Abstract
A decline in skeletal muscle mitochondrial function is associated with the loss of skeletal muscle size and function during knee osteoarthritis (OA). We have recently reported that 12-weeks of dietary rapamycin (Rap, 14ppm), with or without metformin (Met, 1000ppm), increased plasma glucose and OA severity in male Dunkin Hartley (DH) guinea pigs, a model of naturally occurring, age-related OA. The purpose of the current study was to determine if increased OA severity after dietary Rap and Rap+Met was accompanied by impaired skeletal muscle mitochondrial function. Mitochondrial respiration and hydrogen peroxide (H2O2) emissions were evaluated in permeabilized muscle fibers via high-resolution respirometry and fluorometry using either a saturating bolus or titration of ADP. Rap and Rap+Met decreased complex I (CI)-linked respiration and tended to increase ADP sensitivity, consistent with previous findings in patients with end-stage OA. The decrease in CI-linked respiration was accompanied with lower CI protein abundance. Rap and Rap+Met did not change mitochondrial H2O2 emissions. There were no differences between mitochondrial function in Rap versus Rap+Met suggesting that Rap was likely driving the change in mitochondrial function. This is the first inquiry into how lifespan extending treatments Rap and Rap+Met can influence skeletal muscle mitochondria in a model of age-related OA. Collectively, our data suggest that Rap with or without Met inhibits CI-linked capacity and increases ADP sensitivity in DH guinea pigs that have greater OA severity.
Keywords: Aging, mTOR, metformin, mitochondria, healthspan
INTRODUCTION
Osteoarthritis (OA) is among the top 10 diseases that limit human healthspan and lifespan (Murray et al., 2013). However, there are currently no disease modifying therapies for OA. Age is the greatest risk factor for naturally occurring, primary OA. OA affects 250 million people worldwide and is the leading cause of disability in older adults (Hunter et al., 2014). OA is characterized as a disease of the whole-joint, including articular cartilage, subchondral bone and periarticular skeletal muscle (Loeser et al., 2012). The loss of skeletal muscle size (Fink et al., 2007; Serrão et al., 2015), function (Øiestad et al., 2015; Thorstensson et al., 2004), and quality (Kumar et al., 2014; Noehren et al., 2018) are features of OA that contribute to joint pain, physical inactivity, and comorbidities. Although it is difficult to dissociate the loss of muscle size and function with OA from muscle disuse, there is evidence to suggest that loss of quadriceps muscle strength may occur prior to and be predictive of incident radiographic and symptomatic knee OA (Segal & Glass, 2011; Slemenda et al., 1997).
The age-related decline of skeletal muscle mitochondrial function is associated with impaired skeletal muscle contractile function (Gonzalez-Freire et al., 2018), walking speed (Coen et al., 2013), and fatigability (Santanasto et al., 2015). Older adults with high fatigability also had lower mitochondrial respiration compared to older adults with low fatigability. Nearly 50% of older individuals with high fatigability were diagnosed with OA (Santanasto et al., 2015). Consistent with these findings, patients with end-stage OA about to receive knee arthroplasty had lower skeletal muscle mitochondrial content and maximal complex IV activity compared to young and age-matched non-OA controls (Safdar et al., 2010). Additionally, OA patients have impaired redox homeostasis as evident by decreased activity of the antioxidant MnSOD and increased oxidative damage (Safdar et al., 2010).
The onset of naturally occurring (primary) OA in humans is insidious and unpredictable which limits the study of OA to end-stage disease and makes it difficult to study the cellular processes that are involved in the onset, early progression, and treatment of OA. To circumvent this limitation, we use the Dunkin Hartley (DH) guinea pig model of primary OA. The DH guinea pig predictably develops naturally occurring OA by 5 months of age and demonstrates an age-associated progression to moderate OA by 8–9 months of age (Bendele & Hulman, 1988; Radakovich et al., 2018). OA pathology in the DH guinea pig highly resembles OA observed in humans, as evident by cartilage and bone lesions that typically originate in the medial tibia and spread laterally with age (Huebner & Kraus, 2006; Kapadia et al., 2000). In addition, the DH guinea pig displays a number of recognized age-related changes in human skeletal muscle including a decline in skeletal muscle density, muscle fiber size, and mitochondrial protein synthesis rates (Musci et al., 2020). These characteristics make the DH guinea pig a desirable model to study the onset, progression, and treatment of primary, age-related OA.
The FDA approved drug rapamycin (Rap) can extend lifespan in mice (Harrison et al., 2009) and when metformin (Met) is added to Rap (Rap+Met) there may be an even greater effect on lifespan extension (Strong et al., 2016). Treatments that extend lifespan are also intended to extend healthspan, the time spent without chronic age-related disease and disability. However, the therapeutic potential of Rap and Met for specific age-related diseases, like OA, is incompletely understood. A leading notion is that Met inhibits maximal complex I (CI) linked respiration and H2O2 emissions (Kane et al., 2010; Wessels et al., 2014), however, these effects remain equivocal (Kristensen et al., 2013; Larsen et al., 2012; Vytla & Ochs, 2013). In skeletal muscle, the mTOR inhibitor Rap had no effect (Ye et al., 2013) or inhibited (Cunningham et al., 2007) maximal skeletal muscle mitochondrial respiration while the influence on mitochondrial H2O2 emissions remains unexplored. Despite previous work showing protective effects of Rap or Met on injury induced OA in young mice (Caramés et al., 2012; Cheng et al., 2016; Li et al., 2020; Takayama et al., 2014), we have recently shown that dietary Rap (14ppm) treatment with or without Met (1000ppm) increased plasma glucose and worsened age-related OA severity in DH guinea pigs (Minton et al., 2021). Based on these findings, we sought to determine if the detrimental effects of Rap and Rap+Met on OA pathology and glucose homeostasis would be accompanied by impaired skeletal muscle mitochondrial bioenergetics.
METHODS
Animal Use
The Institutional Animal Care and Use Committee at the University of Illinois Urbana-Champaign approved all animal procedures. Data collection and analysis were completed at University of Wisconsin-Madison and William S. Middleton Memorial Veterans Hospital. The details of the experimental design and animal husbandry have been previously described in (Minton et al., 2021). In brief, Dunkin Hartley Guinea Pigs were obtained from Charles River and during acclimation provided a standard chow diet (Envigo 2040) fortified with vitamin C (1050 ppm) and Vitamin D (1.5 IU/kg) ad libitum. At 5 months of age, DH guinea pigs were then randomized to continue the control diet (n=8), or standard chow enriched with encapsulated Rapamycin (14 ppm, n=8) or the combination of encapsulated Rapamycin+Metformin (14 ppm, 1000 ppm, n=7) for 12-weeks. Microencapsulated Rapamycin (Rapamycin Holdings) and Metformin HCl (AK Scientific I506) were compounded into the diet by Envigo. Guinea pigs provided experimental diets enriched with Rap or the combination of Rap+Met were allowed continued ad libitum access to food and water. Food provided to the control group was calculated to match the food consumption of the experimental diet to minimize the influence of food intake on dependent variables.
Tissue Collection
Following 3 months of treatment, two animals were sacrificed daily. Food was removed from cages 2–3 hours prior to euthanasia. Animals were anesthetized in a chamber containing 5% isoflurane and maintained using a mask with 1.5–3% isoflurane. Once reflexively unresponsive, the thoracic cavity was opened and whole blood was collected via cardiac venipuncture in K2EDTA coated tubes for measurement of rapamycin and metformin by the Bioanalytical Pharmacology Core at the San Antonio Nathan Shock Center. The heart was then excised. The right soleus was removed from the hind limb and immediately placed in ice-cold BIOPS solution (2.77 mM CaK2-EGTA, 7.23 mM K2-EGTA, 20 mM imidazole, 20 mM taurine, 50 mM K-MES, 0.5 mM dithiothreitol, 6.56 mM MgCl2, 5.77 mM ATP, and 15 mM phosphocreatine, pH 7.1). The left soleus was frozen in liquid nitrogen and stored at −80°C until processing.
The right hind limb was removed at the coxofemoral joint, fixed in 10% neutral buffered formalin (NBF) for 48 hours, and transferred to 70% ethanol and processed for histology. Toludine blue stained slides were then scored by two blinded reviewers for OA severity following OARSI Modified Mankin guidelines (Kraus et al., 2010). Briefly, toluidine blue stained histology slides were assigned scores for severity of articular cartilage structural damage (0–8), proteoglycan loss as assessed by absence of toluidine blue staining (0–6), disruption of chondrocyte cellularity (0–3), and tidemark integrity (0–1), with a total possible score of 18 per joint compartment (Total OARSI Score). The pathology was most prevalent in the medial tibial plateau.
High-resolution respirometry and fluorometry
High-resolution respirometry and fluorometry measurements were performed in duplicate using two chambers of the Oxygraph-2k (O2K; OROBOROS INSTRUMENTS, Innsbruck, Austria). Air calibration and instrument background oxygen flux were performed before each experiment. After the addition of the permeabilized fibers, the O2K was calibrated to measure to measure H2O2 emissions with three injections of H2O2 (40μM). Temperature within the chambers was maintained at 37°C and oxygen concentrations were kept within the range of 350 – 250 μmol/mL. Reoxygenation was performed when oxygen concentrations approached 250 μmol/mL to ensure oxygen availability was not a limiting factor. Data are presented as the average between duplicates and respiration rates (O2 flux) and H2O2 emissions were expressed relative to tissue wet weight (pmol/s/mg).
Tissue Preparation
Consistent with muscle from patients with knee OA (Noehren et al., 2018), muscle from the Hartley guinea pig contained a visible prevalence of non-contractile tissue. During mechanical permeabilization, connective and adipose tissue were removed. Fibers were then chemically permeabilized in BIOPS with saponin (50μg/mL) for 30 minutes. Permeabilized muscle fibers were rinsed with MiR05 (0.5 mM EGTA, 3 mM MgCl2, 60 mM K-lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose, 1 g/l BSA essentially fatty acid free, pH 7.1) plus 25μM blebbistatin, a myosin II-specific inhibitor to prevent muscle fiber contraction (Perry et al., 2011). Fibers were then blotted on filter paper to remove excess buffer and weighed on a microscale (Mettler Toledo X105). Fibers bundles (1.5–2.5 mg) were then placed into the chambers containing MiR05 plus 12.5μM blebbistatin.
Mitochondrial Bioenergetics
Two different protocols were used to assess mitochondrial function. The first protocol was supported by complex-I linked substrates, pyruvate (5mM), glutamate (10mM) and malate (0.5mM) to evaluate complex I (CI)-linked LEAK (CIL) respiration followed by a bolus of ADP (5mM) to stimulate maximal complex I-linked OXPHOS (CIP). Subsequent additions included cytochrome c (10mM) to test mitochondrial membrane integrity, succinate (10mM) for complex I plus II supported OXPHOS (CI+IIP), and Carbonyl cyanide m-chlorophenyl hydrazone (CCCP) (0.25–0.75 μM) to stimulate maximal uncoupled electron transport system (ETS; CI+IIE) capacity. Next, the complex-I inhibitor rotenone (0.5μM) was added so that the remaining respiration was reflective of CIIE. Mitochondrial respiration was stopped by the complex III inhibitor antimycin A (2.5μM) to measure residual oxygen consumption (ROX). SUIT 1 was used to measure respiration in 4 animals per group.
We next utilized an ADP titration protocol to evaluate mitochondrial respiration and H2O2 emissions in a different set of 4 animals per group. This protocol was also supported by pyruvate (5mM), glutamate (10mM) and malate (0.5mM) to stimulate LEAK respiration and evaluate CI-linked H2O2 emissions. Subsequently, ADP was serially injected to reach concentrations of 0.125, 0.25, 0.56, 1.2, 2.4, 5.6, and 11.8 mM followed by sequential addition of cytochrome c, succinate, and CCCP to determine maximal CI+IIP and CI+IIE as previously performed (Konopka et al., 2017, 2019). Using Michaelis-Menten kinetics, Vmax and the apparent Km for ADP were calculated. We also determined the area under the curve (AUC) for respiration and H2O2 emissions during titration of ADP. Mitochondrial H2O2 was measured using fluorometry by determining the rate of Amplex UltraRed (10uM) oxidation to resorufin in the presence of excess horseradish peroxidase (1U/mL) and superoxide dismutase (5 U/mL). H2O2 emissions were detected simultaneously with respirometry during the ADP titration protocol. We also expressed H2O2 emissions relative to respiration as an indicator of electron leak. With both protocols, the ratio of maximal OXPHOS to maximal uncoupled ETS capacity (P/E) was used to gain insight into intrinsic mitochondrial function independent of changes in mitochondrial abundance.
Western Blotting
Soleus tissue were homogenized in liquid nitrogen cooled mortar and pestle followed by bead homogenization in RIPA lysis buffer (150mM NaCl, 0.1mM EDTA, 50mM Tris, 0.1% wt/vol deoxycholate, 0.1% wt/vol SDS, 1% vol/vol Triton X-100) with 1X Halt Protease and Phosphatase Inhibitor Cocktail (Thermofisher Scientific: 78442) as previously described (Minton et al., 2021) 10 μg of protein was loaded for OXPHOS proteins using a cocktail of subunits from the 5 electron transport system complexes, CI-NDUFB8, CII-SDH8, CIII-UQCRC2, CIV-MTCO1, CV-ATP5A (Abcam, MS604). 20 μg of protein loaded for all other proteins. Samples prepared in reducing conditions with β-mercaptoethanol in 2x Laemmli Sample Buffer (BioRad 1610737) and heated at 95°C. The samples for OXPHOS blots were heated at 50°C to preserve Complex I, II, IV proteins which are sensitive to high heat. Proteins were separated on 4–15% TGX Precast Gels (BioRad, 4561083) and transferred to PVDF membranes (Invitrogen). Membranes were blocked in TBS-Tween 20 (0.1%) (TBST) with 5% BSA and then incubated overnight with primary antibodies for OXPHOS (Abcam MS604), P-RPS6 Ser235/236 (Cell Signaling, 4858), RPS6 (Cell Signaling, 2217), P-AKT S473 (Cell Signaling, 4060), AKT (Cell Signaling, 4685) and B-Actin (Abcam Ab6276) at 1:500, 1:2000, 1:1000, and 1:5000 respectively. Despite reactivity with other guinea pig tissues, we were not able to detect p-AMPK using three different antibodies (Cell Signaling, 50081, 2531, 2535) in DH guinea pig skeletal muscle. OXPHOS was diluted in 1% Nonfat milk/PBS following manufacturer recommendation and all others in 5% BSA/TBST. Membranes were washed with TBST between incubations. Membranes were then incubated with IgG HRP conjugated Anti-Mouse (Abcam, 6728) and Anti-Rabbit (Cell Signaling, 7074) secondary antibodies at 1:10,000. Membranes exposed to SuperSignal Pico Substrate (Fisher, PI34095) and imaged with BioRad ChemiDox XRS+ or UVP Biospectrum 500. Membranes were stripped with Restore Stripping Buffer following manufacturer recommendations (ThermoFisher Scientific 21059). Densitometric calculations determined using Image Lab (BioRad) or VisionWorks (Analytikjena).
Statistics
Data are presented as mean ± standard error of the mean (SEM). Significance was set at P < 0.05. Michaelis-Menten kinetics were used to determine the estimated apparent Km and maximal respiration (Vmax) in Prism 9.0 (GraphPad Software, Inc., La Jolla, CA, USA). One-way ANOVA was used to compare between groups. When significant main effects were present a two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli post-hoc test was chosen to account for multiple comparisons. We also performed covariate analysis to try to account for differences in body weight between groups; however, there was no significant impact of body weight on mitochondrial function.
RESULTS
DH Guinea Pig Characteristics
The DH guinea pig characteristics and OA score from the same cohort of DH guinea pigs have been previously published with a focus on OA pathology (Minton et al., 2021). Here, we summarize those data in Table 1 for ease of readership. Briefly, Rap and Rap+Met animals weighed 15 and 22% (P<0.05) less than control animals. Rap and Rap+Met treated animals had increased fasting plasma glucose by 64 and 39% (P<0.05). Adding Met to Rap attenuated the hyperglycemia compared to Rap alone (P=0.05). OA severity measured by medial tibia OARSI score was nearly 2-fold greater in Rap and Rap+Met compared to age matched controls (P<0.05) and there were no differences between Rap versus Rap+Met.
Table 1.
Physical and Metabolic Characteristics of Dunkin Hartley Guinea Pigs
| CONTROL | RAP | RAP+MET | |
|---|---|---|---|
| Body Weight (g) | 992 ± 90 | 846 ± 49* | 775 ± 100* |
| Medial Tibia OARSI Score | 4.6 ± 1.4 | 8.9 ± 3.0* | 8.7 ± 3.6* |
| Fasted Blood Glucose (mg/dL) | 242 ± 55 | 396 ± 61* | 335 ± 53*† |
| Blood Rapamycin (ng/mL) | 0.4 ± 0 | 72 ± 8 | 78 ± 10 |
| Blood Metformin (ng/mL) | 2 ± 0 | N/A | 282 ± 54 |
Note: Control values for Rap and Met in blood circulation are set to lowest readable value. Data are presented as mean ± SEM
P<0.05 vs Con.
P=0.05 vs Rap.
Mitochondrial Respiration
ADP bolus:
When provided a saturating bolus of ADP there was no statistical differences in mitochondrial respiration between DH guinea pigs treated with Con, Rap or Rap+Met (Figure 1A). There were also no differences between groups in the P/E ratio (Figure 1B).
Figure 1.
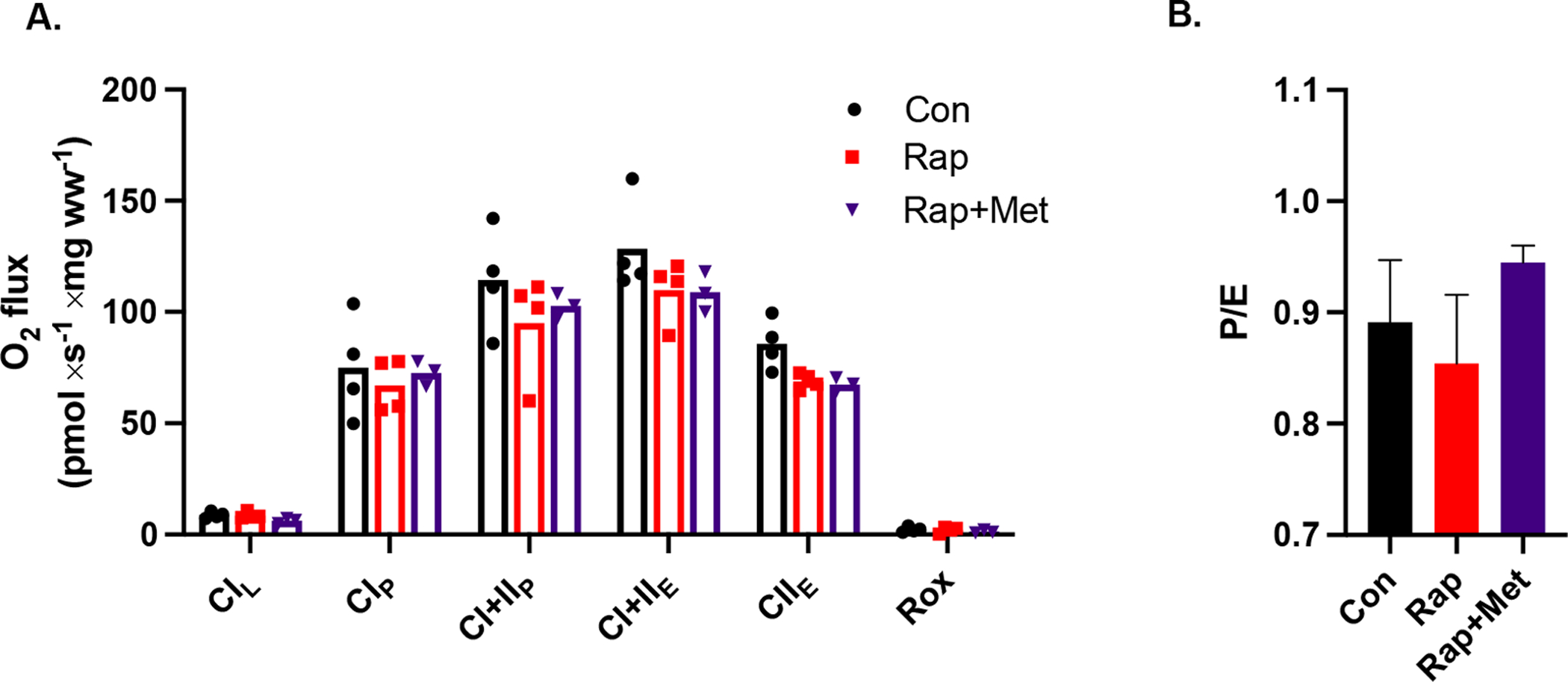
A) Skeletal muscle mitochondrial respiration during a SUIT protocol with an ADP bolus. B) The ratio of coupled OXPHOS to uncoupled ETS capacity (P/E). Con (n=4), Rap (n=4), and Rap+Met (n=3). Data are presented as mean with individual data points or ± SEM.
ADP Titration:
Using the ADP titration protocol revealed statistically significant differences in DH guinea pigs treated with Rap and Rap+Met compared to control. There were no differences between Rap versus Rap+Met. Treatments did not influence CIL respiration. Compared to control, Rap and Rap+Met treated DH guinea pigs had lower (P<0.05) submaximal and maximal CI-linked respiration starting at a sub-saturating dose of 1 mM ADP and continuing until a maximal, saturating dose of 12 mM ADP (Figure 2A). The inhibitory effects of Rap and Rap+Met were further supported by a lower Vmax (P<0.001) and AUC (P<0.01) versus control (Figure 2B, C). Rapamycin also induced a leftward shift in the respiration curve (% of maximum) consistent with a tendency to lower the apparent Km of ADP in the Rap (P=0.08) and Rap+Met (P=0.09) treated DH guinea pigs compared to control (Figure 2D, E). A lower apparent Km of ADP represents a greater ADP sensitivity. The differences in mitochondrial respiration between Rap and Rap+Met versus control were no longer apparent during CI+IIP (P=0.09), CI+IIE, or CIIE (Figure 2F). Furthermore, the ratio of P/E was not significantly different after Rap or Rap+Met treatment (Figure 2G).
Figure 2.
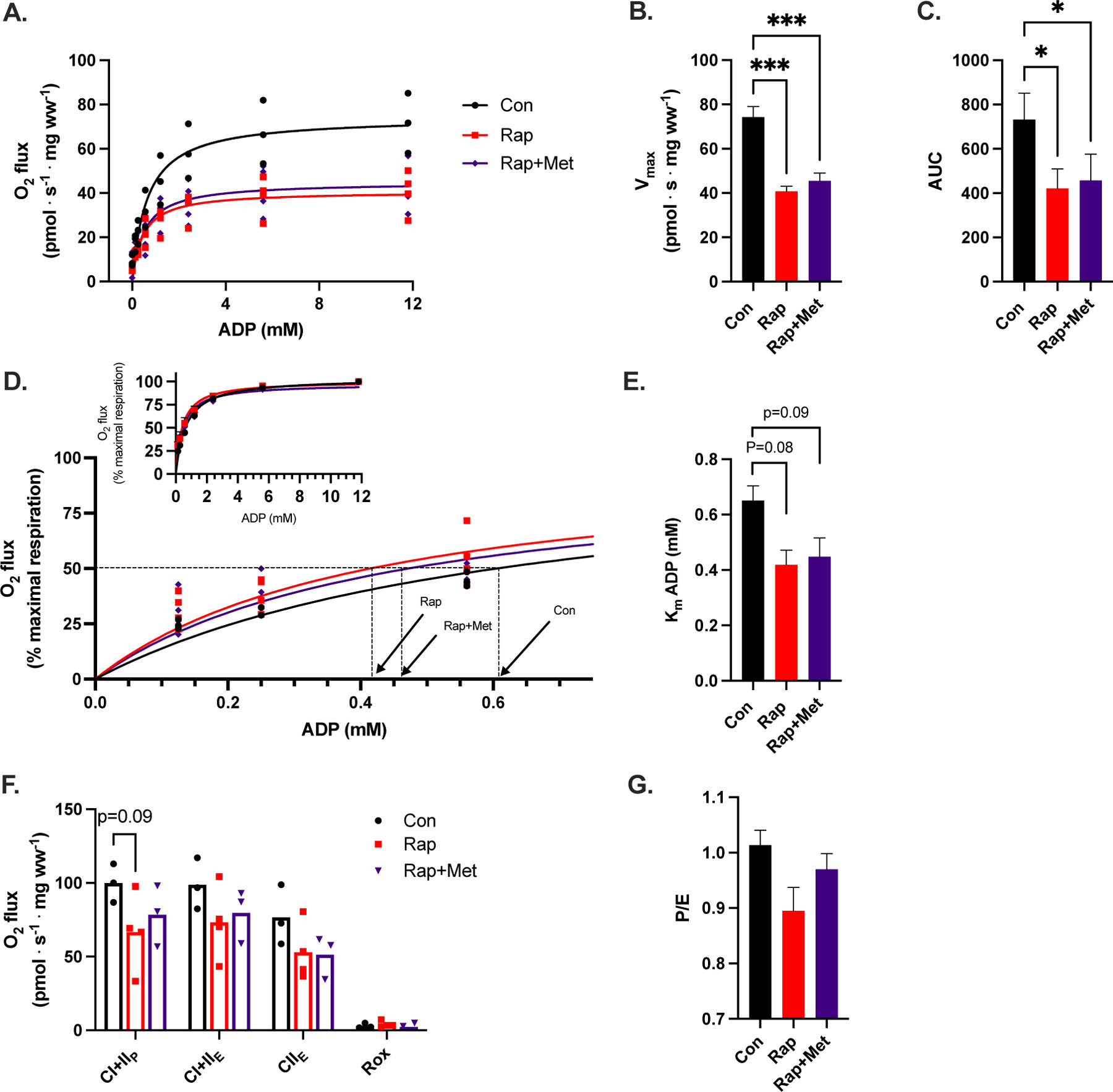
A) Complex I (CI)-linked respiration, B) Vmax and C) area under the curve (AUC) during an ADP titration protocol. D) CI-linked respiration expressed as a percentage of maximal respiration from 0 to 0.6 mM ADP. Inset of respiration from 0 to 12 mM ADP. E) the apparent Km of ADP calculated from panel D. F) CI+II OXPHOS (P), ETS (E) and ROX after the ADP titration protocol and G) the P/E ratio. Con (n=3), Rap (n=4), and Rap+Met (n=4). 1 GP in Rap+Met group did not have a succinate response to stimulate CI+IIP and was not included in F. *P<0.05 vs. Con, **P<0.01 vs Con, ***P<0.001 vs Con. Data are presented as mean with individual data points or ± SEM.
Mitochondrial H2O2 emissions
There was no effect of Rap or Rap+Met on mitochondrial H2O2 emissions during CI-supported LEAK nor during the titration of ADP or AUC (data not shown). When mitochondrial H2O2 emissions were expressed relative to mitochondrial O2 flux, there were also no differences between groups.
Mitochondrial Content
To estimate mitochondrial protein abundance, we evaluated subunit content of complexes I through V of the ETS. CI protein content was lower (P<0.05) in the Rap and tended to be lower (P=0.09) in the Rap+Met treatment groups (Figure 3A). There were no other changes in protein abundance for any other complexes between DH guinea pigs receiving treatment compared to control or Rap versus Rap+Met.
Figure 3. Skeletal muscle OXPHOS and nutrient-sensing signaling proteins.
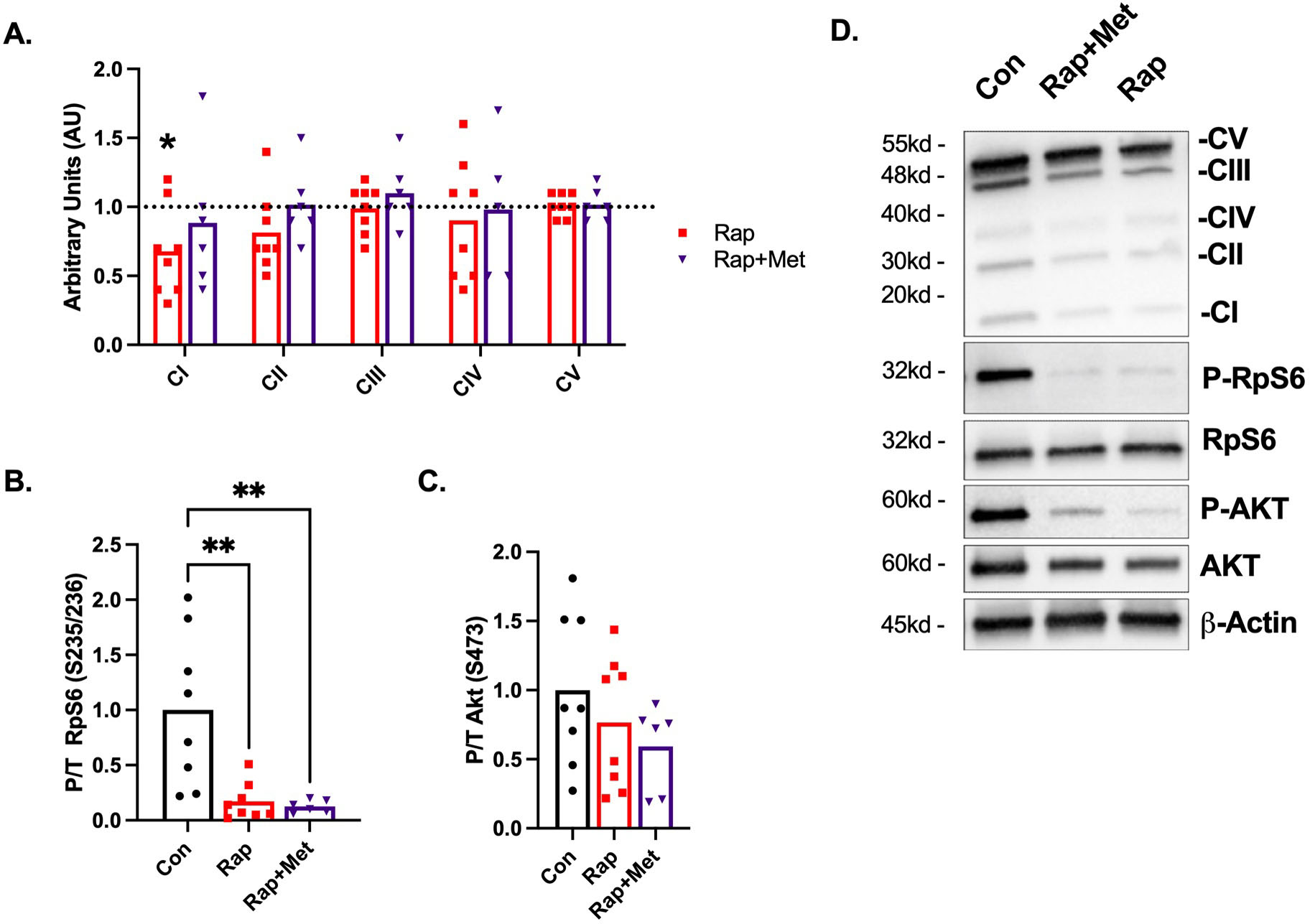
A) Protein content of the ETS complex I-V. Control is indicated by the dotted line and all data is expressed relative to the mean of the control. B) Protein content of P-RPS6/Total and C) P-AKT/Total. D) Representative images. Con (n=8), Rap (n=8), and Rap+Met (n=6).*P<0.05 vs. Con. **P<0.01 vs Con. Data are presented as mean with individual data points.
Nutrient Signaling
To evaluate whether dietary Rap and Rap+Met modified mTOR signaling pathways in skeletal muscle of the DH guinea pig, we investigated phosphorylation of RpS6 S235/236 and AKT S473 as downstream targets of mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). Rap and Rap+Met diminished mTORC1 signaling as evident by an 83 and 87% (P<0.01) decrease in phosphorylated RpS6 (Figure 3B) respectively. Rap and Rap+Met did not inhibit mTORC2 signaling as evident by a non-significant 20 and 40% decrease in AKT S473 phosphorylation (Figure 3C). For unknown reasons, we were unable to detect phosphorylated AMPK in skeletal muscle of DH guinea pigs.
DISCUSSION
The main findings of this study are that at doses previously shown to slow aging in mice, dietary Rap (14ppm) and Rap+Met (14+1000ppm) modified skeletal muscle mitochondrial bioenergetics in the DH guinea pig model of age-related primary OA. DH guinea pigs treated with Rap with or without Met had lower submaximal and maximal CI-linked respiration and less CI protein content. Rap and Rap+Met treated guinea pigs also tended to have greater ADP sensitivity, indicated by a lower apparent Km of ADP. The rapamycin-induced changes in mitochondrial function recapitulate similar findings in human patients with severe, end-stage OA (Eimre et al., 2006; Safdar et al., 2010). Consistent with this notion, the impaired mitochondrial function in Rap treated DH guinea pigs were accompanied by elevated hyperglycemia and increased OA pathology. Collectively our data indicate that dietary Rap with or without Met appears to worsen skeletal muscle mitochondrial function, glycemic control, and OA pathology in DH guinea pigs.
Interventions targeted to improve mitochondrial biogenesis and function have been proposed to increase longevity and delay musculoskeletal disease progression. Rap extends lifespan and healthspan in aging models (Harrison et al., 2009; Miller et al., 2011), but the effects on mitochondria and in models of specific age-related disease are poorly understood. In the current study, titrating ADP revealed that dietary Rap seemed to impair mitochondrial function in DH guinea pigs as evident by decreased submaximal and maximal CI-linked respiration and increased ADP sensitivity. These findings are in line with previous work to suggest that Rap decreased respiration in cultured muscle cells (Ye et al., 2012, 2013) and mice (Cunningham et al., 2007). We were unable to detect any differences between control, Rap or Rap+Met treated DH guinea pigs when using a bolus of ADP. These findings are in agreement with our previous study that showed by using a titration and not a bolus of ADP, we were able to detect subtle differences in skeletal muscle mitochondrial respiration after healthspan extending treatments in older adults (Konopka et al., 2019). In the context of aging, we initially viewed greater ADP sensitivity after Rap and Rap+Met treatment as beneficial since older adults have lower ADP sensitivity (Holloway et al., 2018). However, in patients with severe, end-stage OA about to undergo joint replacement, skeletal muscle mitochondrial ADP sensitivity was elevated and mitochondrial complex IV activity was lower compared to healthy, age-matched controls (Eimre et al., 2006; Safdar et al., 2010). Therefore, our data in DH guinea pigs treated with Rap and Rap+Met are consistent with end-stage human OA and indicate a potential connection between greater OA severity and impaired skeletal muscle mitochondrial bioenergetics.
Alterations to adenylate transport proteins through either change in protein content or post-translational modifications contribute to the regulation of ADP sensitivity (Holloway et al., 2018; Miotto et al., 2018) Transport of adenylates (ADP, ATP, etc.) into the mitochondria is mediated by voltage-dependent anion channels (VDAC), mitochondrial creatine kinase, and adenine nucleotide transport proteins. Previous work indicates that mTORC1 phosphorylates Bcl-lx at serine 62 which complexes with VDAC1 to increase substrate and adenylate transport across the outer mitochondrial membrane (Ramanathan & Schreiber, 2009). Attenuation of mTORC1 signaling by Rap, as observed in the current study, can disassociate Bcl-lx from VDAC limiting ADP/ATP transport and could be one mechanism involved in lowering the apparent Km of ADP and limiting mitochondrial respiratory capacity.
The inhibition of CI-supported respiration after Rap treatment was accompanied by a decrease in CI protein abundance. The P/E ratio is considered a reflection of intrinsic mitochondrial function independent from changes in protein content. No statistical change in P/E ratio after Rap supports the notion that a decrease in CI content contributes to lower CI-linked respiration. Previous work has demonstrated that rapamycin decreases transcription factors involved in mitochondrial biogenesis (Cunningham et al., 2007; Ye et al., 2012, 2013) with no change in OXPHOS proteins. Rap preserves bulk mitochondrial protein synthesis rates (Drake et al., 2013) while proteomic approaches reveal that turnover of proteins within the ETS are subunit specific (Karunadharma et al., 2015; Wolff et al., 2020). Rap increased the half-life of the CI subunit NDFUB8 in skeletal and cardiac muscle (Dai et al., 2014; Karunadharma et al., 2015) which is the specific subunit probed in our western blot analysis for CI. Therefore, changes in NDUFB8 protein turnover may be one factor contributing to lower abundance and changes to mitochondrial respiration. Although it remains unclear how Rap alters mitochondrial content and function, it may be linked to changes in protein turnover and assembly of ETS subunits. Future work is needed to connect ETS proteostasis to functional outcomes to further understand the mechanisms promoting healthspan extension.
Mitochondrial H2O2 emissions in permeabilized muscle fibers are the result of H2O2 production and antioxidant scavenging capacity. Rap and Rap+Met treatment did not change mitochondrial H2O2 emissions in the DH guinea pig. However, it should be noted that mitochondrial H2O2 emissions assessed in this study were in the absence of ADP during CI-linked LEAK respiration. Future studies should consider also using substrates with convergent electron supply to the Q-junction through CII to induce maximal mitochondrial H2O2 emissions to clarify if Rap can improve the sensitivity of ADP to suppress H2O2 emissions.
A common side effect of chronic rapamycin treatment is impaired glucose metabolism and insulin resistance driven by off target inhibition of mTORC2 (Arriola Apelo, Pumper, et al., 2016; Lamming et al., 2012; Ye et al., 2012). Consistent with these findings, we show that 12-weeks of dietary Rap and Rap+Met increased plasma glucose and non-significantly attenuated skeletal muscle mTORC2 signaling as evident by a 20% and 40% decrease in AKT S473 phosphorylation. We have also found that increased plasma glucose was correlated to increased OA severity in DH guinea pigs (Minton et al., 2021). Intermittent rapamycin dosing schedules or alternative rapamycin analogs that selectively inhibit mTORC1 minimize off-target side metabolic effects mediated by inhibition of mTORC2 (Arriola Apelo, Neuman, et al., 2016). Therefore, future work is needed to understand if different rapamycin or rapalog dosing regimens may prevent detrimental side effects such as impaired mitochondrial function and hyperglycemia and be able to successfully delay or prevent age-related OA pathology.
Limitations
Because we used multiple respirometry protocols, our mitochondrial function results include a small sample size. However, it is encouraging that across rapamycin treated groups (Rap and Rap+Met), we observed similar inhibitory effects on mitochondrial CI-linked respiration, which is in agreement with a previous study in Rap-treated mice (Cunningham et al., 2007). We selected one muscle, the soleus, which is predominantly composed of oxidative muscle fibers. Therefore, these data cannot be extrapolated to glycolytic muscles since previous work in mice suggests that Rap did not significantly impact mitochondrial respiration in the gastrocnemius (Cunningham et al., 2007). There are limited commercially available antibodies compatible with the Guinea pig which restricted further investigation into the potential mechanisms by which rapamycin alters skeletal muscle mitochondrial function and nutrient sensing pathways that are associated with aging and age-related OA. We used a single dietary Rap (14ppm) and Met (1000ppm) concentration previously shown to have beneficial health and lifespan extending effects in mice and rats (Harrison et al., 2009; Strong et al., 2016; Van Skike et al., 2020). Alternative dosing schemes may yield different results in DH guinea pigs.
Conclusion
Within the current study we observed that DH guinea pigs treated with Rap (14ppm) either alone or in combination with Met (1000ppm), had lower mitochondrial CI-linked respiration and greater ADP sensitivity. These findings are consistent with previous work in patients with end-stage OA that have lower mitochondrial respiration and elevated ADP sensitivity compared to age-matched non-OA controls. Therefore, future work is warranted to understand if approaches to reverse skeletal muscle mitochondrial dysfunction could be a therapeutic strategy for OA. Taken together with our previous findings that Rap induced hyperglycemia was associated with greater OA severity, our data suggests long-term treatment with dietary rapamycin (14ppm) may not be suitable for the prevention or treatment of age-related OA in DH guinea pigs. Future studies are needed to determine if alternative rapamycin dosing regimens such as lower concentrations, intermittent administration, or rapalogs may be more effective to safely maximize healthspan extending effects on OA pathology by minimizing off-target side effects associated with chronic use of dietary rapamycin.
Highlights.
Impact of lifespan extending treatments on age-related disease is not well known
Osteoarthritis (OA) is one of the most prevalent age-related diseases
Decline in skeletal muscle mitochondrial function is associated with OA severity
Dietary rapamycin impaired muscle mitochondrial function in model of age-related OA
Decreased mitochondrial function by rapamycin was associated with greater OA severity
Acknowledgements
The authors would like to thank Martin Javors and Greg Friesenhahn at the Analytical Pharmacology and Drug Evaluation Core of the San Antonio Nathan Shock Center. We would also like to acknowledge the technical assistance from William Fairfield and Oscar Safairad.
Funding
The Konopka Laboratory is supported by the University of Wisconsin-Madison School of Medicine and Public Health, Department of Medicine, and National Institutes of Health grant R21AG067464 and previously supported by the University of Illinois. This work was supported using facilities and resources from the William S. Middleton Memorial Veterans Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the Department of Veterans Affairs, or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arriola Apelo SI, Neuman JC, Baar EL, Syed FA, Cummings NE, Brar HK, Pumper CP, Kimple ME, & Lamming DW (2016). Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell, 15(1), 28–38. 10.1111/acel.12405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriola Apelo SI, Pumper CP, Baar EL, Cummings NE, & Lamming DW (2016). Intermittent administration of rapamycin extends the life span of female C57BL/6J Mice. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 71(7), 876–881. 10.1093/gerona/glw064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendele AM, & Hulman JF (1988). Spontaneous cartilage degeneration in guinea pigs. Arthritis & Rheumatism, 31(4), 561–565. 10.1002/art.1780310416 [DOI] [PubMed] [Google Scholar]
- Caramés B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, & Lotz M (2012). Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Annals of the Rheumatic Diseases, 71(4), 575–581. 10.1136/annrheumdis-2011-200557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NT, Guo A, & Cui YP (2016). Intra-articular injection of Torin 1 reduces degeneration of articular cartilage in a rabbit osteoarthritis model. Bone and Joint Research, 5(6), 218–224. 10.1302/2046-3758.56.BJR-2015-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW, Manini TM, Wohlgemuth SE, Leeuwenburgh C, Cummings SR, Newman AB, Ferrucci L, Toledo FGS, Shankland E, Conley KE, & Goodpaster BH (2013). Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 68(4), 447–455. 10.1093/gerona/gls196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, & Puigserver P (2007). mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature, 450(7170), 736–740. 10.1038/nature06322 [DOI] [PubMed] [Google Scholar]
- Dai DF, Karunadharma PP, Chiao YA, Basisty N, Crispin D, Hsieh EJ, Chen T, Gu H, Djukovic D, Raftery D, Beyer RP, Maccoss MJ, & Rabinovitch PS (2014). Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell, 13(3), 529–539. 10.1111/acel.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake JC, Peelor FF, Biela LM, Watkins MK, Miller RA, Hamilton KL, & Miller BF (2013). Assessment of mitochondrial biogenesis and mtorc1 signaling during chronic rapamycin feeding in male and female mice. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 68(12 A), 1493–1501. 10.1093/gerona/glt047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimre M, Puhke R, Alev K, Seppet E, Sikkut A, Peet N, Kadaja L, Lenzner A, Haviko T, Seene T, Saks VA, & Seppet EK (2006). Altered mitochondrial apparent affinity for ADP and impaired function of mitochondrial creatine kinase in gluteus medius of patients with hip osteoarthritis. American Journal of Physiology - Regulatory Integrative and Comparative Physiology, 290(5), 1271–1275. 10.1152/ajpregu.00651.2005 [DOI] [PubMed] [Google Scholar]
- Fink B, Egl M, Singer J, Fuerst M, Bubenheim M, & Neuen-Jacob E (2007). Morphologic changes in the vastus medialis muscle in patients with osteoarthritis of the knee. Arthritis and Rheumatism, 56(11), 3626–3633. 10.1002/art.22960 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Freire M, Scalzo P, D’Agostino J, Moore ZA, Diaz-Ruiz A, Fabbri E, Zane A, Chen B, Becker KG, Lehrmann E, Zukley L, Chia CW, Tanaka T, Coen PM, Bernier M, de Cabo R, & Ferrucci L (2018). Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: The Baltimore Longitudinal Study of Aging. Aging Cell, 17(2), 1–11. 10.1111/acel.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, & Miller RA (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460(7253), 392–395. 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway GP, Holwerda AM, Miotto PM, Dirks ML, Verdijk LB, & van Loon LJC (2018). Age-Associated Impairments in Mitochondrial ADP Sensitivity Contribute to Redox Stress in Senescent Human Skeletal Muscle. Cell Reports, 22(11), 2837–2848. 10.1016/j.celrep.2018.02.069 [DOI] [PubMed] [Google Scholar]
- Huebner JL, & Kraus VB (2006). Assessment of the utility of biomarkers of osteoarthritis in the guinea pig. Osteoarthritis and Cartilage, 14(9), 923–930. 10.1016/j.joca.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Schofield D, & Callander E (2014). The individual and socioeconomic impact of osteoarthritis. Nature Reviews Rheumatology, 10(7), 437–441. 10.1038/nrrheum.2014.44 [DOI] [PubMed] [Google Scholar]
- Kane DA, Anderson EJ, Price JW, Woodlief TL, Lin C. Te, Bikman BT, Cortright RN, & Neufer PD (2010). Metformin selectively attenuates mitochondrial H2O2 emission without affecting respiratory capacity in skeletal muscle of obese rats. Free Radical Biology and Medicine, 49(6), 1082–1087. 10.1016/j.freeradbiomed.2010.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia RD, Badger AM, Levin JM, Swift B, Bhattacharyya A, Dodds RA, Coatney RW, & Lark MW (2000). Meniscal ossification in spontaneous osteoarthritis in the guinea-pig. Osteoarthritis and Cartilage, 8(5), 374–377. 10.1053/joca.1999.0312 [DOI] [PubMed] [Google Scholar]
- Karunadharma PP, Basisty N, Chiao YA, Dai DF, Drake R, Levy N, Koh WJ, Emond MJ, Kruse S, Marcinek D, Maccoss MJ, & Rabinovitch PS (2015). Respiratory chain protein turnover rates in mice are highly heterogeneous but strikingly conserved across tissues, ages, and treatments. FASEB Journal, 29(8), 3582–3592. 10.1096/fj.15-272666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka AR, Castor WM, Wolff CA, Musci RV, Reid JJ, Laurin JL, Valenti ZJ, Hamilton KL, & Miller BF (2017). Skeletal muscle mitochondrial protein synthesis and respiration in response to the energetic stress of an ultra-endurance race. Journal of Applied Physiology, 123(6), 1516–1524. 10.1152/japplphysiol.00457.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka AR, Laurin JL, Schoenberg HM, Reid JJ, Castor WM, Wolff CA, Musci RV, Safairad OD, Linden MA, Biela LM, Bailey SM, Hamilton KL, & Miller BF (2019). Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell, 18(1). 10.1111/acel.12880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus VB, Huebner JL, DeGroot J, & Bendele A (2010). The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthritis and Cartilage, 18(SUPPL. 3), S35–S52. 10.1016/j.joca.2010.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen JM, Larsen S, Helge JW, Dela F, & Wojtaszewski JFP (2013). Two Weeks of Metformin Treatment Enhances Mitochondrial Respiration in Skeletal Muscle of AMPK Kinase Dead but Not Wild Type Mice. PLoS ONE, 8(1), 1–10. 10.1371/journal.pone.0053533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Karampinos DC, MacLeod TD, Lin W, Nardo L, Li X, Link TM, Majumdar S, & Souza RB (2014). Quadriceps intramuscular fat fraction rather than muscle size is associated with knee osteoarthritis. Osteoarthritis and Cartilage, 22(2), 226–234. 10.1016/j.joca.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Sabatini DM, & Baur JA (2012). Rapamycin-Induced Insulin Resistance Is Mediated by mTORC2 Loss and Uncoupled from Longevity. Science, 335(March). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S, Rabøl R, Hansen CN, Madsbad S, Helge JW, & Dela F (2012). Metformin-treated patients with type 2 diabetes have normal mitochondrial complex i respiration. Diabetologia, 55(2), 443–449. 10.1007/s00125-011-2340-0 [DOI] [PubMed] [Google Scholar]
- Li H, Ding X, Terkeltaub R, Lin H, Zhang Y, Zhou B, He K, Li K, Liu Z, Wei J, Yang Y, Xie H, Zeng C, & Lei G (2020). Exploration of metformin as novel therapy for osteoarthritis: Preventing cartilage degeneration and reducing pain behavior. Arthritis Research and Therapy, 22(1), 1–11. 10.1186/s13075-020-2129-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser RF, Goldring SR, Scanzello CR, & Goldring MB (2012). Osteoarthritis: A disease of the joint as an organ. Arthritis and Rheumatism, 64(6), 1697–1707. 10.1002/art.34453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, De Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, & Strong R (2011). Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 66 A(2), 191–201. 10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton DM, Elliehausen CJ, Javors MA, Santangelo KS, & Konopka AR (2021). Rapamycin induced hyperglycemia is associated with exacerbated age-related osteoarthritis. BioRxiv. 10.1101/2021.05.21.445179 ; this [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto PM, LeBlanc PJ, & Holloway GP (2018). High-Fat Diet Causes Mitochondrial Dysfunction as a Result of Impaired ADP Sensitivity. Diabetes, 67(11), 2199–2205. 10.2337/db18-0417 [DOI] [PubMed] [Google Scholar]
- Murray CJL, Abraham J, Ali MK, Alvarado M, Atkinson C, Baddour LM, Bartels DH, Benjamin EJ, Bhalla K, Birbeck G, Bolliger I, Burstein R, Carnahan E, Chen H, Chou D, Chugh SS, Cohen A, Colson KE, Cooper LT, … Zabetian A (2013). The State of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA - Journal of the American Medical Association, 310(6), 591–608. 10.1001/jama.2013.13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musci RV, Walsh MA, Konopka AR, Wolff CA, Peelor FF, Reiser RF, Santangelo KS, & Hamilton KL (2020). The Dunkin Hartley Guinea Pig Is a Model of Primary Osteoarthritis That Also Exhibits Early Onset Myofiber Remodeling That Resembles Human Musculoskeletal Aging. Frontiers in Physiology, 11(October), 1–18. 10.3389/fphys.2020.571372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noehren B, Kosmac K, Walton RG, Murach KA, Lyles MF, Loeser RF, Peterson CA, & Messier SP (2018). Alterations in quadriceps muscle cellular and molecular properties in adults with moderate knee osteoarthritis. Osteoarthritis and Cartilage, 26(10), 1359–1368. 10.1016/j.joca.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øiestad BE, Juhl CB, Eitzen I, & Thorlund JB (2015). Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthritis and Cartilage, 23(2), 171–177. 10.1016/j.joca.2014.10.008 [DOI] [PubMed] [Google Scholar]
- Perry CGR, Kane DA, Lin C. Te, Kozy R, Cathey B, Lark DS, Kane CL, Brophy PM, Gavin TP, Anderson EJ, & Neufer PD (2011). Inhibiting myosin-ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. Biochemical Journal, 437(2), 215–222. 10.1042/BJ20110366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radakovich LB, Marolf AJ, Shannon JP, Pannone SC, Sherk VD, & Santangelo KS (2018). Development of a microcomputed tomography scoring system to characterize disease progression in the Hartley guinea pig model of spontaneous osteoarthritis. Connective Tissue Research, 59(6), 523–533. 10.1080/03008207.2017.1409218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan A, & Schreiber SL (2009). Direct control of mitochondrial function by mTOR. Proceedings of the National Academy of Sciences of the United States of America, 106(52), 22229–22232. 10.1073/pnas.0912074106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar A, Hamadeh MJ, Kaczor JJ, Raha S, deBeer J, & Tarnopolsky MA (2010). Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS ONE, 5(5), 31–33. 10.1371/journal.pone.0010778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanasto AJ, Glynn NW, Jubrias SA, Conley KE, Boudreau RM, Amati F, Mackey DC, Simonsick EM, Strotmeyer ES, Coen PM, Goodpaster BH, & Newman AB (2015). Skeletal Muscle Mitochondrial Function and Fatigability in Older Adults. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 70(11), 1379–1385. 10.1093/gerona/glu134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal NA, & Glass NA (2011). Is quadriceps muscle weakness a risk factor for incident or progressive knee osteoarthritis? Physician and Sportsmedicine, 39(4), 44–50. 10.3810/psm.2011.11.1938 [DOI] [PubMed] [Google Scholar]
- Serrão PRMS, Vasilceac FA, Gramani-Say K, Lessi GC, Oliveira AB, Reiff RBM, Mattiello-Sverzut AC, & Mattiello SM (2015). Men with early degrees of knee osteoarthritis present functional and morphological impairments of the quadriceps femoris muscle. American Journal of Physical Medicine and Rehabilitation, 94(1), 70–81. 10.1097/PHM.0000000000000143 [DOI] [PubMed] [Google Scholar]
- Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, & Wolinsky FD (1997). Quadriceps weakness and osteoarthritis of the knee. Annals of Internal Medicine, 127(2), 97–104. 10.7326/0003-4819-127-2-199707150-00001 [DOI] [PubMed] [Google Scholar]
- Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhães JP, Martinez PA, McCord JM, Miller BF, Müller M, Nelson JF, Ndukum J, Rainger GE, … Harrison DE (2016). Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell, 15(5), 872–884. 10.1111/acel.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K, Kawakami Y, Kobayashi M, Greco N, Cummins JH, Matsushita T, Kuroda R, Kurosaka M, Fu FH, & Huard J (2014). Local intra-articular injection of rapamycin delays articular cartilage degeneration in a murine model of osteoarthritis. Arthritis Research and Therapy, 16(1), 1–10. 10.1186/s13075-014-0482-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorstensson CA, Petersson IF, Jacobsson LTH, Boegård TL, & Roos EM (2004). Reduced functional performance in the lower extremity predicted radiographic knee osteoarthritis five years later. Annals of the Rheumatic Diseases, 63(4), 402–407. 10.1136/ard.2003.007583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Skike CE, Lin AL, Roberts Burbank R, Halloran JJ, Hernandez SF, Cuvillier J, Soto VY, Hussong SA, Jahrling JB, Javors MA, Hart MJ, Fischer KE, Austad SN, & Galvan V (2020). mTOR drives cerebrovascular, synaptic, and cognitive dysfunction in normative aging. Aging Cell, 19(1), 1–11. 10.1111/acel.13057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytla VS, & Ochs RS (2013). Metformin increases mitochondrial energy formation in L6 muscle cell cultures. Journal of Biological Chemistry, 288(28), 20369–20377. 10.1074/jbc.M113.482646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels B, Ciapaite J, Van Den Broek NMA, Nicolay K, & Prompers JJ (2014). Metformin impairs mitochondrial function in skeletal muscle of both lean and diabetic rats in a Dose-dependent manner. PLoS ONE, 9(6). 10.1371/journal.pone.0100525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff CA, Lawrence MM, Porter H, Zhang Q, Reid JJ, Laurin JL, Musci RV, Linden MA, Peelor FF, Wren JD, Creery JS, Cutler KJ, Carson RH, Price JC, Hamilton KL, & Miller BF (2020). Sex differences in changes of protein synthesis with rapamycin treatment are minimized when metformin is added to rapamycin. GeroScience. 10.1007/s11357-020-00243-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Varamini B, Lamming DW, Sabatini DM, & Baur JA (2012). Rapamycin has a biphasic effect on insulin sensitivity in C2C12 myotubes due to sequential disruption of mTORC1 and mTORC2. Frontiers in Genetics, 3(SEP), 1–10. 10.3389/fgene.2012.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Widlund AL, Sims CA, Lamming DW, Guan Y, Davis JG, Sabatini DM, Harrison DE, Vang O, & Baur JA (2013). Rapamycin doses sufficient to extend lifespan do not compromise muscle mitochondrial content or endurance. Aging, 12(7), 6486–6487. 10.18632/aging.102976 [DOI] [PMC free article] [PubMed] [Google Scholar]


