Abstract
Introduction
Metastatic urothelial carcinomas are common in lung, liver, and lymph nodes. We present rare secondary tumor of the prostate metastasized from upper tract urothelial carcinoma.
Case presentation
An 87‐year‐old man was diagnosed as urothelial carcinoma of left upper tract and bladder. Only transurethral resection of bladder tumor was performed as palliative therapy to control hematuria. Thereafter, the tumor of left upper tract showed aggressive progression with multiple metastases involving lymph nodes and bilateral lungs. Finally, autopsy revealed swelling of left kidney due to tumor growth and systemic cancer disseminations involving bilateral lungs and renal hilar lymph nodes. In addition, prostate tumor was found incidentally. Histological examination including immunohistochemistry revealed the prostate tumor as metastatic tumor from urothelial carcinoma of left renal pelvis.
Conclusion
We reported rare secondary tumor of the prostate, derived from upper tract urothelial carcinoma. Further consideration would be required to provide better knowledge of the disease.
Keywords: invasive UTUC, metastasis, secondary tumor of the prostate, upper tract urothelial carcinoma
Abbreviations & Acronyms
- AMACR
alpha‐methylacyl‐CoA‐racemase
- CT
computed tomography
- GATA3
GATA‐binding protein 3
- PAS‐AB
periodic acid Schiff‐Alcian blue
- TURBT
transurethral resection of bladder tumor
- UTUC
upper tract urothelial carcinoma
Keynote message.
The secondary tumor involving the prostate is not common, that could be found in autopsy cases with lung cancer, pancreas cancer, or melanoma. Urothelial carcinoma of prostate might be observed as direct invasion or extension from bladder cancer, but distant metastasis from upper tract urothelial carcinoma is rare.
Introduction
UTUC is an uncommon adult malignant tumor that counts 2–3 persons per 100,000 in developed countries. 1 Most of the cases with UTUC are found as advanced tumors which have already accompanied with metastasis. 2 In most cases, urothelial carcinoma found in prostate was observed as direct invasion, extension through prostatic urethra, or transluminal dissemination from bladder cancer. 3 , 4 In this report, we present a rare case of a secondary prostate tumor derived from UTUC that was found incidentally in autopsy.
Case presentation
An 87‐year‐old man consulted our hospital with left hydronephrosis. He had renal dysfunction due to diabetes mellitus. Because no imaging examination using intravenous contrast‐enhanced media was indicated, the unenhanced CT examination was performed and revealed left hydronephrosis (Fig. 1a). In cystoscopy, papillary tumors were found in bladder, suspected as superficial bladder urothelial tumors (Fig. 1b). Retrograde pyelography showed apparent ureteral obstruction of left ureter due to tumor and contrast media could not pass into proximal segment of left ureter (Fig. 1c). Together with the results of radiological examinations, tumor of left ureter was suspected invasive UTUC clinically, and it was diagnosed cytologically as urothelial carcinoma. Because he did not agree with radical nephroureterectomy and postoperative induction of hemodialysis for left ureteral tumor, only TURBT was performed as palliative therapy to control hematuria. The tumors of bladder were diagnosed as noninvasive papillary urothelial carcinoma pathologically (Fig. 1d), and the tumors were completely resected clinically. After TURBT, he had not received any other treatment including systemic chemotherapy against tumors. One month before his death, he consulted our hospital again with general fatigue and fever. CT examination revealed apparent enlargement of the tumor in left renal pelvis (Fig. 1e) and tumor metastasis into renal hilar lymph nodes and bilateral lungs (Fig. 1f). At this point, curative treatment was determined to be difficult and palliative therapy was performed. Four months after his first visit, he had been in terminal cancer illness, especially suffered from severe dyspnea and renal failure, and died. Autopsy revealed swelling of left kidney due to tumor growth, and systemic cancer disseminations involving bilateral lungs and renal hilar lymph nodes (Fig. 2a), as noted on CT examination. Bladder carcinomas were not residual (Fig. 2b). In addition, prostate tumor was found incidentally. Histologically, tumor of left renal pelvis showed papillary growth with fibrovascular core and invaded into muscular layer and renal parenchyma, and diagnosed as invasive urothelial carcinoma of renal pelvis (Fig. 3a,b) On immunohistochemistry, tumor cells of UTUC were positive for cytokeratin 7 (Fig. 3c), cytokeratin 34βE12 (Fig. 3e), and p63 (Fig. 3g), and negative for cytokeratin 20 (Fig. 3d), GATA3 (Fig. 3f), PSA (Fig. 3h), and AMACR (Fig. 3i). In addition, PAS‐AB staining showed no mucin production in UTUC (Fig. 3j). On the other hand, prostate tumor did not appear to be extended from prostatic urethra because the tumor focus was on the middle of prostate isolated from prostatic urethra (Fig. 4a,b) and carcinoma in␣situ or dysplasia was not found in prostatic urethra. Histological and immunohistochemical characters of tumor found in prostate were similar to that of UTUC (Fig. 4c–j). These results suggested that the tumor of prostate was not primary adenocarcinoma but secondary neoplasm originated from UTUC.
Fig. 1.
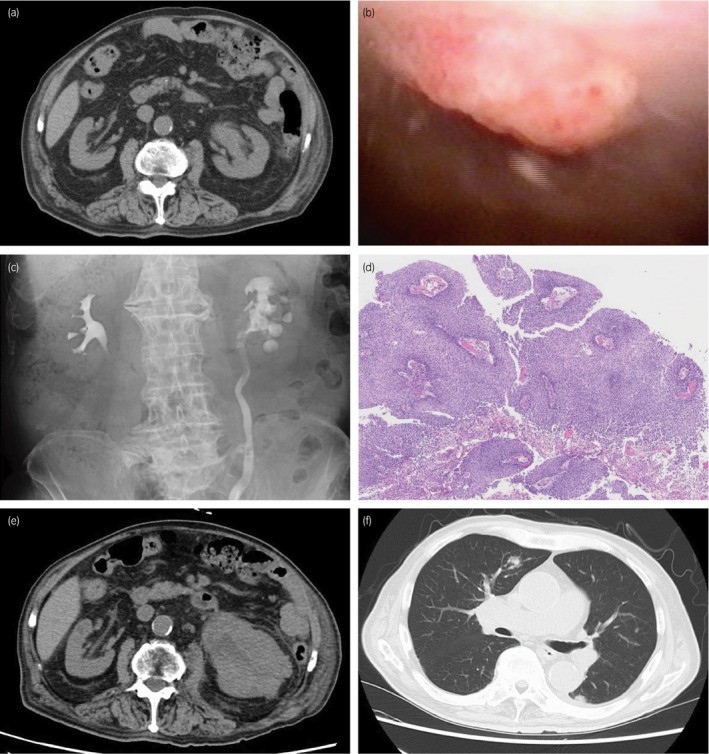
Clinical findings of the case. (a) CT image at the first visit. Left hydronephrosis was evident. (b) Cystoscopy showed papillary bladder tumor. (c) Retrograde pyelography showed left hydronephrosis with filling defect in the renal pelvis. (d) Bladder tumor had papillary architecture with fibromuscular core without invasion to lamina propria. (e) CT examination revealed apparent progression of the tumor in renal pelvis. (f) Bilateral lung metastasis
Fig. 2.
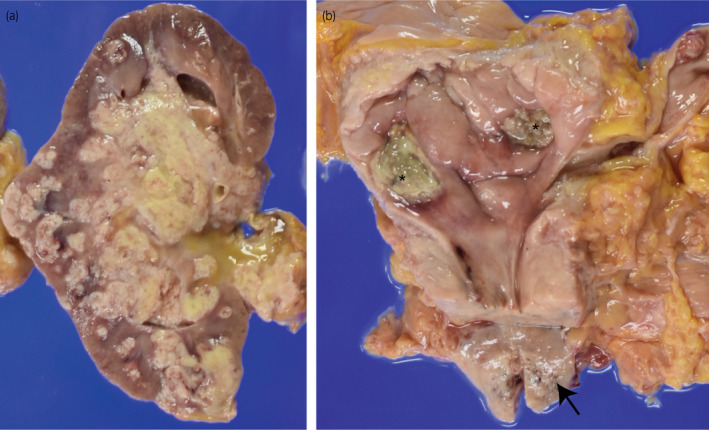
Gross appearance of left kidney, bladder, and prostate. (a) Tumors in left renal pelvis. (b) Scars due to TUR were found in urinary bladder (asterisk) and tumor nodule was evident in the prostate (arrow)
Fig. 3.
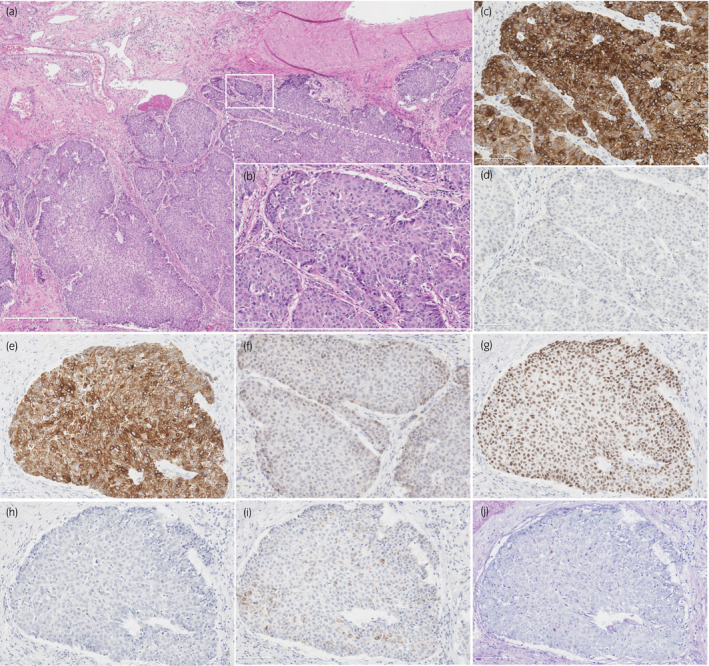
Histological findings of tumor in left kidney. (a) Hematoxylin and eosin staining with lower magnification (x50). Tumor cells had papillary architecture and invading into muscular layer. (b) Tumor in left kidney with high magnification (x200). Immunohistochemistry showed tumor cells were (c) positive for cytokeratin 7, (d) negative for cytokeratin 20, (e) positive for cytokeratin 34βE12, (f) negative for GATA‐binding protein 3 (GATA3), (g) positive for p63, (h) negative for PSA, and (i) negative for AMACR. (h) Periodic acid Schiff‐Alcian blue staining showed no mucin production in tumor. Scale bar indicates 100 µm
Fig. 4.
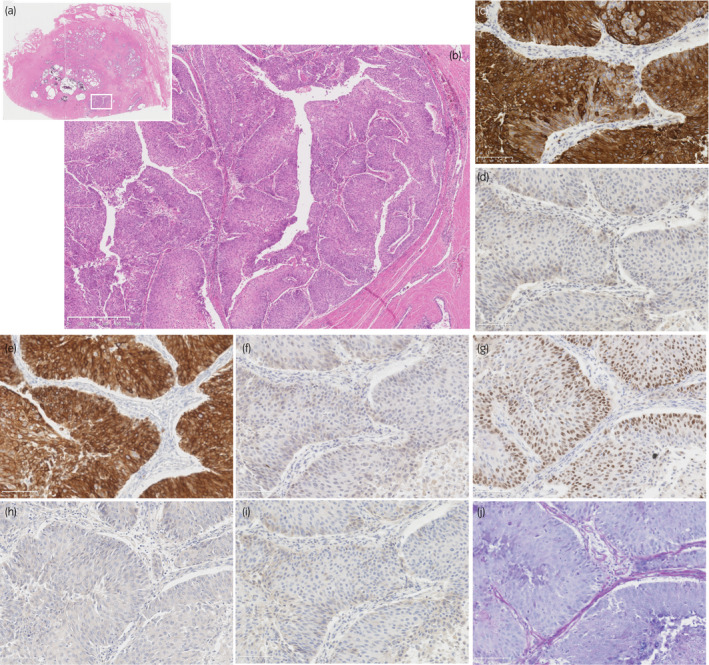
Histological findings of tumor in prostate. (a) Loupe picture of hematoxylin and eosin staining section. Tumor focus was detected in the middle of the prostate. (b) Tumor in prostate with lower magnification (x20). Tumor focus was isolated from prostatic urethra and tumor cells had papillary architecture, similar as the tumor in renal pelvis. (c) Tumor in prostate with high magnification (x200). Immunohistochemistry showed tumor cells were (d) positive for cytokeratin 7, (e) negative for cytokeratin 20, (f) positive for cytokeratin 34βE12, (g) negative for GATA3, (h) positive for p63, (i) negative for PSA, and (j) negative for AMACR. (k) Periodic acid Schiff‐Alcian blue staining showed no mucin production in tumor. Scale bar indicates 100 µm
Discussion
The secondary tumor involving the prostate is rare, which is incidentally found by autopsy in most cases. It was counted only 0.4%–2.0% of autopsy cases. 5 , 6 In other serial reports, about 51 cases of secondary neoplasms involving prostate, 34 cases were regarded as direct invasion from bladder cancer and rest 17 cases were as metastatic disease. 7 Although direct invasion to prostate of urothelial carcinoma from urinary bladder had been regarded as most common secondary tumor of the prostate, it may should have been distinguished from distant metastasis. Recently, prostate tumor of urothelial origin is considered as metastatic tumor if dysplastic epithelium is absent in prostatic urethra. 8 Accordingly, metastatic tumor in the prostate is rare. The most common primary tumors were carcinoma of lung, carcinoma of pancreas and melanoma, meanwhile metastases of tumors of gastrointestinal tract, kidney and endocrine organs, and germ cell tumor were less. 9 , 10 In this case, the tumor was localized in prostate and carcinoma in␣situ or dysplasia was not found in both bladder and prostatic urethra. Therefore, the prostate tumor was considered as secondary tumor metastasized from other organs rather than primary urothelial carcinoma of the prostate. In considering the primary site of prostate tumor, invasive urothelial carcinoma of left renal pelvis was most likely to that because it showed aggressive progression including multiple metastases involving bilateral lungs and lymph nodes. We considered the possibility of metastasis of bladder tumors as very low, because these were noninvasive papillary urothelial carcinoma, clinically completely resected, and not residual in autopsy. Furthermore, it might be unnatural to consider the metastasis happened from noninvasive small tumors rather than from aggressive invasive tumor. Therefore, we concluded the prostate tumor as metastasis from the tumor of renal pelvis.
Immunohistochemical assessment is helpful to determine the origin of the tumor. 11 , 12 Previously, it has been suggested that high molecular weight cytokeratins and GATA3 were positive sensitive marker to differentiate UC from other metastatic cancers. 13 , 14 In this case, tumor cells in both left renal pelvic and prostate tumor were positive for cytokeratin 7 and cytokeratin 34βE12, and negative for cytokeratin 20. GATA3 has been reported as an useful positive marker for UC and breast cancer because of its high expression rate in these organs compared with few positivity in other organs, 15 but the positivity of GATA3 in UC was reported as 70%–90% and some cases could be negative. 15 , 16 In this case, though the tumor cells were negative for GATA3, results of other immunohistochemical positive markers strongly suggested this tumor as UC. Although, patient had noninvasive papillary UCs in the bladder, there was no evidence of direct extension of them through prostatic urethra. Furthermore, tumor cells of the prostate were negative for PSA and AMACR, positive markers for primary adenocarcinoma of prostate. Therefore, in this case, prostate tumor was diagnosed as metastatic tumor of UTUC. In reported literatures, secondary tumors in the prostate were incidentally found in the specimens of cystoprostatectomy or autopsy. Even in this case, the secondary tumor of the prostate was incidentally found in the autopsy. If we clinically found the secondary UC in the prostate alone, nephroureterectomy and cystoprostatectomy might be indicated. However, in this case, because multiple lung metastases were apparent, the treatment policy of systemic chemotherapy might not be changed.
Conclusion
In this time, we reported rare secondary tumor of the prostate found in autopsy diagnosed as metastatic tumor of UTUC. Because it has no specific clinical signs or symptoms, histological evaluation would be required as well as the other secondary prostate tumors. Immunohistochemistry is helpful for differentiation of primary and secondary tumors of the prostate. There were few reports of metastatic urothelial carcinoma involving prostate and all of them were originated not from ureter like this case but from urinary bladder. Further consideration would be required for better knowledge of the disease.
Conflict of interest
The authors declare no conflict of interest.
Approval of the research protocol by an institutional reviewer board
Not applicable.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Registry and registration no. of the study/trial
Not applicable.
Acknowledgments
The authors express the heartfelt condolence to the patient and bereaved families.
Goto K, Kambara T, Kagiyama Y, et␣al. The secondary tumor of the prostate derived from upper tract urothelial carcinoma: An autopsy case. IJU Case Rep. 2021; 4: 397–402. 10.1002/iju5.12358.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Lughezzani G, Burger M, Margulis V et␣al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur. Urol. 2012; 62: 100–14. [DOI] [PubMed] [Google Scholar]
- 3. Matzkin H, Soloway MS, Hardeman S. Transitional cell carcinoma of the prostate. J. Urol. 1991; 146: 1207–12. [DOI] [PubMed] [Google Scholar]
- 4. Njinou Ngninkeu B, Lorge F, Moulin P, Jamart J, van Cangh PJ. Transitional cell carcinoma involving the prostate: a clinicopathological retrospective study of 76 cases. J. Urol. 2003; 169: 149–52. [DOI] [PubMed] [Google Scholar]
- 5. Johnson DE, Chalbaud R, Ayala AG. Secondary tumors of the prostate. J. Urol. 1974; 112: 507–8. [DOI] [PubMed] [Google Scholar]
- 6. Zein TA, Huben R, Lane W, Pontes JE, Englander LS. Secondary tumors of the prostate. J. Urol. 1985; 133: 615–6. [DOI] [PubMed] [Google Scholar]
- 7. Bates A, Baithun S. Secondary solid neoplasms of the prostate: a clinico‐pathological series of 51 cases. Virchows Arch. 2002; 440: 392–6. [DOI] [PubMed] [Google Scholar]
- 8. Fusco N, Sciarra A, Guerini‐Rocco E et␣al. Rediscovering secondary tumors of the prostate in the molecular era. Adv. Anat. Pathol. 2016; 23: 170–9. [DOI] [PubMed] [Google Scholar]
- 9. Bates A, Baithun S. The significance of secondary neoplasms of the urinary and male genital tract. Virchows Arch. 2002; 440: 640–7. [DOI] [PubMed] [Google Scholar]
- 10. Morichetti D, Mazzucchelli R, Lopez‐Beltran A et␣al. Secondary neoplasms of the urinary system and male genital organs. BJU Int. 2009; 104: 770–6. [DOI] [PubMed] [Google Scholar]
- 11. Dennis JL, Hvidsten TR, Wit EC et␣al. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin. Cancer Res. 2005; 11: 3766–72. [DOI] [PubMed] [Google Scholar]
- 12. Chang A, Amin A, Gabrielson E et␣al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am. J. Surg. Pathol. 2012; 36: 1472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Varma M, Morgan M, Amin MB, Wozniak S, Jasani B. High molecular weight cytokeratin antibody (clone 34βE12): a sensitive marker for differentiation of high‐grade invasive urothelial carcinoma from prostate cancer. Histopathology 2003; 42: 167–72. [DOI] [PubMed] [Google Scholar]
- 14. Parker DC, Folpe AL, Bell J et␣al. Potential utility of uroplakin III, thrombomodulin, high molecular weight cytokeratin, and cytokeratin 20 in noninvasive, invasive, and metastatic urothelial (transitional cell) carcinomas. Am. J. Surg. Pathol. 2003; 27: 1–10. [DOI] [PubMed] [Google Scholar]
- 15. Liu H, Shi J, Wilkerson ML, Lin F. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am. J. Clin. Pathol. 2012; 138: 57–64. [DOI] [PubMed] [Google Scholar]
- 16. Liang Y, Heitzman J, Kamat AM, Dinney CP, Czerniak B, Guo CC. Differential expression of GATA‐3 in urothelial carcinoma variants. Hum. Pathol. 2014; 45: 1466–72. [DOI] [PubMed] [Google Scholar]


