Abstract
After being banned by the European Commission in 2018, the use of formaldehyde as a feed amendment in the United States has come into question. Therefore, this study was conducted to explore alternatives to formaldehyde, such as formic acid and monoglycerides, and their effects on poultry production. In total, 1,728 Cobb 700 broilers were randomly assigned to 96-floor pens on day of hatch (18 birds/pen). Using a randomized complete block design (4 blocks), treatments were assigned to pens with blocking based on location within the barn, with the eastern half of the barn designated for digestibility and the western half designated for production (per experiment: 8 control pens and 10 pens per treatment). All diets were based on a negative control (NC), basal diet. Dietary treatments consisted of: NC, NC + 0.25% formalin (F), NC + 0.25 and 0.50% Amasil NA (AML and AMH; 61% formic acid and 20.5% Na-formate), and NC + SILO Health 104L (SILO; mixture of monoglycerides; 0.5% from 0 to 14 d, 0.4% from 14 to 28 d, and 0.2% from 28 to 42 d). Water and feed were provided ad libitum. Performance data were collected during feed changes on d 0, 14, 28, and 42, with digestibility data collected at d 14 (2 per pen) and carcass quality (6 per pen) assessed at d 46 with a randomly selected group of broilers. A one-way ANOVA followed by Dunnett's multiple comparison, where treatments were evaluated against F were conducted using JMP 14.0 (P ≤ 0.05). Main effect of treatment was significant for performance, nutrient digestibility, and carcass quality. Differences in body weight and ADG were observed from d 14 to d 28, resulting in a trending improvement in lysine digestibility on d 14 and carcass quality on d 46 of birds fed AML and AMH in comparison to those fed F (P < 0.05). Whereas birds fed SILO had reduced digestibility of methionine on d 14 and a decrease in meat quality on d 46 in comparison to those fed F (P < 0.05). Therefore, Amasil NA at 0.25 or 0.50% may be an effective alternative to formaldehyde as a feed amendment for poultry production.
Key words: broiler, Cobb 700, formaldehyde, formic acid, monoglyceride
INTRODUCTION
Poor feed hygiene can lead to premature spoilage resulting in significant economic loss as well as blooms of microbial and fungal growth that expose poultry to unnecessary microbial risk (Ricke et al., 2019). Additionally, unrestricted microbial growth in the broiler chicken gastrointestinal tract partitions energy away from the musculoskeletal development of the bird and shunts it to the immune system that is actively restricting microbial growth (Dibner and Richards 2005; Swaggerty et al., 2019). Because of the energetic burden this produces on the bird, feed amendments may be included to control microbial outgrowth (Dittoe et al., 2018; Ricke et al., 2019, 2020). In addition, the subsequent colonization of foodborne microbiota within the gastrointestinal tract can include foodborne pathogens, such as Salmonella enterica and Campylobacter, which can also become a critical concern (Dibner and Richards, 2005; Dunkley et al., 2009; Horrocks et al., 2009; Foley et al., 2011, 2013; Suresh et al., 2018; Ricke et al., 2019; Swaggerty et al., 2019). Ultimately, beyond the preservation of feed, the reduction of foodborne pathogens and the improvement of bird gut health are important keystones to feed management (Maciorowski et al., 2007; Ricke et al., 2019).
Formaldehyde-based feed amendments sanitize the feed and may provide antimicrobial effects to the bird (Wales et al., 2010; Khan and Iqbal, 2016; Ricke et al., 2019). Despite public opinion, formaldehyde and other aldehydes are naturally present in the environment, and governmental food and regulatory agencies have determined they can be used safely (Ricke et al., 2019; Khan and Iqbal, 2016). The inclusion of formaldehyde is a safe and effective antimicrobial that potentially limits vertical transmission of pathogens such as Salmonella to the food supply (Wales et al., 2010; Ricke et al., 2019).
Organic acids are a potential substitute for formaldehyde in feed (Khan et al., 2003; Ricke, 2003; Wales et al., 2010; Dittoe et al., 2018; Ricke et al., 2019, 2020). Although some comparisons of chemical treatments on feed microbiology have been made (Cochrane et al., 2016), there has yet to be a comprehensive study that evaluates the effects of formic acid or monoglycerides against formaldehyde in a longitudinal broiler grow out and processing investigation. Therefore, this study was conceived to assess whether or not feed containing Amasil NA (BASF Corporation, Florham Park, NJ), a non-corrosive formic acid and sodium formate product, and SILO 104L Health (Silo Health, Via San Bartolo a Cintoia, Florence, Italy), a 1-monoglyceride compound consisting predominantly of monobutyrate, have the potential to improve production, efficiency, nutrient digestibility, and carcass quality as compared to feed treated with formaldehyde.
MATERIALS AND METHODS
Animal Husbandry
All Cobb 700 broiler chickens in this study were used in strict accordance with a protocol approved by the University of Arkansas Institutional Care and Use Committee (IACUC Number: 18067). Approximately 21 d prior to hatch, 5,000 fertile Cobb 700 hatching eggs arrived at the University of Arkansas Hatchery. Eggs were set and hatched according to standard industry guidelines and practices (Cobb-Vantress, Siloam Springs, AR). On 21 d of incubation (day of hatch), chicks were vent sexed at hatch, and the female broiler chicks were humanly euthanized by carbon dioxide asphyxiation. The male broilers were orally vaccinated against coccidiosis (Coccivac-B; MSD Animal Health; Summit, NJ) according to manufacturer recommendations and transported in temperature-controlled vehicles to the broiler house, where they were set on fresh pine shavings with ad libitum access to food and water. Upon arrival to the chicken house, 18 chicks per pen were placed in 96 floor pens with an average set weight of 45 ± 1 g per chick. All birds were transferred to the floor pens within minutes of each other. For the production study, the western wing of the barn was used (48 pens or experimental units). Whereas the eastern wing (48 pens) of the barn was used for the digestibility portion of the study. Regardless, each wing of the house consisted of 48 pens with 8 pens being designated for the basal diet (control) fed birds (4 pens per block) and 10 pens being utilized per experimental treatment group (5 pens per block) across 2 blocks.
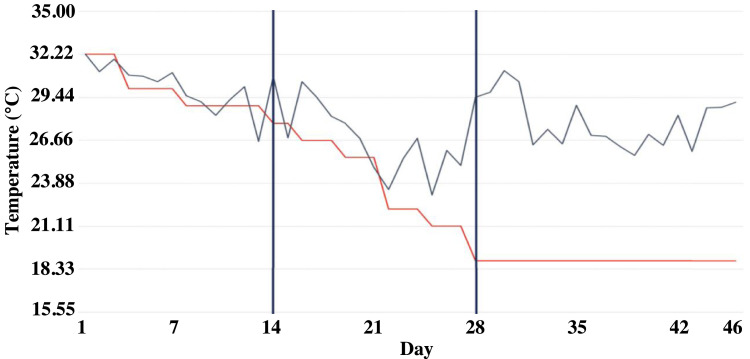
The broiler house containing 96-floor pens with fresh pine shavings was equipped with misters, and negative pressure ventilation fans. The temperature control was implemented per the recommendations of the Cobb 700 broiler management guide (Cobb-Vantress Inc.). However, the house was not equipped with an evaporative cooling system; therefore, there were deviations from the recommended temperature profile, particularly from d 22 through d 46 (Figure 1). As a result, additional 48” box fans were added to the center isles of the production facility to promote air circulation. A concerted effort to reduce the movement of the birds throughout the day occurred, relegating the welfare checks to the early morning and late evening hours.
Figure 1.
Temperature profiles of set temperature (red line) and actual ambient temperature (black line) within poultry production facility throughout a 46 day Cobb 700 growout period.
Feed Preparations
The feed was formulated and produced at the University of Arkansas poultry feed mill (Fayetteville, AR) according to industry standards and guidelines established by the National Research Council (National Research Council, 1994). The researchers were blinded to the treatments throughout the study, with each fed bag categorized with a specific number to the unknown treatment. Treatments were unblinded after the initial analysis was conducted. The diets were isocaloric, with 12 pounds of sand removed per ton and replaced with the respective amendment plus sand up to 12 pounds per 1 ton of feed. The feed was analyzed for pH and formic acid or formaldehyde concentration by a third party (Eurofins, Des Moines, IA; Table 1). The diet formulations are listed in Table 2. Dietary treatments included: a no-treatment control (NC), basal diet; NC + 0.25% of formaldehyde (F); NC + 0.25% of Amasil NA (AML; 61% formic acid and 20.5% Na-formate); NC + 0.5% of Amasil NA (AMH; 61% formic acid and 20.5% Na-formate); and NC + SILOhealth 104L (SILO; mixture of monoglycerides; 0.5% from 0 to 14 d, 0.4% from 14 to 28 d, and 0.2% from 28 to 42 d). All dietary supplements were included in the diet per manufacturer recommendations with the concentrations of SILOhealth 104L being chosen to promote increased carcass characteristics (increased breast weight) as seen in Bedford et al. (2018).
Table 1.
Composition of the experimental diets (pH, % formate, % Amasil NA, % Recovery) after feed manufacturing during the starter (0 to 14 d), grower (14 to 28 d), and withdrawal (28 to 42 d) diets.
| Phase | Diet | pH | Formate (%)1 | Amasil NA (%)2 | Recovery (%)3 |
|---|---|---|---|---|---|
| Starter | NC | 6.35 | < 0.02 | 0.000 | - |
| 0.25% formaldehyde | 6.29 | < 0.02 | 0.000 | - | |
| 0.25% Amasil NA | 6.02 | 0.155 | 0.207 | 83 | |
| 0.5% Amasil NA | 5.65 | 0.318 | 0.424 | 85 | |
| SILO 104L | 6.16 | < 0.02 | 0.000 | - | |
| Grower | NC | 6.31 | < 0.02 | 0.000 | - |
| 0.25% formaldehyde | 6.29 | < 0.02 | 0.000 | - | |
| 0.25% Amasil NA | 5.91 | 0.165 | 0.220 | 88 | |
| 0.5% Amasil NA | 5.66 | 0.328 | 0.437 | 87 | |
| SILO 104L | 6.17 | < 0.02 | 0.000 | - | |
| Withdrawal | NC | 6.27 | < 0.02 | 0.000 | - |
| 0.25% formaldehyde | 6.31 | < 0.02 | 0.000 | - | |
| 0.25% Amasil NA | 5.93 | 0.175 | 0.233 | 93 | |
| 0.5% Amasil NA | 5.58 | 0.347 | 0.463 | 93 | |
| SILO 104L | 6.29 | < 0.02 | 0.000 | - |
Formate recovered from manufactured feed presented as a percentage of feed.
Amasil NA recovered from manufactured feed presented as a percentage of feed.
Percentage of recovered of Amasil NA from manufactured feed supplemented with Amasil NA. Percentage was based on recovered vs. what was supplemented in diet (0.25 or 0.5%).
Table 2.
Basal diet composition (NC) of the starter (0 to 14 d), grower (14 to 21 d), and withdrawal (21 to 42 d) manufactured feed.
| Starter | Grower | Withdrawal | |
|---|---|---|---|
| Ingredient1 | % | % | % |
| Corn | 61.43 | 64.88 | 66.18 |
| Soybean meal (48%) | 30.26 | 27.26 | 25.02 |
| Pro-Plus (54%)2 | 4.00 | 4.00 | 4.00 |
| Poultry Fat | 1.02 | 1.25 | 2.29 |
| Limestone | 0.71 | 0.66 | 0.64 |
| Sand | 0.60 | 0.60 | 0.60 |
| Titanium dioxide | 0.50 | 0.00 | 0.00 |
| NaCl | 0.41 | 0.41 | 0.41 |
| DL-Met | 0.31 | 0.29 | 0.27 |
| L-Lys HCl | 0.22 | 0.23 | 0.23 |
| Dicalcium phosphate | 0.16 | 0.07 | 0.00 |
| Trace mineral premix3 | 0.10 | 0.10 | 0.10 |
| Vitamin premix4 | 0.10 | 0.10 | 0.10 |
| L-Thr | 0.10 | 0.09 | 0.10 |
| Choline CL (60%) | 0.05 | 0.03 | 0.03 |
| Se premix5 | 0.02 | 0.02 | 0.02 |
| OptiPhos20006 | 0.01 | 0.01 | 0.01 |
| Total | 100.00 | 100.00 | 100.00 |
| Calculated nutrient content | |||
| ME, Kcal/kg | 3,015 | 3,075 | 3,150 |
| CP, % | 22.00 | 20.83 | 19.84 |
| Na, % | 0.21 | 0.21 | 0.21 |
| DEB, mEq/kg | 210 | 196 | 185 |
| Ca, % | 0.90 | 0.85 | 0.82 |
| P, available, % | 0.45 | 0.43 | 0.41 |
| Choline, mg/kg | 1,762 | 1,625 | 1,575 |
| TSAA, % digestible | 0.97 | 0.84 | 0.80 |
| Lys, % digestible | 1.31 | 1.24 | 1.06 |
| Thr, % digestible | 0.77 | 0.73 | 0.71 |
| Val, % digestible | 0.90 | 0.85 | 0.81 |
| Ile, % digestible | 0.80 | 0.75 | 0.71 |
| Leu, % digestible | 1.60 | 1.53 | 1.47 |
| Arg, % digestible | 1.30 | 1.21 | 1.14 |
| Trp, % digestible | 0.22 | 0.20 | 0.19 |
Ingredient nutrient composition was analyzed before formulating the diet.
H.J. Baker's ProPlus 55 Animal Protein Concentrate.
Trace Mineral Premix provided the following per kilogram of finished diet: 100 mg manganese, 100 mg zinc, 10 mg copper, 1 mg iodine, 50 mg iron, 27 mg magnesium, 55 mg calcium.
Vitamin Premix provided the following per kilogram of finished diet: 15,432 IU Vitamin A, 11,023 ICU vitamin D3, 110 IU vitamin E, 0.3 mg vitamin B12, 3 mg menadione. 13.23 mg riboflavin, 19.84 mg d-pantothenic acid, 3.09 mg thiamine, 77.16 mg of niacin, 5.51 mg pyridoxine, 1.76 mg folic acid, 0.17 mg biotin.
Se premix provided the following per kilogram of finished diet: 0.20 mg selenium.
OptiPhos2000 from a dietary inclusion of 0.125% provided the following per kilogram of finished diet: 200 FTU (Huvepharma, Inc., Peachtree City, GA).
Three feeding stages were used throughout the trial, with starter crumble (0–14 d), pelleted grower (14–28 d), and pelleted withdrawal feed (28–42 d). The feed was weighed per bag and added to the feeders on a per bag basis to monitor feed consumption. The final feeder weights were recorded prior to feed change, dumped, and wiped down prior to the addition of another feeding stage. The birds were kept on their dietary treatments through d 46 when they were processed for meat quality.
Evaluation of Feed Efficiency
The average of all the bird pen weights (48 pens at the western part of the house) at d 0 was 45 g per broiler chicken. At d 14, 28, and 42, the feed was removed from the feeders, weighed, and the total feed consumed was recorded throughout the study. The broiler chickens were weighed on a per pen basis (including mortalities). The weights were recorded and divided by the pen population to determine the average bird weight per pen. Feed intake (FI), body weight (BW), gain to feed ratio (G:F), average daily feed intake (ADFI), and average daily gain (ADG) were calculated using standard equations.
Digestibility
A titanium dioxide tracer was added to the starter crumble feed (0.5% of diet) to enable the determination of digestibility by the index method. A homogenous aliquot of the starter “crumble” feed (500 g × 2) was collected at the start of the trial and frozen at −20°C until the analysis could occur. At d 14, two randomly selected broiler chickens per pen on the eastern side of the house (48 pens, 96 birds total, 16 control birds, and 20 per experimental treatment) were humanely euthanized prior to the feed change, and the ileum was collected distal to the Meckel's diverticulum. The contents were flushed into screw-top urine collection cups with 10 mL of sterile water and stored at −20°C until shipped to ATS Scientific (ATS Scientific, El Dorado, AR) to be analyzed for digestibility.
Meat Quality Analysis
On d 45, the feed was withdrawn from the birds 12 h prior to processing. The following day (d 46), 6 birds per pen on the western side of the barn (48 pens, 48 control birds, 60 per experiment treatment) were couped and transported to the University of Arkansas Pilot Processing Plant, where they were humanely slaughtered and processed. A total of 60 broilers per treatment, except NC, which had 48 broilers, were processed, and used for meat quality analysis. Birds were processed using commercial methods including stunning, exsanguination, scalding, picking, and evisceration (Mehaffey et al., 2006). Carcasses were immersed in stationary chill tanks for 120 min to achieve an end-point temperature of 4°C. The chilling process included a 15 min pre-chill at 12°C and 105 min chill at 1°C. Carcasses were deboned 2 h postmortem (PM). Prior to deboning, carcasses were weighed, and deboning was performed by trained and experienced staff.
Live weight, chilled carcass without giblets (WOG) weight, wings, breast, tenders, whole legs, and frame weights were collected, with parts yields being determined as a percentage of live weight. Meat quality was also assessed by scoring for myopathies of white striping and woody breast scores after deboning using scales developed by Kuttappan et al. (2012) and Tijare et al. (2016). Approximately 24 h postmortem, fillets were weighed, and drip loss (%) was determined. Muscle pH was measured using a Testo spear tip probe and meter (Model Testo 205, Testo Inc., Sparta, NJ). To assess color changes in the fillet, L*, a*, and b* color values were determined as an average of 3 different sites on the dorsal (bone side) of the fillet using a Minolta colorimeter (CR-300, Konica Minolta, Ramsey, NJ). One side of the butterfly fillet was marinated (0.75% NaCl, 0.45% phosphate, target 15% pickup); marinade pickup and marinade drip loss (% loss after 24 h) were determined. Fillets (marinated and non-marinated) were cooked and sheared using the Muellenet Owens Razor Shear (MORS) method on a texture analyzer (Model TAX-T2, Texture Technologies, Scarsdale, NY; Cavitt et al., 2004; Tijare et al., 2016). Shear energy and force were measured.
Statistical Analysis
All data were analyzed using SAS JMP 14.0 (SAS Institute Inc., Cary, NC). A one-way analysis of variance (ANOVA) was used to test for the significance of the main effects of both block and dietary treatment. The experimental unit of this study was pen, with 10 pens per experimental treatment (AML, AMH, SILO, F) and 8 pens for the NC-treated birds. All comparisons among the treatments were made using Dunnett's multiple comparisons, with the formaldehyde group directly compared against the other dietary treatment groups. Statistical significance was determined at P ≤ 0.05, with important trends noted at P ≤ 0.10 but P > 0.05.
RESULTS
Performance
Prior to the analysis of the data, a homogenous sample representative of the entire tonnage of feed produced for all dietary treatment at each feed stage (starter, grower, and withdrawal) was collected and analyzed for the recoverable levels of formaldehyde and formic acid. Table 1 describes the difference in quantified vs. calculated inclusion rate and the pH changes versus the feed amendment added to the feed. Overall, the consistency between the treatments at each feed stage was maintained.
Production gains were measured by BW, ADG, FI, ADFI, F:G, and mortality for the individual weigh period as well as the full length of the trial. On d 28, there was a main effect of treatment (P = 0.004) on BW with birds fed diets containing NC, AMH, AML, and SILO were significantly (P < 0.05) heavier than those with diets supplemented with F. Correspondingly, there was a main effect of treatment on the ADG from d 14 to d 28 (P = 0.001) with those fed diets containing NC, AMH, and AML being greater than that of those fed F (P < 0.05; Table 3). During that period (14–28 d), the AMH and AML fed groups gained an average of 73.15 and 70.68 g/bird, respectively, as compared to 67 g/bird for those fed diets containing F. Those gains correspond to a 9 and 5% improvement in ADG due to acidification by AML and AMH, respectively. By d 42, however, the significant differences in BW were no longer detected (withdrawal diet; d 28–42).
Table 3.
Production parameters such as body weight, average daily gain, feed intake, average daily feed intake, and gain to feed during each feeding phase, starter (0 to 14), grower (14 to 28), and withdrawal (28 to 42 d), and throughout a 42 d growout (0 to 42 d).
| NC3 |
F4 |
AML4 |
AMH4 |
SILO4 |
Dunnett's multiple comparison2 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Production parameters | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Block1 | Diet1 | NC vs. F | AML vs. F | AMH vs. F | SILO vs. F |
| Body weight (kg) | ||||||||||||||||
| D0 | 0.045 | 0.000 | 0.045 | 0.000 | 0.045 | 0.000 | 0.045 | 0.000 | 0.045 | 0.000 | 0.028 | 0.210 | 0.505 | 0.711 | 0.964 | 0.964 |
| D14 | 0.369 | 0.009 | 0.361 | 0.008 | 0.377 | 0.008 | 0.372 | 0.008 | 0.379 | 0.008 | 0.953 | 0.592 | 0.915 | 0.485 | 0.783 | 0.375 |
| D28 | 1.374 | 0.020 | 1.299 | 0.018 | 1.401 | 0.018 | 1.361 | 0.018 | 1.358 | 0.018 | 0.735 | 0.004 | 0.024 | 0.001 | 0.053 | 0.069 |
| D42 | 2.710 | 0.058 | 2.619 | 0.052 | 2.747 | 0.052 | 2.749 | 0.052 | 2.669 | 0.052 | 0.778 | 0.348 | 0.594 | 0.250 | 0.243 | 0.900 |
| Average daily gain (g/bird) | ||||||||||||||||
| D0 to 14 | 23.125 | 0.671 | 22.570 | 0.601 | 23.700 | 0.601 | 23.330 | 0.601 | 23.840 | 0.601 | 0.928 | 0.597 | 0.929 | 0.489 | 0.786 | 0.386 |
| D14 to 28 | 71.763 | 1.078 | 67.000 | 0.964 | 73.150 | 0.964 | 70.680 | 0.964 | 69.970 | 0.964 | 0.642 | 0.001 | 0.007 | <0.001 | 0.034 | 0.112 |
| D28 to 42 | 95.438 | 3.927 | 94.280 | 3.512 | 96.180 | 3.512 | 99.100 | 3.512 | 93.610 | 3.512 | 0.859 | 0.829 | 0.998 | 0.987 | 0.737 | 1.000 |
| D0 to 42 | 63.438 | 1.380 | 61.280 | 1.235 | 64.350 | 1.235 | 64.360 | 1.235 | 62.490 | 1.235 | 0.789 | 0.355 | 0.602 | 0.251 | 0.249 | 0.897 |
| Feed intake (kg/pen) | ||||||||||||||||
| D0 to 14 | 8.350 | 0.190 | 8.050 | 0.170 | 8.630 | 0.170 | 8.120 | 0.170 | 8.540 | 0.170 | 0.558 | 0.084 | 0.595 | 0.068 | 0.995 | 0.149 |
| D14 to 28 | 26.515 | 0.784 | 27.220 | 0.655 | 28.450 | 0.655 | 26.770 | 0.655 | 27.860 | 0.655 | 0.057 | 0.274 | 0.900 | 0.494 | 0.969 | 0.899 |
| D28 to 42 | 39.813 | 0.828 | 42.530 | 0.740 | 42.913 | 0.781 | 40.569 | 0.781 | 40.090 | 0.740 | 0.956 | 0.017 | 0.063 | 0.990 | 0.227 | 0.082 |
| D0 to 42 | 74.643 | 1.300 | 77.800 | 1.086 | 79.278 | 1.146 | 75.289 | 1.146 | 76.470 | 1.086 | 0.441 | 0.056 | 0.212 | 0.765 | 0.339 | 0.809 |
| Average daily feed intake (g/bird) | ||||||||||||||||
| D0 to 14 | 33.575 | 0.698 | 32.150 | 0.624 | 34.440 | 0.624 | 32.390 | 0.624 | 34.460 | 0.624 | 0.542 | 0.026 | 0.371 | 0.044 | 0.996 | 0.042 |
| D14 to 28 | 108.593 | 3.100 | 109.220 | 2.590 | 115.150 | 2.590 | 108.480 | 2.590 | 114.510 | 2.590 | 0.039 | 0.197 | 1.000 | 0.320 | 0.999 | 0.419 |
| D28 to 42 | 164.913 | 2.598 | 170.730 | 2.324 | 175.613 | 2.452 | 165.846 | 2.452 | 166.760 | 2.324 | 0.404 | 0.022 | 0.296 | 0.420 | 0.420 | 0.577 |
| D0 to 42 | 101.365 | 1.413 | 103.920 | 1.180 | 107.072 | 1.246 | 101.572 | 1.246 | 104.560 | 1.180 | 0.137 | 0.018 | 0.459 | 0.223 | 0.471 | 0.987 |
| Gain to feed | ||||||||||||||||
| D0 to 14 | 0.689 | 0.013 | 0.703 | 0.012 | 0.689 | 0.012 | 0.718 | 0.012 | 0.692 | 0.012 | 0.509 | 0.359 | 0.832 | 0.789 | 0.793 | 0.900 |
| D14 to 28 | 0.656 | 0.016 | 0.615 | 0.013 | 0.638 | 0.013 | 0.653 | 0.013 | 0.615 | 0.013 | 0.109 | 0.114 | 0.179 | 0.566 | 0.161 | 1.000 |
| D28 to 42 | 0.578 | 0.020 | 0.552 | 0.018 | 0.553 | 0.019 | 0.591 | 0.019 | 0.561 | 0.018 | 0.872 | 0.522 | 0.740 | 1.000 | 0.392 | 0.990 |
| D0 to 42 | 0.616 | 0.013 | 0.590 | 0.011 | 0.604 | 0.011 | 0.630 | 0.011 | 0.598 | 0.011 | 0.673 | 0.126 | 0.369 | 0.816 | 0.055 | 0.967 |
| Mortality | ||||||||||||||||
| D0 to 14 | 0.028 | 0.010 | 0.011 | 0.007 | 0.017 | 0.008 | 0.017 | 0.008 | 0.028 | 0.015 | 0.885 | 0.773 | 0.650 | 0.986 | 0.986 | 0.603 |
| D14 to 28 | 0.143 | 0.009 | 0.000 | 0.000 | 0.006 | 0.006 | 0.006 | 0.006 | 0.012 | 0.008 | 0.101 | 0.854 | 0.350 | 0.919 | 0.919 | 0.466 |
| D28 to 42 | 0.029 | 0.015 | 0.006 | 0.006 | 0.011 | 0.007 | 0.040 | 0.019 | 0.040 | 0.012 | 0.224 | 0.137 | 0.558 | 0.994 | 0.173 | 0.186 |
| D0 to 42 | 0.069 | 0.017 | 0.017 | 0.008 | 0.033 | 0.009 | 0.061 | 0.023 | 0.078 | 0.017 | 0.120 | 0.174 | 0.091 | 0.863 | 0.150 | 0.027 |
Main effect of block and diet using one-Way ANOVA.
Pairwise comparisons between dietary treatments were performed using Dunnett's multiple comparison with formaldehyde designated as the control. Significance is denoted in bold with a P ≤ 0.05.
The negative control (NC) fed birds had 8 replicate pens (n = 8).
Experimental diet fed birds, Formalin (F), 0.25% Amasil NA (AML), 0.5% Amasil NA (AMH), and SILO Health 104L (SILO) had 10 replicate pens (n = 10)
There was an effect of dietary treatments on the FI from d 28 to 42 (P = 0.017); however, there were no differences between those fed F and those fed the NC or other experimental diets (P > 0.05). There was a trending difference in FI between those fed NC and F and those fed SILO and F with the FI of those fed F being less than those fed diets containing NC or SILO (P = 0.063 and P = 0.082). In addition, there were significant main effects of diet on ADFI from d 0 to d 14, d 28 to d 42, and d 0 to d 42 (P < 0.05). Birds fed diets supplemented with both AML and SILO consumed approximately 2.3 g/d more than those supplemented with F in the first 14 d. However, despite the significant main effect of diet on ADFI in both periods ending at d 42, there were no significant differences according to the Dunnett multiple comparison (P > 0.05). There were no detected differences in the G:F ratios or mortalities across all periods when evaluating the main effect of diet (P > 0.05).
Digestibility
Significant main effects were seen in the apparent ileal digestibility (AID) of lysine, methionine, cysteine, and tyrosine (P < 0.05; Table 4). For cysteine, lysine, and tyrosine, these main effect differences did not result in significant pairwise comparisons vs. the F treatment (Dunnett's, P > 0.05). For lysine, there was a tendency toward lower AID in the birds fed F supplemented diets relative to those provided both AML and AMH dietary treatments (0.836 vs. 0.887 and 0.898, respectively; P < 0.10). However, birds fed diets supplemented with NC and SILO had a lower digestibility for methionine relative to those fed diets containing F (0.898 and 0.896 vs. 0.942, respectively; P < 0.05), which was in turn numerically similar to the AID of methionine for the birds fed diets containing Amasil NA, regardless of concentration (0.921 and 0.938).
Table 4.
Apparent illeal digestibility of amino acids of 14 d old Cobb 700 broilers fed diets containing either no supplementation or diets containing formaldehyde, Amasil NA (0.25 and 0.5%), or SILO Health 104L.
| NC3 |
F4 |
AML4 |
AMH4 |
SILO4 |
Dunnett's multiple comparison2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amino acid | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Diet1 | NC vs. F | AML vs. F | AMH vs. F | SILO vs. F |
| Alanine | 0.845 | 0.029 | 0.804 | 0.018 | 0.849 | 0.019 | 0.859 | 0.026 | 0.809 | 0.020 | 0.254 | 0.617 | 0.290 | 0.332 | 1.000 |
| Arginine | 0.891 | 0.020 | 0.888 | 0.012 | 0.906 | 0.013 | 0.906 | 0.018 | 0.876 | 0.014 | 0.544 | 1.000 | 0.823 | 0.808 | 0.939 |
| Aspartic Acid | 0.827 | 0.026 | 0.816 | 0.016 | 0.858 | 0.018 | 0.842 | 0.024 | 0.791 | 0.018 | 0.127 | 0.990 | 0.316 | 0.763 | 0.782 |
| Cysteine | 0.681 | 0.041 | 0.744 | 0.025 | 0.787 | 0.028 | 0.772 | 0.037 | 0.669 | 0.029 | 0.035 | 0.540 | 0.704 | 0.928 | 0.212 |
| Dry Matter | 0.777 | 0.040 | 0.706 | 0.025 | 0.754 | 0.027 | 0.764 | 0.037 | 0.728 | 0.028 | 0.480 | 0.420 | 0.532 | 0.581 | 0.956 |
| Glutamic Acid | 0.888 | 0.019 | 0.874 | 0.012 | 0.902 | 0.013 | 0.899 | 0.017 | 0.865 | 0.013 | 0.239 | 0.943 | 0.379 | 0.577 | 0.971 |
| Glycine | 0.792 | 0.039 | 0.726 | 0.024 | 0.804 | 0.027 | 0.827 | 0.036 | 0.748 | 0.028 | 0.103 | 0.471 | 0.141 | 0.100 | 0.952 |
| Histidine | 0.849 | 0.032 | 0.804 | 0.020 | 0.848 | 0.022 | 0.852 | 0.030 | 0.774 | 0.023 | 0.107 | 0.637 | 0.437 | 0.554 | 0.760 |
| Isoleucine | 0.862 | 0.028 | 0.829 | 0.017 | 0.849 | 0.019 | 0.842 | 0.025 | 0.783 | 0.020 | 0.108 | 0.757 | 0.875 | 0.991 | 0.274 |
| Leucine | 0.875 | 0.023 | 0.850 | 0.014 | 0.871 | 0.015 | 0.872 | 0.021 | 0.835 | 0.016 | 0.404 | 0.790 | 0.721 | 0.853 | 0.911 |
| Lysine | 0.872 | 0.023 | 0.836 | 0.014 | 0.887 | 0.015 | 0.898 | 0.021 | 0.846 | 0.016 | 0.050 | 0.520 | 0.076 | 0.066 | 0.979 |
| Methionine | 0.898 | 0.013 | 0.942 | 0.008 | 0.921 | 0.009 | 0.938 | 0.012 | 0.896 | 0.009 | 0.003 | 0.030 | 0.302 | 0.996 | 0.002 |
| Phenylalanine | 0.863 | 0.025 | 0.838 | 0.016 | 0.866 | 0.017 | 0.857 | 0.023 | 0.809 | 0.018 | 0.187 | 0.850 | 0.615 | 0.922 | 0.611 |
| Proline | 0.837 | 0.027 | 0.808 | 0.017 | 0.866 | 0.018 | 0.852 | 0.024 | 0.820 | 0.019 | 0.165 | 0.807 | 0.094 | 0.389 | 0.971 |
| Serine | 0.813 | 0.028 | 0.804 | 0.017 | 0.838 | 0.019 | 0.827 | 0.026 | 0.788 | 0.020 | 0.431 | 0.998 | 0.559 | 0.885 | 0.954 |
| Taurine | 0.648 | 0.072 | 0.549 | 0.045 | 0.626 | 0.049 | 0.615 | 0.066 | 0.525 | 0.051 | 0.466 | 0.651 | 0.710 | 0.799 | 0.996 |
| Threonine | 0.762 | 0.039 | 0.730 | 0.024 | 0.796 | 0.026 | 0.769 | 0.036 | 0.719 | 0.028 | 0.281 | 0.917 | 0.269 | 0.783 | 0.998 |
| Tryptophan | 0.849 | 0.033 | 0.802 | 0.020 | 0.821 | 0.022 | 0.869 | 0.030 | 0.777 | 0.023 | 0.134 | 0.615 | 0.949 | 0.252 | 0.852 |
| Tyrosine | 0.903 | 0.019 | 0.876 | 0.012 | 0.896 | 0.013 | 0.889 | 0.017 | 0.842 | 0.014 | 0.037 | 0.618 | 0.758 | 0.894 | 0.223 |
| Valine | 0.831 | 0.033 | 0.783 | 0.020 | 0.836 | 0.022 | 0.817 | 0.030 | 0.767 | 0.023 | 0.185 | 0.591 | 0.271 | 0.822 | 0.967 |
Main effect of diet using one-Way ANOVA.
Pairwise comparisons between dietary treatments were performed using Dunnett's multiple comparison with formaldehyde designated as the control. Significance is denoted in bold with a P ≤ 0.05.
The negative control (NC) fed birds had 8 replicate pens (n = 8).
Experimental diet fed birds, Formalin (F), 0.25% Amasil NA (AML), 0.5% Amasil NA (AMH), and SILO Health 104L (SILO) had 10 replicate pens (n = 10)
Carcass and Meat Quality
The main effect of diet was significant for the live weight (P < 0.05; Table 5). Wing yield as a percent of live weight was impacted by diet overall (P < 0.05; Table 5) but did not result in other significant pairwise comparisons vs. the F group. There was a tendency for diet to impact the size of the rack as a percent of live weight (P = 0.100) with birds fed diets containing NC having a smaller frame percentage compared to those fed F (20.91 vs. 21.58%, respectively; P < 0.05).
Table 5.
Processing yield of 46 d old Cobb 700 broilers fed diets containing either no supplementation or diets containing formaldehyde, Amasil NA (0.25 and 0.5%), or SILO Health 104L.
| NC4 |
F5 |
AML5 |
AMH5 |
SILO5 |
Dunnett's multiple comparison3 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Processing characteristics | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Diet2 | NC vs. F | AML vs. F | AMH vs. F | SILO vs. F |
| Live weight (g) | 2,968.000 | 34.854 | 2,971.250 | 24.306 | 2,966.500 | 27.925 | 3,039.767 | 26.454 | 2,896.967 | 30.929 | 0.010 | 1.000 | 0.976 | 0.247 | 0.186 |
| WOG1 (%) | 78.219 | 0.323 | 78.433 | 0.200 | 78.657 | 0.179 | 78.957 | 0.186 | 78.658 | 0.169 | 0.133 | 0.893 | 0.663 | 0.210 | 0.852 |
| Whole breast1 (%) | 22.689 | 0.274 | 22.157 | 0.202 | 22.271 | 0.241 | 22.683 | 0.200 | 22.273 | 0.182 | 0.300 | 0.281 | 0.599 | 0.237 | 0.986 |
| Wings1 (%) | 7.519 | 0.075 | 7.678 | 0.054 | 7.640 | 0.051 | 7.687 | 0.053 | 7.788 | 0.049 | 0.030 | 0.166 | 0.999 | 1.000 | 0.424 |
| Tenders1 (%) | 4.462 | 0.065 | 4.488 | 0.041 | 4.493 | 0.047 | 4.462 | 0.037 | 4.410 | 0.052 | 0.719 | 0.986 | 0.660 | 0.982 | 0.547 |
| Legs1 (%) | 22.053 | 0.224 | 22.235 | 0.128 | 22.207 | 0.158 | 22.340 | 0.150 | 22.465 | 0.157 | 0.486 | 0.859 | 0.947 | 0.971 | 0.686 |
| Frame1 (%) | 20.906 | 0.249 | 21.582 | 0.170 | 21.483 | 0.151 | 21.232 | 0.163 | 21.250 | 0.153 | 0.100 | 0.027 | 0.162 | 0.385 | 0.434 |
Yield as a percent of live weight (%).
Main effect of diet using one-Way ANOVA.
Pairwise comparisons between dietary treatments were performed using Dunnett's multiple comparison with formaldehyde designated as the control. Significance is denoted in bold with a P ≤ 0.05.
The negative control (NC) fed birds had 8 replicate pens (n = 8).
Experimental diet fed birds, Formalin (F), 0.25% Amasil NA (AML), 0.5% Amasil NA (AMH), and SILO Health 104L (SILO) had 10 replicate pens (n = 10)
Meat quality was also evaluated (Table 6). There were no differences in the groups concerning woody breast or white striping scores (P > 0.05). There were no differences (P > 0.05) in the color scores (L*, a*, b*) among the groups. Additionally, there was a trending difference of pH between the breast of different treatment groups (P = 0.10) with the pH of the breast meat of the birds fed AML supplemented diets having a pH of 5.83 compared to a pH of 5.88 for those fed the diets containing F (P = 0.042). There was also an effect of treatment on drip loss, cook loss, and MORS force and energy measurements (P < 0.01) of non-marinated fillets. As compared to the F fed broilers, those fed diets supplemented with AML and AMH had lower cook loss (26.8 vs. 22.5 and 23.9%, respectively; P < 0.01), and those fed SILO yielded greater cook loss (30.7%; P < 0.01). Acidification of the diet (AML and AMH) also improved or tended to improve breast MORS Force by 5.8 (AML; P < 0.10) to 7.8% (AMH; P < 0.05) relative to those fed diets supplemented with F for unmarinated fillets.
Table 6.
Quality of skinless boneless breast fillets of 46 d old Cobb 700 broilers fed diets containing either no supplementation or diets containing formaldehyde, Amasil NA (0.25 and 0.5%), or SILO Health 104L.
| NC3 |
F4 |
AML4 |
AMH4 |
SILO4 |
Dunnett's multiple comparison2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meat quality | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Diet1 | NC vs. F | AML vs. F | AMH vs. F | SILO vs. F |
| WB score | 1.438 | 0.128 | 1.392 | 0.111 | 1.358 | 0.122 | 1.325 | 0.103 | 1.483 | 0.111 | 0.874 | 0.996 | 0.999 | 0.982 | 0.944 |
| WS score | 1.073 | 0.098 | 0.892 | 0.080 | 1.083 | 0.098 | 1.025 | 0.090 | 1.075 | 0.094 | 0.537 | 0.476 | 0.372 | 0.684 | 0.412 |
| pH | 5.857 | 0.018 | 5.888 | 0.018 | 5.832 | 0.012 | 5.869 | 0.016 | 5.843 | 0.015 | 0.105 | 0.511 | 0.042 | 0.806 | 0.146 |
| L* | 51.637 | 0.352 | 51.101 | 0.252 | 51.268 | 0.254 | 51.104 | 0.295 | 51.433 | 0.285 | 0.663 | 0.518 | 0.982 | 1.000 | 0.821 |
| a* | 4.393 | 0.137 | 4.390 | 0.077 | 4.328 | 0.098 | 4.365 | 0.117 | 4.409 | 0.098 | 0.984 | 1.000 | 0.980 | 0.999 | 1.000 |
| b* | 1.092 | 0.114 | 0.996 | 0.094 | 0.974 | 0.104 | 0.947 | 0.096 | 1.147 | 0.101 | 0.591 | 0.920 | 1.000 | 0.991 | 0.664 |
| Drip loss (%) | 1.458 | 0.105 | 1.402 | 0.120 | 1.343 | 0.105 | 1.247 | 0.090 | 1.297 | 0.098 | 0.021 | 0.988 | 0.984 | 0.662 | 0.881 |
| Cook loss (%) | 28.575 | 0.831 | 26.795 | 0.752 | 22.505 | 0.620 | 23.870 | 0.545 | 30.742 | 0.649 | < 0.001 | 0.225 | < 0.001 | 0.007 | < 0.001 |
| MORS force (N) | 12.915 | 0.286 | 12.592 | 0.232 | 11.856 | 0.260 | 11.611 | 0.204 | 12.647 | 0.211 | < 0.001 | 0.765 | 0.087 | 0.012 | 1.000 |
| MORS energy (N/mm) | 169.213 | 3.500 | 160.765 | 3.035 | 156.131 | 3.622 | 153.849 | 2.939 | 166.082 | 3.356 | < 0.001 | 0.245 | 0.705 | 0.365 | 0.598 |
Main effect of diet using one-Way ANOVA.
Pairwise comparisons between dietary treatments were performed using Dunnett's multiple comparison with formaldehyde designated as the control. Significance is denoted in bold with a P ≤ 0.05.
The negative control (NC) fed birds had 8 replicate pens (n = 8).
Experimental diet fed birds, Formalin (F), 0.25% Amasil NA (AML), 0.5% Amasil NA (AMH), and SILO Health 104L (SILO) had 10 replicate pens (n = 10).
Marinade uptake and drip loss both exhibited a main effect of treatment (P < 0.05; Table 7). The breast of the birds fed diets supplemented with SILO took up less and retained less of the marinade (P < 0.05). Marinade uptake and retention were not different for the breast of those fed diets containing AML and AMH compared to those fed diets with F, but the cook loss tended to be (AML; P < 0.10) less. The breast of those fed AML saw a tendency toward reduced cook loss in marinated fillets (19.20 vs. 20.97%; P < 0.10). There was a main effect of diet on MORS energy; however, there were no pairwise differences compared to the fillets of those fed diets containing F after marination. There was no effect of diet on MORS force.
Table 7.
Quality parameters (marinade uptake, drip loss, and cook loss, and MORS force and energy) of marinated skinless, boneless breast fillets of 46 d old Cobb 700 broilers fed diets containing either no supplementation or diets containing formaldehyde, Amasil NA (0.25 and 0.5%), or SILO Health 104L.
| NC3 |
F4 |
AML4 |
AMH4 |
SILO4 |
Dunnett's multiple comparison2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meat quality | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Diet1 | NC vs. F | AML vs. F | AMH vs. F | SILO vs. F |
| Marinade uptake (%) | 10.581 | 0.300 | 10.917 | 0.245 | 11.355 | 0.309 | 11.680 | 0.272 | 9.820 | 0.399 | <0.001 | 0.879 | 0.704 | 0.233 | 0.040 |
| Marinade drip loss (%) | 8.723 | 0.308 | 9.113 | 0.248 | 8.993 | 0.304 | 9.328 | 0.222 | 8.148 | 0.290 | 0.025 | 0.738 | 0.994 | 0.948 | 0.041 |
| Marinated cook loss (%) | 21.327 | 0.538 | 20.965 | 0.602 | 19.200 | 0.486 | 19.433 | 0.528 | 21.213 | 0.592 | 0.009 | 0.977 | 0.074 | 0.148 | 0.993 |
| MORS force (N) | 10.408 | 0.255 | 10.360 | 0.234 | 9.945 | 0.273 | 9.829 | 0.253 | 10.710 | 0.233 | 0.077 | 1.000 | 0.575 | 0.356 | 0.709 |
| MORS energy (N/mm) | 139.825 | 3.028 | 133.404 | 2.614 | 131.813 | 3.353 | 129.145 | 3.092 | 141.024 | 2.900 | 0.022 | 0.405 | 0.987 | 0.702 | 0.210 |
Main effect of diet using one-Way ANOVA.
Pairwise comparisons between dietary treatments were performed using Dunnett's multiple comparison with formaldehyde designated as the control. Significance is denoted in bold with a P ≤ 0.05. 3The negative control (NC) fed birds had 8 replicate pens (n = 8). 4Experimental diet fed birds, Formalin (F), 0.25% Amasil NA (AML), 0.5% Amasil NA (AMH), and SILO Health 104L (SILO) had 10 replicate pens (n = 10).
The negative control (NC) fed birds had 8 replicate pens (n = 8). 4Experimental diet fed birds, Formalin (F), 0.25% Amasil NA (AML), 0.5% Amasil NA (AMH), and SILO Health 104L (SILO) had 10 replicate pens (n = 10).
Experimental diet fed birds, Formalin (F), 0.25% Amasil NA (AML), 0.5% Amasil NA (AMH), and SILO Health 104L (SILO) had 10 replicate pens (n = 10).
DISCUSSION
Feed hygiene is essential in any system trying to control the spread of bacterial diseases and foodborne pathogens. In 2012, despite improvements in feed hygiene, Salmonella was detected in 19% of feed ingredient samples and 6% of finished feed samples collected from feed mills (Li et al., 2012). In a more recent study, Shariat et al. (2021) using CRISPR-typing determined that Salmonella was present in 6.2% (24/387) of animal feed samples collected over 11-mo period across the United States. Chemical amendments can be an important supplement to thermal processing steps such as pelleting (Cochrane et al., 2016; Jendza et al., 2018), which have been shown to be inconsistently effective in practice (Davies and Wray, 1997). This, in part, has been attributed to inconsistent temperature control during the pelleting process and the potential for recontamination post-pelleting in the feed mill (Jones and Richardson, 2004; Jones, 2011).
Formaldehyde has been utilized widely in the poultry industry as a preservative and sanitizer in feed and has not been shown to result in adverse effects in the broiler when used in accordance with the FDA approval (Ricke et al., 2019). However, there remain questions about the safety of workers in feed mills due to formaldehyde exposure incidental to their exposure to formaldehyde from equipment and feed (Ricke et al., 2019). There have also been questions raised about the potential negative effects of formaldehyde on nutrient availability in treated feeds (Greiner et al., 2017; Campbell et al., 2018a,b; Williams et al., 2018). Both the worker safety and nutrient availability concerns stem from the way formaldehyde chemically interacts with proteins as it works to preserve feedstuffs by denaturing protein and forming cross-linkages between proteins (Gustafsson et al., 2015) through a process called alkylation. These alkylated cross-linkages interfere with normal enzymatic processes and can inhibit the enzymatic digestion of dietary protein (Greiner et al., 2017). Therefore, the current study was developed to investigate an alternative to formaldehyde, formic acid, and its effect on the production efficiency, nutrient digestibility, and carcass quality as compared to diets treated with formaldehyde.
Production and Relation to Amino Acid Digestibility
Formic acid, an organic acid, works to preserve feedstuffs by acidification. Contrary to formaldehyde, formic acid is not considered a potential cancer risk to workers and has been reported to enhance nutrient digestibility (Tung and Pettigrew, 2006; García et al., 2007; Abdollahi et al., 2020). It has been suggested that the improved digestibility is due to enhanced enzyme activity since many digestive enzymes are more active at lower pH, and crop pH has been shown to be reduced by as much as 1 log when consuming feed acidified with formic acid (Al-Natour and Alshawabkeh, 2005). It is also possible that this enhancement in nutrient availability is due to the reduced formation of Maillard reaction cross-linkages between proteins and carbohydrates in the feed during steam conditioning and pelleting. Ajandouz and Puigserver (1999) showed that the destruction of lysine via Maillard reaction was slowed at a lower pH, with up to 20% degradation of lysine within 20 min of incubation at a pH of 12 and less than 1% at over an hour incubation at a pH of 4.0 in an in vitro test.
In the current study, broilers fed diets supplemented with AML, AMH, and SILO had greater live weights on d 28 and a greater ADG from d 14 to 28 (grower phase) compared to those fed diets containing F. Additionally, broilers fed diets supplemented with AML and AMH tended to have greater digestibility of lysine than those fed diets containing F. The improved performance of birds supplemented with AML, AMH, and SILO compared to those supplemented with F may have occurred due to the potential of formaldehyde to alkylate and reduce its availability. Although lysine cross-linkages due to the Maillard reaction were not explored in the current study, lysine has previously been shown to be highly susceptible to cross-linkage formation due to the Maillard reaction, which can occur during the cooking or drying of feed or food ingredients (Carpenter and Booth, 1973; Mauron, 1981; Hagemeister and Erbersdobler, 1985; Friedman, 1996). Whereas the supplementation of formic acid has the potential to improve amino acid digestibility and subsequent performance due to the decrease in intestinal pH (Hernández et al., 2006; Tung and Pettigrew, 2006; García et al., 2007; Coelho and Ader, 2019, Coelho and Ader, 2019; Abdollahi et al., 2020) which was noted in the current study. In contrast to the current study, Hernández et al. (2006) determined that the addition of formic acid at 10 g/kg (1%) in the diets of male Ross broilers improved apparent ileal digestibility of dry matter at 42 d of age and that this improvement in digestibility did not result in improved performance, intestinal histomorphology, or plasma metabolites of these birds compared to other treatments.
Previous trials have shown that monoglycerides, such as SILO, can improve bird performance under a necrotic enteritis challenge (Coelho and Ader, 2019, Coelho and Ader, 2019). However, the mode of action for this additive is believed to be the targeted delivery of butyric acid to the hindgut. Butyrate in the hindgut has long been associated with improved intestinal histology and gut health (Bedford et al., 2018). It is believed that butyrate is a preferred energy source for enterocytes (Peng et al., 2009), which get first pass access to any absorbed nutrients. Through increased access to butyrate, enterocytes increase the production of tight junction proteins (Peng et al., 2009; Yan and Ajuwon, 2017), thus decreasing paracellular translocation of pathogens into the bird and reduce overall activation of the gut-associated immune response (Feng et al., 2018). This then spares nutrients and energy for use by the bird to deposit additional body mass. Therefore, it is understandable that the supplementation of the SILO product did not impact the digestibility of lysine and reduced the digestibility of methionine, since its mode of action is unrelated to protein metabolism unlike formic acid (Amasil NA). Although there was not a positive impact on amino acid digestibility through the dietary supplementation of SILO, there was an improvement of live weight on d 28 and ADG from d 14 to 28 (grower phase) when compared to those fed F.
Acidification of Diets on Carcass Quality Characteristics
One would expect that with the improved performance seen from d 0 to 28 in the current study that this would translate to improved processing characteristics and carcass on d 46. However, there were no effects of dietary treatment on the processing characteristics of the broilers. Ragaa and Korany (2016a) determined that when Cobb 500 broilers were provided diets supplemented with 5 g/kg (0.5%) of formic acid in the diet, the dressing, breast, and thigh weight were improved in comparison to those fed the basal diet. However, the dressing, breast, and thigh weight of broilers supplemented with formic acid were not different than that of those fed diets supplemented with thyme or the combination of thyme and formic acid (Ragaa and Korany, 2016a). Similarly, Ragaa and Korany (2016b) supplied Cobb 500 broilers with diets containing formic (5 g/kg or 0.5%) or potassium diformate (5 g/kg or 0.5%) had improved live weight and dressing weight and tended to have improved breast weight when compared to those fed basal diets. In the current study, there was a main effect of diet on the live weight and on wing yield as a percentage of the dressed bird; however, this did not translate to improved yield of those fed the experimental treatments compared to those fed diets containing F.
Although there were no improvements in the carcass characteristics on d 46, there were improvements in the cook loss and MORS force of the non-marinated breasts of those fed diets containing AML and AMH compared to those fed diets containing F. Similarly, when Lohmann MB-202 broilers were supplemented diets containing 0.1% formic acid there was no direct effect on carcass characteristics, but there was an effect on the physical and chemical characteristics (Sugiharto et al., 2019). As such, there was an increase the pH of the meat at broth 45 min and 24 h post slaughter, a decrease in drip loss, an increase in water holding capacity, an increase in lightness and yellowness compared to those fed the basal diet only (Sugiharto et al., 2019).
Unlike AML and AMH fed birds, the cook loss and MORS force of the non-marinated breasts and the marinade uptake and retention of the marinated breasts of those fed diets containing SILO were negatively impacted in comparison to that of those fed diets containing F. These differences seen in meat quality of those fed diets containing SILO could potentially be attributed to the reduced digestibility of methionine (0.942 vs. 0.896) on d 14 despite the improvements in body weight and ADG seen on d 28 and d 14 to 28, respectively. In congruence, Zhai et al. (2016a) reported improved carcass, breast, tender, and leg quarter weight of Ross 708 broilers supplemented with increased supplementation of dietary methionine and lysine. Additionally, Zhai et al. (2016b) reported lower cook loss and shear force in Ross 708 broilers consuming increased levels of dietary lysine and methionine with the two amino acids demonstrating an interaction on these parameters. Zhai et al. (2016b) attributed the improved breast quality to increased sarcoplasmic protein concentration, ratio to myofibrillar protein, and solubility.
Formic Acid as a Replacement for Formaldehyde
In conclusion, the 3 dietary treatments, AML, AMH, and SILO, evaluated in the present study differentially affected the growth performance, apparent ileal digestibility of amino acids, processing characteristics, and meat quality in comparison to that of those fed diets containing formaldehyde. The supplementation of AML, AMH, and SILO improved live weight and ADG on d 14 and d 14 to 28, respectively. Those fed AML and AMH tended to exhibit improved lysine digestibility whereas those fed SILO had a decrease in digestibility of methionine compared to those fed F. Downstream, these differences resulted in improved cook loss and MORS force of AML and AMH fed birds and a negative impact on the cook loss and MORS force of the non-marinated breasts and the marinade uptake and retention of the marinated breasts of those fed diets containing SILO in comparison to the fed diets containing F.
Therefore, the supplementation of Amasil NA (61% formic acid and 20.5% Na-formate), regardless of concentration, at 0.25 and 0.50% in Cobb 700 broiler diets can effectively replace formaldehyde in current feed hygienic practices as Amasil NA has the potential to increase apparent ileal digestibility lysine on d 14 and improve the performance from d 14 to 28 that may translate into the improved carcass and meat quality on d 46. However, the supplementation of diets with SILO health 104 L, a mixture of monoglycerides, may not be an advantageous alternative to formaldehyde as birds fed diets supplemented with SILO demonstrated a negative impact on methionine digestibility and meat quality in comparison to those fed diets containing formaldehyde.
ACKNOWLEDGMENTS
First and foremost, this project would have never been completed if it was not for the very competent and hardworking efforts of our Feed Mill crew led by the late Howard Lester, our Farm and Hatchery Crew (the late Doug Yoho, David Reynolds, the late Janet May-Washington, and crew), and our Pilot Plant Crew led by Rodney Wolfe. Without their sheer dedication to poultry science, patience, and absolute service and dedication to educating our crew, we would not have executed this project, and we cannot thank them enough. We would also like to thank Jessica Woitte, Adam Lattin, Julie Atchley, Hao Shi, Aaron Bodie, Dr. Peter Rubinelli, Andrew Miccichi, Nanping Wang, Juan D. Latore, and Mikayla F.A. Baxter for their assistance throughout this trial. The author DKD would like to acknowledge the Graduate College at the University of Arkansas to support the research and the stipend provided through the Distinguished Academy Fellowship.
Author Contribution: KMF, JJ, CMO, MTK, SCR conceived the project. KMF organized, executed the project, and analyzed the data with JJ and DKD. MTK formulated the diets and coordinated the barns. CMO and JPC facilitated the processing of the birds, performed the meat quality assessment, and provided the initial analysis. JJ provided significant statistical support and quality control and analysis throughout the trial and beyond. BAM, DKD, and GTI provided intellectual and technical support throughout the study, with BAM producing the initial analysis for the AID data. BB and KMF compiled and KMF, DKD, and JJ analyzed the data. KMF, DKD, and JJ finalized the analyses and synthesized the story. KMF and DKD wrote the manuscript with SCR, CMO, MTK, and JJ, providing edits and feedback for the first draft. DKD prepared and submitted the manuscript for submission. All authors reviewed the publication prior to submission.
DISCLOSURES
Joshua Jendza was employed by BASF Corporation during the execution of the current project. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- Abdollahi M.R., Zaefarian F., Hall L., Jendza J.A. Feed acidification and steam-conditioning temperature influence nutrient utilization in broiler chickens fed wheat-based diets. Poult. Sci. 2020;99:5037–5046. doi: 10.1016/j.psj.2020.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajandouz E.H., Puigserver A. Nonenzymatic browning reaction of essential amino acids: effect of pH on caramelization and Maillard reaction kinetics. J. Agric. Food Chem. 1999;47:1786–1793. doi: 10.1021/jf980928z. [DOI] [PubMed] [Google Scholar]
- Al-Natour M.Q., Alshawabkeh K.M. Using varying levels of formic acid to limit growth of Salmonella Gallinarum in contaminated broiler feed. Asian-Australas. J. Anim. Sci. 2005;18:390–395. [Google Scholar]
- Bedford A., Yu H., Hernández M., Squires E.J., Leeson S., Gong J. Effects of fatty acid glyceride product SILOhealth 104 on the growth performance and carcass composition of broiler chickens. Poult. Sci. 2018;97:1315–1323. doi: 10.3382/ps/pex440. [DOI] [PubMed] [Google Scholar]
- Campbell J.M., Crenshaw J.D., Polo J., Mellick D., Bienhoff M., Stein H.H. Impact of formaldehyde treated pig feed containing spray dried plasma on weaned pig growth performance. J. Anim. Sci. 2018;96(Suppl. 2):138–139. [Google Scholar]
- Campbell J.M., Crenshaw J.D., Polo J., Mellick D., Bienhoff M., Stein H.H. Chemical analysis of formaldehyde treated spray dried plasma and effects on weaned pig growth performance when included in diets. J. Anim. Sci. 2018;96(Suppl. 2):138. [Google Scholar]
- Carpenter K.J., Booth V.H. Damage to lysine in food processing: its measurement and its significance. Nutrition Abstract and Reviews. 1973;43:423–451. [Google Scholar]
- Cavitt L., Youm G., Meullenet J., Owens C., Xiong R. Prediction of poultry meat tenderness using razor blade shear, Allo-Kramer shear, and sarcomere length. J. Food Sci. 2004;69:SNQ11–SNQ15. [Google Scholar]
- Cochrane R.A., Huss A.R., Aldrich G.C., Stark C.R., Jones C.K. Evaluating chemical mitigation on Salmonella Typhimurium ATCC 14028 in animal feed ingredients. J. Food Protect. 2016;79:672–676. doi: 10.4315/0362-028X.JFP-15-320. [DOI] [PubMed] [Google Scholar]
- Coelho M., Ader P. Comparison of the impact of monobutyrin, other glycerides, and glycerol (SiloHealth 104), sodium formate (Amasil NA) and bacitracin methylene disalicylate (BMD) on performance and necrotic enteritis when fed to broiler chickens challenged with mild coccidia and Clostridium perfringens (Cp) 2019 International Poultry Scientific Forum. 2019:P243. [Google Scholar]
- Coelho M., Ader P. Comparison of the impact of monobutyrin, other glycerides, and glycerol (SiloHealth 104), sodium formate (Amasil NA) and bacitracin methylene disalicylate (BMD) on intestinal and processed parts bacteria count when fed to broiler chickens challenged with mild coccidia and Clostridium perfringens (Cp) 2019 International Poultry Scientific Forum. 2019:M38. [Google Scholar]
- Davies R.H., Wray C. Distribution of Salmonella contamination in ten animal feedmills. Vet. Microbiol. 1997;57:159–169. doi: 10.1016/s0378-1135(97)00114-4. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Dittoe D.K., Ricke S.C., Kiess A.S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 2018;5:216. doi: 10.3389/fvets.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley, K. D., T. R. Callaway, V. I. Chavlova, J. L. McReynolds, M. E. Hume, C. S. Dunkley, L. F. Kubera, D. J. Nisbit, and S. C. Ricke. 2009. Foodborne Salmonella ecology in the avian gastrointestinal tract. 15:26-35. [DOI] [PubMed]
- Feng Y., Wang Y., Wang P., Huang Y., Wang F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell Physiol. Biochem. 2018;49:190–205. doi: 10.1159/000492853. [DOI] [PubMed] [Google Scholar]
- Foley S.L., Johnson T.L., Ricke S.C. Salmonella pathogenicity and host adaptation in chicken associated serovars. Microbiol. Mol. Biol. Rev. 2013;77:582–606. doi: 10.1128/MMBR.00015-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley S.L., Nayak R., Hanning I.B., Johnson T.J., Han J., Ricke S.C. Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Appl. Environ. Microbiol. 2011;77:4273–4279. doi: 10.1128/AEM.00598-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. Nutritional Value of Proteins from Different Food Sources. A Review. Journal of Agricultural and Food Chemistry. 1996;44(1):6–29. doi: 10.1021/jf9400167. [DOI] [Google Scholar]
- García V., Catalá-Gregori P., Hernández F., Megías M.D., Madrid J. Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa, morphology, and meat yield of broilers. J. Appl. Poult. Res. 2007;16:555–562. [Google Scholar]
- Greiner L., Connor J., Knopf B., Graham A.B., Ochoa L., Harrell R.J. Pages 284-285 in Evaluation of nutrient availability when using feed disinfection in nursery diets 48th AASV Annual Meeting; Denver, CO; 2017. [Google Scholar]
- Gustafsson O.J., Arentz G., Hoffmann P. Proteomic developments in the analysis of formalin-fixed tissue. Biochim. Biophys. Acta. 2015;1854:559–580. doi: 10.1016/j.bbapap.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Hagemeister H., Erbersdobler H. Chemical labelling of dietary protein by transformation of lysine to homoarginine: a new technique to follow intestinal digestion and absorption. Proc Nutr Soc. Proceedings of the Nutrition Society. 1985;44(133a) [Google Scholar]
- Hernández F., García V., Madrid J., Oregno J., Catalá P., Megías M.D. Effect of formic acid on performance, digestibility, intestinal histomorphology, and plasma metabolite levels of broiler chickens. Bri. Poult. Sci. 2006;47:50–56. doi: 10.1080/00071660500475574. [DOI] [PubMed] [Google Scholar]
- Horrocks S.M., Anderson R.C., Nisbit D.J., Ricke S.C. Incidence and ecology of Campylobacter jejuni and coli in animals. Anaerobe. 2009;15:18–25. doi: 10.1016/j.anaerobe.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Jendza J.A., Huss A., Jones C., Abdollahi M.R., Hall L. Effect of feed acidification and conditioning temperature on feed hygiene and Salmonella recovery from mash and pelleted broiler feed. Proc. Aust. Poult. Sci. Symp. 2018;29:97–100. [Google Scholar]
- Jones F.T. A review of practical Salmonella control measures in animal feed. J. Appl. Poult. Res. 2011;20(1):102–113. doi: 10.3382/japr.2010-00281. 1, [DOI] [Google Scholar]
- Jones F.T., Richardson K.E. Salmonella in Commercially Manufactured Feeds. Poult. Sci. 2004;83(3):384–391. doi: 10.1093/ps/83.3.384. 1, [DOI] [PubMed] [Google Scholar]
- Khan M.Z., Ali Z., Muhammad G., Khan A., Mahmood F. Pathological effects of formalin (37% formaldehyde) mixed in feed or administered to the crops of white leghorn chickens. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 2003;50:354–358. doi: 10.1046/j.1439-0442.2003.00550.x. [DOI] [PubMed] [Google Scholar]
- Khan S.H., Iqbal J. Recent advances in the role of organic acids in poultry nutrition. J. Appl. Anim. Res. 2016;44:359–369. [Google Scholar]
- Kuttappan V.A., Lee Y.S., Erf G.F., Meullenet J.-F.C., McKee S.R., Owens C.M. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012;91:1240–1247. doi: 10.3382/ps.2011-01947. [DOI] [PubMed] [Google Scholar]
- Li X., Bethune L.A., Jia Y., Lovell R.A., Proescholdt T.A., Benz S.A., Schell T.C., Kaplan G., McChesney D.G. Surveillance of Salmonella prevalence in animal feeds and characterization of the Salmonella isolates by serotyping and antimicrobial susceptibility. Foodborne Path. Dis. 2012;9:692–698. doi: 10.1089/fpd.2011.1083. [DOI] [PubMed] [Google Scholar]
- Maciorowski K.G., Herrera P., Jones F.T., Pillai S.D., Ricke S.C. Effects on poultry and livestock of feed contamination with bacteria and fungi. Anim. Feed Sci. Tech. 2007;133:109–136. [Google Scholar]
- Mauron J. The Maillard reaction in food; a critical review from the nutritional standpoint Prog Food Nutr Sci. 1981;5(1-6):5-35. Progress in Food & Nutrition Science. 1981;5(1–6):5–35. [PubMed] [Google Scholar]
- Mehaffey J.M., Pradhan S.P., Meullenet J.F., Emmert J.L., McKee S.R., Owens C.M. Meat quality evaluation of minimally aged broiler breast fillets from five commercial genetic strains. Poult. Sci. 2006;85:902–908. doi: 10.1093/ps/85.5.902. [DOI] [PubMed] [Google Scholar]
- National Research Council . Nutrient Requirements of Poultry. Ninth Revised Edition. The National Academies Press; Washington, D.C: 1994. [Google Scholar]
- Peng L., Li Z.R., Green R.S., Holzman I.R., Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragaa N.M., Korany R.M.S. Effect of thyme and/or formic acid dietary supplementation on broiler performance and immunity. Agric. Agric. Sci. Proc. 2016;10:270–279. [Google Scholar]
- Ragaa N.M., Korany R.M.S. Studying the effect of formic acid and potassium diformate on performance, immunity and gut health of broiler chickens. Anim. Nutr. 2016;2:296–302. doi: 10.1016/j.aninu.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke S.C. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 2003;82:632–639. doi: 10.1093/ps/82.4.632. [DOI] [PubMed] [Google Scholar]
- Ricke S.C., Dittoe D.K., Richardson K.E. Formic acid as an antimicrobial for poultry production: a review. Front. Vet. Sci. 2020;7:563. doi: 10.3389/fvets.2020.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke S.C., Richardson K., Dittoe D.K. Formaldehydes in feed and their potential interaction with the poultry gastrointestinal tract microbial community—a review. Front. Vet. Sci. 2019;6:188. doi: 10.3389/fvets.2019.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariat N.W., Feye K.M., Richards A.K., Booher B., Flores Z., Rubinelli P.M., Olson E.G. Incidence of Salmonella serovars isolated from commercial animal feed mills in the United States and serovar identification using CRISPR analysis. J. Appl. Microbiol. 2021;130:2141–2146. doi: 10.1111/jam.14933. [DOI] [PubMed] [Google Scholar]
- Sugiharto S., Yudiarti T., Isroli I., Widiastuti E., Wahyuni H.I., Sartono T.A., Nurwantoro N., Al-Baarri A.N. Effect of dietary supplementation of formic acid, butyric acid, or their combination on carcass and meat characteristics of broiler chickens. J. Indones. Trop. Anim. 2019;44:286–294. [Google Scholar]
- Suresh G., Das R.K., Brar S.K., Rouissi T., Ramirez A., Chorfi Y., Godbout S. Alternatives to antibiotics in poultry feed: molecular perspectives. Crit. Rev. Microbiol. 2018;44:318–335. doi: 10.1080/1040841X.2017.1373062. [DOI] [PubMed] [Google Scholar]
- Swaggerty C.L., Callaway T.R., Kogut M.H., Piva A., Grilli E. Modulation of the immune response to improve health and reduce foodborne pathogens in poultry. Microorganisms. 2019;7:65. doi: 10.3390/microorganisms7030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijare V.V., Yang F., Kuttappan V., Alvarado C., Coon C., Owens C. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 2016;95:2167–2173. doi: 10.3382/ps/pew129. [DOI] [PubMed] [Google Scholar]
- Tung, C. M., and J. E. Pettigrew. 2006. Critical review of acidifiers. Accessed May 2017. http://research.pork.org/FileLibrary/ResearchDocuments/05-169-PETTIGREW-UofILL.pdf.
- Wales A.D., Allen V.M., Davies R.H. Chemical treatment of animal feed and water for the control of Salmonella. Foodbor. Path. Dis. 2010;7:3–15. doi: 10.1089/fpd.2009.0373. [DOI] [PubMed] [Google Scholar]
- Williams H.E., Cochrane R.A., Woodworth J.C., DeRouchey J.M., Dritz S.S., Tokach M.D., Jones C.K., Fernando S.C., Burkey T.E., Li Y.S., Goodband R.D., Amachawadi R.G. Effects of dietary supplementation of formaldehyde and crystalline amino acids on gut microbial composition of nursery pigs. Sci. Rep. 2018;8:8164. doi: 10.1038/s41598-018-26540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Ajuwon K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PloS One. 2017;12 doi: 10.1371/journal.pone.0179586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai W., Peebles E.D., Schilling M.W., Mercier Y. Effects of dietary lysine and methionine supplementation on Ross 708 male broilers from 21 to 42 d of age (I): growth performance, meat yield, and cost effectiveness. J. Appl. Poult. Res. 2016;25:197–211. [Google Scholar]
- Zhai W., Schilling M.W., Jackson V., Peebles E.D., Mercier Y. Effects of dietary lysine and methionine supplementation on Ross 708 male broilers from 21 to 42 d of age (II): breast meat quality. J Appl. Poult. Res. 2016;25:212–222. [Google Scholar]