Abstract
Background
Real‐world outcomes of nivolumab treatment for gastric cancer and associated prognostic factors remain unclear; the present study aimed to evaluate both items.
Methods
A total of 278 consecutive patients treated with nivolumab for gastric cancer during 2017‐2019 were enrolled in this multi‐institutional retrospective cohort study. The impact of laboratory findings, immune‐related adverse events (irAEs), and clinicopathological factors on long‐term survival was evaluated using the Cox proportional hazards model.
Results
The response rate was 11.7% in patients with measurable lesions. The overall and progression‐free survival estimates were 6.77 and 2.53 months, respectively. The incidence of irAEs was 30.6% (6.8% for grade ≥3). There were no treatment‐related deaths. Multivariate analysis revealed that C‐reactive protein level of ≤0.5 mg/dL (hazard ratio = 0.476, P < .001), irAE occurrence (hazard ratio = 0.544, P < .001), albumin level of >3.5 g/dL (hazard ratio = 0.688, P = .045), performance status 0 (hazard ratio = 0.711, P = .028), lymphocyte count >1000/μL (hazard ratio = 0.686, P = .027), and differentiated histological type (hazard ratio = 0.740, P = .046) were independently associated with improved survival. The median survival of patients with four or more good prognostic factors was 18.3 months.
Conclusion
Nivolumab showed safety and survival benefits in patients with previously treated unresectable or recurrent gastric cancer. Low C‐reactive protein level, irAE occurrence, high albumin level, high lymphocyte count, and differentiated histological type may affect outcomes. The presence of four or more good prognostic factors may help identify likely long‐term survivors.
Keywords: antineoplastic agents, adenocarcinoma, immunotherapy, nivolumab, stomach neoplasms
Multi‐institutional retrospective cohort study of nivolumab treatment for unresectable or recurrent gastric cancer revealed that the median overall and progression‐free survival estimates were 6.77 and 2.53 months, respectively. Low C‐reactive protein level, immune‐related adverse events, high albumin level, performance status 0, high lymphocyte count, and differentiated pathological type were associated with improved survival.

1. INTRODUCTION
Despite improvements in chemotherapy for unresectable or recurrent gastric cancer (GC), the median overall survival (OS) remains within 11‐14 months. 1 , 2 , 3 , 4 In the last two decades, while the median OS associated with advanced colorectal adenocarcinoma exceeded 30 months, 5 the development of GC chemotherapy didn't make that much progress. Immune checkpoint inhibitors have been recently introduced for GC and are expected to improve the prognosis of unresectable or recurrent GC in later‐line chemotherapy. 6 , 7
A randomized phase 3 trial (ATTRACTION‐2) has demonstrated a significant survival benefit associated with nivolumab, a humanized IgG4 monoclonal antibody of programmed cell death protein 1 (PD‐1), in unresectable or recurrent GC. 7 , 8 In response, the Japanese gastric cancer treatment guidelines recommend the use of nivolumab in third‐line chemotherapy. 9 However, real‐world therapeutic effects of nivolumab remain unclear, as do factors associated with prognosis. Herein, we performed a multi‐institutional retrospective cohort study aimed at evaluating the real‐world therapeutic effects and prognostic factors associated with unresectable or recurrent GC treated with nivolumab.
2. MATERIALS AND METHODS
2.1. Study design
Between October 2017 and December 2019, a total of 282 patients with initially unresectable or recurrent GC underwent nivolumab treatment as a later‐line chemotherapy across 11 institutions around Tokyo Bay. Four patients with other simultaneous active malignancies were excluded; a total of 278 patients were enrolled in this study.
Data were collected retrospectively. In every case, the tumor was histologically confirmed as adenocarcinoma with distant metastasis using imaging techniques or exploratory laparotomy. Tumor response was assessed using computed tomography, magnetic resonance imaging, and gastroscopy, according to the Response Evaluation Criteria in Solid Tumors guidelines version 1.1. 10 Adverse events were assessed according to the Common Terminology Criteria for Adverse Events version 4.0. 11
2.2. Treatment
Patients received 3 mg/kg or 240 mg/body nivolumab intravenously every 2 weeks. Nivolumab treatment was continued until the evidence of progressive disease or onset of severe adverse effects were observed by the attending physician. Three patients with initially unresectable distant metastasis underwent conversion surgery after nivolumab treatment. Treatment after discontinuation of nivolumab was determined by the attending physician.
2.3. Immune‐related adverse events
The present study was retrospective, precluding definitive conclusions regarding the relationship between adverse events and nivolumab treatment. Therefore, we defined the following adverse events as immune‐related adverse events (irAEs): diarrhea (grade 2 or above), pruritus (any grade), rash (any grade), arthritis (any grade), myositis (any grade), intestinal lung disease (any grade), aspartate aminotransferase level increase (grade 2 or above), alanine aminotransferase level increase (grade 2 or higher), renal disorder (any grade), hypothyroidism (any grade), hyperthyroidism (any grade), adrenal deficiency (any grade), diabetes mellitus (any grade), thrombocytopenia (any grade), myocarditis (any grade), myasthenia gravis (any grade).
2.4. Statistical analysis
Continuous variables were expressed as median values (interquartile ranges). We measured OS from the start of nivolumab administration to the date of the last follow‐up. Progression‐free survival (PFS) was measured from the date of nivolumab administration to the date of progressive disease or death. OS and PFS were calculated using the Kaplan‐Meier method, and differences between groups were compared using the log‐rank test. The optimal cut‐off values of neutrophil count, lymphocyte count, monocyte count, platelet count, albumin level, and C‐reactive protein (CRP) level were determined using receiver operating characteristic curve analyses to predict the survival for 1 year. Univariate and multivariate survival analyses of OS were performed using the Cox proportional hazards regression. Multivariate analysis included covariates with P‐values of <.05 in univariate analyses. After identifying factors independently associated with improved OS in multivariate analysis, we calculated the prognostic score based on the overall number of prognostic factors present and overall survival time. All statistical analyses were performed using JMP® 13 (SAS Institute Inc). P‐values of <.05 were considered indicative of a statistically significant difference.
3. RESULTS
3.1. Patient characteristics
Table 1 summarizes the demographic and clinical characteristics of 278 patients that received nivolumab treatment. The sample included 185 (66.6%) men, and the median age was 68.5 (interquartile range, 61‐74) years. Thirteen (4.7%) patients were diagnosed with Eastern Cooperative Oncology Group Performance Status (PS) 2 when nivolumab treatment was started. There were 133 (47.8%) primary unresectable cases and 145 (52.1%) post‐gastrectomy recurrence cases. The most frequent metastatic site was the peritoneum (n = 117, 42.1%). A total of 147 (52.9%) patients had an undifferentiated histological type, and 59 (21.2%) patients were positive for human epidermal growth factor receptor 2. A total of 242 (87.1%) patients had received taxane‐based regimens, and 216 (77.7%) patients had received ramucirumab regimens prior to nivolumab administration. Post‐treatment, 14 (5.0%) patients continued nivolumab treatment, 104 (37.4%) patients received other regimens, and 160 (57.6%) patients received supportive care.
TABLE 1.
Baseline patient characteristics
| Variables | n = 278 (%) |
|---|---|
| Age | 68.5 (61‐74) |
| Sex | |
| Male | 185 (66.6) |
| Female | 93 (33.5) |
| Eastern cooperative oncology group performance status (ECOG PS) | |
| 0 | 143 (51.4) |
| 1 | 122 (43.9) |
| 2 | 13 (4.7) |
| Type of cancer | |
| Primary | 133 (47.8) |
| Recurrence | 145 (52.1) |
| Esophagogastric junction cancer (Siewert type) | |
| Type 1 | 6 (2.2) |
| Type 2 | 29 (10.4) |
| Type 3 | 12 (4.3) |
| Metastatic site | |
| Peritoneum | 117 (42.1) |
| Liver | 82 (29.5) |
| Lung | 23 (8.3) |
| Lymph node | 89 (32.0) |
| Pathological type | |
| Differentiated | 130 (46.8) |
| Undifferentiated | 147 (52.9) |
| Unknown | 1 (0.4) |
| Human epidermal growth factor 2 (HER2) status | |
| Positive | 59 (21.2) |
| Negative | 184 (66.2) |
| Unknown | 35 (12.6) |
| Number of prior regimens a | |
| ≤2 | 183 (65.8) |
| 3 | 72 (25.9) |
| ≥4 | 23 (8.3) |
| Prior regimens | |
| Taxane agents | 242 (87.1) |
| Irinotecan | 33 (11.9) |
| Ramucirumab | 216 (77.7) |
| Post nivolumab treatment | |
| Nivolumab continuation | 14 (5.0) |
| Irinotecan b | 61 (21.9) |
| Trifluridine/Tipiracil b | 25 (9.0) |
| Taxane agents b | 6 (2.2) |
| Other regimens b | 17 (6.1) |
| Best supportive care | 160 (57.6) |
Includes treatments received in the adjuvant setting.
Including overlap.
3.2. Short‐term results
Table 2 summarizes short‐term outcomes of nivolumab treatment. Among 163 patients with measurable lesions, 6 (3.6%), 13 (8.0%) and 26 (16.0%) patients showed complete and partial response and stable disease, respectively. The response and disease control rates were 11.7% and 27.6%, respectively. The overall disease control rate was 27.0% (n = 278), and the median number of treatment cycles was four (interquartile range, 3‐8).
TABLE 2.
Summary of response and treatment cycle of nivolumab treatment
| Patients with measurable lesions | n = 163 (%) |
|---|---|
| Complete response | 6 (3.6) |
| Partial response | 13 (8.0) |
| Stable disease | 26 (16.0) |
| Progressive disease | 115 (70.6) |
| Not evaluated | 3 (1.8) |
| Overall response rate | 19 (11.7) |
| Disease control rate | 45 (27.6) |
| All patients | n = 278 (%) |
|---|---|
| Disease control rate | 75 (27.0) |
| Median treatment cycles (Interquartile range) | 4 (3‐8) |
3.3. Adverse events
Table 3 summarizes all adverse events associated with nivolumab treatment. The most common adverse event was appetite loss (15.8%). Meanwhile, irAEs occurred in 85 (30.6%, including overlaps) patients. The most frequent irAEs were pruritus (6.8%), hypothyroidism (6.8%), skin rash (5.4%), and renal disorder (2.9%). Intestinal lung disease occurred in seven (0.4%, all grades) patients. Grade 3 or above irAEs occurred in 19 (6.8%) patients. One (0.4%) patient had grade 4 myocarditis, and another (0.4%) patient suffered from myasthenia gravis. There were no treatment‐related deaths.
TABLE 3.
Treatment‐related adverse events
| Adverse event | Grade (%) | ||||
|---|---|---|---|---|---|
| Any | 1 | 2 | 3 | 4 | |
| Appetite loss | 44 (15.8) | 26 (9.4) | 15 (5.4) | 3 (1.1) | 0 (0) |
| Fatigue | 42 (15.1) | 31 (11.2) | 10 (3.6) | 1 (0.4) | 0 (0) |
| AST increased | 41 (14.7) | 30 (10.8) | 9 (3.2) | 2 (0.7) | 0 (0) |
| Diarrhea | 28 (10.1) | 22 (7.9) | 4 (1.4) | 2 (0.7) | 0 (0) |
| Nausea | 27 (9.7) | 17 (6.1) | 8 (2.9) | 2 (0.7) | 0 (0) |
| Pruritus | 19 (6.8) | 15 (5.4) | 3 (1.1) | 1 (0.4) | 0 (0) |
| Hypothyroidism | 19 (6.8) | 15 (5.4) | 3 (1.1) | 1 (0.4) | 0 (0) |
| ALT increased | 17 (6.1) | 13 (4.7) | 3 (1.1) | 1 (0.4) | 0 (0) |
| Rash | 15 (5.4) | 9 (3.2) | 5 (1.8) | 1 (0.4) | 0 (0) |
| Renal disorder | 8 (2.9) | 3 (1.1) | 4 (1.4) | 1 (0.4) | 0 (0) |
| Intestinal lung disease | 7 (0.4) | 3 (1.1) | 1 (0.4) | 3 (1.1) | 0 (0) |
| Arthritis | 5 (1.8) | 2 (0.7) | 2 (0.7) | 1 (0.4) | 0 (0) |
| Pyrexia | 4 (14.4) | 2 (0.7) | 2 (0.7) | 0 (0) | 0 (0) |
| Hyperthyroidism | 4 (1.4) | 3 (1.1) | 0 (0) | 1 (0.4) | 0 (0) |
| Diabetes mellitus | 3 (1.1) | 0 (0) | 0 (0) | 3 (1.1) | 0 (0) |
| Adrenal deficiency | 2 (0.7) | 0 (0) | 0 (0) | 2 (0.7) | 0 (0) |
| Thrombocytopenia | 2 (0.7) | 1 (0.4) | 1 (0.4) | 0 (0) | 0 (0) |
| Myocarditis | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 1 (0.4) |
| Myasthenia gravis | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 1 (0.4) |
| Myositis | 1 (0.4) | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase.
3.4. Long‐term results
The median OS was 6.77 months (95% confidence interval [CI]: 5.33‐9.30 months) (Figure 1A). The median PFS was 2.53 months (95% CI = 2.10‐2.83 months) (Figure 1B).
FIGURE 1.
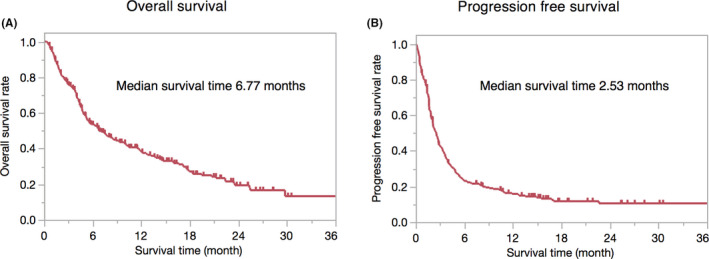
Kaplan‐Meier curve for overall survival and progression free survival. (A) median overall survival was 6.77 (95% confidence interval of 5.33‐9.30) months, (B) median progression‐free survival was 2.53 (95% confidence interval of 2.10‐2.83) months
3.5. Factors associated with overall survival
The cut‐off values of laboratory findings were as follows: neutrophil count of 2850/μL, lymphocyte count of 1000/μL, monocyte count of 400/μL, platelet count of 20 × 104/μL, albumin level of 3.5 g/dL, and CRP level of 0.5 mf/dL, respectively (Figure S1).
Univariate analysis of OS showed that low CRP level, irAEs, high albumin level, PS 0, high lymphocyte count, low platelet count, low neutrophil count, and differentiated histological type were associated with improved OS. However, human epidermal growth receptor 2 status, monocyte count, age, type of cancer (recurrence or primary), number of non‐curable factors, sex, number of prior regimens, and prior use of ramucirumab had no impact on OS. Multivariate analysis showed that low CRP level (hazard ratio = 0.476, 95% CI: 0.336‐0.675, P < .000), irAE occurrence (hazard ratio = 0.544, 95% CI: 0.384‐0.770, P < .001), high albumin level (hazard ratio = 0.688, 95% CI: 0.478‐0.991, P = .045), PS 0 (hazard ratio = 0.711, 95% CI: 0.525‐0.964, P = .028), high lymphocyte count (hazard ratio = 0.686, 95% CI: 0.492‐0.958, P = .027), and differentiated histological type (hazard ratio = 0.740, 95% CI: 0.550‐0.995, P = .046) were independently associated with improved OS (Table 4).
TABLE 4.
Univariate and multivariate analysis of clinicopathological factors for predicting overall survival in the gastric cancer patients who received nivolumab
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Hazard ratio | P‐value | Hazard ratio | 95% confidence interval | P‐value | |
| C‐reactive protein | |||||
| ≤0.5 | 0.358 | <.001 | 0.476 | 0.336‐0.675 | <.001 |
| >0.5 | 1 | 1 | |||
| Immune related adverse events | |||||
| Yes | 0.501 | <.001 | 0.544 | 0.384‐0.770 | <.001 |
| No | 1 | 1 | |||
| Albumin | |||||
| >3.5 | 0.536 | <.001 | 0.688 | 0.478‐0.991 | .045 |
| ≤3.5 | 1 | 1 | |||
| Performance status | |||||
| 0 | 0.590 | <.001 | 0.711 | 0.525‐0.964 | .028 |
| 1, 2 | 1 | 1 | |||
| Lymphocyte count | |||||
| ≥1000 | 0.631 | .005 | 0.686 | 0.492‐0.958 | .027 |
| <1000 | 1 | 1 | |||
| Platelet count | |||||
| <20 × 104 | 0.636 | .003 | 0.851 | 0.608‐1.190 | .346 |
| ≥20 × 104 | 1 | 1 | |||
| Neutrophil count | |||||
| ≤2850 | 0.663 | .007 | 0.922 | 0.655‐1.298 | .643 |
| >2850 | 1 | 1 | |||
| Histological type | |||||
| Differentiated | 0.668 | .007 | 0.740 | 0.550‐0.995 | .046 |
| Undifferentiated | 1 | 1 | |||
| Human epidermal growth receptor 2 status | |||||
| Positive | 0.722 | .079 | |||
| Negative | 1 | ||||
| Monocyte count | |||||
| ≤400 | 0.779 | .091 | |||
| >400 | 1 | ||||
| Age | |||||
| ≥75 | 0.810 | .234 | |||
| <75 | 1 | ||||
| Type of cancer | |||||
| Recurrence | 0.859 | .301 | |||
| Primary | 1 | ||||
| Number of non‐curable factors | |||||
| 2 or more | 0.861 | .415 | |||
| 1 | 1 | ||||
| Sex | |||||
| Male | 0.882 | .419 | |||
| Female | 1 | ||||
| Number of prior regimens | |||||
| ≥3 | 0.919 | .592 | |||
| ≤2 | 1 | ||||
| Prior ramucirumab | |||||
| Yes | 0.950 | .751 | |||
| No | 1 | ||||
P‐values of <.05 were shown in bold type.
OS estimates, stratified by CRP level, irAEs, albumin level, PS, lymphocyte count, and histological type are shown in Figure 2. There were significant differences between groups, as follows: CRP level of ≥0.5 mg/dL and CRP level of <0.5 mg/dL (P < .001), irAE “yes” and “no” (P < .001), albumin level of >3.5 g/dL and albumin of ≤3.5 g/dL (P < .001), PS 0 and PS 1, 2 (P < .001), lymphocyte count of >1000/μL and lymphocyte of ≤1000/μL (P = .004), and undifferentiated and differentiated histological types (P = .006). Except for irAEs, these factors can be assessed ahead of treatment initiation and were thus used to calculate the pre‐treatment prognostic score. The median OS was significantly different between pre‐treatment prognostic score groups; pre‐treatment prognostic scores of 0 (n = 12), 1 (n = 51), 2 (n = 70), 3 (n = 71), 4 (n = 56), and 5 (n = 18) points were associated with OS of 4.7, 2.87, 5.53, 10.1, 17.6, and 22.4 months, respectively (P < .001) (Figure 3A). Patients were divided into low (n = 63, score of 0 and 1 points), medium (n = 141, score of 2 and 3 points), and high (n = 74, score of 4 and 5 points) pre‐treatment prognostic score groups, obtaining median OS estimates of 2.97, 6.63, and 18.3 months, respectively (P < .001) (Figure 3B).
FIGURE 2.
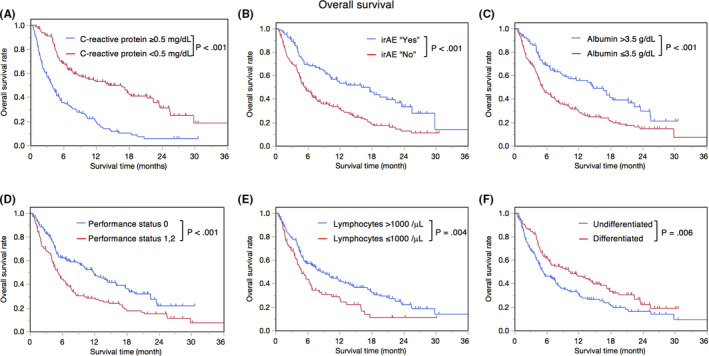
Kaplan‐Meier overall survival for all registered patients, stratified by C‐reactive protein level, immune‐related adverse events occurrence, albumin level, performance status, lymphocyte count, histological type. (A) CRP ≥0.5 vs CRP <0.5 (MST (months) 3.93 vs 16.63, P < .001), (B) irAE “Yes” vs irAE “No” (MST 16.00 vs 5.37, P < .001), (C) Alb >3.5 vs Alb ≤3.5 (MST 14.67 vs 5.20, P < .001), (D) PS 0 vs PS 1, 2 (MST 11.77 vs 5.20, P < .001), (E) Lym >1000 vs Lym ≤1000 (MST 8.43 vs 4.83, P = .004), (F) Undifferentiated vs Differentiated (MST 5.33 vs 10.37, P = .006). CRP, C‐reactive protein; MST, median survival time; irAE, immune‐related adverse event; Alb, albumin; PS, performance status
FIGURE 3.
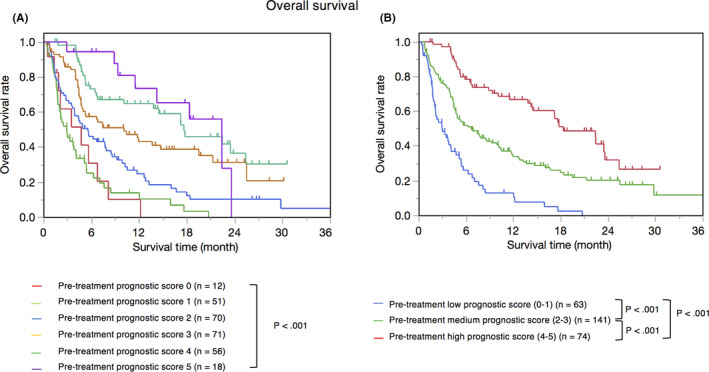
Kaplan‐Meier overall survival according to pre‐treatment prognostic score. (A) Pre‐treatment prognostic score 0 (n = 12) 4.7 months, pre‐treatment prognostic score 1 (n = 51) MST 2.87 months, pre‐treatment prognostic score 2 (n = 70) MST 5.53 months, pre‐treatment prognostic score 3 (n = 71) MST 10.1 months, pre‐treatment prognostic score 4 (n = 56) MST 17.6 months, pre‐treatment prognostic score 5 (n = 18) MST 22.4 months. (B) Pre‐treatment low prognostic score (0‐1) (n = 63) MST 2.97 months, pre‐treatment medium prognostic score (2‐3) (n = 141) MST 6.63 months, pre‐treatment high prognostic score (4‐5) (n = 74) MST 18.3 months. Pre‐treatment high prognostic score group showed significantly better OS compared with pre‐treatment medium prognostic score group (P < .001). Pre‐treatment medium prognostic score group showed significantly better OS compared with low pre‐treatment prognostic score group (P < .001). MST, median survival time; OS, overall survival
3.6. Conversion surgery
Among 133 patients with initially unresectable GC, three (2.3%) underwent conversion surgery (Table 5). One patient presented with a grade 3 pathological response and the pre‐treatment prognostic score of 4 points. Two patients remained alive for >2 years after conversion surgery without recurrence. One patient died 6 months after conversion surgery due to tumor recurrence.
TABLE 5.
The clinicopathological factors of the three cases who underwent conversion surgery after nivolumab treatment
| Case | Sex | Age | PS | Metastatic site | Before nivolumab | After nivolumab | Treatment cycles | Survival | Survival time (months) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cT | cN | Pre‐treatment prognostic score | ypT | ypN | ypStage | ||||||||
| 1 | Male | 63 | 0 | Peritoneum, Lung | 4b | 1 | 4 | 0 | 0 | 0 | 17 | Alive | 27.07 |
| 2 | Female | 65 | 2 | Peritoneum, Lymph node | 3 | 1 | 1 | 4b | 3a | IV | 4 | Dead | 17.60 |
| 3 | Male | 67 | 1 | Peritoneum | 4b | 1 | 2 | 3 | 0 | IIA | 3 | Alive | 26.17 |
Abbreviation: PS, performance status.
4. DISCUSSION
This retrospective multi‐institutional study of patients with unresectable or recurrent GC treated with nivolumab as a late‐line chemotherapy revealed the median OS and PFS estimates of 6.77 and 2.53 months, respectively. The overall response rate was 11.7%. Low CRP level, irAEs, high albumin level, PS 0, high lymphocyte count, and differentiated histological type were prognostic factors associated with improved OS. We found that the pre‐treatment prognostic score, which accounted for all pre‐treatment prognostic factors, was significantly associated with OS. To the best of our knowledge, this study is the first to show the pre‐treatment prognostic score in unresectable or recurrent GC treated by nivolumab.
The present median OS (6.77 months) and PFS (2.53 months) estimates were relatively higher than those reported by the ATTRACTION‐2 trial (corresponding values of 5.26 and 1.61, respectively). 7 This discrepancy might be accounted for by the rates of post‐nivolumab chemotherapy, which were comparable overall (34.8% vs 37.4%, respectively), while trifluridine/tiperacil was administered in 25 cases in the present study. 12 As a result, relatively greater survival estimates may have been achieved. Another reason behind this between‐study discrepancy in estimates may be sample heterogeneity. The rate of PS 0 patients was significantly higher in our study than in the nivolumab group in the ATTRACTION‐2 trial (51.4% vs 29%, P = .001). As PS is associated with survival, the good general condition of the present study patients may have resulted in relatively good prognosis. Third, conversion surgery may have affected survival. Two of three patients undergoing surgery survived for >2 years. Conversion surgery may be paramount to the treatment of unresectable GC; surgery during nivolumab treatment may further improve outcomes in patients with a good response. 13 , 14
The incidence of irAEs may affect the prognosis of patients with GC treated with nivolumab; in the present study, irAEs were more common than they were in a previous study (30.6% vs 21.5%). 15 This discrepancy may be due to the differences in irAE definitions used. As this was a retrospective study, the relationship between irAEs and nivolumab was difficult to confirm. Although intestinal lung disease, endocrine disorder, skin disease, and neuromuscular disease are specific to immune checkpoint inhibitors, liver enzyme elevation and diarrhea are common adverse events during any kind of systemic chemotherapy. Slight liver enzyme elevation often occurs in patients that were heavily pre‐treated with systemic chemotherapy; mild diarrhea often occurs after gastrectomy. Therefore, in the present study, irAEs were defined as grade 2 or higher liver enzyme elevation and diarrhea. Overall, irAEs occurred more frequently in PS 0 patients than in PS 1 or 2 patients (40.6% vs 20.0%, P < .001); overall, irAEs were common in the present study. PS has been reported as an important prognostic factor in other malignancies treated with immune checkpoint inhibitors. PS 0 may help predict survival in patients with unresectable or recurrent GC. 16 , 17 , 18
CRP and albumin levels reflect local and systemic inflammation associated with cancer progression and nutritional status. Namikawa et al demonstrated that GPS, which is a score that combines CRP and albumin levels, is independently associated with survival in patients with GC treated with nivolumab; the present study findings are consistent with those of this previous study. 19 Lymphocytes play a critical role in the host immune response and may suppress cancer progression. 20 Peripheral PD‐1 positive CD4 T‐lymphocyte count has been associated with PFS in non‐small cell lung cancer patients treated with immune checkpoint inhibitors. Peripheral lymphocyte count may be a biomarker of nivolumab treatment efficacy against unresectable or recurrent GC. 21
Differentiated histological type was another factor independently associated with good survival. The direct relationship between histological type and efficacy of nivolumab treatment remains unclear; however, programmed cell death ligand 1 (PD‐L1) expression may account for this suspected link. Yamashita et al reported that the combined positive score—which consists of the number of PD‐L1‐positive tumor cells, lymphocytes, and macrophages—was significantly higher in patients with the differentiated type than in their counterparts. 22 Hagi et al reported that combined positive score was an independent biomarker for nivolumab treatment efficacy in unresectable or recurrent GC; in the present study, the differentiated type was positively associated with survival. 23
In the sub‐analysis of Japanese patients in ATTRACTION‐2 study, patients with prior ramucirumab showed better response rate than those without; however, there were no differences in response rate, disease control rate, OS, and PFS between the two groups in the current study (data not shown). 24 These results may be explained by the frequency of prior ramucirumab between the two studies. In the current study, 77.7% of the patients received ramucirumab prior to nivolumab; however, only 22.3% did in nivolumab group of Japanese patients in ATTRACTION‐2 study.
In multivariate analysis, the prognostic score was significantly associated with OS (Figure S2). IrAEs were absent before nivolumab treatment; thus, they were not accounted for in the pre‐treatment prognostic scores. The high pre‐treatment prognostic score group showed relatively good survival, suggesting this approach to prognostication may be useful in clinical practice.
This study has some limitations. First, this was a retrospective study that may have been subject to selection bias. Although 278 patients treated across 11 institutions were included, nivolumab treatment was mainly performed by surgeons; therefore, recurrent cases were frequently observed. Second, irAE occurrence was evaluated retrospectively; therefore, the relationship between nivolumab and the observed adverse events was difficult to confirm. Third, due to the lack of data, the present study did not account for immunohistochemical analysis. Combined positive score and mismatch repair deficiency have been reported as effective efficacy biomarkers for unresectable or recurrent GC treated with nivolumab; therefore, future studies should account for immunohistochemical analysis. 23
In conclusion, this multi‐institutional study revealed real‐world therapeutic effects of nivolumab in patients with unresectable or recurrent GC. Low CRP level, irAEs, high albumin level, PS 0, high lymphocyte count, and differentiated histological type may help predict good OS. Patients with the pre‐treatment prognostic score of ≥4 points may be suitable for nivolumab treatment as later‐line chemotherapy.
DISCLOSURE
Funding: This study was supported by Toho University research grant.
Conflict of Interest: Authors S.S., Y.S., H.Y., Y.I., and H.S. received lecture fees from Ono Pharmaceutical Co. Ltd. Authors S.S., Y.S., H.Y., and H.S. received lecture fees from Taiho Pharmaceutical Co. Ltd. Author H.Y received lecture fees from Chugai pharmaceutical Co. Author Y.I received lecture fees from NIPRO and Takeda Pharmaceutical Co. Ltd. Authors Y.S., I.E., and H.S. received research grants from Ono Pharmaceutical Co. Ltd. Authors Y.S., C.K., and H.S. received research grants from Taiho Pharmaceutical Co. Ltd. Authors Y.S., C.K., I.E., and Y.I. received research grants from Chugai Pharmaceutical Co. Authors Y.S. and C.K. received research grants from Yakult Honsha Co. Ltd. Author Y.I. received research grant from Takeda Pharmaceutical Co. Ltd and Shionogi Pharmaceutical Co. Ltd. Author I.E. received research grant from Asahi Kasei Pharma corporation.
Ethics: All procedures in this retrospective study were performed following the ethical standards of each institution's committee on human experimentation and were in compliance with the Helsinki Declaration of 1964 and its later versions. Following personal information protection laws, patients were given an opportunity to opt‐out of this study through public announcements published by each institution. The study protocol was approved by the Toho University School of Medicine Institutional Review Board (approval number of the study: A20043_A20007_A19017_A16084). All institutions applied for and obtained study approval from their respective institutional review boards.
Author Contributions: Sho Sato: Conceptualization, investigation, formal analysis, writing‐original draft, and writing‐review and editing. Yoko Oshima: Conceptualization, investigation, and writing‐review and editing. Yu Matsumoto: Conceptualization, investigation, and writing‐review and editing. Yasuyuki Seto: Conceptualization, project administration, supervision, and writing‐review and editing. Hiroharu Yamashita: Conceptualization, investigation, and writing‐review and editing. Koichi Hayano: Conceptualization, investigation, and writing‐review and editing. Masayuki Kano: Conceptualization, investigation, and writing‐review and editing. Hidetaka Andrew Ono: Conceptualization, investigation, and writing‐review and editing. Norio Mitsumori Conceptualization, investigation, project administration, supervision, and writing‐review and editing. Muneharu Fujisaki: Conceptualization, investigation, and writing‐review and editing. Chikara Kunisaki: Conceptualization, investigation, and writing‐review and editing. Hirotoshi Akiyama: Conceptualization, investigation, and writing‐review and editing. Itaru Endo: Conceptualization, investigation, project administration, supervision, and writing‐review and editing. Yasushi Ichikawa: Conceptualization, investigation, and writing‐review and editing. Hidejiro Urakami: Conceptualization, investigation, and writing‐review and editing. Hirokazu Kubo: Conceptualization, investigation, and writing‐review and editing. Sakae Nagaoka: Conceptualization, investigation, and writing‐review and editing. Hideaki Shimada: Conceptualization, project administration, resources, formal analysis, and writing‐review and editing.
Supporting information
Fig S1
Fig S2
ACKNOWLEDGEMENTS
This study was supported by investigators at 11 hospitals (Toho University Ohashi Medical Center, Toho University Omori Medical Center, Japanese Red Cross Medical Center, University of Tokyo Hospital, Jikei University Hospital, Chiba University Hospital, National Hospital Organization Tokyo Medical Center, Yokohama City University Hospital, Yokohama City University Medical Center, Yokosuka Kyosai Hospital, and Yokohama City Minato Red Cross Hospital). We thank all investigators at these institutions.
Sato S, Oshima Y, Matsumoto Y, Seto Y, Yamashita H, Hayano K, et al. The new prognostic score for unresectable or recurrent gastric cancer treated with nivolumab: A multi‐institutional cohort study. Ann Gastroenterol Surg. 2021;5:794–803. 10.1002/ags3.12489
REFERENCES
- 1. Bang Y‐J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet. 2010;376(9742):687–97. [DOI] [PubMed] [Google Scholar]
- 2. Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S‐1 plus cisplatin versus S‐1 alone for first‐line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9(3):215–21. [DOI] [PubMed] [Google Scholar]
- 3. Wilke H, Muro K, Van Cutsem E, Oh S‐C, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro‐oesophageal junction adenocarcinoma (RAINBOW): a double‐blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–35. [DOI] [PubMed] [Google Scholar]
- 4. Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus S‐1 with cisplatin plus S‐1 in chemotherapy‐naive patients with advanced gastric cancer. Ann Oncol. 2015;26(1):141–8. [DOI] [PubMed] [Google Scholar]
- 5. Bekaii‐Saab T, Kim R, Kim TW, O’Connor JM, Strickler JH, Malka D, et al. Third‐ or later‐line therapy for metastatic colorectal cancer: reviewing best practice. Clin Colorectal Cancer. 2019;18(1):e117–e29. [DOI] [PubMed] [Google Scholar]
- 6. Shitara K, Özgüroğlu M, Bang Y‐J, Di Bartolomeo M, Mandalà M, Ryu M‐H, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro‐oesophageal junction cancer (KEYNOTE‐061): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–33. [DOI] [PubMed] [Google Scholar]
- 7. Kang Y‐K, Boku N, Satoh T, Ryu M‐H, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro‐oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO‐4538‐12, ATTRACTION‐2): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71. [DOI] [PubMed] [Google Scholar]
- 8. Chen L‐T, Satoh T, Ryu M‐H, Chao Y, Kato K, Chung HC, et al. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION‐2): 2‐year update data. Gastric Cancer. 2020;23(3):510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Association JGC. Japanese gastric cancer treatment guidelines 2018 (ver. 5). 2018.
- 10. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 11. Common Terminology Criteria for Adverse Events v4.0: Institute NC. https://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed 10 Mar 2021.
- 12. Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau H‐T, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–48. [DOI] [PubMed] [Google Scholar]
- 13. Sato S, Kunisaki C, Tanaka Y, Sato K, Miyamoto H, Yukawa N, et al. Curative‐intent surgery for stage IV advanced gastric cancer: who can undergo surgery and what are the prognostic factors for long‐term survival? Ann Surg Oncol. 2019;26(13):4452–63. [DOI] [PubMed] [Google Scholar]
- 14. Yamaguchi K, Yoshida K, Tanahashi T, Takahashi T, Matsuhashi N, Tanaka Y, et al. The long‐term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. 2018;21(2):315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masuda K, Shoji H, Nagashima K, Yamamoto S, Ishikawa M, Imazeki H, et al. Correlation between immune‐related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer. 2019;19(1):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petrillo LA, El‐Jawahri A, Nipp RD, Lichtenstein MRL, Durbin SM, Reynolds KL, et al. Performance status and end‐of‐life care among adults with non‐small cell lung cancer receiving immune checkpoint inhibitors. Cancer. 2020;126(10):2288–95. [DOI] [PubMed] [Google Scholar]
- 17. Greally M, Chou JF, Chatila WK, Margolis M, Capanu M, Hechtman JF, et al. Clinical and molecular predictors of response to immune checkpoint inhibitors in patients with advanced esophagogastric cancer. Clin Cancer Res. 2019;25(20):6160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dall'Olio FG, Maggio I, Massucci M, Mollica V, Fragomeno B, Ardizzoni A. ECOG performance status ≥2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors‐a systematic review and meta‐analysis of real world data. Lung Cancer. 2020;145:95–104. [DOI] [PubMed] [Google Scholar]
- 19. Namikawa T, Yokota K, Tanioka N, Fukudome I, Iwabu J, Munekage M, et al. Systemic inflammatory response and nutritional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg Today. 2020;50(11):1486–95. [DOI] [PubMed] [Google Scholar]
- 20. Feng F, Sun L, Zheng G, Liu S, Liu Z, Xu G, et al. Low lymphocyte‐to‐white blood cell ratio and high monocyte‐to‐white blood cell ratio predict poor prognosis in gastric cancer. Oncotarget. 2017;8(3):5281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inomata M, Kado T, Okazawa S, Imanishi S, Taka C, Kambara K, et al. Peripheral PD1‐positive CD4 T‐lymphocyte count can predict progression‐free survival in patients with non‐small cell lung cancer receiving immune checkpoint inhibitor. Anticancer Res. 2019;39(12):6887–93. [DOI] [PubMed] [Google Scholar]
- 22. Yamashita K, Iwatsuki M, Harada K, Eto K, Hiyoshi Y, Ishimoto T, et al. Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand‐1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer. 2020;23(1):95–104. [DOI] [PubMed] [Google Scholar]
- 23. Hagi T, Kurokawa Y, Kawabata R, Omori T, Matsuyama J, Fujitani K, et al. Multicentre biomarker cohort study on the efficacy of nivolumab treatment for gastric cancer. Br J Cancer. 2020;123(6):965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kato K, Satoh T, Muro K, Yoshikawa T, Tamura T, Hamamoto Y, et al. A subanalysis of Japanese patients in a randomized, doubleblind, placebo‐controlled, phase 3 trial of nivolumab for patients with advanced gastric or gastro‐esophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO‐4538‐12, ATTRACTION‐2). Gastric Cancer. 2019;22(2):344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2


