millar PJ. Acute beetroot juice supplementation on sympathetic nerve activity: a randomized, double-blind, placebo-controlled proof-of-concept study. Am J Physiol Heart Circ Physiol 313: H59–H65, 2017. doi: 10.1152/ajpheart.00163.2017. [DOI] [PubMed] [Google Scholar]
- 59.Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol 281: H284–H289, 2001. doi: 10.1152/ajpheart.2001.281.1.H284. [DOI] [PubMed] [Google Scholar]
- 60.Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation 105: 213–217, 2002. doi: 10.1161/hc0202.101970. [DOI] [PubMed] [Google Scholar]
- 61.Davenport PW, Thompson FJ. Mechanosensitive afferents of femoral-saphenous vein. Am J Physiol Regul Integr Comp Physiol 252: R367–R370, 1987. doi: 10.1152/ajpregu.1987.252.2.R367. [DOI] [PubMed] [Google Scholar]
- 62.Andrews CJ, Andrews WH, Orbach J. A sympathetic reflex elicited by distension of the mesenteric venous bed. J Physiol 226: 119–131, 1972. doi: 10.1113/jphysiol.1972.sp009976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doe CP, Drinkhill MJ, Myers DS, Self DA, Hainsworth R. Reflex vascular responses to abdominal venous distension in the anesthetized dog. Am J Physiol Heart Circ Physiol 271: H1049–H1056, 1996. doi: 10.1152/ajpheart.1996.271.3.H1049. [DOI] [PubMed] [Google Scholar]
- 64.Cui J, McQuillan P, Moradkhan R, Pagana C, Sinoway LI. Sympathetic responses during saline infusion into the veins of an occluded limb. J Physiol 587: 3619–3628, 2009. doi: 10.1113/jphysiol.2009.173237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park J, Marvar PJ, Liao P, Kankam ML, Norrholm SD, Downey RM, McCullough SA, Le NA, Rothbaum BO. Baroreflex dysfunction and augmented sympathetic nerve responses during mental stress in veterans with post-traumatic stress disorder. J Physiol 595: 4893–4908, 2017. doi: 10.1113/JP274269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marchi A, Bari V, De Maria B, Cerruti S, Heusser K, Tank J, Jordan J, Barbic F, Furlan R, Porta A. . Evaluation of the correlation between cardiac and sympathetic baroreflex sensitivity before orthostatic syncope. Annu Int Conf Proc IEEE Eng Med Biol Soc 37: 2063–2066, 2015. doi: 10.1109/embc.2015.7318793. [DOI] [PubMed] [Google Scholar]
- 67.Adlan AM, Paton JF, Lip GY, Kitas GD, Fisher JP. Increased sympathetic nerve activity and reduced cardiac baroreflex sensitivity in rheumatoid arthritis. J Physiol 595: 967–981, 2017. doi: 10.1113/JP272944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fonkoue IT, Marvar PJ, Norrholm SD, Kankam ML, Li Y, DaCosta D, Rothbaum BO, Park J. Acute effects of device-guided slow breathing on sympathetic nerve activity and baroreflex sensitivity in posttraumatic stress disorder. Am J Physiol Heart Circ Physiol 315: H141–H149, 2018. doi: 10.1152/ajpheart.00098.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferguson DW, Hayes DW. Nifedipine potentiates cardiopulmonary baroreflex control of sympathetic nerve activity in healthy humans. Direct evidence from microneurographic studies. Circulation 80: 285–298, 1989. doi: 10.1161/01.cir.80.2.285. [DOI] [PubMed] [Google Scholar]
- 70.Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res 114: 1004–1021, 2014. doi: 10.1161/CIRCRESAHA.113.302549. [DOI] [PubMed] [Google Scholar]