Abstract
We demonstrate here the first experimental suppression of a premature termination codon in vivo by using an ochre suppressor tRNA acting in an intact mouse. Multicopy tRNA expression plasmids were directly injected into skeletal muscle and into the hearts of transgenic mice carrying a reporter gene with an ochre mutation. A strategy for modulation of suppressor efficiency, applicable to diverse systems and based on tandem multimerization of the tRNA gene, is developed. The product of suppression (chloramphenicol acetyltransferase) accumulates linearly with increases in suppressor tRNA concentration to the point where the ochre-suppressing tRNASer is in four- to fivefold excess over the endogenous tRNASer. The subsequent suppressor activity plateau seems to be attributable to accumulation of unmodified tRNAs. These results define many salient variables for suppression in vivo, for example, for tRNA suppression employed as gene therapy for nonsense defects.
Translation termination is triggered by recognition of one of the three termination codons (UAA [ochre], UGA [opal], or UAG [amber]), followed by hydrolysis of the peptidyl-tRNA. Nonsense mutations that generate termination codons in the coding region of a gene cause premature termination of protein synthesis. Nonsense mutations can be suppressed by mutant tRNAs that can read termination codons as sense codons, restoring the synthesis of an active gene product (33). The efficiency of termination and that of nonsense suppression are influenced by the 3′ codon context, with termination ruled by the base immediately following the termination codon (34). In particular, the efficiency of suppression of an amber codon in human tissue culture cells varies according to the 3′ base in the pattern C < G = U < A (41). In addition, the physical and chemical characteristics of the last two amino acids in the nascent peptide function as additional codon context determinants in both bacteria and Saccharomyces cerevisiae (7, 8, 32). Together, these observations could explain why a very low number of natural termination codons are suppressed in Xenopus oocytes injected with purified suppressor tRNAs (5) and why, for example, some nonsense mutations detected in the cystic fibrosis gene cause a less severe phenotype (12, 26).
Since nonsense mutations are associated with an increasing number of human genetic diseases (3), suppressor tRNAs have also been studied as possible therapeutic agents for both β° thalassemia and xeroderma pigmentosum (38, 50). Even though these reports provided the first promising evidence for a potential clinical use of tRNA-mediated suppression, they did not demonstrate suppression in vivo in mammals.
tRNA transcription appears to have been optimized by cells (48). Therefore high levels of suppressor tRNA have been obtained only by amplifying the copy numbers of suppressor tRNA genes linked to the simian virus 40 (SV40) origin of replication, in cell lines expressing the SV40 T antigen (10, 46). However, in light of the requirement for a viral transforming protein and origin of replication, this approach cannot be used for gene therapy purposes.
More recently, inducible suppressor tRNA genes have been generated by the tetracycline or the lac operator/repressor systems. These approaches induced repression or activation of constitutive suppressor tRNA expression (16, 49, 52) as well as control of suppressor tRNA function by modulation of the aminoacylation process (18, 39).
Based on these previous studies we developed a multimerized suppressor tRNA gene system that can be used to express different amounts of suppressor tRNA. This fine tuning is desirable when contemplating a suppressor tRNA employed as a therapeutic drug. The need for such a balance is underscored by the possibility that tRNA overexpression may have toxic effects on cell metabolism (17, 23, 28). In the present study, the ability of the multicopy suppressor tRNA plasmids to rescue chloramphenicol acetyltransferase (CAT) activity of a transgenic mouse expressing a CAT ochre gene in the heart demonstrates the efficacy of our approach. This is the first example of in vivo suppression in a mammalian organism. (A preliminary report on in vivo suppression purported to come from our laboratory [31a] was published without our knowledge or agreement and has been retracted.)
MATERIALS AND METHODS
Plasmid constructions.
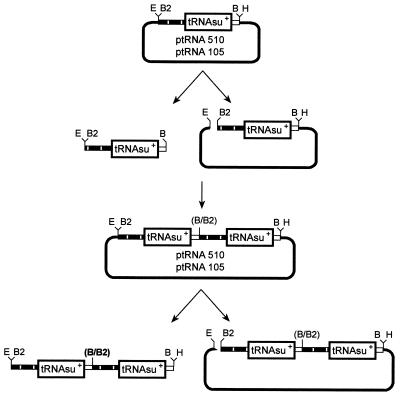
All constructs used in this study were prepared according to standard techniques (45). Plasmids containing 8 or 16 copies of the ochre suppressor tRNA gene were assembled following the scheme depicted in Fig. 1. Two PCR fragments, each containing the human serine ochre suppressor tRNA gene (tRNAsu+ gene) were generated from plasmid pSV1GT3-ser ochre using primers A (5′-ATAGAATTCAGATCTGATGTCTGTGAAAAGACACAT-3′), B (5′-ATAGAATTCAGATCTCGAAACCATCCTCTGCTATAT-3′), and D (5′-ATATAAGCTTGGATCCCCGGATTTCCTCTACCCGAGA-3′). The 5′ primers A and B are complementary to nucleotides (nt) 63 to 83 and 467 to 487, respectively, upstream of the tRNAsu+ gene and carry the EcoRI and BglII restriction sites at their 5′ ends. The 3′ primer D is complementary to nt 16 to 36 downstream of the tRNAsu+ gene and carries the BamHI and HindIII restriction sites at its 5′ end. The two PCR products so obtained contain the tRNAsu+ gene flanked by different 5′ regions (63 and 468 nt, respectively) and by the same 36-nt 3′ region that includes the transcription termination signal. Both have unique EcoRI-BglII and HindIII-BamHI restriction sites flanking the gene. The two PCR fragments were digested with EcoRI and HindIII and cloned into vector pUC18, generating the plasmids ptRNA105 and ptRNA510 (3,299 nt). The subsequent multimerization of the tRNAsu+ gene was obtained as shown in Fig. 1. The final constructs were designated ptRNA8mer/105 (4,134 nt), ptRNA16mer/105 (5,630 nt), ptRNA8mer/510 (7,366 nt), and ptRNA16mer/510 (12,094 nt). Plasmid VR1332 (renamed ViCAT in this study and provided by Vical Incorporated, San Diego, Calif.) contains the wild-type (wt) CAT gene. Plasmid ViCAT(oc27) was constructed by replacing the wt CAT gene of the ViCAT plasmid with the CAT ochre gene derived from plasmid pRSVcat(oc27) (10).
FIG. 1.
tRNAsersu+ ochre gene multimerization strategy. ptRNA 510 and 105 were obtained by recloning the tRNAsu+ gene into plasmid pUC18 as described in Materials and Methods. They carry different 5′ regions, respectively 468 and 63 nt (vertically striped boxes) and the same 36-nt 3′ region (open boxes). Plasmids containing two tRNAsu+ copies (ptRNA2mer/510 and ptRNA2mer/105) were generated after endonuclase digestion and ligation as shown schematically. Since the BamHI/BglII junction [B/B2] becomes resistant to the cleavage of either enzyme, the BamHI-BglII sites can be reused for a new round of multimerization. Unique restriction sites are indicated by the following abbreviations: E, EcoRI; H, HindIII; B, BamHI; B2, BglII. Plasmids containing 8 or 16 tRNAsu+ copies were generated from constructs containing two tRNAsu+ copies after two or three rounds of the described multimerization steps. The final constructs were designated ptRNA8mer/105, ptRNA16mer/105, ptRNA8mer/510, and ptRNA16mer/510 (numbers before the slash represent the copies of tRNAsu+ gene present in each plasmid; numbers after the slash indicate the nucleotide length of the spacer separating each tRNAsu+ gene).
DNA transfection and CAT assay.
COS 7 cells were transfected with different amounts of DNA according to DEAE-dextran (45) or FuGENE (Boehringer Mannheim) procedures. Cells were then incubated at 37°C for 36 h. Transfection efficiency was monitored by (i) CAT activity produced by the ViCAT plasmid and (ii) histochemical staining of β-galactosidase expressed by the pSV-β-gal plasmid (Promega). In all our experiments, 40 or 50% of the cells were consistently transfected with DEAE or FuGENE, respectively. Based on these results and the amount of tRNA produced, we estimate that the FuGENE method introduced ∼2.5-fold more plasmid into COS 7 cells than the DEAE method. Protein extracts were obtained, and the CAT assay (with 10 μg of total protein) was performed, as described by Sambrook et al. (45). The CAT assay of tissue was performed as described by Kass-Eisler et al. (25). Signals were quantified by a Storm 860 image analyzer (Molecular Dynamics).
DNA injections in vivo.
Male 2- and 4-week-old CD-1 mice obtained from Charles River Laboratories (Wilmington, Mass.) and α-CAT ochre transgenic mice were anesthetized by intraperitoneal injection with Avertin (21). Injections into the proximal two-thirds of tibialis anterior (TA) muscles, tongue, and heart were performed as previously described (31, 42, 56). Muscle regeneration was induced by injection of 0.75% bupivacaine hydrochloride (Sigma, St. Louis, Mo.) 5 days before plasmid DNA injection (13). Specific amounts of DNA injected in each muscle are given in the figure legends.
tRNA analysis.
Total RNA was isolated with TRI REAGENT (Molecular Research Center), according to the manufacturer's protocol, 36 h after COS 7 cell transfection. For the detection of serine-tRNA/tRNAsu+ and 5.8S rRNA, 15 μg of total RNAs was separated on 8 M urea–8% polyacrylamide gels. The portion of the gel containing the RNAs of interest was then electroblotted onto a Nytran SuPerCharge nylon membrane (Schleicher & Schuell) in Tris-borate-EDTA buffer for 3 h at 500 mA. The membrane was then UV cross-linked, and tRNAs were detected by Northern blot hybridization using oligonucleotides α (5′-TTTAAAGTCCATCGCC-3′), complementary to nt 23 to 38 of tRNAsu+, and T (5′-GTCGGCAGGATTCGAACCTGCGCGGGGAGACCCCAATGGA-3′), complementary to nt 39 to 78 of serine-tRNA, as described by Buvoli et al. (unpublished data). Hybridization signals were normalized to the level of 5.8S rRNA after membranes were stripped and reprobed with oligonucleotide R (5′-CGAAGTGTCGATGATCAAT-3′), complementary to nt 86 to 104 of the 5.8S rRNA. Prehybridization with oligonucleotide J (5′-AAGCACGCCGTAGTCG) was performed at room temperature in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate–100 mg of denatured salmon sperm DNA/ml. The membrane was then washed at room temperature in 6× SSC for 10 min. In vitro transcription of the tRNAsu+ was carried out on a PCR fragment containing the T7 promoter. The in vitro tRNAsu+ (88 nt) contains three extra nucleotides at the 5′ end (GGG) and the CCA sequence at the 3′ end. Detection of aminoacylated tRNA was achieved by using an 8% polyacrylamide gel containing 8 M urea and 0.1 M sodium acetate buffer (pH 5), as previously described (53).
Northern blot analysis of RNA isolated from α-CAT ochre gene-expressing transgenic mouse hearts.
Total RNA was isolated from α-CAT ochre gene-expressing transgenic mouse hearts using TRI REAGENT according to the manufacturer's protocol. Ten micrograms of total RNA was separated on a 1.5% agarose–6% formaldehyde gel as previously described (45). The gel was blotted onto a Hybond N nylon membrane (Amersham) and UV cross-linked. The membrane was first hybridized with an oligonucleotide (5′-TCAAACTGGTGAAACTCAC-3′) complementary to nt 450 to 470 of the CAT gene. It was subsequently stripped and reprobed with a second oligonucleotide (5′-AGCGGAAGCGCTCGTTGCCAAT-3′) labeled at the same specific activity and complementary to nt 670 to 691 of the adult cardiac muscle α-actin gene (2). Each prehybridization and hybridization reactions were carried out as described by Sambrook et al. (45).
RESULTS
Construction of plasmids carrying multiple copies of the tRNASersu+ ochre gene.
We optimized the efficiency of tRNA-mediated suppression of a nonsense mutation in cultured mammalian cells using readthrough of a premature ochre stop codon contained in the bacterial CAT reporter gene. In our initial assays, COS 7 cells were cotransfected with two plasmids, one expressing the CAT ochre gene and the other expressing a suppressor tRNA gene (tRNAsu+) derived from human serine-tRNA (9, 10). Suppression efficiency was the CAT activity produced by the rescued CAT ochre mRNA expressed as a percentage of the CAT activity obtained from the wt CAT gene. In a preliminary set of experiments we found that the detectable level of rescued CAT was unchanged and was independent of the levels of CAT ochre RNA (data not shown). We therefore hypothesized that the concentration of suppressor tRNA was limiting in our experimental conditions. We then attempted to increase tRNAsu+ expression by constructing plasmids carrying multiple copies of the tRNAsu+ gene. To determine the minimum distance between two tRNAsu+ genes for optimal expression, we tested two different spacer lengths: 105 and 510 nt. The suppressor tRNA gene was multimerized up to 16 copies per plasmid following the strategy shown in Fig. 1.
Abilities of different multimers to suppress ochre codons in cell culture.
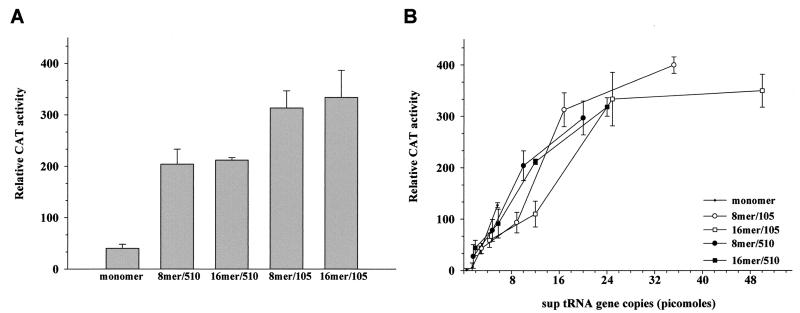
In order to determine the relative suppression activities of plasmids carrying multiple copies of the tRNAsu+ gene, COS 7 cells were cotransfected according to the DEAE-dextran method with a highly active CAT ochre expression plasmid [ViCAT(oc27)] and equal masses of the tRNAsu+ constructs. The results of this analysis are shown in Fig. 2A and are quantified in Table 1. Suppressor activity obtained for each multimer was roughly proportional to the number of tRNA genes transfected into the cells. CAT activities resulting from the 8mer/510 and 16mer/510 constructs (the prefix ptRNA is omitted) were not significantly different (P = 0.76). This was predicted because of the copy numbers of suppressor tRNA genes (Table 1). Activities resulting from 8mer/510 and 8mer/105, however, were significantly different, with the 8mer/105 construct showing its predicted higher activity (P = 0.034) (Table 1). These data suggest that (i) all of the tRNAsu+ genes can be actively transcribed without transcription or termination interference, (ii) the multimers appear to be stable in mammalian cells, and (iii) tRNAsu+ genes are functional when the distance between them is only 105 nt. A dose response experiment was then performed (Fig. 2B) by cotransfecting COS 7 cells with 4 μg of ViCAT(oc27) and 1, 3, 6, and 12 μg of each suppressor tRNA construct. For the monomer, 8mer/510, and 16mer/510 there was a linear correlation between the number of tRNAsu+ genes and the suppression efficiency. In contrast, the 8mer/105 construct and particularly the 16mer/105 construct appeared to approach a plateau at 12 μg of plasmid input, corresponding to ∼51 pmol of suppressor tRNA genes. This resulted in the synthesis of ∼5.2 pmol of tRNAsu+ (this calculation is based on a comparison with the in vitro-transcribed tRNAsu+) (Fig. 3B) per ∼105 transfected cells. This finding suggests that at this high number of tRNAsu+ genes per microgram of DNA, the 8mer/105 and 16mer/105 plasmids can saturate the transcription and/or processing pathways before the other constructs. Alternatively, saturation of suppression could occur. The 8mer/105 construct showed the highest suppression efficiency when the cells were transfected with 35 pmol of suppressor tRNA genes. In these conditions the 8mer/105 construct was able to restore the activity of ViCAT(oc27) to approximately 1.48% that of the wt. For comparison, we determined the ability of the previously described amplifying tRNAsu+ SV40 system (10) to suppress the ViCAT(oc27) mutation. In our experimental conditions we found that, 35 h after transfection, suppressor activity was approximately equivalent to that of the 8mer/510 plasmid (data not shown).
FIG. 2.
CAT activity in COS 7 cells cotransfected with the CAT ochre reporter gene and different tRNAsu+ constructs. (A) COS 7 cells were cotransfected with 4 μg of ViCAT(oc27) and 6 μg of each tRNAsu+ construct. Data represent mean values of CAT activity from four independent transfections. In order to maintain the assay in the linear range, extracts were diluted in 1% bovine serum albumin. Relative CAT activity corresponds to the percentage of acetylated chloramphenicol relative to 10 μg of total protein multiplied by the dilution factor of the cell lysate. tRNAsu+ multimers are designated as in Fig. 1; the monomer corresponds to the plasmid ptRNA 510. By analysis of variance, P < 0.05. By Fisher's protected least-square difference post hoc analysis, for 8mer/510 and 16mer/510 P = 0.76 and for 8mer/510 and 8mer/105 P = 0.034. (B) COS 7 cells were cotransfected with 4 μg of ViCAT(oc27) and 1, 3, 6, and 12 μg of each tRNAsu+ construct. Data points represent mean values of CAT activity from three independent transfections.
TABLE 1.
Relative activities of tRNAsu+ constructsa
| Construct (size [nt]) | Calculated ratio (n-mer/monomer) of tRNA gene copiesb | Measured ratio (n-mer/monomer) of:
|
||
|---|---|---|---|---|
| CAT activity | tRNA expression after transfection with:
|
|||
| DEAE | FuGENE | |||
| 8mer/510 (7,366) | 3.5 | 5 | 4.1 | 1.3 |
| 16mer/510 (12,094) | 4.2 | 5.2 | 3.9 | 1.2 |
| 8mer/105 (4,134) | 6.2 | 7.8 | 5.5 | 1.5 |
| 16mer/105 (5,630) | 9.2 | 8.3 | 7.8 | 1.5 |
COS 7 cells were cotransfected with 4 μg of ViCAT(oc27) and 6 μg of each tRNAsu+ construct. Data are mean values from four independent transfections.
Monomer length, 3,229 nt.
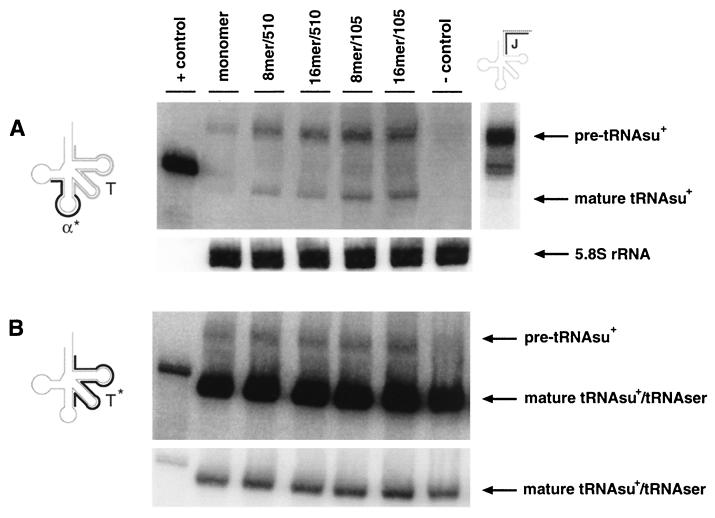
FIG. 3.
Northern blot analysis of RNA purified from COS 7 cells transfected with different tRNAsu+ constructs by the DEAE-dextran procedure. COS 7 cells were transfected according to the DEAE-dextran procedure with 6 μg of different tRNAsu+ constructs. After 36 h RNA was isolated, separated on an 8% polyacrylamide–8 M urea gel, and electroblotted as described in Materials and Methods. tRNAsu+ constructs are designated as in Fig. 1. +control, in vitro-transcribed tRNAsu+ (2 ng), which contains three additional nucleotides (Materials and Methods); −control, RNA purified from untransfected cells. Hybridization signals were normalized to the level of 5.8S rRNA and quantified as described in Materials and Methods. (A) The membrane at the left was probed with the 5′-end-labeled oligonucleotide α (α*) complementary to the anticodon loop of tRNAsu+ in the presence of an excess of unlabeled oligonucleotide T complementary to the TΨC arm and variable arm. Oligonucleotide α* (black line) and oligonucleotide T (gray line) are depicted at the left. RNA purified from COS 7 cells transfected with the 16mer/105 construct was blotted on the membrane on the right. The membrane was hybridized with oligonucleotide J (black line) complementary to the last 8 nt of the 3′ end of the mature tRNA and the subsequent 8 nt located in the tRNA gene. (B) The membrane from panel A was rehybridized using 5′-end-labeled oligonucleotide T* (left) as described in Materials and Methods. A short exposure of the portion of the gel containing the mature tRNAs is shown at the bottom.
Expression of tRNA multimers in cultured cells.
Since it appeared that better suppression was obtained with more copies of the tRNAsu+ gene, it was important to determine whether this was due to increased amounts of tRNAsu+. tRNA expression was assayed by Northern blot hybridization of transfected-cell RNA. In this assay we used an excess of an unlabeled oligonucleotide complementary to the tRNAsu+ TΨC arm and variable arm (oligonucleotide T) to make the anticodon more accessible to hybridization with a 5′-end-labeled oligonucleotide specific for the tRNAsu+ (oligonucleotide α) (Buvoli et al., unpublished data).
Figure 3A shows the results of such analysis for COS 7 cells transfected with 6 μg of each tRNAsu+ construct. In this experiment, a band migrating as expected for the mature 85-nt tRNAsu+ was detected for all the constructs tested. Levels of tRNAsu+ increased proportionately with the number of tRNAsu+ genes used in the transfection, with a good correlation between the calculated values and the measured ones (Table 1).
While oligonucleotide α, specific for the suppressor tRNA, did not recognize the endogenous serine-tRNA or any other RNA (Fig. 3A, lane −control), it detected another more intense band migrating 8 to 10 nt above the mature tRNAsu+. Since pre-tRNAs usually terminate with extra nucleotides that are removed by endo- and exonucleolytic cleavages (15), it seemed likely that these additional nucleotides corresponded to the 3′ trailer of the unprocessed tRNAsu+ carrying the first uridine residues of the transcription terminator (22, 35). This assumption was confirmed by Northern blot analysis using an oligonucleotide complementary to the last 8 nt of the 3′ end of the mature tRNAsu+ and the subsequent 8 nt located in the tRNA gene (Fig. 3A, right, oligonucleotide J).
When the intensities of the upper and lower bands were compared, we found that their ratio was constant among the different tRNAsu+ constructs (approximately 2 to 1). Since the precursor did not show the expected accumulation as tRNAsu+ expression increased, we decided to investigate the hybridization efficiencies of the unprocessed and processed tRNAsu+. It has been shown that, in addition to the higher-order structure of the tRNA, the presence of a modified nucleotide in the anticodon loop can change dramatically the hybridization efficiency of a short oligonucleotide probe (27; Buvoli et al., unpublished data). Although a previous report did not detect any modification in the anticodon loop of tRNAsu+ overexpressed in CV-1 cells (9), the rat liver serine-tRNA carries a (i6A 37) modification (44). Based on the observation that, at least for tRNAs that undergo splicing, the modification at position 37 (i6A 37) is usually found only in tRNAs of mature size (6), we hypothesized a hybridization of oligonucleotide α that was more efficient with the tRNAsu+ precursor than with the mature tRNAsu+.
To test this hypothesis and to determine the relative amounts of tRNAsu+ and endogenous tRNASer, we used a probe whose hybridization efficiency is not affected by the presence of modifications and which recognizes endogenous and suppressor tRNASer. The 40-nt oligonucleotide T shows this property (Buvoli et al., unpublished data) and was used to rehybridize the same filter shown in Fig. 3A. This probe can be used to determine the relative amount of the tRNAsu+ by subtracting the hybridization signal obtained in the untransfected control (endogenous serine-tRNA) from the hybridization signal obtained from cells transfected with the tRNAsu+ constructs. Figure 3B shows the results of such analysis. In this experiment we observed that (i) oligonucleotide T also recognizes the upper band previously detected with oligonucleotide α (pre-tRNA), (ii) the ratio between the upper and lower bands is inverted, with 20 times more mature tRNAsu+ than precursor, and (iii) as detected in Fig. 3A, the different constructs showed levels of expression proportional to the number of tRNA genes. The inversion in the ratio between mature and precursor tRNAsu+ detected in Fig. 3B, clearly shows that only a minority of the mature tRNAsu+ molecules were detected by hybridization using oligonucleotide α (Fig. 3A). This result supports the hypothesis that, when the level of total expression of tRNAsu+ reaches ∼1.5 times the level of endogenous serine-tRNA (Fig. 3B; comparison between lane −control and lanes for the other tRNAsu+ constructs), position 37 of the tRNAsu+ anticodon appears to be modified in the majority of the molecules. In addition it demonstrates that the precursor tRNAsu+ does not represent the predominant tRNAsu+ product as it appears to do in Fig. 3A.
Limiting steps affecting tRNAsu+ overexpression.
When the total expression of tRNAsu+ was increased up to approximately four to five times the level of endogenous serine-tRNA using the more efficient FuGENE transfection method (Fig. 4B; comparison between lane −control and lanes for the other tRNAsu+ constructs), we found that all constructs apparently reached a transcription-processing plateau with small differences in their expression (Fig. 4A; Table 1). In addition, despite higher tRNAsu+ expression, the ratio between the unprocessed and mature species did not increase but surprisingly was inverted, with apparently only ∼2.8-fold more mature tRNAsu+ than the 3′ unprocessed tRNAsu+ (Fig. 4A). When blots shown in Fig. 3A and 4A were quantified, it appeared that tRNAsu+ expression was far higher (∼20-fold) in the cells transfected with FuGENE. However, when the filter shown in Fig. 4A was reprobed with oligonucleotide T (Fig. 4B) and was compared to the filter shown in Fig. 3B, it was clear that the total tRNAsu+ levels changed far less (approximately threefold). Therefore, we hypothesized that much of the tRNAsu+ in Fig. 4A was not modified and consequently would hybridize much more efficiently than modified tRNAsu+.
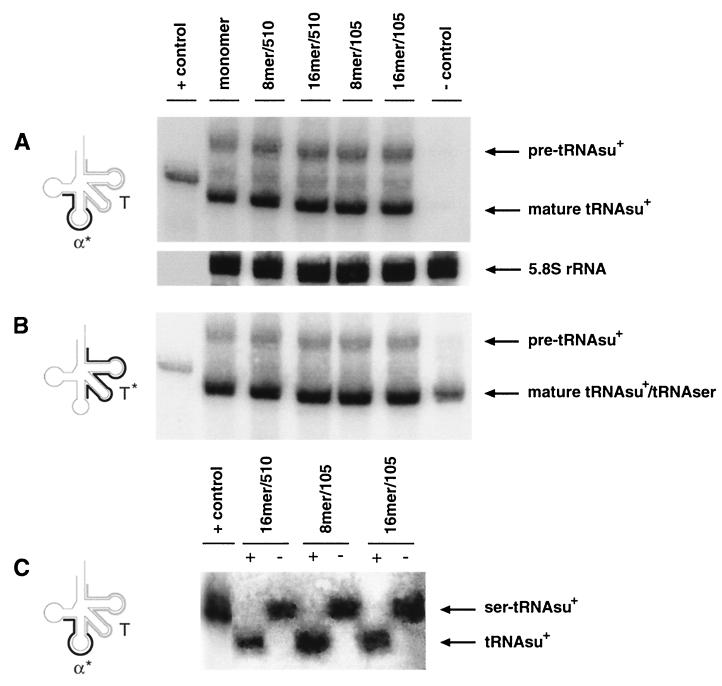
FIG. 4.
Limiting steps affecting tRNAsu+ overexpression. (A) Northern blot analysis of RNA purified from COS 7 cells transfected with different tRNAsu+ constructs by the FuGENE procedure. RNA was isolated and analyzed as for Fig. 3. tRNAsu+ constructs are designated as in Fig. 1. +control, in vitro-transcribed tRNAsu+ (2 ng); −control, RNA purified from untransfected cells. Oligonucleotides α* and T are at the left. (B) The membrane from panel A was rehybridized using the 5′-end-labeled oligonucleotide T* (left) as described in Materials and Methods. (C) Extent of tRNAsu+ in vivo aminoacylation. COS 7 cells were transfected with 12 μg of different tRNAsu+ constructs using the FuGENE method. RNA isolated in acidic conditions (pH 5) was separated on an acid-urea gel, blotted, and analyzed by Northern hybridization as for Fig. 3A. tRNAsu+ constructs are designated as in Fig. 1. +control, nonaminoacylated in vitro-transcribed tRNAsu+; −, samples loaded without any treatment; +, samples treated with 0.2 M Tris-HCl (pH 9) at 37°C for 30 min before gel electrophoresis.
Since other reports have shown that the absence of the (i6A 37) modification affects suppressor tRNA function (24, 29), we measured CAT activity from the same cell extracts used to prepare the RNA analyzed in Fig. 4A. This analysis showed that even though the apparent level of mature tRNAsu+ was 20-fold higher than that shown in Fig. 3A, CAT activity increased by only 1.5-fold (data not shown).
The aminoacylation of serine-tRNA should not be affected by nucleotide changes in the anticodon loop since the two major elements required for this biochemical process are the discriminator base and the long extra arm (1). Although the tRNAsu+ should be fully charged, aminoacylation could become the limiting step when the tRNAsu+ is overexpressed. To rule out this possibility, we prepared RNA under acidic conditions to avoid hydrolysis of the aminoacyl-tRNAsu+ bond and separated the aminoacyl-tRNAsu+ from tRNAsu+ by acid-urea gel electrophoresis. The results of this experiment are shown in Fig. 4C, where 12 μg of the most active constructs (16mer/510 and 8-16mer/105) was transfected in COS 7 cells using FuGENE. This analysis clearly demonstrates that all the constructs tested were fully aminoacylated and excludes the possibility that a change in the level of tRNAsu+ aminoacylation was responsible for the observed discrepancy between the high level of tRNAsu+ and the relatively low suppression activity observed in COS 7 cells transfected with FuGENE. Although the modification at position 37 seems to represent the main limiting step during tRNAsu+ overexpression, we found that the tRNAsu+-processing machinery was also approaching saturation. In fact we observed that the ratio between mature and precursor tRNAsu+ detected in Fig. 4B increased just 2.5-fold instead of the 20-fold increase detected in Fig. 3B.
In vivo tRNA suppression in skeletal muscles.
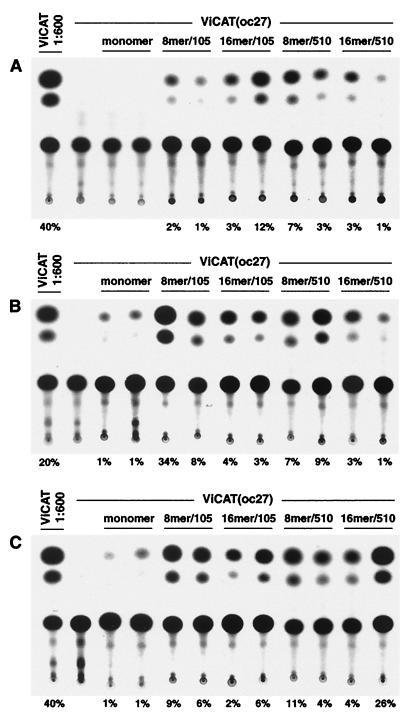
The tRNA suppressor gene constructs were next tested in vivo by direct coinjection into mouse muscle with the CAT ochre plasmid. Described initially by Wolff et al. (55), intramuscular injection of plasmid DNA expression vectors results in cellular uptake and expression of the plasmid. Although only a small portion of the muscle fibers (∼10%) are transfected, this technique provides a powerful way for characterizing the regulation of gene expression under more-physiological conditions than tissue culture allows. Results of these experiments are shown in Fig. 5. The TA muscle under two different conditions (Fig. 5A and B) and the tongue (Fig. 5C) were coinjected with 12.5 μg of the ViCAT(oc27) plasmid and 40 μg of each tRNAsu+ gene-containing plasmid. One group (Fig. 5B) was pretreated with bupivacaine to increase gene transfer by inducing muscle degeneration and regeneration (13). Since saturation of gene expression in mouse muscle occurs at ∼50 μg of plasmid DNA (30), larger amounts of DNA were not tested. Seven days after injection, animals were sacrificed and suppression efficiency was determined as described in Materials and Methods. When the ViCAT(oc27) plasmid was injected alone, no CAT activity was detected in TA muscle or in the tongue. In contrast, as clearly shown in all three panels, when ViCAT(oc27) was coinjected along with the tRNAsu+ constructs, CAT activity was restored to variable levels (for each construct the highest and lowest percentages of chloramphenicol conversion are reported at the bottom of each panel). Plasmids carrying 8 to 16 copies of the tRNAsu+ gene were able to restore the activity of ViCAT(oc27) to a substantially higher level than the monomer. The 16mer/105 construct was the most effective construct in normal skeletal muscle, showing an extent of CAT conversion of up to 12% under the described assay conditions. Variable levels of suppression were also observed in TA muscle pretreated with bupivacaine (Fig. 5B, 8mer/105; 34%) and in tongues (Fig. 5C, 16mer/510; 26%). The suppression efficiencies of the most active constructs were then evaluated by comparing their CAT activities with that produced by the wt CAT plasmid. Compared to that for wt CAT, the efficiencies of suppression were 0.05% for the 16mer/105 construct in TA muscle of 2-week-old animals, 0.28% for the 8mer/105 construct in TA muscle pretreated with bupivacaine, and 0.1% for the 16mer/510 construct injected twice into the tongue. Suppression in vivo was therefore substantially lower than in cultured cells.
FIG. 5.
In vivo suppression of CAT ochre reporter gene coinjected in skeletal muscles with different tRNAsu+ constructs. CAT activity in 10% of muscle extract was determined 1 week after plasmid injection. The highest and lowest percentages of chloramphenicol conversion obtained for each construct are shown at the bottom of the panels (n = 5). Since the CAT activity of ViCAT was out of the linear range, the extracts were diluted in 1% bovine serum albumin. tRNAsu+ constructs are designated as in Fig. 1. (A) Two-week-old mouse TA muscles were injected with 12.5 μg of ViCAT(oc27) and 40 μg of different tRNAsu+ constructs in 50 μl of sterile normal saline. (B) Four-week-old mouse TA muscles undergoing regeneration were injected with 12.5 μg of ViCAT(oc27) and 40 μg of different tRNAsu+ constructs in 100 μl of sterile normal saline. Muscle regeneration was induced 5 days before DNA injections by treatment with 0.75% bupivacaine solution. (C) Four-week-old mouse tongues were injected with 12.5 μg of ViCAT(oc27) and 40 μg of different tRNAsu+ constructs. Tongues were reinjected a second time 30 min after the first injection. Each injection was performed in 60 μl of sterile normal saline.
In vivo suppression in transgenic mice.
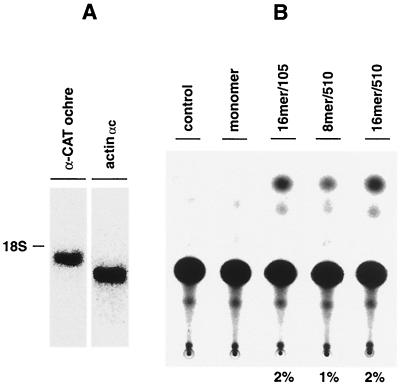
If direct DNA injection of suppressor tRNA is to be contemplated as a therapeutic approach, it is important to apply it to a model where the mutant gene is in the context of a chromosome instead of an extrachromosomal plasmid DNA. Transgenic mice can provide such a model not only for studying the pathological effects of genetic alterations but also for testing the efficacy of gene therapy strategies. To test the possible use of the tRNAsu+ constructs as therapeutic tools, they were injected into transgenic mouse hearts expressing the CAT(oc27) gene under the control of the α-myosin heavy chain promoter (α-CAT ochre gene) (54). The α cardiac actin mRNA, which represents ∼2.8% of the cardiac poly(A)+ RNA and ∼95.8% of total cardiac actin mRNA (19), was used to quantify the level of expression of the α-CAT ochre mRNA (Fig. 6A). The 1,850-nt CAT ochre mRNA corresponds to roughly one-third of α cardiac actin mRNA, therefore representing ∼0.9% of the total cardiac poly(A)+ RNA. Direct injection of each construct into the myocardiums of these transgenic mice was performed as described in Materials and Methods. No CAT activity was found in the heart extracts of animals injected with normal saline alone as a control. While the monomer was unable to rescue this mutation, suppression was obtained with three multimer constructs with CAT activities ranging from 1 to 2% (Fig. 6B). In order to determine the amount of CAT protein produced after the rescue of the CAT ochre mRNA, a standard curve of CAT activity was carried out using different dilutions of purified CAT enzyme (Promega). Approximately 1.89 × 109 molecules of CAT, equivalent to ∼78 pg, were necessary to obtain a 2% CAT conversion (data not shown). Since the CAT assay was carried out using 12.5% of the heart extract, the total amount of CAT protein that was produced after direct gene injection into the mouse hearts corresponds to ∼600 pg.
FIG. 6.
In vivo suppression in the α-CAT ochre gene-expressing transgenic mouse. (A) Northern blot analysis of α-CAT ochre gene mRNA. Total RNA (10 μg) extracted from the heart of an α-CAT ochre gene-expressing transgenic mouse was separated on a 1.5% agarose–6% formaldehyde gel and blotted on a nylon membrane. The filter was sequentially hybridized with α-CAT and actinαc mRNA-specific oligonucleotide probes, labeled at the same specific activity. After each hybridization, the filter was exposed for 6 h and signals were quantified by a Storm 860 image analyzer. Similar results have been obtained by analyzing several transgenic mice. (B) Levels of nonsense suppression in the α-CAT ochre gene-expressing transgenic mouse. Four-month-old transgenic mouse hearts were injected with 50 μg of different tRNAsu+ constructs in 20 μl of normal saline. tRNAsu+ constructs are designated as in Fig. 1. Control, α-CAT ochre gene-expressing transgenic mice injected with normal saline alone (n = 10 for the monomer and 3 for each of the other constructs). CAT activity in 10% of heart extract was measured 1 week after plasmid injection. The highest percentage of chloramphenicol conversion is shown below each construct. tRNAsu+ constructs are designated as in Fig. 1.
DISCUSSION
tRNA suppression and the more recent use of aminoglycoside antibiotics have been suggested as potential gene therapy approaches to restore translation of mRNAs that contain nonsense mutations which cause a large number of human diseases through premature termination of translation (4, 38, 50).
The success of a therapeutic suppression approach lies in the possibility of removing the translation block caused by a nonsense mutation without affecting the termination process at natural stop codons. For this reason, suppression therapy should be restricted to diseases in which the nonsense mutation is surrounded by a weak codon context (3).
The results presented here demonstrate that, in principle, it is possible to achieve controlled levels of suppression and potentially reverse a mutant phenotype without causing toxic effects by selecting the number of tRNA suppressor genes that can be multimerized on a single plasmid. From a corrective point of view, it is important to consider that therapeutic thresholds vary in different diseases. In Duchenne and Becker muscular dystrophy, for example, genetic analysis suggests a minimal target level of about 30 to 40% normal dystrophin expression (20). In canine hemophilia B, 1% of normal factor IX levels results in partial correction of the coagulation defect (47). These observations strengthen the idea that, in particular pathological conditions, suppression therapy could be employed successfully.
Here we show that, when the tRNA modification machinery is not saturated, levels of tRNAsu+ expression and the relative suppression of an ochre stop codon are proportional to the number of multimerized tRNAsu+ genes transfected into cultured cells. This linear correlation shows that plasmids carrying up to 16 tRNAsu+ genes are stable when transfected in COS 7 cells and suggests that additional copies could be added without affecting vector stability or tRNA functionality, if higher levels of suppression are required.
We also show that efficient tRNA expression and processing can occur when two adjacent tRNAsu+ genes are separated by only 105 nt. Since transcription of the human serine-tRNA appears positively controlled by a flanking promoter element located between positions −66 and −18 (11), this distance may approach the minimal functional spacer that allows the transcription and termination process to occur without steric interference. Since 16 functional tRNAsu+ copies span only 3,040 nt, the in vivo delivery of multimerized suppressor tRNAs by small-cloning-capacity viral vectors, such as the adeno-associated virus, should be considered.
The ability of our constructs to introduce more genes per microgram of DNA transfected or injected represents an efficient way to increase and regulate gene expression even if the DNA uptake plateaus. For mouse muscle, for example, saturation of gene expression takes place at DNA doses close to 50 to 75 μg per injection (30). However the use of multicopy tRNAsu+ constructs can easily bypass this gene transfer limitation.
The advantages of our approach become striking when high levels of suppression are required. Since our preamplified system does not rely on the previous SV40-based gene amplification (46), high levels of suppressor tRNA expression can be reached without any accessory factors and in all cell lines and terminally differentiated tissues. We also show that, in COS 7 cells, when the level of suppressor tRNA is four to five times higher than the level of endogenous serine-tRNA, there is accumulation of unmodified (at position 37) and therefore less-active suppressor. Modified nucleotides play an important role in several interactions between tRNAs and other components of the translational machinery. In particular, modifications at positions 34 and 37 are involved in important anticodon-codon interactions. Hydrophobic modifications at position 37, for example, appear to be present when it is necessary to stabilize a U-A base pairing occurring at the first nucleotide of the codon (6). The modified (i6A) nucleotide at position 37 found in rat liver serine-tRNA was the only modification not detected when tRNAsu+ was overexpressed in CV-1 cells at levels 20-fold higher than that of the endogenous serine-tRNA (9). However, our evidence supports, although indirectly, the hypothesis that this modification is present in the tRNAsu+ anticodon loop and plays an important role in suppressor activity.
A comparison of tRNAs containing the (i6A) modification indicates that recognition determinants for the tRNA (i6A 37) synthetase are three As at positions 36 to 38 and a 5-bp anticodon stem (51). Since the mutagenesis of the human serine-tRNA gene employed to generate the tRNAsu+ did not alter these sequences, the tRNAsu+ should still have all the requirements to be efficiently modified at position 37.
Taken together our results strongly suggest that the tRNAsu+ is modified at position 37 and that the absence of this modification represents the major limiting factor affecting tRNAsu+ activity. This finding can also explain why cells overexpressing tRNAsu+ did not show any sign of toxicity and did not lose the ability to replicate (data not shown). However, if the lack of modification plays an important role in limiting a high level of suppression in COS 7 cells, there are several other reasons to believe that, when the tRNAsu+ multimers are delivered to the muscle as a therapeutic agent, the cytoarchitecture of the myofibers may overcome this limitation. A typical striated muscular cell has unique anatomical characteristics, measuring 1 to 40 mm in length and 10 to 50 mm in width and containing up to 100 nuclei. If multiple nuclei may provide a greater target for nuclear transport, their abundance may also dilute the number of plasmids per nucleus. This could eliminate the requirement for a higher level of tRNA (i6A 37) synthetase, which should be associated with tRNAsu+ overexpression.
Both variability and a drop in suppression efficiency (10- to 30-fold reduction) were observed when the multimer constructs were tested in TA and tongue muscles, compared to results obtained in tissue culture cells. Although variability in gene expression after direct DNA injection could reflect, as previously reported, a technical limitation of the procedure (14, 55, 56), the anatomy and physiology of the myofibers probably also contribute to the fluctuating level of suppression we observed in our experiments. In fact, it has been shown that the nuclei in a single fiber do not have equivalent levels of gene expression and that their activity changes during muscle development and regeneration despite the presence of a common cytoplasm (36). In addition, it is still not known if coinjection of two plasmids, as in our experiments, results in their efficient colocalization in the same fiber and subsequently in the same nucleus. Since our approach is based on posttranscriptional genetic therapy, the local concentrations of the mutated mRNA and the suppressor tRNA are critical parameters influencing suppression efficiency. The limited diffusion of gene products in the cytoplasm of multinucleated muscle cells has been reported (37, 40, 43), as well as the finding that mRNA does not migrate a long distance from the site of origin in myotubes (43). Thus we believe that the selective gene expression observed in different nuclei and the poor molecular diffusion through the myofiber cytoplasm are largely responsible for the variability observed in our in vivo experiments.
An obvious goal of our study was to test the efficacy of our approach in pathophysiological conditions. In order to mimic a genetic disease caused by a nonsense mutation and at the same time quantitatively monitor the success of our genetic treatment, we generated a transgenic mouse expressing a CAT ochre gene in the heart. Direct DNA injection has also been proven to be a useful method for transferring genes into the mouse myocardium to study in vivo gene regulation (31). However, it has to be pointed out that in a typical mouse heart injection, only about a hundred cardiac myocytes along the needle track are transfected (31). Here we show that after a single injection of tRNAsu+ multimers into the heart, rescue of CAT ochre mRNA produced a total amount of ∼600 pg of active CAT protein. The comparison between this result and other pathological conditions where levels of gene expression do not need to be restored completely (20, 47) suggests that multimer constructs could be successfully employed for gene therapy. However, additional experiments will be required to determine the impact of long-term high tRNAsu+ expression on cellular metabolism. By combining the advantages of the CATgene-expressing transgenic mouse with the availability of adeno-associated viral vectors that allow prolonged gene expression without causing toxicity, it will be possible to determine the biological limits of our approach.
ACKNOWLEDGMENTS
This work was supported by the Muscular Dystrophy Association (M.B.) and NIHHL50560 to L.A.L.
We thank Olke Uhlenbeck and Bob Thompson for helpful suggestions and discussion and Tom Cech and Mike Yarus for critical reading of the manuscript. We also thank Uttam RajBhandary for providing the pSV1GT3-ser ochre and pRSVcat(oc27) plasmids and Karen Vikstrom for production of the α-myosin heavy chain–CAT ochre gene-expressing mice.
REFERENCES
- 1.Achsel T, Gross H J. Identity determinants of human tRNAser: sequence elements necessary for serylation and maturation of a tRNA with a long arm. EMBO J. 1993;12:3333–3338. doi: 10.1002/j.1460-2075.1993.tb06003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso S, Minty A, Bourlet Y, Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23:11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson J, Martin R. Mutations to nonsense codons in human genetic disease: implications for gene therapy by nonsense suppressor tRNAs. Nucleic Acids Res. 1994;22:1327–1334. doi: 10.1093/nar/22.8.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton-Davis E R, Cordier L, Shoturma D I, Leland S E, Sweeney H L. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J Clin Investig. 1999;104:375–381. doi: 10.1172/JCI7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bienz M, Kubli E, Kohli J, deHenau S, Huez G, Marbaix G, Grosjean H. Usage of the three termination codons in a single eukaryotic cell, the Xenopus laevis oocyte. Nucleic Acids Res. 1981;9:3835–3851. doi: 10.1093/nar/9.15.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Björk G R. Biosynthesis and function of modified nucleotides. In: Söll D, RajBhandary U L, editors. tRNA: structure, biosynthesis, and function. Washington, D.C.: American Society for Microbiology; 1995. pp. 165–205. [Google Scholar]
- 7.Björnsson A, Mottagui-Tabar S, Isaksson L A. Structure of the C-terminal end of nascent peptide influences translation termination. EMBO J. 1996;15:1696–1704. [PMC free article] [PubMed] [Google Scholar]
- 8.Bonetti B, Fu L, Moon J, Bedwell D M. The efficiency of translation termination is determined by a synergistic interplay between upstream and downstream sequences in Saccharomyces cerevisiae. J Mol Biol. 1995;251:334–345. doi: 10.1006/jmbi.1995.0438. [DOI] [PubMed] [Google Scholar]
- 9.Capone J P, Sharp P A, RajBhandary U L. Amber, ochre and opal suppressor tRNA genes derived from a human serine tRNA gene. EMBO J. 1985;4:213–221. doi: 10.1002/j.1460-2075.1985.tb02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capone J P, Sedivy J M, Sharp P A, RajBhandary U L. Introduction of UAG, UAA, and UGA nonsense mutations at a specific site in the Escherichia coli chloramphenicol acetyltransferase gene: use in measurement of amber, ochre, and opal suppression in mammalian cells. Mol Cell Biol. 1986;6:3059–3067. doi: 10.1128/mcb.6.9.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capone J P. Modulation of the phenotypic expression of a human serine tRNA gene by 5′-flanking sequences. DNA. 1988;7:459–468. doi: 10.1089/dna.1.1988.7.459. [DOI] [PubMed] [Google Scholar]
- 12.Cutting G R, Kasch L M, Rosenstein B J, Tsui L C, Kazazian H H, Jr, Antonarakis S E. Two patients with cystic fibrosis, nonsense mutations in each cystic fibrosis gene, and mild pulmonary disease. New Engl J Med. 1990;323:1685–1689. doi: 10.1056/NEJM199012133232407. [DOI] [PubMed] [Google Scholar]
- 13.Danko I, Fritz J D, Jiao S, Hogan K, Latendresse J S, Wolff J A. Pharmacological enhancement of in vivo foreign gene expression in muscle. Gene Ther. 1994;1:114–121. [PubMed] [Google Scholar]
- 14.Davis H L, Whalen R G, Demeneix B A. Direct gene transfer into skeletal muscle in vivo: factors affecting efficiency of transfer and stability of expression. Hum Gene Ther. 1993;4:151–159. doi: 10.1089/hum.1993.4.2-151. [DOI] [PubMed] [Google Scholar]
- 15.Deutscher M P. tRNA processing nucleases. In: Söll D, RajBhandary U L, editors. tRNA: structure, biosynthesis, and function. Washington, D.C.: American Society for Microbiology; 1995. pp. 51–65. [Google Scholar]
- 16.Dingermann T, Werner H, Schutz A, Zundorf I, Nerke K, Knecht D, Marschalek R. Establishment of a system for conditional gene expression using an inducible tRNA suppressor gene. Mol Cell Biol. 1992;12:4038–4045. doi: 10.1128/mcb.12.9.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doerig R E, Suter B, Gray M, Kubli E. Identification of an amber nonsense mutation in the rosy516 gene by germline transformation of an amber suppressor aminoacyl-tRNA gene. EMBO J. 1988;7:2579–2584. doi: 10.1002/j.1460-2075.1988.tb03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drabkin H J, Park H J, RajBhandary U L. Amber suppression in mammalian cells dependent upon expression of an Escherichia coli aminoacyl-tRNA synthetase gene. Mol Cell Biol. 1996;16:907–913. doi: 10.1128/mcb.16.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garner I, Sassoon D, Vandekerckhove J, Alonso S, Buckingham M E. A developmental study of the abnormal expression of α-cardiac and skeletal actins in the striated muscle of a mutant mouse. Dev Biol. 1989;134:236–245. doi: 10.1016/0012-1606(89)90093-6. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman E P, Fischbeck K H, Brown R H, Johnson M, Medori R, Loike J D, Harris J B, Waterston R, Brooke M, Specht L, et al. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne's or Becker's muscular dystrophy. N Engl J Med. 1988;318:1363–1368. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- 21.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 22.Hong H J, Yoo S H, Yoo O J. The nucleotide sequence of a human serine tRNA gene. Nucleic Acids Res. 1987;12:4987. doi: 10.1093/nar/15.12.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudziak R M, Laski F A, RajBhandary U L, Sharp P A, Capecchi M R. Establishment of mammalian cell line containing multiple nonsense mutations and functional suppressor tRNA genes. Cell. 1982;31:137–146. doi: 10.1016/0092-8674(82)90413-5. [DOI] [PubMed] [Google Scholar]
- 24.Janner F, Vogeli G, Fluri R. The antisuppressor strain sin1 of Schizosaccharomyces pombe lacks the modification isopentenyladenosine in transfer RNA. J Mol Biol. 1980;139:207–219. doi: 10.1016/0022-2836(80)90305-8. [DOI] [PubMed] [Google Scholar]
- 25.Kass-Eisler A, Falk-Pedersen E, Alvira M, Rivera J, Buttrick P M, Wittenberg B A, Cipriani L, Leinwand L A. Quantitative determination of adenovirus-mediated gene delivery to rat cardiac myocytes in vitro and in vivo. Proc Natl Acad Sci USA. 1993;90:11498–11502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerem B S, Zielenski J, Markiewicz D, Bozon D, Gazit E, Yahav J, Kennedy D, Riordan J R, Collins F S, Rommens F S, Tsui L C. Identification of mutations in regions corresponding to the two putative nucleotide (ATP)-binding folds of the cystic fibrosis gene. Proc Natl Acad Sci USA. 1990;87:8447–8451. doi: 10.1073/pnas.87.21.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumazawa Y, Yokogawa T, Tsurui H, Miura K, Watanabe K. Effect of the higher-order structure of tRNAs on the stability of hybrids with oligodeoxyribonucleotides: separation of tRNA by an efficient solution hybridization. Nucleic Acids Res. 1992;20:2223–2232. doi: 10.1093/nar/20.9.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laski F A, Ganguly A S, Sharp P A, RajBhandary U L, Rubin G M. Construction, stable transformation, and function of an amber suppressor tRNA gene in Drosophila melanogaster. Proc Natl Acad Sci USA. 1989;86:6696–6698. doi: 10.1073/pnas.86.17.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laten H, Gorman J, Bock R B. Isopentenyladenosine deficient tRNA from an antisuppressor mutant of Saccharomyces cerevisiae. Nucleic Acids Res. 1978;5:4329–4343. doi: 10.1093/nar/5.11.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy M Y, Barron L G, Meyer K B, Szoka F C., Jr Characterization of plasmid DNA transfer into mouse skeletal muscle: evaluation of uptake mechanism, expression and secretion of gene products into blood. Gene Ther. 1996;3:201–211. [PubMed] [Google Scholar]
- 31.Li K, Welikson R E, Vikstrom K L, Leinwand L A. Direct gene transfer into the mouse heart. J Mol Cell Cardiol. 1997;29:1499–1504. doi: 10.1006/jmcc.1997.0389. [DOI] [PubMed] [Google Scholar]
- 31a.Li K, Zhang J, Buvoli M, Yan X D, Leinwand L, Ite H. Ochre suppressor transfer RNA restored dystrophin expression in mdx mice. Life Sci. 1997;61:205–209. doi: 10.1016/s0024-3205(97)00714-5. . (Retraction, 66:83, 1999.) [DOI] [PubMed] [Google Scholar]
- 32.Mottagui-Tabar S, Tuite M F, Isaksson L A. The influence of 5′ codon context on translation termination in Saccharomyces cerevisiae. Eur J Biochem. 1998;257:249–254. doi: 10.1046/j.1432-1327.1998.2570249.x. [DOI] [PubMed] [Google Scholar]
- 33.Murgola E J. Translational suppression: when two wrongs do make one right. In: Söll D, RajBhandary U L, editors. tRNA: structure, biosynthesis, and function. Washington, D.C.: American Society for Microbiology; 1995. pp. 491–509. [Google Scholar]
- 34.Nakamura Y, Ito K, Isaksson L A. Emerging understanding of translation termination. Cell. 1996;87:147–150. doi: 10.1016/s0092-8674(00)81331-8. [DOI] [PubMed] [Google Scholar]
- 35.Nashimoto M. Distribution of both lengths and 5′ terminal nucleotides of mammalian pre-tRNA 3′ trailers reflects properties of 3′ processing endoribonuclease. Nucleic Acids Res. 1997;25:1148–1154. doi: 10.1093/nar/25.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newlands S, Levitt L K, Robinson C S, Karpf A B C, Hodgson V R M, Wade R P, Hardeman E C. Transcription occurs in pulses in muscle fibers. Genes Dev. 1998;12:2748–2758. doi: 10.1101/gad.12.17.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ono T, Ono K, Mizukawa K, Ohta T, Tsuchiya T, Tsuda M. Limitated diffusibility of gene products directed by a single nucleus in the cytoplasm of multinucleated myofibres. FEBS Lett. 1994;337:18–22. doi: 10.1016/0014-5793(94)80621-7. [DOI] [PubMed] [Google Scholar]
- 38.Panchal R G, Wang S, McDermott J, Link C J., Jr Partial functional correction of xeroderma pigmentosum group A cells by suppressor tRNA. Hum Gene Ther. 1999;10:2209–2219. doi: 10.1089/10430349950017194. [DOI] [PubMed] [Google Scholar]
- 39.Park H J, RajBhandary U L. Tetracycline-regulated suppression of amber codons in mammalian cells. Mol Cell Biol. 1998;18:4418–4425. doi: 10.1128/mcb.18.8.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavlath G K, Rich K, Webster S G, Blau H M. Localization of muscle gene products in nuclear domains. Nature. 1989;337:570–573. doi: 10.1038/337570a0. [DOI] [PubMed] [Google Scholar]
- 41.Phillips-Jones M K, Hill L S J, Atkinson J, Martin R. Context effects on misreading and suppression at UAG codons in human cells. Mol Cell Biol. 1995;15:6593–6600. doi: 10.1128/mcb.15.12.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prigozy T, Dalrymple K, Kedes L, Shuler C. Direct DNA injection into mouse tongue muscle for analysis of promoter function in vivo. Somat Cell Mol Genet. 1993;19:111–122. doi: 10.1007/BF01233527. [DOI] [PubMed] [Google Scholar]
- 43.Ralston E, Hall Z W. Transfer of a protein encoded by a single nucleus to nearby nuclei in multinucleated myotubes. Science. 1989;244:1066–1069. doi: 10.1126/science.2543074. [DOI] [PubMed] [Google Scholar]
- 44.Randerath E, Gopalakrishnan A S, Gupta R C, Agrawal H P, Randerath K. Lack of a specific ribose methylation at guanosine 17 in Morris hepatoma 5123D tRNASer1IGA. Cancer Res. 1981;41:2863–2867. [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 46.Sedivy J M, Capone J P, RajBhandary U L, Sharp P A. An inducible mammalian amber suppressor: propagation of a poliovirus mutant. Cell. 1987;50:379–389. doi: 10.1016/0092-8674(87)90492-2. [DOI] [PubMed] [Google Scholar]
- 47.Snyder R O, Miao C, Meuse L, Tubb J, Donahue B A, Lin H F, Stafford D W, Patel S, Thompson A R, Nichols T, Read M S, Bellinger D A, Brinkhous K M, Kay M A. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 48.Sprague K U. Transcription of eukaryotic tRNA genes. In: Söll D, RajBhandary U L, editors. tRNA: structure, biosynthesis, and function. Washington, D.C.: American Society for Microbiology; 1995. pp. 31–49. [Google Scholar]
- 49.Syroid D E, Tapping R I, Capone J P. Regulated expression of a mammalian nonsense suppressor tRNA gene in vivo and in vitro using the lac operator/repressor system. Mol Cell Biol. 1992;12:4271–4278. doi: 10.1128/mcb.12.10.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Temple G F, Dozy A M, Roy K L, Kan Y W. Construction of a functional human suppressor tRNA gene: an approach to gene therapy for β-thalassaemia. Nature. 1982;296:537–540. doi: 10.1038/296537a0. [DOI] [PubMed] [Google Scholar]
- 51.Tsang T H, Buck M, Ames B N. Sequence specificity of tRNA-modifying enzymes. An analysis of 258 tRNA sequences. Biochem Biophys Acta. 1983;741:180–196. doi: 10.1016/0167-4781(83)90058-1. [DOI] [PubMed] [Google Scholar]
- 52.Ulmasov B, Capone J P, Folk W. Regulated expression of plant tRNA genes by the prokaryotic tet and lac repressors. Plant Mol Biol. 1997;35:417–424. doi: 10.1023/a:1005819007549. [DOI] [PubMed] [Google Scholar]
- 53.Varshney U, Lee C P, RajBhandary U L. Direct analysis of aminoacylation levels of tRNA in vivo. J Biol Chem. 1991;266:24712–24718. [PubMed] [Google Scholar]
- 54.Vikstrom K L, Factor S M, Leinwand L A. Mice expressing mutant myosin heavy chains are a model for familial hypertrophic cardiomyopathy. Mol Medicine. 1996;2:556–567. [PMC free article] [PubMed] [Google Scholar]
- 55.Wolff J A, Malone R W, Williams P, Chong W, Acsadi G, Jani A, Felgner P L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 56.Wolff J A, Williams P, Acsadi G, Jiao S, Jani A, Chong W. Conditions affecting direct gene transfer into rodent muscle in vivo. BioTechniques. 1991;11:474–485. [PubMed] [Google Scholar]