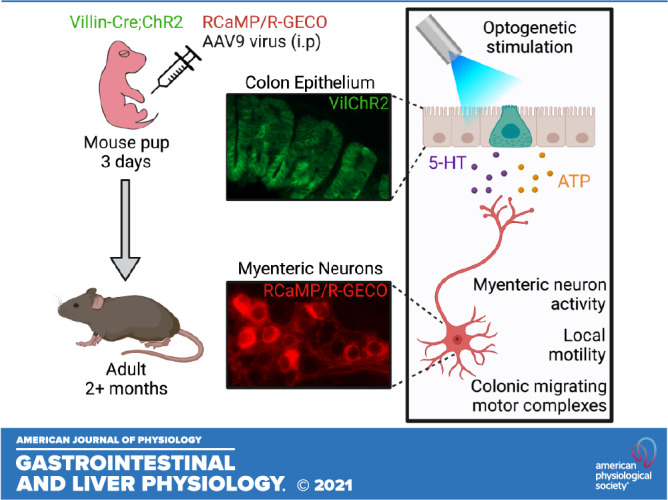
Keywords: channelrhodopsin, colonic migrating motor complex, myenteric neurons, RCaMP, R-GECO
Abstract
Digestive functions of the colon depend on sensory-motor reflexes in the enteric nervous system (ENS), initiated by intrinsic primary afferent neurons (IPANs). IPAN terminals project to the mucosal layer of the colon, allowing communication with epithelial cells comprising the colon lining. The chemical nature and functional significance of this epithelial-neural communication in regard to secretion and colon motility are of high interest. Colon epithelial cells can produce and release neuroactive substances such as ATP and 5-hydroxytryptamine (5-HT), which can activate receptors on adjacent nerve fibers, including IPAN subtypes. In this study, we examined if stimulation of epithelial cells alone is sufficient to activate neural circuits that control colon motility. Optogenetics and calcium imaging were used in ex vivo preparations of the mouse colon to selectively stimulate the colon epithelium, measure changes in motility, and record activity of neurons within the myenteric plexus. Light-mediated activation of epithelial cells lining the distal, but not proximal, colon caused local contractions and increased the rate of colonic migrating motor complexes. Epithelial-evoked local contractions in the distal colon were reduced by both ATP and 5-HT receptor antagonists. Our findings indicate that colon epithelial cells likely use purinergic and serotonergic signaling to initiate activity in myenteric neurons, produce local contractions, and facilitate large-scale coordination of ENS activity responsible for whole colon motility patterns.
NEW & NOTEWORTHY Using an all-optical approach to measure real-time cell-to-cell communication responsible for colon functions, we show that selective optogenetic stimulation of distal colon epithelium produced activity in myenteric neurons, as measured with red genetically encoded calcium indicators. The epithelial-induced neural response led to local contractions, mediated by both purinergic and serotonergic signaling, and facilitated colonic motor complexes that propagate from proximal to distal colon.
INTRODUCTION
The enteric nervous system (ENS) is a network of neural ganglia intrinsic to the gastrointestinal (GI) tract that controls motility, local blood flow, mucosal transport, and secretion. Dysregulation of motility patterns is associated with constipation or diarrhea, which can be a prominent feature of many GI disorders, and oftentimes results in a significant reduction in quality of life. Dysmotility is even recognized as a common symptom of diseases previously thought to only affect the brain, e.g., autism, Parkinson’s (1–3). Proper colon motility is dependent on coordinated sensory-motor reflexes in the ENS. Intrinsic primary afferent neurons (IPANs) are sensory neurons unique to the GI tract that initiate reflexive contractions. IPANs sense mechanical or chemical changes in the intestinal lumen and in turn activate interneurons and motor neurons in the myenteric plexus that generate propulsive contractions (4–6). With cell bodies in the myenteric plexus, they extend processes into the submucosal layer that terminate in close apposition to colon epithelial cells (7), allowing for functional communication. How colon epithelial cells influence activity in ENS circuits is a major unanswered question.
Enteroendocrine epithelial cells (EECs) express membrane receptors that can sense chemical irritants, e.g., transient receptor potential ankyrin 1 (TRPA1) (8) and mechanical stimulation, e.g., Piezo2 (9). In response to receptor activation, EECs release neuroactive substances (e.g., 5-HT, ATP) (10–12) that bind receptors on local neuron terminals (8, 13, 14), providing a means by which the epithelium can directly detect and respond to luminal contents. In previous studies, epithelial-neural communication was shown using an optogenetic approach; we found that light-mediated activation of epithelial cells caused action potential firing in extrinsic primary afferent neurons (ExPANs; located in dorsal root ganglia) and evoked behavioral responses similar to those recorded in response to colon distension (11). A logical extension of these studies is to ask if activation of colon epithelial cells is also sufficient to impact colon motility, hypothetically via activation of IPAN terminals.
Previous studies suggest that mucosal 5-HT is essential for normal propagation of motor patterns in the colon (15–17). This is particularly true for colonic migrating motor complexes (CMMCs), which are rhythmic motor patterns that facilitate the movement of fecal matter through the colon (18). Other studies contend that signaling from the mucosa is not required for generation of motor patterns, and rather that mucosal-derived 5-HT has a modulatory role (19, 20). Given these discrepancies, the aim of this study was to reevaluate the role of the epithelium in colon motility using an optogenetic approach that allows specific activation of the epithelium and subsequent measure of ENS and motility responses.
Ex vivo colon preparations from transgenic mice that express the blue light-activated excitatory channelrhodopsin protein (ChR2) in colon epithelial cells were analyzed. Light activation of epithelial cells was done with simultaneous measure of myenteric neuron responses using red-shifted genetically encoded Ca2+ indicators (GECIs), as well as local colon motility and CMMCs. Light stimulation of distal colon epithelium, but not proximal colon, increased Ca2+ transients in a subpopulation of myenteric neurons, caused changes in local motility, and increased the frequency of CMMCs. Pharmacological antagonism of ATP and 5-HT signaling reduced epithelial-evoked changes in motility. These findings are consistent with previous findings that indicate colon epithelial cells induce neuronally mediated changes in motility through purinergic and serotonergic signaling, and that this induction occurs in a regionally defined manner.
MATERIALS AND METHODS
Animals
Adult male and female mice 2–4 mo of age were used for the majority of experiments, with the exception of three mice that were 12 mo. As described previously (11), a channel rhodopsin (ChR2)-enhanced yellow fluorescent protein (eYFP) fusion protein was genetically targeted to the intestinal epithelium using a villin-Cre driver. Mice with ChR2-eYFP [ChR2(H134R)-EYFP] in the Rosa26 locus downstream of a floxed-STOP cassette (Ai32 mice; RRID: IMSR_JAX:012659) were crossed with villin-Cre mice (RRID: IMSR_JAX004586). Littermates with ChR2-eYFP but lacking Cre were used as controls. Animals were housed in an American Association for the Accreditation of Laboratory Animal Care-approved facility and handled in accordance with protocols approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
RCaMP/R-GECO Expression in Enteric Neurons
P2–P4 neonatal mice were injected intraperitoneally with RCaMP or R-GECO virus to enable expression throughout the peripheral nervous system. Insulin syringes (3/10 mL, 31 G; Allison Medical, Littleton, CO) were used to inject 10 µL of RCaMP virus (pAAV.Syn.NES.jRCaMP1b.WPRE.SV40, Addgene, Watertown, MA) or R-GECO virus (AAV9.Syn.NES-jRGECO1a.WPRE.SV40; Addgene). Mice were analyzed at least 6 wk after injection.
Imaging Ca2+ Transients and Local Motility
After mice were euthanized with isoflurane, distal colons were isolated and placed in a Sylgard-lined dish containing artificial cerebrospinal fluid (ACSF), containing (in mM): 117.9 NaCl, 4.7 KCl, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO4·7H2O, 2.5 CaCl2, 11.1 d-glucose, 2 sodium butyrate, and 20 sodium acetate. Fecal contents were flushed from the lumen and the colon was opened longitudinally and pinned flat with serosal side up. During imaging, the dish was superfused with ACSF and maintained at 35°C. Nifedipine (4 µM, Sigma-Aldrich) was added to the ACSF to prevent spontaneous colon movement. Myenteric neurons were imaged in the distal colon (0–3 cm from the anus). The myenteric plexus was visualized at ×40 using an upright Leica DM6000FS fluorescent microscope (Leica, Buffalo Grove, IL) with a 597/60 nm excitation filter. Activity in myenteric neurons and movements of the colon were recorded using a CMOS camera (Prime 95B Photometrics; Roper Scientific, Tucson, AZ) at a 20-Hz sampling rate, 50-ms exposure time. Image stacks were collected using Metamorph software (Molecular Devices, San Jose, CA). Activity in myenteric neurons was recorded before, during, and after blue light stimulation of lining epithelial cells. Stimulation was delivered using a 473-nm wavelength laser (Laserglow Technologies, Toronto, Canada) with a 1.5-mm optical fiber (ThorLabs, Newton, NJ). The optical fiber was positioned 2–3 mm away from the imaging field, in the aboral direction, and 3 mm above the colon, enabling the optical fiber to deliver 20 mW of laser power to the colon tissue. Light stimulation was delivered for 20 s (11). Local colon motility was also imaged in Vil-ChR2 and control littermate mice that did not express GECIs in the myenteric plexus. In these mice, images were taken of the distal colon (0–3 cm from the anus) and the proximal colon (3–6 cm from the anus) and tissue movement was recorded before, during, and after laser stimulation.
Drugs Used
Tetrodotoxin (TTX) 0.5 µM and hexamethonium (HEX) 300 µM (both from Sigma-Aldrich, St. Louis, MO) were dissolved in oxygenated ACSF on the day of the experiment. Drug concentrations were chosen based on previous studies (21, 22). The ATP receptor antagonist cocktail consisted of the following drugs (all from Tocris Bioscience, Minneapolis, MN): TNP-ATP triethylammonium salt (P2X1, P2X2/3, and P2X3 antagonist, dissolved in H2O for a 100 µM stock solution), 5-(3-bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one (5-BDBD) [P2X4 antagonist, dissolved in dimethylsulfoxide (DMSO, Sigma-Aldrich) for a 30 mM stock solution], MRS 2500 (P2Y1 antagonist, dissolved in H2O for a 1 mM stock solution), AR-C 118925XX (P2Y2 antagonist, dissolved in DMSO for a 5 mM stock solution), and MRS 2578 (P2Y6 antagonist, dissolved in DMSO for a 5 mM stock solution). Drugs were added to ACSF for final concentrations of 20 nM TNP-ATP, 20 µM 5-BDBD, 10 µM MRS 2500, 10 µM AR-C118925XX, and 1 µM MRS 2578. The 5-HT receptor antagonist cocktail consisted of the following drugs: alosetron (Sigma-Aldrich, 5-HT3 antagonist, dissolved in H2O for a 20 mM stock solution), ketanserin (Tocris, 5-HT2 antagonist, dissolved in H2O for a 1 mM stock solution), and GR 113808 (Tocris, 5-HT4 antagonist, dissolved in HCl for a 20 mM stock solution). Drugs were added to ACSF for final concentrations of 20 µM alosetron, 2 µM ketanserin, and 2 µM GR 113808. Colon tissue was imaged after 15 min of antagonist drug incubation.
Whole Colon Motility
After mice were euthanized with isoflurane, whole colons were isolated, placed in a Sylgard-lined dish containing ACSF, fecal contents were flushed, and the colon was gently pinned at the mesentery to the dish. The dish was superfused with ACSF and maintained at 35°C. Measures of motility began as soon as spontaneous colonic migrating motor complexes (CMMCs) were observed (∼10–30 min after dissection from the mouse). Spontaneous contractions were video recorded (Sony, HDR-CX440) for 20 min. In some experiments, colons were connected to a force transducer to measure changes in tension (mN) during CMMC propagation. To test the effects of blue light stimulation of the colon on CMMCs, a fiber optic was secured directly above the colon at 1 cm from the anus for distal colon stimulation and 5 cm from the anus for proximal colon stimulation (total colon length ∼6.5 cm). A sustained 20-s blue laser stimulus was delivered every 2 min, for 20 min total while CMMCs were video recorded. Video analysis done blinded to the mouse genotype counted the number of CMMCs observed during the 20-min videos. CMMCs were defined as circular contractions initiated in the proximal colon that propagated at least 1 cm; only anterograde CMMCs (i.e., those traveling in the oral to aboral direction) were included for analysis.
Data Analysis
Imaging files and video recordings of colon motility were analyzed in a blinded manner. Images collected in Metamorph were exported to ImageJ (National Institutes of Health, Bethesda, MD). Local motility, i.e., colon tissue movement, was quantified using the template matching plugin in ImageJ as previously described (22). The amplitudes of calcium indicator signals were quantified by calculating ΔF/F0 as % = [(F – F0/F0)] × 100, where F is the peak fluorescence signal and F0 is the baseline fluorescence signal. Statistical analyses were performed in Prism (GraphPad, San Diego, CA) and included unpaired Student’s t test, one-way, and two-way analysis of variance (ANOVA) with post hoc tests as indicated in results. Data are represented as means ± SE, and results were considered significant when P ≤ 0.05.
RESULTS
Optogenetic Stimulation of Colon Epithelium Changes Activity in Myenteric Neurons
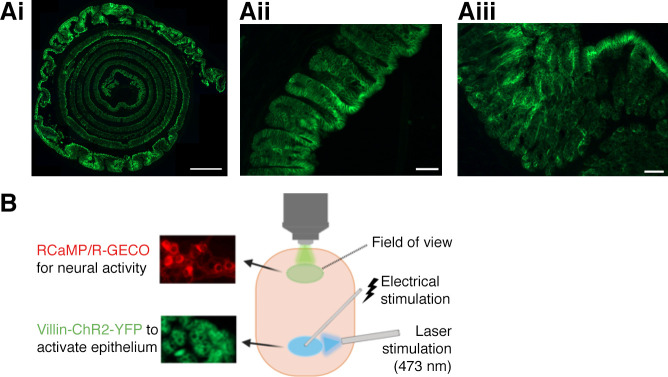
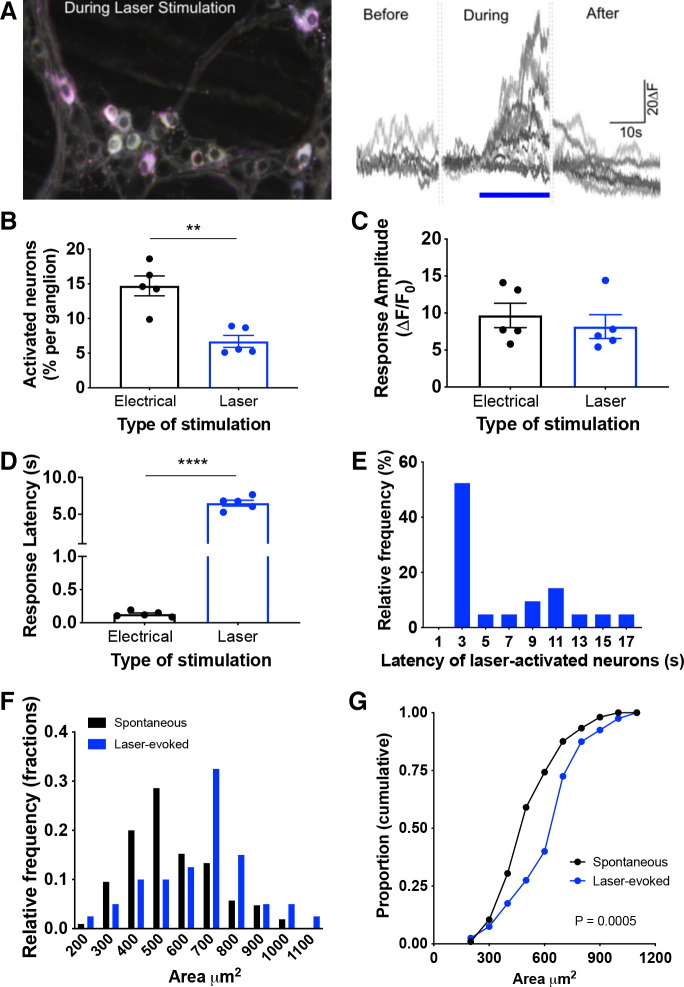
To enable selective optogenetic activation of colon epithelial cells, we generated villin-ChR2 (Vil-ChR2) mice by crossing ChR2-YFP mice with villin-cre mice. We examined the expression of ChR2-YFP in the colons of Vil-ChR2 mice and found that it was restricted to the epithelium and evenly distributed along the colon, as evidenced by Swiss roll histology (Fig. 1Ai). We also examined cross-sections of the colon to ensure that ChR2-YFP expression was abundant in both the distal (Fig. 1Aii) and proximal colon (Fig. 1Aiii). To determine whether stimulation of colon epithelium affects myenteric neuron activity, we virally expressed red calcium indicators (RGECO or RCaMP) in myenteric neurons of Vil-ChR2 mice (n = 5 mice; 3 female, 2 male). This strategy allowed for an all-optical approach to assess intercellular communication in the colon (Fig. 1B). Ca2+ transients, used as a proxy for myenteric neuron activity, were recorded before, during, and after laser stimulation (20 s) of the epithelium (Fig. 2A). Tracking of activity changes in individual neurons revealed a small population of myenteric neurons in the distal colon that were activated by laser stimulation. Activated myenteric neurons initially displayed no activity and only began to exhibit Ca2+ signals after blue light stimulation of the epithelium. Fewer neurons per ganglion responded to laser compared with electrical stimulation applied to the same location (electrical: 14.71 ± 1.43% vs. laser: 6.7 ± 0.89%; P = 0.008, Mann–Whitney U test; Fig. 2B), indicating that a smaller subpopulation of myenteric neurons receives epithelial input. The average amplitude of response was not significantly different between laser and electrical stimulation (electrical: 9.67 ± 1.65 ΔF vs. laser: 8.16 ± 1.61 ΔF; P = 0.53, unpaired t test; Fig. 2C), suggesting individual neuron responses to laser stimulation were comparable to when neural circuits were electrically activated. As would be expected in a multicellular circuit, the latency of response to laser was significantly longer than electrical stimulation (electrical: 0.13 ± 0.02 s vs. laser: 6.51 ± 0.4; P < 0.0001, unpaired t test; Fig. 2D). Latencies ranged from 2 to 16 s with the majority of responses occurring within 4 s from the start of laser stimulation (Fig. 2E). We assessed the size of RCaMP-positive spontaneously active myenteric neurons and compared them to myenteric neurons activated by blue light stimulation of the epithelium (Fig. 2F; spontaneous neurons: n = 105 neurons, laser-evoked neurons: n = 40 neurons). Size analysis shows blue light stimulation of the epithelium preferentially activated large myenteric neurons (Fig. 2G; P = 0.0005; Kolmogorov–Smirnov test), suggesting laser responsive neurons were primarily IPANs, which have Dogiel type II morphology previously characterized as larger than Dogiel type I neurons (i.e., interneurons and motor neurons) (23).
Figure 1.
Technique for optical interrogation of epithelial-myenteric neuron interactions in the mouse colon. Channel rhodopsin (ChR2), conjugated to yellow fluorescent protein (YFP), was expressed under the villin-Cre promoter to enable specific optogenetic activation of colon epithelial cells. Ai: Swiss roll histology showed that expression was restricted to epithelial cells of the colon mucosal layer and expression was even throughout the colon in Vil-ChR2 mice. Cross sections showed that ChR2-YFP was abundant in both the distal (Aii) and proximal colon (Aiii). B: experimental setup: RCaMP/R-GECO was virally expressed in myenteric neurons in Vil-ChR2 mice. Laser and electrical stimulation were applied 3 mm below the imaging field. Scale bars: Ai – 1 mm, Aii, Aiii – 50 µM. Figure was created using Biorender.com.
Figure 2.
Optogenetic stimulation of colon epithelium changes activity in myenteric neurons. A: myenteric neuron activity was recorded before, during, and after laser stimulation (20 s) of the epithelium. B: electrical stimulation in Vil-ChR2 mice (n = 5 mice) initiated Ca2+ transients in a greater percentage of myenteric neurons than laser stimulation (P = 0.008; Mann–Whitney U test). C: the average amplitude of response observed in myenteric neurons was not significantly different between laser and electrical stimulation (P = 0.53; unpaired t test). D: the latency of response to laser was significantly longer than electrical stimulation (P < 0.0001; unpaired t test). E: latencies ranged from 2 to 16 s with the majority of responses within 4 s from the start of laser stimulation. F: area was measured in RCaMP-expressing myenteric neurons, both spontaneously active neurons and neurons activated by blue light stimulation of the epithelium. G: blue light stimulation of the epithelium preferentially activated larger neurons (P = 0.0005; Kolmogorov–Smirnov test). **P < 0.01; ****P < 0.0001. ChR2, channel rhodopsin.
Optogenetic Stimulation of Colon Epithelium Initiates Changes in Local Motility
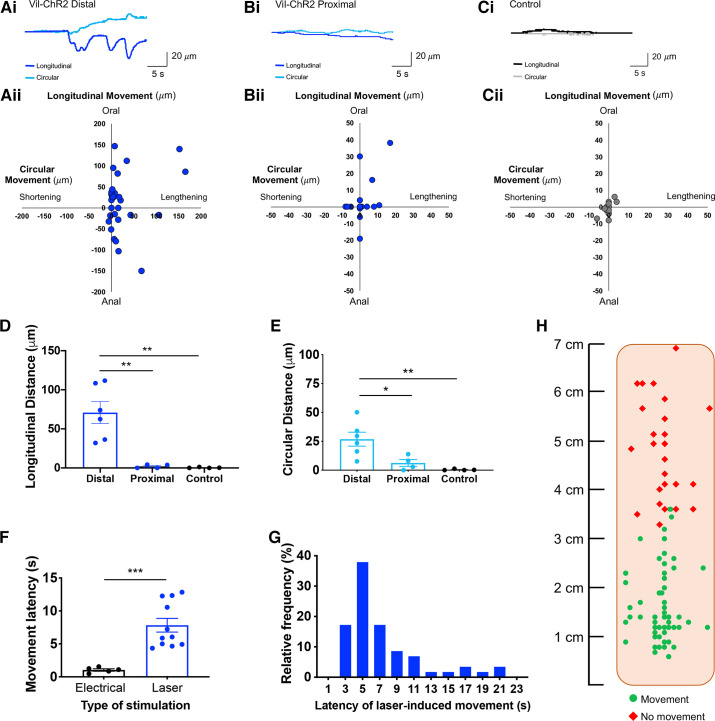
In addition to evoked activity in myenteric neurons, epithelial activation also caused movement in the imaging field, indicating the generation of smooth muscle contractions or active relaxation (22). Movement patterns were quantified by measuring the maximum tissue displacement in the longitudinal and circular directions (normalized to baseline), and coordinates were plotted on scatterplots to compare across conditions. Representative traces of tissue displacement (Fig. 3Ai) and quantification (Fig. 3Aii) illustrate robust epithelium-evoked movement in response to laser stimuli applied to the distal colon of Vil-ChR2 mice (n = 6 mice; 3 female, 3 male). In contrast, epithelial activation in the proximal colon evoked small, if any, movements. Example traces illustrate smaller movement patterns in the proximal colon of Vil-ChR2 mice (Fig. 3Bi), which were quantified in the longitudinal and circular directions (n = 4 mice; 3 female, 1 male; Fig. 3Bii). Control littermate mice showed minimal changes in local motility, as illustrated by the representative traces (Fig. 3Ci) and quantification (n = 4 mice; 2 female, 2 male; Fig. 3Cii). Epithelial stimulation in the distal colon produced local movements in the longitudinal direction (70.84 ± 13.97 µM) that were significantly greater than those produced by proximal colon epithelial stimulation (2.03 ± 1.18 µM; P = 0.002) and in colons from control littermate mice (0.38 ± 0.30 µM; P = 0.002; one-way ANOVA; Fig. 3D). Evoked movements in the circular direction were also significantly larger in response to stimulation of the distal colon (26.83 ± 6.04 µM) compared with proximal colon (6.21 ± 3.05 µM; P = 0.03) and control colon (0.37 ± 0.30 µM; P = 0.006; one-way ANOVA; Fig. 3E).
Figure 3.
Optogenetic stimulation of distal colon epithelium initiates change in local motility. Ai: representative traces illustrate the epithelium-evoked movement in the distal colon of Vil-ChR2 mice. Aii: movement patterns were characterized by tissue movement in the longitudinal and circular directions (n = 6 mice). Bi: example traces show smaller movement patterns in the proximal colon of Vil-ChR2 mice. Bii: movement patterns in the proximal colon were quantified in the longitudinal and circular directions (n = 4 mice). Ci: control littermate mice showed very minimal changes in local motility, demonstrated by the representative traces. Cii: quantification of longitudinal and circular movement also shows minimal movement in control colons (n = 4 mice). D: epithelial stimulation in the distal colon produced local movements in the longitudinal direction that were significantly greater than those produced by proximal colon epithelial stimulation (P = 0.002; one-way ANOVA) and in colons from control littermate mice (P = 0.002; one-way ANOVA). E: evoked movements in the circular direction were also significantly larger in the distal colon compared with proximal colon (P = 0.03; one-way ANOVA) and control colon (P = 0.006; one-way ANOVA). F: there was a latency between the start of blue light stimulation and colon movement which was significantly longer than the latency with electrical stimulation (P = 0.0005; Mann–Whitney test). G: latencies ranged from 3 to 22 s, with the majority of responses occurring within 6 s from the onset of laser. H: schematic of flattened colon preparation indicating where, in Vil-ChR2 mice, blue light stimulation of the epithelium initiated tissue movement above spontaneous baseline movement and where blue light stimulation failed to produce tissue movement. *P < 0.05, **P < 0.01, ***P 0.0005. ChR2, channel rhodopsin.
Tissue movement latency was quantified in Vil-ChR2 distal colons. As for myenteric neuron activation, there was a measurable latency between the start of blue light stimulation in the distal colon and colon movement, which was significantly longer than the latency to movement with electrical stimulation (electrical: 1.04 ± 0.18 s vs. laser: 7.83 ± 1.04 s; P = 0.0005; Fig. 3F). Latencies ranged from 3 to 22 s with the majority of responses occurring within 6 s from the start of laser stimulation (Fig. 3G). The fields that were imaged in the flattened colon preparation of Vil-ChR2 mice are illustrated in Fig. 3H, which indicates where blue-light epithelial stimulation initiated movement of the tissue above spontaneous baseline movement (mostly between 0 and 3 cm from the anus) and where blue light stimulation failed to produce tissue movement (mostly above 3 cm).
Epithelium-Induced Motility is Mediated by Neuronal Activity
We hypothesized that the epithelium, upon activation, signals to enteric neurons to produce the observed movement patterns. Acetylcholine (ACh) is the major neurotransmitter for synaptic transmission in the ENS and is released from enteric motor neurons to produce smooth muscle contractions (24). Therefore, the nicotinic ACh receptor antagonist hexamethonium (HEX, 300 µM) was added to the bath to test whether laser-induced movement was reduced by blocking synaptic transmission in enteric neural circuits. We also used tetrodotoxin (TTX, 0.5 µM), a voltage-gated Na+ channel blocker, to confirm that laser-induced movement depended on action potential firing in neurons. As shown in the example traces, epithelial-evoked movement of the colon (Fig. 4A) was effectively reduced in the presence of HEX (n = 5 mice; 2 female, 3 male; Fig. 4B) and TTX (n = 5 mice; 3 female, 2 male; Fig. 4C). There was a significant decrease in colon movement when either HEX or TTX was applied (vehicle: 60.59 ± 13.42 µM vs. HEX: 8.93 ± 5.35 µM; P = 0.003 and TTX: 7.3 ± 3.66 µM; P = 0.002; one-way ANOVA; Fig. 4D), confirming that blue light-evoked colon movement observed in Vil-ChR2 mice is neuronally mediated.
Figure 4.
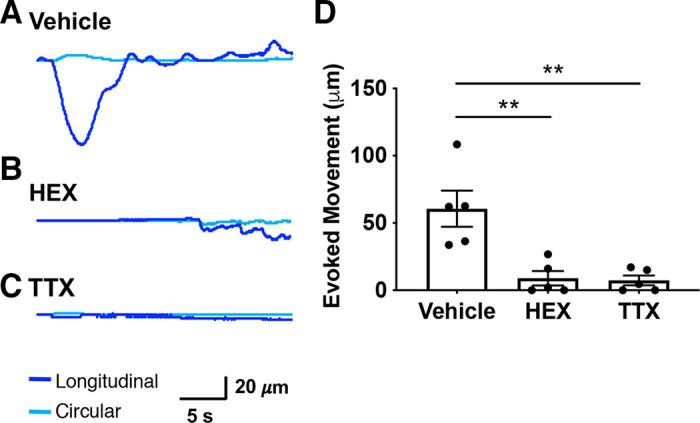
Epithelium-induced motility is mediated by neuronal activity. Epithelial-evoked movement of the colon (A) was effectively reduced by the presence of HEX (B) and TTX (C). D: there was a significant decrease in colon movement when either HEX or TTX was applied (n = 5 mice; HEX: P = 0.003 and TTX: P = 0.002; one-way ANOVA), confirming that blue light-evoked colon movement observed in Vil-ChR2 mice is neuronally mediated. **P < 0.01. ChR2, channel rhodopsin; HEX, hexamethonium; TTX, tetrodotoxin.
5-HT and ATP Mediate Epithelium-ENS Interactions
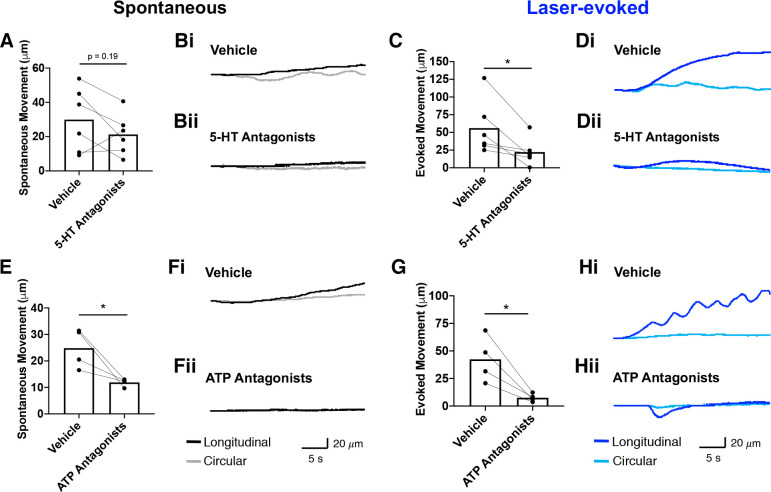
To further define the mechanism by which the epithelium initiates colon motility, we used more specific pharmacological approaches. Two candidate neurotransmitters were tested: 5-HT and ATP, both important signaling molecules in the ENS. A cocktail of 5-HT receptor antagonists (alosetron, ketanserin, and GR113808) was applied to the bath and local motility changes were measured. Spontaneous colon movement and tissue movement evoked by blue light were recorded in the presence of antagonists (n = 6 mice; 2 female, 4 male). The total spontaneous movement (combined x- and y-directions) did not change with the addition of 5-HT receptor antagonists (vehicle: 29.92 ± 7.61 µM vs. 5-HT receptor antagonists: 21.25 ± 4.89 µM; P = 0.19; paired t test; Fig. 5A). Example traces show spontaneous movement in the presence of vehicle (Fig. 5Bi) and 5-HT receptor antagonists (Fig. 5Bii). In contrast, blue light-evoked movement significantly decreased after the addition of 5-HT receptor antagonists (vehicle: 56.01 ± 15.69 µM vs. 22.22 ± 7.72 µM; P = 0.03; paired t test; Fig. 5C). Example traces show evoked movement in the presence of vehicle (Fig. 5Di) is reduced with the addition of 5-HT receptor antagonists (Fig. 5Dii). In a separate set of experiments, we applied an ATP receptor antagonist cocktail (TNP-ATP, 5-BDBD, MRS 2500, AR-C118925XX, and MRS 2578) to examine the effects on local motility (n = 4 mice; 2 female, 2 male). Spontaneous movement significantly decreased after the addition of ATP receptor antagonists (vehicle: 24.81 ± 3.73 µM vs. ATP receptor antagonists: 11.87 ± 0.75 µM; P = 0.05; paired t test; Fig. 5E). Example traces show spontaneous movement with vehicle treatment (Fig. 5Fi) that is decreased in the presence of ATP receptor antagonists (Fig. 5Fii). Evoked movement was also significantly reduced (vehicle: 42.37 ± 10.48 µM vs. ATP receptor antagonists: 7.53 ± 1.86 µM; P = 0.03; paired t test; Fig. 5G). Example traces show evoked movement with vehicle treatment (Fig. 5Hi) that is decreased in the presence of ATP receptor antagonists (Fig. 5Hii).
Figure 5.
ATP and 5-HT mediate epithelium-ENS interactions. A: total spontaneous movement (combined x- and y-directions) slightly decreased with the addition of 5-HT receptor antagonists (n = 6 mice; P = 0.19; paired t test). Example traces show spontaneous movement in the presence of vehicle (Bi) and 5-HT receptor antagonists (Bii). C: blue light-evoked movement decreased after the addition of 5-HT receptor antagonists (P = 0.48; paired t test). Example traces show evoked movement in the presence of vehicle (Di) that is decreased in the presence of 5-HT receptor antagonists (Dii). E: spontaneous movement significantly decreased after addition of ATP receptor antagonists (n = 4 mice; P = 0.05; paired t test). Example traces show spontaneous movement with vehicle treatment (Fi) that is decreased in the presence of ATP receptor antagonists (Fii). G: evoked movement was also significantly reduced with ATP receptor antagonists (P = 0.03; paired t test). Example traces show evoked movement with vehicle treatment (Hi) that is decreased in the presence of ATP receptor antagonists (Hii). *P < 0.05. ENS, enteric nervous system.
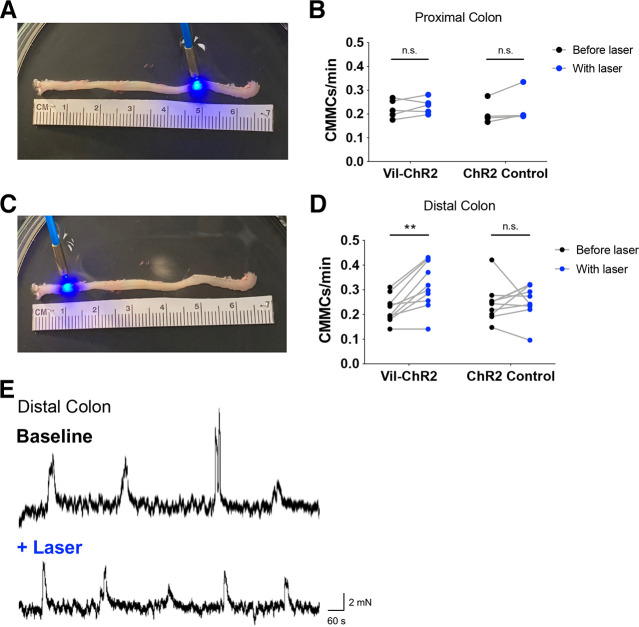
Optogenetic Activation of the Epithelium Facilitates Colonic Migrating Motor Complexes
Given the impact of epithelial activation on local motility in the distal colon, we next tested whether activation of the epithelium could influence whole colon motility patterns. Colonic migrating motor complexes (CMMCs) are rhythmic propulsive contractions mediated by enteric neurons that normally occur every 3–5 min and relate to overall transit time in vivo. Video recordings captured CMMCs at spontaneous baseline levels and in response to blue laser stimulation of the intact colon in proximal and distal regions. Laser stimulation (20 s) was applied every 2 min for 20 min, and changes in CMMC frequency were quantified in Vil-ChR2 and control littermate mice. Because CMMCs are typically initiated in the proximal colon regions and travel in the anal direction, we predicted that activation of the proximal colon epithelium (5 cm from anus, Fig. 6A) would influence CMMC frequency despite the absence of local motility changes. However, similar to our results described above, blue light stimulation of the proximal colon epithelium did not change the frequency of CMMCs in Vil-ChR2 mice (baseline: 0.22 ± 0.02 vs. laser: 0.23 ± 0.01; n = 5 mice, 1 female, 4 male) nor in control mice (baseline: 0.20 ± 0.02 vs. laser: 0.23 ± 0.04; n = 4 mice, 1 female, 3 male). There was no significant interaction between genotype and laser in these experiments (P = 0.49; two-way ANOVA; Fig. 6B). We repeated these experiments in the distal colon to determine whether colon epithelium stimulation and the resulting activation of ENS circuits in distal regions would affect CMMCs. Interestingly, blue light stimulation of the distal colon epithelium (1 cm from anus, Fig. 6C) increased the rate of CMMCs initiated in the proximal colon of Vil-ChR2 mice. There was a significant interaction between genotype and laser in these experiments (P = 0.02; two-way ANOVA). Post hoc analysis revealed that blue light activation of the epithelium increased the rate of CMMCs in Vil-ChR2 mice (baseline CMMC frequency: 0.22 ± 0.02 per min vs. CMMC frequency with laser: 0.32 ± 0.03 per min; n = 10 mice, 5 female, 5 male; P = 0.001; Sidak’s test; Fig. 6D) but not in control mice (baseline: 0.25 ± 0.02 per min vs. laser: 0.26 ± 0.02 per min; n = 10 mice, 6 female, 4 male). CMMC events were verified using a force transducer that measured individual CMMCs (Fig. 6E); example traces show CMMC rate at baseline and with the addition of laser in the distal colon. Similar to epithelial-induced local motility patterns, these results show that epithelial stimulation in the distal, but not proximal colon, facilitates whole colon motility patterns.
Figure 6.
Optogenetic activation of the epithelium in the distal colon facilitates CMMCs. A: video recordings captured CMMCs at spontaneous baseline levels and with the application of blue laser to the proximal colon (right side), 5 cm from the anus. Changes in CMMC rate (No. of events in 20 min) were quantified in Vil-ChR2 (n = 5 mice) and control littermate mice (n = 4 mice). B: blue light stimulation of the proximal colon epithelium did not change the rate of CMMCs in the Vil-ChR2 mice nor in control mice. There was no significant interaction between genotype and laser in these experiments (P = 0.49; two-way ANOVA). C: laser stimulation was repeated in the distal colon (left side), 1 cm from the anus. Changes in CMMC rate were quantified in Vil-ChR2 (n = 10 mice) and control littermate mice (n = 10 mice). D: blue light activation of the epithelium in the distal colon increased the rate of CMMCs in Vil-ChR2 mice (P = 0.001; Sidak’s test). The rate of CMMCs in the control mice did not change (P = 0.88; Sidak’s test). E: CMMC events were verified using a force transducer that measured changes in tension (milli-Newtons) during CMMCs. These example traces show CMMC rate at baseline and then with the addition of laser in the distal colon. **P < 0.01. ChR2, channel rhodopsin; CMMCs, colonic migrating motor complexes; n.s., not significant.
DISCUSSION
The goal of this study was to investigate the role of the epithelium in colon motility. We employed optogenetic stimulation of epithelial cells coupled with optical measurement of neuronal activity, establishing this study as the first to interrogate ENS circuits using all optical techniques. The ability to selectively activate epithelial cells and assess colon activity allowed direct demonstration of epithelial cell communication with myenteric neurons and subsequent changes in colon motility. Results indicate that epithelial activation initiated Ca2+ activity in myenteric neurons in the distal colon and produced a functional outcome, evidenced by local colon motility. Our studies also suggest that 5-HT and ATP underlie local epithelial-evoked movement. In full-length, closed colon preparations, epithelial activation in the distal colon increased the frequency of CMMCs initiated in the proximal colon, indicating that the colon epithelium has the potential to influence the large-scale coordination of ENS activity that is required to generate CMMCs.
Previous work has suggested that colon epithelial cells have reciprocal interactions with myenteric neurons (25). However, because myenteric neurons and epithelial cells express similar receptors and are thus activated by similar physiological stimuli, it has been challenging to detail the mechanisms of neuroepithelial communication. Recent studies have used physical separation of the myenteric plexus and mucosa to confirm that luminal activation of the epithelium drives neuronal activity (26). Here, we used a combination of optogenetic approaches to selectively activate colon epithelium using ChR2 and blue light, while simultaneously recording neuronal responses using red-shifted calcium indicators that require green light as an excitation wavelength. Both of these approaches show that the activation of epithelial cells, whether by physiological or optogenetic stimulation, produces neural activity in the ENS.
Our experimental protocol enabled measurement of neural activity in addition to localized colon tissue movement, which we found was reliably evoked with blue light stimulation of the epithelium. These changes in motility are likely the result of epithelial-IPAN communication, where epithelial stimulation leads to activation of IPAN terminals that project from cell bodies within the myenteric plexus (4). Once activated, IPANs evoke, via synaptic signaling, activity of local interneuron, and motor neuron populations (27). Compared with direct electrical stimulation of ENS circuits, the latency of neural activation and movement evoked by laser stimulation was greater. This increased latency may reflect release of neuroactive chemicals from the activity of epithelial cells, which then contact IPAN nerve terminals to engage the sensory-motor reflex. This is similar to a previous analysis showing ChR2-mediated activation of the colon epithelium initiated action potential (AP) firing in ExPANs, in which the latency between epithelial stimulation and AP firing (mean 15 s) was significantly longer than the latency to AP firing when the ExPANs were directly (electrically) activated (mean 0.34 s) (11).
To determine which neuroactive chemicals were critical for epithelial-myenteric neuron communication, we tested antagonists for 5-HT and ATP receptors. Previous studies have implicated 5-HT released by enterochromaffin (EC) cells as a critical regulator of peristalsis and generation of CMMCs (15, 16, 28), and other studies indicate that epithelial-released ATP also acts on IPANs in the colon (10, 29, 30). The antagonist cocktails we used for 5-HT and ATP showed that blocking receptors for these neurotransmitters significantly reduced local epithelial-evoked colon motility. However, the ability of ATP receptor antagonists to block the spontaneous local contractions suggests ATP is also involved in ongoing, basal contractions that ensure smooth muscle tone in the colon. Studies have shown that IPANs display low levels of activity even in the absence of applied stimuli and these activity levels are diminished without intact mucosa (31). This observation combined with the present study suggests that there is ongoing purinergic signaling from the epithelium, which may contribute to spontaneous movements of the colon. Also, because ATP signaling occurs between ENS neurons in descending pathways (10) and in neuron-glia communication (32), ATP antagonists may have blocked colon motility independent of epithelial signaling. Antagonists for 5-HT receptors, however, did not block spontaneous local contractions but suppressed motility evoked by laser-stimulation of the epithelium, implying a prominent role for 5-HT signaling in producing motility driven by epithelial input to the ENS.
Remarkably, the robust changes in motility we observed occurred when stimulating the distal, but not proximal, colon epithelium. Although it is possible that this observation is an unexplained consequence of our approach or model, it is more likely that this clear region-specific response results from the unique organization of ENS circuits in these two functionally distinct regions. The wiring of neurons, neurochemical properties, receptor expression and input from extrinsic sources varies substantially between proximal and distal colon (33, 34). Therefore, neurons in the distal colon may be more responsive to epithelial input to produce peristaltic reflexes, whereas motor patterns in the proximal colon are primarily driven by hard-wired ENS circuits. In fact, CMMCs are spontaneously generated in the proximal colon even in the absence of mechanical or chemical stimuli, suggesting that epithelial activation is not required to generate motor patterns in this region. CMMCs in the distal colon, by contrast, rely more on mechanical distension shown to activate specialized epithelial cells that secrete serotonin (e.g., enterochromaffin cells). Interestingly, myenteric neurons that express mRNA transcripts for the ionotropic serotonergic receptor, 5-HT3A, which is the likely receptor subtype responsible for epithelial-induced ENS activation, are almost exclusively found in the distal colon (35). Evidence also suggests that the molecular signature of the colon epithelium itself is different in proximal versus distal colon (36), likely reflecting regional specialization of enteroendocrine cells. Importantly, we confirmed that ChR2 expression in our mouse model was present throughout the full length of colon. Therefore, our results strongly support a role for the epithelium, especially in the distal colon, as an initiator of local and long-range ENS network activity.
This study demonstrates the potential for using optical techniques to interrogate intercellular communication between neuronal and nonneuronal cell types in the colon. Optogenetic activation of colon epithelial cells revealed their role in ENS neuron activation and colon motility, but we were not able to parse out the effects of different epithelial cell types in this study, as ChR2 was ubiquitously expressed in the intestinal epithelium under the villin promoter. Future studies will target specific activation to enterochromaffin cells as well as other subpopulations of EECs, as the neurotransmitters released from these cells may have the most direct input to surrounding neurons. Other nonneuronal cell types that are involved in ENS function include enteric glia, immune cells and interstitial cells of Cajal (37). The optical approaches described here should be employed in future studies to better understand how these different cell types works together to maintain colon health and homeostasis.
GRANTS
This work was supported by National Institutes of Health Grants F32 DK120115, K99 DK129708, T32 DK063922-17, OT2 OD023859, and R01AR069951.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M.A., B.M.D., and K.M.S-E. conceived and designed research; S.A.N. and K.M.S-E. performed experiments; S.A.N., B.S.E., and K.M.S-E. analyzed data; S.A.N., K.M.A., B.M.D., and K.M.S-E. interpreted results of experiments; S.A.N. and K.M.S-E. prepared figures; S.A.N. and K.M.S-E. drafted manuscript; S.A.N., B.S.E., K.M.A., B.M.D., and K.M.S-E. edited and revised manuscript; S.A.N., B.S.E., K.M.A., B.M.D., and K.M.S-E. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge and thank Christopher Sullivan for mouse colony maintenance and technical assistance.
REFERENCES
- 1.Bessac A, Cani PD, Meunier E, Dietrich G, Knauf C. Inflammation and gut-brain axis during type 2 diabetes: focus on the crosstalk between intestinal immune cells and enteric nervous system. Front Neurosci 12: 725, 2018. doi: 10.3389/fnins.2018.00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalazonitis A, Rao M. Enteric nervous system manifestations of neurodegenerative disease. Brain Res 1693: 207–213, 2018. doi: 10.1016/j.brainres.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am 46: 77–89, 2017. doi: 10.1016/j.gtc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Costa M, Brookes SJ, Hennig GW. Anatomy and physiology of the enteric nervous system. Gut 47 (Suppl 4): iv15–iv9, 2000; discussion iv26. doi: 10.1136/gut.47.suppl_4.iv15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furness JB, Jones C, Nurgali K, Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol 72: 143–164, 2004. doi: 10.1016/j.pneurobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Bertrand PP, Kunze WA, Bornstein JC, Furness JB. Electrical mapping of the projections of intrinsic primary afferent neurones to the mucosa of the guinea-pig small intestine. Neurogastroenterol Motil 10: 533–541, 1998. doi: 10.1046/j.1365-2982.1998.00128.x. [DOI] [PubMed] [Google Scholar]
- 7.Furness JB, Robbins HL, Xiao J, Stebbing MJ, Nurgali K. Projections and chemistry of Dogiel type II neurons in the mouse colon. Cell Tissue Res 317: 1–12, 2004. doi: 10.1007/s00441-004-0895-5. [DOI] [PubMed] [Google Scholar]
- 8.Nozawa K, Kawabata-Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S, Sano Y, Inamura K, Matsushime H, Koizumi T, Yokoyama T, Ito H. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci USA 106: 3408–3413, 2009. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, Linden DR, Li JH, Leiter AB, Szurszewski JH, Farrugia G, Beyder A. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci USA 115: E7632–E7641, 2018. doi: 10.1073/pnas.1804938115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnstock G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal 10: 3–50, 2014. doi: 10.1007/s11302-013-9397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makadia PA, Najjar SA, Saloman JL, Adelman P, Feng B, Margiotta JF, Albers KM, Davis BM. Optogenetic activation of colon epithelium of the mouse produces high-frequency bursting in extrinsic colon afferents and engages visceromotor responses. J Neurosci 38: 5788–5798, 2018. doi: 10.1523/JNEUROSCI.0837-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gershon MD. 5-hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 20: 14–21, 2013. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grider JR, Kuemmerle JF, Jin JG. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am J Physiol Gastrointest liver Physiol 270: G778–G782, 1996. doi: 10.1152/ajpgi.1996.270.5.G778. [DOI] [PubMed] [Google Scholar]
- 14.Kadowaki M, Wade PR, Gershon MD. Participation of 5-HT3, 5-HT4, and nicotinic receptors in the peristaltic reflex of guinea pig distal colon. Am J Physiol 271: G849–G857, 1996. doi: 10.1152/ajpgi.1996.271.5.G849. [DOI] [PubMed] [Google Scholar]
- 15.Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology 136: 1328–1338, 2009. doi: 10.1053/j.gastro.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heredia DJ, Gershon MD, Koh SD, Corrigan RD, Okamoto T, Smith TK. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol 591: 5939–5957, 2013. doi: 10.1113/jphysiol.2013.256230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith TK, Gershon MD. CrossTalk proposal: 5-HT is necessary for peristalsis. J Physiol 593: 3225–3227, 2015. doi: 10.1113/JP270182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spencer NJ. Control of migrating motor activity in the colon. Curr Opin Pharmacol 1: 604–610, 2001. doi: 10.1016/S1471-4892(01)00103-5. [DOI] [PubMed] [Google Scholar]
- 19.Keating DJ, Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology 138: 659–670.e1–2, 2010. doi: 10.1053/j.gastro.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Spencer NJ, Sia TC, Brookes SJ, Costa M, Keating DJ. CrossTalk opposing view: 5-HT is not necessary for peristalsis. J Physiol 593: 3229–3231, 2015. doi: 10.1113/JP270183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hibberd TJ, Travis L, Wiklendt L, Costa M, Brookes SJH, Hu H, Keating DJ, Spencer NJ. Synaptic activation of putative sensory neurons by hexamethonium-sensitive nerve pathways in mouse colon. Am J Physiol Gastrointest Liver Physiol 314: G53–G64, 2018. doi: 10.1152/ajpgi.00234.2017. [DOI] [PubMed] [Google Scholar]
- 22.Smith-Edwards KM, Najjar SA, Edwards BS, Howard MJ, Albers KM, Davis BM. Extrinsic primary afferent neurons link visceral pain to colon motility through a spinal reflex in mice. Gastroenterology 157: 522–536.e2, 2019. doi: 10.1053/j.gastro.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nurgali K, Stebbing MJ, Furness JB. Correlation of electrophysiological and morphological characteristics of enteric neurons in the mouse colon. J Comp Neurol 468: 112–124, 2004. doi: 10.1002/cne.10948. [DOI] [PubMed] [Google Scholar]
- 24.Sang Q, Young HM. The identification and chemical coding of cholinergic neurons in the small and large intestine of the mouse. Anat Rec 251: 185–199, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 25.Walsh KT, Zemper AE. The enteric nervous system for epithelial researchers: basic anatomy, techniques, and interactions with the epithelium. Cell Mol Gastroenterol Hepatol 8: 369–378, 2019. doi: 10.1016/j.jcmgh.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fung C, Cools B, Malagola S, Martens T, Tack J, Kazwiny Y, Vanden Berghe P. Luminal short-chain fatty acids and 5-HT acutely activate myenteric neurons in the mouse proximal colon. Neurogastroenterol Motil: e14186, 2021. doi: 10.1111/nmo.14186. [DOI] [PubMed] [Google Scholar]
- 27.Smolilo DJ, Hibberd TJ, Costa M, Wattchow DA, De Fontgalland D, Spencer NJ. Intrinsic sensory neurons provide direct input to motor neurons and interneurons in mouse distal colon via varicose baskets. J Comp Neurol 528: 2033–2043, 2020. doi: 10.1002/cne.24872. [DOI] [PubMed] [Google Scholar]
- 28.Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther 288: 93–97, 1999. [PubMed] [Google Scholar]
- 29.Bertrand PP. ATP and sensory transduction in the enteric nervous system. Neuroscientist 9: 243–260, 2003. doi: 10.1177/1073858403253768. [DOI] [PubMed] [Google Scholar]
- 30.Bertrand PP, Bornstein JC. ATP as a putative sensory mediator: activation of intrinsic sensory neurons of the myenteric plexus via P2X receptors. J Neurosci 22: 4767–4775, 2002. doi: 10.1523/JNEUROSCI.22-12-04767.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunze WAA, Bertrand PP, Furness JB, Bornstein JC. Influence of the mucosa on the excitability of myenteric neurons. Neuroscience 76: 619–634, 1997. doi: 10.1016/S0306-4522(96)00408-3. [DOI] [PubMed] [Google Scholar]
- 32.Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology 136: 1349–1358, 2009. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Hao MM, Van den Haute C, Baekelandt V, Boesmans W, Vanden Berghe P. Regional complexity in enteric neuron wiring reflects diversity of motility patterns in the mouse large intestine. Elife 8: e42914, 2019. doi: 10.7554/eLife.42914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith-Edwards KM, Edwards BS, Wright CM, Schneider S, Meerschaert KA, Ejoh LL, Najjar SA, Howard MJ, Albers KM, Heuckeroth RO, Davis BM. Sympathetic input to multiple cell types in mouse and human colon produces region-specific responses. Gastroenterology 160: 1208.– , 2021.doi: 10.1053/j.gastro.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drokhlyansky E, Smillie CS, Van Wittenberghe N, Ericsson M, Griffin GK, Eraslan G, Dionne D, Cuoco MS, Goder-Reiser MN, Sharova T, Kuksenko O, Aguirre AJ, Boland GM, Graham D, Rozenblatt-Rosen O, Xavier RJ, Regev A. The human and mouse enteric nervous system at single-cell resolution. Cell 182: 1606–1622.e23, 2020. doi: 10.1016/j.cell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Billing LJ, Larraufie P, Lewis J, Leiter A, Li J, Lam B, Yeo GS, Goldspink DA, Kay RG, Gribble FM, Reimann F. Single cell transcriptomic profiling of large intestinal enteroendocrine cells in mice – identification of selective stimuli for insulin-like peptide-5 and glucagon-like peptide-1 co-expressing cells. Mol Metab 29: 158–169, 2019. doi: 10.1016/j.molmet.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9: 286–294, 2012. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]