Keywords: ANGPTL proteins, cardiovascular disease, dyslipidemia, lipoprotein lipase, lipoproteins
Abstract
Triglyceride-rich lipoproteins deliver fatty acids to tissues for oxidation and for storage. Release of fatty acids from circulating lipoprotein triglycerides is carried out by lipoprotein lipase (LPL), thus LPL serves as a critical gatekeeper of fatty acid uptake into tissues. LPL activity is regulated by a number of extracellular proteins including three members of the angiopoietin-like family of proteins. In this review, we discuss our current understanding of how, where, and when ANGPTL3, ANGPTL4, and ANGPTL8 regulate lipoprotein lipase activity, with a particular emphasis on how these proteins interact with each other to coordinate triglyceride metabolism and fat partitioning.
LIPOPROTEIN LIPASE AND TRIGLYCERIDE PARTITIONING
Fatty acids are a vital energy source for many oxidative tissues, including heart and skeletal muscle. Most fatty acids are obtained from diet, but fatty acids can also be synthesized de novo, primarily in the liver and adipose tissue. Both dietary and liver-synthesized fatty acids are esterified into triglycerides (TG) and packaged into triglyceride-rich lipoproteins. These lipoproteins, chylomicrons for intestinally packaged dietary fat and VLDL for liver-derived fatty acids, circulate in the bloodstream allowing for the distribution of fatty acids to various peripheral tissues.
In most tissues, extraction of fatty acids from circulating triglyceride-rich lipoproteins is primarily carried out by lipoprotein lipase (LPL). Acting on the luminal surface of capillaries, LPL binds circulating triglyceride-rich lipoproteins and hydrolyses the triglycerides within, releasing fatty acids for uptake into tissues (1). LPL is expressed highly in tissues that consume or store fat, including heart, skeletal muscle, all adipose depots, and the mammary gland (2–5). LPL is secreted by the parenchymal cells of these tissues, i.e., the adipocytes, cardiomyocytes, and myocytes (6) and then transported across capillary endothelial cells by the endothelial cell protein glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1) (7). GPIHBP1 also anchors LPL to the capillary wall during lipolysis of lipoproteins (8, 9). Without active vascular LPL, very little lipoprotein triglyceride is delivered to peripheral tissues. In mice, LPL-deficiency leads to neonatal death due to the inability of pups to properly utilize the triglyceride-rich milk from their mothers (10). Humans deficient in LPL manifest severe hypertriglyceridemia due to the lack of triglyceride clearing (11).
The fatty acids liberated by LPL-mediated lipolysis are largely taken up by the tissue in which LPL is located. Thus, LPL acts as a fatty acid gateway and the relative levels of LPL activity in different tissues are a major determinant of the relative distribution of triglyceride-derived fatty acids to those tissues. The tissue distribution of fatty acids, often referred to as fatty acid partitioning, shifts according to metabolic state and is important for maintaining metabolic homeostasis. Ectopic deposition of fat to tissues such as liver and skeletal muscle can drive metabolic dysfunction and lead to metabolic disease (reviewed in Ref. 3). Thus, proper regulation of LPL activity is critical for maintaining metabolic health. Although LPL activity is regulated on many levels, including transcriptional, translational, and posttranslational, one of the most prominent forms of regulation is inhibition by extracellular proteins. Here, we examine three such extracellular inhibitors, ANGPTL4, ANGPTL3, and ANGPTL8, which have emerged as key regulators of LPL, acting in specific tissues and at specific times to modulate LPL activity and adjust lipid partitioning.
THE ANGPTL FAMILY OF PROTEINS
The angiopoietin-like (ANGPTL) family of proteins are named such because of their structural similarity to angiopoietin proteins. Like angiopoietin proteins, ANGPTL proteins, with the exception of ANGPTL8, contain a coiled-coil domain and a large globular C-terminal fibrinogen-like domain (12, 13). Unlike angiopoietin proteins, ANGPTL proteins do not bind the receptor tyrosine kinase Tie2, but may still contribute to angiogenesis (12, 14). Each of the ANGPTL proteins contain a signal peptide and can be secreted from cells. The family currently has eight known members, ANGPTL1-8, with pleiotropic functions (13). Relevant to this review, several family members contribute to triglyceride metabolism. ANGPTL4, ANGPTL3, and ANGPTL8 have been subject to detailed study using both animal models (Table 1) and human genetics. These three proteins will be discussed in depth below. In addition to these three proteins, ANGPTL5 and ANGPTL6 may also play a role in lipid metabolism. Single nucleotide polymorphisms (SNPs) in ANGPTL5 are associated with plasma triglyceride levels in humans (40). In humans, ANGPTL5 is expressed in several tissues, including high expression in adipose tissue (40). However, ANGPTL5 is not found in mice, which has hampered its further study. Mice deficient in ANGPTL6 (also known as angiopoietin-related growth factor) become obese, insulin resistant, and accumulate lipid in skeletal muscle and liver (41). However, despite these marked changes, ANGPTL6 does not appear to modulate plasma TG levels (40, 41).
Table 1.
Animal models used in ANGPTL studies
| Generated Animal Models | |
|---|---|
| ANGPTL3 | KK/San mice with natural ANGPTL3 mutation (15) Adenovirus overexpression mouse (15) Whole body knockout mice (16–19) ANGPTL3-deficient mouse created with CRISPR base editing (20) ANGPTL3/ANGPTL8 double knockout mouse (21) |
| ANGPTL4 | Adenovirus overexpression mice (22–24) Mouse with adenovirus overexpression of only the fibrinogen-like domain (25) Mouse with transgenic overexpression from the ApoE promoter (16) Mouse with transgenic overexpression from the AP2 promoter (26) Mouse with transgenic overexpression from the α-MHC promoter (27) Mouse with transgenic overexpression from the NPHS2 promoter (28) Whole body knockout mice (16, 29, 30) Mouse with ANGPTL4 E40K mutation (17) Adipose-specific (Adipo-Cre) knockout mice (31, 32) Brown adipose-specific (UCP1-ER-Cre) knockout mouse (33) ANGPTL4 hypomorphic mouse (EUCOMM/KOMP allele) (34) |
| ANGPTL8 | Adenovirus overexpression mice (19, 35) Whole body knockout mice (36, 37) Whole body knockout rat (38) Mouse with human ANGPTL8 (164) Adipose-specific (Adipo-Cre) knockout mice (39) Liver-specific (Alb-Cre) knockout mice (39) ANGPTL3/ANGPTL8 double knockout mouse (21) |
ANGPTL, angiopoietin-like.
In addition to their role in regulating plasma triglyceride levels, ANGPTL3, ANGPTL4, and ANGPTL8 have been associated with numerous physiological roles including stem-cell renewal, angiogenesis, and inflammation (42–45). However, this review will focus specifically on their roles in triglyceride metabolism.
ANGPTL4
Discovery
In 2000, two groups described the discovery of a secreted PPAR target gene expressed highly in adipose tissue (46, 47). Yoon et al. (47) named the protein PGAR (PPARγ angiopoietin related). Kersten et al. (46) named the protein fasting-induced adipose factor (FIAF) owing to their observation that the protein was highly induced by fasting. They also found that fasting induced liver expression of FIAF. Both groups speculated that this newly uncovered protein was involved in metabolism in some way. This conjecture was supported by a study in 2002 from Yoshida et al. (48), who renamed FIAF/PGAR to ANGPTL4 based on its homology to ANGPTL3. They showed that injection of ANGPTL4 could increase plasma triglycerides and that recombinant ANGPTL4 could inhibit LPL in vitro (48). The role of ANGPTL4 in regulating LPL was further solidified in a 2005 study by Köster et al. (16). They also found that ANGPTL4 could inhibit LPL in vitro. Moreover, they reported that transgenic overexpression of ANGPTL4 increased plasma triglycerides and reduced LPL activity measured in postheparin plasma. Conversely, ANGPTL4-deficient mice had lower than normal plasma triglycerides and higher postheparin-plasma LPL activity (16). In the years since, multiple laboratories have confirmed the ability of ANGPTL4 to inhibit LPL (49–55). With the ability of ANGPTL4 to inhibit LPL firmly established, attention has turned to the molecular mechanisms and the physiological roles of this inhibition.
Physiological Roles in Lipid Metabolism
ANGPTL4 clearly influences circulating triglyceride levels. It inhibits LPL (16, 48, 49, 55). ANGPTL4 loss-of-function mutations in humans are associated with lower plasma triglycerides (40, 56–58). ANGPTL4 deficiency in mice also results in lower plasma triglycerides (16, 17, 59) and ANGPTL4 overexpression increases triglycerides (16, 22, 26). However, despite the clear effect of ANGPTL4 on circulating triglycerides and despite the presence of ANGPTL4 in the circulation (26), most evidence suggests that the influence of ANGPTL4 on circulating TGs is a result of it acting locally in the tissue in which it is expressed to control local uptake of triglyceride-derived fatty acids. In fact, several studies have found that vascular LPL, bound to its receptor GPIHBP1, is protected from ANGPTL4 inhibition (51, 52, 60). As this is the context in which circulating ANGPTL4 would encounter LPL, these observations suggest that ANGPTL4 can only effectively inhibit LPL if it does so before it binds to GPIHBP1 on endothelial cells.
The most well characterized example of ANGPTL4 acting locally to control triglyceride partitioning is in adipose tissue during fasting. In 2002, after ANGPTL4 was discovered, but before its characterization as an LPL inhibitor, Bergö et al. (61) found that the decrease in adipose LPL activity that occurs with fasting was mediated by an independent protein that acted on LPL posttranslationally. It is now generally accepted that this protein is ANGPTL4. Angptl4 is highly expressed in adipose tissue (46, 47) and expression in adipose is quickly induced by fasting (16, 46, 62, 63). In adipocytes, ANGPTL4 appears able to inactivate LPL both intracellularly (64, 65) and extracellularly (29, 66). Thus, under fasting conditions, ANGPTL4 would inhibit LPL and reduce triglyceride delivery to adipose tissues. Studies with knockout mice confirm that in the absence of ANGPTL4, LPL activity and TG uptake in adipose increases under fasting conditions (31, 32, 63). In adipose-specific ANGPTL4 knockout mice, LPL activity is increased in adipose tissue but not in other tissues (31, 32), supporting the idea that adipose ANGPTL4 acts almost exclusively in paracrine/autocrine fashion to regulate LPL activity. Recent studies in human subjects confirm that fasting induces ANGPTL4 inhibition of LPL in human adipose as well (67).
Adipose-derived ANGPTL4 also plays a role in the triglyceride partitioning changes that occur with cold exposure. Cold exposure induces large increases in triglyceride uptake into brown adipose tissue (BAT) (68, 69). These increases require LPL activity, suggesting a shift in LPL regulation (68). Angptl4 gene expression decreases in brown adipose tissue in the cold (33, 70, 71). The resulting decrease in ANGPTL4 protein leads to increased LPL activity in BAT, increasing uptake of triglycerides for thermogenic oxidation (71). Cold exposure did not increase LPL activity in white adipose tissue, nor was ANGPTL4 expression decreased (71), indicating that these changes were specific to brown adipose tissue. Consistent with a role for ANGPTL4 in thermogenic triglyceride partitioning, either whole body or BAT-specific deletion of ANGPTL4 resulted in increased LPL activity and triglyceride uptake in BAT (33, 71). It is important to note, however, that modulation of ANGPTL4 expression is not essential for cold-induced thermogenesis or even cold-induced changes in triglyceride partitioning. Dijk et al. (71) found that greater uptake of TGs into BAT was induced by cold both in ANGPTL4-deficient mice and in mice overexpressing ANGPTL4. Dijk et al. (71) also observed that neither whole body knockout of ANGPTL4 nor ANGPTL4 overexpression had a significant effect on body temperature after prolonged cold exposure. Singh et al. (33) found that mice lacking ANGPTL4 specifically in BAT did have slightly higher body temperatures after acute cold exposure, but did not test the effects of prolonged exposure.
ANGPTL4 is also involved in the shifting of metabolic fuel in other tissues and under other conditions. Although Angptl4 is most highly expressed in adipose, it is also expressed in several other tissues including liver, heart, intestine, and skeletal muscle (40, 46, 47, 72). Catoire et al. (73) found that acute exercise increased ANGPTL4 expression in skeletal muscle, but only in nonexercising muscle. Using cell culture models they further found that free fatty acids, which increase in the serum with exercise, induce ANGPTL4 expression in myocytes. However, AMPK activation, as would occur in exercising muscle, counteracted fatty acid induction of ANGPTL4. These data suggested a model in which ANGPTL4 is induced in nonexercising skeletal muscle to prevent lipotoxicity and to divert fuel to exercising muscle where fatty acid oxidation is increased (73).
ANGPTL4 also plays an important role in limiting LPL activity in macrophages. LPL expression is high in macrophages and LPL activity can increase lipid uptake and foam cell formation when macrophages are exposed to triglyceride-rich lipoproteins (74). Strong evidence suggests that ANGPTL4 regulates this activity, blunting lipid uptake into macrophages. ANGPTL4-deficient mice fed a diet high in saturated fat accumulate Touton giant cells, cells that result from the fusion of lipid-laden macrophages, in their mesenteric lymph nodes (34, 59). These mice eventually die, likely as a consequence of the inflammation that results from unfettered lipid uptake into lymphatic macrophages (59). Peritoneal macrophages isolated from ANGPTL4-deficient mice exhibit significantly more lipid uptake and inflammatory cytokine release than those from normal mice when incubated with chyle (chylomicron-containing lymphatic fluid) (59). Treating these cells with either recombinant ANGPTL4 or with LPL inhibitors greatly reduces both lipid uptake and cytokine release, suggesting that ANGPTL4 protects from lipid-induced inflammatory responses (59). microRNA-134m, which suppresses ANGPTL4 expression in macrophages, increases foam cell formation in an LPL-dependent manner (75, 76). ANGPTL4 deficiency specifically in hematopoietic cells also increases both foam cell formation and atherosclerosis (77). Conversely, overexpression of ANGPTL4 in a mouse atherosclerotic model reduces foam cell formation and atherosclerosis (78).
ANGPTL4 may play a role in protecting against lipotoxicity in cardiac tissue as well. In the heart, ANGPTL4 is localized to the surface of cardiomyocytes (79). In mice, overexpression of ANGPTL4 reduces triglyceride uptake into the heart and protects against oxidative stress after either oral fat loading or high-fat diet feeding (27, 79).
ANGPTL4 is also expressed in the intestine (30, 80), where, according to one report, it may regulate fat absorption, possibly by inhibiting pancreatic lipase (81). In the intestine, there also appears to be an interaction between ANGPTL4 and the gut microbiome (30, 82–85). Conventionalizing germ-free mice (colonizing germ-free mice with microorganisms) suppresses intestinal ANGPTL4 expression (30, 83). In some studies, germ-free mice were protected from weight gain, and ANGPTL4 appeared to be necessary for this protection (30, 82). However, in other studies, germ-free mice were not protected from weight gain (83). The microbiota have also been implicated in the improved glucose tolerance observed in ANGPTL4-deficient mice fed a diet high in unsaturated fat (84). Despite these intriguing findings, the interactions between the microbiota and intestinal ANGPTL4 have not been clearly defined, nor have the mechanisms by which modulation of intestinal ANGPTL4 changes metabolic homeostasis been delineated. Future studies, likely employing intestinal-specific ANGPTL4 knockout mice, will be needed.
ANGPTL4 is robustly expressed in the liver in mice, and in humans liver is the tissue with greatest ANGPTL4 expression (86, 87). Yet the role of ANGPTL4 in the liver remains mysterious. Little LPL is expressed in the liver (3–5), and whole body ANGPTL4 deficiency does not alter triglyceride uptake into the liver (63), suggesting that hepatic ANGPTL4 does not target LPL-mediated lipolysis in the liver. Early studies showed that much of liver-derived ANGPTL4 is proteolytically cleaved (88). As proteolytic cleavage is known to increase the ability of ANGPTL4 to inhibit LPL (51, 89, 90), it is possible that cleaved, liver-derived ANGPTL4 is secreted into the circulation and regulates systemic triglyceride metabolism in an endocrine manner. Studies with liver-specific ANGPTL4 knockout mice are likely needed to fully understand the role of ANGPTL4 in this important tissue.
ANGPTL4 deficiency in humans is associated with higher circulating HDL-cholesterol (HDL-C) levels (56, 58, 91, 92), but the mechanism behind this change is not clear. In humans, HDL levels correlate with LPL activity levels (93, 94). Thus, increased HDL levels in ANGPTL4-deficiency individuals could simply be the indirect consequence of increased LPL activity. It is also possible that ANGPTL4 plays a more direct role in regulating HDL levels. ANGPTL4 can be found associated with HDL particles (26, 95), and a study by Yang et al. (95) reported that ANGPTL4 associated with HDL protects HDL phospholipids from hydrolysis by endothelial lipase (EL). However, further investigation is necessary to adequately understand the relationship between ANGPTL4 and HDL metabolism.
Mechanism of Action
The N-terminal coiled-coil domain of ANGPTL4 mediates the formation of oligomers, mostly dimers and tetramers (22, 89, 96). Oligomerization appears to be required for LPL inhibition (22, 89), though the structural basis for this requirement is not known. ANGPTL4 can be cleaved by furin-like proteases, separating the N-terminal coiled-coil domain from the C-terminal fibrinogen-like domain (96). When cleaved, the C-terminal domain dissociates to monomers, but the N-terminal region can remain oligomerized (89, 96). Several studies have shown that not only is the N-terminal region of ANGPTL4 necessary and sufficient for LPL inhibition, but also that, once cleaved from the fibrinogen-like domain, the N-terminal region inhibits LPL more efficiently than full-length ANGPTL4 (49, 51, 72, 89, 90). ANGPTL4 expressed in the liver is cleaved at a much higher rate than ANGPTL4 expressed in the adipose (88), but the physiological role of ANGPTL4 cleavage and how it is regulated are not clear.
The mechanism by which ANGPTL4 inhibits LPL has been the subject to much study and some debate. Several studies have indicated that ANGPTL4 catalytically inactivates LPL (49, 51, 52, 97, 98). For many years, LPL was thought to be active only as a homodimer, and initial studies suggested that ANGPTL4 inactivated LPL by converting active dimers to inactive monomers (49). Recent studies have shown that LPL is catalytically active as a monomer (99), making a dimer-to-monomer mechanism less likely. Indeed, a recent series of studies from the laboratory of Michael Ploug have shown that ANGPTL4 can act on monomeric LPL, and that ANGPTL4 increases the inherent instability of LPL resulting in the unfolding of LPL’s catalytic domain (52, 97, 98). This mechanism has not yet been universally accepted and studies from the laboratory of Saskia Neher have suggested that ANGPTL4 acts as a reversible, uncompetitive inhibitor of LPL (53, 100).
The ability of ANGPTL4 to inhibit LPL is also modulated by the environmental milieu. Vascular LPL is normally bound to its endothelial cell receptor GPIHBP1 (7–9). LPL bound to GPIHBP1 is largely protected from ANGPTL4 (51, 52, 60, 98). LPL is also protected from ANGPTL4 by the presence of fatty acids and lipoproteins (101, 102).
ANGPTL3
Discovery
In 1999, Conklin et al. (103) searched expressed sequence tag (EST) databases for signal sequences and amphipathic helices and found a 460-amino acid protein with the conserved secondary structure of the ANGPTL family (a signal peptide followed by an N-terminal coil-coil domain with a linker leading to a C-terminal fibrinogen-like domain). They found that this protein, which they named ANGPTL3, was expressed almost exclusively in the liver in both mice and humans (103). The role of ANGPTL3 in lipid metabolism was uncovered through a substrain of KK-obese mice. Although KK mice manifest obesity, insulin resistance, and hyperlipidemia (104), this substrain (KK/San) presented with strikingly lower levels of plasma triglycerides (15). Koishi et al. (15) identified a homozygous loss of function mutation in ANGPTL3 as the causal factor. They further showed that overexpression of wild-type ANGPTL3 could increase plasma triglycerides in these mice (15). Soon after, Shimizugawa et al. (105) made a direct connection between ANGPTL3 and LPL, finding that the KK/San mice had increased clearance of triglyceride-rich lipoproteins and showing that ANGPTL3 could inhibit LPL in vitro.
Physiological Roles in Lipid Metabolism
Since its initial discovery, the connection of ANGPTL3 to lipid metabolism has been extensively validated. ANGPTL3 null mice have decreased cholesterol and triglyceride levels and increased LPL activity in postheparin plasma (16, 18, 106). Overexpression or injection of ANGPTL3 into mice increases plasma triglycerides (15, 107). In humans, loss of functional ANGPTL3 protein also leads to decreased triglyceride levels (40, 108–110).
ANGPTL3 is expressed in and secreted from the liver and circulates in the bloodstream (15, 40, 72, 103). The physiological data would therefore suggest that circulating ANGPTL3 encounters and interacts with LPL in the vasculature, inhibiting its lipase activity and reducing clearance of TG-rich lipoprotein particles. In the absence of ANGPTL3, LPL would remain active and clearance of VLDL and chylomicrons would increase. In reality, the action of ANGPTL3 is more nuanced. Wang et al. (106) found that ANGPTL3 deficiency did increase TG-derived fatty acid uptake into heart and skeletal muscle, but that ANGPTL3 deficiency simultaneously decreased uptake into white adipose tissue. Moreover, this effect was observed primarily in the fed state (106). Curiously, although ANGPTL3 expression is regulated by LXR, insulin, leptin, and thyroid hormone (111–114), circulating levels of ANGPTL3 are relatively constant across feeding states (72, 106, 115).
In addition to decreased plasma triglycerides, mice and humans lacking ANGPTL3 also have lower plasma cholesterol levels (16, 18, 106, 108, 109, 116–118). The effect of ANGPTL3 on cholesterol levels is largely attributed to the ability of ANGPTL3 to inhibit endothelial lipase (EL). EL belongs to the same lipase family as LPL, but targets phospholipids on the shell of high-density lipoproteins (HDL), leading to decreased HDL levels (119–122). The relationship between ANGPTL3 and EL was uncovered by Shimamura et al. (123), who found that ANGPTL3 inhibited EL in a dose-dependent manner and that reexpression of ANGPTL3 in ANGPTL3-knockout mice increased plasma HDL-C levels. Subsequent studies confirmed the ability of ANGPTL3 to inhibit EL (124). More recent evidence indicates that EL regulates LDL-cholesterol (LDL-C) levels by hydrolyzing the phospholipids of VLDL remnant particles and reducing their conversion to LDL, suggesting that the lower LDL-C levels observed in ANGPTL3-deficient individuals may also arise from reduced EL inhibition (125, 126). Alternatively, lower LDL-C levels might be mediated by LPL. LPL is known to facilitate the uptake of lipoproteins in the liver (127–130). It is possible that in the absence of ANGPTL3, this nonenzymatic function of LPL might also be increased, thus lowering circulating levels of LDL-C.
Mechanism of Action
ANGPTL3 contains a protease cleavage site, and in vivo can be cleaved into two domains both intracellularly by PCSK3 and extracellularly by PACE4 (107, 124, 131, 132). The N-terminal domain contains the conserved SE1/LPL inhibitory domain (amino acids 32–55), a domain found in ANGPTL3 and ANGPTL4 known to be important for the binding and inhibition of LPL (17). Several studies have identified the N-terminal cleavage product to be necessary and sufficient for lipase inhibition (107, 123, 124). The necessity of proteolytic cleavage remains unclear. In vitro studies of the effect of cleavage on ANGPTL3-mediated inhibition have reported increased inhibition, decreased inhibition, and no effect on inhibition (107, 115, 124). As with ANGPTL4, ANGPTL3 oligomerizes, appearing to form trimers or hexamers, and oligomerization is mediated by the N-terminal domain (72, 100).
Aside from proteolytic cleavage, the ability of ANGPTL3 to inhibit LPL is modulated by a number of environmental factors. LPL’s endothelial cell receptor GPIHBP1 protects LPL from ANGPTL3 inhibition as does heparin (60, 115). The presence of triglyceride-rich lipoproteins also reduces ANGPTL3 inhibition (102).
The mechanism by which ANGPTL3 inhibits EL has not been investigated. The mechanism by which ANGPTL3 inhibits LPL has been studied, but has not yet been identified. Several possible, and somewhat contradictory, mechanisms have been proposed. One proposed mechanism, the dissociation of LPL dimers into inactive monomers (133), seems less likely given recent evidence that LPL is catalytically active as a monomer (99). LPL can be cleaved into an inactive state by furin-like proprotein convertases (134, 135). Liu et al. (132) found that ANGPTL3 increased the cleavage of LPL by PACE4, but not PCKS5. Interestingly, this cleavage was not prevented by the presence of GPIHBP1 or heparan sulfate proteoglycans (HSPGs) (132). Whether the LPL cleavage they observed was the direct mechanism of inhibition or LPL that has already been inhibited by ANGPTL3 is simply more susceptible to cleavage is not clear. Shan et al. (55) found that LPL inhibition by ANGPTL3 was distinct from ANGPTL4-mediated LPL inactivation. They found that ANGPTL3 reduced the catalytic activity of LPL, but did not increase the rate of self-inactivation. They also determined that ANGPTL3 inhibition of LPL was reversible and that heparin protected LPL from inhibition (55). In contrast, Mysling et al. (52) found that, like ANGPTL4, ANGPTL3 could inhibit LPL by unfolding or sterically changing the active domain of LPL, though its potency was far less than that of ANGPTL4. In fact, several studies have reported that ANGPTL3 is a less potent inhibitor than ANGPTL4 (54, 55, 60, 133, 136), and most of the studies showing that ANGPTL3-mediated LPL inhibition in vitro required supraphysiological concentrations of ANGPTL3 to achieve significant inhibition (60, 105, 107, 115). These observations were curious given that ANGPTL3-deficiency lowers plasma TG just as markedly as ANGPTL4 deficiency (16). As discussed below, more recent studies have shown that ANGPTL3 forms of complex with ANGPTL8 and that this complex is most likely the physiologically relevant LPL inhibitor (19, 54, 115, 137, 138). As none of the ANGPTL3 inhibition mechanistic studies discussed here were performed in the presence of ANGPTL8, their significance is unclear.
ANGPTL8
Discovery
ANGPTL8 is the most recently identified member of the ANGPTL family in mammals. In 2012, three groups independently identified ANGPTL8 and published studies indicating its importance in triglyceride metabolism (19, 35, 139). All three studies recognized the homology of ANGPTL8 with other ANGPTL family members especially ANGPTL3. All three studies also found that ANGPTL8 is expressed primarily in the adipose tissue and liver of mice and that expression was much higher in the fed state than in the fasted state. The Smas laboratory named the protein RIFL (refeeding induced fat and liver) based on its expression pattern (139). They reported that mice lacking ANGPTL8 had much lower than normal plasma triglyceride levels and that lipid storage into adipocytes was impaired in the absence of ANGPTL8. The Zhang laboratory first identified ANGPTL8 in an RNA-seq screen looking for nutritionally regulated genes (35). In addition to expression of ANGPTL8 in the adipose and liver of mice, they found that ANGPTL8 was expressed in human liver. Consistent with the decreased plasma TG levels observed in ANGPTL8-deficient mice, they found that overexpression of ANGPTL8 increased plasma TG levels. Moreover, they reported that recombinant ANGPTL8 was able to inhibit LPL activity and thus named the protein lipasin (35). Like the Zhang laboratory, the laboratory of Jonathan Cohen and Helen Hobbs also reported that ANGPTL8 overexpression increased plasma TG levels in mice (19), and also later showed that plasma TG levels were decreased in ANGPTL8-deficient mice (36). They found that similar to mice, ANGPTL8 was expressed in the liver and adipose of humans (19). Importantly, they first reported the interactions of ANGPTL8 with ANGPTL3, a finding that was crucial for the understanding of ANGPTL8 function, as will be discussed below. Based on its homology with other ANGPTL proteins, they named the protein ANGPTL8 and this is the name that has now been adopted in the field. A year after these foundational papers were published, another study renamed ANGPTL8 as betatrophin due to its purported ability to induce β-cell proliferation (140). This finding could not be reproduced (36, 141–143) and the initial manuscript has been retracted. Thus, the name betatrophin should not be used.
Physiological Roles in Lipid Metabolism
ANGPTL8 was initially identified as a regulator of triglyceride metabolism and this remains its clearest and most characterized physiologic function. However, it does not appear to act independently in this role. Instead, ANGPTL8 exerts its effects through its interactions with ANGPTL3 and ANGPTL4.
Interactions with ANGPTL3.
Although ANGPTL8 was initially reported to inhibit LPL on its own (35), later studies found that this not to be the case (115, 137, 138). Instead, ANGPTL8 acts on LPL and on triglyceride metabolism through its interaction with ANGPTL3. ANGPTL8 overexpression only increases plasma triglyceride levels if ANGPTL3 is present (19, 137). ANGPTL8 forms a complex with ANGPTL3 (19, 54, 115, 138) with an apparent stoichiometry of 3 ANGPTL3:1 ANGPTL8 (54). Complex formation is necessary for efficient secretion of ANGPTL8 (115) and appears to require intracellular co-folding, as mixing ANGPTL3 and ANGPTL8 outside the cell does not result in complex formation (115, 138). In vivo, ANGPTL3-ANGPTL8 complexes form in the liver as inferred by the observations that almost all circulating ANGPTL8 comes from the liver (39) and that most circulating ANGPTL8 is complexed with ANGPTL3 (54). Importantly, in vitro several studies have found that the ability of ANGPTL3-ANGPTL8 complexes to inhibit LPL is orders of magnitude better than that of either ANGPTL3 or ANGPTL8 alone (54, 115, 137, 138), suggesting that these complexes are the physiologically relevant inhibitory unit.
The idea that the ANGPTL3-ANGPTL8 complex is the functional unit of LPL inhibition for both ANGPTL3 and ANGPTL8 is supported by several in vivo observations. For example, the necessity of the ANGPTL3-ANGPTL8 complex explains why deletion of either ANGPTL3 or ANGPTL8 results in lower plasma TGs (15, 16, 18, 36, 39, 139), but overexpression of ANGPTL8 in the absence of ANGPTL3, or vice versa, has limited ability to increase plasma triglycerides (19, 115, 137), as absence of either protein would preclude the formation of the complex and limit LPL inhibition. The requirement of the ANGPTL3-ANGPTL8 complex and the strong induction of ANGPTL8 by feeding also explain why disruption of ANGPTL3 primarily effects triglyceride metabolism and lipid partitioning in the fed state despite the fact that ANGPTL3 expression itself is not feeding induced (72, 106).
Recent data suggest that the ANGPTL3-ANGPTL8 functional complex is also the target of the ApoA5. ApoA5 is an atypical apolipoprotein that plays a critical role in regulating plasma triglycerides in mice and humans (144–151). However, the mechanism of its triglyceride-lowering action has remained unknown. Chen et al. (152) recently reported that ApoA5 binds ANGPTL3-ANGPTL8 complexes, but not ANGPTL3, ANGPTL4, or ANGPTL8 alone, and greatly reduces the ability of this complex to inhibit LPL.
The exact mechanism by which the formation of ANGPTL3-ANGPTL8 complexes allows efficient inhibition of LPL is not clear. Although ANGPTL8 was initially reported to promote cleavage of ANGPTL3 (19), follow-up studies showed that this was not the case (36, 39). Part of the increase in LPL inhibition can be attributed to the increased ability of the complex to bind LPL compared with ANGPTL3 alone (54, 115). ANGPTL8 has a domain homologous to the lipase inhibitory domain of ANGPTL3 and ANGPTL4 (137). Haller et al. (137) found that this domain was incapable of inhibiting LPL when ANGPTL8 was alone, but was necessary for LPL inhibition by ANGPTL3-ANGPTL8 complexes. They hypothesized that complex formation unmasks the LPL inhibitory domain of ANGPTL8 allowing ANGPTL3-ANGPTL8 complexes to efficiently inhibit LPL. The precise role of the LPL inhibitory domain in inhibition is not known, so it may be that one of the primary functions of this domain is to facilitate binding to LPL. Thus, unmasking of this domain might also explain why the complex has much higher affinity for LPL than either ANGPTL3 or ANGPTL8 alone. Although it seems likely, based on homology, that ANGPTL3-ANGPTL8 complexes catalytically inactivate LPL in a manner similar to ANGPTL4, such a mechanism has not yet been shown directly.
Interactions with ANGPTL4
Both ANGPTL8 and ANGPTL3 are expressed in the liver, but ANGPTL8 is also expressed in adipose tissue where ANGPTL3 is not expressed (15, 19, 35, 40, 72, 103, 139), suggesting an ANGPTL3-independent role for ANGPTL8. Recently, it has become clear that ANGPTL8 also interacts directly with ANGPTL4, but the consequences of this interaction are quite different from those of ANGPTL3-ANGPTL8 complexes. Like with ANGPTL3, ANGPTL4 can be coimmunoprecipitated with ANGPTL8 in both in vitro and in vivo samples (39, 54, 138), but the stoichiometry of ANGPTL4-ANGPTL8 complexes appears to be 1:1 (54). Unlike the situation with ANGPTL3, where ANGPTL8 greatly enhances LPL inhibition, ANGPTL4-ANGPTL8 complexes are substantially poorer at inhibiting LPL than ANGPTL4 alone (39, 54, 138). These observations suggest that not only is ANGPTL8 unnecessary for ANGPTL4-mediated inhibition, it actively suppresses inhibition by ANGPTL4. The mechanism by which ANGPTL8 suppresses ANGPTL4 inhibition is not yet clear. Oldoni et al. (39) found that ANGPTL8 reduced secretion of ANGPTL4, suggesting that ANGPTL8 reduced ANGPTL4 inhibition simply by reducing levels of ANGPTL4. Chen et al. (54), however, did observe secretion of ANGPTL4-ANGPTL8 complexes and both Chen et al. (54) and Kovrov et al. (138) found that the specific inhibitory activity of ANGPTL4-ANGPTL8 complexes was greatly less than that of ANGPTL4 alone. Interestingly, Chen et al. (54) found that this was not because these complexes could not bind LPL, in fact they bound tightly, but that binding of the complex did not lead to substantial inhibition. Moreover, the binding of ANGPTL4-ANGPTL8 complexes appeared to prevent ANGPTL3-ANGPTL8 complexes and uncomplexed ANGPTL4 from inhibiting LPL (54). The extent to which ANGPTL4-ANGPTL8 complexes regulate LPL activity in vivo remains to be determined. Upon feeding, ANGPTL8 expression is induced, but ANGPTL4 expression is suppressed. The in vivo half-life of ANGPTL4 protein is not known. As ANGPTL4-ANGPTL8 complexes can be immunoprecipitated in vivo (54), there appears to be at least some overlap of ANGPTL4 and ANGPTL8 protein. One possibility is that induction of ANGPTL8 serves to neutralize remaining ANGPTL4 inhibition as the body transitions from fasting to feeding.
REVISITING THE A3-4-8 MODEL OF LPL REGULATION
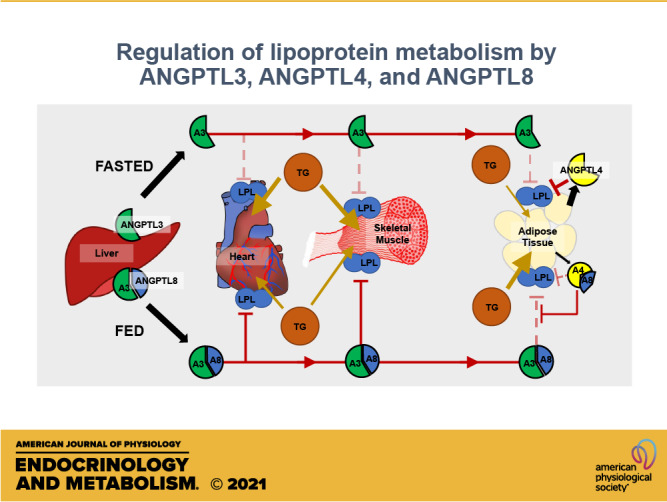
Although ANGPTL3, ANGPTL4, and ANGPTL8 all regulate lipid metabolism, they clearly differ in the location and timing of their expression and their functional activity (summarized in Table 2). In 2016, Ren Zhang (153) outlined what he termed the A3-4-8 model of LPL regulation. In this model, ANGPTL4 is induced by fasting, inhibiting LPL activity locally in adipose tissue, and thus diverting triglycerides to oxidative tissues. Conversely, in the fed state, ANGPTL8 is induced and activates ANGPTL3. Acting as an endocrine factor, ANGPTL3 then inhibits LPL activity in oxidative tissues, thus funneling triglycerides to adipose tissue. Five years later, this model continues to be supported by the data. Moreover, many of the mechanistic and physiological details have come into sharper focus (Fig. 1).
Table 2.
Comparison of ANGPTL3, ANGPTL4, and ANGPTL8
| ANGPTL3 | ANGPTL4 | ANGPTL8 | |
|---|---|---|---|
| Tissue expression | Liver | Widely expressed, particularly high in liver and adipose | Liver Adipose |
| Known targets | EL LPL (w/ANGPTL8) |
LPL PL |
LPL (w/ANGPTL3) |
| Feeding regulation | Not strongly regulated by feeding state | Fasting induced | Feeding induced |
| Likely mode of action | Endocrine | Paracrine/autocrine | Endocrine (liver) Paracrine/autocrine (adipose) |
LPL, lipoprotein lipase.
Figure 1.
The ANGPTL3-4-8 model revisited. In the fasted state (left), ANGPTL4 is expressed in adipose tissue and inhibits lipoprotein lipase (LPL) locally, reducing LPL activity and fatty acid uptake in adipose. ANGPTL3 is secreted by the liver, but has little ability to inhibit LPL on its own. In the fed state (right), both ANGPTL3 and ANGPTL8 are expressed in the liver, forming a complex that is secreted into the circulation and can inhibit LPL in oxidative tissues. In the adipose tissue, ANGPTL4 expression is repressed and ANGPTL8 expression is induced, leading to formation of ANGPTL4-ANGPTL8 complexes that have little ability to inhibit LPL and may also protect LPL from inhibition by circulating ANGPTL3-ANGPTL8 complexes.
Fasted State
As described in detail above, ANGPTL4 in adipose tissue is induced within the first few hours of fasting. ANGPTL4 inhibits LPL by promoting its degradation within adipocytes and/or by inactivating LPL extracellularly. This results in a decrease in LPL activity and reduced adipose uptake of triglyceride-derived fatty acids from the circulation. ANGPTL3 is also expressed during fasting, but without ANGPTL8 it has little ability to inhibit LPL. Expression of ANGPTL8 under fasting conditions is limited. Thus, few ANGPTL3-ANGPTL8 complexes are secreted into the circulation. In the absence of ANGPTL3-ANGPTL8 complexes, LPL is not inhibited in oxidative tissues such as heart and skeletal muscle. The combination of ANGPTL4 inhibition in adipose tissue and the lack of systemic LPL inhibition by ANGPTL3-ANGPTL8 complexes results in the preferential uptake of triglyceride-derived fatty acid fuel in heart and skeletal muscle where it can be used to fuel, allowing the organism to continue to function in the fasted state. It is important to note that although triglyceride delivery to adipose tissue is slowed during fasting, it does not stop.
Fed State
In the fed state, ANGPTL8 is induced both in the liver and in the adipose tissue. In liver, ANGPTL8 forms a complex with ANGPTL3 and this complex is secreted into the circulation as an endocrine factor. Circulating ANGPTL3-ANGPTL8 complexes inhibit LPL in oxidative tissues, such as heart and skeletal muscle, blunting triglyceride uptake into these tissues and thus allowing more uptake into adipose tissue. Circulating ANGPTL3-ANGPTL8 complexes could potentially inhibit LPL in adipose tissue as well, however, there is a concurrent reduction in ANGPTL4-mediated inhibition that results in a net increase in LPL activity in adipose in the fed state. This reduction in ANGPTL4 inhibition is mediated both by a decrease in ANGPTL4 expression and by an increase in adipose ANGPTL8 expression. The induction of ANGPTL8 expression results in the formation of ANGPTL4-ANGPTL8 complexes, which greatly reduce the ability of ANGPTL4 to inhibit LPL. Moreover, ANGPTL4-ANGPTL8 complexes, which retain the ability to bind LPL, appear to protect LPL from inhibition by the much more potent ANGPTL3-ANGPTL8 complexes. In combination, these events increase LPL activity in adipose tissue, allowing increased partitioning of triglyceride-derived fatty acids to adipose tissue for storage. The tissue-specific roles of ANGPTL8 outlined by this model are clearly apparent in adipose- and liver-specific ANGPTL8 knockout mice. Oldoni et al. (39) found that mice lacking liver ANGPTL8 had significantly lower plasma triglycerides than wild-type mice in the fed state, consistent with the absence of the inhibitory ANGPTL3-ANGPTL8 complexes that normally form in the liver. Conversely, in the absence of adipose ANGPTL8, fed-state plasma triglycerides were elevated compared with wild-type mice, consistent with the ability of adipose ANGPTL8 to diminish ANGPTL4 and/or ANGPTL3-ANGPTL8 inhibition in fat depots (39).
Although the model as a whole has come into greater focus, there are still details that remain obscure. For example, fasting induces ANGPTL4 expression in tissues other than adipose, including liver and some oxidative tissues (40, 46, 47, 72). Unlike the situation in adipose tissue, lack of ANGPTL4 does not appear to increase triglyceride partitioning to these other tissues under fasting conditions (63), and the physiological significance of ANGPTL4 induction in these tissues during fasting remains unclear. The C-terminal fibrinogen-like domain of ANGPTL4 has been shown to stimulate adipose tissue lipolysis (25). Therefore, it is possible that fasting induction of ANGPTL4 in tissues like liver serves to increase mobilization of lipid stores. The molecular mechanism by which ANGPTL proteins interact with one another and with lipoprotein lipase remain a work in progress. Understanding these mechanisms and the physiological timeline over which these interactions occur will provide additional insights into feeding-state induced shifts in LPL activity and fat partitioning.
Thermogenesis
LPL-mediated shifts in fat partitioning are also important for nonshivering thermogenesis. With cold exposure, large amounts of triglyceride are funneled to brown adipose tissue in an LPL-dependent manner (68, 69, 71). Although there are many gaps in our understanding of this process, a model for how ANGPTL4, ANGPTL8, and ANGPTL3 participate in facilitating thermogenesis is beginning to take shape. With prolonged cold exposure, expression of ANGPTL4 decreases in BAT and increases in WAT (33, 70, 71). In BAT, this results in increased LPL activity in BAT, increasing uptake of triglycerides for thermogenic oxidation (71). At the same time, ANGPTL8 expression is induced in the brown adipose tissue during cold exposure (70). This would increase ANGPTL4-ANGPTL8 complex formation, further reducing ANGPTL4 inhibition and increasing LPL activity in BAT. Chen et al. (154) showed that ANGPTL4-ANGPTL8 complexes may actually stimulate LPL activity at 22°C. However, the relevance of this observation to thermogenesis is not clear as it seems unlikely that temperatures in BAT ever drop this low. ANGPTL3 likely also plays some role in thermogenesis, as ANGPTL3-ANGPTL8 double knockout mice have higher body temperatures and appear to be more sensitive to sympathetic stimulation than wild-type mice (21). However, the mechanisms and pathways by which ANGPTL3 contributes to thermogenesis have not been elucidated.
It is important to note that ANGPTL proteins are likely not the primary driver of the alterations in lipid partitioning that occur during thermogenesis. The alterations in LPL activity and lipid partitioning observed in ANGPTL4 knockout mice and mice overexpressing ANGPTL4 only occurred after sustained cold exposure, and even then, the cold-induced increase of triglyceride uptake in BAT still occurred even when ANGPTL4 was absent or when it was overexpressed (71). Moreover, even though cold exposure increased ANGPTL4 expression in white adipose tissue, the partitioning of triglycerides to these depots did not appear to decrease (71).
Chronic High-Fat Diet and Obesity
Chronic overfeeding and obesity can lead to shifts in fat partitioning, ectopic lipid deposition, and metabolic disease (155–157). Surprisingly, despite the importance of altered lipid partitioning to the onset of metabolic disease, the contribution of ANGPTL proteins to these obesity-induced alterations have not been well characterized. The ANGPTL-regulated shifts in triglyceride partitioning for feeding and fasting and for thermogenesis have been delineated in nonobese, chow-fed mice. Whether the same shifts occur in the setting of obesity is not clear. As noted above, early experiments testing high-fat feeding in ANGPTL4-deficient mice were hampered by the lethal chylous ascites, intestinal injury, and lymphatic nodule inflammation induced by high-fat feeding in these mice (59). These lethal phenotypes can be avoided with a diet high in unsaturated fatty acids (158). Using such a diet, Janssen et al. (84) found that ANGPTL4-deficient mice gained more weight but were more glucose tolerant than wild-type mice. This finding is consistent with the observation that overexpression of ANGPTL4 in mice impaired glucose intolerance (26). These studies suggested that with high-fat feeding, ANGPTL4 would normally direct triglycerides away from adipose, increasing ectopic lipid deposition and glucose intolerance. More recent studies with tissue-specific ANGPTL4 knockout mice have provided additional insights. Two independent studies found that when high-fat diet was fed to mice without adipose ANGPTL4, these mice had increased adipose triglyceride deposition and improved glucose tolerance compared with wild-type mice (31, 32). Interestingly, both studies found that this effect was lost with chronic (5+ mo) of high-fat feeding (31, 32). Interestingly, even though increased triglyceride uptake in adipose disappeared with chronic high-fat feeding, adipose LPL activity remained higher in adipose-specific ANGPTL4 knockout mice, suggesting that after chronic high-fat feeding, LPL activity is no longer the limiting factor for triglyceride deposition in adipose (32). How the presence or absence of ANGPTL3 or ANGPTL8 effects fat partitioning after chronic high-fat feeding has not been examined but may provide key insights into the progression of ectopic lipid deposition and metabolic disease.
ANGPTL PROTEINS IN DISEASE AND DISEASE TREATMENT
Metabolic diseases such as obesity, metabolic syndrome, and type 2 diabetes mellitus often present with elevated plasma triglycerides and elevated triglyceride levels represent a cardiovascular risk factor (159–162). Recent human genetic evidence, including data from individuals with ANGPTL deficiency, has strengthened the idea that lowering plasma triglycerides could be beneficial for treating dyslipidemia and cardiovascular disease. Like mice, humans deficient in ANGPTL3, ANGPTL4, or ANGPTL8 have lower plasma triglycerides (56–58, 163–166). In the case of ANGPTL3, loss of ANGPTL3 results not only in hypotriglyceridemia, but in familial combined hypolipidemia (108, 109, 116–118). Importantly, multiple studies found that individuals deficient in one of these three ANGPTL proteins have a lower risk of cardiovascular disease (56, 57, 163–166). Interestingly, there is evidence that loss of ANGPTL function can also improve glucose homeostasis (110, 163, 167).
The protective effects of ANGPTL loss of function mutations has prompted studies into whether targeting these proteins could be used to treat dyslipidemia and metabolic disease. Preclinical studies using antibodies or antisense oligos showed that targeting ANGPTL3, ANGPTL4, or ANGPTL8 can lower plasma triglycerides (29, 56, 60, 165, 168–172). Unfortunately, targeting ANGPTL4 with an anti-ANGPTL4 antibody in mice fed a high-fat diet led to intestinal abnormalities, chylous ascites, lymph node inflammation, and premature death (29), consistent with observations for ANGPTL4-deficient mice fed a high-fat diet (29, 59). Lymph node inflammation was also observed in cynomolgus monkeys treated with a monoclonal antibody against ANGPTL4 (56).
Similar detrimental effects have not been observed in preclinical studies targeting ANGPTL3. In fact, an Italian town known for the longevity of its residents was found to have an unusually large number of individuals with the S17X loss-of-function ANGPTL3 mutation (108, 118, 173). Therefore, ANGPTL3 has become a key target for drug therapies that treat dyslipidemia. In particular, two targeting approaches, monoclonal antibodies and antisense oligonucleotides, have shown therapeutic promise.
In initial studies, Evinacumab, a human anti-ANGPTL3 monoclonal antibody, lowered triglyceride levels by 76%, LDL-cholesterol by 23.2%, and HDL-cholesterol by 18.4% in healthy individuals (165). Further studies confirmed the ability of Evinacumab to lower plasma triglyceride levels in healthy individuals (174), hypertriglyceridemic subjects (175), and patients with familial hypercholesterolemia (176–178). As the ANGPTL3-ANGPTL8 complex is the functional unit of LPL inhibition, Evinacumab likely acts to lower plasma triglycerides by binding the ANGPTL3 of ANGPTL3-ANGPTL8 complexes. Indeed, Jin et al. (179) showed that Evinacumab could robustly block inhibition by ANGPTL3-ANGPTL8 complexes. Similar to ANGPTL3-deficient individuals (discussed above in ANGPTL3, Physiological Roles in Lipid Metabolism), Evinacumab may lower plasma LDL-C levels either by preventing inhibition of EL by ANGPTL3 (125) or by preventing inhibition of LPL by ANGPTL3-ANGPTL8 complexes (54). In February 2021, Evinacumab, under the trade name Evkeeza, was approved by the Food and Drug Administration for the treatment of patients with homozygous familial hypercholesterolemia.
Vupanorsen, an antisense oligonucleotide designed to prevent the expression of ANGPTL3 in hepatocytes, represents an independent approach for therapeutically targeting ANGPTL3. In initial studies, treatment with Vupanorsen was associated with a 33%–63% decrease in triglycerides as well as reductions in Apo-C III, remnant and total cholesterol, HDL-C, and LDL-C (169). Vupanorsen also lowered plasma triglycerides in patients with diabetes, hepatic steatosis, and hypertriglyceridemia (180). Although these results are promising, it remains to be seen if targeting ANGPTL3 can improve cardiovascular outcomes.
The cardiovascular protection and the lack of deleterious effects associated with ANGPTL3 deficiency (40, 108, 163, 165, 166) have led to the proposal of a third, more radical method of targeting ANGPTL3 in humans, gene editing (20, 181, 182). Chadwick et al. (20) used CRISPR-mediated base editing to induce ANGPTL3 deficiency in 5-wk-old mice and found that this gene editing reduced ANGPTL3 expression, plasma triglycerides, and total cholesterol levels in both normal and hyperlipidemic mice. However, the bioethics of gene editing in humans remains controversial and both the ability to successfully gene edit ANGPTL3 in adult humans and the ability of ANGPTL3 gene editing to improve cardiovascular outcomes without side effects remain unproven.
The therapeutic benefit of targeting ANGPTL4 and ANGPTL8 remains to be determined. Given the detrimental effects observed in mice and monkeys, targeting ANGPTL4 in humans certainly warrants caution, but it should be noted that thus far these detrimental effects have not been reported in humans with ANGPTL4 deficiency. If targeting ANGPTL4 or ANGPTL8 does prove safe, it is possible that the efficacy in combating metabolic disease that comes from targeting these proteins could be quite different. Lowering ANGPTL3, ANGPTL4, or ANGPTL8 would each be expected to lower plasma triglycerides, but as these proteins act at different times and at different places, systemic effects could vary substantially. For example, targeting ANGPTL4 could potentially increase adiposity and increase glucose tolerance, as it does in mice (31–33, 84), an effect that might not be observed when targeting ANGPTL3 or ANGPTL8.
OPEN QUESTIONS
Despite the rapid progress in ANGPTL research, there is much about their function, their interactions with each other, and their physiological roles that remain unknown. Some of these open questions and how they might be addressed are discussed below.
Many questions regarding the structure and mechanisms of ANGPTL interactions remain unanswered. Although the mechanism of ANGPTL4-mediated LPL inhibition is coming into focus (52, 97, 98), the mechanism by which ANGPTL3-ANGPTL8 complexes inhibit LPL remains to be elucidated. One recent study suggested that ANGPTL3-ANGPTL8 complexes promote furin-mediated cleavage of LPL, but the levels of cleavage observed were not sufficient to explain the level of LPL inhibition (179). The structural basis for ANGPTL8’s interaction with ANGPTL3 and ANGPTL4 is not known, nor is it known why ANGPTL8 has opposite effects on ANGPTL3 and ANGPTL4. Such studies would likely be aided by solved protein structures. However, only the C-terminal fibrinogen domains of ANGPTL3 and ANGPTL4 have been solved (183), leaving the structure of the more relevant N-terminal LPL interacting domains unknown.
The ANGPTL3-4-8 model outlined above details how the interactions between ANGPTL3, ANGPTL4, and ANGPTL8 modulate fat partitioning according to feeding state. There are, however, many more metabolic states that alter fat delivery and could potentially be regulated by ANGPTL proteins. Two of these, cold-induced thermogenesis and high-fat feeding were discussed above. Other states include exercise, starvation, and chronic inflammation. One physiological state that deserves far more attention is age. Triglyceride metabolism changes with age, but the mechanisms behind these changes are largely unknown (reviewed in Ref. 184). The studies that form the basis of the ANGPTL3-4-8 model were largely performed in young mice. However, the risk of metabolic disease increases with age, especially in connection with chronic high-fat feeding. Studies in adipose-specific ANGPTL4-deficient mice, wherein some of the benefits of ANGPTL4 deficiency were lost with age and high-fat feeding (31, 32), highlight the importance of investigating the intersection of age and diet. It is likely that ANGPTL proteins both contribute to and are affected by age-induced metabolic changes.
It is worth noting that despite the profound effects that ANGPTL3, ANGPTL4, and ANGPTL8 have on circulating lipids, deficiency for these proteins has only minor effects on body weight, particularly in animals fed a normal chow diet. In some studies, ANGPTL8-deficient mice fed normal had lower body weights than wild-type littermates (36), but ANGPTL3- and ANGPTL4-deficient mice fed normal chow appear to have normal body weights (16). This is not necessarily unexpected. Even strong disruptions of plasma triglyceride clearance and uptake do not necessarily impact body weight, especially on diets that are not high in fat. For example, GPIHBP1-deficient mice exhibit severe hypertriglyceridemia and have very little LPL-mediated triglyceride uptake (8). Yet, these mice have normal body weights on normal chow diets (185). Why profound changes in circulating triglycerides do not translate to similarly profound changes in body composition is not clear? Likely, compensatory mechanisms help maintain gross body composition. However, additional metabolic challenges may make underlying partitioning defects more apparent. For example, when ANGPTL4 knockout mice are fed a high-fat diet, they gain significantly more weight and adiposity than wild-type littermates (59, 84). Increased study of ANGPTL proteins in the context of different metabolic challenges, including disease states will help unravel the physiological consequences of misregulated triglyceride delivery.
Although this review has largely focused on ANGPTL regulation of lipoprotein lipase, other lipases are also targeted by ANGPTL proteins. ANGPTL4 may inhibit pancreatic lipase (81) and there are conflicting reports of its ability to inhibit hepatic lipase (16, 50). ANGPTL3 is a known inhibitor of endothelial lipase (123, 124). The ability of ANGPTL8 to alter ANGPTL3- or ANGPTL4-mediated inhibition of these other lipases is not yet known. As endothelial lipase, pancreatic lipase, and hepatic lipase all contribute to plasma lipoprotein metabolism, a complete understanding of ANGPTL3, ANGPTL4, and ANGPTL8 in metabolic homeostasis will require incorporating other lipase targets into the ANGPTL3-4-8 model.
In summary, ANGPTL3, ANGPTL4, and ANGPTL8 have emerged as critical regulators of lipid homeostasis and promising therapeutic targets. Investigating their critical role in metabolic health and disease and their interactions with each other continues to produce important insights into the regulation of lipid partitioning and lipid homeostasis.
GRANTS
This work was supported by grants from the National Institutes of Health [R01HL130146 and R01HL134787 (to B.S.J.D.)].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.S.J.D. prepared figures; K.L.S-D. and B.S.J.D. drafted manuscript; K.L.S-D. and B.S.J.D. edited and revised manuscript; K.L.S-D. and B.S.J.D. approved final version of manuscript.
REFERENCES
- 1.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab 297: E271–E288, 2009. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 2.Hamosh M, Clary TR, Chernick SS, Scow RO. Lipoprotein lipase activity of adipose and mammary tissue and plasma triglyceride in pregnant and lactating rats. Biochim Biophys Acta 210: 473–482, 1970. doi: 10.1016/0005-2760(70)90044-5. [DOI] [PubMed] [Google Scholar]
- 3.Olafsen T, Young SG, Davies BSJ, Beigneux AP, Kenanova VE, Voss C, Young G, Wong K-P, Barnes RH, Tu Y, Weinstein MM, Nobumori C, Huang S-C, Goldberg IJ, Bensadoun A, Wu AM, Fong LG. Unexpected expression pattern for glycosylphosphatidylinositol-anchored HDL-binding protein 1 (GPIHBP1) in mouse tissues revealed by positron emission tomography scanning. J Biol Chem 285: 39239–39248, 2010. doi: 10.1074/jbc.M110.171041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenkovich CF, Chen SH, Wims M, Luo CC, Li WH, Chan L. Lipoprotein lipase and hepatic lipase mRNA tissue specific expression, developmental regulation, and evolution. J Lipid Res 30: 423–431, 1989. doi: 10.1016/S0022-2275(20)38369-3. [DOI] [PubMed] [Google Scholar]
- 5.The Human Protein Atlas (Online). https://www.proteinatlas.org/ [2020Jul 29].
- 6.Camps L, Reina M, Llobera M, Vilaró S, Olivecrona T. Lipoprotein lipase: cellular origin and functional distribution. Am J Physiol Cell Physiol 258: C673–C681, 1990. doi: 10.1152/ajpcell.1990.258.4.C673. [DOI] [PubMed] [Google Scholar]
- 7.Davies BSJ, Beigneux AP, Barnes RH 2nd, Tu Y, Gin P, Weinstein MM, Nobumori C, Nyrén R, Goldberg I, Olivecrona G, Bensadoun A, Young SG, Fong LG. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab 12: 42–52, 2010. doi: 10.1016/j.cmet.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beigneux AP, Davies BSJ, Gin P, Weinstein MM, Farber E, Qiao X, Peale F, Bunting S, Walzem RL, Wong JS, Blaner WS, Ding Z-M, Melford K, Wongsiriroj N, Shu X, Sauvage F. D, Ryan RO, Fong LG, Bensadoun A, Young SG. Glycosylphosphatidylinositol-anchored high density lipoprotein–binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab 5: 279–291, 2007. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goulbourne CN, Gin P, Tatar A, Nobumori C, Hoenger A, Jiang H, Grovenor CRM, Adeyo O, Esko JD, Goldberg IJ, Reue K, Tontonoz P, Bensadoun A, Beigneux AP, Young SG, Fong LG. The GPIHBP1-LPL complex is responsible for the margination of triglyceride-rich lipoproteins in capillaries. Cell Metab 19: 849–860, 2014. doi: 10.1016/j.cmet.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstock PH, Bisgaier CL, Aalto-Setälä K, Radner H, Ramakrishnan R, Levak-Frank S, Essenburg AD, Zechner R, Breslow JL. Severe hypertriglyceridemia, reduced high density lipoprotein, and neonatal death in lipoprotein lipase knockout mice. Mild hypertriglyceridemia with impaired very low density lipoprotein clearance in heterozygotes. J Clin Invest 96: 2555–2568, 1995. doi: 10.1172/JCI118319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reina M, Brunzell JD, Deeb SS. Molecular basis of familial chylomicronemia: mutations in the lipoprotein lipase and apolipoprotein C-II genes. J Lipid Res 33: 1823–1832, 1992. doi: 10.1016/S0022-2275(20)41340-9. [DOI] [PubMed] [Google Scholar]
- 12.Oike Y, Yasunaga K, Suda T. Angiopoietin-related/angiopoietin-like proteins regulate angiogenesis. Int J Hematol 80: 21–28, 2004. doi: 10.1532/IJH97.04034. [DOI] [PubMed] [Google Scholar]
- 13.Santulli G. Angiopoietin-like proteins: a comprehensive look. Front Endocrinol (Lausanne) 5: 4, 2014. doi: 10.3389/fendo.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim I, Kwak HJ, Ahn JE, So J-N, Liu M, Koh KN, Koh GY. Molecular cloning and characterization of a novel angiopoietin family protein, angiopoietin-3. FEBS Lett 443: 353–356, 1999. doi: 10.1016/s0014-5793(99)00008-3. [DOI] [PubMed] [Google Scholar]
- 15.Koishi R, Ando Y, Ono M, Shimamura M, Yasumo H, Fujiwara T, Horikoshi H, Furukawa H. Angptl3 regulates lipid metabolism in mice. Nat Genet 30: 151–157, 2002. doi: 10.1038/ng814. [DOI] [PubMed] [Google Scholar]
- 16.Köster A, Chao YB, Mosior M, Ford A, Gonzalez-DeWhitt PA, Hale JE, Li D, Qiu Y, Fraser CC, Yang DD, Heuer JG, Jaskunas SR, Eacho P. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology 146: 4943–4950, 2005. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 17.Lee E-C, Desai U, Gololobov G, Hong S, Feng X, Yu X-C, Gay J, Wilganowski N, Gao C, Du L-L, Chen J, Hu Y, Zhao S, Kirkpatrick L, Schneider M, Zambrowicz BP, Landes G, Powell DR, Sonnenburg WK. Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL). J Biol Chem 284: 13735–13745, 2009. doi: 10.1074/jbc.M807899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimoto K, Koishi R, Shimizugawa T, Ando Y. Angptl3-null mice show low plasma lipid concentrations by enhanced lipoprotein lipase activity. Exp Anim 55: 27–34, 2006. doi: 10.1538/expanim.55.27. [DOI] [PubMed] [Google Scholar]
- 19.Quagliarini F, Wang Y, Kozlitina J, Grishin NV, Hyde R, Boerwinkle E, Valenzuela DM, Murphy AJ, Cohen JC, Hobbs HH. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc Natl Acad Sci USA 109: 19751–19756, 2012. doi: 10.1073/pnas.1217552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chadwick AC, Evitt NH, Lv W, Musunuru K. Reduced blood lipid levels with in vivo CRISPR-Cas9 base editing of ANGPTL3. Circulation 137: 975–977, 2018. doi: 10.1161/CIRCULATIONAHA.117.031335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banfi S, Gusarova V, Gromada J, Cohen JC, Hobbs HH. Increased thermogenesis by a noncanonical pathway in ANGPTL3/8-deficient mice. Proc Natl Acad Sci USA 115: E1249–E1258, 2018. doi: 10.1073/pnas.1717420115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge H, Yang G, Yu X, Pourbahrami T, Li C. Oligomerization state-dependent hyperlipidemic effect of angiopoietin-like protein 4. J Lipid Res 45: 2071–2079, 2004. doi: 10.1194/jlr.M400138-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Xu A, Lam MC, Chan KW, Wang Y, Zhang J, Hoo RLC, Xu JY, Chen B, Chow W-S, Tso AWK, Lam KSL. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc Natl Acad Sci USA 102: 6086–6091, 2005. doi: 10.1073/pnas.0408452102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Liu L-M, Wei L, Ye W-W, Meng X-Y, Chen F, Xiao Q, Chen J-Y, Zhou Y. Angiopoietin-like protein 4 improves glucose tolerance and insulin resistance but induces liver steatosis in high-fat-diet mice. Mol Med Rep 14: 3293–3300, 2016. doi: 10.3892/mmr.2016.5637. [DOI] [PubMed] [Google Scholar]
- 25.McQueen AE, Kanamaluru D, Yan K, Gray NE, Wu L, Li M-L, Chang A, Hasan A, Stifler D, Koliwad SK, Wang J-C. The C-terminal fibrinogen-like domain of angiopoietin-like 4 stimulates adipose tissue lipolysis and promotes energy expenditure. J Biol Chem 292: 16122–16134, 2017. doi: 10.1074/jbc.M117.803973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandard S, Zandbergen F, Straten E. V, Wahli W, Kuipers F, Müller M, Kersten S. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J Biol Chem 281: 934–944, 2006. [Erratum in J Biol Chem 281: 21575, 2006]. doi: 10.1074/jbc.M506519200. [DOI] [PubMed] [Google Scholar]
- 27.Yu X, Burgess SC, Ge H, Wong KK, Nassem RH, Garry DJ, Sherry AD, Malloy CR, Berger JP, Li C. Inhibition of cardiac lipoprotein utilization by transgenic overexpression of Angptl4 in the heart. Proc Natl Acad Sci USA 102: 1767–1772, 2005. doi: 10.1073/pnas.0409564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clement LC, Avila-Casado C, Macé C, Soria E, Bakker WW, Kersten S, Chugh SS. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med 17: 117–122, 2011. doi: 10.1038/nm.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desai U, Lee E-C, Chung K, Gao C, Gay J, Key B, Hansen G, Machajewski D, Platt KA, Sands AT, Schneider M, Van Sligtenhorst I, Suwanichkul A, Vogel P, Wilganowski N, Wingert J, Zambrowicz BP, Landes G, Powell DR. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci USA 104: 11766–11771, 2007. doi: 10.1073/pnas.0705041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101: 15718–15723, 2004. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aryal B, Singh AK, Zhang X, Varela L, Rotllan N, Goedeke L, Chaube B, Camporez J-P, Vatner DF, Horvath TL, Shulman GI, Suárez Y, Fernández-Hernando C. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis. JCI Insight 3: e97918, 2018. doi: 10.1172/jci.insight.97918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spitler KM, Shetty SK, Cushing EM, Sylvers-Davie KL, Davies BSJ. Regulation of plasma triglyceride partitioning by adipose-derived ANGPTL4 in mice. Sci Rep 11: 7873, 2021. doi: 10.1038/s41598-021-87020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh AK, Aryal B, Chaube B, Rotllan N, Varela L, Horvath TL, Suárez Y, Fernández-Hernando C. Brown adipose tissue derived ANGPTL4 controls glucose and lipid metabolism and regulates thermogenesis. Mol Metab 11: 59–69, 2018. doi: 10.1016/j.molmet.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oteng A-B, Ruppert PMM, Boutens L, Dijk W, van Dierendonck XAMH, Olivecrona G, Stienstra R, Kersten S. Characterization of ANGPTL4 function in macrophages and adipocytes using Angptl4-knockout and Angptl4-hypomorphic mice. J Lipid Res 60: 1741–1754, 2019. doi: 10.1194/jlr.M094128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels . Biochem Biophys Res Commun 424: 786–792, 2012. doi: 10.1016/j.bbrc.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Quagliarini F, Gusarova V, Gromada J, Valenzuela DM, Cohen JC, Hobbs HH. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc Natl Acad Sci USA 110: 16109–16114, 2013. doi: 10.1073/pnas.1315292110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang T, Li L, Tang J, Li Y, Lin WY, Martin F, Grant D, Solloway M, Parker L, Ye W, Forrest W, Ghilardi N, Oravecz T, Platt KA, Rice DS, Hansen GM, Abuin A, Eberhart DE, Godowski P, Holt KH, Peterson A, Zambrowicz BP, de Sauvage FJ. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol 28: 749–755, 2010. doi: 10.1038/nbt.1644. [DOI] [PubMed] [Google Scholar]
- 38.Izumi R, Kusakabe T, Noguchi M, Iwakura H, Tanaka T, Miyazawa T, Aotani D, Hosoda K, Kangawa K, Nakao K. CRISPR/Cas9-mediated Angptl8 knockout suppresses plasma triglyceride concentrations and adiposity in rats. J Lipid Res 59: 1575–1585, 2018. doi: 10.1194/jlr.M082099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oldoni F, Cheng H, Banfi S, Gusarova V, Cohen JC, Hobbs HH. ANGPTL8 has both endocrine and autocrine effects on substrate utilization. JCI Insight 5: e138777, 2020. doi: 10.1172/jci.insight.138777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romeo S, Yin W, Kozlitina J, Pennacchio LA, Boerwinkle E, Hobbs HH, Cohen JC. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J Clin Invest 119: 70–79, 2009. doi: 10.1172/JCI37118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oike Y, Akao M, Yasunaga K, Yamauchi T, Morisada T, Ito Y, Urano T, Kimura Y, Kubota Y, Maekawa H, Miyamoto T, Miyata K, Matsumoto S, Sakai J, Nakagata N, Takeya M, Koseki H, Ogawa Y, Kadowaki T, Suda T. Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat Med 11: 400–408, 2005. doi: 10.1038/nm1214. [DOI] [PubMed] [Google Scholar]
- 42.Carbone C, Piro G, Merz V, Simionato F, Santoro R, Zecchetto C, Tortora G, Melisi D. Angiopoietin-like proteins in angiogenesis, inflammation and cancer. Int J Mol Sci 19: 431, 2018. doi: 10.3390/ijms19020431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hato T, Tabata M, Oike Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc Med 18: 6–14, 2008. doi: 10.1016/j.tcm.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Kadomatsu T, Oike Y. Roles of angiopoietin-like proteins in regulation of stem cell activity. J Biochem 165: 309–315, 2019. doi: 10.1093/jb/mvz005. [DOI] [PubMed] [Google Scholar]
- 45.Navaeian M, Asadian S, Ahmadpour Yazdi H, Gheibi N. ANGPTL8 roles in proliferation, metabolic diseases, hypothyroidism, polycystic ovary syndrome, and signaling pathways. Mol Biol Rep 48: 3719–3731, 2021. doi: 10.1007/s11033-021-06270-8. [DOI] [PubMed] [Google Scholar]
- 46.Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem 275: 28488–28493, 2000. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 47.Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, Friedman JM, Holmes WE, Spiegelman BM. Peroxisome proliferator-activated receptor γ target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol 20: 5343–5349, 2000. doi: 10.1128/MCB.20.14.5343-5349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida K, Shimizugawa T, Ono M, Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J Lipid Res 43: 1770–1772, 2002. doi: 10.1194/jlr.c200010-jlr200. [DOI] [PubMed] [Google Scholar]
- 49.Sukonina V, Lookene A, Olivecrona T, Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci USA 103: 17450–17455, 2006. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lichtenstein L, Berbée JFP, Dijk S. V, Dijk K. V, Bensadoun A, Kema IP, Voshol PJ, Müller M, Rensen PCN, Kersten S. Angptl4 upregulates cholesterol synthesis in liver via inhibition of LPL- and HL-dependent hepatic cholesterol uptake. Arterioscler Thromb Vasc Biol 27: 2420–2427, 2007. doi: 10.1161/ATVBAHA.107.151894. [DOI] [PubMed] [Google Scholar]
- 51.Chi X, Shetty SK, Shows HW, Hjelmaas AJ, Malcolm EK, Davies BSJ. Angiopoietin-like 4 modifies the interactions between lipoprotein lipase and its endothelial cell transporter GPIHBP1. J Biol Chem 290: 11865–11877, 2015. doi: 10.1074/jbc.M114.623769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mysling S, Kristensen KK, Larsson M, Kovrov O, Bensadouen A, Jørgensen TJ, Olivecrona G, Young SG, Ploug M. The angiopoietin-like protein ANGPTL4 catalyzes unfolding of the hydrolase domain in lipoprotein lipase and the endothelial membrane protein GPIHBP1 counteracts this unfolding. Elife 5: e20958, 2016. doi: 10.7554/eLife.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lafferty MJ, Bradford KC, Erie DA, Neher SB. Angiopoietin-like protein 4 inhibition of lipoprotein lipase: evidence for reversible complex formation. J Biol Chem 288: 28524–28534, 2013. doi: 10.1074/jbc.M113.497602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen YQ, Pottanat TG, Siegel RW, Ehsani M, Qian Y-W, Zhen EY, Regmi A, Roell WC, Guo H, Luo MJ, Gimeno RE, Van't Hooft F, Konrad RJ. Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids. J Lipid Res 61: 1203–1220, 2020. doi: 10.1194/jlr.RA120000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shan L, Yu X-C, Liu Z, Hu Y, Sturgis LT, Miranda ML, Liu Q. The angiopoietin-like proteins ANGPTL3 and ANGPTL4 inhibit lipoprotein lipase activity through distinct mechanisms. J Biol Chem 284: 1419–1424, 2009. doi: 10.1074/jbc.M808477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dewey FE, Gusarova V, O’Dushlaine C, Gottesman O, Trejos J, Hunt C, Van Hout CV, Habegger L, Buckler D, Lai K-MV, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Ledbetter DH, Penn J, Lopez A, Borecki IB, Overton JD, Reid JG, Carey DJ, Murphy AJ, Yancopoulos GD, Baras A, Gromada J, Shuldiner AR. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med 374: 1123–1133, 2016. doi: 10.1056/NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stitziel NO, Stirrups KE, Masca NGD, Erdmann J, Ferrario PG, König IR, et al. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med 374: 1134–1144, 2016. [Erratum in N Engl J Med 374: 1134–1144, 2016]. doi: 10.1056/NEJMoa1507652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjaerg-Hansen A, Hobbs HH, Cohen JC. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet 39: 513–516, 2007. doi: 10.1038/ng1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lichtenstein L, Mattijssen F, de Wit NJ, Georgiadi A, Hooiveld GJ, van der Meer R, He Y, Qi L, Köster A, Tamsma JT, Tan NS, Müller M, Kersten S. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab 12: 580–592, 2010. doi: 10.1016/j.cmet.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sonnenburg WK, Yu D, Lee E-C, Xiong W, Gololobov G, Key B, Gay J, Wilganowski N, Hu Y, Zhao S, Schneider M, Ding Z-M, Zambrowicz BP, Landes G, Powell DR, Desai U. GPIHBP1 stabilizes lipoprotein lipase and prevents its inhibition by angiopoietin-like 3 and angiopoietin-like 4. J Lipid Res 50: 2421–2429, 2009. doi: 10.1194/jlr.M900145-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bergö M, Wu G, Ruge T, Olivecrona T. Down-regulation of adipose tissue lipoprotein lipase during fasting requires that a gene, separate from the lipase gene, is switched on. J Biol Chem 277: 11927–11932, 2002. doi: 10.1074/jbc.M200325200. [DOI] [PubMed] [Google Scholar]
- 62.Kroupa O, Vorrsjo E, Stienstra R, Mattijssen F, Nilsson SK, Sukonina V, Kersten S, Olivecrona G, Olivecrona T. Linking nutritional regulation of Angptl4, Gpihbp1, and Lmf1 to lipoprotein lipase activity in rodent adipose tissue. BMC Physiol 12: 13, 2012. doi: 10.1186/1472-6793-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cushing EM, Chi X, Sylvers KL, Shetty SK, Potthoff MJ, Davies BSJ. Angiopoietin-like 4 directs uptake of dietary fat away from adipose during fasting. Mol Metab 6: 809–818, 2017. doi: 10.1016/j.molmet.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dijk W, Beigneux AP, Larsson M, Bensadoun A, Young SG, Kersten S. Angiopoietin-like 4 promotes intracellular degradation of lipoprotein lipase in adipocytes. J Lipid Res 57: 1670–1683, 2016. [Erratum in J Lipid Res 57: 1949–1950, 2016]. doi: 10.1194/jlr.M067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dijk W, Ruppert PMM, Oost LJ, Kersten S. Angiopoietin-like 4 promotes the intracellular cleavage of lipoprotein lipase by PCSK3/furin in adipocytes. J Biol Chem 293: 14134–14145, 2018. doi: 10.1074/jbc.RA118.002426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Makoveichuk E, Vorrsjö E, Olivecrona T, Olivecrona G. Inactivation of lipoprotein lipase in 3T3-L1 adipocytes by angiopoietin-like protein 4 requires that both proteins have reached the cell surface. Biochem Biophys Res Commun 441: 941–946, 2013. doi: 10.1016/j.bbrc.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 67.Ruppert PMM, Michielsen CCJR, Hazebroek EJ, Pirayesh A, Olivecrona G, Afman LA, Kersten S. Fasting induces ANGPTL4 and reduces LPL activity in human adipose tissue. Mol Metab 40: 101033, 2020. doi: 10.1016/j.molmet.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmüller A, Gordts PLSM, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med 17: 200–205, 2011. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 69.Khedoe PPSJ, Hoeke G, Kooijman S, Dijk W, Buijs JT, Kersten S, Havekes LM, Hiemstra PS, Berbée JFP, Boon MR, Rensen PCN. Brown adipose tissue takes up plasma triglycerides mostly after lipolysis. J Lipid Res 56: 51–59, 2015. doi: 10.1194/jlr.M052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fu Z, Yao F, Abou-Samra AB, Zhang R. Lipasin, thermoregulated in brown fat, is a novel but atypical member of the angiopoietin-like protein family. Biochem Biophys Res Commun 430: 1126–1131, 2013. doi: 10.1016/j.bbrc.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 71.Dijk W, Heine M, Vergnes L, Boon MR, Schaart G, Hesselink MK, Reue K, Lichtenbelt WD, Olivecrona G, Rensen PC, Heeren J, Kersten S. ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure. eLife 4: e08428, 2015. doi: 10.7554/eLife.08428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ge H, Cha J-Y, Gopal H, Harp C, Yu X, Repa JJ, Li C. Differential regulation and properties of angiopoietin-like proteins 3 and 4. J Lipid Res 46: 1484–1490, 2005. doi: 10.1194/jlr.M500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 73.Catoire M, Alex S, Paraskevopulos N, Mattijssen F, Gogh IE, Schaart G, Jeppesen J, Kneppers A, Mensink M, Voshol PJ, Olivecrona G, Tan NS, Hesselink MKC, Berbée JF, Rensen PCN, Kalkhoven E, Schrauwen P, Kersten S. Fatty acid-inducible ANGPTL4 governs lipid metabolic response to exercise. Proc Natl Acad Sci USA 111: E1043–E1052, 2014. doi: 10.1073/pnas.1400889111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Babaev VR, Fazio S, Gleaves LA, Carter KJ, Semenkovich CF, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo. J Clin Invest 103: 1697–1705, 1999. doi: 10.1172/JCI6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lan G, Xie W, Li L, Zhang M, Liu D, Tan Y-L, Cheng H-P, Gong D, Huang C, Zheng X-L, Yin W-D, Tang C-K. MicroRNA-134 actives lipoprotein lipase-mediated lipid accumulation and inflammatory response by targeting angiopoietin-like 4 in THP-1 macrophages. Biochem Biophys Res Commun 472: 410–417, 2016. doi: 10.1016/j.bbrc.2015.10.158. [DOI] [PubMed] [Google Scholar]
- 76.Ye Q, Tian G-P, Cheng H-P, Zhang X, Ou X, Yu X-H, Tan R-Q, Yang F-Y, Gong D, Huang C, Pan Y-J, Zhang J, Chen L-Y, Zhao Z-W, Xie W, Li L, Zhang M, Xia X-D, Zheng X-L, Tang C-K. MicroRNA-134 promotes the development of atherosclerosis via the ANGPTL4/LPL pathway in apolipoprotein E knockout mice. J Atheroscler Thromb 25: 244–253, 2018. doi: 10.5551/jat.40212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Aryal B, Rotllan N, Araldi E, Ramírez CM, He S, Chousterman BG, Fenn AM, Wanschel A, Madrigal-Matute J, Warrier N, Martín-Ventura JL, Swirski FK, Suárez Y, Fernández-Hernando C. ANGPTL4 deficiency in haematopoietic cells promotes monocyte expansion and atherosclerosis progression. Nat Commun 7: 12313, 2016. doi: 10.1038/ncomms12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Georgiadi A, Wang Y, Stienstra R, Tjeerdema N, Janssen A, Stalenhoef A, van der Vliet JA, de Roos A, Tamsma JT, Smit JWA, Tan NS, Müller M, Rensen PCN, Kersten S. Overexpression of angiopoietin-like protein 4 protects against atherosclerosis development. Arterioscler Thromb Vasc Biol 33: 1529–1537, 2013. doi: 10.1161/ATVBAHA.113.301698. [DOI] [PubMed] [Google Scholar]
- 79.Georgiadi A, Lichtenstein L, Degenhardt T, Boekschoten MV, van Bilsen M, Desvergne B, Müller M, Kersten S. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ Res 106: 1712–1721, 2010. doi: 10.1161/CIRCRESAHA.110.217380. [DOI] [PubMed] [Google Scholar]
- 80.Alex S, Lichtenstein L, Dijk W, Mensink RP, Tan NS, Kersten S. ANGPTL4 is produced by entero-endocrine cells in the human intestinal tract. Histochem Cell Biol 141: 383–391, 2014. doi: 10.1007/s00418-013-1157-y. [DOI] [PubMed] [Google Scholar]
- 81.Mattijssen F, Alex S, Swarts HJ, Groen AK, van Schothorst EM, Kersten S. Angptl4 serves as an endogenous inhibitor of intestinal lipid digestion. Mol Metab 3: 135–144, 2014. doi: 10.1016/j.molmet.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 104: 979–984, 2007. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fleissner CK, Huebel N, Abd El-Bary MM, Loh G, Klaus S, Blaut M. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br J Nutr 104: 919–929, 2010. doi: 10.1017/S0007114510001303. [DOI] [PubMed] [Google Scholar]
- 84.Janssen AWF, Katiraei S, Bartosinska B, Eberhard D, Willems van Dijk K, Kersten S. Loss of angiopoietin-like 4 (ANGPTL4) in mice with diet-induced obesity uncouples visceral obesity from glucose intolerance partly via the gut microbiota. Diabetologia 61: 1447–1458, 2018. doi: 10.1007/s00125-018-4583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Korecka A, de Wouters T, Cultrone A, Lapaque N, Pettersson S, Doré J, Blottière HM, Arulampalam V. ANGPTL4 expression induced by butyrate and rosiglitazone in human intestinal epithelial cells utilizes independent pathways. Am J Physiol Gastrointest Liver Physiol 304: G1025–G1037, 2013. doi: 10.1152/ajpgi.00293.2012. [DOI] [PubMed] [Google Scholar]
- 86.Zandbergen F, Dijk S. V, Müller M, Kersten S. Fasting-induced adipose factor/angiopoietin–like protein 4: a potential target for dyslipidemia? Future Lipidology 1: 227–236, 2006. doi: 10.2217/17460875.1.2.227. [DOI] [Google Scholar]
- 87.Kersten S, Lichtenstein L, Steenbergen E, Mudde K, Hendriks HFJ, Hesselink MK, Schrauwen P, Müller M. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler Thromb Vasc Biol 29: 969–974, 2009. doi: 10.1161/ATVBAHA.108.182147. [DOI] [PubMed] [Google Scholar]
- 88.Mandard S, Zandbergen F, Tan NS, Escher P, Patsouris D, Koenig W, Kleemann R, Bakker A, Veenman F, Wahli W, Müller M, Kersten S. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J Biol Chem 279: 34411–34420, 2004. doi: 10.1074/jbc.M403058200. [DOI] [PubMed] [Google Scholar]
- 89.Yin W, Romeo S, Chang S, Grishin NV, Hobbs HH, Cohen JC. Genetic variation in ANGPTL4 provides insights into protein processing and function. J Biol Chem 284: 13213–13222, 2009. doi: 10.1074/jbc.M900553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lei X, Shi F, Basu D, Huq A, Routhier S, Day R, Jin W. Proteolytic processing of angiopoietin-like protein 4 by proprotein convertases modulates its inhibitory effects on lipoprotein lipase activity. J Biol Chem 286: 15747–15756, 2011. doi: 10.1074/jbc.M110.217638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Talmud PJ, Smart M, Presswood E, Cooper JA, Nicaud V, Drenos F, Palmen J, Marmot MG, Boekholdt SM, Wareham NJ, Khaw K-T, Kumari M, Humphries SE; EARSII ConsortiumHIFMECH Consortium. ANGPTL4 E40K and T266M: effects on plasma triglyceride and HDL levels, postprandial responses, and CHD risk. Arterioscler Thromb Vasc Biol 28: 2319–2325, 2008. doi: 10.1161/ATVBAHA.108.176917. [DOI] [PubMed] [Google Scholar]
- 92.Folsom AR, Peacock JM, Demerath E, Boerwinkle E. Variation in ANGPTL4 and risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Metabolism 57: 1591–1596, 2008. doi: 10.1016/j.metabol.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nikkilä EA, Taskinen M-R, Kekki M. Relation of plasma high-density lipoprotein cholesterol to lipoprotein-lipase activity in adipose tissue and skeletal muscle of man. Atherosclerosis 29: 497–501, 1978. doi: 10.1016/0021-9150(78)90178-8. [DOI] [PubMed] [Google Scholar]
- 94.Taskinen MR, Glueck CJ, Kashyap ML, Srivastava LS, Hynd BA, Perisutti G, Robinson K, Kinnunen PJ, Kuusi T. Post-heparin plasma lipoprotein and hepatic lipases. Relationships to high density lipoprotein cholesterol and to apolipoprotein CII in familial hyperalphalipoproteinemic and in normal subjects. Atherosclerosis 37: 247–256, 1980. doi: 10.1016/0021-9150(80)90010-6. [DOI] [PubMed] [Google Scholar]
- 95.Yang L-Y, Yu C-G, Wang X-H, Yuan S-S, Zhang L-J, Lang J-N, Zhao D, Feng Y-M. Angiopoietin-like protein 4 is a high-density lipoprotein (HDL) component for HDL metabolism and function in nondiabetic participants and type-2 diabetic patients. J Am Heart Assoc 6: e005973, 2017. doi: 10.1161/JAHA.117.005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ge H, Yang G, Huang L, Motola DL, Pourbahrami T, Li C. Oligomerization and regulated proteolytic processing of angiopoietin-like protein 4. J Biol Chem 279: 2038–2045, 2004. doi: 10.1074/jbc.M307583200. [DOI] [PubMed] [Google Scholar]
- 97.Kristensen KK, Leth-Espensen KZ, Mertens HDT, Birrane G, Meiyappan M, Olivecrona G, Jørgensen TJD, Young SG, Ploug M. Unfolding of monomeric lipoprotein lipase by ANGPTL4: insight into the regulation of plasma triglyceride metabolism. Proc Natl Acad Sci USA 117: 4337–4346, 2020. doi: 10.1073/pnas.1920202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leth-Espensen KZ, Kristensen KK, Kumari A, Winther A-ML, Young SG, Jørgensen TJD, Ploug M. The intrinsic instability of the hydrolase domain of lipoprotein lipase facilitates its inactivation by ANGPTL4-catalyzed unfolding. Proc Natl Acad Sci USA 118: e2026650118, 2021. doi: 10.1073/pnas.2026650118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beigneux AP, Allan CM, Sandoval NP, Cho GW, Heizer PJ, Jung RS, Stanhope KL, Havel PJ, Birrane G, Meiyappan M, Gill JE, Murakami M, Miyashita K, Nakajima K, Ploug M, Fong LG, Young SG. Lipoprotein lipase is active as a monomer. Proc Natl Acad Sci USA 116: 6319–6328, 2019. doi: 10.1073/pnas.1900983116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gunn KH, Gutgsell AR, Xu Y, Johnson CV, Liu J, Neher SB. Comparison of angiopoietin-like protein 3 and 4 reveals structural and mechanistic similarities. J Biol Chem 296: 100312, 2021. doi: 10.1016/j.jbc.2021.100312.[33482195] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Robal T, Larsson M, Martin M, Olivecrona G, Lookene A. Fatty acids bind tightly to the N-terminal domain of angiopoietin-like protein 4 and modulate its interaction with lipoprotein lipase. J Biol Chem 287: 29739–29752, 2012. doi: 10.1074/jbc.M111.303529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nilsson SK, Anderson F, Ericsson M, Larsson M, Makoveichuk E, Lookene A, Heeren J, Olivecrona G. Triacylglycerol-rich lipoproteins protect lipoprotein lipase from inactivation by ANGPTL3 and ANGPTL4. Biochim Biophys Acta 1821: 1370–1378, 2012. doi: 10.1016/j.bbalip.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 103.Conklin D, Gilbertson D, Taft DW, Maurer MF, Whitmore TE, Smith DL, Walker KM, Chen LH, Wattler S, Nehls M, Lewis KB. Identification of a mammalian angiopoietin-related protein expressed specifically in liver. Genomics 62: 477–482, 1999. doi: 10.1006/geno.1999.6041. [DOI] [PubMed] [Google Scholar]
- 104.Nakamura M, Yamada K. Studies on a diabetic (KK) strain of the mouse. Diabetologia 3: 212–221, 1967. doi: 10.1007/BF01222198. [DOI] [PubMed] [Google Scholar]
- 105.Shimizugawa T, Ono M, Shimamura M, Yoshida K, Ando Y, Koishi R, Ueda K, Inaba T, Minekura H, Kohama T, Furukawa H. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J Biol Chem 277: 33742–33748, 2002. doi: 10.1074/jbc.M203215200. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y, McNutt MC, Banfi S, Levin MG, Holland WL, Gusarova V, Gromada J, Cohen JC, Hobbs HH. Hepatic ANGPTL3 regulates adipose tissue energy homeostasis. Proc Natl Acad Sci USA 112: 11630–11635, 2015. doi: 10.1073/pnas.1515374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ono M, Shimizugawa T, Shimamura M, Yoshida K, Noji-Sakikawa C, Ando Y, Koishi R, Furukawa H. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J Biol Chem 278: 41804–41809, 2003. doi: 10.1074/jbc.M302861200. [DOI] [PubMed] [Google Scholar]
- 108.Minicocci I, Montali A, Robciuc MR, Quagliarini F, Censi V, Labbadia G, Gabiati C, Pigna G, Sepe ML, Pannozzo F, Lütjohann D, Fazio S, Jauhiainen M, Ehnholm C, Arca M. Mutations in the ANGPTL3 gene and familial combined hypolipidemia: a clinical and biochemical characterization. J Clin Endocrinol Metab 97: E1266–E1275, 2012. doi: 10.1210/jc.2012-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Musunuru K, Pirruccello JP, Do R, Peloso GM, Guiducci C, Sougnez C, Garimella KV, Fisher S, Abreu J, Barry AJ, Fennell T, Banks E, Ambrogio L, Cibulskis K, Kernytsky A, Gonzalez E, Rudzicz N, Engert JC, DePristo MA, Daly MJ, Cohen JC, Hobbs HH, Altshuler D, Schonfeld G, Gabriel SB, Yue P, Kathiresan S. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med 363: 2220–2227, 2010. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Robciuc MR, Maranghi M, Lahikainen A, Rader D, Bensadoun A, Öörni K, Oörni K, Metso J, Minicocci I, Ciociola E, Ceci F, Montali A, Arca M, Ehnholm C, Jauhiainen M. Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arterioscler Thromb Vasc Biol 33: 1706–1713, 2013. [Erratum in Arterioscler Thromb Vasc Biol 33: e124, 2013]. doi: 10.1161/ATVBAHA.113.301397. [DOI] [PubMed] [Google Scholar]
- 111.Kaplan R, Zhang T, Hernandez M, Gan FX, Wright SD, Waters MG, Cai T-Q. Regulation of the angiopoietin-like protein 3 gene by LXR. J Lipid Res 44: 136–143, 2003. doi: 10.1194/jlr.m200367-jlr200. [DOI] [PubMed] [Google Scholar]
- 112.Shimamura M, Matsuda M, Ando Y, Koishi R, Yasumo H, Furukawa H, Shimomura I. Leptin and insulin down-regulate angiopoietin-like protein 3, a plasma triglyceride-increasing factor. Biochem Biophys Res Commun 322: 1080–1085, 2004. doi: 10.1016/j.bbrc.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 113.Nidhina Haridas PA, Soronen J, Sädevirta S, Mysore R, Quagliarini F, Pasternack A, Metso J, Perttilä J, Leivonen M, Smas CM, Fischer-Posovszky P, Wabitsch M, Ehnholm C, Ritvos O, Jauhiainen M, Olkkonen VM, Yki-Järvinen H. Regulation of angiopoietin-like proteins (ANGPTLs) 3 and 8 by insulin. J Clin Endocrinol Metab 100: E1299–E1307, 2015. doi: 10.1210/jc.2015-1254. [DOI] [PubMed] [Google Scholar]
- 114.Fugier C, Tousaint J-J, Prieur X, Plateroti M, Samarut J, Delerive P. The lipoprotein lipase inhibitor ANGPTL3 is negatively regulated by thyroid hormone. J Biol Chem 281: 11553–11559, 2006. doi: 10.1074/jbc.M512554200. [DOI] [PubMed] [Google Scholar]
- 115.Chi X, Britt EC, Shows HW, Hjelmaas AJ, Shetty SK, Cushing EM, Li W, Dou A, Zhang R, Davies BSJ. ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol Metab 6: 1137–1149, 2017. doi: 10.1016/j.molmet.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martín-Campos JM, Roig R, Mayoral C, Martinez S, Martí G, Arroyo JA, Julve J, Blanco-Vaca F. Identification of a novel mutation in the ANGPTL3 gene in two families diagnosed of familial hypobetalipoproteinemia without APOB mutation. Clin Chim Acta 413: 552–555, 2012. doi: 10.1016/j.cca.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 117.Noto D, Cefalù AB, Valenti V, Fayer F, Pinotti E, Ditta M, Spina R, Vigna G, Yue P, Kathiresan S, Tarugi P, Averna MR. Prevalence of ANGPTL3 and APOB gene mutations in subjects with combined hypolipidemia. Arterioscler Thromb Vasc Biol 32: 805–809, 2012. doi: 10.1161/ATVBAHA.111.238766. [DOI] [PubMed] [Google Scholar]
- 118.Pisciotta L, Favari E, Magnolo L, Simonelli S, Adorni MP, Sallo R, Fancello T, Zavaroni I, Ardigò D, Bernini F, Calabresi L, Franceschini G, Tarugi P, Calandra S, Bertolini S. Characterization of three kindreds with familial combined hypolipidemia caused by loss-of-function mutations of ANGPTL3. Circ Cardiovasc Genet 5: 42–50, 2012. doi: 10.1161/CIRCGENETICS.111.960674. [DOI] [PubMed] [Google Scholar]
- 119.Jaye M, Lynch KJ, Krawiec J, Marchadier D, Maugeais C, Doan K, South V, Amin D, Perrone M, Rader DJ. A novel endothelial-derived lipase that modulates HDL metabolism. Nat Genet 21: 424–428, 1999. doi: 10.1038/7766. [DOI] [PubMed] [Google Scholar]
- 120.Ma K, Cilingiroglu M, Otvos JD, Ballantyne CM, Marian AJ, Chan L. Endothelial lipase is a major genetic determinant for high-density lipoprotein concentration, structure, and metabolism. Proc Natl Acad Sci USA 100: 2748–2753, 2003. doi: 10.1073/pnas.0438039100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McCoy MG, Sun G-S, Marchadier D, Maugeais C, Glick JM, Rader DJ. Characterization of the lipolytic activity of endothelial lipase. J Lipid Res 43: 921–929, 2002. doi: 10.1016/S0022-2275(20)30466-1. [DOI] [PubMed] [Google Scholar]
- 122.Takiguchi S, Ayaori M, Yakushiji E, Nishida T, Nakaya K, Sasaki M, Iizuka M, Uto-Kondo H, Terao Y, Yogo M, Komatsu T, Ogura M, Ikewaki K. Hepatic overexpression of endothelial lipase lowers high-density lipoprotein but maintains reverse cholesterol transport in mice. Arterioscler Thromb Vasc Biol 38: 1454–1467, 2018. doi: 10.1161/ATVBAHA.118.311056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shimamura M, Matsuda M, Yasumo H, Okazaki M, Fujimoto K, Kono K, Shimizugawa T, Ando Y, Koishi R, Kohama T, Sakai N, Kotani K, Komuro R, Ishida T, Hirata K, Yamashita S, Furukawa H, Shimomura I. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler Thromb Vasc Biol 27: 366–372, 2007. doi: 10.1161/01.ATV.0000252827.51626.89. [DOI] [PubMed] [Google Scholar]
- 124.Jin W, Wang X, Millar JS, Quertermous T, Rothblat GH, Glick JM, Rader DJ. Hepatic proprotein convertases modulate HDL metabolism. Cell Metab 6: 129–136, 2007. doi: 10.1016/j.cmet.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Adam RC, Mintah IJ, Alexa-Braun CA, Shihanian LM, Lee JS, Banerjee P, Hamon SC, Kim HI, Cohen JC, Hobbs HH, Van Hout C, Gromada J, Murphy AJ, Yancopoulos GD, Sleeman MW, Gusarova V. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J Lipid Res 61: 1271–1286, 2020. doi: 10.1194/jlr.RA120000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu L, Soundarapandian MM, Castoreno AB, Millar JS, Rader DJ. LDL-cholesterol reduction by ANGPTL3 inhibition in mice is dependent on endothelial lipase. Circ Res 127: 1112–1114, 2020. doi: 10.1161/CIRCRESAHA.120.317128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Felts JM, Itakura H, Crane RT. The mechanism of assimilation of constituents of chylomicrons, very low density lipoproteins and remnants - a new theory. Biochem Biophys Res Commun 66: 1467–1475, 1975. [0.1016/0006-291X(75)90524-0] [DOI] [PubMed] [Google Scholar]
- 128.Beisiegel U, Weber W, Bengtsson-Olivecrona G. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein receptor-related protein. Proc Natl Acad Sci USA 88: 8342–8346, 1991. doi: 10.1073/pnas.88.19.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mulder M, Lombardi P, Jansen H, van Berkel TJC, Frants RR, Havekes LM. Heparan sulphate proteoglycans are involved in the lipoprotein lipase-mediated enhancement of the cellular binding of very low density and low density lipoproteins. Biochem Biophys Res Commun 185: 582–587, 1992. doi: 10.1016/0006-291X(92)91664-C. [DOI] [PubMed] [Google Scholar]
- 130.Mulder M, Lombardi P, Jansen H, Berkel T. V, Frants RR, Havekes LM. Low density lipoprotein receptor internalizes low density and very low density lipoproteins that are bound to heparan sulfate proteoglycans via lipoprotein lipase. J Biol Chem 268: 9369–9375, 1993. doi: 10.1016/s0021-9258(18)98359-6. [DOI] [PubMed] [Google Scholar]
- 131.Essalmani R, Susan-Resiga D, Chamberland A, Asselin M-C, Canuel M, Constam D, Creemers JW, Day R, Gauthier D, Prat A, Seidah NG. Furin is the primary in vivo convertase of angiopoietin-like 3 and endothelial lipase in hepatocytes. J Biol Chem 288: 26410–26418, 2013. doi: 10.1074/jbc.M113.501304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu J, Afroza H, Rader DJ, Jin W. Angiopoietin-like protein 3 inhibits lipoprotein lipase activity through enhancing its cleavage by proprotein convertases. J Biol Chem 285: 27561–27570, 2010. doi: 10.1074/jbc.M110.144279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yau M, Wang Y, Lam KSL, Zhang J, Wu D, Xu A. A highly conserved motif within the NH2-terminal coiled-coil domain of angiopoietin-like protein 4 confers its inhibitory effects on lipoprotein lipase by disrupting the enzyme dimerization. J Biol Chem 284: 11942–11952, 2009. doi: 10.1074/jbc.M809802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jin W, Fuki IV, Seidah NG, Benjannet S, Glick JM, Rader DJ. Proprotein covertases are responsible for proteolysis and inactivation of endothelial lipase. J Biol Chem 280: 36551–36559, 2005. [Erratum in J Biol Chem 280: 41124, 2005]. doi: 10.1074/jbc.M502264200. [DOI] [PubMed] [Google Scholar]
- 135.Wu MJ, Wolska A, Roberts BS, Pearson EM, Gutgsell AR, Remaley AT, Neher SB. Coexpression of novel furin-resistant LPL variants with lipase maturation factor 1 enhances LPL secretion and activity. J Lipid Res 59: 2456–2465, 2018. doi: 10.1194/jlr.D086793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Reimund M, Kovrov O, Olivecrona G, Lookene A. Lipoprotein lipase activity and interactions studied in human plasma by isothermal titration calorimetry. J Lipid Res 58: 279–288, 2017. doi: 10.1194/jlr.D071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haller JF, Mintah IJ, Shihanian LM, Stevis P, Buckler D, Alexa-Braun CA, Kleiner S, Banfi S, Cohen JC, Hobbs HH, Yancopoulos GD, Murphy AJ, Gusarova V, Gromada J. ANGPTL8 requires ANGPTL3 to inhibit lipoprotein lipase and plasma triglyceride clearance. J Lipid Res 58: 1166–1173, 2017. doi: 10.1194/jlr.M075689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kovrov O, Kristensen KK, Larsson E, Ploug M, Olivecrona G. On the mechanism of angiopoietin-like protein 8 for control of lipoprotein lipase activity. J Lipid Res 60: 783–793, 2019. doi: 10.1194/jlr.M088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ren G, Kim JY, Smas CM. Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am J Physiol Endocrinol Metab 303: E334–E351, 2012. doi: 10.1152/ajpendo.00084.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yi P, Park J-S, Melton DA. Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell 153: 747–758, 2013. doi: 10.1016/j.cell.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 141.Cox AR, Lam CJ, Bonnyman CW, Chavez J, Rios JS, Kushner JA. Angiopoietin-like protein 8 (ANGPTL8)/betatrophin overexpression does not increase beta cell proliferation in mice. Diabetologia 58: 1523–1531, 2015. doi: 10.1007/s00125-015-3590-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gusarova V, Alexa CA, Na E, Stevis PE, Xin Y, Bonner-Weir S, Cohen JC, Hobbs HH, Murphy AJ, Yancopoulos GD, Gromada J. ANGPTL8/betatrophin does not control pancreatic beta cell expansion. Cell 159: 691–696, 2014. doi: 10.1016/j.cell.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jiao Y, Le Lay J, Yu M, Naji A, Kaestner KH. Elevated mouse hepatic betatrophin expression does not increase human beta-cell replication in the transplant setting. Diabetes 63: 1283–1288, 2014. doi: 10.2337/db13-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baroukh N, Bauge E, Akiyama J, Chang J, Afzal V, Fruchart J-C, Rubin EM, Fruchart-Najib J, Pennacchio LA. Analysis of apolipoprotein A5, c3, and plasma triglyceride concentrations in genetically engineered mice. Arterioscler Thromb Vasc Biol 24: 1297–1302, 2004. doi: 10.1161/01.ATV.0000130463.68272.1d. [DOI] [PubMed] [Google Scholar]
- 145.Evans D, Buchwald A, Beil FU. The single nucleotide polymorphism -1131T>C in the apolipoprotein A5 (APOA5) gene is associated with elevated triglycerides in patients with hyperlipidemia. J Mol Med (Berl) 81: 645–654, 2003. doi: 10.1007/s00109-003-0465-4. [DOI] [PubMed] [Google Scholar]
- 146.Horínek A, Vráblík M, Ceska R, Adámková V, Poledne R, Hubacek JA. T-1131–>C polymorphism within the apolipoprotein AV gene in hypertriglyceridemic individuals. Atherosclerosis 167: 369–370, 2003. doi: 10.1016/s0021-9150(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 147.Kao JT, Wen HC, Chien KL, Hsu HC, Lin SW. A novel genetic variant in the apolipoprotein A5 gene is associated with hypertriglyceridemia. Hum Mol Genet 12: 2533–2539, 2003. doi: 10.1093/hmg/ddg255. [DOI] [PubMed] [Google Scholar]
- 148.Pennacchio LA, Olivier M, Hubacek JA, Krauss RM, Rubin EM, Cohen JC. Two independent apolipoprotein A5 haplotypes influence human plasma triglyceride levels. Hum Mol Genet 11: 3031–3038, 2002. doi: 10.1093/hmg/11.24.3031. [DOI] [PubMed] [Google Scholar]
- 149.Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science 294: 169–173, 2001. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 150.van der Vliet HN, Schaap FG, Levels JHM, Ottenhoff R, Looije N, Wesseling JG, Groen AK, Chamuleau RAFM. Adenoviral overexpression of apolipoprotein A-V reduces serum levels of triglycerides and cholesterol in mice. Biochem Biophys Res Commun 295: 1156–1159, 2002. doi: 10.1016/S0006-291X(02)00808-2. [DOI] [PubMed] [Google Scholar]
- 151.van der Vliet HN, Sammels MG, Leegwater AC, Levels JH, Reitsma PH, Boers W, Chamuleau RA. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J Biol Chem 276: 44512–44520, 2001. doi: 10.1074/jbc.M106888200. [DOI] [PubMed] [Google Scholar]
- 152.Chen YQ, Pottanat TG, Zhen EY, Siegel RW, Ehsani M, Qian Y-W, Konrad RJ. ApoA5 lowers triglyceride levels via suppression of ANGPTL3/8-mediated LPL inhibition. J Lipid Res 62: 100068, 2021. doi: 10.1016/j.jlr.2021.100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang R. The ANGPTL3-4-8 model, a molecular mechanism for triglyceride trafficking. Open Biol 6: 150272, 2016. doi: 10.1098/rsob. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen YQ, Pottanat TG, Siegel RW, Ehsani M, Qian Y-W, Roell WC, Konrad RJ. Angiopoietin-like protein 4(E40K) and ANGPTL4/8 complex have reduced, temperature-dependent LPL-inhibitory activity compared to ANGPTL4. Biochem Biophys Res Commun 534: 498–503, 2021. doi: 10.1016/j.bbrc.2020.11.053. [DOI] [PubMed] [Google Scholar]
- 155.Girousse A, Virtue S, Hart D, Vidal-Puig A, Murgatroyd PR, Mouisel E, Sengenès C, Savage DB. Surplus fat rapidly increases fat oxidation and insulin resistance in lipodystrophic mice. Mol Metab 13: 24–29, 2018. doi: 10.1016/j.molmet.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 1801: 209–214, 2010. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 157.Wende AR, Symons JD, Abel ED. Mechanisms of lipotoxicity in the cardiovascular system. Curr Hypertens Rep 14: 517–531, 2012. doi: 10.1007/s11906-012-0307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Oteng A-B, Bhattacharya A, Brodesser S, Qi L, Tan NS, Kersten S. Feeding Angptl4-/- mice trans fat promotes foam cell formation in mesenteric lymph nodes without leading to ascites. J Lipid Res 58: 1100–1113, 2017. doi: 10.1194/jlr.M074278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Subramanian S, Chait A. Hypertriglyceridemia secondary to obesity and diabetes. Biochim Biophys Acta 1821: 819–825, 2012. doi: 10.1016/j.bbalip.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 160.Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet 45: 1345–1352, 2013. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kannel WB, Vasan RS. Triglycerides as vascular risk factors: new epidemiologic insights. Curr Opin Cardiol 24: 345–350, 2009. doi: 10.1097/HCO.0b013e32832c1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease a scientific statement from the American Heart Association. Circulation 123: 2292–2333, 2011. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 163.Lotta LA, Stewart ID, Sharp SJ, Day FR, Burgess S, Luan J, Bowker N, Cai L, Li C, Wittemans LBL, Kerrison ND, Khaw K-T, McCarthy MI, O'Rahilly S, Scott RA, Savage DB, Perry JRB, Langenberg C, Wareham NJ. Association of genetically enhanced lipoprotein lipase–mediated lipolysis and low-density lipoprotein cholesterol–lowering alleles with risk of coronary disease and type 2 diabetes. JAMA Cardiol 3: 957–966, 2018. doi: 10.1001/jamacardio.2018.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Helkkula P, Kiiskinen T, Havulinna AS, Karjalainen J, Koskinen S, Salomaa V, Daly MJ, Palotie A, Surakka I, Ripatti S; FinnGen. ANGPTL8 protein-truncating variant associated with lower serum triglycerides and risk of coronary disease. PLoS Genet 17: e1009501, 2021. doi: 10.1371/journal.pgen.1009501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med 377: 211–221, 2017. doi: 10.1056/NEJMoa1612790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stitziel NO, Khera AV, Wang X, Bierhals AJ, Vourakis AC, Sperry AE, Natarajan P, Klarin D, Emdin CA, Zekavat SM, Nomura A, Erdmann J, Schunkert H, Samani NJ, Kraus WE, Shah SH, Yu B, Boerwinkle E, Rader DJ, Gupta N, Frossard PM, Rasheed A, Danesh J, Lander ES, Gabriel S, Saleheen D, Musunuru K, Kathiresan S. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol 69: 2054–2063, 2017. doi: 10.1016/j.jacc.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gusarova V, O'Dushlaine C, Teslovich TM, Benotti PN, Mirshahi T, Gottesman O, et al. Genetic inactivation of ANGPTL4 improves glucose homeostasis and is associated with reduced risk of diabetes. Nat Commun 9: 2252, 2018. doi: 10.1038/s41467-018-04611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fu Z, Abou-Samra AB, Zhang R. A lipasin/Angptl8 monoclonal antibody lowers mouse serum triglycerides involving increased postprandial activity of the cardiac lipoprotein lipase. Sci Rep 5: 18502, 2015. doi: 10.1038/srep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Graham MJ, Lee RG, Brandt TA, Tai L-J, Fu W, Peralta R, Yu R, Hurh E, Paz E, McEvoy BW, Baker BF, Pham NC, Digenio A, Hughes SG, Geary RS, Witztum JL, Crooke RM, Tsimikas S. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med 377: 222–232, 2017. doi: 10.1056/NEJMoa1701329. [DOI] [PubMed] [Google Scholar]
- 170.Gusarova V, Banfi S, Alexa-Braun CA, Shihanian LM, Mintah IJ, Lee JS, Xin Y, Su Q, Kamat V, Cohen JC, Hobbs HH, Zambrowicz B, Yancopoulos GD, Murphy AJ, Gromada J. ANGPTL8 blockade with a monoclonal antibody promotes triglyceride clearance, energy expenditure, and weight loss in mice. Endocrinology 158: 1252–1259, 2017. doi: 10.1210/en.2016-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gusarova V, Alexa CA, Wang Y, Rafique A, Kim JH, Buckler D, Mintah IJ, Shihanian LM, Cohen JC, Hobbs HH, Xin Y, Valenzuela DM, Murphy AJ, Yancopoulos GD, Gromada J. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J Lipid Res 56: 1308–1317, 2015. doi: 10.1194/jlr.M054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vatner DF, Goedeke L, Camporez J-PG, Lyu K, Nasiri AR, Zhang D, Bhanot S, Murray SF, Still CD, Gerhard GS, Shulman GI, Samuel VT. Angptl8 antisense oligonucleotide improves adipose lipid metabolism and prevents diet-induced NAFLD and hepatic insulin resistance in rodents. Diabetologia 61: 1435–1446, 2018. doi: 10.1007/s00125-018-4579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fazio S, Sidoli A, Vivenzio A, Maietta A, Giampaoli S, Menotti A, Antonini R, Urbinati G, Baralle FE, Ricci G. A form of familial hypobetalipoproteinaemia not due to a mutation in the apolipoprotein B gene. J Intern Med 229: 41–47, 1991. doi: 10.1111/j.1365-2796.1991.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 174.Harada-Shiba M, Ali S, Gipe DA, Gasparino E, Son V, Zhang Y, Pordy R, Catapano AL. A randomized study investigating the safety, tolerability, and pharmacokinetics of evinacumab, an ANGPTL3 inhibitor, in healthy Japanese and Caucasian subjects. Atherosclerosis 314: 33–40, 2020. doi: 10.1016/j.atherosclerosis.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 175.Ahmad Z, Banerjee P, Hamon S, Chan K-C, Bouzelmat A, Sasiela WJ, Pordy R, Mellis S, Dansky H, Gipe DA, Dunbar RL. Inhibition of angiopoietin-like protein 3 with a monoclonal antibody reduces triglycerides in hypertriglyceridemia. Circulation 140: 470–486, 2019. doi: 10.1161/CIRCULATIONAHA.118.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gaudet D, Gipe DA, Pordy R, Ahmad Z, Cuchel M, Shah PK, Chyu K-Y, Sasiela WJ, Chan K-C, Brisson D, Khoury E, Banerjee P, Gusarova V, Gromada J, Stahl N, Yancopoulos GD, Hovingh GK. ANGPTL3 Inhibition in Homozygous Familial Hypercholesterolemia. N Engl J Med 377: 296–297, 2017. doi: 10.1056/NEJMc1705994. [DOI] [PubMed] [Google Scholar]
- 177.Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJP, Rubba P, Ali S, Banerjee P, Chan K-C, Gipe DA, Khilla N, Pordy R, Weinreich DM, Yancopoulos GD, Zhang Y, Gaudet D; ELIPSE HoFH Investigators. Evinacumab for Homozygous Familial Hypercholesterolemia. N Engl J Med 383: 711–720, 2020. doi: 10.1056/NEJMoa2004215. [DOI] [PubMed] [Google Scholar]
- 178.Rosenson RS, Burgess LJ, Ebenbichler CF, Baum SJ, Stroes ESG, Ali S, Khilla N, Hamlin R, Pordy R, Dong Y, Son V, Gaudet D. Evinacumab in patients with refractory hypercholesterolemia. N Engl J Med 383: 2307–2319, 2020. doi: 10.1056/NEJMoa2031049. [DOI] [PubMed] [Google Scholar]
- 179.Jin N, Matter WF, Michael LF, Qian Y, Gheyi T, Cano L, Perez C, Lafuente C, Broughton HB, Espada A. The angiopoietin-like protein 3 and 8 complex interacts with lipoprotein lipase and induces LPL cleavage. ACS Chem Biol 16: 457–462, 2021. doi: 10.1021/acschembio.0c00954. [DOI] [PubMed] [Google Scholar]
- 180.Gaudet D, Karwatowska-Prokopczuk E, Baum SJ, Hurh E, Kingsbury J, Bartlett VJ, Figueroa AL, Piscitelli P, Singleton W, Witztum JL, Geary RS, Tsimikas S, O’Dea LSL, Vupanorsen Study Investigators. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur Heart J 41: 3936–3945, 2020. doi: 10.1093/eurheartj/ehaa689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.King A. A CRISPR edit for heart disease. Nature 555: S23–S25, 2018. doi: 10.1038/d41586-018-02482-4. [DOI] [PubMed] [Google Scholar]
- 182.Rhee J-W, Wu JC. In vivo genome editing of ANGPTL3: a potential therapeutic strategy for coronary atherosclerosis? Nat Rev Cardiol 15: 259–260, 2018. doi: 10.1038/nrcardio.2018.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Biterova E, Esmaeeli M, Alanen HI, Saaranen M, Ruddock LW. Structures of Angptl3 and Angptl4, modulators of triglyceride levels and coronary artery disease. Sci Rep 8: 6752, 2018. doi: 10.1038/s41598-018-25237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Spitler KM, Davies BSJ. Aging and plasma triglyceride metabolism. J Lipid Res 61: 1161–1167, 2020. doi: 10.1194/jlr.R120000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cushing EM, Sylvers KL, Chi X, Shetty SK, Davies BSJ. Novel GPIHBP1-independent pathway for clearance of plasma TGs in Angptl4−/−Gpihbp1−/− mice. J Lipid Res 59: 1230–1243, 2018. doi: 10.1194/jlr.M084749. [DOI] [PMC free article] [PubMed] [Google Scholar]



