Abstract
This study intended to record a species of feather mite, Neopteronyssus bilineatus Mironov, 2003, (Arachnida: Pteronyssidae), from a grey-capped pygmy woodpecker, Yungipicus canicapillus (Blyth, 1845), in the Republic of Korea. Mite samples were collected from the flight feathers of a woodpecker, preserved directly in 95% ethyl alcohol, and then observed by a light microscope after specimen preparation. Morphology of Neopteronyssus bilineatus is distinguished from other pici group species by opisthosoma part with 2 longitudinal bends, tarsal seta rIII 3 times longer than tarsus III in males, and 2 elongated hysteronotal plates extending beyond the level of setae e2 in females. In the present study, a species of feather mite, N. bilineatus, was newly recorded from Y. canicapillus in Korean fauna.
Keywords: Neopteronyssus bilineatus, grey-capped pygmy woodpecker, feather mite, Korea
Feather mites are generally known as avian commensals and parasites, which inhabit the feathers and/or skin of birds [1–3]. Feather mites usually exhibit a high level of host specificity and spend their entire life cycle in the avian host [2,4,5]. Taxonomically, they belong to the superfamilies Analgoidea and Pterolichoidea within the order Sarcoptiformes. More than 2,500 species have been reported worldwide [1]. In Korea, about 52 species of feather mites have been identified except those of the family Pyroglyphidae [6–24].
The genus Neopteronyssus Mironov, 2003 is one of about 23 genera belonging to the family Pteronyssidae Oudemans, 1941 that comprises 17 species, generally associated with woodpeckers of the family Picidae Leach, 1820 [23,25]. The genus Neopteronyssus is classified into 3 species groups, i.e., pici, picinus, and pycnospilus [25]. The differences of these 3 groups are the transventral sclerite of epiandrium and dorsobasal teeth of tarsus IV in males and the shape of hysteronotal shield in females [23,25,26]. In the pici group, 5 species, i.e., N. bilineatus Mironov, 2003; N. elongatus (Buchholz, 1869); N. koreanus Han et al. 2019; N. pici (Scopoli, 1763); N. yungipicinus (Mironov, 1987), have been reported in worldwide [23,25].
To date, 9 species of woodpecker in the family Picidae are known to inhabit Korea [27,28]. Among these woodpeckers, the grey-headed woodpecker, Picus canus, was reported as the host of N. koreanus in Korea [23]. Therefore, in the present study, we intended to record a species of feather mite, Neopteronyssus bilineatus (Arachnida: Pteronyssidae), from a grey-capped pygmy woodpecker, Yungipicus canicapillus (Blyth, 1845), in Korea.
The carcass of Y. canicapillus (WCC no. 20150389) was provided by the Wildlife Center of Chungbuk (WCC). This woodpecker was initially rescued in Heungdeok-gu (gu=Borough) (36°36′4.84″N, 127°28′44.64″E), Cheongju-si (si=City), Chungcheongbuk-do (do=Province), South Korea in July 2015, but died during the treatment. Mite samples were collected from its flight feathers, preserved directly in 95% ethyl alcohol, and then cleared in 10% lactic acid for a day. The cleared mite samples were mounted on microscope slides in PVA mounting medium (BioQuip, Rancho Dominguez, California, USA) and identified with a Leica DM2500 microscope (Wetzlar, Germany) equipped with DIC (differential interference contrast) optics and microscopic digital camera. Description of the species was given according to the recent format used for species of genus Neopteronyssus [23,25,26]. Morphological terminology and nomenclature of leg and idiosomal setae followed Gaud & Atyeo [29] and Norton [30]. All measurements were in micrometers (μm). The classification and scientific names of birds follow Gill et al. [31]. All examined specimens were deposited in the National Institute of Biological Resources (NIBR) with the specimen numbers NIBRIV 0001043151–0001043155.
Description of Neopteronyssus bilineatus Mironov, 2003
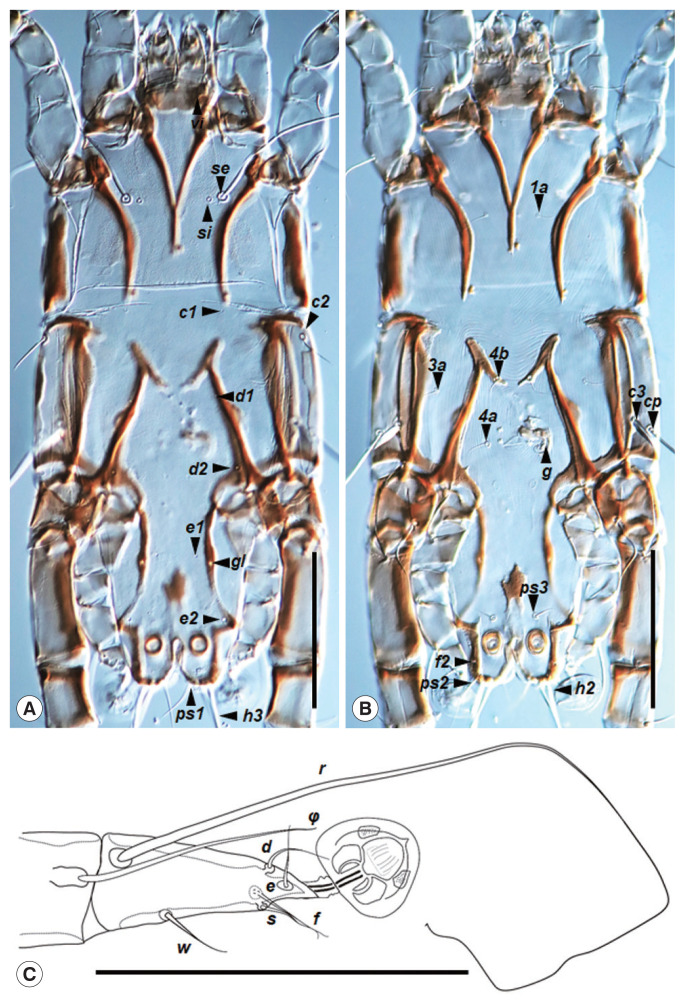
Male (n=2): idiosoma 435–448×200–215 (length×width) in size (Fig. 1A). Hysterosoma 270 in length. Prodorsal shield triangular-shape occupying entire prodorsum, weakly sclerotized, posterior margin straight, 139–150 in length along the midline, 145–150 in width of the posterior part (Fig. 2A). Setae c2 filiform, 29–31 in length, the distance between setae se 70–71. Subhumeral setae c3 lanceolate, 25–28×5.5–6. Distance between prodorsal and hysteronotal shields 1–6 in length along the midline. Hysteronotal shield anterior margin convex, weakly sclerotized, surface without ornamentation, greatest length 280–290, width at the level of anterior margin 150–155 (Fig. 2A). Bases between setae d2 and e2 with a pair of strongly sclerotized longitudinal sclerites, 80–87 in length. Opisthosomal lobes bluntly rounded; Setae h2 and h3 located on the posterior margin of these lobes. Setae ps2 situated posterior to setae f2; setae ps2 setiform. Dorsal measurements: c2: d2 100, d2: e2 110, d2: gl 37–67, e2: h3 48–49, d1: d2 52, e1: gl 5–6, h2: h2 46–49, h3: h3 35–36, ps1: ps1 19–22. Transventral sclerite absent, inner ends of epimerites IIIa shaped as an oblique T, epiandrum absent (Fig. 2B). Genital apparatus 24–28×13–18, with genital setae g at the midlevel of this apparatus. Setae 3a slightly located posterior to setae 4b. Adanal shield irregular form, with the anterior end of the anal opening. Diameter of anal suckers 18–19. Ventral measurements: 3a: 4a 43–50, ps3: ps3 30, ps3: h3 50. Tarsus III 60 in length; setae r 3–3.5 times longer than this segment (Fig. 2C). Tarsus IV with 2 acute dorsobasal teeth.
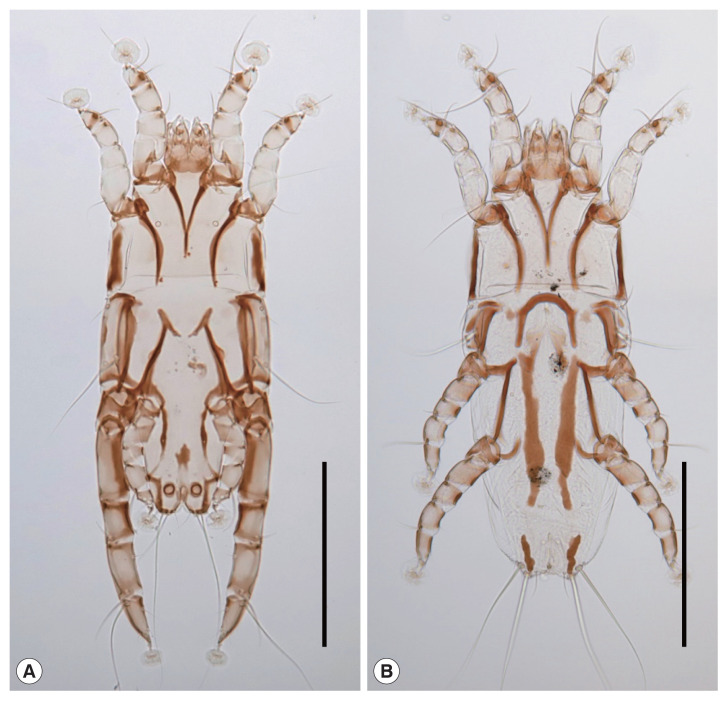
Fig. 1.
Neopteronyssus bilineatus recovered from a grey-capped pygmy woodpecker, Yungipicus canicapillus in Korea. (A) Male. (B) Female. Scale bars=0.2 mm.
Fig. 2.
Neopteronyssus bilineatus, male. (A) Dorsal view of idiosoma, opisthosoma part with 2 longitudinal bends. (B) Ventral view of idiosoma. (C) Tarsus III. Scale bar=0.1 mm.
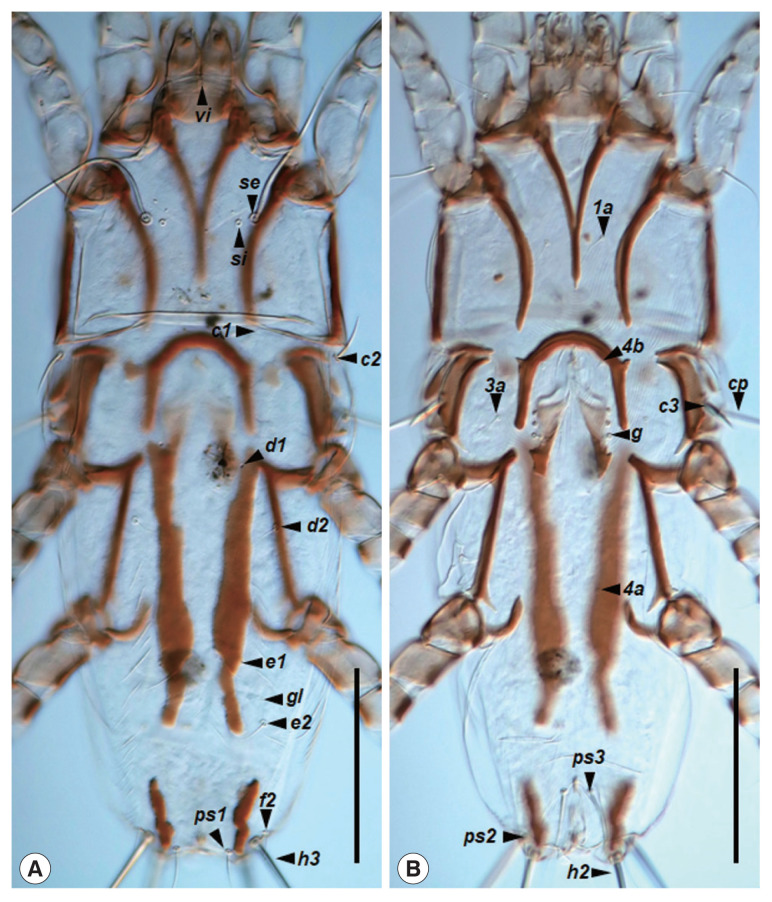
Female (n=3): idiosoma 490–510×195–208 (length×width) in size (Fig. 1B). Hysterosoma 320–355 in length. Prodorsal shield (Fig. 3A): Mostly shaped as in a male, length 150–155, width 145–158. Setae c2 filiform, 32–34 in length; subhumeral setae c3 lanceolate, 26–27×5–6. Paired anterior hysteronotal plates situated at the midlevel of hysterosoma, anterior margins extending to the level of setae c3, posterior margins extending beyond the level of setae e2, usually shaped as large longitudinal bends (severe variation in each individual), greatest length 150–190, greatest width 20–24 (Figs. 3A, 4); setae e1 lateral margins of this plates. Pygidial sclerites small longitudinal shaped, located near the bases of setae h2 and h3, greatest length 13–58, greatest width 8–14. External copulatory tube situated between setae h3, small finger-like. Dorsal measurements: c2: d2 103–113, d2: e2 113–138, d2: gl 82–120, e2: h3 81–90, d2: gl 82–120, h2: ps1 6–8, h2: h2 68–72, h3: h3 54–60. Epigynum semicircular, 63–65 long, 65–75 wide (Fig. 3B). Tarsi III and IV 31–33 and 35–36, respectively.
Fig. 3.
Neopteronyssus bilineatus, female. (A) Dorsal view of idiosoma, hysterosoma with 2 hysteronotal sclerotized sclerites. (B) Ventral view of idiosoma. Scale bars=0.1 mm.
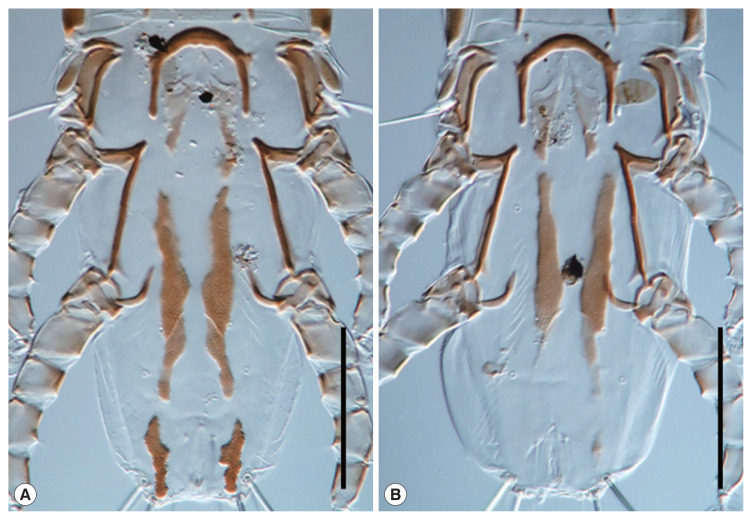
Fig. 4.
Variation of hysteronotal plates in females. (A) NIBRIV0001043154. (B) NIBRIV0001043155. Scale bars=0.1 mm.
Neopteronyssus bilineatus was originally described by Mironov [25] based on specimens collected from Y. canicapillus (=D. canicapillus) in Thailand. In genus Neopteronyssus, males of group pici have common characteristics of opisthosomal lobes small rounded, transventral sclerite and epiandrium absent, tarsi IV with 2 small dorsobasal teeth, and females have the same features as unpaired or paired hysteronotal shields situated at the midlevel of opisthosoma and not extending to poster end of the body [25]. Among 5 species in the pici group, N. bilineatus is most similar to N. koreanus (Han et al., 2019) and N. yungipicinus (Mironov, 1987) regarding the external traits. However, N. bilineatus clearly differs from N. koreanus and N. yungipicinus by the following features: in males, the dorsal surface of opisthosoma has longitudinal sclerites, and seta r of tarsus III is 3 times longer than this segment; in females, the anterior margins of hysteronotal sclerotized bands extend to the level of setae cp and the posterior margins of these sclerites extend beyond the level setae e2 [25]. In contrast, in males of N. yungipicinus, the posterior part of hysteronotal shield lacks ornamentation, and the length of seta rIII is 2.5 times shorter than the length of this tarsus; and in females, 2 elongated hysteronotal plates are situated between setae d2 and e2 [25,32]. In addition, hysteronotal shield in males of N. koreanus is weakly sclerotized without 2 longitudinal sclerites, and tarsal seta rIII is 1.5 times shorter than tarsus III; in females, pair of anterior hysteronotal plates are located at the level of setae d1 and usually shaped as irregular rectangles (in few specimens, these plates fused by thin sclerite) [23].
In general, N. bilineatus found in Korea were morphologically consistent with the original description and illustrations of Mironov [25]. However, the anterior margins of hysteronotal plates in Korean females did not stretch to the level of setae d1. Furthermore, hysteronotal plates of all Korean females are highly varied in length and shape. As the original description was based on 2 female samples, an explanation of individual variation was insufficient. Although females of N. koreanus, a closely related species, show variation in the anterior hysteronotal plates, there was no significant difference in the partial sequences of mitochondrial COI gene (cytochrome c oxidase subunit I) utilized for a DNA barcode [23]. Therefore, we considered this difference as an intraspecific variation in females of N. bilineatus. Accordingly, the hysteronotal plate length of female was not considered a suitable attribute to distinguish the species.
In this study, N. bilineatus was discovered for the first time in Korea. Although some species of feather mites in the genus Neopteronyssus share multiple hosts, most of them show host specificity [23,25]. Until now, N. bilineatus has been detected only in Y. canicapillus, distributed in Southeast Asia and Korea [33,34]. Therefore, identification of N. bilineatus was chiefly made by the morphological characteristics of mites, and the distribution of host and host specificity were additionally considered in this study.
Conclusively, it was confirmed for the first time that a species of feather mite, N. bilineatus, is indigenously infested in Y. canicapillus in Korea. Additional studies such as DNA barcoding and morphological comparison of specimens are needed to confirm the intraspecific variation of N. bilineatus from Southeast Asia.
ACKNOWLEDGMENT
The authors wish to thank Prof. Ki-Jeong Na, Dr. Youngsun Lee (Wildlife Center of Chungbuk) for sample collection, and Dr. Sergey V. Mironov (Zoological Institute, Russian Academy of Sciences, Russia) for advice and help in identifying the species.
Footnotes
The authors declare that they have no conflict of interest with this article.
REFERENCES
- 1.Mironov SV. On some problems in the systematics of feather mites. Acarina. 2003;11:3–29. [Google Scholar]
- 2.Proctor HC. Feather mites (Acari: Astigmata): ecology, behavior and evolution. Annu Rev Entomol. 2003;48:185–209. doi: 10.1146/annurev.ento.48.091801.112725. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor BM. Cohort Astigmatina. In: Krantz GW, Walter DE, editors. A manual of acarology. 3rd ed. Lubbock, Texas: Texas Tech University Press; 2009. pp. 565–657. [Google Scholar]
- 4.Dabert J, Mironov SV. Origin and evolution of feather mites (Astigmata) Exp Appl Acarol. 1999;23:437–454. doi: 10.1023/A:1006180705101. [DOI] [Google Scholar]
- 5.Mironov SV, Dabert J. Phylogeny and coevolutionary trends in feather mites of the subfamily Avenzoariinae (Analgoidea: Avenzoariidae) Exp Appl Acarol. 1999;23:525–549. doi: 10.1023/A:1006132806010. [DOI] [Google Scholar]
- 6.Atyeo WT, Gaud J. A new genus of feather mites near Proctophyllodes Robin, 1877 (Analgoidea: Proctophyllodidae) Georgia Entomol soc. 1971;6:43–50. [Google Scholar]
- 7.Peterson PC. The feather mite family Alloptidae Gaud III. The Echinacarinae, new subfamily (Acarina, Analgoidea) Steenstrupia. 1972;2:197–205. [Google Scholar]
- 8.Atyeo WT. Feather mites. In: McClure HE, Ratanaworabhan N, editors. Some Ectoparasites of the Birds of Asia. Bangkok, Thailand: Jintana Printing Ltd; 1973. pp. 54–78. [Google Scholar]
- 9.Atyeo WT, Peterson PC. The species of the feather mite family Rectijanuidae (Acarina: Analgoidea) [Duck parasites] J Georgia Entomol soc. 1976;2:349–366. [Google Scholar]
- 10.Santana FJ. A review of the genus Trouessartia (Analgoidea: Alloptidae) J Med Entomol Suppl. 1976;1:1–128. doi: 10.1093/jmedent/13.Suppl1.1. [DOI] [PubMed] [Google Scholar]
- 11.Hwang ID, Jong PC, Choi KS. A study on the feather mites (Analgesidae) in Korea. The Chonbuk University Medical Journal. 1986;10:11–21. (in Korean). [Google Scholar]
- 12.Sohn BO, Noh YT. Feather mites of Kramerellidae and Pterolichidae in Korea (Acari, Pterolichoidea) Korean J Parasitol. 1994;32:75–83. doi: 10.3347/kjp.1994.32.2.75. [DOI] [PubMed] [Google Scholar]
- 13.Sohn BO, Noh YT. Systematic studies of feather mites in Korea - Family Analgidae (Acari, Analgoidea) Korean J Entomol. 1994;24:81–91. (in Korean). [Google Scholar]
- 14.Sohn BO. Three new species of the feather mite genus Analges (Analgoidea: Analgidae) from passeriform birds from Korea. Int J Acarol. 1995;21:27–32. doi: 10.1080/01647959508684040. [DOI] [Google Scholar]
- 15.The Korean Society of Systematic Zoology . List of Animals in Korea (excluding insects) Seoul, Korea: Academy Press; 1997. pp. 158–159. [Google Scholar]
- 16.Han YD, Song JH, Min GS. New record of two feather mites (Acari: Saroptiformes: Astigmata) from Korea. J Species Res. 2016;5:324–332. doi: 10.12651/JSR.2016.5.3.324. [DOI] [Google Scholar]
- 17.Han YD, Min GS. New record of four Korean feather mites (Acari: Sarcoptiformes: Pterolichidae) isolated from the birds in the family Rallidae. J Species Res. 2017;6:152–163. doi: 10.12651/JSR.2017.6(S).152. [DOI] [Google Scholar]
- 18.Han YD, Choe S, Eom KS, Min GS. New record of two Korean feather mites (Acari: Sarcoptiformes: Astigmata) isolated from water birds. J Species Res. 2017;6:177–184. doi: 10.12651/JSR.2017.6(S).177. [DOI] [Google Scholar]
- 19.Han YD, Min GS. Three feather mites (Acari: Sarcoptiformes: Astigmata) isolated from Tringa glareola in South Korea. J Species Res. 2019;8:215–224. doi: 10.12651/JSR.2019.8.2.215. [DOI] [Google Scholar]
- 20.Han YD, Min GS. New record of two feather mites (Acari: Sarcoptiformes: Astigmata) isolated from Actitis hypoleucos in South Korea. J Species Res. 2019;8:225–232. doi: 10.12651/JSR.2019.8.2.225. [DOI] [Google Scholar]
- 21.Han YD, Min GS. Four unrecorded species of genus Alloptes (Acari: Sarcoptiformes: Alloptidae) from charadriiform birds in South Korea. Anim Syst Evol Divers. 2019;35:63–72. doi: 10.5635/ASED.2019.35.2.005. [DOI] [Google Scholar]
- 22.Han YD, Min GS. Three feather mites (Acari: Sarcoptiformes) isolated from black-tailed godwit, Limosa limosa in Korea. Anim Syst Evol Divers. 2019;35:105–113. doi: 10.5635/ASED.2019.35.3.006. [DOI] [Google Scholar]
- 23.Han YD, Mironov SV, Min GS. Two new feather mites (Acari: Analgoidea) isolated from the grey-headed woodpecker, Picus canus (Piciformes: Picidae) in Korea. Syst Appl Acarol. 2019;24:2167–2183. doi: 10.11158/saa.24.11.9. [DOI] [Google Scholar]
- 24.Mironov SV. A new species of the feather mite genus Analges Nitzsch, 1818 (Acariformes: Analgidae) from the streaked spiderhunter Arachnothera magna (Passeriformes: Nectariniidae), with a renewed diagnosis and world checklist to the genus. Acarina. 2019;27:19–43. doi: 10.21684/0132-8077-2019-27-1-19-43. [DOI] [Google Scholar]
- 25.Mironov SV. A review of feather mites of the genus Neopteronyssus (Astigmata Pteronyssidae) associated with woodpeckers (Piciformes: Picidae) of the Old World. Belgian J Entomol. 2003;5:37–77. [Google Scholar]
- 26.Mironov SV. Taxonomic corrections to the feather mite genera Pteronyssus Robin, 1877 and Parapteronyssus Faccini et Atyeo, 1981 (Analgoidea, Pteronyssidae) Acarina. 2002;10:137–147. [Google Scholar]
- 27.Lee WS, Koo TH, Park JY. A Field Guide to the Birds of Korea. 2nd ed. Seoul, Korea: LG Evergreen Foundation; 2015. [Google Scholar]
- 28.Park JG. Identification Guide to Birds of Korea. Seoul, Korea: Nature and Ecology Publication; 2014. p. 369. (in Korean). [Google Scholar]
- 29.Gaud J, Atyeo WT. Feather Mites of the World (Acarina, Astigmata): the Supraspecific Taxa. Belgium Musee Royal De L’Africque Central. 1996:1–193. [Google Scholar]
- 30.Norton RA. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes) Exp Appl Acarol. 1998;22:559–594. doi: 10.1023/A:1006135509248. [DOI] [Google Scholar]
- 31.Gill F, Donsker D, Rasmussen P. IOC World Bird List (v. 11. 2) International Ornithologists’ Union; 2021. [DOI] [Google Scholar]
- 32.Mironov SV. Three new feather mite species of the family Avenzoariidae (Sarcoptiformes: Analgoidea) Parazitologiya. 1987;21:528–536. [Google Scholar]
- 33.Bi D, Ding H, Wang Q, Jiang L, Lu W, Wu X, Zhu R, Zeng J, Zhou S, Yang X, Kan X. Two new mitogenomes of Picidae (Aves, Piciformes): Sequence, structure and phylogenetic analyses. Int J Biol Macromol. 2019;133:683–692. doi: 10.1016/j.ijbiomac.2019.04.157. [DOI] [PubMed] [Google Scholar]
- 34.Higuchi H. Colonization and coexistence of woodpeckers in the Japanese Islands. J Yamashina Inst Ornithol. 1980;12:139–156. doi: 10.3312/jyio1952.12.3_139. [DOI] [Google Scholar]