Abstract
The centrosome is a subcellular organelle from which a cilium assembles. Since centrosomes function as spindle poles during mitosis, they have to be present as a pair in a cell. How the correct number of centrosomes is maintained in a cell has been a major issue in the fields of cell cycle and cancer biology. Centrioles, the core of centrosomes, assemble and segregate in close connection to the cell cycle. Abnormalities in centriole numbers are attributed to decoupling from cell cycle regulation. Interestingly, supernumerary centrioles are commonly observed in cancer cells. In this review, we discuss how supernumerary centrioles are generated in diverse cellular conditions. We also discuss how the cells cope with supernumerary centrioles during the cell cycle.
Keywords: cancer cells, cell cycle, centrosome, mitosis, supernumerary centrioles
INTRODUCTION
The centrosome is the major microtubule organizing center in an animal cell and functions as spindle poles to pull a set of chromosomes equally into two daughter cells during mitosis. Centrosomes are comprised of centrioles surrounded by pericentriolar material (PCM). A daughter centriole is assembled next to the mother centriole during S phase and remains associated until the end of mitosis. Once a cell exits mitosis, the daughter centriole departs from the mother centriole and becomes a young mother centriole (Wang et al., 2011). The young mother centriole is now able to recruit PCM on its own and becomes a microtubule organizing center. It also acquires the ability to function as a template for the assembly of a new daughter centriole in the subsequent S phase (Fu et al., 2016; Tsuchiya et al., 2016; Wang et al., 2011). Therefore, an animal cell always includes two centrosomes throughout the cell cycle (Sullenberger et al., 2020).
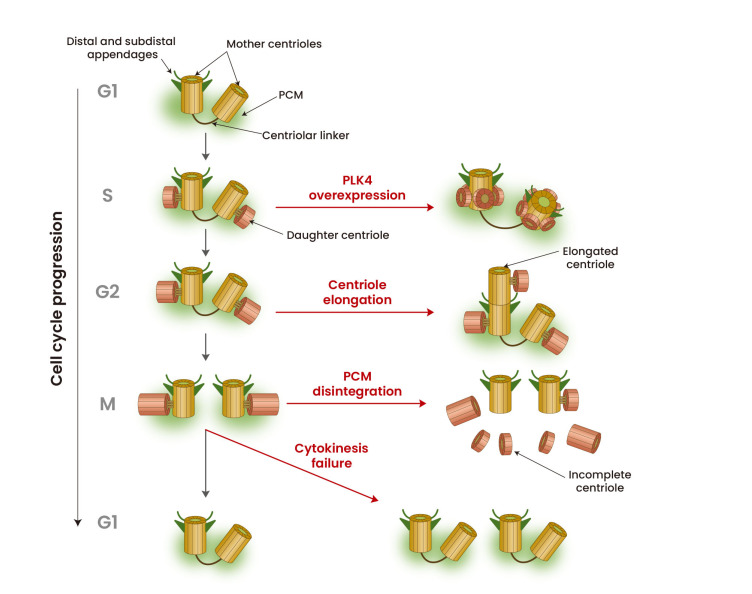
Conserved mechanisms in centriole biogenesis have been extensively studied for the last two decades. Polo-like kinase 4 (PLK4) is a central regulator of centriole duplication and, therefore, the levels and activity of PLK4 are tightly regulated during the cell cycle (Bettencourt-Dias et al., 2005; Habedanck et al., 2005; O’Connell et al., 2001). During G1/S transition period, PLK4 forms a stable complex with SCL/TAL-interrupting locus protein (STIL) and recruits spindle assembly abnormal protein 6 (SAS6) at a single site on the proximal end of the mother centriole where centriole biogenesis occurs (Dzhindzhev et al., 2014; Ohta et al., 2014). SAS6 assembles a nine-fold symmetry cartwheel structure which is the template for a daughter centriole (Hirono, 2014). Mother centrioles are not allowed to form new daughter centrioles in the G2 and M phases, as long as they are associated with daughter centrioles in their vicinity (Cabral et al., 2013; Kim et al., 2015; Tsou and Stearns, 2006; Wong et al., 2003).
Centrosome number should be tightly controlled during the cell cycle, especially for maintaining genomic stability. Extra centrosomes may be experimentally generated by drug treatments as well as genetic manipulations. Under such experimental conditions, extra centrosomes are problematic since they may lead to the formation of multipolar spindles during mitosis, leading to mitotic arrest and aneuploidy (Raff and Basto, 2017). Supernumerary centrioles are also naturally observed in cancer cell lines and highly prevalent in aggressive breast carcinomas (Marteil et al., 2018; Pihan et al., 2003). Supernumerary centrioles often correlate with advanced tumor grade and poor clinical outcome (Godinho and Pellman, 2014; Nigg, 2006; Nigg and Raff, 2009).
In this review, we discuss how supernumerary centrioles are generated in diverse cellular conditions. We also discuss how cells cope with supernumerary centrioles during the cell cycle. We regret that only a selected number of papers are referred to in this review.
GENERATION OF SUPERNUMERARY CENTRIOLES
Cytokinesis failure
In proliferating cells, polyploidy can arise via abnormal cell cycle events, including cytokinesis failure, cell fusion, endoreplication, and mitotic slippage (Davoli and de Lange, 2011; Dikovskaya et al., 2007; Edgar and Orr-Weaver, 2001; Holland and Cleveland, 2009; Larsson et al., 2008; Reider, 2011). Tetraploid cells are commonly found in premalignant lesions and tumors at different stages (Davoli and de Lange, 2011; Galipeau et al., 1996; Olaharski et al., 2006). In fact, whole genome doubling is known to precede aneuploidy in approximately one-third of tumors (Zack et al., 2013). Genome duplication concomitantly generates extra centrosomes in a cell (Fig. 1). However, supernumerary centrioles are not always maintained in a cell population after genome duplication (Krzywicka-Racka and Sluder, 2011). Extra centrosomes are rapidly lost from the cell population after several cell divisions, and normal centriole numbers are detected in most tetraploid or near-tetraploid cell clones (Galofré et al., 2020; Ganem et al., 2009; Godinho et al., 2014; Kuznetsova et al., 2015; Potapova et al., 2016). For multiploid cells to keep extra centrosomes, they should follow additional genetic changes under certain conditions in the tissue microenvironment that favor the presence of extra centrosomes (Baudoin et al., 2020). Loss of p53 may lead to a permissive environment for supernumerary centrioles since it senses mitotic delays caused by defects in bipolar spindle formation (Fukasawa et al., 1996; Lambrus and Holland, 2017; Lambrus et al., 2016). Supernumerary centrioles cause mitotic delays, which stabilize p53 through activation of LATS2 kinase in the Hippo pathway (Ganem et al., 2014). Additional pathways also work for p53 stabilization in response to supernumerary centrosomes (Nigg and Holland, 2018). For example, it was recently known that PIDDosome, an activating platform containing caspase-2, is specifically located at the mother centrioles (Burigotto et al., 2021). Supernumerary centrioles trigger the activation of PIDDosome, leading to caspase-2-mediated MDM2 cleavage, p53 stabilization, and p21-dependent cell cycle arrest (Fava et al., 2017).
Fig. 1. Generation of supernumerary centrioles.
Centrioles in normal cells assemble and segregate at the S and M phases, respectively. Supernumerary centrioles are generated at different cell cycle stages, caused by diverse occasions, depending on the diverse responsible mechanisms, such as PLK4 overexpression, centriole over-elongation, PCM disintegration and cytokinesis failure.
PLK4 overexpression
PLK4 is a central regulator of centriole assembly (Bettencourt-Dias et al., 2005; Habedanck et al., 2005; O’Connell et al., 2001); therefore, the activity of PLK4 has to be tightly regulated during the cell cycle. Overexpression of PLK4 increases centrosome number in the absence of direct effects on cellular ploidy or oncogenes and tumor suppressor genes (Fig. 1; Habedanck et al., 2005; Kleylein-Sohn et al., 2007). PLK4-overexpressing flies form supernumerary centrosomes in larval brain and wing disk tissues but survive (Basto et al., 2008; Castellanos et al., 2008; Sabino et al., 2015). On the other hand, neuronal progenitors in PLK4-overexpressing flies have defects in spindle positioning, resulting in a reduction in the neuronal progenitor pool (Basto et al., 2008).
Elevated levels of PLK4 are detected in a variety of tumor cells (Liao et al., 2019). In fact, genetic variants near the PLK4 locus are closely associated with aneuploidy (McCoy et al., 2015). However, it is controversial whether PLK4 overexpression per se induces tumorigenesis (Raff and Basto, 2017). PLK4 overexpression in the developing epidermis induces centrosome amplification and multipolar divisions, leading to p53 elevation and apoptosis of epidermal progenitors (Serçin et al., 2016). Centrosome amplification in embryonic neural progenitors results in aneuploidy, cell death, and microcephaly but does not promote tumorigenesis (Marthiens et al., 2013). In addition, increasing the centrosome number in the skin of mice fails to promote the formation of spontaneous or carcinogen-induced skin tumors (Kulukian et al., 2015; Vitre et al., 2015). In contrast, centrosome amplification—either globally or in the skin—accelerates the onset of tumors caused by loss of p53 (Coelho et al., 2015; Serçin et al., 2016). A chronic or transient increase in PLK4 promotes aneuploidy and centrosome amplification that drives the development of spontaneous tumors in multiple tissues, indicating that centrosome amplification is sufficient to initiate tumorigenesis in a mouse model (Levine et al., 2017). Accordingly, disruption of Kruppel-like Factor 14, a transcriptional repressor of PLK4, causes centrosome amplification, aneuploidy and spontaneous tumorigenesis (Fan et al., 2015).
Centriole elongation
Cell division failure and dysregulation of the centrosome duplication machinery are considered two main mechanisms to induce centrosome amplification (Godinho and Pellman, 2014). However, additional mechanisms have been recently proposed for the generation of supernumerary centrioles. Centriole structural aberrations, including over-elongation, are frequently observed in various tumors (Chan, 2011; Marteil et al., 2018). Since centrioles do not have an elongation monitoring mechanism, they are prone to over-elongation in cells with prolonged G2 and mitosis (Kong et al., 2020). Several factors lead to over-elongation in certain cellular conditions. For example, overexpression of centrosomal P4.1-associated protein (CPAP) leads to over-elongation of either the parental centriole or procentriole (Schmidt et al., 2009). As one of the kinases that regulates centriole elongation, PLK1 is also critical for G2 and mitotic centriole over-elongation (Kong et al., 2020). Dysregulated phosphorylation of CPAP by PLK2 also contributes to over-elongation of centrioles (Chang et al., 2010). Severe centriole over-elongation can promote amplification through both centriole fragmentation and ectopic procentriole formation along their elongated walls (Fig. 1; Kohlmaier et al., 2009; Marteil et al., 2018). Detailed mechanisms in centriole formation from over-elongated centrioles remain to be investigated.
Disintegration of PCM during mitosis
A daughter centriole assembles and engages at a perpendicular angle to the mother centriole during interphase. Once a cell enters mitosis, a mother and daughter centriole pair immediately disengages but remains associated due to the surrounding PCM (Cabral et al., 2013; Seo et al., 2015). Separase, a cysteine protease responsible for triggering anaphase by hydrolyzing cohesin, also hydrolyzes pericentrin, a PCM protein (Lee and Rhee, 2012; Matsuo et al., 2012). Disintegration of PCM is followed by pericentrin cleavage. If PCM is unexpectedly disintegrated at early mitosis, the mother and daughter centrioles precociously separate from each other (Cabral et al., 2013; Seo et al., 2015). Since cellular PLK4 levels are maintained at high levels during mitosis, liberated centrioles are able to amplify extra centrioles even in M phase (Fig. 1; Kim et al., 2019). A recent study revealed that TP53;PCNT;CEP215 triple knockout cells generate supernumerary centrioles in M phase, which are maintained throughout the cell cycle (Jung and Rhee, 2021). Extra centrioles may be generated when some cell lines are arrested at the S and G2 phases for a prolonged time period (Balczon et al., 1995; Loncarek et al., 2010; Shukla et al., 2015). Accordingly, DNA damage can induce centrosome amplification by increasing the time cells spend in G2 phase (Inanç et al., 2010). If mother centrioles are somehow liberated from the daughter centrioles during cell cycle arrest, the mother centrioles are allowed to generate extra centrioles.
FATES OF THE SUPERNUMERARY CENTRIOLES DURING THE CELL CYCLE
Balancing between generation and removal of extra centrioles
Extra centrioles are problematic in mitosis, and cells with extra centrosomes are selectively removed from the population, possibly by mitotic arrest (Fig. 2; Chiba et al., 2000). For example, cells with supernumerary centrioles generated by PLK4 overexpression go through longer durations of both interphase and mitosis, which overall imparts growth disadvantages in comparison to cells with normal centriole numbers (Sala, et al., 2020). Tetraploid cells that lose extra centrosomes over time may be another example of clonal elimination of cells with extra centrioles (Krzywicka-Racka and Sluder, 2011; Mikeladze-Dvali et al., 2012). Nonetheless, many cancer cell lines maintain a distinct proportion of cells with supernumerary centrioles (Marteil et al., 2018). A few mechanisms have been proposed to explain how cancer cell lines maintain supernumerary centrioles. The first mechanism is that the generation and removal of extra centrioles are balanced in a cell population. Extra centrioles may be continuously generated in a comparable rate, following one of the mechanisms previously described in this review.
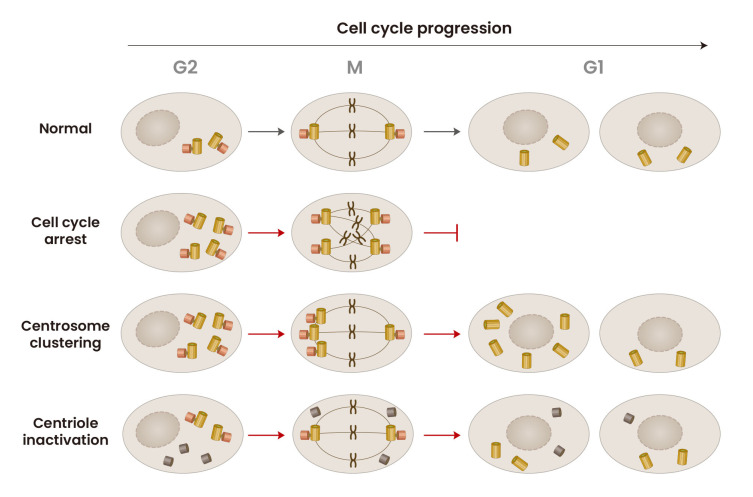
Fig. 2. Fates of the supernumerary centrioles during the cell cycle.
The cells with supernumerary centrioles may be arrested at M phase, due to mitosis failure. In other occasion, the cells may form bipolar spindles with clustered centrioles and undergo mitosis. Finally, biologically inactive forms of supernumerary centrioles hardly affect cell cycle progression.
Centrosome clustering
Centrosome clustering is the best-characterized mechanism to cope with extra centrosomes and is perhaps the most prevalent in cancer (Godinho et al., 2014). Cells can cluster supernumerary centrosomes into two spindle poles to allow the formation of a pseudobipolar spindle, which permits bipolar cell division and survival (Fig. 2; Quintyne et al., 2005). Human cells have an intrinsic ability to cluster centrosomes. This process depends on proteins that directly or indirectly contribute to force generation during mitosis. Within the spindle, centrosome clustering arises from motor proteins located near spindle poles and centrosomes, such as dynein and KIFC1/HSET, as well as proteins localized at the kinetochore or centromere that control microtubule binding and spindle assembly checkpoint signaling (Drosopoulos et al., 2014; Kwon et al., 2008; Leber et al., 2010; Quintyne et al., 2005). Outside of the spindle, at the cell cortex, motor proteins associate with the cortical actin network, such as Myo10 and dynein, and position centrosomes by generating force on astral microtubules (Kwon et al., 2015; Quintyne et al., 2005). Compared with nontransformed cells, centrosome clustering is more efficient in cancer cells (Ganem et al., 2009).
Chromosomal aberration frequently occurs when centrosomes are clustered through the formation of merotelic kinetochore-microtubule attachments (Ganem et al., 2009). At the same time, centrosome clustering may be used to generate daughter cells with a normal centrosome number. For example, tetraploid cells can inherit a normal centrosome number through asymmetric centrosome clustering during cell division (Baudoin et al., 2020). It was recently reported that cancer-associated mutations in the alpha isoform of the scaffolding subunit of protein phosphatase 2A (PP2A-Aα) enhance centrosome clustering (Antao et al., 2019). These observations suggest that mutations in PP2A-A α may be one of the genetic backgrounds favoring supernumerary centrioles in cancer cells.
Centriole inactivation
The number of centrioles doubles after cells pass through the G1/S transition phase. However, supernumerary centrioles are not always doubled, suggesting that a fraction of centrioles may be inactive in centriole assembly (Sala et al., 2020). Heterogeneity in supernumerary centrioles has been reported in TP53;PCNT;CEP215 triple knockout cells (Jung and Rhee, 2021). A couple of centrioles in triple KO cells are assembled in S phase, while the other centrioles are accidentally assembled in M phase (Jung and Rhee, 2021). M-phase-assembled centrioles include structural and functional defects, which hamper daughter-to-mother centriole conversion after mitotic exit (Jung and Rhee, 2021). Daughter-to-mother centriole conversion is critical for centrosome function during the cell cycle (Wang et al., 2011). M-phase-assembled centrioles lack the ability to function as templates for centriole assembly during S phase (Fig. 2). They also lack the ability to organize microtubules in interphase (Jung and Rhee, 2021). On the other hand, the S-phase-assembled centrioles in the same cells are intact. In these cells, centriole assembly occurs only from the S-phase-assembled centrioles as templates. During mitosis, a normal bipolar spindle is formed with intact pairs of centrosomes. Therefore, even if extra centrioles are present in the cells, they hardly disturb cell cycle progression. Nevertheless, a fraction of the extra centrioles may form PCM and function as spindle poles, causing mitotic defects and aneuploidy. Inactive centrioles may be generated in other cases, as can be surmised based on reports concerning PCM inactivation in Drosophila (Sabino et al., 2015).
FUTURE DIRECTIONS
Considerable efforts have been made to elucidate how extra centrioles are generated in dividing cells. Supernumerary centrioles may just be an outcome of abnormal cell cycle defects. Alternatively, supernumerary centrioles are beneficial to some cells that can sufficiently cope with the difficulty of mitotic catastrophe. It would be interesting to investigate what are the positive forces to maintain supernumerary centrioles during the cell cycle. A few lines of evidence indicate that supernumerary centrioles contribute to the metastasis of tumor cells. For example, an increase in microtubule nucleation with extra centrosomes promotes activation of the small GTPase Rac1, which, in turn, initiates actin polymerization and promotes cell migration (Godinho et al., 2014). Structural aberration induced by overexpression of the ninein-like protein promotes budding of mitotic cells from acini in a three-dimensional culture model (Ganier et al., 2018; Schnerch and Nigg, 2016). There may be additional forces to promote the maintenance of supernumerary centrioles.
One should consider numerical as well as structural aberrations of supernumerary centrioles. Several studies have hinted that supernumerary centrioles may be heterogeneous in their structures and biological activities (Jung and Rhee, 2021; Marteil et al., 2018). Therefore, the outcome of supernumerary centrioles may be diverse in cancer cells, depending on the types of centrioles they possess.
It is also interesting to investigate how supernumerary centrioles are discarded from the population. Clonal elimination is a prominent way to remove extra centrioles from cells. However, autophagy may also be involved in the removal of defective centrioles in cells with a normal number of centrioles as well as with supernumerary centrioles. In the very least, dysregulation of autophagy might contribute to the generation of supernumerary centrioles per se via accumulation of components implicated in duplication, such as CEP63 (Watanabe et al., 2016; Wu et al., 2021). We hope that these key questions will be resolved in the near future.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2019R1A2C22002726).
Footnotes
AUTHOR CONTRIBUTIONS
All authors wrote the manuscript. B.S. and K.R. prepared the figures.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Antao N.V., Marcet-Ortega M., Cifani P., Kentsis A., Foley E.A. A cancer-associated missense mutation in PP2A-Aα increases centrosome clustering during mitosis. Science. 2019;19:74–82. doi: 10.1016/j.isci.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balczon R., Bao L., Zimmer W.E., Brown K., Zinkowski R.P., Brinkley B.R. Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J. Cell Biol. 1995;130:105–115. doi: 10.1083/jcb.130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basto R., Brunk K., Vinadogrova T., Peel N., Franz A., Khodjakov A., Raff J.W. Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008;133:1032–1042. doi: 10.1016/j.cell.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudoin N.C., Nicholson J.M., Soto K., Martin O., Chen J., Cimini D. Asymmetric clustering of centrosomes defines the early evolution of tetraploid cells. Elife. 2020;9:e54565. doi: 10.7554/eLife.54565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt-Dias M., Rodrigues-Martins A., Carpenter L., Riparbelli M., Lehmann L., Gatt M.K., Carmo N., Balloux F., Callaini G., Glover D.M. SAK/PLK4 is required for centriole duplication and flagella development. Curr. Biol. 2005;15:2199–2207. doi: 10.1016/j.cub.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Burigotto M., Mattivi A., Migliorati D., Magnani G., Valentini C., Roccuzzo M., Offterdinger M., Pizzato M., Schmidt A., Villunger A., et al. Centriolar distal appendages activate the centrosome-PIDDosome-p53 signalling axis via ANKRD26. EMBO J. 2021;40:e104844. doi: 10.15252/embj.2020104844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral G., Sans S.S., Cowan C.R., Dammermann A. Multiple mechanisms contribute to centriole separation in C. elegans . Curr. Biol. 2013;23:1380–1387. doi: 10.1016/j.cub.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos E., Dominguez P., Gonzalez C. Centrosome dysfunction in Drosophila neural stem cells causes tumors that are not due to genome instability. Curr. Biol. 2008;18:1209–1214. doi: 10.1016/j.cub.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Chan J.Y. A clinical overview of centrosome amplification in human cancers. Int. J. Biol. Sci. 2011;7:1122–1144. doi: 10.7150/ijbs.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Cizmecioglu O., Hoffmann I., Rhee K. PLK2 phosphorylation is critical for CPAP function in procentriole formation during the centrosome cycle. EMBO J. 2010;29:2395–2406. doi: 10.1038/emboj.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Okuda M., Mussman J.G., Fukasawa K. Genomic convergence and suppression of centrosome hyperamplification in primary p53-/- cells in prolonged culture. Exp. Cell Res. 2000;258:310–321. doi: 10.1006/excr.2000.4916. [DOI] [PubMed] [Google Scholar]
- Coelho P.A., Bury L., Shahbazi M.N., Liakath-Ali K., Tate P.H., Wormald S., Hindley C.J., Huch M., Archer J., Skarnes W.C., et al. Over-expression of Plk4 induces centrosome amplification, loss of primary cilia and associated tissue hyperplasia in the mouse. Open Biol. 2015;5:150209. doi: 10.1098/rsob.150209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli T., de Lange T. The causes and consequences of polyploidy in normal development and cancer. Annu. Rev. Cell Dev. Biol. 2011;27:585–610. doi: 10.1146/annurev-cellbio-092910-154234. [DOI] [PubMed] [Google Scholar]
- Dikovskaya D., Schiffmann D., Newton I.P., Oakley A., Kroboth K., Sansom O., Jamieson T.J., Meniel V., Clarke A., Näthke I.S. Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. J. Cell Biol. 2007;176:183–195. doi: 10.1083/jcb.200610099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosopoulos K., Tang C., Chao W.C.H., Linardopoulos S. APC/C is an essential regulator of centrosome clustering. Nat. Commun. 2014;5:3686. doi: 10.1038/ncomms4686. [DOI] [PubMed] [Google Scholar]
- Dzhindzhev N.S., Tzolovsky G., Lipinszki Z., Schneider S., Lattao R., Fu J., Debski J., Dadlez M., Glover D.M. Plk4 phosphorylates Ana2 to trigger Sas6 recruitment and procentriole formation. Curr. Biol. 2014;24:2526–2532. doi: 10.1016/j.cub.2014.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar B.A., Orr-Weaver T.L. Endoreplication cell cycles: more for less. Cell. 2001;105:297–306. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- Fan G., Sun L., Shan P., Zhang X., Huan J., Zhang X., Li D., Wang T., Wei T., Zhang X., et al. Loss of KLF14 triggers centrosome amplification and tumorigenesis. Nat. Commun. 2015;6:8450. doi: 10.1038/ncomms9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava L.L., Schuler F., Sladky V., Haschka M.D., Soratroi C., Eiterer L., Demetz E., Weiss G., Geley S., Nigg E.A., et al. The PIDDosome activates p53 in response to supernumerary centrosomes. Genes Dev. 2017;31:34–45. doi: 10.1101/gad.289728.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Lipinszki Z., Rangone H., Min M., Mykura C., Chao-Chu J., Schneider S., Dzhindzhev N.S., Gottardo M., Riparbelli M.G., et al. Conserved molecular interactions in centriole-to-centrosome conversion. Nat. Cell Biol. 2016;18:87–99. doi: 10.1038/ncb3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa K., Choi T., Kuriyama R., Rulong S., Vande Woude G.F. Abnormal centrosome amplification in the absence of p53. Science. 1996;271:1744–1747. doi: 10.1126/science.271.5256.1744. [DOI] [PubMed] [Google Scholar]
- Galipeau P.C., Cowan D.S., Sanchez C.A., Barrett M.T., Emond M.J., Levine D.S., Rabinovitch P.S., Reid B.J. 17p (p53) allelic losses, 4N (G2/tetraploid) populations, and progression to aneuploidy in Barrett's esophagus. Proc. Natl. Acad. Sci. U. S. A. 1996;93:7081–7084. doi: 10.1073/pnas.93.14.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galofré C., Asensio E., Ubach M., Torres I.M., Quintanilla I., Castells A., Camps J. Centrosome reduction in newly-generated tetraploid cancer cells obtained by separase depletion. Sci. Rep. 2020;10:9152. doi: 10.1038/s41598-020-65975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem N.J., Cornils H., Chiu S.Y., O'Rourke K.P., Arnaud J., Yimlamai D., Théry M., Camargo F.D., Pellman D. Cytokinesis failure triggers hippo tumor suppressor pathway activation. Cell. 2014;158:833–848. doi: 10.1016/j.cell.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem N.J., Godinho S.A., Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganier O., Schnerch D., Oertle P., Lim R.Y., Plodinec M., Nigg E.A. Structural centrosome aberrations promote non-cell-autonomous invasiveness. EMBO J. 2018;37:e98576. doi: 10.15252/embj.201798576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho S.A., Pellman D. Causes and consequences of centrosome abnormalities in cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130467. doi: 10.1098/rstb.2013.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho S.A., Picone R., Burute M., Dagher R., Su Y., Leung C.T., Polyak K., Brugge J.S., Théry M., Pellman D. Oncogene-like induction of cellular invasion from centrosome amplification. Nature. 2014;510:167–171. doi: 10.1038/nature13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habedanck R., Stierhof Y.D., Wilkinson C.J., Nigg E.A. The Polo kinase Plk4 functions in centriole duplication. Nat. Cell Biol. 2005;7:1140–1146. doi: 10.1038/ncb1320. [DOI] [PubMed] [Google Scholar]
- Hirono M. Cartwheel assembly. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130458. doi: 10.1098/rstb.2013.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland A.J., Cleveland D.W. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat. Rev. Mol. Cell Biol. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inanç B., Dodson H., Morrison C.G. A centrosome-autonomous signal that involves centriole disengagement permits centrosome duplication in G2 phase after DNA damage. Mol. Biol. Cell. 2010;21:3866–3877. doi: 10.1091/mbc.E10-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G.I., Rhee K. Triple deletion of TP53, PCNT, and CEP215 promotes centriole amplification in the M phase. Cell Cycle. 2021;20:1500–1517. doi: 10.1080/15384101.2021.1950386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kim J., Rhee K. PCNT is critical for the association and conversion of centrioles to centrosomes during mitosis. J. Cell Sci. 2019;132:jcs225789. doi: 10.1242/jcs.225789. [DOI] [PubMed] [Google Scholar]
- Kim J., Lee K., Rhee K. PLK1 regulation of PCNT cleavage ensures fidelity of centriole separation during mitotic exit. Nat. Commun. 2015;6:10076. doi: 10.1038/ncomms10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleylein-Sohn J., Westendorf J., Le Clech M., Habedanck R., Stierhof Y.D., Nigg E.A. Plk4-induced centriole biogenesis in human cells. Dev. Cell. 2007;13:190–202. doi: 10.1016/j.devcel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Kohlmaier G., Loncarek J., Meng X., McEwen B.F., Mogensen M.M., Spektor A., Dynlacht B.D., Khodjakov A., Gönczy P. Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr. Biol. 2009;19:1012–1018. doi: 10.1016/j.cub.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D., Sahabandu N., Sullenberger C., Vásquez-Limeta A., Luvsanjav D., Lukasik K., Loncarek J. Prolonged mitosis results in structurally aberrant and over-elongated centrioles. J. Cell Biol. 2020;219:e201910019. doi: 10.1083/jcb.201910019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywicka-Racka A., Sluder G. Repeated cleavage failure does not establish centrosome amplification in untransformed human cells. J. Cell Biol. 2011;194:199–207. doi: 10.1083/jcb.201101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulukian A., Holland A.J., Vitre B., Naik S., Cleveland D.W., Fuchs E. Epidermal development, growth control, and homeostasis in the face of centrosome amplification. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E6311–E6320. doi: 10.1073/pnas.1518376112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A.Y., Seget K., Moeller G.K., de Pagter M.S., de Roos J.A., Dürrbaum M., Kuffer C., Müller S., Zaman G.J., Kloosterman W.P., et al. Chromosomal instability, tolerance of mitotic errors and multidrug resistance are promoted by tetraploidization in human cells. Cell Cycle. 2015;14:2810–2820. doi: 10.1080/15384101.2015.1068482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M., Bagonis M., Danuser G., Pellman D. Direct microtubule-binding by Myosin-10 orients centrosomes toward retraction fibers and subcortical actin clouds. Dev. Cell. 2015;34:323–337. doi: 10.1016/j.devcel.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M., Godinho S.A., Chandhok N.S., Ganem N.J., Azioune A., Thery M., Pellman D. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 2008;22:2189–2203. doi: 10.1101/gad.1700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrus B.G., Daggubati V., Uetake Y., Scott P.M., Clutario K.M., Sluder G., Holland A.J. A USP28-53BP1-p53-p21 signaling axis arrests growth after centrosome loss or prolonged mitosis. J. Cell Biol. 2016;214:143–153. doi: 10.1083/jcb.201604054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrus B.G., Holland A.J. A new mode of mitotic surveillance. Trends Cell Biol. 2017;27:314–321. doi: 10.1016/j.tcb.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L.I., Bjerregaard B., Talts J.F. Cell fusions in mammals. Histochem. Cell Biol. 2008;129:551–561. doi: 10.1007/s00418-008-0411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber B., Maier B., Fuchs F., Chi J., Riffel P., Anderhub S., Wagner L., Ho A.D., Salisbury J.L., Boutros M., et al. Proteins required for centrosome clustering in cancer cells. Sci. Transl. Med. 2010;2:33ra38. doi: 10.1126/scitranslmed.3000915. [DOI] [PubMed] [Google Scholar]
- Lee K., Rhee K. Separase-dependent cleavage of pericentrin B is necessary and sufficient for centriole disengagement during mitosis. Cell Cycle. 2012;11:2476–2485. doi: 10.4161/cc.20878. [DOI] [PubMed] [Google Scholar]
- Levine M.S., Bakker B., Boeckx B., Moyett J., Lu J., Vitre B., Spierings D.C., Lansdorp P.M., Cleveland D.W., Lambrechts D., et al. Centrosome amplification is sufficient to promote spontaneous tumorigenesis in mammals. Dev. Cell. 2017;40:313–322.e5. doi: 10.1016/j.devcel.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z., Zhang H., Fan P., Huang Q., Dong K., Qi Y., Song J., Chen L., Liang H., Chen X., et al. High PLK4 expression promotes tumor progression and induces epithelial-mesenchymal transition by regulating the Wnt/β-catenin signaling pathway in colorectal cancer. Int. J. Oncol. 2019;54:479–490. doi: 10.3892/ijo.2021.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncarek J., Hergert P., Khodjakov A. Centriole reduplication during prolonged interphase requires procentriole maturation governed by Plk1. Curr. Biol. 2010;20:1277–1282. doi: 10.1016/j.cub.2010.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteil G., Guerrero A., Vieira A.F., de Almeida B.P., Machado P., Mendonça S., Mesquita M., Villarreal B., Fonseca I., Francia M.E., et al. Over-elongation of centrioles in cancer promotes centriole amplification and chromosome missegregation. Nat. Commun. 2018;9:1258. doi: 10.1038/s41467-018-03641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthiens V., Rujano M.A., Pennetier C., Tessier S., Paul-Gilloteaux P., Basto R. Centrosome amplification causes microcephaly. Nat. Cell Biol. 2013;15:731–740. doi: 10.1038/ncb2746. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Ohsumi K., Iwabuchi M., Kawamata T., Ono Y., Takahashi M. Kendrin is a novel substrate for separase involved in the licensing of centriole duplication. Curr. Biol. 2012;22:915–921. doi: 10.1016/j.cub.2012.03.048. [DOI] [PubMed] [Google Scholar]
- McCoy R.C., Demko Z., Ryan A., Banjevic M., Hill M., Sigurjonssen S., Robinowitz M., Fraser H., Petrov D.A. Common variants spanning PLK4 are associated with mitotic-origin aneuploidy in human embryos. Science. 2015;348:235–238. doi: 10.1126/science.aaa3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikeladze-Dvali T., von Tobel L., Strnad P., Knott G., Leonhardt H., Schermelleh L., Gönczy P. Analysis of centriole elimination during C. elegans oogenesis. Development. 2012;139:1670–1679. doi: 10.1242/dev.075440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E.A. Origins and consequences of centrosome abberations in human cancers. Int. J. Cancer. 2006;119:2717–2723. doi: 10.1002/ijc.22245. [DOI] [PubMed] [Google Scholar]
- Nigg E.A., Holland A.J. Once and only once: mechanisms of centriole duplication and their deregulation in disease. Nat. Rev. Mol. Cell Biol. 2018;19:297–312. doi: 10.1038/nrm.2017.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E.A., Raff J.W. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139:663–678. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- O'Connell K.F., Caron C., Kopish K.R., Hurd D.D., Kemphues K.J., Li Y., White J.G. The C. elegans zyg-1 gene encodes a regulator of centrosome duplication with distinct maternal and paternal roles in the embryo. Cell. 2001;105:547–558. doi: 10.1016/s0092-8674(01)00338-5. [DOI] [PubMed] [Google Scholar]
- Ohta M., Ashikawa T., Nozaki Y., Kozuka-Hata H., Goto H., Inagaki M., Oyama M., Kitagawa D. Direct interaction of Plk4 with STIL ensures formation of a single procentriole per parental centriole. Nat. Commun. 2014;5:5267. doi: 10.1038/ncomms6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaharski A.J., Sotelo R., Solorza-Luna G., Gonsebatt M.E., Guzman P., Mohar A., Eastmond D.A. Tetraploidy and chromosomal instability are early events during cervical carcinogenesis. Carcinogenesis. 2006;27:337–343. doi: 10.1093/carcin/bgi218. [DOI] [PubMed] [Google Scholar]
- Pihan G.A., Wallace J., Zhou Y., Doxsey S.J. Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res. 2003;63:1398–1404. [PubMed] [Google Scholar]
- Potapova T.A., Seidel C.W., Box A.C., Rancati G., Li R. Transcriptome analysis of tetraploid cells identifies cyclin D2 as a facilitator of adaptation to genome doubling in the presence of p53. Mol. Biol. Cell. 2016;27:3065–3084. doi: 10.1091/mbc.E16-05-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne N.J., Reing J.E., Hoffelder D.R., Gollin S.M., Saunders W.S. Spindle multipolarity is prevented by centrosomal clustering. Science. 2005;307:127–129. doi: 10.1126/science.1104905. [DOI] [PubMed] [Google Scholar]
- Raff J.W., Basto R. Centrosome amplification and cancer: a question of sufficiency. Dev. Cell. 2017;40:217–218. doi: 10.1016/j.devcel.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Reider C.L. Mitosis in vertebrates: the G2/M and M/A transitions and their associated checkpoints. Chromosome Res. 2011;19:291–306. doi: 10.1007/s10577-010-9178-z. [DOI] [PubMed] [Google Scholar]
- Sabino D., Gogendeau D., Gambarotto D., Nano M., Pennetier C., Dingli F., Arras G., Loew D., Basto R. Moesin is a major regulator of centrosome behavior in epithelial cells with extra centrosomes. Curr. Biol. 2015;25:879–889. doi: 10.1016/j.cub.2015.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala R., Farrell K.C., Stearns T. Growth disadvantage associated with centrosome amplification drives population-level centriole number homeostasis. Mol. Biol. Cell. 2020;31:2646–2656. doi: 10.1091/mbc.E19-04-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T.I., Kleylein-Sohn J., Westendorf J., Le Clech M., Lavoie S.B., Stierhof Y.D., Nigg E.A. Control of centriole length by CPAP and CP110. Curr. Biol. 2009;19:1005–1011. doi: 10.1016/j.cub.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Schnerch D., Nigg E.A. Structural centrosome aberrations favor proliferation by abrogating microtubule-dependent tissue integrity of breast epithelial mammospheres. Oncogene. 2016;35:2711–2722. doi: 10.1038/onc.2015.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M.Y., Jang W., Rhee K. Integrity of the pericentriolar material is essential for maintaining centriole association during M phase. PLoS One. 2015;10:e0138905. doi: 10.1371/journal.pone.0138905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serçin Ö., Larsimont J.C., Karambelas A.E., Marthiens V., Moers V., Boeckx B., Le Mercier M., Lambrechts D., Basto R., Blanpain C. Transient PLK4 overexpression accelerates tumorigenesis in p53-deficient epidermis. Nat. Cell Biol. 2016;18:100–110. doi: 10.1038/ncb3270. [DOI] [PubMed] [Google Scholar]
- Shukla A., Kong D., Sharma M., Magidson V., Loncarek J. Plk1 relieves centriole block to reduplication by promoting daughter centriole maturation. Nat. Commun. 2015;6:8077. doi: 10.1038/ncomms9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullenberger C., Vasquez-Limeta A., Kong D., Loncarek J. With age comes maturity: biochemical and structural transformation of a human centriole in the making. Cells. 2020;9:1429. doi: 10.3390/cells9061429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou M.F., Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006;442:947–951. doi: 10.1038/nature04985. [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y., Yoshiba S., Gupta A., Watanabe K., Kitagawa D. Cep295 is a conserved scaffold protein required for generation of a bona fide mother centriole. Nat. Commun. 2016;7:12567. doi: 10.1038/ncomms12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitre B., Holland A.J., Kulukian A., Shoshani O., Hirai M., Wang Y., Maldonado M., Cho T., Boubaker J., Swing D.A., et al. Chronic centrosome amplification without tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E6321–E6330. doi: 10.1073/pnas.1519388112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.J., Soni R.K., Uryu K., Tsou M.F. The conversion of centrioles to centrosomes: essential coupling of duplication with segregation. J. Cell Biol. 2011;193:727–739. doi: 10.1083/jcb.201101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Honda S., Konishi A., Arakawa S., Murohashi M., Yamaguchi H., Torii S., Tanabe M., Tanaka S., Warabi E., et al. Autophagy controls centrosome number by degrading Cep63. Nat. Commun. 2016;7:13508. doi: 10.1038/ncomms13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Stearns T. Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nat. Cell Biol. 2003;5:539–544. doi: 10.1038/ncb993. [DOI] [PubMed] [Google Scholar]
- Wu Q., Yu X., Liu L., Sun S., Sun S. Centrosome-phagy: implications for human diseases. Cell Biosci. 2021;11:49. doi: 10.1186/s13578-021-00557-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack T.I., Schumacher S.E., Carter S.L., Cherniack A.D., Saksena G., Tabak B., Lawrence M.S., Zhsng C.Z., Wala J., Mermel C.H., et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 2013;45:1134–1140. doi: 10.1038/ng.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]