Abstract
Background
Moderate physical activity is associated with an improved prognosis and psychosocial outcome in breast cancer patients. Although exercise and physical activity are associated with multiple physiological and psychological effects, many of the underlying mechanisms remain obscure. The BEGYN study (Influence of physical activity in breast cancer patients on physiological and psychological parameters and on biomarkers) aims at identifying potential associations between the extent of physical activity, fitness, body composition, immunological biomarkers, psycho-emotional parameters, and the course of treatment during the first year after diagnosis of breast cancer.
Methods
The prospective observational BEGYN study will include 110 non-metastatic breast cancer patients. The patients will be assessed during a base line visit prior to the initiation of the antineoplastic therapy and after 3, 6, 9 and 12 months. The physical activity will be measured using a fitness tracker and a self-assessment diary during the entire study. Each visit will include the assessment of (i) cardiorespiratory fitness measured by spiroergometry, (ii) body composition, (iii) psycho-emotional parameters (quality of life, mental health, fatigue, depression, distress, anxiety, well-being), and (iv) extensive blood tests including routine laboratory, vitamin D, selenium and immunologically relevant biomarkers (e.g., leukocyte subpopulations and cytokine profiles).
Discussion
Whereas most studies investigating the influence of physical activity in breast cancer patients focus on specific activities for three months or less, the BEGYN study will quantify the daily physical activity and cardiorespiratory fitness of breast cancer patients based on objective measurements in the context of the oncological therapy for 12 months after diagnosis. The study will reveal potential associations between exercise, immune status and physical as well as psycho-emotional outcome and the clinical course of the disease. Moreover, complementary therapies such as Vit D and Selenium supplementation and parameters investigating the motivation of the patients are part of the study. Due to this holistic approach, the BEGYN study will guide towards confirmatory studies on the role of physical activity in breast cancer patients to develop individualized counselling regarding the recommended type and extent of exercise.
Trial Registration
This study has been registered at the German Clinical Trials Register DRKS00024829.
Keywords: breast cancer, physical activity, spiroergometry, psychological parameters, body composition, chemotherapy, immune monitoring, observational study
1 Introduction
Historic recommendations to avoid physical activity during cancer treatment to save all energy for fighting the disease have proven wrong (1–6). Breast cancer is the most common cancer in women, accounting for more than 680.000 deaths per year worldwide (7). Although modern breast cancer treatment such as improved diagnostic and staging procedures, advanced systemic therapy, surgery and radiotherapy increases long-term survival and clinical outcome of breast cancer patients, there is still a deficiency of supportive and psychosocial care (8). Breast cancer survivors are at risk of suffering potentially disabling physical and psychological sequelae, such as lymphedema, axillary web syndrome, chronic pain, osteoporosis and fractures, arthralgia, chronic fatigue syndrome and depression (9). During and after antineoplastic therapy, rehabilitation and complementary therapies are crucial to improve the quality of life and overall prognosis of breast cancer survivors (10–12).
Multiple studies in cancer patients have demonstrated that physical activity and exercise correlate with an improved outcome regarding the course of the underlying disease and with a better tolerance to the antineoplastic treatments (13). For example, exercise had positive effects on the fatigue syndrome and quality of life in cancer (12, 14), the course of lymphedema (13, 15) and osteoporosis in breast cancer (16) and prostate cancer (17). Moreover, physical activity influences various functions of the immune system, such as the proportions of circulating leukocyte subsets and the expression of cytokines (4, 18–21). Moderate sporting activity has an immune-protective effect, whereas excessive sporting activity is associated with an increased susceptibility to infections - possibly mediated by a reduction in circulating natural killer cells (22). Natural killer cells and other leukocyte subsets play a crucial role in the physiological attempts of the organism to control cancer cells (23). Thus, Ashcraft et al. put forth the hypothesis that exercise-induced modulations of the immune status do not only alter the susceptibility to infections, but also the immune response to neoplastic diseases and the effectivity of antineoplastic therapies (24). Current data suggest that a complex interplay of the above-mentioned factors contributes to a positive correlation between the quantity of physical activities and event-free survival in cancer patients (69% reduced hazard of mortality among highly active patients) (25). In consequence, more prospective studies were recommended to characterize the influence of sporting activities on the immune system and on potential individualized rehabilitation approaches in cancer patients (9, 26, 27).
According to Mehnert et al. (28) the prevalence of any mental disorder among the major tumor entities is 32% and breast cancer has the highest prevalence of mental disorders with 42%. 17% of breast cancer patients are afflicted with anxiety disorders and 9% with affective disorders. Physical activity can affect anxiety and depression in breast cancer patients. Interestingly, leisure time physical activity was negatively related to depression, whereas occupational physical activity related positively to anxiety (29).
30% of disease-free breast cancer survivors suffer from cancer related fatigue syndrome, causing a massive reduction of psycho-emotional wellbeing and quality of life (30–32). Regular exercise plays an important role in the management of cancer-related fatigue (33–35). Clinical trials have shown that individual and patient-adapted exercise programs yield the best outcome regarding physical functioning and health among breast cancer patients (25, 32, 36–38).
The body composition strongly correlates with physical activity and is a relevant prognostic factor for breast cancer patients (39). On average, overweight patients have a higher risk to develop breast cancer, a higher rate of relapses and a shorter recurrence-free period (40). The loss of muscle mass and the gain of fat mass (sarcopenia) can be detected with a bioelectrical impedance analysis, a simple, non-invasive technique (41). A lower muscle index can be associated with a higher toxicity of the chemotherapy (42).
Since the physical activity plays a key role in determining the prognosis of breast cancer patients, it is useful to combine exercise diaries with objective measurements such as pedometers or fitness trackers (43, 44). Moreover, this yields continuous information on vital parameters, including the pulse and resting heart rate as an estimate for overall cardiopulmonary fitness (45).
Studies that combine fitness tests, physical assessments, immunological markers, and psychological tests in breast cancer patients are still scarce, thus it is highly difficult to understand potential cross-links between these aspects. Filling this gap of knowledge could allow conclusions on individualized prevention and rehabilitation of the multiple sequelae that are potentially associated with breast cancer (9). Clinical studies in cancer patients must consider that according to current guidelines, all breast cancer patients are to be advised to exercise endurance and muscle strength (39). However, little is known on the extent to which patients adhere to this recommendation. Moreover, it would be unethical to withhold motivation for sportive activity from breast cancer patients for experimental purposes as a control. Thus, the BEGYN study was designed as an observational study, using validated methods to assess physical activity, cardiopulmonary fitness, body composition, psychological parameters, and extensive blood tests (e.g., immune status, vitamin D and selenium) during the first year of antineoplastic therapy after diagnosis of non-metastatic breast cancer. To gain information even after a longer period the BEGYN study is designed to collect data over one year after diagnosis. This study will shed light into the dynamics of physiological and psychological variables and the course of the disease during the first year after initiation of antineoplastic therapy in breast cancer patients in correlation with the physical activity. Ultimately, this study will lay the basis to develop individualized recommendations for exercise in breast cancer patients to improve the quality of life and prognosis.
2 Materials and Methods
2.1 Study Population
The BEGYN study will assess 110 female patients with non-metastatic invasive breast cancer prior to the initiation of antineoplastic therapy. The patients will be followed for one year after diagnosis. Since the study focuses on variables that are heavily influenced by gender (e.g., physical fitness and body composition), we did not include male breast cancer patients. Inclusion and exclusion criteria ensure that patients can undergo spiroergometry, blood tests, assessment of physical activities and psychological parameters during the first year after the diagnosis of breast cancer. The detailed inclusion criteria and exclusion criteria are given in Table 1 .
Table 1.
Inclusion criteria and exclusion criteria of the BEGYN study.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
2.2 Study Schedule
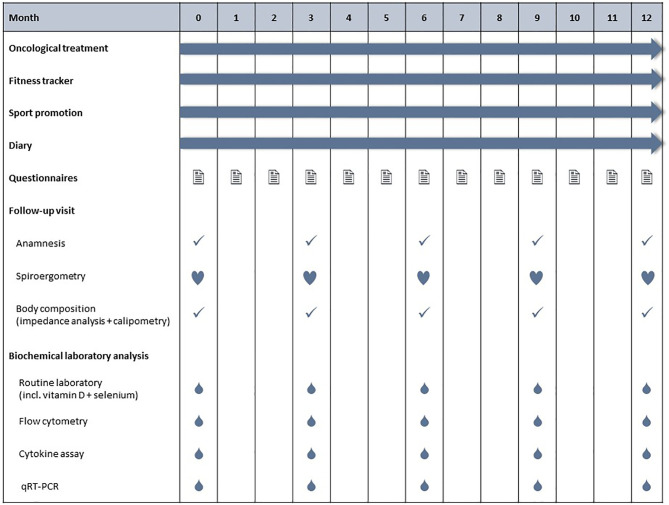
The patients are enrolled to the BEGYN study after initial diagnosis. The baseline study visit is scheduled before the initiation of any antineoplastic therapy, followed by quarterly follow up visits. During each visit, patients undergo a clinical assessment, spiroergometry on a treadmill, blood tests, measurement of the body composition by bioimpedance analysis, plicometry, validated psychological questionnaires and other assessments ( Figure 1 ).
Figure 1.
Schedule for the BEGYN study. All patients are asked to write down their physical activities in a diary (type and duration of exercise) and to continuously wear a fitness tracker. Study visits are scheduled quarterly, with the first visit taking place before initiation of antineoplastic therapy.
2.3 Clinical Assessment
Routine assessment during quarterly follow-up visits includes an anamnesis focused on potential side effects of antineoplastic therapies, measuring the body weight and blood pressure.
2.4 Assessment of Physical Activity and Exercise
2.4.1 Patient Self-Assessment Diary
Each patient will be asked to document her daily sporting activities in a standardized self-assessment study diary. The patients will be instructed by study personnel how to use the diary during recruitment and during each follow-up visit. The study personnel check the completeness and accuracy of the diary and discuss potential improvements with the patients on each follow-up visit. The diary will be used to document the following points:
Medically relevant information (change of medication or nutritional supplements, fever, symptoms etc.)
Daily sportive activities
Weekly read outs of the fitness tracker
Weekly psychological questionnaires
Questions and notes that the study participants might have.
As a standardized measure of physical activity the metabolic equivalent task (MET) will be used to describe the metabolic turnover of the patients even when performing different sportive activities (46, 47).
2.4.2 Spiroergometry
During each of the five study visits the patients perform a spiroergometry on a treadmill (XRCISE RUNNER MED™ by Cardiowise, ERGO-FIT™, Pirmasens, Germany) for the assessment of cardiopulmonary fitness (48). After a technical introduction for the patient, the calibration of the aeroman™ and a baseline spirometry in seated position, the patients start walking at an individually determined speed, typically 4 kmph. Subsequently, the patient is challenged according to a standardized, validated protocol that ensures a linear increase in Oxygen uptake response (49). For inter- and intraindividual comparison, the time course of work rate in watts (WR(t)) is calculated using the formula published by Porszasz et al. (49) WT(t) = m * g * ν(t) * sin (α), where m is body mass in kg, g is the gravitational acceleration (9.81 m*s-2), ν(t) is the time course of velocity in meters per second, and α is the angle of inclination. The speed or gradient of the treadmill is increased by 0.5 kmph or 1 percent every 2 minutes to intensify the level of exertion. After each interval, the patient breathes through a tightly fitting spirometer mouthpiece (aeroman™ professional, ACEOS GmbH, Fürth, Germany) according to a validated protocol (50). The measurement will be terminated once the patient reaches a maximum heart rate defined as 220 – age (bpm) or a respiratory quotient >1. Furthermore, the subjective perception of exertion according to Borg >17 (51), dizziness, dyspnea, nausea or pain will lead to termination of the spiroergometry (52). The patient can interrupt the measurement due to subjective exhaustion at any time. This will reveal intraindividual longitudinal changes during the first year after the diagnosis of breast cancer. The ventilatory threshold (VT) will be used as a submaximal indicator of general cardiopulmonary fitness (53). VT represents the excessive increase of carbon dioxide output compared to oxygen uptake. In addition, heart rate (HR), oxygen uptake (VO2), carbon dioxide release (VCO2), respiratory quotient (RQ), breathing frequency (BF) and respiratory minute volume (VE) will be assessed to allow the analysis of endurance performance. The measurements obtained with the spiroergometry are given in Table 2 .
Table 2.
Spiroergometry.
| Description | Abbreviation | Unit |
|---|---|---|
| Heart rate | HR | Beats per minute |
| Oxygen uptake | VO2 | L/min |
| Carbon dioxide release (l/min) | VCO2 | L/min |
| Respiratory quotient | RQ | VO2/VCO2 |
| Breathing frequency | BF | Breaths per minute |
| Respiratory minute volume | VE | L/min |
2.4.3. Fitness Tracker
Physical activity is assessed daily by supplying each patient uses a commercial fitness tracker (Fitbit charge 3™ (Fitbit Inc., San Francisco) that will be linked to her smartphone (54). Study personnel assists the patients and – if required – their associate to install the smartphone app and to setup the measurements. The patients are requested to transcribe the measurements of their fitness tracker into their study diary weekly. Those values are shown in Table 3 .
Table 3.
Measurements with the fitness tracker.
| Description | Abbreviation | Registration interval | Unit |
|---|---|---|---|
| Core stats | Daily | ||
| Steps taken | Daily | Count | |
| Resting heart rate | RHR | Daily | Beats per minute |
| Calories burned | Cal | Daily | Kcal |
| Workout stats | Real-time | ||
| Elapsed time | Real-time | Minute | |
| Distance covered | Real-time | Km | |
| Calories burned | Real-time | Kcal | |
| Average heart rate | øHR | Real-time | Beats per minute |
| Maximum heart rate | HRmax | Real-time | Beats per minute |
2.5 Body Composition
2.5.1. Bioelectrical Impedance Analysis
The body composition will be determined based on bioelectrical impedance analysis (BIA) (TANITA scale™, Tanita Europe BV, Stuttgart). The test person stands barefoot on a body scale and holds sensors in both hands. Using the impedance between the four measuring points, numerous data can be collected that provide information on the muscle, fat, bone and water content of the whole body and individual compartments. Bioimpedance analysis is routinely used in the context of nutritional advice and sports medicine, but it can also shed light into disease processes, such as hemodialysis patients (55) ( Table 4 ). When accessible, the body composition will also be estimated by using routine CT scans of the study patients as previously published (56).
Table 4.
Measurements by scale and bioimpedance analysis.
| Item | Unit |
|---|---|
| Weight | kg |
| Total body fat | % |
| Total muscle mass | kg |
| Bone mass | kg |
| Body-Mass-Index | Kg/m² |
| Basal metabolic rate | kcal |
| Metabolic age | years |
| Total body water | % |
| Visceral fat | kg |
| Segmental muscle mass in arms, legs, and torso | kg |
| Segmental body fat in arms, legs, and torso | % |
2.5.2 Plicometry (Calipometry)
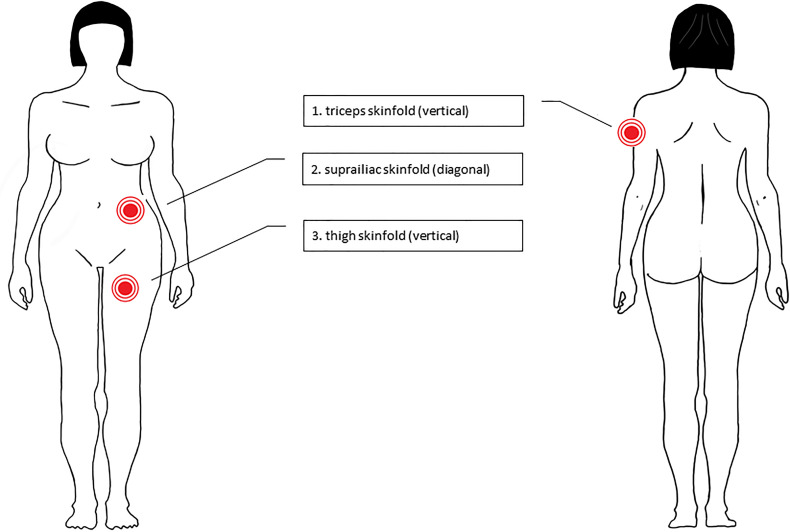
Plicometry allows a standardized estimate of the body fat status which can change under influences such as sport, chemotherapy or cancer (57–60). In the BEGYN study, fat distribution is assessed using the skinfold measurement using the 3 point-method (triceps, suprailiac skinfold, and thigh) according to Jackson and Pollock (61). ( Figure 2 ). The total body subcutaneous fat tissue (in kg) was estimated from the plicometry measurements using the formula (61):
Figure 2.
Plicometry sites of measurement (58, 59). Skinfold measurement using the 3 point-method (1 = triceps, 2 = suprailiac skinfold; 3 = thigh).
S is the sum of the skinfold thicknesses measured at the triceps, the suprailiac skinfold and the thigh.
2.6 Nutritional Habits, Intake Of Food Supplements, Self-Medications, and Lifestyle
According to the guideline, a Mediterranean diet was recommended to all patients (39). Nutritional habits were assessed using a questionnaire with a focus on characteristic dietary patterns. In addition, sleeping, smoking, and drinking habits, the use of food supplements and self-medication as well as exposure to sunlight and use of sun protection were assessed using questionnaires and the self-assessment diary, respectively.
2.7 Biomarkers
Cancer, antineoplastic therapy as well as physical activity are closely related with biomarkers (22, 62–66).
2.7.1 Blood Count, Biochemical Laboratory Markers
For disease monitoring and adjustment of the antineoplastic therapy, multiple biomarkers are routinely assessed during the quarterly study visits ( Table 5 ). In addition, the BEGYN study will include vitamin D and selenium concentrations since they are often discussed as potentially relevant to cancer biology (69–72).
Table 5.
Laboratory values, intervals of assessment and reference values.
| Parameter | Assessment interval | Vial | Unit | reference value |
|---|---|---|---|---|
| Blood count | ||||
| Erythrocytes | quarterly | EDTA | 10\S\12/l | 4.00 – 5.20 |
| Hb | quarterly | EDTA | g/dl | 12.0 – 16.0 |
| Leukocytes | quarterly | EDTA | 10\S\9/l | 3.9 – 10.2 |
| Thrombocytes | quarterly | EDTA | 10\S\9/l | 140 – 400 |
| Differential leukocyte count | ||||
| Neutrophils rel. | quarterly | EDTA | % | 42.0 – 77.0 |
| Neutrophils abs. | quarterly | EDTA | 10\S\9/l | 1.5 – 7.7 |
| Lymphocytes | quarterly | EDTA | % | 25.0 – 45.0 |
| Monocytes | quarterly | EDTA | % | 2.0 – 10.0 |
| Eosinophils | quarterly | EDTA | % | 0.0 – 5.0 |
| Basophils | quarterly | EDTA | % | 0.0 – 1.0 |
| Biochemical parameters | ||||
| Sodium | quarterly | Li-Heparin-Plasma | mmol/l | 135 – 145 |
| Potassium | quarterly | Li-Heparin-Plasma | mmol/l | 3.5 – 5.1 |
| Calcium | quarterly | Li-Heparin-Plasma | mmol/l | 2.2 – 2.6 |
| Magnesium | quarterly | Li-Heparin-Plasma | mmol/l | 0.66 – 1.07 |
| Iron | quarterly | Li-Heparin-Plasma | µg/dl | 33 – 193 |
| Creatinin | quarterly | Li-Heparin-Plasma | mg/dl | 0.50 – 0.90 |
| Urea | quarterly | Li-Heparin-Plasma | mg/dl | 17 – 48 |
| Uric acid | quarterly | Li-Heparin-Plasma | mg/dl | 2.5 – 5.7 |
| Glucose | quarterly | Li-Heparin-Plasma | mg/dl | 60 – 100 |
| Protein | quarterly | Serum | g/l | 66 – 87 |
| Albumin | quarterly | Serum | g/l | 35 – 52 |
| Cholesterol | quarterly | Li-Heparin-Plasma | mg/dl | <200 |
| Triglycerides | quarterly | Li-Heparin-Plasma | mg/dl | <150 |
| CK | quarterly | Li-Heparin-Plasma | U/l | 0 – 170 |
| ASAT | quarterly | Li-Heparin-Plasma | U/l | 0 – 35 |
| ALAT | quarterly | Li-Heparin-Plasma | U/l | 0 – 35 |
| gamma-GT | quarterly | Li-Heparin-Plasma | U/l | <40 |
| Alkaline phosphatase | quarterly | Li-Heparin-Plasma | U/l | 35 – 104 |
| Bilirubin | quarterly | Li-Heparin-Plasma | mg/dl | <1.2 |
| Lipase | quarterly | Li-Heparin-Plasma | U/l | 13 – 60 |
| LDH | quarterly | Li-Heparin-Plasma | U/l | 0 – 262 |
| CRP | quarterly | Li-Heparin-Plasma | mg/l | 0.0 – 5.0 |
| HbA1c | quarterly | EDTA | % | <6.0 |
| LDL-Cholesterol | quarterly | Li-Heparin-Plasma | mg/dl | <130 |
| HDL-Cholesterol | quarterly | Li-Heparin-Plasma | mg/dl | 45 – 65 |
| VLDL-Cholesterol | quarterly | Li-Heparin-Plasma | mg/dl | <35 |
| Interleukin-6 | quarterly | Li-Heparin-Plasma | pg/ml | <7 |
| Selenium | quarterly | Serum | µg/l | 50 – 120 (67) |
| Vitamin D-25-OH | quarterly | Serum | ng/ml | 30 – 100 (68) |
| Hormones | ||||
| TSH | quarterly | Li-Heparin-Plasma | µIU/ml | 0.27 – 4,2 |
| fT3 | quarterly | Li-Heparin-Plasma | pg/ml | 2.0 – 4.4 |
| fT4 | quarterly | Li-Heparin-Plasma | ng/dl | 0.93 – 1.7 |
| Cortisol | quarterly | Serum | µg/dl | 4.82 – 19.5* |
| Insulin | quarterly | Serum | µIU/ml | <29.1 |
| ß-hCG | quarterly | Serum | mIU/ml | <1.0 |
| Tumor marker | ||||
| CA 15-3 | optional | Serum | U/ml | <26.2 |
*Reference values for Cortisol vary depending on the time of blood collection. As in the BEGYN study, samples were routinely taken in the morning these standard values were used.
2.7.2 Leukocyte Subsets
Peripheral blood mononuclear cells (PBMCs) will be obtained from 9,6 ml anticoagulated blood samples by Ficoll density gradient centrifugation and resuspended in 90% fetal calf serum + 10% dimethylsulphoxide. Plasma supernatant will be collected and all biosamples will be cryopreserved at -80°C until further processing (73).
The underlying disease, the antineoplastic therapies and physical activity can have a profound impact on the immune system, respectively (26, 74–77). The number of 36 circulating leukocyte subsets will be assessed by flow cytometry using a panel of validated antibody stainings ( Table 6 ), based on previous studies (73, 78). Cells will be analyzed with FACSCelesta™ (BD Biosciences, Franklin Lakes, New Jersey, USA) (79).
Table 6.
Leukocyte subsets.
| population | Surface antigens |
|---|---|
| B cells | |
| innate B cells | CD19+ CD27- IgD- IgM- |
| naïve B cells | CD19+ CD27- IgD+ IgM+ |
| memory B cells | CD19+ CD27+ |
| marginal zone memory B cells | CD19+ CD27+ IgD+ IgM+ |
| IgM memory B cells | CD19+ CD27+ IgD- IgM+ |
| class switched memory B cells | CD19+ CD27+ IgD- IgM- |
| late memory B cells | CD19+ CD27+ CD38+ IgM+ |
| plasmablasts | CD19+ CD27+ CD38bright IgM- |
| transitonal B cells | CD19+ CD20+ CD27- CD38+ |
| pre-naïve B cells (B1 cells) | CD20+ CD27+ CD43+ CD70- |
| B2 cells | CD20+ CD27+ CD43- |
| T cells | |
| Th cells with αβ-TCR | TCRαβ+ CD4+ |
| cytotoxic Th cells with αβ-TCR | TCRαβ+ CD8+ |
| memory effector Th cells | CD3+ CD4+ CD62L- CD45RO+ |
| memory central Th cells | CD3+ CD4+ CD62L+ CD45RO+ |
| naïve effector Th cells | CD3+ CD4+ CD62L- CD45RO- |
| naive central Th cells | CD3+ CD4+ CD62L+ CD45RO- |
| memory effector cytotoxic T cells | CD3+ CD8+ CD62L- CD45RO+ |
| memory central cytotoxic T cells | CD3+ CD8+ CD62L+CD45RO+ |
| naïve effector cytotoxic T cells | CD3+ CD8+ CD62L- CD45RO- |
| naïve central cytotoxic T cells | CD3+ CD8+ CD62L+ CD45RO- |
| T cells with γδ-TCR | TCR γδ+ CD3+ CD5+ |
| naïve thymus negative Th cells | CD3+ CD4+ CD31- CD45RO- |
| naïve thymus negative Th cells | CD3+ CD4+ CD31+ CD45RO- |
| Th1 cells | CD3+ CD4+ CD183+ CCR6+ |
| Th2 cells | CD3+ CD4+ CCR4+ CRTH2+ |
| naïve Th1 cells | CD3+ CD4+ CD183+ CD45RO- |
| memory Th1 cells | CD3+ CD4+ CD183+ CD45RO+ |
| naïve Th2 cells | CD3+ CD4+ CD45RO- CRTH2+ |
| memory Th2 cells | CD3+ CD4+ CD45RO+ CRTH2+ |
| regulatory T cells | CD3+ CD4+ CD25+ CD127- |
| NK cells | |
| natural killer cells | Lin- CD335+ CD56+ CD16+ |
| memory-like natural killer cells | Lin- CD335+ CD56dim CD16+ |
| intermediate natural killer cells | Lin- CD335+ CD56bright CD16- |
| innate natural killer cells | Lin- CD335+ CD56- CD16- |
2.7.3 Cytokine Profiles
Plasma cytokine profiles will be measured by Luminex® xMAP™ technology (Austin Texas, USA) since they reflect the activity of various immune cells ( Table 7 ). Luminex® xMAP™ technology performed on MAGPIX™ instruments, enables the simultaneous quantification of up to 50 target proteins or nucleic acids (80) and have been validated for numerous immunological studies, including breast cancer (20, 62, 81) and infections (82). Superparamagnetic microsphere beads are conjugated with a distinct monoclonal antibody. The beads themselves are dyed with varying amounts of red and infrared fluorophores to allow clear assignment to a specific bead region. MAGPIX™ fluorescence imager-based instruments use a LED illumination/CCD camera detection system for both bead region identification and reporter fluorophore-based analyte quantification (83).
Table 7.
Cytokine profiles measures by Luminex™ (MAGPIX®).
| Cytokine | abbreviation |
|---|---|
| Tumor necrosis factor α | TNFα |
| Interferon γ | IFNγ |
| Interleukin 1α | IL-1α |
| Interleukin 1β | IL-1β |
| Interleukin 2 | IL-2 |
| Interleukin 4 | IL-4 |
| Interleukin 6 | IL-6 |
| Interleukin 10 | IL-10 |
| Interferon γ -induced protein 10 | IP10 |
| Monocyte chemoattractant protein 1 | MCP-1 |
| Granulocyte-macrophage colony-stimulating factor | GM-CSF |
2.7.4 Gene Expression Profiles
The expression of selected mRNA transcripts related to breast cancer and inflammation will be measured by a quantitative real-time PCR (qPCR). The method used is based on a previously published technique (73). Total RNA will be isolated from PMBCs using the High Pure RNA Isolation Kit (Roche, Basel, Suisse). Reverse transcription will be performed using SuperScript™ VILO IV cDNA Synthesis Kit (Invitrogen, Thermo Fisher Scientific) followed by the cDNA purification using QIAquick™ PCR Purification Kit (Qiagen, Hilden). Concentration and purity determination of the isolated RNA will be performed with NanoDrop™ (Thermo Fisher Scientific, Waltham, Massachusetts, USA). TaqMan™ Gene Expression Assays (Applied Biosystems, Thermo Fisher Scientific, Waltham, Massachusetts, USA) will be used to perform qRT-PCR of immunological key transcription factors TBX21, RORC, GATA3, FOXP3 (84–88). The Δct-values will be calculated using ACTB, EEF1A1, and 18S as a standard. Values will be normalized to the data from the samples taken at the baseline visit.
2.8 Assessment of Psychological Parameters
The assessment of psychological parameters will be performed by using standardized questionnaires that were validated for (breast) cancer patients, respectively (89–98). A list of questionnaires is shown in Table 8 . Moreover, the patients have the opportunity to report individual thoughts as free texts and during the study visits.
Table 8.
Assessment of the psychological parameters (used questionnaires).
| Abbreviation | Name | Nmber of items | Content | Reference |
|---|---|---|---|---|
| EORTC QLQ-C30 | European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 | 30 items | health-related quality of life (QoL) | (95) |
| EORTC QLQ-BR23 | European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-BReast cancer module 23 | 23 items | health-related quality of life (QoL) in breast cancer patients: systemic therapy side-effects, arm symptoms, breast symptoms, body image and sexual functioning | (95) |
| MHS | Mental Health Scales | 76 items | willpower, acceptance of life, self-reflection, finding of a meaning, naturalness and social integration | (92) |
| HADS | Hospital Anxiety and Depression Scale | 14 items | level of anxiety and level of depression | (89) |
| DT | Distress Thermometer | DT: 1 item | Psychosocial distress and possibly associated problems (practical, family, emotional, spiritual/religious and physical) | (94) |
| Problem list: 39 items | ||||
| MDWQ | MultiDimensional Well-being Questionnaire | 12 items | mood, level of alertness and level of calmness | (91) |
2.8.1 Quality of Life
The overall quality of life is assessed using the Quality of Life Questionnaire (QLQ-C30 version 3.0) which has been developed and validated by the European Organisation for Research and Treatment of Cancer (EORTC) to explore quality of life among cancer patients (90). The supplementary questionnaire QLQ-BR23 will be used to record symptoms specific for breast cancer (95). In sum, a score from 0 – 100 can be calculated allowing the quantification of the impairments regarding the patient’s health and quality of life.
2.8.2 Mental Health
The questionnaire “Mental health scales” (MHS) (92) was used to assess the psychological integrity of the study participants, in order to evaluate the development of the patients’ personality throughout the study duration. The questionnaire consists out of seven scales (autonomy (17 items), willpower (14 items), acceptance of live (8 items), self-reflection (12 items), finding of a meaning (7 items), naturalness (10 items) and social integration (8 items)), which yields a total of 76 items. The questionnaire is answered with the help of a five-point Likert-scale (1 = I fully agree; 5 = I fully disagree) and an example item is “Generally I am confident”.
2.8.3 Chronic Fatigue Syndrome
Symptoms of chronic fatigue syndrome (CFS) (93) that affects more than 50% of breast cancer patients and the extent of psychological stress will be assessed using the Distress Thermometer (DT) (96).
2.8.4 Anxiety and Depression
The self reported anxiety and distress will be assessed using the HADS (Hospital Anxiety and Depression Scale) questionnaire (89, 97) and the German adaptation of the Distress Thermometer (DT) (94, 96–98).
2.8.5 Well-Being
To get an overview of the mental state of the patients, the German questionnaire “Multidimensional Well-being Questionnaire” (MDBQ) (91) has been used in its short version with twelve items (short version A). This questionnaire captures three bipolar dimensions of the current mental state (good mood – bad mood, alertness – fatigue, tranquility – inquietude) of the breast cancer patients. The questionnaire presents twelve adjectives (e.g., satisfied, flabby, good etc.) and with the help of a five-point Likert-scale (1 = not at all; 5 = very much) the patients rate their instant feeling. Cronbachs’α is between 0.86 and 0.94, indicating a good consistency of the scale.
2.9 Sample Size Calculation and Statistical Analyses
The Institute for Medical Biometry, Epidemiology and Medical Informatics (IMBEI) Saarland University is supporting the sample size calculation, study design, data management and evaluation using PASS 2019 (NCSS, LLC, Kaysville, Utah, USA) and SPSS (Version 27 IBM SPSS Statistics, Armonk, New York, USA). Data were collected by using Excel 2019 (Microsoft, Redmond, USA). A sample size of 110 produces a two-sided 95% confidence interval with a distance from the mean to the limits that is equal to 2.835 when the estimated standard deviation is 15.0. A sample size of 110 produces a two-sided 95% confidence interval with a width equal to 0.187 when the sample proportion is 0.50.
Continuous measures are presented as means ± standard deviations (SD) or medians (range). Categorical variables are presented as frequencies (percentage). For continuous variables normality is tested using Shapiro-Wilk-Test. In case of non-rejection of normality two-group comparisons are due to the t-test for two independent samples. For more than two groups, comparisons are due to one-way Analysis of Variance (ANOVA). Comparing two repeated measurements, t-test for 2 dependent samples is used and for more than two we use repeated measures ANOVA. In case of rejection of normality, Mann-Whitney U-test, Kruskal-Wallis-test, Wilcoxon test for two dependent samples and Friedman-test are used, respectively. For group comparisons considering categorical variables the chi-squared test and for repeated measures McNemars’s test are applied. Considering possible confounding we use subgroup analyses for categorical subgroups or propensity-score-matching otherwise. Statistical significance will be calculated using appropriate statistical methods with two-sided p-values < 0.05. The statistical analyses are explorative, so there will be no correction for multiple testing.
Data cleanup will include tests for missing data and plausibility. Depending on the degree of incompleteness or lack of accuracy, all, or some of the data from a patient will be excluded from further evaluation. In the case of a study drop out prior the last follow up visit, the patient’s data will only be included, and tests will be performed to identify potential predictors for a study drop-out (e.g., age or weight) that might introduce a bias. The risk of bias will be discussed in any publications of the data.
3 Discussion
The BEGYN study will provide a holistic insight into the physical activity in relation to the physiological and psychological dynamics during the first year after diagnosis of non-metastatic breast cancer. The complex interplay between the underlying disease, antineoplastic therapy and individual constitution affects the patient in toto. Thus, therapeutic approaches must not be limited to surgery, antineoplastic medication, radiotherapy and psychological intervention alone, but should include the motivation for supportive activities such as exercise, which has great effect on the quality of life and prognosis (35, 99–101). A better knowledge of biomarkers is a prerequisite to assess the effects of complementary therapies and to develop personalized strategies for rehabilitation, e.g., regarding the type and dose of exercise (9). Therefore, the BEGYN study unites multiple validated assessments, allowing cross-linking analyses between the level of physical activity, cardiopulmonary fitness, body composition, biochemical measurements, an in-depth immune status including quantification of circulating lymphocyte subpopulations, mRNA expression and cytokine profiles and an extensive evaluation of psychological factors.
3.1 Novelty of the Approach
Whereas most studies investigating the influence of physical activity in breast cancer patients focus on specific activities for three months or less, the BEGYN study will quantify the daily physical activity and cardiorespiratory fitness of breast cancer patients based on objective measurements in the context of the oncological therapy for 12 months after diagnosis. Multiple studies have shown that physical activity can have positive effects in breast cancer patients (4–6, 33–35, 37). Guidelines recommend physical activity during breast cancer treatment, in example in the United States (102), in Great Britain (103), in Germany (39) and others. In particular, physical activity appears to have a positive effect on breast cancer associated fatigue syndrome (3) and on the overall quality of life (104). However, multiple aspects including the underlying mechanisms remain poorly understood. The BEGYN study will contribute important data to understand the influence of physical activity on the dynamics during the first year of breast cancer treatment and may reveal potential modifiers of the patient’s well-being.
3.1.1 Holistic Approach
To our knowledge, the BEGYN study is one of the largest studies with a very broad approach in breast cancer patients that will allow to identify associations between physical, psychological and laboratory variables, thus linking aspects that are often studied separately in breast cancer patients. Regarding the assessment of physical activity, it will be of particular interest to discuss the results of the BEGYN study in the light of other ongoing highly innovative studies such as the PROTECT study which also includes patients with colorectal cancer and lung cancer (6). One of the major strengths is the continuous recording of the patient’s physical activity and well-being by diary and by use of a fitness tracker since physical activity encompasses not only explicit sports activities, which may last a few hours per week, but also activity during everyday activities. Various forms of physical activity have been proposed, such as yoga, Tai Chi Chuan, Nordic walking, jogging, weight training, cycling, dancing and many others (20, 36, 37, 47). However, it has been claimed that individualized recommendations are needed to meet the needs of the patient (6, 105). By using the concept of metabolic equivalents (MET), the BEGYN study will allow comparing effects of physical activities of similar intensities independent of the type of exercise. Since nutrition, food supplements and lifestyle may significantly influence the effects of exercise and antineoplastic therapies, these variables are assessed by using questionnaires and the self-assessment diary.
3.1.2 One Year Study Schedule
The BEGYN study will yield an overview on a relatively long period of 12 months after diagnosis, starting before any specific antineoplastic therapy. Thus, the patient will typically be observed beyond the initial steps of therapy, which are also often associated with psychoemotional distress. This may give insight into predictors of a more favorable long-term management towards a new physical and psychological balance. Moreover, the 12 months study period will reduce potential artifacts due to seasonal effects which can, in example, influence outdoor activities and Vitamin D concentrations. The role of Vitamin D in carcinogenesis and cancer therapy is still under debate. Importantly, serial measurements of 2,5 OH Vitamin D concentrations in peripheral blood and assessment of therapeutic Vitamin D intake may give insight into the role of Vitamin D metabolism and may also provide data on the interaction between antineoplastic therapy and Vitamin D. In addition, the BEGYN study will yield serial selenium concentrations and insight on the consumption of selenium by the patients, which often is provided as a self-medication, causing significant financial burden for some patients.
3.1.3 Comparison of Different Antineoplastic Therapies
The BEGYN study will allow to compare patients with various antineoplastic therapies, i.e., endocrine therapy, chemotherapy, surgery, and radiotherapy. Due to the strict adherence to the national guideline, therapy concepts are to be expected representative for the German national standard.
3.1.4 Immunophenotyping
The BEGYN study will yield a deep insight into the innate and adaptive immune system during the first year after diagnosis of non-metastatic breast cancer (62). Inflammatory processes are a crucial part of the physiological response to cancer cells. Simultaneously, the BEGYN study will shed light on the effects of endocrine therapy and of chemotherapy on the immune system. Moreover, it is well recognized that moderate physical activity is associated with an improved immune status whereas excessive physical activity leads to an increase susceptibility towards (viral) infections (22). Interestingly, natural killer (NK) cells play a key role in sports physiology and in restricting tumor growth (64). Thus, the BEGYN study will be among the first studies to quantify four distinct subsets of NK cells in relation not only to breast cancer and antineoplastic therapy, but also in relation to the extent and type of physical activity.
The BEGYN study also has some limitations, such as the single center approach. However, the data will serve as a basis for designing future multicenter studies. In example, the results of the BEGYN study might help focusing the highly detailed immunophenotyping regarding the characterization of leukocyte subsets, mRNA transcripts and cytokines to the most promising variables in future study protocols. One further limitation of the study is that the quality of self-assessments may underlie intra-individual and inter-individual variations when filling out the self-assessment diary (e.g. daily documentation of physical activities, weekly read outs of the measurements of the fitness tracker etc.). Due to the need of serial measurements of the body composition, ethical concerns on X-ray exposure were the reason to use bioimpedance analysis and plicometry rather than Dual-energy X-ray absorptiometry, which is regarded as the gold standard (106). With regard to the known limitations of bioimpedance analysis and plicometry, we will compare the intra-individual relative changes over time instead of absolute values (107). In addition, we will further extend the data by estimating the body composition from the routine CT scans of the study patients (56). The BEGYN study is an observational study, thus potential causal relationships must be interpreted carefully. Ethical concerns would not allow randomizing patients into a group that would not be encouraged to exercise since this would conflict with the high evidence guidelines. However, the BEGYN study might help defining valid methods of continuous registration of physical activity for future studies to compare the role of various types of exercise, e.g., training of strength versus endurance.
3.2 Conclusion
The holistic approach over 12 months including physiological, psychological, and immunological data is the main strength of the BEGYN study. By including a homogeneous group of 110 female non-metastatic breast cancer patients, the study will provide highly valid data on the complex interplay between physical activity, underlying disease, type of therapy and psychological parameters.
The BEGYN study provides a uniquely thorough analysis of non-metastatic breast cancer patient during the first year after diagnosis.
3.3 Ethics and Dissemination
The study is carried out at the Department for Gynecology, Saarland University Medical Center and has been approved by the ethics committee of the Medical Association of Saarland (study # 229/18). Written consent is obtained from the patient in accordance with the Declaration of Helsinki. Any amendment to the protocol will require the formal modification and approval by the same local ethics committee that approved the study prior to implementation and will be described transparently in subsequent reports. This study is registered at German Clinical Trials Register (DRKS) (DRKS00024829). Patient recruitment took place between September 2019 and January 2021 and data collection will continue until March 2022.
Ethics Statement
The studies involving human participants were reviewed and approved by ethics committee of the Medical Association of Saarland (study # 229/18). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CZ designed the study and wrote the first draft of the manuscript. GW performed sample size calculations, gave advice for statistical analyses and helped drafting the manuscript. CS, JS, CW, CM, LA, ML, L-SS, LK, and IT performed the clinical experiments, helped with the study design, and helped writing the manuscript. CM helps to raise funding and helped writing the manuscript. EK, RS, and SG-F performed the laboratory experiments and helped writing the manuscript. MZ, GS, and E-FS gave advice for the study design, supervised the study, and helped writing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by miteinander gegen Krebs e.V. and by the intramural funds of the Saarland University Medical Center.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Dr. Maria Cacacciola-Ketter, Cross against Cancer – miteinander gegen Krebs e.V., Bernd Neuhardt (Laufschule Saarpfalz, Runners Gym Zweibrücken), Ellen Maurer.
References
- 1. Baumann F, Bloch W, Jäger E. Sport Und Körperliche Aktivität in Der Onkologie. 1st ed. Berlin Heidelberg: Springer-Verlag Berlin Heidelberg; (2012). [Google Scholar]
- 2. Stene GB, Helbostad JL, Balstad TR, Riphagen II, Kaasa S, Oldervoll LM. Effect of Physical Exercise on Muscle Mass and Strength in Cancer Patients During Treatment–A Systematic Review. Crit Rev Oncol Hematol (2013) 88:573–93. doi: 10.1016/j.critrevonc.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 3. Furmaniak AC, Menig M, Markes MH. Exercise for Women Receiving Adjuvant Therapy for Breast Cancer. Cochrane Database Syst Rev (2016) 9:CD005001. doi: 10.1002/14651858.CD005001.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmidt T, Jonat W, Wesch D, Oberg H-H, Adam-Klages S, Keller L, et al. Influence of Physical Activity on the Immune System in Breast Cancer Patients During Chemotherapy. J Cancer Res Clin Oncol (2018) 144:579–86. doi: 10.1007/s00432-017-2573-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holmen Olofsson G, Jensen AWP, Idorn M, Thor Straten P. Exercise Oncology and Immuno-Oncology; A (Future) Dynamic Duo. Int J Mol Sci (2020) 21:E3816. doi: 10.3390/ijms21113816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mallard J, Hucteau E, Schott R, Petit T, Demarchi M, Belletier C, et al. Evolution of Physical Status From Diagnosis to the End of First-Line Treatment in Breast, Lung, and Colorectal Cancer Patients: The PROTECT-01 Cohort Study Protocol. Front Oncol (2020) 10:1304. doi: 10.3389/fonc.2020.01304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 8. Fallowfield L, Osborne S, Langridge C, Monson K, Kilkerr J, Jenkins V. Implications of Subcutaneous or Intravenous Delivery of Trastuzumab; Further Insight From Patient Interviews in the PrefHer Study. Breast (2015) 24:166–70. doi: 10.1016/j.breast.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 9. Invernizzi M, de Sire A, Venetis K, Cigna E, Carda S, Borg M, et al. Quality of Life Interventions in Breast Cancer Survivors: State of the Art in Targeted Rehabilitation Strategies. Anticancer Agents Med Chem (2021). doi: 10.2174/1871520621666210609095602 [DOI] [PubMed] [Google Scholar]
- 10. Zaidi S, Hussain S, Verma S, Veqar Z, Khan A, Nazir SU, et al. Efficacy of Complementary Therapies in the Quality of Life of Breast Cancer Survivors. Front Oncol (2017) 7:326. doi: 10.3389/fonc.2017.00326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo X-C, Liu J, Fu J, Yin H-Y, Shen L, Liu M-L, et al. Effect of Tai Chi Chuan in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Front Oncol (2020) 10:607. doi: 10.3389/fonc.2020.00607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Invernizzi M, de Sire A, Lippi L, Venetis K, Sajjadi E, Gimigliano F, et al. Impact of Rehabilitation on Breast Cancer Related Fatigue: A Pilot Study. Front Oncol (2020) 10:556718. doi: 10.3389/fonc.2020.556718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wirtz P, Baumann FT. Physical Activity, Exercise and Breast Cancer - What Is the Evidence for Rehabilitation, Aftercare, and Survival? A Review. Breast Care (Basel) (2018) 13:93–101. doi: 10.1159/000488717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, et al. Screening, Assessment, and Management of Fatigue in Adult Survivors of Cancer: An American Society of Clinical Oncology Clinical Practice Guideline Adaptation. J Clin Oncol (2014) 32:1840–50. doi: 10.1200/JCO.2013.53.4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Invernizzi M, Lopez G, Michelotti A, Venetis K, Sajjadi E, De Mattos-Arruda L, et al. Integrating Biological Advances Into the Clinical Management of Breast Cancer Related Lymphedema. Front Oncol (2020) 10:422. doi: 10.3389/fonc.2020.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bailey S, Lin J. The Association of Osteoporosis Knowledge and Beliefs With Preventive Behaviors in Postmenopausal Breast Cancer Survivors. BMC Womens Health (2021) 21:297. doi: 10.1186/s12905-021-01430-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uth J, Fristrup B, Haahr RD, Brasso K, Helge JW, Rørth M, et al. Football Training Over 5 Years Is Associated With Preserved Femoral Bone Mineral Density in Men With Prostate Cancer. Scand J Med Sci Sports (2018) 28:61–73. doi: 10.1111/sms.13242 [DOI] [PubMed] [Google Scholar]
- 18. Dieli-Conwright CM, Parmentier J-H, Sami N, Lee K, Spicer D, Mack WJ, et al. Adipose Tissue Inflammation in Breast Cancer Survivors: Effects of a 16-Week Combined Aerobic and Resistance Exercise Training Intervention. Breast Cancer Res Treat (2018) 168:147–57. doi: 10.1007/s10549-017-4576-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zimmer P, Schmidt ME, Prentzell MT, Berdel B, Wiskemann J, Kellner KH, et al. Resistance Exercise Reduces Kynurenine Pathway Metabolites in Breast Cancer Patients Undergoing Radiotherapy. Front Oncol (2019) 9:962. doi: 10.3389/fonc.2019.00962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loo LWM, Nishibun K, Welsh L, Makolo T, Chong CD, Pagano I, et al. Using a Cultural Dance Program to Increase Sustainable Physical Activity for Breast Cancer Survivors-A Pilot Study. Complement Ther Med (2019) 47:102197. doi: 10.1016/j.ctim.2019.102197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu Y, Rogers CJ. Physical Activity and Breast Cancer Prevention: Possible Role of Immune Mediators. Front Nutr (2020) 7:557997. doi: 10.3389/fnut.2020.557997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rooney BV, Bigley AB, LaVoy EC, Laughlin M, Pedlar C, Simpson RJ. Lymphocytes and Monocytes Egress Peripheral Blood Within Minutes After Cessation of Steady State Exercise: A Detailed Temporal Analysis of Leukocyte Extravasation. Physiol Behav (2018) 194:260–7. doi: 10.1016/j.physbeh.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 23. Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res (2019) 79:4557–66. doi: 10.1158/0008-5472.CAN-18-3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ashcraft KA, Warner AB, Jones LW, Dewhirst MW. Exercise as Adjunct Therapy in Cancer. Semin Radiat Oncol (2019) 29:16–24. doi: 10.1016/j.semradonc.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cannioto RA, Hutson A, Dighe S, McCann W, McCann SE, Zirpoli GR, et al. Physical Activity Before, During, and After Chemotherapy for High-Risk Breast Cancer: Relationships With Survival. JNCI: J Natl Cancer Institute (2021) 113:54–63. doi: 10.1093/jnci/djaa046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmidt T, van Mackelenbergh M, Wesch D, Mundhenke C. Physical Activity Influences the Immune System of Breast Cancer Patients. J Cancer Res Ther (2017) 13:392–8. doi: 10.4103/0973-1482.150356 [DOI] [PubMed] [Google Scholar]
- 27. van der Leeden M, Huijsmans RJ, Geleijn E, de Rooij M, Konings IR, Buffart LM, et al. Tailoring Exercise Interventions to Comorbidities and Treatment-Induced Adverse Effects in Patients With Early Stage Breast Cancer Undergoing Chemotherapy: A Framework to Support Clinical Decisions. Disability Rehabil (2018) 40:486–96. doi: 10.1080/09638288.2016.1260647 [DOI] [PubMed] [Google Scholar]
- 28. Mehnert A, Brähler E, Faller H, Härter M, Keller M, Schulz H, et al. Four-Week Prevalence of Mental Disorders in Patients With Cancer Across Major Tumor Entities. J Clin Oncol (2014) 32:3540–6. doi: 10.1200/JCO.2014.56.0086 [DOI] [PubMed] [Google Scholar]
- 29. Ribeiro FE, Palma MR, Silva DTC, Tebar WR, Vanderlei LCM, Fregonesi CEPT, et al. Relationship of Anxiety and Depression Symptoms With the Different Domains of Physical Activity in Breast Cancer Survivors. J Affect Disord (2020) 273:210–4. doi: 10.1016/j.jad.2020.03.110 [DOI] [PubMed] [Google Scholar]
- 30. Alexander S, Minton O, Stone PC. Evaluation of Screening Instruments for Cancer-Related Fatigue Syndrome in Breast Cancer Survivors. J Clin Oncol (2009) 27:1197–201. doi: 10.1200/JCO.2008.19.1668 [DOI] [PubMed] [Google Scholar]
- 31. Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K. Effects of Resistance Exercise on Fatigue and Quality of Life in Breast Cancer Patients Undergoing Adjuvant Chemotherapy: A Randomized Controlled Trial. Int J Cancer (2015) 137:471–80. doi: 10.1002/ijc.29383 [DOI] [PubMed] [Google Scholar]
- 32. Duijts SFA, Faber MM, Oldenburg HSA, van Beurden M, Aaronson NK. Effectiveness of Behavioral Techniques and Physical Exercise on Psychosocial Functioning and Health-Related Quality of Life in Breast Cancer Patients and Survivors–A Meta-Analysis. Psychooncology (2011) 20:115–26. doi: 10.1002/pon.1728 [DOI] [PubMed] [Google Scholar]
- 33. Lucía A, Earnest C, Pérez M. Cancer-Related Fatigue: Can Exercise Physiology Assist Oncologists? Lancet Oncol (2003) 4:616–25. doi: 10.1016/s1470-2045(03)01221-x [DOI] [PubMed] [Google Scholar]
- 34. Barsevick AM, Newhall T, Brown S. Management of Cancer-Related Fatigue. Clin J Oncol Nurs (2008) 12:21–5. doi: 10.1188/08.CJON.S2.21-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cramp F, Byron-Daniel J. Exercise for the Management of Cancer-Related Fatigue in Adults. Cochrane Database Syst Rev (2012) 11:CD006145. doi: 10.1002/14651858.CD006145.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, et al. Weight Lifting in Women With Breast-Cancer-Related Lymphedema. N Engl J Med (2009) 361:664–73. doi: 10.1056/NEJMoa0810118 [DOI] [PubMed] [Google Scholar]
- 37. Baumann FT, Reike A, Reimer V, Schumann M, Hallek M, Taaffe DR, et al. Effects of Physical Exercise on Breast Cancer-Related Secondary Lymphedema: A Systematic Review. Breast Cancer Res Treat (2018) 170:1–13. doi: 10.1007/s10549-018-4725-y [DOI] [PubMed] [Google Scholar]
- 38. Baumann FT, Reike A, Hallek M, Wiskemann J, Reimer V. Does Exercise Have a Preventive Effect on Secondary Lymphedema in Breast Cancer Patients Following Local Treatment? - A Systematic Review. Breast Care (Basel) (2018) 13:380–5. doi: 10.1159/000487428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF). S3-Leitlinie Früherkennung, Diagnose, Therapie Und Nachsorge Des Mammakarzinoms, Version 4.4 (2021). Available at: http://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom (Accessed July 24, 2021). AWMF Registernummer: 032-045OL.
- 40. Deluche E, Leobon S, Desport JC, Venat-Bouvet L, Usseglio J, Tubiana-Mathieu N. Impact of Body Composition on Outcome in Patients With Early Breast Cancer. Support Care Cancer (2018) 26:861–8. doi: 10.1007/s00520-017-3902-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khalil SF, Mohktar MS, Ibrahim F. The Theory and Fundamentals of Bioimpedance Analysis in Clinical Status Monitoring and Diagnosis of Diseases. Sensors (Basel) (2014) 14:10895–928. doi: 10.3390/s140610895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cespedes Feliciano E, Chen WY. Clinical Implications of Low Skeletal Muscle Mass in Early-Stage Breast and Colorectal Cancer. Proc Nutr Soc (2018) 77:382–7. doi: 10.1017/S0029665118000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Migueles JH, Cadenas-Sanchez C, Ekelund U, Delisle Nyström C, Mora-Gonzalez J, Löf M, et al. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sports Med (2017) 47:1821–45. doi: 10.1007/s40279-017-0716-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Skender S, Ose J, Chang-Claude J, Paskow M, Brühmann B, Siegel EM, et al. Accelerometry and Physical Activity Questionnaires - A Systematic Review. BMC Public Health (2016) 16:515. doi: 10.1186/s12889-016-3172-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reimers AK, Knapp G, Reimers C-D. Effects of Exercise on the Resting Heart Rate: A Systematic Review and Meta-Analysis of Interventional Studies. J Clin Med (2018) 7:E503. doi: 10.3390/jcm7120503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, et al. Compendium of Physical Activities: A Second Update of Codes and MET Values. Med Sci Sports Exerc (2011) 43:1575–81. doi: 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 47. Baumann FT, Bieck O, Oberste M, Kuhn R, Schmitt J, Wentrock S, et al. Sustainable Impact of an Individualized Exercise Program on Physical Activity Level and Fatigue Syndrome on Breast Cancer Patients in Two German Rehabilitation Centers. Support Care Cancer (2017) 25:1047–54. doi: 10.1007/s00520-016-3490-x [DOI] [PubMed] [Google Scholar]
- 48. Saemann L, Lachner K, Wenzel F. Cardiac Frequency and Cutaneous Microcirculation During and After Exercising in the View of Physical Condition. Clin Hemorheol Microcirc (2017) 67:221–7. doi: 10.3233/CH-179203 [DOI] [PubMed] [Google Scholar]
- 49. Porszasz J, Casaburi R, Somfay A, Woodhouse LJ, Whipp BJ. A Treadmill Ramp Protocol Using Simultaneous Changes in Speed and Grade. Med Sci Sports Exerc (2003) 35:1596–603. doi: 10.1249/01.MSS.0000084593.56786.DA [DOI] [PubMed] [Google Scholar]
- 50. Gerhardt O. Evaluation Unterschiedlicher Spirometriesysteme (3) Für Den Einsatz Der Stoffwechselmessung in Der Fitnessbranche (2010). Available at: http://www.telmed.de/files/siehe_zusammenfassung_diplomarbeit_gerhardt.pdf.
- 51. Borg G. Psychophysical Scaling With Applications in Physical Work and the Perception of Exertion. Scand J Work Environ Health (1990) 16 Suppl 1:55–8. doi: 10.5271/sjweh.1815 [DOI] [PubMed] [Google Scholar]
- 52. Löllgen H, Leyk D. Exercise Testing in Sports Medicine. Dtsch Arztebl Int (2018) 115:409–16. doi: 10.3238/arztebl.2018.0409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scharhag-Rosenberger F. Spiroergometrie Zur Ausdauerleistungsdiagnostik. Deutsche Z für Sportmedizin (2010) 61:146–7. [Google Scholar]
- 54. Singh B, Spence RR, Sandler CX, Tanner J, Hayes SC. Feasibility and Effect of a Physical Activity Counselling Session With or Without Provision of an Activity Tracker on Maintenance of Physical Activity in Women With Breast Cancer - A Randomised Controlled Trial. J Sci Med Sport (2020) 23:283–90. doi: 10.1016/j.jsams.2019.09.019 [DOI] [PubMed] [Google Scholar]
- 55. Souweine J-S, Kuster N, Chenine L, Rodriguez A, Patrier L, Morena M, et al. Physical Inactivity and Protein Energy Wasting Play Independent Roles in Muscle Weakness in Maintenance Haemodialysis Patients. PloS One (2018) 13:e0200061. doi: 10.1371/journal.pone.0200061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Solomayer E-F, Braun E-M, Zimmermann JSM, Radosa JC, Stroeder J, Endrikat J, et al. Muscle Mass Loss in Patients With Metastatic Breast Cancer. Arch Gynecol Obstet (2019) 300:201–6. doi: 10.1007/s00404-019-05168-y [DOI] [PubMed] [Google Scholar]
- 57. Kuo F-C, Lu C-H, Wu L-W, Kao T-W, Su S-C, Liu J-S, et al. Comparison of 7-Site Skinfold Measurement and Dual-Energy X-Ray Absorptiometry for Estimating Body Fat Percentage and Regional Adiposity in Taiwanese Diabetic Patients. PloS One (2020) 15:e0236323. doi: 10.1371/journal.pone.0236323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Busse M, Dräger M, Schätzel M, Thomas M, Schulze A, Falz R. Estimation of Subcutaneous Fat in Men – Part 1: Accuracy of 3 to 9 Point Measurements. Clin Sports Med Int (2013) 6:21–3. [Google Scholar]
- 59. Busse M, Schätzel M, Dräger M, Thomas M, Schulze A, Falz R. Estimation of Subcutaneous Fat in Men – Part 2: Comparison of Caliper and Ultrasound Measuring. Clin Sports Med Int (2013) 6:24–7. [Google Scholar]
- 60. Ketel IJG, Volman MNM, Seidell JC, Stehouwer CDA, Twisk JW, Lambalk CB. Superiority of Skinfold Measurements and Waist Over Waist-to-Hip Ratio for Determination of Body Fat Distribution in a Population-Based Cohort of Caucasian Dutch Adults. Eur J Endocrinol (2007) 156:655–61. doi: 10.1530/EJE-06-0730 [DOI] [PubMed] [Google Scholar]
- 61. Jackson AS, Pollock ML, Ward A. Generalized Equations for Predicting Body Density of Women. Med Sci Sports Exerc (1980) 12:175–81. doi: 10.1249/00005768-198023000-00009 [DOI] [PubMed] [Google Scholar]
- 62. Jabeen S, Espinoza JA, Torland LA, Zucknick M, Kumar S, Haakensen VD, et al. Noninvasive Profiling of Serum Cytokines in Breast Cancer Patients and Clinicopathological Characteristics. Oncoimmunology (2019) 8:e1537691. doi: 10.1080/2162402X.2018.1537691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gustafson CE, Jadhav R, Cao W, Qi Q, Pegram M, Tian L, et al. Immune Cell Repertoires in Breast Cancer Patients After Adjuvant Chemotherapy. JCI Insight (2020) 5:134569. doi: 10.1172/jci.insight.134569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Spiliopoulou P, Gavriatopoulou M, Kastritis E, Dimopoulos MA, Terzis G. Exercise-Induced Changes in Tumor Growth via Tumor Immunity. Sports (Basel) (2021) 9:46. doi: 10.3390/sports9040046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Alberts E, Wall I, Calado DP, Grigoriadis A. Immune Crosstalk Between Lymph Nodes and Breast Carcinomas, With a Focus on B Cells. Front Mol Biosci (2021) 8:673051. doi: 10.3389/fmolb.2021.673051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hanson ED, Bates LC, Harrell EP, Bartlett DB, Lee JT, Wagoner CW, et al. Exercise Training Partially Rescues Impaired Mucosal Associated Invariant T-Cell Mobilization in Breast Cancer Survivors Compared to Healthy Older Women. Exp Gerontol (2021) 152:111454. doi: 10.1016/j.exger.2021.111454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Robert Koch-Institut . Selen in Der Umweltmedizin: Mitteilung Der Kommission, Methoden Und Qualitätssicherung in Der Umweltmedizin. In: Bundesgesundheitsbl - Gesundheitsforsch - Gesundheitsschutz, vol. 49. Cham: Springer Nature; (2006). p. 88–102. doi: 10.1007/s00103-005-1185-4 [DOI] [Google Scholar]
- 68. DiaSorin Inc . LIAISON® 25 OH Vitamin D TOTAL Assay. Available at: https://zentrallaborweb.uniklinikum-saarland.de/labor/data/Beipackzettel/Liaison/25DS6/Vitamin_D_2016-08.pdf (Accessed August 7, 2021).
- 69. Bittenbring JT, Neumann F, Altmann B, Achenbach M, Reichrath J, Ziepert M, et al. Vitamin D Deficiency Impairs Rituximab-Mediated Cellular Cytotoxicity and Outcome of Patients With Diffuse Large B-Cell Lymphoma Treated With But Not Without Rituximab. J Clin Oncol (2014) 32:3242–8. doi: 10.1200/JCO.2013.53.4537 [DOI] [PubMed] [Google Scholar]
- 70. Vaughan-Shaw PG, O’Sullivan F, Farrington SM, Theodoratou E, Campbell H, Dunlop MG, et al. The Impact of Vitamin D Pathway Genetic Variation and Circulating 25-Hydroxyvitamin D on Cancer Outcome: Systematic Review and Meta-Analysis. Br J Cancer (2017) 116:1092–110. doi: 10.1038/bjc.2017.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vinceti M, Filippini T, Del Giovane C, Dennert G, Zwahlen M, Brinkman M, et al. Selenium for Preventing Cancer. Cochrane Database Syst Rev (2018) 1:CD005195. doi: 10.1002/14651858.CD005195.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mao J, Yin H, Wang L, Wu J-Z, Xia Y, Zhu H-Y, et al. Prognostic Value of 25-Hydroxy Vitamin D in Extranodal NK/T Cell Lymphoma. Ann Hematol (2021) 100:445–53. doi: 10.1007/s00277-020-04320-y [DOI] [PubMed] [Google Scholar]
- 73. Schindler TI, Wagner J-J, Goedicke-Fritz S, Rogosch T, Coccejus V, Laudenbach V, et al. TH17 Cell Frequency in Peripheral Blood Is Elevated in Overweight Children Without Chronic Inflammatory Diseases. Front Immunol (2017) 8:1543. doi: 10.3389/fimmu.2017.01543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zamarron BF, Chen W. Dual Roles of Immune Cells and Their Factors in Cancer Development and Progression. Int J Biol Sci (2011) 7:651–8. doi: 10.7150/ijbs.7.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tsuda B, Miyamoto A, Yokoyama K, Ogiya R, Oshitanai R, Terao M, et al. B-Cell Populations Are Expanded in Breast Cancer Patients Compared With Healthy Controls. Breast Cancer (2018) 25:284–91. doi: 10.1007/s12282-017-0824-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gonzalez H, Hagerling C, Werb Z. Roles of the Immune System in Cancer: From Tumor Initiation to Metastatic Progression. Genes Dev (2018) 32:1267–84. doi: 10.1101/gad.314617.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang L, Simons DL, Lu X, Tu TY, Avalos C, Chang AY, et al. Breast Cancer Induces Systemic Immune Changes on Cytokine Signaling in Peripheral Blood Monocytes and Lymphocytes. EBioMedicine (2020) 52:102631. doi: 10.1016/j.ebiom.2020.102631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cossarizza A, Chang H-D, Radbruch A, Akdis M, Andrä I, Annunziato F, et al. Guidelines for the Use of Flow Cytometry and Cell Sorting in Immunological Studies. Eur J Immunol (2017) 47:1584–797. doi: 10.1002/eji.201646632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Varchetta S, Mele D, Oliviero B, Mantovani S, Ludovisi S, Cerino A, et al. Unique Immunological Profile in Patients With COVID-19. Cell Mol Immunol (2021) 18:604–12. doi: 10.1038/s41423-020-00557-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mazhari R, Brewster J, Fong R, Bourke C, Liu ZSJ, Takashima E, et al. A Comparison of non-Magnetic and Magnetic Beads for Measuring IgG Antibodies Against Plasmodium Vivax Antigens in a Multiplexed Bead-Based Assay Using Luminex Technology (Bio-Plex 200 or MAGPIX). PloS One (2020) 15:e0238010. doi: 10.1371/journal.pone.0238010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gómez AM, Martínez C, Fiuza-Luces C, Herrero F, Pérez M, Madero L, et al. Exercise Training and Cytokines in Breast Cancer Survivors. Int J Sports Med (2011) 32:461–7. doi: 10.1055/s-0031-1271697 [DOI] [PubMed] [Google Scholar]
- 82. Tamayo-Velasco Á, Peñarrubia-Ponce MJ, Álvarez FJ, Gonzalo-Benito H, de la Fuente I, Martín-Fernández M, et al. Evaluation of Cytokines as Robust Diagnostic Biomarkers for COVID-19 Detection. J Pers Med (2021) 11:681. doi: 10.3390/jpm11070681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wild D, John R, Sheehan C, Binder S, He J. eds. The Immunoassay Handbook: Theory and Applications of Ligand Binding. In: ELISA and Related Techniques, 4th ed. Oxford, Waltham, Amsterdam: Elsevier. [Google Scholar]
- 84. Zheng W, Flavell RA. The Transcription Factor GATA-3 Is Necessary and Sufficient for Th2 Cytokine Gene Expression in CD4 T Cells. Cell (1997) 89:587–96. doi: 10.1016/s0092-8674(00)80240-8 [DOI] [PubMed] [Google Scholar]
- 85. Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T Helper Cell Fate Specified by Kinase-Mediated Interaction of T-Bet With GATA-3. Science (2005) 307:430–3. doi: 10.1126/science.1103336 [DOI] [PubMed] [Google Scholar]
- 86. Kanhere A, Hertweck A, Bhatia U, Gökmen MR, Perucha E, Jackson I, et al. T-Bet and GATA3 Orchestrate Th1 and Th2 Differentiation Through Lineage-Specific Targeting of Distal Regulatory Elements. Nat Commun (2012) 3:1268. doi: 10.1038/ncomms2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yakushina VD, Vasil’eva OA, Ryazantseva NV, Novitsky VV, Tashireva LA. The Effects of Galectin-1 on the Gene Expression of the Transcription Factors TBX21, GATA-3, FOXP3 and RORC. Mol Cell Biochem (2015) 398:245–9. doi: 10.1007/s11010-014-2227-8 [DOI] [PubMed] [Google Scholar]
- 88. Lin Z-W, Wu L-X, Xie Y, Ou X, Tian P-K, Liu X-P, et al. The Expression Levels of Transcription Factors T-Bet, GATA-3, Rorγt and FOXP3 in Peripheral Blood Lymphocyte (PBL) of Patients With Liver Cancer and Their Significance. Int J Med Sci (2015) 12:7–16. doi: 10.7150/ijms.8352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 90. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J Natl Cancer Inst (1993) 85:365–76. doi: 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 91. Steyer R, Schwenkmezger P, Notz P, Eid M. Testtheoretische Analysen Des Mehrdimensionalen Befindlichkeitsfragebogen (MDBF). Diagnostica (1994) 40(4):320–8. [Google Scholar]
- 92. Tönnies S, Plöhn S, Krippendorf U. Skalen Zur Psychischen Gesundheit (SPG). Testmanual. Heidelberg: Roland Asanger Verlag; (1996). [Google Scholar]
- 93. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring Fatigue and Other Anemia-Related Symptoms With the Functional Assessment of Cancer Therapy (FACT) Measurement System. J Pain Symptom Manage (1997) 13:63–74. doi: 10.1016/s0885-3924(96)00274-6 [DOI] [PubMed] [Google Scholar]
- 94. Roth AJ, Kornblith AB, Batel-Copel L, Peabody E, Scher HI, Holland JC. Rapid Screening for Psychologic Distress in Men With Prostate Carcinoma: A Pilot Study. Cancer (1998) 82:1904–8. doi: [DOI] [PubMed] [Google Scholar]
- 95. Fayers P, Aaronson NK, Bjordal K, groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 Scoring Manual. Brussels: European Organisation for Research and Treatment of Cancer; (2001). [Google Scholar]
- 96. Mehnert-Theuerkauf A, Müller D, Lehmann C, Koch U. Die Deutsche Version Des NCCN Distress-Thermometers: Empirische Prüfung Eines Screening-Instruments Zur Erfassung Psychosozialer Belastung Bei Krebspatienten. Z für Psychiatr Psychol und Psychotherapie (2006) 54:213–23. doi: 10.1024/1661-4747.54.3.213 [DOI] [Google Scholar]
- 97. Riba MB, Donovan KA, Andersen B, Braun II, Breitbart WS, Brewer BW, et al. Distress Management, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2019) 17:1229–49. doi: 10.6004/jnccn.2019.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yeh M-L, Chung Y-C, Hsu M-YF, Hsu C-C. Quantifying Psychological Distress Among Cancer Patients in Interventions and Scales: A Systematic Review. Curr Pain Headache Rep (2014) 18:399. doi: 10.1007/s11916-013-0399-7 [DOI] [PubMed] [Google Scholar]
- 99. Speed-Andrews AE, Courneya KS. Effects of Exercise on Quality of Life and Prognosis in Cancer Survivors. Curr Sports Med Rep (2009) 8:176–81. doi: 10.1249/JSR.0b013e3181ae98f3 [DOI] [PubMed] [Google Scholar]
- 100. Segal R, Zwaal C, Green E, Tomasone JR, Loblaw A, Petrella T. The Exercise for People With Cancer Guideline Development Group. Exercise for People With Cancer: A Systematic Review. Curr Oncol (2017) 24:290–315. doi: 10.3747/co.24.3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jackisch C, Kreienberg R, Blettner M, Harbeck N, Lück H-J, Haidinger R, et al. Assessment of Quality of Life in Postmenopausal Women With Early Breast Cancer Participating in the PACT Trial: The Impact of Additional Patient Information Material Packages and Patient Compliance. Breast Care (Basel) (2020) 15:236–45. doi: 10.1159/000500771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lyman GH, Greenlee H, Bohlke K, Bao T, DeMichele AM, Deng GE, et al. Integrative Therapies During and After Breast Cancer Treatment: ASCO Endorsement of the SIO Clinical Practice Guideline. J Clin Oncol (2018) 36:2647–55. doi: 10.1200/JCO.2018.79.2721 [DOI] [PubMed] [Google Scholar]
- 103. NICE Guidline: Advanced Breast Cancer: Diagnosis and Treatment (2009). Available at: https://www.nice.org.uk/guidance/cg81 (Accessed July 31, 2021).
- 104. Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise Interventions on Health-Related Quality of Life for People With Cancer During Active Treatment. Cochrane Database Syst Rev (2012) 8:CD008465. doi: 10.1002/14651858.CD008465.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Carayol M, Bernard P, Boiché J, Riou F, Mercier B, Cousson-Gélie F, et al. Psychological Effect of Exercise in Women With Breast Cancer Receiving Adjuvant Therapy: What Is the Optimal Dose Needed? Ann Oncol (2013) 24:291–300. doi: 10.1093/annonc/mds342 [DOI] [PubMed] [Google Scholar]
- 106. Minetto MA, Busso C, Gamerro G, Lalli P, Massazza G, Invernizzi M. Quantitative Assessment of Volumetric Muscle Loss: Dual-Energy X-Ray Absorptiometry and Ultrasonography. Curr Opin Pharmacol (2021) 57:148–56. doi: 10.1016/j.coph.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 107. Ballesteros-Pomar MD, González-Arnáiz E, Pintor-de-la Maza B, Barajas-Galindo D, Ariadel-Cobo D, González-Roza L, et al. Bioelectrical Impedance Analysis as an Alternative to Dual-Energy X-Ray Absorptiometry in the Assessment of Fat Mass and Appendicular Lean Mass in Patients With Obesity. Nutrition (2021) 93:111442. doi: 10.1016/j.nut.2021.111442 [DOI] [PubMed] [Google Scholar]