Abstract
Reversion mutations are associated with clinical resistance to poly(ADP-ribose) polymerase inhibitors (PARPi). Here, we describe the detection of a BRCA1 reversion mutation in a 39-year-old woman with metastatic breast cancer harboring a heterozygous germline BRCA1 exons 7–8 deletion who received PARPi olaparib combined with immune checkpoint inhibitor camrelizumab as third-line therapy. During progression from the olaparib and camrelizumab combination therapy, we identified via genomic sequencing a novel 7-base pair somatic deletion in BRCA1 (c.617_623delACAAATC). Sequence analyses indicated that this mutation realigned the reading frame of BRCA1, which potentially led to the reversal of its normal function and conferred resistance to PARPi.
Keywords: Breast neoplasms, Poly(ADP-ribose) polymerase inhibitors, High-throughput nucleotide sequencing, Circulating tumor DNA
INTRODUCTION
Germline mutations in the breast cancer susceptibility genes BRCA1 and BRCA2 are associated with an increased risk of developing breast cancer due to increased DNA damage and accumulation of mutations; however, they also impart sensitivity to platinum-based chemotherapy and poly(ADP-ribose) polymerase inhibitors (PARPi) [1]. Secondary somatic mutations in BRCA1/2, referred to as reversion mutations, are associated with acquired resistance to platinum and PARPi [2,3,4,5,6,7]. A majority of the reversion mutations emerging from patients who progressed on platinum or PARPi are unique 1–100 base pairs (bp) truncating mutations located downstream of the original pathogenic mutation, which realigns the gene sequence to its native reading frame and restores its normal protein expression and function [2,3,4,5,6,7].
In this report we describe the detection of a BRCA1 reversion mutation in a plasma sample from a patient with germline BRCA1 metastatic breast cancer after clinical progression from olaparib and camrelizumab therapy.
CASE REPORT
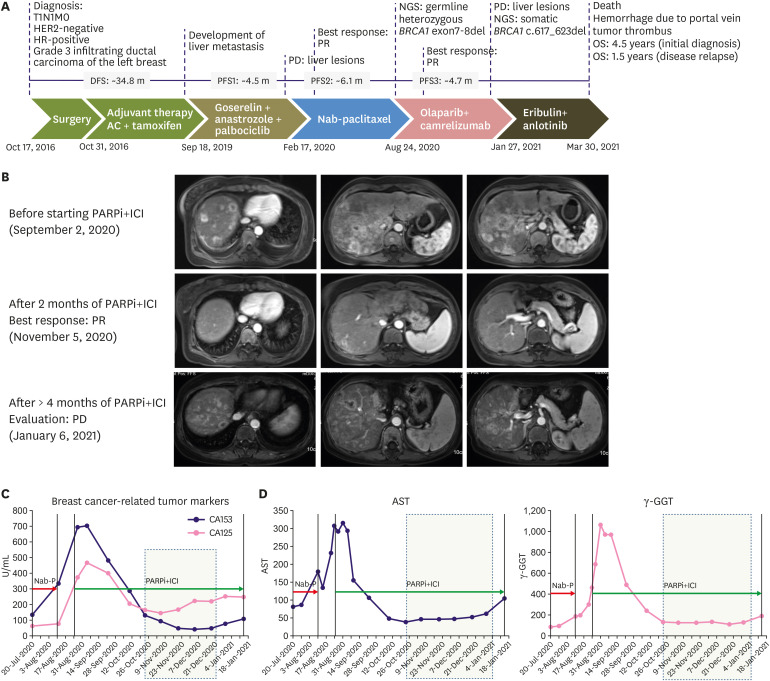
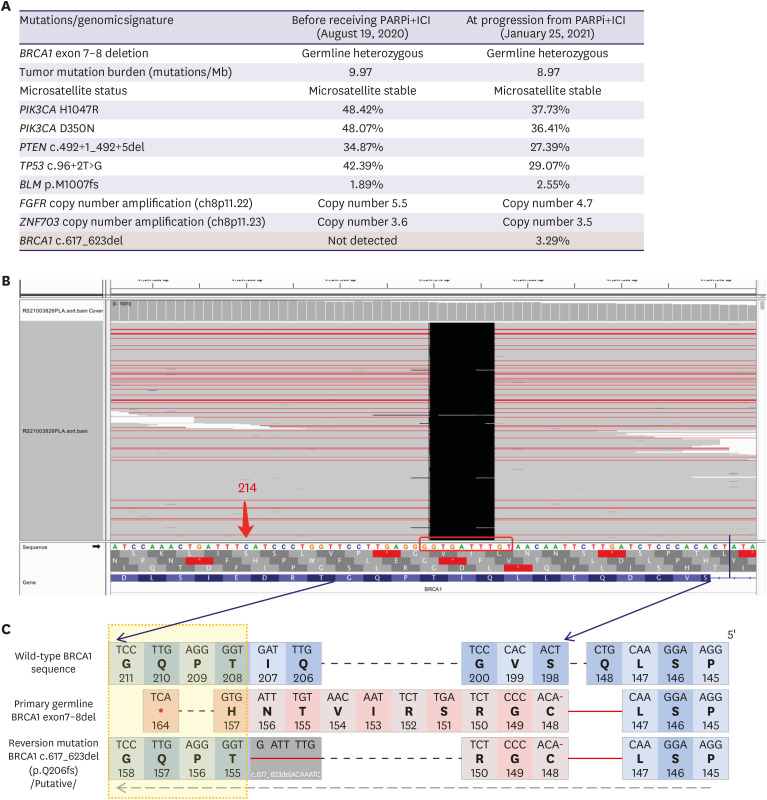
In October 2016, a 39-year-old woman was referred to our hospital because of a lump that had been present in her left breast for a year. She had undergone partial thyroidectomy for papillary thyroid carcinoma four years previously but denied any family history of cancer. Figure 1A summarizes the treatment history of our patient. On October 17, 2016, she underwent mastectomy of the left breast, in which a 2-cm nodule was removed, and sentinel lymph node biopsy was performed, as well as tissue expander installation. The four left axillary lymph nodes were negative for malignancy. The results of immunohistochemistry of surgical breast tissue samples were as follows: CerbB2 (1+), ER (2+, 70%), PR (3+, 90%), and Ki67 (1+, 30%). The patient was diagnosed with pathological stage I (T1N0M0), luminal B (human epidermal growth factor receptor 2 [HER2]-negative) subtype, and invasive ductal carcinoma (grade 3) of the breast. Adjuvant therapy consisted of 4 cycles of doxorubicin and cyclophosphamide (AC) regimen followed by tamoxifen (10 mg twice daily). In May 2017, the tissue expander was removed, and breast reconstruction was completed using the latissimus dorsi myocutaneous flap technique. The patient was followed up regularly. On September 11, 2019, after the patient had been disease-free for 34.8 months, ultrasonography revealed multiple nodules in the liver; these were confirmed via computed tomography scanning as three nodules with a maximum diameter of 1.2 cm. Ultrasound-guided needle biopsy of the liver mass revealed HER2-negative, hormone receptor (HR)-positive, invasive adenocarcinoma. The first-line regimen comprised goserelin (3.6 mg every 4 weeks), anastrozole (1 mg once daily [QD]), and palbociclib (125 mg QD, 3 weeks on/1 week off) for 4.5 months until progressive disease (PD) was evaluated for the presence of multiple enlarged liver lesions by computed tomography. The patient then received nanoparticle albumin-bound paclitaxel monotherapy (200 mg on days 1, 8, and 15 every 4 weeks), achieving partial response lasting for 6.1 months (Figure 1B, top). Genomic profiling of her blood samples at PD stage was performed using a commercial 520-gene panel and sequenced in a paired-end system, which achieved an actual coverage of 95% and an actual average sequencing depth of 18,209× (OncoScreen Plus; Burning Rock Biotech, Guangzhou, China). Analysis of the genomic profiling results identified the heterozygous germline BRCA1 deletion of exons 7–8 and various somatic mutations, including PIK3CA H1047R and D350N (Figure 2A). On August 24, 2020, camrelizumab (200 mg intravenously every 2 weeks) combined with olaparib (150 mg orally twice daily) was administered. Treatment-related toxicities, including grade 3 leukopenia, grade 2 anemia, and grade 2 skin rashes were observed and managed by decreasing one dose level of olaparib. Abdominal magnetic resonance imaging revealed shrinkage of liver lesions within 2.4 months of combination therapy, evaluated as partial response (Figure 1B, middle). The dynamics of the blood tumor markers CA125 and CA153 were also associated with the therapeutic response (Figure 1C). Liver chemistries measured using serum aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT) also showed near normal levels, indicating disease control (Figure 1D). After 4.7 months of response, the liver lesions progressed again (Figure 1B, bottom). Genomic profiling of blood samples at PD stage using the OncoScreen Plus panel (actual coverage of 94% and actual average sequencing depth of 14,496×) identified a new 7-bp somatic deletion in BRCA1 (c.617_623delACAAATC) (Figure 2A and B). Sequence alignment revealed that BRCA1 c.617_623del induced realignment of the reading frame starting at position T155 (Figure 2C). The patient then received a combination of eribulin (2 mg on days 1 and 8) and anlotinib (12 mg orally on day 1–14) as a fourth-line regimen. She died on March 30, 2021, due to hemorrhage caused by portal vein tumor thrombus with an overall survival of 4.5 years from the initial diagnosis and 1.5 years from disease relapse.
Figure 1. Metastatic liver lesions responded to olaparib and camrelizumab combination therapy (PARPi+ICI). (A) Diagram summarizing the various treatment modalities received by our patient. (B) Abdominal magnetic resonance imaging scans of the liver lesions before, during, and at clinical progression with PARPi+ICI therapy. (C-D) Longitudinal monitoring of serum levels of breast cancer-related tumor biomarkers CA125 and CA153 (C) and liver function biomarkers AST and GGT (D) from the start of PARPi+ICI therapy until disease progression.
PARPi = poly(ADP-ribose) polymerase inhibitors; ICI = immune checkpoint inhibitors; AST = aspartate aminotransferase; GGT = gamma-glutamyl transferase.
Figure 2. Reversion mutation BRCA1 c.617_623del mediates resistance to olaparib and camrelizumab combination therapy (PARPi+ICI). (A) Tabulated summary of the mutations and genomic signatures (such as tumor mutation burden and microsatellite status) detected from blood samples before receiving and after progression from PARPi+ICI. (B) Screenshot of integrated genome viewer showing the sequencing reads for BRCA1 c.617_623delACAAATC detected at progression from PARPi+ICI. (C) Diagram showing the amino acid sequences corresponding to wild-type BRCA1, the germline BRCA1 exon 7-8 deletion, and the BRCA1 c.617_623delACAAATC.
PARPi = poly(ADP-ribose) polymerase inhibitors; ICI = immune checkpoint inhibitors.
DISCUSSION
Germline BRCA1 mutations are detected in 1%–5% of all breast cancers [1]. Consistent with olaparib monotherapy [8], early clinical trials have demonstrated promising antitumor activity and safety of the combination of PARPi and immune checkpoint inhibitors (ICI) in patients with germline BRCA1/2 metastatic breast cancer [9,10]. Despite an initial response to systemic PARPi or platinum chemotherapy, reversion and non-reversion mechanisms of acquired resistance would result in disease progression [2,3,4,5,6,7].
Our case report describes the detection of a 7-bp deletion in BRCA1 from plasma circulating tumor DNA, which mediated the clinical resistance of our patient to olaparib and camrelizumab therapy. The germline heterozygous BRCA1 deletion of exons 7–8 harbored by our patient resulted in a frameshift and potentially yielded a truncated non-functional protein and a deficiency of its function, which conferred sensitivity to olaparib. Our patient clinically benefited from olaparib and camrelizumab therapy, as shown by the dramatic shrinkage of the hepatic lesions as well as the near-normal serum levels of tumor markers and liver function biomarkers. The treatment-related adverse events observed in our patient were consistent with the reported toxicities for PARPi and ICI as monotherapy or in combination [9,10]. Despite the additional ICI therapy, the clinical benefit to our patient was not sustained, possibly due to the 7-bp BRCA1 reversion mutation. The selective pressure imposed by olaparib potentially compelled the hepatic lesions to develop a 7-bp deletion upstream of critical functional domains in the C-terminal region of BRCA1. This small deletion resulted in the reversion of the germline out-of-frame BRCA1 sequence to its correct reading frame, which presumably enabled BRCA1 to regain its normal function, leading to clinical olaparib resistance and subsequent disease progression. We speculate that the reversion mutation that led to a restoration of BRCA1 function and the homologous recombination repair pathway could also contribute to the resistance to ICI by mediating DNA repair; however, because a combination therapy was administered to our patient, we could not identify the specific therapeutic agent that elicited the response or resistance. Notably, the reversion mutation we detected was also a unique short deletion—a 2-nucleotide (AC) flanking microhomology site—located in the N-terminal, and 3′ from the original germline mutation, and this description is consistent with the features of reversion mutations described in previous reports [5]. Reversion mutations emerging from patients who progressed on platinum or PARPi were more commonly unique pathogenic mutations (68/91, 75%), predominantly deletions between 1–100 bp, containing short microhomology sites, and arise at the 3′ flanking sequence of the original pathogenic mutation [5]. The acquisition of BRCA2 reversion mutations was demonstrated by in vitro experiments to restore their normal interaction with other proteins such as PALB2 and RAD51, similar to wild-type BRCA2-expressing cells, which were absent in the cells expressing the BRCA2 pathogenic mutation [11].
Our patient was also diagnosed with somatic PIK3CA H1047R, which is the most commonly reported PIK3CA mutation among Chinese patients with breast cancer [12]. PIK3CA mutations are associated with poor outcomes in endocrine therapy, HER2 targeted therapy, and chemotherapy [13]. Despite the selection of treatment strategies that are shown by various clinical trials to prolong progression-free survival [8,9,10,14,15,16], our patient had a non-sustained response to most therapeutic regimens, possibly due to the activation of the PI3K pathway.
Our study is limited by the lack of transcriptomic analysis of the patient sample or in vitro functional assay to elucidate the mechanisms by which the 7-bp deletion could mediate PARPi resistance. Due to the low abundance of the BRCA1 7-bp deletion reversion mutation (3.29%), we could not prove whether this somatic mutation was treatment-emergent or was undetected at baseline due to its ultra-low abundance. It is possible that there were other resistance mechanisms or other mutations that were not covered by the 520-gene panel that we used for mutational profiling. However, based on the fact that BRCA1/2 reversion mutations are reported to emerge during PARPi/platinum-based chemotherapy and are implicated in mediating disease progression, we are associating the BRCA1 7-bp reversion mutation detected in plasma samples collected during progression from olaparib and camrelizumab therapy to the therapeutic resistance and disease progression in our patient.
In conclusion, our report provides clinical evidence that a unique BRCA1 reversion mutation detected in plasma circulating tumor DNA is involved in the acquired resistance to PARPi and ICI combination therapy in a patient with metastatic breast cancer harboring a germline heterozygous BRCA1 mutation. Our report also highlights the importance of genomic sequencing in understanding breast cancer biology in order to guide therapeutic decisions and elucidate the molecular resistance mechanism.
ACKNOWLEDGMENTS
We thank the family of our patient for their cooperation. We also thank Drs. Analyn Lizaso, Songan Chen, Jin Niu, and Jianxing Xiang of Burning Rock Biotech for their support.
Footnotes
Funding: This work was funded by grants from the Key Research-Development Program of Zhejiang Province (grant numbers: 2019C04001 to WMC and 2020C04012 to XJW) and the Natural Science Foundation of Zhejiang Province (grant number: LY21H160005 to WMC). The funders had no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Pan JN, Cao WM.
- Data curation: Pan JN, Lei L, Ye WW, Wang XJ, Cao WM.
- Formal analysis: Pan JN, Lei L, Ye WW, Wang XJ, Cao WM.
- Funding acquisition: Wang XJ, Cao WM.
- Investigation: Pan JN, Lei L, Ye WW, Wang XJ, Cao WM.
- Methodology: Lei L, Ye WW, Wang XJ, Cao WM.
- Project administration: Cao Wm.
- Supervision: Cao WM.
- Validation: Wang XJ.
- Visualization: Wang XJ.
- Writing - original draft: Pan JN, Cao WM.
- Writing - review & editing: Pan JN, Lei L, Ye WW, Wang XJ, Cao WM.
References
- 1.Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12:68–78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakai W, Swisher EM, Karlan BY, Agarwal MK, Higgins J, Friedman C, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, et al. Resistance to therapy caused by intragenic deletion in BRCA2 . Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Bernhardy AJ, Cruz C, Krais JJ, Nacson J, Nicolas E, et al. The BRCA1-Δ11q alternative splice isoform bypasses germline mutations and promotes therapeutic resistance to PARP inhibition and cisplatin. Cancer Res. 2016;76:2778–2790. doi: 10.1158/0008-5472.CAN-16-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pettitt SJ, Frankum JR, Punta M, Lise S, Alexander J, Chen Y, et al. Clinical BRCA1/2 reversion analysis identifies hotspot mutations and predicted neoantigens associated with therapy resistance. Cancer Discov. 2020;10:1475–1488. doi: 10.1158/2159-8290.CD-19-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobalina L, Armenia J, Irving E, O'Connor MJ, Forment JV. A meta-analysis of reversion mutations in BRCA genes identifies signatures of DNA end-joining repair mechanisms driving therapy resistance. Ann Oncol. 2021;32:103–112. doi: 10.1016/j.annonc.2020.10.470. [DOI] [PubMed] [Google Scholar]
- 7.Waks AG, Cohen O, Kochupurakkal B, Kim D, Dunn CE, Buendia Buendia J, et al. Reversion and non-reversion mechanisms of resistance to PARP inhibitor or platinum chemotherapy in BRCA1/2-mutant metastatic breast cancer. Ann Oncol. 2020;31:590–598. doi: 10.1016/j.annonc.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 9.Domchek SM, Postel-Vinay S, Im SA, Park YH, Delord JP, Italiano A, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020;21:1155–1164. doi: 10.1016/S1470-2045(20)30324-7. [DOI] [PubMed] [Google Scholar]
- 10.Vinayak S, Tolaney SM, Schwartzberg L, Mita M, McCann G, Tan AR, et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. 2019;5:1132–1140. doi: 10.1001/jamaoncol.2019.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weigelt B, Comino-Méndez I, de Bruijn I, Tian L, Meisel JL, García-Murillas I, et al. Diverse BRCA1 and BRCA2 reversion mutations in circulating cell-free DNA of therapy-resistant breast or ovarian cancer. Clin Cancer Res. 2017;23:6708–6720. doi: 10.1158/1078-0432.CCR-17-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao Z, Li T, Feng Z, Liu C, Shao Y, Zhu M, et al. Characterizations of cancer gene mutations in chinese metastatic breast cancer patients. Front Oncol. 2020;10:1023. doi: 10.3389/fonc.2020.01023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartzberg LS, Vidal GA. Targeting PIK3CA alterations in hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: new therapeutic approaches and practical considerations. Clin Breast Cancer. 2020;20:e439–e449. doi: 10.1016/j.clbc.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 15.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 16.Gradishar WJ, Krasnojon D, Cheporov S, Makhson AN, Manikhas GM, Clawson A, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009;27:3611–3619. doi: 10.1200/JCO.2008.18.5397. [DOI] [PubMed] [Google Scholar]