Abstract
Aim: Familial hypercholesterolaemia (FH) is the most common autosomal dominant lipid disorder, leading to severe hypercholesterolaemia. Early detection and treatment with lipid-lowering medications may reduce the risk of premature coronary artery disease in FH patients. However, there is scarcity of data on FH prevalence, detection rate, treatment and control with lipid-lowering therapy in the Malaysian community.
Methods: Community participants ( n =5130) were recruited from all states in Malaysia. Blood samples were collected for lipid profiles and glucose analyses. Personal and family medical histories were collected by means of assisted questionnaire. Physical examination for tendon xanthomata and premature corneal arcus were conducted on-site. FH were clinically screened using Dutch Lipid Clinic Network Criteria.
Results: Out of 5130 recruited community participants, 55 patients were clinically categorised as potential (Definite and Probable) FH, making the prevalence FH among the community as 1:100. Based on current total population of Malaysia (32 million), the estimated number of FH patients in Malaysia is 320,000, while the detection rates are estimated as 0.5%. Lipid-lowering medications were prescribed to 54.5% and 30.5% of potential and possible FH patients, respectively, but none of them achieved the therapeutic LDL-c target.
Conclusion: Clinically diagnosed FH prevalence in Malaysian population is much higher than most of the populations in the world. At community level, FH patients are clinically under-detected, with majority of them not achieving target LDL-c level for high-risk patients. Therefore, public health measures are warranted for early detection and treatment, to enhance opportunities for premature CAD prevention.
Keywords: Familial hypercholesterolaemia, Malaysia, Prevalence, Under-detected, Under-treated
Introduction
Familial hypercholesterolaemia (FH), an inherited lipid disorder, is the most common autosomal dominant disease affecting mankind 1) . The most common gene mutations were found in LDLR , followed by APOB and PCSK9 , whilst other minor genes have also been implicated 2) . Individuals with FH will experience severely elevated low-density lipoprotein cholesterol (LDL-c) level and accelerated atherogenesis that leads to premature coronary artery disease (CAD) 3) . Mutations in LDLR is generally more severe, causing higher LDL-c level than that by other gene mutations 4) . Statin was introduced in 1976 and since then it became the first-line medication for hypercholesterolaemia 5) . Administration of statin in FH patients has been demonstrated to reduce CAD risk by about 76% 6) , hence early detection and treatment will provide opportunities for prevention of premature CAD and better quality of life.
Despite many decades of statin introduction at global scale, cardiovascular disease is still the number one cause for non-communicable disease (NCD) death worldwide, including Malaysia 7) . Countries with advanced health system such as Japan, Canada and France have lower rate of cardiovascular-related death, partly due to their elaborated screening programme, treatment and prevention for hypercholesterolaemia 8) . Unlike non-FH hypercholesterolaemic patients, genetically confirmed FH patients have additional four folds of CAD risk and require more extensive lipid-lowering therapy, compared to hypercholesterolaemia patients without genetic mutation 9) . Early detection and commencement of lipid-lowering therapy may extend CAD-free life up to 18 years 10) when compared to untreated FH patients.
The prevalence of clinical FH in Caucasian populations was reported to be between 1:500 to 1:200 11) , while globally it was estimated to be about 1:250 12 , 13) . Out of six World Health Organization (WHO) global regions, more than half of the individuals affected by FH are located in just two regions, namely, Western Pacific and South East Asia regions, which has been estimated to contain about 7.2 million individuals with FH 14) . Within these regions, the FH prevalence of some countries were well-documented. Using Dutch Lipid Clinic Network criteria (DLCN), Australia has reported an FH prevalence of 1:353 – 1:229 15) , while Japan, using their own FH diagnostic criteria 16) , has reported FH prevalence of 1:208 17) . China has recently developed their own FH diagnostic criteria, and has reported a staggering FH prevalence of 1:58 18) . Taiwan, which has started their national FH registry in 2015, has estimated their FH prevalence as ≈1:200 19) . However, the reports on prevalence of FH in some Western Pacific and South East Asia countries are scarce. Due to the possible founder effect from non-native immigrating ethnics, which is especially true in the racially diversified countries, the prevalence of FH in these regions can be higher than that of Caucasian population.
Singapore, the smallest nation within Western Pacific region with a total population of only 5.9 million people, is uniquely diverse with different ethnics, but has only reported 50 patients with molecularly confirmed LDLR mutations 20) by next-generation sequencing method. Thailand and the Philippines have also respectively reported 45 and 20 molecularly confirmed FH patients using PCR and denaturing high-performance liquid chromatography methods to detect LDLR mutations, but their nationwide FH prevalences were still yet to be investigated 21 , 22) . Vietnam shared the same dedication of reporting the molecularly confirmed FH patients, but only managed to discover 20 individuals with APOB R3500Q mutations 23) . The clinical and genetic prevalence of FH in the rest of Western Pacific and Southeast Asia countries, to the best of our knowledge, were yet to be reported 24) .
Regional collaborative study to investigate the clinical and epidemiological aspects of FH, including prevalence of FH in the community, has been initiated by the ‘10 Countries Study’ programme in 2016 which consists of 15 countries, including Malaysia 25 , 26) . With the hypercholesterolaemia prevalence of 35.0% in 2011, 47.7% in 2015, and 38.1% in 2019, and a very high frequency of hypercholesterolaemia at 67.0% in certain rural areas 27 , 28) , Malaysia has placed itself above the average prevalence of raised total cholesterol among Western Pacific nations 29) , which further support the possibility of high prevalence of FH among Malaysian population. Thus far, The Malaysian Study on Health and Wellbeing Assessment for Familial Hypercholesterolaemia (MyHEBAT - FH) has completed the FH patient recruitment across the country. Therefore, the study group aimed to report the first nation-wide investigation on the prevalence of FH in the Malaysian population, their detection rate, proportion on lipid-lowering treatment and control within the Malaysian community.
Methods
Study Design and Population
This was a cross-sectional population-based study involving participants attending the Health Screening Programmes across East and West Malaysia over 9 years duration, from 2011 to 2019. Inclusion criteria were Malaysian adults aged ≧18 years old. Those with secondary hypercholesterolaemia (hypothyroidism, chronic kidney disease, nephrotic syndrome and cholelithiasis) or pregnancy were excluded.
Definition of Terms
FH was clinically diagnosed using the DLCN 11) . The minimal pre-treatment LDL-c cut-offs for FH with and without premature CAD were set at LDL-c ≧4.0 and ≧4.9 mmol/L respectively. DLCN score of ≧6 (Probable FH: 6-8; Definite FH >8) were categorised as “Potential FH” 30) , and those with DLCN score of 3 – 5 were categorised as “Possible FH”. Those with DLCN score of <3 were categorised as “Unlikely” FH. Prevalence of clinically diagnosed FH was defined as those with Potential FH (Probable or Definite FH).
Participants Recruitment
Participants who attended the Health Screening Programmes in almost all states in Malaysia were recruited via convenience sampling. The Health Screening Programmes were mainly held in school halls or municipal general-purpose halls on weekends, where the events were advertised by the respective local authority by means of banners and social media, at least two weeks before the event day. At the registration desk, all participants were given the study information sheet and screened for eligibility according to the inclusion and exclusion criteria. Written informed consent was obtained from those who were eligible and recruited into the study.
Demographic and Anthropometric Data Collection
Data were collected by research assistants who were trained regarding the study procedures prior to the conduct of the study in order to standardise and minimise variability of the data collection methods. Demographic information (age, gender and ethnicity) and clinical history (presence of co-morbidities, smoking status and lipid-lowering therapy) were collected via face-to-face interview using standardised questionnaire. Data on premature CAD was obtained from the history taken based on past record of angina, myocardial infarction, percutaneous coronary intervention and/or coronary artery bypass graft surgery, onset at age <55 in men and <60 years old in women. Diabetes mellitus (DM) was defined as past history of known DM with/without anti-diabetic medication regardless of the glucose level; or having fasting plasma glucose ≧7.0 mmol/L and/or random plasma glucose ≧11.1 mmol/L for newly diagnosed cases.
Blood pressure (BP) was measured twice, two minutes apart on the right arm in sitting position, using an Omron HEM-8712 automatic digital blood pressure monitor (Omron, Tokyo, Japan). Participants were made to rest for at least 5 minutes before the measurements were taken. They were seated upright with their right arm supported at the heart level. The mean of the first and second systolic and diastolic measurements was reported as the BP value for the individual participant. Waist Circumference (WC) was measured to the nearest 0.1 cm using non-stretchable measuring tape with the subjects standing in a relaxed position and arms at the side. The measurement was taken at the midpoint between the lower rib margin (12 th rib) and the iliac crest. Physical examination for tendon xanthomata and premature corneal arcus were conducted by the clinicians. Tendon xanthomata were examined by visually identifying the presence of nodules or thickening of tendons at Achilles tendons, dorsal extensor of hands, elbows or knees 31) . Premature corneal arcus was visually identified by the presence of white opaque ring at the peripheral of cornea 32) in participants with age <45 years old, while participants with corneal arcus but age ≧45 years old were asked for the age of onset for the corneal arcus. Those with corneal arcus onset at age ≧45 years old were not considered as premature corneal arcus.
Blood Sampling and Laboratory Analysis
A total of 9 mL venous blood samples were collected following non-traumatic venepuncture for fasting lipid profiles and glucose level. The blood samples were stood for 30 – 60 min at 4℃ before being centrifuged on-site at 4000 rpm for 15 minutes. The serum and plasma samples were aliquoted into secondary tubes and kept at −20℃ until analysis. Serum lipid profile which includes total cholesterol (TC), triglyceride (TG) and high-density lipoprotein (HDL) and glucose level were analysed on automated analyser (Roche COBAS Integra® 400, Germany). Serum TC, TG and HDL were measured enzymatically with the use of commercially available reagents while LDL-c level was derived by calculation using the Friedewald equation 33) . For subjects whose samples were collected whilst on lipid-lowering medications, the baseline pre-treatment LDL-c level was calculated using the conversion algorithm 34) . The conversion algorithm requires the information of statin type and dose in order to determine the baseline LDL-c. If the statin information is not available, the treated LDL-c value were multiplied with a general conversion factor of 1.43 35) .
Plasma glucose was measured by using enzymatic hexokinase reference method. Calibration and internal quality control (QC) were performed as per work instruction set up by the institutional Pathology Laboratory which is ISO 15189 certified by the National Laboratory Accreditation Scheme, Skim Akreditasi Makmal Malaysia (SAMM), and recognised by APLAC Asia-Pacific Laboratory Accreditation Cooperation) and ILAC (International Laboratory Accreditation Cooperation).
Statistical Analyses
Data were analysed using the IBM SPSS Statistics version 22 (IBM, NY, USA). Categorical variables were reported as frequency and percentage (%). Continuous variables were reported using means and standard deviation (SD) for normally distributed data and as median with interquartile range (IQR) for non-normally distributed data. Normally distributed continuous data were analysed using one-way ANOVA, non-normally distributed data were analysed using Kruskal Wallis test followed by Mann-Whitney test. Chi-squared test were performed to analyse the association between categorical data. Statistical value of p <0.05 indicated significant difference.
Ethical Consideration
The ethical approval was obtained from the Institutional Research Ethics Committee (Ref: UiTM 600-IRMI [5/1/6]), which is in accordance to the Declaration of Helsinki. Written informed consent was obtained from all participants prior to recruitment. The study information sheet highlighted the rights of the participants to voluntarily participate in the study and to withdraw from the study at any time without any reason and without penalty.
Results
A total of 5130 participants were recruited. Table 1 shows the distribution of participants into Definite, Probable, Possible and Unlikely FH according to the DLCN criteria. Malay was the major ethnic across all categories of FH. Out 55 participants with Potential FH, 41 and 14 had Probable and Definite FH respectively, whilst another 374 were found to have Possible FH. The mean age of participants in the Definite FH group was higher compared to those in the other FH groups (59.8±10.3 vs 58.7±10.1 vs 50.5±13.1 vs 40.3±15.4 years, definite vs probable vs possible vs unlikely FH, p <0.05). The LDL-c and TC levels were significantly different ( p <0.05) across all FH groups (LDL-c: 7.4±1.4 vs 5.8±1.1 vs 5.6±0.7 vs 3.1±0.9 mmol/L; TC: 8.3±1.7 vs 7.2±1.5 vs 7.3±1.1 vs 5.1±1.1 mmol/L). Presence of tendon xanthomata, personal history of CAD, premature corneal arcus and hypertension were significantly associated with positive FH, with odds ratio of 9.77, 5.55, 2.93 and 2.37 respectively, compared to Unlikely FH individuals.
Table 1. Distribution of Participants into Definite, Probable, Possible and Unlikely FH according to the DLCN criteria ( n = 5130) .
|
† Potential FH (Definite and Probable FH, n =55) |
Definite FH ( n =14) | Probable FH ( n =41) | Possible FH ( n =374) | Unlikely FH ( n =4701) | |
|---|---|---|---|---|---|
| Age (years) #A | 59.0±10.1 | 59.8±10.3 @ | 58.7±10.1 * ^ | 50.5±13.1 * ^ | 40.3±15.4 *@ |
| Gender ^ | |||||
| Male | 25 (45.5%) | 5 (35.7%) | 20 (48.8%) | 170 (45.5%) | 1753 (37.3%) |
| Female | 30 (54.5%) | 9 (64.3%) | 21 (51.2%) | 204 (54.5%) | 2948 (62.7%) |
| Ethnicity | |||||
| Malay | 50 (90.9%) | 14 (100.0%) | 36 (87.8%) | 292 (78.1%) | 3409 (72.5%) |
| Chinese | 0 | 0 | 0 | 4 (1.1%) | 161 (3.4%) |
| Indian | 0 | 0 | 0 | 12 (3.2%) | 87 (1.9%) |
| Others | 5 (9.1%) | 0 | 5 (12.2%) | 66 (17.6%) | 1044 (22.2%) |
| LDL-c (mmol/L) (Baseline) #A | 6.2±1.3 | 7.4±1.4 * | 5.8±1.1 * | 5.6±0.7 * | 3.1±0.9 * |
| TC (mmol/L) A | 7.5±1.6 | 8.3±1.7 * | 7.2±1.5 * | 7.3±1.1 * | 5.1±1.1 * |
| TG (mmol/L) B | 1.7, IQR 0.9 | 1.8, IQR 2.4 @ | 1.6, IQR 3.4^ | 1.8, IQR 1.1 * | 1.4, IQR 1.1 * ^ @ |
| HDL (mmol/L) #A | |||||
| Males | 1.3±0.4 | 1.1±0.2 | 1.4±0.5^ | 1.2±0.3 * | 1.1±0.3 * ^ |
| Females | 1.6±0.5 | 1.9±0.6 * ^ @ | 1.4±0.3 * | 1.4±0.4^ | 1.4±0.4 @ |
| Waist circumference (cm) #A | |||||
| Males | 89.2±12.0 | 90.9±7.2 | 88.7±13.1 | 92.3±12.2 | 90.1±13.1 |
| Females | 88.8±12.6 | 81.8±9.7 | 91.7±12.7 | 88.7±10.7 | 82.9±12.9 |
| Tendon xantomata # | |||||
| Yes | 6 (13.6%) | 6 (50.0%) | 0 (0.0%) | 0 (0.0%) | 5 (0.4%) |
| No | 38 (86.4%) | (50.0%) | 32 (100.0%) | 87 (100.0%) | 1222 (99.6%) |
| Premature corneal arcus # | |||||
| Yes | 41 (95.3%) | 10 (90.9%) | 31 (96.9%) | 6 (7.0%) | 201 (16.3%) |
| No | 2 (4.7%) | 1 (9.1%) | 1 (3.1%) | 80 (93.0%) | 1029 (83.7%) |
| Diabetes mellitus #C | |||||
| Yes | 9 (17.3%) | 4 (7.7%) | 8 (20.5%) | 44 (12.3%) | 374 (8.2%) |
| No | 43 (82.7%) | 12 (92.3%) | 31 (79.5%) | 314 (87.7%) | 4204 (91.8%) |
| Hypertension #C | |||||
| Yes | 29 (52.7%) | 4 (28.6%) | 23 (56.1%) * | 176 (47.7%) * | 1317 (28.3%) * |
| No | 26 (47.3%) | 10 (71.4%) | 18 (43.9%) * | 176 (52.3%) * | 3329 (71.7%) |
| Blood pressure | |||||
| (Systolic/Diastolic mmHg) #A |
132.2 (±18.8)/ 77.7 (±10.4) |
127.4 (±12.2)/ 75.8 (±10.2) |
133.8 (±20.3)/ 78.4 (±10.5)^ |
132.1 (±20.9)/ 80.3 (±12.0) * |
123.2 (±19.1)/ 76.4 (±11.7) * ^ |
| History of premature CAD #C | |||||
| Yes | 6 (10.9%) | 2 (14.3%) * | 4 (9.8%) * | 22 (6.2%) * | 60 (1.3%) * |
| No | 49 (89.1%) | 12 (85.7%) | 37 (90.2%) | 333 (93.8%) | 4539 (98.7%) |
| Smoking status # | |||||
| Current smoker | 5 (9.3%) | 1 (7.7%) | 4 (9.8%) | 59 (16.3%) | 572 (12.5%) |
| Ever smoker | 10 (18.5%) | 2 (15.4%) | 8 (19.5%) | 78 (21.5%) | 435 (9.5%) |
| Non-smoker | 39 (72.2%) | 10 (76.9%) | 29 (70.7%) | 225 (62.2%) | 3575 (78.0%) |
| On lipid-lowering therapy #C | |||||
| Yes | 30 (56.6%) | 10 (71.4%) * | 20 (51.3%) * | 114 (32.2%) * | 228 (5.1%) * |
| No | 23 (43.4%) | 4 (28.6%) * | 19 (48.7%) * | 240 (67.8%) * | 4258 (94.9%) |
† = Potential FH is a combination of Definite and Probable FH
Normally distributed continuous data are presented as mean±standard deviation.
Non-normally distributed continuous data are presented as median with interquartile range (IQR).
# Subjects with no data were excluded A = One-way ANOVA
B = Kruskal Wallis followed by Mann Whitney tests C = Chi-squared test
* ^ @ = p < 0.05. Statistical tests with same symbols in a same row are significantly different with each other.
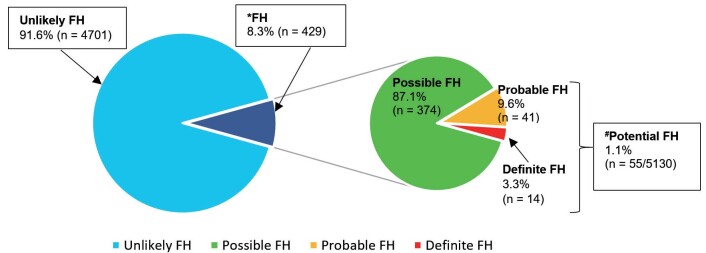
Fig.1 shows the prevalence of FH among the study participants. Out of 5130, 429 (8.3%) were found to have Possible, Probable or Definite FH. The prevalence of clinically diagnosed FH as defined by having Potential FH (Probable and Definite FH) was about 1 in 100 individuals (1.1%). Based on the current Malaysian total population of 32 million and the prevalence of 1:100, it was estimated that there were approximately 320,000 of individuals with Potential FH in Malaysia. Assuming that all FH cases in Malaysia were reported 36 - 43) , and taking 1:500 global prevalence of FH, this translates to an estimated detection rate of 2.3% and 0.3% for all categories of FH and potential FH respectively.
Fig.1. Prevalence of FH in the Malaysian community ( N =5130) .
* FH was defined as those with Possible, Probable and Definite FH by DLCN.
# Prevalence of clinically diagnosed FH was defined as those with Potential FH (Probable or Definite FH)
Table 2 shows the distribution of participants on lipid-lowering therapy and proportion achieving therapeutic target according to the FH categories. The percentage of participants who received lipid-lowering medication increased from 32.2 to 71.4% across Possible to Definite FH categories. All treated FH participants in this study acquired their treatment at their local primary healthcare centres. Virtually all who received the lipid-lowering therapy were treated with statin or statin combination. None of the participants with FH on lipid-lowering medications achieved the therapeutic target of LDL-c <1.4 mmol/L or <1.8 mmol/L, the recommended therapeutic target of post-lipid-lowering treatment for very high-risk group 44 , 45) .
Table 2. Distribution of participants on lipid-lowering therapy and proportion achieving therapeutic target according to the FH groups.
| Therapeutic target 44 - 47) | *# Potential FH ( n =53) | Definite FH ( n =14) | # Probable FH ( n =39) | # Possible FH ( n =354) |
|---|---|---|---|---|
| On lipid-lowering therapy # | 30 (56.6%) | 10 (71.4%) | 20 (51.3%) | 114 (32.2%) |
| LDL-c <3.4 mmol/L | 1 (3.3%) | 0 | 1 (5.0%) | 8 (7.0%) |
| (% achieving therapeutic target) | ||||
| LDL-c <3.0 mmol/L | 0 | 0 | 0 | 1 (0.9%) |
| (% achieving therapeutic target) | ||||
| LDL-c ≤ 2.6 mmol/L | 0 | 0 | 0 | 0 |
| (% achieving therapeutic target) | ||||
| LDL-c <1.8 mmol/L | 0 | 0 | 0 | 0 |
| (% achieving therapeutic target) | ||||
| LDL-c <1.4 mmol/L | 0 | 0 | 0 | 0 |
| (% achieving therapeutic target) |
* Potential FH is a combination of Definite and Probable FH
# Subjects with no data were excluded
Discussion
This study is the first to report the prevalence of clinically-diagnosed FH in the Malaysian community, as defined by having Potential FH (Definite and Probable FH) according to the DLCN criteria. This definition was used in order to standardise the reporting method with other Western countries.
Our study shows that the prevalence of Potential FH in the Malaysian population was 1.1% i.e. about 1 in 100 individuals. This is no doubt higher than the reported global prevalence of 1 in 250 12) , and surprisingly six times higher than what has been estimated two decades ago 48) , based on the data acquired from Singapore Lipid Clinic 49) . This high prevalence of FH has put Malaysia among the highest reported FH prevalence in the Western Pacific region, just below China, and above Russia that respectively have reported FH prevalence of 1 in 58 and 1 in 108 18 , 50) . The high prevalence of FH in these three countries could be explained by the fact that physical examinations to identify premature corneal arcus and tendon xanthomata were performed in these studies. Besides high level of LDL-c, premature corneal arcus and tendon xanthomata are the cardinal signs for FH that contribute towards high scores in DLCN scoring. Omitting these physical signs in FH clinical assessment may lead to under-detection of FH patients, as indicated in this study where people with tendon xanthomata has almost 10-fold chance of being identified as Potential FH. Therefore, there is a possibility that other countries such as Australia and Taiwan may obtain higher prevalence of FH than what they currently reported if physical examination had been incorporated into their studies. On the other hand, there were only a few countries that have reported FH prevalence of more frequent than 1% (1 in 100 individuals), such as in South Africa who had molecularly confirmed FH prevalence of 1:83 among 1612 Afrikaners 51) . A meta-analysis on global data of FH in patients on statin treatment also discovered very high prevalence of FH such as in Egypt (5.9%), Baltic States (4.9%) and Slovenia (3.4%) 52) . The high FH prevalence were expected in these populations as they predominantly included statin-treated patients with CAD.
The majority of the Potential FH in this study was comprised of Probable FH patients, where the DLCN scores were substantially contributed by the presence corneal arcus. However, none of the Probable FH patients in this study has tendon xanthomata, which raise the concern of overdiagnosis due to DLCN overscoring by the presence of corneal arcus (4 points). However, extra care has been taken where only patients with premature corneal arcus which appeared at age of <45 years old were recorded as positive corneal arcus. The absence of tendon xanthomata in Probable FH patients could possibly be due to the fact that tendon xanthomata can be regressed by lipid-lowering medications 53) , which had been prescribed in half of the Probable FH patients in this study, while for corneal arcus, the manifestation is non-regressible 54) . Another possible explanation for this observation is that this present study was an epidemiological study based in the community, where corneal arcus can be easily detected whilst tendon xanthomata may be easily missed by the less experience doctors. This is particularly so when the signs are subtle, especially in the in the statin-treated patients.
Another possibility that could explain the high prevalence of FH in the Malaysian population is the founder effect and consanguineous marriages. Founder effect is known to contribute towards high prevalence of FH in some countries with high composition of none native-ethnics, where those ethnics may have carried the pathogenic FH genetic variants from their countries of origin and introduced the founder effect for FH after thriving for several generations in the new countries 3 , 55) . Malaysia is an ethnically diversified country, where >30% of the Malaysian citizens are non-native (Chinese or Indians ethnicities), who may introduce founder effect for FH in the Malaysian population. In this study, although more than 90% of Potential FH were found in native Malays, the other ethnic groups were under-represented, hence the possibility of founder effect cannot be totally excluded. Therefore, future studies should address the possible founder effect of FH in the Malaysian community. Consanguineous marriages are also noted to be prevalent in certain states in Malaysia which parallels the high prevalence of FH in those states (data not shown).
The high mean TC and LDL-c levels across the FH groups in our study could be explained by the unhealthy diet among the Malaysian population. Culturally, Malaysian traditional foods such as ‘nasi lemak’ (coconut oil rice) and ‘roti canai’ (Indian-style flatbread) are inherently high in saturated fat content 56) . Together with the gradual transition of Malaysian dietary pattern to urban fast food consumption 57) , Malaysian population are generally consuming more fat compared to other Western Pacific Countries, which subsequently may have caused the high level of mean TC and LDL-c in the Malaysian population, and ultimately may tip the Malaysian DLCN score to the higher side.
Genetic testing is not a routine test for FH even in an advanced Western Pacific countries like Japan, where their national FH guideline extensively relies on clinical assessment such as LDL-c level and examination of tendon xanthomata rather than genetic finding 16) . The Japanese guideline only utilise the genetic testing as a differential test to distinguish between FH and autosomal recessive hypercholesterolaemia, where clinical manifestation for both disorders are similar, but the latter is caused by different genetic mutations (ATP-binding cassette transporter G5 or G8) than that in FH 58) . Given that FH is an inherited genetic disorder, the ultimate way to diagnose it is supposedly by genetic testing. However, diagnosis of FH, even in Malaysia, are usually made clinically due to limited financial resources to conduct genetic testing. Based on the current FH prevalence of 1:100, Malaysia has clinically detected only 0.5% of the total estimated individuals with FH, which is substantially lower when compared to other Western Pacific countries with advance healthcare system, such as Japan (26%) and Australia-New Zealand (4%) 59) . In countries where genetic testing for FH is funded by the government, such as the Netherlands, a commendable genetic testing rate of 71% has been reported 60) . Financial constraints, lack of human resources and workforce experience, in addition to lack of widely available genetic testing facilities are among barriers to FH genetic testing in the Asia-Pacific region including Malaysia 61 , 62) . Currently, genetic testing is still not a routine protocol for FH management in Malaysia and most Asian countries, and is only possibly conducted as part of clinical research, funded by the universities, government or industrial grants. There are several Malaysian studies which reported various FH genetic variants from LDLR to PCSK9 38 , 63 - 67) . However, some pathogenic LDLR variants such as LDLR c.763T>A 37 , 42 , 64 , 68) and c.301G>A 37 , 64 , 69) were detected in multiple FH patients by unrelated Malaysian study groups, which give a glimpse of potential utilisation of genetic testing as standard diagnostic protocol in detecting common pathogenic FH genetic variants in Malaysia Healthcare system.
Enhancing genetic testing and implementation of family cascade screening may increase FH detection. Data has shown that some FH genetic mutations are found in individuals with moderate LDL-c level (3.4 - 4.8 mmol/L) 9) , hence the reported FH prevalence in our study could be under-reported as some individuals with moderate level of LDL-c were categorised as Unlikely or Possible FH by DLCN. The probability of under-detection of FH by clinical diagnosis without genetic testing was evidenced by a Dutch population study, where an FH prevalence of 1:137 was reported when over 60,000 of community participants were genetically screened for pathogenic LDLR and APOB variants 35) . In contrast, another Dutch community-based meta-analysis which had solely rely on clinical diagnosis, reported a lower FH prevalence of 1:232 70) . Furthermore, genetic testing for FH, an autosomal dominant disorder with high penetrance, is valuable to enhance diagnostic precision, enable cascade testing, improve risk prediction, treatment adherence, and justify use of potent expensive medications 61) . The use and wider application of genetic testing for FH will be enhanced by rapidly advancing technology, but cost-effectiveness, patient preferences and health care professional training in genomic medicine remain key issues that need to be addressed.
The lipid-lowering treatment coverage for individuals with Potential FH in the Malaysian community was well over 50%. This is comparable to other countries such as Australia and the Netherlands that have the lipid-lowering therapy coverage of 67% and 48% in their respective communities 15 , 35) . However, the achievement for therapeutic target of the lipid-lowering therapy in our study population did not share the same optimism, where none of the individuals with Potential and Possible FH in the community achieved the therapeutic LDL-c targets. In contrast to our finding, the Centralized Pan-Asian Survey on the Under Treatment of Hypercholesterolemia (CEPHEUS) reported a therapeutic target achievement ( n =112) of 36.6% 71) . However, individuals with FH recruited in our study included newly diagnosed FH, whereas FH patients in the CEPHEUS were previously diagnosed and aggressively treated. This may explain why the LDL-c target achievement in CEPHEUS was better than that in this present study. Poor therapeutic LDL-c target achievement among individuals with FH in the Malaysian community could be due to the lack of awareness about FH among primary care physicians and the patients themselves. This is supported by a study by Azraii et al. where less than half of Malaysian primary care physicians were familiar with FH, and only about a quarter of them were aware of FH diagnostic criteria 72) . Therefore, increasing awareness and knowledge regarding FH among primary care physicians in Malaysia through educational training should be a priority. Primary care physicians need to be more vigilant in identifying and managing FH. Individuals with Potential FH should have been clinically identified earlier by primary care physicians and treated more aggressively to target LDL-c levels for high-risk category patients. Referral to lipid specialist and/or cardiologist in secondary care setting should also be done early for diagnostic confirmation, treatment optimisation and family cascade screening for early detection of affected family members. Another barrier to lipid-lowering treatment among individuals with FH is the treatment cost, especially among those with uncontrolled LDL-c. Aside from subsidised oral lipid-lowering agents, the treatment modalities for patients with uncontrolled severely elevated LDL-c are lipoprotein apheresis and injectable PCSK9 inhibitors 73) . However, both of these therapeutic modalities are expensive and are therefore not subsidised in Malaysia 13) , making the management of FH in patients with severely elevated LDL-c difficult.
Improving FH management starts with accurate early clinical detection and treatment, and where possible genetic testing, in addition to family cascade screening. However, even without molecular testing, additional inexpensive assay such as measurement of Lp(a) may improve the accuracy of DLCN and Simon Broome FH clinical diagnostic criteria. Clinical diagnosis of FH without genetic confirmation is heavily dependent on LDL-c. Indirect LDL-c measurement, which is derived from Friedewald equation, includes TC in the calculation that may be contributed by Lp(a), where Lp(a) level is highly varied among individuals 74) . A recent study also demonstrated that the accuracy of DLCN and Simon Broome criteria can be affected by Lp(a) level, and thus has recommended that all suspected FH patients to have their Lp(a) measured 75) . In addition, elevated Lp(a) serum level which is predominantly genetically determined, has great value in risk prediction. However, at present, genetic testing for identification of LPA gene copy number variants has no clinical diagnostic role 61) .
Strengths and Limitations
The strength of this study include the application of physical examination to identify premature corneal arcus and tendon xanthomata for more accurate clinical diagnosis of FH. This study also recruited the largest number of FH patients, covering various states and regions in Malaysia, compared to other previous smaller scale FH studies ever conducted in Malaysia. Therefore, the prevalence of FH reported in this study is the best representation of the Malaysian population which we have to date. However, this study has several limitations. Firstly, majority of the participants in this study were Malays, Bidayuh and Dusun. The Malaysian Chinese and Indians were under-represented in this study. Ideally, prevalence study in a multi-ethnic country such as Malaysia requires proportional coverage of all major ethnic groups in the country. Second, the participants were recruited using convenient sampling method, which may introduce selection bias when the demographic nature of any of the Health Screening sites were not heterogenous. However, the possibility of sampling bias in this study is remedied by the fact that the Health Screening Programmes were carried out at multiple sites in almost all states, which may sufficiently be representative to the whole Malaysian population. Third, this study also acquired participant’s clinical history through face-to-face interview, where the participants may not give the most accurate answer, especially the medical history of their family members. More often than not, participants did not remember the exact age of onset for CAD in their family members, or the baseline LDL-c level in their statin-treated family members. Lastly, due to majority of the participants not being able to recall the type and dose of their lipid-lowering medication, half of the reported baseline LDL-c for potential FH were estimated using the LDL-c conversion algorithm, which may affect the accuracy of the reported FH prevalence. The only way to obtain the actual baseline LDL-c level were to access the patients’ medical record in their respective clinics, but this was technically and logistically not feasible to be done by our research team at that time.
Implications on Future Research and Clinical Practice
The findings of this study suggest that clinically diagnosed FH is highly prevalent, and is under-detected and under-treated in the Malaysian community. However, Malaysia is a unique multiracial country. Epidemiological study of disease that can be implicated by genetic diversity should take the participants racial proportion into consideration. Therefore, future study on FH prevalence which includes all the major ethnic groups by probability sampling in Malaysia is recommended. Further research should also include identification of FH by genetic testing among the Malaysian population.
Our study shows poor therapeutic target achievement among FH patients treated with lipid-lowering medications. Majority of them received their lipid lowering prescriptions from their local primary healthcare centres. This indicates lack of awareness, knowledge and skills to optimally manage FH among primary care physicians. These Potential FH patients should be referred to the Lipid Specialists for confirmation of diagnosis and further management which include cascade screening of family members. Primary care physicians are ideally suited to play a pivotal role in the early detection and treatment of FH in the community. To increase awareness and knowledge regarding FH among primary care physicians in Malaysia, FH training module and clinical guideline need to be developed. Additionally, the community itself has to be educated on health consequences of FH through government-organised health campaign or FH family support group 76 , 77) , which are yet to be initiated in Malaysia. Well-informed community are more prone to self-report their condition, or adhere to their lipid-lowering medications if they are aware of the consequences of FH 78) . Lastly, a national FH registry has to be established in Malaysia in order to integrate the FH data collection, which is not only useful for epidemiological study, but essential in standardising the FH management throughout the country.
Conclusion
The prevalence of clinically diagnosed FH in the Malaysian community is 1:100 which is much higher than that of the global general population, with low detection rate of <1.0%. Molecular testing for FH may improve detection rate and family cascade screening, but cost-effectiveness, patient preferences and health care professional training in genomic medicine remain key issues that need to be addressed. FH is under-detected and under-treated with poor achievement of LDL-c targets in Malaysian community. Increasing awareness and knowledge regarding FH among primary care physicians and the community may improve early detection and optimal treatment. Timely referral to secondary care for further management of poorly controlled LDL-c and family cascade screening is also pivotal to reduce complications including premature CAD. Public Health policies should include strategies including public education, development of FH training module and clinical practice guidelines for clinicians. A national FH registry needs to be further supported to enhance clinical research and improvement in comprehensive FH management.
Acknowledgement
This study was funded by University Teknologi MARA MITRA Grant [600-IRMI/MYRA 5/3/MITRA (003/2017)] and Malaysia Ministry of Higher Education Long Term Research Grant Scheme (RMI/ST/LRGS5/3 (2/2011), which were awarded to the corresponding author. Special appreciation to the Malaysian Professorial Council (MPN) for support in the National Health Screening Programmes; all Council and Committee Members; and Fadlullah Jili Fursani, Ku Izani Che Ku Yusoff, along with other staff of I-PPerForM and Centre for Pathology Diagnostic and Research Laboratories (CPDRL) for their assistance rendered in performing the routine laboratory tests.
Conflict of Interest
There is no other conflict of interest to declare.
Appendix
Malaysian Health and Wellbeing Assessment for Familial Hypercholesterolaemia (MyHEBAT - FH)
1. Hapizah Nawawi
2. Noor Alicezah Mohd Kasim
3. Noor Shafina Mohd Nor
4. Anis Safura Ramli
5. Salmi Razali
6. Suraya Abdul Razak
7. Alyaa Al-Khateeb
8. Yung-An Chua
9. Thuhairah Hasrah Abdul Rahman
10. Sazzli Shahlan Kasim
11. Suhaila Muid
12. Azhari Rosman
13. Ahmad Bakhtiar Md Radzi
14. Khairul Shafiq Ibrahim
15. Sukma Azureen Nazli
16. Aimi Zafira Razman
References
- 1).Defesche JC, Gidding SS, Harada-Shiba M, Hegele RA, Santos RD, and Wierzbicki AS: Familial hypercholesterolaemia. Nat Rev Dis Primers, 2017; 3: 17093 [DOI] [PubMed] [Google Scholar]
- 2).Truong PK, Lao TD, and Le THA: The Major Molecular Causes of Familial Hypercholesterolemia. Asian J Pharma Res Health Care, 2018; 10: 60-68 [Google Scholar]
- 3).Austin MA, Hutter CM, Zimmern RL, and Humphries SE: Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol, 2004; 160: 407-420 [DOI] [PubMed] [Google Scholar]
- 4).Mabuchi H: Half a century tales of familial hypercholesterolemia (FH) in Japan. J Atheroscler Thromb, 2017; 24: 189-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Goldstein JL and Brown MS: A century of cholesterol and coronaries: from plaques to genes to statins. Cell, 2015; 161: 161-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Versmissen J, Oosterveer DM, Yazdanpanah M, Defesche JC, Basart DC, Liem AH, Heeringa J, Witteman JC, Lansberg PJ, and Kastelein JJ: Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ, 2008; 337: a2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).World Health Organization, Noncommunicable Diseases (NCD) Country Profiles. 2018 [Google Scholar]
- 8).Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, and Murray CJ: Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation, 2015; 132: 1667-1678 [DOI] [PubMed] [Google Scholar]
- 9).Khera AV, Won H-H, Peloso GM, Lawson KS, Bartz TM, Deng X, Van Leeuwen EM, Natarajan P, Emdin CA, and Bick AG: Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol, 2016; 67: 2578-2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, Cuchel M, Ose L, Averna M, Boileau C, and Borén J: Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J, 2015; 36: 2425-2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, and Defesche JC: Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease. Eur Heart J, 2013; 34: 3478-3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Akioyamen LE, Genest J, Shan SD, Reel RL, Albaum JM, Chu A, and Tu JV: Estimating the prevalence of heterozygous familial hypercholesterolaemia: a systematic review and meta-analysis. BMJ Open, 2017; 7: e016461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Vallejo-Vaz AJ, De Marco M, Stevens CaT, Akram A, Freiberger T, Hovingh GK, Kastelein JJP, Mata P, Raal FJ, Santos RD, Soran H, Watts GF, Abifadel M, Aguilar-Salinas CA, Al-Khnifsawi M, Alkindi FA, Alnouri F, Alonso R, Al-Rasadi K, Al-Sarraf A, Ashavaid TF, Binder CJ, Bogsrud MP, Bourbon M, Bruckert E, Chlebus K, Corral P, Descamps O, Durst R, Ezhov M, Fras Z, Genest J, Groselj U, Harada-Shiba M, Kayikcioglu M, Lalic K, Lam CSP, Latkovskis G, Laufs U, Liberopoulos E, Lin J, Maher V, Majano N, Marais AD, März W, Mirrakhimov E, Miserez AR, Mitchenko O, Nawawi HM, Nordestgaard BG, Paragh G, Petrulioniene Z, Pojskic B, Postadzhiyan A, Reda A, Reiner Ž, Sadoh WE, Sahebkar A, Shehab A, Shek AB, Stoll M, Su T-C, Subramaniam T, Susekov AV, Symeonides P, Tilney M, Tomlinson B, Truong T-H, Tselepis AD, Tybjærg-Hansen A, Vázquez-Cárdenas A, Viigimaa M, Vohnout B, Widén E, Yamashita S, Banach M, Gaita D, Jiang L, Nilsson L, Santos LE, Schunkert H, Tokgözoğlu L, Car J, Catapano AL, and Ray KK: Overview of the current status of familial hypercholesterolaemia care in over 60 countries - The EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Atherosclerosis, 2018; 277: 234-255 [DOI] [PubMed] [Google Scholar]
- 14).Pang J, Sullivan DR, Harada-Shiba M, Ding PYA, Selvey S, Ali S, and Watts GF: Significant gaps in awareness of familial hypercholesterolemia among physicians in selected Asia-Pacific countries: A pilot study. J Clin Lipidol, 2015; 9: 42-48 [DOI] [PubMed] [Google Scholar]
- 15).Watts GF, Shaw JE, Pang J, Magliano DJ, Jennings G, and Carrington MJ: Prevalence and treatment of familial hypercholesterolaemia in Australian communities. Int J Cardiol, 2015; 185: 69 [DOI] [PubMed] [Google Scholar]
- 16).Harada-Shiba M, Arai H, Oikawa S, Ohta T, Okada T, Okamura T, Nohara A, Bujo H, Yokote K, and Wakatsuki A: Guidelines for the management of familial hypercholesterolemia. J Atheroscler Thromb, 2012; 19: 1043-1060 [DOI] [PubMed] [Google Scholar]
- 17).Mabuchi H, Nohara A, Noguchi T, Kobayashi J, Kawashiri M-A, Tada H, Nakanishi C, Mori M, Yamagishi M, and Inazu A: Molecular genetic epidemiology of homozygous familial hypercholesterolemia in the Hokuriku district of Japan. Atherosclerosis, 2011; 214: 404-407 [DOI] [PubMed] [Google Scholar]
- 18).Cao Y-X, Sun D, Liu H-H, Jin J-L, Li S, Guo Y-L, Wu N-Q, Zhu C-G, Gao Y, and Dong Q-T: A Novel Modified System of Simplified Chinese Criteria for Familial Hypercholesterolemia (SCCFH). Mol Diagn Ther, 2019; 23: 547-553 [DOI] [PubMed] [Google Scholar]
- 19).Li Y-H, Ueng K-C, Jeng J-S, Charng M-J, Lin T-H, Chien K-L, Wang C-Y, Chao T-H, Liu P-Y, Su C-H, Chien S-C, Liou C-W, Tang S-C, Lee C-C, Yu T-Y, Chen J-W, Wu C-C, and Yeh H-I: 2017 Taiwan lipid guidelines for high risk patients. J Formos Med Assoc, 2017; 116: 217-248 [DOI] [PubMed] [Google Scholar]
- 20).Pek SLT, Dissanayake S, Fong JCW, Lin MX, Chan EZL, Justin I, Tang S, Lee CW, Ong HY, and Sum CF: Spectrum of mutations in index patients with familial hypercholesterolemia in Singapore: Single center study. Atherosclerosis, 2018; 269: 106-116 [DOI] [PubMed] [Google Scholar]
- 21).Pongrapeeporn K-U, Leowattana W, Nuchpramool W, Kerdsaeng K, Thepsuriyanon P, Kiartivich S, Yamwong P, Ong-Ajyooth S, Amornrattana A, and Kasemsuk L: Screening for mutations in exons encoding the ligand-binding domain of the LDL receptor gene using PCR-CFLP and PCR-SSCP. J Med Assoc Thailand, 2001; 84: S619-27 [PubMed] [Google Scholar]
- 22).Punzalan FER, Sy RG, Santos RS, Cutiongco EM, Gosiengfiao S, Fadriguilan E, George P, and Laurie A: Low Density Lipoprotein− Receptor (LDL-R) Gene Mutations among Filipinos with Familial Hypercholesterolemia. J Atheroscler Thromb, 2005; 12: 276-283 [DOI] [PubMed] [Google Scholar]
- 23).Truong PK, Van Bui C, Lao TD, and Le THA: Detection of Defective Apolipoprotein B-100 R3500Q Mutation Caused Familial Hypercholesterolemia in Vietnamese Patients. in International Conference on the Development of Biomedical Engineering in Vietnam. 2017. Ho Chi Minh: Springer [Google Scholar]
- 24).Charng M-J and Huang C-C: Genetic Diagnosis of Familial Hypercholesterolemia in Asia. Frontiers in Genetics, 2020; 11: 833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Watts GF, Ding PY, George P, Hagger MS, Hu M, Lin J, Khoo KL, Marais AD, Miida T, and Nawawi HM: Translational research for improving the care of familial hypercholesterolemia: The “Ten Countries Study” and beyond. J Atheroscler Thromb, 2016; 23: 891-900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Pang J, Hu M, Lin J, Miida T, Nawawi HM, Park JE, Wu X, Ramli AS, Kim NT, and Gonzalez-Santos LE: An enquiry-based on a standardised questionnaire into knowledge, awareness and preferences concerning the care of familial hypercholesterolaemia among primary care physicians in the Asia-Pacific region: The “Ten Countries Study”. BMJ Open, 2017; 7: e017817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Ministry of Health Malaysia, National Health and Morbidity Survey 2019. 2020 [Google Scholar]
- 28).Nawawi HM, Nor IM, Noor IM, Karim NA, Arshad F, Khan R, and Yusoff K: Current status of coronary risk factors among rural Malays in Malaysia. Journal of cardiovascular risk, 2002; 9: 17-23 [DOI] [PubMed] [Google Scholar]
- 29).World Health Organization. Global Health Observatory (GHO) data - Raised cholesterol. 2008 [accessed 13 Jul, 2019]; Available from: https://www.who.int/gho/ncd/risk_factors/cholesterol_text/en/ [Google Scholar]
- 30).De Backer G, Besseling J, Chapman J, Hovingh GK, Kastelein JJP, Kotseva K, Ray K, Reiner Ž, Wood D, and De Bacquer D: Prevalence and management of familial hypercholesterolaemia in coronary patients: An analysis of EUROASPIRE IV, a study of the European Society of Cardiology. Atherosclerosis, 2015; 241: 169-175 [DOI] [PubMed] [Google Scholar]
- 31).Rekha A and Rai DK: Tendon xanthomas. The Foot, 2010; 20: 85-86 [DOI] [PubMed] [Google Scholar]
- 32).Zech LA and Hoeg JM: Correlating corneal arcus with atherosclerosis in familial hypercholesterolemia. Lipids in Health and Disease, 2008; 7: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Friedewald WT, Levy RI, and Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 1972; 18: 499-502 [PubMed] [Google Scholar]
- 34).Ellis KL, Pang J, Chan DC, Hooper AJ, Bell DA, Burnett JR, and Watts GF: Familial combined hyperlipidemia and hyperlipoprotein (a) as phenotypic mimics of familial hypercholesterolemia: Frequencies, associations and predictions. J Clin Lipidol, 2016; 10: 1329-1337 [DOI] [PubMed] [Google Scholar]
- 35).Benn M, Watts GF, Tybjaerg-Hansen A, and Nordestgaard BG: Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab, 2012; 97: 3956-3964 [DOI] [PubMed] [Google Scholar]
- 36).Al-Khateeb A, Al-Talib H, Mohamed MS, Yusof Z, and Zilfalil BA: Phenotype-genotype analyses of clinically diagnosed Malaysian familial hypercholestrolemic patients. Adv Clin Exp Med, 2013; 22: 57-67 [PubMed] [Google Scholar]
- 37).Khoo KL, Page MM, Liew YM, Defesche JC, and Watts GF: Ten years of lipoprotein apheresis for familial hypercholesterolemia in Malaysia: A creative approach by a cardiologist in a developing country. J Clin Lipidol, 2016; 10: 1188-1194 [DOI] [PubMed] [Google Scholar]
- 38).Lye S-H, Chahil JK, Bagali P, Alex L, Vadivelu J, Ahmad WaW, Chan S-P, Thong M-K, Zain SM, and Mohamed R: Genetic polymorphisms in LDLR, APOB, PCSK9 and other lipid related genes associated with familial hypercholesterolemia in Malaysia. PloS One, 2013; 8: e60729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Khoo KL, Van Acker P, Tan H, and Deslypere J: Genetic causes of familial hypercholesterolaemia in a Malaysian population. Med J Malaysia, 2000; 55: 409-418 [PubMed] [Google Scholar]
- 40).Choong M-L, Koay ES, Khoo K-L, Khaw M-C, and Sethi SK: Denaturing gradient-gel electrophoresis screening of familial defective apolipoprotein B-100 in a mixed Asian cohort: two cases of arginine3500® tryptophan mutation associated with a unique haplotype. Clin Chem, 1997; 43: 916-923 [PubMed] [Google Scholar]
- 41).Kyi WM, Isa MN, Rashid FA, Osman JaM, and Mansur MA: Absence of Apo B R3500Q Mutation among Kelantanese Malays with Hyperlipidaemia. Malays J Med Sci, 2000; 7: 16 [PMC free article] [PubMed] [Google Scholar]
- 42).Azian M, Hapizah M, Khalid B, Khalid Y, Rosli A, and Jamal R: Use of the denaturing gradient gel electrophoresis (DGGE) method for mutational screening of patients with familial hypercholesterolaemia (FH) and familial defective apolipoprotein B100 (FDB). Malays J Pathol, 2006; 28: 7-15 [PubMed] [Google Scholar]
- 43).Masaany M, Siti HS, Nurliza I, and Mazita A: Bilateral middle ear cholesterol granuloma in familial hypercholesterolemia. Otolaryngology—Head and Neck Surgery, 2008; 138: 803-804 [DOI] [PubMed] [Google Scholar]
- 44).Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O, and Group ESD: 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J, 2019; 41: 111-188 [Google Scholar]
- 45).Ministry of Health Malaysia, Management of Dyslipidaemia 2017, M.o.H. Malaysia, Editor. 2017, Ministry of Health: Malaysia [Google Scholar]
- 46).Rajadurai J, Wan Ahmad WA, Nawawi H, Choo GH, Ng WK, Mohd Ali R, Omar AF, Kasim S, Maskon O, and Quek DKL: Updates in the management of dyslipidaemia in the high and very high risk individual for CV risk reduction. Med J Malaysia, 2018; 73: 154 [PubMed] [Google Scholar]
- 47).Stone NJ, Robinson JG, Lichtenstein AH, Merz CNB, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, and Lloyd-Jones DM: 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 2014; 63: 2889-2934 [DOI] [PubMed] [Google Scholar]
- 48).Khoo KL: Hereditary Hyperlipidemia in Malaysia: A historical Perspective of Six Decade of Research and Treatment. Med J Malaysia, 2014; 69: 57-59 [PubMed] [Google Scholar]
- 49).World Health Organization, Familial Hypercholesterolaemia (FH) - Report of a second WHO Consultation. 1999 [Google Scholar]
- 50).Ershova AI, Meshkov AN, Bazhan SS, Storozhok MA, Efanov AY, Medvedeva IV, Indukaeva EV, Danilchenko YV, Kuzmina OK, and Barbarash OL: The prevalence of familial hypercholesterolemia in the West Siberian region of the Russian Federation: A substudy of the ESSE-RF. PloS One, 2017; 12: e0181148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Steyn K, Goldberg YP, Kotze MJ, Steyn M, Swanepoel AS, Fourie JM, Coetzee GA, and Van Der Westhuyzen DR: Estimation of the prevalence of familial hypercholesterolaemia in a rural Afrikaner community by direct screening for three Afrikaner founder low density lipoprotein receptor gene mutations. Hum Genet, 1996; 98: 479-484 [DOI] [PubMed] [Google Scholar]
- 52).Catapano AL, Lautsch D, Tokgözoglu L, Ferrieres J, Horack M, Farnier M, Toth PP, Brudi P, Tomassini JE, and Ambegaonkar B: Prevalence of potential familial hypercholesteremia (FH) in 54,811 statin-treated patients in clinical practice. Atherosclerosis, 2016; 252: 1-8 [DOI] [PubMed] [Google Scholar]
- 53).Tsouli S, Kiortsis D, Argyropoulou M, Mikhailidis D, and Elisaf M: Pathogenesis, detection and treatment of Achilles tendon xanthomas. Eur J Clin Invest, 2005; 35: 236-244 [DOI] [PubMed] [Google Scholar]
- 54).Pietroleonardo L and Ruzicka T: Skin manifestations in familial heterozygous hypercholesterolemia. Acta Dermatovenerol Alp Panonica Adriat, 2009; 18: 183-187 [PubMed] [Google Scholar]
- 55).Mszar R, Buscher S, Taylor HL, Rice-Defosse MT, and Mccann D: Familial Hypercholesterolemia and the Founder Effect Among Franco-Americans: A Brief History and Call to Action. CJC Open, 2020; 2: 161-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Lipoeto NI, Lin KG, and Angeles-Agdeppa I: Food consumption patterns and nutrition transition in South-East Asia. Public health nutrition, 2013; 16: 1637-1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Noor MI: The nutrition and health transition in Malaysia. Public health nutrition, 2002; 5: 191-195 [DOI] [PubMed] [Google Scholar]
- 58).Harada-Shiba M, Arai H, Ishigaki Y, Ishibashi S, Okamura T, Ogura M, Dobashi K, Nohara A, Bujo H, and Miyauchi K: Guidelines for diagnosis and treatment of familial hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 751-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Nordestgaard BG and Benn M: Genetic testing for familial hypercholesterolaemia is essential in individuals with high LDL cholesterol: who does it in the world? Eur Heart J, 2017; 38: 1580-1583 [DOI] [PubMed] [Google Scholar]
- 60).Louter L, Defesche J, and Van Lennep JR: Cascade screening for familial hypercholesterolemia: Practical consequences. Atheroscler Suppl, 2017; 30: 77-85 [DOI] [PubMed] [Google Scholar]
- 61).Nawawi HM, Chua Y-A, and Watts GF: The brave new world of genetic testing in the management of the dyslipidaemias. Curr Opin Cardiol, 2020; 35: 226-233 [DOI] [PubMed] [Google Scholar]
- 62).Pang J, Chan DC, Hu M, Muir LA, Charng M-J, Florkowski CM, George PM, Lin J, Marais AD, and Nawawi HM: Comparative aspects of the care of familial hypercholesterolemia in the “Ten Countries Study”. J Clin Lipidol, 2019; 13: 287-300 [DOI] [PubMed] [Google Scholar]
- 63).Khoo K, Van Acker P, Defesche J, Tan H, Van De Kerkhof L, Heijnen-Van Eijk S, Kastelein J, and Deslypere J: Low-density lipoprotein receptor gene mutations in a Southeast Asian population with familial hypercholesterolemia. Clinical genetics, 2000; 58: 98-105 [DOI] [PubMed] [Google Scholar]
- 64).Al-Khateeb A, Hamzan NS, Razali R, Froemming GA, Rahman T, Peng HB, and Nawawi H: Genetic Study of Low-Density Lipoprotein Receptor Gene and Apolipoprotein B-100 Gene among Malaysian Patients with Familial Hypercholesterolaemia. Int Arch Med, 2016; 9: 1-12 [Google Scholar]
- 65).Al-Khateeb AR: Genetic researches among Malaysian familial hypercholesterolaemic population. J Health Transl Med, 2016; 19: 1-11 [Google Scholar]
- 66).Mohd Nor NS, Al-Khateeb AM, Chua Y-A, Mohd Kasim NA, and Nawawi H: Heterozygous familial hypercholesterolaemia in a pair of identical twins: a case report and updated review. BMC pediatrics, 2019; 19: 106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Al-Khateeb A, Hamzan NS, Razali R, Froemming GA, Rahman T, Peng HB, and Nawawi H: Genetic Study of Low-Density Lipoprotein Receptor Gene and Apolipoprotein B-100 Gene among Malaysian Patients with Familial Hypercholesterolaemia. Int Arch Med, 2016; 9: 1-12 [Google Scholar]
- 68).Mohd Kasim A, Razali R, Rahman T, Hoh B, Hamzan NS, Muid S, Nawawi H, and Koshy M: Homozygous familial hypercholesterolemia. Malays J Pathol, 2014; 36: 131-137 [PubMed] [Google Scholar]
- 69).Muhd Nur NI, Al-Khateeb AR, Wan Hitam WH, Alwi Z, and Ahmad Tajudin L-S: Low density lipoprotein receptor (LDLR) gene and ocular manifestation in Malay patients with familial hypercholesterolaemia. Malaysian Journal of Ophthalmology, 2019; 1: 98-113 [Google Scholar]
- 70).Lansberg P, Tuzgöl S, Defesche J, and Kastelein J: Higher prevalence of familial hypercholesterolemia than expected in adult patients of four family practices in Netherlands. Ned Tijdschr Genees, 2000; 144: 1437-1440 [PubMed] [Google Scholar]
- 71).Park JE, Chiang C-E, Munawar M, Pham GK, Sukonthasarn A, Aquino AR, Khoo KL, and Chan HWR: Lipid-lowering treatment in hypercholesterolaemic patients: the CEPHEUS Pan-Asian survey. Eur J Prev Cardiol, 2012; 19: 781-794 [DOI] [PubMed] [Google Scholar]
- 72).Azraii AB, Ramli AS, Ismail Z, Abdul-Razak S, Mohd-Kasim NA, Ali N, Watts GF, and Nawawi H: Knowledge, awareness and practice regarding familial hypercholesterolaemia among primary care physicians in Malaysia: The importance of professional training. Atherosclerosis, 2018; 277: 508-516 [DOI] [PubMed] [Google Scholar]
- 73).Makino H, Koezuka R, Tamanaha T, Ogura M, Matsuki K, Hosoda K, and Harada-Shiba M: Familial Hypercholesterolemia and Lipoprotein Apheresis. J Atheroscler Thromb, 2019; 26: 679-687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74).Li KM, Wilcken D, and Dudman N: Effect of serum lipoprotein (a) on estimation of low-density lipoprotein cholesterol by the Friedewald formula. Clin Chem, 1994; 40: 571-573 [PubMed] [Google Scholar]
- 75).Chan DC, Pang J, Hooper AJ, Bell DA, Burnett JR, and Watts GF: Effect of Lipoprotein (a) on the Diagnosis of Familial Hypercholesterolemia: Does it Make a Difference in the Clinic? Clin Chem, 2019; 65: 1258-1266 [DOI] [PubMed] [Google Scholar]
- 76).Watts GF, Gidding S, Wierzbicki AS, Toth PP, Alonso R, Brown WV, Bruckert E, Defesche J, Lin KK, and Livingston M: Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Int J Cardiol, 2014; 171: 309-325 [DOI] [PubMed] [Google Scholar]
- 77).Hagger MS, Hardcastle SJ, Hu M, Kwok S, Lin J, Nawawi HM, Pang J, Santos RD, Soran H, Su T-C, Tomlinson B, and Watts GF: Health literacy in familial hypercholesterolemia: A cross-national study. Eur J Prev Cardiol, 2018; 25: 936-943 [DOI] [PubMed] [Google Scholar]
- 78).Razali S, Ismail Z, Abdullah N, and Nawawi H: Illness perception of patients with familial hypercholesterolaemia varies with level of education and presence of cardiovascular disease. Env Behav Proc J, 2019; 4 [Google Scholar]