Abstract
Aim: The Japan Diet (JD) recommended by the Japan Atherosclerosis Society based on the traditional Japanese diet is presumably favorable for preventing atherosclerotic cardiovascular diseases, but few high-quality controlled clinical trials have examined its benefits as compared with other diets. We studied effects of nutrition education for JD intake as compared with partial JD (PJD) intake on serum lipids and inflammatory parameters in subjects with dyslipidemia.
Methods: A randomized parallel controlled clinical trial was conducted on outpatients with dyslipidemia. Participants were randomly divided into the JD or the PJD group. Face-to-face nutrition education based on each diet at baseline and at 3 months, as well as monthly counseling by mail during the intervening 3-month period, were provided and participants practiced up to 6 months. Both groups were advised to reduce consumptions of animal fat/ fatty meat/poultry, confections, and alcoholic drinks. Additionally, the JD group participants were recommended to consume more fish, soybean products especially natto, vegetables, and seaweed/mushrooms/konjak, and to switch from refined to unrefined cereals or barley.
Results: Mean LDL-cholesterol was 125 +/- 29 mg/dL at baseline, and the JD group ( n =49) showed a greater mean LDL-cholesterol decrease than the PJD group ( n =49) [- 8 mg/dL in JD vs 1 mg/dL in PJD, difference, -9 mg/dL (95%CI, -17 to 0) p =0.043)], and triglyceride ( p =0.023) and insulin ( p =0.033) reductions were larger in the JD group than in the PJD group at 6 months.
Conclusion: Nutrition education for JD intake was suggested to improve serum lipid and metabolic parameters in patients with dyslipidemia.
Keywords: LDL-cholesterol, Diet, Triglyceride, Fish, Soy
See editorial vol. 28: 1023-1024
Introduction
The importance of primary prevention and the urgent need for amelioration of cardiovascular disease risks such as obesity, dyslipidemia, hypertension and impaired glucose tolerance/diabetes are global issues, impacting Asia as well as other regions. The Japanese dietary pattern has been considered to be anti-atherosclerotic as compared to those of western countries 1 - 4) . However, the increasing westernization of eating habits with higher intakes of meat and animal fats as compared with fish and vegetable oils has been noted for nearly half a century, in parallel with rising cardiovascular disease risks, in Japan 5 , 6) . The Dietary Approach to Stop Hypertension (DASH) diet and Mediterranean diet have been highly recommended in Western countries based on efficacy in preventing life-style related diseases 7 - 10) . In a recent meta-analysis, a prudent diet pattern was related to a low incidence of coronary heart disease 11) . These diets generally emphasize higher intakes of fruits, vegetables, nuts and whole grains, and moderate intakes of fish, poultry, and low/nonfat dairy products, as well as low intakes of sodium, processed meats, added sugar, and saturated fat, while the Japan Atherosclerosis Society (JAS) has recommended the so-called Japan Diet (JD) for the prevention of atherosclerotic cardiovascular diseases in its guidelines 12 , 13) .
Compared to the other healthy diets described above, the JD is rich in (1) oily fish which contains eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), (2) soy and Japanese traditional soy-products containing isoflavones and dietary fiber, and (3) energy free and fiber rich plant foods such as seaweed, mushrooms and konjak. These healthy components combined with reduced consumptions of animal fat, fatty meat, poultry, and alcoholic drinks, constitute the specific advantages of the JD. However, to our knowledge, no intervention study focusing on benefits of the JD for the prevention of atherosclerotic cardiovascular disease risks has been reported. Moreover, traditional Japanese dietary patterns have been pointed out to have the disadvantage of containing high sodium concentrations which contribute to a high incidence of strokes including hemorrhagic stroke 14) .
We previously conducted a 6-week pilot study of a nutrition education program and reported the beneficial effects of the JD, as defined by the JAS, on body weight reduction and lipid parameters, including oxidized low-density lipoprotein (LDL), as well as effectively changing serum phospholipid fatty acids to an anti-atherosclerotic profile in middle-aged Japanese men who had been brought up in the highly westernized dietary environment of modern Japan 15 , 16) . Clinical dietary interventions for lifestyle-related diseases should be implemented in an ongoing manner, but the effects of the JD on patients with risk factors for atherosclerotic cardiovascular diseases are unknown.
In addition, as atherosclerosis is a systemic chronic inflammatory disease, effective dietary interventions for cardiovascular risk factors aimed at preventing chronic inflammation are also needed 17) . Epidemiological and clinical intervention studies suggested healthy diets comprised of a variety of foods to be beneficial for protecting against chronic life-style related diseases associated with subclinical inflammation 14) .
We conducted a randomized controlled trial of a 6-month nutrition education program comparing the effects of JD intake and partial JD (PJD) intake on metabolic and inflammatory parameters in patients with dyslipidemia. As the PJD emphasizes reducing consumption of animal fat, fatty meat and poultry, sweets, confections, and alcoholic drinks, this diet is expected to improve metabolic parameters based on overall dietary improvement for subjects. The PJD is therefore ethically acceptable.
Aim
Our study aimed to further clarify beneficial effects, in terms of improving metabolic and inflammatory parameters, in subjects adhering to the JD. The primary outcome was the between-group difference in LDL cholesterol (LDL-C) level changes from baseline to the 6-month follow-up. The secondary outcomes were between-group differences in other physical, biochemical and inflammatory marker changes.
Methods
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human patients were approved by The Ethics Committee for Experimental Research involving Human Subjects of Japan Women’s University (No. 246). All study participants provided written informed consent. This study was registered at Umin.ac.jp/ctr Identifier: UMIN000022955.
Study Participants
Outpatients with dyslipidemia who had not received dietary counseling were recruited from the Teramoto Medical and Dental Clinic (Tokyo, Japan), Shizuoka City Shizuoka Hospital (Shizuoka, Japan) and Tokorozawa Heart Center (Saitama, Japan). Inclusion criteria were being a Japanese patient with dyslipidemia, between 30 and 65 years of age, a body mass index (BMI) over 18.5 kg/m 2 , non-smoker, and being permitted to participate in the program by doctors certified by the JAS. Exclusion criteria were as follows: smoker, pregnant, use of dietary supplements or health food products, homozygous familial hypercholesterolemia, past history of atherosclerotic cardiovascular disease, renal dysfunction (estimated glomerular filtration rate lower than 60 mL/min/1.73m 2 ), hemoglobin A1c over 8%, impaired thyroid function and any changes in prescribed drugs within 3 months prior to enrollment.
Study Design, Randomization, and Masking
This was a randomized parallel-group clinical trial. Participants were allocated to either the JD group or the PJD group at a 1:1 ratio by stratified permuted block randomization; stratification factors were sex, age, diabetes and medications for EPA and/or DHA. Randomized allocation was implemented by a research associate working independently of the outcome assessments and intervention delivery. Participants did not learn of their diet group assignment until they had completed all baseline measurements and attended their first face-to-face personal counseling session. Blinding of the participants was not possible after group allocation due to the impracticality of blinding for the diets. Data were anonymized. Study doctors and staff members who analyzed the dietary records were blinded to the diet assignments.
Interventions
Interventions were started and baseline data were obtained at the next clinic visit after the patients had agreed to participate in the study with provision of informed consent. Face-to-face nutrition education for each diet, at baseline and at 3 months, was provided by three registered dietitians from Japan Women’s University who were specially trained for this study. Monthly counseling was provided by mail for the first 3 months by one of the trained dietitians.
Recommended nutrient and food compositions for the JD and PJD, with a total intake of 1800 kcal/day, are shown in Table 1 . Diet plans were individualized for energy levels or to maintain a healthy body weight (a BMI of approximately 22 kg/m 2 ). Energy percentages derived from fat, saturated fatty acid (SFA) and carbohydrate were 20-25%, 4.5-7.0% and 50-60%, respectively, for both diet groups. In addition, cholesterol, dietary fiber and n-3 polyunsaturated fatty acid (PUFA) intakes were recommended to be less than 200 mg, over 25 g and 2.1 g, respectively, in the JD group.
Table 1. Recommended daily food and nutrient intake for 1800 kcal prescription in the Partial Japan Diet group and the Japan Diet Group.
| Partial Japan Diet | Japan Diet | |||
|---|---|---|---|---|
| <Food group> | Highly recommended food | Highly recommended food | ||
| Cereals (g) | Boiled rice | 510 | Boiled rice with barley and/or unrefined cereals | 510 |
| Potatoes (g) | 50 ~ 150 | 50 ~ 150 | ||
| Fruit (g) | 100 ~ 200 | 100 ~ 200 | ||
| Fish (g) | 80 | Fatty fish | 80 | |
| Soy and soy-products (g) | Tofu | 100 | Natto | 40 |
| Meat and/or chiken (g) | Lean and/or white | 80 | Lean and/or white | 80 |
| Egg (g) | 50 | 15 | ||
| Milk and/or Yogurt (g) | Low fat | 150 | Low fat | 150 |
| Vegetable oil (g) | 25 | Soy and cotton seed mixed oil | 25 | |
| Green and yellow vegetable (g) | 120 | 120 | ||
| Other vegetable (g) | 230 | Other vegetable | 230 | |
| Seaweed/mashroom/konjak | 50 | |||
| <Nutrient> | ||||
| Energy (kcal) | 1800 | 1800 | ||
| Protein (% Energy) | 15 ~ 20 | 15 ~ 20 | ||
| Fat (% Energy) | 20 ~ 25 | 20 ~ 25 | ||
| SFA (% Energy) | 4.5 ~ 7.0 | 4.5 ~ 7.0 | ||
| n-3 PUFA (g) | 2.1 | |||
| Cholesterol (mg) | <200 | |||
| Carbohydrate (% Energy) | 50 ~ 60 | 50 ~ 60 | ||
| Dietary fiber (g) | 25 | |||
| Sodium (mg) | <2360 | <2360 | ||
The both groups were recommended to consume lean and white meat insted of red meat and poultry with skin, avoid animal fat, sweets and alcoholic drinks. The Japan Diet group was recommended to consume fatty fish, Japanese fermented soy-products; Natto, and energy free and fiber rich plant foods such as seaweed, mushrooms and konjak, as additional vegetables.
Patients received counselling from the trained registered dietitians focusing on prescribed energy and nutrient intakes and food items classified as constituting each diet at baseline. For both diet groups, reductions in the intakes of animal fat, meat and poultry with fat, sweets, desserts and snacks, and alcoholic drinks, as compared with the individual’s usual dietary intakes, were recommended. In addition, consuming more fish (especially fatty fish), soybeans and soy products (especially natto, a soy product fermented with Bacillussubtilis ), vegetables including green and yellow vegetables, seaweed, mushrooms, konjak and unrefined cereals including barley, as compared with usual dietary intakes, were recommended for the JD group. Participants were recommended to cook dishes light taste and avoid, or at least minimize, consumptions of Japanese high-salt foods such as salted vegetables and fishes. Participants compared their nutrient and food consumptions based on their weighed dietary records with those recommended, and thereby became aware of problems in selecting food items for their own diets that needed to be changed. The patients were then encouraged to set body weight and performance goals in terms of intakes of the foods comprising the PJD and the JD diets for 3 months. They were then encouraged to keep daily body weight and performance records in terms of the intakes of 3 food items comprising the PJD and 9 food items comprising the JD for 3 months, employing a group-tailored self-monitoring sheet. The records had to be mailed to the registered dietitian at 1 and 2 months, and each patient then received supportive individual advice by mail. After the baseline nutrition counselling, patients in the JD group received boiled rice with barley for microwave-oven cooking (150 g of Mochimugi-gohan (energy 195 kcal, protein 4.2 g, carbohydrate 44.1 g, fat 0.75 g, dietary fiber 2.7 g) ) from Hakubaku Co. Ltd.: 24 packs, and 150 g of Omugi Seikatsu (energy 209 kcal, protein 5.4 g, carbohydrate 46.7 g, fat 1.1 g, dietary fiber 4.5 g) from Otsuka Pharmaceutical Co., Ltd.: 2 packs) and 190 g of canned mackerel in brine (energy 317 kcal, protein 26.8 g, carbohydrate 0.0 g, fat 23.4 g, EPA 2071 mg, DHA 2717 mg) from Maruha Nichiro Co., Ltd., 24 cans) to facilitate obtaining the recommended cereals and fish consumptions. Participants were not urged by the dieticians to use these foods, instead choosing them of their own volition.
At 3 months, patients came to an individual diet counselling session to review their 3-month self-monitoring records and to compare their food consumption volumes based on their weighed dietary records with those recommended, and thus recognized their own dietary problems requiring modification in terms of selecting food items and precise intake volume. They were encouraged to keep daily self-monitoring records for another 3 months and handed these into the nutrition counselors at 6 months.
The dietitians were trained for this program according to a nutrition counseling manual to standardize the intervention, and nutrition counseling procedures were reported just after each patient’s visit to the Japan Women’s University laboratory, and if any deviations from the manual were detected, the reason was described. Monthly data from the self-monitoring sheet and questionnaires on habitual food intake frequency and self-efficacy, which complied with recommended food intakes, were used to assess adherence to the diet at 3 and 6 months.
The participants were instructed to maintain their habitual physical activities during the study period. They answered a physical activity questionnaire, and the responses were used to calculate energy expenditure 18) .
Dietary Data Collection
On the day a team doctor obtained informed consent from the patients, a dietitian explained in detail how to record their diets. Three-day (two weekdays and 1 weekend day) weighted dietary records were kept at baseline, and at the ends of the 3- and 6-month study periods, and were then collected after confirmation by interview with a trained dietitian. Patients were encouraged to submit photos of meals, as well as food and nutrition labeling of the prepared foods that they had consumed. Nutrient intakes were calculated employing Excel-Eiyokun Ver.8.0, (Kenpaku-sha Co., Ltd., Tokyo, Japan) software. The volumes of foods consumed were summarized into food groups: refined cereals (consisting of refined rice, wheat and corn), unrefined cereals (consisting of unrefined rice, wheat and corn, barley and millet), potato/starches and sugars, soybean and soy products including natto, nuts, green and yellow vegetables, other vegetables, seaweed, mushrooms, konjak, fish, other seafood (consisting of shellfish, prawns, shrimp, squid, octopus, crab, fish eggs and viscera and fish paste products), meats and poultry and processed meat products (including internal organs), fruits, eggs, milk and other dairy products, fats and hydrogenated fats (consisting of meat fat, lard, beef tallow, butter, margarine, shortening and fat spread), seasonings (including miso: fermented soybean paste), vegetable oils, dressings and mayonnaise, confections (consisting of sweets, desserts, snacks, and sweet buns), sugar sweetened beverages and alcoholic drinks based on the Standard Table of Food Composition in Japan 2015 (the Science and Technology Agency of Japan), partially modified in accordance with nutrient content similarities and dietary counseling instructions. Alcoholic drinks were summarized as total ethanol intake. All dietary evaluations were performed by a nutrition study team at the Laboratory of Clinical Nutrition and Nutrition Education in Japan Women’s University.
Outcomes
Anthropometric measurements and fasting blood collection were conducted in the morning following an at least 12-hour fast at baseline, and again at 3 months and 6 months. Body height and weight were measured, and BMI was calculated as weight (kg) divided by the square of height (m). Waist circumference was determined with a tape measure during the late exhalation phase in the standing position. Blood pressure was measured using an automatic blood pressure manometer with the participant in the seated position. Plasma and serum samples were obtained and the following concentrations were measured: serum total cholesterol (cholesterol dehydrogenase UV method), LDL-C (enzymatic direct method), high-density lipoprotein (HDL) cholesterol (enzymatic direct method), triglyceride (TG) (enzymatic method), hemoglobin A1c (latex coagulating method), malondialdehyde modified (MDA)-LDL (enzyme-linked immunosorbent assay (ELISA) method), plasma glucose (hexokinase UV method), serum insulin (chemiluminescent enzyme immunoassay method), and high sensitivity C-reactive protein (hs-CRP) (latex nephelometry method) at the laboratory of BML Inc., Tokyo, Japan, and leptin (ELISA), high-molecular-weight (HMW) adiponectin (ELISA), high sensitivity tumor necrosis factor α (hs-TNFα) (ELISA) and monocyte chemotactic protein-1 (ELISA) were analyzed simultaneously in the laboratory of Mibyou Marker Co. Ltd., Osaka, Japan. Interleukin-6 (IL-6) (chemiluminescent enzyme immunoassay (CLEIA)) was analyzed in the laboratory of LSI Medience Co. Ltd., Osaka, Japan.
Sample Size
The sample size in this study was based on what was considered to be feasible, i.e. the recruitment of 120 participants. We calculated the sample size needed to detect the intervention effect of an 11 mg/dL change with a standard deviation (SD) of 19 mg/dL on the serum LDL-C concentration within a group and between the groups based on a previous study 19) , using 0.05 for the alpha (1.96) and a power of 80%, Z 0.20 =0.84. The necessary sample size was 46 participants per arm. Taking into account a possible drop-out rate of up to 20% and a 5% exclusion rate, the number of participants at randomization was increased to 60 per arm.
Statistical Analysis
Statistical analyses were carried out using IBM SPSS for Windows (version 26.0; SPSS Japan, Inc.). The primary outcomes were analyzed according to the intention-to-treat principle. Imputations were used to replace missing data, employing the last observation carried forward (LOCF) method. We used descriptive statistics with the means +/- SD, median and interquartile range values or the mean and 95% confidence intervals (CI), which are also presented for each continuous value. Categorical variables are expressed as percentages.
Between-group comparisons of variables at baseline were conducted using the chi-square test or Fisher’s exact test, and the t test or the Mann-Whitney test was applied for categorical, continuous or non-normally distributed quantitative variables, respectively. Differential changes between the diet groups were examined employing unpaired t tests based on equal variances or the Mann-Whitney test. Effect sizes were calculated as Cohen’s d (the post-test intervention mean minus the post-test control mean divided by the common standard deviation). Changes within diet groups were determined by the paired t test or the Wilcoxon signed rank test. All statistical tests were two-tailed with significance set at P <0.05.
Results
Recruitment
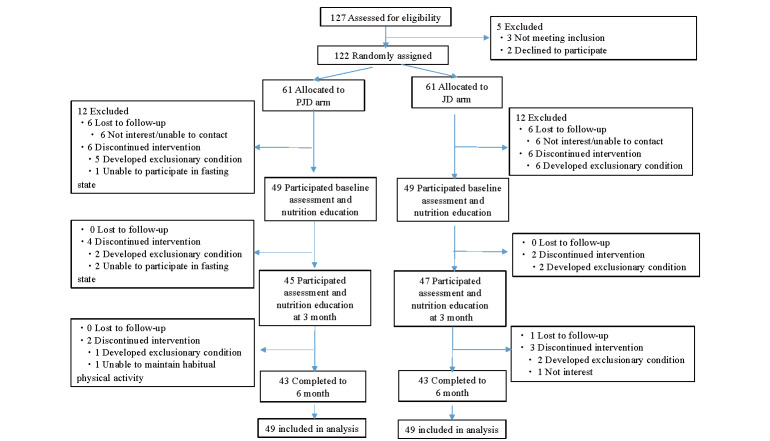
Participant enrollment began on September14, 2016, and continued through September 6, 2018. The date of final follow-up was March 30, 2019. As shown in Fig.1 , after being given a detailed explanation of the program, 127 patients provided written informed consent and 122 were randomized to the JD or the PJD group. Allocation rates did not differ among the 3 medical institutions. A total of 24 participants withdrew prior to study commencement and diet assignment disclosure, such that 98 patients participated in the intervention at baseline and were included in the analysis for all measurements except those of inflammation markers. Inflammation markers were analyzed for 46 and 44 participants in the PJD and JD groups, respectively, excluding those who had fever, allergy symptoms, and dental or joint pain at the time of blood collection. The mean delays between enrollment and baseline intervention were 37 +/- 30 (mean +/- SD) days and 37 +/- 26 days for the JD and PJD groups, respectively. The compliance with baseline medications was poor in 3 PJD and 1 JD group participant, such that these data were regarded as being missing. There were no changes in pharmacotherapy for lipid abnormalities during the study period. At the 6-month follow-up, a total of 86 participants had completed the study and the retention rate was 87.8% for both groups.
Fig.1.
Participant flow diagram
Baseline Data
In total, 42 (42.9%), 18 (18.4%) and 27 (27.6%) participants were taking LDL-C-lowering, TG-lowering and both types of drugs, respectively, and these percentages did not differ between the JD and PJD groups ( Table 2 ) .
Table 2. Baseline characteristics of the participants.
| Partial Japan Diet ( n = 49) | Japan Diet ( n = 49) | p | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (year) | 54.1 (7.6) § | 53.5 (8.2) | 0.68 | ||
| Men/Women | 25/24 | 21/28 | 0.42 | ||
| Type 2 diabetes | 8 | 16.3 | 7 | 14.3 | 0.78 |
| Hypertention | 20 | 40.8 | 25 | 51.0 | 0.31 |
| Medications | |||||
| LDL-cholesterol agent | 21 | 42.9 | 21 | 42.9 | 1.00 |
| Statin | 18 | 36.7 | 19 | 38.8 | 0.83 |
| Intestinal cholesterol transporter inhibitor | 6 | 12.2 | 3 | 6.1 | 0.49 |
| Probucol | 0 | 0.0 | 2 | 4.1 | 0.49 |
| TG-lowering agent | 9 | 18.4 | 9 | 18.4 | 1.00 |
| Fibrate | 7 | 14.3 | 6 | 12.2 | 0.77 |
| n-3 polyunsaturated fatty acid | 4 | 8.2 | 3 | 6.1 | 1.00 |
| Oral hypoglycemic agent | 7 | 14.3 | 3 | 6.1 | 0.32 |
| Antihypertensive agent | 14 | 28.6 | 13 | 26.5 | 0.82 |
| Energy expenditure (METs/day) | 39.2 (3.0) § | 39.8 (4.2) | 0.90 | ||
P values were between group differences analyzed by the unpaired t -test for the mean difference, and by the chi- square test or Fisher's exact test for the percentage.
§ Values are expressed as mean +/- SD.
METs, metabolic equivalents
At baseline, the participants had a mean LDL-C of 125 +/- 29 mg/dL and no differences in any of the following measurements were observed between the JD and PJD groups: anthropometric variables, blood pressure, biochemical and inflammatory parameters, food and nutrient intake.
Primary Outcome
From baseline to after 6-month intervention, the JD group showed a greater mean LDL-C decrease than the PJD group [- 8 mg/dL (95%CI, -13 to -2) in JD vs 1 mg/dL (95%CI, -6 to 8) in PJD, difference, -9 mg/dL (95%CI, -17 to 0), p =0.043] ( Table 3 , Fig.2 ) . The percentage of participants with LDL-C exceeding 140 mg/dL did not change in the PJD group (from 34.7% at baseline to 36.7% at 6 months), while tending to decrease from 28.5% at baseline to 10.2% at 6 months in the JD group ( p =0.066).
Table 3. Changes in anthropometric variables, blood pressure and circulating metabolic parameters in the Partial Japan Diet and Japan Diet groups.
|
Partial Japan Diet
( n = 49) |
Japan Diet
( n = 49) |
Between-group comparisons at 3 and 6 months (Japan Diet - Partial Japan Diet) | ||||||
|---|---|---|---|---|---|---|---|---|
| Difference (95% CI) § | p | effect size | ||||||
| Body weight (kg) # | Baseline | 64.6 | (12.9) | 64.5 | (13.4) | |||
| 3-month | 63.3 | (12.5) | 63.5 | (13.6) | 0.3 (-0.4, 1.0) | 0.44 | 0.16 | |
| 6-month | 63.0 | (12.9) | 63.3 | (13.5) | 0.4 (-0.6, 1.3) | 0.41 | 0.17 | |
| Body Mass Index (kg/m 2 ) # | Baseline | 23.9 | (3.3) | 24.4 | (3.7) | |||
| 3-month | 23.5 | (3.2) | 24.0 | (3.8) | 0.1 (-0.2, 0.4) | 0.50 | 0.14 | |
| 6-month | 23.3 | (3.3) | 24.0 | (3.7) | 0.1 (-0.2, 0.5) | 0.43 | 0.16 | |
| Waist circumference (cm) # | Baseline | 86.1 | (9.7) | 88.8 | (9.8) | |||
| 3-month | 85.6 | (9.2) | 87.2 | (9.8) | -1.0 (-2.1, 0.0) | 0.053 | 0.41 | |
| 6-month | 84.8 | (9.3) | 86.3 | (10.0) | -1.2 (-2.5, 0.2) | 0.091 | 0.35 | |
| Systolic blood pressure (mmHg) # | Baseline | 127 | (16) | 127 | (18) | |||
| 3-month | 124 | (14) | 123 | (16) | -1 (-5, 4) | 0.82 | 0.05 | |
| 6-month | 123 | (15) | 122 | (14) | 0 (-5, 5) | 0.94 | 0.02 | |
| Diastolic blood pressure (mmHg) # | Baseline | 79 | (12) | 79 | (13) | |||
| 3-month | 78 | (11) | 78 | (12) | 1 (-2, 4) | 0.56 | 0.12 | |
| 6-month | 76 | (12) | 76 | (11) | 0 (-3, 3) | 0.98 | 0.00 | |
| Total cholesterol (mg/dL) # | Baseline | 224 | (38) | 213 | (29) | |||
| 3-month | 219 | (38) | 204 | (25) | -4 (-14, 5) | 0.38 | 0.18 | |
| 6-month | 223 | (36) | 202 | (27) | -10 (-19, -1) | 0.033 | 0.44 | |
| LDL-cholesterol (mg/dL) # | Baseline | 128 | (33) | 123 | (24) | |||
| 3-month | 124 | (30) | 116 | (20) | -3 (-11, 5) | 0.45 | 0.15 | |
| 6-month | 129 | (32) | 115 | (22) | -9 (-17, 0) | 0.043 | 0.41 | |
| MDA-LDL (U/L) † | Baseline | 94 | (71, 126) | 88 | (74, 107) | |||
| 3-month | 86 | (71, 107) | 86 | (69, 105) | 4.3 (-7.0, 15.7) | 0.76 | 0.15 | |
| 6-month | 91 | (67, 122) | 79 | (69, 98) | -3.7 (-15.7, 8.3) | 0.29 | 0.12 | |
| HDL-cholesterol (mg/dL) # | Baseline | 63 | (18) | 61 | (17) | |||
| 3-month | 63 | (21) | 60 | (15) | -2 (-4, 1) | 0.26 | 0.23 | |
| 6-month | 64 | (20) | 60 | (16) | -2 (-4, 1) | 0.25 | 0.23 | |
| Triglyceride (mg/dL) † | Baseline | 90 | (63, 173) | 103 | (77, 155) | |||
| 3-month | 91 | (61, 152) | 92 | (68, 127) | -9 (-30, 13) | 0.21 | 0.16 | |
| 6-month | 87 | (64, 153) | 86 | (60, 131) | -10 (-33, 13) | 0.023 | 0.17 | |
| Glucose (mg/dL) † | Baseline | 93 | (88, 104) | 95 | (90, 101) | |||
| 3-month | 92 | (89, 101) | 95 | (88, 101) | 2 (-1, 5) | 0.20 | 0.30 | |
| 6-month | 93 | (87, 100) | 94 | (88, 101) | 0 (-3, 4) | 0.98 | 0.03 | |
| Insulin (µU/mL) † | Baseline | 4.7 | (3.5, 7.8) | 6.2 | (3.4, 10.7) | |||
| 3-month | 4.6 | (3.1, 8.3) | 5.4 | (3.1, 9.7) | -0.9 (-2.1, 0.3) | 0.29 | 0.29 | |
| 6-month | 4.5 | (3.2, 7.3) | 5.1 | (3.2, 7.7) | -1.2 (-2.3, 0.0) | 0.033 | 0.40 | |
| HbA1c (%) † | Baseline | 5.5 | (5.3, 5.8) | 5.5 | (5.4, 5.9) | |||
| 3-month | 5.6 | (5.3, 5.8) | 5.5 | (5.4, 5.8) | -0.01 (-0.10, 0.08) | 0.96 | 0.05 | |
| 6-month | 5.6 | (5.3, 5.9) | 5.6 | (5.3, 5.8) | -0.04 (-0.14, 0.05) | 0.23 | 0.19 | |
MDA-LDL, Malondialdehyde modified-low density cholesterol.
P values between-group comparisons were analyzed by unpaired t test or Mann–Whitney U test.
# Values are expressed as mean (SD).
† Values are expressed as median (25 percentile, 75 percentile).
§ Values are expressed as means (95% CI)
There were missing data for 3 of PJD and 2 of JD participants at 3 month, and 6 participants each in both group at 6 months.
Fig.2. Mean LDL-C concentration changes during the intervention.
Partial Japan diet (PJD) group (dotted line) n =49, Japan diet (JD) group (continuous line)=49. The mean between-group difference was significant at 6 months ( p =0.043).
There were missing data for 3 PJD and 2 JD participants at 3 months, and 6 participants in each of the two groups at 6 months.
Secondary Outcomes
After the 6-month intervention, body weight, BMI, waist circumference and systolic blood pressure were decreased, with activity maintained at stable levels, in both groups ( p <0.001). Between-group differences in changes were observed in TG and insulin, with mean between-group differences for change of -10 mg/dL (95%CI, -33 to 13) ( p =0.023) and -1.2 µU/mL (95%CI, -2.3 to 0.0) ( p =0.033), respectively, at 6 months.
Among adipocytokine markers, HMW-adiponectin was increased early in the JD group as compared with the PJD group with a between-group difference for change of 0.21 µg/ml at 3 months ( p =0.040). The between-group difference had disappeared due to a late HMW-adiponectin increase in the PJD group at 6 months. None of the inflammation markers showed changes between the two groups ( Table 4 ) .
Table 4. Changes in adipo-cytokine and inflammatory parameters in the Partial Japan Diet and Japan Diet groups.
| Partial Japan Diet | Japan Diet | Between-group comparisons at 3 and 6 months (Japan Diet - Partial Japan Diet) | ||||||
|---|---|---|---|---|---|---|---|---|
| Difference (95% CI) § | p | effect size | ||||||
| Leptin † (µg/L) | Baseline | 7.9 | (4.4, 12.2) | 8.1 | (4.2, 16.0) | |||
| 3-month | 6.2 | (3.6, 9.4) | 5.8 | (4.0, 11.3) | 0.4 (-1.3, 2.0) | 0.54 | 0.09 | |
| 6-month | 6.4 | (3.6, 9.1) | 6.8 | (3.8, 11.3) | -0.2 (-1.7, 1.3) | 0.86 | 0.05 | |
| HMW adiponectin † (µg/mL) | Baseline | 3.05 | (1.05, 7.04) | 2.87 | (1.60, 5.29) | |||
| 3-month | 2.84 | (1.37, 7.01) | 3.14 | (1.63, 6.07) | 0.21 (-0.10, 0.53) | 0.040 | 0.27 | |
| 6-month | 3.33 | (1.33, 8.27) | 3.70 | (1.70, 5.67) | -0.01 (-0.50, 0.48) | 0.95 | 0.01 | |
| hs-TNFα # (pg/mL) | Baseline | 1.92 | (0.79) | 1.77 | (0.64) | |||
| 3-month | 1.86 | (0.82) | 1.80 | (0.73) | 0.09 (-0.06, 0.23) | 0.22 | 0.26 | |
| 6-month | 1.85 | (0.78) | 1.77 | (0.66) | 0.08 (-0.04, 0.20) | 0.19 | 0.28 | |
| hs-CRP † (mg/dL) | Baseline | 0.033 | (0.018, 0.071) | 0.029 | (0.013, 0.074) | |||
| 3-month | 0.036 | (0.014, 0.068) | 0.030 | (0.013, 0.102) | -0.018 (-0.081, 0.045) | 0.53 | 0.12 | |
| 6-month | 0.035 | (0.014, 0.076) | 0.026 | (0.012, 0.064) | -0.001 (-0.033, 0.030) | 0.92 | 0.02 | |
| IL-6 † (pg/mL) | Baseline | 1.20 | (0.90, 1.50) | 1.09 | (0.83, 1.46) | |||
| 3-month | 1.07 | (0.76, 1.34) | 1.18 | (0.82, 1.44) | 0.17 (-0.07, 0.42) | 0.10 | 0.30 | |
| 6-month | 1.04 | (0.90, 1.37) | 1.15 | (0.82, 1.62) | 0.13 (-0.12, 0.39) | 0.052 | 0.21 | |
| MCP-1 † (pg/mL) | Baseline | 113 | (86, 137) | 115 | (81, 161) | |||
| 3-month | 114 | (84, 138) | 121 | (90, 177) | 15 (2, 27) | 0.13 | 0.50 | |
| 6-month | 117 | (84, 143) | 115 | (73, 157) | 2 (-13, 16) | 0.66 | 0.04 | |
HMW-adiponectin, High molecular weight adiponectin; hs-TNFα, High sensitivity tumor necrosis factor alpha; hs-CRP, High sensitivity C-reactive protein; IL-6, interleukin 6; MCP-1, Monocyte chemotactic protein 1.
Leptin and HMW-adiponectin were analyzed in the 49 participants in both groups.
Hs-CRP, hs-TNFα, IL-6 and MCP-1 were analyzed in the participants of 46 and 44 in the PJD and JD group, respectively. P values between- group comparisons were analyzed by unpaired t test or Mann–Whitney U test.
# Values are expressed as mean (SD).
† Values are expressed as median (25 percentile, 75 percentile).
§ Values are expressed as means (95% CI)
There were missing data for 3 of PJD and 2 of JD participants at 3 months, and 6 participants each in both group at 6 months.
No adverse events were observed in either group during the study.
Food Intake Volumes
As to the foods for which reduced consumptions had been recommended to participants, between-group differences in changes were observed in refined cereals and confections at 6 months, with mean between-group differences for changes of -70 g ( p =0.021) and -16 g ( p =0.04), respectively.
The JD group additionally showed increased consumptions of recommended foods such as unrefined cereals, fish, green and yellow vegetables and the sum of seaweed, mushrooms and konjak at 3 months, and these improvements were maintained throughout the study period. The mean between-group difference was significant for unrefined cereals at 30 g ( p =0.021), natto at 9 g ( p =0.018), green and yellow vegetables at 61 g ( p =0.002), fish at 24 g ( p =0.005) and fatty fish contain EPA/docosapentaenoic acid (DPA)/DHA over 1.0 g per 100 g at 22 g ( p =0.001).
Reductions in meat consumption were essentially the same as the sum of increased amounts of fish and soybean and soy-products consumed in the JD group. Seaweed intake was doubled in the JD group ( Table 5 , Supplementary Table 1 ) .
Table 5. Changes in daily food intakes in the Partial Japan Diet and Japan Diet groups.
|
Partial Japan Diet
( n = 49) |
Japan Diet
( n = 49) |
Between-group comparisons at 3 and 6 months (Japan Diet - Partial Japan Diet) | |||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Difference (95% CI) | p | ||
| Refined cereals | Baseline | 325 (153) | 359 (154) | ||
| 3-month | 315 (161) | 288 (135) | -62 (-107, -17) | 0.008 | |
| 6-month | 307 (159) | 271 (155) | -70 (-122, -18) | 0.02 | |
| Unrefined cereals | Baseline | 34 (44) | 30 (61) | ||
| 3-month | 34 (38) | 75 (67) | 45 (19, 71) | <0.001 | |
| 6-month | 42 (51) | 68 (63) | 30 (1, 59) | 0.021 | |
| Soy and soy products | Baseline | 59 (54) | 71 (75) | ||
| 3-month | 65 (65) | 89 (85) | 12 (-12, 35) | 0.11 | |
| 6-month | 86 (94) | 86 (101) | -12 (-48, 24) | 0.90 | |
| Natto | Baseline | 10 (13) | 8 (11) | ||
| 3-month | 11 (15) | 15 (16) | 6 (0, 11) | 0.073 | |
| 6-month | 12 (16) | 19 (19) | 9 (2, 15) | 0.018 | |
| Green and yellow vegetables | Baseline | 103 (70) | 81 (54) | ||
| 3-month | 97 (62) | 123 (82) | 48 (20, 77) | 0.002 | |
| 6-month | 107 (79) | 146 (98) | 61 (28, 93) | 0.002 | |
| Other vegetables | Baseline | 162 (71) | 165 (68) | ||
| 3-month | 168 (68) | 196 (78) | 25 (-7, 57) | 0.24 | |
| 6-month | 164 (105) | 186 (80) | 19 (-21, 59) | 0.131 | |
| Seaweed, mushrooms and konjak | Baseline | 33 (26) | 27 (20) | ||
| 3-month | 30 (27) | 39 (27) | 16 (3, 29) | 0.013 | |
| 6-month | 31 (27) | 40 (33) | 15 (0, 29) | 0.053 | |
| Fish | Baseline | 44 (37) | 46 (36) | ||
| 3-month | 54 (46) | 80 (58) | 23 (1, 46) | 0.030 | |
| 6-month | 37 (32) | 64 (46) | 24 (8, 41) | 0.005 | |
| Fatty fish | Baseline | 22 (25) | 25 (26) | ||
| 3-month | 29 (34) | 58 (52) | 26 (7, 45) | 0.023 | |
| 6-month | 17 (21) | 43 (41) | 22 (10, 35) | <0.001 | |
| Meat | Baseline | 96 (52) | 97 (58) | ||
| 3-month | 89 (51) | 66 (38) | -23 (-43, -4) | 0.052 | |
| 6-month | 79 (44) | 69 (45) | -12 (-30, 7) | 0.22 | |
| Fats and hydrogenated fat | Baseline | 2 (3) | 2 (3) | ||
| 3-month | 2 (2) | 2 (2) | 0 (-2, 1) | 0.46 | |
| 6-month | 2 (4) | 2 (2) | 0 (-2, 1) | 0.72 | |
| Alcoholic beverages § | Baseline | 8 (15) | 10 (18) | ||
| 3-month | 6 (13) | 7 (13) | -1 (-5, 2) | 0.46 | |
| 6-month | 8 (18) | 7 (14) | -3 (-8, 2) | 0.76 | |
| Confections | Baseline | 45 (50) | 40 (36) | ||
| 3-month | 37 (51) | 22 (28) | -10 (-26, 5) | 0.20 | |
| 6-month | 41 (54) | 20 (29) | -16 (-35, 2) | 0.040 | |
Values are expressed in grams.
P values between-group comparisons were analyzed by unpaired t test or Mann–Whitney U test. Soy and soy products: Soy and soy products including natto.
Fish: Total fish amount including fatty fish which contain sum of eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA) over 1.0 g per 100g edible portion.
§ Values are calculated as pure ethanol amount in grams.
There were missing data for 3 of PJD and 2 of JD participants at 3 months, and 6 participants each in both group at 6 months.
Supplementary Table 1. Changes in all daily food intakes in the Partial Japan Diet and Japan Diet groups.
|
Partial Japan Diet ( n = 49) |
Japan Diet
( n = 49) |
Between-group comparisons at 3 and 6 months (Japan Diet - Partial Japan Diet) | |||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Difference (95% CI) | p | ||
| Refined cereals | Baseline | 325 (153) | 359 (154) | ||
| 3-month | 315 (161) | 288 (135) | -62 (-107, -17) § | 0.008 | |
| 6-month | 307 (159) | 271 (155) | -70 (-122, -18) | 0.02 | |
| Unrefined cereals | Baseline | 34 (44) | 30 (61) | ||
| 3-month | 34 (38) | 75 (67) | 45 (19, 71) | <0.001 | |
| 6-month | 42 (51) | 68 (63) | 30 (1, 59) | 0.021 | |
| Potatoes, starches, sugar | Baseline | 36 (28) | 38 (35) | ||
| 3-month | 44 (36) | 33 (27) | -13 (-28, 2) | 0.069 | |
| 6-month | 35 (27) | 32 (25) | -5 (-18, 9) | 0.65 | |
| Soy and soy products | Baseline | 59 (54) | 71 (75) | ||
| 3-month | 65 (65) | 89 (85) | 12 (-12, 35) | 0.11 | |
| 6-month | 86 (94) | 86 (101) | -12 (-48, 24) | 0.90 | |
| Natto | Baseline | 10 (13) | 8 (11) | ||
| 3-month | 11 (15) | 15 (16) | 6 (0, 11) | 0.073 | |
| 6-month | 12 (16) | 19 (19) | 9 (2, 15) | 0.018 | |
| Nuts | Baseline | 2 (6) | 3 (7) | ||
| 3-month | 3 (6) | 3 (6) | 0 (-3, 3) | 0.96 | |
| 6-month | 2 (4) | 2 (4) | 0 (-3, 3) | 0.58 | |
| Green and yellow vegetables | Baseline | 103 (70) | 81 (54) | ||
| 3-month | 97 (62) | 123 (82) | 48 (20, 77) | 0.002 | |
| 6-month | 107 (79) | 146 (98) | 61 (28, 93) | 0.002 | |
| Other vegetables | Baseline | 162 (71) | 165 (68) | ||
| 3-month | 168 (68) | 196 (78) | 25 (-7, 57) | 0.24 | |
| 6-month | 164 (105) | 186 (80) | 19 (-21, 59) | 0.131 | |
| Seaweed, mushrooms and konjak | Baseline | 33 (26) | 27 (20) | ||
| 3-month | 30 (27) | 39 (27) | 16 (3, 29) | 0.013 | |
| 6-month | 31 (27) | 40 (33) | 15 (0, 29) | 0.053 | |
| Fish | Baseline | 44 (37) | 46 (36) | ||
| 3-month | 54 (46) | 80 (58) | 23 (1, 46) | 0.030 | |
| 6-month | 37 (32) | 64 (46) | 24 (8, 41) | 0.005 | |
| Fatty fish | Baseline | 22 (25) | 25 (26) | ||
| 3-month | 29 (34) | 58 (52) | 26 (7, 45) | 0.023 | |
| 6-month | 17 (21) | 43 (41) | 22 (10, 35) | <0.001 | |
| Other seafood | Baseline | 20 (23) | 24 (29) | ||
| 3-month | 20 (17) | 18 (18) | -5 (-17, 7) | 0.75 | |
| 6-month | 17 (21) | 17 (16) | -4 (-14, 7) | 0.50 | |
| Meat | Baseline | 96 (52) | 97 (58) | ||
| 3-month | 89 (51) | 66 (38) | -23 (-43, -4) | 0.052 | |
| 6-month | 79 (44) | 69 (45) | -12 (-30, 7) | 0.22 | |
| Fruits | Baseline | 77 (68) | 61 (88) | ||
| 3-month | 63 (63) | 47 (81) | 0 (-28, 28) | 0.78 | |
| 6-month | 69 (74) | 41 (56) | -12 (-39, 15) | 0.29 | |
| Eggs | Baseline | 37 (25) | 36 (28) | ||
| 3-month | 39 (26) | 31 (27) | -7 (-18, 4) | 0.22 | |
| 6-month | 33 (23) | 31 (23) | -2 (-13, 10) | 0.73 | |
| Milk and dairy products | Baseline | 102 (96) | 99 (97) | ||
| 3-month | 99 (99) | 92 (88) | -4 (-37, 29) | 0.72 | |
| 6-month | 88 (92) | 94 (104) | 9 (-23, 42) | 0.92 | |
| Fats and hydrogenated fat | Baseline | 2 (3) | 2 (3) | ||
| 3-month | 2 (2) | 2 (2) | 0 (-2, 1) | 0.46 | |
| 6-month | 2 (4) | 2 (2) | 0 (-2, 1) | 0.72 | |
| Alcoholic beverages | Baseline | 8 (15) | 10 (18) | ||
| 3-month | 6 (13) | 7 (13) | -1 (-5, 2) | 0.46 | |
| 6-month | 8 (18) | 7 (14) | -3 (-8, 2) | 0.76 | |
| Sugar sweetened beverages | Baseline | 77 (152) | 52 (94) | ||
| 3-month | 29 (71) | 42 (127) | 39 (2, 75) | 0.17 | |
| 6-month | 39 (138) | 29 (98) | 16 (-35, 67) | 0.54 | |
| Seasonings | Baseline | 44 (22) | 49 (19) | ||
| 3-month | 48 (23) | 48 (21) | -5 (-14, 3) | 0.38 | |
| 6-month | 42 (21) | 52 (23) | 5 (-3, 13) | 0.26 | |
| Vegetable oils, dressing and mayonnaise | Baseline | 18 (10) | 18 (9) | ||
| 3-month | 19 (11) | 17 (11) | -2 (-6, 3) | 0.35 | |
| 6-month | 18 (13) | 17 (10) | -1 (-5, 4) | 0.63 | |
| Confections | Baseline | 45 (50) | 40 (36) | ||
| 3-month | 37 (51) | 22 (28) | -10 (-26, 5) | 0.20 | |
| 6-month | 41 (54) | 20 (29) | -16 (-35, 2) | 0.040 | |
Values are expressed in grams. P values between-group comparisons were analyzed by unpaired t test or Mann–Whitney U test. Soy and soy products: Soy and soy products including natto. Fish: Total fish amount including fatty fish which contain sum of eicosapentaenoic acid (EPA), docosapentaenoic acid
(DPA) and docosahexaenoic acid (DHA) over 1.0 g per 100g edible portion. § Values are calculated as pure ethanol amount in grams.
There were missing data for 3 of PJD and 2 of JD participants at 3 months, and 6 participants each in both group at 6 months.
Participants randomized to the JD diet answered that the numbers of the provided boiled rice with barley and canned mackerel products that they had used were 18.9 +/- 7.2 and 14.5 +/- 7.5, respectively, and those who found these supplies to be useful accounted for 77.6% and 53.1%, respectively, of subjects at 3 months.
Nutrient Intakes
Both groups decreased total energy intake to 29 kcal per standard body weight with lowered SFA, resulting in the percentage of energy derived from SFA in the PJD and JD groups being 8.1% and 7.7%, respectively, at 6 months. These changes were observed earlier in the JD group than in the PJD group. Cholesterol intake decreased, to less than 300 mg at 6 months, in both groups. Total EPA, DPA and DHA increased in the JD group to 1666 mg at 3 months and then fell to 1188 mg at 6 months in the JD group. Dietary fiber intake, which was low at baseline, increased slightly in the JD group. Sodium intake did not change during the intervention in the either group, but potassium, calcium and magnesium intakes were increased at 3 months, remaining elevated throughout the study period, in the JD group. Between-group differences in changes at 6 months were observed in EPA/DPA/DHA, dietary fiber, calcium and magnesium ( Supplementary Table 2 ) .
Supplementary Table 2. Changes in daily energy and nutrient intakes in the Partial Japan Diet and Japan Diet groups.
|
Partial Japan Diet
( n = 49) |
Japan Diet
( n = 49) |
Between-group comparisons at 3 and 6 months (Japan Diet - Partial Japan Diet) | |||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Difference ( 95% CI ) | p | ||
| Energy (kcal ) | Baseline | 1873 (494) | 1919 (440) | ||
| 3-month | 1786 (446) | 1749 (303) | -83 (-220, 54) § | 0.23 | |
| 6-month | 1726 (503) | 1650 (415) | -121 (-258, 16) | 0.25 | |
| Protein (%E) | Baseline | 18.4 (19.3) | 15.2 (2.4) | ||
| 3-month | 19.1 (19.3) | 16.9 (2.8) | 1.0 (-0.3, 2.2) | 0.071 | |
| 6-month | 18.3 (19.3) | 17.2 (3.0) | 2.1 (0.9, 3.2) | 0.001 | |
| Fat (%E) | Baseline | 30.4 (5.9) | 30.1 (5.0) | ||
| 3-month | 30.2 (6.6) | 29.8 (6.7) | -0.2 (-2.7, 2.4) | 0.90 | |
| 6-month | 28.7 (6.5) | 29.6 (5.3) | 1.2 (-1.2, 3.6) | 0.22 | |
| SFA (g) | Baseline | 18.8 (6.8) | 18.3 (6.8) | ||
| 3-month | 16.8 (6.7) | 14.8 (5.3) | -1.5 (-4.1, 1.1) | 0.25 | |
| 6-month | 15.6 (6.4) | 14.3 (6.2) | -0.8 (-0.3, 1.5) | 0.50 | |
| MUFA (g) | Baseline | 23.9 (8.0) | 25.2 (8.5) | ||
| 3-month | 22.3 (7.7) | 20.8 (7.3) | -2.8 (-6.2, 0.6) | 0.11 | |
| 6-month | 20.5 (8.0) | 20.1 (8.2) | -1.6 (-4.6, 1.3) | 0.27 | |
| n-6PUFA (g) | Baseline | 11.3 (4.1) | 11.1 (3.4) | ||
| 3-month | 11.0 (4.0) | 10.9 (3.7) | 0.1 (-1.5, 1.6) | 0.88 | |
| 6-month | 10.5 (4.1) | 10.6 (4.1) | 0.4 (-1.1, 1.8) | 0.70 | |
| n-3PUFA (g) | Baseline | 2.6 (1.3) | 2.8 (1.7) | ||
| 3-month | 2.8 (1.8) | 3.5 (1.8) | 0.5 (-0.4, 1.3) | 0.085 | |
| 6-month | 2.3 (1.3) | 2.9 (1.2) | 0.3 (-0.3, 1.0) | 0.051 | |
| EPA+DPA+DHA (mg) | Baseline | 709 (637) | 919 (804) | ||
| 3-month | 854 (760) | 1666 (1317) | 601 (132, 1071) | 0.013 | |
| 6-month | 607 (514) | 1188 (919) | 371 (33, 710) | 0.033 | |
| Cholesterol (mg) | Baseline | 325 (133) | 341 (152) | ||
| 3-month | 323 (139) | 298 (133) | -41 (-99, 18) | 0.17 | |
| 6-month | 282 (124) | 272 (127) | -26 (-81, 28) | 0.87 | |
| Carbohydrate (%E) | Baseline | 47.4 (8.8) | 46.8 (8.2) | ||
| 3-month | 47.2 (9.5) | 46.1 (7.2) | -0.6 (-3.3, 2.2) | 0.69 | |
| 6-month | 48.8 (9.1) | 45.4 (7.2) | -2.8 (-5.5, -0.1) | 0.042 | |
| Dietary fiber (g) | Baseline | 15.5 (5.0) | 14.0 (4.3) | ||
| 3-month | 15.0 (3.8) | 17.9 (6.2) | 4.4 (2.5, 6.4) | <0.001 | |
| 6-month | 15.6 (4.9) | 17.7 (6.3) | 3.7 (1.7, 5.8) | <0.001 | |
| Sodium (mg) | Baseline | 3817 (1108) | 4077 (923) | ||
| 3-month | 3786 )(1053) | 3881 (1043) | -165 (-566, 236) | 0.13 | |
| 6-month | 3575 (1093) | 3975 (1032) | 140 (-280, 560) | 0.56 | |
| Potassium (mg) | Baseline | 2663 (736) | 2501 (700) | ||
| 3-month | 2576 (559) | 2740 (689) | 326 (55, 596) | 0.049 | |
| 6-month | 2513 (719) | 2620 (762) | 269 (-1, 538) | 0.16 | |
| Calcium (mg) | Baseline | 501 (209) | 470 (168) | ||
| 3-month | 469 (210) | 543 (256) | 105 (31, 180) | 0.008 | |
| 6-month | 472 (229) | 513 (191) | 72 (11, 132) | 0.021 | |
| Magnesium (mg) | Baseline | 283 (79) | 276 (73) | ||
| 3-month | 283 (77) | 307 (95) | 32 (1, 63) | 0.044 | |
| 6-month | 279 (98) | 296 (88) | 24 (-5, 53) | 0.038 | |
| α-Tocopherol (mg) | Baseline | 7.9 (2.3) | 7.7 (2.7) | ||
| 3-month | 8.1 (3.5) | 8.4 (3.3) | 0.5 (-1.0, 1.9) | 0.39 | |
| 6-month | 7.6 (2.5) | 8.2 (3.3) | 0.7 (-0.4, 1.8) | 0.28 | |
| Vitamin K (µg) | Baseline | 262 (143) | 216 (102) | ||
| 3-month | 248 (131) | 338 (177) | 134 (75, 194) | <0.001 | |
| 6-month | 266 (142) | 363 (184) | 142 (83, 202) | <0.001 | |
| Vitamin C (mg) | Baseline | 111 (52) | 98 (39) | ||
| 3-month | 102 (38) | 115 (53) | 26 (9, 43) | 0.018 | |
| 6-month | 117 (74) | 116 (54) | 12 (-12, 35) | 0.059 | |
(DHA: docosahexaenoic acid, DPA: docosapentaenoic acid, EPA: eicosapentaenoic acid, MUFA: monounsaturated fatty acids, PUFA: polyunsaturated fatty acids, SFA: saturated fatty acids, %E: % energy. P values within-group comparisons from baseline were analyzed by paired t test or Wilcoxon signed-rank test. P values between-group comparisons were analyzed by unpaired t test or Mann–Whitney U test.
There were missing data for 3 of PJD and 2 of JD participants at 3 months, and 6 participants each in both group at 6 months.
Discussion
Nutrition education for the JD had a significant effect on serum LDL-C reduction. We recommended to both groups of participants in this study that they decrease their intakes of meat, animal fats, confections and alcoholic drinks to reduce energy and/or SFA intake. Reduced total energy intake resulted in weight loss, though the percentage of energy derived from SFA was 8.5% at baseline, already lower than those in western countries 20) , and the recommended target of SFA under 7% was not achieved in either group. We previously reported the baseline food and nutrient intakes of the participants, “meat, poultry and processed meat products”, “confections and sweets”, “milk and dairy products”, and “animal fats, SFA-rich vegetable oils and margarine” contributed to SFA intake at 34%, 12%, 12%, and 5%, respectively 21) . As animal fat intake was already very low at baseline, additional reduction of SFA from animal fat was difficult to achieve. If we hope to achieve significant LDL-C decreases in response to energy and SFA reductions, western type confections and dairy products must be more aggressively restricted.
The serum LDL-C reduction obtained in the JD group might have been a result of combined consumption of the recommended Japanese foods. Consuming fish has no significant effect on LDL-C according to a meta-analysis 22) , while PUFAs from a combination of increased fish and soy intakes might have contributed to lower LDL-C. As for soy, according to a meta-analysis, soy protein at a median amount of 25 g/day was reported to decrease LDL-C by 4.76 mg/dL, and this effect was seen even in patients with hyper-cholesterolemia 23) . Our participants already consumed 65 g of soy consisting of approximately 20 g of soy protein at baseline, and consumption of soy and soy products tended to further increase in both groups. Soy and soy-products contain isoflavones, plant sterols/stanols and PUFA as well as being free of cholesterol, with all of these factors being considered to contribute to lowering the LDL-C level 24 , 25) , though LDL-C was not decreased in our PJD group. In this study, the JD group increased soy consumption by eating more of the recommended natto, containing an abundance of viscose fiber, than soy and other soy products. We cannot speculate as to the additional effects of natto on LDL-C due to the lack of a human intervention study on this issue. Replacing meats with fish and soy in the JD group apparently contributed to LDL-C reduction with insufficient limitation of SFA intake in this study, even in the relatively short time period of this study.
Moreover, increasing intakes of unrefined cereals, vegetables, seaweed/mushrooms/konjak, all rich in dietary fiber, were recommended in the JD group. This study does not address the effects of dietary fiber on metabolic parameters, because mean unrefined cereals and barley expected to supply dietary fiber increased only 40 g, and the fiber intake level did not meet the recommended volume in the JD group. Seaweed, mushrooms and konjak are widely consumed in Japan and are essentially non-caloric. Furthermore, seaweed and mushrooms are used for achieving the umami taste. The participants already consumed about 30 g of these foods daily at baseline, and seaweed intake was doubled in the JD group. As these non-caloric traditional foods contributed to increasing the volumes of dishes without raising the caloric load, the JD may have the advantage of providing greater meal satisfaction.
As to secondary outcomes, body weight reduction resulting from decreased energy intake was expected to be associated with decreased TG and insulin in both groups. However, such reductions were not observed in the PJD group. Regarding TG reduction, fish was reportedly even more effective than foods such as whole grains and vegetables 26) . Increased intakes of EPA, DPA and DHA, exceeding 1000 mg per day, from fatty fish in the JD group might have contributed to the observed TG reduction.
Circulating MDA-LDL is considered to be a useful prognostic marker for future cardiac events 27) , especially in patients with low LDL-C 28) and aggressive use of LDL-lowering medications reportedly impacts MDA-LDL reduction 29) . We reported that JD consumption produced significant MDA-LDL reductions in healthy middle-aged men in a pilot study. However, the low level at baseline resulted in there being no between-group difference at 6 months in this study. Jenkins et al. reported consumption of high-isoflavone foods to be associated with reduced levels of circulating oxidized LDL in hyperlipidemic patients 30) . Iwai et al. reported natto to exert an inhibitory effect on LDL oxidation in vitro and in an animal study 31 , 32) . Mediterranean food patterns are reportedly associated with decreases in plasma oxidized LDL, despite a lack of change in plasma LDL-C, and plasma oxidized LDL is associated with serving sizes of fruits and vegetables 33) . We suspected favorable changes in MDA-LDL in the JD group based partly on improvements in dyslipidemia, but further study is needed to determine which foods comprising the JD are effective for reducing oxidative stress levels under low LDL-C conditions.
An inverse relationship of hs-CRP with healthy dietary patterns characterized by high intakes of vegetables, fruit, soy-products and fish, was reported in a Japanese cohort study 34) . EPA and DHA have anti-inflammatory or inflammation-resolving properties, whereas SFA influences pro-inflammatory effects 35 , 36) , and weight loss is well known to be accompanied by decreased concentrations of CRP, TNF-α and IL-6 37) . The JD group showed body weight decreases with reduced SFA and increased EPA/DPA/DHA intakes, while no changes were observed in these inflammatory markers. Adherence to the Mediterranean or the DASH diet is considered to improve circulating inflammatory biomarkers as compared with a routine diet 38 - 40) . However, these dietary interventions aimed at concurrently achieving body weight reduction, and many studies included intensive approaches and/or additional food supplements such as virgin olive oil and nuts 39 , 40) . The lack of changes in inflammatory parameters in this study, despite decreased body weight and improved circulating lipid levels, might largely reflect the already low baseline levels in our participants as compared with other studies 39 - 41) . Furthermore, the volumes and types of foods recommended were not extreme and the participants were readily able to access and consume these foods.
With regard to adipose tissue-derived cytokines, leptin reportedly up-regulates pro-inflammatory factors like TNF-α and IL-6, while adiponectin down-regulates these cytokines 42 , 43) . Leptin decreased and HMW-adiponectin increased, in both of our study groups, possibly suggesting beneficial effects of reducing fatty meat and confection consumptions.
As to concerns about JD effects on blood pressure, sodium intake did not change but potassium, magnesium and calcium intakes were increased and might have contributed to lower blood pressure in parallel with body fat reduction 44) . We recommend avoiding Japanese high-salt foods such as salted vegetables and fishes 45) , instead encouraging light tastes. This study demonstrated that the subjects adhering to the JD succeeded in changing their diets without increasing salt consumption.
This study has limitations. First, the JD group participants were given boiled rice with barley and canned mackerel to help them access these foods at baseline, such that the results at 3 months did not necessarily represent the effects of nutrition education. The rebound in the results of these food intake volumes at 6 months might suggest difficulties in consuming these foods by Japanese participants with highly westernized dietary habits. Skill training and environmental assistance to adopt behaviors needed for consuming the JD are warranted for highly westernized Japanese patients with dyslipidemia. Second, given the 6-week delay in starting this intervention from the day informed consent was obtained, some participants were allowed to begin restricting dietary intakes in advance. This might have improved the baseline data. Third, missing data were imputed by the LOCF method which gives a biased estimate of the treatment effect and underestimates the variability of the estimated results. However, we believe that the power was sufficient to allow evaluation of the dietary intervention in free-living outpatients.
While we acknowledge these limitations, we believe that this randomized controlled trial demonstrates for the first time the benefits of the JD in patients with dyslipidemia.
Conclusion
Nutritional education for the JD reduced serum LDL-C and improved metabolic risk factors more effectively than the PJD, though the latter was also beneficial in terms of body weight reduction in Japanese patients with dyslipidemia. Further long-term study is needed to clarify the effects of an improved education program designed to optimize consumption of the recommended JD.
Acknowledgements
The authors thank all patients who participated in the present study, and all staff members who supported the survey. The authors also appreciate all nutrition study team members of the laboratory of Nutrition Education and Clinical Nutrition of Japan Women’s University, including Ms. Kanako Kamoshita, Ms. Seina Komine, Ms. Sayaka Hasegawa, Ms. Rina Ichiki, Ms. Kanako Chibai, Ms. Chieko Fukuda, Ms. Miyu Oshika, Ms. Sia SuHuai, Ms. Moe Matsumoto, Ms. Saaya Yamada, Ms. Mariko Nakazawa, Ms. Yui Nishikata, Ms. Sayuri Igawa, Ms. Hazuki Kitayama, Ms. Mayuka Kodama, Ms. Kirika Fujitani, Ms. Aoi Tokunaga, Ms. Akari Yasuda, Ms. Hinako Omata, Ms. Saori Toyota, Ms. Kana Kinugawa, Ms. Mai Murano, Ms. Chisato Ogawa, and Ms. Nana Mihara, all of whom made major contributions to calculating the nutrient intakes in this study.
Financial Support
This study was supported by a research grant from the SKYLARK Food Science Institute and Rice Stable Supply Support Organization. This study was also supported by gifts of canned mackerel from Maruha Nichiro corporation, Tokyo, retort-packed rice with barley, Mochimugi Gohan from Hakubaku Co., Ltd. Tokyo and Omugi Gohan from Otsuka Pharmaceutical Co., Ltd. The SKYLARK Food Science Institute, Rice Stable Supply Support Organization, Maruha Nichiro corporation and Hakubaku Co., Ltd. had no role in the design, analysis or writing of this article.
Conflicts of Interest
The authors report the following disclosures: Masako Waki, has received clinical research funding from AstraZeneca KK, Sanofi KK and Eli Lilly Japan KK and; Tamio Teramoto has received honoraria from Bayer Pharma KK and Takeda Pharma KK, and clinical research funding from Dai-ichi Sankyo KK and Astellas Pharma KK. Chizuko Maruyama has received clinical research funding from SKYLARK Food Science Institute and Rice Stable Supply Support Organization. Yuri Shijo, Noriko Kameyama, Ariko Umezawa, Aisa Sato, Ai Nishitani, Makoto Ayaori, Katsunori Ikewaki have no conflicts of interest.
References
- 1).Kromhout D, Keys A, Aravanis C, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, and Pekkarinen M: Food consumption patterns in the 1960s in seven countries. Am J Clin Nutr, 1989; 49: 889-894 [DOI] [PubMed] [Google Scholar]
- 2).Menotti A, Kromhout D, Blackburn H, Fidanza F, Buzina R, and Nissinen A: Food intake patterns and 25-year mortality from coronary heart disease: cross-cultural correlations in the Seven Countries Study. The Seven Countries Study Research Group. Eur J Epidemiol, 1999; 15: 507-515 [DOI] [PubMed] [Google Scholar]
- 3).Tada N, Maruyama C, Koba S, Tanaka H, Birou S, Teramoto T, and Sasaki J: Japanese dietary lifestyle and cardiovascular disease. J Atheroscler Thromb, 2011; 18: 723-734 [DOI] [PubMed] [Google Scholar]
- 4).Shimazu T, Kuriyama S, Hozawa A, Ohmori K, Sato Y, Nakaya N, Nishino Y, Tsubono Y, and Tsuji I: Dietary patterns and cardiovascular disease mortality in Japan: a prospective cohort study. Int J Epidemiol, 2007; 36: 600-609 [DOI] [PubMed] [Google Scholar]
- 5).Teramoto T: Is “The Japan Diet” cardioprotective?. J Atheroscler Thromb, 2017; 24: 388-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Yokoyama S: Beneficial effect of retuning to “Japan Diet” for the Japanese. J Atheroscler Thromb, 2019; 26: 1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, Karanja N, Lin PH, and DASH-Sodium Collaborative Research Group: Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med, 2001; 344: 3-10 [DOI] [PubMed] [Google Scholar]
- 8).Vincent-Baudry S, Defoort C, Gerber M, Bernard M-C, Verger P, Helal O, Portugal H, Planells R, Grolier P, Amiot-Carlin M-J, Vague P, and Lairon D: The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am J Clin Nutr, 2005; 82: 964-971 [DOI] [PubMed] [Google Scholar]
- 9).The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): In: 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Editor-in-Chief: Professor Arnold von Eckardstein, pp33-35, The European Society of Cardiology, 2016 [Google Scholar]
- 10).Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Zachary D, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, William McEvoy J, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams Sr KA, Yeboah J, and Ziaeian B: 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2019; 140: e-605-607(e596-e646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Steffen LM, and Hootman KC: A posteriori data-derived dietary patterns and incident coronary heart disease: Making sense of inconsistent findings. Curr Nutr Rep, 2016; 5: 168-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, and Yokote K: Treatment A) lifestyle modification: executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan--2012 version. J Atheroscler Thromb, 2013; 20: 835-849 [DOI] [PubMed] [Google Scholar]
- 13).Committee for Epidemiology and Clinical Management of Atherosclerosis: Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Mozaffarian D, Appel LJ, and Van Horn L: Components of a cardioprotective diet new insights. Circulation, 2011; 123: 2870-2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Maruyama C, Nakano R, Shima M, Mae A, Shijo Y, Nakamura E, Okabe Y, Park S, Kameyama N, Hirai S, Nakanishi M, Uchida K, and Nishiyama H: Effects of a Japan diet intake program on metabolic parameters in middle-aged men. J Atheroscler Thromb, 2017; 24: 393-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).ShijoY, Maruyama C, Nakamura E, Nakano R, Shima M, Mae A, Okabe Y, Park S, Kameyama N, and Hirai S: Japan diet intake changes serum phospholipid fatty acid compositions in middle-aged men: a pilot study. J Atheroscler Thromb, 2019; 26: 3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Calder PC, Albers R, Antoine J-M, Blum S, Bourdet-Sicard R, Ferns GA, Folkerts G, Friedmann PS, Frost GS, Guarner F, Løvik M, Macfarlane S, Meyer PD, M’Rabet L, Serafini M, Van Eden W, Van Loo J, Vas Dias W, Vidry S, Winklhofer-Roob BM, and Zhao J: Inflammatory disease processes and interactions with nutrition. Br J Nutr, 2009; 101 Suppl. 1: S1-45 [DOI] [PubMed] [Google Scholar]
- 18).Takeda-Imai F, Yamamoto S, Fujii H, Noda M, Inoue M. Tsugane S: Validity and reproducibility of the self-administered shorter version of the physical activity questionnaire used in the JPHC study. 2010; 12: 1-10 [in Japanese] [Google Scholar]
- 19).Arao T, Oida Y, Maruyama C, Mutou T, Sawada S, Matsuzuki H, and Nakanishi Y: Impact of lifestyle intervention on physical activity and diet of Japanese workers. Prev Med, 2007; 45: 146-152 [DOI] [PubMed] [Google Scholar]
- 20).Drouin-Chartier J-P, Tremblay AJ, Lépine M-C, Lemelin V, Lamarche B, and Couture P: Substitution of dietary ω-6 polyunsaturated fatty acids for saturated fatty acids decreases LDL apolipoprotein B-100 production rate in men with dyslipidemia associated with insulin resistance: A randomized controlled trial. Am J Clin Nutr, 2018; 107: 26-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Kameyama N, Maruyama C, Shijo Y, Umezawa A, Sato A, Ayaori M, Ikewaki K, Waki M, and Teramoto T: Comparison of food and nutrient intakes between Japanese dyslipidemic patients with and without low-density lipoprotein cholesterol lowering drug therapy: a cross-sectional study. J Atheroscleror Thromb, 2020; 27: 683-694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Alhassan A, Young J, Lean MEJ, and Lara J: Consumption of fish and vascular risk factors: A systematic review and meta-analysis of intervention studies. Atherosclerosis, 2017; 266: 87-94 [DOI] [PubMed] [Google Scholar]
- 23).Mejia SB, Messina M, Li SS, Viguiliouk E, Chiavaroli L, Khan TA, Srichaikul K, Mirrahimi A, Sievenpiper JL, Kris-Etherton P, and Jenkins DJA: A meta-analysis of 46 studies identified by the FDA demonstrates that soy protein decreases circulating LDL and total cholesterol concentrations in adults. J Nutr, 2019; 149: 968-981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Taku K, Umegaki K, Sato Y, Taki Y, Endoh K, and Watanabe S: Soy isoflavones lower serum total and LDL cholesterol in humans: A meta-analysis of 11 randomized controlled trials. Am J Clin Nutr, 2007; 85: 1148-1156 [DOI] [PubMed] [Google Scholar]
- 25).Sedaghat A, Shahbazian H, Rezazadeh A, Haidari F, Jahanshahi A, Latfi SM, and Shirbeigi E: The effect of soy nut on serum total antioxidant, endothelial function and cardiovascular risk factors in patients with type 2 diabetes. Diabetes Metab Syndr, 2019; 13: 1387-1391 [DOI] [PubMed] [Google Scholar]
- 26).Schwingshackl L, Hoffmann G, Iqbal K, Schwedhelm C and Boeing H: Food groups and intermediate disease markers: a systematic review and network meta-analysis of randomized trials. Am J Clin Nutr, 2018; 108: 576-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Kotani K, Tashiro J, Yamazaki K, Nakamura Y, Miyazaki A, Bujo H, Saito Y, Kanno T, and Maekawa M: Investigation of MDA-LDL (Malondialdehyde-Modified Low-Density Lipoprotein) as a prognostic marker for coronary artery disease in patients with type 2 diabetes mellitus. Clin Chim Acta, 2015; 450: 145-150 [DOI] [PubMed] [Google Scholar]
- 28).Moriyama K, and Takahashi E: Evaluation of malondialdehyde low-density lipoprotein stratified by low-density lipoprotein cholesterol. Clin Lab, 2017; 63: 1179-1186 [DOI] [PubMed] [Google Scholar]
- 29).Nishikido T, Oyama J, Keida T, Ohira H, and Node K: High-dose statin therapy with rosuvastatin reduces small dense LDL and MDA-LDL: The Standard Versus high-Dose therApy with Rosuvastatin for lipiD Lowering (SARD) trial. J Cardiol, 2016; 67: 340-346 [DOI] [PubMed] [Google Scholar]
- 30).Jenkins DJA, Kendall CWC, Chung-Ja C Jackson CC, Connelly PW, Parker T, Faulkner D, Vidgen E, Cunnane SC, Leiter LA, and Josse RG: Effects of high- and low-isoflavone soyfoods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am J Clin Nutr, 2002; 76: 365-372 [DOI] [PubMed] [Google Scholar]
- 31).Iwai K, Nakaya N, Kawasaki Y, and Matsue H: Inhibitory effect of natto, a kind of fermented soybeans, on LDL oxidation in vitro. J Agric Food Chem, 2002; 50: 3592-3596 [DOI] [PubMed] [Google Scholar]
- 32).Iwai K, Nakaya N, Kawasaki Y, and Matsue H: Antioxidative functions of natto, a kind of fermented soybeans: effect on LDL oxidation and lipid metabolism in cholesterol-fed rats. J Agric Food Chem, 2002; 50: 3597-3601 [DOI] [PubMed] [Google Scholar]
- 33).Lapointe A, Goulet J, Couillard C, Lamarche B, and Lemieux S: A nutritional intervention promoting the Mediterranean food pattern is associated with a decrease in circulating oxidized LDL particles in healthy women from the Québec City metropolitan area. J Nutr, 2005; 135: 410-415 [DOI] [PubMed] [Google Scholar]
- 34).Nanri A, Yoshida D, Yamaji T, Mizoue T, Takayanagi R, and Kono S: Dietary patterns and C-reactive protein in Japanese men and women. Am J Clin Nutr, 2008; 87: 1488-1496 [DOI] [PubMed] [Google Scholar]
- 35).de Roos B, Mavrommatis Y, and Brouwer IA: Long-chain n-3 polyunsaturated fatty acids: new insights into mechanisms relating to inflammation and coronary heart disease. Br J Pharmacol, 2009; 158: 413-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour D, McArdle HJ, Kremer BHA, Sterkman L, Vafeiadou K, Benedetti MM, Williams CM, and Calder PC: Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr, 2015; 114: 999-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, Esposito K, Jönsson LS, Kolb H, Lansink M, Marcos A, Margioris A, Matusheski N, Nordmann H, O’Brien J, Pugliese G, Rizkalla S, Schalkwijk C, Tuomilehto J, Wärnberg J, Watzl B, and Winklhofer-Roob BM: Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr, 2011; 106: Suppl. 3, S5-78 [DOI] [PubMed] [Google Scholar]
- 38).Soltani S, Chitsazi MJ, and Salehi-Abargouei A: The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: a systematic review and meta-analysis of randomized trials. Clin Nutr, 2018; 37: 542-550 [DOI] [PubMed] [Google Scholar]
- 39).Mena M-P, Sacanella E, Vazquez-Agell M, Morales M, Fitó M, Escoda R, Serrano-Martínez M, Salas-Salvadó J, Benages N, Casas R, Lamuela-Raventós RM, Masanes F, Ros E, and Estruch R: Inhibition of circulating immune cell activation: a molecular antiinflammatory effect of the Mediterranean diet. Am J Clin Nutr, 2009; 89: 248-256 [DOI] [PubMed] [Google Scholar]
- 40).Casas R, Sacanella E, Urpí-Sardà M, Corella D, Castañer O, Lamuela-Raventos R-M, Salas-Salvadó J, Martínez-González M-A, Ros E, and Estruch R: Long-term immunomodulatory effects of a mediterranean diet in adults at high risk of cardiovascular disease in the PREvención con Dleta MEDiterránea (PREDIMED) randomized controlled trial. J Nutr, 2016; 146: 1684-1693 [DOI] [PubMed] [Google Scholar]
- 41).Jaceldo-Siegl K, Haddad E, Knutsen S, Fan J, Lloren L, Bellinger D, and Fraser GE: Lower C-reactive protein and IL-6 associated with vegetarian diets are mediated by BMI. Nutr Metab Cardiovasc Dis, 2018; 28: 787-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Choi HM, Doss HM, and Kim KS: Multifaceted physiological roles of adiponectin in inflammation and diseases. Int J Mol Sci, 2020; 21: 1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Woodward L, Akoumianakis I, Antoniades C: Unravelling the adiponectin paradox: novel roles of adiponectin in the regulation of cardiovascular disease. Br J Pharmacol, 2017; 174: 4007-4020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Morikawa Y, Nakagawa H, Okayama A, Mikawa K, Sakata K, Miura K, Ishizaki M, Yoshita K, Naruse Y, Kagamimori S, Hashimoto T, and Ueshima H: A Cross-sectional study on association of calcium intake with blood pressure in Japanese population. J Hum Hypertens, 2002; 16: 105-110 [DOI] [PubMed] [Google Scholar]
- 45).Okuda N, Okayama A, Miura K, Yoshita K, Saito S, Nakagawa H, Sakata K, Miyagawa N, Chan Q, Elliott P, Ueshima H, and Stamler J: Food sources of dietary sodium in the Japanese adult population: the international study of macro-/micronutrients and blood pressure (INTERMAP). Eur J Nutr, 2017; 56: 1269-1280 [DOI] [PMC free article] [PubMed] [Google Scholar]