Abstract
Lipocalin-2 (LCN2) is an inflammatory mediator best known for its role as an innate acute-phase protein. LCN2 mediates the innate immune response to pathogens by sequestering iron, thereby inhibiting pathogen growth. Although LCN2 and its bacteriostatic properties are well studied, other LCN2 functions in the immune response to inflammatory stimuli are less well understood, such as its role as a chemoattractant and involvement in the regulation of cell migration and apoptosis. In the lungs, most studies thus far investigating the role of LCN2 in the immune response have looked at pathogenic inflammatory stimuli. Here, we compile data that explore the role of LCN2 in the immune response to various inflammatory stimuli in an effort to differentiate between protective versus detrimental roles of LCN2.
Keywords: LCN2, lipocalin-2, lung inflammation, neutrophil gelatinase-associated lipocalin, NGAL
INTRODUCTION
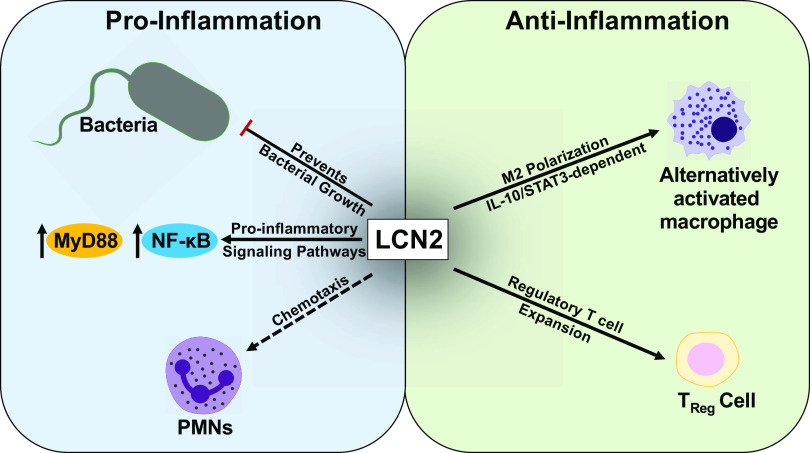
Lipocalin-2 (LCN2), also known as neutrophil gelatinase-associated lipocalin (NGAL), is an acute-phase protein that is secreted by immune and epithelial cells in various mucosal tissues. LCN2 was first discovered in 1989 in granules of neutrophils as a 25-kDa protein covalently linked to matrix metalloproteinase 9 (MMP9) but is also expressed in macrophages, eosinophils, basophils, and epithelial cells. LCN2 is associated with acute inflammation and is involved in the regulation of host responses to inflammation (1). Thus, it likely plays an inflammatory role at mucosal surfaces and is associated with a wide range of pathologies. Common pathologies associated with LCN2 inflammatory roles include heart failure, kidney disease, and gut inflammation, and LCN2 has been proposed as a biomarker for the identification of each of these diseases (2–6). LCN2 is also found in certain tissues such as the lungs under normal physiological conditions and has been shown to play a critical role in controlling the pathogenesis of bacterial infections (7–9). Current literature suggests that LCN2 plays paradoxical roles in lung inflammation by recruiting neutrophils and inducing proinflammatory cytokine signaling, while also promoting anti-inflammatory, alternatively activated or “M2-like” macrophage polarization (10). Given these seemingly contradictory roles that LCN2 plays in the lung immune response (Fig. 1), the role of LCN2 in lung inflammation remains to be better characterized.
Figure 1.
Lipocalin 2 (LCN2) roles in mucosal surfaces. In response to inflammation induced by various stimuli, LCN2 levels increase and have been shown to mediate both pro- and anti-inflammatory responses. Left: proinflammatory responses: 1) LCN2 is most commonly known for its innate response to bacteria and iron scavenging abilities, thereby preventing bacterial growth. 2) Additionally, LCN2 increases proinflammatory signaling pathways. 3) LCN2 has also been shown to recruit polymorphonuclear leukocytes (PMNs) to the site of inflammation. Right: anti-inflammatory responses: 1) LCN2 skews toward alternatively activated, “M2-like” macrophage polarization through an IL-10/STAT3-dependent manner. 2) LCN2 also plays a role in the expansion of regulatory T cells.
In this review, we compile information on LCN2 homeostasis and LCN2-mediated effects during lung inflammatory states. The structure and function of LCN2, as well as its association with lung inflammation and iron homeostasis in the lungs, are discussed. Additionally, the use of LCN2 as a diagnostic biomarker of various pathologies as well as its potential as a biomarker in lung inflammatory conditions is explored. Together, this review aims to highlight our current knowledge regarding the protective versus harmful roles LCN2 may play in lung inflammation and immunity, as well as identify gaps in knowledge with an aim of spurring future research in this area.
LIPOCALIN 2 STRUCTURE AND FUNCTION
Lipocalin 2 Structure
Lipocalin 2 (LCN2) is also known as NGAL, 24p3, siderocalin, and uterocalin (11). LCN2 is a member of the lipocalin family, which consists of proteins that generally bind small hydrophobic ligands (12). The lower region of LCN2’s cuplike structure (calyx) contains hydrophobic amino acid residues that act as binding sites for small lipophilic molecules. Additionally, unlike other lipocalins, the calyx of LCN-2 consists of a larger and shallower mouth lined with positively charged and polar residues that allow the binding of macromolecules and hydrophilic molecules (12, 13). In the lungs, the secretion of LCN2 by immune cells, such as neutrophils and macrophages, and airway epithelial cells is induced during an inflammatory response, while LCN2 secretion by various types of cells also occurs in response to oxidative stress (10, 14, 15). LCN2 can be secreted in three different forms: as a monomer, homodimer, or as a heterodimer with MMP9; the secreted conformation is typically dependent on the cell type secreting it (13). Due to the relatively small and stable nature of LCN2, it has been proposed as a candidate diagnostic marker for inflammatory lung conditions such as lung cancer (13, 14).
Lipocalin 2 Receptors
There are five known LCN2 receptors: megalin (16), 24p3R (Slc22a17), melanocortin 4 receptor (MC4R) (17), melanocortin 1 receptor (MC1R) (18), and melanocortin 3 receptor (MC3R) (17, 18). Megalin is an endocytic receptor expressed primarily on the apical membrane of epithelial cells in several tissues including the lungs, brain, kidney, placenta, endocrine glands, and the genital system (19, 20). Megalin is also expressed in nonepithelial cells such as oligodendrocytes in the spinal cord and neurons in the cerebellum of mice (21). The expression of megalin can be regulated by the nuclear receptor hepatic nuclear factor 4α (22) as well as peroxisomal proliferator-activated receptors (PPARs) (16). The expression of megalin is critical in brain development and in various tissues of adults such as the blood-brain barrier, nervous system, and gallbladder. PPARs serve as protective agents in all of the different physiological systems, highlighting the importance of understanding the mechanisms controlling the expression of megalin (16).
The receptor 24p3R belongs to the organic cation transporter family, is ubiquitously expressed, and plays a crucial role in regulating the expression of iron-responsive genes via its binding to LCN2 and subsequent endo- and exocytosis of LCN2. The endocytosis of both iron-bound and -free LCN2 can be promoted by 24p3R (13). 24p3R has been implicated in LCN2-mediated apoptosis of human epithelial HeLa cells (2). In this study, HeLa cells were transfected with recombinant iron-loaded or iron-lacking 24p3R. The latter resulted in induced expression of the proapoptotic protein Bim and consequently, apoptosis. Furthermore, addition of intracellular iron was able to block the Bim induction and suppress apoptosis. Expression of 243pR receptor can be regulated by the Wnt signaling pathway at the transcriptional level, leading to alternative spliced isoforms of the receptor. Thus, the biological role of 24p3R in cells with an active Wnt pathway may differ from its role in cells without an active Wnt pathway (23).
The melanocortin receptors are seven-transmembrane G protein-coupled receptors (18). These receptors are well known for their roles in steroidogenesis and skin pigmentation but have also been shown to be potential modifiers of inflammatory diseases (18). The receptors are expressed throughout the body and exhibit a myriad of downstream effects, but all are known to activate MAPK and signal through the JAK-STAT pathways (18). Recently, it was discovered that bone-derived LCN2 can regulate food intake by binding to the MC4R (17). LCN2 crosses the blood-brain barrier and binds to MC4R in the paraventricular ventromedial neurons in the hypothalamus (17), which results in suppression of appetite. It is important to note that MC1R and MC3R are also receptors that LCN2 can bind; however, findings from this study showed that MC1R and MC3R do not regulate appetite, unlike the metabolic regulatory effects seen with MC4R. Several studies have shown that the expression of LCN2 receptors increases during inflammation, such as Shao et al. (24) who showed increased levels of 24p3R expression in neutrophils of patients with psoriasis versus normal neutrophils. Most of the findings on changes of LCN2 receptor expression during inflammation have been shown at other mucosal sites and remain to be investigated in the lungs.
Lipocalin 2 Function
LCN2 is involved with inflammatory response in multiple diseases and during infections; however, whether this modulation is beneficial or detrimental is context dependent. For example, LCN2 can regulate chemokine levels during lung inflammation and the recruitment of polymorphonuclear leukocytes (PMNs) (11). Additionally, LCN2 can limit inflammatory responses, as seen in several studies including a systemic LPS challenge where LCN2−/− mice exhibited higher TNFα and IL-6 levels relative to wild-type (WT) controls (25). LCN2 is also involved in apoptosis modulation (12, 13, 26) and has been shown to play a role in cancer progression and chemoresistance depending on the type of tumor (14). Physiologically, monomeric LCN2 is cleared from circulation more rapidly than the dimeric form (27). Epithelial cells can secrete only the monomeric form, and immune cells predominantly secrete dimeric LCN2. In one study aimed at assessing the contribution of immune versus nonimmune cells on systemic LCNs, LCN2 bone marrow chimeras were used to measure LCN2 expression, and they showed that nonimmune cells were the major sources of systemic LCN2 during basal and inflammatory states (28). The two different forms of LCN2 have been used diagnostically to differentiate between acute kidney injury, chronic kidney injury, and urinary tract infection (UTI). Studies have shown that monomeric and dimeric LCN2 as well as their two sources can have different functions. For example, LCN2 is known to facilitate mucosal regeneration by promoting cell migration and forming a heterodimer with MMP-9, and one study showed that immune cell-derived LCN2 molecules are the main contributors to mucosal protection again chronic colitis (28). The roles of immune- versus nonimmune cell-derived and monomeric versus dimeric forms of LCN2 remain to be better characterized, however. LCN2 levels have been shown to be upregulated in both direct and indirect lung injuries, but its exact role in the different lung injury types remains elusive. The levels of LCN2 in direct versus indirect lung injury have been investigated and shown to be increased in direct lung injury; Kangelaris et al. (29) compared LCN2 gene expression levels in patients who had sepsis with and without acute respiratory distress syndrome (29), and found that the former had higher LCN2 gene expression levels . One of the roles that LCN2 is thought to have on direct lung injuries is the deactivation of macrophages (10). LCN2 was shown to skew toward M2-macrophage polarization, thus impacting host defense against bacterial pathogens. Mesenchymal stem cells (30) have been shown to enhance bacterial clearance in pneumonia, and Gupta et al. showed that bacterial clearance was mediated by LCN2, which is upregulated in MSCs in response to inflammatory stimuli (30). Several studies have shown that in sepsis-induced lung injury, increased LCN2 expression induced by epithelial cells as well as plasma NGAL levels can be predictive of multiple organ injuries induced by sepsis (31). Although LCN2 has been shown to increase in indirect injuries, is capable of binding to inflammatory mediators such as LPS, and binds MMP-9, its direct roles remain elusive. In the next sections, we highlight several of the key functions of LCN2 in the context of immune response, iron homeostasis, and microbial pathogenesis.
LIPOCALIN 2 AND INFLAMMATION
LCN2 and Its Role in Inflammation and Immune Response
LCN2 is an important inflammatory mediator in both acute and chronic inflammation. In the lung, LCN2 can be rapidly induced and secreted into the alveolar spaces by airway epithelial cells, alveolar macrophages, and neutrophils in response to inflammation (9). LCN2 enhances and sustains the inflammatory response by serving as a chemoattracting factor for the recruitment of neutrophils (32). LCN2 recruitment of neutrophils has been shown in chronic inflammatory pain, alcoholic liver disease, and psoriasis studies (4, 6, 33). Upon recruitment, the PMNs then stimulate the production of proinflammatory cytokines and chemokines, including LCN2, creating a feedback loop that drives the innate immune response. Additionally, during inflammation, the increased levels of LCN2 expression lead to increased neutrophil infiltration and activation, which then further promote epithelial cell responses including the activation of MyD88 and NF-κB (33). LCN2 reacts with its receptors on various cell types in the lung to exert its biological effects including apoptosis, cell migration, morphological changes, and the amplification of inflammatory responses (33). LCN2 regulates iron metabolism and the Bim (2) pathway, allowing it to also regulate cell death and cell survival (34). The clearance of apoptotic immune cells such as neutrophils in response to inflammation is necessary for immune resolution, thus highlighting the importance of the functional role of LCN2 on apoptosis and the prevention of a prolonged inflammatory response.
LCN2 also plays a role in the expansion of regulatory T cells (35). The aim of this study was to investigate how LCN2 modulates T-cell response, specifically, via its regulation of the human leukocyte antigen G complex (35). Peripheral blood mononuclear cells from healthy donors were treated with different concentrations of NGAL in iron-loaded and/or iron-free forms and the regulatory T-cell population was identified as CD4 + CD25+FoxP3+ cells using flow cytometry. The findings showed that LCN2 increases HLA-G expression during acute and chronic inflammatory responses and expansion of regulatory T cells, thus indicating a potential protective role of LCN2 as an immune activator.
Several studies have shown that the induction of LCN2 expression in response to inflammation is mediated by the MyD88 signaling pathway (11, 28, 33). One form of neutrophil activation is via the formation of neutrophil extracellular traps (33), a process of cell death. This process of cell death is known as ETosis and was first observed in neutrophils; however, other immune cells such as macrophages, eosinophils, and basophils have all been reported to undergo ETosis. ETosis consists of two major events: chromatin unfolding and the production of reactive oxygen species (ROS), which lead to the release of the decondensed chromatin along with the granular contents into the extracellular space (36). In keratinocytes of psoriasis patients, neutrophil extracellular traps (NETs) stimulate epithelial cells to produce high levels of inflammatory mediators such as LCN2 and IL-36γ. IL-36γ then induces Toll-like receptor 4 (TLR4) expression, and both synergize and signal through MyD88 and NF-κB to induce further production of LCN2 and IL-36γ. Consequently, the upregulated LCN2 levels modulate neutrophil migration and NET formation, thus contributing to the sustainment or enhancement of the inflammatory response, which is referred to as amplification loops in psoriasis (33).
Interestingly, MyD88 knockout (KO) mice were found to be more susceptible to dextran sulfate sodium (37)-induced colitis than WT mice and to have an impaired LCN2 response, suggesting that MyD88-mediated LCN2 induction may actually help protect against inflammation in the gut (11). Microbiota present in a given tissue, such as the gut microbiota, can also regulate LCN2 expression in a MyD88-dependent manner. For example, a recent investigation measured LCN2 levels from germ-free and conventional WT mice before and after the introduction of fecal contents of conventional mice to germ-free mice. The investigators identified significantly increased LCN2 levels in the conventional WT and conventionalized germ-free mice compared with the germ-free mice, highlighting a role for the gut microbiota in maintaining normal levels of LCN2. Singh et al. (28) showed that in MyD88 KO mice lacking signaling through all TLRs except TLR3, dextran sulfate sodium (DSS)-induced colitis resulted in lower levels of colonic and systemic LCN2. In comparison, they showed that when using adaptive immune response-deficient Rag1 KO mice, colonic and systemic LCN2 levels were unaltered (28). These results highlight the potential protective role of LCN2 against gut inflammation, as shown by the increased susceptibility to DSS-induced colitis and impaired LCN2 response in the MyD88 KO mice. These data are seemingly contrary to other data indicating that LCN2 plays a detrimental or pathological role in response to inflammation, e.g., as seen by the amplification loop in psoriasis mentioned previously (33). A pathological role of LCN2 has also been observed in the brain, where LCN2 contributed to neuronal damage in the brain caused by the HIV viral glycoprotein, gp120. Here, the researchers showed that the ablation of LCN2 leads to the amelioration of neuronal damage, prevention of behavioral deficits, and an increase in the expression of a neuroprotective CCR5 ligand (38). Taken together, there is evidence to suggest that LCN2 could be playing either a protective or detrimental role in the immune response to inflammation. The role of LCN2 in the lungs has been studied primarily using pathogenic and carcinogenic models, as outlined in the microbial pathogenesis section below. However, other known insults of lung inflammation include allergens and particulate matter exposures. Future studies looking specifically at the role of LCN2 in both the innate and adaptive arms of the immune system across different types of inflammation in the lungs are needed. In addition, it is important to consider that LCN2 could potentially be involved in the resolution of the immune response to inflammation, thus emphasizing the need for studies looking into its impact on resolution markers as well as pathological changes at different time points.
LCN2 and Its Role in Microbial Pathogenesis
One of the best-known roles of LCN2 is its impact on microbial pathogenesis. LCN2 inhibits bacterial growth by chelating bacterial siderophores and sequestering iron. Some of the most well-studied bacteria in LCN2 investigations are Escherichia coli and Mycobacterium tuberculosis, which cause various conditions including UTIs and respiratory illnesses (22, 39, 40). While studies of bacterial infection in the lung have provided very useful information that help elucidate the role of LCN2, bacterial studies looking at other organs can also provide insight into the role of LCN2 in the lung. For example, Moschen et al. (41) showed that an LCN2 deficiency in IL10−/− mice, leads to the compensatory expression of antimicrobial peptides. They also showed that altered microbial signatures led to increased growth of pathogenic bacteria and to the progression from colitis to colorectal cancer.
Siderophores are iron chelators essential for the interaction of iron with LCN2. Siderophores are classified into three groups according to their functional chemical group: catecholates, carboxylates, and hydroxamates (13). Endogenous siderophores such as lactoferrin and transferrin help transport iron throughout the body. Bacteria have evolved siderophores that have a higher affinity for iron than host endogenous molecules, such as various Enterobacter species (13). As a result, the host has evolved to produce proteins such as LCN2, which bind to bacterial siderophores heavily loaded with iron and limit the iron availability needed for bacterial growth. During certain bacterial infections in the lungs, host cells can sequester iron and help prevent bacterial growth, which is often described as nutritional immunity and refers to the ability of a host to sequester trace minerals to prevent pathogenicity (20). When the iron supply is scarce, bacteria increase siderophore production to meet their iron requirements and outcompete host siderophores. In response to this, host cells such as airway epithelial cells and neutrophils secrete additional LCN2 to sequester and neutralize the bacteria (13). Interestingly, several reports show that bacteria can evolve to express modified siderophores that can evade recognition by LCN2 (13).
Furthermore, the effects of LCN2 in the pathogenesis of bacterial infections have been shown to be particularly important in the lungs and specifically alveolar epithelial cell responses. Saiga et al. (9) showed that LCN2-deficient mice infected with M. tuberculosis have higher levels of bacteria in alveolar epithelial cells than WT mice, suggesting LCN2 limits the growth of M. tuberculosis (7). In contrast, Guglani et al. (8) showed that LCN2 KO mice did not exhibit a higher bacterial burden than WT mice when infected with M. tuberculosis. However, LCN2 KO mice had reduced PMN levels and increased granuloma sizes, thus LCN2 played a protective role by driving PMN infiltration, as well as reducing the size of granulomas. As a result, animals expressing LCN2 had a lower mortality rate than LCN2-deficient mice (8). Additionally, this group also showed that LCN2 can regulate the infiltration of lymphocytes by inhibiting inflammatory chemokines and that LCN2 promoted the accumulation of neutrophils during mycobacterial infection (8). Another M. tuberculosis chronic infection model provides additional support for the innate role of LCN2, where the investigators found an increased inflammatory response in LCN2 KO mice compared with WT mice as shown with increased number and size of granulomatous lesions in the LCN2 KO mice, possibly due to the progression of M. tuberculosis (9). Furthermore, in the highly susceptible LCN2 KO mice, the impaired mycobacterial clearance was from alveolar epithelial cells, not alveolar macrophages, as assessed by measuring mRNA levels of lung fibroblasts, alveolar macrophages, and tracheal epithelial cells (7, 8).
Similar effects of LCN2 on lung inflammation and bacterial growth were observed in studies using E. coli and Klebsiella pneumonia infection models (39, 40). Chan et al. (40) showed that in response to K. pneumonia, LCN2 KO mice had impaired lung clearance, but that reconstitution with LCN2 rescued the phenotype. Additionally, LCN2 also contributes to skewing toward M2-like polarization and worsens pneumococcal pneumonia outcomes, as shown with elevated levels of LCN2 and IL-10, which were associated with detrimental outcomes (10). In one study looking at the effects of a specialized proresolving lipid mediator (SPM), aspirin-triggered resolvin D1 (42), in the resolution of E.coli-induced pneumonia (42), LCN2 bronchoalveolar lavage (BAL) levels were elevated in mice infected with E. coli. Moreover, AT-RvD1 further increased LCN2 levels in BAL. In addition to these observations, reduced proinflammatory cytokine levels and improved bacterial clearance were observed, thus highlighting potential protective roles in mucosal host defense of LCN2 and SPMs. The roles of LCN2 in lung infections outside of M. tuberculosis, Klebsiella pneumonia, and E. coli remain largely unexplored, and even less is known regarding its role in noninfectious, inflammatory lung conditions, such as asthma, chronic obstructive pulmonary disease, and cancer. For example, LCN2 has been shown to promote lung metastasis of murine breast cancer cells as well as to induce cancer cell apoptosis (2). Lastly, LCN2 levels are also elevated in response to dust-induced lung inflammation, with one example being elevated serum LCN2 levels in patients exposed to house dust mite extracts (43). After undergoing sublingual allergen immunotherapy, LCN2 serum levels were restored to normal levels, and the authors concluded that clinical reactivity in allergic patients may be assessed by serum LCN2 (43). Investigations into the role of LCN2 in these other settings could yield important biological insights considering the roles LCN2 plays in inflammation and the immune response in other inflammatory settings.
LIPOCALIN 2 AND IRON HOMEOSTASIS
Nearly all aerobic animals require iron for cell growth and proliferation, and the required iron is derived from dietary sources and recycled daily to maintain iron homeostasis. Enterocytes in the duodenum of the small intestine absorb nutritional iron. Ferric reductases in the lumen of the intestine reduce the ferric iron (Fe3+) to ferrous iron (Fe2+), which is then transported across the apical membrane of the duodenal enterocytes (44). Ferrous iron can be oxidized by ferric protein within the enterocytes, which leads to storage of iron. Iron can also be exported across the basolateral membrane of enterocytes to the bloodstream depending on the body’s requirements of iron (5, 44). Iron plays a crucial role in providing oxygen to all cells in the body, which is needed for oxidative metabolism and is essential for the proper functioning of many iron-containing proteins. LPS-induced hypoferremia has been shown to be facilitated by LCN2, resulting in the ability of a host to regulate its iron levels based on inflammatory conditions (45). Srinivasin et al. (45) showed that LCN2 acts as an antioxidant by regulating iron homeostasis, which was evident by the protection of an iron chelator known as desferroxamine from LPS-induced toxicity in LCN2 KO mice. Another example showing the regulation of LCN2 on iron homeostasis was shown by Lim et al. (46) who showed that in the brains of patients with dementia, LCN2 regulates iron homeostasis. Authors speculate that as a consequence of LCN2 modulation on iron transport and cell death signaling, LCN2 likely regulates cell differentiation (46). Because iron is required throughout the body, transport of iron to various tissues can be mediated by endogenous siderophores. Lung cells are able to acquire iron from the circulation and exogenously (44). The ability of LCN2 to serve as a scavenger for iron via its binding to siderophores allows for its accumulation of iron, impacting iron equilibrium during both homeostatic and inflammatory/infectious settings. The rapid increase of LCN2 seen in various tissues in response to acute inflammation modulates iron homeostasis, consequently affecting the function of neutrophils (47).
In addition to regulating tissue-specific iron availability, LCN2 also plays a role in preventing deleterious impacts of having high free iron levels. Specifically, iron has the ability to shift between oxidation states and is therefore known as a transition metal. However, this also results in the potential high reactivity and toxicity of free iron. The production of ROS is catalyzed by iron and these free radicals can damage tissue. The binding of iron to proteins can counteract iron’s chemical reactivity. Iron levels are carefully balanced and necessary to ensure the systemic and cellular metabolic needs as well as in the prevention of harmful iron excess (44).
The role of LCN2 in iron homeostasis may be particularly relevant in the respiratory and cardiovascular systems because gas exchange between the atmosphere and the bloodstream occurs through a collaborative effort between numerous cell types in the lungs and vasculature (44). Here, the lungs play a crucial role in the transport of oxygen to all other tissues in the body via the diffusion of oxygen that is transported by red blood cells (RBCs) containing heme groups that bind oxygen through a central iron atom. Iron is needed for not only RBC production and oxygen transport, but for energy production in the mitochondria, DNA synthesis and repair, and transcription (44). Lung cells, like many others, require iron to maintain their metabolic demands and ensure their survival and function. Imbalances of iron levels are known to lead to respiratory problems such as chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and acute respiratory distress syndrome (ARDS) (29, 44). Abnormal iron metabolism and catalytically active metals can cause oxidative stress and lead to cell and tissue damage. Common symptoms of ARDS include severe shortness of breath and rapid breathing, and it is important to note that patients with ARDS have higher levels of iron and iron-related proteins in their lower respiratory tracts (48). One hallmark characteristic in patients with CF is the colonization at an early age by Pseudomonas aeruginosa in the lungs. Airways secretions/sputum of CF patients as described in Ref. 49 consist of high levels of iron, thought to be of importance in the bacterial load. Interestingly, high iron levels are also observed in CF patients without P. aeruginosa infection, suggesting that the CF lungs may potentially be primed for the bacterial infection (49). Additionally, iron accumulation in the lungs of COPD patients has also been observed which is postulated to contribute to the generation of ROS and to bacterial pathogenesis and COPD exacerbation (50, 51).
LCN2 USE AS A BIOMARKER
LCN2 is rapidly induced in response to stress and inflammation, while also being constitutively produced by certain cell types in different tissues. In the heart and kidneys, LCN2 is constitutively produced by preadipocytes and mature adipocytes; thus LCN2 has been proposed to be a reliable biomarker in the diagnosis of obesity-related metabolic complications, kidney disease, and heart failure (5). LCN2 has been shown to play a protective role during kidney inflammation through its activation of autophagy, which Qui et al. (52) showed is mediated by hypoxia-inducible factor (HIF)-1alpha. This group also showed that LCN2 reduced the NF-κB-p65 signaling pathways, suggesting a potential cross talk between HIF-1alpha and NF-κB (52). Mishra et al. (53) showed that in acute kidney injury, LCN2 can tilt the fate of tubule cells toward survival, thus making it a desirable candidate for therapeutic interventions.
Immune cell infiltrates are widely used to diagnose inflammatory conditions; in “low-grade inflammation,” modest elevations in proinflammatory genes are observed. LCN2 is stable, and fecal LCN2 is an effective and noninvasive biomarker in diagnosing various low-grade inflammatory conditions such as intestinal inflammation (6) as well as Crohn’s inflammatory bowel disease (54). Additionally, urinary LCN2 is used as a biomarker to diagnose Lupus nephritis (4). Plasma levels of LCN2 have been postulated as a biomarker to detect mild cognitive impairments in Alzheimer’s disease (55) and to predict clinical outcomes in ischemic stroke patients (56). Additionally, LCN2 levels in the cerebrospinal fluid have been shown to be a sensitive biomarker for vascular dementia (57) and neuropsychiatric lupus (58). Lastly, LCN2 levels are useful in detecting multiple types of cancers such as colorectal cancer, breast cancer, ovarian cancer, and pancreatic cancer (2, 4). In terms of lung inflammation, LCN2 has been proposed as a potential biomarker candidate to diagnose different types of lung cancers (11, 14, 59). The role of LCN2 in lung inflammation and as a potential marker of inflammatory lung diseases is a less well-defined research area that warrants further evaluation.
In cancer, the function of LCN2 appears to be context dependent. In breast cancer, LCN2 contributes to tumor growth, and higher levels of metalloproteinase-9 (MMP9) (60). Interestingly, LCN2 promotes lung metastasis of breast cancer possibly by inhibiting the PI3K/Akt pathway (61). In the context of lung cancer, Steiling et al. (62) the identification of COPD-related processes that potentially impact lung carcinogenesis was done by investigating the effects of COPD on lung cancer-specific gene expression. From this study, LCN2 was one of the genes associated by COPD in smokers. By using mice with the G protein-coupled receptor family C type 5 A (Gprc5a) gene knockout and exposing them to tobacco carcinogen, Treekitkarnmongko et al. (63) showed that the Gprc5a knockout mice develop lung tumors with somatic driver mutations in Kras, unlike WT mice. Furthermore, they showed that early in lung adenocarcinoma (LUAD) development, LCN2 was increased. Additionally, Treekitkarnmongko et al. (63) found that LCN2 was elevated in human LUAD, however, not in lung squamous cell carcinoma compared with normal lung tissues. This study also found a strong association between LCN2 and COPD among patients with LUAD and lung cancer-free smokers (63). Overall, the study showed that LUAD development was increased in Gprc5a knockout mice and that mice exhibited elevated protumor inflammatory signaling and reduced antitumor immunity, suggesting that LCN2 antagonizes LUAD development and can be identified as a molecular feature of COPD (63). Consistent with an antitumor effect of LCN2, a proteomic analysis analyzing the bronchoalveolar lavage (BAL) of patients with COPD, COPD and lung cancer, lung cancer without COPD, and healthy controls found that LCN2 was significantly downregulated in patients with lung cancer. Although contrary to Treekitkarnmongko et al. (63), this publication found that LCN2 was also significantly decreased in COPD patients (64). However, the mechanism driving LCN2’s antitumor effect in lung cancer and whether the LCN2-PI3K/Atk pathway is also involved in this disease reminds to be elucidated.
CONCLUSIONS
LCN2 is an important inflammatory mediator at mucosal surfaces throughout the body. It is well known for its iron-chelating properties that prevent bacterial growth, while also serving as an important regulator of iron transport, tissue-specific iron availability, and ROS production. LCN2 has also been shown to mediate inflammation and the immune response in different ways. Increased levels of LCN2 that result from inflammatory conditions lead to the recruitment and thus sustained activities of neutrophils, while emerging evidence suggests additional roles for LCN2 in regulating immune response, including the regulation of lymphocyte activation and functioning. Clinically, LCN2 has proven to be a promising biomarker candidate for various inflammatory conditions and cancers, warranting continued studies to better understand LCN2’s roles in each of these pathologies. Since the lung inflammatory role of LCN2 in response to nonbacterial insults remains understudied, research investigating the role of LCN2 in lung inflammation using different insults is needed. These types of studies, such as ones done by our laboratory using organic agricultural dust extracts (65), will help us gain a better understanding of the underlying role of LCN2 in the lung immune response to different type of exposures and help identify any differences (if any) in LCN2 roles observed using different types of exposures. Overall, the upregulated levels of LCN2 in direct and indirect lung injuries play a protective role against bacterial pathogenesis, although macrophage deactivation worsens infections in the lungs and other organs. Moreover, the ability of LCN2 in binding to other microbes or microbial components, such as LPS, could also determine injury outcomes. In any case, additional research in this area is needed. Taken together, it will be critical to identify both the protective and detrimental roles of LCN2 in the lung comparable to what has been found elsewhere in the body. These investigations will support a deeper understanding of LCN2 functions, including potential translational opportunities that could improve the treatment and/or diagnosis of inflammatory lung pathologies.
GRANTS
This work was supported in part by National Institute of Environmental Health Sciences Grant R00ES025819 (to T.M.N.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.G. prepared figure; S.G. drafted manuscript; S.G., D.O., M.K., and T.M.N. edited and revised manuscript; S.G., D.O., M.K., and T.M.N. approved final version of manuscript.
REFERENCES
- 1.Wu H, Santoni-Rugiu E, Ralfkiaer E, Porse BT, Moser C, Hoiby N, Borregaard N, Cowland JB. Lipocalin 2 is protective against E. coli pneumonia. Respir Res 11: 96, 2010. doi: 10.1186/1465-9921-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell 123: 1293–1305, 2005. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Ellermann M, Arthur JC. Siderophore-mediated iron acquisition and modulation of host-bacterial interactions. Free Radic Biol Med 105: 68–78, 2017. doi: 10.1016/j.freeradbiomed.2016.10.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Shahawy MS, Hemida MH, Abdel-Hafez HA, El-Baz TZ, Lotfy AM, Emran TM. Urinary neutrophil gelatinase-associated lipocalin as a marker for disease activity in lupus nephritis. Scand J Clin Lab Invest 78: 264–268, 2018. doi: 10.1080/00365513.2018.1449242. [DOI] [PubMed] [Google Scholar]
- 5.Li D, Yan Sun W, Fu B, Xu A, Wang Y. Lipocalin-2-The myth of its expression and function. Basic Clin Pharmacol Toxicol 127: 142–151, 2020. doi: 10.1111/bcpt.13332. [DOI] [PubMed] [Google Scholar]
- 6.Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, Cani PD, Theodorou V, Dekker J, Meheust A, de Vos WM, Mercenier A, Nauta A, Garcia-Rodenas CL. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol 312: G171–G193, 2017. doi: 10.1152/ajpgi.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahl SL, Woodworth JS, Lerche CJ, Cramer EP, Nielsen PR, Moser C, Thomsen AR, Borregaard N, Cowland JB. Lipocalin-2 functions as inhibitor of innate resistance to Mycobacterium tuberculosis. Front Immunol 9: 2717, 2018. doi: 10.3389/fimmu.2018.02717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guglani L, Gopal R, Rangel-Moreno J, Junecko BF, Lin Y, Berger T, Mak TW, Alcorn JF, Randall TD, Reinhart TA, Chan YR, Khader SA. Lipocalin 2 regulates inflammation during pulmonary mycobacterial infections. PLoS One 7: e50052, 2012. e50052. doi: 10.1371/journal.pone.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saiga H, Nishimura J, Kuwata H, Okuyama M, Matsumoto S, Sato S, Matsumoto M, Akira S, Yoshikai Y, Honda K, Yamamoto M, Takeda K. Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J Immunol 181: 8521–8527, 2008. doi: 10.4049/jimmunol.181.12.8521. [DOI] [PubMed] [Google Scholar]
- 10.Warszawska JM, Gawish R, Sharif O, Sigel S, Doninger B, Lakovits K, Mesteri I, Nairz M, Boon L, Spiel A, Fuhrmann V, Strobl B, Muller M, Schenk P, Weiss G, Knapp S. Lipocalin 2 deactivates macrophages and worsens pneumococcal pneumonia outcomes. J Clin Invest 123: 3363–3372, 2013. doi: 10.1172/JCI67911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moschen AR, Adolph TE, Gerner RR, Wieser V, Tilg H. Lipocalin-2: a master mediator of intestinal and metabolic inflammation. Trends Endocrinol Metab 28: 388–397, 2017. doi: 10.1016/j.tem.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Goetz DH, Willie ST, Armen RS, Bratt T, Borregaard N, Strong RK. Ligand preference inferred from the structure of neutrophil gelatinase associated lipocalin. Biochemistry 39: 1935–1941, 2000. doi: 10.1021/bi992215v. [DOI] [PubMed] [Google Scholar]
- 13.Xiao X, Yeoh BS, Vijay-Kumar M. Lipocalin 2: an emerging player in iron homeostasis and inflammation. Annu Rev Nutr 37: 103–130, 2017. doi: 10.1146/annurev-nutr-071816-064559. [DOI] [PubMed] [Google Scholar]
- 14.Rahimi S, Roushandeh AM, Ahmadzadeh E, Jahanian-Najafabadi A, Roudkenar MH. Implication and role of neutrophil gelatinase-associated lipocalin in cancer: lipocalin-2 as a potential novel emerging comprehensive therapeutic target for a variety of cancer types. Mol Biol Rep 47: 2327–2346, 2020. doi: 10.1007/s11033-020-05261-5. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Jia M, Yan X, Cao L, Barnes PJ, Adcock IM, Huang M, Yao X. Increased neutrophil gelatinase-associated lipocalin (NGAL) promotes airway remodelling in chronic obstructive pulmonary disease. Clin Sci (Lond) ) 131: 1147–1159, 2017. doi: 10.1042/CS20170096. [DOI] [PubMed] [Google Scholar]
- 16.Cabezas F, Lagos J, Cespedes C, Vio CP, Bronfman M, Marzolo MP. Megalin/LRP2 expression is induced by peroxisome proliferator-activated receptor-alpha and -gamma: implications for PPARs’ roles in renal function. PLoS One 6: e16794, 2011. doi: 10.1371/journal.pone.0016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z, Huang Y, Zong H, Friedman RA, Barasch J, Lanzano P, Deng L, Leibel RL, Rubin M, Nickolas T, Chung W, Zeltser LM, Williams KW, Pessin JE, Kousteni S. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature 543: 385–390, 2017. doi: 10.1038/nature21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moscowitz AE, Asif H, Lindenmaier LB, Calzadilla A, Zhang C, Mirsaeidi M. The importance of melanocortin receptors and their agonists in pulmonary disease. Front Med (Lausanne) 6: 145, 2019. doi: 10.3389/fmed.2019.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK, Borregaard N. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett 579: 773–777, 2005. doi: 10.1016/j.febslet.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 20.Chella Krishnan K, Sabir S, Shum M, Meng Y, Acin-Perez R, Lang JM, Floyd RR, Vergnes L, Seldin MM, Fuqua BK, Jayasekera DW, Nand SK, Anum DC, Pan C, Stiles L, Peterfy M, Reue K, Liesa M, Lusis AJ. Sex-specific metabolic functions of adipose Lipocalin-2. Mol Metab 30: 30–47, 2019. doi: 10.1016/j.molmet.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wicher G, Larsson M, Fex Svenningsen A, Gyllencreutz E, Rask L, Aldskogius H. Low density lipoprotein receptor-related protein-2/megalin is expressed in oligodendrocytes in the mouse spinal cord white matter. J Neurosci Res 83: 864–873, 2006. doi: 10.1002/jnr.20774. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki S, Hara A, Sakaguchi M, Nangaku M, Inoue Y. Hepatocyte nuclear factor 4alpha regulates megalin expression in proximal tubular cells. Biochem Biophys Rep 17: 87–92, 2019. doi: 10.1016/j.bbrep.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler S, Rohrs S, Tickenbrock L, Langerak A, Chu ST, Feldmann I, Jakubowski N, Muller O. Lipocalin 24p3 is regulated by the Wnt pathway independent of regulation by iron. Cancer Genet Cytogenet 174: 16–23, 2007. doi: 10.1016/j.cancergencyto.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Shao S, Cao T, Jin L, Li B, Fang H, Zhang J, Zhang Y, Hu J, Wang G. Increased lipocalin-2 contributes to the pathogenesis of psoriasis by modulating neutrophil chemotaxis and cytokine secretion. J Invest Dermatol 136: 1418–1428, 2016. doi: 10.1016/j.jid.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Kang SS, Ren Y, Liu CC, Kurti A, Baker KE, Bu G, Asmann Y, Fryer JD. Lipocalin-2 protects the brain during inflammatory conditions. Mol Psychiatry 23: 344–350, 2018. doi: 10.1038/mp.2016.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao GH, Ho CT, Barasch J. The ligands of neutrophil gelatinase-associated lipocalin. RSC Adv 5: 104363–104374, 2015. doi: 10.1039/C5RA18736B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Axelsson L, Bergenfeldt M, Ohlsson K. Studies of the release and turnover of a human neutrophil lipocalin. Scand J Clin Lab Invest 55: 577–588, 1995. doi: 10.3109/00365519509110257. [DOI] [PubMed] [Google Scholar]
- 28.Singh V, Yeoh BS, Chassaing B, Zhang B, Saha P, Xiao X, Awasthi D, Shashidharamurthy R, Dikshit M, Gewirtz A, Vijay-Kumar M. Microbiota-inducible innate immune, siderophore binding protein lipocalin 2 is critical for intestinal homeostasis. Cell Mol Gastroenterol Hepatol 2: 482–498, 2016. e486. doi: 10.1016/j.jcmgh.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kangelaris KN, Prakash A, Liu KD, Aouizerat B, Woodruff PG, Erle DJ, Rogers A, Seeley EJ, Chu J, Liu T, Osterberg-Deiss T, Zhuo H, Matthay MA, Calfee CS. Increased expression of neutrophil-related genes in patients with early sepsis-induced ARDS. Am J Physiol Lung Cell Mol Physiol 308: L1102–L1113, 2015. doi: 10.1152/ajplung.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 67: 533–539, 2012. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Yang W, Zhao X, Zhang R. Experimental study of the protective effect of simvastatin on lung injury in rats with sepsis. Inflammation 41: 104–113, 2018. doi: 10.1007/s10753-017-0668-4. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh S, Stepicheva N, Yazdankhah M, Shang P, Watson AM, Hose S, Liu H, Weiss J, Zigler JS Jr, Valapala M, Watkins SC, Sinha D. The role of lipocalin-2 in age-related macular degeneration (AMD). Cell Mol Life Sci 77: 835–851, 2020. doi: 10.1007/s00018-019-03423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao S, Fang H, Dang E, Xue K, Zhang J, Li B, Qiao H, Cao T, Zhuang Y, Shen S, Zhang T, Qiao P, Li C, Gudjonsson JE, Wang G. Neutrophil extracellular traps promote inflammatory responses in psoriasis via activating epidermal TLR4/IL-36R crosstalk. Front Immunol 10: 746, 2019. doi: 10.3389/fimmu.2019.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang W, Ma J, Gu R, Lei B, Ding X, Xu G. Light-induced lipocalin 2 facilitates cellular apoptosis by positively regulating reactive oxygen species/Bim signaling in retinal degeneration. Invest Ophthalmol Vis Sci 59: 6014–6025, 2018. doi: 10.1167/iovs.18-25213. [DOI] [PubMed] [Google Scholar]
- 35.La Manna G, Ghinatti G, Tazzari PL, Alviano F, Ricci F, Capelli I, Cuna V, Todeschini P, Brunocilla E, Pagliaro P, Bonsi L, Stefoni S. Neutrophil gelatinase-associated lipocalin increases HLA-G(+)/FoxP3(+) T-regulatory cell population in an in vitro model of PBMC. PLoS One 9: e89497, 2014. e89497. doi: 10.1371/journal.pone.0089497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radic M. NETosis and ETosis: incompletely understood types of granulocyte death and their proposed adaptive benefits and costs. In: Apoptosis and Beyond. Hoboken, NJ: Wiley: 2018, p. 511–534. [Google Scholar]
- 37.Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One 7: e44328, 2012. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ojeda-Juarez D, Shah R, Fields JA, Harahap-Carrillo IS, Koury J, Maung R, Gelman BB, Baaten BJ, Roberts AJ, Kaul M. Lipocalin-2 mediates HIV-1 induced neuronal injury and behavioral deficits by overriding CCR5-dependent protection. Brain Behav Immun 89: 184–199, 2020. doi: 10.1016/j.bbi.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bachman MA, Lenio S, Schmidt L, Oyler JE, Weiser JN. Interaction of lipocalin 2, transferrin, and siderophores determines the replicative niche of Klebsiella pneumoniae during pneumonia. mBio 3: e00224-11, 2012. doi: 10.1128/mBio.00224-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan YR, Liu JS, Pociask DA, Zheng M, Mietzner TA, Berger T, Mak TW, Clifton MC, Strong RK, Ray P, Kolls JK. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol 182: 4947–4956, 2009. doi: 10.4049/jimmunol.0803282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moschen AR, Gerner RR, Wang J, Klepsch V, Adolph TE, Reider SJ, Hackl H, Pfister A, Schilling J, Moser PL, Kempster SL, Swidsinski A, Orth Holler D, Weiss G, Baines JF, Kaser A, Tilg H. Lipocalin 2 protects from inflammation and tumorigenesis associated with gut microbiota alterations. Cell Host Microbe 19: 455–469, 2016. doi: 10.1016/j.chom.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Abdulnour RE, Sham HP, Douda DN, Colas RA, Dalli J, Bai Y, Ai X, Serhan CN, Levy BD. Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal Immunol 9: 1278–1287, 2016. doi: 10.1038/mi.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roth-Walter F, Schmutz R, Mothes-Luksch N, Lemell P, Zieglmayer P, Zieglmayer R, Jensen-Jarolim E. Clinical efficacy of sublingual immunotherapy is associated with restoration of steady-state serum lipocalin 2 after SLIT: a pilot study. World Allergy Organ J 11: 21, 2018. doi: 10.1186/s40413-018-0201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neves J, Haider T, Gassmann M, Muckenthaler MU. Iron homeostasis in the lungs–a balance between health and disease. Pharmaceuticals (Basel) 12, 2019. doi: 10.3390/ph12010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srinivasan G, Aitken JD, Zhang B, Carvalho FA, Chassaing B, Shashidharamurthy R, Borregaard N, Jones DP, Gewirtz AT, Vijay-Kumar M. Lipocalin 2 deficiency dysregulates iron homeostasis and exacerbates endotoxin-induced sepsis. J Immunol 189: 1911–1919, 2012. doi: 10.4049/jimmunol.1200892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim D, Jeong JH, Song J. Lipocalin 2 regulates iron homeostasis, neuroinflammation, and insulin resistance in the brains of patients with dementia: evidence from the current literature. CNS Neurosci Ther 27: 883–894, 2021. doi: 10.1111/cns.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cronin SJF, Woolf CJ, Weiss G, Penninger JM. The role of iron regulation in immunometabolism and immune-related disease. Front Mol Biosci 6: 116, 2019. doi: 10.3389/fmolb.2019.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghio AJ, Carter JD, Richards JH, Richer LD, Grissom CK, Elstad MR. Iron and iron-related proteins in the lower respiratory tract of patients with acute respiratory distress syndrome. Crit Care Med 31: 395–400, 2003. doi: 10.1097/01.CCM.0000050284.35609.97. [DOI] [PubMed] [Google Scholar]
- 49.Reid DW, Carroll V, O’May C, Champion A, Kirov SM. Increased airway iron as a potential factor in the persistence of Pseudomonas aeruginosa infection in cystic fibrosis. Eur Respir J 30: 286–292, 2007. doi: 10.1183/09031936.00154006. [DOI] [PubMed] [Google Scholar]
- 50.McKeever TM, Lewis SA, Smit HA, Burney P, Cassano PA, Britton J. A multivariate analysis of serum nutrient levels and lung function. Respir Res 9: 67, 2008. doi: 10.1186/1465-9921-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Obase Y, Mouri K, Shimizu H, Ohue Y, Kobashi Y, Kawahara K, Oka M. Nutritional deficits in elderly smokers with respiratory symptoms that do not fulfill the criteria for COPD. Int J Chron Obstruct Pulmon Dis 6: 679–683, 2011. doi: 10.2147/COPD.S25293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiu S, Chen X, Pang Y, Zhang Z. Lipocalin-2 protects against renal ischemia/reperfusion injury in mice through autophagy activation mediated by HIF1alpha and NF-kappab crosstalk. Biomed Pharmacother 108: 244–253, 2018. doi: 10.1016/j.biopha.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 53.Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 15: 3073–3082, 2004. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 54.Hsieh H, Morin J, Filliettaz C, Varada R, LaBarre S, Radi Z. Fecal lipocalin-2 as a sensitive and noninvasive biomarker in the TNBS Crohn’s inflammatory bowel disease model. Toxicol Pathol 44: 1084–1094, 2016. doi: 10.1177/0192623316665927. [DOI] [PubMed] [Google Scholar]
- 55.Song J, Kim OY. Perspectives in lipocalin-2: emerging biomarker for medical diagnosis and prognosis for Alzheimer’s disease. Clin Nutr Res 7: 1–10, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hochmeister S, Engel O, Adzemovic MZ, Pekar T, Kendlbacher P, Zeitelhofer M, Haindl M, Meisel A, Fazekas F, Seifert-Held T. Lipocalin-2 as an infection-related biomarker to predict clinical outcome in ischemic stroke. PLoS One 11: e0154797, 2016. doi: 10.1371/journal.pone.0154797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Llorens F, Hermann P, Villar-Piqué A, Diaz-Lucena D, Nägga K, Hansson O, Santana I, Schmitz M, Schmidt C, Varges D, Goebel S, Dumurgier J, Zetterberg H, Blennow K, Paquet C, Baldeiras I, Ferrer I, Zerr I. Cerebrospinal fluid lipocalin 2 as a novel biomarker for the differential diagnosis of vascular dementia. Nat Commun 11: 619–611, 2020. doi: 10.1038/s41467-020-14373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mike EV, Makinde HM, Gulinello M, Vanarsa K, Herlitz L, Gadhvi G, Winter DR, Mohan C, Hanly JG, Mok CC, Cuda CM, Putterman C. Lipocalin-2 is a pathogenic determinant and biomarker of neuropsychiatric lupus. J Autoimmun 96: 59–73, 2019. doi: 10.1016/j.jaut.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun B, Guo W, Hu S, Yao F, Yu K, Xing J, Wang R, Song H, Liao Y, Wang T, Jiang P, Han B, Deng J. Gprc5a-knockout mouse lung epithelial cells predicts ceruloplasmin, lipocalin 2 and periostin as potential biomarkers at early stages of lung tumorigenesis. Oncotarget 8: 13532–13544, 2017. doi: 10.18632/oncotarget.14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berger T, Cheung CC, Elia AJ, Mak TW. Disruption of the Lcn2 gene in mice suppresses primary mammary tumor formation but does not decrease lung metastasis. Proc Natl Acad Sci USA 107: 2995–3000, 2010. doi: 10.1073/pnas.1000101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi H, Gu Y, Yang J, Xu L, Mi W, Yu W. Lipocalin 2 promotes lung metastasis of murine breast cancer cells. J Exp Clin Cancer Res 27: 83–83, 2008. doi: 10.1186/1756-9966-27-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steiling K, Jiwani A, Gosselink JV, Liu G, Alekseyev Y, Lam W, Sin D, Lam S, Pare P, Hogg JC, Spira A, Lenburg ME. A distinct lung cancer gene-expression signature in smokers with COPD (Abstract). Am J Respir Crit Care Med 187: A1041, 2013. doi: 10.1164/ajrccm-conference.2013.187.1_MeetingAbstracts.A1041. [DOI] [Google Scholar]
- 63.Treekitkarnmongkol W, Hassane M, Sinjab A, Chang K, Hara K, Rahal Z, et al. Augmented lipocalin-2 is associated with chronic obstructive pulmonary disease and counteracts lung adenocarcinoma development. Am J Respir Crit Care Med 203: 90–101, 2021. doi: 10.1164/rccm.202004-1079OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pastor MD, Nogal A, Molina-Pinelo S, Meléndez R, Salinas A, González De la Peña M, Martín-Juan J, Corral J, García-Carbonero R, Carnero A, Paz-Ares L. Identification of proteomic signatures associated with lung cancer and COPD. J Proteomics 89: 227–237, 2013. doi: 10.1016/j.jprot.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 65.Guardado S, Ojeda-Juárez D, Ulu A, Sveiven S, Dominguez E, Kaul M, Nordgren TM. The role of lipocalin-2 (LCN2) in organic dust-induced lung inflammation (Abstract). Am J Respir Crit Care Med 203: A3045, 2021. doi: 10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A3045. [DOI] [Google Scholar]