Keywords: fasting, high-fat diet, lipoprotein lipase, lipoprotein metabolism, triglyceride
Abstract
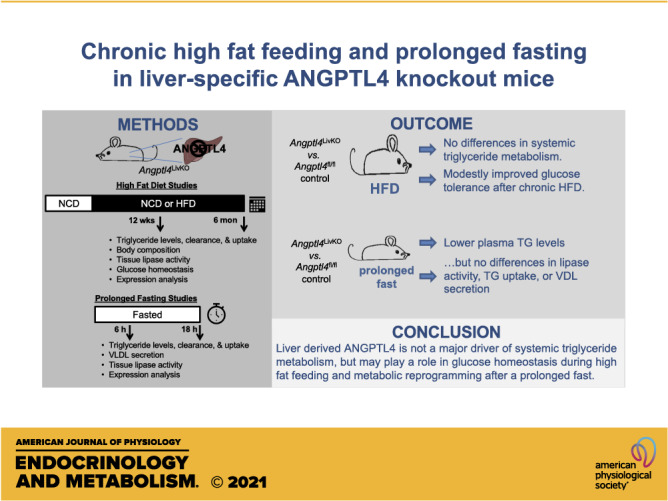
Obesity is associated with dyslipidemia, ectopic lipid deposition, and insulin resistance. In mice, the global or adipose-specific loss of function of the protein angiopoietin-like 4 (ANGPTL4) leads to decreased plasma triglyceride levels, enhanced adipose triglyceride uptake, and protection from high-fat diet (HFD)–induced glucose intolerance. ANGPTL4 is also expressed highly in the liver, but the role of liver-derived ANGPTL4 is unclear. The goal of this study was to determine the contribution of hepatocyte ANGPTL4 to triglyceride and glucose homeostasis in mice during a high-fat diet challenge. We generated hepatocyte-specific ANGPTL4 deficient (Angptl4LivKO) mice, fed them a 60% kcal/fat diet (HFD) for 6 mo and assessed triglyceride, liver, and glucose metabolic phenotypes. We also explored the effects of prolonged fasting on Angptl4LivKO mice. The loss of hepatocyte-derived ANGPTL4 led to no major changes in triglyceride partitioning or lipoprotein lipase activity compared with control mice. Interestingly, although there was no difference in fasting plasma triglyceride levels after a 6 h fast, after an 18-h fast, normal chow diet-fed Angptl4LivKO mice had lower triglyceride levels than control mice. On a HFD, Angptl4LivKO mice initially showed no difference in glucose tolerance and insulin sensitivity, but improved glucose tolerance emerged in these mice after 6 mo on HFD. Our data suggest that hepatocyte ANGPTL4 does not directly regulate triglyceride partitioning, but that loss of liver-derived ANGPTL4 may be protective from HFD-induced glucose intolerance and influence plasma triglyceride (TG) metabolism during prolonged fasting.
NEW & NOTEWORTHY 1) Angiopoietin-like 4 deficiency in hepatocytes (Angptl4LivKO) does not improve triglyceride phenotypes during high-fat feeding. 2) Angptl4LivKO mice have improved glucose tolerance after chronic high-fat diet. 3) Angptl4LivKO mice have decreased fasting plasma triglyceride levels after an 18-h fast, but not after a 6-h fast.
INTRODUCTION
The prevalence of obesity is increasing worldwide and has become a serious public health problem in the United States. During obesity, an imbalance in lipid storage and release can lead to an increase in ectopic fat accumulation into tissues such as muscle and liver and has been linked to the development of cardiovascular and metabolic diseases (1). The liver is a major regulator of systemic glucose and lipid metabolism, and pathological changes within the liver contribute to dyslipidemia and insulin resistance (2). Hepatokines, molecules secreted by the liver into the circulation, can influence not only liver metabolic health but also whole body lipid and glucose homeostasis (3).
One hepatokine that could potentially regulate lipid and glucose homeostasis is angiopoietin-like 4 (ANGPTL4). ANGPTL4 has emerged as a possible therapeutic target for the treatment of metabolic and cardiovascular diseases, as ANGPTL4 deficiency exhibits beneficial effects on plasma triglyceride levels and glucose homeostasis (4–7). These beneficial effects are due, at least in part, to the ability of ANGPTL4 to inhibit lipoprotein lipase (LPL). Circulating plasma triglycerides are hydrolyzed by LPL, releasing free fatty acids to be taken up by tissues for energy use or storage. Mice lacking Angptl4 or receiving monoclonal antibodies against ANGPTL4 have increased LPL activity and lower plasma triglyceride levels than control mice (8–10). Angptl4–/– mice also have increased uptake of triglyceride-derived fatty acids into adipose tissue (8, 11). In a mouse model of diet-induced obesity, Angptl4–/– mice exhibited increased fat mass but improved glucose tolerance compared with control mice (12). Mice lacking ANGPTL4 specifically in adipose tissue also show increased uptake of triglycerides into adipose and display improved glucose tolerance and insulin sensitivity when challenged with a short-term high-fat diet (HFD) (13, 14). However, in both studies, glucose tolerance and insulin sensitivity improvements were mostly lost after long-term high-fat diet administration. It remains unclear if the beneficial cardiovascular and metabolic effects of ANGPTL4 deficiency in humans are due to loss of ANGPTL4 strictly in adipose or if loss of ANGPTL4 in other tissues also contributes.
Although ANGPTL4 is highly expressed in the adipose tissue of both mice and humans, it is also expressed in the liver (15–17). Currently, the role of liver-derived ANGPTL4 in lipid metabolism and glucose homeostasis is unclear. Given the importance of the liver in regulating systemic glucose and lipid homeostasis, we sought to define the actions of hepatocyte-derived ANGPTL4 during obesity. We generated hepatocyte-specific Angptl4 deficient mice and assessed triglyceride and glucose homeostasis when these mice were challenged with a high-fat diet for 6 mo. Additionally, as ANGPTL4 is largely induced by fasting, we further went on to characterize the role of hepatocyte-derived Angptl4 in prolonged fasting conditions.
METHODS
Mice
Mice with floxed alleles of the Angptl4 gene (Angptl4fl) were generated by the University of Iowa genome editing facility using CRISPR/Cas9 as previously described (14). Mice utilized in these studies were confirmed for the presence of LoxP sites through genotyping PCR. Primers used for genotyping: Forward TAGGCGCATCTACTAGGACTC; Reverse AGATATGCAAGGCTAGTGAAGAC to detect the 5' LoxP site and primers: Forward CCTCCAACATCTCTTGATGTAAC; Reverse TATGTGTATGTGACTGGATGG to detect the 3' LoxP site. Hepatocyte-specific knockout mice (Angptl4LivKO) were generated by breeding Angptl4fl/fl mice with C57BL/6 transgenic mice containing the albumin promoter-driven Cre recombinase [kindly provided by Dr. Eric Taylor, Jackson stock 003574 (18)]. Primers used to confirm albumin-Cre by genotyping PCR were TGCAAACATCACATGCACAC (forward to detect wild type), GAAGCAGAAGCTTAGGAAGATGG (forward to detect albumin Cre), and TTGGCCCCTTACCATAACTG (common reverse). Cre-negative Angptl4fl/fl littermates were used as control mice in these studies.
Mice were maintained on a 12:12-h light-dark cycles at 25°C with free access to water and food, except during specified fasts. During fasting, food was removed from the cage but free access to water remained. All mice were housed with littermates (1–5 mice per cage). For 6-mo-diet studies, cage density was matched between genotypes. Before beginning special diet studies, mice were fed a normal chow diet (NCD; NIH 7951). Starting at 8 wk of age, mice were randomized to either remain on the normal chow diet (NIH 7951) or be placed on a high-fat diet (60% by kcal; Research Diets, Cat. No. 12592). Mice were maintained on their respective diets for either 12 wk or 6 mo. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Iowa and were carried out in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals.
For terminal procedures, mice were fasted for either 6 h or overnight. The mice were anesthetized (isoflurane) and euthanized by exsanguination and thoracic dissection. For RNA, LPL activity, and liver triglyceride measurements, tissues were rapidly excised, flash frozen in liquid nitrogen and stored in a −80°C freezer. For radiolabeled uptake experiments, see methods.
Analysis of Fasting Plasma Parameters
Mice were fasted for either 6 h or overnight, and blood was collected into EDTA-coated capillary tubes following a tail-nick and immediately placed on ice. Plasma was collected following centrifugation and stored at −80°C until used for assays. Plasma glucose was assessed via glucometer (One Touch Ultra). Plasma triglyceride levels were assessed using the Infinity Triglyceride Reagent (Thermo Scientific, Cat. No. TR22421). Free fatty acids were assessed by using the colorimetric kit from Wako (HR Series NEFA-HR 999–34691/995–34791 and NEFA-HR 991–34891/993–35191). Amyloid A levels were measured in plasma by ELISA (Crystal Chem, Cat. No. 80659) according to manufacturer’s protocols. Aspartate aminotransferase (AST, Sigma-Aldrich, Cat. No. MAK055) and alanine aminotransferase (ALT, Sigma-Aldrich, Cat. No. MAK052) activities were measured in the plasma using commercial kits according to manufacturer’s protocols.
RNA Extraction and qPCR Analysis
Snap frozen mouse tissues were homogenized with a tissue pulverizer. Tissues were lysed, and total RNA was extracted using Trizol reagent (Invitrogen, Cat. No. 15596026) according to the manufacturer’s instructions. For cDNA synthesis, 2 μg of RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Cat. No. 4368813) according to the manufacturer’s instructions. Prepared diluted cDNA, primers, and SYBR Green ER qPCR Supermix reagent (Invitrogen, Cat. No. 11762100) were combined, and PCR was performed on the QuantStudio 6 Flex system (3 technical replicates per mouse; Applied Biosystems, Iowa Institute of Human Genetics). Gene expression was calculated using the ΔΔCt method (19) using either CycloA or U36B4 as the reference gene. The primer sequences utilized in the study are listed in Supplemental Tables S1 and S2 (All Supplementary material is available at https://doi.org/10.6084/m9.figshare.14377031).
Body Composition
Body composition [fat mass (g) and lean mass (g)] was measured by nuclear magnetic resonance (NMR) in mice after 11 or 25 wk on diet. Mice were weighed immediately before being placed in a restraint tube. Mice weighing less than 50 g were placed into a Bruker LF50, and mice weighing more than 50 g were placed into a Bruker LF90. The NMR measurements were performed in the University of Iowa Metabolic Phenotyping Core.
Triglyceride Clearance and Uptake Assay
3H-labeled chylomicrons were obtained from Gpihbp1–/– mice as previously described (8). The chylomicrons contained 3H-labeled Triolein (PerkinElmer, Cat. No. NET431001MC), metabolically incorporated into triglycerides, and used to measure fatty acid uptake. Triglyceride clearance and tissue triglyceride uptake assays were performed in mice as previously described (8). Briefly, female and male mice were fasted (6 h or overnight), anesthetized with isoflurane, and injected retro-orbitally with 3H-labeled chylomicrons (100 µL). Triglyceride clearance was assessed by collecting blood (10 µL) from the tail 1, 5, 10, and 15 min following injection. Mice had recovered from isoflurane anesthetization by the 1-min time point and were awake and ambulatory for all blood draws. Radioactivity in the blood was measured in scintillation fluid on a scintillation counter. Following the final blood draw at 15 min, the mice were anesthetized (isoflurane) and perfused with PBS containing tyloxapol (1%). Tyloxapol is a nonionic liquid polymer that prevents the hydrolysis of lipoprotein triglycerides by LPL and is used in this case to prevent further hydrolysis during tissue processing. Tissues were rapidly excised and 40–90 mg of tissues were combined with a chloroform:methanol solution (2:1) and stored overnight at 4°C. Organic and aqueous phases were separated by adding 1 mL of 2 M CaCl2. Organic fractions were subsequently dried and resuspended in scintillation fluid. The aqueous fraction was also combined with scintillation fluid and radioactivity in both fractions was measured on a scintillation counter. Counts-per-minute (CPMs) from both aqueous and organic fractions were combined to determine total tissue uptake of radioactivity. To normalize radiolabel across mice, CPM values were normalized to the CPMs of the injected dose (measured by assaying 10% of the chylomicron suspension injected into the mouse).
Liver Triglyceride Measurements
Liver triglyceride measurement was performed using a modified Folch extraction method (20). Liver tissues were weighed (∼100 mg) and homogenized in ice-cold PBS. Note that, PBS, and 50 µL of homogenate was removed to determine protein concentration. Lipids were extracted in chloroform:methanol (2:1) and separated via centrifugation (2,050 g at 4°C, 10 min). The lower organic phase was pipetted into a new vial and dried. The dried lipid was then dissolved in chloroform containing 2% Triton X-100 and dried again. The dried lipid was resuspended in molecular grade water. Triglyceride levels were measured using the Infinity Triglyceride Reagent (Thermo Scientific, Cat. No. TR22421), and concentrations were determined using a standard curve prepared from Triolein standard (Nu-Chek Prep, Lot T-235-N13-Y). Protein concentrations were determined using the DC assay (Bio-Rad, Cat. No. 5000111). Triglyceride levels were normalized to protein concentration.
Lipoprotein Lipase Activity Assays
Triglyceride lipase activity in tissues was measured as previously described (8). Frozen tissues collected following exsanguination were crushed and homogenized in LPL assay buffer [25 mM NH4Cl, 5 mM EDTA, 0.01% SDS, 45 U/mL heparin, 0.05% 3-(N,N-dimethylmyristylammonio) propanesulfonate zwittergent detergent (Acros Organics, Cat. No. 427740050)] containing protease inhibitor (Mammalian ProteaseArrest APExBIO K1008). The homogenate was clarified by centrifugation (15,000 g at 4°C, 15 min). Protein concentration was determined using the DC assay (Bio-Rad), and homogenate protein concentrations were equalized. Supernatants were added to a 96-well black, clear bottom plate. Assay buffer [0.6 M NaCl, 80 mM Tris-HCl, pH 8, 6% fatty-acid free BSA, and 1% of the EnzChek lipase fluorescent substrate (Molecular Probes, Cat. No. E33955)] was added to each well. Fluorescence was measured (technical duplicates, 30 min, 37°C) on a SpectraMax i3 plate reader (Molecular Devices). Relative lipase activity was determined following calculation of the linear slope of the curve and subtraction of background (assay buffer) slope readings. For liver lipase activity assays, each lysate was treated with either vehicle (PBS) or NaCl (1 M final concentration) to differentiate the roles of hepatic lipase (NaCl insensitive) from LPL (NaCl sensitive) before the addition of the assay buffer. Although we refer to this assay as an LPL activity assay, it should be noted that the assay likely registers the activity of any triglyceride lipase present in the sample.
Glucose and Insulin Tolerance Tests
Glucose tolerance tests (GTT) were performed after 10 or 24 wk on diet. Mice were fasted for 6 h and then given an intraperitoneal injection with glucose (12-wk HFD study: 2 g/kg for mice fed NCD, 1.3 g/kg for mice fed HFD and 6-mo HFD study: 1 g/kg for all mice). Blood samples were collected at 0, 30, 60, 90, and 120 min following injection. For mice fed a HFD for 12 wk, blood was collected into an EDTA-coated capillary tube and stored on ice. Plasma was collected following centrifugation of sample tubes at 1,500 g for 20 min. Plasma glucose concentration was assessed using the Autokit Glucose kit (Wako-997–03001). For mice fed a HFD for 6 mo, blood glucose readings were measured at 0, 30, 60, 90, and 120 min following glucose injection using a glucometer (OneTouch Ultra). Insulin tolerance tests (ITT) were performed at week 11 or 25 on the diet study. Mice were fasted for 4 h and then given an intraperitoneal injection with insulin (0.75 U/kg, Humalin-R 100). Glucose readings were measured at 0, 15, 30, 60, and 90 min after injection using a glucometer (OneTouch Ultra).
VLDL-TG Secretion
Plasma triglyceride accumulation with time was assessed following retroorbital injection of tyloxapol (500 mg/kg; Sigma, Cat. No. T8761) in male Angptl4fl/fl and Angptl4LivKO mice that had been fasted 18 h. Tyloxapol (10% w/v) was prepared in sterile saline. Blood was collected into EDTA-coated capillary tubes following a tail-nick immediately before and 1, 2, and 3 h following injection of tyloxapol. At the conclusion of 3 h, the mice were euthanized, the liver was rapidly excised, weighed, and flash frozen in liquid nitrogen before being stored at −80°C. Plasma was collected by centrifugation at 1,500 g for 15 min. Plasma triglyceride levels were measured as described above. Liver triglyceride levels were measured as described above.
Statistics and Outlier Identification
Results are expressed as means ± SE. Bar graphs also show individual values. Outlier identification was performed on all mouse datasets using Robust regression and outlier removal (ROUT) analysis in GraphPad Prism. An unpaired Student’s t test with Welch’s correction was used to determine statistical significance of samples with two groups (Figs. 1 and 2). For groups of three or more, statistical significance was determined by two-way ANOVA followed by multiple comparisons with Tukey’s correction. Repeated measured ANOVA was utilized for body weights, chylomicron clearance, GTT, and ITT assays. Statistical analysis was performed in GraphPad Prism.
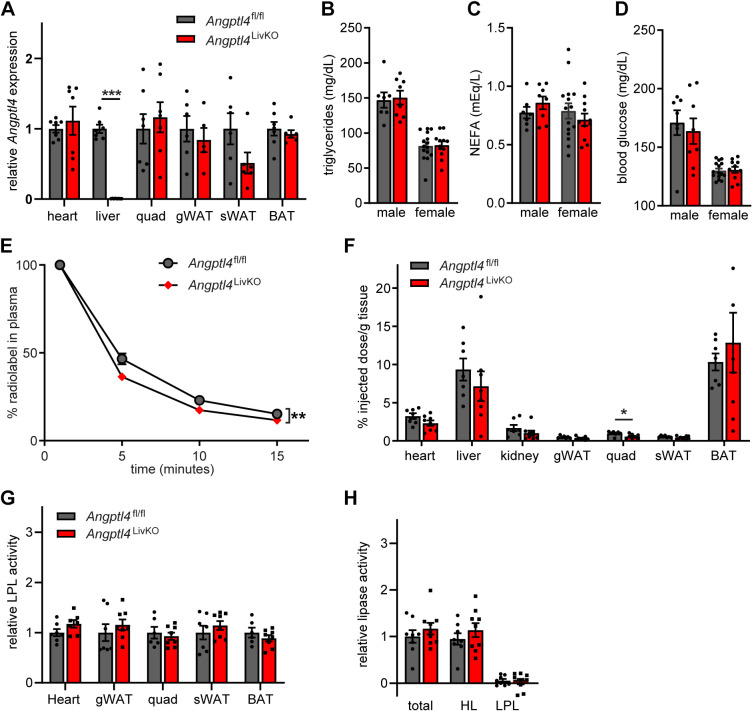
Figure 1.
Generation and characterization of mice with liver-specific deletion of Angptl4. A: mRNA expression of Angptl4 in heart, liver, quadriceps muscle (quad), gonadal white adipose tissue (gWAT), subcutaneous white adipose tissue (sWAT), and brown adipose tissues (BAT) from 8- to 12-wk-old female Angptl4fl/fl and Angptl4LivKO mice following a 6 h fast (means ± SE; n = 5–8 mice). B, C, and D: fasted (6 h) plasma triglyceride (B), nonesterified fatty acids (C), and blood glucose levels (D) in 8- to 12-wk-old male and female Angptl4fl/fl and Angptl4LivKO mice (means ± SE; n = 7–15). E and F: fasted (6 h) female Angptl4fl/fl and Angptl4LivKO mice were injected intravenously with chylomicrons containing radiolabeled 3H-triglycerides. E: clearance of 3H-radiolabel from the plasma 1, 5, 10, and 15 min after injection. Points represent percentage of radiolabel remaining in the plasma at each time point as a percentage of the 1 min time point (means ± SE; n = 7–8). **P < 0.01 by repeated measures ANOVA. F: 3H-radiolabeled uptake into indicated tissues after 15 min (% injected dose/g tissue; means ± SE; n = 7–8). *P < 0.05 vs. Angptl4fl/fl mice by Student’s t test. G: lipoprotein lipase activity from fasted (6 h) female Angptl4fl/fl and Angptl4LivKO tissues [heart, gonadal white adipose tissue (gWAT), quadriceps muscle (Quad), subcutaneous adipose tissue (sWAT), and brown adipose (BAT); means ± SE; n = 6–9/group]. H: liver was harvested from fasted (6 h) female Angptl4fl/fl and Angptl4LivKO mice (n = 8–9/group). Lipase activity was measured in the presence or absence of 1 M NaCl to distinguish between hepatic and lipoprotein lipase. Bars show relative lipase activity in each tissue normalized to Angptl4fl/fl (means ± SE). HL, hepatic lipase; LPL, lipoprotein lipase.
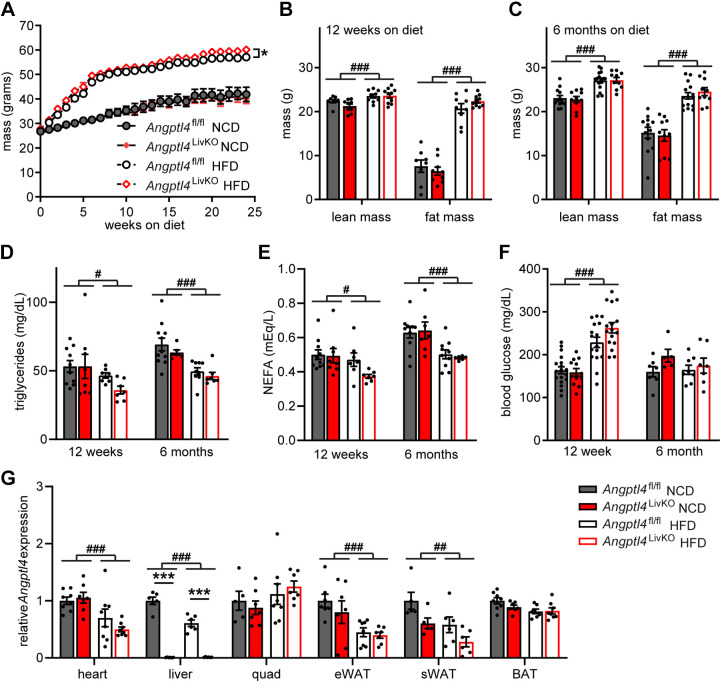
Figure 2.
Body weights, fat mass, and metabolic phenotypes in Angptl4LivKO mice after high-fat diet feeding. A: weekly body weights of male Angptl4LivKO and Angptl4fl/fl mice fed either a normal chow diet (NCD) or a high-fat diet (HFD; 60% by kcal) for 25 wk starting at 8 wk of age (means ± SE; n = 12–20 mice/group). *P < 0.05 by repeated measures ANOVA. B and C: lean mass and fat mass of Angptl4LivKO and Angptl4fl/fl after 12 wk (B) or 25 wk (C) on diet (means ± SE; n = 6–9/group). D, E, and F: fasted (6 h) plasma triglycerides (D), plasma non-esterified fatty acids (E), and blood glucose (F) from male Angptl4LivKO and Angptl4fl/fl mice after either 12 or 25 wk of either normal chow diet (NCD) or high-fat diet (HFD; means ± SE; n = 6–15). #P < 0.05, ###P < 0.001 for dietary differences by two-way ANOVA (Tukey’s correction). G: fasted (6 h) mRNA expression of Angptl4 from heart, liver, quadriceps muscle (quad), gonadal white adipose tissues (gWAT), subcutaneous white adipose tissue (sWAT), and brown adipose tissues (BAT) from male Angptl4LivKO and Angptl4fl/fl mice after 6 mo on respective diets (means ± SE; n = 5–8/group). ##P < 0.01, ###P < 0.001 for dietary differences by two-way ANOVA. ***P < 0.001 for individual genotype-specific differences by multiple comparisons after two-way ANOVA (Tukey’s correction).
RESULTS
Assessment of Triglyceride Phenotypes in Angptl4LivKO Mice
To explore the role of liver-derived ANGPTL4, we generated hepatocyte-specific Angptl4 knockout mice. We have recently reported generation of Angptl4-floxed mice (Angptl4fl/fl) (14). After recombination, which eliminates exons 2 and 3 of Angptl4, the remaining coding sequence did not result in expression of any protein, at least in cell culture (14). This observation contrasts with an independent floxed allele used in some published studies that leads to a generation of a hypomorphic ANGPTL4 protein (13, 21, 22). The floxed allele itself, before recombination, had no effect on tissue LPL activity, indicating that the introduction of loxP sites did not significantly alter ANGPTL4 function before Cre-mediated recombination (Supplemental Fig. S1A). Hepatocyte-specific (Angptl4LivKO) mice were generated by crossing mice homozygous for the floxed allele (Angptl4fl/fl mice) with albumin-Cre mice. Currently, there is no murine-specific antibody that can measure protein levels of ANGPTL4; therefore, we performed qPCR analysis utilizing seven different primer sets that span coding region of the mouse ANGPTL4 gene to confirm the impact of Cre-mediated recombination on Angptl4 expression in liver tissue from Angptl4LivKO and Angptl4fl/fl mice (Supplemental Fig. S1B). Expression of Angptl4 was greatly reduced in all seven primer sets of the Angptl4LivKO mice compared with Angptl4fl/fl mice, and no expression was seen in primers sets amplifying across exons 2 or 3 (Supplemental Fig. S1, B and C). These data demonstrate successful Cre-mediated recombination within the liver of the Angptl4LivKO mice. qPCR analysis of Angptl4 in heart, liver, quadriceps muscle, gonadal white adipose tissue, subcutaneous white adipose tissue (sWAT), and brown adipose tissue (BAT) of Angptl4fl/fl and Angptl4LivKO mice revealed that loss of Angptl4 expression occurred only in the liver of Angptl4LivKO mice (Fig. 1A).
Interestingly, fasting plasma triglyceride levels were not different in either male or female Angptl4LivKO mice compared with Angptl4fl/fl mice (Fig. 1B). These results suggest that the decreased fasting plasma triglyceride (TG) levels observed in Angptl4 whole body knockout (Angptl4–/–) mouse (8, 9, 23) are not due to the loss of hepatocyte-derived ANGPTL4. Additionally, there was no difference in free fatty acid levels or fasting blood glucose in either male or female Angptl4LivKO mice compared with Angptl4fl/fl mice (Fig. 1, C and D).
In our previous work with the whole body Angptl4–/– mice (8) and adipose-specific Angptl4 deficient mice (Angptl4AdipoKO) (14), we observed increased plasma triglyceride clearance, increased adipose uptake of triglycerides, and increased adipose LPL activity. To determine if hepatocyte-derived Angptl4 may contribute to these triglyceride phenotypes, we performed triglyceride clearance and uptake assays, as well as LPL activity assays on female Angptl4LivKO and Angptl4fl/fl mice. Following intraorbital injection of radiolabeled chylomicrons, there was a modest increase in the plasma clearance rate of the radiolabel in Angptl4LivKO mice compared with Angptl4fl/fl mice (Fig. 1E). However, the only significant difference in radiolabel tissue uptake was a decrease in TG uptake in the quadriceps of Angptl4LivKO mice compared with Angptl4fl/fl mice (Fig. 1F). There were also no differences in tissue LPL activity in female Angptl4LivKO mice compared with female Angptl4fl/fl mice (Fig. 1, G and H). These data support the idea that liver ANGPTL4 does not contribute significantly to the increased adipose TG uptake or LPL activity in the Angptl4–/– mouse (8, 9, 23).
Assessment of Chronic High-Fat Feeding in Angptl4LivKO Mice
Given the role of ANGPTL4 in triglyceride partitioning (8, 12, 13, 22), we sought to determine how short- or long-term administration of a high-fat diet would alter metabolic phenotypes in Angptl4LivKO and Angptl4fl/fl mice. Starting at 8 wk of age, male Angptl4LivKO and Angptl4fl/fl mice were fed either a normal chow diet (NCD) or high-fat diet (HFD; 60% kcal/fat) for 12 wk (short term) or 6 mo (long term). Although 12 wk of high-fat-diet administration is widely used to assess the effects of HFD in mouse models on metabolic and lipid homeostasis, we also wanted to assess the long-term effects of ANGPTL4 deficiency in the liver in the setting of obesity and therefore also fed mice NCD or HFD for 6 mo. As many of our experimental procedures were terminal, the 12-wk and 6-mo diet studies were performed in separate cohorts of mice. Mice were weighed weekly over a period of 25 wk. At the end of 25 wk of high-fat feeding, Angptl4LivKO mice weighed slightly more than their high-fat-fed Angptl4fl/fl controls (Fig. 2A). Despite this modest elevation in weight compared with high-fat-fed Angptl4fl/fl control mice, Angptl4LivKO mice had no significant differences in total lean muscle or fat mass as determined by NMR after either 12 wk (Fig. 2B) or 6 mo of high-fat feeding (Fig. 2C). There were no genotype-specific differences in fasting plasma triglyceride, free fatty acid, or glucose levels between Angptl4LivKO and Angptl4fl/fl mice at either time point (Fig. 2, D–F); however, HFD alone lowered fasting TG and free fatty levels, while increasing plasma glucose levels (Fig. 2, D–F). Other than the expected lack of Angptl4 gene expression in the liver, there were no genotype-specific differences in Angptl4 expression at either time point or on either diet (Fig. 2G, Supplemental Fig. S2). HFD led to reduced Angptl4 expression in heart and white adipose tissue and increased expression in quadriceps muscle in both genotypes after 12 wk on diet (Supplemental Fig. S2). After 6 mo of HFD, Angptl4 expression remained lower in the heart and white adipose tissue and was also lower in liver (Fig. 2G).
To further assess metabolic consequences of HFD, we placed Angptl4LivKO and Angptl4fl/fl mice into the Promethion metabolic caging system to assess energy expenditure, oxygen consumption (VO2), carbon dioxide production (VCO2), and respiratory quotient (RQ; VCO2/VO2). After 12 wk of high-fat-diet feeding, there was an increase in energy expenditure, VO2 and VCO2, in the Angptl4LivKO mice compared with HFD-fed Angptl4fl/fl mice during the dark cycle (6 PM to 6 AM; Supplemental Fig. S3). No significant genotype-specific differences were seen in mice on NCD (Supplemental Fig. S3). As expected, HFD decreased RQ in both genotypes during both the light and dark cycles (Supplemental Fig. S3).
Assessment of Liver Phenotypes in Angptl4LivKO Mice during Chronic High-Fat Feeding
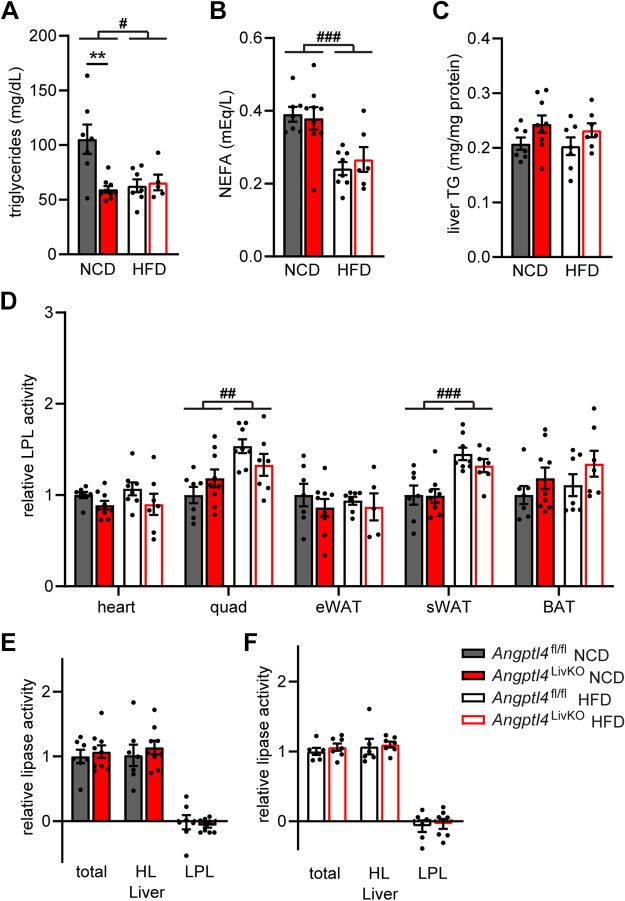
To assess liver health in our mice, we measured plasma activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), two enzymes commonly used to assess liver damage (24). All mice fed a HFD for 6 mo had elevated AST (Fig. 3A) and ALT (Fig. 3B) activity compared with NCD-fed mice, but no genotype-specific differences were observed. We also measured inflammatory protein amyloid A in the plasma of our mice. Although HFD-fed mice had an increase in plasma amyloid A, there were no genotype-specific differences (Fig. 3C). Visually, livers of mice fed a HFD were larger than those from mice fed NCD, but there were no observable genotype-specific differences (Fig. 3D). Likewise, increases in liver weights were observed in mice fed a HFD, but no genotype-specific differences emerged after either 12 wk (Supplemental Fig. S4A) or 6 mo (Fig. 3E) on either diet. Neither did we observe genotype-specific weight differences in other metabolically active tissues [heart, kidney, quadriceps muscle, epididymal white adipose tissue (eWAT), sWAT, and BAT] after either 12 wk or 6 mo of NCD or HFD feeding (Supplemental Fig. S4, A and B). Liver gene expression levels of steatosis and fibrosis markers collagen, type 1, alpha 1 (Col1a1), collagen, type III, alpha 1 (Col3a1), matrix metalloproteinase 2 (Mmp2), and transforming growth factor, beta 1 (Tgfb1) were increased in all HFD-fed mice, but the only genotype-specific difference was decreased Col1a1 expression in HFD-fed Angptl4LivKO mice compared with HFD-fed Angptl4fl/fl mice (Fig. 3F). Liver triglyceride content was increased following 12 wk of HFD in both male Angptl4LivKO and Angptl4fl/fl compared with NCD-fed mice (Fig. 3G). However, these diet-induced differences in liver triglyceride content disappeared after 6 mo of HFD feeding (Fig. 3G). No genotype-specific differences in liver triglyceride content were observed (Fig. 3G). Liver inflammation has been shown to contribute to the development of insulin resistance during metabolic diseases such as obesity and type 2 diabetes (25). We measured liver expression of inflammatory markers C–C motif chemokine ligand 2 (Ccl2), Cd68, and tumor necrosis factor α (Tnfα). No major genotype-specific differences were observed, but as expected there, was an increase in expression of liver inflammatory genes in all HFD-fed mice (Fig. 3H).
Figure 3.
Liver phenotypic measurements from Angptl4LivKO and Angptl4fl/fl mice after chronic high-fat feeding. Male Angptl4LivKO and Angptl4fl/fl mice were fed either a normal chow diet (NCD) or a high-fat diet (HFD; 60% by kcal) for 6 mo and at the conclusion of the study liver phenotypic measurements were assessed. Liver triglyceride content was also assessed 12 wk on diet. A, B, and C: plasma from 6 (h) fasted mice was used to assess AST activity (A), ALT activity (B), and plasma amyloid A (C; means ± SE; n = 4–5 mice/group). D: representative pictures of livers. E: liver mass (means ± SE; n = 8–17/group). F: fasted (6 h) mRNA expression of steatosis and fibrosis markers Col1a1, Col3a1, Tgfb1, and Mmp2 from liver tissue (means ± SE, n = 8/group). G: liver triglyceride content normalized to protein content after 12 wk (means ± SE; n = 6–7/group) or 6 mo (means ± SE; n = 7–13/group) on diet study. H: fasted (6 h) mRNA expression of inflammatory markers Ccl2, Cd68, and Tnfα from liver tissue (means ± SE; n = 7–8/group). ##P < 0.01, ###P < 0.001 for dietary differences by two-way ANOVA. *P < 0.05 for individual genotype-specific differences by multiple comparisons after two-way ANOVA (Tukey’s correction). ALT, alanine aminotransferase; AST, aspartate aminotransferase; TG, triglyceride.
Assessment of Triglyceride Partitioning Phenotypes in Angptl4LivKO Mice during Chronic High-Fat Feeding
To assess if loss of hepatocyte-derived ANGPTL4 altered triglyceride partitioning, we performed radiolabeled chylomicron plasma triglyceride clearance and tissue uptake assays in male Angptl4LivKO and Angptl4fl/fl mice after either 12 wk or 6 mo of NCD or HFD feeding. No significant difference in triglyceride clearance was observed in NCD-fed mice (Fig. 4A, Supplemental Fig. S5A). HFD-fed mice cleared radiolabeled chylomicrons faster than NCD-fed mice, but there were no genotype-specific differences in clearance rates (Fig. 4A, Supplemental Fig. S5A). An increase in radiolabel uptake was seen in the livers of Angptl4LivKO mice compared with Angptl4fl/fl mice fed a NCD in the 12-wk-diet cohort, but not the 6-mo cohort (Fig. 4B, Supplemental Fig. S5B). In other metabolically active tissues (heart, kidney, quadriceps muscle, eWAT, sWAT, and BAT) assessed, there were no significant genotype-specific differences in uptake of radiolabeled triglycerides after 12 or 6 mo of NCD or HFD (Fig. 4B, Supplemental Fig. S5B). Interestingly, diet-induced differences in triglyceride uptake were seen in most of the measured tissues in the 12-wk-diet groups, but these differences largely disappeared after 6 mo on diet (Supplemental Fig. S5B, Fig. 4B).
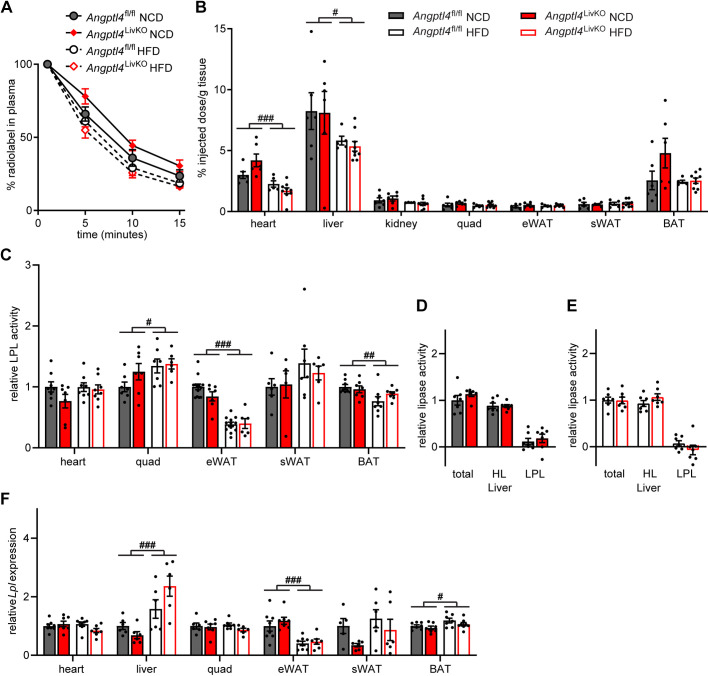
Figure 4.
Triglyceride phenotypes after chronic high-fat feeding. At the conclusion of 6 mo of either normal chow diet (NCD) or high-fat diet (HFD) Angptl4LivKO and Angptl4fl/fl mice (n = 5–9 mice/group) were fasted (6 h) and injected intravenously with 3H-triglyceride containing chylomicrons. A: clearance of radiolabel from the plasma 1, 5, 10, and 15 min after injection. Points represent percentage of radiolabel remaining in the plasma at the indicated time points compared with the 1-min time point (means ± SE). B: uptake of radiolabel (% injected dose/g tissue) into the indicated tissues 15 min after injection of 3H-chylomicrons (means ± SE). C: heart, quadriceps muscle (Quad), epididymal adipose tissue (eWAT), subcutaneous adipose tissue (sWAT), and brown adipose (BAT) tissue from fasted (6 h) male Angptl4LivKO and Angptl4fl/fl mice (n = 5–13/group) were harvested and lipase activity was measured. D and E: liver was harvested from fasted (6 h) male Angptl4LivKO and Angptl4fl/fl mice [NCD groups (D) and HFD groups (E)] (n = 6–7/group). To distinguish between hepatic versus lipoprotein lipase activity, lipase activity was measured in the presence or absence of 1 M NaCl. Bars show relative lipase activity in each tissue normalized to Angptl4fl/fl (means ± SE). F: fasted (6 h) mRNA expression of Lpl in heart, liver, quadriceps muscle (quad), epididymal white adipose tissue (eWAT), subcutaneous white adipose tissue (sWAT), and brown adipose tissues (BAT) of male Angptl4LivKO and Angptl4fl/fl mice fed either a normal chow diet (NCD) or a high-fat diet (HFD; 60% by kcal) for 6 mo (means ± SE; n = 5–8/group). #P < 0.05, ##P < 0.01, ###P < 0.001 for dietary differences by two-way ANOVA. HL, hepatic lipase; LPL, lipoprotein lipase.
As LPL is a major determinant of triglyceride partitioning and the target of ANGPTL4 inhibition, we also measured tissue lipase activity in metabolically active tissues from male Angptl4LivKO and Angptl4fl/fl mice after either 12 wk or 6 mo of NCD or HFD feeding. There were no genotype-specific differences in tissue LPL activity between Angptl4LivKO mice and Angptl4fl/fl mice (Fig. 4C, Supplemental Fig. S5C). Interestingly, high-fat diet alone altered LPL activity in mice fed a HFD for 12 wk. LPL activity increased in the heart, quadriceps muscle, and brown adipose tissue whereas activity decreased in the epididymal white adipose tissue (Supplemental Fig. S5C). After 6 mo of high-fat feeding, LPL activity remained increased in quadriceps and decreased in epididymal white adipose tissue (Fig. 4C). Although there is generally very little LPL activity in liver, it is possible that the absence of ANGPTL4 in the liver would lead to an increase in hepatic LPL activity. We therefore measured liver lipase activity in male Angptl4LivKO and Angptl4fl/fl mice after either 12 wk or 6 mo of NCD of HFD feeding. As expected, the contribution of LPL activity in the liver was minimal compared with hepatic lipase. Interestingly, in mice in the 12-wk diet cohort, total lipase activity was slightly decreased in Angptl4LivKO mice compared with Angptl4fl/fl mice on both diets, and LPL activity was slightly decreased in Angptl4LivKO mice fed a HFD (Supplemental Fig. S5D). These differences were not apparent after 6 mo on diet (Fig. 4, D and E). Lpl gene expression in tissues was not different between genotypes after either 12 wk or 6 mo on diet (Fig. 4F and Supplemental Fig. 5F). Neither the triglyceride uptake data nor the LPL activity data support a role for hepatic ANGPTL4 in the increased adipose TG uptake or LPL activity observed in the whole body Angptl4–/– mouse.
Assessment of Glucose Tolerance and Insulin Sensitivity in Angptl4LivKO Mice during Chronic High-Fat Feeding
To assess if the loss of hepatocyte-derived ANGPTL4 altered glucose metabolism in our Angptl4LivKO mice, we performed glucose (GTT) and insulin tolerance (ITT) tests. After 12 wk of either a NCD or HFD, no genotype-specific differences were observed in glucose tolerance or insulin sensitivity in Angptl4LivKO mice compared with Angptl4fl/fl mice (Fig. 5, A and C). Interestingly, despite no evidence of altered triglyceride partitioning, Angptl4LivKO mice displayed improved glucose tolerance compared with littermate Angptl4fl/fl mice after 6 mo of high-fat feeding (Fig. 5B). Insulin sensitivity also appeared slightly improved, but this improvement did not reach statistical significance (Fig. 5D). As adipose tissue inflammation can play a role in the development of insulin resistance, we measured tissue expression (eWAT, sWAT, and BAT) of inflammatory markers Ccl2, Cd68, and Tnfα. Once again, there was an increase in inflammatory gene expression in HFD-fed mice, but consistent genotype-specific differences were not observed (Supplemental Fig. S6).
Figure 5.
Glucose tolerance and insulin sensitivity of Angptl4LivKO mice. A and B: glucose tolerance tests were performed on fasted (6 h) male Angptl4LivKO and Angptl4fl/fl mice after either 11 wk (A) or 24 wk (B) of either a normal chow diet (NCD) or high-fat diet (HFD). Mice were injected with glucose (2 g/kg for NCD; 1.3 g/kg HFD) and blood glucose concentrations were measured over 2 h. Points represent glucose levels (means ± SE; n = 7–11 mice) at each respective time point. Bar graphs represent area under the curve (means ± SE) for all time points. *P < 0.05 by repeated measures ANOVA. C and D: insulin tolerance tests were performed on fasted (4 h) male an Angptl4LivKO mice after either 12 wk (C) or 25 wk (D) of either a normal chow diet (NCD) or high-fat diet (HFD). Mice were injected with 0.75 U/mL of human insulin (Humalin-R) and blood glucose concentrations were measured over 90 min. Points represent glucose levels (means ± SE; n = 7–11) at each respective time point. Bar graphs represent area under the curve (means ± SE) for all time points. #P < 0.05, ###P < 0.001 for dietary differences by two-way ANOVA. *P < 0.05, for individual genotype-specific differences by repeated measures ANOVA.
Assessment of Prolonged Fasting in Angptl4LivKO Mice
Hepatic ANGPTL4 expression is highly induced by fasting. Currently, the physiological function of fasting-induced liver ANGPTL4 is unknown. During the completion of this work, another study investigating the role of liver ANGPTL4 appeared in preprint (26). Singh et al. used the KOMP flox allele to generate a liver-ANGPTL4 deficient mouse model. Contrary to our observations, they observed lower levels of fasting plasma TG levels in their liver-deficient Angptl4 mice fed either a normal chow or a Western diet for 16 wk (26). Because Singh et al. measured plasma TG levels after an overnight fast and we measured TG levels after a 6 h fast, we asked if fasting our Angptl4LivKO mice for 18 h would uncover a change in plasma TG levels. Indeed, after prolonged fasting, 8- to 12-wk-old Angptl4LivKO mice had decreased plasma TG levels compared with Angptl4fl/fl mice, but when plasma TG levels were measured in the same mice after a 6-h fast, no difference was observed, consistent with our previous observations (Fig. 6A). To further investigate the consequences of an overnight fast on triglyceride partitioning, we performed a TG clearance and uptake assay in 8- to 12-wk-old male Angptl4LivKO and Angptl4fl/fl mice after an overnight fast. Although there was a trend toward increased radiolabeled TG clearance in the Angptl4LivKO mice compared with Angptl4fl/fl mice (Fig. 6B), no differences in tissue TG uptake were observed (Fig. 6C). We asked if the decreased triglycerides in Angptl4LivKO mice after an overnight fast were due to a change in very-low-density lipoprotein (VLDL) secretion. To address this possibility, we performed VLDL secretion assays in mice fasted overnight. No significant differences were seen in VLDL-TG secretion between Angptl4LivKO and Angptl4fl/fl mice (Fig. 6D). Furthermore, liver triglyceride content at the end of the secretion assay did not differ between genotypes (Fig. 6E).
Figure 6.

Triglyceride levels and partitioning in Angptl4LivKO mice after overnight fast. A: triglyceride levels on fasted (6 and 18 h) 8- to 12-wk-old male Angptl4fl/fl and Angptl4LivKO mice (means ± SE; n = 8–10 mice). B: clearance of radiolabel from the plasma 1, 5, 10, and 15 min after injection in 8- to 12-wk-old male Angptl4fl/fl and Angptl4LivKO mice fasted for 18 h (means ± SE; n = 7–9). Points represent percentage of radiolabel remaining in the plasma at the indicated time points compared with the 1-min time point (means ± SE). C: uptake of radiolabel (% injected dose/g tissue) into the indicated tissues after 15 min (means ± SE). D: plasma triglyceride levels following tyloxapol (500 mg/kg, intravenous) injection in 8- to 12-wk-old male Angptl4fl/fl and Angptl4LivKO mice following an overnight fast (means ± SE; n = 6/group). E: liver triglyceride content in mice 3 h after tyloxapol injection from mice in E (means ± SE; n = 6/group). **P < 0.01 vs. Angptl4fl/fl mice by Student’s t test. TG, triglyceride.
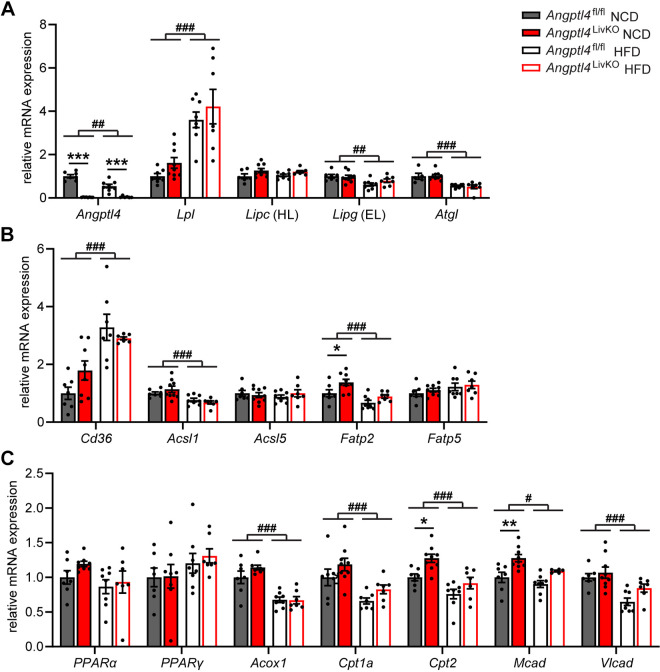
We also examined the effect of overnight fasting on the plasma TG levels of Angptl4LivKO and Angptl4fl/fl after 6 mo of high-fat feeding (Fig. 7A). After 6 mo on diet, the decreased plasma TG levels after an overnight fast persisted in Angptl4LivKO fed a NCD, but there were no genotype-specific differences on HFD, nor were there genotype-specific differences in nonesterified fatty acid (NEFA) levels on either diet (Fig. 7, A and B). As we observed after a 6-h fast, liver triglyceride content was not different between groups following an overnight fast (Fig. 7C). Neither did we observe any significant changes in LPL activity in metabolically active tissues that would explain the lower plasma TG levels (Fig. 7, D–F). We asked if transcriptional changes in the liver might underlie the decreased plasma TG levels seen in Angptl4LivKO mice after a prolonged fast. As expected, liver expression of Angptl4 was absent in the Angptl4LivKO mice, and just as we saw with a 6-h fast, HFD-fed Angptl4fl/fl mice had reduced liver Angptl4 expression compared with NCD-fed mice (Fig. 8A). HFD feeding increased hepatic expression of Lpl, had no effect on Lipc (hepatic lipase expression), and decreased hepatic Lipg (endothelial lipase) and Atgl (adipose triglyceride lipase) expression, but there were no genotype-specific differences (Fig. 8A). There were also no genotype-specific alterations in the expression levels of fatty acid transport and synthesis genes Cd36 (Cd36), Acyl-Coa Synthetase 1 (Acsl1), Acyl-Coa Synthetase 5 (Acsl5), or Fatty acid transport protein 5 (Fatp5; Fig. 8B). We did observe a slight increase in Fatp2 expression in the livers of Angptl4LivKO mice fed a NCD compared with control mice (Fig. 8B). Peroxisome proliferator-activated receptor α is a major regulator of lipid metabolism during fasting in the liver (27). However, there were no significant differences in Ppara gene expression in the livers of Angptl4LivKO mice on either diet; neither were there significant differences in the gene expression of the related metabolic transcription factor, peroxisome proliferator-activated receptor γ (Fig. 8C). Because activation of PPAR transcription factors does not require changes in Ppar expression, we assessed gene expression of several downstream targets of PPARα that play a role in lipid metabolism. Although HFD feeding caused a reduction in mRNA expression from genes coding peroxisomal straight-chain acyl-CoA oxidase (Acox1), carnitine palmitoyltransferase 1 A (Cpt1a), carnitine palmitoyltransferase 2 (Cpt2), medium-chain acyl-CoA dehydrogenase (Mcad), and very long-chain acyl-CoA dehydrogenase (Vlcad) in the livers of Angptl4LivKO and Angptl4fl/fl mice fasted for 18 h, the only genotype-specific differences were increases in Cpt2 and Mcad expression in the livers of Angptl4LivKO mice fed a NCD (Fig. 8C).
Figure 7.
Plasma measures and lipase activity in chronic HFD-fed Angptl4LivKO mice after overnight fast. Following 6 mo of either NCD or HFD, Angptl4fl/fl and Angptl4LivKO mice were fasted for 18 h and plasma triglyceride levels (A), plasma NEFA levels (B), and liver triglyceride content (C) were assessed (means ± SE; n = 7–9 mice/group). D: heart, quadriceps muscle (Quad), epididymal adipose tissue (eWAT), subcutaneous adipose tissue (sWAT), and brown adipose (BAT) tissue from fasted (18 h) male Angptl4LivKO and Angptl4fl/fl mice (n = 7–9/group) were harvested and lipase activity was measured. E and F: liver was harvested from fasted (18 h) male Angptl4LivKO and Angptl4fl/fl mice [NCD groups (E) and HFD groups (F)] mice (n = 6–9/group). To distinguish between hepatic versus LPL activity, lipase activity was measured in the presence or absence of 1 M NaCl. Bars show relative lipase activity in each tissue normalized to Angptl4fl/fl (means ± SE). #P < 0.05, ##P < 0.01, ###P < 0.001 for dietary differences by two-way ANOVA. **P < 0.01 for individual genotype-specific differences by multiple comparisons after two-way ANOVA (Tukey’s correction). HFD, high-fat diet; LPL, lipoprotein lipase; NCD, normal chow diet; HL, hepatic lipase; NEFA, nonesterified fatty acid.
Figure 8.
Gene expression in chronic high-fat diet-fed Angptl4LivKO mice after overnight fast. Fasted (18 h) mRNA expression of Angptl4 and lipases (Lpl, Lipc, Lipg, Atgl; A), fatty acid transport/synthesis markers (Cd36, Acsl1, Acsl5, Fatp2, Fatp5; B), and Ppara and downstream targets (Ppara, Pparg, Acox1, Cpt1a, Cpt2, Mcad, Vlcad; C) in livers of male Angptl4LivKO and Angptl4fl/fl mice fed either a normal chow diet (NCD) or a high-fat diet (HFD; 60% by kcal) for 6 mo (means ± SE; n = 5–8 mice/group). #P < 0.05, ##P < 0.01, ###P < 0.001 for dietary differences by two-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001 for individual genotype-specific differences by multiple comparison after two-way ANOVA (Tukey’s correction). EL, endothelial lipase; HL, hepatic lipase.
DISCUSSION
Proteins made and secreted by the liver (hepatokines) play a vital role in lipid and glucose homeostasis during obesity. ANGPTL4 is highly expressed by both adipose tissue and liver in mice and humans (16, 17, 28). In this study, we investigated triglyceride and metabolic phenotypes in Angptl4LivKO mice during a 6-mo high-fat feeding study. Loss of hepatocyte-derived ANGPTL4 did not alter 6-h fasted plasma triglyceride levels, triglyceride tissue uptake, or LPL activity during the diet study when compared with Angptl4fl/fl control mice. Initially, Angptl4LivKO mice showed no difference in glucose tolerance and insulin sensitivity, but after 6 mo on HFD, these mice had improved glucose tolerance compared with HFD-fed Angptl4fl/fl mice. On NCD, both young (2–3 mo) and old (8 mo) Angptl4LivKO mice fasted for 18 h had lower plasma triglyceride levels compared with NCD-fed Angptl4fl/fl mice. However, despite this decrease in plasma triglyceride levels, there were no differences in triglyceride tissue uptake or tissue LPL activity in Angptl4LivKO compared with Angptl4fl/fl mice.
Both mice and humans deficient in ANGPTL4 have reduced plasma triglyceride levels (5, 12, 29), and Angptl4AdipoKO mice similarly have lower plasma TG levels (13, 14, 22). Part of our objective in this study was to determine the contribution of liver ANGPTL4 to triglyceride metabolism. Our initial studies on mice that had been fasted 6 h suggested no role for liver ANGPTL4 in systemic triglyceride metabolism. Compared with control mice, we observed no differences in plasma triglyceride levels or triglyceride uptake into tissues in Angptl4LivKO mice fed either HFD or normal chow. We also observed no increases in LPL or hepatic lipase activity in these mice. However, when Angptl4LivKO mice were subjected to a prolonged, 18-h fast, plasma TG in these mice were significantly lower than control mice. Fasting alters enzyme activities and expression of many genes important to lipid metabolism (30), and it has been shown that liver weights, liver triglyceride content, liver enzyme activities, and expression of genes related to lipogenesis and lipolysis can differ significantly depending on the length of fasting (31). In mice, 4- to 6-h fasts are similar to overnight fast in humans, whereas fasting mice overnight or longer induces a catabolic, starvation-like state (32, 33). Thus, although withholding food for 6 or 18 h both constitute fasting, they represent very different physiological states.
To gain insight into why a prolonged fast resulted in genotype-specific differences in plasma TG levels, we attempted to ascertain the mechanism by which TGs were decreased in Angptl4LivKO mice. Remarkably, the lower plasma TG levels we observed in Angptl4LivKO mice after an 18-h fast occurred in the absence of any observed increase in TG uptake or triglyceride lipase activity in any of the metabolically active tissues that we assayed. This is in contrast to Angptl4–/– and Angptl4AdipoKO mice, where lower plasma TG levels are accompanied by increased lipase activity and increased TG uptake into adipose (8, 13, 14). It has been shown that ANGPTL4 can stimulate lipolysis in adipocytes during fasting (34). Therefore, one possibility was that the absence of liver ANGPTL4 alters adipose lipolysis and in turn the hepatic incorporation of circulating fatty acids into VLDL. However, after an 18-h fast, we observed no differences in plasma NEFA levels or VLDL secretion between Angptl4LivKO and Angptl4fl/fl mice. As neither TG clearance nor secretion seemed to be significantly altered, the mechanism by which Angptl4LivKO mice manifest lower TG levels after an 18-h fast remains unclear. One possibility is the lower TG levels result from small changes in TG clearance that are obscured by the biological variability in our assays. For example, although not reaching statistical significance, TG clearance appears to be somewhat faster in Angptl4LivKO mice after an 18-h fast (see Fig. 6B). A small increase in clearance to multiple tissues or to tissues not represented in our assay might be sufficient to lower plasma TG levels. The metabolic pathways regulated by liver ANGPTL4 after an 18-h fast likewise remains obscure. Although ANGPTL4 expression is greatly induced by fasting (28), previous work has shown that Angptl4 expression in mouse tissues, including the liver, is induced within 2 h of fasting (8). The fact that we did not observe changes in plasma triglyceride levels until much later in a fast and the absence of any differences in lipase activity between genotypes after prolonged fasting suggest that liver ANGPTL4 may not be acting directly on lipases to lower plasma triglyceride levels. Because fasting alters expression of many genes key to lipid metabolism, we measured gene expression of several hepatic metabolic enzyme, fatty acid transport/synthesis, and PPAR-related genes. However, we observed few differences that would explain the decreased plasma triglyceride levels in Angptl4LivKO mice after a prolonged fast. Therefore, the mechanism by which hepatic ANGPTL4 regulated plasma TG levels after a prolonged fast and the physiological consequences of this regulation remain unclear.
Human ANGPTL4 deficiency not only reduces plasma triglyceride levels but is also associated with lower risk of metabolic syndrome and type 2 diabetes (6, 35). Moreover, Angptl4–/– mice fed a high-fat diet high in unsaturated fatty acids have improved glucose tolerance (12). Together these observations suggest that ANGPTL4 might contribute to glucose homeostasis. We and others have reported short-term improvement in glucose tolerance and insulin sensitivity in high-fat-fed Angptl4AdipoKO mice (13, 14, 22). Our data suggest that the role of liver ANGPTL4 in glucose homeostasis is not clear-cut. Initially, we observed no improvement in glucose homeostasis in high-fat-fed Angptl4LivKO mice, but after 6 mo of HFD feeding, glucose tolerance was improved and there was a trend toward improved insulin sensitivity. These data suggest that loss of Angptl4 from both adipose tissue and the liver may contribute to the improved glucose tolerance seen in whole body Angptl4–/– mice and the resistance to type 2 diabetes in ANGPTL4-deficient humans. The most well-characterized role of ANGPTL4 is the inactivation of LPL through unfolding of the catalytic domain (36–38). However, unlike in Angptl4AdipoKO or Angptl4–/– mice, the improved glucose tolerance in Angptl4LivKO mice occurred in the absence of differences in fasting plasma TG levels, increases in LPL activity, or alterations in tissue TG uptake, suggesting that an LPL-independent mechanism may be at work. Although the mechanism behind improved glucose tolerance in these mice is unclear, metabolic caging data indicated that Angptl4LivKO mice had increased energy expenditure. Given that the improved glucose tolerance emerged only in mice fed a chronic HFD, it would be interesting to determine if an even longer period of HFD feeding would increase the difference in glucose tolerance between Angptl4LivKO mice and controls, and if the increased energy expenditure we observed in Angptl4LivKO mice would persist at older ages.
Independent of ANGPTL4 deficiency, chronic high-fat feeding had effects on Angptl4 and Lpl gene expression not observed in more typical high-fat feeding regimes. Using Northern blot analysis, Kersten et al. previously found no difference in Angptl4 expression in either the liver or white adipose tissue following 15 wk of high-fat feeding (28). We likewise saw no differences in Angptl4 expression in the liver or subcutaneous white adipose tissue of Angptl4fl/fl mice after 12 wk of normal chow or high-fat feeding, though we did observe a decrease in expression in epidydimal white adipose tissue. After 6 mo on diet, however, HFD-fed mice had significantly lower Angptl4 expression in liver, subcutaneous white adipose tissue, and epididymal white adipose tissue compared with NCD-fed mice. Likewise, we observed no diet-induced differences in Lpl expression in the liver after 12 wk, but after 6 mo of high-fat feeding, Lpl expression was significantly increased compared with chow-fed animals. Both Lpl expression and triglyceride lipase activity decreased in the epididymal white adipose tissue of mice fed a HFD for 6 mo. These data demonstrate that duration on diet, not just diet type, can have significant effects on Lpl and Angptl4 expression.
Although the use of a 60% kcal/fat diet is a common laboratory diet used to induce obesity and glucose intolerance in mice, the use of other diets might be necessary for exploring the importance of liver-derived ANGPTL4. For example, the Western diet (high fat, high sucrose) has emerged as a tool to study pathologies specific to the liver such as nonalcoholic fatty liver disease (NAFLD) (39), and future studies with Angptl4LivKO mice could unravel a role for liver-derived ANGPTL4 in the pathogenesis of NAFLD. Methionine- and choline-deficient diets have been used to study nonalcoholic steatohepatitis (NASH), and using this diet, Teratani et al. found that Angptl4–/– mice have increased liver fibrosis and activated hepatic stellate cells with no increase in hepatocellular injury or inflammation compared with control mice (40). They found that this was partially due to an increase in free cholesterol levels in the HSCs of the Angptl4–/– mice. Liver-specific Angptl4 knockout mice, such as those described here, could be used to explore the role of Angptl4 in NASH and other metabolic liver diseases.
In summary, our data support the idea that liver ANGPTL4 is not a primary driver of plasma triglyceride levels, LPL activity, or lipid partitioning in mice. We find that liver-derived ANGPTL4 may play a role in glucose homeostasis during chronic high-fat-diet feeding and in metabolic adaptation to prolonged fasting in mice. However, the mechanisms of these observations remain to be elucidated, and the primary physiological role of liver ANGPTL4 remains an open question.
SUPPLEMENTAL DATA
Supplemental Tables S1 and S2 and Supplemental Figs. S1–S6: https://doi.org/10.6084/m9.figshare.14377031.
GRANTS
This work was supported by grants from the National Institutes of Health (R01HL130146 to B.S.J.D.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M.S. and B.S.J.D. conceived and designed research; K.M.S., S.K.S., E.M.C., and K.L.S.-D performed experiments; K.M.S., S.K.S., E.M.C., K.L.S.-D., and B.S.J.D. analyzed data; K.M.S. and B.S.J.D. interpreted results of experiments; K.M.S. and B.S.J.D. prepared figures; K.M.S. drafted manuscript; K.M.S., S.K.S., K.L.S.-D, and B.S.J.D. edited and revised manuscript; K.M.S., S.K.S., E.M.C., K.L.S.-D, and B.S.J.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the University of Iowa Genome Editing Facility for assistance in generating the ANGPTL4 floxed allele and the Fraternal Order of Eagles Diabetes Research Center Metabolic Phenotyping Core Laboratory for assistance in collecting metabolic caging data.
REFERENCES
- 1.Tchernof A, Després J-P. Pathophysiology of human visceral obesity: an update. Physiol Rev 93: 359–404, 2013. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 2.Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 56: 952–964, 2012. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 3.Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol 13: 509–520, 2017. doi: 10.1038/nrendo.2017.56. [DOI] [PubMed] [Google Scholar]
- 4.Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjaerg-Hansen A, Hobbs HH, Cohen JC. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet 39: 513–516, 2007. doi: 10.1038/ng1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewey FE, Gusarova V, O'Dushlaine C, Gottesman O, Trejos J, Hunt C, Van Hout CV, Habegger L, Buckler D, Lai K-MV, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Ledbetter DH, Penn J, Lopez A, Borecki IB, Overton JD, Reid JG, Carey DJ, Murphy AJ, Yancopoulos GD, Baras A, Gromada J, Shuldiner AR. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med 374: 1123–1133, 2016. doi: 10.1056/NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gusarova V, O'Dushlaine C, Teslovich TM, Benotti PN, Mirshahi T, Gottesman O, et al. Genetic inactivation of ANGPTL4 improves glucose homeostasis and is associated with reduced risk of diabetes. Nat Commun 9: 2252, 2018. doi: 10.1038/s41467-018-04611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stitziel NO, Stirrups KE, Masca NGD, Erdmann J, Ferrario PG, König IR; Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators, et al. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med 374: 1134–1144, 2016. [Erratum in N Engl J Med 374: 1898, 2016]. doi: 10.1056/NEJMoa1507652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cushing EM, Chi X, Sylvers KL, Shetty SK, Potthoff MJ, Davies BSJ. Angiopoietin-like 4 directs uptake of dietary fat away from adipose during fasting. Mol Metab 6: 809–818, 2017. doi: 10.1016/j.molmet.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Köster A, Chao YB, Mosior M, Ford A, Gonzalez-DeWhitt PA, Hale JE, Li D, Qiu Y, Fraser CC, Yang DD, Heuer JG, Jaskunas SR, Eacho P. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology 146: 4943–4950, 2005. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 10.Desai U, Lee E-C, Chung K, Gao C, Gay J, Key B, Hansen G, Machajewski D, Platt KA, Sands AT, Schneider M, Van Sligtenhorst I, Suwanichkul A, Vogel P, Wilganowski N, Wingert J, Zambrowicz BP, Landes G, Powell DR. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci USA 104: 11766–11771, 2007.doi: 10.1073/pnas.0705041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dijk W, Heine M, Vergnes L, Boon MR, Schaart G, Hesselink MK, Reue K, van W, Lichtenbelt DM, Olivecrona G, Rensen PC, Heeren J, Kersten S. ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure. eLife 4: e08428, 2015. doi: 10.7554/eLife.08428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen AWF, Katiraei S, Bartosinska B, Eberhard D, Willems van Dijk K, Kersten S. Loss of angiopoietin-like 4 (ANGPTL4) in mice with diet-induced obesity uncouples visceral obesity from glucose intolerance partly via the gut microbiota. Diabetologia 61: 1447–1458, 2018. doi: 10.1007/s00125-018-4583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aryal B, Singh AK, Zhang X, Varela L, Rotllan N, Goedeke L, Chaube B, Camporez J-P, Vatner DF, Horvath TL, Shulman GI, Suárez Y, Fernández-Hernando C. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis. JCI Insight 3: e97918, 2018. doi: 10.1172/jci.insight.97918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitler KM, Shetty SK, Cushing EM, Sylvers-Davie KL, Davies BSJ. Regulation of plasma triglyceride partitioning by adipose-derived ANGPTL4 in mice. Sci Rep 11: 7873, 2021. doi: 10.1038/s41598-021-87020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T; Mouse ENCODE Consortium, et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature 515: 355–364, 2014. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, Friedman JM, Holmes WE, Spiegelman BM. Peroxisome proliferator-activated receptor γ target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol 20: 5343–5349, 2000. doi: 10.1128/MCB.20.14.5343-5349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics 13: 397–406, 2014. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem 274: 305–315, 1999. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 19.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C T method. Nat Protoc 3: 1101–1108, 2008. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 20.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- 21.Oteng A-B, Ruppert PMM, Boutens L, Dijk W, van Dierendonck XAMH, Olivecrona G, Stienstra R, Kersten S. Characterization of ANGPTL4 function in macrophages and adipocytes using Angptl4-knockout and Angptl4-hypomorphic mice. J Lipid Res 60: 1741–1754, 2019. doi: 10.1194/jlr.M094128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh AK, Aryal B, Chaube B, Rotllan N, Varela L, Horvath TL, Suárez Y, Fernández-Hernando C. Brown adipose tissue derived ANGPTL4 controls glucose and lipid metabolism and regulates thermogenesis. Mol Metab 11: 59–69, 2018. doi: 10.1016/j.molmet.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee E-C, Desai U, Gololobov G, Hong S, Feng X, Yu X-C, Gay J, Wilganowski N, Gao C, Du L-L, Chen J, Hu Y, Zhao S, Kirkpatrick L, Schneider M, Zambrowicz BP, Landes G, Powell DR, Sonnenburg WK. Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL). J Biol Chem 284: 13735–13745, 2009. doi: 10.1074/jbc.M807899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stranges S, Dorn JM, Muti P, Freudenheim JL, Farinaro E, Russell M, Nochajski TH, Trevisan M. Body fat distribution, relative weight, and liver enzyme levels: a population-based study. Hepatology 39: 754–763, 2004. doi: 10.1002/hep.20149. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh AK, Chaube B, Canfrán-Duque A, Zhang X, Price NL, Aryal B, Sun J, Citrin KM, Rotllan N, Lee RG, Suárez Y, Fernández-Hernando C. Liver-specific suppression of ANGPTL4 improves obesity-associated diabetes and mitigates atherosclerosis in mice. bioRxiv 2020.06.02.130922, 2020. 10.1101/2020.06.02.130922 [DOI] [PMC free article] [PubMed]
- 27.Kersten S, Stienstra R. The role and regulation of the peroxisome proliferator activated receptor alpha in human liver. Biochimie 136: 75–84, 2017. doi: 10.1016/j.biochi.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem 275: 28488–28493, 2000. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 29.Lichtenstein L, Mattijssen F, de Wit NJ, Georgiadi A, Hooiveld GJ, van der Meer R, He Y, Qi L, Köster A, Tamsma JT, Tan NS, Müller M, Kersten S. Angptl4 protects against severe pro-inflammatory effects of dietary saturated fat by inhibiting lipoprotein lipase-dependent uptake of fatty acids in mesenteric lymph node macrophages. Cell Metab 12: 580–592, 2010. doi: 10.1016/j.cmet.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palou M, Priego T, Sánchez J, Villegas E, Rodríguez AM, Palou A, Picó C. Sequential changes in the expression of genes involved in lipid metabolism in adipose tissue and liver in response to fasting. Pflugers Arch 456: 825–836, 2008. doi: 10.1007/s00424-008-0461-1. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda I, Metoki K, Yamahira T, Kato M, Inoue N, Nagao K, Yanagita T, Shirakawa H, Komai M. Impact of fasting time on hepatic lipid metabolism in nutritional animal studies. Bioscience. Biosci Biotechnol Biochem 78: 1584–1591, 2014. doi: 10.1080/09168451.2014.923297. [DOI] [PubMed] [Google Scholar]
- 32.Jensen TL, Kiersgaard MK, Sørensen DB, Mikkelsen LF. Fasting of mice: a review. Lab Anim 47: 225–240, 2013. doi: 10.1177/0023677213501659. [DOI] [PubMed] [Google Scholar]
- 33.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- 34.Gray NE, Lam LN, Yang K, Zhou AY, Koliwad S, Wang J-C. Angiopoietin-like 4 (Angptl4) protein is a physiological mediator of intracellular lipolysis in murine adipocytes. J Biol Chem 287: 8444–8456, 2012. [Erratum in J Biol Chem 292: 16135, 2017]. doi: 10.1074/jbc.M111.294124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q, Oliver-Williams C, Raitakari OT, Viikari J, Lehtimäki T, Kähönen M, Järvelin M-R, Salomaa V, Perola M, Danesh J, Kettunen J, Butterworth AS, Holmes MV, Ala-Korpela M. Metabolic profiling of angiopoietin-like protein 3 and 4 inhibition: a drug-target Mendelian randomization analysis. Eur Heart J 42: 1160–1169, 2020. doi: 10.1093/eurheartj/ehaa972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kristensen KK, Leth-Espensen KZ, Mertens HDT, Birrane G, Meiyappan M, Olivecrona G, Jørgensen TJD, Young SG, Ploug M. Unfolding of monomeric lipoprotein lipase by ANGPTL4: insight into the regulation of plasma triglyceride metabolism. Proc Natl Acad Sci USA 117: 4337–4346, 2020. doi: 10.1073/pnas.1920202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leth-Espensen KZ, Kristensen KK, Kumari A, Winther A-ML, Young SG, Jørgensen TJD, Ploug M. The intrinsic instability of the hydrolase domain of lipoprotein lipase facilitates its inactivation by ANGPTL4-catalyzed unfolding. Proc Natl Acad Sci USA 118: e2026650118, 2021. doi: 10.1073/pnas.2026650118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mysling S, Kristensen KK, Larsson M, Kovrov O, Bensadouen A, Jørgensen TJ, Olivecrona G, Young SG, Ploug M. The angiopoietin-like protein ANGPTL4 catalyzes unfolding of the hydrolase domain in lipoprotein lipase and the endothelial membrane protein GPIHBP1 counteracts this unfolding. eLife 5: e20958, 2016. doi: 10.7554/eLife.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephenson K, Kennedy L, Hargrove L, Demieville J, Thomson J, Alpini G, Francis H. Updates on dietary models of nonalcoholic fatty liver disease: current studies and insights. Gene Expr 18: 5–17, 2018. doi: 10.3727/105221617X15093707969658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teratani T, Tomita K, Wada A, Sugihara N, Higashiyama M, Inaba K, Horiuchi K, Hanawa Y, Nishii S, Mizoguchi A, Tanemoto R, Ito S, Okada Y, Kurihara C, Akita Y, Narimatsu K, Watanabe C, Komoto S, Oike Y, Miura S, Hokari R, Kanai T. Angiopoietin-like protein 4 deficiency augments liver fibrosis in liver diseases such as nonalcoholic steatohepatitis in mice through enhanced free cholesterol accumulation in hepatic stellate cells. Hepatol Res 51: 580–592, 2020. doi: 10.1111/hepr.13603. [DOI] [PubMed] [Google Scholar]