Abstract
Aims
Digital health can transform the management of atrial fibrillation (AF) and enable patients to take a central role in detecting symptoms and self-managing AF. There is a gap in understanding factors that support sustained use of digital health tools for patients with AF. This study identified predictors of Alivecor® KardiaMobile ECG monitor usage among patients with AF enrolled in the iPhone®Helping Evaluate Atrial fibrillation Rhythm through Technology (iHEART) randomized controlled trial.
Methods and results
We analysed data from 105 English and Spanish-speaking adults with AF enrolled in the intervention arm of the iHEART trial. The iHEART intervention included smartphone-based electrocardiogram self-monitoring with Alivecor® KardiaMobile and triweekly text messages for 6 months. The primary outcome was use of Alivecor® categorized as: infrequent (≤5 times/week), moderate (>5 times and ≤11 times/week), and frequent (>11 times/week). We applied multinomial logistic regression modelling to characterize frequency and predictors of use. Of the 105 participants, 25% were female, 75% were White, and 45% were ≥65 years of age. Premature atrial contractions (PACs) [adjusted odds ratio (OR): 1.23, 1.08–1.40, P = 0.002] predicted frequent as compared to infrequent use. PACs (adjusted OR: 1.17, 95% confidence interval 1.06–1.30, P = 0.003), lower symptom burden (adjusted OR: 1.06, 1.01–1.11, P = 0.02), and less treatment concern (adjusted OR: 0.96, 0.93–0.99, P = 0.02) predicted moderate as compared to infrequent use.
Conclusions
Frequent use of AliveCor® is associated with AF symptoms and potentially symptomatic cardiac events. Symptom burden and frequency should be measured and incorporated into analyses of future digital health trials for AF management.
Keywords: Atrial fibrillation, Atrial premature complexes, Smartphone, Mobile health, Text messaging, Logistic models, Self-management, Electrocardiography, Risk assessment, Remote monitoring
Implications for practice.
Symptom burden and frequency should be measured and incorporated into analyses of future digital health trials for atrial fibrillation management.
Additional clinical resources include the 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons: https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000665.
Introduction
Atrial fibrillation (AF) is highly prevalent, affecting an estimated 33.5 million people globally.1,2 AF is a complex cardiac condition to manage because recurrence is high (41–54%), even after procedures to restore normal sinus rhythm.3,4 Optimal management of AF is important because it is the most common cause of stroke,5,6 and it doubles the risk of mortality. In addition, higher AF symptom severity is associated with lower quality of life (QOL),7–9 and a high burden of psychological distress.10
Digital health is transforming the management of AF and empowering individuals to assume a more central role in detecting symptoms and self-managing their disease. Digital health represents the convergence between healthcare and emerging digital technologies, and includes tools intended for healthcare providers, for patients, or both. Technology used in everyday life is increasingly incorporating cardiac digital health elements. Many smartphone applications enable heart rate and rhythm monitoring. For example, the Apple Watch allows individuals to record a single-lead electrocardiogram (ECG) in an easy, accurate, and timely manner.11 As mobile devices improve in precision, they are also facilitating earlier time to diagnose AF recurrence12 and population screening for AF among currently undiagnosed (estimated to be 13%)13 and asymptomatic patients with AF.
Across many health domains, sustained patient engagement with digital health technologies has been low.14,15 The optimal frequency and duration of engagement with digital health are generally driven by clinical necessity. For example, in AF, frequent use is important because the unpredictable and spontaneous nature of AF makes capturing recurrent AF challenging. Moreover, sustained use for greater than 3 months after a catheter ablation is necessary to clear the ‘blanking period’ when the heart is remodelling and healing, to determine whether an ablation has been successful.16 Sustained use of digital health tools is important because timely recording and transmission of ECG data to healthcare providers has the potential to facilitate earlier diagnosis and treatment of AF. Earlier diagnosis and treatment has the potential to reduce risk of stroke,17 heart failure,18 myocardial infarction,19,20 hospitalizations, and sudden cardiac death.21–23 To date, low sustained use of digital health tools for AF has been a barrier to evaluating effectiveness.
In the iPhone®Helping Evaluate Atrial fibrillation Rhythm through Technology (iHEART) intervention, participants received an Alivecor® KardiaMobile ECG monitor (hereby termed Alivecor®) and behavioural altering motivational (BAM) text messages three times a week for 6 months. The primary hypothesis for the iHEART study was that participants randomized to receive the mobile ECG monitoring intervention would have shorter time to detection of recurrent AF episodes after an intervention to restore normal sinus rhythm versus usual cardiac care. Secondary hypotheses related to more timely treatment for recurrent AF, improved QOL, and increased knowledge of AF and self-management in the intervention as compared to the usual care group. Results from the iHEART study have been reported.12,24,25 Participants in the intervention arm had shorter time to detecting AF recurrence than those in the usual care group.12 However, hypotheses related to more timely treatment for recurrence and improved QOL were not supported.12,24 Moreover, there were no differences in AF disease-specific symptom severity between the two groups.24 In this analysis of the iHEART trial, we describe predictors of moderate and frequent use of AliveCor® among intervention participants. This analysis provides insights into the factors that predict use of the AliveCor® digital health tool over 6 months.
Methods
Study design
As previously published,23 participants were recruited for the study between March 2014 and May 2017, from a large urban hospital and randomized into two study arms: (i) usual cardiac care, or (ii) daily AliveCor® use and BAM text messages three times a week for 6 months. All patients provided written informed consent. This study was approved by the Columbia University Medical Center Institutional Review Board, and the analysis of data was approved by the Weill Cornell Medicine Institutional Review Board.
Intervention: AliveCor®and Behavioural Altering Messages
Participants randomized to the iHEART intervention received an iPhone® and cellular service plan with unlimited data/text messaging, AliveCor®, and BAM text messages three times per week for 6 months.23 AliveCor® is an FDA-cleared device that works through the free accompanying KardiaMobile® application. AliveCor® captures highly sensitive, specific, and accurate single-lead 30-s ECG recordings through two non-adhesive electrodes.26 The ECG recording begins automatically when the patient opens the KardiaMobile® application and the electrodes come in contact with the patient’s fingers. ECG recordings are automatically uploaded via Wi-Fi or cellular network transmission to the HIPAA-compliant, secure AliveCor® cloud. An algorithm in the KardiaMobile® app uses the regularity of R-to-R intervals and presence or absence of p-waves in an ECG to identify the rhythm of each recording as either normal sinus rhythm, AF, or ‘unclassified’, meaning the algorithm could not identify the rhythm.27
Patients were instructed to record an ECG twice daily and additional ECGs whenever they experienced cardiac symptoms. Patients were told to contact their cardiac healthcare provider for subsequent treatment, management, and follow-up if AliveCor® detected possible AF. The iHEART study staff, including a nurse practitioner and cardiac electrophysiologist, reviewed and interpreted ECG strips transmitted to the AliveCor® cloud during the previous 24 h daily. Any clinically significant arrhythmias were immediately referred to the patient’s nurse practitioner or cardiac electrophysiologist and followed up with the patient.
The BAM text messages were automated and sent in English or Spanish on Monday, Wednesday, and Friday of each week. We developed a bank of brief educational and motivational messages based on the American Heart Association (AHA) Life’s Simple Seven educational materials.28 Messages from this bank were sent to participants based on the presence of AF-associated risk factors in the patient’s problem list in the electronic health record (EHR). A sample of messages is included in Supplementary material online, Table S1.
Participants
The study eligibility criteria have been published23 and are summarized here. We recruited English and Spanish-speaking patients ≥18 years of age with documented AF and at least one AF-related risk factor, who were undergoing either direct current cardioversion or radiofrequency ablation as treatment to restore normal sinus rhythm.23 Patients were excluded from participating in the study if they had a history of severe cognitive impairment (based on a diagnosis of dementia from the EHR) or were unwilling to use AliveCor®, agree to data collection, or receive text messages three times a week.
If participants owned a smartphone compatible with AliveCor®, they had the option to use AliveCor® with their own phone; otherwise, an iPhone and cellular service plan were provided to them. Prior to use, participants randomized to the iHEART intervention were trained in-person on how to use the KardiaMobile® application. A return demonstration from the participant to research staff was performed on how to capture a daily ECG.23
Measures and data sources
Our primary outcome was average weekly AliveCor® use over the 6-month period, which was categorized into three groups: infrequent (≤5 times/week), moderate (>5 times and ≤11 times/week), and frequent (>11 times/week) use. These categories were selected based on the 25th and 75th quartiles of weekly usage.
Demographic and clinical characteristics were assessed at baseline, including age, sex, race, ethnicity, technology experience, pre-intervention ECG rhythm, and AF procedures performed, using both patient-self report and the patient’s EHR. Technology experience was evaluated using a 10-item questionnaire that asked about comfort and use of cellphones, smartphones, computers, the Internet, and text messaging, and perceived likelihood of adopting of new technologies in general. Weekly episodes of AF or atrial flutter and total number of premature atrial contractions (PACs) were recorded with AliveCor® and reviewed by a clinical member of the study team, including a cardiac electrophysiologist. Other study measures are listed in Table 1.
Table 1.
Baseline demographic and clinical characteristics of iHEART study participants
| Variable | All users in intervention arm (n = 105) | Group 1: Infrequent (<5 times/week) (n = 33) | Group 2: Moderate (>5 and <11 times/week) (n = 44) | Group 3: Frequent (>11 times/week) (n = 28) | P-value |
|---|---|---|---|---|---|
| Demographic | |||||
| Age (years) | 0.53 | ||||
| <65 | 58 (55%) | 24 (41%) | 20 (35%) | 14 (24%) | |
| 65–75 | 36 (34%) | 4 (11%) | 19 (53%) | 13 (36%) | |
| >75 | 11 (11%) | 5 (45%) | 5 (45%) | 1 (10%) | |
| Female | 26 (25%) | 8 (31%) | 13 (50%) | 5 (19%) | 0.54 |
| Race | 0.99 | ||||
| White | 80 (76%) | 28 (35%) | 29 (36%) | 23 (29%) | |
| Black/African-American | 2 (2%) | 1 (50%) | 0 (0%) | 1 (50%) | |
| Asian | 1 (1%) | 0 (0%) | 1 (100%) | 0 (0%) | |
| Unknown/not reported | 22 (21%) | 4 (18%) | 14 (64%) | 4 (18%) | |
| Ethnicity | 0.13 | ||||
| Hispanic/Latino | 10 (10%) | 1 (10%) | 9 (90%) | 0 (0%) | |
| Not Hispanic/Latino | 55 (52%) | 22 (40%) | 19 (35%) | 14 (25%) | |
| Unknown/not reported | 40 (38%) | 10 (25%) | 16 (40%) | 14 (35%) | |
| Clinical characteristics | |||||
| Type of AF | 0.99 | ||||
| Paroxysmal | 70 (67%) | 22 (31%) | 30 (43%) | 18 (26%) | |
| Persistent | 32 (30%) | 10 (31%) | 13 (41%) | 9 (28%) | |
| Unknown/not reported | 3 (3%) | 1 (33%) | 1 (33%) | 1 (33%) | |
| Family history of AF | 17 (16%) | 7 (41%) | 7 (41%) | 3 (18%) | 0.54 |
| Baseline rhythm | 0.96 | ||||
| AF | 68 (65%) | 22 (32%) | 28 (41%) | 18 (27%) | |
| AF/atrial flutter | 37 (35%) | 11 (30%) | 16 (43%) | 10 (27%) | |
| Procedure at enrolment | 0.92 | ||||
| Cardioversion | 49 (47%) | 15 (31%) | 20 (41%) | 14 (29%) | |
| Ablation | 56 (53%) | 18 (32%) | 24 (43%) | 14 (25%) | |
| AF recurrencea | 0.87 | ||||
| Recurrence | 64 (61%) | 19 (30%) | 28 (44%) | 17 (27%) | |
| No recurrence | 41 (39%) | 14 (34%) | 16 (39%) | 11 (27%) | |
| Median weekly number of AF episodesa | 1.4 ± 3.2 | 0.6 ± 1.0 | 1.1 ± 2.0 | 2.8 ± 5.3 | 0.26 |
| Median number of PACsa | 18.8 ± 8.3 | 14.1 ± 7.2 | 19.7 ± 8.6 | 23.1 ± 6.0 | <0.0001 |
| Diabetes | 12 (11%) | 3 (35%) | 9 (75%) | 0 (0%) | 0.03 |
| Hypertension | 61 (58%) | 15 (35%) | 30 (49%) | 16 (26%) | 0.14 |
| Obesity | 35 (33%) | 10 (29%) | 18 (51%) | 7 (20%) | 0.35 |
| History of smoking | 0.79 | ||||
| Current | 5 (5%) | 3 (60%) | 1 (20%) | 1 (20%) | |
| Past | 33 (31%) | 7 (21%) | 17 (52%) | 9 (27%) | |
| None | 67 (64%) | 23 (34%) | 26 (39%) | 18 (27%) | |
| Sleep apnoea | 24 (23%) | 9 (37%) | 10 (42%) | 5 (21%) | 0.69 |
| Heart failure | 18 (17%) | 3 (17%) | 11 (61%) | 4 (22%) | 0.17 |
| TIA or stroke | 10 (10%) | 2 (20%) | 5 (50%) | 3 (30%) | 0.71 |
| Symptoms and QOL | |||||
| AF Effect on QOL (AFEQT) | |||||
| Overall | 65.2 ± 20.5 | 67.2 ± 19.8 | 67.6 ± 19.8 | 60.7 ± 21.9 | 0.48 |
| Symptom burden | 71.6 ± 23.9 | 71.4 ± 22.9 | 80.3 ± 18.0 | 62.0 ± 27.3 | 0.03 |
| Treatment concern | 63.2 ± 27.1 | 70.2 ± 24.4 | 58.2 ± 29.0 | 61.9 ± 27.0 | 0.23 |
| Daily activities | 63.6 ± 26.7 | 62.7 ± 24.3 | 68.3 ± 26.8 | 59.2 ± 28.8 | 0.45 |
| Treatment satisfaction | 72.7 ± 24.0 | 78.8 ± 17.5 | 71.8 ± 29.5 | 67.9 ± 21.9 | 0.23 |
| AF severity (AFSS) | 10.0 ± 7.0 | 9.8 ± 6.8 | 8.9 ± 6.5 | 11.5 ± 7.9 | 0.52 |
| Depressive symptoms | 4.9 ± 5.8 | 4.9 ± 6.8 | 3.9 ± 4.2 | 6.0 ± 6.4 | 0.64 |
| Anxiety symptoms | |||||
| State | 31.5 ± 12.8 | 32.1 ± 14.9 | 31.0 ± 12.0 | 31.5 ± 11.9 | 0.89 |
| Trait | 31.4 ± 11.0 | 31.1 ± 12.7 | 30.3 ± 9.2 | 32.8 ± 11.5 | 0.54 |
| Self-management skills | |||||
| AF knowledge | 7.3 ± 2.0 | 6.9 ± 2.1 | 7.7 ± 1.4 | 7.4 ± 2.3 | 0.38 |
| Controls-attitudes | 26.5 ± 6.1 | 25.5 ± 6.3 | 27.3 ± 6.2 | 26.6 ± 5.9 | 0.55 |
| Self-efficacy in medication adherence | 35.2 ± 5.2 | 34.8 ± 5.4 | 35.4 ± 5.3 | 35.2 ± 5.2 | 0.74 |
| Reported medication adherence | 2.0 ± 0.9 | 1.9 ± 0.9 | 2.0 ± 0.8 | 2.1 ± 1.0 | 0.55 |
| Technology experience | |||||
| Cellphone owner | 83 (99%) | 25 (30%) | 31 (37%) | 27 (33%) | 0.44 |
| Smartphone owner | 75 (89%) | 23 (31%) | 27 (36%) | 25 (33%) | 0.52 |
| Use Internet on smartphone | 68 (82%) | 21 (31%) | 25 (37%) | 22 (32%) | 0.95 |
| Use email on smartphone | 68 (82%) | 21 (31%) | 25 (37%) | 22 (32%) | 0.95 |
| Downloaded apps on smartphone | 66 (80%) | 21 (32%) | 26 (39%) | 19 (29%) | 0.26 |
| Send/receive text messages | 80 (94%) | 25 (31%) | 30 (38%) | 25 (31%) | 0.86 |
| Followed link from text messages | 63 (74%) | 21 (33%) | 23 (37%) | 19 (30%) | 0.65 |
| Use computer at home | 78 (94%) | 23 (29%) | 31 (40%) | 24 (31%) | 0.68 |
| Internet access at home | 82 (96%) | 25 (30%) | 31 (38%) | 26 (32%) | 0.99 |
| Comfort using Internet on computer | 83 (99%) | 25 (30%) | 32 (39%) | 26 (31%) | 0.35 |
Data were collected with AliveCor™ device; all other data came from surveys or the electronic health record.
Measures: AF knowledge: AF Knowledge Scale (AFKS); Anxiety symptoms: State-Trait Anxiety Inventory (STAI); Controls-attitudes: Controls-Attitudes Scale-Revised (CAS-R); Depressive symptoms: Patient Health Questionnaire (PHQ-9); Reported medication adherence: Morisky Medication Adherence Scale (MMAS); Self-efficacy in medication adherence: Self-Efficacy in Medication Adherence scale (SEAMS).
We defined AF recurrence as any occurrence of AF or atrial flutter during the 6-month study duration that was captured by AliveCor®, a standard 12-lead ECG, Holter monitor, or other type of external recording mechanism for detection of AF. Differences in AF detection by AliveCor® compared to other methods are reported elsewhere.12 We used the University of Toronto AF Severity Scale (AFSS) to assess the severity of AF in terms of healthcare utilization, frequency and duration of AF episodes, and symptom severity.29,30 The AFSS is a disease-specific measure of QOL in AF that measures both subjective and objective AF disease burden. In this study, we analysed the AFSS symptom subscale, which measures seven symptoms (palpitations, shortness of breath at rest, shortness of breath during physical activity, exercise intolerance, fatigue at rest, lightheadedness/dizziness, and chest pain or pressure), each on a Likert scale of 0 to 5 (most burdensome). The total range of scores for the AFSS symptom subscale is 0 to 35.29
The impact of AF on QOL was measured by the Atrial Fibrillation Effect on QOL (AFEQT) instrument,31 which assesses QOL across four domains: symptom burden, daily activities, treatment concerns, and treatment satisfaction. Individual scores for each of the subscales and the global score range from zero (complete disability) to 100 (highest level of QOL).31 The AFEQT asks respondents how bothered they have been by four symptoms (palpitations, irregular heartbeat, a pause in heart activity, and lightheadedness/dizziness) in the context of these symptoms affecting their QOL.
Statistical analyses
We conducted bivariate analyses to assess whether each baseline characteristic differed among level of usage (infrequent, moderate, or frequent) using Kruskal–Wallis tests. We further used multivariate multinomial logistic regression to examine the relationship between baseline characteristics and AliveCor® use (comparing frequent and moderate to infrequent users). First, we constructed a preliminary main effects model using all independent variables where P < 0.25 in bivariate analyses. Then, we used SAS version 9.4 to perform model selection using Akaike Information Criteria for model comparison.
Results
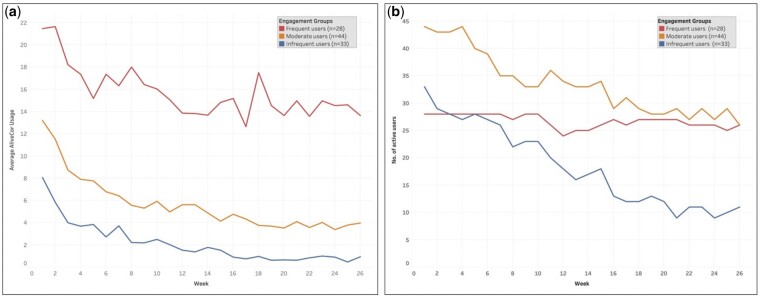
The comparison of baseline characteristics overall and by usage group is presented in Table 1. The mean use of AliveCor® was 8.8 (SD 7.4) times per week among all 105 participants. Among the participants, 28 (27%) met the criteria for frequent use of AliveCor®, 44 (42%) were classified as moderate users, and 33 (31%) were infrequent users. Within each of the groups, there was general consistency in the use of Alivecor (i.e. frequent users remained the highest users over 6 months) (Figure 1A); however, the number of active AliveCor® users transmitting declined over 6 months within the moderate and infrequent engagement groups (Figure 1B).
Figure 1.
Frequent, moderate, and infreqruent Alivecor users.
Participants had an average age of 62 years (range 26–87); 34% were between the ages of 65–75 years and 11% were over age 75. A quarter of the participants were female, three-quarters were White and 10% were Hispanic. The majority of participants had paroxysmal AF (67%, n = 70) and 61% (n = 64) had AF or atrial flutter recurrence within 6 months. Among the three user groups, there were no differences in gender, age, race, ethnicity, or technology experience. There were differences in median number of PACs, median weekly number of AF episodes, and AF symptom burden impact on QOL measured by the AFEQT symptom sub-scale at baseline.
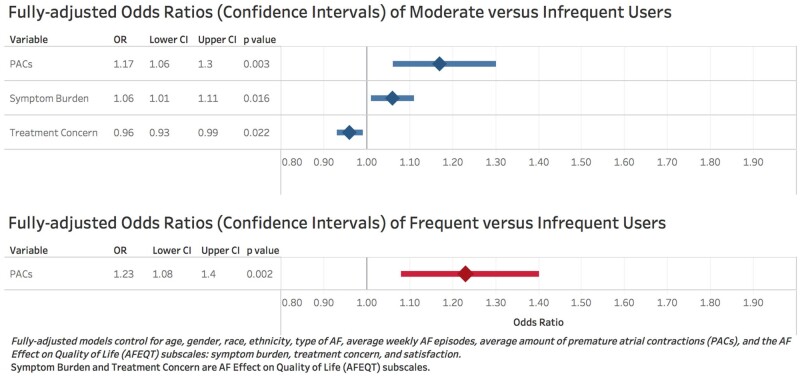
As reported in Table 2 and Figure 2, comparing moderate to infrequent users, the moderate users were 17% more likely to have more PACs (adjusted odds ratio (OR): 1.17, 95% confidence interval 1.06–1.30, P = 0.003), 6% more likely to have higher AFEQT symptom burden scores indicating less symptom burden (adjusted OR: 1.06, 1.01–1.11, P = 0.016), and 4% less likely to have higher treatment concern scores indicating less treatment concern (adjusted OR: 0.96, 0.93–0.99, P = 0.022). Comparing frequent to infrequent users, the frequent users were 23% more likely to have PACS (adjusted OR: 1.23, 1.08–1.40, P = 0.002).
Table 2.
Multinomial regression model
| Variable | Odds ratios (Moderate vs. Infrequent)a | P-value | Odds ratios (Frequent vs. Infrequent)a | P-value |
|---|---|---|---|---|
| Age | 2.00 (0.68–5.90) | 0.21 | 2.47 (0.73–8.37) | 0.15 |
| Gender | 0.90 (0.14–5.67) | 0.91 | 0.26 (0.03–2.53) | 0.24 |
| Race | 1.46 (0.18–12.02) | 0.72 | 0.19 (0.02–1.57) | 0.12 |
| Ethnicity | 0.30 (0.07–1.24) | 0.10 | 1.95 (0.47–8.07) | 0.36 |
| Type of AF | 1.83 (0.34–9.96) | 0.49 | 1.25 (0.23–6.98) | 0.80 |
| Average weekly AF episodes | 1.03 (0.68–1.58) | 0.89 | 1.30 (0.91–1.87) | 0.15 |
| Average count of PACs | 1.17 (1.06–1.30) | 0.003 | 1.23 (1.08–1.40) | 0.002 |
| AFEQT: Symptom burden | 1.06 (1.01–1.11) | 0.02 | 0.99 (0.95–1.02) | 0.43 |
| AFEQT: Treatment concern | 0.96 (0.93–0.99) | 0.02 | 0.98 (0.95–1.01) | 0.20 |
| AFEQT: Treatment satisfaction | 0.99 (0.95–1.03) | 0.58 | 0.98 (0.94–1.01) | 0.20 |
Controlling for age, gender, race, ethnicity, type of AF, average weekly AF episodes, average amount of premature atrial contractions (PACs), and the AF Effect on Quality of Life (AFEQT) subscales: symptom burden, treatment concern, and satisfaction.
Figure 2.
Multinomial logistic regression model results.
Overall, the average number of BAM text messages sent to each participant over 6 months was 87.2 (±31.5). All patients received AF-related messages; in addition, 79% received messages on eating well, 55% on exercise, and 26% on managing stress.
Discussion
The iHEART trial is one of the first randomized clinical trials to evaluate the effect of smartphone-based ECG monitoring coupled with AF knowledge- and behavioural altering text messages in a post-ablation and post-cardioversion patient population. One of the greatest challenges for the ongoing monitoring and management of AF using digital health is sustaining use. A greater understanding of factors associated with sustained use can inform the development of future digital health tools and guide clinicians and researchers in identifying subgroups of patients who may be best suited to use digital health for the management of AF. In this study, we identified three distinct categories of Alivecor usage over 6 months. We found evidence that AF symptoms and symptomatic cardiac events are associated with more frequent Alivercor use over time.
The 2019 clinical guidelines acknowledge the potential role for digital health tools, including ‘smart’ worn or handheld Wi-Fi-enabled devices with remote electrocardiographic acquisition and interpretation.32 Two questions for future research using digital health tools specifically are: what is the optimal length of time for use, and what intensity of engagementis appropriate and clinically beneficial? Questions of the appropriate frequency and duration, or persistence, of digital health tools for monitoring AF are of paramount importance for their clinical utility. In our study, usage declined within each group over 6 months; however, this may still represent clinically appropriate engagement if AF did not recur during this period. The clinical benefit of very frequent active monitoring 6 months after an ablation is currently unknown. Although previous work has determined the ideal time frame for monitoring using implantable devices (diagnostic yield),33–35 this question has yet to be explored for digital health tools. Nonetheless, it is well worth exploring, as the future potential for the optimization of digital health tools is that they could be used to reduce inefficiencies and costs, improve access and quality, and make healthcare more personalized for patients with AF.
Overall, we found that participants who experience more frequent PACs were more likely to have sustained use (five times per week) of AliveCor® over the course of 6 months compared to other AF-related clinical characteristics. A high burden of PACs may have created symptoms that simulated AF, cueing moderate and frequent use. It is unlikely that PACs incorrectly interpreted by the AliveCor algorithm as AF drove more frequent use, given that AF episodes themselves were not associated with use in the final multinomial models. Moderate users also had less symptom burden but greater treatment concern than infrequent users. These moderate users may have been conscientious patients who engaged with AliveCor® as a way to allay AF-related anxiety, even despite the lack of heavy symptom burden. Alternatively, lack of symptom burden could have enabled, rather than inhibited, more regular use of AliveCor®. AFEQT scores demonstrate that the burden of symptoms on QOL was heaviest among infrequent and frequent users, which suggests that symptom burden either drove extremely high usage (>11 times per week on average), or overwhelmed participants’ willingness or abilities to engage with AliveCor® altogether, leading to infrequent use.
Our findings are supported by a recent review which found that symptoms are an independent driver of QOL regardless of the frequency or duration of AF episodes.36 Even if patients are not frequently in AF, they may be highly symptomatic during AF episodes which may adversely impact their QOL.36,37 In a related qualitative analysis of AliveCor® use in the iHEART trial,38 many of the patients who were more frequent users appropriately focused on their actual ECG data instead of their symptoms when determining whether to continue using AliveCor®. Conversely, lack of use among infrequent users was largely driven by either an absence of symptoms, which may have been interpreted as a sign of wellness, or confusion about AF symptoms and how they changed over time.38 While the complex psychosomatic relationship between AF episodes and symptoms has been well-documented, our results confirm early exploratory analyses of this trial which suggested that the complexity of this relationship influences use of digital health.39
While the relationship between symptoms and AF episodes is complex and not well elucidated,36,40,41 symptoms nonetheless impact QOL and are the primary indication for catheter ablation. Digital health tools are well positioned to quantify symptom burden and possible improvements from different treatment modalities. As previously reported, although AliveCor enables voice memos during recording to report symptoms and other details, it was only used by 11 (10%) of patients in the iHEART trial.12 Future studies should capture repeated assessments of ECG rhythm concurrent with symptoms, for example through ecological momentary assessment (EMA), an approach which involves repeatedly sampling an individuals’ behaviours and experiences in real-time.42 By repeatedly measuring behaviours and experiences in real-time in patients’ daily lives, EMA interventions that use digital health technology are increasingly popular for their responsiveness to patient-reported behaviours and outcomes. These interventions may offer a more detailed understanding of the dynamic interplay between physiology, psychology, patient-reported symptoms, and functioning in daily life,36 and may also be useful in the context of motivating self-monitoring in response to symptoms, functioning, and physiology.43 In the context of AF, individuals’ symptoms and precipitating behaviours could be captured in real-time alongside ECG rhythm to understand associations in greater detail and with less recall bias. Further research using EMA will be helpful for gaining knowledge on behaviours that we need to assess to strengthen active AF monitoring and holistic management.
Neither age nor technology experience were associated with use of AliveCor® over 6 months. This finding aligns with growing acceptance of digital health tools for self-monitoring and management among seniors. According to Pew reports, the use of the digital tools among adults over 65 years has increased fourfold in 5 years.44 In 2017, it was estimated that 42% of seniors owned smartphones.44 Relatively younger (<65 years), affluent, and educated seniors are driving the growth in technology adoption.44 One explanation for sustained frequent use among older adults is that they reported finding AliveCor® easy to use; its simplicity made repeated use easy and less burdensome.38 Moreover, there was no difference in frequency of AliveCor® use based on technology experience. Together, these findings are critical because they challenge assumptions that older or less tech-savvy patients will not engage with digital health, and expand potential users of the tool to those with low technology experience.
Limitations
One of the limitations of this study was lack of gender, racial, ethnic, and sociodemographic diversity. The inclusion criteria of access to an ablation or cardioversion could partially explain this because there are known racial, ethnic, and gender disparities in access to interventional cardiac procedures.45–49 The overall iHEART trial was also a single-study from one electrophysiology unit at a large urban, academic medical centre. As such, we recognize the limitations of study generalizability, this population was not representative of many patients with AF. In addition, the symptom reporting feature of the AliveCor device was not required for iHEART trial participation and was used by only a few participants. Therefore, we were unable to evaluate exactly which symptoms participants experienced during AF and other cardiac events (such as PACs). Studying associations between cardiac rhythm and specific symptoms is an important area of future research.
The largest limitation of the study was missing data for the follow-up survey at 6 months in the intervention arm, which meant that we could only use baseline data for some variables and could not examine the impact that variability in predictor variables from baseline to 6 months had upon AliveCor® use. Consequently, while the variables that yielded the largest predictions came from AliveCor®, we may have failed to detect important self-reported predictors. We also summarized usage as average weekly usage over the study period, thus our analysis did not account for fluctuations in engagement over time.
Conclusion
This study was able to address the question of what factors at baseline and during use predict sustained use of AliveCor® over 6 months. Frequent use of AliveCor®, a digital health monitoring tool, was associated with symptom burden and potentially symptomatic cardiac events (PACs). Future digital health interventions should consider using the AFEQT to be able to measure the influence of symptom burden on digital health usage over time in order to differentiate the impact of an intervention on symptom burden. In addition, providers and researchers should be aware of the complex role that symptoms play in driving digital health usage as they develop digital health tools and implement them in clinical practice. Future research should consider novel approaches to encourage self-monitoring in ways that account for symptoms, such as EMA interventions.
Supplementary material
Supplementary material is available at European Journal of Cardiovascular Nursing online.
Supplementary Material
Acknowledgements
We thank the other members of the iHEART team, including Teresa Riga, Paul Gonzalez, Robert R. Sciacca, and all of the patients who enrolled as participants in the iHEART trial.
Funding
We would also like to acknowledge our funding support. The iHEART trial was funded by the National Institute of Nursing Research (NINR) of the National Institutes of Health (NIH) under award number R01NR014853. M.R.T. is funded by NINR under award number K99NR019124. R.M.C. is funded by NINR under award number R00NR016275. B.C., T.K., and S.B. are funded by NINR under award number P30NR016587.
Conflict of interest: none declared.
References
- 1. Patel NJ, Atti V, Mitrani RD, Viles-Gonzalez JF, Goldberger JJ.. Global rising trends of atrial fibrillation: a major public health concern. Heart 2018;104:1989–1990. [DOI] [PubMed] [Google Scholar]
- 2. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim Y-H, McAnulty JH, Zheng Z-J, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJL.. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease Study. Circulation 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sultan A, Lüker J, Andresen D, Kuck KH, Hoffmann E, Brachmann J, Hochadel M, Willems S, Eckardt L, Lewalter T, Senges J, Steven D.. Predictors of atrial fibrillation recurrence after catheter ablation: data from the German Ablation Registry. Sci Rep 2017;7:16678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verma A, Jiang C-y, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque J-P, Nardi S, Menardi E, Novak P, Sanders P.. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–1822. [DOI] [PubMed] [Google Scholar]
- 5. Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey J-Y, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc J-J, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006;114:e257–e354. [DOI] [PubMed] [Google Scholar]
- 6. Chugh SS, Blackshear JL, Shen W-K, Hammill SC, Gersh BJ.. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol 2001;37:371–378. [DOI] [PubMed] [Google Scholar]
- 7. Zhang L, Gallagher R, Neubeck L.. Health-related quality of life in atrial fibrillation patients over 65 years: a review. Eur J Prev Cardiol 2015;22:987–1002. [DOI] [PubMed] [Google Scholar]
- 8. Kochhäuser S, Joza J, Essebag V, Proietti R, Koehler J, Tsang B, Wulffhart Z, Pantano A, Khaykin Y, Ziegler PD, Verma A.. The impact of duration of atrial fibrillation recurrences on measures of health-related quality of life and symptoms. Pacing Clin Electrophysiol 2016;39:166–172. [DOI] [PubMed] [Google Scholar]
- 9. Serpytis R, Navickaite A, Serpytiene E, Barysiene J, Marinskis G, Jatuzis D, Petrulioniene Z, Laucevicius A, Serpytis P.. Impact of atrial fibrillation on cognitive function, psychological distress, quality of life, and impulsiveness. Am J Med 2018;131:703.e1–703.e5. [DOI] [PubMed] [Google Scholar]
- 10. Tan HC, Koh KWL, Wu VX, Lim TW, Wang W.. Health-related quality of life, psychological distress, and symptom burden in an Asian population of outpatients with atrial fibrillation. Heart Lung 2018;47:322–328. [DOI] [PubMed] [Google Scholar]
- 11. Wasserlauf J, You C, Patel R, Valys A, Albert D, Passman R.. Smartwatch performance for the detection and quantification of atrial fibrillation. Circ Arrhythm Electrophysiol 2019;12:e006834. [DOI] [PubMed] [Google Scholar]
- 12. Goldenthal IL, Sciacca RR, Riga T, Bakken S, Baumeister M, Biviano AB, Dizon JM, Wang D, Wang KC, Whang W, Hickey KT, Garan H.. Recurrent atrial fibrillation/flutter detection after ablation or cardioversion using the AliveCor KardiaMobile device: iHEART results. J Cardiovasc Electrophysiol 2019;30:2220–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turakhia MP, Shafrin J, Bognar K, Trocio J, Abdulsattar Y, Wiederkehr D, Goldman DP.. Estimated prevalence of undiagnosed atrial fibrillation in the United States. PLoS One 2018;13:e0195088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Michie S, Yardley L, West R, Patrick K, Greaves F.. Developing and evaluating digital interventions to promote behavior change in health and health care: recommendations resulting from an international workshop. J Med Internet Res 2017;19:e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhavnani SP, Narula J, Sengupta PP.. Mobile technology and the digitization of healthcare. Eur Heart J 2016;37:1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D; Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 2012;9:632–696. [DOI] [PubMed] [Google Scholar]
- 17. Loomba RS, Buelow MW, Aggarwal S, Arora RR, Kovach J, Ginde S.. Arrhythmias in adults with congenital heart disease: what are risk factors for specific arrhythmias? Pacing Clin Electrophysiol 2017;40:353–361. [DOI] [PubMed] [Google Scholar]
- 18. Ruddox V, Sandven I, Munkhaugen J, Skattebu J, Edvardsen T, Otterstad JE.. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: A systematic review and meta-analysis. Eur J Prev Cardiol 2017;24:1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, Herrington DM, Cushman M.. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med 2014;174:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soliman EZ, Lopez F, O’Neal WT, Chen LY, Bengtson L, Zhang Z-M, Loehr L, Cushman M, Alonso A.. Atrial fibrillation and risk of ST-segment-elevation versus non-ST-segment-elevation myocardial infarction: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2015;131:1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA.. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ 2016;354:i4482. [DOI] [PubMed] [Google Scholar]
- 22. Steinhubl SR, Mehta RR, Ebner GS, Ballesteros MM, Waalen J, Steinberg G, Van Crocker P, Felicione E, Carter CT, Edmonds S, Honcz JP, Miralles GD, Talantov D, Sarich TC, Topol EJ.. Rationale and design of a home-based trial using wearable sensors to detect asymptomatic atrial fibrillation in a targeted population: The mHealth Screening To Prevent Strokes (mSToPS) trial. Am Heart J 2016;175:77–85. [DOI] [PubMed] [Google Scholar]
- 23. Hickey KT, Hauser NR, Valente LE, Riga TC, Frulla AP, Creber RM, Whang W, Garan H, Jia H, Sciacca RR, Wang DY.. A single-center randomized, controlled trial investigating the efficacy of a mHealth ECG technology intervention to improve the detection of atrial fibrillation: the iHEART study protocol. BMC Cardiovasc Disord 2016;16:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caceres BA, Hickey KT, Bakken SB, Biviano AB, Garan H, Goldenthal IL, Koleck TA, Masterson-Creber R, Turchioe MR, Jia H.. Mobile ECG monitoring and health-related quality of life in patients with atrial fibrillation: findings from the iHEART study. J Cardiovasc Nurs 2020;35:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koleck TA, Mitha SA, Biviano A, Caceres BA, Corwin EJ, Goldenthal I, Creber RM, Turchioe MR, Hickey KT, Bakken S.. Exploring depressive symptoms and anxiety among patients with atrial fibrillation and/or flutter at the time of cardioversion or ablation. J Cardiovasc Nurs 2020;doi:10.1097/JCN.0000000000000723. https://pubmed.ncbi.nlm.nih.gov/32675627/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lau JK, Lowres N, Neubeck L, Brieger DB, Sy RW, Galloway CD, Albert DE, Freedman SB.. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol 2013;165:193–194. [DOI] [PubMed] [Google Scholar]
- 27. Chan P-H, Wong C K, Poh YC,. et al. Diagnostic Accuracy of a Smartphone-Based Atrial Fibrillation Detection Algorithm. Boston, MA: Heart Rhythm Society; 2018. [Google Scholar]
- 28.Association AH. Life's Simple 7. https://www.heart.org/en/professional/workplace-health/lifes-simple-7 (2 February 2021).
- 29. Dorian P, Paquette M, Newman D, Green M, Connolly SJ, Talajic M, Roy D.. Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. Am Heart J 2002;143:984–990. [DOI] [PubMed] [Google Scholar]
- 30. Dorian P, Guerra PG, Kerr CR, O’Donnell SS, Crystal E, Gillis AM, Mitchell LB, Roy D, Skanes AC, Rose MS, Wyse DG.. Validation of a new simple scale to measure symptoms in atrial fibrillation: the Canadian Cardiovascular Society Severity in Atrial Fibrillation scale. Circ Arrhythm Electrophysiol 2009;2:218–224. [DOI] [PubMed] [Google Scholar]
- 31. Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR, Lakkireddy DR, Wimmer AP, Bhandari A, Burk C.. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) Questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4:15–25. [DOI] [PubMed] [Google Scholar]
- 32. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW.. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Heart Rhythm 2019;16:e66–e93. [DOI] [PubMed] [Google Scholar]
- 33. Deering TF, Hindricks G, Marrouche NF.. Digital health: present conundrum, future hope or hype? Heart Rhythm 2019;16:1303–1304. [DOI] [PubMed] [Google Scholar]
- 34. Bumgarner JM, Lambert CT, Hussein AA, Cantillon DJ, Baranowski B, Wolski K, Lindsay BD, Wazni OM, Tarakji KG.. Smartwatch algorithm for automated detection of atrial fibrillation. J Am Coll Cardiol 2018;71:2381–2388. [DOI] [PubMed] [Google Scholar]
- 35. Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C, Gravenor MB.. Assessment of remote heart rhythm sampling using the AliveCor Heart Monitor to Screen for Atrial Fibrillation. Circulation 2017;136:1784–1794. [DOI] [PubMed] [Google Scholar]
- 36. Heidt S, Kratz A, Najarian K, Hassett AL, Oral H, Gonzalez R, Nallamothu BK, Clauw D, Ghanbari H.. Symptoms in atrial fibrillation: a contemporary review and future directions. J Atr Fibrillation 2016;9:1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, Noseworthy PA, Perez MV, Turakhia MP.. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation 2018;137:e623–e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reading M, Baik D, Beauchemin M, Hickey K, Merrill J.. Factors influencing sustained engagement with ECG self-monitoring: perspectives from patients and health care providers. Applied Clinical Informatics 2018;09:772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reading M, Biviano AB, Mitrani L, et al. Abstract 14561: The role of symptoms in adherence to mHealth ECG monitoring for atrial fibrillation. Circulation 2018;136:A14561. https://www.ahajournals.org/doi/10.1161/circ.136.suppl_1.14561. [Google Scholar]
- 40. Verma A, Champagne J, Sapp J, Essebag V, Novak P, Skanes A, Morillo CA, Khaykin Y, Birnie D.. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Intern Med 2013;173:149–156. [DOI] [PubMed] [Google Scholar]
- 41. Simantirakis EN, Papakonstantinou PE, Chlouverakis GI, Kanoupakis EM, Mavrakis HE, Kallergis EM, Arkolaki EG, Vardas PE.. Asymptomatic versus symptomatic episodes in patients with paroxysmal atrial fibrillation via long-term monitoring with implantable loop recorders. Int J Cardiol 2017;231:125–130. [DOI] [PubMed] [Google Scholar]
- 42. Shiffman S, Stone AA, Hufford MR.. Ecological momentary assessment. Ann Rev Clin Psychol 2008;4:1–32. [DOI] [PubMed] [Google Scholar]
- 43. Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkiewitz K, Tewari A, Murphy SA.. Just-in-time adaptive interventions (JITAIs) in mobile health: key components and design principles for ongoing health behavior support. Ann Behav Med 2018;52:446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pew Research Center. Tech Adoption Climbs Among Older Adults. 2017. http://www.pewinternet.org/2017/05/17/tech-adoption-climbs-among-older-adults/ (2 February 2021).
- 45. Alkhouli M, Alqahtani F, Holmes DR, Berzingi C.. Racial disparities in the utilization and outcomes of structural heart disease interventions in the United States. J Am Heart Assoc 2019;8:e012125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ugowe FE, Jackson LR, Thomas KL.. and ethnic differences in the prevalence, management, and outcomes in patients with atrial fibrillation: a systematic review. Heart Rhythm 2018;15:1337–1345. [DOI] [PubMed] [Google Scholar]
- 47. Hoyt H, Nazarian S, Alhumaid F, Dalal D, Chilukuri K, Spragg D, Henrikson CA, Sinha S, Cheng A, Edwards D, Needleman M, Marine JE, Berger R, Calkins H.. Demographic profile of patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:994–998. [DOI] [PubMed] [Google Scholar]
- 48. Bhave PD, Lu X, Girotra S, Kamel H, Vaughan Sarrazin MS.. Race- and sex-related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm 2015;12:1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schnabel RB, Pecen L, Ojeda FM, Lucerna M, Rzayeva N, Blankenberg S, Darius H, Kotecha D, Caterina RD, Kirchhof P.. Gender differences in clinical presentation and 1-year outcomes in atrial fibrillation. Heart 2017;103:1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.