Abstract
Background:
Posttraumatic stress disorder (PTSD) is associated with hyperarousal and stress reactivity, features consistent with behavioral sensitization. In this Phase 1b, parallel-arm, randomized, double-blind, placebo-controlled trial, we tested whether the selective low-trapping N-methyl-D-aspartate receptor (NMDAR) antagonist [Lanicemine (BHV-5500)] blocks expression of behavioral sensitization.
Methods:
Twenty-four participants with elevated anxiety potentiated startle (APS) and moderate-to-severe PTSD symptoms received 3 infusions of lanicemine 1.0 mg/ml (100 mg) or matching placebo (0.9% saline) (1:1 ratio), over a 5-day period. The primary outcome was change in APS from baseline to end of third infusion. We also examined changes in EEG gamma-band oscillatory activity as measures of NMDAR target engagement and explored Clinician-Administered PTSD Scale (CAPS-5) hyperarousal scores.
Results:
Lanicemine was safe and well-tolerated with no serious adverse events. Using Bayesian statistical inference, the posterior probability that lanicemine outperformed placebo on APS T-score after three infusions was 38%. However, after the first infusion, there was a 90% chance that lanicemine outperformed placebo in attenuating APS T-score by a standardized effect size > 0.4.
Conclusion:
We demonstrated successful occupancy of lanicemine on NMDAR using gamma-band EEG and effects on hyperarousal symptoms (Cohen’s d=0.75). While lanicemine strongly attenuated APS following a single infusion, differential changes from placebo after three infusions was likely obscured by habituation effects. To our knowledge, this is the first use of APS in the context of an experimental medicine trial of a NMDAR antagonist in PTSD. These findings support selective NMDAR antagonism as a viable pharmacological strategy for salient aspects of PTSD.
INTRODUCTION
Exposure to traumatic stressors in vulnerable individuals can lead to chronic, debilitating, and life-threatening conditions, including suicidality, functional impairment, and increased susceptibility to posttraumatic stress disorder (PTSD) 1. PTSD is characterized by the emergence of several symptom clusters following a traumatic event, including intrusive memories and re-experiencing of trauma; avoidance of trauma reminders; negative cognitions and mood; and hyperarousal (i.e., anger outbursts, hypervigilance, exaggerated startle).2 Despite an estimated 8.7% prevalence of PTSD in the U.S,2 only two drugs have received Food and Drug Administration approval (the selective serotonin reuptake inhibitors paroxetine and sertraline). These drugs have small effect sizes and low remission rates.3–5. Thus, there is a need to develop new candidate drugs that demonstrate efficacy in the treatment of PTSD. The experimental therapeutics approach to early phase clinical trials advocated by NIMH focuses on rigorous testing of target engagement and functional activity prior to engaging in larger studies with traditional clinical endpoints. 6,7
Behavioral sensitization refers to a process whereby trauma-associated stressors sensitize behavioral, motivational, and stress systems, increasing reactivity to subsequent stressors even after prolonged delay. Individuals with clinically significant PTSD symptoms and dysregulation in the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) domains of Arousal and Negative Valence Systems may have behavioral sensitization to repeated stressors, rendering them susceptible to recurrent depressive episodes and addictions.8 Preclinical studies have found that N-methyl-D-aspartate receptor (NMDAR) antagonists block mechanisms underlying expression of behavioral sensitization,9 while clinical studies with the NMDAR antagonist ketamine have reported rapid benefit for varied conditions associated with elevated arousal and anxious reactivity, including PTSD,10–12 depression,13,14 and suicidal ideation.15 While specific mechanisms underlying the therapeutic effects in PTSD are unclear, evidence from ketamine studies suggest that a transient surge in post-synaptic glutamate activation can lead to persistent upregulation of neurotrophic factors and increased synapse formation, reversing neuronal effects of stress.10,16–18
Here, we investigated the selective NMDAR antagonist lanicemine (BHV-5500, formerly AZD6765) in individuals with PTSD symptoms and human laboratory evidence of hyperarousal. Lanicemine infusion was extensively studied in preclinical and early-phase clinical studies in patients with stroke, sleep apnea, and more recently, in treatment-resistant depression (TRD).19–21 A pharmacological fMRI study found that lanicemine impacts neural circuits integral to stress- and fear-related psychiatric disorders.22 While the largest trial to date in TRD did not significantly reduce depressive symptoms on the primary endpoint, in a post-hoc analyses, the 100 mg dose demonstrated efficacy in patients with greater depression severity and suicidal ideation.21 In contrast to other NMDAR channel blockers such as ketamine and MK801, lanicemine is rapidly reversible (fast off-rate) and “low-trapping”, properties associated with a favorable safety and tolerability profile.20
The primary hypothesis of this Phase 1b, parallel-arm, randomized, double-blind, placebo-controlled study was that three infusions of lanicemine (100 mg delivered over 60 min) would block the expression of behavioral sensitization, operationalized as a pronounced startle response to an unpredictable aversive stimulus, in individuals with PTSD symptoms and exaggerated psychophysiological responses to a laboratory probe of anxiety. Anxiety-potentiated startle (APS) was measured using the NPU (Neutral, Predictable, and Unpredictable) threat task, a validated probe of behavioral sensitization to contextual threat previously used in the context of experimental interventional studies.23–27 To measure APS, the change in magnitude of the eyeblink startle reflex measured during the unpredictable threat condition was compared to a safe no-threat condition. A previous report by Grillon et al.26 found that an elevated APS amplitude derived from the NPU-threat test reliably discriminated individuals with PTSD from healthy controls. Furthermore, pharmacological dissociation of responses to cued fear and contextual anxiety was observed using the NPU-test in a repeated measures study in healthy controls.28 We thus hypothesized that relative to placebo, lanicemine would show normalization of the APS response following three treatments.
As secondary outcomes, we measured resting-state spontaneous gamma band EEG (resting-gamma), building on dose-related NMDAR target engagement by lanicemine in healthy volunteers.20 We also evaluated 40-Hz auditory steady state response (ASSR), a robust measure of the integrity of inhibitory interneuron synchronization implicating cortical NMDA receptor function.29 We hypothesized that gamma band power (resting and ASSR) would increase with lanicemine compared to placebo. Finally, we used the Clinician-Administered PTSD Scale (CAPS-5), to explore the impact of lanicemine on the hyperarousal symptom cluster as well as overall PTSD symptoms.
MATERIALS AND METHODS
Trial Oversight
This Phase 1b, parallel-arm, randomized, double-blind, placebo-controlled single center study was conducted between September 2018 and November 2019. Participants provided written informed consent prior to study entry. The trial protocol was approved by the Baylor College of Medicine (BCM) Institutional Review Board with additional local oversight by the Michael E. Debakey VA Medical Center (MEDVAMC) Research and Development Committee. A Data Safety and Monitoring Board organized by the NIMH provided trial oversight, and NIMH personnel conducted on-site monitoring. Biohaven Pharmaceuticals provided the investigational drug (BHV-5500) and supporting data for an investigator-initiated IND from the FDA (IND#134304). A description of the trial protocol has been previously reported.30 The trial was registered at clinicaltrials.gov, NCT03166501.
Participants
Patients were recruited by advertisement or clinician referral from the greater Houston region. Male and female outpatients (21 to 65 years of age) were eligible for inclusion if they had experienced an index traumatic event, endorsed significant PTSD symptoms (as defined below), and had an APS T-score greater than or equal to 2.8 at screening and at baseline on the day of first infusion. The a priori minimum required APS T-score ensured that patients enrolled would have at least two standard deviations above the mean APS T-score of healthy controls.26 No dose adjustments or modifications to existing medications or study drug were permitted. Complete inclusion and exclusion criteria are in Supplementary Materials - Section I.
Randomization, Masking, and Treatment Sessions
Following APS-T score assessment (see Primary Outcome Measure below) and CAPS-5 (last week version), eligible patients who fasted overnight received either lanicemine or placebo at approximately 11:00 a.m. three times (Mon, Wed, Fri) over a 5-day period. The randomization list used a computer-generated random number list with a permuted block procedure. Only the research pharmacist and study statistician had access to the randomization code; clinicians, raters, and data analysts were masked to treatment group.
Twenty-four patients were randomized (1:1 ratio) to either a lanicemine solution 1.0 mg/ml (100 mg) or matching placebo (0.9% saline) by intravenous (IV) infusion (Supplementary Materials - Section II, Figure S1: CONSORT flowchart). The infusion was administered via an infusion pump in a volume of 100 mL at 1.67 mL/min (1.667 mg/min) over 60 min. After the first and third infusion, patients remained on site for at least 4.5 hours after the end of the infusion for APS and EEG. At each visit, a study psychiatrist assessed suicidal ideation and behavior using the Columbia Suicide Severity Rating Scale (C-SSRS),31 adverse events (AEs), and changes in concomitant medications. On Day 8, the CAPS-5 (last week version) was administered by telephone by the same rater who conducted the baseline CAPS-5. Laboratory measurements and clinical assessments were repeated 14 days following the last dosing day. (See Figure 1: Study Schematic).
Figure 1:
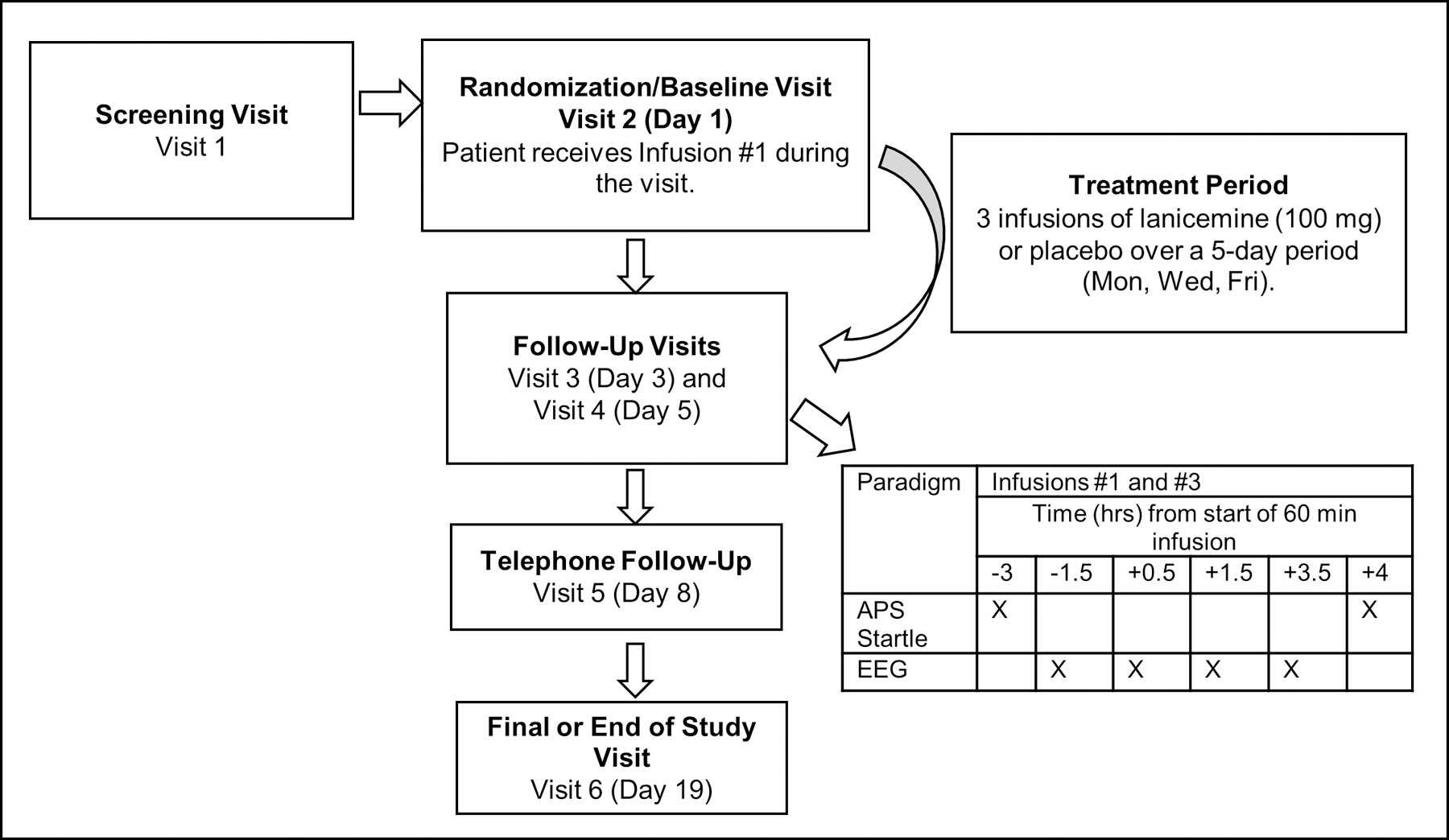
Study Schematic
Primary Outcome Measure
The NPU threat test follows descriptions by Schmitz and Grillon,23 and consists of neutral (N), predictable (P), and unpredictable (U) threat conditions (See Supplementary Materials-Section III). Trials within these blocks are marked by the presentation of a colored shape. During the neutral blocks (N), subjects are not at risk. During the predictable blocks (P), subjects are at risk of receiving presentations of an aversive stimulus, but only when cued by the shape. During the unpredictable blocks (U), subjects are at risk of receiving presentations of the aversive stimulus throughout. Throughout the task, text at the top of the screen informed subjects of the current threat contingencies. Periodic presentation of mild air puffs to the forehead were administered during the cues and intertrial intervals (ITIs) throughout the task to elicit an eyeblink startle reflex, which was recorded using electromyography (EMG) signals from just below the left eye. Methods of processing EMG signals and computing APS-T score are described in Supplementary Materials - Section III.
Secondary Outcome Measures
EEG was recorded at 4 time points (−1.5 hrs (pre-infusion), +0.5 hrs, +1.5hrs and +3.5 hrs) on the first and third infusion days using Curry 7.0.10 software in combination with a SynAmps-RT 64-channel amplifier (Compumedics Neuroscan, Charlotte, NC, USA) and a 64-channel actiCAP (Brain Vision, Morrisville, NC, USA). Data was sampled at 1000 Hz and filtered online between 0.1 Hz and 400 Hz. Resting state EEG was recorded as two 1-minute blocks with the patient’s eyes closed and open, respectively. Gamma power (resting-gamma) was extracted using the Welch’s power spectral density method (PSD) 32. Resting-gamma was computed on the relative PSD after combining eyes closed and open blocks. The 40Hz ASSR consisted of 100 1-second trains of 1ms duration, 80dB 1kHz carrier frequency clicks. Interstimulus interval was 500ms. ASSR-gamma was computed in the steady state period of 300 ms to 900 ms post stimulus onset and between 38Hz and 42Hz. Full details of preprocessing and feature extraction procedures are described in Supplementary Materials - Section IV.
Exploratory Outcomes
Clinical measures are considered exploratory because of the small sample size, brevity of the trial (3 infusions over 5 days) and the primary focus on target engagement. The clinical endpoint was PTSD symptom severity, using the past week CAPS-5 score, at Day 8 (3 days after infusion #3). Three days after infusion #3 was selected as an endpoint based on Sanacora et al.20 who found that the trend for antidepressant effect of lanicemine peaked at 72 hours. We also examined individual PTSD symptom clusters, primarily focusing on changes in Criterion E (alterations in arousal and reactivity). In addition, we administered the Posttraumatic Stress Disorder Checklist (PCL-5) at baseline, Day 8 and Day 19, 14 days following the last dosing day.
Statistical Analysis
We performed Bayesian analyses using generalized linear modeling to provide an estimate of treatment effect using the formulation of the posterior probability, the probability that an effect exists. For the primary endpoint of change in APS from baseline to end of third infusion, K = 500 Monte Carlo simulations indicated a 96% chance of identifying benefit (i.e. a posterior probability of > 0.80 of a Cohen’s d < 0) for the 100 mg dose of lanicemine. Cohen’s d was calculated as d = (M1 -M2)/ S.D. where M is the mean of each condition and S.D is the pooled standard deviation. If we found a large probability of an effect, to provide sufficient evidence to inform the go-no go decision for a larger proof-of-concept clinical trial in the same population, we aimed to use a higher threshold (i.e., a posterior probability of > 0.75 of a Cohen’s d < −0.4). EEG and clinical outcomes did not use standardized effect sizes. The sample size justification is reported in Supplementary Materials - Section V.
The sample appeared comparable across baseline covariates except for baseline APS T-Scores, CAPS-5 Total and CAPS-5 Criterion E. Evaluation of these measures at later time-points as a function of treatment included the baseline index as a covariate such that treatment predicted the residual change score. Bayesian parameter estimates are taken from the posterior distribution that captures the uncertainty surrounding the magnitude of an effect.33 Models used weakly informative priors (b = ~Normal [µ = 0, σ2 = 1e3], sd and sigma = ~Student-t [µ = 0, σ2 = 1e3] to maximize the influence of the present data on posterior probabilities. Gaussian-distributed, dichotomous, and count outcomes were modeled using the Student-t distribution, the binomial distribution, and the negative binomial distribution. Analyses were performed in the R statistical computing environment34 using the packages, brms v. 2.6–035 and rstan v. 2.18.1.36
RESULTS
Subject Demographics
Table 1 summarizes the demographic and clinical characteristics of patients randomly assigned to receive lanicemine or placebo. The groups were generally comparable; the posterior probability that there was a non-zero difference between baseline CAPS-5 total severity scores was 0.97.
Table 1. Subject Demographics:
Demographic and clinical characteristics of patients randomly assigned to receive lanicemine (100mg) or placebo. CAPS-5, Clinician-Administered PTSD Scale, PCL-5, Posttraumatic Stress Disorder Checklist; MDD, Major Depressive disorder; SD, standard deviation
| Characteristic | Lanicemine (n=12) |
Placebo (n=12) |
|---|---|---|
| Age in Years, mean (SD) | 41.0 (10.8) | 40.7 (7.0) |
| Female, n (%) | 6 (50.0) | 8 (66.7) |
| Race, Caucasian, n (%) | 7 (58.3) | 6 (50.0) |
| Veteran Status, n (%) | 4 (33.0) | 7 (58.3) |
| Criterion A Trauma, n (%) | ||
| Combat-related | 3 (25.0) | 3 (25.0) |
| Adult physical assault | 2 (16.7) | 2 (16.7) |
| Adult sexual assault | 3 (25.0) | 3 (25.0) |
| Childhood sexual assault | 2 (16.7) | 3 (25.0) |
| Other | 2 (16.7) | 1 (8.3) |
| Mean Time since Trauma, yr. (SD) | 14.5 (9.7) | 17.8 (12.2) |
| Co-morbid MDD, n (%) | 9 (75.0) | 11 (91.7) |
| Psychotropic Medications, n (%) | 4 (33.3) | 5 (41.7) |
| Baseline CAPS-5 (past week), mean (SD) | 44.8 (10.1) | 52.4 (8.2) |
| Baseline CAPS-5: Sub-Scale E – Arousal and Reactivity Symptom Severity, mean (SD) | 11.4 (2.4) | 14.7 (3.0) |
| Baseline PCL-5, mean (SD) | 53.8 (14.1) | 59.4 (9.9) |
Safety and Tolerability
Supplementary Materials – Section VI, Table ST1 summarizes the adverse events. No severe or unexpected adverse events occurred. No patients discontinued infusions prematurely due to intolerability. One patient in the placebo group reported mild dissociation. Lanicemine was associated with modest and transient increases in systolic and diastolic blood pressure (See Supplementary Materials - Section VI, Table ST2). No patient experienced an increase in suicidal ideation or behavior, as documented by the C-SSRS.
Primary Outcome: APS
Posterior probabilities are presented for each outcome, and for each simple effect, magnitude and direction is described by a point estimate and 95% credible interval. Figure 2 shows the results from infusion #1 and #3. Controlling for pre-infusion #1 baseline levels, the posterior probability that the effect for APS T-score was < 0 is 0.38 (b=0.70, 95% Crl = [−4.33–5.44], Figure 2B) after Infusion #3; lanicemine compared to placebo had a 38% chance of decreasing APS after Infusion #3, our primary endpoint. Following infusion #1, there was a 90% chance that lanicemine outperformed placebo in attenuating APS T-score by a standardized effect size < −0.4 (b=−0.98; 95% Crl = [−1.86 −0.08], Figure 2D). Therefore, APS attenuation by lanicemine at infusion #1 was no longer evident at infusion #3.
Figure 2:
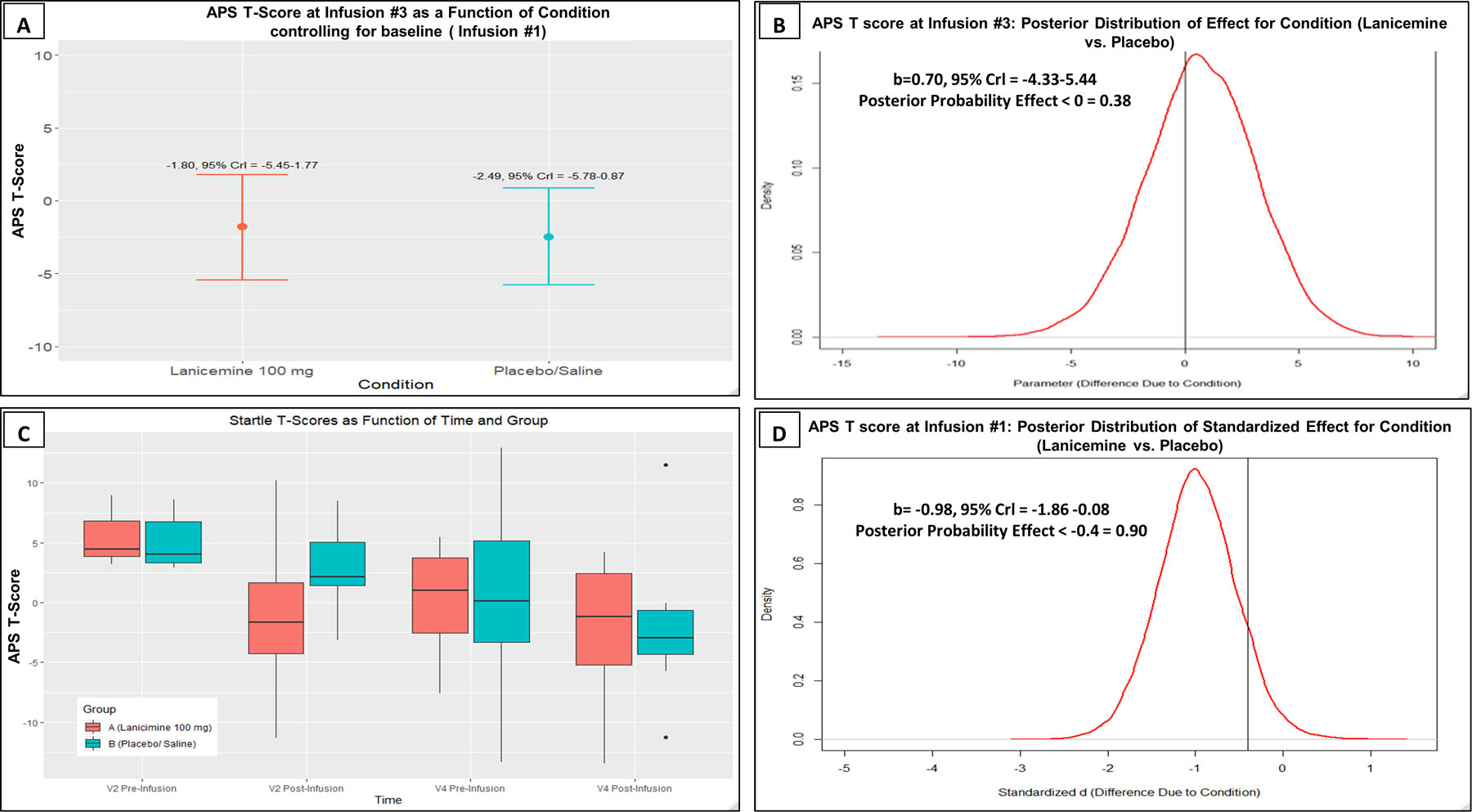
A) APS T-score at Infusion #3 as a function of condition controlling for baseline (Infusion #1). Red indicates Lanicemine group and Blue indicates Placebo group. B) Bayesian results for APS T-score at Infusion #3 (Lanicemine vs Placebo), including the median and Credible Interval (Crl) of the posterior distribution as well as the posterior probability that an effect of treatment exists. C) APS T score as a function of Time and Group. D) Bayesian results for APS T-score (Lanicemine vs Placebo) at Infusion #1.
EEG results
The resting-gamma and ASSR-gamma results are shown in Figures 3 and 4, respectively. Analyses used pre-infusion values at infusion #1 as baseline control for all measures. There was an 80% chance that lanicemine increased resting-gamma at the 1.5-hour assessment (30 min following the infusion) after infusion #3 (b=1.19; 95% Crl =[0.78–1.83], Figure 3D). The most robust effects of lanicemine on higher resting-gamma compared to placebo (96% posterior probability) were found at the 0.5-hour assessment during infusion #1 (b=0.79; 95% Crl =[0.40–1.19], Figure 3C), similar to the case with APS. ASSR-gamma results had a lower probability of a drug effect, with a 78% chance of higher ASSR-gamma in lanicemine than placebo at the 1.5-hour assessment at infusion #1 (b=0.90; 95% Crl = [−1.55–3.40], Figure 4C). See Supplementary Materials - Section X for additional exploratory EEG analyses, including phase locking factor (PLF) analyses.
Figure 3:
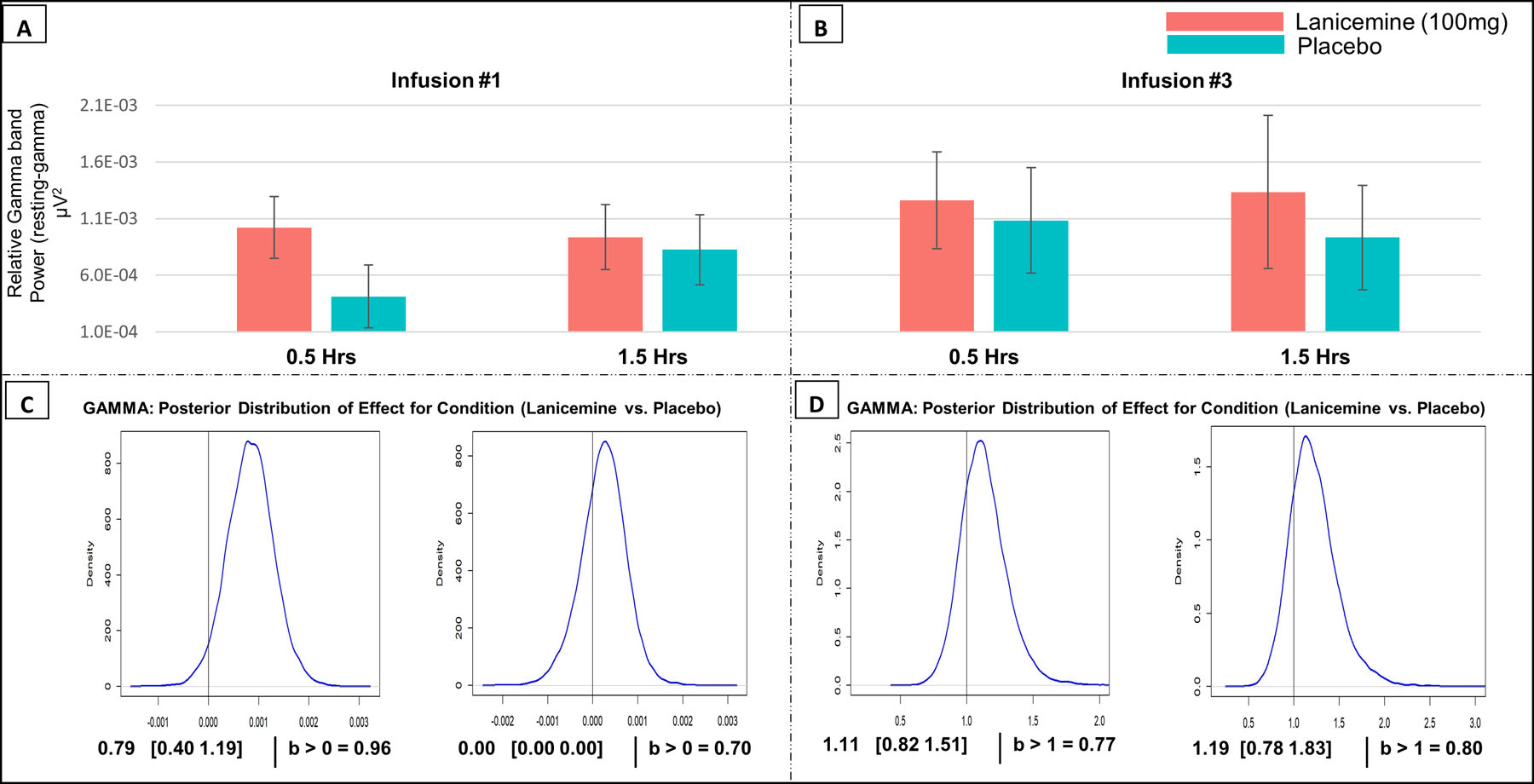
A) resting-gamma at Infusion #1 at 0.5- and 1.5-hour measurements. Bar plots are relative to pre-infusion (before infusion #1 values using subtraction). Red indicates Lanicemine group and Blue indicates Placebo group. B) resting-gamma at Infusion #3. Bar plots are relative to pre-infusion values (before Infusion #1 using subtraction). C) Bayesian results for resting-gamma at Infusion #1 (Lanicemine vs Placebo) at 0.5- and 1.5-hour measurements, including the median and Credible Interval (Crl) of the posterior distribution as well as the posterior probability that an effect of treatment exists. Analysis controlled for pre-infusion values before infusion #1. D) Bayesian results for resting-gamma at Infusion #3 at 0.5- and 1.5-hour measurements. Analysis controlled for pre-infusion values before infusion #1.
Figure 4:
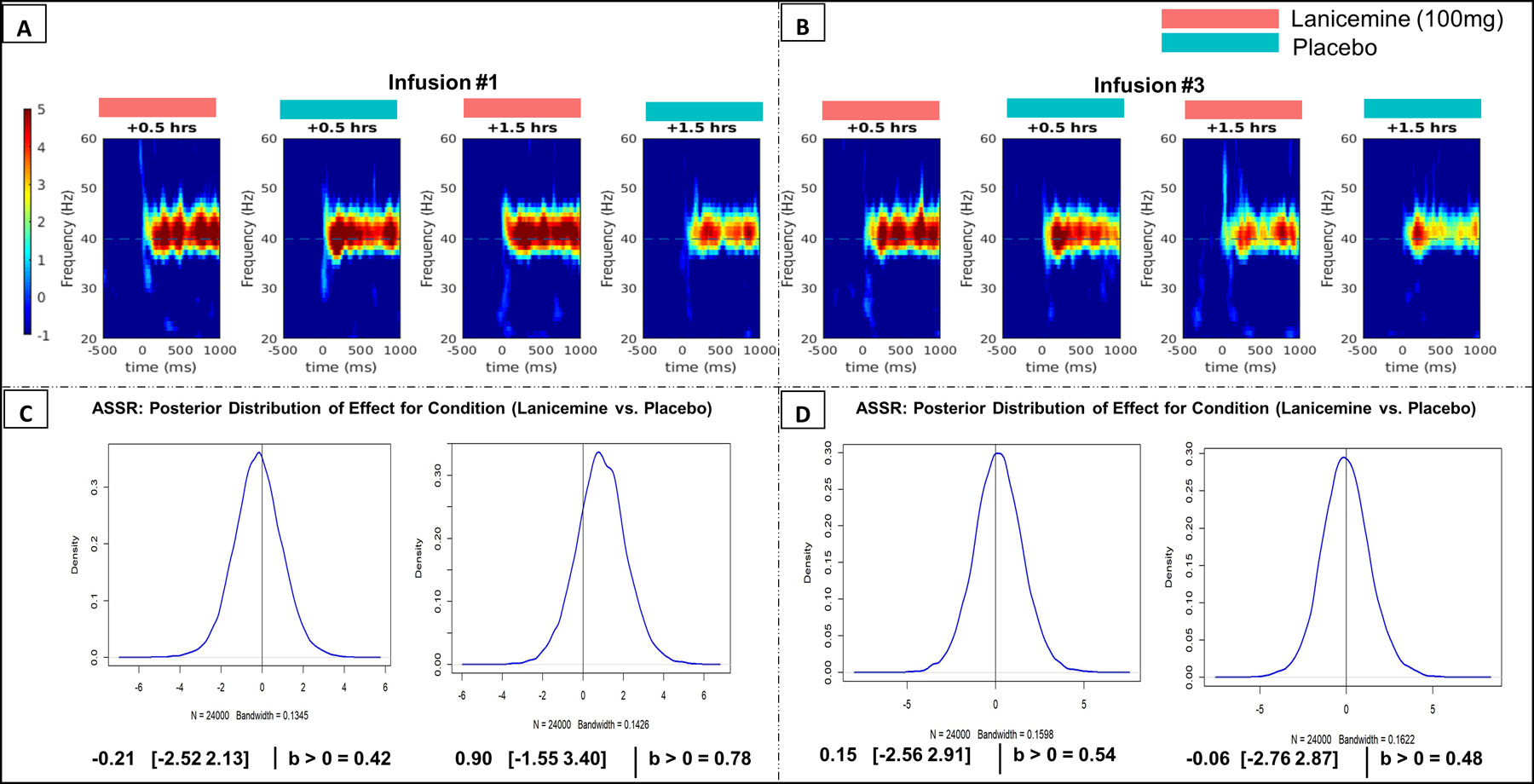
A) ASSR Time Frequency plots at Infusion #1 at 0.5- and 1.5-hour measurements. Red indicates Lanicemine group and Blue indicates Placebo group. B) ASSR Time Frequency plots at Infusion #3 at 0.5- and 1.5-hour measurements. C) Bayesian results for ASSR at Infusion #1 (Lanicemine vs Placebo), including the median and Credible Interval (Crl) of the posterior distribution as well as the posterior probability that an effect of treatment exists. Analysis controlled for pre-infusion values before 1st infusion. D) Bayesian results for ASSR at Infusion #3. Analysis controlled for pre-infusion values before infusion #1.
Impact on PTSD Symptoms
At Day 8 (3 days following infusion #3, the final administration of lanicemine/placebo), there was a 93% chance that lanicemine reduced CAPS-5 Criterion E (hyperarousal) scores compared to placebo, and a 77% chance that lanicemine outperformed placebo by a standardized effect size > 0.4. The Cohen’s d effect size was −0.75 (Supplementary Materials-Section VII, Table ST3). Lanicemine was more effective than placebo in 5 of the 6 individual items comprising the hyperarousal cluster. The clinical effect of lanicemine therefore persisted after the first infusion, consistent with the mechanism described above where a relatively transient increase in glutamatergic activity could have persistent behavioral effects 9 involving sensitization 37.
For CAPS-5 total score at Day 8, there was a 53% chance that lanicemine [30.8 (95% Crl = 23.2–37.7) was more effective than placebo [31.1 (95%Crl 23.6–38)]. When controlled for screening CAPS-5 score, which included symptoms over the past month instead of just the past week, there was a 67% chance that lanicemine [30.1 (95%Crl 22.7–36.8)] reduced CAPS-5 total score compared to placebo [32.2 (95%Crl 24.7–38.7)]. Lanicemine was associated with small effect sizes for Criterion C (Avoidance) and D (Negative Alterations in Cognition and Mood) (Supplementary Materials - Section VII, Table ST3). Individual CAPS-5 total scores and Criterion E (hyperarousal) scores for Baseline, Day 8 and Day 19 (end of study visit) are available in Supplementary Materials - Section VIII. PCL-5 scores are available in Supplementary Materials - Section IX. Pharmacokinetic results and methods are available in Supplementary Materials Section XI.
DISCUSSION
This Phase 1b study investigated whether the selective NMDAR channel blocker lanicemine impacted the expression of behavioral sensitization in individuals with PTSD and physiological evidence of hyperarousal, manifested by exaggerated APS. Three infusions of lanicemine (100 mg) were well-tolerated, with no serious or severe adverse events and no dropouts. Our primary outcome measure was negative, in that there was only a 38% chance that lanicemine outperformed placebo in reducing APS T-score after three infusions. However, we found that following a single infusion of lanicemine, relative to placebo, there was a strong probability of a reduction in APS. We also found a high probability of an increase in resting-gamma due to lanicemine during the first infusion and a high probability that lanicemine reduced CAPS-5 hyperarousal symptom cluster scores compared to placebo.
To our knowledge, this is the first investigation of NMDAR engagement using APS in human subjects. APS assesses tonic negative emotional states via activation of the bed nucleus of the stria terminalis (BNST), in response to a context that signals threat of an uncontrollable and unpredictable aversive stimulus.38,39 The startle reflex is known to be modulated by hyperexcitability of the corticotropin-releasing hormone (CRH) system of the BNST, a component of the extended amygdala, to uncontrollable stressors.40,41 Preclinical work has shown that behavioral sensitization, and specifically BNST reactivity may be mediated by NMDARs.42 NMDAR antagonists infused directly into the amygdala of rats demonstrated blocking of acquisition of startle in a fear potentiated acoustic startle protocol.43 Further, blockade of sensitization-related arousal by NMDAR antagonists is consistent with results in animal models44,45 on expression of the sensitized locomotor response. Long-lasting reduction in sensitized response to stimulants with single (8a) or repeated administration of NMDAR antagonist MK-801 has also been observed.37 In our patient sample 83.3% presented with co-morbid major depressive disorder. Depression can be mixed with symptoms of mania or symptoms of anxiety and are both related to activation and behavioral sensitization 46,47Our results demonstrated that after a single dose of lanicemine there was a 90% chance that APS T-score would be normalized relative to placebo which suggests that our outcomes could generalize to patients with activated or mixed depression.
Consistent with this work and our hypothesis, we observed an effect on reduction of APS following a single infusion of lanicemine. However, contrary to expectations, the acute effect on APS was not sustained following three infusions of lanicemine, possibly owing to habituation effects across multiple sessions. In addition to receiving the NPU task at screening and baseline (pre-infusion), participants underwent the task an additional three times over 5 days. It is known that repeated exposure to an aversive stimulus can lead to a reduction in the unconditioned response elicited by the stimulus, which ultimately limits the level of anxiety that the stimulus can maintain.48 Although the aversive auditory stimuli were chosen based on previous work showing sensitivity to auditory stimuli in PTSD, other work in different populations has shown that painful tactile stimuli, like mild shocks or heat pain may be less susceptible to habituation.49–51 Another possible explanation for the lack of lanicemine effect in the later session could be habituation to the startle stimulus (air puff) itself. Although the potentiated startle during the NPU task has previously been shown to have adequate temporal stability across sessions 52,53, it is currently unclear how the temporal stability in this task is affected by the modality of the noxious stimuli used to evoke anxiety. While studies have demonstrated that APS with low intensity air puff probes can sufficiently elicit startle response with less disruption of ongoing emotional and attentional processes,54–56 it is possible that the intensity (40 psi) was insufficient to elicit a reflex in all trials. Evidence from fear conditioning studies suggests that a wide variety of stimulus types can be used to potentiate defensive responses, but that shocks may potentiate startle to a greater degree when compared to auditory stimuli, and may be more resistant to habituation processes57,58. Future pharmacotherapy studies assessing functional target engagement could employ more noxious stimuli such as shocks in a repeated measures design28, and should explore the impact of noxious stimulus modality on the temporal stability of the startle responses in the NPU task.
As a secondary outcome measure, we measured EEG gamma band oscillations, which provide a marker of the relationship between fast-spiking parvalbumin (PV) interneurons and glutamatergic pyramidal neurons.59,60 Inhibition of the NMDAR channel at the interneuron level alters the dynamic E/I balance within the circuit causing an extended state of disinhibition of excitatory pyramidal neurons.61 The increase of glutamate and subsequent activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR)62,63 reflects a net increase in excitatory drive recorded as increased power in the EEG gamma band.60 In our study, we found a high probability of an increase in resting-gamma in the lanicemine group compared to the placebo group during the first infusion, controlling for baseline (pre-infusion) values. This result is consistent with a prior study of lanicemine in healthy volunteers20 and provides evidence of target engagement. Interestingly, shortly following the first infusion, there was a smaller probability of an increase in resting-gamma due to lanicemine, suggesting a fast normalization in synaptic glutamate at this dose.29 There was also a reduced probability of effect of lanicemine during and following the third infusion. By accounting for pre-infusion baseline prior to the first infusion, the results suggest a lack of sustained effect over time. While little is known on the longitudinal effects of NMDAR antagonists on gamma oscillatory function, one study using prolonged ketamine exposure demonstrated acute but not sustained enhancement of gamma.64
In both human and animal models, ASSR amplitude and PLF has been used to assess the functional integrity of neural circuits that support the synchronization during sensory processing.65–67 The utility of studying gamma oscillations as an indicator of clinical engagement is their ability to index the rapid coordination of E/I balance at both the general cortical level (resting) and within a specific functional context (e.g., ASSR). Whereas the former is useful for studying macro-level properties of the cortical system, the latter approach can provide insight into functional specificity that is useful as a pharmacodynamic biomarker for cortical NMDA function. While we would expect similar results, we found a smaller probability that lanicemine increased ASSR-gamma compared to resting-gamma following the first and third infusions. We speculate this to reflect a reduction in S/N ratio and evoked gamma activity due to increased baseline gamma levels.68
Hyperarousal and reactivity belong to a core symptom cluster of PTSD. In an exploratory analysis, we found a high probability (93%) that lanicemine reduced CAPS-5 hyperarousal symptom cluster scores compared to placebo, with a moderate-large effect size (Cohen’s d=0.75) at the rating conducted 3 days after the third infusion. There was some specificity for lanicemine’s impact on this cluster (Table 3), as its effects on other PTSD symptom clusters were either small (e.g., reexperiencing, negative alterations in cognition/mood) or failed to distinguish from placebo (e.g., reexperiencing). In humans, intraoperative administration of NMDAR antagonist ketamine may reduce PTSD risk69,70 and a single and repeated infusions of ketamine, compared to midazolam in patients with PTSD, resulted in rapid and sustained and improvement in PTSD.10–12 Our results are also consistent with evidence of improvement in hyperarousal symptoms in individuals with PTSD following treatment with low-affinity NMDAR antagonists such as memantine.71
Changes in cortical excitability72 may provide a unifying link between the EEG and startle findings. The reduction in probability of higher resting-gamma with lanicemine after the first infusion corresponded with a high chance of attenuation of APS. Recent data-driven approaches have shown that elevation in cortical excitability occurs during elevated state anxiety, measured by APS.72 One study in healthy volunteers using inhibitory low frequency repetitive transcranial magnetic stimulation (rTMS) demonstrated reduction in APS.24 It is possible that for several hours after infusion there is a shift in cortical E/I balance towards increased inhibitory tone, as reflected by reduced gamma oscillatory activity, demonstrating a normalizing effect on hyperexcitability in PTSD patients.73 While speculative, this “normalization” could contribute to beneficial effects in clinical symptoms.
A limitation of the study is the lack of inclusion of stress hormonal measurements (e.g., cortisol) in the study design. Recent studies have found that individual differences in startle response could be attributed to cortisol and the sulfate ester of dehydroepiandrosterone (DHEA-S) levels.74 Another limitation was that the confounding effects of specific classes of psychotropic medications was not analyzed. While there was approximately the same proportion of patients in each group receiving psychotropic medications (Lanicemine = 33.3%, Placebo = 41.7%) and no modifications were permitted to existing medications for the duration of the study, there is some evidence of a potential effect of psychotropics such as sertraline on habituation of startle response.75 Our study highlights that habituation effects might be occurring at the central level, however, the etiology of this is unclear. To establish whether changes are occurring at the central level as a result of our treatment regimen, future investigations should manipulate the time between infusions over a short period akin to what has been studied in ketamine infusion trials (e.g. 2 weeks 12,76). This would help to determine the optimum strategy to elicit and maintain a clinically relevant behavioral effect, as well as understanding the physiological state of the system during APS at each time point. A final limitation of our study is the lack of scope to control for the details of trauma exposure, or trauma-dosage. Future studies should be mindful of this to improve our understanding of lanicemine’s effects across different dimensions of PTSD experience.
In conclusion, we demonstrated preliminary evidence of novel effects of lanicemine on behavioral sensitization, expressed as an exaggerated startle response, a phasic autonomic response to sudden intense stimuli. Using changes in gamma band oscillation, we showed successful occupancy of NMDAR by lanicemine, and subsequent facilitation of acute reductions in interneuron and/or increased AMPA receptor activity. These preliminary findings, coupled with a robust drug effect on hyperarousal symptoms, support further study of selective NMDAR modulators in subgroups of PTSD and related disorders.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R61MH110540. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
S.J.M, M.L and A.C.S conceptualized the study. N.R wrote the manuscript and made subsequent revisions. C.E.G performed the statistical analysis. N.R, M.L, B.V.L, T.I, N.M, N.L.B and C.G performed data analysis. N.R and C.E.G prepared figures. S.J.M provided scientific oversight. All authors contributed to the drafting and revising of the manuscript.
We thank Biohaven Pharmaceuticals for providing the investigational drug for this study, and Robert Berman, M.D., Lia Donahue, M.A., and Mitch Seymour, Ph.D. for their valuable collaboration. We thank Meghan Atkinson, M.D. for assistance with insertion of intravenous lines, the study medical monitor Asim Shah, M.D., and the NIH Data Safety and Monitoring Board.
S.J.M is supported through the use of facilities and resources at the Michael E. Debakey VA Medical Center, Houston, Texas, and receives support from The Menninger Clinic, Houston, Texas. He has served as a consultant to Alkermes, Allergan, Axsome Therapeutics, BioXcel Therapeutics, Clexio Biosciences, Engrail Therapeutics, Greenwich Biosciences, Intra-Cellular Therapies, Janssen, Neurocrine, Perception Neurosciences, Praxis Precision Medicines, Relmada Therapeutics, Signant Health, and Sage Therapeutics. He has received research support from Biohaven Pharmaceuticals and VistaGen Therapeutics. M.L. has served as principal investigator for trials funded by NeuroRx and Vistagen Therapeutics. A.C.S. is supported through the Michael E. DeBakey VA Medical Center and receives support from the American Foundation for Suicide Prevention. In the past 5 years, J.W.M. has provided consultation services and/or served on advisory boards for Allergan, Boehreinger Ingelheim, Clexio Biosciences, Fortress Biotech, FSV7, Global Medical Education (GME), Impel Neuropharma, Janssen Research and Development, Medavante-Prophase, Novartis, Otsuka, Sage Therapeutics, and Engrail Therapeutics. Dr. Murrough is named on a patent pending for neuropeptide Y as a treatment for mood and anxiety disorders and on a patent pending for the use of KCNQ channel openers to treat depression and related conditions. All the other authors report no financial relationships with commercial interests.
Footnotes
Previous presentation. The results included in the present paper have been presented in part at the American Society for Clinical Psychopharmacology, May 2020, and as an e-poster at the Society of Biological Psychiatry 2020 e-poster gallery.
Location of work and address for reprints. Mood and Anxiety Disorders Program, Menninger Department of Psychiatry & Behavioral Sciences, Baylor College of Medicine, Houston, Texas, USA.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier NCT03166501
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1.Sareen J Posttraumatic stress disorder in adults: impact, comorbidity, risk factors, and treatment. Can J Psychiatry 2014; 59: 460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic And Statistical Manual Of Mental Disorders, Fifth Edition. Arlington, VA: Am Psychiatr Publ; 2013. [Google Scholar]
- 3.Liriano F, Hatten C, Schwartz TL. Ketamine as treatment for post-traumatic stress disorder: a review. Drugs Context 2019; 8: 212305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander W Pharmacotherapy for Post-traumatic Stress Disorder In Combat Veterans: Focus on Antidepressants and Atypical Antipsychotic Agents P T 2012; 37: 32–38. [PMC free article] [PubMed] [Google Scholar]
- 5.Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry 2002; 159: 1777–1779. [DOI] [PubMed] [Google Scholar]
- 6.Morgan P, Van Der Graaf PH, Arrowsmith J, Feltner DE, Drummond KS, Wegner CD et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov Today 2012; 17: 419–424. [DOI] [PubMed] [Google Scholar]
- 7.Soares HD. The use of mechanistic biomarkers for evaluating investigational CNS compounds in early drug development. Curr Opin Investig Drugs 2010; 11: 795–801. [PubMed] [Google Scholar]
- 8.Lijffijt M, Hu K, Swann AC. Stress modulates illness-course of substance use disorders: a translational review. Front psychiatry 2014; 5: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia LSB, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F et al. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33: 450–455. [DOI] [PubMed] [Google Scholar]
- 10.Albott CS, Lim KO, Forbes MK, Erbes C, Tye SJ, Grabowski JG et al. Efficacy, safety, and durability of repeated ketamine infusions for comorbid posttraumatic stress disorder and treatment-resistant depression. J Clin Psychiatry 2018; 79. [DOI] [PubMed] [Google Scholar]
- 11.Feder A, Parides M, Murrough JW, Perez AM, Morgan J, Saxena S et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: A randomized clinical trial. JAMA Psychiatry 2014; 71: 681–688. [DOI] [PubMed] [Google Scholar]
- 12.Feder A, Costi S, Rutter SB, Collins AB, Govindarajulu U, Jha MK et al. A Randomized Controlled Trial of Repeated Ketamine Administration for Chronic Posttraumatic Stress Disorder. Am J Psychiatry 2021; : appi.ajp.2020.20050596. [DOI] [PubMed] [Google Scholar]
- 13.Murrough J, Iosifescu D, Chang L, Al Jurdi R, Green C, Perez A et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am J Psychiatry 2013; 170: 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am J Psychiatry 2015; 172: 950–966. [DOI] [PubMed] [Google Scholar]
- 15.Witt K, Potts J, Hubers A, Grunebaum MF, Murrough JW, Loo C et al. Ketamine for suicidal ideation in adults with psychiatric disorders: A systematic review and meta-analysis of treatment trials. Aust N Z J Psychiatry 2020; 54: 29–45. [DOI] [PubMed] [Google Scholar]
- 16.Krystal JH, Abdallah CG, Averill LA, Kelmendi B, Harpaz-Rotem I, Sanacora G et al. Synaptic Loss and the Pathophysiology of PTSD: Implications for Ketamine as a Prototype Novel Therapeutic. Curr Psychiatry Rep 2017; 19: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdallah CG, Averill LA, Akiki TJ, Raza M, Averill CL, Gomaa H et al. The Neurobiology and Pharmacotherapy of Posttraumatic Stress Disorder. Annu Rev Pharmacol Toxicol 2019; 59: 171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riaza Bermudo-Soriano C, Perez-Rodriguez MM, Vaquero-Lorenzo C, Baca-Garcia E. New perspectives in glutamate and anxiety. Pharmacol Biochem Behav 2012; 100: 752–774. [DOI] [PubMed] [Google Scholar]
- 19.Zarate CAJ, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I et al. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry 2013; 74: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ et al. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry 2014; 19: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanacora G, Johnson MR, Khan A, Atkinson SD, Riesenberg RR, Schronen JP et al. Adjunctive Lanicemine (AZD6765) in Patients with Major Depressive Disorder and History of Inadequate Response to Antidepressants: A Randomized, Placebo-Controlled Study. Neuropsychopharmacology 2017; 42: 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Downey D, Dutta A, McKie S, Dawson GR, Dourish CT, Craig K et al. Comparing the actions of lanicemine and ketamine in depression: Key role of the anterior cingulate. Eur Neuropsychopharmacol 2016; 26: 994–1003. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nat Protoc 2012; 7: 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balderston NL, Beydler EM, Goodwin M, Deng Z De, Radman T, Luber B et al. Low-frequency parietal repetitive transcranial magnetic stimulation reduces fear and anxiety. Transl Psychiatry 2020; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grillon C, Hale E, Lieberman L, Davis A, Pine DS, Ernst M. The CRH 1 Antagonist GSK561679 Increases Human Fear but Not Anxiety as Assessed by Startle. Neuropsychopharmacology 2015; 40: 1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol Psychiatry 2009; 66: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grillon C, Heller R, Hirschhorn E, Kling MA, Pine DS, Schulkin J et al. Acute hydrocortisone treatment increases anxiety but not fear in healthy volunteers: a fear-potentiated startle study. Biol Psychiatry 2011; 69: 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grillon C, Chavis C, Covington MF, Pine DS. Two-week treatment with the selective serotonin reuptake inhibitor citalopram reduces contextual anxiety but not cued fear in healthy volunteers: A fear-potentiated startle study. Neuropsychopharmacology 2009; 34: 964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sivarao DV, Chen P, Senapati A, Yang Y, Fernandes A, Benitex Y et al. 40 Hz auditory steady-state response is a pharmacodynamic biomarker for cortical NMDA receptors. Neuropsychopharmacology 2016; 41: 2232–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lijffijt M, Green C, Balderston N, Iqbal T, Atkinson M, Vo-Le B et al. A proof-of-mechanism study to test effects of the NMDA receptor antagonist lanicemine on behavioral sensitization in individuals with symptoms of PTSD. Front psychiatry 2019; 10: 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry 2007; 164: 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welch P The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoust 1967; 15: 70–73. [Google Scholar]
- 33.Gelman A Comment: Bayesian Checking of the Second Levels of Hierarchical Models. Stat Sci 2007; 22: 349–352. [Google Scholar]
- 34.R Development Core Team R. R: A language and environment for statistical computing 2013.
- 35.Bürkner P-C. Advanced Bayesian Multilevel Modeling with the R Package Brms. Adv Bayesian Multilevel Model with R Packag Brms 2017; : 1–28.
- 36.Team S development. RStan: the R interface to Stan. R package version 2.18.2 2018.
- 37.Sripada S, Gaytan O, Swann A, Dafny N. The role of MK-801 in sensitization to stimulants. Brain Res Rev 2001; 35: 97–114. [DOI] [PubMed] [Google Scholar]
- 38.Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol 2003; 114: 1557–1579. [DOI] [PubMed] [Google Scholar]
- 39.Vaidyanathan U, Patrick CJ, Cuthbert BN. Linking dimensional models of internalizing psychopathology to neurobiological systems: affect-modulated startle as an indicator of fear and distress disorders and affiliated traits. Psychol Bull 2009; 135: 909–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weston CSE. Posttraumatic stress disorder: a theoretical model of the hyperarousal subtype. Front psychiatry 2014; 5: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dedic N, Kühne C, Gomes KS, Hartmann J, Ressler KJ, Schmidt MV et al. Deletion of CRH From GABAergic Forebrain Neurons Promotes Stress Resilience and Dampens Stress-Induced Changes in Neuronal Activity. Front. Neurosci 2019; 13: 986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glangetas C, Massi L, Fois GR, Jalabert M, Girard D, Diana M et al. NMDA-receptor-dependent plasticity in the bed nucleus of the stria terminalis triggers long-term anxiolysis. Nat Commun 2017; 8: 14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miserendino MJD, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature 1990; 345: 716–718. [DOI] [PubMed] [Google Scholar]
- 44.Broadbent J, Kampmueller KM, Koonse SA. Expression of behavioral sensitization to ethanol by DBA/2J mice: the role of NMDA and non-NMDA glutamate receptors. Psychopharmacology (Berl) 2003; 167: 225–234. [DOI] [PubMed] [Google Scholar]
- 45.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev 1991; 16: 223–244. [DOI] [PubMed] [Google Scholar]
- 46.Swann AC, Steinberg JL, Lijffijt M, Moeller GF. Continuum of depressive and manic mixed states in patients with bipolar disorder: quantitative measurement and clinical features. World Psychiatry 2009; 8: 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swann AC, Moeller FG, Steinberg JL, Schneider L, Barratt ES, Dougherty DM. Manic symptoms and impulsivity during bipolar depressive episodes. Bipolar Disord 2007; 9: 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cevik MÖ. Habituation, sensitization, and Pavlovian conditioning. Front Integr Neurosci 2014; 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious Responses to Predictable and Unpredictable Aversive Events. Behav. Neurosci 2004; 118: 916–924. [DOI] [PubMed] [Google Scholar]
- 50.Nelson BD, Hajcak G. Defensive motivation and attention in anticipation of different types of predictable and unpredictable threat: A startle and event-related potential investigation. Psychophysiology 2017; 54: 1180–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferry RA, Nelson BD. Differential impact of threat type on defensive motivation and attention during the NPU-threat task. Motiv Emot 2020; 44: 670–685. [Google Scholar]
- 52.Kaye JT, Bradford DE, Curtin JJ. Psychometric properties of startle and corrugator response in NPU, affective picture viewing, and resting state tasks. Psychophysiology 2016; 53: 1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V et al. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol Psychiatry 2006; 60: 760–766. [DOI] [PubMed] [Google Scholar]
- 54.Grillon C, Ameli R. Effects of threat of shock, shock electrode placement and darkness on startle. Int. J. Psychophysiol 1998; 28: 223–231. [DOI] [PubMed] [Google Scholar]
- 55.Miller MW, Curtin JJ, Patrick CJ. A startle-probe methodology for investigating the effects of active avoidance on negative emotional reactivity. Biol Psychol 1999; 50: 235–257. [DOI] [PubMed] [Google Scholar]
- 56.Lissek S, Baas JMP, Pine DS, Orme K, Dvir S, Nugent M et al. Airpuff startle probes: an efficacious and less aversive alternative to white-noise. Biol Psychol 2005; 68: 283–297. [DOI] [PubMed] [Google Scholar]
- 57.Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J et al. Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci Biobehav Rev 2017; 77: 247–285. [DOI] [PubMed] [Google Scholar]
- 58.Glenn CR, Lieberman L, Hajcak G. Comparing electric shock and a fearful screaming face as unconditioned stimuli for fear learning. Int J Psychophysiol 2012; 86: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uhlhaas PJ, Singer W. High-frequency oscillations and the neurobiology of schizophrenia. Dialogues Clin Neurosci 2013; 15: 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buzsáki G, Wang X-J. Mechanisms of gamma oscillations. Annu Rev Neurosci 2012; 35: 203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 2007; 27: 11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Routley BC, Singh KD, Hamandi K, Muthukumaraswamy SD. The effects of AMPA receptor blockade on resting magnetoencephalography recordings. J Psychopharmacol 2017; 31: 1527–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE et al. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry 2019; 24: 1040–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye T, Bartlett MJ, Schmit MB, Sherman SJ, Falk T, Cowen SL. Ten-Hour Exposure to Low-Dose Ketamine Enhances Corticostriatal Cross-Frequency Coupling and Hippocampal Broad-Band Gamma Oscillations. Front. Neural Circuits 2018; 12: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Picton TW, John MS, Dimitrijevic A, Purcell D. Human auditory steady-state responses. Int J Audiol 2003; 42: 177–219. [DOI] [PubMed] [Google Scholar]
- 66.O’Donnell BF, Vohs JL, Krishnan GP, Rass O, Hetrick WP, Morzorati SL. The auditory steady-state response (ASSR): a translational biomarker for schizophrenia. In: Supplements to Clinical neurophysiology Elsevier, 2013, pp 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murphy N, Ramakrishnan N, Walker CP, Polizzotto NR, Cho RY. Intact Auditory Cortical Cross-Frequency Coupling in Early and Chronic Schizophrenia. Front Psychiatry 2020; 11: 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Honda S, Matsumoto M, Tajinda K, Mihara T. Enhancing Clinical Trials Through Synergistic Gamma Power Analysis. Front. Psychiatry 2020; 11: 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McGhee LL, Maani CV, Garza TH, Gaylord KM, Black IH. The Correlation Between Ketamine and Posttraumatic Stress Disorder in Burned Service Members. J Trauma Acute Care Surg 2008; 64. [DOI] [PubMed] [Google Scholar]
- 70.McGhee LL, Maani CV, Garza TH, Slater TM, Petz LN, Fowler M. The Intraoperative Administration of Ketamine to Burned U.S. Service Members Does Not Increase the Incidence of Post-Traumatic Stress Disorder. Mil Med 2014; 179: 41–46. [DOI] [PubMed] [Google Scholar]
- 71.Battista MA, Hierholzer R, Khouzam HR, Barlow A, O’Toole S. Pilot Trial of Memantine in the Treatment of Posttraumatic Stress Disorder. Psychiatry Interpers Biol Process 2007; 70: 167–174. [DOI] [PubMed] [Google Scholar]
- 72.Balderston NL, Hale E, Hsiung A, Torrisi S, Holroyd T, Carver FW et al. Threat of shock increases excitability and connectivity of the intraparietal sulcus. Elife 2017; 6: e23608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Centonze D, Palmieri MG, Boffa L, Pierantozzi M, Stanzione P, Brusa L et al. Cortical hyperexcitability in post-traumatic stress disorder secondary to minor accidental head trauma: a neurophysiologic study. J Psychiatry Neurosci 2005; 30: 127–132. [PMC free article] [PubMed] [Google Scholar]
- 74.Grillon C, Pine D, Baas JMP, Lawley M, Ellis V, Charney D. Cortisol and DHEA-S are associated with startle potentiation during aversive conditioning in humans. Psychopharmacology (Berl) 2006; 186: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quednow BB, Kühn K-U, Stelzenmuelle R, Hoenig K, Maier W, Wagner M. Effects of serotonergic and noradrenergic antidepressants on auditory startle response in patients with major depression. Psychopharmacology (Berl) 2004; 175: 399–406. [DOI] [PubMed] [Google Scholar]
- 76.Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry 2016; 173: 816–826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


