Keywords: internally specified movements, 6-OHDA, rate model, stimulus-guided movements, substantia nigra pars reticulata
Abstract
Parkinsonian motor deficits are associated with elevated inhibitory output from the basal ganglia (BG). However, several features of Parkinson’s disease (PD) have not been accounted for by this simple “classical rate model” framework, including the observation in patients with PD that movements guided by external stimuli are less impaired than otherwise identical movements generated based on internal goals. Is this difference due to divergent processing within the BG itself or due to the recruitment of extra-BG pathways by sensory processing? In addition, surprisingly little is known about precisely when, in the sequence from selecting to executing movements, BG output is altered by PD. Here, we address these questions by recording activity in the substantia nigra pars reticulata (SNr), a key BG output nucleus, in hemiparkinsonian mice performing a well-controlled behavioral task requiring stimulus-guided and internally specified directional movements. We found that hemiparkinsonian mice exhibited a bias ipsilateral to the side of dopaminergic cell loss that was stronger when movements were internally specified rather than stimulus guided, consistent with clinical observations in patients with Parkinson’s disease. We further found that changes in parkinsonian SNr activity during movement preparation were consistent with the ipsilateral behavioral bias, as well as its greater magnitude for internally specified movements. Although these findings are inconsistent with some aspects of the classical rate model, they are accounted for by a related “directional rate model” positing that SNr output phasically overinhibits motor output in a direction-specific manner. These results suggest that parkinsonian changes in BG output underlying movement preparation contribute to the greater deficit in internally specified than stimulus-guided movements.
NEW & NOTEWORTHY Movements of patients with Parkinson’s disease are often less impaired when guided by external stimuli than when generated based on internal goals. Whether this effect is due to distinct processing in the basal ganglia (BG) or due to compensation from other motor pathways is an open question with therapeutic implications. We recorded BG output in behaving parkinsonian mice and found that BG activity during movement preparation was consistent with the differences between these forms of movement.
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disease of the basal ganglia (BG) in which motor impairments arise from disordered—typically, elevated—inhibitory BG output resulting from the loss of dopaminergic tone (1–9). One predominant theoretical framework for BG pathology in PD is the “classical rate model,” which posits that motor centers downstream of the BG are overinhibited, leading to disordered movements (2, 6, 7, 10–12). However, it is not clear whether the classical rate model can account for context-dependent PD motor phenomena, including the intriguing clinical observation that not all forms of movement are equally affected by PD: when movements are guided by external stimuli (e.g., gait matching with a rhythmic auditory stimulus or visually patterned flooring), kinematics are less impaired than for otherwise identical movements made in the absence of guiding stimuli (13–18). The primary question raised by this observation is whether parkinsonian BG output is similarly disrupted for these “stimulus-guided” and “internally specified” movements. If so, we might infer that stimulus-guided movements are protected from PD via the recruitment of extra-BG pathways (1, 19–22). However, differences in parkinsonian BG output between these forms of movements—particularly differences that are consistent with rate model predictions—would implicate BG processing itself in this behavioral phenomenon. Given that understanding the neural basis for this clinical observation could be leveraged to improve treatment for PD, we sought to develop an experimental paradigm for examining parkinsonian BG output during stimulus-guided and internally specified movements.
We focused on parkinsonian BG output during movement preparation, a key motor phase in which several sensory and cognitive variables are integrated to select and plan the most valuable movement (23), and which when disrupted may contribute to bradykinesia (24–31). Under normal conditions, the substantia nigra pars reticulata (SNr), a BG output nucleus, is strongly engaged by the preparation of directional movements (32–34). However, although numerous studies have examined parkinsonian changes in SNr activity under passive conditions or rhythmic locomotion (3, 5, 8, 9, 35–39), this approach is insufficient for differentiating how SNr activity during distinct motor phases, including movement preparation, is affected by PD. To address this question, we recorded SNr activity during a behavioral task in which mice with unilateral dopaminergic cell loss prepare, and subsequently initiate, SNr-engaging directional (left or right) movements that are either stimulus guided or internally specified (34, 40, 41). Crucially, by requiring that mice wait for a go signal before initiating their movement to the reward port, movement preparation is temporally isolated from initiation, allowing the dissociation of PD impact on BG output underlying these sets of processes.
We found that mice exhibited a directional bias ipsilateral to the hemisphere with dopaminergic cell loss that was more prominent in internally specified than stimulus-guided trials, accordant with clinical observations of context-dependent motor effects in PD. Furthermore, we found that SNr activity during movement preparation was altered in a manner consistent with the relationship between BG output and behavior predicted by a simple extension of the classical rate model—a “directional rate model”—suggesting that reorganization of BG processing by dopaminergic cell loss contributes to the greater deficit in performance of internally specified than stimulus-guided movements. These findings inform our understanding of BG pathophysiology and can contribute to refining neuromodulatory PD treatments.
MATERIALS AND METHODS
Animal Subjects
All experiments were performed according to protocols approved by the University of Colorado Anschutz Medical Campus Institutional Animal Care and Use Committee. Subjects were adult C57BL/6J mice (aged 7–14 mo at the start of experiments; Jackson Labs) housed in a vivarium with a 12-h light/dark cycle with lights on at 5:00 AM. Only male mice were used due to practical challenges in training mice of both sexes in the same behavioral apparatuses on olfactory tasks. Food (Teklad Global Rodent Diet No. 2918; Harlan) was available ad libitum. Access to water was restricted before the behavioral session to motivate performance; however, if mice did not obtain 1.5 mL of water during the behavioral session, additional water was provided for ∼2–5 min following the behavioral session. All mice (n = 13 total) were weighed daily and received sufficient water during behavioral sessions to maintain >85% of prewater restriction weight.
Our initial data set consisted of six hemiparkinsonian (hemi-PD) mice and seven control mice. All hemi-PD mice received an injection of 6-OHDA (Sigma, H4381-1G), were implanted with a tetrode drive, performed the behavioral task, and performed the rotation assay (all procedures described in Experimental Methods). Four of the control mice were implanted with a tetrode drive and performed the behavioral task, whereas the remaining three control mice only received a saline injection and performed the rotation assay. Data from a given mouse were excluded from specific sets of analyses for failing to meet relevant criteria: one mouse was excluded from the hemi-PD group due to insufficient dopaminergic cell loss evaluated histologically (see Immunohistochemistry), one hemi-PD mouse was excluded from behavioral analyses for failing to complete at least 15 presurgery and 15 postsurgery sessions, and one hemi-PD mouse was excluded from electrophysiological analyses due to yielding too few well-isolated units. Thus, behavioral and electrophysiological analyses each included data from four hemi-PD and four control mice. Some behavioral and electrophysiological data from control mice were previously published using different analyses than the current study (34).
Experimental Methods
Behavioral task.
Mice were trained on a task requiring stimulus-guided (SG) and internally specified (IS) movements (Figs. 1 and 2, A and B) (34). All behavior was performed in the dark in the absence of visual cues. Training stages are described in detail below. Once trained, on each trial, the mouse entered the odor port, triggering the delivery of an odor, waited 488 ± 104 ms (means ± SD) for an auditory go signal, exited the odor port, and entered one of the reward ports (Fig. 2A). Premature exit from the odor port resulted in the unavailability of reward on that trial. We focus our analyses of neural activity on two epochs defined by task events: the movement preparation epoch (from 100 ms after the odor valve was opened until the go signal), during which the upcoming movement can be planned, and the movement initiation epoch (from the go signal until 100 ms after odor port exit), during which the planned movement can be initiated.
Figure 1.
Overview of experimental timeline. Box lengths represent average durations for each stage in control and hemi-PD mice. “Surgery & Pharmacology” includes recovery from surgery and the initial time for the hemi-PD condition to develop following 6-OHDA injection. SNr recordings (“Behavior & Neurophysiology”) began after recovery from surgery and stabilization of postsurgery behavioral performance (based on number of trials performed), which on average occurred later in hemi-PD than control mice but varied within both groups. PD, Parkinson’s disease; SNr, substantia nigra pars reticulata.
Figure 2.
Behavioral task and baseline performance. A: port locations (top) and timing of task events (bottom). Ipsilateral (I) and contralateral (C) are defined relative to the side of the brain targeted for surgery (always left). Cyan represents ipsilateral choices and magenta represents contralateral choices. B: odor mixtures on SG (stimulus-guided) and IS (internally specified) trials (top) and interleaving of SG and IS blocks within a session (bottom). On SG trials, the odor A/odor B mixture presented was 5/95, 20/80, 40/60, 50/50, 60/40, 80/20, or 95/5. On IS trials, the odor A/odor B mixture presented was always 50/50. Horizontal cyan and magenta lines indicate which port(s) were rewarded in each block. On SG trials, the ipsilateral side was rewarded when odor A > odor B (cyan), the contralateral side was rewarded when odor B > odor A (magenta), and either side was equally likely to be rewarded when odor A = odor B; on all IS trials, odor A = odor B and only one side was rewarded throughout the block. C: baseline (presurgery) performance on SG trials for representative mouse subsequently assigned to the hemi-PD group. Gray lines show best-fit logistic functions (p = 1/1 + e(−a − bx), where x is the proportion of odor A, p is the fraction of ipsilateral choices, and a and b are the best-fit free parameters) for each session (n = 17 sessions). Circles show average across sessions for the binary odor mixtures presented; solid line shows best-fit logistic function to all choices across sessions. D: baseline (presurgery) performance on IS trials for representative mouse subsequently assigned to hemi-PD group. Gray lines link ipsilateral- and contralateral-rewarded IS blocks within the same session (n = 15 sessions). Black circles indicate medians. Mouse chose the ipsilateral port more often on ipsilateral-rewarded blocks (*P = 3.05 × 10−5, one-tailed Wilcoxon signed-rank test). PD, Parkinson’s disease.
Odors comprised binary mixtures of (−)-carvone (“odor A,” Acros Organics, No. 154591000) and (+)-carvone (“odor B,” Acros Organics, No. 150680250). On each SG trial, one of seven odor mixtures was equally likely to be presented via an olfactometer (Island Motion, Model No. 109-024[B]): odor A/odor B = 95/5, 80/20, 60/40, 50/50, 40/60, 20/80, or 5/95. Mixtures in which odor A > odor B indicated reward availability only at the left port, mixtures in which odor B > odor A indicated reward availability only at the right port, and for mixtures in which odor A = odor B (i.e., the 50/50 mixture), reward was equally likely (probability = 0.5) at both ports (Fig. 2B). Since we surgically targeted the left hemisphere in all mice, we refer to odor A as the “ipsilateral odor” and odor B as the “contralateral odor” (e.g., Fig. 2C). Similarly, we refer to the directions “left” and “right” as “ipsilateral” and “contralateral,” respectively. On trials in which odor A = odor B (odor A/odor B = 50/50), the probability of reward at the ipsilateral and contralateral ports, independently, was 0.5. Reward, consisting of 4 µL of water, was delivered by transiently opening a calibrated water valve (Lee Valve Co., No. LHDA123115H) 10–100 ms after reward port entry. Odor and water delivery were controlled, and port entries and exits were recorded, using custom software (available upon request; adapted from C. D. Brody) written in MATLAB (MathWorks).
Mice were trained to perform SG trials using the following steps (42): First, mice were restricted to 1.5 mL water/day for 5 days. Second, with the center port blocked, mice were water-rewarded for entering either the left or right reward ports. These sessions were repeated until the mice obtained 1.5 mL of water from the side ports within one session. Third, with only the right port blocked, the mice were required to enter the center port before receiving a reward at the left port. Once mice received 1.5 mL of water, the barrier was switched to the left port and the process was repeated with water delivery to the right port. Fourth, in alternating blocks of 30 rewarded trials, one reward port was blocked and 95/5 (corresponding to open left port) or 5/95 (corresponding to open right port) odor A/odor B mixtures were presented upon entry to the center port. A minimum required odor sampling time, indicated by a go signal (auditory tone), began at 0.01 s and increased in 0.01 s increments upon completion (i.e., successfully waiting for the go signal and subsequently entering the reward port) of 80% of the previous 20 trials. This process was repeated until the mice achieved a required odor sampling time of 488 ms. Fifth, all ports were open and delivery of 95/5 and 5/95 mixtures was randomized trial by trial. Upon completing >65% of trials in the session at >80% correct, the 80/20 and 20/80 mixtures were introduced. This process was repeated for the 60/40 and 40/60, and then the 50/50 mixtures. Mice were typically trained on SG trials in ∼48 sessions (1 session/day).
On each IS trial, the 50/50 mixture was presented, and reward was available only at one side throughout the block. To train mice on IS trials, they were first introduced to interleaved blocks, each of which required 25 correct trials to advance to the next block. Once they performed ∼70% of trials in the session correctly, the number of correct trials required per block was increased to 50 (34). Mice required an additional ∼5 sessions to learn to perform interleaved blocks of SG and IS trials.
Upon completing training, mice performed at least 15 sessions to establish presurgery baseline behavior, underwent surgery (see below), and subsequently resumed task performance, beginning with sessions consisting of SG trials only (postsurgery behavior; Fig. 1).
Rotation assay.
The direction of spontaneous movement was assessed before and after surgery using a standard rotation assay (43, 44). Following intraperitoneal (ip) administration of d-amphetamine (2.5 mg/kg, Sigma, A5880-1G), mice were placed in a transparent beaker with a diameter of 11.5 cm. Mice were monitored for the next 90 min and behavior was recorded using an overhead camera (Supercircuits, Inc., No. PC6EX4). Rotations were analyzed from 10 to 30 min after intraperitoneal injection. A rotation score was calculated by counting the total number of complete ipsilateral (left) rotations and subtracting the total number of complete contralateral (right) rotations. Repeated testing was carried out with at least 1 wk between d-amphetamine injections to allow for recovery. All mice (hemi-PD, n = 5; control, n = 3) completed at least 3 presurgery and 3 postsurgery rotation assay sessions.
Substantia nigra pars compacta surgery: unilateral 6-OHDA and saline injections.
The mouse was anesthetized with isoflurane and secured in a stereotaxic device (Kopf Instruments, Model 963), the skull was exposed with a midline incision, and a craniotomy targeting the left SNc (substantia nigra pars compacta) was performed, centered at 3.07 mm posterior from bregma, 1.25 mm lateral from the midline, and 4.35 mm deep from cortical surface (45). Injection volume totaled 2 µL was injected at target (2.43 mg 6-OHDA/mL 0.02% ascorbic acid). After suturing the incision, a topical triple antibiotic ointment (Major Pharmaceuticals, No. 10095168) mixed with 2% lidocaine hydrochloride jelly (Akorn Pharmaceuticals, No. 3498367) was applied to the scalp, the mouse was removed from the stereotaxic device, the isoflurane was turned off, and oxygen alone was delivered to the mouse to gradually alleviate anesthetic state. Mice were administered sterile isotonic saline (0.9%) for rehydration and an analgesic (Ketofen, MWI Animal Supply; 5 mg/kg) for pain management. Analgesic and topical antibiotic administration was repeated daily for up to 5 days, and mice were closely monitored for any signs of distress.
This procedure was identical for those control mice assessed on the rotation assay but with saline injected instead of 6-OHDA. These mice did not undergo further surgery and were used solely for rotation assay testing.
SNr surgery: tetrode implantation.
Details of the surgical procedure are provided in Thompson and Felsen (40). Briefly, following establishment of presurgery baseline behavior and (in hemi-PD mice) following unilateral 6-OHDA injections (Fig. 1), the mouse was anesthetized with isoflurane and secured in a stereotaxic device, the scalp was incised and retracted, two small screws were attached to the skull, and a craniotomy targeting the left SNr was performed, centered at 3.07 mm posterior from bregma and 1.25 mm lateral from the midline (45). A VersaDrive 4 microdrive (Neuralynx), containing four independently adjustable tetrodes, was affixed to the skull via the screws, luting (3 M), and dental acrylic (A-M Systems). A second small craniotomy was performed to place the ground wire in direct contact with the brain. After the acrylic hardened, a topical triple antibiotic ointment mixed with 2% lidocaine hydrochloride jelly was applied to the scalp, the mouse was removed from the stereotaxic device, the isoflurane was turned off, and oxygen alone was delivered to the mouse to gradually alleviate anesthetic state. Mice were administered sterile isotonic saline (0.9%) for rehydration and an analgesic (Ketofen; 5 mg/kg) for pain management. Analgesic and topical antibiotic administration was repeated daily for up to 5 days, and mice were closely monitored for any signs of distress.
Electrophysiology.
Neural recordings began after recovery from surgery and stabilization of behavioral performance (∼4 wk and ∼13 wk following surgery in control and hemi-PD mice, respectively; Fig. 1). Recordings were collected using four tetrodes, wherein each tetrode consisted of four polyimide-coated nichrome wires (Sandvik, PX000001; single-wire diameter 12.5 μm) gold plated to 0.2–0.4 MΩ impedance. Electrical signals were amplified and recorded using the Digital Lynx S multichannel acquisition system (Neuralynx) in conjunction with Cheetah data acquisition software (Neuralynx). Tetrode depths were adjusted ∼23 h before each recording session to sample an independent population of neurons across sessions. To estimate tetrode depths during each session, we calculated distance traveled with respect to the rotation fraction of the screw that was affixed to the shuttle holding the tetrode. One full rotation moved the tetrode ∼250 μm and tetrodes were moved ∼62.5 μm between sessions. The final tetrode location was confirmed through histological assessment (see below).
Offline spike sorting and cluster quality analysis were performed using MClust software (MClust-4.3, 47) in MATLAB. Briefly, for each tetrode, single units were isolated by manual cluster identification based on spike features derived from sampled waveforms (see example mean waveforms recorded in control mice in Ref. 34). Identification of single units through examination of spikes in high-dimensional feature space allowed us to refine the delimitation of identified clusters by examining all possible two-dimensional combinations of selected spike features. We used standard spike features for single-unit extraction: peak amplitude, energy (square root of the sum of squares of each point in the waveform, divided by the number of samples in the waveform), and the first principal component normalized by energy. Spike features were derived separately for individual leads. To assess the quality of identified clusters, we calculated two standard quantitative metrics: L-ratio and isolation distance (47). Clusters with an L-ratio of less than 0.75 and isolation distance greater than 6.5 were deemed single units, which resulted in the exclusion of 7% of the identified clusters. Only clusters with few interspike intervals less than 1.5 ms were considered for further examination. Furthermore, we excluded the possibility of including data from the same neuron twice by ensuring that both the waveforms and response properties sufficiently changed across sessions. If they did not, we conservatively assumed that we were recording from the same neuron, and only included data from one session.
Immunohistochemistry.
Final tetrode locations were verified by producing electrolytic lesions (100 µA, ∼1.5 min/lead) after the last recording session (see example lesions in control mice in Ref. 34). Mice were then overdosed with an intrapertinoneal injection of sodium pentobarbital (100 mg/kg) and transcardially perfused with saline followed by ice-cold 4% paraformaldehyde (PFA, Electron Microscopy Sciences) in 0.1 M phosphate buffer (PB). After perfusion, brains were submerged in 4% PFA in 0.1 M PB for 24 h for postfixation and then cryoprotected for 24 h by immersion in 30% sucrose in 0.1 M PB. The brain was encased in the same sucrose solution and frozen rapidly on dry ice.
Serial coronal sections (60 μm) were cut on a sliding microtome (Leica, SM2010R). Fluorescent Nissl (NeuroTrace, Invitrogen) was used to identify cytoarchitectural features of the SNr and verify tetrode tracks and lesion damage within or below the SNr (34). In addition, the coronal sections were stained for tyrosine hydroxylase (TH). Following repeated soaks in PBS and blocking solution, the sections were exposed to primary antibody overnight [anti-tyrosine hydroxylase (rabbit) antibody, 1:1,000, Rockland]. Next, the sections were washed in carrier solution (2 × 10 min) and exposed to a secondary antibody for 2 h [goat anti-rabbit IgG (H + L) secondary antibody, 1:500]. Images were captured with a ×10 objective lens, using an LSM 5 Pascal series Axioskop 2 FS MOT confocal microscope (Zeiss). For each mouse, a representative coronal section including the SNc (45) was used to quantify dopaminergic cell loss by comparing the number of TH+ neurons ipsilateral and contralateral to the injection.
Dopaminergic cell loss was quantified by calculating the percent decrease of red (TH+) pixel intensity in the SNc on the injected side relative to the SNc on the noninjected side, after accounting for background differences in red pixel intensity between the two sides. One of the six hemi-PD mice exhibited <70% dopaminergic cell loss and was therefore excluded from the group, consistent with previously published thresholds (36, 49, 50). Average TH+ loss of the five remaining hemi-PD mice ranged from 73% to 88%, with a median of 78%. Secondary confirmation of dopaminergic cell loss was quantified in the same manner using coronal sections containing the striatum (Fig. 3A).
Figure 3.
Validation of hemi-PD mouse model. A: representative coronal section (0.97 mm anterior to Bregma) in hemi-PD mouse. 6-OHDA was delivered to the ipsilateral SNc. Green, Nissl; red, tyrosine hydroxylase. B: overall activity (odor port entry to reward port exit in each trial) of SNr neurons during task performance in hemi-PD (dark gray, n = 4 mice) and control (light gray, n = 4 mice) mice. Black lines, medians. Median overall SNr activity was higher in hemi-PD mice (*P = 0.0170, one-tailed Wilcoxon rank-sum test). PD, Parkinson’s disease; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata.
Data Analysis
Behavioral directional bias.
Behavioral directional bias was quantified as the difference between the fraction of ipsilateral choices on ipsilaterally rewarded trials (i.e., trials for which reward was available at the ipsilateral port) and the fraction of contralateral choices on contralaterally rewarded trials (i.e., trials for which reward was available at the contralateral port):
Positive values reflect an ipsilateral behavioral bias and negative values reflect a contralateral bias. The 50/50 SG trials were evenly split between ipsilaterally rewarded and contralaterally rewarded and either choice was considered correct. To compare directional bias between presurgery and postsurgery sessions (Figs. 4 and 5), we calculated a single presurgery bias for each mouse based on all presurgery trials performed by that mouse (i.e., as if all trials had been performed in a single “super-session”). To compare directional bias between SG and IS trials within the same session (Fig. 6), to account for ceiling effects on ipsilaterally rewarded IS trials, the bias calculation was modified to quantify ipsilateral choices on contralaterally rewarded trials only. Sessions were included in this analysis if the mouse completed at least 25 trials of each direction (ipsilateral and contralateral) and type (SG and IS) trials. We did not observe a systematic change in bias as a function of time from the 6-OHDA injection, and therefore analyzed all postsurgery sessions as a single group.
Figure 4.
Effect of unilateral dopaminergic cell loss on stimulus-guided (SG) movements. A: thin lines show best-fit logistic functions (as in Fig. 2C) for SG trials for each presurgery session (gray, n = 30 sessions) and postsurgery session (black, n = 42 sessions) for a representative hemi-PD mouse. Thick lines show fits for SG trials combined across all presurgery (gray) and postsurgery (black) sessions. B: as in A, for a representative control mouse (tetrode drive implanted to SNr; n = 33 presurgery and 17 postsurgery sessions). C: directional bias (relative to the presurgery baseline for each mouse) on SG trials for each postsurgery session (138 sessions in 4 hemi-PD mice; 57 sessions in 4 control mice). Each row of symbols corresponds to sessions from one mouse; histograms show all sessions across mice. Hemi-PD mice exhibited an ipsilateral bias (P = 8.87 × 10−8, two-tailed Wilcoxon signed-rank test); control mice did not (P = 0.3480, two-tailed t test; postsurgery bias differed between hemi-PD than control mice, P = 5.64 × 10−5, two-tailed Wilcoxon rank-sum test; black and white arrows indicate median values for hemi-PD and control mice, respectively). D: reaction times (from go signal to reward port entry) on ipsilateral (left) and contralateral (right) SG trials in presurgery (gray) and postsurgery (black) trials for a representative hemi-PD mouse (ipsilateral: 1,957 trials presurgery, 5,393 trials postsurgery; contralateral: 2,193 trials presurgery, 3,143 trials postsurgery). E: as in D, for a representative control mouse (ipsilateral: 1,386 trials presurgery, 2,340 trials postsurgery; contralateral: 1,621 trials presurgery, 1,760 trials postsurgery). F: change (from the presurgery median baseline for each mouse) in median reaction time for each postsurgery session (same mice and sessions as in C) on ipsilateral (left) and contralateral (right) SG trials. Each row of symbols corresponds to sessions from one mouse; histograms show all sessions across mice. Hemi-PD mice exhibited larger changes in reaction times than control mice (ipsilateral trials: P = 9.90 × 10−3, contralateral trials: P = 8.19 × 10−22, two-tailed Wilcoxon rank-sum tests; black and white arrows indicate median values for hemi-PD and control mice, respectively) and exhibited a larger change on contralateral than on ipsilateral trials (P = 1.66 × 10−12, two-tailed Wilcoxon signed-rank test). PD, Parkinson’s disease; SNr, substantia nigra pars reticulata.
Figure 5.
Effect of unilateral dopaminergic cell loss on internally specified (IS) movements. A: connected symbols show fraction of ipsilateral choices on ipsilateral and contralateral blocks of IS trials for each presurgery session (n = 63 sessions) and postsurgery session (n = 16 sessions) for a representative hemi-PD mouse. Filled symbols show means. B: as in A, for a representative control mouse (tetrode drive implanted; n = 14 presurgery and 14 postsurgery sessions). C: directional bias (relative to the presurgery baseline for each mouse) on IS trials for each postsurgery session (65 sessions in 4 hemi-PD mice; 48 sessions in 4 control mice). Each row of symbols corresponds to sessions from one mouse; histograms show all sessions across mice. Hemi-PD mice exhibited an ipsilateral bias (P = 1.49 × 10−6, two-tailed Wilcoxon signed-rank test); control mice did not (P = 0.3855, two-tailed t test; postsurgery bias differed between hemi-PD and control mice, P = 7.74 × 10−3, two-tailed Wilcoxon rank-sum test; black and white arrows indicate median values for hemi-PD and control mice, respectively). D: reaction times (from go signal to reward port entry) on ipsilateral (left) and contralateral (right) IS trials in presurgery (gray) and postsurgery (black) trials for a representative hemi-PD mouse (ipsilateral: 874 trials presurgery, 2,328 trials postsurgery; contralateral: 789 trials presurgery, 1,783 trials postsurgery). E: as in D, for a representative control mouse (ipsilateral: 764 trials presurgery, 839 trials postsurgery; contralateral: 820 trials presurgery, 804 trials postsurgery). F: change (from the presurgery median baseline for each mouse) in median reaction time for each postsurgery session (same mice and sessions as in C) on ipsilateral (left) and contralateral (right) IS trials. Each row of symbols corresponds to sessions from one mouse; histograms show all sessions across mice. Hemi-PD mice exhibited larger changes in reaction times than control mice (ipsilateral trials: P = 9.38 × 10−5, two-tailed Wilcoxon rank-sum test, contralateral trials: P = 3.90 × 10−18, two-tailed t test; black and white arrows indicate median values for hemi-PD and control mice, respectively) and exhibited a larger change on contralateral than on ipsilateral trials (P = 1.09 × 10−10, two-tailed Wilcoxon signed-rank test). PD, Parkinson’s disease.
Figure 6.
Direct comparison between stimulus-guided (SG) and internally specified (IS) behavior in hemi-PD mice. A: connected symbols show fraction of ipsilateral choices on SG and IS contralaterally rewarded trials for each presurgery session (n = 128 sessions) and postsurgery session (n = 65 sessions) for hemi-PD mice (n = 4 mice). Hemi-PD mice were more ipsilaterally biased on IS than SG trials presurgery (P = 1.99 × 10−21, two-tailed Wilcoxon signed-rank test), but not postsurgery (P = 0.0924, two-tailed Wilcoxon signed-rank test). B: within-session ipsilateral bias differences between SG and IS trials changed between presurgery and postsurgery sessions (P = 3.62 × 10−11, two-tailed Wilcoxon rank-sum test). Each row of symbols corresponds to presurgery or postsurgery sessions from one mouse; histograms show all sessions across mice. C: as in A, for control mice (n = 148 presurgery sessions, n = 48 postsurgery sessions, n = 4 mice). Control mice were more ipsilaterally biased on IS than SG trials presurgery (P = 1.23 × 10−8, two-tailed Wilcoxon signed-rank test) and postsurgery (P = 4.72 × 10−3, two-tailed Wilcoxon signed-rank test). D: as in B, for control mice. Within-session ipsilateral bias differences between SG and IS trials did not change between presurgery and postsurgery sessions (P = 0.643, two-tailed Wilcoxon rank-sum test). PD, Parkinson’s disease.
Neuronal direction preference.
We used a firing rate-based receiver operating characteristic (ROC)-based analysis to quantify the selectivity of single neurons for movement direction (34, 51). This analysis calculates the ability of an ideal observer to classify whether a given firing rate was recorded in one of two conditions [i.e., preceding ipsilateral (left) or contralateral (right) movement]. We defined “preference” as 2(ROCarea − 0.5), a measure ranging from −1 to 1, where −1 signifies the strongest possible preference for ipsilateral, 1 signifies the strongest possible preference for contralateral, and 0 signifies no preference (34, 52). For example, if the firing rate of a given neuron is generally higher preceding ipsilateral than contralateral movements, that neuron is assigned a preference <0. Statistical significance was determined with a permutation test: we recalculated the preference after randomly reassigning all firing rates to either of the two groups, repeating this procedure 500 times to obtain a distribution of values, and calculated the fraction of random values exceeding the actual value. We tested for significance at α = 0.05. To ensure that movements were unidirectional, trials in which the movement time (between odor port exit and reward port entry) was >1.5 s were excluded from all analyses. To ensure a sufficient number of trials and spikes for neural analyses, neurons with <25 trials of each trial type under comparison (ipsilateral SG, contralateral SG, ipsilateral IS, or contralateral IS) or with a firing rate <2.5 spikes/s for either trial type under comparison were excluded from all analyses (34, 40).
Shift function.
We used a shift function to quantify if and how two distributions differ (53). Briefly, using a Harrell–Davis quantile estimator (54), distributions were divided into 10 equal parts by 9 “deciles.” For example, the 1st decile is the value below which 10% of the values lie, whereas the 9th decile is the value below which 90% of values lie. The shift function compares a given decile in distribution A with its corresponding decile in distribution B. Corresponding deciles were determined to be significantly different if the confidence interval of their differences, calculated by sampling the difference between bootstrapped distributions 200 times, did not cross 0.
Activity change during epoch of interest.
We calculated the normalized response (NR) for each neuron as ,, where Ft is the median firing rate in the “test” window (either movement preparation epoch or movement initiation epoch) and Fo is the median overall firing rate from odor port entry to reward port exit [i.e., the duration of the entire trial (34); note that the structure of our task does not include a natural “baseline” epoch in which the mouse is in a motionless state unaffected by task demands]. Neurons with Fo < 2.5 spikes/s were excluded from analyses. Statistical significance was determined using a pairwise t test to compare Ft and Fo from the same trial. Neurons with NR < 1 (P < 0.05) were defined as “Decreasing” and neurons with NR > 1 (P < 0.05) were defined as “Increasing”; all other neurons were categorized as “No Δ” (Fig. 8; Table 1). Note that, by convention, a decreasing neuron that decreases more for contralateral than ipsilateral movement would be considered to have an ipsilateral direction preference (as calculated above) because firing rate is higher for ipsilateral movement (33, 34).
Figure 8.

Functional classes of SNr neurons during movement preparation and initiation in behaving hemi-PD and control mice. A: fraction of neurons with a given direction preference segregated by whether their average activity during the movement preparation epoch (beginning 100 ms after the odor valve opens and ending with the go signal) significantly increased (Inc.), decreased (Dec.), or did not change (No Δ) relative to overall firing rate, for hemi-PD (top) and control (bottom) mice. Proportion of neurons exhibiting an increase, a decrease, and no change differs between hemi-PD and control mice (P = 1.32 × 10−12, χ2 test = 52.96, df = 2). Similarly, proportion of ipsilateral, contralateral, and no direction preference neurons differed between hemi-PD and control mice (P = 2.2 × 10−16, χ2 test = 149.83, df = 2). B: fraction of neurons with a given direction preference segregated by whether their activity during the movement initiation epoch (beginning with the go signal and ending 100 ms after odor port exit) increased, decreased, or did not change relative to presurgery, for hemi-PD (top) and control (bottom) mice. Proportion of neurons exhibiting an increase, a decrease, and no change did not differ between hemi-PD and control mice (P = 0.372, χ2 test = 1.98, df = 2; n = 181 neurons in 4 hemi-PD mice; 285 neurons in 4 control mice); proportion of ipsilateral, contralateral, and no direction preference neurons differed between hemi-PD and control mice (P = 6.68 × 10−5, χ2 test = 19.23, df = 2). FR, firing rate; PD, Parkinson’s disease; SNr, substantia nigra pars reticulata.
Table 1.
Relationship between activity change and direction preference for all SNr neurons recorded from all hemi-PD (upper) and control (lower) mice
| Decreased FR | No Δ FR | Increased FR | Total | |
|---|---|---|---|---|
| Hemi-PD | ||||
| Ipsilateral | 7 | 9 | 3 | 19 (10.5%) |
| Nonselective | 67 | 34 | 29 | 130 (71.8%) |
| Contralateral | 25 | 5 | 2 | 32 (17.7%) |
| Total | 99 (54.7%) | 48 (26.5%) | 34 (18.8%) | 181 neurons |
| Control | ||||
| Ipsilateral | 42 | 18 | 34 | 94 (33.0%) |
| Nonselective | 36 | 10 | 40 | 86 (30.2%) |
| Contralateral | 36 | 15 | 54 | 105 (36.8%) |
| Total | 114 (40%) | 43 (15.1%) | 128 (44.9%) | 285 neurons |
FR, firing rate; PD, Parkinson’s disease; SNr, substantia nigra pars reticulata.
Statistics.
MATLAB was used for all statistical analyses except for χ2 analyses, which were performed in R. Distributions were tested for normality using the Lilliefors test (lillitest MATLAB function) and, unless all data sets under comparison were normally distributed, nonparametric statistical tests were used for that analysis. For consistency, all graphical representations of central tendency are medians, independent of whether parametric or nonparametric statistical tests were used. We used two-tailed tests unless testing for a predicted direction of an effect, in which case one-tailed tests were appropriate. Since we are interested in the neural basis of parkinsonian behavior, and particularly in comparing behavior and neural activity between IS and SG trials within sessions, we conservatively analyze our behavioral data with both mice and sessions as the units of analysis, and we analyze our neural data with individual neurons, reported by mouse, as the unit of analysis (55).
RESULTS
Effect of Unilateral Dopaminergic Cell Loss on Stimulus-Guided and Internally Specified Movements
To examine how parkinsonian conditions affect stimulus-guided (SG) and internally specified (IS) movements and their underlying BG output, we first trained mice on a behavioral task designed to elicit these forms of movements (Figs. 1 and 2, A and B) (34). Briefly, on each trial of the task, the mouse was presented with a binary mixture of odors A and B at a central odor port, waited for an auditory go signal, and moved to the ipsilateral (left) or contralateral (right) reward port for a water reward (Fig. 2A). Each daily experimental session consisted of interleaved blocks of SG and IS trials; unsignaled transitions between blocks occurred upon completion of ∼50 rewarded trials (Fig. 2B; materials and methods) (34). On SG trials, the dominant component of the odor mixture—which varied by trial—determined the side at which reward was delivered. When odor A was dominant, reward was available at the left port, when odor B was dominant, reward was available at the right port, and when odor A = odor B, reward was equally likely at both ports (Fig. 2B). On IS trials, only the odor A = odor B mixture was presented and reward was delivered at only one port (left or right) throughout the block (Fig. 2B; materials and methods); thus, the odor mixture on IS trials did not inform which side would be rewarded. Critically, although for both SG and IS trials the same auditory go signal indicated when mice were permitted to exit the odor port, the olfactory stimulus cued the direction of movement only on SG, and not IS, trials. Consistent with previous results (34), mice quickly inferred block transitions based on the mixtures presented and the outcomes of their choices, such that the direction of movement on SG trials was selected primarily based on the stimulus (Fig. 2C) while the direction of movement on IS trials was selected primarily based on recent trial history (Fig. 2D).
Upon achieving proficient performance on the task (Fig. 2, C and D; materials and methods), mice received 6-OHDA injections to one SNc to unilaterally ablate dopaminergic neurons (8, 9, 43, 44, 56–59) and were implanted with a chronic tetrode drive targeting the ipsilateral SNr to record BG output (Fig. 1). Only mice with >70% histologically confirmed dopaminergic cell loss (examined following all behavioral and recording experiments; Figs. 1 and 3A) were included in the “hemi-PD” group for subsequent analyses (materials and methods). Confirming the validity of our hemi-PD model, hemi-PD mice exhibited a greater ipsilateral bias than control mice (saline delivered to SNc) on a standard rotation assay (postsurgery change in net ipsilateral rotations for five hemi-PD mice: 16, 53, −6, 151 and 61; for three control mice: 6, −11 and −14; P = 0.0357, one-tailed Wilcoxon rank-sum test; materials and methods) and exhibited higher mean SNr activity (P = 0.0170, one-tailed Wilcoxon rank-sum test; Fig. 3B), consistent with previous findings in hemi-PD models (5, 8, 35, 36, 58, 60). The bimodality of the distributions of mean SNr activity suggests that the difference between control and hemi-PD mice may be driven by a larger proportion of high-activity neurons, rather than a uniform shift toward a higher firing rate across all SNr neurons, in hemi-PD mice.
Before directly comparing the effects of unilateral dopaminergic cell loss on the SG and IS movements required by our task, we sought to characterize the effects on each trial type individually. Given the classical rate-model prediction that SNr-recipient nuclei for contralateral movement are overinhibited (2, 6, 7, 10–12) and supported by our findings of greater ipsilateral bias on the rotation assay and an increase in mean SNr activity (Fig. 3B), we expected hemi-PD mice (as determined by the extent of dopaminergic cell loss; Fig. 3A) to exhibit an ipsilateral bias for both SG and IS movements.
We first examined SG movements. Qualitatively, we found that the psychometric functions fit to postsurgery behavioral sessions (as in Fig. 2) were shifted ipsilaterally relative to presurgery sessions in hemi-PD (Fig. 4A), but not control (Fig. 4B), mice. To quantify how bias changed following surgery, we calculated the behavioral directional bias for each postsurgery session and subtracted the presurgery bias for that mouse (materials and methods). In sessions performed by hemi-PD mice, despite typical levels of variability consistent with other behavioral assays of this 6-OHDA model (44), we found that choices after surgery were biased ipsilaterally (P = 8.87 × 10−8, two-tailed Wilcoxon signed-rank test), whereas no postsurgery bias was observed in control mice (P = 0.3480, two-tailed t test), and postsurgery bias differed between hemi-PD and control mice (P = 5.64 × 10−5, two-tailed Wilcoxon rank-sum test; Fig. 4C). We obtained the same pattern of results when we analyzed the data with respect to mice rather than sessions by comparing the overall presurgery bias to postsurgery bias within mice [hemi-PD (n = 4): P = 0.0470; control (n = 4): P = 0.3227, two-tailed t tests]. Hemi-PD mice also exhibited changes in reaction time (defined as the time from go signal to reward port entry) consistent with their ipsilateral directional bias. Figure 4D shows the distribution of reaction times for each trial of all presurgery and postsurgery sessions for the same example mouse shown in Fig. 4A. Reaction times are longer postsurgery, most notably for contralateral trials (Fig. 4A, right). Control mice also exhibited longer reaction times postsurgery, as expected given the additional weight of the chronic recording drive, but for the example mouse, the increase does not appear to be greater for contralateral than ipsilateral movements (Fig. 4E). To quantify the change in reaction time across all mice, we calculated the median reaction time for each postsurgery session and subtracted the overall median presurgery reaction time for each mouse (Fig. 4F). As exhibited by the example mice (Fig. 4, D and E), reaction times were longer postsurgery, but hemi-PD mice exhibited larger changes in reaction times than control mice (ipsilateral trials: P = 9.90 × 10−3, contralateral trials: P = 8.19 × 10−22, two-tailed Wilcoxon rank-sum tests; Fig. 4F) and exhibited a larger change on contralateral than on ipsilateral trials (P = 1.66 × 10−12, two-tailed Wilcoxon signed-rank test). Analyzing the data with respect to mice yielded complementary results: hemi-PD mice exhibited a larger increase in reaction time postsurgery for contralateral than ipsilateral movements (P = 0.0286, two-tailed Wilcoxon rank-sum test), whereas in control mice, the increase was not greater for contralateral than ipsilateral movements (P = 0.770, two-tailed t test). This greater slowing of contralateral movements in hemi-PD mice is consistent with their ipsilateral directional bias (Fig. 4C).
We observed similar effects of unilateral dopaminergic cell loss on IS movements (Fig. 5). For these trials, we first examined bias separately during blocks in which reward was delivered at the ipsilateral port (“ipsilateral blocks”) and the contralateral port (“contralateral blocks”). Figure 5, A and B, shows behavior on IS trials for all presurgery and postsurgery sessions for representative hemi-PD and control mice. On ipsilateral blocks, both mice frequently chose the ipsilateral port. On contralateral blocks, however, the hemi-PD mouse was more likely to choose the ipsilateral port postsurgery than presurgery (Fig. 5A), whereas the control mouse did not exhibit this change. Across all mice, we quantified directional bias as we did for SG trials (materials and methods), and again found that choices after surgery were biased ipsilaterally in hemi-PD mice (P = 1.49 × 10−6, two-tailed Wilcoxon signed-rank test) while no postsurgery bias was observed in control mice (P = 0.3855, two-tailed t-test); this postsurgery bias differed between hemi-PD and control mice (P = 7.74 × 10−3, two-tailed Wilcoxon rank-sum test; Fig. 5C). We again obtained similar results when we analyzed the data with respect to mice rather than sessions: on contralateral blocks, hemi-PD mice were more likely to choose the ipsilateral port after than before surgery (P = 0.0230, two-tailed t test; Fig. 5, A and C), whereas control mice did not exhibit this change (P = 0.200, two-tailed t test). We also found similar effects on reaction times on IS trials as we did on SG trials: hemi-PD mice exhibited larger changes in reaction times than control mice (ipsilateral trials: P = 9.38 × 10−5, two-tailed Wilcoxon rank-sum test, contralateral trials: P = 3.90 × 10−18, two-tailed t test; Fig. 5, D–F) and exhibited a larger change on contralateral than on ipsilateral trials (P = 1.09 × 10−10, two-tailed Wilcoxon signed-rank test), again consistent with their ipsilateral directional bias (Fig. 5C). Analyzing the data with respect to mice, hemi-PD mice exhibited a larger increase in reaction time postsurgery for contralateral than ipsilateral movements (P = 0.0286, two-tailed Wilcoxon rank-sum test) while control mice did not (P = 0.868, two-tailed t test). Thus, although hemi-PD mice retained the ability to perform SG and IS trials, unilateral dopaminergic cell loss resulted in fewer and slower contralateral movements on both trial types, consistent with classical rate-model predictions.
Next, we sought to examine whether this directional effect was greater in magnitude, indicative of greater impairment, on SG or IS trials. To do so we directly compared, within each session, directional bias on contralaterally rewarded SG and IS trials. We found that presurgery, hemi-PD mice were more ipsilaterally biased on IS than SG trials (P = 1.99 × 10−21, two-tailed Wilcoxon signed-rank test; Fig. 6A), as expected since on some SG trials an ambiguous stimulus cue was presented (Fig. 2C). However, after surgery, ipsilateral bias differed little between IS and SG trials (P = 0.0924, two-tailed Wilcoxon signed-rank test; Fig. 6A), resulting in a shift of the distribution of within-session ipsilateral bias differences postsurgery (P = 3.62 × 10−11, two-tailed Wilcoxon rank-sum test; Fig. 6B). In contrast, control mice exhibited a greater ipsilateral bias on SG than IS trials both before (P = 1.23 × 10−8, two-tailed Wilcoxon signed-rank test) and after (P = 4.72 × 10−3, two-tailed Wilcoxon signed-rank test) surgery (Fig. 6C), and the magnitude of this relative bias did not differ between sessions before and after surgery (P = 0.643, two-tailed Wilcoxon rank-sum test; Fig. 6D). Together, these analyses demonstrate that, despite the behavioral variability typical in hemi-PD mice, IS trials are relatively more affected than SG trials by unilateral dopaminergic cell loss.
Effect of Unilateral Dopaminergic Cell Loss on Basal Ganglia Output
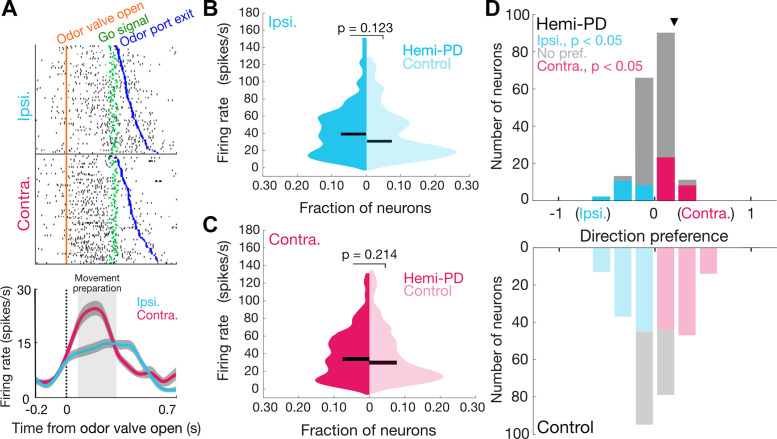
We sought to determine whether behavioral differences between SG and IS trials could be accounted for by activity in the SNr, which is known to play a role in the movements elicited by this task (34). In a subset of the mice described in the above behavioral results, we used chronically implanted tetrodes to record from 181 SNr neurons in hemi-PD mice (n = 4) and 285 neurons in control mice (n = 4) that met our analysis criteria (materials and methods); each neuron was recorded in only one session. We focused on activity underlying movement preparation, which has been implicated in parkinsonian motor deficits (24–31). Consistent with previous results (34), we observed that SNr activity recorded from hemi-PD mice was often modulated during the “movement preparation epoch” (from 100 ms after the odor valve was opened until the go signal) and depended on movement direction (Fig. 7A). We first asked whether the firing rate during this epoch differed between hemi-PD and control mice, similar to the difference we and others have observed in overall activity (Fig. 3B) (3, 5, 8, 9, 35–39). As described in the introduction, the classical rate model would predict elevated SNr activity in hemi-PD mice, consistent with the ipsilateral bias that we observed given that SNr activity inhibits downstream motor centers that primarily mediate contralateral movement. However, there was not a clear overall difference between groups for movement in either direction (Pipsi= 0.123; Pcontra= 0.214, one-tailed Wilcoxon rank-sum tests; Fig. 7, B and C). The ipsilateral bias exhibited by hemi-PD mice on SG and IS trials therefore cannot be explained by an absolute increase in SNr activity during movement preparation, as would be predicted by the classical rate model.
Figure 7.
SNr activity during movement preparation in behaving hemi-PD and control mice. A: rasters (top) and peri-event time histograms (bottom) for an example neuron from a hemi-PD mouse aligned to odor valve open and segregated by choice. Histograms are smoothed with a Gaussian filter; shading, ±SE. B and C: mean firing rate during movement preparation epoch (between odor valve open and go signal) did not differ between populations of neurons in hemi-PD and control mice on ipsilateral (B) or contralateral (C) trials (Pipsi= 0.1227; Pcontra= 0.2138, one-tailed Wilcoxon rank-sum tests; n = 181 neurons in 4 hemi-PD mice; 285 neurons in 4 control mice). Horizontal bars, medians. D: distribution of direction preferences for population of neurons in hemi-PD mice (top) had a smaller range than in control mice (bottom) (P = 5.73 × 10−4, two-sample Kolmogorov–Smirnov test), and more neurons exhibited a significant preference in control than in hemi-PD mice (P = 2.20 × 10−16, χ2 test). Arrowhead, example neuron shown in A. PD, Parkinson’s disease; SNr, substantia nigra pars reticulata.
We next examined whether unilateral dopaminergic cell loss affected the relative activity of individual neurons between ipsilateral and contralateral movements (Fig. 7A), which could also potentially account for the ipsilateral behavioral bias. Specifically, if the SNr on the side with dopaminergic cell loss exhibits a higher firing rate for contralateral than ipsilateral movements, as compared with the SNr in control mice (and, presumably, to the SNr on the intact side in hemi-PD mice), then the affected SNr would overinhibit those downstream motor structures on contralateral trials, resulting in an ipsilateral behavioral bias. These results would be consistent with a “directional rate model” in which movement direction is determined by the relative firing rate of both SNrs for ipsilateral and contralateral movements. We therefore calculated the “direction preference” of each neuron, which quantifies the difference in firing rate during a specified epoch between ipsilateral and contralateral movements, and ranges from −1 (higher firing rates for ipsilateral movements) to 1 (higher firing rates for contralateral movements), where 0 represents no preference (materials and methods) (34, 51, 52). We found that the populations of neurons recorded in hemi-PD and control mice each exhibited a range of preferences, with some neurons preferring ipsilateral movement (Fig. 7D, cyan; P < 0.05, permutation test; materials and methods) and some preferring contralateral movement (Fig. 7D, magenta; P < 0.05, permutation test). However, the distribution of preferences exhibited by neurons recorded in hemi-PD and control mice differ (P = 5.73 × 10−4, two-sample Kolmogorov–Smirnov test) in two key respects.
First, the population of SNr neurons in hemi-PD mice, but not control mice, exhibited a slight bias toward contralateral preferences. Although the entire distribution of preferences in hemi-PD mice was not contralaterally skewed (median = 0.138; P = 0.372, one-tailed Wilcoxon signed-rank test), of the neurons that exhibited a significant preference (Fig. 7D, magenta and cyan, Table 1), more were contralateral preferring (32/51, magenta; Table 1) than ipsilateral preferring (19/51, cyan; P = 0.0132, χ2 test = 4.83, df = 1; Table 1; 3/4 hemi-PD mice exhibited more contralateral- than ipsilateral-preferring neurons; Table 2). In contrast, in control mice, we found roughly equal proportions of contralateral-preferring (105/199, magenta; Table 1) and ipsilateral-preferring (94/199, cyan; Table 1) neurons (P = 0.158, χ2 test = 1.01, df = 1; Fig. 7D, Table 1). This analysis indicates that, in the population of SNr neurons in which direction preference is spared, unilateral dopaminergic cell loss results in more neurons exhibiting higher activity for contralateral than ipsilateral movements, consistent with the ipsilateral behavioral bias that we observed (Figs. 4 and 5, Table 1) and with the directional rate model.
Table 2.
Activity change, direction of preference and strength of preference for SNr neurons recorded from individual hemi-PD (upper) and control (lower) mice
| Mouse No. | Dec. FR | No Δ FR | Inc. FR | Ipsi. | Nonselective | Contra. | Fraction Pref. | Mean Pref. | Mean|pref.| |
|---|---|---|---|---|---|---|---|---|---|
| Hemi-PD | |||||||||
| 1 | 28 | 17 | 10 | 9 | 34 | 12 | 0.38 | 0.0014 | 0.14 |
| 2 | 54 | 17 | 7 | 5 | 60 | 13 | 0.23 | 0.020 | 0.075 |
| 3 | 14 | 7 | 4 | 5 | 15 | 5 | 0.40 | −0.0045 | 0.14 |
| 4 | 3 | 7 | 13 | 0 | 21 | 2 | 0.09 | 0.020 | 0.16 |
| Total | 99 | 48 | 34 | 19 | 130 | 32 | |||
| Control | |||||||||
| 1 | 41 | 15 | 40 | 23 | 28 | 45 | 0.71 | 0.073 | 0.22 |
| 2 | 27 | 11 | 27 | 30 | 23 | 12 | 0.65 | −0.11 | 0.23 |
| 3 | 20 | 9 | 41 | 19 | 12 | 39 | 0.83 | 0.11 | 0.24 |
| 4 | 26 | 8 | 20 | 22 | 23 | 9 | 0.57 | −0.062 | 0.12 |
| Total | 114 | 43 | 128 | 94 | 86 | 105 | |||
Contra., contralateral; Dec., decreased; FR, firing rate; Inc., increased; Ipsi, ipsilateral; PD, Parkinson’s disease; SNr, substantia nigra pars reticulata.
Second, the population of SNr neurons in hemi-PD mice exhibited weaker direction preference than the population in control mice. We quantified this difference with several complementary analyses. A smaller proportion of neurons in hemi-PD mice (51/181, 28%; Table 1) than control mice (199/285, 70%; Table 1) exhibited a significant direction preference (P = 2.20 × 10−16, χ2 test = 150, df = 1). This relationship was held within individual mice (Table 2). When the entire population in hemi-PD and control mice is considered (i.e., including neurons with and without a significant direction preference), the strength of the preference, independent of sign, was smaller in hemi-PD mice (median = 0.0809) than in control mice (median = 0.179; P = 1.25 × 10−12, two-tailed Wilcoxon rank-sum tests; Table 2). Finally, we used a shift function analysis to identify the deciles in which the distributions differed (materials and methods) (53). This analysis revealed that preferences were closer to 0 in hemi-PD than control mice in the three deciles representing the most ipsilateral preferences and in the three deciles representing the most contralateral preferences, consistent with weaker direction preference in hemi-PD mice. Together, although these results are not predicted by the classical or directional rate model, they indicate that unilateral dopaminergic cell loss results in a fundamental disruption of the representation of movement direction that is normally observed in the SNr (24, 32, 34).
To gain insight into the reorganization of BG output induced by unilateral dopaminergic cell loss, we next examined these systematic differences in preferences between hemi-PD and control mice within functional classes of SNr neurons (Fig. 8, Table 1). Separate subpopulations of SNr neurons are known to exhibit increases or decreases in activity as movements are prepared and initiated (32–34); these subpopulations presumably play different functional roles. We therefore categorized neurons into one of three classes based on whether their activity during movement preparation increased, decreased, or did not change, relative to overall activity (Table 1; materials and methods). We first noticed a clear difference in the proportion of neurons in each class between hemi-PD and control groups (P = 1.32 × 10−12, χ2 test = 50.96, df = 2; n = 181 neurons, 4 hemi-PD mice; n = 285 neurons, 4 control mice; Fig. 8A, Table 1). Specifically, we found that a higher proportion of neurons recorded in hemi-PD mice (54.7%) than in control mice (40.0%) exhibited decreased activity, and a corresponding lower proportion of neurons recorded in hemi-PD mice (18.8%) than in control mice (44.9%) exhibited increased activity (Fig. 8A, Table 1; materials and methods). In addition, our finding that fewer neurons in hemi-PD than control mice exhibited a significant direction preference held true across all three subpopulations of neurons (Fig. 8A, Table 1). Finally, we found that the contralateral bias in the hemi-PD group among neurons exhibiting a direction preference was largely due to those that exhibited decreased activity during movement preparation (contralateral:ipsilateral ratio = 25:7, P = 1.07 × 10−5, χ2 test = 18.1, df = 1; Fig. 8A, Table 1); this subpopulation in the control group did not show this effect (contralateral:ipsilateral ratio = 36:42; P = 0.788, χ2 test = 0.641, df = 1; Fig. 8A, Table 1). Thus, unilateral dopaminergic cell loss resulted in a larger proportion of SNr neurons that release downstream motor centers from inhibition, and they do so to a greater extent for ipsilateral than contralateral movements, consistent with the ipsilateral behavioral bias.
Thus far, we have focused on SNr activity during the movement preparation epoch. As our task is designed to separate movement preparation from initiation, we were able to extend our analyses to the movement initiation epoch, which we defined as the period from the go signal until 100 ms after odor port exit (results were unchanged when we instead defined this epoch as the 150 ms preceding odor port exit). We expected to observe similar changes during movement initiation, consistent with previous studies (61–64). We therefore repeated our firing rate, direction preference, and functional class analyses for the movement initiation epoch. As with the movement preparation epoch, we found no overall difference in firing rate during movement initiation between the hemi-PD and control groups for movement in either direction (Pipsi= 0.389; Pcontra= 0.211, one-tailed Wilcoxon rank-sum tests). Next, as with movement preparation, we found that a smaller proportion of neurons in hemi-PD than control mice exhibited a direction preference during movement initiation (P = 6.68 × 10−5, χ2 test = 19.23, df = 1; Fig. 8B), but our shift function analysis comparing the distributions of direction preferences in hemi-PD and control mice revealed only one differing decile (8th decile). Consistent with this result, we found no difference between hemi-PD and control mice in the proportion of neurons that exhibited increased or decreased activity during movement initiation (P = 0.372, χ2 test = 1.98, df = 1; n = 181 neurons, 4 hemi-PD mice; n = 285 neurons, 4 control mice; Fig. 8B, Table 1). Thus, dopaminergic cell loss appears to affect BG output more during movement preparation than during movement initiation in the context of our behavioral task.
Finally, we examined whether the changes in SNr activity during movement preparation associated with unilateral dopaminergic cell loss were consistent with the stronger ipsilateral behavioral bias on IS compared with SG trials (Fig. 6). For example, the representative neuron shown in Fig. 9A appears to exhibit a contralateral preference, consistent with an ipsilateral behavioral bias predicted by the directional rate model, on IS but not SG trials. To examine this phenomenon across the population, we compared direction preference during the movement preparation epoch between IS and SG trials within the same session for neurons with a direction preference in IS or SG trials. In the hemi-PD group, we found that preference was significantly greater (i.e., more contralateral) on IS than SG trials (P = 0.0066, one-tailed Wilcoxon signed-rank test, n = 79 neurons; Fig. 9B). Consistent with this difference in direction preference, we found that firing rate during the movement preparation epoch was higher for IS than SG trials on contralateral trials (P = 8.45 × 10−5, two-tailed paired t test), but not ipsilateral trials (P = 0.733, two-tailed paired t test). We also observed a significant difference in direction preference in control mice (P = 0.0134, two-tailed Wilcoxon signed-rank test; n = 237 neurons), but in the opposite direction (i.e., more ipsilateral). Together, these analyses of SNr activity show that changes in BG output in hemi-PD mice are consistent with their overall ipsilateral behavioral bias (Figs. 4 and 5), as well as their stronger bias on IS than SG trials (Fig. 6).
Figure 9.
SNr activity on stimulus-guided (SG) and internally specified (IS) trials in hemi-PD mice. A: rasters (top) and peri-event histograms (bottom), on stimulus-guided (SG, left) and internally specified (IS, right) trials, for an example neuron from a hemi-PD mouse aligned to odor valve open and segregated by choice. Histograms are smoothed with a Gaussian filter; shading, ±SE. B: direction preference on SG and IS trials for population of SNr neurons in hemi-PD mice. Each neuron is represented by a pair of connected symbols; each symbol represents one mouse; only neurons with significant preference (P < 0.05) on SG or IS trials are shown. Preference was more contralateral on IS than SG trials (*P = 0.0066, one-tailed Wilcoxon signed-rank test, n = 79 neurons). Result was the same if all neurons recorded under IS and SG conditions are included in the comparison (P = 0.00390, one-tailed Wilcoxon signed-rank test, n = 158 neurons). Black-filled symbols, medians. PD, Parkinson’s disease; SNr, substantia nigra pars reticulata.
DISCUSSION
In this study, we sought to determine whether BG output could explain the greater impairment in IS than SG movements under parkinsonian conditions. By recording SNr activity in hemi-PD mice performing SG and IS movements, we found that unilateral dopaminergic cell loss alters the relationship between SNr activity and movement direction as movements are prepared (Figs. 7D and 8, Table 1), consistent with the ipsilateral bias in behavior (Figs. 4 and 5). Although we did not observe an absolute increase in preparation-related SNr activity in hemi-PD mice (Fig. 7, B and C), as predicted by the classical rate model of parkinsonian BG activity (2, 6), our findings are consistent with a direction-sensitive form of the rate model—a “directional rate model”—in which SNr output inhibits contralateral more than ipsilateral movements. In contrast, neural activity during movement initiation was little changed between hemi-PD and control groups (Fig. 8B), suggesting that parkinsonian conditions affect BG output subserving processes associated with movement preparation more than initiation, consistent with studies in patients with PD (26, 28–31, 65).
Although unilateral dopaminergic cell loss resulted in an ipsilateral bias on both SG and IS trials (Figs. 4 and 5), we found that the effect was larger on IS trials (Fig. 6). This difference was reflected in the activity of SNr neurons, which exhibited a stronger contralateral preference (i.e., higher activity on contralateral than ipsilateral trials) on IS than SG trials (Fig. 9). This finding is consistent with our previous work showing that SNr activity is more sensitive to movement direction under IS than SG conditions (34), which suggests that the cognitive processes associated with preparing IS movements are more BG dependent than those associated with SG movements. One key factor that may explain the difference in BG dependence between IS and SG trials is the influence of prior choices and outcomes, which are crucial for determining the correct direction of movement on IS, but not SG, trials. The SNr receives direct excitatory input from the pedunculopontine tegmental nucleus (66, 67), which encodes prior choices and outcomes (40); SNr activity itself reflects prior choices (34); and in general, the BG are thought to bias activity in downstream motor centers toward movements associated with larger rewards (33, 68–71). The disruption of BG signaling by unilateral dopaminergic cell loss may therefore affect the (adaptive) influence of priors on movement selection (72), resulting in a greater deficit on IS than SG trials. The importance of the BG in representing priors is also consistent with our finding that SNr activity related to movement preparation, which is influenced by priors, was more affected than activity related to movement initiation, which is not.
Notably, the critical distinction between SG and IS trials was that the direction of rewarded movement was or was not guided by the (olfactory) stimulus. However, both forms of the movement were temporally cued by an auditory go signal. Although this identical temporal cueing could contribute to our finding of similar SNr activity during movement initiation, it also warrants caution in directly translating between our results in mice making movements with a direction cued and uncued by olfactory stimuli and those in patients with PD performing movements cued and uncued by external (and often visual) stimuli (13–18). A related consideration is that, by design, on IS trials, mice learned which side was rewarded in that block and could potentially position themselves in the odor port to efficiently move to the rewarded side. Such positioning could provide a postural cue, in addition to the internal specification, for movement direction on IS trials, and may contribute to the differences in behavior and BG activity on SG and IS trials.
In addition to the shift in the population of neurons in hemi-PD mice toward contralateral preference consistent with the ipsilateral behavioral bias, a large proportion of neurons exhibited no relationship between activity and movement direction (gray bars in Figs. 7D and 8A, Table 1). Further, we found that more neurons in hemi-PD mice exhibited a decrease than an increase in activity during movement preparation compared with overall activity, which was not the case in control mice (Fig. 8A, Table 1), perhaps due to the increase in overall activity in hemi-PD mice (Fig. 3B). Although these findings do not directly relate to our primary behavioral readout of directional bias and are not predicted by either the classical or directional rate models, they suggest a profound reorganization of BG output under parkinsonian conditions. How this reorganization could contribute to other features of parkinsonian behavior can be examined in future studies.
Although we have demonstrated a link between the electrophysiological and behavioral effects of unilateral dopaminergic cell loss, it is worth considering potential caveats in interpreting our results. First, it is possible that compensatory mechanisms unrelated to the electrophysiological effects of unilateral dopaminergic cell loss are responsible for the behavioral bias we observed, particularly since the hemi-PD mice included in our study all exhibited a behavioral bias in the same direction (ipsilateral). Although our electrophysiological results are consistent with this bias, one way we could draw stronger conclusions about the relationship between BG output and behavior is with a set of mice exhibiting a wider range of behavioral biases. Indeed, one mouse that was excluded from our hemi-PD group due to insufficient dopaminergic cell loss (<70%) exhibited a significant contralateral bias on both SG (P = 3.10 × 10−3, two-tailed Wilcoxon signed-rank test, median bias = −0.223) and IS trials (P = 5.41 × 10−4, two-tailed Wilcoxon signed-rank test, median bias = −0.0798). When we analyzed the SNr recordings from this mouse, we found that the neurons with a significant direction preference (74/83 neurons) largely exhibited an ipsilateral preference during the movement preparation epoch (ipsilateral:contralateral ratio = 59:15, P = 7.74 × 10−13, χ2 test = 50.0, df = 1, see Fig. 8A, Table 1 for comparisons). Although we can only speculate about the potential compensatory adaptations that emerge in the BG with relatively moderate dopaminergic cell loss, and we cannot rule out contributions of additional mechanisms to the behavioral bias, the fact that the direction of behavioral bias remains consistent with neural direction preference further supports our finding that BG output mediates the effect of parkinsonian conditions on behavior. Another way to strengthen our conclusions about the causal relationship between parkinsonian BG output and behavior, in future studies, is with perturbation experiments (64, 73, 74). Since unilateral dopaminergic cell loss shifted the proportions of SNr neurons exhibiting increased versus decreased activity and ipsilateral versus contralateral direction preference during movement preparation (Figs. 7D and 8, Table 1), the clearest effects on behavior would result from perturbing specific functional classes of SNr neurons, which may become possible with activity-based and intersectional optogenetic strategies (75, 76). In general, though, we would expect that unilaterally inhibiting parkinsonian SNr activity during movement preparation would facilitate contralateral movements and therefore counteract the ipsilateral behavioral bias we observed (Figs. 4 and 5).
Second, olfactory deficits are a known hallmark of PD (77–80). Given our use of an olfactory task, is it possible that the behavioral effects under parkinsonian conditions are due to sensory and not motor deficits? We suggest that this is unlikely for two reasons: 1) on IS trials, the olfactory cue was not informative about reward location and 2) on SG trials, a deficit in olfactory discrimination would be reflected in a flattening, rather than a shift, in the psychometric function, which we did not observe.
Finally, while the hemi-PD model provides a powerful approach for examining the neural basis of parkinsonian movement (8, 38, 43, 56, 57, 81), PD typically presents with bilateral dopaminergic cell loss. It is possible that dopaminergic input from the spared SNc may compensate for the unilateral insult, and other differences between the model and the clinical condition must be considered. However, our comparison between ipsilateral and contralateral movements, at the behavioral and neurophysiological levels, was well suited to the hemi-PD model, and we suggest that our results can cautiously inform the reorganization of BG output that occurs in PD.
In conclusion, we found that the behavioral effects of unilateral dopaminergic cell loss, including differences between stimulus-guided and internally specified movements, were consistent with changes in SNr activity during movement preparation. Although our results could not be explained by the classical rate model prediction that BG output is tonically elevated by dopaminergic cell loss, they were nevertheless consistent with a directional rate model in which movement direction is influenced by the rate of BG output during movement preparation. Future studies can examine how the changes in SNr activity observed here affect the activity of SNr-recipient structures, as well as how the BG interact with other motor systems to differentially mediate stimulus-guided and internally specified movements under parkinsonian conditions.
GRANTS
This work was supported by the National Institutes of Health (R01NS079518, P30NS048154) and the Boettcher Foundation (Webb-Waring Biomedical Research Award).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.F. conceived and designed research; A.T. and M.J.L. performed experiments; A.T. analyzed data; A.T., M.J.L., J.A.T., and G.F. interpreted results of experiments; A.T. prepared figures; A.T., J.A.T., and G.F. drafted manuscript; A.T., J.A.T., and G.F. edited and revised manuscript; A.T., M.J.L., J.A.T., and G.F. approved final version of manuscript.
ACKNOWLEDGMENTS
Technical support was provided by the Optogenetics and Neural Engineering Core at the University of Colorado School of Medicine, funded in part by the National Institutes of Health (P30NS048154). We thank Quang Dang and Ben Peterson for data collection and members of the Felsen lab for comments on the manuscript.
REFERENCES
- 1.Filyushkina V, Popov V, Medvednik R, Ushakov V, Batalov A, Tomskiy A, Pronin I, Sedov A. Hyperactivity of basal ganglia in patients with Parkinson's disease during internally guided voluntary movements. Front Neurol 10: 847, 2019. doi: 10.3389/fneur.2019.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13: 281–285, 1990. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Zhang QJ, Liu J, Ali U, Gui ZH, Hui YP, Chen L, Wang T. Changes in firing rate and pattern of GABAergic neurons in subregions of the substantia nigra pars reticulata in rat models of Parkinson's disease. Brain Res 1324: 54–63, 2010. doi: 10.1016/j.brainres.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Ibanez-Sandoval O, Carrillo-Reid L, Galarraga E, Tapia D, Mendoza E, Gomora JC, Aceves J, Bargas J. Bursting in substantia nigra pars reticulata neurons in vitro: possible relevance for Parkinson disease. J Neurophysiol 98: 2311–2323, 2007. doi: 10.1152/jn.00620.2007. [DOI] [PubMed] [Google Scholar]
- 5.Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res 125: 397–409, 1999. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]
- 6.McGregor MM, Nelson AB. Circuit mechanisms of Parkinson's disease. Neuron 101: 1042–1056, 2019. doi: 10.1016/j.neuron.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Utter AA, Basso MA. The basal ganglia: an overview of circuits and function. Neurosci Biobehav Rev 32: 333–342, 2008. doi: 10.1016/j.neubiorev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Brazhnik E, Novikov N, McCoy AJ, Cruz AV, Walters JR. Functional correlates of exaggerated oscillatory activity in basal ganglia output in hemiparkinsonian rats. Exp Neurol 261: 563–577, 2014. doi: 10.1016/j.expneurol.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeger-Armbruster S, von Ameln-Mayerhofer A. Short- and long-term unilateral 6-hydroxydopamine lesions in rats show different changes in characteristics of spontaneous firing of substantia nigra pars reticulata neurons. Exp Brain Res 224: 15–24, 2013. doi: 10.1007/s00221-012-3285-3. [DOI] [PubMed] [Google Scholar]
- 10.Vitek JL, Johnson LA. Understanding Parkinson's disease and deep brain stimulation: role of monkey models. Proc Natl Acad Sci USA 116: 26259–26265, 2019. doi: 10.1073/pnas.1902300116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obeso JA, Rodriguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M. Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease. Mov Disord 23, Suppl 3: S548–S559, 2008. doi: 10.1002/mds.22062. [DOI] [PubMed] [Google Scholar]
- 12.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 13.Ballanger B, Thobois S, Baraduc P, Turner RS, Broussolle E, Desmurget M. “Paradoxical kinesis” is not a hallmark of Parkinson's disease but a general property of the motor system. Mov Disord 21: 1490–1495, 2006. doi: 10.1002/mds.20987. [DOI] [PubMed] [Google Scholar]
- 14.Daroff RB. Paradoxical kinesia. Mov Disord 23: 1193, 2008. doi: 10.1002/mds.22060. [DOI] [PubMed] [Google Scholar]
- 15.Distler M, Schlachetzki JC, Kohl Z, Winkler J, Schenk T. Paradoxical kinesia in Parkinson's disease revisited: anticipation of temporal constraints is critical. Neuropsychologia 86: 38–44, 2016. doi: 10.1016/j.neuropsychologia.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Glickstein M, Stein J. Paradoxical movement in Parkinson's disease. Trends Neurosci 14: 480–482, 1991. doi: 10.1016/0166-2236(91)90055-y. [DOI] [PubMed] [Google Scholar]
- 17.McDonald LM, Griffin HJ, Angeli A, Torkamani M, Georgiev D, Jahanshahi M. Motivational modulation of self-initiated and externally triggered movement speed induced by threat of shock: experimental evidence for paradoxical kinesis in Parkinson's disease. PLoS One 10: e0135149, 2015. doi: 10.1371/journal.pone.0135149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McIntosh GC, Brown SH, Rice RR, Thaut MH. Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 62: 22–26, 1997. doi: 10.1136/jnnp.62.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, de Hemptinne C, Miller AM, Leibbrand M, Little SJ, Lim DA, Larson PS, Starr PA. Prefrontal-subthalamic hyperdirect pathway modulates movement inhibition in humans. Neuron 106: 579–588.e3, 2020. doi: 10.1016/j.neuron.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hackney ME, Lee HL, Battisto J, Crosson B, McGregor KM. Context-dependent neural activation: internally and externally guided rhythmic lower limb movement in individuals with and without neurodegenerative disease. Front Neurol 6: 251, 2015. doi: 10.3389/fneur.2015.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drucker JH, Sathian K, Crosson B, Krishnamurthy V, McGregor KM, Bozzorg A, Gopinath K, Krishnamurthy LC, Wolf SL, Hart AR, Evatt M, Corcos DM, Hackney ME. Internally guided lower limb movement recruits compensatory cerebellar activity in people with Parkinson's disease. Front Neurol 10: 537, 2019. doi: 10.3389/fneur.2019.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis MM, Slagle CG, Smith AB, Truong Y, Bai P, McKeown MJ, Mailman RB, Belger A, Huang X. Task specific influences of Parkinson's disease on the striato-thalamo-cortical and cerebello-thalamo-cortical motor circuitries. Neuroscience 147: 224–235, 2007. doi: 10.1016/j.neuroscience.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci 33: 269–298, 2010. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- 24.Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson's disease. Brain 124: 2131–2146, 2001. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- 25.Hess CW, Hallett M. The phenomenology of Parkinson's disease. Semin Neurol 37: 109–117, 2017. doi: 10.1055/s-0037-1601869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jahanshahi M, Brown RG, Marsden CD. Simple and choice reaction time and the use of advance information for motor preparation in Parkinson’s disease. Brain 115: 539–564, 1992. doi: 10.1093/brain/115.2.539. [DOI] [PubMed] [Google Scholar]
- 27.Dick JP, Cowan JM, Day BL, Berardelli A, Kachi T, Rothwell JC, Marsden CD. The corticomotoneurone connection is normal in Parkinson's disease. Nature 310: 407–409, 1984. doi: 10.1038/310407a0. [DOI] [PubMed] [Google Scholar]
- 28.Wu T, Hallett M, Chan P. Motor automaticity in Parkinson's disease. Neurobiol Dis 82: 226–234, 2015. doi: 10.1016/j.nbd.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moroney R, Heida C, Geelen J. Increased bradykinesia in Parkinson's disease with increased movement complexity: elbow flexion-extension movements. J Comput Neurosci 25: 501–519, 2008. doi: 10.1007/s10827-008-0091-9. [DOI] [PubMed] [Google Scholar]
- 30.Cutsuridis V, Perantonis S. A neural network model of Parkinson's disease bradykinesia. Neural Netw 19: 354–374, 2006. doi: 10.1016/j.neunet.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Suri RE, Albani C, Glattfelder AH. Analysis of double-joint movements in controls and in parkinsonian patients. Exp Brain Res 118: 243–250, 1998. doi: 10.1007/s002210050278. [DOI] [PubMed] [Google Scholar]
- 32.Handel A, Glimcher PW. Quantitative analysis of substantia nigra pars reticulata activity during a visually guided saccade task. J Neurophysiol 82: 3458–3475, 1999. doi: 10.1152/jn.1999.82.6.3458. [DOI] [PubMed] [Google Scholar]
- 33.Sato M, Hikosaka O. Role of primate substantia nigra pars reticulata in reward-oriented saccadic eye movement. J Neurosci 22: 2363–2373, 2002. doi: 10.1523/JNEUROSCI.22-06-02363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lintz MJ, Felsen G. Basal ganglia output reflects internally-specified movements. eLife 5: e13833, 2016. doi: 10.7554/eLife.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutchison WD, Lozano AM, Davis KD, Saint-Cyr JA, Lang AE, Dostrovsky JO. Differential neuronal activity in segments of globus pallidus in Parkinson's disease patients. Neuroreport 5: 1533–1537, 1994. doi: 10.1097/00001756-199407000-00031. [DOI] [PubMed] [Google Scholar]
- 36.Lobb CJ, Jaeger D. Bursting activity of substantia nigra pars reticulata neurons in mouse parkinsonism in awake and anesthetized states. Neurobiol Dis 75: 177–185, 2015. doi: 10.1016/j.nbd.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aristieta A, Ruiz-Ortega JA, Miguelez C, Morera-Herreras T, Ugedo L. Chronic L-DOPA administration increases the firing rate but does not reverse enhanced slow frequency oscillatory activity and synchronization in substantia nigra pars reticulata neurons from 6-hydroxydopamine-lesioned rats. Neurobiol Dis 89: 88–100, 2016. doi: 10.1016/j.nbd.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Galati S, D'Angelo V, Olivola E, Marzetti F, Di Giovanni G, Stanzione P, Stefani A. Acute inactivation of the medial forebrain bundle imposes oscillations in the SNr: a challenge for the 6-OHDA model? Exp Neurol 225: 294–301, 2010. doi: 10.1016/j.expneurol.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Willard AM, Isett BR, Whalen TC, Mastro KJ, Ki CS, Mao X, Gittis AH. State transitions in the substantia nigra reticulata predict the onset of motor deficits in models of progressive dopamine depletion in mice. eLife 8: e42746, 2019. doi: 10.7554/eLife.42746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson JA, Felsen G. Activity in mouse pedunculopontine tegmental nucleus reflects action and outcome in a decision-making task. J Neurophysiol 110: 2817–2829, 2013. doi: 10.1152/jn.00464.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci 6: 1224–1229, 2003. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- 42.Stubblefield EA, Costabile JD, Felsen G. Optogenetic investigation of the role of the superior colliculus in orienting movements. Behav Brain Res 255: 55–63, 2013. doi: 10.1016/j.bbr.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ungerstedt U. 6-Hydroxydopamine-induced degeneration of the nigrostriatal dopamine pathway: the turning syndrome. Pharmacol Ther B 2: 37–40, 1976. doi: 10.1016/0306-039x(76)90016-7. [DOI] [PubMed] [Google Scholar]
- 44.Smith GA, Heuer A. 6-OHDA toxin model in mouse. In: Animal Models of Movement Disorders, edited by Lane EL, Dunnett SB.. New York: Humana, 2011, p. 281–297. Neuromethods vol. 61. doi: 10.1007/978-1-61779-298-4_14. [DOI] [Google Scholar]
- 45.Franklin KBJ, Paxinos G. Paxinos and Franklin's The Mouse Brain in Stereotaxic Coordinates (2nd ed.). San Diego, CA: Elsevier Academic Press, 2004. [Google Scholar]
- 47.Schmitzer-Torbert N, Jackson J, Henze D, Harris K, Redish AD. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131: 1–11, 2005. doi: 10.1016/j.neuroscience.2004.09.066. [DOI] [PubMed] [Google Scholar]
- 49.Hornykiewicz O. Biochemical aspects of Parkinson's disease. Neurology 51: S2–S9, 1998. doi: 10.1212/wnl.51.2_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 50.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20: 415–455, 1973. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 51.Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York, NY: John Wiley & Sons, 1966. [Google Scholar]
- 52.Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF. Representation of spatial goals in rat orbitofrontal cortex. Neuron 51: 495–507, 2006. doi: 10.1016/j.neuron.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 53.Rousselet GA, Pernet CR, Wilcox RR. Beyond differences in means: robust graphical methods to compare two groups in neuroscience. Eur J Neurosci 46: 1738–1748, 2017. doi: 10.1111/ejn.13610. [DOI] [PubMed] [Google Scholar]
- 54.Harrell FE, Davis CE. A new distribution-free quantile estimator. Biometrika 69: 635–640, 1982. doi: 10.1093/biomet/69.3.635. [DOI] [Google Scholar]
- 55.Makin TR, Orban de Xivry JJ. Ten common statistical mistakes to watch out for when writing or reviewing a manuscript. eLife 8: e48175, 2019. doi: 10.7554/eLife.48175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Avila I, Parr-Brownlie LC, Brazhnik E, Castaneda E, Bergstrom DA, Walters JR. Beta frequency synchronization in basal ganglia output during rest and walk in a hemiparkinsonian rat. Exp Neurol 221: 307–319, 2010. doi: 10.1016/j.expneurol.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brazhnik E, Cruz AV, Avila I, Wahba MI, Novikov N, Ilieva NM, McCoy AJ, Gerber C, Walters JR. State-dependent spike and local field synchronization between motor cortex and substantia nigra in hemiparkinsonian rats. J Neurosci 32: 7869–7880, 2012. doi: 10.1523/JNEUROSCI.0943-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang JY, Shi LH, Luo F, Woodward DJ. Neural responses in multiple basal ganglia regions following unilateral dopamine depletion in behaving rats performing a treadmill locomotion task. Exp Brain Res 172: 193–207, 2006. doi: 10.1007/s00221-005-0312-7. [DOI] [PubMed] [Google Scholar]
- 59.Israel Z, Bergman H. Pathophysiology of the basal ganglia and movement disorders: from animal models to human clinical applications. Neurosci Biobehav Rev 32: 367–377, 2008. doi: 10.1016/j.neubiorev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 60.Sanderson P, Mavoungou R, Albe-Fessard D. Changes in substantia nigra pars reticulata activity following lesions of the substantia nigra pars compacta. Neurosci Lett 67: 25–30, 1986. doi: 10.1016/0304-3940(86)90202-8. [DOI] [PubMed] [Google Scholar]
- 61.Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of basal ganglia output by direct and indirect pathway projection neurons. J Neurosci 33: 18531–18539, 2013. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Zhang QJ, Liu J, Ali U, Gui ZH, Hui YP, Chen L, Wu ZH, Li Q. Noradrenergic lesion of the locus coeruleus increases apomorphine-induced circling behavior and the firing activity of substantia nigra pars reticulata neurons in a rat model of Parkinson's disease. Brain Res 1310: 189–199, 2010. doi: 10.1016/j.brainres.2009.10.070. [DOI] [PubMed] [Google Scholar]
- 63.Abedi PM, Delaville C, De Deurwaerdere P, Benjelloun W, Benazzouz A. Intrapallidal administration of 6-hydroxydopamine mimics in large part the electrophysiological and behavioral consequences of major dopamine depletion in the rat. Neuroscience 236: 289–297, 2013. doi: 10.1016/j.neuroscience.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 64.Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626, 2010. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beiser DG, Houk JC. Model of cortical-basal ganglionic processing: encoding the serial order of sensory events. J Neurophysiol 79: 3168–3188, 1998. doi: 10.1152/jn.1998.79.6.3168. [DOI] [PubMed] [Google Scholar]
- 66.Beninato M, Spencer RF. A cholinergic projection to the rat substantia nigra from the pedunculopontine tegmental nucleus. Brain Res 412: 169–174, 1987. doi: 10.1016/0006-8993(87)91455-7. [DOI] [PubMed] [Google Scholar]
- 67.Scarnati E, Campana E, Pacitti C. Pedunculopontine-evoked excitation of substantia nigra neurons in the rat. Brain Res 304: 351–361, 1984. doi: 10.1016/0006-8993(84)90339-1. [DOI] [PubMed] [Google Scholar]
- 68.Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80: 953–978, 2000. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- 69.Kawagoe R, Takikawa Y, Hikosaka O. Reward-predicting activity of dopamine and caudate neurons—a possible mechanism of motivational control of saccadic eye movement. J Neurophysiol 91: 1013–1024, 2004. doi: 10.1152/jn.00721.2003. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe K, Lauwereyns J, Hikosaka O. Neural correlates of rewarded and unrewarded eye movements in the primate caudate nucleus. J Neurosci 23: 10052–10057, 2003. doi: 10.1523/JNEUROSCI.23-31-10052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol 95: 567–584, 2006. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- 72.Perugini A, Ditterich J, Basso MA. Patients with Parkinson's disease show impaired use of priors in conditions of sensory uncertainty. Curr Biol 26: 1902–1910, 2016. doi: 10.1016/j.cub.2016.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morrissette AE, Chen PH, Bhamani C, Borden PY, Waiblinger C, Stanley GB, Jaeger D. Unilateral optogenetic inhibition and excitation of basal ganglia output affect directional lick choices and movement initiation in mice. Neuroscience 423: 55–65, 2019. doi: 10.1016/j.neuroscience.2019.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sakamoto M, Hikosaka O. Eye movements induced by microinjection of GABA agonist in the rat substantia nigra pars reticulata. Neurosci Res 6: 216–233, 1989. doi: 10.1016/0168-0102(89)90061-8. [DOI] [PubMed] [Google Scholar]
- 75.DeNardo L, Luo L. Genetic strategies to access activated neurons. Curr Opin Neurobiol 45: 121–129, 2017. doi: 10.1016/j.conb.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fenno LE, Ramakrishnan C, Kim YS, Evans KE, Lo M, Vesuna S, Inoue M, Cheung KYM, Yuen E, Pichamoorthy N, Hong ASO, Deisseroth K. Comprehensive dual- and triple-feature intersectional single-vector delivery of diverse functional payloads to cells of behaving mammals. Neuron 107: 836–853.e11, 2020. doi: 10.1016/j.neuron.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tekriwal A, Kern DS, Tsai J, Ince NF, Wu J, Thompson JA, Abosch A. REM sleep behaviour disorder: prodromal and mechanistic insights for Parkinson's disease. J Neurol Neurosurg Psychiatry 88: 445–451, 2017. doi: 10.1136/jnnp-2016-314471. [DOI] [PubMed] [Google Scholar]
- 78.Tarakad A, Jankovic J. Anosmia and ageusia in Parkinson's disease. Int Rev Neurobiol 133: 541–556, 2017. doi: 10.1016/bs.irn.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 79.Hawkes CM. Diagnosis and treatment of Parkinson's disease. Anosmia is a common finding. BMJ 310: 1668, 1995. doi: 10.1136/bmj.310.6995.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hawkes CH, Shephard BC. Selective anosmia in Parkinson's disease? Lancet 341: 435–436, 1993. [PubMed] [Google Scholar]
- 81.Dorval AD, Grill WM. Deep brain stimulation of the subthalamic nucleus reestablishes neuronal information transmission in the 6-OHDA rat model of parkinsonism. J Neurophysiol 111: 1949–1959, 2014. doi: 10.1152/jn.00713.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]