Keywords: calcium imaging, cortical pyramidal neurons, dantrolene, Huntington’s disease, R6/2 mice, two-photon laser scanning microscopy
Abstract
Huntington’s disease (HD) is a fatal, hereditary neurodegenerative disorder that predominantly affects striatal medium-sized spiny neurons and cortical pyramidal neurons (CPNs). It has been proposed that perturbations in Ca2+ homeostasis could play a role in CPN alterations. To test this hypothesis, we used the R6/2 mouse model of juvenile HD at different stages of disease progression; presymptomatic, early symptomatic, and late symptomatic. We combined whole-cell patch-clamp recordings of layer 2/3 CPNs with two-photon laser scanning microscopy to image somatic and dendritic Ca2+ transients associated with evoked action potentials (APs). We found that the amplitude of AP-induced Ca2+ transients recorded at the somata of CPNs was significantly reduced in presymptomatic and late symptomatic R6/2 mice compared with wild-type (WT) littermates. However, reduced amplitudes were compensated by increases in decay times, so that Ca2+ transient areas were similar between genotypes. AP-induced Ca2+ transients in CPN proximal dendrites were variable and differences did not reach statistical significance, except for reduced areas in the late symptomatic group. In late symptomatic mice, a specific store-operated Ca2+ channel antagonist, EVP4593, reduced somatic Ca2+ transient amplitude similarly in WT and R6/2 CPNs. In contrast, dantrolene, a ryanodine receptor (RyR) antagonist, and nifedipine, an L-type Ca2+ channel blocker, significantly reduced both somatic Ca2+ transient amplitude and area in R6/2 but not WT CPNs. These findings demonstrate that perturbations of Ca2+ homeostasis and compensation occur in CPNs before and after the onset of overt symptoms, and suggest RyRs and L-type Ca2+ channels as potential targets for therapeutic intervention.
NEW & NOTEWORTHY We used two-photon microscopy to examine calcium influx induced by action potentials in cortical pyramidal neurons from a mouse model of Huntington’s disease (HD), the R6/2. The amplitude of somatic calcium transients was reduced in R6/2 mice compared with controls. This reduction was compensated by increased decay times, which could lead to reduced calcium buffering capacity. L-type calcium channel and ryanodine receptor blockers reduced calcium transient area in HD neurons, suggesting new therapeutic avenues.
INTRODUCTION
Huntington’s disease (HD) is a progressive, debilitating, and fatal neurodegenerative disorder that manifests with motor (e.g., chorea), psychiatric, and cognitive disturbances (1, 2). The etiology of the disease lies in the abnormal expansion of CAG trinucleotide repeats in the first exon of the Huntingtin (HTT) gene (3). Postmortem brain assessments from patients with HD demonstrate that major neuronal atrophy and death occur in striatum and cerebral cortex, predominantly affecting medium-sized spiny neurons (MSNs) and cortical pyramidal neurons (CPNs). However, it has been hypothesized that abnormal cortical signaling precedes striatal dysfunction (4, 5), as functional studies revealed that somatosensory, attention, and cognitive deficits precede chorea and other motor abnormalities (6, 7). Thus, disruptions in excitatory cortical inputs to the striatum set the stage for the emergence of the cardinal HD behavioral phenotype. Furthermore, evidence from postmortem HD tissue reveals that cortical degeneration and subsequent loss of CPNs are the earliest neuropathological features of HD (8–10). In addition, a significant correlation between motor dysfunction and loss of CPNs in the primary motor cortex, as well as mood-associated clinical symptoms and CPN loss in the cingulate cortex have been documented in HD patients (11). As such, examining early cortical alterations is of vital importance in understanding HD etiology and manifestations.
A growing body of evidence indicates that imbalance of intracellular neuronal Ca2+ signaling plays a pivotal role in HD progression (12–14). Ca2+ dyshomeostasis leads to degeneration of MSNs in the YAC128 mouse model of HD, as store-operated Ca2+ channel (SOC) activity is enhanced in neurons expressing mutant huntingtin (mHTT) and drugs that target the SOC pathway rescue spine loss (15). In addition, altered Ca2+ signaling induced by human mHTT fragments has been reported in cultured striatal neurons and striatal mitochondria from transgenic R6/2 and YAC128 mice, respectively (16, 17). Furthermore, decreased density and survival of dendritic spines have been shown in neuronal cultures from R6/2 mice (18). Notably, genetically reducing mHTT expression in cortical neuronal populations from BACHD mice partially alleviates motor and psychiatric-like behavioral deficits (19, 20).
Previous studies in our laboratory demonstrated early alterations in glutamate receptor-mediated currents, as well as augmented voltage-gated Ba2+ currents in CPNs from late symptomatic R6/2 mice (21). Thus, it is likely that neuronal Ca2+ signaling in CPNs of the motor cortex is fundamentally disturbed and its alteration contributes to neurological and behavioral abnormalities observed in HD. In the present study, we used the R6/2 transgenic mouse model of juvenile HD (22) to examine electrophysiological properties and Ca2+ signaling in CPNs from the primary motor cortex in R6/2 mice at different time points of the disease. These mice are presymptomatic at 3–4 wk; symptoms start at ∼5 wk, and are manifested by reduced mobility and tremor. In the late, fully symptomatic stage (>9 wk), mice are hypokinetic, tremors become more pronounced, they lose weight and usually die during an epileptic seizure (23, 24). Our findings demonstrate that perturbations of Ca2+ homeostasis occur very early in the disease and can be modulated in symptomatic animals by agents that reduce Ca2+ influx and intracellular Ca2+ release.
MATERIALS AND METHODS
Mice
Experiments were performed on R6/2 transgenic mice and their wild-type (WT) littermates as controls. Mice were obtained from our breeding colonies at UCLA and every effort was made to minimize pain, discomfort, and the number of mice used. All experimental procedures were performed in accordance with the United States Public Health Service Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at UCLA. Three age groups of R6/2 mice and WT littermates of either sex were studied: 3–4.5 wk (presymptomatic; 7 WT, P24 ± 0.7 days and 7 R6/2, P24 ± 0.7 days), 5–6 wk (early symptomatic; 7 WT, P39 ± 0.3 days and 6 R6/2, P38 ± 0.6 days), and 9–13 wk (late symptomatic; 21 WT, P78 ± 1.5 days and 22 R6/2, P78 ± 1.2 days). CAG repeat length analysis for all mice used was performed by Laragen Inc (Culver City, CA). The average length of CAG repeats was 158 ± 0.92 and did not differ significantly among the three age groups.
Brain Slice Electrophysiology
Mice were deeply anesthetized with isoflurane and perfused transcardially with ice-cold, high-sucrose slicing solution containing (in mM): 26 NaHCO3, 1.25 NaH2PO4, 208 sucrose, 10 glucose, 2.5 KCl, 1.3 MgCl2, and 8 MgSO4. Mice were swiftly decapitated, brains extracted, and immediately placed in oxygenated high-sucrose slicing solution. Coronal slices from primary motor cortex (M1) were cut at 300 μm and transferred to an incubating chamber containing artificial cerebrospinal fluid (ACSF) (in mM): 130 NaCl, 5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2 MgCl2, 2 CaCl2, and 10 glucose oxygenated with 95% O2–5% CO2 (pH 7.2–7.4, osmolarity 290–310 mosmol/L, 32°C–34°C). Slices were allowed to recover for an additional 30 min at room temperature. Electrophysiological recordings were performed at room temperature.
Whole-cell patch-clamp recordings in voltage- and current-clamp modes were obtained from visually identified layer 2/3 (L2/3) CPNs within the primary motor cortex (M1) using a MultiClamp 700B Amplifier (Molecular Devices) and the pCLAMP 10.4 acquisition software (Molecular Devices). Intracellular K-gluconate based solution contained (in mM): 112.5 K-gluconate, 4 NaCl, 17.5 KCl, 0.5 CaCl2, 1 MgCl2, 5 ATP (dipotassium salt), 1 NaGTP, and 10 HEPES (pH 7.2, 270–280 mosmol/L). For slice recordings, we used ACSF containing a slightly higher concentration of K+ (5 mM KCl) and a slightly lower concentration of Ca2+ (1 mM CaCl2) to increase excitability. Cells were voltage clamped at −70 mV to assess basic membrane properties. Membrane capacitance, input resistance and time constant were measured by applying a 10 mV depolarizing step voltage command and using the membrane test function integrated in the pClamp10 software. Action potential (AP) properties were measured using Clampfit and included AP amplitude, area, half-amplitude duration, and decay time. Electrode resistance was typically 4–5 MΩ in the bath. Cells with access resistance (Ra) exceeding 25 MΩ were excluded from analysis. Voltage responses were recorded at a sampling rate of 5 kHz.
To investigate activity-dependent Ca2+ influx, the Ca2+-sensitive dye, Oregon Green BAPTA-1 (OGB-1, 150 µM, Thermo Fisher Scientific, Waltham, MA), dissolved in the intracellular solution, was allowed to diffuse through the patch pipette for at least 20 min before imaging. Single or multiple APs were evoked by a series of 50 ms depolarizing current steps (50 pA increments, 5 s interstimulus interval) from the resting membrane potential (RMP), and the evoked somatic and dendritic Ca2+ transients were recorded.
Two-Photon Ca2+ Imaging
Ca2+ imaging in cortical slices was performed using a Scientifica two-photon laser-scanning microscope (2PLSM) equipped with MaiTai laser (Spectra-physics). To image Ca2+ signals, the laser was tuned to 920 nm wavelength. The laser power measured at the sample was less than 30 mW with a ×40 (0.8 NA) water-immersion objective lens (Olympus). Ca2+ transients were sampled at a rate of 3.81 Hz using SciScan software (Scientifica). Optical signal amplitudes were expressed as ΔF/F, where F represents the baseline light intensity at the beginning of the optical trace, and ΔF represents the change in fluorescence during the biological signal. These proportion values were then multiplied by 100 to obtain percentage values. ImageJ (NIH) was used to process raw fluorescence values, which were then exported to Excel (Microsoft) and Clampfit to calculate % ΔF/F. Regions of interest (ROI) were drawn at both soma and proximal segment of the apical dendrite (∼20 µm from the apex). For somatic measurements, in ImageJ, we made a box that covered the largest portion of the soma while minimizing the background and the average fluorescence value was calculated within the box for each video frame (Fig. 1). As the somatic areas of CPNs from WT and R6/2 mice vary in size and those from symptomatic R6/2 mice are generally smaller than those from WTs, we customized the box to fit the largest portion of the soma (range 25–30 pixels width × 35–40 pixels height). For measurements of apical dendrites, the same box size was used (15 × 15 pixels) regardless of age or genotype. All n’s provided for somatic data indicate number of measured somata and all n’s given for the dendritic data indicate the number of measured dendrites. Although we attempted to measure Ca2+ activity in the dendrites of all measured somata, this was not feasible as the apical dendrite was not always visible. This occurred because it had a peculiar angle outside the observable plane of section or because it was cut during slicing. To determine background fluorescence, we created a maximum z-stack image using ImageJ and placed a 20 × 20 pixel box in the darkest area of the video (total image frame was 256 × 256 pixels). The mode fluorescence within the box was used as the background signal. The background signal for each video was then subtracted from the soma or dendrite fluorescence. Given the nonphysiological fluctuations in neuron fluorescence throughout the recording session due to slight changes in focus on the z plane, we calculated the baseline fluorescence for each Ca2+ transient individually. The baseline for each transient was calculated as the mean fluorescence of the 4 frames before transient initiation. Percent ΔF/F was then calculated as (fluorescence at a given time point – baseline fluorescence)/(baseline fluorescence) × 100%. Amplitude, area, and decay time of Ca2+ transients were calculated using custom written programs in Python. Amplitude was calculated as the peak % ΔF/F. Area was calculated as the area under the curve of the Ca2+ transient up until the Ca2+ transient reached a % ΔF/F of 0. Finally, decay time was calculated as the time between the Ca2+ transient reaching 90% of the peak % ΔF/F, postamplitude peak, until the Ca2+ transient fell to 10% of the peak % ΔF/F. If the Ca2+ transient did not reach 10% of the amplitude within 8 s (the time in which the next AP was generated), the decay time was not calculated for the transient. All transients were manually inspected, and signals in which the signal variability was indistinguishable from the background variability (no transient >1.5 SD above noise level) were removed from analysis.
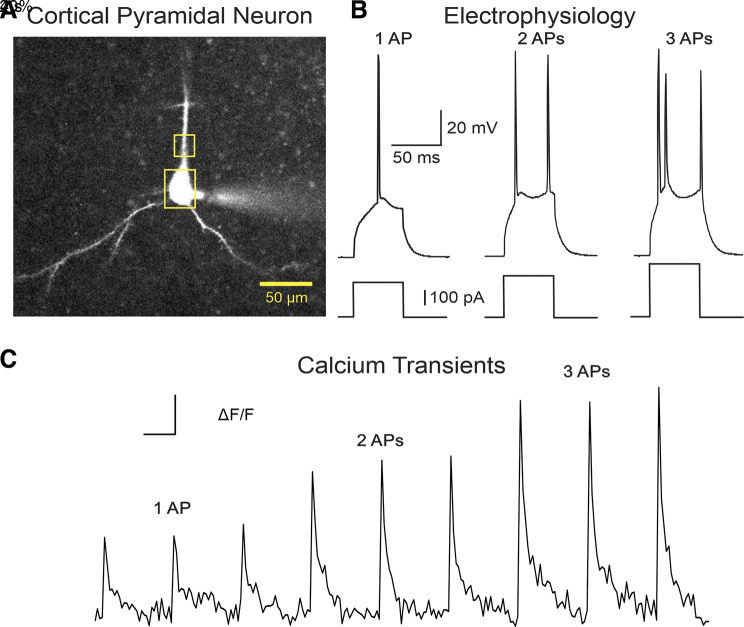
Figure 1.
A: L2/3 cortical pyramidal neuron (CPN) patched and filled with OGB-1. Yellow boxes depict the two regions of interest where Ca2+ transients were measured at the soma or proximal apical dendrite. B: single, double, and triple action potentials (APs) were evoked by a series of 50 ms depolarizing current steps (50 pA increments, 8-s interstimulus interval) from the resting membrane potential. C: representative traces of a train of single, double, and triple AP-induced Ca2+ transients. Ca2+ responses in CPNs were recorded and compared between R6/2 mice and their WT age-matched littermates. WT, wild type.
Drugs
Stock solutions of EVP4593 and nifedipine (Sigma-Aldrich) were dissolved in dimethylsulfoxide. Dantrolene (Sigma-Aldrich) was dissolved in water. Final concentrations in the bath: EVP4593 = 3 µM (25), nifedipine = 10 µM (26) and dantrolene = 10 µM (27).
Statistical Analyses
All statistical analyses were performed using SigmaPlot (v14). Differences between group means were assessed with appropriate Student’s t tests (unpaired) or Mann–Whitney Rank Sum tests and appropriately designed two-way ANOVAs with post hoc Holm-Sidak t tests. Values in the table are presented as means ± SE. In the figures, values are expressed as box and whisker plots. Differences were considered statistically significant if P < 0.05 and are indicated with asterisks in the figures (*P < 0.05, **P < 0.01, ***P < 0.001).
RESULTS
Altered Passive and Active Membrane Properties of CPNs in R6/2 Mice
We first assessed intrinsic membrane properties of L2/3 CPNs within the primary motor cortex from R6/2 mice at pre-, early- and late symptomatic stages compared with age-matched WT littermates. In voltage clamp mode, changes in passive membrane properties between genotypes were detected. CPN capacitance was significantly smaller in R6/2 mice relative to WT littermates for early symptomatic [main effect of genotype: F(1,129) = 22.0, P < 0.001, post hoc: t = 3.2, P = 0.002] and late symptomatic (post hoc: t = 3.3, P = 0.001) age groups, but not for presymptomatic mice. Input resistance was significantly higher in the late symptomatic age group only [main effect of genotype: F(1,121) = 19.7, P < 0.001, post hoc: t = 6.3, P < 0.001; Table 1]. In contrast, there was no significant interaction of genotype and age of the decay time constant. As a consequence of increased input resistance in early- and late-symptomatic mice, a significantly lower stimulus intensity was required to evoke an action potential (rheobase) in CPNs from R6/2 mice compared to WTs [main effect of genotype: F(1,116) = 17.6, P < 0.001, post hoc: t = 2.0, P = 0.05 for early symptomatic and t = 4.4, P < 0.001 for late symptomatic; Table 1].
Table 1.
CPN membrane properties
| Cm, pF | Rm, MΩ | τ, ms | RMP, mV | Rheo, pA | |
|---|---|---|---|---|---|
| WT, n = 20 | 219.6 ± 12.2 | 51.8 ± 10.0 | 3.1 ± 0.2 | −68.1 ± 1.0 | 774.6 ± 57.2 |
| R6/2 Pre, n = 11 | 187.2 ± 11.5 | 65.9 ± 9.8 | 2.7 ± 0.2 | −68.0 ± 1.1 | 640.0 ± 50.4 |
| WT, n = 11 | 256.7 ± 15.9 | 47.8 ± 13.4 | 3.7 ± 0.3 | −70.8 ± 1.5 | 789.0 ± 67.7 |
| R6/2 Early, n = 15 | 191.3 ± 13.0** | 77.2 ± 11.3 | 3.2 ± 0.2 | −67.8 ± 1.2 | 608.8 ± 61.8* |
| WT, n = 36 | 176.4 ± 8.3 | 76.3 ± 7.6 | 2.8 ± 0.1 | −68.7 ± 0.8 | 658.1 ± 38.4 |
| R6/2 Late, n = 37 | 138.1 ± 8.2** | 142.2 ± 7.1*** | 2.9 ± 0.1 | −67.3 ± 0.8 | 427.7 ± 35.2*** |
Cm, membrane capacitance; CPN, cortical pyramidal neuron; Early, early symptomatic; Late, late symptomatic; Pre, presymptomatic; rheo, rheobase; Rm, input resistance; RMP, resting membrane potential; WT, wild type; τ, decay time constant. *P < 0.05; **P < 0.01, ***P < 0.001, significant genotype effect compared to WT littermates (two-way ANOVA). Numbers in parentheses indicate number of CPNs recorded.
The main properties of APs evoked by depolarizing current pulses were measured in the 3 age groups of WT and R6/2 mice. We selected only the traces that induced a single AP. Interestingly, we found AP areas were reduced in R6/2 CPNs at all stages [main effect of genotype: F(1,414) = 28.2, P < 0.001; post hoc: t = 2.3, P = 0.02 for presymptomatic, t = 4.2, P < 0.001 for early symptomatic, and t = 2.8, P = 0.006 for late symptomatic; Table 2]. Also, there was a strong trend for reduced AP amplitudes but only in the late symptomatic group. Half-amplitude duration was significantly reduced in the early symptomatic group but this effect inverted in the late symptomatic group [main effect of genotype: F(2,415) = 6.1, P = 0.002, post hoc: t = 2.1, P = 0.04 for early symptomatic and t = 2.5, P = 0.01 for late symptomatic]. Decay time of APs was reduced in the presymptomatic and early symptomatic group [interaction of genotype and age: F(2,412) = 3.4, P = 0.03, post hoc: t = 2.2, P = 0.03 for presymptomatic and t = 3.1, P = 0.002 for early symptomatic]. In a previous study on striatal MSNs (28) we reported similar decreases in AP amplitude and increased half-amplitude duration in late symptomatic R6/2 mice. The widening of APs could be due to reduced voltage-gated K+ currents (29), as well as diminished Ca2+ buffering capacity.
Table 2.
CPN action potential properties
| Amp, mV | Area, mV/s | Half-Width, ms | Decay Time, ms | |
|---|---|---|---|---|
| WT, n = 49 | 89.4 ± 1.0 | 221.0 ± 6.4 | 1.96 ± 0.05 | 3.38 ± 0.17 |
| R6/2 Pre, n = 40 | 89.6 ± 1.0 | 201.6 ± 4.5* | 1.87 ± 0.04 | 2.99 ± 0.13* |
| WT, n = 33 | 89.4 ± 1.5 | 221.8 ± 7.2 | 2.06 ± 0.07 | 3.46 ± 0.14 |
| R6/2 Early, n = 47 | 86.9 ± 1.1 | 183.1 ± 5.2*** | 1.89 ± 0.03* | 2.88 ± 0.08** |
| WT, n = 102 | 88.8 ± 0.7 | 206.7 ± 4.1 | 1.90 ± 0.03 | 2.79 ± 0.08 |
| R6/2 Late, n = 153 | 86.5 ± 0.8 | 192.4 ± 3.4** | 2.01 ± 0.03** | 2.73 ± 0.06 |
Amp, amplitude; CPN, cortical pyramidal neuron; Early, early symptomatic; Late, late symptomatic; Pre, presymptomatic; WT, wild type. *P < 0.05, **P < 0.01, ***P < 0.001, significant genotype effect compared to WT littermates (two-way ANOVA). Numbers in parentheses indicate number of action potentials measured.
Reduced Amplitudes but Longer Decay Times of Evoked Ca2+ Transients in the Somata of CPNs from R6/2 Mice at All Ages Examined
To date, there have been no studies systematically assessing Ca2+ changes associated with intrinsically evoked APs in CPNs of R6/2 mice at ages representing different stages of disease. The amplitude, area, and decay time of Ca2+ transients evoked by 1, 2, or 3 APs were examined. The number of APs significantly predicted the amplitude [R2 = 0.32, F(1,364) = 173.0, P < 0.001], area [R2 = 0.37, F(1,349) = 203.1, P < 0.001], and decay time [R2 = 0.11, F(1,343) = 44.0, P < 0.001] of Ca2+ transients independent of age and genotype. Overall, generation of 2 or 3 action potentials resulted in differences in AP-associated Ca2+ transients between CPNs from either pre-, early- or late symptomatic R6/2 and WT mice whereas the generation of 1 AP was insufficient to produce significant genotype differences (Figs. 2, 3, and 4). This suggests that Ca2+ disequilibrium becomes accentuated as a function of Ca2+ influx load.
Figure 2.
Ca2+ transients in cortical pyramidal neuron (CPN) somata of presymptomatic R6/2 mice: A: Ca2+ transients evoked in CPNs by 1, 2, and 3 action potentials (APs) in WT (black; n = 39) and R6/2 presymptomatic (red; n = 37) mice. B: voltage traces show APs induced by increasing intensities of 50 ms depolarizing current injections in CPNs. C: box and whisker plots of amplitude, decay time, and area between WT and R6/2 mice. The horizontal line indicates the median, the box represents the 25–75 percentile, the whiskers represent the range, and the diamonds represent outliers (data points outside of the 25–75 percentile). *P < 0.05 and ***P < 0.001 as determined by a two-way ANOVA and post hoc Holm–Sidak tests. WT, wild type.
Figure 3.
Ca2+ transients in cortical pyramidal neuron (CPN somata of early symptomatic R6/2 mice: A: Ca2+ transients evoked in CPNs by 1, 2, and 3 action potentials (APs) in WT (black; n = 12) and R6/2 early symptomatic (red; n = 17) mice. B: voltage traces show APs induced by increasing intensities of 50 ms depolarizing current injections in CPNs. C: box and whisker plots of amplitude, decay time, and area between WT and R6/2 mice. The horizontal line indicates the median, the box represents the 25–75 percentile, the whiskers represent the range, and the diamonds represent outliers (data points outside of the 25–75 percentile). WT, wild type.
Figure 4.
Ca2+ transients in cortical pyramidal neuron (CPN) somata of late symptomatic R6/2 mice: A: Ca2+ transients evoked in CPNs by 1, 2, and 3 action potentials (APs) in WT (black; n = 33) and R6/2 late symptomatic (red; n = 32) mice. B: voltage traces show APs induced by increasing intensities of 50 ms depolarizing current injections in CPNs. C. Box and whisker plots of amplitude, decay time, and area between WT and R6/2 mice. The horizontal line indicates the median, the box represents the 25–75 percentile, the whiskers represent the range, and the diamonds represent outliers (data points outside of the 25-75 percentile). *P < 0.05, **P < 0.01, and ***P < 0.001 as determined by a two-way ANOVA and post hoc Holm–Sidak tests. WT, wild type.
In the presymptomatic group, differences in amplitude and kinetics of Ca2+ transients at the somata of CPNs were already evident (Fig. 2). Ca2+ transients had significantly lower amplitudes compared to those from age-matched WTs [R6/2 n = 37, WT n = 39, main effect of genotype on amplitude: F(1,90) = 20.2, P < 0.001]. Significant differences in the amplitude of Ca2+ transients associated with 2 (post hoc: t = 2.6, P = 0.01) and 3 APs (post hoc: t = 4.0, P < 0.001) were observed. Due to a significant increase in the main effect of genotype on decay time [main effect of genotype on decay time: F(1,90) = 4.4, P = 0.04] that was most pronounced at 3 APs (post hoc: t = 2.3, P = 0.02, no significant post hoc effects with 1 or 2 APs) in R6/2 compared with WTs, there was no significant difference in area under the curve while there was a trend towards decreased area in R6/2 compared to WTs [main effect of genotype on area: F(1,90) = 3.3, P = 0.07].
In the early symptomatic group, despite a main effect of genotype on decreased amplitude [R6/2 n = 17, WT n = 12, main effect of genotype on amplitude: F(1,75) = 4.0, P = 0.048], we did not find any significant differences with any individual AP generation (Fig. 3). Decay times of Ca2+ signals associated with 1, 2, or 3 APs were generally longer in CPNs from early symptomatic R6/2 mice relative to WTs but did not reach statistical significance [main effect of genotype on decay time: F(1,75)=3.7, P = 0.06]. Given the lack of significant effects on decay time or amplitude, there was no significant effect of genotype on area [main effect of genotype on area: F(1,75)=1.0, P = 0.3].
In contrast, in the late symptomatic group, differences in amplitude and decay time were more pronounced and some measures became statistically significant (Fig. 4). Although no differences were seen with 1 AP, significant decreases in amplitude with 2 and 3 APs in R6/2 compared with WT CPNs [R6/2 n = 32, WT n = 33, main effect of genotype on amplitude: F(1,183) = 19.4, P < 0.001; post hoc 2 APs: t = 2.9, P = 0.004; post hoc 3 APs: t = 3.5, P < 0.001] and increases in decay time were observed with 2 and 3 APs in R6/2 compared with WT CPNs [main effect of genotype on decay time: F(1,162) = 12.4, P < 0.001; post hoc 2 APs: t = 2.6, P = 0.01; post hoc 3 APs: t = 2.1, P = 0.04]. This suggests compensatory changes for reduced amplitudes in early and late-symptomatic groups, so that the areas remained relatively constant between genotypes.
No Significant Changes in Amplitude or Kinetics of Evoked Ca2+ Transients in the Proximal Dendrites of CPNs at Pre- and Early Symptomatic Stages of the Disease
No consistent genotype changes were observed in amplitude, area, or decay time of AP-associated Ca2+ transients in proximal dendritic compartments of CPNs at pre- or early symptomatic stages (R6/2 Presymptomatic n = 23, WT Presymptomatic n = 35, R6/2 Early Symptomatic n = 26, WT Early Symptomatic n = 18; Fig. 5). Decay time of dendritic Ca2+ signals from late symptomatic R6/2 CPNs was reduced compared to WTs, however this effect was only seen with 1 AP [R6/2 n = 57, WT n = 31, main effect of genotype on decay time: F(1,82) = 9.2, P = 0.003; post hoc 1 AP: t = 2.4, P = 0.02]. Interestingly, the area in the dendrites was significantly decreased in R6/2 mice compared to WTs and this was due primarily to a reduction in decay time [main effect of genotype on area: F(1,82) = 9.2, P = 0.003], however there were no significant differences at a specific number of APs as determined by post hoc tests. This is opposite to changes found in the somata, where decay times were consistently increased, which countered the decrease in amplitude resulting in no significant change in area.
Figure 5.
Ca2+ transients in cortical pyramidal neuron (CPN) proximal dendrites of presymptomatic, early symptomatic and late symptomatic R6/2 mice: No differences in amplitude, decay time, and area of Ca2+ transients were observed in the proximal apical dendrites of CPNs between genotypes at presymptomatic (R6/2: n = 23, WT: n = 35; A) or early symptomatic (R6/2: n = 26, WT: n = 18; B) stages. C: in the late symptomatic stage (C; R6/2: n = 57, WT: n = 31) the changes in amplitude and decay time of Ca2+ transients evoked by 2 or 3 action potentials (APs) were opposite to those occurring in the somata of CPNs. With 3 APs, the area of the Ca2+ transients was significantly reduced. The horizontal line indicates the median, the box represents the 25–75 percentile, the whiskers represent the range, and the diamonds represent outliers (data points outside of the 25–75 percentile). * indicates P < 0.05, as determined by a two-way ANOVA and post hoc Holm–Sidak tests. WT, wild type.
Role of Ca2+ Entry through SOC Channels in Ca2+ Mishandling in Symptomatic R6/2 Mice
In this and the following experiments, we tested the effects of various pharmacological treatments on Ca2+ transients evoked by 2 APs only. Neuronal store-operated channels (SOCs) are membrane-bound Ca2+-conducting channels that activate in response to depletion of Ca2+ stores (30). EVP4593, a specific SOC antagonist, ameliorates the motor phenotype of flies in a Drosophila HD model and rescues YAC128 mice from glutamate-induced apoptosis both in vitro and in vivo (15). Therefore, in the following set of experiments, we tested the effects of blocking SOC channels on somatic AP-driven Ca2+ transients in symptomatic R6/2 mice and their WT littermates. We used a drug concentration of 3 µM, as this concentration has been shown to significantly reduce the peak amplitude of SOC entry in RBL-2H3 cells (25). In CPNs from both WT and R6/2 mice, EVP4593 produced a reduction (∼20%–30%) in Ca2+ transient amplitude. In general, the amplitudes were significantly decreased in R6/2 compared to WT both before and after the application of EVP7593 [R6/2 n = 13, WT n = 12, main effect of genotype: F(1, 35) = 7.9, P = 0.008; post hoc WT compared to R6/2 Pre-EVP: t = 2.3, P = 0.03; post hoc WT compared with R6/2 Post-EVP: t = 2.3, P = 0.03] but there were no significant genotype-dependent differences in the effects of EVP4593 on Ca2+ transient amplitude as there were no significant differences in percent change in amplitude in WT compared to R6/2 (Fig. 6). EVP7593 increased decay times of Ca2+ transients in both WT and R6/2 CPNs [main effect of drug: F(1,33) = 50.2, P < 0.001; post hoc WT: t = 7.1, P < 0.001; post hoc R6/2: t = 3.0, P = 0.006]. Area of Ca2+ transients was not significantly changed before EVP4593 application but the area was decreased in R6/2 mice compared with WT mice after application of EVP4593 [main effect of genotype: F(1,35) = 7.9, P = 0.002; post hoc WT compared to R6/2 Post-EVP: t = 2.4, P = 0.02], however EVP4593 itself had no genotype-dependent effect on the percent change in area. As decay times are significantly increased in symptomatic R6/2 mice, this treatment may not be suitable for reducing intracellular Ca2+ load in HD models.
Figure 6.
Effects of EVP4593 on somatic Ca2+ transients: A: somatic Ca2+ transients evoked by 2 action potentials (APs) in WT (n = 12) and R6/2 (n = 13) cortical pyramidal neurons (CPNs) before and after application of the store-operated Ca2+ channel (SOC) blocker EVP4593. In both genotypes the amplitude of the response was reduced. Box-whisker plots show raw and percent changes in amplitude (B), decay time (C), and area (D) of Ca2+ transients evoked by 2 APs before and after bath application of EVP4593. This SOC blocker reduced the amplitude similarly in CPNs from both genotypes. In contrast, decay times were increased significantly but again no difference between genotypes was observed. Area of Ca2+ transients did not change significantly. The horizontal line indicates the median, the box represents the 25–75 percentile, the whiskers represent the range, and the diamonds represent outliers (data points outside of the 25–75 percentile). *P < 0.05, **P < 0.01, and ***P < 0.001 as determined by a two-way ANOVA and post hoc Holm–Sidak tests. WT, wild type.
Ryanodine Receptor Block Reduces Intracellular Ca2+ Load in CPNs from Symptomatic R6/2 Mice
Membrane depolarization-induced changes in intracellular Ca2+ stimulate RyRs through Ca2+-induced Ca2+ release, which subsequently amplifies the Ca2+ signal. It has been reported that signaling through RyRs is significantly altered by the presence of mHTT, as demonstrated by elevated intracellular Ca2+ levels in YAC128 MSNs (27). To investigate the role of RyRs on AP-driven Ca2+ responses in symptomatic R6/2 mice we used dantrolene, a selective RyR antagonist that inhibits the release of intracellular Ca2+. We used a concentration of 20 μM, as dantrolene concentrations between 10–50 μM have been reported to protect YAC128 MSNs from glutamate-induced toxicity (27). We found that the amplitude of AP-associated Ca2+ transients at the somata of CPNs from late symptomatic R6/2 mice was significantly attenuated relative to WTs with or without the presence of dantrolene [R6/2 n = 9, WT n = 11; main effect of genotype: F(1,32)=15.6, P < 0.001; post hoc WT compared with R6/2 pre-dantrolene: t = 2.4, P = 0.02; post hoc WT compared with R6/2 post-dantrolene: t = 3.2, P = 0.003; Fig. 7]. However, when the percent decrease in amplitude of Ca2+ transients was measured, there was a significantly greater percent decrease in symptomatic R6/2 mice compared with WTs following addition of dantrolene (t = 2.9, P = 0.01). Pre-dantrolene, there was a significant increase in decay time in R6/2 mice compared to WT [F(1,23)=5.6, P = 0.03; post hoc WT compared with R6/2 pre-dantrolene: t = 2.6, P = 0.02] and this effect was abolished with dantrolene as there was no significant difference in decay time post-dantrolene in R6/2 mice compared to WT. Dantrolene produced negligible effects on decay time in WT CPNs (6% reduction), whereas in R6/2 CPNs, the effect was greater (26% reduction), although statistical significance was not reached. There was no significant difference between genotypes in raw area pre- and post-dantrolene, however, the percent decrease of Ca2+ area was highly significant in R6/2 CPNs compared to WTs (t = 3.8, P = 0.002), due to a combination of reduced amplitude and decay time. This finding suggests that dantrolene could be more efficacious at reducing Ca2+ load in HD CPNs compared to EVP4593.
Figure 7.
Effects of dantrolene on somatic Ca2+ transients: A: somatic Ca2+ transients evoked by 2 action potentials (APs) in WT (n = 11) and R6/2 (n = 9) cortical pyramidal neurons (CPNs) before and after application of the ryanodine receptor (RyR) antagonist dantrolene. B: in both genotypes the raw amplitude of the response was reduced. However, the percent reduction was much larger and statistically significant in CPNs from R6/2 compared with WT mice. C: box-whisker plots show that while in CPNs from WT animals the decay time was practically identical after dantrolene, in CPNs from R6/2 mice the raw and percent change in decay time was reduced, although the reduction did not reach statistical significance. D: however, a combination of reduced amplitude and decay time produced a highly and statistically significant reduction in Ca2+ transient area after dantrolene. The horizontal line indicates the median, the box represents the 25–75 percentile, the whiskers represent the range, and the diamonds represent outliers (data points outside of the 25–75 percentile). *P < 0.05, **P < 0.01, and *** P < 0.001 as determined by a two-way ANOVA and post hoc Holm–Sidak tests. WT, wild type.
L-type Ca2+ Channels Could Also Contribute to Ca2+ Dyshomeostasis in Symptomatic R6/2 Mice
Previous studies in our laboratory demonstrated increases in voltage-gated Ba2+ currents in CPNs from symptomatic R6/2 mice (21). In addition, increased Cav1.2 channels and L-type peak Ca2+ current density have been reported in BACHD cortical but not striatal neurons (31). Thus, we also investigated the contribution of L-type Ca2+ channels on AP-induced Ca2+ responses in CPNs from symptomatic R6/2 mice. We used nifedipine at a concentration of 10 µM, as this concentration has been shown to enhance the survival of CPN and substantia nigra neurons (26, 31).
We observed that the amplitudes of AP-driven Ca2+ transients at the somata of CPNs from both late symptomatic R6/2 and WT mice were effectively diminished after introduction of nifedipine [R6/2 n = 9; WT n = 9, main effect of drug: F(1,25)= 11.6, P = 0.002] and a genotype-dependent decrease in amplitude pre-nifedipine [main effect of genotype: F(1,25) = 6.6, P = 0.02; post hoc WT compared with R6/2 pre-nifedipine: t = 2.2, P = 0.04; Fig. 8]. There was a significantly greater percent decrease in the amplitude of Ca2+ responses from R6/2 CPNs following nifedipine relative to WTs (t = 4.3, P = 0.002). The decay time was significantly increased in R6/2 CPNs compared to WTs before administration of nifedipine [main interaction of genotype and drug: F(1,25)=6.3, P = 0.02; post hoc WT compared with R6/2 pre-nifedipine: t = 2.8, P = 0.01], and this difference was abolished post-nifedipine (Fig. 8). However, there was no significant genotype effect of nifedipine on percent change in decay time. The area of Ca2+ transients was significantly decreased following nifedipine [main effect of drug: F(1,24)=4.3, P = 0.05], but there were no differences between genotypes. However, the percent change in area was significantly decreased in R6/2 compared to WT [t = 2.5, P = 0.03]. This suggests that L-type Ca2+ channels located at the soma of CPNs from R6/2 mice are more abundant or more sensitive to nifedipine block. Significant reductions in amplitude and a greater, though not significant, reduction in decay time indicate that nifedipine could also be useful in reducing Ca2+ load in HD CPNs.
Figure 8.
Effects of nifedipine on somatic Ca2+ transients: A: somatic Ca2+ transients evoked by 2 APs in WT (n = 9) and R6/2 (n = 9) cortical pyramidal neurons (CPNs) before and after application of the L-type Ca2+ channel blocker nifedipine. B: in both genotypes, the amplitude of the response was reduced. However, the percent reduction was significantly greater in R6/2 CPNs. C: the median decay time changed in opposite ways after nifedipine, slightly increased in WT CPNs but slightly reduced in R6/2 CPNs. D: a decrease in amplitude with no change in decay time led to a significant reduction in Ca2+ transient area in CPNs from R6/2 mice compared to WTs. The horizontal line indicates the median, the box represents the 25–75 percentile, the whiskers represent the range, and the diamonds represent outliers (data points outside of the 25–75 percentile). *P < 0.05 and **P < 0.01 as determined by a two-way ANOVA and post hoc Holm–Sidak tests. WT, wild type.
DISCUSSION
Altered Ca2+ signaling in HD contributes to progressive degeneration of MSNs and CPNs, as well as to neurological abnormalities observed in the disease. In the present study, we used the transgenic R6/2 mouse model of juvenile HD (22) to examine intrinsic passive and active membrane properties and AP-associated Ca2+ elevations in L2/3 CPNs of the primary motor cortex at different time points of disease progression. Our findings demonstrate that alterations in Ca2+ homeostasis occur before the emergence of the overt phenotype. Some of these changes lead to compensatory mechanisms. Additionally, the present study examined the effects of delivering pharmacological agents that could potentially reinstate intracellular Ca2+ buffering capacity.
In agreement with previous studies from our laboratory (32), we observed altered passive and active membrane properties in L2/3 CPNs of R6/2 motor cortex as early as 3 wk, before the onset of overt symptoms, and these became more pronounced with disease progression. Such alterations included decreased cell membrane capacitance and increased membrane input resistance, suggestive of smaller somatic areas, dendritic thinning and reduced neurite arborization, as previously documented using the Golgi technique (28). These results support the idea that motor cortex CPNs exhibit signs of cellular dysfunction and degeneration even before the appearance of motor symptoms. In addition, CPNs from pre-, early-, and late symptomatic R6/2 mice displayed a significant reduction in rheobasic current, indicating hyperexcitability of R6/2 CPNs at all ages, as well as changes in some AP properties. Reduced AP areas appear to reflect reduced Ca2+ transient amplitudes observed in R6/2 mice, while increased half-amplitude duration of APs only in the late symptomatic group implicate diminished Ca2+ buffering capacity. Similar alterations were reported in CPNs of the perirhinal cortex in R6/1 mice (33) and in striatal MSNs of R6/2 mice (28).
Neuronal Ca2+ influx and accumulation can occur via voltage-gated channels, glutamate receptor channels, or SOCs. Disturbances in intracellular Ca2+ homeostasis have been documented in several experimental mouse models of HD. As the main culprit of HD manifestations is the aggregation of mHTT, Ca2+ signaling can be severely affected in a variety of ways given the close interaction of mHtt with Ca2+-binding proteins, postsynaptic receptors, intracellular stores and mitochondrial membranes (31, 34, 35). For example, the presence of mHTT increases expression of functional NMDA receptors at the cell surface, which in turn enhances NMDA receptor-mediated current density by leading to an excessive increase in intracellular Ca2+ concentration (36–39). Further, studies in mitochondria from whole brain of presymptomatic YAC72 full-length mice provide evidence that mHTT encourages perturbations in Ca2+ signaling (40, 41). In addition, direct binding of mHTT to the cytosolic portion of the type 1 IP3R’s carboxy-terminus increases the receptor responsiveness and induces a persistent ER Ca2+ leak (42).
Our findings agree with the general consensus of Ca2+ dyshomeostasis in HD (43). We demonstrate compensatory changes in the form of reduced activity-dependent Ca2+ influx, in agreement with other studies (14). It could be asked, how is it that augmented intracellular Ca2+ can lead to reduced amplitudes of Ca2+ transients. The classic assumption is that cell loss is directly related to accumulation of intracellular Ca2+. Although this may be true in acute conditions, in pathologies with slow, progressive degeneration there is room and time for compensation to prevent deleterious Ca2+ accumulation. In HD models, CPNs and striatal neurons possess homeostatic mechanisms to prevent such Ca2+ overload (14). Indeed, L-type Ca2+ channels in particular display fast Ca2+-dependent inactivation (CDI), which could prevent excessive intracellular Ca2+. This CDI operates via multiple mechanisms and calmodulin plays a critical role (44). In further support, a recent study from our laboratory demonstrated reduced amplitude of Ca2+ transients associated with spontaneous firing in CPNs from awake, behaving R6/2 and Q175 mice (45). However, with disease progression CPNs seem to lose the capacity to buffer increases in somatic Ca2+ as reflected by increased decay times. Thus, drugs that reduce decay time could bestow better protection from Ca2+ overload. As the present data showed that the decay time of Ca2+ transients was significantly longer in presymptomatic and late symptomatic R6/2 CPNs compared to age-matched WTs, we can assume underlying impairments in Ca2+ efflux mechanisms. Similar impairments have been demonstrated in ischemia (46, 47) and in temporal lobe epilepsy (48).
Overall, in both genotypes, differences in AP-driven Ca2+ responses in either somata or dendrites appeared more prominent as the number of evoked APs increased. We also observed a statistically significant reduction in the area of Ca2+ responses occurring in proximal dendrites at the late symptomatic stage, and this effect was due primarily to a non-significant reduction in decay time, which is opposite to changes in the somatic compartment. This suggests that Ca2+ disturbances are more prominent at the somata of CPNs and that backpropagating APs may not significantly affect Ca2+ levels in dendritic and spine compartments of R6/2 mice. Thus, in terms of toxicity caused by Ca2+ elevations, spine loss and dendritic thinning may be the consequence of altered Ca2+ influx through NMDA or Ca2+-permeable AMPA receptors (14, 49, 50).
Numerous reports have extensively documented that abnormalities in intracellular Ca2+ are closely associated with increased expression of voltage-gated Ca2+ channels (51–54). Such dysregulation has been implicated in neurodegenerative disorders, including Alzheimer’s and Parkinson’s diseases (55, 56). Therefore, we investigated whether high-voltage-activated L-type Ca2+ channels, preferentially situated postsynaptically at somatodendritic locations, played a role in Ca2+ mishandling in CPNs from R6/2 mice. Our findings demonstrate that antagonizing L-type Ca2+ channels with nifedipine, a short-acting dihydropyridine, significantly reduced amplitude and area of AP-evoked Ca2+ transients in R6/2 CPNs compared to WTs. These results are consistent with a recent study showing that treatment with L-type Ca2+ channel blockers, namely nifedipine and isradipine, were able to considerably diminish Ca2+ peak current from both WT and BACHD neurons, with higher nifedipine-resistant Ca2+ current density in BACHD neurons (31).
Recent evidence points toward increased and persistent Ca2+ leak mediated via RyRs, due to the binding of mHTT to these receptors. This discovery can shed light into the Ca2+-centered mechanisms underlying mHTT-induced neuronal death (57). In support, numerous studies have documented the neuroprotective effects of dantrolene, a potent RyR antagonist and clinically relevant Ca2+ signaling stabilizer (58). Long-term treatment of cultured YAC128 MSNs with dantrolene protected against glutamate-induced apoptosis, ameliorated motor coordination deficits, and reduced the formation of mHTT aggregates (27). Our data demonstrate that administration of dantrolene significantly restored augmented somatic AP-driven Ca2+ accumulations in symptomatic R6/2 mice, as reflected by reduced Ca2+ transient decay times.
Experiments in YAC128-derived corticostriatal co-cultures indicate that the persistent Ca2+ leakage from the ER, due to the intimate interaction between IP3R and mHTT, ultimately leads to elevated SOC response, which subsequently facilitates ER refilling and provokes dendritic spine loss (35, 59, 60). Further, treatment of co-cultures with EVP4593, a quinazoline-derived compound used to inhibit SOC, has been shown to reverse dendritic spine loss in HD MSNs (35). In addition, EVP4593 ameliorates the motor phenotype in a Drosophila HD model and rescues YAC128 mice from glutamate-induced apoptosis both in vitro and in vivo (15). In contrast, we found that although EVP4593 slightly reduced enhanced Ca2+ levels in cortical brain slices of symptomatic R6/2 mice, this effect occurred similarly in both WT and R6/2 CPNs. Therefore, our data indicate that Ca2+ entry through SOC channels does not appear to be significantly involved in Ca2+ mishandling in R6/2 mice. One possibility is that only inhibition of intracellular Ca2+ release from RyRs, but not IP3Rs, can mitigate Ca2+ mishandling. Our findings are in agreement with those showing that 2-aminoethoxydiphenyl borate (2APB), an inhibitor of IP3Rs, failed to protect striatal neurons but, in contrast, inhibitors of RyRs, namely dantrolene and 1,1′-diheptyl-4, 4′-bipyridinium dibromide (DHBP) ruthenium red (RR), were able to effectively attenuate neuronal death in primary cultured cortical and striatal neurons of R6/2 mice (57).
In summary, neuronal Ca2+ homeostasis in CPNs of the primary motor cortex is fundamentally disturbed in HD. In addition, perturbations in Ca2+ dynamics preceding the onset of overt behavioral deficits (45) reiterate that Ca2+-oriented therapies may need to be delivered early in the time course of the disease. Although our data suggest that dysregulation of L-type voltage-gated Ca2+ channels and RyRs contribute to abnormal Ca2+ influx in R6/2 mice, we cannot exclude that additional mechanisms may also play a role (34, 38, 61). Overall, the present study provides new information about the role of Ca2+ in CPN perturbations and establishes a framework for a rational approach to examine progressive CPN neurodegeneration in HD.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
GRANTS
This work was supported by the National Institutes of Health (NS 96994 to MSL) and the Hereditary Disease Foundation (Award Number: 20194204 to KDO).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S.L., C.C., and K.D.O. conceived and designed research; K.D.O. performed experiments; K.D.O., E.J.D., and M.T.N.B. analyzed data; K.D.O., E.J.D., C.C., and M.S.L. interpreted results of experiments; K.D.O. and E.J.D. prepared figures; K.D.O., E.J.D., C.C., and M.S.L. drafted manuscript; K.D.O., E.J.D., C.C., and M.S.L. revised manuscript; K.D.O., E.J.D., M.T.N.B., C.C., and M.S.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Drs. Baljit Khakh and Xinzhu Yu for their generous help sharing equipment and providing training on use of two-photon confocal microscope. We also thank Dr. Joshua Barry for his help with the illustrations.
REFERENCES
- 1.Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, Nance M, Ross CA, Scahill RI, Wetzel R, Wild EJ, Tabrizi SJ. Huntington disease. Nat Rev Dis Primers 1: 15005, 2015. doi: 10.1038/nrdp.2015.5. [DOI] [PubMed] [Google Scholar]
- 2.Tabrizi SJ, Reilmann R, Roos RA, Durr A, Leavitt B, Owen G, Jones R, Johnson H, Craufurd D, Hicks SL, Kennard C, Landwehrmeyer B, Stout JC, Borowsky B, Scahill RI, Frost C, Langbehn DR; TRACK-HD Investigators. Potential endpoints for clinical trials in premanifest and early Huntington's disease in the TRACK-HD study: analysis of 24 month observational data. Lancet Neurol 11: 42–53, 2012. doi: 10.1016/S1474-4422(11)70263-0. [DOI] [PubMed] [Google Scholar]
- 3.The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72: 971–983, 1993. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 4.Reedeker W, van der Mast RC, Giltay EJ, Kooistra TA, Roos RA, van Duijn E. Psychiatric disorders in Huntington's disease: a 2-year follow-up study. Psychosomatics 53: 220–229, 2012. doi: 10.1016/j.psym.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Thompson JC, Harris J, Sollom AC, Stopford CL, Howard E, Snowden JS, Craufurd D. Longitudinal evaluation of neuropsychiatric symptoms in Huntington's disease. J Neuropsychiatry Clin Neurosci 24: 53–60, 2012. doi: 10.1176/appi.neuropsych.11030057. [DOI] [PubMed] [Google Scholar]
- 6.Beglinger LJ, O'Rourke JJ, Wang C, Langbehn DR, Duff K, Paulsen JS; Huntington Study Group Investigators. Earliest functional declines in Huntington disease. Psychiatry Res 178: 414–418, 2010. doi: 10.1016/j.psychres.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boecker H, Ceballos-Baumann A, Bartenstein P, Weindl A, Siebner HR, Fassbender T, Munz F, Schwaiger M, Conrad B. Sensory processing in Parkinson's and Huntington's disease: investigations with 3D H(2)(15)O-PET. Brain 122: 1651–1665, 1999. doi: 10.1093/brain/122.9.1651. [DOI] [PubMed] [Google Scholar]
- 8.Hedreen JC, Peyser CE, Folstein SE, Ross CA. Neuronal loss in layers V and VI of cerebral cortex in Huntington's disease. Neurosci Lett 133: 257–261, 1991. doi: 10.1016/0304-3940(91)90583-F. [DOI] [PubMed] [Google Scholar]
- 9.Cudkowicz M, Kowall NW. Degeneration of pyramidal projection neurons in Huntington's disease cortex. Ann Neurol 27: 200–204, 1990. doi: 10.1002/ana.410270217. [DOI] [PubMed] [Google Scholar]
- 10.Heinsen H, Strik M, Bauer M, Luther K, Ulmar G, Gangnus D, Jungkunz G, Eisenmenger W, Gotz M. Cortical and striatal neurone number in Huntington's disease. Acta Neuropathol 88: 320–333, 1994. doi: 10.1007/BF00310376. [DOI] [PubMed] [Google Scholar]
- 11.Thu DC, Oorschot DE, Tippett LJ, Nana AL, Hogg VM, Synek BJ, Luthi-Carter R, Waldvogel HJ, Faull RL. Cell loss in the motor and cingulate cortex correlates with symptomatology in Huntington's disease. Brain 133: 1094–1110, 2010. doi: 10.1093/brain/awq047. [DOI] [PubMed] [Google Scholar]
- 12.Bezprozvanny I. Role of inositol 1,4,5-trisphosphate receptors in pathogenesis of Huntington's disease and spinocerebellar ataxias. Neurochem Res 36: 1186–1197, 2011. doi: 10.1007/s11064-010-0393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller BR, Bezprozvanny I. Corticostriatal circuit dysfunction in Huntington's disease: intersection of glutamate, dopamine and calcium. Future Neurol 5: 735–756, 2010. doi: 10.2217/fnl.10.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackay JP, Nassrallah WB, Raymond LA. Cause or compensation?-Altered neuronal Ca(2+) handling in Huntington's disease. CNS Neurosci Ther 24: 301–310, 2018. doi: 10.1111/cns.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Shih HP, Vigont V, Hrdlicka L, Diggins L, Singh C, Mahoney M, Chesworth R, Shapiro G, Zimina O, Chen X, Wu Q, Glushankova L, Ahlijanian M, Koenig G, Mozhayeva GN, Kaznacheyeva E, Bezprozvanny I. Neuronal store-operated calcium entry pathway as a novel therapeutic target for Huntington's disease treatment. Chem Biol 18: 777–793, 2011. doi: 10.1016/j.chembiol.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton J, Brustovetsky T, Brustovetsky N. Oxidative metabolism and Ca(2+) handling in striatal mitochondria from YAC128 mice, a model of Huntington's disease. Neurochem Int 109: 24–33, 2017. doi: 10.1016/j.neuint.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton J, Pellman JJ, Brustovetsky T, Harris RA, Brustovetsky N. Oxidative metabolism and Ca2+ handling in isolated brain mitochondria and striatal neurons from R6/2 mice, a model of Huntington's disease. Hum Mol Genet 25: 2762–2775, 2016. doi: 10.1093/hmg/ddw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murmu RP, Li W, Holtmaat A, Li JY. Dendritic spine instability leads to progressive neocortical spine loss in a mouse model of Huntington's disease. J Neurosci 33: 12997–13009, 2013. doi: 10.1523/JNEUROSCI.5284-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estrada-Sanchez AM, Burroughs CL, Cavaliere S, Barton SJ, Chen S, Yang XW, Rebec GV. Cortical efferents lacking mutant huntingtin improve striatal neuronal activity and behavior in a conditional mouse model of Huntington's disease. J Neurosci 35: 4440–4451, 2015. doi: 10.1523/JNEUROSCI.2812-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang N, Gray M, Lu XH, Cantle JP, Holley SM, Greiner E, Gu X, Shirasaki D, Cepeda C, Li Y, Dong H, Levine MS, Yang XW. Neuronal targets for reducing mutant huntingtin expression to ameliorate disease in a mouse model of Huntington's disease. Nat Med 20: 536–541, 2014. doi: 10.1038/nm.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andre VM, Cepeda C, Venegas A, Gomez Y, Levine MS. Altered cortical glutamate receptor function in the R6/2 model of Huntington's disease. J Neurophysiol 95: 2108–2119, 2006. doi: 10.1152/jn.01118.2005. [DOI] [PubMed] [Google Scholar]
- 22.Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87: 493–506, 1996. doi: 10.1016/S0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 23.Cepeda C, Cummings DM, Andre VM, Holley SM, Levine MS. Genetic mouse models of Huntington's disease: focus on electrophysiological mechanisms. ASN Neuro 2: e00033, 2010. doi: 10.1042/AN20090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cepeda C, Levine MS. Synaptic dysfunction in Huntington's disease: lessons from genetic animal models. Neuroscientist 1073858420972662, 2020. doi: 10.1177/1073858420972662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chauvet S, Jarvis L, Chevallet M, Shrestha N, Groschner K, Bouron A. Pharmacological characterization of the native store-operated calcium channels of cortical neurons from embryonic mouse brain. Front Pharmacol 7: 486, 2016. [Erratum in Front Pharmacol 8: 152, 2017]. doi: 10.3389/fphar.2016.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daschil N, Humpel C. Nifedipine and nimodipine protect dopaminergic substantia nigra neurons against axotomy-induced cell death in rat vibrosections via modulating inflammatory responses. Brain Res 1581: 1–11, 2014. doi: 10.1016/j.brainres.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Wu J, Lvovskaya S, Herndon E, Supnet C, Bezprozvanny I. Dantrolene is neuroprotective in Huntington's disease transgenic mouse model. Mol Neurodegener 6: 81, 2011. doi: 10.1186/1750-1326-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klapstein GJ, Fisher RS, Zanjani H, Cepeda C, Jokel ES, Chesselet MF, Levine MS. Electrophysiological and morphological changes in striatal spiny neurons in R6/2 Huntington's disease transgenic mice. J Neurophysiol 86: 2667–2677, 2001. doi: 10.1152/jn.2001.86.6.2667. [DOI] [PubMed] [Google Scholar]
- 29.Ariano MA, Cepeda C, Calvert CR, Flores-Hernandez J, Hernandez-Echeagaray E, Klapstein GJ, Chandler SH, Aronin N, DiFiglia M, Levine MS. Striatal potassium channel dysfunction in Huntington's disease transgenic mice. J Neurophysiol 93: 2565–2574, 2005. doi: 10.1152/jn.00791.2004. [DOI] [PubMed] [Google Scholar]
- 30.Putney JW Jr. A model for receptor-regulated calcium entry. Cell Calcium 7: 1–12, 1986. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Miranda AS, Cardozo PL, Silva FR, de Souza JM, Olmo IG, Cruz JS, Gomez MV, Ribeiro FM, Vieira LB. Alterations of calcium channels in a mouse model of Huntington's disease and neuroprotection by blockage of CaV1 channels. ASN Neuro 11: 1759091419856811, 2019. doi: 10.1177/1759091419856811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings DM, Andre VM, Uzgil BO, Gee SM, Fisher YE, Cepeda C, Levine MS. Alterations in cortical excitation and inhibition in genetic mouse models of Huntington's disease. J Neurosci 29: 10371–10386, 2009. doi: 10.1523/JNEUROSCI.1592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cummings DM, Milnerwood AJ, Dallerac GM, Waights V, Brown JY, Vatsavayai SC, Hirst MC, Murphy KP. Aberrant cortical synaptic plasticity and dopaminergic dysfunction in a mouse model of Huntington's disease. Hum Mol Genet 15: 2856–2868, 2006. doi: 10.1093/hmg/ddl224. [DOI] [PubMed] [Google Scholar]
- 34.Rosenstock TR, Bertoncini CR, Teles AV, Hirata H, Fernandes MJ, Smaili SS. Glutamate-induced alterations in Ca2+ signaling are modulated by mitochondrial Ca2+ handling capacity in brain slices of R6/1 transgenic mice. Eur J Neurosci 32: 60–70, 2010. doi: 10.1111/j.1460-9568.2010.07268.x. [DOI] [PubMed] [Google Scholar]
- 35.Wu J, Ryskamp DA, Liang X, Egorova P, Zakharova O, Hung G, Bezprozvanny I. Enhanced store-operated calcium entry leads to striatal synaptic loss in a Huntington's disease mouse model. J Neurosci 36: 125–141, 2016. doi: 10.1523/JNEUROSCI.1038-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen N, Luo T, Wellington C, Metzler M, McCutcheon K, Hayden MR, Raymond LA. Subtype-specific enhancement of NMDA receptor currents by mutant huntingtin. J Neurochem 72: 1890–1898, 1999. doi: 10.1046/j.1471-4159.1999.0721890.x. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Savanenin A, Reddy PH, Liu YF. Polyglutamine-expanded huntingtin promotes sensitization of N-methyl-D-aspartate receptors via post-synaptic density 95. J Biol Chem 276: 24713–24718, 2001. doi: 10.1074/jbc.M103501200. [DOI] [PubMed] [Google Scholar]
- 38.Cepeda C, Ariano MA, Calvert CR, Flores-Hernandez J, Chandler SH, Leavitt BR, Hayden MR, Levine MS. NMDA receptor function in mouse models of Huntington disease. J Neurosci Res 66: 525–539, 2001. doi: 10.1002/jnr.1244. [DOI] [PubMed] [Google Scholar]
- 39.Zeron MM, Hansson O, Chen N, Wellington CL, Leavitt BR, Brundin P, Hayden MR, Raymond LA. Increased sensitivity to N-methyl-D-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington's disease. Neuron 33: 849–860, 2002. doi: 10.1016/S0896-6273(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 40.Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat Neurosci 5: 731–736, 2002. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 41.Choo YS, Johnson GV, MacDonald M, Detloff PJ, Lesort M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum Mol Genet 13: 1407–1420, 2004. doi: 10.1093/hmg/ddh162. [DOI] [PubMed] [Google Scholar]
- 42.Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, Hayden MR, Bezprozvanny I. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron 39: 227–239, 2003. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends Mol Med 15: 89–100, 2009. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Budde T, Meuth S, Pape HC. Calcium-dependent inactivation of neuronal calcium channels. Nat Rev Neurosci 3: 873–883, 2002. doi: 10.1038/nrn959. [DOI] [PubMed] [Google Scholar]
- 45.Donzis EJ, Estrada-Sanchez AM, Indersmitten T, Oikonomou K, Tran CH, Wang C, Latifi S, Golshani P, Cepeda C, Levine MS. Cortical network dynamics is altered in mouse models of Huntington's disease. Cereb Cortex 30: 2372–2388, 2020. doi: 10.1093/cercor/bhz245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell 120: 275–285, 2005. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 47.Lehotsky J, Kaplan P, Racay P, Mezesova V, Raeymaekers L. Distribution of plasma membrane Ca2+ pump (PMCA) isoforms in the gerbil brain: effect of ischemia-reperfusion injury. Neurochem Int 35: 221–227, 1999. doi: 10.1016/s0197-0186(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 48.Raza M, Pal S, Rafiq A, DeLorenzo RJ. Long-term alteration of calcium homeostatic mechanisms in the pilocarpine model of temporal lobe epilepsy. Brain Res 903: 1–12, 2001. doi: 10.1016/s0006-8993(01)02127-8. [DOI] [PubMed] [Google Scholar]
- 49.Mandal M, Wei J, Zhong P, Cheng J, Duffney LJ, Liu W, Yuen EY, Twelvetrees AE, Li S, Li XJ, Kittler JT, Yan Z. Impaired alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking and function by mutant huntingtin. J Biol Chem 286: 33719–33728, 2011. doi: 10.1074/jbc.M111.236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raymond LA, Andre VM, Cepeda C, Gladding CM, Milnerwood AJ, Levine MS. Pathophysiology of Huntington's disease: time-dependent alterations in synaptic and receptor function. Neuroscience 198: 252–273, 2011. doi: 10.1016/j.neuroscience.2011.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueda K, Shinohara S, Yagami T, Asakura K, Kawasaki K. Amyloid beta protein potentiates Ca2+ influx through L-type voltage-sensitive Ca2+ channels: a possible involvement of free radicals. J Neurochem 68: 265–271, 1997. doi: 10.1046/j.1471-4159.1997.68010265.x. [DOI] [PubMed] [Google Scholar]
- 52.Cano-Abad MF, Villarroya M, Garcia AG, Gabilan NH, Lopez MG. Calcium entry through L-type calcium channels causes mitochondrial disruption and chromaffin cell death. J Biol Chem 276: 39695–39704, 2001. doi: 10.1074/jbc.M102334200. [DOI] [PubMed] [Google Scholar]
- 53.Luo CX, Zhu XJ, Zhang AX, Wang W, Yang XM, Liu SH, Han X, Sun J, Zhang SG, Lu Y, Zhu DY. Blockade of L-type voltage-gated Ca channel inhibits ischemia-induced neurogenesis by down-regulating iNOS expression in adult mouse. J Neurochem 94: 1077–1086, 2005. doi: 10.1111/j.1471-4159.2005.03262.x. [DOI] [PubMed] [Google Scholar]
- 54.Swayne LA, Chen L, Hameed S, Barr W, Charlesworth E, Colicos MA, Zamponi GW, Braun JE. Crosstalk between huntingtin and syntaxin 1A regulates N-type calcium channels. Mol Cell Neurosci 30: 339–351, 2005. doi: 10.1016/j.mcn.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 55.Min D, Guo F, Zhu S, Xu X, Mao X, Cao Y, Lv X, Gao Q, Wang L, Chen T, Shaw C, Hao L, Cai J. The alterations of Ca2+/calmodulin/CaMKII/CaV1.2 signaling in experimental models of Alzheimer's disease and vascular dementia. Neurosci Lett 538: 60–65, 2013. doi: 10.1016/j.neulet.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Hurley MJ, Brandon B, Gentleman SM, Dexter DT. Parkinson's disease is associated with altered expression of CaV1 channels and calcium-binding proteins. Brain 136: 2077–2097, 2013. doi: 10.1093/brain/awt134. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki M, Nagai Y, Wada K, Koike T. Calcium leak through ryanodine receptor is involved in neuronal death induced by mutant huntingtin. Biochem Biophys Res Commun 429: 18–23, 2012. doi: 10.1016/j.bbrc.2012.10.107. [DOI] [PubMed] [Google Scholar]
- 58.Frandsen A, Schousboe A. Dantrolene prevents glutamate cytotoxicity and Ca2+ release from intracellular stores in cultured cerebral cortical neurons. J Neurochem 56: 1075–1078, 1991. doi: 10.1111/j.1471-4159.1991.tb02031.x. [DOI] [PubMed] [Google Scholar]
- 59.Czeredys M, Maciag F, Methner A, Kuznicki J. Tetrahydrocarbazoles decrease elevated SOCE in medium spiny neurons from transgenic YAC128 mice, a model of Huntington's disease. Biochem Biophys Res Commun 483: 1194–1205, 2017. doi: 10.1016/j.bbrc.2016.08.106. [DOI] [PubMed] [Google Scholar]
- 60.Czeredys M. Dysregulation of neuronal calcium signaling via store-operated channels in Huntington's disease. Front Cell Dev Biol 8: 611735, 2020. doi: 10.3389/fcell.2020.611735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeron MM, Fernandes HB, Krebs C, Shehadeh J, Wellington CL, Leavitt BR, Baimbridge KG, Hayden MR, Raymond LA. Potentiation of NMDA receptor-mediated excitotoxicity linked with intrinsic apoptotic pathway in YAC transgenic mouse model of Huntington's disease. Mol Cell Neurosci 25: 469–479, 2004. doi: 10.1016/j.mcn.2003.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.