Abstract
The purpose of this project was to provide a profile of DNA, RNA, and protein content in adipose tissue, which is relatively understudied in humans, to gain more insight into the amount of tissue that may be required for various analyses. Skeletal muscle tissue was also investigated to provide a direct comparison into potential differences between these two highly metabolically active tissues. Basal adipose and skeletal muscle tissue samples were obtained from 10 (7 M, 3 W) recreationally active participants [25 ± 1 yr; 84 ± 3 kg, maximal oxygen consumption (V̇o2max): 3.5 ± 0.2 L/min, body fat: 29 ± 2%]. DNA, RNA, and protein were extracted and subsequently analyzed for quantity and quality. DNA content of adipose and skeletal muscle tissue was 52 ± 14 and 189 ± 44 ng DNA·mg tissue−1, respectively (P < 0.05). RNA content of adipose and skeletal muscle tissue was 46 ± 14 and 537 ± 72 ng RNA·mg tissue−1, respectively (P < 0.05). Protein content of adipose and skeletal muscle tissue was 4 ± 1 and 177 ± 10 µg protein·mg tissue−1, respectively (P < 0.05). In summary, human adipose had 28% of the DNA, 9% of the RNA, and 2% of the protein found in skeletal muscle per mg of tissue. This information should be useful across a wide range of human clinical investigation designs and various laboratory analyses.
NEW & NOTEWORTHY This investigation studied DNA, RNA, and protein contents of adipose and skeletal muscle tissues from young active individuals. A series of optimization steps were investigated to aid in determining the optimal approach to extract high-yield and high-quality biomolecules. These findings contribute to the knowledge gap in adipose tissue requirements for molecular biology assays, which is of increasing importance due to the growing interest in adipose tissue research involving human exercise physiology research.
Keywords: adipose, DNA, protein, RNA, skeletal muscle
INTRODUCTION
In 1962, Jonas Bergström reintroduced the skeletal muscle biopsy technique for use in medical research, which was quickly adapted for human exercise physiology research (1). This paved the way for assay development in the exercise biochemistry era (late 1960s to the 1980s) (2–6) and evolved into the molecular biology era (1990s to present) (7–10), providing a rich understanding of human skeletal muscle alterations with various perturbations. With the advancement of molecular biology tools and various -omics platforms, the amount of skeletal muscle tissue required continues to be reduced, and in some cases, only a few milligrams or even nanograms are needed (11–18). In contrast, the adipose biopsy technique is relatively new to the field of exercise physiology and much less information has been established regarding the amount of adipose tissue needed for various biochemical and molecular biology assays.
The importance of this fundamental knowledge gap is highlighted by the fact that skeletal muscle and adipose tissue are two of the most metabolically active tissues before, during, and after exercise (19–22). It has recently become evident that these tissues communicate via cytokines and likely have, at least partially, a coordinated response to exercise (23–26). Previously considered as a passive energy-storing organ for triacylglycerols, adipose tissue is now recognized as a complex and highly active metabolic and endocrine organ (27, 28). The increased attention to the biology of adipose tissue has proven to be of key importance for the research of diabetes, metabolic syndrome, and obesity, all of which make up rising epidemics in the United States and many parts of the world (29). Adipose tissue biology is of increasing interest when it comes to understanding the beneficial adaptations that occur with chronic exercise training and how they influence overall health (22, 30–34). The high lipid content of adipose tissue is known to provide some challenges in obtaining total DNA, RNA, and protein content, thus further optimization may improve their isolation from adipose tissue (35–37).
There have been previous reports of the DNA, RNA, and protein contents of adipose and skeletal muscle tissue in the literature; however, methodologies of tissue sampling and biomolecule extraction differ (14, 38), and in some cases, the literature guidance is more than 50 years old (39, 40). In addition, a fairly large range in DNA, RNA, and protein content is evident in adipose and skeletal muscle tissues, which may impact the amount of starting material needed for downstream analysis. DNA content has previously been reported with ranges from 74 to 200 ng DNA·mg tissue−1 for adipose (41, 42) and 14 to 250 ng DNA·mg tissue−1 for skeletal muscle (38). RNA content is reported to range from 12 to 44 ng RNA·mg tissue−1 for adipose (43, 44) and ∼200 to 650 ng RNA·mg tissue−1 for skeletal muscle (45–47), whereas protein content ranges from 10 to 79 µg protein·mg adipose tissue−1 and 152 to 219 µg protein·mg skeletal muscle tissue−1 in the literature (48–53). Advances in technology involved in extraction and quantification methods provide an opportunity to reexamine the various approaches for biomolecule extraction and analysis for use in human research.
The purpose of this investigation was to provide a basic profile of DNA, RNA, and protein content in adipose tissue. Skeletal muscle tissue was also investigated to provide a direct comparison into potential differences between these two tissues. To examine the contents of these tissues, basal adipose and skeletal muscle tissue biopsies were obtained from young, healthy individuals. We hypothesized that adipose tissue would have lower yields of DNA, RNA, and protein per mg of tissue compared with skeletal muscle tissue.
METHODS
Subjects and Overall Study Design
Ten recreationally active individuals (7 M, 3 W) participated in this investigation (Table 1). Subjects completed detailed medical history, exercise, and dietary habit questionnaires. All subjects were physically active (i.e., regular aerobic and/or resistance exercise 3–5 days·wk−1), nonsmokers, and apparently healthy. None of the subjects chronically consumed prescription or nonprescription analgesics, anti-inflammatory drugs, or dietary supplements. Measurements of height and body mass were obtained from each subject before assessment of body composition using a dual-energy X-ray absorptiometry (DXA) scan (Lunar iDXA full body scanner, GE Healthcare, Madison WI).
Table 1.
Subject characteristics (n = 10)
| Age, yr | 25 ± 2 |
| Height, cm | 176 ± 11 |
| Body mass, kg | 84 ± 8 |
| BMI, kg·m−2 | 27 ± 2 |
| Body Fat, % | 29 ± 7 |
| V̇o2max, L·min−1 | 3.5 ± 0.6 |
| V̇o2max, ml·kg−1·min−1 | 41.6 ± 4.7 |
| HRmax, beats/min | 195 ± 8 |
Values are means ± SD. BMI, body mass index; V̇o2max, maximal oxygen consumption.
After this initial screening, subjects completed an assessment of maximal aerobic capacity (maximal oxygen consumption, V̇o2max), as well as a biopsy trial to collect basal adipose and skeletal muscle biopsies after adhering to dietary and physical activity controls. All procedures, as well as risks and benefits associated with the experimental testing, were explained to the subjects before providing written consent according to the guidelines of the Institutional Review Board of Ball State University.
Maximal Oxygen Consumption Test
Subjects performed a continuous cycle ergometer (Lode Excalibur Sport, Lode BV, Groningen, The Netherlands) test with 12-lead ECG (Philips ST80i Stress Testing System; Philips, Andover, MA) to volitional exhaustion. Oxygen uptake was determined every 30 s through an automated open-circuit indirect calorimetry system incorporating electronic O2 and CO2 analyzers (S-3A/I and CD-3A, AEI Technologies, Pittsburgh, PA). The gas analyzers were calibrated with gases of known concentration. Subjects completed a 2-min warm up (men: 100 W, women: 50 W) followed by a ramped increase in workload (men: 25 W·min−1, women: 20 W·min−1) until the subject reached volitional fatigue. Successful testing criteria included a plateau in the volume of oxygen consumed (V̇o2), a respiratory exchange ratio (RER) of ≥1.10, and/or a rating of perceived exertion (RPE) ≥19 at the completion of the test.
Prebiopsy Trial Controls
Diet and physical activity were controlled for several days before the basal muscle and adipose tissue collections. Subjects were instructed to refrain from taking any COX inhibitors (e.g., aspirin, acetaminophen, ibuprofen) for 7 days before their trial. For the 48 h leading into the trial, subjects were instructed to refrain from alcohol consumption and exercise training and to maintain normal dietary habits. For 24 h preceding the biospecimen collections, subjects were instructed to refrain from caffeine consumption. The evening before the trial, subjects were instructed to consume their evening meal no later than 7:00 PM. In addition, subjects were provided a liquid nutrition supplement (Ensure Plus, Abbott Laboratories, Columbus, OH; 8 oz, 350 kcal, 57% carbohydrate, 15% protein, and 28% fat) to consume 10 h before their scheduled muscle and adipose collections the following morning (∼7:00 AM). This supplement allowed for the final nutrient intake and fast duration to be standardized across subjects. Only water was allowed after consumption of the standardized nutrition supplement until the completion of the trial the next day. All women were biopsied between days 3 and 7 of their menstrual cycle.
Muscle Biopsy
After at least 30 min of supine rest, subjects underwent a skeletal muscle biopsy of the vastus lateralis following local anesthetic (Lidocaine HCl 1%) with a 6-mm Bergström needle with suction (1). Following the muscle biopsy, excess blood, visible fat, and connective tissue were removed from the sample, and the muscle was divided and frozen in liquid nitrogen (<6 min) until analysis.
Adipose Biopsy
Shortly following the muscle biopsy, subjects underwent a subcutaneous adipose tissue biopsy from the abdominal region from an area centering on ∼5 cm lateral to the umbilicus. After administration of local anesthetic (1% lidocaine HCl), an incision was made ∼8–10 cm lateral to the umbilicus, followed by administration of 40–60 mL of tumescent solution (0.09% lidocaine HCl in 0.9% NaCl) with a blunt-tipped 16-gauge cannula (54). A 4-mm Mercedes biopsy needle with suction applied via a 10-mL syringe and syringe lock were used to remove adipose tissue from the tumesced area. The adipose tissue was immediately cleansed over 255-µm mesh (Component Supply Company, U-CMN-255, Maiden, NC) with cold PBS. Blood vessels and connective tissues were removed from the sample, and the adipose tissue was divided and frozen in liquid nitrogen (<6 min) until analysis.
Preliminary DNA and Protein Isolation
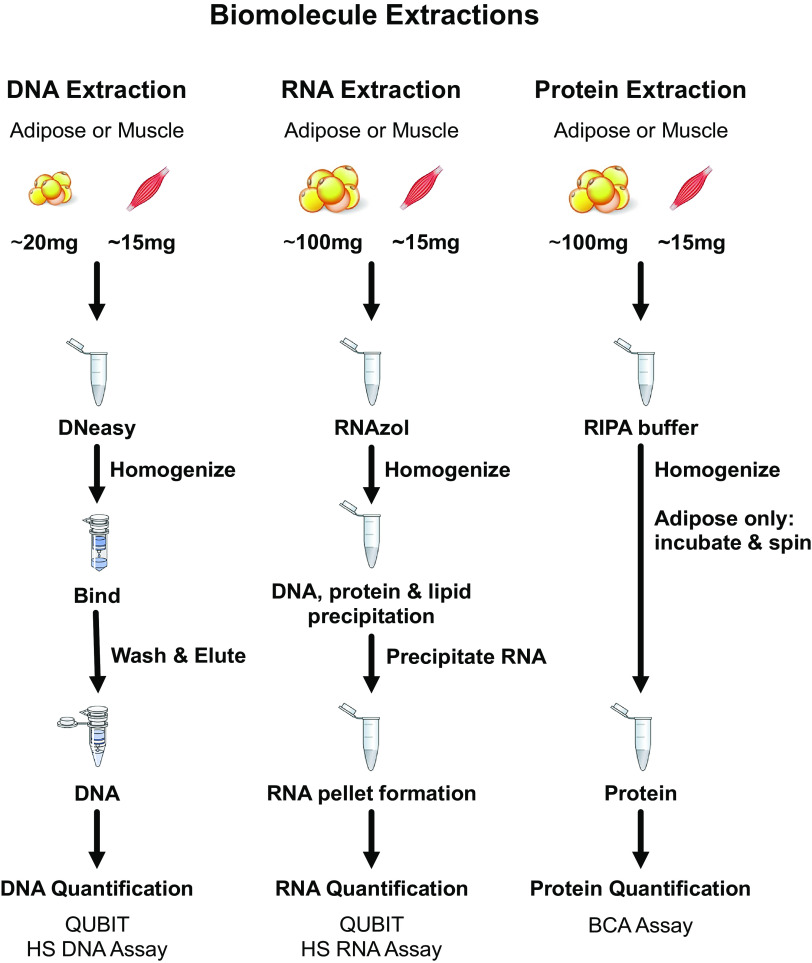
Before deciding on the methods for DNA and protein isolation, which are presented in more detail in the following sections and overviewed in Fig. 1, a variety of protocols were tested. These protocols included various homogenization techniques and extraction buffers for both DNA and protein isolation. Briefly, two homogenization techniques (plastic tube and pestle and the Omni soft tissue probe) and three extraction methods (TRIzol, DNAzol, and DNeasy Blood & Tissue kit) were tested for DNA isolation. For protein isolation, six homogenization techniques (glass homogenizer, plastic tube and pestle, PowerGen, glass tube and pestle, Omni hard tissue probe, and Omni soft tissue probe) and three extraction methods [House buffer, radioimmunoprecipitation assay (RIPA) buffer, and Minute Total Protein Extraction Kit for Adipose Tissues] were tested. Data from these preliminary methods are in Tables 2 and 3, respectively.
Figure 1.
Overview of experimental approaches for DNA, RNA, and protein extractions. For additional details, refer to text. RIPA, radioimmunoprecipitation assay.
Table 2.
Preliminary DNA isolation data
| Extraction Method | Tissue | Homogenization Technique |
|
|---|---|---|---|
| Tube and Pestle* | Omni Soft Tissue Probe | ||
| TRIzol | Muscle | 30 | 27 |
| Adipose | 0.9 | 3.8 | |
| DNAzol | Muscle | 24 | 66 |
| Adipose | NA | NA | |
| DNeasy Blood & Tissue Kit* | Muscle | 225 | NA |
| Adipose | 55 | NA | |
All units are in (ng DNA·mg tissue−1). NA, method pairing was not attempted. *Final methods used for this investigation were a tube and pestle for homogenization and the DNeasy Blood & Tissue Kit for DNA extraction.
Table 3.
Preliminary protein isolation data
| Buffer | Tissue | Homogenization Technique |
|||||
|---|---|---|---|---|---|---|---|
| Glass Homogenizer | Plastic Tube and Pestle | PowerGen | Glass Tube and Pestle | Omni Hard Tissue Probe | Omni Soft Tissue Probe* | ||
| House | Muscle | NA | 80 | 48 | 89 | 116 | NA |
| Adipose | NA | 1.5 | 2.3 | 1.4 | 2.3 | NA | |
| RIPA* | Muscle | 125 | 103 | 246 | 156 | 151 | 187 |
| Adipose | 2.6 | 3.2 | 2.9 | 2.6 | 3.4 | 3.5 | |
| Minute Protein | Muscle | NA | NA | NA | NA | NA | NA |
| Extraction Column | Adipose | NA | 1.9 | NA | NA | NA | NA |
All units are in (µg protein·mg tissue−1). NA, method pairing was not attempted. *Final methods used for this investigation were the Omni Soft Tissue Probe for homogenization and radioimmunoprecipitation assay (RIPA) buffer for protein extraction.
The preliminary DNA extraction methods produced varied results ranging from 1 to 55 ng DNA·mg tissue−1 for adipose tissue and from 24 to 225 ng DNA·mg tissue−1 for skeletal muscle tissue (Table 2). The combination of the DNeasy Blood & Tissue kit with manual plastic tube and pestle homogenization yielded the highest quantity of DNA for both adipose and skeletal muscle tissues. The various preliminary protein extraction methods produced a myriad of results ranging from 1.4 to 3.5 µg protein·mg tissue−1 for adipose tissue and 48 to 246 µg protein·mg tissue−1 for skeletal muscle (Table 3). To attempt to extract more proteins from adipose tissue, a total protein extraction column specific for adipose tissue was tested. This yielded 1.9 µg protein·mg tissue−1 which was similar to the other methods tested. The protocol chosen for protein extraction from both tissues was homogenization with the Omni Tissue homogenizer with the plastic soft tissue homogenizing probe in RIPA buffer, as this yielded the highest protein content for adipose and the second highest for skeletal muscle.
DNA Isolation, Quantification, and Quality
Frozen tissue samples were weighed at −30°C with a Cahn C-35 ultra-microbalance (Thermo Electron Corporation, Waltham, MA) (adipose: 18.1 ± 3.2 mg, range 14.1–22.9 mg; skeletal muscle: 12.7 ± 3.7 mg, range 7.7–20.4 mg) and total DNA was extracted using the DNeasy Blood & Tissue kit (Cat. No. 69504, QIAGEN, Austin, TX) according to the manufacturer’s instructions (Fig. 1). Once samples were placed in the buffer, they equilibrated to room temperature for 3 min and were then homogenized with a disposable plastic pestle (Bel-Art SP Scienceware, South Wayne, NJ) for 3 min. This was followed by a 15-s vortex at speed 7 (Fisherbrand Analog Vortex Mixer, Waltham, MA) and 10-s pulse centrifugation at 4,000 rpm (Elmi Fugamix Mixer CM70M-09, Riga, Latvia) before periodic vortexing and pulse centrifugation and an overnight incubation at 56°C. After the overnight incubation, only adipose samples were centrifuged at 10,000 g for 10 min at 20°C, forming a lipid layer at the top. The adipose lysate was transferred to a new tube while avoiding the transfer of the lipid layer.
The tissue lysate was added to the DNeasy Mini Spin Column and centrifuged, allowing for the DNA to bind to the column; the flow through was discarded. The column was washed twice, discarding the flow through each time. The DNA was then eluted in two 200-µL elutions per sample as multiple elutions maximized DNA extraction. Each elution was treated separately for quantification and quality analysis. These elutions were stored at 4°C until DNA quantification and then aliquoted for storage at −20°C until DNA quality measurements.
DNA concentration was analyzed using a Qubit DNA HS Assay Kit with the Qubit 2.0 fluorometer (Cat No. Q32851, Life Technologies, Carlsbad, CA). The concentration values for the DNA samples were calculated by the Qubit software based on the standard calibration curve and the volume of sample DNA was analyzed. The two elutions were quantified separately, these values were then added together to get the total DNA concentration from the sample. This was then divided by the weight of the starting tissue sample which provided the DNA content per mg of tissue. To determine the quality of the extracted DNA, A260/280 ratios were obtained using an Eppendorf BioSpectrometer (Eppendorf AG, Hamburg, Germany).
RNA Isolation, Quantification, and Quality
Given our laboratory’s experience with RNA from human tissue samples over the past 20 years (16, 17, 55–58), we applied that knowledge for the current investigation and outlined the approach in Fig. 1. Frozen tissue samples were weighed at −30°C (adipose: 96.0 ± 2.8 mg, range 91.9–99.8 mg; skeletal muscle: 13.9 ± 2.6 mg, range 10.3–17.2 mg), and total RNA was extracted using RNAzol RT (Molecular Research Center, Cincinnati, OH) reagent according to the manufacturer’s instructions (Fig. 1). Polyacryl carrier (Molecular Research Center, Cincinnati, OH) was added to the RNAzol before frozen tissue was homogenized for 30 s using a motorized Polytron PT 1200 homogenizer (Kinematica, Bohemia, NY) set to high.
The homogenate was then incubated at room temperature for ∼10 min. Each tissue homogenate was treated with an additional centrifugation at 12,000 g for 5 min at 4°C producing a lipid layer only at the top of the adipose sample tubes, which was not removed. After DNA, protein, and polysaccharide precipitation, the RNA-containing supernatant was removed. RNA was precipitated with isopropanol to form a pellet, which was washed and solubilized in 30 µL of RNase-free water. After overnight storage at 4°C, the samples were transferred and stored at −80°C until quantification and quality analyses.
RNA concentration was analyzed by fluorometry using a Qubit RNA HS Assay Kit with the Qubit 2.0 fluorometer (Life Technologies, Carlsbad, CA). RNA concentration was then used to calculate total RNA, which was then divided by the starting weight of the sample to provide the RNA content per mg of tissue. To determine the quality and integrity of the extracted RNA, an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA) was used to obtain a RNA Integrity Number (RIN) following the manufacturer’s protocol for RNA 6000 Nano LabChip kits as previously described (17, 20, 59).
Protein Isolation and Quantification
Frozen tissue samples were weighed at −30°C (adipose: 100.6 ± 8.8 mg, range 93.5–119.7 mg; skeletal muscle: 12.3 ± 3.3 mg, range 8.3–17.9 mg). Total protein was extracted using RIPA buffer (Pierce Biotechnology, Rockford, IL) (Fig. 1). Protease and phosphatase inhibitors (Pierce Biotechnology, Rockford, IL) were mixed with RIPA buffer in a 1:100 ratio to create the homogenization buffer. Frozen tissue was added to the solution and homogenized with the Omni Tissue Homogenizer and the plastic soft tissue homogenizing probe (Omni International, Kennesaw, GA). Skeletal muscle lysate was stored at −20°C until protein quantification later the same day. Adipose tissue lysate then underwent a modified RELi (Removal of Excess Lipids) protocol, beginning with a 1-h incubation on ice (60). During this incubation, the lysate was disturbed with 15-s vortex at speed 7 every 5 min. After the incubation, the adipose tissue lysate was centrifuged three times at 20,000 g for 15 min at 4°C. The lysate was transferred to a new tube between each centrifugation while avoiding the transfer of the lipid layer. After the final centrifugation, the lysate was transferred once more and stored at −20°C until protein quantification later the same day.
Protein concentration was analyzed by colorimetric spectrophotometry using a Pierce BCA Protein Assay Kit (Cat. No. 23227, Pierce Biotechnology, Rockford, IL) with the BioTek plate reader (BioTek ELx808, BioTek Instruments, Winoski, VT). Sample concentrations were determined using a standard curve, and this was then used to calculate total protein of the starting tissue sample. Once the total µg protein was calculated, this was divided by the starting weight of the tissue to provide the protein content per milligram of tissue.
Statistical Analysis
DNA, RNA, and protein in adipose and skeletal muscle were compared with a two-tailed paired samples t test and values of P < 0.05 were considered statistically significant. All data are presented as means ± SD, unless otherwise noted.
RESULTS
DNA
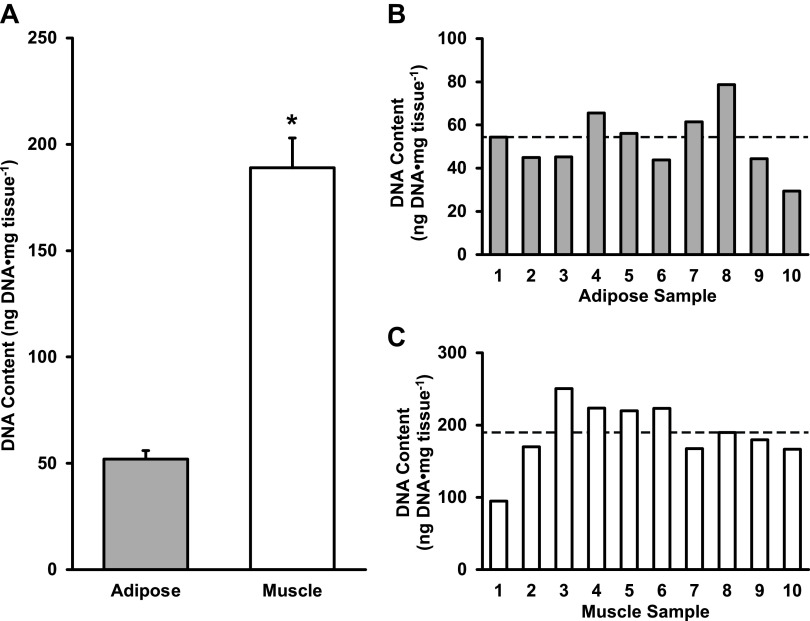
Using the DNeasy Blood & Tissue DNA extraction column, human adipose tissue had 52 ± 14 ng DNA·mg tissue−1, which was 28% of the DNA found in skeletal muscle (189 ± 44 ng DNA·mg tissue−1) (P < 0.05, Fig. 2A). Individual DNA content from adipose (ranging from 29 to 79 ng DNA·mg tissue−1) and skeletal muscle (ranging from 95 to 250 ng DNA·mg tissue−1) tissues are shown in Fig. 2, B and C, respectively. DNA purity A260/280 ratio for the first elution of adipose tissue averaged 2.4 ± 0.2, whereas the second elution averaged 2.9 ± 0.9. A260/280 ratios for the first elution of skeletal muscle averaged 2.1 ± 0.1 and 1.5 ± 0.3 for the second elution. The ranges of DNA content for skeletal muscle and adipose tissue are presented in Fig. 3, A and D, respectively.
Figure 2.
Average DNA content of adipose and skeletal muscle tissues (A), individual DNA content of adipose (B), and skeletal muscle tissues (C). Data are shown as means ± SD. *P < 0.05 vs. adipose. Dotted line represents group mean.
Figure 3.
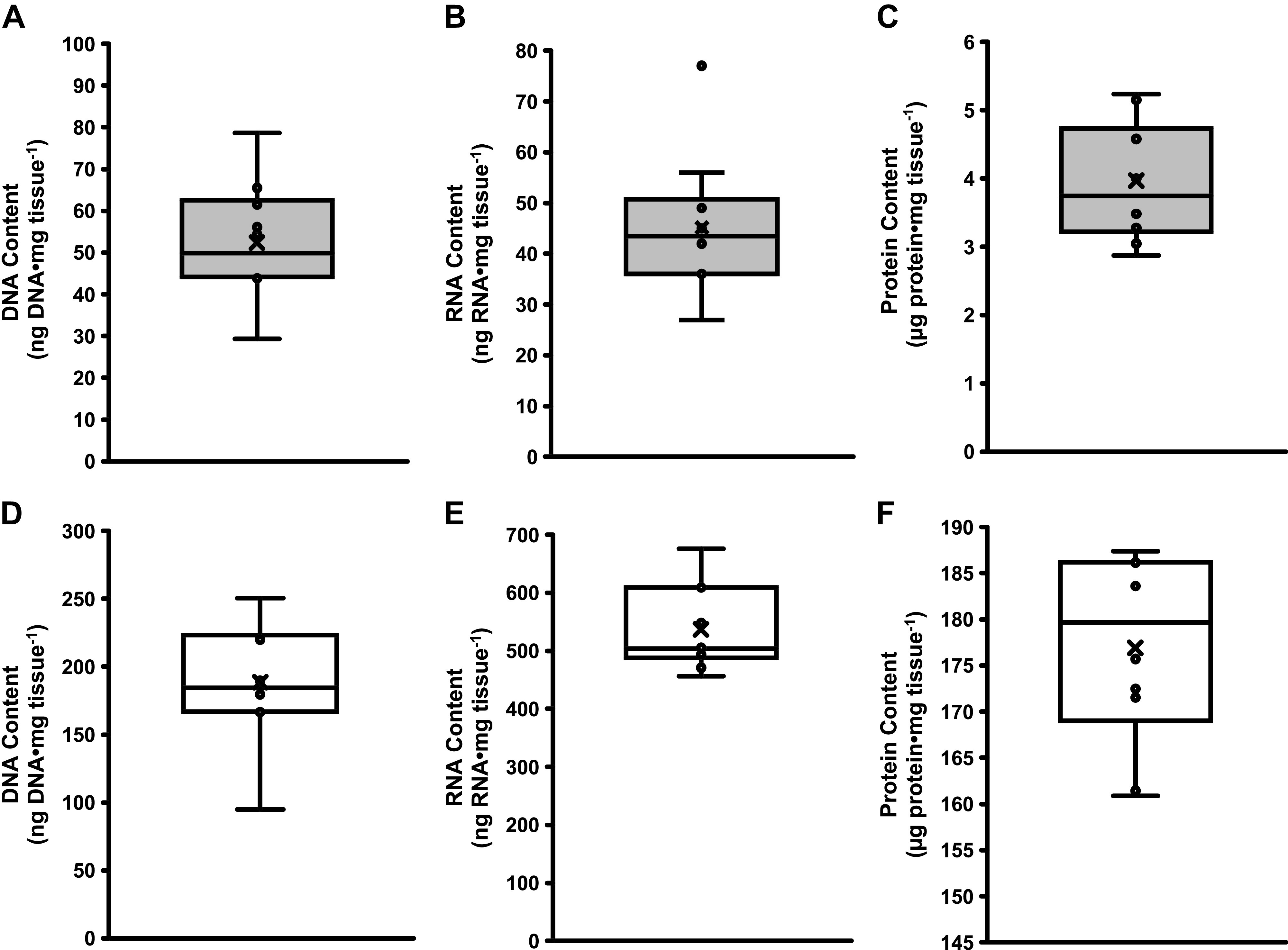
Box and whisker plots for adipose tissue (gray bars) DNA content (A), RNA content (B), and protein content (C) and skeletal muscle (open bars) DNA content (D), RNA content (E), and protein content (F).
RNA
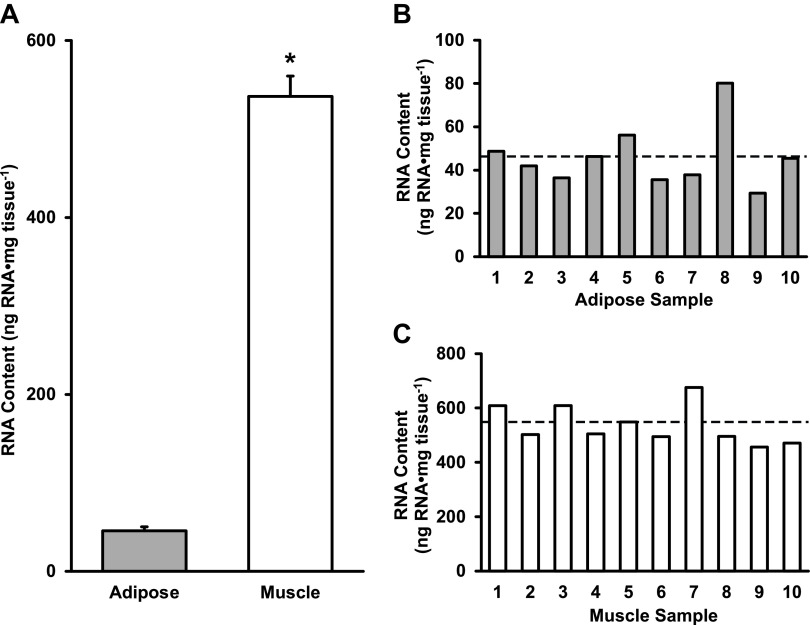
Using the RNAzol extraction protocol, human adipose tissue had 46 ± 14 ng RNA·mg tissue−1, which was 9% of the RNA found in skeletal muscle (537 ± 72 ng RNA·mg tissue−1) (P < 0.05, Fig. 4A). Individual RNA content from adipose (ranging from 29 to 80 ng RNA·mg tissue−1) and skeletal muscle (ranging from 456 to 676 ng RNA·mg tissue−1) tissues are shown in Fig. 4, B and C, respectively. RNA quality measurements yielded RIN values of 7.8 ± 1.0 for adipose and 7.9 ± 0.6 for muscle tissue. The ranges of RNA content for skeletal muscle and adipose tissue are presented in Fig. 3, B and E, respectively.
Figure 4.
Average RNA content of adipose and skeletal muscle tissues (A), individual RNA content of adipose (B), and skeletal muscle tissues (C). Data are shown as means ± SD. *P < 0.05 vs. adipose. Dotted line represents group mean.
Protein
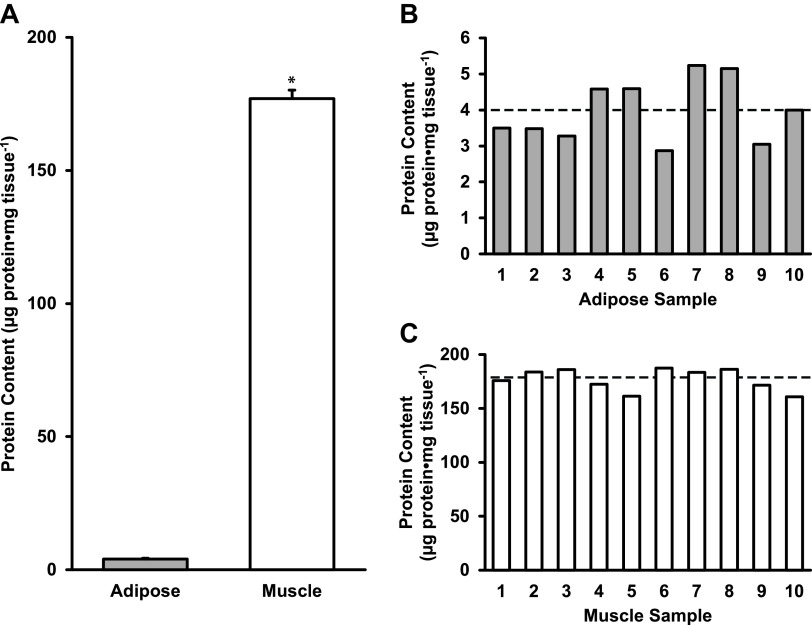
Using RIPA buffer and a modified RELi protocol, human adipose tissue had 4 ± 1 µg protein·mg tissue−1, which was 2% of the protein found in skeletal muscle (177 ± 10 µg protein·mg tissue−1) (P < 0.05, Fig. 5A). Individual protein content from adipose (ranging 3 to 5 µg protein·mg tissue−1) and skeletal muscle (ranging from 161 to 187 µg protein·mg tissue−1) tissues are shown in Fig. 5, B and C, respectively. The ranges of protein content for skeletal muscle and adipose tissue are presented in Fig. 3, C and F, respectively.
Figure 5.
Average protein content of adipose and skeletal muscle tissues (A), individual protein content of adipose (B), and skeletal muscle tissues (C). Data are shown as means ± SD. *P < 0.05 vs. adipose. Dotted line represents group mean.
DISCUSSION
This study examined profiles of DNA, RNA, and protein contents in human adipose tissue. Skeletal muscle was included as a comparison tissue that is commonly studied in exercise physiology and to provide insight into potential differences between these two metabolic tissues. The primary findings from this investigation show that human adipose tissue has 28% of the DNA, 9% of the RNA, and 2% of the protein found in skeletal muscle per mg of tissue. The variability of DNA content between individuals was similar for both adipose and skeletal muscle tissue. The interindividual variability in RNA and protein contents were larger for adipose tissue than skeletal muscle, which should be taken into consideration when isolating appropriate amounts of RNA or protein from adipose tissue in future studies. This information can be applied across a wide range of human clinical investigation study designs and various laboratory analyses, including large-scale -omics applications.
When isolating DNA, RNA, or protein, most studies have a goal of obtaining enough high-quality material for downstream assays, and thus, there are few with the goal of quantifying total DNA, RNA, or protein content in the tissue. More specifically, the studies that did aim to quantify total DNA in tissues reported a range of results depending on the extraction method used. When comparing methods, it was apparent in the values obtained for DNA content of tissue that the yield was method dependent. This was shown in studies of skeletal muscle (14, 38), as well as in the preliminary work completed for the current study (Table 2). Values for adipose tissue DNA content using methodology from the late 1960s were reported to range from 74 to 200 ng DNA·mg tissue−1 (41, 42). The results from this study fall below these values with an average of 52 ± 14 ng DNA·mg tissue−1 and range of 29 to 79 ng DNA·mg tissue−1. These lower adipose tissue DNA content values could be a result of loss within the transfer of the homogenate to the DNeasy Blood & Tissue column while avoiding the lipid layer or as a result of lipid contamination during lysis and extraction while using the column method. The discrepancies in adipose tissue DNA contents in previous literature generated more than 50 years ago compared with the present study may be due to advances in methodologies used today that are considered more precise and accurate, even when contaminants are present in a given sample (39, 40, 61–63). The DNA content of skeletal muscle from this study averaged 189 ± 44 ng DNA·mg tissue−1 and ranged from 95 to 250 ng DNA·mg tissue−1, which falls within the range of values reported in previous literature (14, 42).
The RNAzol protocol for total extraction of RNA yielded high-quality RNA that also allowed for calculation of total RNA quantity within tissue samples. To date, few studies have attempted to determine the total quantity of RNA within a given human adipose tissue sample (43, 44). By using the RNAzol protocol and correction calculations, we were able to report total RNA contents of tissue samples in relation to the starting weight of the tissue. Values reported here are approximately two times that of what others have reported when using commercially available RNA extraction kits (43, 44). However, others who have modified their RNA extraction protocols to optimize high-quality RNA yields from high lipid tissues have reported values similar to the findings of the current investigation. Viguerie et al. (44) reported 44 ± 16 ng RNA·mg adipose tissue−1 (means ± SE) using in-house modified RNeasy Lipid Tissue Mini Kit, which aligns with the value currently being reported of 46 ± 14 ng RNA·mg adipose tissue−1 using the RNAzol protocol. Our skeletal muscle RNA content is comparable with the most recent reports that have isolated and quantified RNA using a range of methods (47, 64).
There are protein content values reported for both adipose and skeletal muscle tissues in the literature, but these studies have their limitations of subject demographics and sample size. Human adipose tissue total protein content has been reported to range from 10 to 79 µg protein·mg tissue−1 (48, 49, 51–53). The results from this study fall below this range with an average of 4 ± 1 μg protein·mg tissue−1 and range from 2.9 to 5.2 µg protein·mg tissue−1. This inconsistency with the literature could be due to different methods used for protein extraction. The protocol used in this study was a modified version of the RELi protocol proposed by Diaz Marin et al. (60) for protein extraction from white, brown, and beige adipose tissues. However, the homogenization used in this study was not as vigorous. Therefore, it is possible that some proteins were not extracted from the lipids in the adipose tissue, meaning the proteins became encapsulated in the lipids and were removed during processing, thus they were not accounted for in the total content. The values for human skeletal muscle protein content that have been reported in literature range from 152 to 219 µg protein·mg tissue−1 (48–51, 53). This current study reported an average skeletal muscle protein content of 177 ± 10 μg protein·mg tissue−1 and a range of 161 to 187 µg protein·mg tissue−1, aligning with previously reported values in literature (48–51, 53).
Adipose tissue is known to provide challenges for clean extraction of biomolecules due to its high lipid content (35–37). To aid in this, additional steps can be added to standard protocols. In this study, additional centrifugations were used to further separate a lipid layer during extraction while being cautious to avoid carrying over any lipid to prevent contamination. Use of phase separation such as chloroform and phenol or a more vigorous homogenization may also assist in providing cleaner extractions. However, compatibility with downstream assays should be considered when using additional steps in the extraction protocols to increase yield or to clean up biomolecules.
In summary, we present data on adipose and skeletal muscle DNA, RNA, and protein contents, as well as an overview of various extraction methods for these tissues. These data help fill a fundamental knowledge gap in adipose tissue requirements for molecular biology assays and show that more adipose tissue is required than skeletal muscle to achieve reasonable concentrations for downstream assays. Importantly, both adipose and skeletal muscle tissue needs for DNA, RNA, and protein content is well within the expected range of yields that are easily attainable with current human adipose and skeletal muscle biopsy procedures. From our experience and that of others (35–37), the lipid enrichment of the adipose tissue does provide some challenges in obtaining total DNA, RNA, and protein content and further optimization may improve upon the content from adipose tissue. However, the data presented here provide strong guidance for adipose and skeletal muscle content for use across a wide range of applications. Moving forward, these adipose and skeletal muscle characteristics will be applicable to numerous human studies, such as the Molecular Transducers of Physical Activity Consortium (MoTrPAC) initiative (65), to probe the molecular responses of muscle and adipose tissues to help elucidate how exercise improves health and ameliorates disease.
GRANTS
This research was supported by National Institutes of Health (U01AR071133) and Ball State University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.M.S., C.E.L., B.E.L., K.M., T.A.T., and S.W.T. conceived and designed research; A.M.S., C.E.L., B.E.L., K.M., T.L.C., C.F.M., C.C.M., W.A.F., T.A.T., and S.W.T. performed experiments; A.M.S., C.E.L., B.E.L., K.M., and S.W.T. analyzed data; A.M.S., C.E.L., B.E.L., K.M., and S.W.T. interpreted results of experiments; A.M.S., C.E.L., and S.W.T. prepared figures; A.M.S., C.E.L., and S.W.T. drafted manuscript; A.M.S., C.E.L., B.E.L., K.M., T.L.C., C.F.M., C.C.M., W.A.F., T.A.T., and S.W.T. edited and revised manuscript; A.M.S., C.E.L., B.E.L., K.M., T.L.C., C.F.M., C.C.M., W.A.F., T.A.T., and S.W.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We are appreciative of the volunteers who gave their time and participated in this study. We thank the students and staff in the Human Performance Lab (HPL) who helped with the various aspects of data collection and interaction with the volunteers.
REFERENCES
- 1.Bergström J. Muscle electrolytes in man. Scand J Clin Lab Invest 14: 511–513, 1962. [Google Scholar]
- 2.Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Arch Neurol 23: 369–379, 1970. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- 3.Gollnick PD, Armstrong RB, Saubert CW, Piehl K, Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol 33: 312–319, 1972. doi: 10.1152/jappl.1972.33.3.312. [DOI] [PubMed] [Google Scholar]
- 4.Gollnick PD, Armstrong RB, Sembrowich WL, Shepherd RE, Saltin B. Glycogen depletion pattern in human skeletal muscle fibers after heavy exercise. J Appl Physiol 34: 615–618, 1973. doi: 10.1152/jappl.1973.34.5.615. [DOI] [PubMed] [Google Scholar]
- 5.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242: 2278–2282, 1967. doi: 10.1016/S0021-9258(18)96046-1. [DOI] [PubMed] [Google Scholar]
- 6.Barnard RJ, Edgerton VR, Peter JB. Effect of exercise on skeletal muscle. I. Biochemical and histochemical properties. J Appl Physiol 28: 762–766, 1970. doi: 10.1152/jappl.1970.28.6.762. [DOI] [PubMed] [Google Scholar]
- 7.Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Pedersen BK. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529: 237–242, 2000. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmons JA, Knudsen S, Rankinen T, Koch LG, Sarzynski M, Jensen T, Keller P, Scheele C, Vollaard NB, Nielsen S, Akerström T, MacDougald OA, Jansson E, Greenhaff PL, Tarnopolsky MA, van Loon LJ, Pedersen BK, Sundberg CJ, Wahlestedt C, Britton SL, Bouchard C. Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J Appl Physiol (1985) 108: 1487–1496, 2010. doi: 10.1152/japplphysiol.01295.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab 279: E806–E814, 2000. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman NJ, Parker BL, Chaudhuri R, Fisher-Wellman KH, Kleinert M, Humphrey SJ, Yang P, Holliday M, Trefely S, Fazakerley DJ, Stöckli J, Burchfield JG, Jensen TE, Jothi R, Kiens B, Wojtaszewski JF, Richter EA, James DE. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metab 22: 922–935, 2015. [Erratum in Cell Metab 22: 948, 2015]. doi: 10.1016/j.cmet.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coggan AR, Spina RJ, Rogers MA, King DS, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic characteristics of skeletal muscle in master athletes. J Appl Physiol (1985) 68: 1896–1901, 1990. doi: 10.1152/jappl.1990.68.5.1896. [DOI] [PubMed] [Google Scholar]
- 12.Luden N, Hayes E, Galpin A, Minchev K, Jemiolo B, Raue U, Trappe TA, Harber MP, Bowers T, Trappe S. Myocellular basis for tapering in competitive distance runners. J Appl Physiol (1985) 108: 1501–1509, 2010. doi: 10.1152/japplphysiol.00045.2010. [DOI] [PubMed] [Google Scholar]
- 13.Gries KJ, Raue U, Perkins RK, Lavin KM, Overstreet BS, D'Acquisto LJ, Graham B, Finch WH, Kaminsky LA, Trappe TA, Trappe S. Cardiovascular and skeletal muscle health with lifelong exercise. J Appl Physiol (1985) 125: 1636–1645, 2018. doi: 10.1152/japplphysiol.00174.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begue G, Raue U, Jemiolo B, Trappe S. DNA methylation assessment from human slow- and fast-twitch skeletal muscle fibers. J Appl Physiol (1985) 122: 952–967, 2017. doi: 10.1152/japplphysiol.00867.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brocca L, McPhee JS, Longa E, Canepari M, Seynnes O, De Vito G, Pellegrino MA, Narici M, Bottinelli R. Structure and function of human muscle fibres and muscle proteome in physically active older men. J Physiol 595: 4823–4844, 2017. doi: 10.1113/JP274148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol 112: 1625–1636, 2012. doi: 10.1152/japplphysiol.00435.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jemiolo B, Trappe S. Single muscle fiber gene expression in human skeletal muscle: validation of internal control with exercise. Biochem Biophys Res Commun 320: 1043–1050, 2004. doi: 10.1016/j.bbrc.2004.05.223. [DOI] [PubMed] [Google Scholar]
- 18.Chi MM, Hintz CS, Coyle EF, Martin WH 3rd, Ivy JL, Nemeth PM, Holloszy JO, Lowry OH. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol Cell Physiol 244: C276–C287, 1983. doi: 10.1152/ajpcell.1983.244.3.C276. [DOI] [PubMed] [Google Scholar]
- 19.Coggan AR, Kohrt WM, Spina RJ, Bier DM, Holloszy JO. Endurance training decreases plasma glucose turnover and oxidation during moderate-intensity exercise in men. J Appl Physiol (1985) 68: 990–996, 1990. doi: 10.1152/jappl.1990.68.3.990. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol (1985) 98: 1745–1752, 2005. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]
- 21.Keller C, Keller P, Marshal S, Pedersen BK. IL-6 gene expression in human adipose tissue in response to exercise–effect of carbohydrate ingestion. J Physiol 550: 927–931, 2003. doi: 10.1113/jphysiol.2003.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riis S, Christensen B, Nellemann B, Møller AB, Husted AS, Pedersen SB, Schwartz TW, Jørgensen JOL, Jessen N. Molecular adaptations in human subcutaneous adipose tissue after ten weeks of endurance exercise training in healthy males. J Appl Physiol (1985) 126: 569–577, 2019. doi: 10.1152/japplphysiol.00989.2018. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8: 457–465, 2012. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 24.Li F, Li Y, Duan Y, Hu CA, Tang Y, Yin Y. Myokines and adipokines: involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor Rev 33: 73–82, 2017. doi: 10.1016/j.cytogfr.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol 536: 329–337, 2001. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carson BP. The potential role of contraction-induced myokines in the regulation of metabolic function for the prevention and treatment of type 2 diabetes. Front Endocrinol (Lausanne) 8: 97, 2017. doi: 10.3389/fendo.2017.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ottaviani E, Malagoli D, Franceschi C. The evolution of the adipose tissue: a neglected enigma. Gen Comp Endocrinol 174: 1–4, 2011. doi: 10.1016/j.ygcen.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Stanford KI, Goodyear LJ. Exercise regulation of adipose tissue. Adipocyte 5: 153–162, 2016. doi: 10.1080/21623945.2016.1191307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, Bahalim AN, McIntire RK, Gutierrez HR, Cowan M, Paciorek CJ, Farzadfar F, Riley L, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr 10: 22, 2012. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanford KI, Middelbeek RJW, Goodyear LJ. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes 64: 2361–2368, 2015. [Erratum in Diabetes 64: 3334, 2015]doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab 20: 396–407, 2014. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Crampes F, Beauville M, Riviere D, Garrigues M. Effect of physical training in humans on the response of isolated fat cells to epinephrine. J Appl Physiol (1985) 61: 25–29, 1986. doi: 10.1152/jappl.1986.61.1.25. [DOI] [PubMed] [Google Scholar]
- 33.You T, Wang X, Yang R, Lyles MF, Gong D, Nicklas BJ. Effect of exercise training intensity on adipose tissue hormone sensitive lipase gene expression in obese women under weight loss. J Sport Health Sci 1: 184–190, 2012. doi: 10.1016/j.jshs.2012.10.001. [DOI] [Google Scholar]
- 34.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463–468, 2012. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zakharchenko O, Greenwood C, Alldridge L, Souchelnytskyi S. Optimized protocol for protein extraction from the breast tissue that is compatible with two-dimensional gel electrophoresis. Breast Cancer (Auckl) 5: 37–42, 2011. doi: 10.4137/BCBCR.S6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sajic T, Hopfgartner G, Szanto I, Varesio E. Comparison of three detergent-free protein extraction protocols for white adipose tissue. Anal Biochem 415: 215–217, 2011. doi: 10.1016/j.ab.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Fabre O, Ingerslev LR, Garde C, Donkin I, Simar D, Barrès R. Exercise training alters the genomic response to acute exercise in human adipose tissue. Epigenomics 10: 1033–1050, 2018. doi: 10.2217/epi-2018-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majumdar G, Vera S, Elam MB, Raghow R. A streamlined protocol for extracting RNA and genomic DNA from archived human blood and muscle. Anal Biochem 474: 25–27, 2015. doi: 10.1016/j.ab.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 39.Le Pecq JB. Use of ethidium bromide for separation and determination of nucleic acids of various conformational forms and measurement of their associated enzymes. Methods Biochem Anal 20: 41–86, 1971. doi: 10.1002/9780470110393.ch2. [DOI] [PubMed] [Google Scholar]
- 40.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem 102: 344–352, 1980. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 41.Baker GL. Human adipose tissue composition and age. Am J Clin Nutr 22: 829–835, 1969. doi: 10.1093/ajcn/22.7.829. [DOI] [PubMed] [Google Scholar]
- 42.Martinsson A. On the composition of human adipose tissue. Acta Med Scand 182: 795–803, 1967. doi: 10.1111/j.0954-6820.1967.tb10909.x. [DOI] [PubMed] [Google Scholar]
- 43.Cashion AK, Umberger RA, Goodwin SB, Sutter TR. Collection and storage of human blood and adipose for genomic analysis of clinical samples. Res Nurs Health 34: 408–418, 2011. doi: 10.1002/nur.20448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viguerie N, Montastier E, Maoret JJ, Roussel B, Combes M, Valle C, Villa-Vialaneix N, Iacovoni JS, Martinez JA, Holst C, Astrup A, Vidal H, Clément K, Hager J, Saris WH, Langin D. Determinants of human adipose tissue gene expression: impact of diet, sex, metabolic status, and cis genetic regulation. PLoS Genet 8: e1002959, 2012. doi: 10.1371/journal.pgen.1002959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gibson JN, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond) 72: 503–509, 1987. doi: 10.1042/cs0720503. [DOI] [PubMed] [Google Scholar]
- 46.Gamrin L, Berg HE, Essén P, Tesch PA, Hultman E, Garlick PJ, McNurlan MA, Wernerman J. The effect of unloading on protein synthesis in human skeletal muscle. Acta Physiol Scand 163: 369–377, 1998. doi: 10.1046/j.1365-201X.1998.t01-1-00391.x. [DOI] [PubMed] [Google Scholar]
- 47.Stec MJ, Mayhew DL, Bamman MM. The effects of age and resistance loading on skeletal muscle ribosome biogenesis. J Appl Physiol (1985) 119: 851–857, 2015. doi: 10.1152/japplphysiol.00489.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forbes GB, Lewis AM. Total sodium, potassium and chloride in adult man. J Clin Invest 35: 596–600, 1956. doi: 10.1172/JCI103313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forbes RM, Cooper AR, Mitchell HH. The composition of the adult human body as determined by chemical analysis. J Biol Chem 203: 359–366, 1953. doi: 10.1016/s0021-9258(19)52646-1. [DOI] [PubMed] [Google Scholar]
- 50.Haus JM, Carrithers JA, Carroll CC, Tesch PA, Trappe TA. Contractile and connective tissue protein content of human skeletal muscle: effects of 35 and 90 days of simulated microgravity and exercise countermeasures. Am J Physiol Regul Integr Comp Physiol 293: R1722–R1727, 2007. doi: 10.1152/ajpregu.00292.2007. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell HH, Hamilton TS, Steggerda FR, Bean HW. The chemical composition of the adult human body and its bearing on the biochemistry of growth. J Biol Chem 158: 625–637, 1945. doi: 10.1016/S0021-9258(19)51339-4. [DOI] [Google Scholar]
- 52.Thomas LW. The chemical composition of adipose tissue of man and mice. Q J Exp Physiol Cogn Med Sci 47: 179–188, 1962. doi: 10.1113/expphysiol.1962.sp001589. [DOI] [PubMed] [Google Scholar]
- 53.Woodard HQ, White DR. The composition of body tissues. Br J Radiol 59: 1209–1218, 1986. doi: 10.1259/0007-1285-59-708-1209. [DOI] [PubMed] [Google Scholar]
- 54.Klein JA. Tumescent Technique: Tumescent Anesthesia & Microcannular Liposuction. St. Louis, MO: Mosby, 2000. [Google Scholar]
- 55.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 103: 1744–1751, 2007. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 56.Raue U, Jemiolo B, Yang Y, Trappe S. TWEAK-Fn14 pathway activation after exercise in human skeletal muscle: insights from two exercise modes and a time course investigation. J Appl Physiol (1985) 118: 569–578, 2015. doi: 10.1152/japplphysiol.00759.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trappe TA, Carroll CC, Jemiolo B, Trappe SW, Døssing S, Kjaer M, Magnusson SP. Cyclooxygenase mRNA expression in human patellar tendon at rest and after exercise. Am J Physiol Regul Integr Comp Physiol 294: R192–R199, 2008. doi: 10.1152/ajpregu.00669.2007. [DOI] [PubMed] [Google Scholar]
- 58.Sullivan BE, Carroll CC, Jemiolo B, Trappe SW, Magnusson SP, Døssing S, Kjaer M, Trappe TA. Effect of acute resistance exercise and sex on human patellar tendon structural and regulatory mRNA expression. J Appl Physiol 106: 468–475, 2009. doi: 10.1152/japplphysiol.91341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 300: R655–R662, 2011. doi: 10.1152/ajpregu.00611.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diaz Marin R, Crespo-Garcia S, Wilson AM, Sapieha P. RELi protocol: Optimization for protein extraction from white, brown and beige adipose tissues. MethodsX 6: 918–928, 2019. doi: 10.1016/j.mex.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gallagher SR. Quantitation of DNA and RNA with absorption and fluorescence spectroscopy. Curr Protoc Mol Biol 93: A.3D.1–A.3D.14, 2011. doi: 10.1002/0471142727.mba03ds93. [DOI] [PubMed] [Google Scholar]
- 62.Gallagher S, Desjardins P. Quantitation of nucleic acids and proteins. Curr Protoc Essential Lab Tech 5: 2.2.1–2.2.36, 2011. doi: 10.1002/9780470089941.et0202s5. [DOI] [Google Scholar]
- 63.Molecular Probes Life Technologies. Qubit® dsDNA HS Assay Kits User Guide. Thermo Fisher Scientific Inc., 2015, p. 1–10. [Google Scholar]
- 64.Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Phillips BE, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J Physiol 594: 7399–7417, 2016. doi: 10.1113/JP272857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanford JA, Nogiec CD, Lindholm ME, Adkins JN, Amar D, Dasari S, Drugan JK, Fernández FM, Radom-Aizik S, Schenk S, Snyder MP, Tracy RP, Vanderboom P, Trappe S, Walsh MJ; Molecular Transducers of Physical Activity Consortium . Molecular Transducers of Physical Activity Consortium (MoTrPAC): mapping the dynamic responses to exercise. Cell 181: 1464–1474, 2020. doi: 10.1016/j.cell.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]