Abstract
The prolonged, postweaning fast of northern elephant seal (Mirounga angustirostris) pups is characterized by a reliance on lipid metabolism and reversible, fasting-induced insulin resistance, providing a unique model to examine the effects of insulin on lipid metabolism. We have previously shown that acute insulin infusion induced a shift in fatty acid metabolism dependent on fasting duration. This study complements the previous study by examining the effects of fasting duration and insulin infusion on circulating levels of oxylipins, bioactive metabolites derived from the oxygenation of polyunsaturated fatty acids. Northern elephant seal pups were studied at two postweaning periods (n = 5/period): early fasting (1–2 wk postweaning; 127 ± 1 kg) and late fasting (6–7 wk postweaning; 93 ± 4 kg). Different cohorts of pups were weighed, sedated, and infused with 65 mU/kg of insulin. Plasma was collected prior to infusion (T0) and at 10, 30, 60, and 120 min postinfusion. A profile of ∼80 oxylipins was analyzed by UPLC-ESI-MS/MS. Nine oxylipins changed between early and late fasting and eight were altered in response to insulin infusion. Fasting decreased prostaglandin F2α (PGF2α) and increased 14,15-dihydroxyicosatrienoic acid (14,15-DiHETrE), 20-hydroxyeicosatetraenoic acid (20-HETE), and 4-hydroxy-docosahexaenoic acid (4-HDoHE) (P < 0.03) in T0 samples, whereas insulin infusion resulted in an inverse change in area-under-the-curve (AUC) levels in these same metabolites (P < 0.05). In addition, 12-12-hydroperoxyeicosatetraenoic acid (HpETE) and 12-HETE decreased with fasting and insulin infusion, respectively (P < 0.04). The oxylipins altered during fasting and in response to insulin infusion may contribute to the manifestation of insulin resistance and participate in the metabolic regulation of associated cellular processes.
Keywords: insulin resistance, lipid mediators, lipid metabolism, lipids, oxylipins
INTRODUCTION
Northern elephant seals (Mirounga angustirostris) have evolved robust physiological mechanisms that support their natural, prolonged fasts, with pups experiencing postweaning fasts of ∼2–3 mo (1). The postweaning fasts are characterized by a reliance on lipid metabolism (RQ = 0.73) (2, 3), elevated plasma free fatty acids (FFAs), and reversible insulin resistance (1). The changes and behavior of FFAs in fasting elephant seal pups are dynamic. We have shown that bolus glucose infusion suppressed FFAs in early-fasted pups but stimulated FFA levels in late-fasted pups, demonstrating a potential shift in the Randle cycle (1). Furthermore, insulin suppressed FFAs in late-fasted pups, suggesting a decrease in triglyceride hydrolysis (4) and providing an explanation for the decrease in plasma insulin with fasting (5, 6). Fasting duration and insulin infusion also induced differential endocannabinoid responses (4), suggesting that lipids respond dynamically in fasting elephant seals. However, the effects of extended fasting duration or food deprivation and insulin on oxylipins, another dynamic lipid mediator, have not been examined.
Oxylipins are derivatives of polyunsaturated fatty acids produced through three main pathways of oxygenation and include isoprostanes, prostanoids, and dihydroxy-, hydroxy-, and epoxy-polyunsaturated fatty acids (PUFAs) (7). Enzymes in the cyclooxygenase, lipoxygenase, and cytochrome P450 (CYP450) families facilitate these transformative reactions. Once produced, oxylipins can activate G protein-coupled receptors, ligand-activated transcription factors, and peroxisome proliferator-activated receptors, among others (8). Oxylipins are generally considered short-range signaling molecules with a vast array of effects linked to cardiovascular disease and type 2 diabetes (9); however, their incorporation and delivery in lipoprotein particles may extend these functions to lipase-mediated endocrine actions. For instance, diet-induced changes in low-density lipoprotein oxylipin composition have been shown to influence inflammatory marker expression in endothelial cells and TNFα-stimulated inflammatory responses in diabetic primary adipocyte cultures (10–12). Their inflammatory effects (8) suggest that oxylipins could potentially contribute to the onset and/or maintenance of insulin resistance in seals. An overnight fast in humans reduced the variability in oxylipins compared with that reported during postprandial analyses, suggesting that fasted samples provide an accurate assessment of oxylipin biology (7) and that these lipid mediators can be influenced by either nutrient intake or postprandial factors responding to them. Although there is extensive literature on the changes in oxylipins with insulin resistance and diabetic conditions, to the best of our knowledge, no studies have examined the behavior of oxylipins in response to prolonged fasting or exogenous insulin infusion.
Therefore, the primary objective of this study was to determine the effects of prolonged fasting and exogenous insulin on oxylipin concentrations to better understand the biology of oxylipins. Furthermore, this study has the unique opportunity to simultaneously examine the effects of prolonged fasting and insulin infusion on oxylipin biology to assess their potential influence on fasting-associated insulin resistance. Because plasma insulin is reduced with fasting duration, we hypothesized that natural fasting duration alters insulin-resistance-mediated oxylipins and that insulin reverses these levels as adaptive responses to facilitate the development of insulin resistance in northern elephant seals.
MATERIALS AND METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committees of both the University of California, Merced and Sonoma State University. All work was realized under the National Marine Fisheries Service marine mammal permit no. 87-1743.
The present study complements our previous study regarding insulin’s effects on metabolomic profiles (4, 13) and represents a refined analysis of the data by focusing on oxylipin profile responses to provide a more thorough examination of these lipid mediators that was beyond the scope of the original study (4). Details of animal and sample handling have been published previously (4, 13) but are mentioned here briefly for quick reference.
Study Subjects
Northern elephant seal pups of two different cohorts at Año Nuevo State Reserve were studied at two postweaning periods (n = 5 animals/period): early fasting (1–2 wk postweaning; 127 ± 1 kg) and late fasting (6–7 wk postweaning; 93 ± 4 kg). Pups were rapidly weighed to minimize stress, sedated, and infused with a bolus of insulin (65 mU/kg; early: 8.3 ± 0.1 U; late: 6.1 ± 0.3 U). Plasma was collected before infusion (T0) and at 10, 30, 60, and 120 min postinsulin infusion (4, 13). We have previously demonstrated that our established protocol to rapidly weigh pups without sedation does not induce a cortisol response (14).
Analytical Chemistry
Although samples used in the present study were collected previously (1, 4, 13), aliquots of those samples were saved specifically for later analyses and protected from thawing and refreezing cycles until the time of processing at the National Institutes of Health West Coast Metabolomics Center at UC Davis. Lipids and lipid mediators were quantified against authentic standards using isotopically labeled surrogates and mass spectrometry-based methods. Oxylipins and endocannabinoids were isolated by solid-phase extraction and quantified against authentic standards using ultra-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (API 4000 QTrap, AB Sciex, Framingham, MA) (15). Nonesterified fatty acids (NEFAs) were extracted, derivatized, and analyzed as fatty acid methyl esters by gas chromatography-electron impact ionization mass spectrometry (Agilent 5973N, Santa Clara, CA) (4). Although the endocannabinoids and NEFAs were reported earlier (4), they are included here as well to complement the analyses of oxylipins and to provide a more thorough examination of these lipids. These novel analyses allow for direct comparisons with the oxylipins examined here. Moreover, their inclusion facilitates a more comprehensive networking map to better understand the shifts in certain metabolic pathways as they relate to fasting duration and insulin infusion.
Statistics
Final concentrations were corrected for changes in plasma volume (16) as previously described and applied for these samples (4). For multivariate analyses, principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (O-PLS-DA) were used as data reduction techniques to distinguish the early and late fasting groups using pareto-scaled data. For univariate analyses, outliers were assessed using the robust fit outlier test. Missing values were imputed using multivariate normal imputation. Normality was assessed using goodness of fit with P < 0.05. Normally distributed data were compared between groups using Student’s t tests. Non-normally distributed data were transformed using Box-Cox transformations and then re-evaluated for normality. Transformed data that became normally distributed were analyzed using t tests, and data that remained not normal were analyzed using the Mann–Whitney U test. Adjustment for multiple hypothesis testing, according to the Benjamini & Hochberg method with a false discovery rate (FDR), was used at 5%. The Cohen’s D effect size statistic was calculated to quantify the magnitude of the difference between the two groups, representing the extent to which the null hypothesis is false. To assess group × time interactions for early and late fasting, a full factorial repeated-measures ANOVA with Tukey’s post hoc test was conducted. The area under the curve (AUC) was calculated based on summing polygons from concentration versus the sample time points (t = 0, 10, 30, 60, and 120 min) for each metabolite as a function of time after insulin infusion at each fasting period. Areas were determined relative to the T0 value. AUC values were used to summarize the relative changes in metabolite concentrations as a function of time for each sample. The AUC was used to create a single continuous variable as opposed to having several variables (e.g., group and time). Although there is no assumed biochemical context encoded by the AUC method, the values are mathematical representations of the original data and enable comparisons between samples’ metabolite concentrations over time. One late fasting seal was not included in the AUC calculation due to missing time-point 60 (T60) values. Univariate and multivariate analyses were conducted using JMP Pro 13 and 15.1 (SAS Institute Inc., Cary, NC), MetaboAnalyst 3.0 (http://www.metaboanalyst.ca/, Canada) (17), and R version 3.0.1 (https://www.r-project.org/, Austria) (18). Biochemical networks were constructed with Cytoscape 2.8.1 and Cytoscape 3.8.0 (19).
Temporal data were scaled such that metabolite levels for each animal were expressed as a percentage of the sum of the postinfusion metabolite concentrations. Missing data from a single time point for one animal in early and late groups were estimated by calculating the average percent response at that time point relative to the remaining four time points for the four remaining seals. These percent responses were then used to calculate four independent estimates of the missing data point, and the average of these estimates was used. The 72 detected metabolites including oxylipins, endocannabinoids, and fatty acids were condensed into 12 cluster components using variable clustering in JMP Pro 15.1 (SAS Institute), an implementation of the principal components analysis (PCA)-based VARCLUS procedure for SAS. Contrast posttests were used to evaluate mean differences between early and late groups at each time. Similarity in cluster components was evaluated using K-nearest neighbors.
All raw data can be accessed at https://doi.org/10.6084/m9.figshare.13577909.
RESULTS
Fasting-Induced Changes in Oxylipins
Fasting induced changes in 9 of the 42 detected oxylipins, as indicated by comparing baseline (T0) concentrations between early and late fast (Table 1). A biochemical network map of altered metabolites can be visualized in Fig. 1. The cyclooxygenase (COX) oxylipins, TXB2 and PGF2α, decreased by 79% and 51%, respectively, from early to late fast (P = 0.004 and P = 0.005). In contrast, 3 of the 10 detected cytochrome P450 (CYP450)-dependent products increased over the fast, ranging from ∼35% for 11,12-DiHETrE (P = 0.045) and 14,15-dihydroxyicosatrienoic acid (14,15-DiHETrE) (P = 0.023) to 149% for 20-hydroxyeicosatetraenoic acid (20-HETE) (P = 0.003). The hydroperoxide, 12-hydroperoxyeicosatetraenoic acid (12-HpETE), generated directly by 12/15-lipoxygenase (12/15-LOX) metabolism, was lower after fasting (P = 0.009), whereas the glutathione peroxidase products of these lipids (i.e., 12-HETE and 15-HETE) were unchanged. Conversely, the 5-LOX-dependent 5-HEPE (P = 0.017) and 4-hydroxy-docosahexaenoic acid (4-HDoHE) (P = 0.0009) increased by 144% and 278%, respectively, across the fast, as did linoleate-derived trihydroxy fatty acids (i.e., Sum-TriHOME; P = 0.009) by 97%, which appear to be markers of auto-oxidation (20).
Table 1.
T0 plasma oxylipins affected by fasting in northern elephant seal pups
| Metabolites, nM | Early Fasted, nM | Late Fasted, nM | Cohen’s Dpooled (95% CI) | P Value | FDR |
|---|---|---|---|---|---|
| 4-HDoHE | 1.86 ± 0.409 | 7.03 ± 4.13 | −13 (−25.8, 0) | 0.00087 | 0.036 |
| 5-HEPE | 2.53 ± 0.362 | 6.17 ± 3.22 | −9.5 (−19.3, 0.2) | 0.017 | 0.10 |
| SUM_TriHOME | 4.73 ± 1.35 | 9.33 ± 2.65 | −3.3 (−7.5, 1.0) | 0.0085 | 0.062 |
| 20-HETE | 3.19 ± 1.51 | 7.95 ± 2.11 | −3.0 (−7.1, 1.0) | 0.0034 | 0.051 |
| 14,15-DiHETrE | 0.556 ± 0.132 | 0.763 ± 0.0979 | −1.4 (−4.5, 1.7) | 0.023 | 0.12 |
| 11,12-DiHETrE | 0.506 ± 0.117 | 0.684 ± 0.120 | −1.3 (−4.4, 1.7) | 0.045 | 0.21 |
| 12-HpETE | 18.1% ± 13.5 | 1.88% ± 2.26 | 0.12 (−2.7, 2.9) | 0.0088 | 0.062 |
| TXB2 | 45.6 ± 36.8 | 9.58 ± 2.93 | 0.99 (−1.9, 3.9) | 0.0037 | 0.051 |
| PGF2α | 4.58 ± 1.81 | 2.25 ± 0.520 | 1.4 (−1.7, 4.4) | 0.0049 | 0.051 |
Values are means ± SD; n = 5 animals/period. Cohen’s D effect sizes: 0.2 = small; 0.5 = medium; 0.8 = large.
12-HpETE levels were estimated based on the peak area relative response ratio response (12-HpETE peak area/internal standard peak area) and expressed as the relative abundance across all analyzed samples. While significant, the observed effect is exceedingly small.
Figure 1.
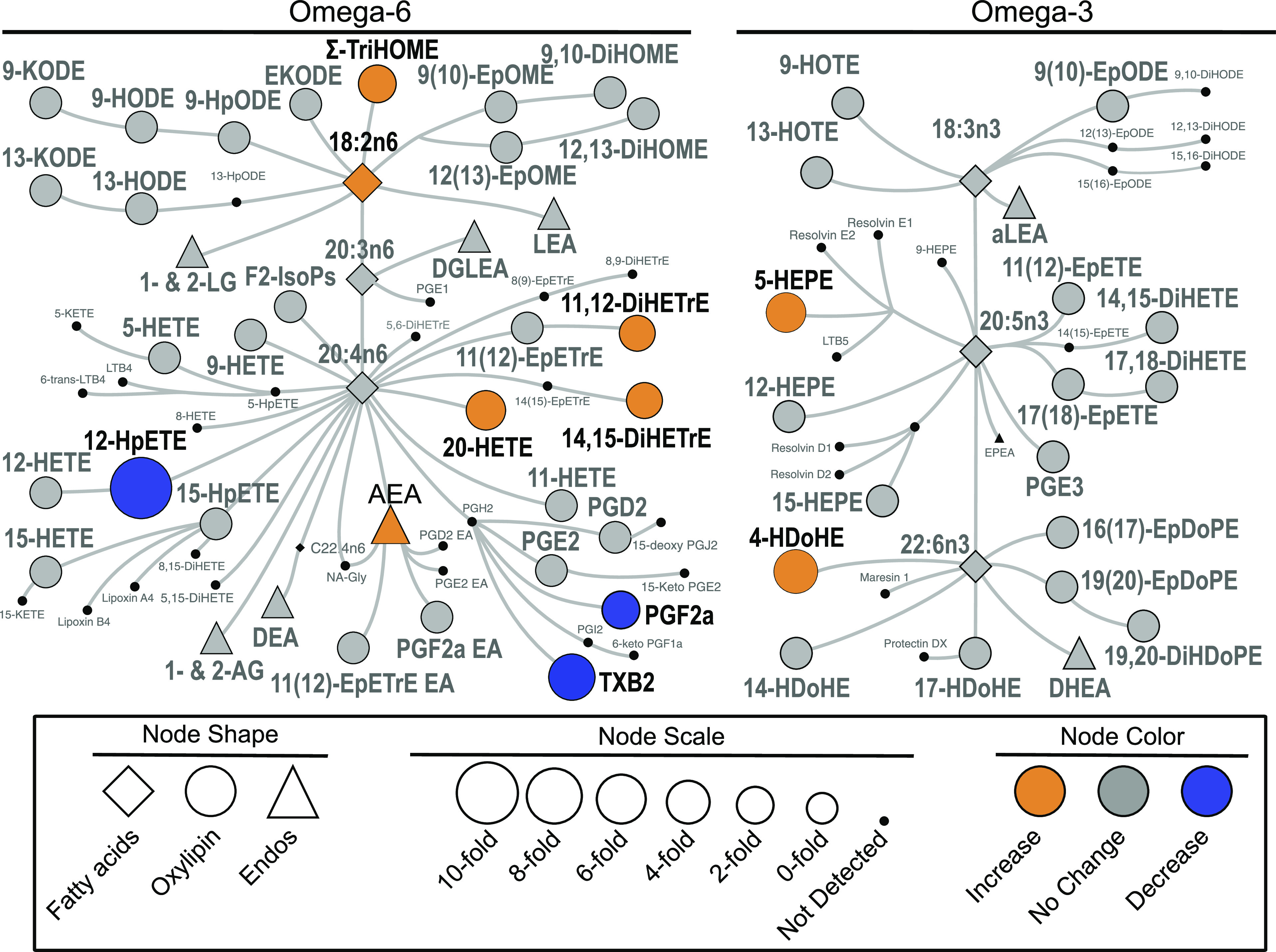
Biochemical network map showing statistically significant changes between early and late fast T0 plasma of northern elephant seal pups (n = 5 animals/period). Lipid mediators derived from omega-6 and omega-3 fatty acids are delineated on the left versus the right of the figure. Derivatives include oxylipins such as prostaglandins, thromboxanes, hydroxy acids, ketones, epoxides, and diols, in addition to endocannabinoids and endocannabinoids-like compounds such as mono-acylglycerols and N-acylethanolamides. Statistically significant changes are shown, with orange indicating an increase in concentration in late-fasted seals, whereas blue denotes a decreased concentration in late-fasted seals. Gray color indicates there was no difference between early and late fast. Fatty acid and endocannabinoid data are from previously published analysis of the same samples (4).
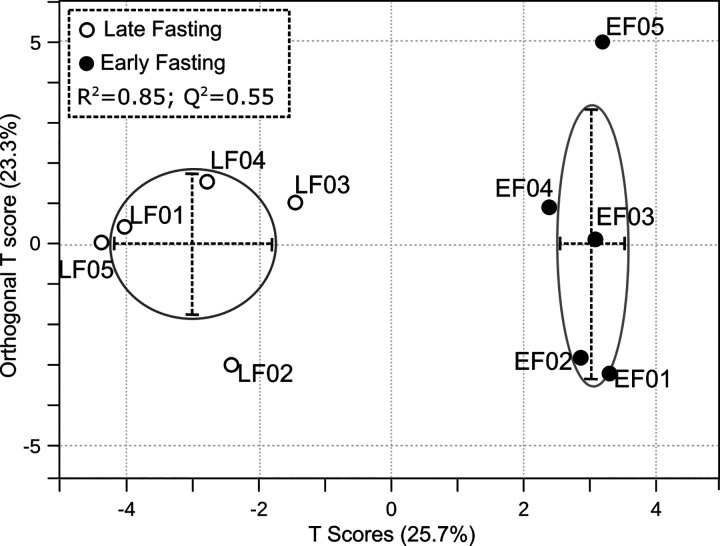
Multivariate analysis methods of PCA and O-PLS-DA were run to assess all the oxylipins together between early and late fast (Fig. 2). A two-tailed t test showed that the PCA component 1 scores from the early and late fast samples were different (P = 0.038). Similarly, O-PLS-DA model cross-validation produced a Q2 = 0.55, which is greater than the empirically inferred value of acceptance >0.4 (21). Furthermore, an R2 = 0.85 indicates the model fits the input data.
Figure 2.
Multivariate analysis of oxylipin values of T0 plasma oxylipins affected by fasting in northern elephant seal pups (n = 5 animals/period). Orthogonal partial least squares discriminant analysis (O-PLS-DA) enabled visualization of group discrimination in a two-dimensional scores plot showing a separation of early and late fasting T0 seals. Each point represents an individual seal.
Insulin-Induced Changes in Oxylipins
The magnitude of the response to insulin was reduced by fasting duration for seven oxylipins and increased for one oxylipin, as determined by total AUC (Fig. 3). Oxylipin responses to insulin are shown in Table 2. Oxylipins altered by insulin, differentially between early and late fasting, were primarily derived from 5-, 12-, and 15-LOX and CYP450. Many oxylipins altered at baseline (T0) with fasting duration were also altered by insulin infusion with fasting duration, including PGF2α, 20-HETE, 4-HDoHE, and 14,15-DiHETrE. PGF2α baseline levels decreased, whereas AUC values were higher at late fast. Conversely, 20-HETE, 4-HDoHE, and 14,15-DiHETrE baseline levels increased, whereas AUC values were lower at late fasting.
Figure 3.
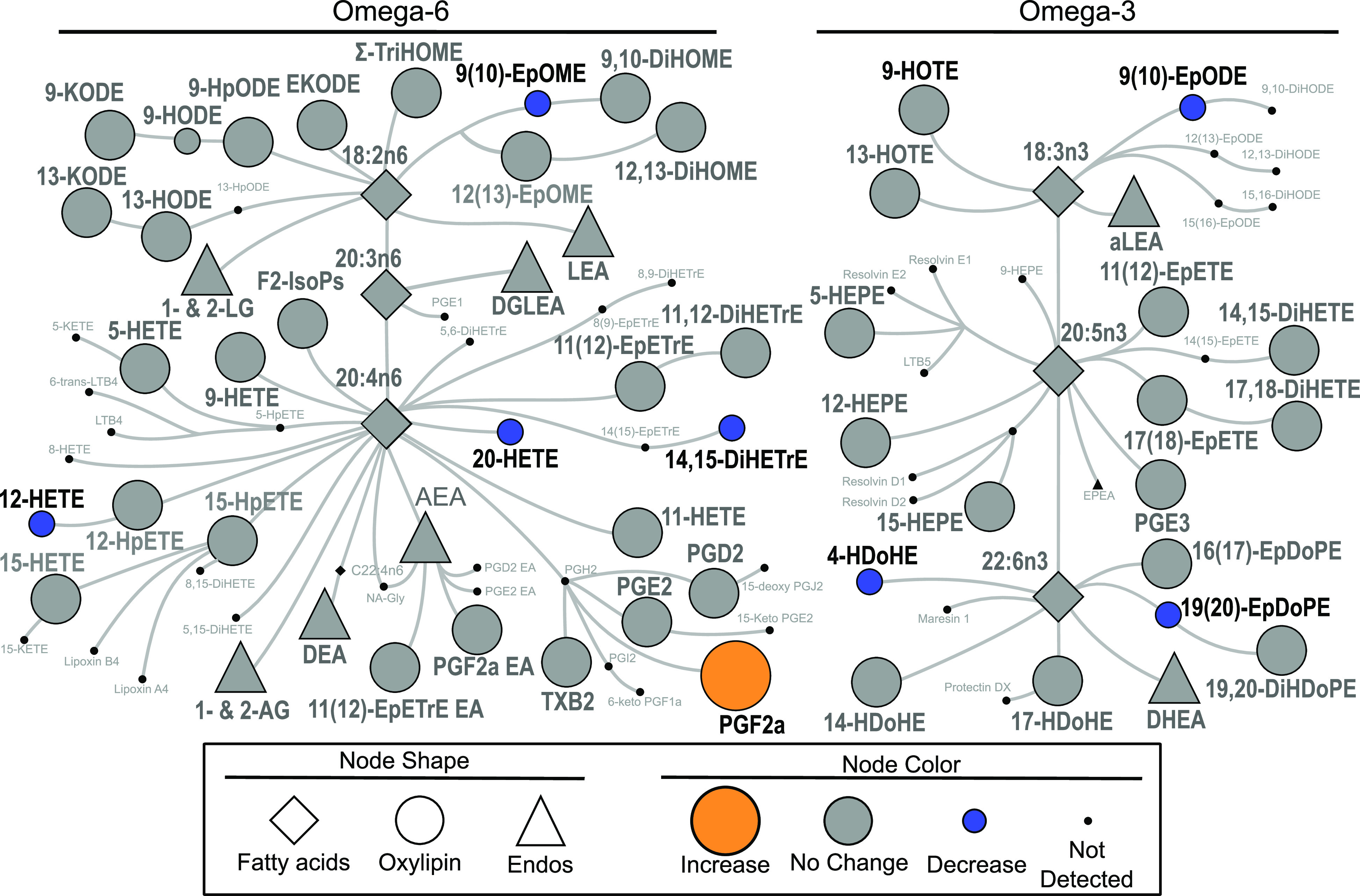
Biochemical network map showing statistically significant changes from insulin infusion between early and late fast AUC levels in the plasma of northern elephant seal pups (n = 5 animals/period). Lipid mediators derived from omega-6 and omega-3 fatty acids are delineated on the left versus the right of the figure. Derivatives include oxylipins such as prostaglandins, thromboxanes, hydroxy acids, ketones, epoxides, and diols, in addition to endocannabinoids and endocannabinoids-like compounds such as mono-acylglycerols and N-acylethanolamides. Statistically significant changes are shown, with orange indicating a higher AUC in late-fasted seals, whereas blue denotes a lower AUC in late-fasted seals. Gray color indicates there was no difference between early and late fast. Fatty acid and endocannabinoid data are from previously published analysis of the same samples (4).
Table 2.
Oxylipin insulin responses affected by fasting in northern elephant seal pup
| Metabolites | Early AUC (nM × min) | Late AUC (nM × min) | Cohen’s Dpooled (95% CI) | P Value | FDR |
|---|---|---|---|---|---|
| 19,20-EpDPE | 916 ± 746 | −521 ± 196 | 2.6 (0.74, 4.5) | 0.030 | 0.20 |
| 9,10-EpOME | 287 ± 270 | −241 ± 146 | 2.4 (0.6, 4.3) | 0.014 | 0.20 |
| 4-HDoHE | 755 ± 750 | −466 ± 319 | 2.1 (0.1, 3.8) | 0.024 | 0.20 |
| PGF2α | −273 ± 242 | 92.3 ± 82.3 | 2.0 (0.32, 3.7) | 0.029 | 0.20 |
| 14,15-DiHETrE | 34.7 ± 42.5 | −26.2 ± 8.83 | 2 (0.29, 3.7) | 0.031 | 0.20 |
| 12-HETE | −265 ± 4.86e3 | −1.02e4 ± 5.41e3 | 1.9 (0.26, 3.6) | 0.034 | 0.20 |
| 9,10-EpODE | 96.4 ± 135 | −160 ± 135 | 1.9 (0.23, 3.6) | 0.036 | 0.20 |
| 20-HETE | −114 ± 312 | −645 ± 291 | 1.8 (0.13, 3.4) | 0.047 | 0.20 |
| 9-HODE | −69.9 ± 460 | −886 ± 486 | 1.7 (0.1, 3.7) | 0.051 | 0.20 |
Values are means ± SD; n = 5 animals/period. Area under the curves (AUCs) were calculated based on summing polygons from concentration versus the sample time points for each metabolite as a function of time postinsulin infusion at each fasting period. One late fasting seal was not included due to missing time-point 60 (T60) values.
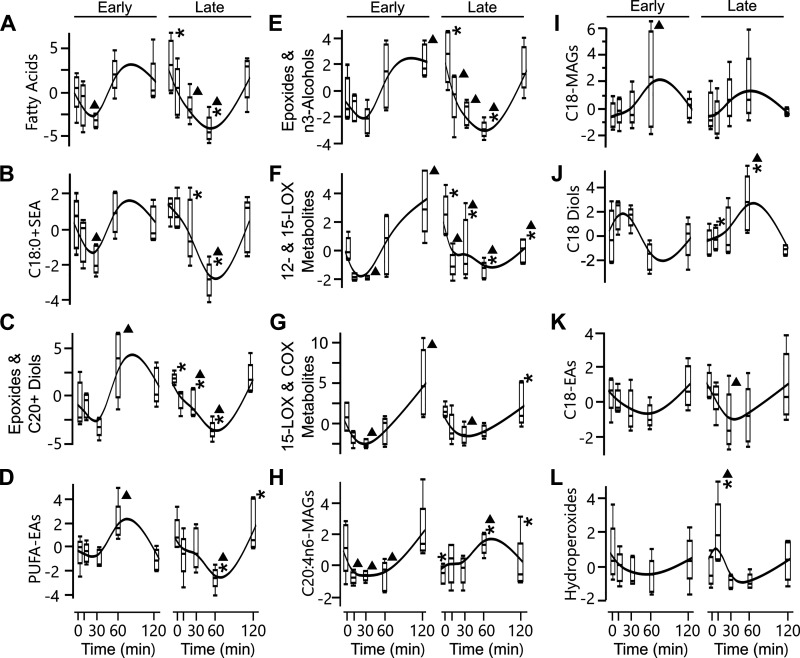
Cluster analysis was performed on the oxylipins and their precursors (n-6 and n-3 fatty acids) and endocannabinoids (related lipid mediators with similar functions). Precursor and endocannabinoid data were previously published in another format (4). Insulin infusion induced temporal changes in fatty acids and lipid mediators in early- and late-fasted seal pups. The 72 metabolites were condensed into 12 cluster components using K-nearest neighbors (Fig. 4, Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.13577909). The emerging clusters included groups characterized by elevated late fasting baseline and a lower T60 such as epoxides and n-3 alcohols, C20+ diols, 12- and 15-LOX metabolites, and fatty acids. Clusters with late-fasting groups, characterized by lower T0 or T10 and elevated T60, were C18 diols and C20:4n6-MAGs. Lipid mediator precursor fatty acids were exclusively clustered in cluster 1, joined only by three other fatty acids and the oxylipin 9,12,13-TriHOME.
Figure 4.
Insulin induced temporal changes in fatty acids and lipid mediators in early- and late-fasted seal pups (n = 5 animals/period). The 72 metabolites were condensed into 12 cluster components using variable clustering in JMP Pro 15.1, an implementation of the principal components analysis-based VARCLUS procedure for SAS. The cluster components were clustered into 3 groups of 4 (A–D, E–H, I–L) using K-nearest neighbors. Clusters are named by representative suites of metabolites contained within each cluster. Contrast posttests were used to evaluate mean differences between early and late groups at each time, as well as differences across time. Differences detected between early and late fast are indicated at P <0.05 with an asterisk (*). Differences detected across time in comparison to T0 are indicated at P <0.05 with a triangle (▴).
DISCUSSION
The current study analyzed plasma oxylipin concentrations in northern elephant seals undergoing prolonged fasting to enhance our understanding of potential contributing mechanisms to their unique physiological adaptations to protracted food deprivation. A further understanding of oxylipin biology was promoted by comparing the chronic, static changes between early and late fasting and the acute, dynamic changes in response to exogenous insulin in fasting northern elephant seals. Nine oxylipins changed significantly between early and late fasting, including a decrease in PGF2α and an increase in 14,15-DiHETrE, 20-HETE, and 4-HDoHE. Interestingly, these same oxylipins, changed in an opposite manner in response to insulin infusion, as determined by total AUC. In addition, 12-HpETE decreased with fasting, and 12-HETE had a lower AUC value in response to insulin during late fast. Many of these oxylipins that changed with prolonged fasting or after insulin infusion have documented metabolic effects on insulin signaling and inflammation (8, 9). Based on k-means cluster analysis, the altered oxylipins appeared to respond to insulin differently than their respective fatty acid precursors. For example, PGF2α shown in cluster E of Fig. 4, 20-HETE shown in cluster F, and 12-HETE shown in cluster G had different temporal responses to insulin compared with their fatty acid precursor, arachidonic acid, shown in cluster A. In addition, 4-HDoHE’s temporal response to insulin, condensed in cluster J, is not a K-nearest neighbor with its precursor, docosahexaenoic acid, in cluster A (Fig. 4).
The development of reversible insulin resistance in northern elephant seals remains a topic of great interest from both comparative and basic biological perspectives. Generally, the evolutionary adaptation in elephant seals to reduce cellular absorption of glucose through insulin resistance is beneficial as the fast progresses since glycogen stores are quickly reduced, contributing minimally to metabolism, and glycerol contributions to gluconeogenesis in fasting pups (<7%) (5) and lactating (fasting) adult females (<3%) (22) are relatively low, suggesting that the capacity to synthesize glucose from by-products of lipolysis is limited (2, 5, 22, 23). Accordingly, there is a decline in the production of insulin in response to glucose concurrently with a stimulation of lipolysis (5, 6, 23). In lactating northern elephant seals, a 150-g injection of glucose did not stimulate insulin production during late lactation compared with a 129% increase during early lactation (24), indicating a decrease in glucose-stimulated insulin secretion (GSIS) in the lactating female with fasting. It appears that the insulin-producing β-cells in the pancreas lose their sensitivity to plasma glucose as levels of NEFAs rise (25). This increase in peripheral insulin resistance has also been observed in fasting pups (26), suggesting that it is the fast itself, and not lactation stress, that promotes these changes. Previous studies in these mammals indicate that the peripheral insulin resistance is associated with reduced phosphorylation of the insulin receptor (IR), insulin receptor substrate-1 (IRS-1), and expression of Akt2 (1). Under normal conditions, insulin binds its receptor to phosphorylate IRS-1, which associates with phosphatidylinositol 3-kinase (PI3K) to phosphorylate Akt2 (27). This signaling cascade enhances translocation of Glut4 to the plasma membrane; thus, a decrease in the activation of IR, IRS-1, and/or Akt2 may contribute to the insulin resistance in northern elephant seals (27). In Znt7 knockout mice, which are susceptible to diet-induced insulin resistance and fatty acid accumulation in skeletal muscle, myotubes treated with 12-HETE and 12,13-DiHOME had a 35% and 40% reduction, respectively, in insulin-dependent Akt phosphorylation (28). Consequently, the myotubes treated with these two oxylipins had a reduction in insulin-stimulated glucose uptake (28), suggesting that 12-HETE and 12,13-DiHOME contribute to insulin resistance. However, this relationship does not elucidate if it holds true with an inverse sequence of oxylipin and insulin provision (28). In the current study, during late fasting when the seals present with insulin resistance, insulin infusion decreased 12-HETE compared with early fasting. This supports the hypothesis that the fasting-induced reduction in plasma insulin is associated with an increase in these 12-lipoxygenase-derived oxylipins, which may facilitate the development of insulin resistance in northern elephant seals.
The altered oxylipin profile in late fasting may contribute to the fasting-associated insulin resistance and effective lipid metabolism. 20-HETE is an extensively studied oxylipin associated with regulation of insulin production, vascular tone, and inflammation (29). For example, 20-HETE may inhibit Glut 2 expression, limit Akt phosphorylation, and reduce GSIS (30). Furthermore, 20-HETE diminishes the vasodilatory effects of insulin by impairing insulin’s effect on the IRS-1/PI3K/Akt2 pathway (31). Diabetics with cardiac ischemia, related to vascular inflammation and endothelial damage, had higher levels of 20-HETE (32), demonstrating the relationship of 20-HETE on diabetic vascular impairment. In addition, 20-HETE levels are greater in type 2 diabetics compared with nondiabetics (29). 20-HETE was increased at late fasting and decreased in response to insulin, suggesting that this oxylipin may contribute to the development and maintenance of the fasting-associated insulin resistance in elephant seal pups. The 20-HETE-associated contribution to insulin resistance in these seals may be mediated in part through its effects on the renin-angiotensin system (RAS). Inappropriately elevated angiotensin II (Ang II) and overactivation of RAS are contributing factors to the development and maintenance of insulin resistance in different models of deranged metabolism (33–35). Plasma Ang II is elevated in late-fasted pups (36) and 20-HETE increased the transcription and activity of angiotensin-converting enzyme (ACE) in human microvascular endothelial cells (37), suggesting that 20-HETE may promote insulin resistance via increased RAS. Because 20-HETE also contributes to regulating vascular tone, this oxylipin may be physiologically significant for such deep-diving mammals that experience extremely long bouts of apnea.
Another oxylipin that differentially changed with fasting duration and with insulin infusion was PGF2α. Baseline levels of PGF2α decreased between early and late fasting; however, PGF2α was the only detected oxylipin that had a higher AUC value in response to insulin infusion. The decrease in basal PGF2α with fasting duration is associated with a nonsignificant decrease in 8-iso-PGF2α (38) and a significant decrease in plasma insulin (1, 4–6, 23). This correlation between PGF2α and insulin corresponds with that found in obese men (r = 0.487) (39). In addition, reduced baseline levels of PGF2α may facilitate the maintenance of lipid over glucose metabolism, especially gluconeogenesis. Treatment with PGF2α in fasting and diabetic mice with and without F-prostanoid receptors (FP) amplified hepatic glucose production by upregulating gluconeogenic genes during fasted conditions particularly in the mice with FP overexpression (40). Fasting in elephant seal pups is associated with a decrease in endogenous glucose production and sustained levels of gluconeogenesis (5). Thus, reduced PGF2α levels during late fasting may facilitate efficient fatty acid oxidation by contributing to the decrease in endogenous glucose production (5).
The effects of glucocorticoids on oxylipin biosynthesis are not well described and incongruent (41). Data suggest that elevated glucocorticoids (GCs) suppress cyclooxygenase 2 (COX2) transcription, which reduces prostaglandins and thromboxanes (42, 43). In rats exposed to behavioral stress (i.e., maternal separation) and chronically provided dietary n-3 PUFA, corticosterone was reduced associated with modest reductions in PGE2, suggesting that reduced prostaglandin synthesis was independent of elevated GCs (44) and contrary to the lipocortin theory of glucocorticoid suppression of prostaglandins (45). In addition, GCs had no effect on PGE2 secretion but substantially increased leukotriene B4 (LTB4), suggesting that elevated GCs had no effect on prostaglandin synthesis but did stimulate LOX-mediated oxylipins in human fetal membranes (46). In the present study, fasting-associated increase in cortisol (1) was associated with reductions of nearly twofold in PGF2α and fivefold in TXB2, suggesting that GCs suppress prostaglandin and thromboxane synthesis and consistent with most in vivo data (47). However, the previously reported insulin-induced increase in cortisol in both early- and late-fasted pups (1) was associated with an increased response in PGF2α in early-fasted pups and decreased response in late-fasted pups, suggesting that the effects of GCs on prostaglandins are modified by fasting duration, whereas there was no effect on TXB2. Thus, during early fasting, tissues are resistant to GC-induced suppression of prostaglandins, which may reflect the lack of a need to initiate anti-inflammatory mechanisms.
Inflammation may contribute to the manifestation of insulin resistance (48); therefore, assessing the potential contributions of oxylipins to inflammation may help elucidate their association to the insulin resistance that develops in northern elephant seal pups (49). The proinflammatory factor, TNF-α, can induce insulin resistance (50). Epoxyeicosatrienoic acids (EET) such as 14,15 EpETrE can mediate anti-inflammatory mechanisms by blocking activation of NF-κB (51) and reducing TNF-α (52), effects reduced by sEH-dependent hydrolysis of EETs into their corresponding diols (DiHETrE). Although 11,12- and 14,15-DiHETrE were increased with fasting, plasma TNF-α was not significantly increased with fasting in elephant seal pups (38), suggesting that DiHETrEs may not contribute to systemic inflammation. However, muscle-specific TNF-α was increased in late-fasted elephant seal pups (53), suggesting that increased DiHETrEs may contribute to local inflammation independent of robust, systemic inflammation. 12-HETE and 12-HpETE may increase TNF-α, which may be a contributing factor in 12-HETE-induced insulin resistance in ZnT7-null mice (8, 28). The fasting-associated decrease in 12-HpETE in the present study may contribute to the lack of a robust increase in plasma TNF-α in these seal pups. As mentioned previously, the pro-inflammatory oxylipin, 20-HETE, was increased with fasting duration, and although we contend that this increase may contribute to the manifestation of insulin resistance in these seal pups, it is not likely through a pro-inflammatory mechanism at a systemic level. We have shown that plasma markers of inflammation (TNF-α, C-reactive peptide, and 8-iso-PGF2α) are not elevated in late-fasted elephant seal pups (38). Therefore, the contributions of 20-HETE to the development of insulin resistance in these pups is likely through induction of local (or tissue-specific) mechanisms independent of profound, systemic inflammation.
The changes in some of the other oxylipins such as TXB2 and 12-HpETE, which are associated with type 2 diabetes mellitus (T2DM) and insulin resistance (8, 54), suggest that these are less likely to contribute to the metabolic adaptations associated with prolonged fasting in elephant seal pups. Nonetheless, we acknowledge the limitations in interpreting changes in static measurements and recognize the need to perform additional studies to further elucidate the contributions of oxylipins in the regulation of substrate-level metabolism in fasting seals. However, many of the relationships revealed here such as the decrease in PGF2α with fasting are substantiated by similar studies in other mammals, suggesting that some of these mechanisms are evolutionarily conserved.
Although we recognize the potential limitations of the study, there are also many strengths worth emphasizing. The use of a large mammal with a unique metabolism experiencing a protracted bout of absolute food deprivation is rare for many reasons. Large mammals such as seals provide the opportunity to collect multiple time-point samples from the same animal over a defined period. The low sample sizes may have limited our ability to detect more differences but incorporating the multiple time points allowed us to overcome much of these limitations. Overall, it appears the oxylipin responses to exogenous insulin independent of fasting may be more intentional via altered enzymatic activity than simply consequences of variations in the amount of their precursor fatty acid since oxylipins from the same fatty acid precursor and pathway had different responses.
Perspectives and Significance
The present study identifies oxylipins altered during fasting and sensitive to insulin infusion that may contribute to the manifestation of insulin resistance and participate in the metabolic regulation of these conditions. The specific effects of these oxylipins on these processes require further investigation. Nonetheless, the contributions of different classes of oxylipins, a group of lipid mediators, which have not been examined in a prolonged-fasted mammal or in response to exogenous insulin infusion, to the adaption to protracted-fasting metabolism have not been previously examined. The present study reveals that oxylipins may contribute to substrate-level metabolism as well as the manifestation of insulin resistance in prolonged-fasted elephant seal pups and likely other age-classes of this species and probably other pinnipeds. Furthermore, the unique responses of the detectable oxylipins to insulin in the present study provide insights to the fasting-associated reduction in plasma insulin and related mechanisms that manifest the insulin resistance in these animals (5, 6). Thus, the reduction in insulin with fasting duration observed in these seals may serve two primary purposes: 1) facilitate the maintenance of relative hyperglycemia and 2) contribute to the onset and/or maintenance of the temporary insulin resistance during the fast via oxylipin-mediated mechanisms. These data further substantiate the importance of oxylipins in the development and maintenance of insulin resistance and ultimately T2DM in other models and humans.
SUPPLEMENTAL DATA
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.13577909.
GRANTS
J.A.V. was supported by a National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) Supplement to Support Diversity (R01HL09176-S). R.M.O. was partially supported by NHLBI Career Development Award (K02HL103787). Research was also funded by NHLBI (R01HL09176), NIH West Coast Metabolomics Center (U24 DK097154), the Cal Poly Summer Undergraduate Research Program, and a Fogarty Training Grant 1D43TW009318-010 provided support to K.G.H.K a Medium-term trainee, Program in International and Community Nutrition, University of California, Davis. Additional support was provided to J.W.N by the United States Department of Agriculture (USDA) (Intramural Projects 2032-51530-022-00D and 2032-51530-025-00D). The USDA is an equal-opportunity employer and provider.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A.V., D.E.C., J.W.N., M.R.L.F., and R.M.O. conceived and designed research; K.G.H.K., J.A.V., D.E.C., J.W.N., M.R.L.F., and R.M.O. performed experiments; D.N.W., J.A.V., D.E.C., J.W.N., M.R.L.F., and R.M.O. analyzed data; D.N.W., J.W.N., M.R.L.F., and R.M.O. interpreted results of experiments; D.N.W., J.W.N., and M.R.L.F. prepared figures; D.N.W., M.R.L.F., and R.M.O. drafted the manuscript; D.N.W., K.G.H.K., J.W.N., M.R.L.F., and R.M.O. edited and revised manuscript; D.N.W., J.A.V., D.E.C., J.W.N., M.R.L.F., and R.M.O. approved the final version of the manuscript.
ACKNOWLEDGMENTS
Present address of J. A. Viscarra: Nutritional Sciences & Toxicology, University of California, Berkeley, CA.
REFERENCES
- 1.Viscarra JA, Vázquez-Medina JP, Crocker DE, Ortiz RM. Glut4 is upregulated despite decreased insulin signaling during prolonged fasting in northern elephant seal pups. Am J Physiol Regul Integr Comp Physiol 300: R150–R154, 2011. doi: 10.1152/ajpregu.00478.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houser DS, Crocker DE, Tift MS, Champagne CD. Glucose oxidation and nonoxidative glucose disposal during prolonged fasts of the northern elephant seal pup (Mirounga angustirostris). Am J Physiol Regul Integr Comp Physiol 303: R562–R570, 2012. doi: 10.1152/ajpregu.00101.2012. [DOI] [PubMed] [Google Scholar]
- 3.Ortiz CL, Costa D, Le Boeuf BJ. Water and energy flux in elephant seal pups fasting under natural conditions Physiol Zool 51: 166–178, 1978.PMC] doi: 10.1086/physzool.51.2.30157864. [DOI] [Google Scholar]
- 4.Olmstead KI, la Frano MR, Fahrmann J, Grapov D, Viscarra JA, Newman JW, Fiehn O, Crocker DE, Filipp F. V, Ortiz RM. Insulin induces a shift in lipid and primary carbon metabolites in a model of fasting-induced insulin resistance. Metabolomics 13: 60, 2017. doi: 10.1007/s11306-017-1186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Champagne CD, Houser DS, Fowler MA, Costa DP, Crocker DE. Gluconeogenesis is associated with high rates of tricarboxylic acid and pyruvate cycling in fasting northern elephant seals. Am J Physiol Regul Integr Comp Physiol 303: R340–R352, 2012. doi: 10.1152/ajpregu.00042.2012. [DOI] [PubMed] [Google Scholar]
- 6.Ortiz R, Noren D, Ortiz C, Talamantes F. GH and ghrelin increase with fasting in a naturally adapted species, the northern elephant seal (Mirounga angustirostris). J Endocrinol 178: 533–539, 2003. doi: 10.1677/joe.0.1780533. [DOI] [PubMed] [Google Scholar]
- 7.Ostermann AI, Greupner T, Kutzner L, Hartung NM, Hahn A, Schuchardt JP, Schebb NH. Intra-individual variance of the human plasma oxylipin pattern: low inter-day variability in fasting blood samples: versus high variability during the day. Anal Methods 10: 4935–4944, 2018. doi: 10.1039/C8AY01753K. [DOI] [Google Scholar]
- 8.Tourdot BE, Ahmed I, Holinstat M. The emerging role of oxylipins in thrombosis and diabetes. Front Pharmacol 4: 176–179, 2014. doi: 10.3389/fphar.2013.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv Nutr 6: 513–540, 2015. doi: 10.3945/an.114.007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shearer GC, Newman JW. Impact of circulating esterified eicosanoids and other oxylipins on endothelial function. Curr Atheroscler Rep 11: 403–410, 2009. doi: 10.1007/s11883-009-0061-3. [DOI] [PubMed] [Google Scholar]
- 11.Borkowski K, Yim SJ, Holt RR, Hackman RM, Keen CL, Newman JW, Shearer GC. Walnuts change lipoprotein composition suppressing TNFα-stimulated cytokine production by diabetic adipocyte. J Nutr Biochem 68: 51–58, 2019. doi: 10.1016/j.jnutbio.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajamani A, Borkowski K, Akre S, Fernandez A, Newman JW, Simon SI, Passerini AG. Oxylipins in triglyceride-rich lipoproteins of dyslipidemic subjects promote endothelial inflammation following a high fat meal. Sci Rep 9: 8655, 2019. doi: 10.1038/s41598-019-45005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viscarra JA, Rodriguez R, Vázquez-Medina JP, Lee A, Tift MS, Tavoni SK, Crocker DE, Ortiz RM. Insulin and GLP-1 infusions demonstrate the onset of adipose-specific insulin resistance in a large fasting mammal: potential glucogenic role for GLP-1. Physiol Rep 1: e00023, 2013. doi: 10.1002/phy2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortiz RM, Wade CE, Ortiz CL. Effects of prolonged fasting on plasma cortisol and TH in postweaned northern elephant seal pups. Am J Physiol Regul Integr Comp Physiol 280: R790–R795, 2001. doi: 10.1152/ajpregu.2001.280.3.R790. [DOI] [PubMed] [Google Scholar]
- 15.Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res 51: 2074–2081, 2010. doi: 10.1194/M900193-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somo DA, Ensminger DC, Sharick JT, Kanatous SB, Crocker DE. Development of dive capacity in northern elephant seals (Mirounga angustirostris): reduced body reserves at weaning are associated with elevated body oxygen stores during the postweaning fast. Physiol Biochem Zool 88: 471–482, 2015. doi: 10.1086/682386. [DOI] [PubMed] [Google Scholar]
- 17.Xia J, Wishart DS. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr Protoc Bioinformat 55: 14.10.1–14.10.91, 2016. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- 18.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. http://www.R-project.org. [Google Scholar]
- 19.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs D, Hamberg M, Sköld CM, Wheelock ÅM, Wheelock CE. An LC–MS/MS workflow to characterize 16 regio- and stereoisomeric trihydroxyoctadecenoic acids. J Lipid Res 59: 2025–2033, 2018. doi: 10.1194/jlr.D087429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Worley B, Powers R. Multivariate analysis in metabolomics. Curr Metab 1: 92–107, 2013. doi: 10.2174/2213235X11301010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houser DS, Champagne CD, Crocker DE. Lipolysis and glycerol gluconeogenesis in simultaneously fasting and lactating northern elephant seals. Am J Physiol Regul Integr Comp Physiol 293, 2007. doi: 10.1152/ajpregu.00403.2007. [DOI] [PubMed] [Google Scholar]
- 23.Houser DS, Champagne CD, Crocker DE. A non-traditional model of the metabolic syndrome: the adaptive significance of insulin resistance in fasting-adapted seals. Front Endocrinol (Lausanne) 4: 164–110, 2013. doi: 10.3389/fendo.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fowler MA, Champagne CD, Houser DS, Crocker DE. Hormonal regulation of glucose clearance in lactating northern elephant seals (Mirounga angustirostris). J Exp Biol 211: 2943–2949, 2008. doi: 10.1242/jeb.018176. [DOI] [PubMed] [Google Scholar]
- 25.Dubois M, Kerr-Conte J, Gmyr V, Bouckenooghe T, Muharram G, D’Herbomez M, Martin-Ponthieu A, Vantyghem MC, Vandewalle B, Pattou F. Non-esterified fatty acids are deleterious for human pancreatic islet function at physiological glucose concentration. Diabetologia 47: 463–469, 2004. doi: 10.1007/s00125-004-1347-1. [DOI] [PubMed] [Google Scholar]
- 26.Kirby VL, Ortiz CL. Hormones and fuel regulation in fasting elephant seals (Online). In: Elephant Seals: Population Ecology, Behavior, and Physiology, edited by Le Boeuf BJ, Laws RM. Berkeley; Los Angeles; Oxford: University of California Press, 1994, 374–386. http://www.escholarship.org/editions/view?docId=ft7b69p131&chunk.id=d0e29854&toc.depth=1&toc.id=d0e29854&brand=ucpress. [Google Scholar]
- 27.Leney SE, Tavaré JM. The molecular basis of insulin-stimulated glucose uptake: signalling, trafficking and potential drug targets. J Endocrinol 203: 1–18, 2009. doi: 10.1677/JOE-09-0037. [DOI] [PubMed] [Google Scholar]
- 28.Huang L, Tepaamorndech S, Kirschke CP, Newman JW, Keyes WR, Pedersen TL, Dumnil J. Aberrant fatty acid metabolism in skeletal muscle contributes to insulin resistance in zinc transporter 7 (znt7)-knockout mice. J Biol Chem 293: 7549–7563, 2018. doi: 10.1074/jbc.M117.817692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Issan Y, Hochhauser E, Guo A, Gotlinger KH, Kornowski R, Leshem-Lev D, Lev E, Porat E, Snir E, Thompson CI, Abraham NG, Laniado-Schwartzman M. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostagland Other Lipid Mediat 100–101: 15–21, 2013. doi: 10.1016/j.prostaglandins.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B, Lai G, Wu J, Sun R, Xu R, Yang X, Qi Y, Zhao Y. 20-HETE attenuates the response of glucose-stimulated insulin secretion through the AKT/GSK-3β/Glut2 pathway. Endocrine 54: 371–382, 2016. doi: 10.1007/s12020-016-1031-5. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Zhao G, Ma B, Li R, Hong J, Liu S, Wang DW. 20-Hydroxyeicosatetraenoic acid impairs endothelial insulin signaling by inducing phosphorylation of the insulin receptor substrate-1 at Ser616. PLoS One 9: e95841, 2014. doi: 10.1371/journal.pone.0095841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoopes SL, Garcia V, Edin ML, Schwartzman ML, Zeldin DC. Vascular actions of 20-HETE. Prostagland Other Lipid Mediat 120: 9–16, 2015. doi: 10.1016/j.prostaglandins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lastra-Lastra G, Sowers JR, Restrepo-Erazo K, Manrique-Acevedo C, Lastra-González G. Role of aldosterone and angiotensin II in insulin resistance: an update. Clin Endocrinol (Oxf) 71: 1–6, 2009. doi: 10.1111/j.1365-2265.2008.03498.x. [DOI] [PubMed] [Google Scholar]
- 34.Olivares-Reyes JA, Arellano-Plancarte A, Castillo-Hernandez JR. Angiotensin II and the development of insulin resistance: implications for diabetes. Mol Cell Endocrinol 302: 128–139, 2009. doi: 10.1016/j.mce.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez R, Viscarra JA, Minas JN, Nakano D, Nishiyama A, Ortiz RM. Angiotensin receptor blockade increases pancreatic insulin secretion and decreases glucose intolerance during glucose supplementation in a model of metabolic syndrome. Endocrinology 153: 1684–1695, 2012. doi: 10.1210/en.2011-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vázquez-Medina JP, Sonanez-Organis JG, Rodriguez R, Viscarra JA, Nishiyama A, Crocker DE, Ortiz RM. Prolonged fasting activates Nrf2 in post-weaned elephant seals. J Exp Biol 216: 2870–2878, 2013. doi: 10.1242/jeb.081927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia V, Shkolnik B, Milhau L, Falck JR, Schwartzman ML. 20-HETE activates the transcription of angiotensin-converting enzyme via nuclear factor-B translocation and promoter binding. J Pharmacol Exp Ther 356: 525–533, 2016. doi: 10.1124/jpet.115.229377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vázquez-Medina JP, Crocker DE, Forman HJ, Ortiz RM. Prolonged fasting does not increase oxidative damage or inflammation in postweaned northern elephant seal pups. J Exp Biol 213: 2524–2530, 2010. doi: 10.1242/jeb.041335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, Maruyama N, Kitagawa N, Tanaka T, Hori Y, Nakatani K, Yano Y, Adachi Y. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab 88: 4673–4676, 2003. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Yan S, Xiao B, Zuo S, Zhang Q, Chen G, Yu Y, Chen D, Liu Q, Liu Y, Shen Y, Yu Y. Prostaglandin F2α facilitates hepatic glucose production through CaMKIIγ/p38/FOXO1 signaling pathway in fasting and obesity. Diabetes 67: 1748–1760, 2018. doi: 10.2337/db17-1521. [DOI] [PubMed] [Google Scholar]
- 41.Duval D, Freyss-Beguin M. Glucocorticoids and prostaglandin synthesis: we cannot see the wood for the trees. Prostagland, Leukotr Essent Fatty Acids 45: 85–112, 1992. doi: 10.1016/0952-3278(92)90225-8. [DOI] [PubMed] [Google Scholar]
- 42.Masferrer JL, Seibert K. Regulation of prostaglandin synthesis by glucocorticoids. Receptor 4: 25–30, 1994. [PubMed] [Google Scholar]
- 43.Goppelt-Struebe M. Molecular mechanisms involved in the regulation of prostaglandin biosynthesis by glucocorticoids. Biochem Pharmacol 53: 1389–1395, 1997. doi: 10.1016/S0006-2952(97)00018-X. [DOI] [PubMed] [Google Scholar]
- 44.Choi J-E, Borkowski K, Newman JW, Park Y. N-3 PUFA improved post-menopausal depression induced by maternal separation and chronic mild stress through serotonergic pathway in rats—effect associated with lipid mediators. J Nutr Biochem 91: 108599, 2021. doi: 10.1016/j.jnutbio.2021.108599. [DOI] [PubMed] [Google Scholar]
- 45.Flower RJ. Lipocortin and the mechanism of action of the glucocorticoids. Br J Pharmacol 94: 987–1015, 1988. doi: 10.1111/j.1476-5381.1988.tb11614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zicari A, Ticconi C, Pontieri G, Loyola G, Piccione E. Effects of glucocorticoids and progesterone on prostaglandin E2 and leukotriene B4 release by human fetal membranes at term gestation. Prostaglandins 54: 539–547, 1997. doi: 10.1016/S0090-6980(97)00124-X. [DOI] [PubMed] [Google Scholar]
- 47.Russo-Marie F, Seillan C, Duval D. Glucocorticoids as inhibitors of prostaglandin synthesis. Bull Eur Physiopathol Respir 17: 587–594, 1981. [PubMed] [Google Scholar]
- 48.Shoelson SE, Lee J, Goldfine AB. Review series inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006. [Erratum in J Clin Invest 116: 2308, 2006]. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 132: 2169–2180, 2007. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 50.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor- (alpha): direct role in obesity-linked insulin resistance. Science 259: 87–92, 1993. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 51.Schuck RN, Zha W, Edin ML, Gruzdev A, Vendrov KC, Miller TM, Xu Z, Lih FB, DeGraff LM, Tomer KB, Jones HM, Makowski L, Huang L, Poloyac SM, Zeldin DC, Lee CR. The cytochrome p450 epoxygenase pathway regulates the hepatic inflammatory response in fatty liver disease. PLoS One 9: e110162, 2014. doi: 10.1371/journal.pone.0110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Dang H, Li D, Pang W, Hammock BD, Zhu Y. Inhibition of soluble epoxide hydrolase attenuates high-fat-diet-induced hepatic steatosis by reduced systemic inflammatory status in mice. PLoS One 7: e39165, 2012. doi: 10.1371/journal.pone.0039165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki M, Vázquez-Medina JP, Viscarra JA, Soñanez-Organis JG, Crocker DE, Ortiz RM. Activation of systemic, but not local, renin-angiotensin system is associated with upregulation of TNF- during prolonged fasting in northern elephant seal pups. J Exp Biol 216: 3215–3221, 2013. doi: 10.1242/jeb.085225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pulcinelli FM, Biasucci LM, Riondino S, Giubilato S, Leo A, di Renzo L, Trifiro E, Mattiello T, Pitocco D, Liuzzo G, Ghirlanda G, Crea F. COX-1 sensitivity and thromboxane A2 production in type 1 and type 2 diabetic patients under chronic aspirin treatment. Eur Heart J 30: 1279–1286, 2009. doi: 10.1093/eurheartj/ehp097. [DOI] [PubMed] [Google Scholar]