With more than 225 million cases of SARS-CoV-2 infection and more than 4,650,000 deaths from COVID-19 worldwide, identifying those individuals at risk of severe disease becomes an incredibly valuable pursuit when resources are limited and medical care is costly. Several COVID-19 risk measures, including the Veterans Health Administration COVID-19 (VACO) Index for COVID-19 Mortality,1 have demonstrated substantial predictive capacity for severe COVID-19 and death. This well-validated index utilizes age, sex, and preexisting comorbidities to estimate risk of 30-day mortality in inpatients and outpatients after SARS-CoV2 infection. COVID-19 has enabled the scientific community to reexamine risk stratification with a comprehensive vision of disease susceptibility that leverages deep immunologic data.
In the November 2021 issue of the Journal of Allergy and Clinical Immunology, Lee et al described a novel attribute, termed immunologic resilience (IR), to predict COVID-19 disease severity and death independent of age, while also informing outcomes for individuals with other infectious and noninfectious diseases.2 The mechanistic rationale for this marker rests on the theory that survival and complications of infectious diseases are dependent on the ability of the host to effectively eliminate pathogens (ie, immunocompetence [IC]) while controlling the associated inflammatory cascade. Thus, those individuals with the highest levels of IC and the lowest levels of inflammation (IF) (IChigh-IFlow) possessed an optimal IR, correlating with survival and milder COVID-19. Their data suggest that IR states, which precede infection and are altered in response to it, influence COVID-19 outcomes. Moreover, these IR attributes were associated with mortality in the Framingham Heart Study and provided an explanation for sex-based differences in health and survival outcomes.
To initially characterize IR, the investigators proposed 4 immune health grades (IHGs) and evaluated their distribution within 10 non–COVID-19 cohorts (N = 13,389 patients) with a diverse history of antigenic (infectious) exposures. These cohorts include the Framingham cohort, SardiNIA cohort, several influenza cohorts, a lupus cohort, the US Military HIV Natural History Study, and health care workers exposed to SARS-CoV-2. Thereafter, the IHGs were applied to 522 hospitalized and nonhospitalized patients with SARS-CoV-2 infection. The primary end point was 30-day mortality; 120-day mortality, hospitalization, length of stay, respiratory progression, and several biomarkers were examined as robust secondary end points.
The framework for IHGs was based on the principle that the equilibrium between peripheral blood CD8+ and CD4+ T-cell counts is a more precise metric of IR than either alone. The metric uses a CD4+/CD8+ ratio stratified at 1.0, followed hierarchically by the CD4 count at 800 cells/μL, with further substratifications below this threshold, resulting in 8 IHG categories. IHG-I was designated as the ideal IR category, with a ratio of 1.0 or higher and a CD4 count of at least 800 cells/μL. This group was the most common overall, and it was overrepresented in the SardiNIA cohort, women, individuals with asymptomatic HIV infection, and individuals with nonsevere COVID-19. Compared with its prevalence in the SardiNIA cohort, IHG-I was substantially underrepresented (21% vs 73% [P < .001]), whereas IHG-II and IHG-IV were substantially overrepresented (64% vs 22% and 13% vs 3%, respectively [P < .001]) among patients with COVID-19. Although IHG-I appears to decline with age in the SardiNIA cohort, this decline is speculated to be driven by life exposure to pathogens rather than by chronologic aging (Fig 1 , A). In addition to pathogen exposure, other environmental (eg diet, radiation) and genetic (ie intrinsic capacity) factors influence the rate of this decline. If correct, this may suggest, as Lee et al2 note, that age is an imperfect proxy for antigenic experience, and it raises the possibility that a metric for antigenic life history might be more informative for disease trajectory than chronologic age alone. The degradation of IHG-I was generally associated with chronologic aging and may in part reflect immunosenescence—both as a generic decline in immune function with aging and as an outcome of cellular senescence. From this perspective, chronic exposure to antigens driving loss in IHG-I may represent a novel “gerodriver” that has been underappreciated in the field of geroscience and especially in the context of immune aging. Because the SardiNIA cohort is a somewhat genetically homogeneous population, albeit well-characterized immunologically,3 it will be essential to “pressure-test” the generalizability of the IHGs in demographically distinct populations.
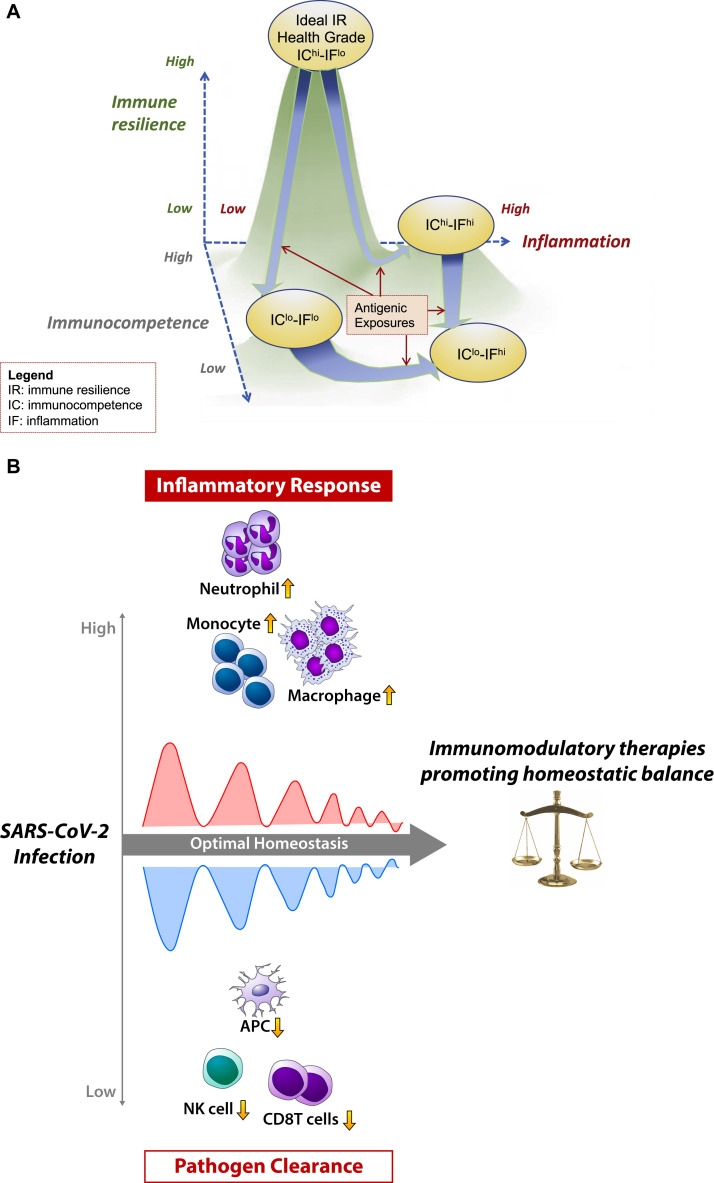
Fig 1.
A, This 3-dimensional model is a depiction of immune resilience (IR) as a composite landscape of immunocompetence (IC) and inflammation (IF). In the landscape, a history of antigenic exposures is represented as arrow trajectories that flow along the contours of this landscape. Over the life course, these trajectories are also influenced by genetic factors and the environment. In terms of clinical interpretation, those individuals with the highest levels of IC and the lowest levels of IF possess the greatest immune resilience within a given population (ie, the ideal IHG of IChi-IFlo). Conversely, those individuals with the lowest levels of IC and the highest levels of IF (ie, IClo-IFhi) would be at greatest risk of complications from infectious diseases, including COVID-19, HIV-1, and influenza. B, Dynamic balance model of immunomodulation. The arrow and direction of the wave form depict an idealized dynamic transition in a person with severe COVID-19 at the leftmost time point, who then improves with therapy toward the right side (homeostatic balance). The cellular changes in severe COVID-19 align with decreased IC driven by reduced antigen-presenting dendritic cells and reduced NK cells (lower waves). Increased viral load–induced IF is driven by neutrophils, monocytes, and macrophage cells (upper waves). Additional aspects of COVID-19 pathogenesis, including the humoral response and coagulation cascade, are not featured in this model. Immune modulating interventions for COVID-19 will need to balance the reduction in sequelae from an increased inflammatory burden while maintaining robust viral clearance pathways.
In the COVID-19 cohort, it is conceivable that IHGs before infection may differ from the IHGs observed at hospitalization. Convalescent T-cell counts appeared to recover for most patients, except for those in the IHG-I group. This implies that IHGs dictate the trajectory of early infection (predicated on IC) and are altered by infection that subsequently influences outcomes (via IF). These data are encouraging because with convalescent return to IHG-I, a critical litmus test is met for a resilience phenotype. Those individuals with the least resilient IHG categories at presentation tended to be older, multimorbid, and cytomegalovirus seropositive. Interestingly, the duration from symptom onset to presentation did not differ by baseline IHG. Mortality at day 30, independent of age and sex, was substantially reduced for those with IHG-I at presentation compared with for those with all other grades (hazard ratio = 0.12 [95% CI = 0.02-0.87]), a finding that held up at 120 days. Finally, sex-based differences in COVID-19 outcomes appear to be largely explained by IHG categories.
Peripheral blood transcriptomic analyses were performed on patients with COVID-19, patients with influenza, and patients in the Framingham cohort to identify mechanistic associations with IHGs, mortality, and disease severity. A high-IC state was associated with traits linked to T-cell costimulation, signaling, and homeostasis, whereas high IF was associated with type I and type II interferon signaling and other antiviral responses. Overall, these gene expression states tracked with survival, mechanical ventilation, age, and IHG category.
The finding that autoantibodies against type I interferons and related dysfunction in interferon receptor signaling in responsive neutrophils can cause increased risk of SARS-CoV-2 underscores the importance of IR.4 These results are consistent with key observations of dysfunctional neutrophils, excess immature monocytes, and dysfunctional natural killer (NK) cells along with a reduction in antigen-presenting dendritic cells in patients with severe COVID-195 (Fig 1, B). Evaluation of sera from patients with a range of mild to severe cases of COVID-19 revealed that systemic biomarkers correlated with these aberrant cells were dysregulated. Furthermore, changes in these biomarkers correlated with changes in ordinal scale, suggesting a hyperinflammatory stage that leads to severe complications in COVID-19. These markers included cytokines such as IL-6 and MCP-3, and their signaling may be attenuated by interventions such as corticosteroids, JAK inhibitors, and IL-6 blockade. Notably, in these patients, the levels of serum markers associated with robust antigen-presenting dendritic cells (eg, TRANCE, SCF, FLT3L) were reduced, whereas the levels of markers associated with vascular IF (eg, CTSL1, PTX-3, IL-6) were increased.6
The viral expansion phase in COVID-19 occurs before symptom presentation and continues as patients progress to more severe disease states. IR was robust in patients who experienced mild symptoms. One feature of innate immunity is a subset of NK cells expressing KLDR1 that respond to the influenza virus. The presence of these cells may predict increased IR and protect from severe symptoms in influenza.7 The observation that serum level of SCF was significantly reduced in patients with severe COVID-19 may suggest that early NK cell development is compromised in patients with severe disease. Collectively, these findings support an increased role played by the viral load–driven increase in inflammatory cytokines in patients with moderate-to-severe COVID-19. Anti-inflammatory interventions for COVID-19 will be tasked with balancing the reduction in sequelae from the increased inflammatory burden while maintaining robust viral clearance pathways. This delicate balance is required to engage crucial adaptive responses that offer long-term protection.
The opportunity to survey biomarkers and sensitive whole body viral load assessments such as droplet PCR, from vaccinated individuals with breakthrough infections and compare to unvaccinated individuals exposed to SARS-CoV-2 may help explore the importance of vaccination in IR. These longitudinal analyses may converge on features of the immune system and serve as a bellwether for a variety of infections across populations.
Because ideal IR was exemplified by IHG-I and an IChigh-IFlow state and because transcriptomic data supported the transition from IHG-I to other states independently of age, it will be interesting to see whether geroscience-focused clinical studies (eg, the TAME trial and others) benefit from measuring associations between IHGs and comorbid burden. Importantly, although the Lee et al2 have begun cross-validating their scoring system with earlier immune age signatures (eg, IMM-AGE),8 it will be important to see how the IHG system complements newer approaches (eg, iAGE).9
A key objective going forward will be to better understand antecedent conditions that lead to the differing IHG states. Because collection of samples was initiated only at presentation to the hospital, inference of IR before infection is difficult, which will likely necessitate longitudinal sampling of other ongoing COVID-19 natural history studies that include preinfection sampling and, ideally, deep immune phenotyping, including via systems serotyping (which was not conducted in the study by Lee et al2). Also, prior studies have identified epigenetic imprinting, termed trained immunity, in response to prior infections.10 Whether historic antigenic exposure trains IHGs will be important to ascertain in future studies. Because the primary outcomes were 30-day mortality, whether IHGs are informative for postacute COVID-19 sequelae is currently unclear. Finally, whether it will be possible to find pathogen-specific signatures that inform treatment of patients toward an IHG-I state remains to be determined.
Beyond COVID-19, there remains a need for a contextual holistic understanding of immunologic vulnerability that could explain the heterogeneity observed in multiple disease manifestations throughout the aging process. In this elegant study by Lee et al,2 immunologic resilience was defined as a capacity to preserve and restore IC and to control IF in the face of pathogenic challenges throughout life, with the IChigh-IFlow phenotype as a hallmark of resilience. As such, the IHG system is a pragmatic clinical tool for staging, monitoring, differential diagnosis, and (potentially) directed therapies. The study by Lee et al2 provides a new lens for understanding immune aging in terms of immunologic life history, and further, for understanding that resilience in the face of these exposures can be categorized in terms of health grades. This newly defined dialectic interaction between IC and IF is an immunologic resilience fitness landscape that is shaped by a person's or population's history of antigenic exposure and is a testable model that will no doubt influence the discussion and interpretation of pathogenic trajectories and treatment strategies going forward.
Footnotes
Supported by the Emory Center for AIDS Research (grant P30AI050409 [to V.C.M.]), Boston Pepper Center (grant P30AG031679 [to M.M.]), and Harvard University Center for AIDS Research (grant P30AI060354 [to M.M.]).
Disclosure of potential conflict of interest: V. C. Marconi has received investigator-initiated research grants (to his institution) and consultation fees (both unrelated to the current work) from Eli Lilly, Bayer, Gilead Sciences, and ViiV Healthcare. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.King J.T., Jr., Yoon J.S., Rentsch C.T., Tate J.P., Park L.S., Kidwai-Khan F., et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee G.C., Restrepo M.I., Harper N., Manoharan M.S., Smith A.M., Meunier J.A., et al. Immunologic resilience and COVID-19 survival advantage. J Allergy Clin Immunol. 2021;148:1176–1191. doi: 10.1016/j.jaci.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramos P.S. Unravelling the complex genetic regulation of immune cells. Nat Rev Rheumatol. 2021;17:131–132. doi: 10.1038/s41584-020-00563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Combes A.J., Courau T., Kuhn N.F., Hu K.H., Ray A., Chen W.S., et al. Global absence and targeting of protective immune states in severe COVID-19. Nature. 2021;591:124–130. doi: 10.1038/s41586-021-03234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathew D., Giles J.R., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E., et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369 doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sims J.T., Krishnan V., Chang C.Y., Engle S.M., Casalini G., Rodgers G.H., et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J Allergy Clin Immunol. 2021;147:107–111. doi: 10.1016/j.jaci.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bongen E., Vallania F., Utz P.J., Khatri P. KLRD1-expressing natural killer cells predict influenza susceptibility. Genome Med. 2018;10:45. doi: 10.1186/s13073-018-0554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alpert A., Pickman Y., Leipold M., Rosenberg-Hasson Y., Ji X., Gaujoux R., et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat Med. 2019;25:487–495. doi: 10.1038/s41591-019-0381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayed N., Huang Y., Nguyen K., Krejciova-Rajaniemi Z., Grawe A.P., Gao T., et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nature Aging. 2021;1:598–615. doi: 10.1038/s43587-021-00082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Netea M.G., Dominguez-Andres J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]