INTRODUCTION
The 30th meeting of the Society for the Neural Control of Movement (NCM) was originally scheduled to take place in Dubrovnik, Croatia, in April of 2020. Due to the COVID-19 pandemic, the in-person meeting was canceled and replaced by a virtual symposium showcasing the work of the society’s 2020 scholarship winners (https://ncm-society.org/symposium/). By the spring of 2021 (April 20th-22nd), the annual meeting was ready to return, although moving for the first time to a completely virtual setting on the online platforms Pheedloop (https://pheedloop.com) and Gathertown (https://gather.town) (1).
More than ever before, this virtual format facilitated participation and social media engagement (e.g., the popular hashtag #NCM2021) from the research community all around the world (Fig. 1). Indeed, the forced choice of moving online had several positive side effects. First, rebounding from a concerning dip in academic participation from female researchers during the COVID-19 pandemic (2), with respect to previous in-person meetings, NCM 2021 showed an uptick in the percentage of female panelists (34% female attendees, 44% female speakers, and 30% female submissions). Second, early career researchers had more opportunities to present their work thanks to the addition of “data blitz” sessions on top of the traditional posters, individual talks, and panel discussions. This novel category allowed 5 min for presentation and 1 min for questions, followed by separate breakout rooms. Third, to enhance discussions during the meeting, panels, and posters were prerecorded and posted online in advance. Live recordings of every session allowed delegates to catch up on missed talks or rewatch talks later. These were welcome additions, as indicated by many respondents to the meeting’s feedback survey.
Figure 1.
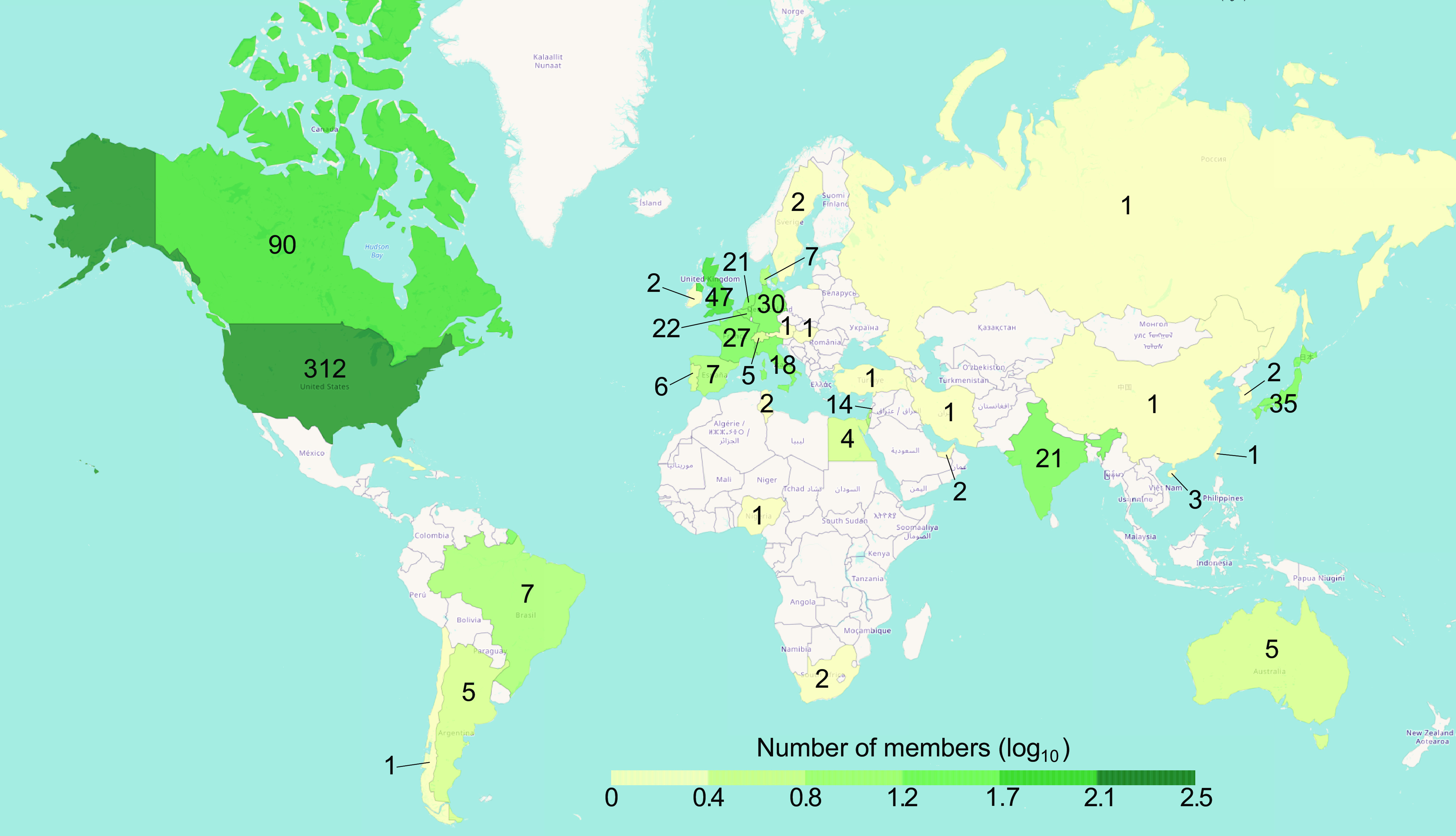
World countries color-coded by the number of people attending Neural Control of Movement (NCM) 2021 affiliated with a university located in that country. Colors are represented on a log10 scale. The number insets in each country show the exact number of attendees.
As done in previous years (3–5), here we present highlights from the meeting. These highlights revolve around four central themes: 1) neuroplasticity, 2) complex motor skills, 3) multimodal sensory integration, and 4) the role of descending spinal tracts in motor control.
NEUROPLASTICITY FOLLOWING ALTERED SENSORY INPUT
A particular focus of NCM 2021 was on studies using multimodal neuroimaging to understand brain plasticity resultant from exposure to body augmentation technologies or abnormal sensory input. Tamar Makin, the 2020 Early Career Award winner, was commended for their innovative work examining the drivers and limitations of plasticity in the human brain as well as their contributions to the Society—including the organization of the first all-female NCM panel in 2018. Investigating embodiment of prostheses using fMRI, Makin and colleagues found that two-handed participants represented cosmetic prostheses more like hands and functional prostheses more like tools. However, among prosthesis users, both functional and cosmetic prostheses were not represented either as hands or as tools, but instead constituted a separate category (6, 7). Makin and collaborators explored a similar effect in another group of expert tool users, London litter pickers (7). Similar to amputees, litter pickers viewed their tool as a separate entity and not as an extension of their hand. Together, these results suggest that the human brain is plastic enough to create representations that are distinct from those shaped by evolution.
A PhD student in Makin’s laboratory, Elena Amoruso, explicitly tested this hypothesis in their “third thumb” project (8). Participants wore an artificial thumb controlled by their toes. Coordination rapidly improved with practice and led to successful learning transfer when the controllers were switched (e.g., the toe that controlled flexion/extension now controlled abduction/adduction). When using local anesthesia to block proprioceptive and somatosensory input of the toes controlling the artificial thumb, early training was not affected, but retention of learning on the second day was smaller compared to a sham anesthesia control group, suggesting that sensory input played a critical role in learning to control the additional finger. The third thumb was also tested in a real-world context (9), where participants wore the device for ∼4.5 h a day for 5 days. Participants rapidly improved their performance and reported increasing sensations that the thumb was part of their body. Participants thus were able to develop a sense of proprioception of the thumb’s position relative to their biological fingers. Together, Makin and colleagues’ work demonstrates the remarkable ability of the human nervous system to undergo plastic changes—an ability Makin believes we should leverage in the development of prostheses. Similarly, Robert Nickl characterized the stability of neuronal responses to movement intention in a patient with incomplete tetraplegia who was bilaterally implanted with multi-unit electrode arrays in the primary motor (M1) and primary somatosensory (S1) cortices (10). Across 12 sessions, the number of active units was more stable in S1 than M1. However, in both areas, the number of overall active units showed a nearly exponential decline. With respect to recorded neuronal activity, in a single channel stability declined within minutes to hours. Interestingly, contralateral activity was more stable than activity in ipsilateral units. This characterization of the stability of neural activity is important for the development of brain-machine interface devices capable of decoding this representation into motor output.
The brain’s ability to form new representations can also be probed by studying the neuroplastic changes driven by exposure to a completely novel environment. For example, Grant Tays investigated the impact of microgravity on fifteen astronauts who spent ∼6 months onboard the International Space Station (11–13). Participants completed MRI and behavioral testing multiple times pre- and postflight. Their data suggested little cognitive change from pre- to postflight but pronounced postflight impairments to mobility, balance, and bimanual coordination (14). These performance declines extended beyond only peripheral changes such as disuse muscle atrophy, suggesting that centrally mediated processes might also contribute to these effects. Further probing central nervous system changes with spaceflight, Kathleen Hupfeld discussed their work on vestibular processing in astronauts. Hupfeld and colleagues applied vestibular stimulation (pneumatic cheekbone taps; 15) to measure brain activity during vestibular processing at multiple times pre- and postflight. As previously demonstrated on Earth (12, 16), preflight, vestibular stimulation elicited activation of the parietal opercular area (i.e., the so-called “vestibular cortex”) and deactivation of somatosensory and visual cortices. Postflight, astronauts showed widespread reductions in somatosensory and visual cortical deactivation. In addition, greater reductions in the deactivation of visual brain regions were associated with smaller declines in standing balance. These findings suggest that microgravity exposure results in cortical plasticity in the form of sensory reweighting, i.e., down-weighting of vestibular inputs (due to this system’s altered signaling in the absence of gravity), and concurrent up-weighting of other sensory processing regions, such as the somatosensory and visual cortices. This reweighting may facilitate more adaptable postflight standing balance when crewmembers readjust to normal vestibular inputs on Earth.
To better characterize the mechanisms underlying cortical plasticity, Caroline Nettekoven presented their data on the relation between motor cortical γ-aminobutyric acid (GABA), previously shown to play a role in motor learning and visuomotor adaptation (17–19). Although participants adapted to a stepwise increasing rotation (or performed a control task) in the MRI scanner, Nettekoven and colleagues measured GABA concentration in the left M1 hand area. GABA concentration before adapting predicted retention of the adapted movement but not the extent of adaptation, suggesting a role for M1 GABA in maintaining but not acquiring the adapted state. This relationship between GABA and retention of the adapted movement was mediated by the change in functional connectivity between the left M1 hand area and the right cerebellar hand area. Participants with higher M1 GABA concentration before adapting showed a decrease in functional connectivity between M1 and the cerebellum during adaptation, and they better retained the adaptive movement. These findings imply a link between motor performance, motor network connectivity, and cortical inhibition, and shed light on the neurochemical bases of human motor adaptation (20). Chris Horton and colleagues provided additional evidence that region-specific cortical GABA concentrations predict aspects of human motor performance. Measuring GABA concentrations in S1 and thalamus during “go” and “stop” tasks, they found no association between GABA and “stopping” performance. However, in the “go” task, higher ipsilateral thalamic GABA correlated with faster reaction time. These data suggest that thalamic GABA concentration supports speeded selection and execution of cued choice responses.
Together, these lines of research contribute to our understanding of how the brain adapts to perturbations or novel sensory conditions (e.g., prolonged prosthesis or tool use, microgravity, and visuomotor perturbations) and have numerous applications for improving human health, such as designing more effective prostheses and maintaining astronaut health during future missions to Mars.
EXPLORING COMPLEX MOTOR SKILLS BEYOND UNIMANUAL REACHING
Another emergent theme of NCM 2021 was a renewed push to study more complex motor skills. Although the definition of a “complex motor skill” may seem arbitrary, here we consider studies that examined naturalistic multijoint movements that tend to involve interactions with tools and/or the control of many degrees of freedom (DOFs).
One example is sophisticated finger control. As Tamar Makin’s work shows, besides prompting questions about artificial limb embodiment and neural plasticity, augmentation technologies can open new avenues for studying complex behaviors by enabling previously impossible actions. In addition, within the clinical setting, we can leverage assistive technologies to measure hand function and monitor the progress of rehabilitation. For example, Jing Xu introduced a novel device, the HAND (Hand Actuation Neural-training Device), that is equipped with force sensors capable of detecting even the smallest isometric forces from a near-plegic hand in 3-D (21). They used this device to characterize finger coactivation patterns in healthy participants and stroke patients. The importance of such research is most evident in manual activities that demand finger individuation, such as producing a chord when playing the piano. By comparing naïve participants and expert musicians using a foot-controlled supernumerary robotic thumb to play the piano, Aldo Faisal asked what determines our ability to learn and use augmentation in skilled tasks. They showed that foot dexterity (and not task-relevant piano expertise) is the best predictor of future performance (22). In addition, the observation of highly idiosyncratic learning curves prompted new questions for future research: can everyone be augmented equally? Or should we design personalized training? Regardless, it is clear that we need real-world complex tasks to improve the training of real-world sensorimotor skills. According to Ilana Nisky, 2021 Early Career Award winner, robot-assisted surgery can bridge the gap between laboratory-based research and real-life applications. Currently, surgical training is not optimized, partly due to a lack of haptic feedback and partly due to limited knowledge on how to measure surgical skills (23–27). Nisky’s research used a teleoperated needle driving task and integrated data measuring the dynamics of the robotic manipulandum and modeling human kinematics to describe the quality of surgical skill throughout the learning process. A key aspect of surgical skill is that it requires extremely precise control of external tools via the coordination of multiple effectors. Similarly, watchmaking is a complex craft that includes bimanual control of 44 DOFs. To understand and model such dexterity, Aude Billard and colleagues examined cohorts of apprentices and expert watchmakers using a combination of motion capture and tactile sensing systems. They found that experts consistently used distinctive hand poses that optimized manipulability and made use of longer preparation times to reduce possible mistakes during execution time (28, 29). Moreover, their research revealed that the two hands can work together distributing control of different variables to achieve better precision than a single hand.
Combining tool use and whole body movements is another way to increase realism and complexity in the study of motor skills. For example, Antonella Maselli presented their work on ball throwing in which they applied spatiotemporal principal component analysis and Hessian-based decomposition to whole body kinematics to obtain compact descriptions of unconstrained throwing. They used these descriptions to quantitatively characterize performance, individual strategies, gender differences, and common patterns from a heterogeneous sample of nontrained throwers (30–32). Zhaoran Zhang also investigated ball throwing but compared movements in a virtual and a real set-up. Tolerance-Noise-Covariation decomposition revealed distinct stages of learning, indicating that subjects reached the stage of fine-tuning throwing variability in the real but not in the virtual task. These findings resonate with the reported problems in transferring therapeutic benefits from virtual to real environments (33). Expanding the research on tool use to the more exotic example of manipulating a bullwhip, Marta Russo investigated how humans can achieve dexterity in manipulating the wave dynamics of the whip's infinite DOFs. Their experimental and simulation results suggested that humans may represent control of this prodigiously complex dynamic object in terms of low-dimensional dynamic primitives. Thus, in the same set of studies, Moses Nah tested whether a distant target could be reached with a whip using a controller composed of only motor primitives. This approach was able to manage 54 DOFs by means of a single submovement in joint space. A detailed model of the whip dynamics was not needed for this approach. This may be a key simplification that humans leverage to learn complex motor skills, avoiding the need to internalize the detailed dynamic properties of the object being manipulated (34, 58).
If details regarding objects’ properties do not need to be internalized, then what are the neural mechanisms underlying tool use? Simon Thibault showed that there is a functional overlap between tool-use planning and complex syntactic processing in the basal ganglia. Behaviorally, this is reflected by bidirectional cross-domain learning transfer, where tool use benefits syntax and vice-versa (35). In addition, Raeed Chowdhury studied the neural underpinnings of highly feedback-driven tasks, such as balancing a stick on a palm. During the task, monkeys displayed multiple control schemes within each trial, suggesting that they might have had multiple goals, and thus corresponding neural strategies, in different phases of the arm motion.
Primates are not the only species that use strategies to control objects. Indeed, it is possible to perform complex tool manipulations without the benefit of specialized hands. New Caledonian crows are an excellent nonprimate animal model for studying complex object manipulation, as shown by Christian Rutz’s work. These animals exhibit a striking degree of dexterity with their beak when manufacturing tools from raw plant materials, using these tools to extract insect prey from hiding places in deadwood, and storing tools for future use in holes or behind tree bark (36).
Such sophisticated control of our bodies extends beyond hand and upper-limb dexterity and is also exemplified by walking. Jacqueline Palmer presented their results on the relationship between motor cortical activity and circuit-specific cortico-cortical interactions during a whole body dual-task involving balance and cognition in older adults. Consistent with findings in younger adults, their results support motor cortical β activity as a potential biomarker for individual levels of balance challenge in older adults (37). To capture individual differences in gait dynamics, Taniel Winner used a data-driven dynamical model: a recurrent neural network (RNN) with long short-term memory. Measuring how the internal parameters of the model discriminated individuals with or without stroke, their work showed that using advanced models over discrete summary variables increased the accuracy of group classification. The ability to discriminate between different individuals may lead to the development of individually tailored rehabilitation to improve balance and gait in elderly or impaired individuals.
Finally, speech is another example of a complex motor skill that requires the fine control of several muscles to produce a sound. To better understand this complexity, two talks focused on the effects of different perturbations during speech production. First, Zoe Swann assessed how a startling acoustic stimulus might affect word repetition in individuals with poststroke aphasia and apraxia. Startle exposure resulted in faster and louder speech. These results were analogous to the finding that a startle during upper extremity movement produced a higher probability of muscle activity onset in severe poststroke subjects who were unable to activate their arm muscles on their own (38). Second, Ding-lan Tang examined movement variability during speech production perturbed by auditory feedback. Motor variability increased with auditory perturbation, and this higher variability persisted even after removing the perturbation.
The breadth of research highlighted in this section confirms a renewed interest in understanding the neural control of complex motor skills across species, body parts, interacting tools, and artificial limbs. Future work should look into transferring these abilities to robotic devices, as part of a continuing effort to close the loop between biological capability in humans and technical capability in robots.
MULTIMODAL SENSORIMOTOR INTEGRATION IN HEALTH AND INJURY
In motor tasks, sensory and motor circuits interact to adjust motor commands to changes in the environment and to modulate the sensory experience to optimize task performance. Here we discuss talks from this year’s meeting that investigated sensory processing and sensorimotor interactions in both healthy and injured systems.
Sliman Bensmaia and colleagues tackled the question of how multimodal sensory information is represented in the cuneate nucleus, a brainstem structure that receives sensory input from primary afferents in the forelimbs. They recorded single-unit activity in the S1, cuneate, and cutaneous primary afferents in response to skin stimulation and found that responses of cuneate neurons resembled those of S1, more so than those of primary afferents. Moreover, by using their novel simulation model (39), Bensmaia’s group was able to use the activity of 5–9 primary afferents from different cutaneous modalities to faithfully predict the response of single cells in the cuneate to skin stimulation. This study demonstrates that integration of multimodal sensory information occurs at the level of the cuneate, well before sensory input reaches the brain. A study led by Nofar Ozeri-Engelhard also demonstrated that multimodal integration of sensory inputs happens in the spinal cord. Using intersectional genetics, they isolated the parvalbumin-expressing interneurons located in the deep dorsal horn of the spinal cord (dPVs) to study their role in sensory processing and motor performance. With functional and histological assays, they provided evidence that dPVs form a circuit that integrates multimodal sensory information to directly communicate with motor neurons. To test whether this circuit plays a role in motor performance, they ablated dPVs and showed that mouse locomotion was perturbed. These results suggest that peripheral sensory circuits directly modulate motor output in the spinal cord to adjust motor performance to changes in the sensory environment.
As we have seen, sensory processing affects movement. However, the opposite is also true: motor circuits interact with sensory pathways to modify sensory input, and consequently, the sensory experience. Kazuhiko Seki tested the idea that a copy of the motor command (i.e., efference copy) modulates sensory-evoked potentials (SEPs) recorded in the cuneate nucleus of monkeys, in response to sensory stimulation of primary afferents innervating the forelimb (40). SEPs were recorded during active, passive, and no movement (i.e., hold) conditions. SEP amplitude was attenuated during active movement compared to hold, suggesting that the motor command’s efference copy modulated cuneate sensory responses. Although to a lesser degree, attenuation was also observed during passive movements, demonstrating that other descending sensory inputs are involved in modulating the sensory response. In support of these conclusions, Seki presented anatomical evidence that the cuneate nuclei receive top-down projections from both the somatosensory and motor (new M1) cortices, proposing these descending projections as the source of attenuation.
Eiman Azim and colleagues studied the role of descending cortico-cuneate pathways in the execution of tactile-guided movements. Using genetic tools in mice, they identified local inhibitory neurons in the cuneate that bidirectionally regulate the activity of cuneolemniscal neurons (which project from the cuneate to the thalamus). Optogenetic manipulation of these neurons altered the gain of the activity of the cuneoleminiscal neurons, and accordingly, the performance of dexterous movements (41). Anatomical experiments showed that both the inhibitory neurons and the cuneolemniscal neurons receive input from the sensory cortex. This supports Azim’s hypothesis that the cortex indirectly disinhibits, and directly inhibits cuneolemniscal neurons to augment sensory information necessary for optimal task performance while attenuating unnecessary information. In contrast to Seki’s findings, Azim’s work showed that descending pathways mostly originated from sensory areas. This discrepancy might be due to differences in species since new M1, which projects to the cuneate in non-human primates, does not exist in mice.
In the cerebellum, the efference copy of motor commands is believed to be used by internal models to predict the sensory consequences of active movements. Sensory prediction can be used to distinguish between a sensory state arising from active (i.e., self-generated) versus passive (i.e., externally generated) movement. For example, although vestibular-spinal reflexes are essential for maintaining balance in response to a passive perturbation, these are counterproductive during active movements. Indeed, Kathleen Cullen’s group showed that the responses of vestibulospinal neurons in deep cerebellar nuclei (DCN) are suppressed during active compared to passive movement (42). Omid Zobeiri investigated whether Purkinje cells in the vestibular cerebellum, which inhibit DCN, can predict the sensory consequences of efference copy and in turn suppress DCN responses during active head movements. Although single Purkinje cells did not encode sensory prediction, they showed heterogeneous responses to vestibular and proprioceptive sensory inputs and motor efference copy. Simulation data suggested that combining the responses of ∼40 Purkinje cells is sufficient to generate sensory predictions that suppress DCN responses during active movements, providing evidence that cerebellar internal models are encoded by Purkinje cell sub-populations.
During voluntary movements, when sensory prediction matches motor output (i.e., no sensory prediction error, SPE), DCN downregulate their responses to externally applied perturbations. But how does DCN sensitivity change in the presence of an SPE? Robyn Mildren studied how varying degrees of SPE impact the suppressed DCN response during active head movements. DCN responses were recorded from the rostral fastigial nucleus of one rhesus monkey. Different magnitudes of assistive and resistive torques were externally applied to introduce SPEs. As SPE increased, suppression of DCN responses during active movements gradually decreased, suggesting a gradual shift in encoding from self-generated to externally applied motion.
In addition to head movements, primates use eye movements to sample the visual environment. Ehsan Sedaghat-Nejad investigated the role of the oculomotor cerebellum in encoding SPEs by recording Purkinje cell activity while marmosets performed saccades to a target presented in random locations. Occasionally, the target location was quickly changed, to introduce a prediction error. They showed that Purkinje cells can be divided into subpopulations, according to their encoding of SPEs, resulting in a population activity that was predictive of the saccade termination (43).
Our brain’s ability to perform two sequential saccades has been long studied by Michael Goldberg, commended for his lifelong contributions to science and to the NCM community, and selected by NCM for the distinguished career award lecture. Although the first saccadic eye movement is an “easy” task for the brain, the second requires an updated representation of the initial position from which the movement starts. This position could be estimated from proprioceptive information about the eye, or from the efference copy of the first movement. To distinguish between these possibilities, the Goldberg laboratory investigated the lateral intraparietal area (LIP), a region that evokes saccades. In a series of experiments, they showed that although LIP receives proprioceptive information regarding eye position, this information arrives too late to alter second saccade planning (44). However, they found that LIP neurons fired before the second saccade, even though the second movement was not in their initial receptive field (45). This observation implies that the efference copy of the first saccade re-maps LIP receptive fields to generate the second saccade. What then is the role of proprioception in saccadic eye movements? Interestingly, with an increase in the number of sequential saccades, the brain shifts from an efference copy to proprioceptive-based encoding (46, 47).
Modulation of bottom-up sensory input by top-down signals is important to maintain movement accuracy. But what role do these interactions serve in functional recovery? Corinna Darian-Smith and their laboratory investigated this question by performing incomplete lesions of dorsal roots innervating forelimb fingers. Although these lesions produced severe deficits in prehension tasks, monkeys exhibited an impressive recovery over 1 to 3 mo. To uncover the mechanism underlying recovery, the Darian-Smith laboratory investigated plasticity in the cuneate nucleus. Using anterograde tracers, they showed that by 5 mo postlesion, spared fibers from deafferented digits sprouted new terminals in the cuneate, which then consolidated in number by 1 yr postlesion. Similar to their work in the spinal cord (48), they demonstrated specific changes to cuneate micro circuitry following injury and recovery, in the connections between S1, primary afferents, and local interneurons. This work suggests that interactions between sensory and motor circuits in the cuneate nucleus are plastic and play a role in functional recovery from injury.
In conclusion, multiple talks demonstrated complex sensory processing throughout the brain—even early in the sensory pathway at the level of the spinal cord and dorsal column nuclei. These processes included integration of multimodal sensory information and top-down modulation of sensory signals via the efference copy of motor commands. Combining the descending efference copy with ascending sensory input allows the brain to coordinate movement sequences, predict the consequences of motor commands, and alter its response to active and passive movement. These sensorimotor interactions are critical for accurate motor performance and functional recovery following injury to sensory fibers.
THE ROLE OF DESCENDING SPINAL TRACTS IN THE NEURAL CONTROL OF MOVEMENT
Throughout the meeting, several talks discussed the organization of descending tracts to the spinal cord, how these participate in motor control, their development, and their role in rehabilitation from injury. Ariel Levine’s group focused on a particular structure projecting onto the spinal cord from the deep cerebellar nuclei: the cerebellospinal tract (CeST). Historically, the CeST has been poorly studied, and its existence debated. Levine’s team leveraged modern genetic tools to better understand the role of this tract as well as spinal cord subpopulations for integrating coordinated motor behaviors. Focusing on the contralateral CeST, they found that the interposed and fastigial nuclei project onto the cervical spinal cord and are specifically involved in motor learning on a rotarod, but not in basic locomotion. In addition, Levine and colleagues found that coordinated control of the hindlimbs is mediated by additional projections from the target cervical interneurons in lamina VII and VIII to the lumbar spinal cord (49). These results provide some of the first insight into the descending CeST’s organization and motor function. In a complementary approach to characterize cell populations, Levine used transcriptomics in mice to develop an atlas with a complete characterization of the molecular profile of spinal cord neurons (50).
Further investigating the descending pathways, Julien Bouvier highlighted the role of the reticulospinal tract for the control of coordinated motor behaviors. To explore whether the reticulospinal tract contains different functional organizations (51), Bouvier’s team focused on the V2a neurons in the mouse reticular formation (52). They found that optogenetic stimulation of V2a neurons provoked complex changes in locomotor behavior including pausing, reorientation of the head, and changes in direction. The Bouvier laboratory was able to distinguish V2a neurons that project to the lumbar and the cervical spinal cord. Crucially, stimulation applied to lumbar-projecting neurons only arrested locomotion, whereas head rotation was observed only after stimulation of cervical-projecting neurons. This study demonstrates the benefits of combining genetic and viral tools to understand the functional heterogeneity of neural circuits.
Vibhu Sahni investigated the molecular processes during development that determine the somatotopic organization of corticospinal tracts innervation of the spinal cord. In previous work, their group had discovered anatomically and genetically distinct bulbar-cervical and thoracic-lumbar projecting populations (53). The origins of these descending axons appear to separate along the medial-lateral axis, with lateral motor cortex containing exclusively bulbar-cervical projecting axons. Recently, they also discovered a similar boundary zone between the brainstem and cervical projecting axons. The targeting of the axon terminations of these populations appears to be under specific molecular control: the Crim1 gene extends axons past the cervical-thoracic boundary, whereas the Klhl14 gene restricts the axons to the bulbar-cervical region. In addition, their team investigated regeneration of the spinal cord after injury. They found that regenerative ability appears to be tied to the growing end of the axons during development. Lesions carried out at the growing end rather than at the upper part of the spinal cord experienced more regrowth and greater functional locomotion recovery.
On restoring lower limb motor control after spinal cord injury (SCI), Grégoire Courtine discussed their work using epidural stimulation in patients with SCI. By patterning this stimulation to match the spatiotemporal activity patterns in the intact spinal cord, they demonstrated remarkable recovery of stepping motion after SCI (54, 55). In addition, in pilot clinical studies, they found that after months of training with spinal stimulation, patients experienced some functional recovery even with the stimulation turned off. To test the role of the motor cortex in functional recovery, Courtine’s team optogenetically inactivated this region in rats during a swimming task. They demonstrated that before SCI, inactivation had no effect, but after SCI and subsequent recovery with epidural stimulation, cortical inactivation caused the animal to lose the recovered swimming ability (56). Histological evaluation revealed axonal sprouting within spared reticulospinal fibers projecting onto the lumbar spinal cord, suggesting a potential cortico-reticulo-spinal pathway mediating this functional recovery.
Several blitz talks expanded upon research focusing on the spinal circuits themselves. Rune Berg presented multielectrode spinal recordings in turtles during scratching and found spinal neurons active across all phases of the scratch cycle. They demonstrated rotational structure in the population activity and presented a new theoretical framework, called the balanced sequence generator, to model these experimental findings. In humans, Samuele Contemori demonstrated that the fast muscle responses observed within 100 ms after visual stimulus onset (stimulus-locked responses, or SLRs, see Ref. 57) are modulated by visual cues and are likely controlled by the tecto-reticulo-spinal pathway.
Overall, this year’s NCM meeting provided a window into the cutting-edge research on the spinal cord and the descending tracts’ role in the control of movement. The panel and talks covered a wide range of techniques and topics, encompassing molecular techniques, histology, electrophysiology, and modeling, and included work on many different species, ranging from rodents to turtles to humans.
CONCLUSIONS
Despite lacking some of the excitement and networking opportunities of an in-person conference, this year’s virtual meeting had several positive outcomes. The most obvious upside of going online was greater inclusivity: a record number of attendees (706 participants), a doubling up in the percentage of female submissions from 2019, and a sharp increase in participation from early-career scientists and underrepresented countries. To continue this trend, the NCM board expressed willingness to consider a hybrid format combining virtual and in-person presentations for future meetings.
The studies highlighted in this article tackled motor control questions at different levels and from multiple perspectives (Fig. 2). One of the emerging themes was that investigating the brain’s plastic abilities is a key asset to develop more effective prostheses and novel, individually tailored rehabilitation protocols for patients or even astronauts. Likewise, to extend our research to real-world applications it is important to design experiments that explore more complex natural movements, such as those engaging multiple joints or involving objects and robotic interfaces. Several studies highlighted how descending pathways carry information about sensory prediction and interact with motor commands. Last, with new genetic and viral tools, we are beginning to uncover the roles of specific supraspinal populations that descend to the spinal cord. This approach is expected to gain momentum and reveal new understandings of spinal cord function in motor control and recovery from spinal cord injury.
Figure 2.
The most common keywords reported by the attendees of Neural Control of Movement (NCM) 2021 representing what was discussed or learned during the meeting.
GRANTS
Marta Russo was supported by the Italian Ministry of Health Starting Grant (SG-2018-12366101) and NSF (M3X-1825942). Giacomo Ariani’s work was supported by a NSERC Discovery Grant (RGPIN-2016–04890) and the Canada First Research Excellence Fund (BrainsCAN). Nofar Ozeri-Engelhard was supported by the New Jersey Commission on Spinal Cord Research (NJCSCR). Kathleen Hupfeld was supported by a fellowship from the National Institute on Aging (F99 AG068440-01).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.N., E.S-.N., and G.A. prepared figures; M.R., N.O.-E., K.H., C.N., S.T., E.S-.N., D.B., D.X., O.Z., K.K., S.T.A., and G.A. drafted manuscript; M.R., N.O.-E., K.H. and G.A. edited and revised manuscript; M.R., N.O.-E., K.H., C.N., S.T., E.S-.N., D.B., D.X., O.Z., K.K., S.T.A., and G.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the memory and the scientific legacy of Rodrigo Maeda, a PhD student at Western University who passed away from cancer on January 1st, 2021. He was a brilliant young scientist and a wonderful all-around person (https://superlab.ca/readinglist/list100.html). He was also a regular attendee of the NCM annual meeting, which he loved, and an author of the 2019 NCM highlights article (https://doi.org/10.1152/jn.00484.2019).
REFERENCES
- 1.Knopf A. New MIT startup makes virtual conferencing more appealing. Alcohol Drug Abus Wkly 33: 5–6, 2021. doi: 10.1002/adaw.32964. [DOI] [Google Scholar]
- 2.Viglione G. Are women publishing less during the pandemic? Here’s what the data say. Nature 581: 365–366, 2020. doi: 10.1038/d41586-020-01294-9. [DOI] [PubMed] [Google Scholar]
- 3.Gallego JA, Hardwick RM, Oby ER. Highlights from the 2017 meeting of the Society for Neural Control of Movement (Dublin, Ireland). Eur J Neurosci 46: 2141–2148, 2017. doi: 10.1111/ejn.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathis A, Pack AR, Maeda RS, McDougle SD. Highlights from the 29th Annual Meeting of the Society for the Neural Control of Movement. J Neurophysiol 122: 1777–1783, 2019. doi: 10.1152/jn.00484.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazurek KA, Berger M, Bollu T, Chowdhury RH, Elangovan N, Kuling IA, Sohn MH. Highlights from the 28th annual meeting of the society for the neural control of movement. J Neurophysiol 120: 1671–1679, 2018. doi: 10.1152/jn.00475.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maimon-Mor RO, Makin TR. Is an artificial limb embodied as a hand? Brain decoding in prosthetic limb users. PLoS Biol 18: e3000729, 2020. doi: 10.1371/journal.pbio.3000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schone HR, Maimon-Mor RO, Baker CI, Makin TR. Expert tool users show increased differentiation between visual representations of hands and tools. J Neurosci 41: 2980–2989, 2021. doi: 10.1523/JNEUROSCI.2489-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amoruso E, Dowdall L, Kollamkulam MT, Ukaegbu O, Kieliba P, Ng T, Dempsey-Jones H, Clode D, Makin TR. Somatosensory signals from the controllers of an extra robotic finger support motor learning. bioRxiv, 2021. doi: 10.1101/2021.05.18.444661. [DOI]
- 9.Kieliba P, Clode D, Maimon-Mor R, Makin T. Neurocognitive consequences of hand augmentation. bioRxiv, 2020. doi: 10.1101/2020.06.16.151944. [DOI]
- 10.Nickl R, Anaya M, Thomas T, Fifer M, McMullen D, Thompson M, Candrea D, Anderson W, Wester B, Tenore F, Crone N, Celnik P, Cantarero G. Characteristics and stability of sensorimotor activity driven by isolated-muscle group activation in a human with tetraplegia. medRxiv, 2020. doi: 10.1101/2020.06.02.20117036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hupfeld KE, McGregor HR, Lee JK, Beltran NE, Kofman IS, De Dios YE, Reuter-Lorenz PA, Riascos RF, Pasternak O, Wood SJ, Bloomberg JJ, Mulavara AP, Seidler RD; Alzheimer’s Disease Neuroimaging Initiative. The impact of 6 and 12 months in space on human brain structure and intracranial fluid shifts. Cereb Cortex Commun 1: tgaa023, 2020. doi: 10.1093/texcom/tgaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hupfeld KE, McGregor HR, Reuter-Lorenz PA, Seidler RD. Microgravity effects on the human brain and behavior: dysfunction and adaptive plasticity. Neurosci Biobehav Rev 122: 176–189, 2021. doi: 10.1016/j.neubiorev.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koppelmans V, Erdeniz B, De Dios YE, Wood SJ, Reuter-Lorenz PA, Kofman I, Bloomberg JJ, Mulavara AP, Seidler RD. Study protocol to examine the effects of spaceflight and a spaceflight analog on neurocognitive performance: extent, longevity, and neural bases. BMC Neurol 13: 205, 2013. doi: 10.1186/1471-2377-13-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tays G, Hupfeld K, McGregor H, Salazar A, De Dios Y, Beltran N, Kofman I, Wood S, Bloomberg J, Mulavara A, Seidler R. The effects of long duration spaceflight on sensorimotor 1 control and cognition. bioRxiv, 2021. doi: 10.1101/2021.06.22.449414. [DOI] [PMC free article] [PubMed]
- 15.Noohi F, Kinnaird C, DeDios Y, Kofman IS, Wood S, Bloomberg J, Mulavara A, Seidler R. Functional brain activation in response to a clinical vestibular test correlates with balance. Front Syst Neurosci 11: 11, 2017. doi: 10.3389/fnsys.2017.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan P, Koppelmans V, Reuter-Lorenz P, De Dios Y, Gadd N, Wood S, Riascos R, Kofman I, Bloomberg J, Mulavara A, Seidler R. Vestibular brain changes within 70 days of head down bed rest. Hum Brain Mapp 39: 2753–2763, 2018. doi: 10.1002/HBM.24037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachtiar V, Stagg CJ. The role of inhibition in human motor cortical plasticity. Neuroscience 278: 93–104, 2014. doi: 10.1016/j.neuroscience.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 18.Kolasinski J, Hinson EL, Divanbeighi Zand AP, Rizov A, Emir UE, Stagg CJ. The dynamics of cortical GABA in human motor learning. J Physiol 597: 271–282, 2019. doi: 10.1113/JP276626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Curr Biol 21: 480–484, 2011. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nettekoven C, Brady S, Clarke WT, Emir U, Levenstein J, Petitet P, Panouilleres MTN, Bachtiar V, O’shea J, Johansen-Berg H, Jenkinson N, Stagg C. GABA relates to functional connectivity changes and retention in visuomotor adaptation. bioRxiv, 2021. doi: 10.1101/2020.12.22.423981. [DOI]
- 21.Mawase F, Cherry-Allen K, Xu J, Anaya M, Uehara S, Celnik P. Pushing the rehabilitation boundaries: hand motor impairment can be reduced in chronic stroke. Neurorehabil Neural Repair 34: 733–745, 2020. doi: 10.1177/1545968320939563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafti A, Haar S, Mio R, Guilleminot P, Faisal AA. Playing the piano with a robotic third thumb: assessing constraints of human augmentation. bioRxiv, 2020. doi: 10.1101/2020.05.21.108407. [DOI] [PMC free article] [PubMed]
- 23.Bitton G, Nisky I, Zarrouk D. A novel grip force measurement concept for tactile stimulation mechanisms - design, validation, and user study. IEEE Trans Haptics 14: 396–408, 2021. doi: 10.1109/TOH.2020.3037175. [DOI] [PubMed] [Google Scholar]
- 24.Farajian M, Leib R, Kossowsky H, Nisky I. Visual feedback weakens the augmentation of perceived stiffness by artificial skin stretch. IEEE Trans Haptics, 2021. doi: 10.1109/TOH.2021.3052912. [DOI] [PubMed] [Google Scholar]
- 25.Kossowsky H, Farajian M, Milstein A, Nisky I. The effect of variability in stiffness on perception and grip force adjustment. IEEE Trans Haptics, 2021. doi: 10.1109/TOH.2021.3052136. [DOI] [PubMed] [Google Scholar]
- 26.Milstein A, Alyagon L, Nisky I. Grip force control during virtual interaction with deformable and rigid objects via a haptic gripper. IEEE Trans Haptics, 2021. doi: 10.1109/TOH.2021.3060507. [DOI] [PubMed] [Google Scholar]
- 27.Sharon Y, Jarc AM, Lendvay TS, Nisky I. Rate of orientation change as a new metric for robot-assisted and open surgical skill evaluation. IEEE Trans Med Robot Bionics 3: 414–425, 2021. doi: 10.1109/tmrb.2021.3073209. [DOI] [Google Scholar]
- 28.Yao K, Billard A. An inverse optimization approach to understand human acquisition of kinematic coordination in bimanual fine manipulation tasks. Biol Cybern 114: 63–82, 2020. doi: 10.1007/s00422-019-00814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao K, Sternad D, Billard A. Hand pose selection in a bimanual fine-manipulation task. J Neurophysiol 126: 195–212, 2021. doi: 10.1152/jn.00635.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maselli A, Dhawan A, Cesqui B, Russo M, Lacquaniti F, d'Avella A. Where are you throwing the ball? I better watch your body, not just your arm! Front Hum Neurosci 11: 505–519, 2017. doi: 10.3389/fnhum.2017.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maselli A, Dhawan A, Russo M, Cesqui B, Lacquaniti F, d'Avella A. A whole body characterization of individual strategies, gender differences, and common styles in overarm throwing. J Neurophysiol 122: 2486–2503, 2019. doi: 10.1152/JN.00011.2019. [DOI] [PubMed] [Google Scholar]
- 32.Tommasino P, Maselli A, Campolo D, Lacquaniti F, d'Avella A. A Hessian-based decomposition characterizes how performance in complex motor skills depends on individual strategy and variability. PLoS One 16: e0253626, 2021. doi: 10.1371/journal.pone.0253626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Sternad D. Back to reality: differences in learning strategy in a simplified virtual and a real throwing task. J Neurophysiol 125: 43–62, 2021. doi: 10.1152/JN.00197.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nah MC, Krotov A, Russo M, Sternad D, Hogan N. Dynamic primitives facilitate manipulating a whip. 2020 8th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), 2020, p. 685–691. doi: 10.1109/BioRob49111.2020.9224399. [DOI] [Google Scholar]
- 35.Brozzoli C, Roy AC, Lidborg LH, Lövdén M. Language as a tool: motor proficiency using a tool predicts individual linguistic abilities. Front Psychol 10: 1639, 2019. doi: 10.3389/fpsyg.2019.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klump BC, Cantat M, Rutz C. Raw-material selectivity in hook-tool-crafting New Caledonian crows. Biol Lett 15: 20180836, 2019. doi: 10.1098/rsbl.2018.0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer JA, Payne AM, Ting LH, Borich MR. Prefrontal-motor and somatosensory-motor cortical network interactions during reactive balance are associated with distinct aspects of balance behavior in older adults. bioRxiv, 2021. doi: 10.1101/2021.01.30.428951. [DOI] [PMC free article] [PubMed]
- 38.Rahimi M, Swann Z, Honeycutt CF. Does exposure to startle impact voluntary reaching movements in individuals with severe-to-moderate stroke? Exp Brain Res 239: 745–753, 2021. doi: 10.1007/s00221-020-06005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saal HP, Delhaye BP, Rayhaun BC, Bensmaia SJ. Simulating tactile signals from the whole hand with millisecond precision. Proc Natl Acad Sci USA 114: E5693–E5702, 2017. doi: 10.1073/pnas.1704856114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oya T, Takei T, Seki K. Distinct sensorimotor feedback loops for dynamic and static control of primate precision grip. Commun Biol 3: 156, 2020. doi: 10.1038/s42003-020-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conner JM, Bohannon A, Igarashi M, Taniguchi J, Baltar N, Azim E. Modulation of tactile feedback for the execution of dexterous movement. bioRxiv, 2021. doi: 10.1101/2021.03.04.433649. [DOI] [PMC free article] [PubMed]
- 42.Brooks JX, Cullen KE. The primate cerebellum selectively encodes unexpected self-motion. Curr Biol 23: 947–955, 2013. doi: 10.1016/j.cub.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sedaghat-Nejad E, Herzfeld DJ, Hage P, Karbasi K, Palin T, Wang X, Shadmehr R. Behavioral training of marmosets and electrophysiological recording from the cerebellum. J Neurophysiol 122: 1502–1517, 2019. doi: 10.1152/jn.00389.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Zhang M, Cohen IS, Goldberg ME. The proprioceptive representation of eye position in monkey primary somatosensory cortex. Nat Neurosci 10: 640–646, 2007. doi: 10.1038/nn1878. [DOI] [PubMed] [Google Scholar]
- 45.Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science 255: 90–92, 1992. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- 46.Poletti M, Listorti C, Rucci M. Stability of the visual world during eye drift. J Neurosci 30: 11143–11150, 2010. doi: 10.1523/JNEUROSCI.1925-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang M, Wang X, Goldberg ME. A spatially nonselective baseline signal in parietal cortex reflects the probability of a monkey’s success on the current trial. Proc Natl Acad Sci USA 111: 8967–8972, 2014. doi: 10.1073/pnas.1407540111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher KM, Garner JP, Darian-Smith XC. Reorganization of the primate dorsal horn in response to a deafferentation lesion affecting hand function. J Neurosci 40: 1625–1639, 2020. doi: 10.1523/JNEUROSCI.2330-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sathyamurthy A, Barik A, Dobrott CI, Matson KJE, Stoica S, Pursley R, Chesler AT, Levine AJ. Cerebellospinal neurons regulate motor performance and motor learning. Cell Rep 31: 107595, 2020. doi: 10.1016/j.celrep.2020.107595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sathyamurthy A, Johnson KR, Matson KJE, Dobrott CI, Li L, Ryba AR, Bergman TB, Kelly MC, Kelley MW, Levine AJ. Massively parallel single nucleus transcriptional profiling defines spinal cord neurons and their activity during behavior. Cell Rep 22: 2216–2225, 2018. doi: 10.1016/j.celrep.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Humphries MD, Gurney K, Prescott TJ. Is there a brainstem substrate for action selection? Philos Trans R Soc Lond B Biol Sci 362: 1627–1639, 2007. doi: 10.1098/rstb.2007.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Usseglio G, Gatier E, Heuzé A, Hérent C, Bouvier J. Control of orienting movements and locomotion by projection-defined subsets of brainstem V2a neurons. Curr Biol 30: 4665–4681.e6, 2020. doi: 10.1016/j.cub.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 53.Itoh Y, Sahni V, Shnider SJ, Macklis JD. Lumican regulates cervical corticospinal axon collateralization via non-autonomous crosstalk between distinct corticospinal neuron subpopulations. bioRxiv, 2021. doi: 10.1101/2021.03.26.437104. [DOI] [Google Scholar]
- 54.Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot JB, Buse N, Gandar J, Barraud Q, Xing D, Rey E, Duis S, Jianzhong Y, Ko WKD, Li Q, Detemple P, Denison T, Micera S, Bezard E, Bloch J, Courtine G. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 539: 284–288, 2016. doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner FB, Mignardot JB, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563: 65–93, 2018. doi: 10.1038/s41586-018-0649-2. [DOI] [PubMed] [Google Scholar]
- 56.Asboth L, Friedli L, Beauparlant J, Martinez-Gonzalez C, Anil S, Rey E, Baud L, Pidpruzhnykova G, Anderson MA, Shkorbatova P, Batti L, Pagès S, Kreider J, Schneider BL, Barraud Q, Courtine G. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat Neurosci 21: 576–588, 2018. doi: 10.1038/s41593-018-0093-5. [DOI] [PubMed] [Google Scholar]
- 57.Contemori S, Loeb GE, Corneil BD, Wallis G, Carroll TJ. The influence of temporal predictability on express visuomotor responses. J Neurophysiol 125: 731–747, 2021. doi: 10.1152/JN.00521.2020. [DOI] [PubMed] [Google Scholar]
- 58.Krotov A. Human Control of a Flexible Object: Hitting a Target with a Bull-Whip (Master’s thesis). Boston, MA: Northeastern University, 2020. [Google Scholar]



