Abstract
Indonesia’s HIV epidemic is concentrated among key populations. While prevalence among men who have sex with men (MSM) is high, transmission among young MSM (15–24-years-old) remains poorly understood. We conducted a respondent driven sampling survey of 211 young MSM in urban Bandung, Indonesia in 2018–2019 to estimate HIV prevalence and associated risk factors. Thirty percent of young MSM were HIV antibody positive. This is nearly 100-fold greater than Indonesia’s population prevalence and sevenfold higher than average estimates for young MSM across Asia and the Pacific Region. Individual risk factors associated with HIV infection were being 20–24 years old, having a steady partner and preferring the receptive position during sex. Issues of stigma, discrimination and social exclusion were common. Few young MSM who were open with friends and family members about their sexual identity. Among those that were, close to half reported experiencing feelings of aversion from these groups. Wider structural factors that reduce social tolerance, restrict the rights of young MSM and compel concealment of sexual identity are likely to fuel high-risk behaviors and limit access to essential testing care and support services including pre-exposure prophylaxis which is not yet widely available. Urgent health, social, legal and political actions are required to respond to these factors and reduce the disproportionate contribution of young MSM to Indonesia’s HIV epidemic.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10461-021-03347-0.
Keywords: HIV, Epidemiology, Key populations, Men-who-have-sex-with-men (MSM), Indonesia
Introduction
While countries in Asia and the Pacific have witnessed declining HIV infections over the past decade, transmission among key populations remains a serious concern [1]. Men who have sex with men (MSM) account for nearly one-third of new infections in the region, with several countries confronting high prevalence and increasing trends [2].
Young MSM (15–24 years old) are at high-risk of HIV infection [1]. Recent evidence from China and Thailand document higher HIV incidence among young MSM relative to older cohorts [3, 4]. In Malaysia, a 2.5-fold increase in prevalence among young MSM was documented between 2014 and 2017 [1].
Factors contributing to HIV transmission among MSM in the region include the use of social media and entertainment venues (i.e. bath houses) to seek casual sexual partners, high-risk sex associated with alcohol and recreational drug use [5–9] and concurrent sexually transmitted infections [10–12]. While specific vulnerabilities to HIV infection experienced by young MSM are less well understood, lower levels of HIV awareness and self-perceived risk, peer influence from older MSM and the exchange of sex for resources have been identified as transmission drivers [5]. Furthermore, well-documented vulnerabilities to HIV infection experienced by young people globally including limited life skills and agency, sexual coercion and feelings of stigma and social exclusion may contribute to transmission among young MSM in Asia and the Pacific [13].
Indonesia’s HIV epidemic is concentrated among key populations. Apart from two provinces in eastern Indonesia with a low-prevalence generalized epidemic (2.4%), adult HIV prevalence has stabilized at around 0.4%, and new infections decreased by one-third over the past decade [14, 15]. Among key populations, while transmission among female sex workers and people who inject drugs has been stable or declining, HIV prevalence among MSM increased at least three-fold in the past decade—from 5.3% in 2007 to 17.9% in 2019 [15].
Despite concerns of increasing transmission, Indonesia’s HIV epidemic among young MSM remains poorly understood. Data disaggregated from national assessments among young MSM in urban centers found a fourfold increase in HIV prevalence between 2011 and 2015 (from 3.8% to 15.6%) [16]. The most recent national assessment (2019), which found high prevalence among urban MSM (17.9%), has not been disaggregated for young MSM [17]. However, as these estimates are drawn from convenience samples they should be interpreted cautiously.
To better understand young MSM’s contribution to Indonesia’s key population HIV epidemic, we conducted a survey using respondent driven sampling (RDS) among young MSM in Bandung city. We present estimates of HIV prevalence and risk behaviors alongside factors associated with HIV infection and compare these to HIV prevalence estimates among young MSM from countries across the wider Asia and the Pacific region.
Methods
Data were collected using a cross sectional design over a three-month period between 2018 and 2019 in Bandung, Indonesia’s third most populous urban centre (population 2.4 million). Eligible young MSM were defined as males 15–24 years old who had anal sex with a male in the past six months and lived, worked, or studied in Bandung. This work was embedded within a wider risk assessment of key populations conducted by the University of Padjadjaran (Bandung) and UNICEF Indonesia in partnership with National and Provincial Ministries of Health [18]. The Institutional Review Board at the University of Padjadjaran reviewed and approved the study protocol (08/UN6.C10/PN/2019).
Respondent Driven Sampling and Survey Procedures
Respondent driven sampling has been widely used to sample socially networked key populations and is described in detail elsewhere [19, 20]. Briefly, RDS is a chain referral sampling method where hard-to-reach populations are asked to refer other members of their social network to enrol in a survey. Respondents provide their social network size (i.e., how many people they know who know them, who fulfil the eligibility and they have seen in the previous two weeks) which is used during analysis to mitigate biases associated with chain referral methods. The sample size was calculated at 300 based on ever having had an HIV test, with 95% confidence, 80% power and a design effect of 2 (a correction factor to increase the sample size to account for the sampling method not being a simple random sample).
Three ‘seeds’ (i.e., initial recruits) were non-randomly selected based on their ability to recruit diverse members of their social networks. Once seeds completed the survey process, they received three uniquely coded coupons to use for recruiting other eligible young MSM. Recruits presenting a coupon at one of two interview sites completed eligibility screening, informed consent and a supervised, self-administered interview with responses entered directly into a tablet. Questionnaires were extensively pre-tested and appropriately revised. Final survey questions recorded socio-demographic details, HIV-related knowledge, beliefs, stigma and discrimination, violence and risk behavior relevant for MSM. Respondents received compensation of approximately 5 USD for survey enrollment and completion and an additional compensation (a maximum of three) for each recruit who enrolled in and completed the survey.
Laboratory Analysis
All respondents received HIV antibody testing after informed written consent and pre-test counselling. Venous blood samples were collected and analyzed at an accredited national laboratory using standardized protocols. Test results were linked using respondents' unique identification number, a laboratory code and collection date. Respondents received vouchers with identification numbers to use for calling the testing center to receive their test results. Negative antibody results were provided over the phone, while those with positive antibody results were asked to present for further counseling, repeat testing and treatment.
Measures
The survey instrument was developed using questions from HIV Integrated Behavioural and Biological Surveys (IBBS) conducted in the Asia Pacific region and the Global AIDS indicators 2020 [21]. Some indicators were collapsed due to the small category size. For instance, the question asking participants how they would best describe their sexual identity had responses for “homosexual”, “heterosexual”, “bi-sexual”, “transgender” and “other”. Given that few MSM responded as being “heterosexual” (n = 8), this category was collapsed with “bisexual”. Comprehensive HIV knowledge was measured by having correct answers about whether “HIV transmission can be reduced by having sex with only one uninfected partner who has no other partners”, “a person can reduce their risk of HIV infection by using a condom every time they have sex”, “a healthy-looking person can still have HIV”, “a person can become HIV infected from mosquito bites” and “a person can get HIV by sharing food with someone who is infected” [21]. A paying partner was described to participants as someone from whom they sold anal sex in exchange for money or goods. A steady partner was described to participants as a husband or wife, boyfriend or girlfriend or someone considered to be a permanent partner. A non-regular, casual partner was described as someone whom they did not consider to be a steady partner, with whom they had sexual intercourse occasionally or once. Participants were asked to assess their own risk for HIV infection to which they could respond “high risk”, “some risk”, “low risk”, “already living with HIV” or “do not know”. “High risk” and “some-risk” were collapsed together and living with HIV or do not know were removed from the denominator.
To contextualize our findings, estimates of HIV prevalence for MSM were compiled for countries in the Asia and the Pacific region from the UNAIDS Key Population Atlas [22]. HIV prevalence estimates are reported by countries to UNAIDS based on findings from bio-behavioral surveillance surveys and may reflect sub-national estimates, rather than national estimates.
Statistical Analysis
All variables were assessed for biases related to RDS assumptions using convergence and bottleneck plots and homophily values [23] in RDS Analyst (www.hpmrg.org), an open-source software based in R program with a graphical user interface specific to RDS analysis [21]. All analyses were conducted using the Gile successive sampling estimator in RDS Analyst using the following cascade of questions to measure network size: (1) “How many males do you know and they know you who are between the ages of 15–24 years who had anal sex with a male in the past six months?”, (2) How many of them live, work, or study in Bandung?”, and (3) How many of them have you seen in the last 2 weeks? The response to the third question was used as the participant’s social network size. Bivariate regression to assess associations between socio-demographic and behavioral variables with HIV status was conducted in STATA v.13 using exported successive sampling weights.
Variables associated with HIV status (p < 0.2) in bivariate analysis or considered important confounders were included in the multivariable model. Adjusted odds ratios (aOR) and 95% confidence intervals (CI) are presented in the final model. Missing values were omitted from the analyses.
Results
Characteristics of Young MSM
Over the course of 34 days, 211 young MSM were sampled. The final sample had three seeds and a maximum number of ten recruitment waves. All variables reached convergence in advance of the final estimates, had neither high homophily nor heterophily and resulted in no bottlenecks. Baseline characteristics are presented in Table 1. Most young MSM are between the ages of 20–24 years old and were employed. Equal proportions of respondents (half) reported having a steady and/or casual partners. Two-thirds used a condom during their last anal sex with steady, casual and paying partners. Roughly one-third have comprehensive knowledge of HIV and report knowing that their steady or casual partners have other partners. One third received money for sex. Equal percentages (one-third) preferred receptive intercourse, insertive intercourse or had no preference for either position. Among half of young MSM reporting alcohol use, two-thirds had sexual intercourse while intoxicated. Of the few that use drugs, the majority used sedatives or cannabis. Just 13% have disclosed that they had sex with men to their families, among which one-third experience stigma and discrimination from friends or family. One fifth experienced sexually transmitted infections symptoms in the past year. Thirty percent tested HIV antibody positive. Among all young MSM, 13% knew their HIV status in advance and 17% learned their HIV status during the survey.
Table 1.
Characteristics of young MSM (total and by HIV status) in Bandung, Indonesia
| Total young MSM = 211 | HIV negative young MSM = 146 | HIV positive Young MSM = 63 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n/N | % | % (95% CI) | n/N | % | 95% CI | n/N | % | 95% CI | |
| Socio-demographic characteristics | |||||||||
| Median age (mean, median, range) | 20.6, 21 (15–24) | 20, 22 (15–24) | 22, 23(17–24) | ||||||
| Age group | |||||||||
| 15–19 | 75/209 | 32.4 | 25.3, 40.4 | 68 | 41.7 | 32.3, 51.7 | 6 | 11.0 | 3.9, 27.1 |
| 20–24 | 134/209 | 67.6 | 59.6, 74.7 | 76 | 58.3 | 48.3, 67.7 | 57 | 89.0 | 72.9, 96.1 |
| Currently enrolled in school | 77/205 | 37.4 | 30, 44.9 | 60 | 39.9 | 30.4, 50.2 | 17 | 32.8 | 19.4, 49.7 |
| Currently employed | 143/209 | 64.8 | 56.0, 72.7 | 93 | 62.8 | 52.3, 72.3 | 48 | 68.4 | 51.3, 81.6 |
| Sexual identity | |||||||||
| Homosexual | 115/211 | 56.5 | 48.1, 64.6 | 76 | 54.7 | 44.5, 64.6 | 38 | 61.8 | 46.6, 75.0 |
| Bisexual | 87/211 | 38.5 | 30.7, 46.8 | 61 | 38.4 | 29.2, 48.6 | 25 | 38.2 | 25.0, 53.4 |
| Other | 9/211 | 5 | 2.3, 10.7 | 7 | 6.9 | 3.0, 15.0 | 0 | ||
| Alcohol and substance use | |||||||||
| Alcohol past 6 months | 105/211 | 48.7 | 40, 57.5 | 77 | 51.2 | 41.0, 61.4 | 26 | 41.5 | 27.3, 57.3 |
| Drug use (ever) | 29/209 | 15.4 | 10.3, 22.5 | 22 | 16.6 | 10.5, 25.2 | 5 | 10.7 | 3.7, 27.0 |
| Injectable drugs among those who have used drugs | 2/29 | 5.3 | 1.1, 21.5 | 0 | 1 | 9.7 | 0.01, 55.3 | ||
| Knowledge and perceptions | |||||||||
| Ever received information on HIV prevention | 154/202 | 78.8 | 71.3, 84.8 | 98 | 72.8 | 64.4, 81.1 | 54 | 91.8 | 86.2, 97.6 |
| Self-perceived HIV risk (high or some) | 115/194 | 64 | 55.5, 71.8 | 79 | 59.0 | 48.9, 68.5 | 35 | 78.1 | 63.3, 88.1 |
| Comprehensive knowledge of HIV | 63/211 | 33.7 | 25.9, 42.6 | 29 | 23.2 | 15.2, 33.9 | 34 | 59.2 | 44.0, 72.7 |
| HIV risk behaviour | |||||||||
| Age of first anal sex (mean, median, range) | 18, 18 (8–24) | 17.8, 18 (8–24) | 18.3, 18 (12–22) | ||||||
| Type of sex partner (past year) | |||||||||
| Non-paying, steady partner | 112/209 | 52.5 | 44.4, 60.9 | 59 | 37.9 | 28.6, 48.3 | 52 | 85.8 | 74.4, 92.7 |
| Non paying, non-regular, casual partner | 97/209 | 49.2 | 40.7, 57.7 | 64 | 46.5 | 36.5, 56.8 | 31 | 54.1 | 38.8, 68.6 |
| Transgender | 48/209 | 24.3 | 17.9, 32.1 | 45 | 32.4 | 23.8, 42.4 | 3 | 6.0 | 1.8, 18.3 |
| Exchange of sex for resources | |||||||||
| Paid for sex | 34/209 | 16.9 | 11.4, 24.5 | 28 | 19.9 | 12.9, 29.2 | 5 | 10.2 | 3.4, 26.7 |
| Received payment for sex | 74/203 | 31.4 | 24.2, 39.6 | 52 | 32.2 | 23.7, 42.1 | 20 | 27.8 | 15.8, 44.0 |
| On-line/social media client identificationb | 43/74 | 61 | 46.2, 73.6 | 26 | 47.3 | 31.5, 63.7 | 15 | 92.4 | 74.1, 97.5 |
| Preferred sexual position | |||||||||
| Insertive | 74/209 | 35 | 27.4, 43.5 | 59 | 40.7 | 31.1, 51.1 | 14 | 22.1 | 11.8, 37.5 |
| Receptive | 76/209 | 33.7 | 26.2, 42.1 | 46 | 28.5 | 20.4, 38.4 | 30 | 46.5 | 31.8, 61.8 |
| No preference | 59/209 | 31.3 | 23.8, 39.7 | 39 | 30.8 | 21.9, 41.3 | 19 | 31.4 | 19.2, 46.9 |
| Sexual violence | |||||||||
| First sexual experience forced | 60/185 | 35.7 | 27.5, 44.9 | 41 | 36.5 | 26.4, 47.9 | 17 | 32.6 | 19.6, 49.0 |
| Used condom at last sexa | |||||||||
| Steady partner | 71/111 | 66.2 | 54.3, 76.4 | 35 | 60.2 | 43.1, 75.1 | 35 | 71.7 | 54.3, 84.3 |
| Casual partner | 66/97 | 67.4 | 54.7, 78.0 | 41 | 62.1 | 45.8, 76.1 | 23 | 76.0 | 56.1, 88.7 |
| Paying partner | 47/72 | 66.9 | 53.0, 78.4 | 28 | 60.7 | 44.0, 75.1 | 17 | 79.5 | 51.4, 93.4 |
| Transgender partner | 23/48 | 49.5 | 35.3, 64.2 | 20 | 45.6 | 29.0, 63.2 | 3 | 100.0 | |
| Partner concurrencea | |||||||||
| Steady partner has other partners | 16/71 | 29.5 | 17.1, 42.6 | 11 | 33.5 | 18.6, 52.7 | 5 | 27.3 | 10.6, 54.3 |
| Casual partner has other partners | 36/95 | 37.9 | 27.1, 48.7 | 25 | 44.4 | 29.6, 60.3 | 10 | 25.2 | 12.3, 44.8 |
| Sex while under the influence of alcohol | 70/211 | 34.5 | 26.9, 42.8 | 53 | 37.6 | 28.4, 47.8 | 15 | 26.0 | 14.3, 42.5 |
| Sexually transmitted infections | |||||||||
| STI symptoms in past 12 months | 47/209 | 21.4 | 15.6,28.6 | 29 | 20.8 | 13.8, 30.1 | 17 | 21.6 | 12.8, 34.2 |
| Stigma and discrimination | |||||||||
| Family knows participant has sex with males | 29/209 | 13.1 | 8.3, 19.7 | 14 | 11.1 | 5.8, 20.2 | 15 | 17.7 | 9.9, 29.8 |
| Experience of aversion from family/friends | 11/29 | 41.9 | 20.4, 67.0 | 4 | 46.3 | 16.6, 78.8 | 7 | 35.7 | 14.2, 65.1 |
| Experience of aversion from health staff | 9/209 | 2.9 | 1.4, 5.9 | 5 | 2.3 | 1.7, 4.4 | 3 | 3.6 | 0, 7.7 |
| Access to HIV prevention services | |||||||||
| Received counselling on condom use/safe sex in past 3 months | 86/195 | 42.5 | 34.1, 51.4 | 53 | 38.5 | 28.7, 49.4 | 31 | 50.1 | 34.8, 65.4 |
| Received HIV test and test results in the last 12 months | 63/211 | 28.6 | 21.7,36.6 | 35 | 23.8 | 16.1, 33.6 | 26 | 38.1 | 24.9, 53.4 |
| Tested for sexually transmitted infections in past three months | 48/196 | 22.1 | 16.0, 29.8 | 27 | 16.5 | 10.5, 25.0 | 19 | 34.2 | 21.0, 50.5 |
| HIV PREVALENCE | |||||||||
| Total | 63/211 | 30.3 | 22.2, 38.4 | ||||||
aAmong those who had respective partners in the last 12 months
bAMONG those who sold anal sex for money or goods in past year
Associations with HIV Positivity
The risk factor analysis is presented in Table 2. HIV antibody positive young MSM have higher odds of being older (20–24 years old versus 15–19 years old), having received information on HIV, having higher comprehensive knowledge, perceiving themselves to have some or high risk of infection, having a steady partner, using on-line resources to identify clients to exchange sex for resources, preferring the receptive position during anal sex, having test for an sexually transmitted infections in the past three months and lower odds of having had sex with a transgender person in the previous year.
Table 2.
Bivariate and multivariate analysis of the association between study variables and HIV infection among young MSM, Bandung, Indonesia
| Bivariate | Multivariate | |||
|---|---|---|---|---|
| OR | 95% CI | Adjusted OR | 95% CI | |
| Socio-demographic characteristics | ||||
| Median age (mean, median, range) | 1.5*** | 1.22, 1.86 | 1.38** | 1.12, 1.70 |
| Age group | ||||
| 15–19 | Ref | |||
| 20–24 | 5.82** | 1.79, 18.89 | ||
| Currently enrolled in school | 0.73 | 0.32, 1.67 | ||
| Currently employed | 1.28 | 0.55, 2.96 | ||
| Knowledge and perceptions | ||||
| Ever received information on HIV prevention | 4.27** | 1.60, 11.41 | ||
| Self-perceived HIV risk (high or some) | 2.45* | 1.06, 5.67 | ||
| Comprehensive knowledge of HIV | 4.79*** | 2.14, 10.72 | ||
| HIV risk behaviour | ||||
| Type of sex partner (past year) | ||||
| Non-paying, steady partner | 9.92*** | 4.27, 23.06 | 6.01*** | 2.37,15.20 |
| Transgender | 0.13** | 0.04, 0.50 | ||
| Preferred sexual position | ||||
| Insertive | Ref | Ref | ||
| Receptive | 3.01* | 1.17, 7.71 | 3.02* | 1.10, 8.33 |
| No preference | 1.88 | 0.70, 5.06 | 1.82 | 0.56, 5.91 |
| Access to hiv prevention services | ||||
| Tested for sexually transmitted infections in past three months | 2.63* | 1.12, 6.19 | ||
*p < 0.05
**p < 0.01
***p < 0.001
In the multivariable model, being 20–24 years old, having a steady partner and preference for the receptive position are associated with HIV positivity. No differences were observed in a sub-analysis comparing young MSM who had prior knowledge of their HIV antibody positive status to those who did not (data not shown).
Asia and the Pacific Country Comparison
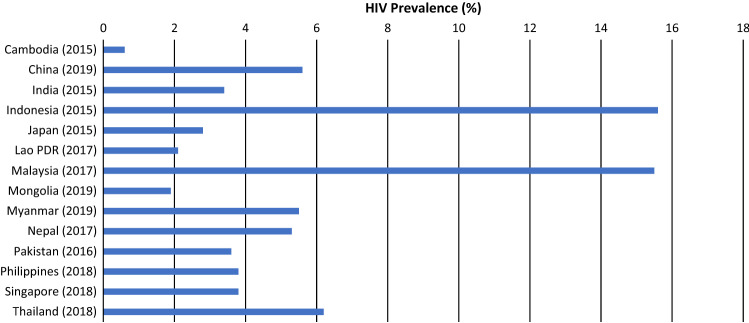
To contextualize our findings, we compiled estimates of HIV prevalence among MSM from 26 countries in Asia and the Pacific (web appendix). Figure 1 presents data reported by 18 countries in the region where recent national estimates of HIV prevalence among young MSM are available (2015 onwards). HIV prevalence averaged (unweighted) 6.2% for all MSM, and 3.9% for young MSM. Prevalence among MSM aged 25 years or older was 2.2-fold higher than for young MSM.
Fig. 1.
HIV prevalence among young MSM (15–24 years old) in Asia and the Pacific Region*
Discussion
Nearly one third of young MSM in Bandung city are living with HIV, which is among the highest prevalence reported globally for this age group. HIV prevalence among young MSM in Bandung is nearly two-fold higher than reported prevalence for MSM of all ages from a national assessment conducted during approximately the same period, and 100-fold higher than national prevalence estimates among the general population. With stable or declining prevalence in other key population groups, high HIV prevalence among young MSM reflects recent infections which likely contribute disproportionately to the country’s HIV epidemic [15]. Indonesia’s escalating HIV infections among young MSM take place on a backdrop of relatively flat trends for MSM across Asia and the Pacific, with prevalence estimates observed in this study seven-fold higher than regional averages from countries where recent data are available.
Few socio-demographic or behavioral factors explain differences between HIV antibody positive and negative young MSM. Young MSM older than 20 years, those with a steady partner and who prefer the receptive position during sex were at higher risk of HIV infection. Findings regarding higher levels of knowledge, self-perceived risk and testing for sexually transmitted infections which were significant in the unadjusted analysis only are likely mediated by the effects of age and/or HIV status. Our findings related to low comprehensive HIV knowledge, low drug and alcohol use, high perception of infection risk and high levels of condom use among young MSM are largely consistent with recent national estimates for MSM of all ages [15].
Partner concurrency and sexually transmitted infections may play an important role in transmission. While not associated with HIV antibody positivity in our study, one-third of young MSM reported their steady or casual partners had other partners. Our findings that one-fifth of young MSM experienced sexually transmitted infections symptoms in the past 6 months align with recent biological estimates of high rates of gonorrhea (18.7%), chlamydia (27.9%) and syphilis (9.6%) among MSM country-wide [15]. Two thirds of young MSM reported condom use at last sex with steady or casual partners.
The relative absence of individual-level associations points to the role of wider structural factors in shaping the risk environment for young MSM [24]. Among the just 13 percent of young MSM were open with friends and family members about their sexual identity, close to half reported experiencing aversion from these groups. Recent qualitative research in Indonesia highlights pervasive bullying of sexual and gender minority youth in school settings [25], with concealment of sexual identity among MSM being an important contributor to high-risk behavior [26, 27]. These findings are re-enforced by an assessment of levels of acceptance of homosexuality among 34 countries, where Indonesia is ranked consistently near the bottom [28]. Persistent calls for criminalization of homosexuality and police crackdowns on MSM are a serious concern [29].
Structural barriers also limit access to critical testing and support services. Despite high perceived HIV risk, less than one third of young MSM had an HIV test in the past year which is half the national levels for all MSM [15]. This may be partly due to legal requirements for parent or guardian consent for those under 18 years old and conflicting laws and regulations that are punitive towards MSM including the criminalization of HIV transmission, exposure, and nondisclosure of HIV status [30]. Fortunately, young MSM interacting with the health sector report experiencing little stigma and discrimination. Among young MSM aware of their HIV antibody positive status in our study almost all had initiated anti-retroviral therapy. Pre-exposure prophylaxis (PrEP) has not yet been widely implemented in Indonesia.
While this study is among the first RDS assessments of young MSM in Indonesia, there are several limitations. First, respondents in Bandung do not represent young MSM in all urban centers. However, among six major cites included in Indonesia’s 2015 national assessment, HIV prevalence among MSM (all ages) in Bandung was in the middle range at 28% (range 13.2–36.9%). Comparisons with other existing data from the region should be interpreted with some caution as they were collected during different time periods and many used non comparable sampling methods, including convenience sampling. Second, the projected sample size of 300 respondents was not reached, potentially because it was too large relative to the young MSM population. Nonetheless, population saturation appears to have been reached near survey completion. Although young MSM were given a number to call to get their HIV test results, many did not. This is especially concerning since many were found to be living with HIV still be unaware.
Conclusion
In summary, urgent health, social and legal measures are required to improve access to essential services and address the structural drivers of accelerating HIV transmission among young MSM in urban Indonesia. These include improving national and sub-national coordination to ensure counselling, testing and treatment are widely available including the removal of age-related restrictions; sexually transmitted infections screening and management; accelerating efforts to pilot and scale PrEP; mobilizing peer outreach and referrals; optimizing the use of social media; enhancing cross-ministerial efforts between health and education to strengthen school-based primary prevention efforts to improve sexual health awareness; revising legal frameworks to reduce stigma and discrimination; and responding to the mental health and psycho-social needs of young MSM.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We appreciate the support of Indonesia’s National Ministry of Health and Provincial Ministry of Health of West Java for their assistance in carrying out this study. We also acknowledge the contributions of Rudi Wisaksana, Zahrotur Rusyda Hinduan, Mawar Nita Pohan, Eka Riyanti Purboningsih, Reynie Purnama Raya and Agnes R. Indrati from the University of Padjadjaran who assisted with the field and laboratory work. This work was supported by UNAIDS UBRAF funding 2018-2019.
Author Contributions
LGJ, ASW, TAP, FFR, RAN, AC, PMP designed the study, oversaw field work and reviewed relevant literature. LJ and PS performed the analysis. PMP and LGJ led the drafting of the manuscript. All authors supported interpretation of results. All authors have read and approved the final manuscript.
Funding
This study was partially funded through UNICEF.
Declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical Approval
This research was approved by the Research Ethics Committee, University of Padjadjaran 2019 (08/UN6.C10/PN/2019). All subjects provided informed consent to participate in interviews and undergo biological sampling. Informed consent from the parents of participants under 18 years old were obtained.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.UNAIDS. UNAIDS Data 2019. Geneva; 2019.
- 2.AIDSDataHub. Men who have sex with men slides 2019 [Internet]. 2019. Available from: https://www.aidsdatahub.org/resource/men-who-have-sex-men-msm-slides. Accessed 10 Jan 2021.
- 3.Mao X, Wang Z, Hu Q, Huang C, Yan H, Wang Z, et al. HIV incidence is rapidly increasing with age among young men who have sex with men in China: a multicentre cross-sectional survey. HIV Med. 2018;19(8):513–522. doi: 10.1111/hiv.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thienkrua W, Van GF, Mock PA, Dunne EF, Wimonsate W, Howteerakul N, et al. Young men who have sex with men at high risk for HIV, Bangkok MSM cohort study, Thailand 2006–2014. AIDS Behav. 2018;22(7):2137–2146. doi: 10.1007/s10461-017-1963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chemnasiri T, Beane CR, Varangrat A, Chaikummao S, Chitwarakorn A, Van GF, et al. Risk behaviors among young men who have sex with men in Bangkok: a qualitative study to understand and contextualize high HIV incidence. AIDS Behav. 2019;66(4):533–548. doi: 10.1080/00918369.2017.1422941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai Y, Musumari PM, Chen H, Huang Y, Techasrivichien T, Suguimoto SP, et al. Recreational drug use, polydrug use and sexual behaviors among men who have sex with men in Southwestern China: a cross-sectional study. Behav Med. 2019;45(4):314–322. doi: 10.1080/08964289.2018.1538099. [DOI] [PubMed] [Google Scholar]
- 7.Dong MJ, Peng B, Liu ZF, Ye QN, Liu H, Lu XL, et al. The prevalence of HIV among MSM in China: a large-scale systematic analysis. BMC Infect Dis. 2019;19(1):1–20. doi: 10.1186/s12879-018-3567-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunne EF, Pattanasin S, Chemnasiri T, Varangrat A, Raengsakulrach B, Wichuda S, et al. Selling and buying sex in the city: men who have sex with men in the Bangkok men who have sex with men cohort study. Int J STD AIDS. 2019;30(3):212–222. doi: 10.1177/0956462418796440. [DOI] [PubMed] [Google Scholar]
- 9.Xu W, Zheng Y, Wiginton JM, Kaufman MR. Alcohol use and binge drinking among men who have sex with men in China: Prevalence and correlates. Drug Alcohol Depend. 2019;202(2019):61–68. doi: 10.1016/j.drugalcdep.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Holtz T, Wimonsate W, Mock P, Pattanasin S, Chonwattana W, Thienkrua W, et al. Why we need pre-exposure prophylaxis: incident HIV and syphilis among men, and transgender women, who have sex with men, Bangkok, Thailand, 2005–2015. Int J STD AIDS. 2019;30(5):430–439. doi: 10.1177/0956462418814994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong JJ, Liao M, Lee A, Fu H, Pan SW, Tang W, et al. Bridging the HIV-syphilis testing gap: dual testing among men who have sex with men living in China. Sex Transm Infect. 2019;95(4):251–253. doi: 10.1136/sextrans-2018-053527. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Wu G, Lu R, Xia W, Hu L, Xiong Y, et al. What has changed HIV and syphilis infection among men who have sex with men (MSM) in Southwest China: a comparison of prevalence and behavioural characteristics (2013–2017) BMC Public Health. 2019;19(1):1–11. doi: 10.1186/s12889-018-6343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. UN-Interagency-Task-Team-on-Young-People. Preventing HIV/AIDS in young people: a systematic review of the evidence from developing countries. Geneva; 2006.
- 14.Ministry-of-Health. HIV Integrated Biological and Behavioral Surveillance Survey among the general population in Tanah Papua, Indonesia. Jakarta; 2014.
- 15.Ministry-of-Health. Integrated biological and behavioral surveillance (2019–2020). Jakarta; 2020.
- 16.UNICEF. HIV among Adolescent and Young Key Populations (Aged 15–19 and 20–24) in Indonesia. Jakarta; 2018.
- 17.Rao A, Stahlman S, Hargreaves J, Weir S, Edwards J, Rice B, et al. Sampling key populations for HIV surveillance: results from eight cross-sectional studies using respondent-driven sampling and venue-based snowball sampling. JMIR Public Heal Surveill. 2017;3(4):e72. doi: 10.2196/publichealth.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UNICEF. Integrated Biological-Behavioral Surveillance Survey Among Adolescent and Young People Who Inject Drugs, Female Sex Workers, Males Who Have Sex With Males and Male to Female Transgender Persons [Internet]. 2021. Available from: https://www.unicef.org/indonesia/reports/growing-hiv-epidemic-young-msm-indonesia. Accessed 23 Mar 2021.
- 19.Johnston LG, Sabin K. Sampling hard-to-reach populations with respondent driven sampling. Methodol Innov Online. 2010;5(2):38–48. [Google Scholar]
- 20.Heckathorn DD. Extensions of respondent driven sampling: analysing continuous variables and controlling for differential recruitment. Sociol Methodol. 2007;37(1):151–207. doi: 10.1111/j.1467-9531.2007.00188.x. [DOI] [Google Scholar]
- 21.UNAIDS. Indicators for monitoring the 2016 Political Declaration on Ending AIDS — Global AIDS Monitoring 2021. Geneva, Switzerland; 2021. Available from: https://www.unaids.org/sites/default/files/media_asset/global-aids-monitoring_en.pdf. Accessed 1 May 2021.
- 22.UNAIDS. UNAIDS Key Population Atlas [Internet]. 2020. Available from: https://kpatlas.unaids.org/dashboard. Accessed 17 Mar 2021.
- 23.Gile KJ, Handcock MS. Respondent-driven sampling: an assessment of current methodology. Sociol Methodol. 2010;40(1):285–327. doi: 10.1111/j.1467-9531.2010.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker RG, Easton D, Klein CH. Structural barriers and facilitators in HIV prevention: a review of international research. AIDS. 2000;14(Supl 1):S22–32. doi: 10.1097/00002030-200006001-00004. [DOI] [PubMed] [Google Scholar]
- 25.Morineau G, Nugrahini N, Riono P, Nurhayati GP, Mustikawati DE, et al. Sexual risk taking STI and HIV prevalence among men who have sex with men in six Indonesian cities. AIDS Behav. 2011;15(5):1033–1044. doi: 10.1007/s10461-009-9590-6. [DOI] [PubMed] [Google Scholar]
- 26.Fauk NK, Merry MS, Sigilipoe MA, Putra S, Mwanri L. Culture, social networks and HIV vulnerability among men who have sex with men in Indonesia. PLoS ONE. 2017;12(6):1–14. doi: 10.1371/journal.pone.0178736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UNICEF. Formative Assessment of Needs for Adolescents and Youth at Risk of HIV in East Asia and the Pacific. 2019.
- 28.Poushter J, Kent NO. The global divide on homosexuality persists: but increasing acceptance in many countries over the past two decades [Internet]. 2020. Available from: https://www.pewresearch.org/global/2020/06/25/global-divide-on-homosexuality-persists/. Accessed 10 Jan 2021
- 29.Wieringa SE. Criminalisation of homosexuality in Indonesia: the role of the Constitution and Civil Society. Aust J Asian Law. 2019;20(1):227. [Google Scholar]
- 30.The Global Fund. Baseline assessment Indonesia: Scaling up programs to reduce human rights-related barriers to HIV and TB services [Internet]. Geneva; 2019. Available from: https://www.theglobalfund.org/media/8721/crg_humanrightsbaselineassessmentindonesia_report_en.pdf?u=637165999520000000. Accessed 10 Jan 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.