Abstract
IL-10+ regulatory B (Breg) cells play a vital role in regulating the immune responses in experimental autoimmune encephalomyelitis, colitis, and contact hypersensitivity (CHS). Several sti-mulants such as lipopolysaccharide (LPS), CD40 ligand, and IL-21 spur the activation and maturation of IL-10+ Breg cells, while the epigenetic mechanism for the IL-10 expression remains largely unknown. It is well accepted that the histone acetylation/deacetylation is an important mechanism that regulates the expression of IL-10. We found that entinostat, an HDAC inhibitor, stimulated the induction of IL-10+ Breg cells by LPS in vitro and the formation of IL-10+ Breg cells to suppress CHS in vivo. We further demonstrated that entinostat inhibited HDAC1 from binding to the proximal region of the IL-10 expression promoter in splenic B cells, followed by an increase in the binding of NF-κB p65, eventually enhancing the expression of IL-10 in Breg cells.
Keywords: Contact hypersensitivity, Entinostat, Histone deacetylase inhibitor, Interleukin-10, Regulatory B cells
INTRODUCTION
B cells are progenitor cells of plasma cells that synthesize and secrete antibody. They are also known to perform the function of antigen-presenting cells. The inhibitory role of B cells was reported in a delayed hypersensitivity animal model (1). Since then, Mizoguchi et al. reported an inhibitory role in the murine model of inflammatory bowel disease and first named IL-10+ B cells as a regulatory B (Breg) cell (2). Many other studies further reported that Breg cells suppress symptoms in various immune diseases such as experimental autoimmune encephalomyelitis (EAE), contact hypersensitivity (CHS), and arthritis. The Breg cells’ inhibitory function was mostly IL-10 dependent (3). However, it is still largely unknown how the expression and secretion of IL-10 from Breg cells are regulated.
The histone acetylation/deacetylation is an important epigenetic mechanism that regulates specific gene expression (4). Histone acetylation and deacetylation require two types of enzymes to perform: histone acetyltransferase and histone deacetylase (HDAC), respectively. HDAC is usually classified as HDAC class 1, 2, and 4 (NAD+-dependent HDAC) and HDAC class 3 (Zn2+-dependent HDAC). The anomalisms of histone acetylation and deacetylation are closely associated with cancer incidence. Consequently, HDAC inhibitors have been developed as an anti-cancer drug (5, 6). Meanwhile, several reports revealed that HDAC inhibitors can control immune response by regulating the activity of various immune cells. For example, it was reported that ACY-1215 (rico-linostat), an HDAC6 inhibitor, inhibits the function of CD8+ T cells in CHS (7). In addition, trichostatin A, a class 1 and 2 HDAC inhibitor, inhibits allergic contact dermatitis by suppressing epidermal Langerhans cells (8). BML-281, an HDAC6 inhibitor, inhibits the infiltration of B cells into the inflammatory site of colitis in mice (9). Another study demonstrated that the HDAC1 and HDAC2 inhibitors of valproic acid and butyrate upregulate microRNAs involved in the expression of activation-induced cytidine deaminase and B lymphocyte-induced maturation protein-1 (10). However, the role of HDAC inhibitors on the induction of IL-10+ Breg cells remains yet to be known.
This study found that entinostat stimulated the differentiation of IL-10+ Breg cells from splenic B cells. Notably, entinostat displayed the effect of inhibiting the disease symptoms by increasing the IL-10+ Breg cell population in mice with CHS. As a mechanism, the study first demonstrated that entinostat inhibits the binding of HDAC1 in the proximal domain of the promoter region to express IL-10 in B cells, which leads to the increase of the NF-κB p65 binding and ends up stimulating the expression of IL-10 from B cells.
RESULTS
Entinostat stimulates the formation of IL-10+ regulatory B cells
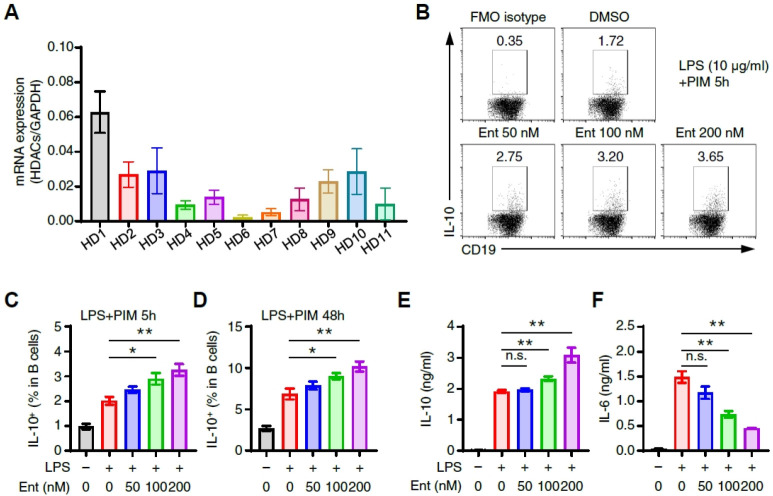
We first investigated the degree of various HDAC isoform expressions in B cells. The expression of HDAC1 was ranked highest, followed by HDAC 2, 3, 9, and 10 in the order of expression (Fig. 1A), with HDAC6 and HDAC7 ranked the lowest (Fig. 1A). Next, the population of IL-10+ Breg cells was increased by entinostat (Fig. 1C, D), suggesting that HDCA1 is potentially critical in regulating the IL-10 expression in B cells. A previous study reported that LPS and CD40 ligand stimulate the maturation and expansion of Breg progenitor cells to express IL-10 (11). We next investigated if entinostat influences the IL-10 expression in B cells by LPS, which, consequently, revealed that entinostat increased the LPS-induced IL-10 expression in B cells in a dose-dependent manner (Fig. 1B, C). We also found that the IL-10+ Breg cell population increased from 2% to 6% by LPS stimulation for 48 h (Fig. 1D). It revealed that the simultaneous treatment of LPS and entinostat increased the secretion of IL-10 from Breg cells (Fig. 1E). Every interestingly, the secretion of IL-6, a typical inflammatory cytokine, was suppressed by the treatment of entinostat (Fig. 1F). These results suggest that entinostat inhibits HDAC1 to stimulate the formation of IL-10+ Breg cells by LPS.
Fig. 1.
Entinostat induces IL-10+ B cells from splenic B cells in vitro. (A) The expressions of various isoforms of HDAC isoforms in splenic CD19+ B cells. (B, C) Flow cytometry analysis of IL-10+ splenic B cells. Splenic B cells (3 × 106) were incubated in 10 μg/ml LPS + PIM with or without entinostat (0-200 nM) for 5 h. Representative dot plot images (B) and frequencies (C) are shown. (D) The histograms show the frequencies of IL-10+ B cells (for LPS 43 h + last PIM 5 h) with or without entinostat (0-200 nM). (E, F) Quantitative analysis of secreted IL-10 and IL-6 in culture supernatant from LPS-stimulated B cells with or without entinostat (0-200 nM) for 48 h. Representative plot images (B) and graphs (A, C, D, E) are the mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01, n.s., not significant by Student’s t-test. Ent, entinostat.
Entinostat suppresses contact hypersensitivity in mice
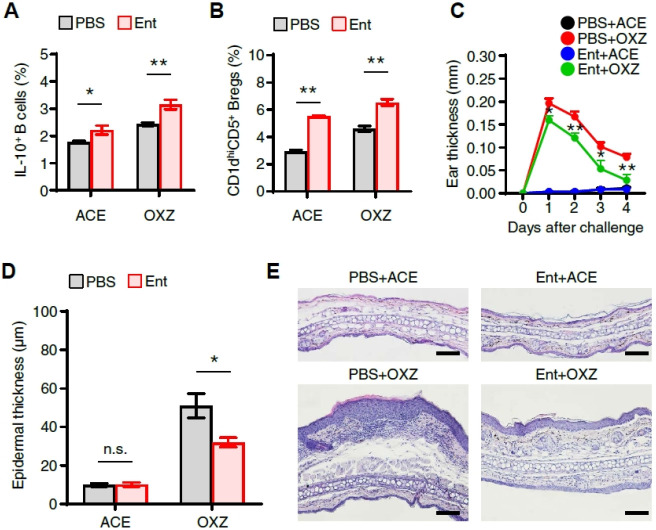
Next, an experiment was then conducted to find if entinostat induces IL-10+ Breg cells in vivo and has an inhibitory effect on CHS symptoms. Notably, entinostat increased CD1dhiCD5+ Breg precursor cells and IL-10+ Breg cells (Fig. 2A, B). The study further had an intriguing discovery that entinostat suppressed CHS (Fig. 2C) with the significant diminishment of the number of cells infiltrating into the ear tissue and the epidermal thickness (Fig. 2D, E) in mice. Collectively, our findings suggest that the inhibition of CHS by entinostat was closely associated with the increase of IL-10+ Breg cells.
Fig. 2.
Entinostat suppresses oxazolone-induced contact hypersensitivity and increases IL-10+ regulatory B cells in vivo. (A) Flow cytometry analysis of splenic IL-10+ B cells and (B) CD1dhiCD5+ B Breg subsets in CHS mice with or without entinostat treatment were performed. (C) The ear thickness of CHS mice was measured daily for 4 days after the challenge with oxazolone. The ear epidermal thickness (D) and representative images (E) of ear tissues are shown after being stained with H&E (scale bar, 100 μm). All values are presented as the mean ± SEM or representative images (E) from three independent experiments (n = 4 per group for each experiment). *P < 0.05, **P < 0.01, n.s., not significant by Student’s t-test. OXZ, oxazolone. Ent, entinostat. ACE, acetone.
Entinostat does not have any effect on the population of Foxp3+ regulatory T cells in mice with CHS
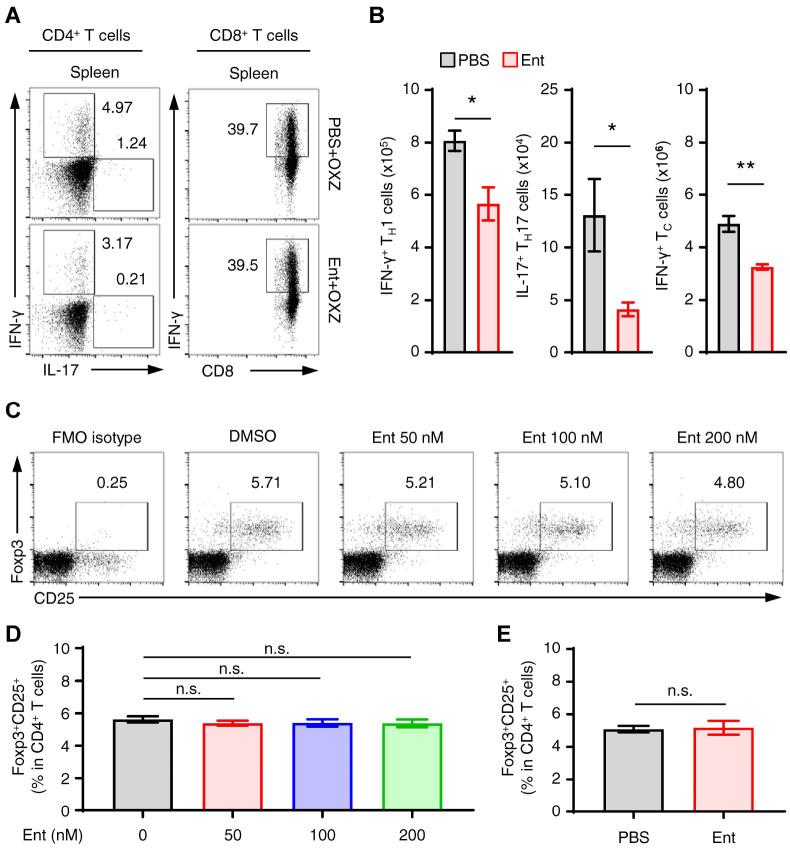
Th1, Th17, and IFN-γ+CD8+ T cells as effector cells and Foxp3+ Treg cells as regulatory cells are widely acknowledged to regulate CHS (12-14). Entinostat significantly suppressed the populations of Th1, Th17, and IFN-γ+CD8+ T cells in spleen during the induction of CHS (Fig. 3A, B). However, entinostat had no effect on the induction of the Foxp3+ Treg cells in vitro (Fig. 3C, D) and in vivo (Fig. 3E), which collectively implies that the inhibition of CHS symptoms by entinostat was most plausibly not associated with the Foxp3+ Treg cells.
Fig. 3.
The population changes of effector and regulatory T cells by entinostat in CHS mice. (A) Representative dot plot images show splenic IFN-γ+CD3+CD4+ (Th1), IL-17+CD3+CD4+ (Th17) T cells, or IFN-γ+CD3+CD8+ (cytotoxic T cells) T cells in mice with or without entinostat. (B) The histograms show the numbers of subsets in each T cell for panel A. (C, D) Splenocytes (3 × 106) were incubated in PIB with or without entinostat (0-200 nM) for 4 h. Representative dot plot images (C) and frequencies (D) of splenic Foxp3+ CD25+ (Treg) cells in CD4+ T cells are shown. (E) The frequencies of Foxp3+ CD25+ Treg cells in spleen from CHS mice with or without entinostat treatment were analyzed by flow cytometry. Representative images (A, C) or all values are shown as the mean ± SEM (B, D, E) from at least three independent experiments. *P < 0.05, **P < 0.01, n.s., not significant by Student’s t-test. Ent, entinostat. OXZ, oxazolone.
Entinostat inhibits the binding of HDAC1 to IL-10 promoter region
We then conducted a ChIP assay to identify an epigenetic mechanism that regulates IL-10 expression in Breg cells by entinostat. LPS suppressed the binding of HDAC1 in the proximal promoter site in B cells, while increasing the binding of NF-κB p65 (Fig. 4A, B), which suggests that HDAC1 regulates the function of NF-κB in IL-10 expression at the IL-10 promoter region. In the ensuing experiment, entinostat in LPS-stimulated B cells further enhanced the unbinding of HDAC1 and the subsequent binding of NF-κB p65 at the IL-10 proximal promoter region (Fig. 4C), suggesting that entinostat inhibits the binding of HDAC1 to the proximal promoter of the IL-10 promoter by LPS, thereby increasing the binding of NF-κB p65 to the proximal promoter.
Fig. 4.
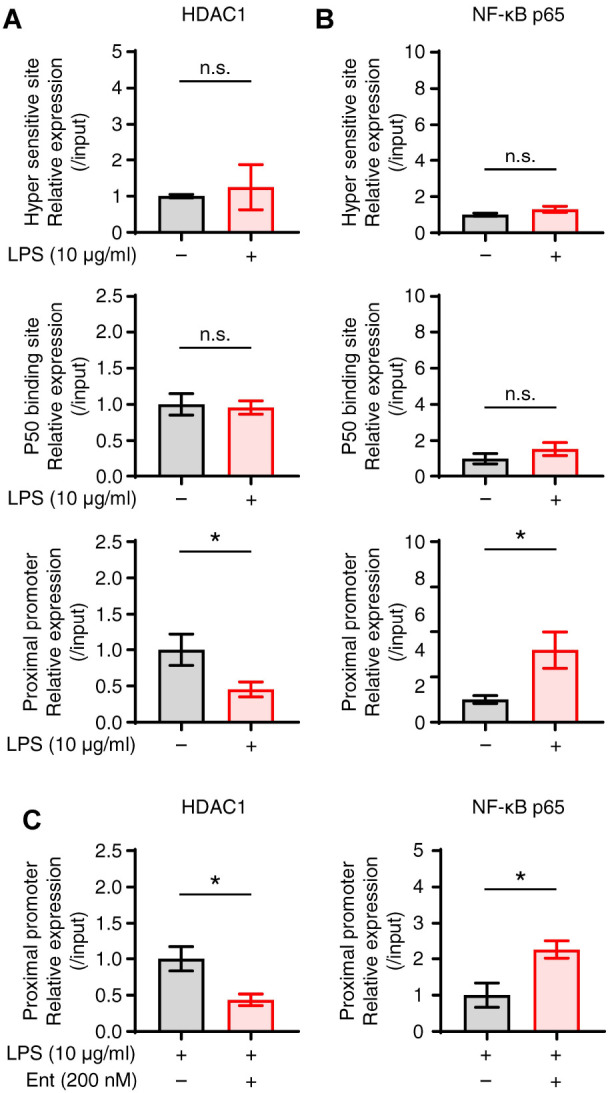
Entinostat enhances the binding of NF-κB at the proximal promoter region for IL-10 expression. Splenic B cells stimulated by LPS for 1 h. Chromatin immunoprecipitation assay was performed with anti-HDAC1 or anti-NF-κB p65 antibody, and respective proteins binding at the hyper sensitive site, p50 binding site, and proximal promoter regions for IL-10 expression were analyzed by real-time PCR. (A, B) Graphs show the relative binding amount of HDAC1 and NF-κB to three promoter regions. (C) Splenic B cells stimulated by LPS with or without 200 nM of entinostat for 1 h. Chromatin immunoprecipitation assay was performed with anti-HDAC1 or anti-NF-κB p65 antibody at the proximal promoter region as in panel A. The relative expression data are the mean ± SEM from at least three independent experiments. *P < 0.05. n.s., not significant by Student’s t-test. Ent, entinostat.
DISCUSSION
Breg progenitor cell initiates the development of IL-10+ Breg cells with the aid of CD40 ligand, LPS, IL-6, IL-10 or other stimulants (11, 15, 16). Still yet to be known is the epigenetic mechanism involved in the formation of IL-10+ Breg cells. In this study, we found that entinostat, an HDAC1 inhibitor, stimulates the formation of IL-10+ Breg cells by LPS (Fig. 1C, D). The study further observed that entinostat increased the IL-10+ Breg cells in mice with CHS (Fig. 2A). Collectively, our findings suggest that HDAC1 is associated with the generation of IL-10+ Breg cells from Breg precursor cells.
The CHS mouse is an animal model that resembles human allergic contact dermatitis (17, 18). Several immune cells, such as T cells, NK cells, neutrophils, and macrophages cause local inflammatory reactions in mice with CHS (19, 20). Meanwhile, IL-10+ Breg cells (3) and Treg cells (21, 22) suppress the immune response in mice with CHS. In this study, we found that entinostat suppressed the inflammatory symptoms in the oxazolone-induced CHS mice (Fig. 2C, D). Notably, entinostat did not alter the Treg cell population in mice with CHS (Fig. 3D, E), which suggests that entinostat does not have any effect on the Treg cell population. We further found that entinostat induces IL-10+ Breg cells in vitro and in vivo (Fig. 1C, D). Collectively, our findings suggest that the inhibitory effect of entinostat on the CHS symptoms is strongly associated with the induction of IL-10+ Breg cells, but not Treg cells.
IL-10 is a typical cytokine that suppresses effector immune cells in various inflammatory diseases. It was reported that the expression of IL-10 is associated with several transcription factors such as Sp1, CREB, c-Maf, and NF-κB (23). The binding of these transcription factors to DNA for IL-10 expression is largely regulated by balancing acetylation and deacetylation of DNA-binding histones (24). It was reported that the binding of NF-κB regulates IL-10 expression at the hyper sensitivity site of the IL-10 promoter, the proximal promoter site, and the p50 binding site in macrophages (25). Entinostat (MS-275) controls IL-10 expression by regulating the binding of NF-κB p65 to the IL-10 gene promoter in macrophages (26). LAQ824, another HDAC inhibitor, controls the expression of IL-10 through a mechanism that regulates the recruitment of PU.1 and HDAC11 to the IL-10 gene promoter (27). However, no study has been conducted to identify the relationship between the expressions of IL-10 in Breg cells and HDAC. This study attempted to clarify the role of HDAC1 in the production of IL-10+ Breg cells, using entinostat, an HDAC1 inhibitor, which demonstrated that LPS stimulates the transcription of IL-10 in B cells by regulating the binding of HDAC1 and NF-κB p65 in the proxi-mal promoter region of IL-10 (Fig. 4).
A recent report indicated that HDAC inhibitors have anti-cancer effects in various types of cancer, notably with suberoylanilide hydroxamic acid proven for anti-cancer effects on cutaneous T-cell lymphoma and approved for an anti-cancer drug by the United States Food and Drug Administration (FDA). Since then, the FDA has approved panobinostat for the treatment of multiple myeloma, and belinostat for the treatment of peripheral T cell lymphoma, with some HDAC inhibitors currently under clinical trials (28). Among them, entinostat is currently undergoing clinical trials with pembrolizumab administered simultaneously for the treatment of uveal melanoma and breast cancer (29, 30). It was reported that entinostat has an anti-cancer effect by promoting CD8+ T cell activation in mice with ovarian cancer (31). On the other hand, in experimental autoimmune neuritis and experimental autoimmune prostatitis using mice, it was reported that entinostat alleviates the disease by increasing the population of Foxp3+ T cells and M2 macrophages in the sciatic nerve (32, 33). Also, entinostat inhibited the CHS symptoms by increasing Foxp3+ T cells in DNFB-induced CHS mice (34). However, we observed that entinostat had no effect on the population of Foxp3+ Treg in the OXZ-induced CHS mice (Fig. 3E). The results suggest that entinostat could regulate differentially immune cells in a disease-specific manner, but the detail mechanism is left for future research.
In this study, we demonstrated for the first time that entinostat induces IL-10+ Breg cells by inhibiting HDAC1 and eventually increasing the binding of NF-κB p65 to the proximal promoter region of IL-10 in splenic B cells and suppress CHS in mice. Altogether, our results suggest that entinostat has a potential use for a therapeutic application to suppress CHS symptoms in human.
MATERIALS AND METHODS
Mice
5-6 week old male C57BL/6 mice were purchased from Orient Bio Inc. (Seongnam-si, Gyeonggi, Korea). Mice were maintained at the pathogen-free facility in Konkuk University (Seoul, Korea). All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Konkuk University (Approval No. KU18127).
Contact hypersensitivity (CHS) model
To induce CHS symptoms, mice were sensitized with 25 μl of a solution containing 100 mg/ml oxazolone (OXZ; Sigma-Aldrich, St. Louis, MO, USA) in acetone/olive oil (4:1, v/v) on shaved hind flank skin twice for 2 consecutive days. Four days after sensitization (on Day 5), the mice were challenged with 10 μl of 10 mg/ml OXZ in acetone/olive oil (4:1, v/v) to both ears (5 μl of the dorsal side and 5 μl on the ventral side). Mice were measured for ear thickness daily for 4 days after the OXZ challenge, using micrometer gauge. For entinostat treatment, the mice were injected intraperitoneally with vehicle (PBS) or entinostat (2.5 mg/kg) twice on day 0 and day 5 (1 hr before the challenge). On Day 7 after the challenge with OXZ, mice were sacrificed to isolate cells from spleen for a flow cytometric analysis.
Flow cytometry analysis
Single-cell suspensions isolated from the spleen were preincubated with anti-CD16/32 (93) to block the surface Fc receptors, followed by surface staining with antibodies specific to CD19 (eBio1D3), CD1d (1B1), CD5 (53-7.3), CD3 (17A2), CD4 (RM4-5), CD25 (PC61.5), and CD8 (53-6.7). To stain intracellular proteins, cells were fixed and permeabilized by using the Foxp3/Transcription factor staining buffer set (eBioscience, San Diego, CA, USA), and antibodies against IL-10 (JES5-16E3), Foxp3 (FJK-16s), INF-γ (XMG1.2), and IL-17 (eBio17B7) were used. To analyze IL-10+ B cells and CD1dhiCD5+ B cells, cells were stimulated by the mixture solution of lipopolysaccharide (LPS; 10 μg/ml; sigma-Aldrich), phorbol 12-myristate 13-acetate (PMA; 50 ng/ml; Sigma-Aldrich), ionomycin (500 ng/ml; Sigma-Aldrich), and monensin (2 μM; eBioscience). To detect Treg, Th1, and Th17 cells, the splenocytes were stimulated by the mixture solution of phorbol 12-myristate 13-acetate (PMA; 50 ng/ml; Sigma-Aldrich), ionomycin (500 ng/ml; Sigma-Aldrich), and brefeldin A (3 μg/ml; eBioscience).
Measurement of IL-10 by ELISA
Splenic CD19+ B cells were pre-sorted by the CD19 mAb-micro-beads (Miltenyi Biotec, Bergisch Gladbach, Germany). The sorted B cells (3 × 106) were stimulated with or without LPS (10 μg/ml) and with or without entinostat for 48 h. The supernatants were harvested and measured by using the IL-10 Mouse Uncoated ELISA Kit (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions.
Histological analysis
After the induction of CHS in mice, their ear tissues were fixed in 4% paraformaldehyde in phosphate-buffered saline for 24 h and then embedded in paraffin. Serial paraffin sections (5 μm) of the ear were stained for hematoxylin and eosin (H&E).
RNA extraction and real-time PCR
Total RNA was extracted from splenic B cells using the easy-spin Total RNA Extraction Kit (iNtRON Biotechnology, Gyeonggi, Korea) and transcribed with the Tetro cDNA Synthesis Kit (Bioline, London, UK) in accordance with the manufacturer’s instructions. Real-time PCR was performed on LightCyclerⓇ 480II (Roche, Basel, Swiss) using LightCyclerⓇ 480 SYBR Green I Master (Roche). Primers were used as follows: mouse HDAC1 (forward 5’-TGGTCTCTACCGAAAAATGGAG-3’, reverse 5’-TCATCACTGTGGTACTTGGTCA-3’); mouse HDAC2 (forward 5’-AAAGGAGCAAAGAAGGCTAGG-3’, reverse 5’-GTCCT TG GATTTGTCTTCTTCC-3’); mouse HDAC4 (forward 5’-CACAC CTCTTGGAGGGT ACAA-3’, reverse 5’-AGCCCATCAGCTGT TTTGTC-3’); mouse HDAC5 (forward 5’-GAGTCCAGTGCTG GTTACAAAA-3’, reverse 5’-TACACCTGGAGGGGCTGTAA-3’); mouse HDAC6 (forward 5’-CGCTGTGTGTCCTTTCAGG-3’, reverse 5’-CAGATCAAT GTATTCCAGGCTGT-3’); mouse HDAC7 (forward 5’-CCATGGGGGATCCTGAGT-3’, reverse 5’-GCAA ACTCTCGGGCAATG-3’); mouse HDAC8 (forward 5’-GGTGA TGAGGACCATCCAGA-3’, reverse 5’-TCCTATAGCTGCTGCA TAGTCAA-3’); mouse HDAC9 (forward 5’-TTGCACACAGAT GGAGTGG-3’, reverse 5’-GGCCCATAGGAACCTCT GAT-3’); mouse HDAC10 (forward 5’-TTCCAGGATGAGGATCTTGC-3’, reverse 5’-ACATCCAATGTTGCTGCTGT-3’); mouse HDA11 (forward 5’-GACGTGCTGGAGGG AGAC-3’, reverse 5’-AAAA CCACTTCATCCCTCTTCA-3’).
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed in accordance with Upstate Biotechnology’s instructions. Splenic B cells (2 × 107 cells) were collected and fixed with 37% formaldehyde for 10 min, followed by shearing cells with sonication, pre-clearing with protein A magnetic beads, and finally precipitating of DNA (50 μg) with antibodies against HDAC1 (Cell Signaling Technology, Danvers, MA, USA) and NF-κB p65 (Cell Signaling Technology). After immunoprecipitation, chromatin fragments were subjected to real-time PCR. The IgG control experiment was performed for all ChIP assays and incorporated into the IP/Input (1%) by presenting the results as (IP-IgG)/(Input-IgG). PCR primers were used as follows: 5’-CTGAGGCCTGTCTGTAAGCTTTGA-3’ and 5’-CGGAAGGGCTGATCGCT-3’ for IL-10 hyper sensitivity site; 5’-TAGAAGAGGGAGGAGGAGCC-3’ and 5’-TGTGGCTTTG GTAGTGCAAG-3’ for IL-10 p50 binding site; 5’-ACGAAGA AGCTCAGATCCCAGC-3’ and 5’-GTTGCTTGCCCAGGGTACA GAA-3’ for IL-10 proximal promoter.
Statistical analysis
The data are presented as the mean ± standard error (SEM) from at least three independent experiments. All animal experiments were performed with five or more mice per group. Statistical analysis was performed using unpaired two-tailed Student’s t-test or the Mann-Whitney test. One-way analysis of variance (ANOVA) with Tukey’s post hoc test was performed to compare multiple experimental groups. Statistical significance (*P < 0.05 and **P < 0.01) was performed using the software Prism version 7.0 (GraphPad, San Diego, CA).
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2021R1A2B5B03002157, NRF-2020R1C1C1003676, and NRF-2016R1A5A2012284).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
References
- 1.Neta R, Salvi SB. Specific suppression of delayed hypersensitivity : the possible presence of a suppressor B cell in the regulation of delayed hypersensitivity. J Immunol. 1974;113:1716–1725. [PubMed] [Google Scholar]
- 2.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d up-regulation. Immunity. 2002;16:219–230. doi: 10.1016/S1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 3.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Lee Junghee, Ryu Hoon. Epigenetic modification is linked to Alzheimer's disease: is it a maker or a marker? BMB Rep. 2010;43:649–655. doi: 10.5483/BMBRep.2010.43.10.649. [DOI] [PubMed] [Google Scholar]
- 5.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 6.Hull EE, Montgomery MR, Leyva KJ. HDAC inhibitors as epigenetic regulators of the immune system: impacts on cancer therapy and inflammatory diseases. Biomed Res Int. 2016;2016:8797206. doi: 10.1155/2016/8797206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuji G, Okiyama N, Villarroel VA, Katz SI. Histone deacetylase 6 inhibition impairs effector CD8 T-cell functions during skin inflammation. J Allergy Clin Immunol. 2015;135:1228–1239. doi: 10.1016/j.jaci.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi YL, Gu J, Park JJ, et al. Histone deacetylases inhibitor Trichostatin A ameliorates DNFB-induced allergic contact dermatitis and reduces epidermal Langerhans cells in mice. J Dermatol Sci. 2012;68:99–107. doi: 10.1016/j.jdermsci.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do A, Reid RC, Lohman RJ, Sweet MJ, Fairlie DP, Iyer A. An HDAC6 inhibitor confers protection and selectively inhibits B-cell infiltration in DSS-induced colitis in mice. J Pharmacol Exp Ther. 2017;360:140–151. doi: 10.1124/jpet.116.236711. [DOI] [PubMed] [Google Scholar]
- 10.White CA, Pone EJ, Lam T, et al. Histone deacetylase inhibitors upregulate B cell microRNAs that silence AID and Blimp-1 expression for epigenetic modulation of anti-body and autoantibody responses. J Immunol. 2014;193:5933–5950. doi: 10.4049/jimmunol.1401702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, Fujisawa H, Zhuang L, et al. CD4+ Th1 and CD8+ type 1 cytotoxic T cells both play a crucial role in the full development of contact hypersensitivity. J Immunol. 2000;165:6783–6790. doi: 10.4049/jimmunol.165.12.6783. [DOI] [PubMed] [Google Scholar]
- 13.Peiser M. Role of Th17 cells in skin inflammation of allergic contact dermatitis. Clin Dev Immunol. 2013;2013:261037. doi: 10.1155/2013/261037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ring S, Oliver SJ, Cronstein BN, Enk AH, Mahnke K. CD4+CD25+ regulatory T cells suppress contact hypersensitivity reactions through a CD39, adenosine-dependent mechanism. J Allergy Clin Immunol. 2009;123:1287–1296.e2. doi: 10.1016/j.jaci.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Rosser EC, Oleinika K, Tonon S, et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat Med. 2014;20:1334–1339. doi: 10.1038/nm.3680. [DOI] [PubMed] [Google Scholar]
- 16.Kim HS, Lee JH, Han HD, et al. Autocrine stimulation of IL-10 is critical to the enrichment of IL-10-producing CD40hiCD5+ regulatory B cells in vitro and in vivo. BMB Rep. 2015;48:54–59. doi: 10.5483/BMBRep.2015.48.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan CH, Rasool S, Johnston GA. Contact dermatitis: allergic and irritant. Clin Dermatol. 2014;32:116–124. doi: 10.1016/j.clindermatol.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 18.Peiser M, Tralau T, Heidler J, et al. Allergic contact dermatitis: epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Cell Mol Life Sci. 2012;69:763–781. doi: 10.1007/s00018-011-0846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kripke ML, Munn CG, Jeevan A, Tang JM, Bucana C. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J Immunol. 1990;145:2833–2838. [PubMed] [Google Scholar]
- 20.Honda T, Egawa G, Grabbe S, Kabashima K. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol. 2013;133:303–315. doi: 10.1038/jid.2012.284. [DOI] [PubMed] [Google Scholar]
- 21.Ring S, Schäfer SC, Mahnke K, Lehr HA, Enk AH. CD4+CD25+ regulatory T cells suppress contact hypersensitivity reactions by blocking influx of effector T cells into inflamed tissue. Eur J Immunol. 2006;36:2981–2992. doi: 10.1002/eji.200636207. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz A, Maeda A, Wild MK, et al. Ultraviolet radiation-induced regulatory T cells not only inhibit the induction bur can suppress the effector phase of contact hypersensitivity. J Immunol. 2004;172:1036–1043. doi: 10.4049/jimmunol.172.2.1036. [DOI] [PubMed] [Google Scholar]
- 23.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 24.Mosser MD, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rec. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saraiva M, Christensen JR, Tsytsykova AV, et al. Identification of a macrophage-specific chromatin signature in the IL-10 locus. J Immunol. 2005;175:1041–1046. doi: 10.4049/jimmunol.175.2.1041. [DOI] [PubMed] [Google Scholar]
- 26.Leus NG, van den Bosch T, van der Wouden PE, et al. HDAC1-3 inhibitor MS-275 enhances IL10 expression in RAW264.7 macrophages and reduces cigarette smoke-induced airway inflammation in mice. Sci Rep. 2017;7:45047. doi: 10.1038/srep45047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Cheng F, Woan K, et al. Histone deacetylase inhibitor LAQ824 augments inflammatory responses in macrophages through transcriptional regulation of IL-10. J Immunol. 2011;186:3986–3996. doi: 10.4049/jimmunol.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suraweera A, O'Byrne KJ, Richard DJ. Combination therapy with histone deacetylase inhibitors (HDACi) for the treatment of cancer: achieving the full therapeutic potential of HDACi. Front Oncol. 2018;8:92. doi: 10.3389/fonc.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jespersen H, Olofsson Bagge R, Ullenhag G, et al. Concomitant use of pembrolizumab and entinostat in adult patients with metastatic uveal melanoma (PEMDAC study): protocol for a multicenter phase II open label study. BMC Cancer. 2019;19:415. doi: 10.1186/s12885-019-5623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeruva SLH, Zhao F, Miller KD, et al. E2112: randomized phase iii trial of endocrine therapy plus entinostat/placebo in patients with hormone receptor-positive advanced breast cancer. NPJ Breast Cancer. 2018;4:1. doi: 10.1038/s41523-017-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCaw TR, Goel N, Brooke DJ, et al. Class I histone deacetylase inhibition promotes CD8 T cell activation in ovarian cancer. Cancer Med. 2020;10:709–171. doi: 10.1002/cam4.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang ZY, Zhang Z, Schluesener HJ. MS-275, an histone deacetylase inhibitor, reduces the inflammatory reaction in rat experimental autoimmune neuritis. Neuroscience. 2010;169:370–377. doi: 10.1016/j.neuroscience.2010.04.074. [DOI] [PubMed] [Google Scholar]
- 33.Zhang ZY, Schluesener HJ. HDAC inhibitor MS-275 attenuates the inflammatory reaction in rat experimental autoimmune prostatitis. Prostate. 2012;72:90–99. doi: 10.1002/pros.21410. [DOI] [PubMed] [Google Scholar]
- 34.Lucas JL, Mirshahpanah P, Stapleton EH, Asadullah K, Zollner MT, Numerof RP. Induction of Foxp3+ regulatory T cells with histone deacetylase inhibitors. Cell Immunol. 2009;257:97–104. doi: 10.1016/j.cellimm.2009.03.004. [DOI] [PubMed] [Google Scholar]