Abstract
Abetalipoproteinemia (ABL) is a rare autosomal recessive disorder caused by biallelic pathogenic mutations in the MTTP gene. Deficiency of microsomal triglyceride transfer protein (MTTP) abrogates the assembly of apolipoprotein (apo) B-containing lipoprotein in the intestine and liver, resulting in malabsorption of fat and fat-soluble vitamins and severe hypolipidemia. Patients with ABL typically manifest steatorrhea, vomiting, and failure to thrive in infancy. The deficiency of fat-soluble vitamins progressively develops into a variety of symptoms later in life, including hematological (acanthocytosis, anemia, bleeding tendency, etc.), neuromuscular (spinocerebellar ataxia, peripheral neuropathy, myopathy, etc.), and ophthalmological symptoms (e.g., retinitis pigmentosa). If left untreated, the disease can be debilitating and even lethal by the third decade of life due to the development of severe complications, such as blindness, neuromyopathy, and respiratory failure. High dose vitamin supplementation is the mainstay for treatment and may prevent, delay, or alleviate the complications and improve the prognosis, enabling some patients to live to the eighth decade of life. However, it cannot fully prevent or restore impaired function. Novel therapeutic modalities that improve quality of life and prognosis are awaited. The aim of this review is to 1) summarize the pathogenesis, clinical signs and symptoms, diagnosis, and management of ABL, and 2) propose diagnostic criteria that define eligibility to receive financial support from the Japanese government for patients with ABL as a rare and intractable disease. In addition, our diagnostic criteria and the entry criterion of low-density lipoprotein cholesterol (LDL-C) <15 mg/dL and apoB <15 mg/dL can be useful in universal or opportunistic screening for the disease. Registry research on ABL is currently ongoing to better understand the disease burden and unmet needs of this life-threatening disease with few therapeutic options.
Keywords: Abetalipoproteinemia, MTTP, Fat-soluble vitamin, Chylomicron, VLDL, Hypolipidemia
1. Introduction
Abetalipoproteinemia (ABL; OMIM 200100) is a rare inherited disease characterized by the absence of plasma apolipoprotein (apo) B-containing lipoproteins and fat-soluble vitamins in the plasma. In 1950, Bassen and Kornzweig first described the syndrome, which is characterized by acanthocytes (“star-shaped” erythrocytes with irregular cytoplasmic projections, i.e., acantha, “thorn” in Greek), retinitis pigmentosa, and ataxia 1) . In 1960, the absence of beta-lipoprotein in the plasma of the syndrome was reported 2) . Later, in 1992, the activity of microsomal triglyceride transfer protein (MTTP) was found to be absent in the intestinal mucosa of ABL patients 3) . In 1993, mutations in the MTTP gene, which encodes the large subunit of MTTP, were identified in ABL patients ( Fig.1 ) 4 , 5) . In this review, we summarize the pathogenesis, clinical signs and symptoms, diagnosis, and management of ABL. We also propose diagnostic criteria for ABL, which have been used to determine the eligibility to receive financial aid from the Japanese government for patients with ABL as a rare and intractable disease. The financial aid is provided by The Program for Designated Intractable Diseases under the Japanese Public Healthcare system. Pediatric ABL patients can be supported separately under The program of Medical Aid for Chronic Pediatric Diseases of Specified Categories.
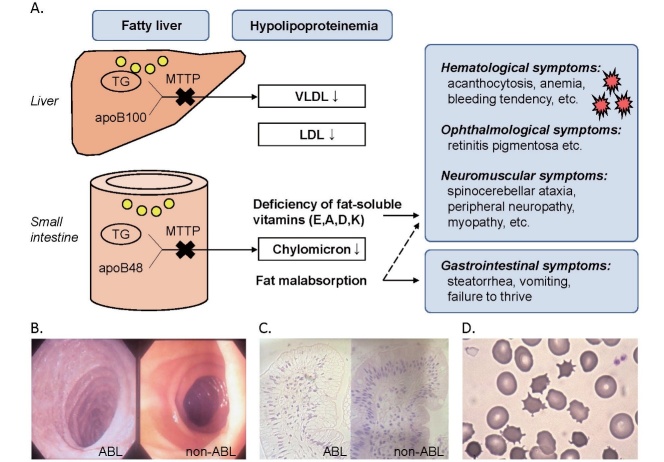
Fig.1. Overview of abetalipoproteinemia.
(A) MTTP is a prerequisite for the assembly and secretion of VLDL and CM by the liver and small intestine, respectively. Homozygous MTTP deficiency causes fat malabsorption, steatorrhea, vomiting, failure to thrive, hypolipoproteinemia, fatty liver, as well as symptoms related to deficiencies of fat-soluble vitamins. (B, C) Endoscopic examination and histological analysis of the duodenal mucosa of an ABL patient and a non-ABL control. Accumulation of intracellular lipids in epithelial cells (C) results in a snowy appearance (B), called snow-white duodenum, a gelee blanche, or white hoar frosting. (D) Acanthocytes of an ABL patient (Patient 1 in Ref 29). Figures 1B, 1C, and 1D are reproduced with permission from Ishibashi S and Ohashi K (The Lipid, 2014; 25: 200-203).
2. Genetic and Molecular Basis
ABL is an autosomal recessive disorder caused by biallelic mutations in the MTTP gene. The estimated frequency of ABL is as rare as less than 1 in 1,000,000 6) . Approximately 100 cases and at least 74 different MTTP mutations have been reported, including five (c.61+1G>C, c.1237-1G>A, c.1389del, p.I564T, p.N780Y) in four Japanese patients ( Fig.2 ) 7 - 35) . About one-third of patients were the progeny of consanguineous marriages 7) . The male-to-female sex ratio is reportedly 1:1 7) or 3:2 2) , although both males and females should theoretically be equally affected. Genetic and clinical features of Japanese ABL patients are listed in Table 1 . As the number of patients is limited, it is difficult to clarify the characteristics of Japanese cases of ABL.
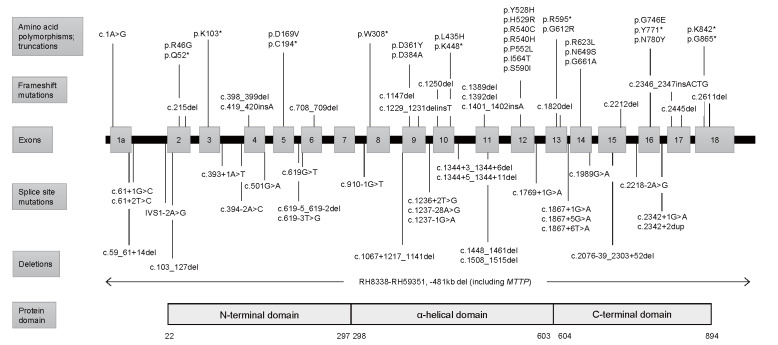
Fig.2. Mutations in the MTTP gene .
At least 74 MTTP mutations have been reported. The type of mutation may influence the severity of the disease 29) . The exon-intron structure of the MTTP gene encoded by exons 1a to 18 has been described 52) . Gray boxes represent exons. Lines represent the position of each mutation and polymorphism. Adapted from Zamel R, et al. 41) , Narcisi TM et al. 27) , and Suzuki T et al. 52)
Table 1. Genetic and clinical features of Japanese patients with abetalipoproteinemia.
| Authors | Age | Gender | Mutation ( MTTP ) | Type | Consanguinity | Biochemical parameters (mg/dL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | TG | LDL-C | HDL-C | cLDL-C | apoB | Vitamin E | ||||||
| Yang XP et al. 28) | 29 | M | c.1237-1G>A | Ho | No (Uniparental disomy) | 33 | 0 | N.D. | 28 | 5 | 0 | <0.1 |
| Ohashi K et al. 29) | 32 | F | c.1389del | Ho | Yes | 42 | 0.2 | N.D. | 36 | 6.0 | 0.9 | <0.1 |
| Ohashi K et al. 29) | 27 | M | c.2338A>T (p.N780Y) | Ho | Yes | 34 | 2.6 | N.D. | 23 | 10.5 | 0.6 | <0.1 |
| Sakamoto O et al. 30) | 15mo | M | c.61+1G>C c.1691T>C (p.I564T) | C. het | N.D. | 46-92 | 10-100 | N.D. | N.D. | - | <7.0 | 0.43 |
| Authors | Clinical features | |||||||||||
| Gastrointestinal | Neuromuscular | Ophthalmological | Hematological | |||||||||
| Yang XP et al. 28) | Frequent diarrhea, fat malabsorption with malnutrition, and short stature (from childhood); lipid-laden enterocytes by intestinal biopsy (29 years old). | Cerebellar and posterior spinal column dysfunction, decreased deep tendon reflexes, impaired vibratory sense and proprioception, dysmetria, ataxia, spastic gait, and positive Chaddock sign (29 years old). | Suspected loss of night vision (3 years old); descreased vision in dim light, visual field defects, and pigmentary retinal degeneration (29 years old). | Acanthocytosis | ||||||||
| Ohashi K et al. 29) | Intolerance for fat-rich meals; snow- white duodenum and lipid-laden enterocytes by biopsy (32 years old). | Absent ankle and knee jerks, positive Romberg’s sign (32 years old). | Fine mottling in the retina (32 years old). | Acanthocytosis (32 years old). | ||||||||
| Ohashi K et al. 29) | Mild fatty liver, no history of steatorrhea | Normal | Normal | Acanthocytosis | ||||||||
| Sakamoto O et al. 30) | Hepatomegaly and fatty liver, no steatorrhea. | Normal | Normal | No acanthocytosis | ||||||||
* Age (years or months (mo)) at molecular diagnosis; M = Males; F = Females; Ho = homozygous; C. het = compound heterozygous; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; cLDL-C, calculated LDL-C; N.D., Not described.
MTTP is localized in the lumen of the endoplasmic reticulum of hepatocytes and intestinal epithelial cells. By transferring triglyceride (TG) and cholesterol ester to apoB, MTTP is essential for the formation of very low-density lipoproteins (VLDLs) and chylomicrons (CMs). A lack of MTTP abrogates the secretion of apoB-containing lipoproteins, which results in malabsorption of dietary fat and fat-soluble vitamins as well as accumulation of intracellular lipids in hepatocytes and intestinal epithelial cells. MTTP is a heterodimer of a large 97 kDa subunit containing 894 amino acids (encoded by MTTP ) and a 55 kDa protein disulfide isomerase (PDI) subunit (encoded by P4HB ) 36) . The MTTP gene consists of 18 coding exons. Crystal structure analysis reveals three structural domains in the large subunit of MTTP: an N-terminal β-barrel domain (amino acids 22-297), which interacts with the N-terminus of apoB; a central α-helical domain (298-603), which interacts with apoB as well as PDI; and a C-terminal domain (604-894) 7 , 37 - 39) . The interaction between the α-helical domain and PDI is required for lipid-transfer activity, and the C-terminal domain mediates lipid-binding and lipid-transfer activity ( Fig.2 ) 37) .
3. Clinical Manifestations
Gastrointestinal Symptoms (Fat Malabsorption and Failure to Thrive):
Symptoms of ABL typically develop in infancy after breastfeeding, including vomiting, steatorrhea due to fat malabsorption, and failure to thrive 2) . Patients often avoid dietary fat to relieve these gastrointestinal symptoms 2) . Endoscopic examination of the intestinal mucosa may reveal a snowy appearance, which is also called snow-white duodenum 40) , a gelee blanche, or white hoar frosting ( Fig.1B and 1C ) 7 , 8) .
4. Diagnosis
ABL is typically suspected in infants who have steatorrhea, vomiting, and failure to thrive. However, the severity of the disease varies depending on the type of mutation in MTTP , and the diagnosis of ABL may be delayed until adulthood 2 , 29) . ABL patients may be found opportunistically at health examinations in adulthood because of extremely low plasma cholesterol levels 29) . For early diagnosis and treatment of ABL, simple diagnostic criteria are warranted. Clinicians across multiple disciplines, including pediatricians, primary care physicians, neurologists, ophthalmologists and gastroenterologists, should consult lipidologists regarding further diagnostic tests when they suspect ABL.
Typical Levels of Plasma Lipids and Lipoproteins:
Plasma levels of total cholesterol (TC) in ABL patients are typically less than half normal, ranging from 20 to 50 mg/dL, with most of TC derived from high-density lipoprotein (HDL). Plasma levels of HDL are typically decreased by ~50%. The decrease in HDL may result in part from absence of phospholipid transfer from VLDL to HDL during the lipolysis of VLDL-TG. Catabolism of HDL, particularly apoE-containing HDL, may be increased, contributing to the apparently normal delivery of cholesterol to peripheral tissues in the absence of apoB-containing lipoproteins in ABL patients 7) . Plasma TG levels in ABL are typically less than 10 mg/dL 7) and do not increase after a dietary fat load 2 , 7 , 48) . Although the levels of TC and TG are variable in ABL patients, plasma levels of low-density lipoprotein (LDL) and apoB are consistently absent or extremely low. We searched PubMed for all previously reported cases and found that ABL patients have LDL-C <15 mg/dL and/or apoB <15 mg/dL (typically apoB <5 mg/dL) 7 , 9 , 28 , 29) , except for three cases of the mild-moderate phenotype 34) . Therefore, patients who have such levels of hypolipidemia should be suspected of having ABL.
Diagnostic Criteria:
A definitive diagnosis of ABL requires genetic testing of MTTP . Clinical diagnosis of ABL based on diagnostic criteria would help identify suspected cases for early diagnosis and treatment. Based on lipid levels of ABL and the clinical manifestations described above, we propose diagnostic criteria for ABL ( Table 2 ) . Our criteria have been used to define the eligibility of ABL patients to receive financial support from the Japanese government as a rare and intractable disease. The entry criterion (LDL-C <15 mg/dL and/or apoB <15 mg/dL) of the criteria will also be useful for identifying suspected cases at health checkups or opportunistic blood testing (i.e., universal or opportunistic screening) for further referral to lipidology specialty clinics.
Table 2. Diagnostic criteria for ABL in Japan.
・Plasma LDL-C level <15 mg/dL AND/OR plasma apoB level <15 mg/dL.
Familial hypobetalipoproteinemia 1 (FHBL1)(OMIM 615558), chylomicron retention disease (Anderson disease) (OMIM 246700), hyperthyroidism. * ABL and homozygous FHBL (Ho-FHBL) can not be distinguished only from the clinical manifestations and laboratory findings of a proband. Family history is helpful. As FHBL1 is an autosomal dominant disorder, obligate heterozygote parents of Ho-FHBL1 patients have <50% of normal LDL-C and apoB plasma levels. On the other hand, obligate heterozygote parents of ABL patients have normal plasma lipid levels. Plasma levels of lipids, apoB, and fat-soluble vitamin of other family members may be helpful.
Pathogenic mutations in the MTTP gene <Diagnosis> Definite ABL: Entry criterion (A) is associated with at least one item of B or C AND exclusion of differential diagnosis (D) AND genetic diagnosis (E). Probable ABL: Entry criterion (A) is associated with at least two items of B or C AND exclusion of differential diagnosis (D). |
Differential Diagnosis:
Hypocholesterolemia in combination with fat malabsorption may result from the following diseases.
・ Familial hypobetalipoproteinemia 1(FHBL1; OMIM 615558) is caused by mutations in APOB (mostly nonsense or frameshift) with an autosomal dominant mode of inheritance. The homozygous type of FHBL1 (Ho-FHBL1) presents with similar biochemical and clinical characteristics to ABL. Ho-FHBL1 can be differentiated from ABL only by family history. As FHBL1 is an autosomal dominant disorder, obligate heterozygote parents of Ho-FHBL1 patients have <50% of normal plasma levels of LDL-C and apoB. On the other hand, obligate heterozygote parents of ABL patients have normal plasma lipid levels. The estimated frequency of Ho-FHBL1 is as rare as less than 1 in 1,000,000, and that of heterozygote FHBL1 is 1 in 1,000 to 3,000 42 , 49) .
・ Chylomicron retention disease (CMRD; OMIM 246700), also referred to as Anderson disease, is a rare autosomal recessive disorder caused by biallelic mutations in the SAR1B gene encoding Sar1b (secretion-associated and Ras-related GTPase 1B). The deficiency of Sar1b, which is a prerequisite for the secretion of CMs, causes severe hypocholesterolemia as well as steatorrhea, vomiting, and failure to thrive 50) . As VLDL secretion is preserved, CMRD can be differentiated from ABL and FHBL1 by plasma lipid levels: In CMRD, plasma levels of total cholesterol, LDL-C, and HDL-cholesterol (HDL-C) are more than 50% decreased, whereas the plasma TG level is normal.
5. Assessment, Treatment, and Management
The current strategy and recommendations for the treatment and management of ABL, which are adapted and modified from reviews by Hegele et al. and others, are summarized below 2 , 6 , 7 , 39 , 41 , 42) .
Assessment:
The recommended assessments for ABL patients include 2 , 6 , 7 , 39 , 41 , 42) :
・ Evaluation of growth at every visit.
・ Annual blood analysis including lipid profiles (TC, TG, LDL-C, HDL-C, apoB, apoA-I), liver function tests (aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transferase (GGT), total and direct bilirubin, alkaline phosphatase, albumin), fat-soluble vitamins (Vitamin A (retinol), β-carotene, 25-OH vitamin D, vitamin E, vitamin K), other micronutrients (vitamin B12, iron, folate), complete blood count, PT-INR, reticulocyte count, ESR, calcium, phosphate, uric acid, and thyroid stimulating hormone (TSH).
・ Ophthalmological and neurological evaluation every 6-12 months.
・ Hepatic ultrasonography, bone mineral density measurements, echocardiography every 3 years.
Treatment and Management:
For the treatment of ABL patients, the standard of care includes 2 , 6 , 7 , 39 , 41 , 42) :
・ Restriction of fat intake is necessary to prevent steatorrhea. Total fat intake should be restricted to less than 30% of the total energy intake, or less than 15 to 20 g per day, or even less than 5 g per day in children 2 , 6 , 7 , 39 , 42) . Increased fat in the stool may induce oxalate urolithiasis by binding dietary calcium and increasing dietary oxalate absorption. This might be prevented by providing sufficient dietary calcium, fluid intake, and reducing dietary oxalate 2) .
・ Adequate calorie intake is essential to avoid growth retardation. It should be noted that fat malabsorption may lead to malabsorption of carbohydrates proteins, and other nutrients 7) . A fat-restricted diet may mitigate such secondary malabsorption.
・ Medium-chain triglyceride (MCT) administration can help correct malnutrition, particularly in infants, though not absolutely necessary. MCTs are absorbed and then transported in the circulation not by CMs but by albumin. Since hepatic fibrosis is a potential adverse effect of MCTs, liver enzymes should be monitored in infants who are administered MCTs, and long-term administration is better avoided 2 , 7) .
・ Oral essential fatty acid supplementation. The daily requirement for essential fatty acids, e.g., up to 1 teaspoon per day of oil rich in polyunsaturated fatty acids (e.g., soybean or olive oil) is recommended 7 , 39 , 42) .
・ High dose oral vitamin E supplementation (100-300 IU/kg/day 6 , 7 , 39 , 42) ; 1,000-2,000 mg/day (infant), 5,000-10,000 mg/day (older children and adults) 2) ; 2,400-12,000 IU/day 41) ; 1IU=1mg tocopheryl acetate) delays or prevents progression of neurological complications 2 , 6) . Even with such high dose vitamin E supplementation, serum vitamin E levels increase to at most 30% of the lower limit of normal serum levels of vitamin E 7 , 41) . However, serum vitamin E levels may not correlate with tissue vitamin E levels 7 , 39 , 41) . Better methods of monitoring tissue vitamin E concentrations are awaited. Vitamin E may be administered via alternative routes (intravenous, intramuscular, etc.). However, oral supplementation is favored due to: 1) feasibility for life-long supplementation, 2) no apparent inferiority in increasing tissue vitamin E levels compared to other methods, 3) no apparent toxicity (Other routes of supplementation may induce fatty liver and other complications 42) ). It should be noted that absorption of large doses of vitamin E may induce or exacerbate vitamin K deficiency 6 , 7 , 41)
・ High dose oral vitamin A supplementation (100-400 IU/kg/day 39 , 41 , 42) ) and vitamin E supplementation can prevent or arrest ophthalmological complications 2 , 6 , 7 , 39) .
・ Supplementation of vitamin D (800-1,200 IU/day 39 , 42) ) should be considered in cases of vitamin D deficiency.
・ Supplementation of vitamin K (5-35 mg/week 39 , 41 , 42) ) should be considered in cases of vitamin K deficiency with hypothrombinemia and prolonged PT-INR. Supplementation of vitamin K will normalize its blood levels 2 , 41) .
・ Supplementation of iron, folate, or vitamin B12 may be necessary in the case of anemia 2 , 6 , 41 , 42) .
・ Multidisciplinary care for neurological complications involving neurologists, physiatrists, physical therapists, occupational therapists, and speech therapists 39) .
Particular caution should be taken to avoid vitamin A toxicity 2 , 39 , 41) , which can be seen even in those who have a normal plasma vitamin A concentration 39) . To avoid toxicity, it is recommended that the target vitamin A concentration goal should be set at the lower limit of normal levels 41) , and the dose of vitamin A supplementation should be titrated by monitoring blood concentrations of vitamin A and β-carotene 39 , 41 , 42) . Women who are pregnant or planning to conceive should receive 50% of the dose of vitamin A supplementation to avoid vitamin A toxicity with careful monitoring of the blood concentrations of vitamin A and β-carotene 39 , 42) . Supplementation of vitamin A should be continued in pregnancy as its deficiency could induce lethal complications in pregnant women 39 , 41) .
6. Burden of Disease and Unmet Needs
If left untreated, ABL patients start manifesting systemic complications related to fat-soluble vitamin deficiencies as early as in the first decade of life, gradually developing into lethal conditions in the third decade 7 , 41) . Early diagnosis and adequate supplementation of vitamin E, A, and other fat-soluble vitamins may prevent, delay, or alleviate the complications and improve the prognosis, enabling some patients to live to the eighth decade of life 7 , 39) . Successful pregnancies in ABL patients have been reported 2 , 7 , 41) . This review and our simple diagnostic criteria aim to contribute to the early diagnosis and treatment of ABL by facilitating cooperation among various medical specialists.
However, the growth potential of patients may not be fully restored by dietary therapy 39 , 42) . High dose vitamin therapy is insufficient for most patients, and even ineffective for some, to recover from vitamin deficiencies and their complications 42) . Deficiency of other lipids or nutrients, such as essential fatty acids, could also contribute to the pathogenesis of ABL. Therefore, a good understanding of its pathogenesis and keeping abreast of novel therapeutic developments are necessary. Gene therapies that correct MTTP deficiency in the liver and small intestine may be promising therapeutic candidates 51) .
More studies are needed to unravel the pathogenesis, genotype-phenotype relationship, burden of disease, and unmet needs. Considering the paucity of patients, a nation-wide registry for a long enough period to evaluate the prognosis would help clarify these issues. To this end, a registry study for rare and intractable lipid disorders including ABL (the PROLIPID study) is ongoing in Japan.
Acknowledgments and Notice of Grant Support
This work has been supported by Health, Labour and Welfare Sciences Research Grant for Research on Rare and Intractable Diseases (H30-nanji-ippan-003).
Conflicts of Interest
Atsushi Nohara has nothing to disclose. Hayato Tada has nothing to disclose. Masatsune Ogura has received honoraria from Amgen Inc., Astellas Pharma Inc. Sachiko Okazaki has received scholarship grants from Minophagen Pharmaceutical Co., Ltd., Kowa Company, Ltd. Koh Ono has nothing to disclose. Hitoshi Shimano has nothing to disclose. Hiroyuki Daida has received honoraria from Amgen Inc., Daiichi-Sankyo Co., Ltd., Kowa Co., Ltd., and MSD K.K., Novartis Pharma K.K., Bayer Yakuhin, Ltd. and received clinical research funding from Canon Medical Systems Corporation, Philips Japan, Ltd., Toho Holdings Co., Ltd., Asahi Kasei Corporation, and Inter Reha Co., Ltd. HD has also received scholarship grants from Nippon Boehringer Ingelheim Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sanofi K.K., MSD K.K., Daiichi-Sankyo Co., Ltd., Pfizer Co., Ltd., Mitsubishi Tanabe Pharma Corp., Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Teijin Pharma, Ltd., Shionogi & Co., Ltd., Actelion Pharmaceuticals, Ltd., Actelion Ltd., Kowa Co., Ltd., Bayer Yakuhin, Ltd. HD has also courses endowed by companies, including Philips Japan, Ltd., ResMed, Fukuda Denshi Co., Ltd., and Paramount Bed Co., Ltd. Kazushige Dobashi has nothing to disclose. Toshio Hayashi has nothing to disclose. Mika Hori has nothing to disclose. Kota Matsuki has nothing to disclose. Tetsuo Minamino has nothing to disclose. Shinji Yokoyama has nothing to disclose. Mariko Harada-Shiba has received stock holdings or options from Liid Pharma, honoraria from Amgen Inc., Astellas Pharma Inc., Sanofi, and scholarship grants from Aegerion Pharmaceuticals, Inc., Recordati Rare Diseases Japan, and Kaneka Corporation. Katsunori Ikewaki has nothing to disclose. Yasushi Ishigaki has nothing to disclose. Shun Ishibashi has received honoraria from Kowa Co., Ltd., and a scholarship grant from Ono Pharmaceutical Co., Ltd. Kyoko Inagaki has nothing to disclose. Hirotoshi Ohmura has nothing to disclose. Hiroaki Okazaki has received scholarship grants from Minophagen Pharmaceutical Co., Ltd., Kowa Company, Ltd. Masa-aki Kawashiri has nothing to disclose. Masayuki Kuroda has nothing to disclose. Masahiro Koseki has received clinical research funding from Kowa Company, Ltd., Rohto Pharmaceutical Co., Ltd. Takanari Gotoda has nothing to disclose. Shingo Koyama has nothing to disclose. Yoshiki Sekijima has nothing to disclose. Manabu Takahashi has nothing to disclose. Yasuo Takeuchi has nothing to disclose. Misa Takegami has nothing to disclose. Kazuhisa Tsukamoto has received honoraria from Bayer Yakuhin, Ltd., MSD Ltd., Takeda Pharmaceutical Company Ltd., and scholarship grants from Mitsubishi Tanabe Pharma Corporation., Bayer Yakuhin, Ltd., Sanofi K.K. Atsuko Nakatsuka has nothing to disclose. Kimitoshi Nakamura has nothing to disclose. Satoshi Hirayama has nothing to disclose. Hideaki Bujo has nothing to disclose. Daisaku Masuda has received clinical research funding from MSD K.K., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Kowa Co., Ltd. Takashi Miida has nothing to disclose. Yoshihiro Miyamoto has nothing to disclose. Takeyoshi Murano has nothing to disclose. Takashi Yamaguchi has nothing to disclose. Shizuya Yamashita has received honoraria from Kowa Company, Ltd., MSD K.K. Masashi Yamamoto has nothing to disclose. Koutaro Yokote has received honoraria from Kowa Company, Ltd., MSD K.K., Astellas Pharma Inc., Mitsubishi Tanabe Pharma Corp., Amgen K.K., Takeda Pharmaceutical Company Limited, Sanofi K.K., Ono Pharmaceutical Co., Ltd., AstraZeneca K.K., Daiichi-Sankyo Co., Ltd., Novartis Pharma K.K., Sumitomo Dainippon Pharma Co., Ltd., Kyowa Kirin Co., Ltd., Pfizer Japan Inc., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly Japan K.K., Taisho Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., and received clinical research funding from Taisho Pharmaceutical Co., Ltd. KY has also received scholarship grants from Mitsubishi Tanabe Pharma Corp., Takeda Pharmaceutical Co., Ltd., MSD K.K., Pfizer Japan Inc., Novo Nordisk Pharma Ltd., Taisho Pharmaceutical Co., Ltd., Kao Corporation, Ono Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Daiichi-Sankyo Co., Ltd., Teijin Pharma, Ltd., Shionogi Co., Ltd., Bayer Yakuhin, Ltd. Jun Wada has nothing to disclose. Ken Ohashi has nothing to disclose.
References
- 1).Bassen FA, Kornzweig AL. Malformation of the erythrocytes in a case of atypical retinitis pigmentosa. Blood, 1950; 5: 381-387 [PubMed] [Google Scholar]
- 2).Kane JP, Havel R. Disorders of the biogenesis and secretion of lipoproteins containing the B apolipoproteins., In: Scriver CR, Beaudet AL, Sly WS, Valle D, Vogelstein B, eds. The Metabolic and Molecular Bases of Inherited Disease. 8 ed. Vol 2. New York, NY: McGraw-Hill; 2001: 2717-2752 [Google Scholar]
- 3).Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, Gay G, Rader DJ, Gregg RE. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science, 1992; 258: 999-1001 [DOI] [PubMed] [Google Scholar]
- 4).Sharp D, Blinderman L, Combs KA, Kienzle B, Ricci B, Wager-Smith K, Gil CM, Turck CW, Bouma ME, Rader DJ, Aggerbeck LP, Gregg RE, Gordon DA, Wetterau JR. Cloning and gene defects in microsomal triglyceride transfer protein associated with abetalipoproteinaemia. Nature, 1993; 365: 65-69 [DOI] [PubMed] [Google Scholar]
- 5).Shoulders CC, Brett DJ, Bayliss JD, Narcisi TM, Jarmuz A, Grantham TT, Leoni PR, Bhattacharya S, Pease RJ, Cullen PM, Levi S, Byfield PG, Purkiss P, Scott J. Abetalipoproteinemia is caused by defects of the gene encoding the 97 kDa subunit of a microsomal triglyceride transfer protein. Hum Mol Genet, 1993; 2: 2109-2116 [DOI] [PubMed] [Google Scholar]
- 6).Burnett JR, Bell DA, Hooper AJ, Hegele RA. Clinical utility gene card for: Abetalipoproteinaemia--Update 2014. Eur J Hum Genet, 2015; 23: 890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Berriot-Varoqueaux N, Aggerbeck LP, Samson-Bouma M, Wetterau JR. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu Rev Nutr, 2000; 20: 663-697 [DOI] [PubMed] [Google Scholar]
- 8).Pons V, Rolland C, Nauze M, Danjoux M, Gaibelet G, Durandy A, Sassolas A, Levy E, Terce F, Collet X, Mas E. A severe form of abetalipoproteinemia caused by new splicing mutations of microsomal triglyceride transfer protein (MTTP). Hum Mutat, 2011; 32: 751-759 [DOI] [PubMed] [Google Scholar]
- 9).Di Filippo M, Moulin P, Roy P, Samson-Bouma ME, Collardeau-Frachon S, Chebel-Dumont S, Peretti N, Dumortier J, Zoulim F, Fontanges T, Parini R, Rigoldi M, Furlan F, Mancini G, Bonnefont-Rousselot D, Bruckert E, Schmitz J, Scoazec JY, Charriere S, Villar-Fimbel S, Gottrand F, Dubern B, Doummar D, Joly F, Liard-Meillon ME, Lachaux A, Sassolas A. Homozygous MTTP and APOB mutations may lead to hepatic steatosis and fibrosis despite metabolic differences in congenital hypocholesterolemia. J Hepatol, 2014; 61: 891-902 [DOI] [PubMed] [Google Scholar]
- 10).Aminoff A, Gunnar E, Barbaro M, Mannila MN, Duponchel C, Tosi M, Robinson KL, Hernell O, Ehrenborg E. Novel mutations in microsomal triglyceride transfer protein including maternal uniparental disomy in two patients with abetalipoproteinemia. Clin Genet, 2012; 82: 197-200 [DOI] [PubMed] [Google Scholar]
- 11).Gunduz M, Ozaydin E, Atar MB, Koc N, Kirsaclioglu C, Kose G, Cefalu AB, Averna M, Tarugi P. Microsomal triglyceride transfer protein gene mutations in Turkish children: A novel mutation and clinical follow up. Indian J Gastroenterol, 2016; 35: 236-241 [DOI] [PubMed] [Google Scholar]
- 12).Zeissig S, Dougan SK, Barral DC, Junker Y, Chen Z, Kaser A, Ho M, Mandel H, McIntyre A, Kennedy SM, Painter GF, Veerapen N, Besra GS, Cerundolo V, Yue S, Beladi S, Behar SM, Chen X, Gumperz JE, Breckpot K, Raper A, Baer A, Exley MA, Hegele RA, Cuchel M, Rader DJ, Davidson NO, Blumberg RS. Primary deficiency of microsomal triglyceride transfer protein in human abetalipoproteinemia is associated with loss of CD1 function. J Clin Invest, 2010; 120: 2889-2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Sani MN, Sabbaghian M, Mahjoob F, Cefalu AB, Averna MR, Rezaei N. Identification of a novel mutation of MTP gene in a patient with abetalipoproteinemia. Ann Hepatol, 2011; 10: 221-226 [PubMed] [Google Scholar]
- 14).Di Filippo M, Crehalet H, Samson-Bouma ME, Bonnet V, Aggerbeck LP, Rabes JP, Gottrand F, Luc G, Bozon D, Sassolas A. Molecular and functional analysis of two new MTTP gene mutations in an atypical case of abetalipoproteinemia. J Lipid Res, 2012; 53: 548-555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Magnolo L, Najah M, Fancello T, Di Leo E, Pinotti E, Brini I, Gueddiche NM, Calandra S, Slimene NM, Tarugi P. Novel mutations in SAR1B and MTTP genes in Tunisian children with chylomicron retention disease and abetalipoproteinemia. Gene, 2013; 512: 28-34 [DOI] [PubMed] [Google Scholar]
- 16).Najah M, Youssef SM, Yahia HM, Afef S, Awatef J, Saber H, Fadhel NM, Sassolas A, Naceur SM. Molecular characterization of Tunisian families with abetalipoproteinemia and identification of a novel mutation in MTTP gene. Diagn Pathol, 2013; 8: 54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Hammer MB, El Euch-Fayache G, Nehdi H, Feki M, Maamouri-Hicheri W, Hentati F, Amouri R. Clinical features and molecular genetics of two Tunisian families with abetalipoproteinemia. J Clin Neurosci, 2014; 21: 311-315 [DOI] [PubMed] [Google Scholar]
- 18).Miller SA, Burnett JR, Leonis MA, McKnight CJ, van Bockxmeer FM, Hooper AJ. Novel missense MTTP gene mutations causing abetalipoproteinemia. Biochim Biophys Acta, 2014; 1842: 1548-1554 [DOI] [PubMed] [Google Scholar]
- 19).Walsh MT, Iqbal J, Josekutty J, Soh J, Di Leo E, Ozaydin E, Gunduz M, Tarugi P, Hussain MM. Novel Abetalipoproteinemia Missense Mutation Highlights the Importance of the N-Terminal beta-Barrel in Microsomal Triglyceride Transfer Protein Function. Circ Cardiovasc Genet, 2015; 8: 677-687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Walsh MT, Di Leo E, Okur I, Tarugi P, Hussain MM. Structure-function analyses of microsomal triglyceride transfer protein missense mutations in abetalipoproteinemia and hypobetalipoproteinemia subjects. Biochim Biophys, 2016; 1861: 1623-1633 [DOI] [PubMed] [Google Scholar]
- 21).Aers XP, Leroy BP, Defesche JC, Shadid S. Abetalipoproteinemia From Previously Unreported Gene Mutations. Ann Intern Med, 2019; 170: 211-213 [DOI] [PubMed] [Google Scholar]
- 22).Di Filippo M, Collardeau Frachon S, Janin A, Rajan S, Marmontel O, Decourt C, Rubio A, Nony S, Dumont S, Cuerq C, Charriere S, Moulin P, Lachaux A, Hussain MM, Bozon D, Peretti N. Normal serum ApoB48 and red cells vitamin E concentrations after supplementation in a novel compound heterozygous case of abetalipoproteinemia. Atherosclerosis, 2019; 284: 75-82 [DOI] [PubMed] [Google Scholar]
- 23).Prachasitthisak N, Tanpowpong P, Tim-Aroon T, Treepongkaruna S, Chongviriyaphan N, Vithayasai N, Iamopas O, Wattanasirichaigoon D. Two infants with abetalipoproteinemia: Classic versus atypical presentation. Pediatr Int, 2019; 61: 508-509 [DOI] [PubMed] [Google Scholar]
- 24).Al-Mahdili HA, Hooper AJ, Sullivan DR, Stewart PM, Burnett JR. A mild case of abetalipoproteinaemia in association with subclinical hypothyroidism. Ann Clin Biochem, 2006; 43: 516-519 [DOI] [PubMed] [Google Scholar]
- 25).Di Filippo M, Varret M, Boehm V, Rabès JP, Ferkdadji L, Abramowitz L, Dumont S, Lenaerts C, Boileau C, Joly F, Schmitz J, Samson-Bouma ME, Bonnefont-Rousselot D. Postprandial lipid absorption in seven heterozygous carriers of deleterious variants of MTTP in two abetalipoproteinemic families. J Clin Lipidol, 2019; 13: 201-212 [DOI] [PubMed] [Google Scholar]
- 26).Khan AO, Basamh O, Alkatan HM. Ophthalmic diagnosis and optical coherence tomography of abetalipoproteinemia, a treatable form of pediatric retinal dystrophy. J AAPOS, 2019; 23: 176-177 [DOI] [PubMed] [Google Scholar]
- 27).Narcisi TM, Shoulders CC, Chester SA, Read J, Brett DJ, Harrison GB, Grantham TT, Fox MF, Povey S, de Bruin TW, Erkelens DW, Muller DP, Lloyd JK, Scott J. Mutations of the microsomal triglyceride-transfer-protein gene in abetalipoproteinemia. Am J Hum Genet, 1995; 57: 1298-1310 [PMC free article] [PubMed] [Google Scholar]
- 28).Yang XP, Inazu A, Yagi K, Kajinami K, Koizumi J, Mabuchi H. Abetalipoproteinemia caused by maternal isodisomy of chromosome 4q containing an intron 9 splice acceptor mutation in the microsomal triglyceride transfer protein gene. Arterioscler Thromb Vasc Biol, 1999; 19: 1950-1955 [DOI] [PubMed] [Google Scholar]
- 29).Ohashi K, Ishibashi S, Osuga J, Tozawa R, Harada K, Yahagi N, Shionoiri F, Iizuka Y, Tamura Y, Nagai R, Illingworth DR, Gotoda T, Yamada N. Novel mutations in the microsomal triglyceride transfer protein gene causing abetalipoproteinemia. J Lipid Res, 2000; 41: 1199-1204 [PubMed] [Google Scholar]
- 30).Sakamoto O, Abukawa D, Takeyama J, Arai N, Nagano M, Hattori H, Egashira T, Sakai N, Yamashita S, Iinuma K, Ohura T. An atypical case of abetalipoproteinaemia with severe fatty liver in the absence of steatorrhoea or acanthocytosis. Eur J Pediatr, 2006; 165: 68-70 [DOI] [PubMed] [Google Scholar]
- 31).Vidanapathirana DM, Jasinge E, Waidyanatha S, Hooper AJ, Burnett JR. Identification of a novel MTTP splice variant c. 394-2A>C in an infant with abetalipoproteinemia. J Rare Dis Res Treat, 2019; 4: 25-27 [Google Scholar]
- 32).Isa H, Mohamed A. Abetalipoproteinemia: three case reports, a novel microsomal triglyceride transfer protein gene mutation and a literature review. J Clin Case Rep, 2016; 6: 9 [Google Scholar]
- 33).Rafique M, Zia S. Abetalipoproteinemia in a Saudi infant. J Coll Physicians Surg Pak, 2011; 21: 117-118 [PubMed] [Google Scholar]
- 34). Rodríguez Gutiérrez PG, González García JR, Castillo De León YA, Zárate Guerrero JR, Magaña Torres MT. A novel p.Gly417Valfs * 12 mutation in the MTTP gene causing abetalipoproteinemia: Presentation of the first patient in Mexico and analysis of the previously reported cases. J Clin Lab Anal, 2020; e23672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Tada H, Usui S, Sakata K, Takamura M, Kawashiri MA. Low-Density Lipoprotein Cholesterol Level cannot be too Low: Considerations from Clinical Trials, Human Genetics, and Biology. J Atheroscler Thromb, 2020; 27: 489-498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Wetterau JR, Combs KA, Spinner SN, Joiner BJ. Protein disulfide isomerase is a component of the microsomal triglyceride transfer protein complex. J Biol Chem, 1990; 265: 9800-9807 [PubMed] [Google Scholar]
- 37).Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res, 2003; 44: 22-32 [DOI] [PubMed] [Google Scholar]
- 38).Biterova EI, Isupov MN, Keegan RM, Lebedev AA, Sohail AA, Liaqat I, Alanen HI, Ruddock LW. The crystal structure of human microsomal triglyceride transfer protein. Proc Natl Acad Sci USA, 2019; 116: 17251-17260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Burnett JR, Hooper AJ, Hegele RA. Abetalipoproteinemia. 2018. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Stephens K, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https: //www.ncbi.nlm.nih.gov/books/NBK532447/ [PubMed] [Google Scholar]
- 40).Mitchell CJ, Scott BB, Bullen AW, Losowsky MS. Snow-white duodenum: a new endoscopic sign in a patient with hypobetalipoproteinemia. Gastrointest Endosc, 1978; 24: 123-124 [DOI] [PubMed] [Google Scholar]
- 41).Zamel R, Khan R, Pollex RL, Hegele RA. Abetalipoproteinemia: two case reports and literature review. Orphanet J Rare Dis, 2008; 3: 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Lee J, Hegele RA. Abetalipoproteinemia and homozygous hypobetalipoproteinemia: a framework for diagnosis and management. J Inherit Metab Dis, 2014; 37: 333-339 [DOI] [PubMed] [Google Scholar]
- 43).Dische MR, Porro RS. The cardiac lesions in Bassen-Kornzweig syndrome. Report of a case, with autopsy findings. Am J Med, 1970; 49: 568-571 [DOI] [PubMed] [Google Scholar]
- 44).Lange Y, Steck TL. Mechanism of red blood cell acanthocytosis and echinocytosis in vivo. J Membr Biol, 1984; 77: 153-159 [DOI] [PubMed] [Google Scholar]
- 45).Forsyth CC, Lloyd JK, Fosbrooke AS. A-BETA-LIPOPROTEINAEMIA. Arch Dis Child, 1965; 40: 47-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Triantafillidis JK, Kottaras G, Peros G, Merikas E, Gikas A, Condilis N, Konstantellou E. Endocrine function in abetalipoproteinemia: a study of a female patient of Greek origin. Ann Ital Chir, 2004; 75: 683-690 [PubMed] [Google Scholar]
- 47).Black DD, Hay RV, Rohwer-Nutter PL, Ellinas H, Stephens JK, Sherman H, Teng BB, Whitington PF, Davidson NO. Intestinal and hepatic apolipoprotein B gene expression in abetalipoproteinemia. Gastroenterology, 1991; 101: 520-528 [DOI] [PubMed] [Google Scholar]
- 48).Kawashiri MA, Tada H, Hashimoto M, Taniyama M, Nakano T, Nakajima K, Inoue T, Mori M, Nakanishi C, Konno T, Hayashi K, Nohara A, Inazu A, Koizumi J, Ishihara H, Kobayashi J, Hirano T, Mabuchi H, Yamagishi M. Extreme Contrast of Postprandial Remnant-Like Particles Formed in Abetalipoproteinemia and Homozygous Familial Hypobetalipoproteinemia. JIMD Rep, 2015; 22: 85-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Burnett JR, Bell DA, Hooper AJ, Hegele RA. Clinical utility gene card for: Familial hypobetalipoproteinaemia (APOB)--Update 2014. Eur J Hum Genet, 2015; 23: 890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Peretti N, Sassolas A, Roy CC, Deslandres C, Charcosset M, Castagnetti J, Pugnet-Chardon L, Moulin P, Labarge S, Bouthillier L, Lachaux A, Levy E. Guidelines for the diagnosis and management of chylomicron retention disease based on a review of the literature and the experience of two centers. Orphanet J Rare Dis, 2010; 5: 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Kassim SH, Wilson JM, Rader DJ. Gene therapy for dyslipidemia: a review of gene replacement and gene inhibition strategies. Clin Lipidol, 2010; 5: 793-809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Suzuki T, Swift LL. Discovery of Novel Splice Variants and Regulatory Mechanisms for Microsomal Triglyceride Transfer Protein in Human Tissues. Sci Rep, 2016; 6: 27308 [DOI] [PMC free article] [PubMed] [Google Scholar]