Abstract
Smad proteins play a key role in the intracellular signaling of the transforming growth factor β (TGF-β) superfamily of extracellular polypeptides that initiate signaling from the cell surface through serine/threonine kinase receptors. A subclass of Smad proteins, including Smad6 and Smad7, has been shown to function as intracellular antagonists of TGF-β family signaling. We have previously reported the identification of a WD40 repeat protein, STRAP, that associates with both type I and type II TGF-β receptors and that is involved in TGF-β signaling. Here we demonstrate that STRAP synergizes specifically with Smad7, but not with Smad6, in the inhibition of TGF-β-induced transcriptional responses. STRAP does not show cooperation with a C-terminal deletion mutant of Smad7 that does not bind with the receptor and consequently has no inhibitory activity. STRAP associates stably with Smad7, but not with the Smad7 mutant. STRAP recruits Smad7 to the activated type I receptor and forms a complex. Moreover, STRAP stabilizes the association between Smad7 and the activated receptor, thus assisting Smad7 in preventing Smad2 and Smad3 access to the receptor. STRAP interacts with Smad2 and Smad3 but does not cooperate functionally with these Smads to transactivate TGF-β-dependent transcription. The C terminus of STRAP is required for its phosphorylation in vivo, which is dependent on the TGF-β receptor kinases. Thus, we describe a mechanism to explain how STRAP and Smad7 function synergistically to block TGF-β-induced transcriptional activation.
The transforming growth factor β (TGF-β) family of polypeptides controls a broad spectrum of biological processes including proliferation, differentiation, apoptosis, and extracellular matrix production (2, 15). TGF-β family members initiate signaling from the cell surface by binding to a heteromeric complex of two distinct but related serine/threonine kinase receptors (17, 22, 43). Binding of the ligand to the type II receptor (TβR-II) results in the recruitment and phosphorylation of the type I receptor (TβR-I). This activates the type I receptor, which propagates the signal to a family of intracellular signaling mediators known as Smads (22, 43).
Smad proteins are classified according to their structure and function in signaling by TGF-β family members. Receptor-regulated Smads (R-Smads), which include Smad1 to -3, -5, and -8, act as direct substrates of specific type I receptors and are activated by phosphorylation on serine residues at the carboxy terminus. Thus, Smad2 and Smad3 mediate signaling by TGF-β and activin (1, 37, 40, 42, 48, 53). Smad1, -5, and -8 are targets of bone morphogenetic protein (BMP) receptors and propagate BMP signals (8, 24, 34, 46). Smad4 is a common mediator of TGF-β, activin, and BMP signals (37, 51). Upon phosphorylation by type I receptors, R-Smads form complexes with Smad4 and translocate to the nucleus, where they activate transcription of target genes through cooperative interactions with DNA, other transcription factors, and coactivators (7, 18, 28, 36, 52, 54).
A distinct class of distantly related Smads, including Smad6 (25) and Smad7 (21, 44), has been identified as consisting of inhibitors of these signaling pathways, and these inhibitors function by interfering with the activation of R-Smads. Smad7 forms stable associations with activated type I receptors, thereby preventing R-Smads from binding to and being phosphorylated by these receptors (21, 27, 44, 47). Smad7 inhibits BMP signaling by blocking the association and phosphorylation of Smad1 and Smad5. A distinct mechanism of inhibition for Smad6 and its primary role in regulating BMP signals have been proposed in which Smad6 specifically competes with Smad4 for binding to receptor-activated Smad1, producing an inactive Smad1-Smad6 complex (20, 26). Thus, Smad7 may function as a general inhibitor of TGF-β family signaling, and Smad6 preferentially antagonizes the BMP signaling pathway.
The inhibitory Smads diverge structurally from other Smad family members. They have sequence similarity with other Smads in the Mad homology 2 (MH2) domain, and their N-terminal regions have limited sequence similarity with those of other Smads (22, 27). Receptor-mediated phosphorylation of the C domain of signal-transducing Smads relieves the inhibitory activity of the N domain. Antagonistic Smads are not substrates for TGF-β family receptors, and the function of the N domain is less clear. A short C-terminal region of Smad7 is required for interaction with the receptor and for its inhibitory function (21). Smad7 has been shown to be predominantly localized in the nucleus in the absence of a ligand, and its MH2 domain is important for nuclear localization. Smad7 accumulates in the cytoplasm upon TGF-β receptor activation (27). This suggests that Smad7 may have a functional role in the nucleus separate from its inhibitory effect on TGF-β signaling.
In addition to Smads, other proteins that interact with TGF-β receptors have been identified, and some of them are involved in TGF-β signaling (17, 22, 30, 43). We have previously reported the identification of a WD40 domain-containing protein, STRAP, which interacts with both TβR-I and TβR-II and which negatively regulates gene expression from TGF-β-responsive promoters (13). Two other WD40 domain-containing proteins, TRIP-1 (6, 10) and the Bα subunit of protein phosphatase 2A (19), that interact with TGF-β receptors and that appear to have a role in TGF-β signaling have been identified. The associations of WD40 repeat proteins with the receptors may allow the repeat proteins to play a role in signaling by the serine/threonine kinase receptors. These WD40 domain-containing proteins appear to serve regulatory functions in various cellular processes, such as signal transduction, transcriptional regulation, RNA processing, vesicular trafficking, and cell cycle progression (45). Some of them consist only of WD40 repeats; others contain N- or C-terminal extensions of various lengths (45). The WD40 repeat structure appears to be a functional motif that facilitates defined protein-protein interactions, sometimes leading to multiprotein complexes, as shown for the β subunit of heteromeric G proteins (11). WD domains contain amino acid residues in a three-strand β sheet. It is not clear whether the conserved core of each repeat binds to any common structure, although it has been shown that some F box proteins contain WD40 repeats that allow them to bind to proteins with phosphorylated serine or sometimes threonine residues (3, 41).
Phosphorylation of R-Smads by TβR-I is required for activating the TGF-β signaling pathway. Smad7 forms stable associations with activated TβR-I. This is critical for preventing R-Smads from being phosphorylated by these receptors and consequently for the inhibitory activity of Smad7 in TGF-β signaling (21). However, little is known about how the Smad7 interaction with the receptors is regulated and how Smad7 blocks the binding of R-Smads with the receptor complex. In the present investigation, we have characterized the negative regulation of STRAP on TGF-β-mediated transcriptional activation. STRAP, in concert with Smad7, shows synergistic inhibition of TGF-β-dependent transcription from several reporters. This synergy in the inhibition of TGF-β signaling is not observed with Smad6 or with a nonfunctional mutant of Smad7, Smad7-Δ408. STRAP is present in a complex with Smad7 and activated type I receptor and stabilizes this complex. STRAP also binds with Smad2 and Smad3 but does not enhance their transactivating function. The C terminus of STRAP is required for its phosphorylation, which depends on the kinase activities of the TGF-β receptors. Our results suggest a mechanism to explain how STRAP and Smad7 can cooperate synergistically to inhibit TGF-β-dependent transactivation.
MATERIALS AND METHODS
Cell lines and transfections.
Mv1Lu and HepG2 cells were obtained from the American Type Culture Collection and were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and nonessential amino acids. COS-1 cells were grown in DMEM containing 10% FBS. For transient transfections, HepG2 cells were seeded at 20% confluency and transfected overnight using the calcium phosphate DNA precipitation method as described previously (12). For transfections in COS-1, cells were seeded at 50% confluency and were transfected using the calcium phosphate DNA precipitation method for 5 h or using FuGENE 6 (Boehringer Mannheim) transfection reagents, according to the manufacturer's instructions. Mv1Lu cells were transfected using a DEAE-dextran transfection method (Promega) by following the manufacturer's instructions.
Plasmid constructs.
The complete region encoding STRAP was amplified by PCR and subcloned into a mammalian expression vector, pcDNA3 (Invitrogen), with one copy of the coding sequence of the epitope in frame to the C terminus of STRAP to generate pcDNA3-STRAP-Flag or pcDNA3-STRAP-HA. The truncation mutant, pcDNA3-STRAP(1-294)-Flag, was constructed similarly by amplifying the sequence encoding STRAP from amino acids 1 to 294 by PCR. All constructs were verified by sequencing.
Immunoprecipitation and immunoblot analyses.
COS-1 cells were transfected with expression constructs. After 40 h, cells were washed, scraped, and solubilized in lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 10 mM EDTA, 0.5% Nonidet P-40, 0.5 mM dithiothreitol, 5 mM sodium fluoride, 0.5 mM sodium orthovanadate, 1.0 mM phenylmethylsulfonyl fluoride, 2 μg [each] of leupeptin, pepstatin, and aprotinin/ml). Cleared cell lysates were incubated with anti-Flag M2 monoclonal antibody (Sigma), antihemagglutinin (anti-HA) polyclonal antibody (Y11; Santa Cruz Biotechnology), or anti-Myc 9E10 monoclonal antibody (Santa Cruz Biotechnology) for 2 h at 4°C, followed by incubation with protein G-Sepharose (Sigma) for 1 h. Immunoprecipitates were washed four times with lysis buffer. The immune complexes were eluted by boiling for 3 min in sodium dodecyl sulfate (SDS) sample buffer and were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE). Proteins were electrotransferred to polyvinylidene fluoride membranes (Millipore Corporation) and immunoblotted with either anti-Flag antibody or anti-HA antibody, followed by detection using an enhanced chemiluminescence system. Expression of different proteins was monitored by immunoblotting after SDS-PAGE and electrotransfer of proteins in total cell lysates.
In vivo phosphorylation.
COS-1 cells were cotransfected with expression plasmids. After 40 h, cells were washed and preincubated with phosphate-free media containing 0.2% FBS. The cells were then incubated with media containing 1 mCi of [32P]orthophosphate per ml for 2 h at 37°C. The cells were washed and solubilized in lysis buffer. 32P-labeled proteins were immunoprecipitated with anti-Flag antibody, and the immunoprecipitates were washed six times with lysis buffer containing 1% Nonidet P-40 and 0.1% SDS (wash buffer). Phosphorylated proteins in the immunoprecipitates were detected by SDS-PAGE and autoradiography. For double immunoprecipitation of phosphorylated STRAP, the immune complexes from the first immunoprecipitations were eluted by boiling for 3 min in SDS sample buffer and the eluants were diluted 20-fold by lysis buffer for the second immunoprecipitation. The immunoprecipitates were washed thoroughly with wash buffer containing 0.1% sodium deoxycholate and then analyzed by SDS-PAGE. Quantitation of STRAP phosphorylation was performed using ImageQuant software (Molecular Dynamics).
Transcriptional response assays.
Mv1Lu or HepG2 cells were transiently transfected with various constructs and pCMV-βgal. In each experiment equal amounts of total DNA were transfected. Twenty hours after transfection, cells were incubated in appropriate media containing 0.2% FBS with or without TGF-β1 (100 pM) for 20 h. Luciferase activity and β-galactosidase activity was measured in an Analytical Luminescence Labs Monolight 2010 luminometer. Luciferase activity was normalized to β-galactosidase activity for determining transfection efficiency.
RESULTS
STRAP synergizes with Smad7, not with Smad6, in inhibiting TGF-β-induced transcription.
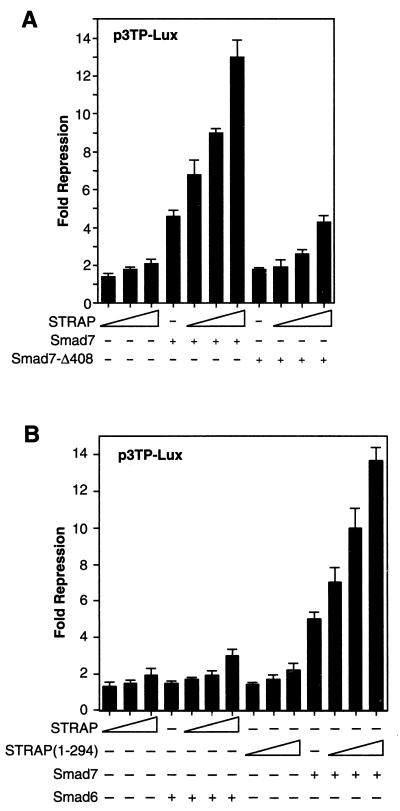
The induction of extracellular matrix protein genes is one of the best-characterized responses to TGF-β (31). This response can be used to evaluate the involvement of a gene in TGF-β signal transduction using transient transfection assays. Transcription of a luciferase reporter containing a PAI-I promoter fragment is frequently used to measure the induction of extracellular matrix protein synthesis in response to TGF-β (31). We tested the potential role of STRAP in Smad7-mediated inhibition of transcriptional responses in Mv1Lu and HepG2 cells, which are highly TGF-β responsive. Initially, we focused our analyses on a TGF-β-responsive reporter, p3TP-Lux (49), which contains elements from the PAI-1 promoter and which drives expression of a luciferase reporter gene. Transient transfection of p3TP-Lux into Mv1Lu cells resulted in low basal levels of transcription, which was strongly induced in response to TGF-β signaling. Overexpression of STRAP suppressed the TGF-β-induced increase in luciferase activity moderately in a dose-dependent manner. Smad7 showed appreciable inhibition of TGF-β-induced transcription as expected (21, 47). Coexpression of Smad7 and STRAP synergistically inhibited the p3TP promoter activity in response to TGF-β (Fig. 1A). In contrast, only a slight inhibition was observed in the absence of TGF-β signaling. To examine whether this synergy in inhibiting TGF-β signals was specific, we used a mutant of Smad7, Smad7-Δ408, in our experiments. This mutant cannot bind the receptor complex and has little effect in blocking TGF-β signals (21, 47). Consistent with these observations, Smad7-Δ408 had little effect on the p3TP promoter activity, either in the absence or presence of STRAP, in response to TGF-β. Importantly, STRAP did not show any synergy with Smad6 (an antagonist of BMP signaling) in suppressing TGF-β-induced transcription (Fig. 1B). We constructed a mutant of STRAP, STRAP(1-294), by deleting the C-terminal 57 amino acids and keeping all WD40 domains intact. This mutant was not phosphorylated in vivo, whereas STRAP was phosphorylated through its C terminus. STRAP(1-294) showed the same inhibitory effect as wild-type STRAP, either in the absence or presence of Smad7 (Fig. 1B), suggesting that phosphorylation of STRAP is dispensable for this transcriptional response.
FIG. 1.
Synergy between STRAP and Smad7 in the inhibition of p3TP promoter activity in response to TGF-β. (A) STRAP synergizes with Smad7 but not with mutant Smad7-Δ408. Mv1Lu cells were transiently transfected with p3TP-Lux (0.3 μg), the β-galactosidase reporter (30 ng), and TβR-I(TD) (0.43 μg) and with Smad7 constructs (0.3 μg) and increasing amounts of STRAP (0.2, 0.5, and 1 μg) as indicated. In each experiment equal amounts of total DNA were transfected. Luciferase activity was normalized to β-galactosidase activity. The mean of triplicate luciferase values from the TGF-β-treated control was considered 100%, and this was then divided by values for three replicates of each point to get the fold repressions. The means of these fold repressions ± standard deviations are plotted. These experiments were performed four times in triplicate with similar results. (B) STRAP does not synergize with Smad6, but the STRAP(1-294) mutant shows synergy with Smad7. Mv1Lu cells were transfected as described above with Smad7 or Smad6 (0.52 μg) and increasing amounts of STRAP or STRAP(1-294). Luciferase assays were performed as described for panel A.
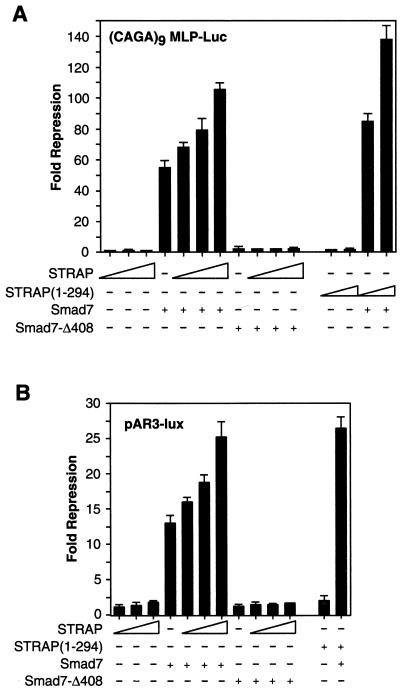
To further examine the synergistic inhibition by STRAP and Smad7, we used another TGF-β-responsive reporter (CAGA)9MLP-Luc, which contains multiple copies of a Smad3- and Smad4-binding CAGA box element upstream of a minimal adenovirus major-late promoter (14). This reporter was induced by 150-fold in response to TGF-β signaling. STRAP alone had little effect on (CAGA)9MLP-Luc promoter activity (Fig. 2A). Smad7 alone showed 57-fold repression of the promoter activity, but in the presence of STRAP it showed dose-dependent repression, reaching a maximum of 105-fold repression, in the presence of TGF-β signaling (Fig. 2A). However, in cells expressing STRAP and Smad7-Δ408, there was no synergy in the inhibition of TGF-β-mediated transcriptional activation of the promoter activity. The phosphorylation-incompetent mutant of STRAP showed synergy with Smad7, similar to wild-type STRAP. These data suggest that STRAP synergistically inhibits TGF-β signaling with Smad7 but not with the Smad7 mutant or Smad6.
FIG. 2.
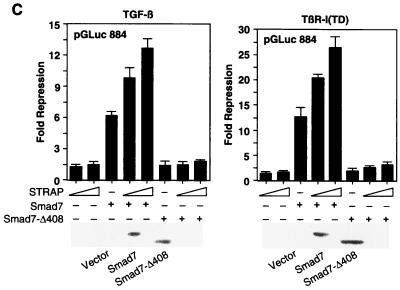
Functional synergy between STRAP and Smad7. (A) Synergistic inhibition of (CAGA)9 MLP-Luc reporter activity in response to TGF-β. HepG2 cells were transfected with a (CAGA)9 MLP-Luc reporter (0.3 μg) containing nine copies of Smad3/Smad4 binding sites, Smad7 constructs, increasing amounts of STRAP, and increasing amounts of STRAP(1-294) (0.5 and 1 μg). TGF-β signaling was initiated by expression of TβR-I(T204D). Luciferase assays were performed as described for Fig. 1A. (B) STRAP and Smad7 synergistically block an immediate-early response to TGF-β. HepG2 cells were cotransfected with pAR3-lux (0.3 μg), FAST2 (15 ng), Smad7 constructs, STRAP(1-294) (1 μg), and increasing amounts of STRAP as indicated. Cells were treated with or without TGF-β (100 pM) for 20 h prior to lysis and then analyzed for luciferase activity. (C) Synergistic inhibition of TGF-β-induced PAI-1 promoter activity by STRAP and Smad7. HepG2 cells were transiently transfected with pGLuc 884 reporter (0.25 μg) (9), HA-tagged Smad7 constructs, and increasing amounts of STRAP. TGF-β signaling was initiated either by treatment of the cells with 100 pM TGF-β (left) or by coexpression of TβR-I(TD) (right). Luciferase assays were performed as described for Fig. 1A. Expression of Smad7 proteins were confirmed by direct immunoblotting of total cell lysates, made for luciferase assays from cells transfected with either vector or coding sequences for Smad7 or the Smad7-Δ408 construct, with anti-HA antibodies.
To determine whether this inhibitory effect of STRAP in cooperation with Smad7 was direct in TGF-β signaling, we used a reporter, pAR3-lux (21), that contains three copies of the activin response element from the Xenopus Mix.2 promoter (7). This construct had minimal basal activity in HepG2 cells due to the lack of endogenous FAST-like activity (36, 39). Since activin and TGF-β activate common downstream signaling pathways to regulate common biological processes (4, 35), we utilized this reporter to investigate STRAP-mediated synergy with Smad7 in blocking immediate-early responses to TGF-β. pAR3-lux was activated approximately 32-fold by FAST2 (36) when transfected HepG2 cells were treated with 100 pM TGF-β. Although STRAP alone had little effect on the promoter activity, it suppressed the TGF-β-dependent activation strongly in concert with Smad7 (Fig. 2B). However, coexpression of STRAP and Smad7-Δ408 did not show any synergy in the inhibition of pAR3-lux transactivation in response to TGF-β. We used both amino- and carboxy-terminal tags in Smad7. These tagged versions of Smad7 were the same as the untagged protein in blocking TGF-β-dependent signaling (21). Similarly, tagged and untagged versions of STRAP are indistinguishable in inhibitory function.
To investigate whether STRAP has a similar effect on a natural promoter, we performed transient transfection assays with a reporter plasmid (pGLuc 884) (9) containing the luciferase gene under the control of the TGF-β-inducible PAI-1 gene promoter. This reporter was strongly induced in HepG2 cells in response to TGF-β signaling initiated either by treatment of the cells with 100 pM TGF-β (Fig. 2C, left) or by coexpression with a constitutively active version of TGF-β type I receptor, TβR-I(TD) (right). We observed a weak suppression of TGF-β-dependent induction of the PAI-1 promoter by STRAP. STRAP showed a synergy in the inhibition of the PAI-1 promoter with wild-type Smad7 but not with the mutant Smad7-Δ408 (Fig. 2C). Taken together, these results show a functional synergy between STRAP and Smad7 in the negative regulation of transcription mediated by TGF-β, and a mutant of Smad7 that fails to associate with the receptor does not synergize with STRAP.
STRAP interacts with Smad6 and Smad7.
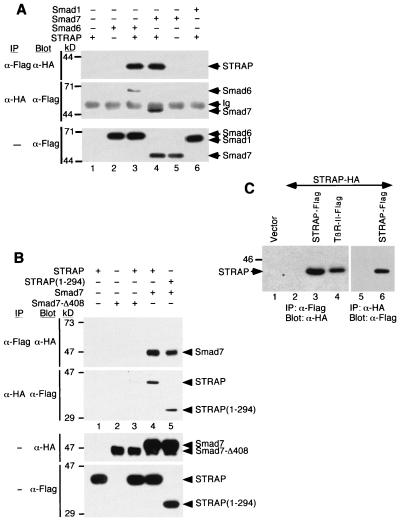
Smad6 and Smad7 are known to be intracellular antagonists of signaling by TGF-β family members. To explore the mechanism by which STRAP exhibits the synergistic inhibition of TGF-β signaling with Smad7, we tested whether STRAP could interact with the inhibitory Smads by using coimmunoprecipitation and immunoblot analyses. STRAP-HA was transiently transfected into COS-1 cells alone or in combination with Flag-tagged Smads. STRAP was detected specifically in the immune complex of either Smad7 or Smad6 (Fig. 3A, lanes 3 and 4). In a reciprocal experiment, we observed that Smad7 or Smad6 coimmunoprecipitated with STRAP (Fig. 3A, middle), demonstrating the association of STRAP with Smad7 or Smad6. We were unable to detect any physical association of STRAP with Smad1(AAVA) (Fig. 3A, top, lane 6). Under similar conditions, STRAP can bind with Smad2 and Smad3 (see below), but not with Smad4 (data not shown).
FIG. 3.
Association of STRAP with Smad7, but not with Smad7-Δ408, and oligomerization of STRAP. (A) Interaction of STRAP with Smad6 and Smad7 in mammalian cells. COS-1 cells were transfected with HA-tagged STRAP either alone or together with the indicated Flag-tagged Smad constructs, including Smad1(AAVA), Smad6, and Smad7. Cell lysates were subjected to an anti-Flag immunoprecipitation (IP), and coprecipitating STRAP was detected by immunoblotting (Blot) with anti-HA antibodies (top section). In the middle section, total lysates were immunoprecipitated using anti-HA antibodies and then immunoblotted with anti-Flag antibodies. To confirm expression of Smads, aliquots of total cell lysates were immunoblotted with anti-Flag antibodies (bottom section). Ig, immunoglobulin. (B) STRAP(1-294) interacts with Smad7, and Smad7-Δ408 does not interact with STRAP. COS-1 cells were transiently transfected with the indicated combinations of Flag-tagged STRAP constructs and HA-tagged Smad7 constructs. Cell lysates were immunoprecipitated with an anti-Flag antibody, and the immunoprecipitates were analyzed by anti-HA antibody immunoblotting (top section). In the second section from the top, cell lysates were subjected to immunoprecipitation with an anti-HA antibody and the precipitates were analyzed with an anti-Flag antibody. Expression of the proteins was confirmed by the direct immunoblotting of the total cell lysates (bottom two sections). (C) Homo-oligomerization of STRAP. Cells were transfected with STRAP-HA alone or together with STRAP-Flag or TβR-II-Flag (serves as a positive control) as indicated. Cell lysates were subjected to immunoprecipitation with a Flag antibody, and coprecipitated proteins were detected by immunoblotting with an HA antibody (lanes 1 to 4). Reciprocal experiments were also performed (lanes 5 and 6).
STRAP showed functional synergy with Smad7 and not with the mutant of Smad7, Smad7-Δ408, in the inhibition of TGF-β-dependent transcription (Fig. 1A and 2). To examine whether this truncation in Smad7 has any effect on the association with STRAP, we coexpressed HA-tagged Smad7-Δ408 with Flag-tagged STRAP in COS-1 cells. Cell lysates were then subjected to immunoprecipitation with an anti-Flag antibody, and the immunoprecipitates were analyzed by immunoblotting with an anti-HA antibody (Fig. 3B, top) and vice versa (Fig. 3B, second from top). STRAP stably associated with Smad7 (lane 4), but only a very low level of interaction between STRAP and Smad7-Δ408 could be detected (lane 3). This supports the specificity of the interaction between STRAP and Smad7. On the other hand, Smad7 was detected in the immune complex of STRAP(1-294), and this was coimmunoprecipitated with Smad7 (lane 5), indicating that the association of STRAP with Smad7 was not affected by deleting the C-terminal 57 amino acids from STRAP. These findings were consistent with our demonstration that STRAP does not synergize with Smad7-Δ408 and that STRAP(1-294) behaves like wild-type STRAP in the transcriptional responses. We used both Flag- and HA-tagged STRAP and Smad7 in the coimmunoprecipitation experiments, demonstrating that the association was independent of the epitope tag employed and that the amino- or carboxy-terminal tags did not alter the association of the proteins. Together, these data indicate that STRAP interacts with Smad6 and Smad7 but not with the mutant of Smad7 and that a C-terminal deletion for STRAP does not affect its association with Smad7.
Homo-oligomerization of STRAP.
Several components of the TGF-β signaling cascade, including receptors and Smad proteins, are known to homo- and hetero-oligomerize (5, 23, 29, 37, 50). WD40 repeat proteins homo- and hetero-oligomerize presumably to stabilize their structure and to serve regulatory functions in various cellular processes (45). STRAP has six WD40 domains (13). To determine whether it can form homo-oligomers, we cotransfected COS-1 cells with two different STRAP constructs, one tagged with a Flag epitope and the other tagged with an HA epitope. Cell lysates were subjected to immunoprecipitation with antibodies to Flag, and each immunoprecipitate was then probed with antibodies to HA (Fig. 3C, lanes 1 to 4). Reciprocal experiments in which proteins immunoprecipitated by antibodies to HA were blotted with an anti-Flag antibody (Fig. 3C, lanes 5 and 6) confirmed the association of STRAP with itself in a ligand-independent manner. As described previously (13), STRAP was detected in the immune complex of TβR-II under similar conditions (lane 4). This illustrates that different epitope tags do not affect the homo-oligomerization and the overall tertiary structure of STRAP.
STRAP stabilizes the complex between Smad7 and activated type I TGF-β receptor.
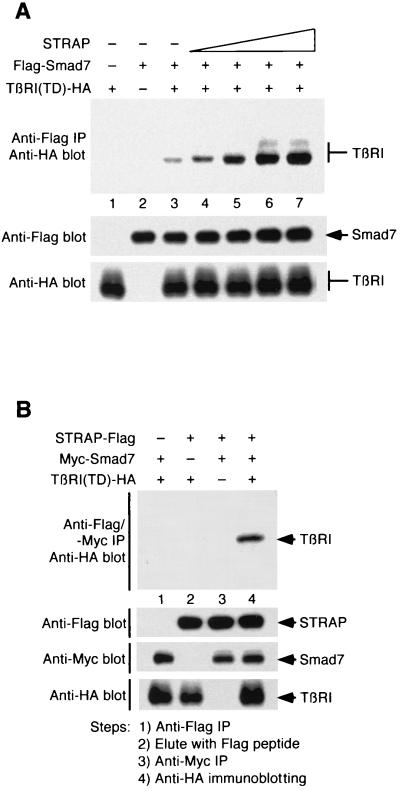
Smad7 blocks TGF-β signaling by preventing heteromeric complex formation between Smad2 or Smad3 and Smad4 and nuclear accumulation of Smad2 or Smad3 in response to TGF-β signaling (21, 44). Smad7 is also known to block BMP signaling by inhibiting the phosphorylation of Smad1 and Smad5 (47). Smad6 has been shown to inhibit BMP signaling by a distinct mechanism (20). It prevents the formation of an active Smad4–Smad1 signaling complex by directly competing with Smad4 for binding to Smad1. The mechanism of inhibition is not well known, although inhibition seems to be primarily mediated through the ability of Smad6 and Smad7 to interact with the type I receptor. Smad7 functions by associating stably with the activated type I receptor to block the interaction, phosphorylation, and subsequent activation of Smad2 and Smad3 (21, 44). Therefore, stable association of Smad7 with the type I receptor is critical for blocking TGF-β family signaling. To explore the mechanism of STRAP function in the synergistic inhibition of TGF-β signaling, we tested whether STRAP could stabilize the complex between Smad7 and activated TβR-I. COS-1 cells were transiently transfected with Flag-Smad7, TβR-I(TD)–HA, and increasing amounts of STRAP. Cell lysates were subjected to immunoprecipitation with antibodies to Flag followed by immunoblotting with anti-HA antibodies. In cells expressing Smad7 and TβR-I(TD), association between these two proteins was detected, similar to previous observations (Fig. 4A, lane 3) (21). Interestingly, Smad7–TβR-I heteromeric complex formation was increased strongly with increasing amounts of STRAP in a dose-dependent manner in the presence of TGF-β signals (lanes 4 to 7). We observed a pronounced stimulation of Smad7–TβR-I(TD) interaction when the STRAP-to-Smad7 concentration ratio was less than 1 or 1 (lanes 4 and 5). Similarly, Smad7 increases the interaction between STRAP and TβR-I(TD) (data not shown). Together, these data demonstrate that STRAP associates stably with Smad7 and that it stabilizes complexes between Smad7 and TβR-I in the presence of TGF-β signaling.
FIG. 4.
STRAP stabilizes the association between Smad7 and activated TβR-I and forms a complex with Smad7 and TβR-I(TD). (A) STRAP stabilizes Smad7–TβR-I(TD) complexes. COS-1 cells were transiently transfected with plasmids encoding Flag-Smad7 (0.4 μg), TβR-I(TD)-HA (0.6 μg), and STRAP (in increasing amounts of 0.2, 0.4, 1, and 2 μg). Cell lysates were subjected to immunoprecipitation (IP) with an anti-Flag antibody, and the presence of TβR-I(TD) in the immunoprecipitates was detected by immunoblotting with an anti-HA antibody (top). To confirm equivalent expression of Smad7 and TβR-I(TD), aliquots of total cell lysates were immunoblotted with an anti-Flag antibody (middle) and an anti-HA antibody (bottom). (B) STRAP is present in a complex with Smad7 and TβR-I(TD). Cells were transfected with indicated combinations of STRAP-Flag, Myc-Smad7, and TβR-I(TD)-HA. Cell lysates were immunoprecipitated with an anti-Flag antibody, proteins were eluted with a Flag peptide, and the eluate was reprecipitated by an anti-Myc antibody followed by anti-HA antibody immunoblotting (top). Expression of the proteins was monitored by immunoblotting.
STRAP forms a ternary complex with Smad7 and TβR-I(TD).
The data presented above show that STRAP binds to Smad7, and our previous data (13) showed the interaction between STRAP and the receptor complex. To determine whether these components were present in the same complex, COS-1 cells were cotransfected with Flag-tagged STRAP, HA-tagged TβR-I(TD), and Myc-tagged Smad7. Cell lysates were subjected to immunoprecipitation with an anti-Flag antibody. Immune complexes were then eluted with a Flag peptide, and the eluate was used in the second immunoprecipitation with an anti-Myc antibody. Finally, the immunoprecipitate was analyzed by immunoblotting with an anti-HA antibody. According to Fig. 4B, a ternary complex was detected when cells were cotransfected with all three constructs (lane 4), but not when any one construct was omitted (lanes 1 to 3). Thus, both STRAP and Smad7 can coexist in the same receptor-containing complex. Taken together, these results suggest that STRAP functions to recruit Smad7 to the activated receptor, forming a ternary complex, and to stabilize the Smad7–receptor complex, thus assisting Smad7 to prevent Smad2 and Smad3 access to the receptor.
Association of STRAP with Smad2 and Smad3.
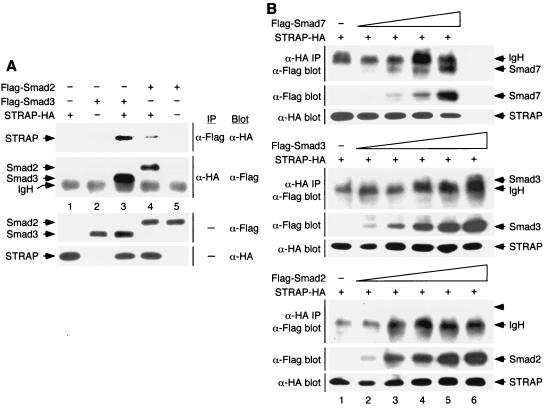
Smad2 and Smad3 are substrates of TGF-β or activin receptors and mediate signaling by these ligands (22, 43). Both of these Smads stably associate with kinase-inactive TβR-I in a complex. Our previous studies showed that STRAP bound with TβR-I constitutively (13). To test whether STRAP can interact with Smad2 and Smad3 in mammalian cells, we expressed HA-tagged STRAP in COS-1 cells together with Flag-tagged versions of Smad2 and Smad3. Cell lysates were subjected to anti-Flag immunoprecipitation followed by immunoblotting with anti-HA antibodies. Efficient coprecipitation of STRAP with either Smad2 or Smad3 was observed (Fig. 5A, top section). Reciprocal experiments showed the coprecipitation of either Smad with STRAP (Fig. 5A, second section from top), demonstrating the association of STRAP with Smad2 or Smad3.
FIG. 5.
Interaction between STRAP and Smad7 is stronger than that between STRAP and Smad2 or between STRAP and Smad3. (A) STRAP interacts with Smad2 and Smad3. COS-1 cells were transiently transfected with combinations of HA-tagged STRAP and Flag-tagged Smad2 or Smad3 as indicated. Cell lysates were subjected to immunoprecipitation (IP) with anti-Flag antibodies and then immunoblotted (Blot) using anti-HA antibodies (top section). In the second section from the top, total lysates were immunoprecipitated using anti-HA antibodies and immunoblotted with anti-Flag antibodies. To confirm the expression of the proteins, aliquots of total cell lysates were immunoblotted with anti-Flag antibodies (third section from the top) and anti-HA antibodies (bottom section). IgH, immunoglobulin H. (B) Relative strengths of binding of STRAP with Smad2, Smad3, and Smad7. COS-1 cells were transfected with a constant amount of the STRAP-HA construct together with increasing amounts of Flag-Smad7 (top), Flag-Smad3 (middle), or Flag-Smad2 (bottom). Cell lysates were subjected to immunoprecipitation with anti-HA antibodies, and the precipitates were analyzed by blotting with anti-Flag antibodies (all three sections). Expression of Smad proteins was monitored by analyzing aliquots of total cell lysate by immunoblotting with anti-Flag antibodies (all sections). Smad7, Smad3, and Smad2 were expressed in equivalent levels in lanes 2 to 5. In lane 6 (middle and bottom), Smad3 and Smad2 were expressed at levels about twofold higher than the corresponding levels of these proteins in lane 5. Expression of STRAP-HA was determined by immunoblotting total cell lysates using anti-HA antibodies. The migration of each protein is indicated on the right by an arrow, and the arrowhead points to the expected position of Smad2 in the bottom section.
To gain insights into the relative strengths of binding of STRAP with Smad2, Smad3, and Smad7, we compared the interaction of STRAP with these Smads over a range of Smad concentrations by coimmunoprecipitation and immunoblot experiments. Briefly, a fixed amount of STRAP-HA plasmid was transfected into COS-1 cells alone or in combination with increasing amounts of coding sequences for Flag-tagged Smads. Flag-tagged Smads were expressed in low and equivalent levels (Fig. 5B, lanes 2 to 5). To recover STRAP itself, plus associated proteins, lysates were immunoprecipitated with antibodies to HA and each immunoprecipitate was then probed with antibodies to Flag. A low level of interaction between STRAP and Smad7 could be detected with the low level of expression of Smad7 (lane 2), and a strong increase in the amount of Smad7 coprecipitated with STRAP was observed with increasing Smad7 expression in a dose-dependent manner (Fig. 5B, top section, lanes 2 to 5). However, no association, either between STRAP and Smad3 or between STRAP and Smad2, was observed when the expression levels of Smad3 and Smad2 were similar to that of Smad7 (Fig. 5B, middle and bottom sections, lanes 2 to 5). We did observe some coprecipitation of Smad3, but not of Smad2, with STRAP when the concentration of these proteins was about twofold higher (middle and bottom sections, lane 6) than the highest concentration used in lane 5. Interaction between STRAP and Smad2 was detected with further increases (twofold) in the expression of Smad2 (data not shown and Fig. 5A). Collectively, these results suggest that the interaction between STRAP and Smad7 is stronger than that between STRAP and Smad3 or STRAP and Smad2.
Effect of STRAP on TGF-β-induced and Smad-dependent transcriptional activation.
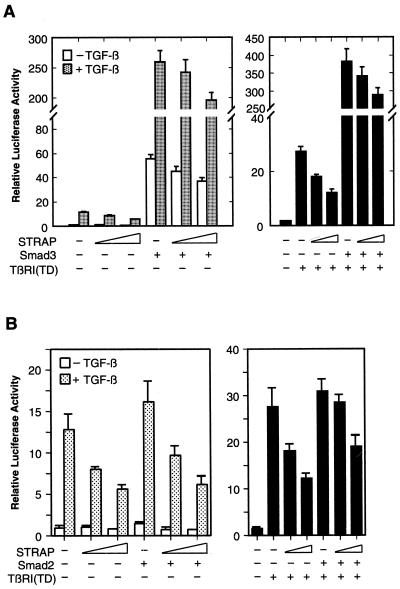
Smad2 and Smad3 play an important role in mediating TGF-β-induced transcriptional activation of downstream genes. To investigate whether binding of STRAP to Smad2 or Smad3 has any functional consequences on transcriptional activation, HepG2 cells were transiently transfected with the TGF-β-responsive p3TP-Lux reporter, an internal lacZ control, the Smad3 coding sequence, and increasing amounts of STRAP expression vector (Fig. 6A, left). Similar to our previous observations, STRAP inhibited TGF-β-dependent induction of the promoter activity. Coexpression of Smad3 alone resulted in a dramatic increase in the luciferase activity both in the presence and absence of TGF-β, as expected (14, 53). Coexpression of STRAP with Smad3 did not potentiate Smad3-dependent transcriptional activation. In contrast, only a slight inhibition of the promoter activity by STRAP was observed. Analogous results were seen when TGF-β signaling was initiated by expression of the constitutively active version of the TGF-β type I receptor (Fig. 6A, right). We saw a similar inhibitory effect of STRAP when the transcriptional activation by Smad3 was low at lower levels of its expression (data not shown). In similar experiments, Smad2 increased the TGF-β-induced promoter activity weakly, as shown previously (42). Coexpression of STRAP with Smad2 did not cooperate with Smad2 in the induction of the p3TP promoter; rather, STRAP showed moderate inhibitory activity (Fig. 6B). These results suggest that STRAP does not cooperate with Smad2 or Smad3 in the induction of the TGF-β-mediated transcriptional responses.
FIG. 6.
Inhibition of TGF-β-induced and Smad3- or Smad2-dependent transcription by STRAP. (A) Smad3-dependent transcriptional activation of the p3TP promoter is not enhanced by STRAP. HepG2 cells were transiently transfected with p3TP-Lux, Smad3, and increasing amounts of STRAP as indicated. Left, cells were treated with or without TGF-β (100 pM) for 20 h prior to lysis and then analyzed for luciferase activity. Right, TGF-β signaling was initiated by expression of TβR-I(TD). Luciferase activity was normalized to β-galactosidase activity and expressed as the mean ± standard deviation of triplicate measurements from a representative experiment. These experiments were performed four times in triplicate with similar results. (B) STRAP does not cooperate with Smad2 in its transactivation function. This experiment was same as that in panel A except that Smad2 was expressed instead of Smad3.
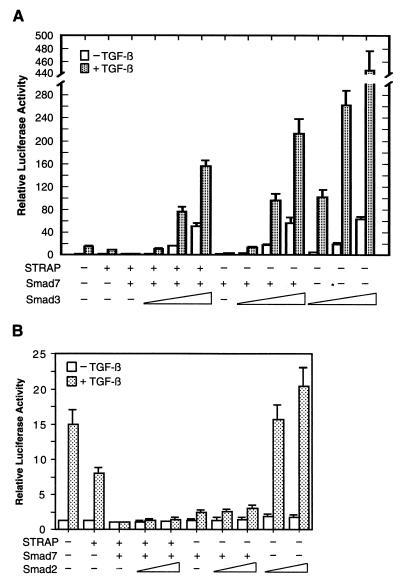
Since Smad2 and Smad3 are centrally involved in mediating TGF-β signals, we examined whether Smad2 or Smad3 might affect the synergistic inhibition of TGF-β-induced transcription by STRAP and Smad7. As shown in Fig. 7A, cotransfection of STRAP and Smad7 in HepG2 cells strongly repressed the p3TP promoter activity. This inhibition of the TGF-β-dependent activation of the promoter was reversed by Smad3 in a dose-dependent manner (maximum induction, 125-fold). The expression of Smad7 was kept between the lowest and the highest levels of Smad3 expression (data not shown). Smad3 showed a somewhat stronger effect in reversing the Smad7-mediated abrogation of TGF-β-induced promoter activity (maximum induction, 170-fold). Overexpression of Smad3 alone with the reporter construct strongly increased luciferase expression both in the presence (maximum induction, 358-fold) and absence of TGF-β, when there was no suppressive effect of either Smad7 alone or STRAP and Smad7 together. In spite of their 92% sequence identity, Smad2 and Smad3 are not functionally equivalent (14). In contrast to Smad3, Smad2 increased the p3TP promoter activity weakly in response to TGF-β as shown previously (42) (Fig. 7B). Smad2, which was expressed efficiently (data not shown), showed little effect in reversing the inhibition of TGF-β-induced transcriptional responses by either Smad7 alone or STRAP and Smad7 together. These experiments suggest that Smad3, unlike Smad2, is able to strongly reverse the synergistic inhibition of TGF-β-dependent transcription by STRAP and Smad7.
FIG. 7.
Smad3, and not Smad2, strongly reverses the inhibition of TGF-β-induced transcription by either Smad7 alone or STRAP and Smad7 together. (A) Reversing the synergistic inhibition of TGF-β-mediated transcription by Smad3. HepG2 cells were transfected with p3TP-Lux and with coding sequences for Smad7, STRAP, and increasing amounts of Smad3 as indicated and were treated with or without TGF-β (100 pM) for 20 h. The relative luciferase activity in cell lysates was measured. Luciferase activity was normalized to β-galactosidase activity and expressed as the mean ± standard deviation of triplicate measurements from a representative experiment. These experiments were performed four times in triplicate with similar results. (B) Smad2 shows a weak effect on reversing the inhibition of the promoter activity by STRAP and Smad7. The experiment was performed as described for panel A except that Smad2 was transfected in two doses instead of Smad3.
Phosphorylation of STRAP in vivo requires its C terminus.
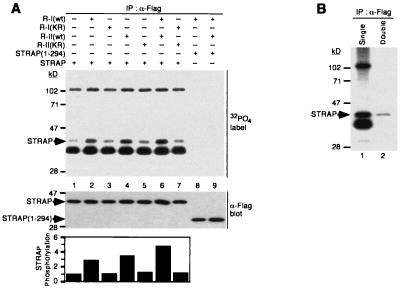
For downstream signaling from receptor kinases to culminate in transcriptional regulation of target genes, the phosphorylation of some signaling components is often essential. To test whether STRAP is a substrate for the serine/threonine kinase receptors, we analyzed the phosphorylation of STRAP in vivo in COS-1 cells where it was coexpressed with different combinations of TGF-β receptors. Metabolic labeling of transfected cells with [32P]orthophosphate followed by immunoprecipitation of STRAP with an anti-Flag antibody indicated a low basal level of STRAP phosphorylation in COS-1 cells without exogenous receptor expression (Fig. 8A, lane 1). An increase in STRAP phosphorylation was detected in cells expressing TβR-I (lane 2). This increase was dependent on TβR-I kinase activity because a point mutation (K232R) that abolishes TβR-I kinase activity prevented the increase in STRAP phosphorylation (lane 3). Coexpression of STRAP with TβR-II resulted in a significant increase in STRAP phosphorylation (lane 4), but a kinase-inactive mutant (K277R) was unable to induce the phosphorylation of STRAP (lane 5). It is possible that the type I receptor was mediating the enhancement of STRAP phosphorylation in vivo and that in lane 4 the overexpressed type II receptor was increasing STRAP phosphorylation through low levels of endogenous type I receptor (21, 36). A further increase in the phosphorylation of STRAP in cells expressing both TβR-I and TβR-II was observed (lane 6), and the kinase activity of both was required for this increase (lane 7). Deletion of the C-terminal 57 amino acids of STRAP abolished both its basal and receptor-induced phosphorylation (lanes 8 and 9), indicating that the C terminus of STRAP is required for its phosphorylation. Double immunoprecipitation from [32P]orthophosphate-labeled cells with an anti-Flag antibody confirmed the identity of phosphorylated STRAP as the 40-kDa band (Fig. 8B). At least two other phosphoproteins were detected in the STRAP immunoprecipitates and not in the STRAP(1-294) immunoprecipitates. A low level of phosphorylation of STRAP was observed in R1B/L17 mink lung epithelial cells deficient in TβR-I. STRAP phosphorylation in these cells was stimulated when TβR-I was coexpressed with STRAP (data not shown). Finally, we evaluated whether STRAP phosphorylation could be regulated by TGF-β. We observed only a marginal increase in STRAP phosphorylation in transfected Mv1Lu cells when cells were stimulated by TGF-β (data not shown). These data suggest that the increase in STRAP phosphorylation in vivo may be mediated by either TGF-β receptors, a receptor-associated kinase, or a STRAP-associated kinase that is activated by TGF-β receptors in a multimeric complex.
FIG. 8.
The C terminus of STRAP is required for its TGF-β receptor-dependent phosphorylation. (A) Phosphorylation of STRAP through its C terminus. COS-1 cells were transiently transfected with STRAP-Flag or STRAP(1-294)-Flag in combination with wild-type (wt) or kinase-defective HA-tagged TβR-I and/or hexahistidine-tagged TβR-II as indicated. Cells were metabolically labeled with [32P]orthophosphate, and equal amounts of extracts were immunoprecipitated with an anti-Flag antibody. Phosphorylated STRAP was detected by SDS-PAGE and autoradiography (top). Equivalent levels of expression of STRAP-Flag and STRAP(1-294)-Flag proteins was confirmed by immunoblotting total cell lysates (middle). Phosphate incorporated into STRAP is plotted in relative units (bottom). The result is representative of five independent experiments. (B) Confirmation of the phosphorylated band as STRAP. The immunoprecipitate from lane 6 of panel A (lane 1) was boiled with Laemmli sample buffer to disrupt the complex and then was subjected to a second immunoprecipitation with anti-Flag antibody (lane 2).
DISCUSSION
TGF-β family members initiate their cellular actions by binding to a heteromeric complex of type I and type II serine/threonine kinase receptors. The multifunctional nature of this family of ligands clearly implies the need for tight control of their biological activity by positive and negative regulation of signaling (17, 22, 35). Smad proteins play a key role in mediating TGF-β signals at the intracellular level. R-Smads are activated by specific activated type I receptors and form heteromeric complexes with the common mediator Smad4. A distinct subfamily of Smads which function to directly inhibit TGF-β family signaling by preventing the formation of an active signal-transducing Smad complex has been identified. Smad7 has been shown to inhibit signaling from TGF-β, activin, and BMP by blocking the receptor-mediated activation of R-Smads (21, 44, 47). Therefore, a stable association between the receptor and Smad7 is critical for it to function as an inhibitor. A distinct mechanism of action for Smad6 in blocking BMP signals, in which Smad6 competes with Smad4 for binding to Smad1 and forms an inactive Smad6–Smad1 complex, has been reported (20). We have previously shown that the novel WD40 repeat protein STRAP associates with both TβR-I and TβR-II and has a role in TGF-β signaling (13). Here we have characterized the molecular mechanism by which STRAP inhibits the transcriptional responses mediated by TGF-β. We demonstrate a synergistic relationship between STRAP and Smad7, but not Smad6, in the inhibition of TGF-β-dependent transcription. A mutant of Smad7, Smad7-Δ408, that fails to associate with the type I receptor does not inhibit TGF-β signaling (21). STRAP does not show any cooperation with this mutant of Smad7, demonstrating the specificity in the synergy between Smad7 and STRAP in the inhibition of TGF-β signaling. Moreover, STRAP does not enhance the transactivating function of Smad2 and Smad3. STRAP forms a ternary complex with Smad7 and the type I receptor in response to TGF-β signaling, and the association between Smad7 and the activated type I receptor is stabilized by STRAP. These studies suggest a mechanism to explain how STRAP functions synergistically with Smad7 to block TGF-β-mediated transcriptional responses.
Functional synergy between STRAP and Smad7.
Smad7 has previously been shown to inhibit signal transduction downstream of TGF-β, activin, and BMP receptors (21, 47). Overexpression of STRAP alone has little effect on the repression of TGF-β-induced p3TP-Lux, (CAGA)9 MLP-Luc, pAR3-lux, and pGLuc 884 reporter activities. Coexpression of STRAP and Smad7 showed a synergistic relationship in the inhibition of these reporter activities in the presence of TGF-β signaling. The fold repression by STRAP and Smad7 acting together is greater than the sum or product of the fold repressions caused by proteins acting alone. Importantly, STRAP did not show any synergistic cooperation with a deletion mutant of Smad7, Smad7-Δ408, which has previously been shown not to inhibit TGF-β signaling (21). In contrast, STRAP was inactive in inhibitory cooperation with Smad6, an antagonist of BMP signaling. This is consistent with both a distinct mechanism of inhibition for Smad6 and its primary role in regulating BMP signals (20, 26). These results suggest that the synergistic relationship between STRAP and Smad7 is specific, and it is possible that STRAP can also inhibit BMP signaling in cooperation with Smad7.
WD40 repeat proteins appear to serve regulatory functions in various cellular processes including cell division, gene transcription, cell fate determination, signal transduction, mRNA modification, and vesicle fusion. These proteins are sometimes stabilized by forming intramolecular dimers or tetramers, and some WD40 repeat proteins require all repeats for their stability (45). We found that STRAP can homo-oligomerize, as assessed by coimmunoprecipitation analyses. Several mutants of STRAP were constructed by deleting one or two WD repeats with or without intervening regions from both the N terminus and C terminus. Two of them did not express the proteins, three mutants showed 10- to 15-fold less expression than the wild-type protein, and the C-terminal mutant, STRAP(1-294), having all the WD40 repeats intact, showed comparable expression of the protein (data not shown and Fig. 8A). These results suggest that all WD40 repeats of STRAP may participate in pairwise interactions within the molecule for its stability. This is consistent with the structure and stability of WD40 repeat proteins (45). STRAP(1-294) exhibited synergistic inhibition of the promoter activities in response to TGF-β signaling, thus having an effect resembling the effect of full-length STRAP protein. These findings suggest that the functional cooperation between STRAP and Smad7 could be an important mechanism for controlling the activity of TGF-β.
STRAP stabilizes the complex between Smad7 and type I receptor: a mechanism for the synergy between STRAP and Smad7.
Our results demonstrate that STRAP synergizes with Smad7 and not with Smad6 or a mutant of Smad7. Previous studies have shown that Smad7 functions as an inhibitor at a very early step in the TGF-β signaling by associating stably with activated type I receptor to block the interaction and subsequent activation of Smad2 and Smad3 (21, 44). There could be several possible mechanisms by which such synergy between STRAP and Smad7 might be achieved. STRAP might bind with Smad7 and recruit it to the receptor to form an inhibitory complex, STRAP might stabilize the association of Smad7 with the receptor, or STRAP and Smad7 might act synergistically without physical interactions.
Many WD40 repeat proteins form multiprotein complexes, sometimes interacting with other proteins through the WD40 repeat region. Such proteins present a changeable surface for protein-protein interaction and are capable of protein-induced conformational changes. Interaction of WD40 repeat proteins with partner proteins may require residues that are distributed along the length of the protein but that may come close together in the folded protein, as described previously for Tup-1 and the Gβ subunit (33, 38). We observed that STRAP associates with Smad7 and not with the mutant of Smad7 Smad7-Δ408, which is consistent with the functional cooperation of STRAP with Smad7, and not with the mutant, in transcriptional repression. This is expected because this mutation in Smad7 interferes with receptor binding and disrupts its inhibitory activity. STRAP(1-294) can also associate with Smad7 and shows synergistic inhibition. However, it is possible that other regions of Smad7, including the C terminus, or the overall three-dimensional structure of this protein might be required for binding with STRAP. In contrast, STRAP also binds with Smad6 but shows no cooperation with Smad6 in transcriptional repression of TGF-β-responsive reporters. These observations suggest that direct protein-protein interaction is required for, but is not the only possible explanation for, the observed functional cooperation between STRAP and Smad7.
STRAP forms a ternary complex with Smad7 and the type I receptor in the presence of TGF-β signaling. This suggests that the binding of Smad7 to the receptor occurs cooperatively with STRAP. In the absence of ligand, Smad7 is found to be predominantly localized in the nucleus and accumulates in cytoplasm upon TGF-β receptor activation (27). Thus, it is likely that STRAP recruits Smad7 from the cytosol to facilitate its association with the activated receptor complex. Furthermore, STRAP stabilizes the interaction between Smad7 and the activated type I receptor in a dose-dependent manner. Similarly, Smad7 can also enhance the binding between STRAP and the receptor (data not shown). This is supported by the observation that Smad7 strongly induces receptor-mediated phosphorylation of STRAP in vivo (data not shown). Our studies indicate that, by interacting with the receptor complex and Smad7, STRAP recruits Smad7 to the activated receptor to form a complex and stabilizes Smad7–receptor complexes, which is critical for Smad7 to prevent access of Smad2 and Smad3 to the receptor. This could be a mechanism to explain how STRAP synergizes with Smad7 to block TGF-β-mediated transcriptional responses. As the numbers of serine/threonine kinase receptors per cell are low depending on the cell type and as only a small fraction must be activated for biological responses (16), facilitating interactions between the receptor complex and Smad7 may be critical in vivo. Many WD40 repeat proteins are involved in signal transduction, such as the β-subunit of heterotrimeric G proteins, RACK1, FAN, PLAP, the Bα subunit of protein phosphatase 2A, and TRIP-1 (6, 19, 32). Sometimes these proteins help to assemble the macromolecular complexes necessary for signaling, as shown for the Gβ subunit (11). Analogous to the recruitment of signaling components to receptor tyrosine kinases, STRAP may be involved generally in recruiting downstream regulatory molecules to receptor serine/threonine kinases.
STRAP does not potentiate the Smad2- or Smad3-dependent transcriptional responses.
We observed that STRAP stably associates with Smad7. In contrast, STRAP did not show any interaction with either Smad2 or Smad3 when expressed at low levels. With elevated levels of Smad2 or Smad3 expression, STRAP showed some interaction with these proteins. This weak interaction between STRAP and Smad2 or Smad3 raises the possibility of influencing the signaling function of these Smads. But it is clear from Fig. 6 that STRAP does not functionally cooperate with Smad2 or Smad3 in the induction of TGF-β-responsive reporters. In contrast, STRAP shows moderate inhibition of TGF-β-induced and Smad-dependent transcriptional activation. Our results suggest that, unlike Smad2, Smad3 has the ability to strongly reverse the inhibition of the TGF-β-mediated transactivation of the reporter by either Smad7 alone or STRAP and Smad7 together, and this may be due to the strong transactivating properties of Smad3 (14, 53). Therefore, STRAP cooperates functionally with Smad7 in the inhibition of TGF-β-mediated transcription but does not cooperate with Smad2 or Smad3 to enhance their transcriptional activation activity. However, we do not rule out the possibility that STRAP might have an effect on other biological functions of Smad2 and Smad3.
C terminus of STRAP is required for its phosphorylation in vivo.
The physical interaction of STRAP with the receptor complex raises the possibility that STRAP is a substrate of the receptors. Our findings show that an increase in the phosphorylation of STRAP requires the kinase activity of receptors in vivo, but STRAP does not appear to be a direct substrate of the receptors in in vitro kinase assays (data not shown). It is possible that only TβR-I is capable of enhancing this phosphorylation in vivo and that the increase in STRAP phosphorylation by TβR-II is through activation of the endogenous type I receptor. Smad7 alone has little effect on the phosphorylation of STRAP, but it can strongly stimulate this receptor-mediated phosphorylation, perhaps by stabilizing the complex between the receptors and STRAP (data not shown). The C terminus of STRAP is required for its phosphorylation and for binding with other phosphoproteins, suggesting the presence of an alternate kinase that might phosphorylate STRAP. STRAP(1-294) interacts with Smad7 and synergizes with it in the inhibition of TGF-β signaling. Thus, the phosphorylation of STRAP is dispensable for this function. These data suggest that the increase in STRAP phosphorylation may be mediated by either TGF-β receptors indirectly in vivo, receptor associated kinases, or STRAP-associated kinases that are activated by TGF-β receptors in a multimeric complex involving Smad7. Future studies will investigate the involvement of STRAP phosphorylation in other TGF-β-mediated responses.
Further functional implications.
STRAP synergizes with Smad7, and not with Smad6, for blocking the transcriptional responses initiated by TGF-β. This may be an important mechanism to maintain specificity and to suppress cross talk between signaling pathways. However, we do not rule out the possibility that this protein may function differently with Smad6 and that it may also cooperate with Smad7 for inhibiting activin and BMP signaling. Although STRAP is expressed in a wide variety of tissues and cell lines, its expression level varies significantly. It is possible that Smad7 requires STRAP for its natural inhibitory activity. Recently, Smad7 has been reported to be predominantly localized in the nucleus in the absence of ligand (27), but its nuclear functions are not known. It will be interesting to determine whether STRAP may also cooperate with Smad7 for accomplishing its putative nuclear functions. Furthermore, STRAP might also modulate the activity of the receptor complex by interacting with it. Alternatively, STRAP may form complexes with other components, known or as yet unidentified, of the TGF-β signaling pathway and may recruit them to the activated receptor complex. This scaffolding function of STRAP may play a critical role in regulating the biological functions of TGF-β.
ACKNOWLEDGMENTS
We thank J. Massagué, J. L. Wrana, L. Attisano, P. ten Dijke, M. Kawabata, J.-M. Gauthier, Douglas E. Vaughan, and Millennium Pharmaceuticals for their generous gifts of plasmids. We also thank Anna Chytil for technical assistance. We are grateful to Brian K. Law, Neil A. Bhowmick, and Peng Liang for critical reading of the manuscript.
This work was supported by National Institute of Health grant CA42572 and the Frances Williams Preston Laboratories of the T. J. Martell Foundation. Sequencing was carried out by the DNA Sequencing Shared Resource supported by P30 CA68485.
REFERENCES
- 1.Abdollah S, Macias-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana J L. TβRI phosphorylation of Smad2 on serine 465 and 467 is required for Smad2/Smad4 complex formation and signaling. J Biol Chem. 1997;272:27678–27685. doi: 10.1074/jbc.272.44.27678. [DOI] [PubMed] [Google Scholar]
- 2.Attisano L, Wrana J L, López-Casillas F, Massagué J. TGF-β receptors and actions. Biochim Biophys Acta. 1994;1222:71–80. doi: 10.1016/0167-4889(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 3.Barinaga M. New clues to how proteins link up to run the cell. Science. 1999;283:1247–1249. doi: 10.1126/science.283.5406.1247. [DOI] [PubMed] [Google Scholar]
- 4.Caŕcamo J, Weis F M B, Ventura F, Wieser R, Wrana J L, Attisano L, Massagué J. Type I receptors specify growth inhibitory and transcriptional responses to TGF-β and activin. Mol Cell Biol. 1994;14:3810–3821. doi: 10.1128/mcb.14.6.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen R-H, Derynck R. Homomeric interactions between type II transforming growth factor-β receptors. J Biol Chem. 1994;269:22868–22874. [PubMed] [Google Scholar]
- 6.Chen R-H, Miettinen P J, Maruoka E M, Choy L, Derynck R. The WD-domain protein that is associated with and phosphorylated by the type II TGF-β receptor. Nature. 1995;377:548–552. doi: 10.1038/377548a0. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Rubock M J, Whitman M. A transcriptional partner for MAD proteins in TGF-β signaling. Nature. 1996;383:691–696. doi: 10.1038/383691a0. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Bhushan A, Vale W. Smad8 mediates the signaling of the receptor serine kinase. Proc Natl Acad Sci USA. 1997;94:12938–12943. doi: 10.1073/pnas.94.24.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y-Q, Su M, Walia R R, Hao Q, Covington J W, Vaughan D E. Sp1 sites mediate activation of the plasminogen activator inhibitor-1 promoter by glucose in vascular smooth muscle cells. J Biol Chem. 1998;273:8225–8231. doi: 10.1074/jbc.273.14.8225. [DOI] [PubMed] [Google Scholar]
- 10.Choy L, Derynck R. The type II transforming growth factor (TGF)-β receptor-interacting protein TRIP-1 acts as a modulator of the TGF-β response. J Biol Chem. 1998;273:31455–31462. doi: 10.1074/jbc.273.47.31455. [DOI] [PubMed] [Google Scholar]
- 11.Clapham D E, Neer E J. New roles for G-protein βγ-dimers in transmembrane signaling. Nature. 1993;365:403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- 12.Datta P K, Bagchi S. Repression of the transforming growth factor β1 promoter by the adenovirus oncogene E1A: identification of a unique GC-rich sequence as a target for E1A repression. J Biol Chem. 1994;269:25392–25399. [PubMed] [Google Scholar]
- 13.Datta P K, Chytil A, Gorska A E, Moses H L. Identification of STRAP, a novel WD domain protein in transforming growth factor-β signaling. J Biol Chem. 1998;273:34671–34674. doi: 10.1074/jbc.273.52.34671. [DOI] [PubMed] [Google Scholar]
- 14.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier J-M. Direct binding of Smad3 and Smad4 to critical TGFβ-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derynck R, Feng X-H. TGF-β receptor signaling. Biochim Biophys Acta. 1997;1333:F105–F150. doi: 10.1016/s0304-419x(97)00017-6. [DOI] [PubMed] [Google Scholar]
- 16.Dyson S, Gurdon J B. The interpretation of position in a morphogen gradient as revealed by occupancy of activin receptors. Cell. 1998;93:557–568. doi: 10.1016/s0092-8674(00)81185-x. [DOI] [PubMed] [Google Scholar]
- 17.Engel M E, Datta P K, Moses H L. Signal transduction by transforming growth factor-β: a cooperative paradigm with extensive negative regulation. J Cell Biochem. 1998;30–31(Suppl.):111–122. [PubMed] [Google Scholar]
- 18.Feng X-H, Zhang Y, Wu R-Y, Derynck R. The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for Smad3 in TGF-β-induced transcriptional activation. Genes Dev. 1998;12:2153–2163. doi: 10.1101/gad.12.14.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griswold-Prenner I, Kamibayashi C, Maruoka E M, Mumby M C, Derynck R. Physical and functional interactions between type I transforming growth factor β receptors and Bα, a WD-40 subunit of phosphatase 2A. Mol Cell Biol. 1998;18:6595–6604. doi: 10.1128/mcb.18.11.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hata A, Lagna G, Massagué J, Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998;12:186–197. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi H, Abdollah S, Qiu Y, Cai J, Xu Y-Y, Grinnell B W, Richardson M A, Topper J N, Gimbrone M A, Jr, Wrana J L, Falb D. The MAD-related protein Smad7 associates with the TGF-β receptor and functions as an antagonist of TGFβ signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 22.Heldin C-H, Miyazono K, ten Dijke P. TGF-β signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 23.Henis Y I, Moustakas A, Lin H Y, Lodish H F. The types II and III transforming growth factor-β receptors form homo-oligomers. J Cell Biol. 1994;126:139–154. doi: 10.1083/jcb.126.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoodless P A, Haerry T, Abdollah S, Stapleton M, O'Connor M B, Attisano L, Wrana J L. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996;85:489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- 25.Imamura T, Takase M, Nishihara A, Oeda E, Hanai J-I, Kawabata M, Miyazono K. Smad6 inhibits signaling by the TGF-β superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- 26.Ishisak A, Yamato K, Hashimoto S, Nakao A, Tamaki K, Nonaka K, ten Dijke P, Sugino H, Nishihara T. Differential inhibition of Smad6 and Smad7 on bone morphogenetic protein- and activin-mediated growth arrest and apoptosis in B cells. J Biol Chem. 1999;274:13637–13642. doi: 10.1074/jbc.274.19.13637. [DOI] [PubMed] [Google Scholar]
- 27.Itoh S, Landstrom M, Hermansson A, Itoh F, Heldin C-H, Heldin N-E, ten Dijke P. Transforming growth factor β1 induces nuclear export of inhibitory Smad7. J Biol Chem. 1998;273:29195–29201. doi: 10.1074/jbc.273.44.29195. [DOI] [PubMed] [Google Scholar]
- 28.Janknecht R, Wells N J, Hunter T. TGF-β-stimulated cooperation of Smad proteins with the coactivators CRP/p300. Genes Dev. 1998;12:2114–2119. doi: 10.1101/gad.12.14.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawabata M, Inoue H, Hanyu A, Imamura T, Miyazono K. Smad proteins exist as monomers in vivo and undergo homo- and hetero-oligomerization upon activation by serine/threonine kinase receptors. EMBO J. 1998;17:4056–4065. doi: 10.1093/emboj/17.14.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawabata M, Imamura T, Miyazono K, Engel M E, Moses H L. Interaction of the transforming growth factor-β type I receptor with farnesyl-protein transferase-α. J Biol Chem. 1995;270:29628–29631. doi: 10.1074/jbc.270.50.29628. [DOI] [PubMed] [Google Scholar]
- 31.Keeton M R, Curriden S A, van Zonneveld A J, Loskutoff D J. Identification of regulatory sequences in the type I plasminogen activator inhibitor gene responsive to transforming growth factor β. J Biol Chem. 1991;266:23048–23052. [PubMed] [Google Scholar]
- 32.Klages S A, Adam D, Wiegmann K, Struve S, Kolanus W, Schneider-Mergener J, Kronke M. FAN, a novel WD-repeat protein, couples the p55 TNF-receptor to neutral sphingomyelinase. Cell. 1996;86:937–947. doi: 10.1016/s0092-8674(00)80169-5. [DOI] [PubMed] [Google Scholar]
- 33.Komachi K, Johnson A D. Residues in the WD repeats of Tup1 required for interaction with α2. Mol Cell Biol. 1997;17:6023–6028. doi: 10.1128/mcb.17.10.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kretzschmar M, Liu F, Hata A, Doody J, Massagué J. The TGF-family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 1997;11:984–995. doi: 10.1101/gad.11.8.984. [DOI] [PubMed] [Google Scholar]
- 35.Kretzschmar M, Massagué J. Smads: mediators and regulators of TGF-β signaling. Curr Opin Genet Dev. 1998;8:103–111. doi: 10.1016/s0959-437x(98)80069-5. [DOI] [PubMed] [Google Scholar]
- 36.Labbé E, Silvestri C, Hoodless P A, Wrana J L, Attisano L. Smad2 and Smad3 positively and negatively regulate TGFβ-dependent transcription through the forkhead DNA-binding protein FAST2. Mol Cell. 1998;2:109–120. doi: 10.1016/s1097-2765(00)80119-7. [DOI] [PubMed] [Google Scholar]
- 37.Lagna G, Hata A, Hemmati-Brivanlou A, Massagué J. Partnership between DPC4 and SMAD proteins in TGF-β signaling pathways. Nature. 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- 38.Lambright D G, Sondek J, Bohm A, Skiba N P, Hamm H E, Singler P B. The 2.0 Å crystal structure of a heterotrimeric G protein. Nature. 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 39.Liu F, Pouponnot C, Massagué J. Dual role of the Smad4/DPC4 tumor suppressor in TGF-β-inducible transcriptional complexes. Genes Dev. 1997;11:3157–3167. doi: 10.1101/gad.11.23.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Sun Y, Constantinescu S N, Karam E, Weinberg R A, Lodish H F. Transforming growth factor β-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc Natl Acad Sci USA. 1997;94:10669–10674. doi: 10.1073/pnas.94.20.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu P-J, Zhou X Z, Shen M, Lu K P. Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science. 1999;283:1325–1328. doi: 10.1126/science.283.5406.1325. [DOI] [PubMed] [Google Scholar]
- 42.Macias-Silva M, Abdollah S, Hoodless P A, Pirone R, Attisano L, Wrana J L. MADR2 is a substrate of the TGFβ receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- 43.Massagué J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 44.Nakao A, Afrakhte M, Morén A, Nakayama T, Christian J L, Heuchel R, Itoh S, Kawabata M, Heldin N-E, Heldin C-H, ten Dijke P. Identification of Smad7, a TGF-β-inducible antagonist of TGF-β signaling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 45.Neer E J, Schmidt C J, Nambudripad R, Smith T F. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371:297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- 46.Nishimura R, Kato Y, Chen D, Harris S E, Mundy G R, Yoneda T. Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J Biol Chem. 1998;273:1872–1879. doi: 10.1074/jbc.273.4.1872. [DOI] [PubMed] [Google Scholar]
- 47.Souchelnytskyi S, Nakayama T, Nakao A, Morén A, Heldin C-H, Christian J L, ten Dijke P. Physical and functional interaction of murine and Xenopus Smad7 with bone morphogenetic protein receptors and transforming growth factor-β receptors. J Biol Chem. 1998;273:25364–25370. doi: 10.1074/jbc.273.39.25364. [DOI] [PubMed] [Google Scholar]
- 48.Souchelnytskyi S, Tamaki K, Engstrom U, Wernstedt C, ten Dijke P, Heldin C-H. Phosphorylation of Ser465 and Ser467 in the C terminus of Smad2 mediates interaction with Smad4 and is required for transforming growth factor-β signaling. J Biol Chem. 1997;272:28107–28115. doi: 10.1074/jbc.272.44.28107. [DOI] [PubMed] [Google Scholar]
- 49.Wieser R, Wrana J L, Massagué J. GS domain mutations that constitutively activate TβR-I, the downstream signaling component in the TGF-β receptor complex. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamashita H, ten Dijke P, Frazén P, Miazono K, Heldin C-H. Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-β. J Biol Chem. 1994;269:20172–20178. [PubMed] [Google Scholar]
- 51.Zhang Y, Musci T, Derynck R. The tumor suppressor Smad4/DPC4 as a central mediator of Smad function. Curr Biol. 1997;7:270–276. doi: 10.1016/s0960-9822(06)00123-0. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Feng X-H, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-β-induced transcription. Nature. 1998;394:909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Feng X-H, Wu R-W, Derynck R. Receptor-associated Mad homologues synergize as effectors of the TGF-β response. Nature. 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]
- 54.Zhou S, Zawel L, Lengauer C, Kinzler K W, Vogelstein B. Characterization of human FAST-1, a TGFβ and activin signal transducer. Mol Cell. 1998;2:121–127. doi: 10.1016/s1097-2765(00)80120-3. [DOI] [PubMed] [Google Scholar]