Abstract
Mainstream genetic testing in routine oncology care requires implementation research to inform intervention design. In Australia, funding is available for oncology health professionals (OHP) to organise genetic testing (GT) for eligible colorectal and endometrial cancer patients as part of their routine care. To assess the health system ability to incorporate this practice change, we conducted an implementation survey using the Consolidated Framework for Implementation Research (CFIR). The online survey was available from April to September 2020 to OHP and genetic health professional (GHP). In total, 198 respondents attempted the survey, with 158 completed and 27 partial responses: 26% were GHP, 66% OHP and 8% pathologists. Of all responders, 50% were female, mainly practicing in public hospital settings (57%) in an urban location (80%) and with an 18–60 years plus age range. The majority of respondents saw the relative advantage of aligning GT to abnormal universal tumour screening (UTS) results, with 77% of GHP and 78% of OHP agreeing it would streamline care for patients. There was disagreement across healthcare professional groups about knowledge and self-efficacy, with 45% of GHP not viewing oncologists as ‘feeling confident’ to use genetic test results for treatment management decisions, while 62% of OHP felt confident in their ability. Both OHP and GHP’s indicated embedding a genetic counsellor in oncology or having a genetics point of contact to support integrating of GT through UTS as favourable interventions. Implementation research findings allow for the design of targeted interventions and a model for GT integration into oncology.
Subject terms: Cancer genetics, Genetic counselling
Introduction
Colorectal cancer (CRC) is the third most common cancer in men and the second most common cancer in women worldwide [1, 2]. In Australia, CRC is the second most common cancer in men and endometrial cancer (EC) affects 1 in 44 women [3]. The majority of sporadic CRC occurs due to the accumulation of multiple mutations in the adenoma–carcinoma pathway [4]. The microsatellite instability (MSI) pathway mainly causes rarer hereditary CRC [4] like Lynch syndrome (LS) through pathogenic variants in MLH1, MSH2, MSH6, PMS2 or EPCAM genes. Other rarer hereditary CRC causes are attributed to pathogenic variants in APC, SMAD4, BMPR1A, STK11, GREM1 and MUTYH. Even though the majority of CRC is sporadic, the rarer heritable cancer accounts for 5–10% due to germline pathogenic variants in the above genes [5]. LS accounts for 3% of CRC and up to 6% of EC [6–8]. For these reasons, recent approval of Australian public funding allows CRC and EC medical specialists to order panel genetic testing (GT) for eligible patients [9].
Previously access to GT for LS was through family history and tumour-based screening of CRC or other LS-related cancers with the Amsterdam and Amsterdam II criteria [10, 11], Bethesda and revised Bethesda criteria [12, 13], which now have proven limitations in detecting LS [14]. To circumvent these limitations recommendations to screen all CRC and EC tumours for deficient mismatch repair (dMMR) proteins (MLH1, MSH2, PMS2 and MSH6)—through UTS utilising immunohistochemical (IHC) staining or MSI testing were made in 2009 [15]. As dMMR protein function is an indication for germline GT [16] it has the potential to increase the identification of LS. However, to streamline the UTS strategy the 10–15% of sporadic CRCs with the somatic loss of MLH1 need to be excluded by reflex testing for BRAF mutation or MLH1 methylation prior to referral to genetics for LS GT [17] (Supplementary Fig. 1).
Even with the above triage screening strategies, evidence highlights that the identification of LS remains suboptimal [18, 19], citing healthcare professional barriers, such as the lack of familiarity of LS and the structure and function of genetic services and negative attitude towards GT [20, 21]. Changes in Australian public funding for GT [9], with EC and CRC medical specialists able to request GT instead of referral to genetic services could circumvent these barriers. The new approach requires UTS for dMMR protein function analysis to be performed in combination with reflex BRAF or MLH1 methylation (excluding sporadic cases). This approach then directs panel GT through the CRC and EC medical specialist for appropriate patients instead of referral to genetic services—defined as CRC and EC mainstreaming (Supplementary Fig. 1). The benefit of improving LS identification through mainstreaming GT is important as cancer prevention strategies exist through annual or biennial colonoscopy, daily aspirin intake and risk-reducing total abdominal hysterectomy [22, 23] in those with an LS-related cancer or an unaffected relative through cascade GT in families [24, 25]. Cancer prevention through cascade GT is one of the premises of an Australian UTS guided GT screening strategies for cost-effectiveness [26].
International studies show challenges with UTS implementation and can inform lessons about how to align UTS with GT for CRC and EC clinical care. A 2012 United States of America study found 51% of 100 community hospitals had adopted UTS [27] citing, lack of a tracking system for genetic referrals and patient follow-up as one of the barriers to adoption. GT integration with UTS would also rely on physician knowledge, continued patient engagement, and efficient pre-test genetic counselling [25], all recognised barriers. Further implementation research and understanding of the barriers to mainstreaming for CRC and EC in routine oncology practice is needed to inform multi-stakeholder implementation. Therefore, the aim of this study was to understand the views of different professional groups and implementation factors that will influence the adoption of mainstream GT for CRC and EC in the Australian oncology health system.
Materials and methods
Survey development
The study survey was developed using the Consolidated Framework for Implementation Research (CFIR) five domains; ‘intervention characteristics’, ‘outer setting’, ‘inner setting’, ‘characteristics of the individuals involved’ and the ‘process of implementation’ [28]. All CFIR domains were included and the survey design was informed from our BRCA qualitative mainstreaming study [29] along with qualitative literature [19–21, 27, 30–32] about the barriers and facilitators of UTS for CRC and EC. The commonalities between BRCA mainstreaming themes [33] and the LS qualitative literature [19–21, 27, 30–32] were mapped to the CFIR domains and constructs to identify key attributes to focus on in the survey design. Agree–disagree statements were designed to address the key attributes identified. The Australian Genetic Testing Mainstreaming Collaborative Group, the internal research team, a consumer representative, an OHP and an international LS genetic counselling expert were consulted. Their review led to the refinement and consensus selection of statements and questions to include. The final survey consists of 18, 26 and 31 agree–disagree statements for pathologists, GHP and OHP, respectively. These statements facilitated data collection on the:
relative advantage of mainstreaming GT for CRC and EC into oncology practice
individual characteristics, such as knowledge and beliefs about the intervention and self-efficacy
inner setting relating to implementation climate and readiness to implement
processes of planning and engaging the main stakeholders in the oncology setting.
The final survey was approved (Ref: 2019/1027) by the University of Sydney (USYD) Human Research Ethics Committee, pilot tested with three G/OHP and distributed (between April and September 2020) via G/OHP professional organisations, taking between 10 and 15 min to complete.
Survey recruitment
Two approaches were used to recruit:
GHP (genetic counsellors and clinical geneticists) through the Australian Society of Genetic Counsellors and the Human Genetics Society of Australasia; OHP (medical oncologists, surgeon, nurses, gastroenterologists and pathologists) through the Medical Oncology Group of Australasia, the Clinical Oncology Society of Australasia, the Colorectal and Surgical Society of Australia and New Zealand, the Cancer Nurses Society of Australia and the Gastrointestinal Society of Australasia; and
disseminated through heads of cancer genetics, oncology, nursing, surgery and gastroenterology departments to snowball the invitation to professionals in their network likely to affected by GT mainstreaming for CRC and EC.
A direct recruitment approach to organisation membership is not permitted by governing bodies and it is not possible to know subspecialty and denominator numbers for all the relevant professionals targeted.
Data analysis
Survey data were collected and managed in Research Electronic Data Capture Version 10.0.1 [33] hosted at the USYD. Descriptive analyses were performed in Excel (Microsoft) by including complete and partial survey data with n varying by statement. The survey was exploratory and not hypothesis driven. To assess differences in views across GHP and OHP for each CFIR implementation factor category, proportion and percentages were used to summarise responses to statements and to assess barriers and intervention suggestions. Consultation with a biostatistician was undertaken and due to the small sample sizes for each health professional, the data did not lend to robust statistical testing. Participants were asked open-ended questions on implementation strategies or factors to determine the success of GT mainstreaming for CRC and EC. After data collection, the answers were separated according to the G/OHP or pathologist in Excel. Thematic analysis was used to interpret the free text comments [34] and coding resulted in emerging patterns and categories in relation to CFIR domains. CFIR category frequencies that appeared most frequently were reported and compared to the quantitative responses. Coding and analysis were checked by SL and NR with consensus agreement reached.
Results
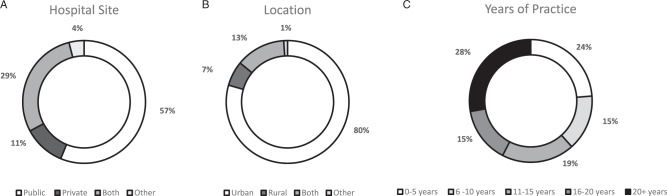
There were 198 attempts to complete the survey. From this pool, there were 158 completed survey responses and 27 partial responses (48 GHP, 123 OHP and 14 pathologists) and all of these were included in the analysis. As complete and partial responses were included in the analysis, n varies by question response. The overall participant demographics are described in Table 1 and Fig. 1A, B, C. An accurate response rate was unable to be determined due to professional organisations privacy regulations and the snowball sample approach taken. However, an estimate of representativeness of the samples reveals 42% (40/96) practicing cancer genetic counsellors, 40% (8/20) geneticists from a possible 20 Familial Cancer Clinics. Forty-nine pathology laboratories exist in Australia, giving an estimate response rate for pathologists of 29% (14/49) as respondents came from all five Australian states. Pathologists survey responses are presented where applicable as the survey questions per health professional group varied due to discipline-specific roles. For all oncologists and trainees, a potential response rate of 41/568 (7%) and for colorectal surgeons 43% (53/122), 1% (9/773) for gastroenterologists and 2% (21/1100) for nurses was estimated. Some estimate response rates are lower as information on denominator numbers for subspecialty groups of interest are not available.
Table 1.
Participant demographics and characteristics for all responders.
| Demographic | Respondents n = 198 (%)a |
|---|---|
| Profession | |
| Medical oncologist | 29 (15) |
| Oncology trainee | 5 (3) |
| Gynaecological oncologist | 7 (4) |
| Nurse | 21 (11) |
| Surgeon | 53 (27) |
| Gastroenterologist | 9 (5) |
| Pathologist | 15 (8) |
| Geneticist | 8 (4) |
| Genetic counsellor | 43 (22) |
| Other | 9 (5) |
| Gender | |
| Male | 95 (48) |
| Female | 100 (50) |
| Prefer not to say | 3 (2) |
| Age range | |
| 18–29 | 3 (2) |
| 30–39 | 58 (29) |
| 40–49 | 49 (25) |
| 50–59 | 56 (28) |
| 60+ | 32 (16) |
aRounding of percentages means they do not always add to 100%.
Fig. 1. Demographics of total respondents.
A Hospital site. B Location. C Years of practice.
Implementation factors of ‘intervention characteristics’
Relative advantage and cost
The majority of respondents (93% of GHP and pathologists; 87% of OHP) were aware of the UTS recommendations for CRC and EC and about three-quarters (77% of GHP; 78% of OHPs) agreed that mainstreaming GT with abnormal UTS would streamline care for patients. OHP saw the relative advantage of GT informing treatment management (85%) and cancer prevention (91%) and that it was relevant to their clinical practice (90%). Despite the perceived benefits of using GT in their practice, OHP and GHP had different views on how this could be achieved. Overall, 39% of GHP and 68% of OHP agreed that aligning GT with abnormal UTS results would be a practical fit with current processes but GHP and OHP disagreed (27% and 13%, respectively) with oncologists and surgeon direct ordering of GT for abnormal UTS and would still prefer referral to GC. Overall, 40% of both oncologists and surgeons thought that pre-test genetic counselling would be too time consuming for their current practice. Fifty-four percent and 67% respectively agreed that referral to GC for GT would be preferred rather than ordering GT after abnormal UTS results. OHP (67%), GHP (82%) and pathologists (86%) agreed support via designated staff funding for GT mainstreaming is needed. Many comments aligning with CFIR constructs of cost (in relation to reimbursement of IHC testing especially in the context of EC), advantage and complexity were found in OHP and GHP open text survey responses aligning with the quantitative findings (Table 2).
Table 2.
Implementation factors for mainstreaming genetic testing for colorectal and endometrial cancer.
| CFIR (Domain—construct) | Genetics health professional (GHP) responses | Oncology health professional (OHP) responses |
|---|---|---|
|
Intervention characteristics—relative advantage Stakeholders’ perception of the advantage of implementing the intervention vs. an alternative solution [28]. |
Relative advantage | |
| “It seems inevitable and likely will occur in other cancers too” | “This is the obvious next step for the identified patients with dMMR tumours having access to appropriate assessment for hereditary cancer risk vs. acquired change” | |
| Intervention characteristics—complexity | Complexity | |
| Perceived difficulty of implementation, reflected by duration, scope, radicalness, disruptiveness, centrality and intricacy and number of steps required to implement [28]. | “I think mainstreaming panel testing for CRC and EC has many more complexities associated with it compared to mainstreaming for breast or ovarian cancer. Also associated treatment implications are less obvious than with breast and ovarian cancer” | “Whilst integrated testing can occur at diagnosis - results are not available before surgery and may not be made available prior to commencement of adjuvant chemotherapy. A better approach may be to automate familial cancer clinic/genetic counsellor consultations (rather that knee-jerk panel testing) to coincide with oncology appointments to ensure patients have access to informed discussion and can adequately consent to panel testing.” |
|
Intervention characteristics—cost Costs of the intervention and costs associated with implementing the intervention including investment, supply and opportunity costs [28]. |
Cost | |
| “Pathology flags and recommendations/notifications on histopathology reports and Medicare funding for MMR panels” | "Routine reimbursement and automatic test once the diagnosis made." | |
|
Inner setting—networks and communication The nature and quality of webs of social networks and the nature and quality of formal and informal communications within an organisation [28]. |
Communication networks and collaboration | |
| “Mainstreaming genetic testing programmes like those used effectively for many years in breast and ovarian cancer should be referred to as they have successfully allowed for a co-ordinated approach between oncology staff and genetics” | “There are several sites with a lot of experience in mainstreaming for ovarian and breast cancer. It works very well with a close collaboration with a genetic department who supported and monitored the implementation for ovarian cancer. I would recommend the same with CRC and EC” | |
| Genetics point of contact | ||
| “Having genetics staff physically co-located with cancer services, to build relationships and increase communication between the specialities” | “Adequate support to clinician in initial phase and also ongoing support from genetics. I think a clinician can learn the needed process—such as we did for BRCA mainstreaming—quite readily. The issue with panel testing is that I suspect it will change over time. There needs to be a mechanism where clinicians can get support from genetics readily and also be able to refer to genetics at any stage for review of case” | |
|
Individuals involved—other personal attributes A broad construct to include other personal traits, such as tolerance of ambiguity, intellectual ability, motivation, values, competence, capacity and learning style [28]. |
Role delineation | |
| “Genetic testing by oncology team and referral to genetics if patient is positive” |
“I think the counselling is best brought in at surgical level as it will capture all colorectal cancer patients. Oncologists only see the proportion of colorectal cancer patients that have high-risk stage II/stage III /stage IV cancer. We need testing done on all of the early-stage I and II cancers as well.” “The family of patients are NOT under the responsibility of clinicians beyond general pre- consent information. Genetics need to take on all family for counselling and testing and the responsibility for ensuring this occurs this should not fall on the clinician” |
|
Implementation factors of individuals and inner setting
Knowledge, beliefs and self-efficacy associated with integrating genetic testing
Overall, 70% of GHP did not believe that OHP in general have the knowledge to take on pre-test genetic counselling and consent, and 45% of GHP did not view oncologists as feeling confident to use genetic test results for treatment management decisions. In contrast, 62% of OHP felt confident in their ability to use genetic results in treatment management, with 60% viewing previous training playing a part in this preparedness. Overall, 44% of oncologists and 41% of surgeons believed they have the knowledge to undertaken pre-test genetic counselling, with nurses (69%) disagreeing more with this statement. The majority of OHP still recognised the need for ongoing support from the genetics team (97%) and for genetics to be a required part of curriculum training (90%). Genetics support and education were frequently found in open text comments from many of the OHP and GHP survey responses (Table 2) highlighting these as important inner setting implementation factors for successful integration of GT.
Implementation climate and readiness to implement
A similar proportion of GHP (38%), OHP (42%) and pathologists (36%) agreed that current GC referral rates for dMMR UTS results are low, with GHP (68%), OHP (77%) and pathologists (64%) agreeing that mainstream GT after abnormal UTS results through the surgeon or oncologist would increase access. These responses may reflect an emerging tension for change in the system. Participants were asked about the networks and communication in their system that could facilitate this change. Two-thirds of GHP, 87.5% of OHP and 100% of pathologists indicated that oncology or genetic staff are a key part of their team. Communication channels were viewed positively with GHP (60%), OHP (72%) and pathologists (93%) responding that good communication exists between genetics, surgery, oncology and pathology. However, there were mixed views on which professional was best placed to ensure GT mainstreaming occurred over the long term (Table 3). For example, 40% of OHP identified surgeons as suitable but not having the time capacity to undertake this role (47%). Time capacity (61%) was also a factor for oncologists (86%) who identified themselves as being suitable to take on the role to sustain GT mainstreaming over the long term. However, many respondents were neutral in preference or uncertain as to who is the best and has sufficient time to initiate and sustain this practice change. Communication networks, and collaboration of CFIR inner setting (networks and communication) construct were frequent in comments from both OHP and GHP open text survey responses, adding further emphasis on the importance of these factors for implementation success (Table 2).
Table 3.
Champions and role delineation to mainstream genetic testing for colorectal and endometrial cancer in the oncology setting.
| Statement agreement | GHP (%) | OHP (%) |
|---|---|---|
| Implementation climate and readiness to implement | ||
| Genetic Counsellors are best placed to facilitated initial adoption of routine genetic testing | 35/43 (81%) | 66/109 (61%) |
| Oncologists are best placed to integrate genetic testing over the long term | 26/44 (59%) | 58/109 (53%) |
| Surgeons are best placed to integrate genetic testing over the long term | 13/44 (30%) | 42/109 (39%) |
| Nurses are best placed to integrate genetic testing over the long term | 11/44 (25%) | 27/109 (25%) |
| Planning and engaging relevant stakeholders | ||
| Oncologists can take on the role of pre-test genetic counselling, consent and ordering genetic testing | 23/42 (55%) | 61/107 (57%) |
| Surgeons can take on the role of pre-test genetic counselling, consent and ordering genetic testing | 14/42 (33%) | 52/107 (49%) |
| Nurses can take on the role of pre-test genetic counselling, consent and ordering genetic testing | 16/40 (40%) | 38/107 (36%) |
| Pathologists can take on the role to trigger an alert from pathology reports for genetic testing | 34/42 (81%) | 65/107 (61%) |
| Genetic counsellor can facilitate tracking of results and follow-up patients | 28/42 (67%) | 94/107 (88%) |
| Any oncology staff can facilitate tracking of results and follow-up patients | 17/41 (41%) | 56/106 (55%) |
Implementation process factors
Planning and engaging relevant stakeholders
There was consensus across all groups (OHP 93%, GHP 85% and pathologists 100%) that multi-stakeholder consultation is required ahead of mainstreaming GT for CRC and EC. Similarly, all groups agreed (85% of OHP and GHP and 100% of pathologists) with the need for surgical, oncology or pathology champions to sustain GT mainstreaming over the long term. Those identified as initiators (genetic counsellors) or sustainors (oncologist) in the results above could be potential collaborative champions for each professional group. Separately, views on role delineation and responsibility for pre-test genetic counselling, consenting and ordering of GT were varied (Table 3). GHP viewed oncologists more favourable to take on these roles and surgeons and nurses less so. OHP viewed oncologists and surgeons favourably to take on these role and nurses less so. The majority of pathologists (84%) and GHP (81%) viewed pathologists as the best person to trigger alerts from pathology reports to initiate GT. Genetic counsellors were viewed as the appropriate professional to take on the role of tracking and follow-up of abnormal UTS and GT results compared to oncology staff (Table 3). Within the individuals involved construct of CFIR, role delineation was indicated as important as evidenced from comments from both OHP and GHP open text survey responses. This further highlights the need for process planning and consultation with relevant stakeholders to understand their role, responsibility and collaboration for UTS alignment with LS GT (Table 2).
Implementation barriers and interventions
Informing intervention and implementation strategies
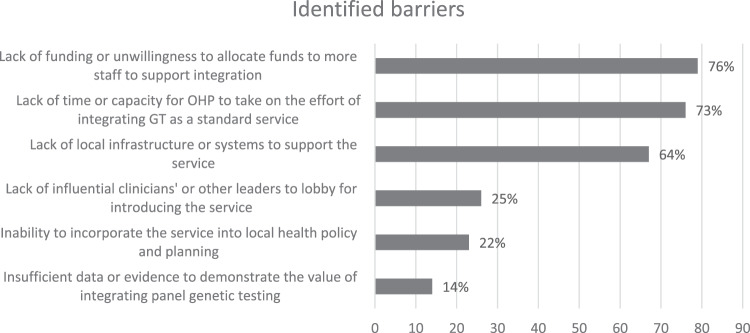
Similar health system barriers to mainstream GT were identified by GHP (80%), OHP (62%) and pathology (70%). The main barrier identified by three-quarters of all respondents across all groups was the lack of funding or unwillingness to allocate funds for increased staff to support GT mainstreaming. The next main barrier was a lack of time or capacity to take on GT mainstreaming (73% of respondents, across each group). Overall, 64% collectively identified the barrier of lack of local infrastructure or systems to support GT mainstreaming for CRC and EC. Other barriers identified are listed in Fig. 2.
Fig. 2. System barriers identified for mainstreaming genetic testing.
Collective responses from G/OHP and pathologists.
The majority of GHPs (85%) and OHPs (82%) respondents nominated suitable interventions that could potentially overcome the identified barriers. Embedding a genetic counsellor in the oncology setting and having a point of contact in the genetics team to support the alignment of GT with abnormal UTS, were indicated as favourable interventions from both OHP and GHP. Some differences emerged among GHPs and OHPs choice of mainstreaming intervention in relation to ordering GT through the Electronic Medical Record (EMR) system, with 79% of OHP preferring this option compared to 59% of GHP. In addition, OHP choose web (62% vs. 44%) and app-based (46% vs. 27%) interventions more than GHP. OHP (81%) chose written material as a source of information for their patients with LS pathogenic variants identified. Table 4 shows the suggested interventions to overcome barriers between OHP and GHP. Less frequent comments in the open text responses related to streamlining the mainstreaming process, communication and appointments between oncology and genetics to benefit patients, with monitoring of outcomes throughout.
Table 4.
Suggested interventions to overcome some barriers in mainstreaming genetic testing for colorectal and endometrial cancer into oncology services.
| GHP n = 41 (%) | OHP n = 102 (%) | |
|---|---|---|
| Intervention (Systems and engaging) to address barrier | ||
| Lack of funding or unwillingness to allocate funds to more staff to support integration | ||
| Lack of time or capacity for OHP to take on the effort of integrating GT as a standard service | ||
| An app with all the relevant information to integrate genetic testing into my practice | 11 (27%) | 47 (46%) |
| An app with patient friendly information about genetic testing | 15 (37%) | 50 (49%) |
| A website with all the relevant information to integrate genetic testing into my practice | 18 (44%) | 63 (62%) |
| Lack of local infrastructure or systems to support the service | ||
| Multidisciplinary team meeting template to include genetic tests ordered and need for follow-up discussed at meeting | 34 (83%) | 68 (67%) |
| Checklist or flowchart of the process for integrating genetic panel testing as standard practice | 30 (73%) | 61 (60%) |
| Patient tracking system in the EMR (electronic medical record) to ensure genetic results are followed up | 28 (68%) | 63 (62%) |
| An easy way to order genetic tests and log the test order in the EMR | 24 (59%) | 81 (79%) |
| Integration of genetic information into the main EMR system | 16 (39%) | 48 (47%) |
| Intervention (Personnel or education) to address barrier | ||
| Lack of time or capacity for OHP to take on the effort of integrating GT as a standard service | ||
| A genetics contact person available via telephone for ongoing support when integrating panel genetic testing into routine practice | 32 (78%) | 74 (73%) |
| Embedded genetic counsellor in oncology to do pre-test genetic counselling | 29 (71%) | 69 (68%) |
| Information for oncology health professionals (OHP) on how to talk with their patients about genetics and genetic testing | 27 (66%) | 60 (59%) |
| Insufficient data or evidence to demonstrate the value of integrating panel genetic testing | ||
| Online training regarding panel genetic testing and adoption as standard practice | 26 (63%) | 56 (55%) |
| Face to face education on genetics and panel genetic test adoption | 25 (61%) | 38 (37%) |
| Genetics specific training in medical school or oncology training | 33 (81%) | 53 (52%) |
| Information for OHP about patient management if a test is positive | 25 (61%) | 70 (69%) |
| Information for OHP about how to manage questions from family members about their genetic risk | 21 (51%) | 63 (62%) |
| Handouts for OHP to give their patients with specific information if a test is positive | 21 (51%) | 83 (81%) |
Discussion
Our study sought to ascertain the implementation factors and needs of the stakeholders who will be involved in GT mainstreaming for abnormal CRC and EC UTS results. Our findings indicate that OHP and GHP recognise the relative advantage of GT mainstreaming in this context, but have differing views about how it would practically fit within current processes. All professional groups recognise the need for funding support via designated staff and continued support from genetics and ongoing education to OHP to initiate and sustain GT mainstreaming. OHP were more confident of their knowledge and skills than the GHP in relation to pre-test genetic counselling, consent and to use genetic test results for treatment management and differing opinions on role delineation indicate the need for consultation to plan this process. Many barriers were recognised in the system and suggested interventions of education, embedded genetic counsellors in oncology, genetics point of contact and multidisciplinary team (MDT) meeting and documentation among others were favoured to overcome barriers.
The relative advantage of GT mainstreaming is supported by other international studies to reduce barriers and ensure optimal GT access in the context of ovarian cancer [35–37] with PARPi personalised medicine strategies available for carriers of dominantly expressed BRCA variants [38]. In the context of LS, the identification of dMMR CRC tumours can impact the choice of treatment, with immunotherapy shown to improve progression-free survival in dMMR metastatic CRC and as a first-line treatment over standard chemotherapy options [39–41]. There is also emerging evidence to support immunotherapy in the treatment of early-stage primary dMMR CRC [42]. Identifying dMMR UTS could tailor treatment regimens and potentially drive the adoption of GT mainstreaming programmes in CRC and EC. Currently, there is no difference in the treatment regimen of either LS-related dMMR CRC or sporadic CRC. However, using UTS as a tool to streamline therapeutic options brings a secondary advantage of guiding GT for LS and optimising identification to aid in cancer prevention in the patient and their relatives [24, 25].
Our study showed that the majority of OHP recognise both the need for ongoing support from genetics and for genomic medicine to be a required part of curriculum training. A systematic review showed in 50% of the reviewed studies that OHP are not confident in the use of GT information in their practice, due to a lack of knowledge in genetic concepts [43]. Our study demonstrated that GHP were less confident about OHP knowledge but OHP had more confidence in themselves. Oncologists engaging with somatic genetics to target treatments and inform care [44], may explain the emerging OHP confidence or through OHP survey participant bias with more genomic confident OHP likely to respond. GHP reflecting lower confidence could be attributed to response bias with potential responders having experience with other mainstreaming programmes implementation challenges. One way to instil confidence is through ongoing education and support and both GHP and OHP in this study identified mainstreaming interventions through online, face-to-face or core curriculum training education of OHP and a point of contact in genetics as useful. Similar educational interventions were identified in the Australian [29] and the international BRCA mainstreaming context [35–37].
Most participants indicated a need to change the system to optimise GT mainstreaming as most agreed that the current low rates of GC referral after abnormal UTS exist. More than half agreed that GT mainstreaming would improve access but differing views existed regarding role delineation for pre-test genetic counselling and ordering of GT. International BRCA mainstreaming programmes for epithelial ovarian cancer (EOC) have resulted in increased access and uptake of GT compared to pre-mainstreaming [35–37]. Qualitative views of key UTS stakeholders revealed the importance of collaboration and role delineation at three UTS stages [30]. Similarly, pathologists were viewed as initiators of the UTS screen (stage 1), oncologist as key in communicating UTS results to patients (stage 2) and the genetic counsellor and oncologist contributing to following up the family and interpreting GT results (stage 3) [30]. West et al. did not interview surgeons; thus, our findings add new knowledge for surgical roles as not all CRC patients will consult an oncologist. Other studies have shown the need for clear role delineation of OHP responsible for treatment management decisions and GHP responsible for positive GT and familial implications [45]. However, in order for CRC and EC mainstreaming programmes to be successful, clearly aligning the views of OHP and GHP on role delineation requires ongoing collaboration for GHP to support and gain confidence in OHPs roles in the process. The first step is for OHP to take the responsibility for embedding GT into practice and embedding genetic counsellors into the oncology setting for support an advantage as seen in international EOC mainstreaming programmes [46]. Future research focusing on multi-stakeholder qualitative consultation should focus on understanding the acceptability of roles and process planning for GT mainstreaming for CRC and EC.
Findings from our study recognise many GT mainstreaming system barriers for CRC and EC. Previous studies identified UTS adoption barriers as cost, the need for significant infrastructure and resource support [31], providing clear rationale to medical staff of the necessity of UTS [47], collaboration [31, 47] and communication [47], and patient and provider/staff education [31]. Similar barriers were identified in our study and the main interventions suggested to overcome these were embedding genetic counsellors in the oncology setting, support from genetics, education, and the use of MDT and EMR tracking. This concurs with previous literature in relation to embedding a genetic counsellor [46] and the use of the EMR to stream line the process [36]. Similarly, improvement strategies in 35 UTS programmes with a high level of involvement of genetic counsellors resulted in improved results tracking and communication [48].
Another potential barrier in Australia is the lack of reimbursement for BRAF/MSI testing and a national testing policy for UTS in CRC and EC. This is evidenced by the wide variation in pathology services practices in 2018 [49]. Future barriers to implementing LS mainstreaming could emerge through existing recommendations to include somatic tumour testing subsequent to a LS negative GT result [50] to inform Lynch-like syndrome and the need for further surveillance follow up. When this aspect of testing is added to the mainstreaming pathway, future implementation research needs to focus on the existing identified barriers, along with understanding role responsibility to trigger such testing, communication through MDT and for family follow-up.
There are several limitations of our findings. Most participants in this study were OHP, reflecting our recruitment strategies that targeted more avenues through OHP groups and snowballing. The lack of denominator numbers for health professional groups, due to the privacy regulations of professional organisations does not allow for reporting accurate response rates, estimates are presented to inform sample representativeness. Lower responses from nurses, gastroenterologists and pathologists limits the generalisability of views from these groups. Small professional group sample sizes did not allow for inferential statistical analysis to be performed. The data represent a single point in time cross-section and may not be reflective of changing health professionals’ views over time. The survey was disseminated 1 month before public funding approval for GT in May 2020 and is not representative of pre-implementation research. Response bias exists from urban areas and OHP and GHP selecting to complete the survey, which may affect our results. Given the limitations with knowledge of exact group denominators and some small sample group sizes, future research is planned to carry out consultation with all stakeholders to inform CRC and EC mainstreaming. Increasing participation from pathologist and gynaecologists in conjunction with other OHP and GHP will be a focus of this work.
In conclusion, our study contributes to the Australian oncology genomics implementation field, in the context of GT mainstreaming for LS related CRC and EC. Views from the various disciplines on knowledge, skill, capacity, process planning and role delineation will likely influence the adoption of routine GT for CRC and EC. Identification of the needs and implementation factors important to OHP, GHP and pathologists in an early phase, along with barriers and recommended interventions can guide sustainable CRC and EC mainstreaming implementation strategies.
Supplementary information
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-021-00871-4.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer. 2014.
- 2.Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, et al. Cancer incidence in five continents. Vol. X. International Agency for Research on Cancer; 2014. [DOI] [PubMed]
- 3.Australian Institute of Health and Welfare (AIHW). Cancer in Australia. Canberra: AIHW; 2017. Cancer series no.101. Cat. no. CAN 100.
- 4.Raskov H, Pommergaard HC, Burcharth J, Rosenberg J. Colorectal carcinogenesis-update and perspectives. World J Gastroenterol. 2014;20:18151–64. doi: 10.3748/wjg.v20.i48.18151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoffel EM, Mangu PB, Gruber SB, Hamilton SR, Kalady MF, WanYee Lau M, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol. 2015;33:209–17. doi: 10.1200/JCO.2014.58.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Win AK, Young JP, Lindor NM, Tucker KM, Ahnen DJ, Young GP, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol. 2012;30:958–64. doi: 10.1200/JCO.2011.39.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan NAJ, McMahon R, Tobi S, Snowsill T, Esquibel S, Wallace AJ, et al. The proportion of endometrial tumours associated with Lynch syndrome (PETALS): A prospective cross-sectional study. PLoS Med. 2020;17:e1003263. doi: 10.1371/journal.pmed.1003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ring KL, Bruegl AS, Allen BA, Elkin EP, Singh N, Hartman AR, et al. Germline multi-gene hereditary cancer panel testing in an unselected endometrial cancer cohort. Mod Pathol. 2016;29:1381–9. doi: 10.1038/modpathol.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medicare benefit schedule. Australian Government Department of Health 2020. Available from http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/20200501-News.
- 10.Vasen HFA, Mecklin JP, Meera Khan P, Lynch HT. The International Collaborative Group on hereditary nonpolyposis colorectal cancer (ICG-HNPCC) Dis Col Rectum. 1991;34:424–5. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 11.Vasen HFA, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453–6. doi: 10.1016/S0016-5085(99)70510-X. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Bigas MA, Boland CR, Hamilton SR, Henson DE, Jass JR, Khan PM, et al. A National Cancer Institute workshop on hereditary nonpolyposis colorectal cancer syndrome: meeting highlights and Bethesda Guidelines. J Natl Cancer Inst. 1997;89:1758–62. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 13.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch Syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syngal S, Fox EA, Eng C, Kolodner RD, Garber JE. Sensitivity and specificity of clinical criteria for hereditary non-polyposis colorectal cancer associated mutations in MSH2 and MLH1. J Med Genet. 2000;37:641–5. doi: 10.1136/jmg.37.9.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Recommendations from the EGAPP Working Group. genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11:35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hegde M, Ferber M, Mao R, Samowitz W, Ganguly A. ACMG technical standards and guidelines for genetic testing for inherited colorectal cancer (Lynch syndrome, familial adenomatous polyposis, and MYH-associated polyposis) Genet Med. 2014;16:101–16. doi: 10.1038/gim.2013.166. [DOI] [PubMed] [Google Scholar]
- 17.Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11:42–65. doi: 10.1097/GIM.0b013e31818fa2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh H, Schiesser R, Anand G, Richardson P, El- Serag HB. Underdiagnoses of Lynch syndrome involves more than family history criteria. Clin Gastroenterol Hepatol. 2010;8:523–9. doi: 10.1016/j.cgh.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pi S, Nap-Hill E, Telford J, Enns R. Recognition of Lynch syndrome amongst newly diagnosed colorectal cancers at St. Paul’s Hospital. Can J Gastroenterol Hepatol. 2017. 10.1155/2017/9625638. [DOI] [PMC free article] [PubMed]
- 20.Tan YY, Fitzgerald LJ. Barriers and motivators for referral of patients with suspected Lynch syndrome to cancer genetic services: a qualitative Study. J Pers Med. 2014;4:20–34. doi: 10.3390/jpm4010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prochniak CF, Martin LJ, Miller EM, Knapke SC. Barriers to and motivations for physician referral of patients to cancer genetics clinics. J Genet Couns. 2012;21:305–25. doi: 10.1007/s10897-011-9401-x. [DOI] [PubMed] [Google Scholar]
- 22.Vasen HF, Abdirahman M, Brohet R, Langers AM, Kleibeuker JH, van Kouwen M, et al. One to 2-year surveillance intervals reduce risk of colorectal cancer in families with Lynch syndrome. Gastroenterology. 2010;138:2300–6. doi: 10.1053/j.gastro.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 23.Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol. 2014;109:1159–79. doi: 10.1038/ajg.2014.186. [DOI] [PubMed] [Google Scholar]
- 24.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N. Engl J Med. 2005;352:1851–60. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 25.Hampel H. Genetic counseling and cascade genetic testing in Lynch syndrome. Fam Cancer. 2016;15:423–7. doi: 10.1007/s10689-016-9893-5. [DOI] [PubMed] [Google Scholar]
- 26.Kang YJ, Killen J, Caruana M, Simms K, Taylor N, Frayling IM, et al. The predicted impact and cost-effectiveness of systematic testing of people with incident colorectal cancer for Lynch syndrome. Med J Aust. 2020;212:72–81. doi: 10.5694/mja2.50356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beamer LC, Grant ML, Espenschied CR, Blazer KR, Hamplel HL, Weitzel JN, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058–63. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damschroder L, Aron D, Keith R, Kirsh S, Alexander J, Lowery J. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Shea R, Rankin NM, Kentwell M, Gleeson M, Salmon L, Tucker KM, et al. How can Australia integrate routine genetic sequencing in oncology: a qualitative study through an implementation science lens. Genet Med. 2020;22:1507–16. doi: 10.1038/s41436-020-0838-x. [DOI] [PubMed] [Google Scholar]
- 30.West KM, Burke W, Korngiebel DM. Identifying “ownership” through role descriptions to support implementing universal colorectal cancer tumor screening for Lynch syndrome. Genet Med. 2017;19:1236–44. doi: 10.1038/gim.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider JL, Davis J, Kauffman TL, Reiss JA, McGinley C, Arnold K, Zepp J, et al. Stakeholder perspectives on implementing a universal Lynch syndrome screening program: a qualitative study of early barriers and facilitators. Genet Med. 2016;18:152–61. doi: 10.1038/gim.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palter VN, Baker NA, Pollett A, Daly C, Facey M, Roteberg C, Rabeneck L, Baxter NN. Learning by example: an international perspective on reflex-testing for Lynch Syndrome. Ann Surg Oncol. 2019;26:425–36. doi: 10.1245/s10434-018-6809-7. [DOI] [PubMed] [Google Scholar]
- 33.Harris R, Taylor BL, Minor V, Elliott M, Fernandez L, O’Neal L, REDCap consortium et al. The REDCap consortium: building an international community of software partners, J Biomed Inform. 2019. 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed]
- 34.Pope C, Mays N. Qualitative research in healthcare. 3rd ed. Oxford, United Kingdom: Blackwell Publishing Ltd; 2006.
- 35.George A, Riddell D, Seal S, Talukdar S, Mahamdallie S, Ruark E, et al. Implementing rapid, robust, cost effective, patient-centred, routine genetic testing in ovarian cancer patients. Sci Rep. 2016;6:1–8. doi: 10.1038/srep29506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uyar D, Neary J, Monroe A, Nugent M, Simpson P, Geurts JL. Implementing a quality improvement project for universal genetic testing in women with ovarian cancer. Gynecol Oncol. 2018;149:565–9. doi: 10.1016/j.ygyno.2018.03.059. [DOI] [PubMed] [Google Scholar]
- 37.Colombo N, Huang G, Scambia G, Chalas E, Pignata S, Fiorica J, et al. Evaluation of a streamlined oncologist-Led BRCA mutation testing and counseling model for patients with ovarian cancer. J Clin Oncol. 2018;36:1300–7. doi: 10.1200/JCO.2017.76.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482–90. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38:11–19. doi: 10.1200/JCO.19.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an openlabel, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–91. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, KEYNOTE-177 Investigators et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl J Med. 2020;383:2207–18. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 42.Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26:566–76. doi: 10.1038/s41591-020-0805-8. [DOI] [PubMed] [Google Scholar]
- 43.White S, Jacobs C, Phillips J. Mainstreaming genetics and genomics: a systematic review of the barriers and facilitators for nurses and physicians in secondary and tertiary care. Genet Med. 2020;22:1149–55. doi: 10.1038/s41436-020-0785-6. [DOI] [PubMed] [Google Scholar]
- 44.de Moor JS, Gray SW, Mitchell SA, Klabunde CN, Freedman AN. Oncologist confidence in genomic testing and implications for using multimarker tumor panel tests in practice. JCO Precis Oncol. 2020;4:620–31. doi: 10.1200/PO.19.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hallowell N, Wright S, Stirling D, Gourley C, Young O, Porteous M. Moving into the mainstream: healthcare professionals’ views of implementing treatment focussed genetic testing in breast cancer care. Fam Cancer. 2019;18:293–301. doi: 10.1007/s10689-019-00122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rana HQ, Kipnis L, Hehir K, Cronin A, Jaung T, Stokes SM, Fekrmandi F, et al. Embedding a genetic counselor into oncology clinics improves testing rates and timeliness for women with ovarian cancer. Gynecol Oncol. 2020. 10.1016/j.ygyno.2020.11.003. [DOI] [PubMed]
- 47.Cohen SA. Current Lynch syndrome tumor screening practices: a survey of genetic counselors. J Genet Couns. 2014;23:38–47. doi: 10.1007/s10897-013-9603-5. [DOI] [PubMed] [Google Scholar]
- 48.Cragun D, DeBate RD, Vadaparampil ST, Baldwin J, Hampel H, Pal T. Comparing universal Lynch Syndrome tumor screening programs to evaluate associations between implementation strategies and patient follow through. Genet Med. 2014;16:773–82. doi: 10.1038/gim.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mascarenhas L, Shanley S, Mitchell G, Spurdle A, Macrae F, Pachter N, et al. Current mismatch repair deficiency tumor testing practices and capabilities: a survey of Australian pathology providers. Asia-Pac J Clin Oncol. 2018;14:417–25. doi: 10.1111/ajco.13076. [DOI] [PubMed] [Google Scholar]
- 50.Monahan KJ, Bradshaw N, Dolwani S, Hereditary CRC. guidelines eDelphi consensus group, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG) Gut. 2020;69:411–44. doi: 10.1136/gutjnl-2019-319915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.