Abstract
Respiratory depression is a potentially fatal side effect of opioid analgesics and a major limitation to their use. G protein-biased opioid agonists have been proposed as “safer” analgesics with less respiratory depression. These agonists are biased to activate G proteins rather than β-arrestin signaling. Respiratory depression has been shown to correlate with both G protein bias and intrinsic efficacy, and recent work has refuted the role of β-arrestin signaling in opioid-induced respiratory depression. In addition, there is substantial evidence that G proteins do, in fact, mediate respiratory depression by actions in respiratory-controlling brainstem neurons. Based on these studies, we provide the perspective that protection from respiratory depression displayed by newly developed G protein-biased agonists is due to factors other than G protein versus arrestin bias.
Keywords: β-arrestin, G protein, opioid, respiratory
Opioid analgesics are the pharmacological gold standard to treat pain. However, abuse liability and potentially lethal respiratory depression limit their clinical safety and therapeutic potential. For example, a recent study found that nearly half of hospitalized patients experienced opioid-induced respiratory depression (1). Moreover, the incidence of opioid abuse and overdose are increasing and constitute a public health crisis (2).
Opioid-induced respiratory depression is due to activation of mu-opioid receptors (MORs) (3), which are expressed on respiratory control neurons in the brainstem. Conditional deletion of MORs from neurons in two of these respiratory control areas—the Kölliker-Fuse nucleus or preBötzinger Complex—attenuates opioid-induced respiratory depression (4). MORs inhibit these neurons by activating G protein-coupled inwardly rectifying potassium (GIRK) conductance (5, 6) and inhibiting neurotransmitter release (7).
Currently, only the opioid antagonist naloxone (Narcan) is used to reverse opioid-induced respiratory depression. Although this is lifesaving, it reverses all opioid effects and leads to withdrawal and uncontrolled pain. Therefore, several alternate strategies are being developed to prevent or avoid respiratory depression while maintaining analgesia, including: 1) immunopharmacotherapies (vaccines and monoclonal antibodies) (8), 2) nonopioid respiratory stimulants (e.g., ampakines, serotonin agonists, intranasal leptin, or orexin agonists) (9), and 3) opioid analgesics with minimal respiratory depression. It is this last point that we would like to address more fully.
Affinity, efficacy, and target are characteristics that distinguish drug-receptor interactions from each other. Through the decades, our understanding of these concepts has greatly expanded. R. P. Stephenson (10) first defined agonist efficacy as linear and postulated that an agonist produced uniform activation at the receptor. Present understanding is that efficacy can be pluridimensional, meaning that ligands can have many receptor-based efficacies. Furthermore, agonists can be “functionally selective” or “biased” and stabilize different receptor conformations that preferentially activate certain effectors (11). Substantial research has aimed at understanding the potential of biased MOR agonists and will be reviewed below.
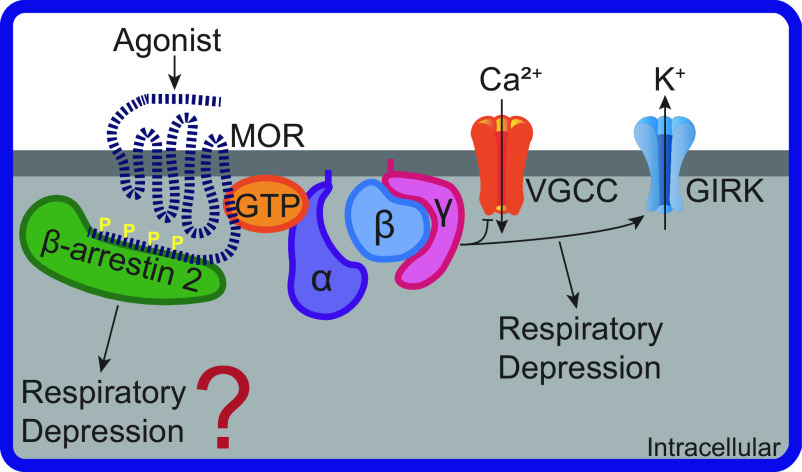
MORs are members of the G protein-coupled receptor (GPCR) superfamily. Stimulation of MORs activates heterotrimeric G proteins to inhibit adenylyl cyclase, modulate ion channel activity, and activate second-messenger signaling cascades (Fig. 1). MOR activity is regulated by a highly conserved process partly involving G protein-coupled receptor kinases (GRKs)—or other second-messenger kinases such as protein kinase C (PKC)—which phosphorylate the intracellular loops and carboxy-terminal tail of MORs and facilitate β-arrestin binding (12). Through this mechanism, MOR signaling is attenuated, MOR desensitization and internalization is initiated, and β-arrestin 2 signaling—independent of G proteins—is activated (13).
Figure 1.
Mu opioid receptor cellular signaling pathways involved in respiratory depression. Agonist binding to mu-opioid receptor (MOR) activates heterotrimeric G protein subunits: alpha (α), beta (β), and gamma (γ). Gβγ inhibits voltage-gated calcium channels (VGCC) and activates G protein-coupled inwardly rectifying potassium channels (GIRK). β-arrestin 2 binds to MOR with carboxy-terminal phosphorylation sites (P). GIRK, but likely not β-arrestin 2, activity can lead to respiratory depression.
β-arrestin 2 knockout mice exhibit impaired MOR desensitization and, consequently, increased morphine analgesia with very little morphine tolerance (14). Subsequent studies have confirmed the critical role β-arrestin 2 plays in MOR desensitization and development of tolerance (15). These initial findings sparked an interest in the regulatory role of β-arrestin 2 in MOR signaling and opioid pharmacology. The finding that β-arrestin 2 knockout mice had attenuated morphine-induced respiratory suppression kindled the flame (16). From this study, stemmed the hypothesis that β-arrestin 2 signaling—more so than G protein signaling—was responsible for deleterious opioid effects, including respiratory depression.
The goal of developing a MOR G protein-biased agonist devoid of respiratory depression has led to the development of several novel compounds (17–19). The first potential drug candidate was oliceridine (TRV-130). Preclinical studies demonstrated less respiratory depression and a threefold preference for G protein signaling compared with morphine and fentanyl (17). However, a 2018 FDA brief summarizing phase 3 clinical trials concluded that oliceridine and morphine caused comparable respiratory depression (20). Recently, reanalysis of pharmacokinetics and pharmacodynamics data from these clinical trials found oliceridine to have a safer profile and lower probability of causing respiratory depression compared with morphine (21). With the recent FDA approval of oliceridine, further testing of its safety profile can be performed. Subsequent drug discoveries had similar challenges. PZM21, another biased-MOR agonist, was developed computationally and found to activate G protein signaling, recruit minimal β-arrestin 2, and have limited respiratory depression (18). However, it was later reported to cause respiratory depression similar to morphine (22).
These G protein-biased drugs, despite the controversy, illustrate a correlation between ligands that preferentially activate G proteins and cause limited respiratory depression (19). However, a causal relationship between G protein bias and limited respiratory depression has not been established. An alternate hypothesis is that attenuated respiratory depression is due to low intrinsic efficacy (ability of a drug-receptor interaction to produce a maximal effect) rather than G protein bias (22, 23). For example, the clinically used agonist buprenorphine has a low intrinsic efficacy and produces significantly less respiratory depression (24, 25).
The absence of mechanistic evidence for how β-arrestin 2 signaling could mediate opioid-induced respiratory depression has led several groups to reexamine the β-arrestin 2 hypothesis. The first study used mice expressing phosphorylation-deficient, G protein-biased MORs that were generated by mutating MOR carboxyl-terminal residues from serine and threonine to alanine (15). This prevented β-arrestin 2 recruitment and subsequent signaling, rendering the receptor G protein biased. Interestingly, phosphorylation-deficient, G protein-biased MOR mice still exhibited respiratory depression, suggesting that G protein signaling contributes to opioid-induced side effects (15). This conclusion was substantiated by several laboratories that were unable to replicate an attenuation of morphine or fentanyl-induced respiratory depression in β-arrestin 2 knockout mice (26, 27). They concluded that their findings “do not support the original suggestion that β-arrestin 2 signaling plays a key role in opioid-induced respiratory depression and call into question the concept of developing G protein-biased MOR agonists as a strategy for the development of safer opioid analgesic drugs” (27). And most recently, He et al. (25) demonstrated that genetic and pharmacological manipulations that enhance MOR-mediated β-arrestin 2 signaling do not influence respiratory depression (but in fact reduces analgesic tolerance), corroborating preexisting evidence. This suggests that a balanced MOR agonist with low intrinsic efficacy may be “safest” because it can retain both physiological G protein function and arrestin-mediated receptor regulation and tolerance development. In addition, He et al. (25) found that 129SvJ mice are less sensitive to morphine-induced respiratory depression than C57BL6/J mice, illustrating that background strain matters in opioid-induced respiratory depression measurements. Since the original β-arrestin 2 knockout mice were on a mixed background of C57BL6/129SvJ, a difference in background could account for the different phenotypes observed by various research groups.
Furthermore, mechanistic evidence demonstrates that G protein signaling can, in fact, mediate opioid-induced respiratory depression (Fig. 1). Activation of MORs stimulates heterotrimeric G proteins, initiating the separation of the Gα-subunit from the Gβγ-subunits for subsequent G protein-signaling transduction. Gβγ-subunits activate GIRK channels, thereby decreasing neuronal excitability. Kölliker-Fuse and preBötzinger Complex neurons, which are critical to opioid-induced respiratory depression, are hyperpolarized by MOR-mediated activation of GIRK conductance (4–6). MOR activation of GIRK channels in the Kölliker-Fuse is similar in wild-type and phosphorylation-deficient G protein-biased MOR mice, illustrating that GIRK channel activation is G protein, not β-arrestin 2 mediated (15). Furthermore, GIRK channel knockout mice experience significantly less opioid-induced respiratory depression than wild-type mice (28).
Considering the mechanistic evidence that G proteins can mediate respiratory depression and the existence of multiple contradictory studies for β-arrestin 2-dependent respiratory depression, we question the utility of future studies to develop G protein-biased MOR agonists as safer analgesics on the basis of G protein versus β-arrestin 2 bias alone. Instead, other factors, such as intrinsic efficacy, receptor selectivity, or bias among G protein subunits or other downstream effectors should be considered. Despite this, the first agonist to come out of this line of work, oliceridine, was recently FDA approved and has been shown to have a better safety profile than morphine in humans (21). Whether this is due to biased signaling or other factors such as intrinsic efficacy remains an active debate (29, 30).
GRANTS
This work was supported by National Institutes of Health Grants R00DA038069 and R01DA047978. J. T. Bateman was supported by NIH Grant F31DA053798.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.T.B. and E.S.L. drafted manuscript; J.T.B. and E.S.L. edited and revised manuscript; J.T.B. and E.S.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. David Baekey and Sandy Saunders for comments on the manuscript.
REFERENCES
- 1.Khanna AK, Bergese SD, Jungquist CR, Morimatsu H, Uezono S, Lee S, Ti LK, Urman RD, McIntyre R Jr, Tornero C, Dahan A, Saager L, Weingarten TN, Wittmann M, Auckley D, Brazzi L, Le Guen M, Soto R, Schramm F, Ayad S, Kaw R, Di Stefano P, Sessler DI, Uribe A, Moll V, Dempsey SJ, Buhre W, Overdyk FJ; PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY (PRODIGY) Group Collaborators. Prediction of opioid-induced respiratory depression on inpatient wards using continuous capnography and oximetry: an international prospective, observational trial. Anesth Analg 131: 1012–1024, 2020. doi: 10.1213/ANE.0000000000004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson N, Kariisa M, Seth P, Smith H 4th, Davis NL. Drug and opioid-involved overdose deaths — United States, 2017–2018. MMWR Morb Mortal Wkly Rep 69: 290–297, 2020. doi: 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahan A, Sarton E, Teppema L, Olievier C, Nieuwenhuijs D, Matthes HWD, Kieffer BL. Anesthetic potency and influence of morphine and sevoflurane on respiration in μ-opioid receptor knockout mice. Anesthesiology 94: 824–832, 2001. doi: 10.1097/00000542-200105000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Varga AG, Reid BT, Kieffer BL, Levitt ES. Differential impact of two critical respiratory centres in opioid-induced respiratory depression in awake mice. J Physiol 598: 189–205, 2020. doi: 10.1113/JP278612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science 286: 1566–1568, 1999. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levitt ES, Abdala AP, Paton JFR, Bissonnette JM, Williams JT. μ opioid receptor activation hyperpolarizes respiratory-controlling Kölliker-Fuse neurons and suppresses post-inspiratory drive. J Physiol 593: 4453–4469, 2015. doi: 10.1113/JP270822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei AD, Ramirez JM. Presynaptic mechanisms and KCNQ potassium channels modulate opioid depression of respiratory drive. Front Physiol 10: 1407, 2019. doi: 10.3389/fphys.2019.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pravetoni M, Comer SD. Development of vaccines to treat opioid use disorders and reduce incidence of overdose. Neuropharmacology 158: 107662, 2019. doi: 10.1016/j.neuropharm.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bateman JT, Saunders SE, Levitt ES. Understanding and countering opioid-induced respiratory depression. Br J Pharmacol, 2021. doi: 10.1111/bph.15580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephenson RP. A modification of receptor theory. Br J Pharmacol Chemother 11: 379–393, 1956. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenakin T. Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther 336: 296–302, 2011. doi: 10.1124/jpet.110.173948. [DOI] [PubMed] [Google Scholar]
- 12.Williams JT, Ingram SL, Henderson G, Chavkin C, Zastrow MV, Schulz S, Koch T, Evans CJ, Christie MJ. Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmocol Rev 65: 223–254, 2013. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science 308: 512–517, 2005. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 14.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408: 720–723, 2000. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 15.Kliewer A, Schmiedel F, Sianati S, Bailey A, Bateman JT, Levitt ES, Williams JT, Christie MJ, Schulz S. Phosphorylation-deficient G-protein-biased μ-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun 10: 367, 2019. doi: 10.1038/s41467-018-08162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raehal KM, Walker JKL, Bohn LM. Morphine side effects in beta-arrestin-2 knockout mice. J Pharmacol Exp Ther 314: 1195–1201, 2005. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 17.DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, Koblish M, Lark MW, Violin JD. A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344: 708–717, 2013. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- 18.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hubner H, Huang X-P, Sassano MF, Giguere PM, Lober S, Duan D, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK. Structure-based discovery of opioid analgesics with reduced side effects. Nature 537: 185–190, 2016. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, Cameron MD, Bannister TD, Bohn LM. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 171: 1165–1175.e13, 2017. doi: 10.1016/j.cell.2017.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hertz S. FDA Briefing Document, Overview of the October 11, 2018. AADPAC Meeting to Discuss NDA 210730, 2018. [Google Scholar]
- 21.Dahan A, van Dam CJ, Niesters M, van Velzen M, Fossler MJ, Demitrack MA, Olofsen E. Benefit and risk evaluation of biased μ-receptor agonist oliceridine versus morphine. Anesthesiology 133: 559–568, 2020. doi: 10.1097/ALN.0000000000003441. [DOI] [PubMed] [Google Scholar]
- 22.Hill R, Disney A, Conibear A, Cutcliffe K, Dewey W, Husbands S, Bailey C, Kelly E, Henderson G. The novel μ-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br J Pharmacol 175: 2653–2661, 2018. doi: 10.1111/bph.14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillis A, Gondin AB, Kliewer A, Sanchez J, Lim HD, Alamein C, Manandhar P, Santiago M, Fritzwanker S, Schmiedel F, Katte TA, Reekie T, Grimsey NL, Kassiou M, Kellam B, Krasel C, Halls ML, Connor M, Lane JR, Schulz S, Christie MJ, Canals M. Low intrinsic efficacy for G protein activation can explain the improved side effect profiles of new opioid agonists. Sci Signal 13: eaaz3140, 2020. doi: 10.1126/scisignal.aaz3140. [DOI] [PubMed] [Google Scholar]
- 24.Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth 94: 825–834, 2005. doi: 10.1093/bja/aei145. [DOI] [PubMed] [Google Scholar]
- 25.He L, Gooding SW, Lewis E, Felth LC, Gaur A, Whistler JL. Pharmacological and genetic manipulations at the µ-opioid receptor reveal arrestin-3 engagement limits analgesic tolerance and does not exacerbate respiratory depression in mice. Neuropsychopharmacology, 2021. doi: 10.1038/s41386-021-01054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachmutsky I, Wei XP, Kish E, Yackle K. Opioids depress breathing through two small brainstem sites. eLife 9: e52694, 2020. doi: 10.7554/elife.52694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kliewer A, Gillis A, Hill R, Schmiedel F, Bailey C, Kelly E, Henderson G, Christie MJ, Schulz S. Morphine-induced respiratory depression is independent of β-arrestin 2 signalling. Br J Pharmacol 177: 2923–2931, 2020. doi: 10.1111/bph.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montandon G, Ren J, Victoria NC, Liu H, Wickman K, Greer JJ, Horner RL. G-protein-gated inwardly rectifying potassium channels modulate respiratory depression by opioids. Anesthesiology 124: 641–650, 2016. doi: 10.1097/ALN.0000000000000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillis A, Kliewer A, Kelly E, Henderson G, Christie MJ, Schulz S, Canals M. Critical assessment of G protein-biased agonism at the μ-opioid receptor. Trends Pharmacol Sci 41: 947–959, 2020. doi: 10.1016/j.tips.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Grim TW, Acevedo-Canabal A, Bohn LM. Toward directing opioid receptor signaling to refine opioid therapeutics. Biol Psychiatry 87: 15–21, 2020. doi: 10.1016/j.biopsych.2019.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]