Abstract
Ageing is a natural process causing alterations in the neuromuscular system, which contributes to reduced quality of life. Motor unit (MU) contributes to weakness, but the mechanisms underlying reduced firing rates are unclear. Persistent inward currents (PICs) are crucial for initiation, gain control and maintenance of motoneuron firing, and are directly proportional to the level of monoaminergic input. Since concentrations of monoamines (i.e. serotonin and noradrenaline) are reduced with age, we sought to determine if estimates of PICs are reduced in older (>60 years old) compared to younger adults (<35 years old). We decomposed MU spike trains from high-density surface electromyography over the biceps and triceps brachii during isometric ramp contractions to 20% of maximum. Estimates of PICs (ΔFrequency; or simply ΔF) were computed using the paired MU analysis technique. Regardless of the muscle, peak firing rates of older adults were reduced by ∼1.6 pulses per second (pps) (P = 0.0292), and ΔF was reduced by ∼1.9 pps (P < 0.0001), compared to younger adults. We further found that age predicted ΔF in older adults (P = 0.0261), resulting in a reduction of ∼1 pps per decade, but there was no relationship in younger adults (P = 0.9637). These findings suggest that PICs are reduced in the upper limbs of older adults during submaximal isometric contractions. Reduced PIC magnitude represents one plausible mechanism for reduced firing rates and function in older individuals, but further work is required to understand the implications in other muscles and during a variety of motor tasks.
Keywords: ageing, motoneuron, motor unit decomposition, neuromodulation, persistent inward current
Graphical Abstract
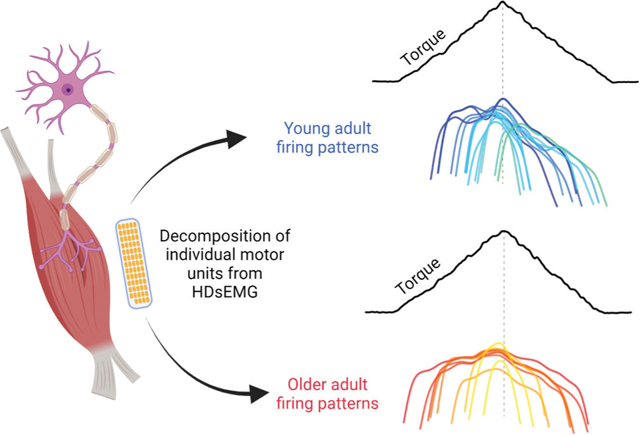
Decomposition of motor unit spike trains from young and older adults reveal vast differences in firing patterns used to achieve triangular isometric torque ramp. Young adults (blue) display asymmetric firing with clear hysteresis. Older adults (red) tend to have symmetrical firing patterns, which indicates reduced PIC contributions in the control of isometric torque.
Introduction
Ageing is a natural process that causes alterations within the neuromuscular system, which can have severe consequences for health and quality of life in older adults (McNeil & Rice, 2018). Even in the absence of disease, there is age-related loss of muscle or lean mass (i.e. sarcopenia), and, perhaps more importantly, age-related loss of strength (i.e. dynapenia). Emerging evidence suggests that dynapenia is a significant contributor to quality of life in the elderly (Mitchell et al. 2012). Indeed, biophysical properties of the muscle play a role in the reduced force-generating capacity, but neural factors are likely to contribute as well.
There is a progressive age-related loss in the number of motor units (MUs) (McNeil et al. 2005), comprising the muscle fibres and their parent motoneurons (Heckman & Enoka, 2012). As such, death of motoneurons is widely accepted as a precursor for many of the age-related adaptations in the nervous system (McNeil & Rice, 2018). Following death of motoneurons, the nervous system displays astounding plasticity, as evidenced by the reinnervation of orphaned muscle fibres by axonal sprouting (Gordon et al. 2004), a process known as MU remodelling (Hepple & Rice, 2016). Since it is speculated that the loss of larger/faster motoneurons precedes the loss of smaller/slower type motoneurons (Kanda & Hashizume, 1989), reductions in MU firing rates are typically ascribed to this mechanism. Dalton et al. (2010) previously showed that the firing rates of both biceps brachii (BIC) and triceps brachii (TRI) MUs are reduced across a wide range of contraction intensities in older, compared to young, adults. They suggested that the relatively higher proportional loss of higher threshold motoneurons may play a role in the age-related decline in firing rates, but alterations in the biophysical properties of the motoneurons are also likely to contribute.
Altered intrinsic motoneuron excitability may play a major role in age-related changes in motoneuron firing patterns. Although motoneurons were once believed to integrate their synaptic inputs passively, many studies have demonstrated that this integration is a highly active process due to voltage-sensitive ion channels in their dendrites (Heckman et al. 2008a,b). Persistent inward currents (PICs) amplify and prolong excitatory synaptic input to the motoneuron (Lee & Heckman, 1998, 2000), which are the result voltage-gated slow activating L-type Ca2+ and fast activating persistent Na+ currents (Heckman et al. 2008b). PICs are activated near threshold and can amplify synaptic currents by as much as 3- to 5-fold (Binder & Powers, 2001), and the level of PIC activation is highly dependent on the neuromodulatory drive from the monoaminergic system (i.e. serotonergic and noradrenergic drive) (Lee & Heckman, 1998, 2000). In addition, PICs are reduced with antagonist muscle afferent input (i.e. reciprocal inhibition), illustrating a role for inhibition in the control of PIC activity (Heckman et al. 2008a; Powers et al. 2012). Therefore, changes in levels of monoaminergic drive, intrinsic motoneuron excitability (i.e. monoamine receptor or ion channel function), and inhibition may alter MU firing patterns, as well as estimates of PICs, (Johnson et al. 2017) with age.
Furthermore, recent work has called for the investigation of PIC estimates in the ageing neuromuscular system (Latella, 2021). The function of two primary monoaminergic nuclei in the brainstem, the raphe nuclei and locus coeruleus, have been shown to deteriorate with age (Shibata et al. 2006; Pagano et al. 2017). This deterioration likely results in reduced monoaminergic drive, and consequently reduced activation of PICs.
Fortuitously, recent advances in technology have enabled us to sample from large populations of concurrently active MUs, by using high-density surface electromyography (HDsEMG) array electrodes and blind source separation algorithms (Holobar & Zazula, 2007; Negro et al. 2016) with great success (Yavuz et al. 2015; Del Vecchio et al. 2018; Thompson et al. 2018; Cogliati et al. 2020; Del Vecchio et al. 2020; Hassan et al. 2020; Kim et al. 2020; Martinez-Valdes et al. 2020). This non-invasive technology has created an opportunity to further study the age-related alterations in the neuromuscular system by sampling from many concurrently active MUs. Using this technology allows us to gain better appreciation of the population behaviour and provide more insights into the control of large portions of the motor pool, which was difficult to achieve with intramuscular EMG approaches.
In this study, we examined whether the MU firing characteristics in a large population of concurrently active MUs differed between a group of younger and older adults. More specifically, we compared MU firing patterns of the elbow flexor and extensors during isometric ramp contractions. We hypothesized that since previous work has shown MU firing rates are reduced in BIC and TRI MUs of older adults, we would observe reductions in peak firing rates of both BIC and TRI MUs, as well as estimates of PIC magnitude (i.e. ΔFrequency; or simply ΔF), in the older group. In addition, since healthy younger adults are unlikely to have an impairment in PIC function, we investigated the relationship between age and estimates of PICs in both groups separately. We hypothesized that there would be no relationship between age and estimates of PICs in the younger group, however there would be a negative relationship between age and estimates of PICs in the older group.
Methods
Participants
In order to compare MU firing behaviour between healthy younger and older individuals, we recruited 10 younger (26 (2.87) years old, 3 female) and 10 older (67 (4.40) years old, 2 female) adults. At the time of testing, all participants were free of neurological, motor and muscular impairments. All participants provided written informed consent and the study was in accordance with the Declaration of Helsinki except for registration in a database. It was approved (STU00084502-CR0003) by the Institutional Review Board of Northwestern University.
Experimental apparatus
The experimental apparatus, protocol and data processing methods utilized are similar to those used in a previous experiment in our lab (Hassan et al. 2020). Participants were secured in a Biodex chair (Biodex Medical Systems, Shirley, NY, USA) with their dominant upper limb rigidly fixed to a six degrees of freedom load cell with a fibreglass cast (JR3, Inc., Woodland, CA, USA). The casted arm was positioned at a shoulder abduction angle of 75° and an elbow flexion angle of 90°. An illustration of the experimental set-up is shown in Fig. 1.
Figure 1. An illustration of the experimental set-up.
High-density surface electromyography (HDsEMG) arrays were placed on the lateral head of the TRI and along the muscle belly of the BIC.
High-density surface EMG (HDsEMG) arrays (64 electrodes, 13 × 5, 8 mm inter-electrode distance, GR08MM1305, OT Bioelettronica, Inc., Turin, Italy) were placed on the BIC and the lateral head of the TRI on the casted limb. HDsEMG data were sampled (2048 Hz), amplified (×150), and band-pass filtered (10–500 Hz) using a Quattrocentro signal amplifier (OT Bioelettronica, Inc.). A reference electrode was placed on the acromion process of the casted arm. Prior to collecting experimental data, real-time HDsEMG recordings were checked visually to ensure high signal-to-noise ratios.
Torque was sampled at 1024 Hz with a forearm-load cell interface. The limb segment lengths and joint angles were converted into elbow flexion (EF) and elbow extension (EE) torques using a Jacobian based algorithm implemented by custom MATLAB software (The MathWorks, Inc., Natick, MA, USA). As EMG and force/torque recordings were collected using separate computers, a 1 s transistor–transistor logic (TTL) pulse was transmitted to both computers for data alignment. Each trial was temporally synced offline using cross-correlation of the TTL pulses.
Experimental protocol
Participants were initially asked to produce their maximum voluntary torques (MVTs) in the directions of elbow flexion or extension. A wall-mounted computer monitor placed directly in front of participants provided real-time feedback of torque performance. MVT trials were repeated until three trials in which the peak torque was within 10% of each other were collected. If the last trial had the highest peak torque, an additional MVT trial was collected. Participants were verbally encouraged during MVT trials to ensure peak torque performance and were given approximately 2 min rest between trials to prevent muscle fatigue.
Each of the subsequent experimental trials consisted of three triangular isometric elbow extension or flexion torque ramps, separated by 10 s of rest within each trial. The order of trials (i.e. 3 torque ramps) was randomized between flexion and extension. Participants performed three to five trials in each direction to ensure at least eight ramps without substantial deviations from the intended torque target. Each ramp required participants to increase torque (2% MVT/s) to 20% MVT over 10 s, and then decrease (−2% MVT/s) to 0% MVT over the next 10 s. During all trials, real-time torque feedback, as well as the desired experimental torque profile, was provided to the participants. Participants were asked to increase and decrease torque as smoothly as possible, and to avoid large corrections when errors did occur. To rectify errors, participants were asked to slowly meet back up with the target, rather than making a jerky correction. In order to avoid fatigue, a minimum of 2 min of rest was given to participants between trials. Trials that did not exhibit a relatively smooth increase of torque from 0% to 20% MVT and a smooth decrease of torque from 20% to 0% MVT over the desired time frame were discarded, as were trials that displayed any sudden jerks in torque. This resulted in the analysis of 8–15 ramps across participants (younger 11.9 (3.07) ramps per muscle; older 12.1 (2.02) ramps per muscle).
Data analysis
MU decomposition and variables of interest.
After data acquisition, each EMG channel of the surface array was manually inspected and any channels with substantial artefacts, noise or analogue to digital saturation were removed. A convolutive blind-source separation algorithm (Negro et al. 2016) with a silhouette threshold of 0.85 was used to decompose HDsEMG into individual MU spike trains. All decomposed MU spike trains were visually inspected for each participant and trial through a custom-made graphical user interface in MATLAB. More specifically, automatic decomposition results were improved through iteratively re-estimating the spike train and correcting for missed spikes or substantial deviations in the discharge profile (Boccia et al. 2019; Afsharipour et al. 2020; Del Vecchio et al. 2020; Hassan et al. 2020; Martinez-Valdes et al. 2020). This process of manual editing has been shown to be highly reliable across different operators (Hug et al. 2021). Instantaneous MU firing rates were calculated as the inverse of the interspike intervals of each MU spike train and smoothed using a 2 s Hanning window using a custom-written MATLAB script.
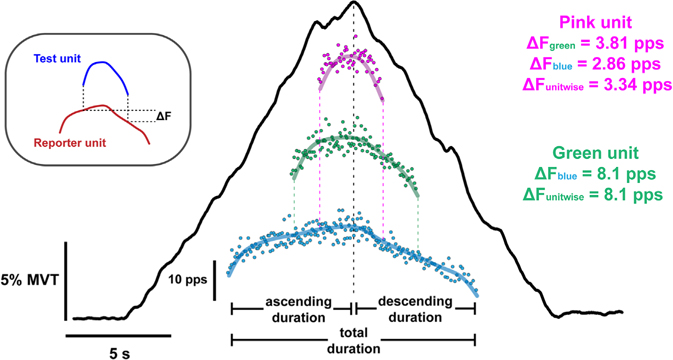
Peak, total duration, and total range of smoothed decomposed MU firing rates were extracted through custom-written MATLAB scripts for each muscle. MU firing rate range and duration during the ascending and descending phases of torque production were also extracted (see Fig. 2). Further, to provide a measure of the symmetry of MU firing throughout the ascending and descending phases of torque, we calculated a ratio of the MU firing duration using the following formula:
| (1) |
Figure 2. Motor unit data obtained from a single isometric ramp contraction.
Top left shows the paired MU analysis method used to estimate persistent inward current magnitude, which quantifies the onset–offset hysteresis (i.e. ΔF) of a higher threshold (test unit; blue) MU with respect to a lower threshold (reporter unit; red). In the centre, a typical extension torque trace (black line) from one younger participant is shown. Underneath the torque ramp, 3 out of the 12 decomposed MU firing patterns are shown. Each point indicates the instantaneous firing rate for each interstimulus interval, and the thick coloured lines indicate the smoothed firing rates of each MU. At the ends of the green and pink unit are vertical dashed lines that extend downward to the units below them (i.e. recruited at lower torque), which helps indicate the onset and offset of firing with respect to their reporter units. Individual ΔF values obtained from each reporter unit and the mean of those values (ΔF unitwise) are shown for each test unit. The vertical black dashed line indicates peak torque in this ramp for ease of viewing the time point between ascending and descending duration.
The duration ratio produced values between −1 and 1, with a MU that was only active on the ascending limb having a value of 1 (leftward shift; less hysteresis), and a MU that was only active on the descending limb producing a value of −1 (rightward shift; more hysteresis).
Estimating persistent inward currents.
The effects of PICs on motoneuronal firing patterns can be appreciated through MU onset–offset hysteresis. The best approximation of this hysteresis, ΔF, is calculated as the difference in the smoothed firing rate of a reporter (lower threshold; often referred to as the control unit in previous studies) MU between the times of recruitment/derecruitment of a test (higher threshold) MU (Gorassini et al. 2002). All ΔF values used throughout this study are ‘unitwise’ mean values. That is, the mean ΔF values obtained for all test–reporter unit pairs that meet our inclusion criteria (i.e. averaged for one test unit or ‘unitwise’). In this way, a motor unit can only have one ΔF value, rather than many (see Fig. 2). Criteria for inclusion of ΔF values from MU pairs were that (1) the test MU was recruited at least 1 s after the control unit to ensure full activation of PIC, (2) the test MU was derecruited at least 1.5 s prior to the control MU to prevent ΔF overestimation, and (3) test unit firing duration was ≥2 s (see Hassan et al. (2020) for more details on utilizing ΔF for paired MU analysis). In our primary analysis, we did not exclude pairs of motor units based on the following criteria: test unit–reporter unit pairs with rate–rate correlations of r2 < 0.7 (Gorassini et al. 2004; Udina et al. 2010; Stephenson & Maluf, 2011; Vandenberk & Kalmar, 2014), or test unit–reporter unit pairs in which the reporter unit firing range (FRmax – FRmin) was <0.5 pps while the test unit was active (Stephenson & Maluf, 2011) because our previous work showed that these criteria did not necessarily reduce variability in the estimation of ΔF (Hassan et al. 2020). Nevertheless, we did also compute ΔF with these criteria, similar to others in the past. That is, ΔF in this case refers to values from MU pairs such that: (1) the test MU was recruited ≥1 s after the control unit to ensure full activation of PIC, (2) there were rate–rate correlations of r2 ≥ 0.7 between test unit–reporter unit pairs, (3) for test unit–reporter unit pairs, the reporter unit firing range (FRmax – FRmin) was ≥0.5 pps while the test unit was active, and (4) the test unit firing duration was ≥2 s (Gorassini et al. 2004; Udina et al. 2010; Stephenson & Maluf, 2011; Vandenberk & Kalmar, 2014). This was done to ensure the criteria we chose did not bias our results in comparison to the findings of past studies.
Statistical analysis
All data were imported into GraphPad (version 9.0.1 for Windows, GraphPad Software, Inc., San Diego, CA, USA) where descriptive statistical analyses were performed. Hedge’s g effect sizes (ES) were calculated to provide a standardized effect for the mean differences between the younger and older subjects for each variable. Mean and standard deviation values for each variable reported are group means, which represent the average and error of the individual means computed for each participant.
We detail the effects of healthy ageing on MVT and MU firing characteristics using linear mixed effects models. More specifically, we took into consideration all of our data points rather than averaging across them and based our analysis on the mean within an individual trial or subject (Giboin et al. 2020). All of these analysis were performed in R (R Core Team 2020, R Foundation for Statistical Computing, Vienna, Austria) using the lme4 package (Bates et al., 2015) and significance was calculated using the lmerTest package (Kuznetsova et al. 2017), which applies Satterthwaite’s method to estimate degrees of freedom and generate P-values for mixed effects models by comparing the full model including the effect of interest against a null model excluding the effect of interest.
We used linear mixed effects models to determine if age group (categorical) and muscle were significant predictors for MVT and our MU variables. We employed age group (younger vs. older), muscle (BIC vs. TRI), and their interaction as fixed effects. As random effects, we included a random intercept for each subject as well as a random slope accounting for the muscle within each subject. Effects estimated from the linear mixed effects models are presented as parameter estimates ± SE.
In order to avoid the bimodal distribution of ages created by our selective sampling of healthy younger and older adults, we used separate generalized linear mixed effects models (computed by the MuMIn R package; Barton, 2018) to identify significant relationships between ΔF and age in the younger and older groups. This was done to assess the degree to which the age of the subjects in each group could account for variance in ΔF. Specifically, we analysed whether age was able to predict ΔF. For each age group, we included age (continuous variable), muscle (TRI vs. BIC), and their interaction as fixed effects. As random effects, we included a random intercept for each subject as well as a random slope accounting for the muscle within each subject. Variance accounted for by the model is reported as conditional R2GLMM values, whereas variance accounted for by only the fixed effects is reported as marginal R2GLMM values (Nakagawa & Schielzeth, 2013; Johnson, 2014; Nakagawa et al., 2017).
We further attempted to identify whether significant relationships between ΔF and MVT were present in the younger and older groups, both as a whole and separately. To do this, we used we used three separate generalized linear mixed effects models. This was done to assess the degree to which the MVT of the subjects as a whole and within in each group could account for variance in ΔF. Specifically, we analysed whether MVT was able to predict ΔF. For the entire group, we included age group, MVT, muscle (TRI vs. BIC) and their interactions as fixed effects. As random effects, we included a random intercept for each subject as well as a random slope accounting for the muscle within each subject. In the separate models for both younger and older individuals, we used the same fixed and random effects but omitted the fixed effect of age group. Variance accounted for by the models and variance accounted for by only the fixed effects are reported as conditional R2GLMM and marginal R2GLMM values, respectively, as described in the previous paragraph.
Results
Comparison of isometric elbow flexion and extension strength between younger and older adults
Younger adults produced 69.9 (30.87) and 46.2 (16.31) N m of torque during isometric elbow flexion and extension, respectively, whereas older adults produced 61.3 (21.40) N m during elbow flexion and 36.7 (12.77) N m during elbow extension. During flexion, the effect size of the difference in MVT between younger and older participants was 0.32, and during flexion the effect size was 0.65. However, age group was not a significant predictor (P = 0.242) nor was the interaction between muscle and age group (P = 0.8714). Contraction type was the only significant predictor of MVT (χ2 (1) = 55.5582, P = 0.0007). Across all participants, the flexion MVT was 23.15 (3.11) N m higher than the flexion MVT (P = 0.0007).
Comparison of MU firing patterns during submaximal ramp contractions
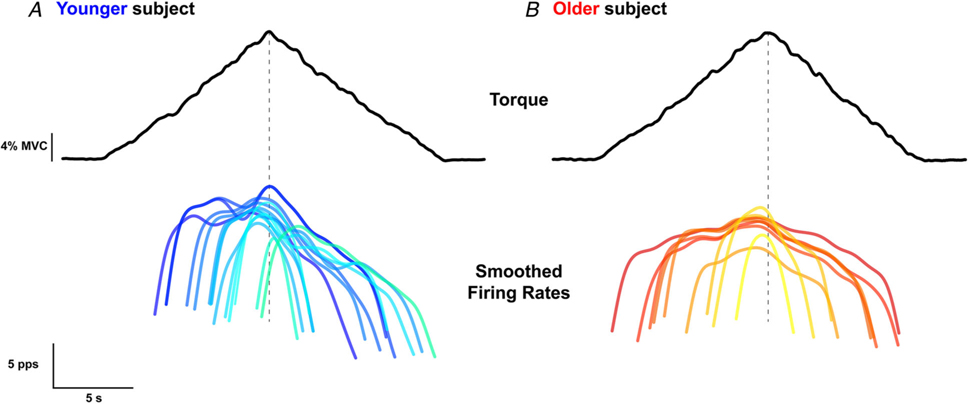
In the 10 younger participants, decomposition yielded 1002 MU spike trains from the BIC, and 1211 MU spike trains from the TRI. In the 10 older participants, decomposition yielded 533 MU spike trains from the BIC, and 827 from the TRI. All participants completed a minimum of 10 submaximal torque ramps in the directions of EF and EE. An average of 6.2 (3.81) and 9.0 (4.99) MUs per trial were decomposed from the BIC and TRI, respectively, of younger participants, and 4.4 (1.60) and 6.7 (3.39) MUs per trial were decomposed from the BIC and TRI, respectively, of older participants. Following the visual inspection and removal of erroneous spike times, the mean silhouette values of the decomposed motor units were 0.91 (0.05) from the BIC and 0.90 (0.05) from the TRI of the younger participants. The mean silhouette values from the older participants were 0.91 (0.04) from the BIC and 0.93 (0.05) from the TRI. An example of smoothed MU firing rate patterns of decomposed TRI MUs in a single trial from one younger and older individual is shown in Fig. 3, which shows many of the features that will be quantified below.
Figure 3. Typical data from a younger and older participant.
Single trial elbow extension torque (top, black traces) and smoothed firing rates of all decomposed triceps brachii MUs (bottom, coloured traces) for a younger (A) and an older (B) participant. Smoothed firing rates are shown darker at lower thresholds and lighter for higher thresholds for both participants.
Comparison of peak MU firing rates during submaximal ramp contractions
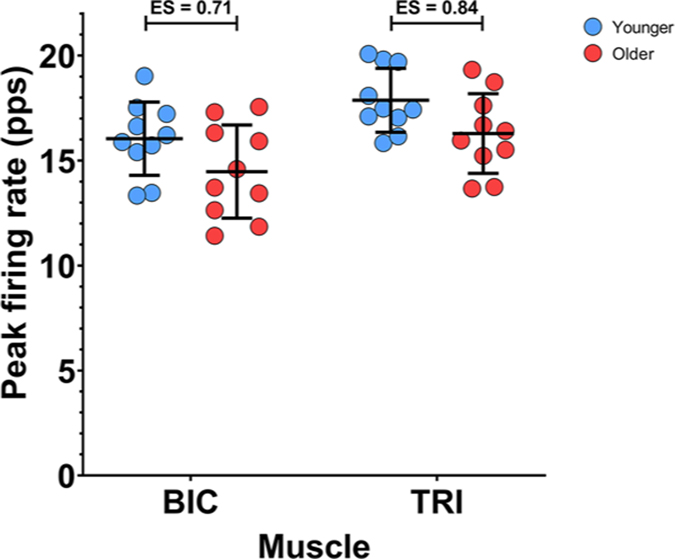
The peak firing rates during submaximal ramp contractions from BIC and TRI MUs are shown in Fig. 4. In this, and in the following figures, each data point represents the mean value from all MUs collected from one subject during the submaximal EF torque ramps (BIC data) or submaximal EE torque ramps (TRI data). In congruence with previous findings (Dalton et al. 2010), older participants had reduced peak firing rates during submaximal isometric contractions compared to younger participants. Group mean peak firing rates for the younger participants were higher than observed in the older participants in both the BIC (16.0 (1.74) vs. 14.5 (2.22) pps, ES = 0.71) and the TRI (17.9 (1.52) vs. 16.3 (1.90) pps, ES = 0.84). A linear mixed effects model revealed that both age group (χ2 (1) = 4.7564, P = 0.0292) and muscle (χ2 (1) = 15.731, P < 0.0001) were significant predictors of peak firing rate, but the interaction between the two variables was not significant (P = 0.9980). Peak MU firing rates were lowered by 1.6 (0.72) pps (P = 0.0412) in older participants, and were 1.8 (0.38) pps (P = 0.0001) higher in TRI, compared to BIC.
Figure 4. Peak motor unit firing rates during isometric ramp contractions.
Individual participant means (younger: blue; older: red) and group mean and SD (black) for the peak firing rate of biceps brachii (BIC) MUs during a 20% elbow flexion ramp, and triceps brachii (TRI) MUs during a 20% elbow extension ramp
Recruitment thresholds
Older participants had slightly lower recruitment thresholds (i.e. percentage of MVT) of TRI, but not BIC, MUs decomposed during submaximal isometric contractions compared to younger participants. Group mean recruitment thresholds for the younger participants were higher than those observed in the older participants in the TRI (11.3 (2.38) vs. 9.1 (1.78)% MVT, ES = 0.73) but not the BIC (11.8 (2.02) vs. 11.7 (2.63) pps, ES = 0.03). A linear mixed effects model revealed that muscle (χ2 (1) = 4.3356, P < 0.03732) was a significant predictor of recruitment threshold, but the age group (P = 0.06392) and the interaction between the two variables were not significant (P = 0.9980). Recruitment thresholds were lowered by 1.9 (0.78)% MVT in the TRI compared to BIC (P = 0.0204).
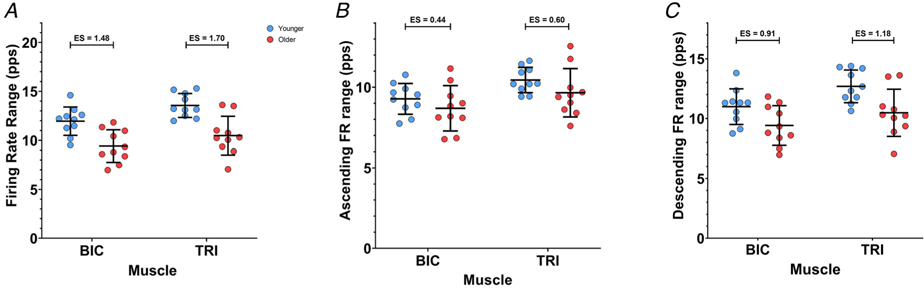
Range of MU firing rates
Figure 5A shows the range of MU firing rates from BIC and TRI MUs. The group mean ranges of firing rate from the younger participants were higher than the MU firing rate ranges in the older participants in both BIC (12.0 (1.45) vs. 9.4 (1.66) pps, ES = 1.48) and TRI (13.6 (1.22) vs. 10.5 (1.97) pps, ES = 1.70). Age group (χ2 (1) = 7.7509, P = 0.0053) and muscle (χ2 (1) = 15.570, P < 0.0001) were both significant predictors of firing rate range; the interaction of age group and muscle was not significant (P = 0.8687). The range of MU firing rates was 1.7 (0.60) pps (P = 0.0091) higher in younger participants than in older participants, and 1.5 (0.32) pps (P = 0.0001) higher in MUs from the TRI than the BIC.
Figure 5. Ranges of motor unit firing rates during isometric ramp contractions.
Participant means (younger: blue; older: red), along with group mean and SD (black), for the range of MU firing rates during the full ramp (A), the ascending limb of the ramp (B), and the descending limb of the ramp (C). Biceps brachii (BIC) data are from elbow flexion ramps, and triceps brachii (TRI) data are from elbow extension ramps.
The observed ranges of MU firing rates for the ascending and descending limbs of the torque ramps are displayed in Fig. 5B and C, respectively. During the ascending torque phase, the group mean firing rate range in the younger participants was 9.3 (0.96) pps in the BIC and 10.4 (0.79) pps in the TRI, while in the older participants, the group mean firing rate range was 8.7 (1.41) pps in the BIC and 9.7 (1.50) pps in the TRI. In the BIC, the effect size of the difference in firing rate range on the ascending limb between younger and older participants was 0.44, and in the TRI the effect size was 0.60. However, age group was not a significant predictor (P = 0.1112) nor was the interaction between muscle and age group (P = 0.7546). Muscle was the only significant predictor of MU firing rate range over the ascending limb of the torque ramp (χ2 (1) = 10.9590, P = 0.0009). The ascending limb firing rate range was 1.1 (0.29) pps higher in the TRI than in the BIC (P = 0.0014).
During the descending torque phase, the group mean MU firing rate ranges were higher in the younger participants compared to the older participants for both muscles (BIC: 11.0 (1.49) vs. 9.4 (1.66) pps, ES = 0.91; TRI: 12.7 (1.37) vs. 10.5 (1.97) pps, ES = 1.18). Both age group (χ2 (1) = 7.7199, P = 0.0055) and muscle (χ2 (1) = 10.856, P = 0.0010) were significant predictors of descending limb firing rate range, in our model. The interaction between age group and muscle was not significant (P = 0.4002). During the descending torque ramps, the firing rate range was 1.9 (0.62) pps higher in younger participants (P = 0.0083), as compared to older participants, and 1.4 (0.37) pps higher in the TRI (P = 0.0015), as compared to the BIC.
Similar to the peak firing rates, older participants showed a reduced range of MU firing rates overall, as well as a reduction in firing rate range on the descending limb. However, the firing rate range during the ascending portion of the torque ramp was not significantly affected by ageing. The difference in firing rate range between younger and older participants can be appreciated in an example of smoothed firing rates from the TRI of one younger and one older participant in Fig. 3.
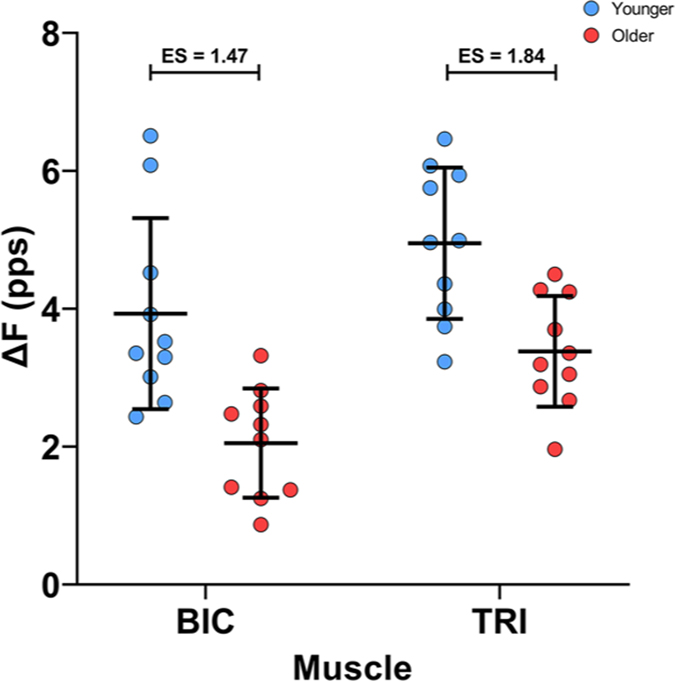
Estimates of persistent inward currents using ΔF
Subject mean values for the ΔF calculation are shown in Fig. 6. Group means for ΔF were substantially higher in the younger participants than in the older participants in the BIC (4.1 (1.35) vs. 2.3 (0.84) pps, ES = 1.47) and in the TRI (5.2 (0.94) vs. 3.2 (1.10) pps, ES = 1.84). Age group (χ2 (1) = 18.326, P < 0.0001) and muscle (χ2 (1) = 17.796, P < 0.0001) were both significant predictors for ΔF in our model, but the interaction between those variables was not significant (P= 0.2848). ΔF was reduced by 1.9 (0.36) pps in the older participants (P < 0.0001), and was 1.3 (0.24) pps lower in the BIC than in the TRI (P < 0.0001).
Figure 6. Estimates of persistent inward current magnitude during isometric ramp contractions.
ΔF values from the biceps brachii (BIC; left) during elbow flexion and triceps brachii (TRI; right) during elbow extension. Participant means in colour (younger: blue; older: red), with the black bars denoting group mean and SD.
Estimates of persistent inward currents using ΔF with previous inclusion criteria
Similar to the ΔF values computed by excluding pairs that had test unit and reporter unit recruitment time differences of ≥1 s and derecruitment time differences ≥1.5 s, and test units that were active for ≥2 s (Hassan et al. 2020), ΔF values computed with previously used inclusion criteria (i.e. rate–rate correlation r2 ≥ 0.7, reporter unit rate modulation ≥0.5 pps, test unit duration ≥2 s; Gorassini et al. 2004; Udina et al. 2010; Stephenson & Maluf, 2011; Vandenberk & Kalmar, 2014) were higher in TRI (5.55 (1.06) vs. 3.56 (0.79) pps, ES = 1.91) and BIC (5.26 (1.39) vs. 2.44 (0.86) pps, ES = 2.22) in younger compared to older participants. Age group (χ2 (1) = 12.91, P = 0.0003268) and muscle (χ2 (1) = 12.909, P = 0.0003268) were both significant predictors for ΔF in our model, but the interaction between those variables was not significant (P = 0.0879). ΔF was reduced by 2.2 (0.46) pps in the older participants (P = 0.0002), and was 0.83 (0.19) pps lower in the BIC than in the TRI (P = 0.0004).
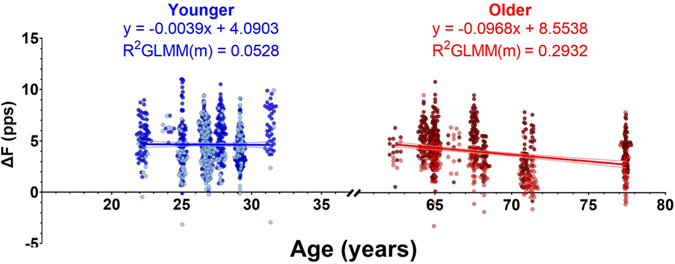
Relationships between ΔF and age
We then determined whether any relationship existed between the reported age of participants and ΔF within each of these age groups. In Fig. 7, ΔF is plotted as a function of participant age along with regression lines from our model. In the younger participants, the generalized linear mixed effects model accounted for 31.96% of the variance in ΔF, with the fixed effects of muscle and age accounting for 5.28% of the variance. Muscle was a significant predictor of ΔF (χ2 (1) = 5.3981, P = 0.0202), but age was not a significant predictor of ΔF in the younger participants (χ2 (1) = 0.0021, P = 0.9637). The interaction between age and muscle was also not a significant predictor (χ2 (1) = 0.3742, P = 0.5407).
Figure 7. The relationship between ΔF and age.
Motor units from the younger (blue, left) and older (red, right) groups of participants are shown, along with lines indicating the individual generalized linear mixed effects models. We show only the overall slope of the model, which includes fixed effects of age and muscle. For clarity of display, individual participants are not distinguished from one another, but the random effect in the model does account for the variability within each participant. Dark and light data points indicate ΔF values from triceps brachii (TRI) and biceps brachii (BIC) MUs, respectively. Some jitter was added to the data point x-value (age) for clarity of display. Equations derived from the model are displayed for the younger and older participants along with the marginal R2GLMM, which indicates the variance accounted for by our fixed effects of muscle and age.
In the older participants, the model accounted for 45.98% of the observed variance in ΔF, with the fixed effects accounting for 29.32% of the variance. Both muscle (χ2 (1) = 14.75, P = 0.0001) and age (χ2 (1) = 4.9504, P = 0.0261) were significant predictors of ΔF, but the interaction between them was not (χ2 (1) = 0.8031, P = 0.3702). Greater reductions in ΔF were associated with increasing age in the older participants (−0.097 (0.041) pps/year, P = 0.0473). In summary, ΔF was reduced in older participants compared to younger participants and a negative relationship existed between age and ΔF in the older participants, but not in the younger participants.
Relationships between ΔF and strength
We also determined whether ΔF was predicted by muscular strength of the individuals, both as a whole and by age group. Overall, MVT was not a significant predictor of ΔF in the younger (χ2 (1) = 0.7069, P = 0.4005), older (χ2 (1) = 0.0093, P = 0.9233) or entire group (χ2 (1) = 0.6245, P = 0.4294).
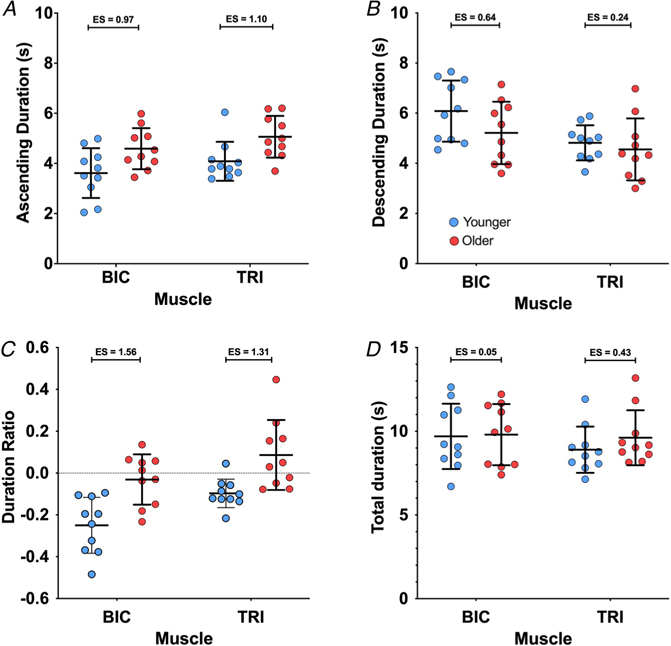
MU firing duration
Provoked by the reduction in firing rate hysteresis in older participants (i.e. reduced ΔF), we investigated whether the duration of the MU firing differed between age groups; the subject and group means for all MU duration variables are plotted in Fig. 8. On the ascending limb of the ramp (Fig. 8A), the group means for MU firing duration were shorter in the younger participants than in the older participants (see Fig. 3 for example) in both the BIC (3.6 (0.99) vs. 4.6 (0.82) s, ES = 0.97) and the TRI (4.1 (0.78) vs. 5.1 (0.83) s, ES = 1.10). Our model found age group (χ2 (1) = 9.3990, P = 0.0022) and muscle (χ2 (1) = 4.5555, P = 0.0328) were both significant predictors of firing duration on the ascending limb of the torque ramp, while the interaction between the two variables was not significant (P = 0.9224). Ascending limb firing duration was increased by 1.0 (0.30) s in the older participants (P = 0.0039), compared to younger participants, and was 0.5 (0.22) s longer in TRI MUs than BIC MUs (P = 0.0401).
Figure 8. Motor unit firing durations during isometric ramp contractions.
Participant means (colour) and group means (black), showing the firing duration of MUs for the full torque ramp (A), the ascending limb of the torque ramp (B), and the descending limb of the torque ramp (C).
On the descending limb of the torque ramps (Fig. 8B), the group means for MU firing duration were longer for the younger participants than the older participants in both muscles (BIC: 6.1 (1.22) vs. 5.2 (1.24) s, ES = 0.64; TRI: 4.8 (0.70) vs. 4.6 (1.24) s, ES = 0.24). Muscle was revealed to be a significant predictor of firing duration on the decreasing torque ramp (χ2 (1) = 7.4705, P = 0.0063), but age group was not a significant predictor (P = 0.1765) and the interaction of age group and muscle was not significant (P = 0.3589). Descending limb firing duration was 1.0 (0.33) s longer in BIC MUs than in TRI MUs.
The duration ratios (Fig. 8C) were lower for the younger adults than older adults in both the BIC (−0.25 (0.13) vs. −0.03 (0.12) s, ES = 1.56) and the TRI (−0.10 (0.07) vs. 0.09 (0.17) s, ES = 1.31), indicative of more symmetry of firing durations between the ascending and descending limbs of the torque ramps in older adults (i.e. leftward shift or less hysteresis). Our model found that both age group (χ2 (1) = 14.003, P = 0.0002) and muscle (χ2 (1) = 13.090, P = 0.0003) were significant predictors of duration ratio, but the interaction between age and muscle was not significant (P = 0.5528). The duration ratio was 0.20 (0.05) higher in the older adults than in the younger adults, and 0.14 (0.03) higher in TRI MUs than in BIC MUs.
Total MU firing duration was similar between age groups and muscles (Fig. 8D). The group means for total MU firing duration in the younger participants were 9.7 (1.95) s in the BIC and 8.9 (1.38) s in the TRI. In the older participants, the group mean for MU firing duration was 9.8 (1.83) s in the BIC and 9.6 (1.64) s in the TRI. The effect sizes between younger and older participants were 0.05 for the BIC and 0.43 in the TRI. However, the linear mixed effects model found that age group (P = 0.3534), muscle (P = 0.3182), and the interaction between age group and muscle (P = 0.5084) were not significant predictors of MU firing duration.
In summary, older participants had an increased duration of firing on the ascending limb of the torque ramp and an increased ratio of firing during the torque ramp, but the overall firing duration and the firing duration on the descending limb of the torque ramp were similar to younger participants. As shown in Fig. 3, a longer duration of firing during the ascending phase of the ramp without a difference during the descending phase of the ramp or total duration of firing indicates a leftward shift, and more symmetrical pattern of firing.
Discussion
The present study aimed to investigate the effects of healthy ageing on the MU firing patterns from the BIC and TRI by comparing younger and older healthy adults. In agreement with previous literature (Dalton et al. 2010), we have found lower peak firing rates during isometric submaximal ramp contractions in both the BIC and TRI of older adults. Further, and perhaps most novel, we found substantial and significant reductions in estimates of persistent inward currents (PICs; ΔF) in older adults irrespective of whether estimated from the BIC or TRI. In older adults, we also found that age was a significant predictor of ΔF (i.e. ΔF decreased with respect to age); however, there was no such relationship in the younger group. Additional characteristics of MU firing patterns support the notion of reduced onset–offset hysteresis, such as reduced firing rate range during the descending phase of the ramp and a leftward shift in MU firing in older compared to younger adults. These findings suggest that MU firing patterns in older people exhibit less PIC activity, which may have implications for motor control.
When compared to younger adults, MU firing rates are typically reduced in older adults (Kamen et al., 1995; Connelly et al., 1999; Kamen & Knight, 2004; Barry et al., 2007; Dalton et al., 2010; Kirk et al., 2018; Kirk et al., 2019), although some evidence has shown no differences with ageing (Roos et al. 1999; Dalton et al. 2009; Kirk et al. 2016). Firing rates are highly dependent upon the biophysical properties of the parent motoneurons, and age-related changes of such properties can lead to reductions in firing rates. For example, spike after-hyperpolarization (AHP) duration is increased in aged rodents (Cameron et al. 1991; Kalmar et al. 2009) and cats (Morales et al. 1987). Similarly, AHP duration increases gradually with age (Piotrkiewicz et al. 2007), and when compared directly, AHP is longer in older compared to younger adults (Christie & Kamen, 2010). In the current investigation, not only were peak firing rates reduced but, older adults showed a compressed range of MU firing rates. This compressed range of firing can arise from similar mechanisms that underlie the reductions in peak firing rates, but it is interesting to note that the firing rate range during the ascending phase of the contraction was similar for both younger and older adults. That is, the rate modulation from the onset of firing to peak firing was similar (see Fig. 5B). On the contrary, the reduction in overall firing rate range (∼1.7 pps) seen in older participants is primarily attributed to the reduction in firing rate range seen on the descending limb of the torque ramp (∼1.9 pps). The reduced firing range seen on the descending limb (i.e. reduced hysteresis) of the torque ramps is most likely related to a decrease in PIC activity, which brings us to the main topic of our discussion: reduced estimates of PICs in older adults.
Estimates of PICs are reduced in older people
We have shown that ΔF is substantially lower in both the elbow flexor and extensor muscles of older adults, compared to younger adults. Further, in our older group, we found that increasing age was associated with reductions in ΔF (see Fig. 7). In addition, the relative firing duration of motor units is shifted to the left in older adults, such that the duration of firing is symmetrical during the ascending and descending phases of the ramp contraction, indicating less onset–offset hysteresis. Although ΔF and the leftward shift in firing patterns are an indirect estimate of PIC activity, they do support our hypothesis that PICs are reduced in older people. Such age-related changes in estimates of PICs are most likely due to changes in (1) monoaminergic input to the motoneurons, (2) the amount or pattern of inhibition, and/or (3) Na+ or Ca2+ channel function.
The overall leftward shift in the firing patterns of older individuals provides further insights into the effects of ageing on MU firing. Based on the reduced ΔF and reduced firing rate range on the descending limb in the older adults, we expected to see a reduced duration of firing on the descending limb in those participants. Instead, we found an increased duration ratio, which was driven by a duration of firing on the ascending limb of the torque ramps in older participants, without significant changes to the overall duration and descending duration of firing. This could suggest that motor units in older participants were recruited earlier than those from younger participants. Although there did appear to be a slight reduction in the average recruitment threshold of decomposed motor units in the TRI, this was not the case in BIC, and age group was not a significant predictor of recruitment threshold in our linear mixed effects model. There are other reports that have shown a lower average recruitment threshold for motor units recorded from older compared to younger adults (Erim et al. 1999; Klass et al. 2005, 2008; Fling et al. 2009; Pascoe et al. 2011), but methodological considerations (i.e. HDsEMG decomposition is biased toward larger and more superficial MUs; Hassan et al. 2019) may skew interpretations deduced from comparisons of recruitment thresholds between heterogeneous groups of people (i.e. younger vs. older herein). Most intriguing in the current study, however, is the fact that the firing patterns of older individuals were more symmetrical (approximately equal time on the ascending and descending portion of the ramps – see Figs 3 and 8C), indicating less hysteresis, a behaviour that is a hallmark of PICs.
Initial attempts to understand PIC effects of human MU firing behaviour focused on self-sustained firing or ‘bi-stability’ (Kiehn & Eken, 1997). In such experiments, MUs are tracked during low-level voluntary efforts and an additional source of synaptic input (i.e. vibration) causes the recruitment of an additional MU (test unit) that maintains firing after the additional input is removed (Gorassini et al. 1998). MUs can then be classified as either having PICs or not based on the occurrence of test units that maintain firing after the additional synaptic input is removed. Using this approach, Kamen et al. (2006) showed that older individuals have a similar occurrence (23.1%) of MUs that exhibit self-sustained firing as younger adults (22.8%). As such, and contrary to the findings in our current investigation, they concluded that PIC-like behaviour does not seem to be affected by the ageing process. They did, however, report that the mean drop-out torque of newly recruited MUs was slightly higher for older adults (3.26% vs. 2.43% maximal voluntary contraction), although variability was high and therefore no statistical differences were reported. It is important to note that the ‘occurrence’ of self-sustained firing may not be the be-all and end-all method to quantify whether PICs are present during voluntary motor behaviour in humans. This is because PICs almost certainly contribute to motoneuron firing during all voluntary behaviours, and without the amplification effects of PICs, the small currents produced by descending and sensory inputs are too weak to have much of an effect on motoneuron firing (Binder & Powers, 2001). More important to the understanding of human motor output is the magnitude of PICs, rather than the relative number of motor units that exhibit a single characteristic mediated by PICs.
Hysteresis of MU firing rates, on the other hand, has proven to be the most consistent hallmark for non-invasive estimation of the magnitude of PICs in humans, as was first realized by Gorassini, Bennett and colleagues (Bennett et al. 2001a; Gorassini et al. 2002). The now standard paired-MU analysis technique (ΔF) has been subject to rigorous investigations interested in the accuracy of these estimates (Bennett et al. 2001a,b; Powers et al. 2008; Revill & Fuglevand, 2011; Powers & Heckman, 2015; Afsharipour et al. 2020; Hassan et al. 2020). Bennett and colleagues (Bennett et al. 2001a,b) used parallel MU and intracellular recordings in rat motoneurons to clearly demonstrate that ΔF reflects features of PICs. With advances in technology, these estimates of PICs have been obtained across hundreds of MUs (Afsharipour et al. 2020; Hassan et al. 2020; Kim et al. 2020; Trajano et al. 2020), which likely provides a better overall estimation of PIC magnitude across the entire motor pool. Even though MUs of older adults in our experiment certainly displayed onset–offset hysteresis (i.e. positive ΔF values overall), the magnitude of this hysteresis was markedly reduced compared to the sample of younger adults that were recruited. In fact, estimates of PICs (ΔF) were reduced by ∼40%, with very large effect sizes (all ES >1.45). In addition, the age of individuals was a significant predictor of ΔF, suggesting that PICs may deteriorate with age at a rate of ∼1 pps/decade, but only in older adults.
The magnitude of PICs is directly proportional to the level of noradrenaline (NA) and serotonin (5-HT) (Lee & Heckman, 1998, 2000), which are monoamines released from the from the caudal raphe nucleus and locus coeruleus, respectively. These monoaminergic nuclei of the brainstem deteriorate with age (Shibata et al. 2006; Pagano et al. 2017), and in particular, the age-related reduction in locus coeruleus structural integrity is associated with impaired cognitive and behavioural function (Liu et al. 2020), as well as reductions in central pain modulation (Grashorn et al. 2013; Damien et al. 2018). Deterioration of these nuclei could also lead to reductions in neuromodulatory drive to motorneurons, reducing PIC activity, which would ultimately explain some of the reductions observed in ΔF. NA-mediated effects are likely predominantly due to degradation of the locus coeruleus because older rodents maintain only ∼30% NA nuclei compared to ∼90% 5-HT nuclei (Tatton et al. 1991). Despite the evidence that a greater proportion of raphe nuclei are maintained with age, spinal 5-HT is greatly reduced (Johnson et al. 1993; Ko et al. 1997). Therefore, 5-HT-mediated effects in the ageing process are more likely to occur peripherally. With ageing, there is increased circulation of cytokines (so-called ‘inflamm-ageing’) (Michaud et al. 2013), which affect 5-HT receptors and increase re-uptake of 5-HT (Steinbusch et al. 2021). In sum, less availability of monoamines would result in reduced PIC magnitude at the same relative effort, which is what we observed as a reduction in ΔF.
PICs are also highly sensitive to inhibitory inputs (Hultborn et al. 2003; Kuo et al. 2003; Heckman et al. 2008a; Hyngstrom et al. 2008; Revill & Fuglevand, 2017). Thus, changes to the amount or pattern of inhibition may lead to reduced estimates of PICs as estimated by ΔF. Indeed, there are age-related alterations in spinal and supraspinal inhibitory circuits (Butchart et al. 1993; Kido et al. 2004; Hortobagyi et al. 2006) that could modify the synaptic input to motoneurons. Modulation of Ia presynaptic inhibition with increasing contraction intensity is reduced in older adults (Butchart et al. 1993), which would lead to differences in the pattern of inhibition and have profound effects on the balance of excitation, inhibition and neuromodulation (Johnson et al. 2017) required to perform a task. The imbalance of inhibition and excitation could therefore play a role in the profound effects on PICs observed in older adults. While difficult to measure, the temporal pattern of inhibitory commands is likely to affect ΔF (Powers et al. 2012; Johnson et al. 2017). Push–pull inhibition, where inhibition varies inversely with excitation, can lead to reductions in MU hysteresis (Powers et al. 2012). It therefore remains possible that the pattern of the inhibitory commands is altered with age to compensate for the various structural and functional changes in the neuromuscular system (Hepple & Rice, 2016; McNeil & Rice, 2018) associated with the ageing process and may contribute to our observed reductions in ΔF.
Alterations in the integrity and function of 5-HT/NA receptors and voltage sensitive ion channels must also be considered in relation to age-related changes in the nervous system. Basic (i.e. larger and longer AHP, lower rheobase, greater input resistance) and rhythmic (i.e. slower minimum and maximum steady-state firing frequencies and lower f–I slopes) motoneuron properties are consistent with reduced motoneuron excitability in very old (>30 months) rodents. However, Kalmar et al. (2009) also showed an increased incidence of PIC-like behaviour in very old rodent motoneurons, which they suggested to have resulted from increased 5-HT and NA receptor sensitivity to residual endogenous monoamines (Harvey et al. 2006) as a compensatory mechanism to counteract the reduced motoneuron excitability. Although this increased incidence of PIC may seem to contradict our findings, this type of analysis simply determines the relative number of motoneurons that have hysteresis in response to current injection to the soma, whereas we quantified the average magnitude of hysteresis during voluntary activation (i.e. axo-dendritic synaptic input). L-type Ca2+ channels are concentrated in the dendritic tree, far away from the soma, meaning that the levels of injected current may have underestimated PICs in a healthy younger motoneuron due to the inability to activate PICs from the soma (Bennett et al. 1998; Lee & Heckman, 1998). Ageing may also result in changes in the expression of receptor subtypes or the downstream signalling of various receptors. Indeed, there are age-related reductions in the duration of Ca2+-mediated plateau potentials in striatal neurons (Dunia et al. 1996), and more generally, deregulated Ca2+ is an active component of healthy ageing that can increase the risk of cell death and neurodegenerative disorders (Nikoletopoulou & Tavernarakis, 2012). As such, it is difficult to pinpoint the exact monoaminergic receptor or ion channel dysfunction that may contribute to the observed reductions in estimated PICs during voluntary contractions with ageing.
Methodological considerations
Since this experiment was conducted in a non-invasive fashion, we were unable to directly determine the PIC magnitude. Instead, we relied on the best estimation of PICs available in humans (i.e. ΔF), which has undergone rigorous scrutiny to ensure accuracy of the estimates (Bennett et al. 2001a,b; Powers et al. 2008; Revill & Fuglevand, 2011; Powers & Heckman, 2015; Afsharipour et al. 2020; Hassan et al. 2020). Nonetheless, it is difficult to determine whether the reduction in ΔF is the result of alterations in monoaminergic drive, the amount or pattern of inhibition, and/or changes to the monoaminergic receptor sensitivity or ion channel function. This delineation will require further work.
Overall, the MUs decomposed in the older participants had longer durations on the ascending phase of the ramp, suggesting a lower relative threshold of units decomposed for older adults. This relatively lower recruitment torque could lead to a ceiling effect in terms of how much hysteresis those units could exhibit. However, as the average duration of MU firing on the descending limb was 5.2 and 4.6 s for the BIC and TRI, respectively, in the older participants with the descending limb of the torque ramp being 10 s long, we do not believe the reduced ΔF observed was due to early derecruitment due to the time constraints of the task. Indeed, age group was not a significant predictor of the recruitment thresholds in our linear mixed effects model, further suggesting that recruitment threshold did not cause reduced estimates of PICs in older adults. It is very important to note, however, that due to the heterogeneity of subjects (i.e. anatomy, electrode placement, subcutaneous tissue, etc.) the comparison of recruitment thresholds of decomposed MUs across subjects may not reflect whether there is truly a shift in the recruitment of all MUs in older adults. For example, the decomposition algorithm is biased toward MUs close to the recording site (i.e. closer to the skin surface) and MUs with larger fibres (i.e. larger MUs; Hassan et al. 2019), which has the potential to skew the findings, especially in older adults who have undergone MU remodelling.
Since the mean age of the older participants in this group is only 67.8 years, some of our sample may not have substantial loss of motoneurons, and the subsequent reorganization of the motor pool would lead to only modest reductions of firing rates. Since our older group was in the mid-seventh decade on average, this might help explain why our older group was not significantly weaker than our younger group. It remains possible that further ageing may have more severe effects on MU firing behaviour and specifically estimates of PICs.
Practical considerations
In the words of Power and colleagues (2016), ‘If you don’t use it, you’ll likely lose it’. Whether this holds true for PICs is unclear at the moment, although Latella (2021) recently made a compelling argument for studying the efficacy of strength training to mitigate the effects of ageing on MU firing behaviour. Indeed, the work of Power and colleagues (2010) suggests that estimates of MU numbers are greater in masters runners compared to their sedentary counter-parts, though contrasting evidence has since shown that masters runners may not be spared from MU remodelling (Piasecki et al. 2016). Strength training-induced plasticity of motoneurons is certainly not limited to younger adults. AHP duration is longer in older compared to younger adults, but that duration can be reduced with strength training in both age groups (Christie & Kamen, 2010). Thus, it remains possible that strength training, which necessitates high levels of effort (likely utilizing high levels of monoaminergic drive; Orssatto et al. 2021), could mitigate deterioration of monoaminergic function and/or PIC behaviour seen in older adults.
Conclusion
The present study compared the firing patterns of MUs from the elbow extensors and flexors of healthy younger and older adults during isometric ramp contractions. Irrespective of muscle, age was a significant predictor of peak firing rate and firing rate hysteresis, such that both were reduced in older adults. In addition to the differences observed between age groups, the age of individuals within the older group predicted a ∼1 pps per decade reduction in ΔF, a non-invasive estimate of PIC magnitude across the motor pool. This reduced estimate of PIC magnitude likely arises from reductions in monoaminergic input, alterations in the amount or pattern of inhibition, and/or alterations in monoamine receptor or ion channel function. It remains unclear whether alterations in firing rate hysteresis are a compensatory adjustment or impairment that occurs with ageing, but it remains possible that physical training may be able to mitigate such changes. However, since we did not observe any relationships between MVT and estimates of PICs (i.e. ΔF), a couple of questions remain: (1) what role do PICs play in normal human function? And (2) do reductions in PICs precede reductions in function?
Supplementary Material
Key points.
Persistent inward currents play an important role in the neural control of human movement and are influenced by neuromodulation via monoamines originating in the brainstem.
During ageing, motor unit firing rates are reduced, and there is deterioration of brainstem nuclei, which may reduce persistent inward currents in alpha motoneurons.
Here we show that estimates of persistent inward currents (ΔF) of both elbow flexor and extensor motor units are reduced in older adults.
Estimates of persistent inward currents have a negative relationship with age in the older adults, but not in the young.
This novel mechanism may play a role in the alteration of motor firing rates that occurs with ageing, which may have consequences for motor control.
Acknowledgements
The authors would like to thank Sabeen Admani and Ahalya Mandana for technical assistance with the design of the apparatus used in the study.
Funding
This study was supported by the National Institute of Neurological Disorders and Stroke (R01NS098509), the National Institute of Child Health and Human Development (R01HD039343) and the Natural Sciences and Engineering Research Council of Canada (NSERC PDF).
Biography

Altamash Hassan is a biomedical engineer who earned his PhD from Northwestern University. During his time at Northwestern, he was mentored by Dr Julius P. A. Dewald, and his research investigates the neural control of movement through analyses of motor unit firing patterns in both healthy and pathological states. Investigating the effects of ageing on motor unit behaviour stemmed from an informal discussion with Dr Gregory Pearcey and provides the foundation for future investigations of interventions aimed at ameliorating motor deficits associated with ageing, and providing appropriate age-matched controls for those deficits seen in individuals post-stroke.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Supporting information
Additional supporting information can be found online in the Supporting Information section at the end of the HTML view of the article. Supporting information files available:
The peer review history is available in the Supporting Information section of this article (https://doi.org/10.1113/JP282063#support-information-section).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
References
- Afsharipour B, Manzur N, Duchcherer J, Fenrich KF, Thompson CK, Negro F, Quinlan KA, Bennett DJ & Gorassini MA (2020). Estimation of self-sustained activity produced by persistent inward currents using firing rate profiles of multiple motor units in humans. J Neurophysiol 124, 63–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry BK, Pascoe MA, Jesunathadas M & Enoka RM (2007). Rate coding is compressed but variability is unaltered for motor units in a hand muscle of old adults. J Neurophysiol 97, 3206–3218. [DOI] [PubMed] [Google Scholar]
- Barton K (2018). Multi-model inference. R package version 1.42.1. https://cran.r-project.org/web/packages/MuMIn/index.html. [Google Scholar]
- Bates D, Mächler M, Bolker B & Walker S (2015). Fitting linear mixed-effects models using lme4. J Stat Softw 67, 1–48. [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B & Gorassini M (1998). Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80, 2023–2037. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ & Gorassini M (2001a). Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol 86, 1972–1982. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y & Siu M (2001b). Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86, 1955–1971. [DOI] [PubMed] [Google Scholar]
- Binder MD & Powers RK (2001). Relationship between simulated common synaptic input and discharge synchrony in cat spinal motoneurons. J Neurophysiol 86, 2266–2275. [DOI] [PubMed] [Google Scholar]
- Boccia G, Martinez-Valdes E, Negro F, Rainoldi A & Falla D (2019). Motor unit discharge rate and the estimated synaptic input to the vasti muscles is higher in open compared with closed kinetic chain exercise. Journal of Applied Physiology 127, 950–958. [DOI] [PubMed] [Google Scholar]
- Butchart P, Farquhar R, Part NJ & Roberts RC (1993). The effect of age and voluntary contraction on presynaptic inhibition of soleus muscle Ia afferent terminals in man. Exp Physiol 78, 235–242. [DOI] [PubMed] [Google Scholar]
- Cameron WE, Jodkowski JS, Fang H & Guthrie RD (1991). Electrophysiological properties of developing phrenic motoneurons in the cat. J Neurophysiol 65, 671–679. [DOI] [PubMed] [Google Scholar]
- Christie A & Kamen G (2010). Short-term training adaptations in maximal motor unit firing rates and afterhyperpolarization duration. Muscle Nerve 41, 651–660. [DOI] [PubMed] [Google Scholar]
- Cogliati M, Cudicio A, Martinez-Valdes E, Tarperi C, Schena F, Orizio C & Negro F (2020). Half marathon induces changes in central control and peripheral properties of individual motor units in master athletes. J Electromyogr Kinesiol 55, 102472. [DOI] [PubMed] [Google Scholar]
- Connelly DM, Rice CL, Roos MR & Vandervoort AA (1999). Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol 87, 843–852. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Harwood B, Davidson AW & Rice CL (2009). Triceps surae contractile properties and firing rates in the soleus of young and old men. J Appl Physiol 107, 1781–1788. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Jakobi JM, Allman BL & Rice CL (2010). Differential age-related changes in motor unit properties between elbow flexors and extensors. Acta Physiol 200, 45–55. [DOI] [PubMed] [Google Scholar]
- Damien J, Colloca L, Bellei-Rodriguez CE & Marchand S (2018). Pain modulation: from conditioned pain modulation to placebo and nocebo effects in experimental and clinical pain. Int Rev Neurobiol 139, 255–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio A, Holobar A, Falla D, Felici F, Enoka RM & Farina D (2020). Tutorial: Analysis of motor unit discharge characteristics from high-density surface EMG signals. J Electromyogr Kinesiol 53, 102426. [DOI] [PubMed] [Google Scholar]
- Del Vecchio A, Negro F, Felici F & Farina D (2018). Distribution of muscle fibre conduction velocity for representative samples of motor units in the full recruitment range of the tibialis anterior muscle. Acta Physiol 222, e12930. [DOI] [PubMed] [Google Scholar]
- Dunia R, Buckwalter G, Defazio T, Villar FD, McNeill TH & Walsh JP (1996). Decreased duration of Ca2+-mediated plateau potentials in striatal neurons from aged rats. J Neurophysiol 76, 2353–2363. [DOI] [PubMed] [Google Scholar]
- Erim Z, Beg MF, Burke DT & de Luca CJ (1999). Effects of aging on motor-unit control properties. J Neurophysiol 82, 2081–2091. [DOI] [PubMed] [Google Scholar]
- Fling BW, Knight CA & Kamen G (2009). Relationships between motor unit size and recruitment threshold in older adults: implications for size principle. Exp Brain Res 197, 125–133. [DOI] [PubMed] [Google Scholar]
- Giboin LS, Tokuno C, Kramer A, Henry M & Gruber M (2020). Motor learning induces time-dependent plasticity that is observable at the spinal cord level. J Physiol 598, 1943–1963. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Bennett DJ & Yang JF (1998). Self-sustained firing of human motor units. Neurosci Lett 247, 13–16. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ & Yang JF (2004). Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127, 2247–2258. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M & Bennett DJ (2002). Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87, 1850–1858. [DOI] [PubMed] [Google Scholar]
- Gordon T, Hegedus J & Tam SL (2004). Adaptive and maladaptive motor axonal sprouting in aging and motoneuron disease. Neurol Res 26, 174–185. [DOI] [PubMed] [Google Scholar]
- Grashorn W, Sprenger C, Forkmann K, Wrobel N & Bingel U (2013). Age-dependent decline of endogenous pain control: exploring the effect of expectation and depression. PLoS One 8, e75629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y & Bennett DJ (2006). Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96, 1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A, Kim E, Khurram O, Cummings M, Thompson C, McPherson L, Heckman C, Dewald J & Negro F (2019). Properties of motor units of elbow and ankle muscles decomposed using high-density surface EMG. In 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp. 3874–3878. IEEE, Berlin, Germany. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A, Thompson CK, Negro F, Cummings M, Powers RK, Heckman CJ, Dewald JPA & McPherson LM (2020). Impact of parameter selection on estimates of motoneuron excitability using paired motor unit analysis. J Neural Eng 17, 016063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ & Enoka RM (2012). Motor unit. Comp Physiol 2, 2629–2682. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Hyngstrom AS & Johnson MD (2008a). Active properties of motoneurone dendrites: diffuse descending neuromodulation, focused local inhibition. J Physiol 586, 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Michael Johnson, Mottram Carol, Jenna Schuster (2008) Persistent Inward Currents in Spinal Motoneurons and Their Influence on Human Motoneuron Firing Patterns. The Neuroscientist 14, 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT & Rice CL (2016). Innervation and neuromuscular control in ageing skeletal muscle. J Physiol 594, 1965–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holobar A & Zazula D (2007) Multichannel blind source separation using convolution kernel compensation. IEEE Trans Signal Process 55, 4487–4496. [Google Scholar]
- Hortobágyi T, delOlmo MF & Rothwell JC (2006). Age reduces cortical reciprocal inhibition in humans. Exp Brain Res 171, 322–329. [DOI] [PubMed] [Google Scholar]
- Hug F, Avrillon S, Del Vecchio A, Casolo A, Ibanez J, Nuccio S, Rossato J, Holobar A & Farina D (2021). Analysis of motor unit spike trains estimated from high-density surface electromyography is highly reliable across operators. J Electromyogr Kinesiol 58, 102548. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Denton ME, Wienecke J & Nielsen JB (2003). Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J Physiol 552, 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyngstrom AS, Johnson MD & Heckman CJ (2008). Summation of excitatory and inhibitory synaptic inputs by motoneurons with highly active dendrites. J Neurophysiol 99, 1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H, Ulfhake B, Dagerlind A, Bennett GW, Fone KC & Hokfelt T (1993). The serotoninergic bulbospinal system and brainstem-spinal cord content of serotonin-, TRH-, and substance P-like immunoreactivity in the aged rat with special reference to the spinal cord motor nucleus. Synapse 15, 63–89. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Thompson CK, Tysseling VM, Powers RK & Heckman CJ (2017). The potential for understanding the synaptic organization of human motor commands via the firing patterns of motoneurons. J Neurophysiol 118, 520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PC (2014). Extension of Nakagawa & Schielzeth’s R2GLMM to random slopes models. Methods Ecol Evol 5, 944–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar JM, Button DC, Gardiner K, Cahill F & Gardiner PF (2009). Caloric restriction does not offset age-associated changes in the biophysical properties of motoneurons. J Neurophysiol 101, 548–557. [DOI] [PubMed] [Google Scholar]
- Kamen G & Knight CA (2004). Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci 59, 1334–1338. [DOI] [PubMed] [Google Scholar]
- Kamen G, Sison SV, Du CC & Patten C (1995). Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol 79, 1908–1913. [DOI] [PubMed] [Google Scholar]
- Kamen G, Sullivan R, Rubinstein S & Christie A (2006). Evidence of self-sustained motoneuron firing in young and older adults. J Electromyogr Kinesiol 16, 25–31. [DOI] [PubMed] [Google Scholar]
- Kanda K & Hashizume K (1989). Changes in properties of the medial gastrocnemius motor units in aging rats. J Neurophysiol 61, 737–746. [DOI] [PubMed] [Google Scholar]
- Kido A, Tanaka N & Stein RB (2004). Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol 82, 238–248. [DOI] [PubMed] [Google Scholar]
- Kiehn O & Eken T (1997). Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol 78, 3061–3068. [DOI] [PubMed] [Google Scholar]
- Kim EH, Wilson JM, Thompson CK & Heckman CJ (2020). Differences in estimated persistent inward currents between ankle flexors and extensors in humans. J Neurophysiol 124, 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk EA, Copithorne DB, Dalton BH & Rice CL (2016). Motor unit firing rates of the gastrocnemii during maximal and sub-maximal isometric contractions in young and old men. Neuroscience 330, 376–385. [DOI] [PubMed] [Google Scholar]
- Kirk EA, Gilmore KJ & Rice CL (2018). Neuromuscular changes of the aged human hamstrings. J Neurophysiol 120, 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk EA, Gilmore KJ, Stashuk DW, Doherty TJ & Rice CL (2019). Human motor unit characteristics of the superior trapezius muscle with age-related comparisons. J Neurophysiol 122, 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass M, Baudry S & Duchateau J (2005). Aging does not affect voluntary activation of the ankle dorsiflexors during isometric, concentric, and eccentric contractions. J Appl Physiol 99, 31–38. [DOI] [PubMed] [Google Scholar]
- Klass M, Baudry S & Duchateau J (2008). Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol 104, 739–746. [DOI] [PubMed] [Google Scholar]
- Ko ML, King MA, Gordon TL & Crisp T (1997). The effects of aging on spinal neurochemistry in the rat. Brain Res Bull 42, 95–98. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Johnson MD, Heckman HM & Heckman CJ (2003). Active dendritic integration of inhibitory synaptic inputs in vivo. J Neurophysiol 90, 3617–3624. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB & Christensen RH (2017). lmerTest package: tests in linear mixed effects models. J Stat Softw 82, doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- Latella C (2021). Pick me, Pick me! Rationale for investigating persistent inward currents (PICs) and associated exercise effects in the ageing neuromuscular system. J Physiol 599, 1957–1959. [DOI] [PubMed] [Google Scholar]
- Lee RH & Heckman CJ (1998). Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol 80, 583–593. [DOI] [PubMed] [Google Scholar]
- Lee RH & Heckman CJ (2000). Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20, 6734–6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KY, Kievit RA, Tsvetanov KA, Betts MJ, Duzel E, Rowe JB, Cam CAN, Howard R & Hammerer D (2020). Noradrenergic-dependent functions are associated with age-related locus coeruleus signal intensity differences. Nat Commun 11, 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Valdes E, Negro F, Falla D, Dideriksen JL, Heckman CJ & Farina D (2020). Inability to increase the neural drive to muscle is associated with task failure during submaximal contractions. J Neurophysiol 124, 1110–1121. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Doherty TJ, Stashuk DW & Rice CL (2005). Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 31, 461–467. [DOI] [PubMed] [Google Scholar]
- McNeil CJ & Rice CL (2018). Neuromuscular adaptations to healthy aging. Appl Physiol Nutr Metab 43, 1158–1165. [DOI] [PubMed] [Google Scholar]
- Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M & Nourhashemi F (2013). Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc 14, 877–882. [DOI] [PubMed] [Google Scholar]
- Mitchell WK, Williams J, Atherton P, Larvin M, Lund J & Narici M (2012). Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 3, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales FR, Boxer PA, Fung SJ & Chase MH (1987). Basic electrophysiological properties of spinal cord motoneurons during old age in the cat. J Neurophysiol 58, 180–194. [DOI] [PubMed] [Google Scholar]
- Nakagawa S & Schielzeth H (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4, 133–142. [Google Scholar]
- Nakagawa S, Johnson PCD & Schielzeth H (2017). The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface 14, 20170213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro F, Muceli S, Castronovo AM, Holobar A & Farina D (2016). Multi-channel intramuscular and surface EMG decomposition by convolutive blind source separation. J Neural Eng 13, 026027. [DOI] [PubMed] [Google Scholar]
- Nikoletopoulou V & Tavernarakis N (2012). Calcium homeostasis in aging neurons. Front Genet 3, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orssatto LBR, Mackay K, Shield AJ, Sakugawa RL, Blazevich AJ & Trajano GS (2021). Estimates of persistent inward currents increase with the level of voluntary drive in low-threshold motor units of plantar flexor muscles. J Neurophysiol 125, 1746–1754. [DOI] [PubMed] [Google Scholar]
- Pagano G, Niccolini F, Fusar-Poli P & Politis M (2017). Serotonin transporter in Parkinson’s disease: a meta-analysis of positron emission tomography studies. Ann Neurol 81, 171–180. [DOI] [PubMed] [Google Scholar]
- Pascoe MA, Holmes MR & Enoka RM (2011). Discharge characteristics of biceps brachii motor units at recruitment when older adults sustained an isometric contraction. J Neurophysiol 105, 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrkiewicz M, Kudina L, Mierzejewska J, Jakubiec M & Hausmanowa-Petrusewicz I (2007). Age-related change in duration of afterhyperpolarization of human motoneurones. J Physiol 585, 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki M, Ireland A, Coulson J, Stashuk DW, Hamilton-Wright A, Swiecicka A, Rutter MK, McPhee JS & Jones DA (2016). Motor unit number estimates and neuromuscular transmission in the tibialis anterior of master athletes: evidence that athletic older people are not spared from age-related motor unit remodeling. Physiol Rep 4(19), e12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power GA, Dalton BH, Behm DG, Vandervoort AA, Doherty TJ & Rice CL (2010). Motor unit number estimates in masters runners: use it or lose it? Med Sci Sports Exerc 42, 1644–1650. [DOI] [PubMed] [Google Scholar]
- Power GA, Dalton BH, Doherty TJ & Rice CL (2016). If you don’t use it you’ll likely lose it. Clin Physiol Funct Imaging 36, 497–498. [DOI] [PubMed] [Google Scholar]
- Powers RK, Elbasiouny SM, Rymer WZ & Heckman CJ (2012). Contribution of intrinsic properties and synaptic inputs to motoneuron discharge patterns: a simulation study. J Neurophysiol 107, 808–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK & Heckman CJ (2015). Contribution of intrinsic motoneuron properties to discharge hysteresis and its estimation based on paired motor unit recordings: a simulation study. J Neurophysiol 114, 184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Nardelli P & Cope TC (2008). Estimation of the contribution of intrinsic currents to motoneuron firing based on paired motoneuron discharge records in the decerebrate cat. J Neurophysiol 100, 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revill AL & Fuglevand AJ (2011). Effects of persistent inward currents, accommodation, and adaptation on motor unit behavior: a simulation study. J Neurophysiol 106, 1467–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revill AL & Fuglevand AJ (2017). Inhibition linearizes firing rate responses in human motor units: implications for the role of persistent inward currents. J Physiol 595, 179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos MR, Rice CL, Connelly DM & Vandervoort AA (1999). Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve 22, 1094–1103. [DOI] [PubMed] [Google Scholar]
- Shibata E, Sasaki M, Tohyama K, Kanbara Y, Otsuka K, Ehara S & Sakai A (2006). Age-related changes in locus ceruleus on neuromelanin magnetic resonance imaging at 3 Tesla. Magn Reson Med Sci 5, 197–200. [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM, Dolatkhah MA & Hopkins DA (2021). Anatomical and neurochemical organization of the serotonergic system in the mammalian brain and in particular the involvement of the dorsal raphe nucleus in relation to neurological diseases. Prog Brain Res 261, 41–81. [DOI] [PubMed] [Google Scholar]
- Stephenson JL & Maluf KS (2011). Dependence of the paired motor unit analysis on motor unit discharge characteristics in the human tibialis anterior muscle. J Neurosci Methods 198, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatton WG, Greenwood CE, Verrier MC, Holland DP, Kwan MM & Biddle FE (1991). Different rates of age-related loss for four murine monoaminergic neuronal populations. Neurobiol Aging 12, 543–556. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Negro F, Johnson MD, Holmes MR, McPherson LM, Powers RK, Farina D & Heckman CJ (2018). Robust and accurate decoding of motoneuron behaviour and prediction of the resulting force output. J Physiol 596, 2643–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajano GS, Taylor JL, Orssatto LBR, McNulty CR & Blazevich AJ (2020). Passive muscle stretching reduces estimates of persistent inward current strength in soleus motor units. J Exp Biol 223, jeb229922. [DOI] [PubMed] [Google Scholar]
- Udina E, D’Amico J, Bergquist AJ & Gorassini MA (2010). Amphetamine increases persistent inward currents in human motoneurons estimated from paired motor-unit activity. J Neurophysiol 103, 1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberk MS & Kalmar JM (2014). An evaluation of paired motor unit estimates of persistent inward current in human motoneurons. J Neurophysiol 111, 1877–1884. [DOI] [PubMed] [Google Scholar]
- Yavuz US, Negro F, Sebik O, Holobar A, Frommel C, Turker KS & Farina D (2015). Estimating reflex responses in large populations of motor units by decomposition of the high-density surface electromyogram. J Physiol 593, 4305–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.