Abstract
We report a patient with severe neurological deterioration due to leptomeningeal metastases involving brain and spinal cord from anaplastic lymphoma kinase (ALK)-positive lung adenocarcinoma, managed rapidly and successfully with lorlatinib therapy. A 48-year-old male patient presented with acute mental deterioration, severe headache, and weakness of both legs. The patient’s previous medical history included cerebral metastases from ALK-positive lung adenocarcinoma, which had been successfully managed via whole brain radiation therapy and gamma knife radiosurgery one year and three months before, respectively. Physical examination revealed neck stiffness and paraparesis with motor grade I. Gadolinium-enhanced brain MRI showed newly developed leptomeningeal enhancement along cerebellar folia, and whole spine MRI revealed similar leptomeningeal metastasis along the whole spinal axis. Lorlatinib was started orally with a dose of 100 mg/day. The patient showed rapid clinical improvement after one week. The patient was alert and the headache disappeared, while the paraparesis improved to normal ambulatory status. Two months of lorlatinib treatment resulted in almost complete disappearance of previous leptomeningeal enhancement of brain and spinal cord, and absence of newly developed metastatic lesions in the central nervous system, based on MRI results. The patient had been regularly followed with ongoing lorlatinib therapy for 5 months without any systemic complications or neurological abnormality. Conclusively, lorlatinib could be a rapid and effective treatment for patients with central nervous system leptomeningeal metastases arising from ALK-positive lung cancer.
Keywords: Anaplastic lymphoma kinase, Brain metastases, Leptomeningeal carcinomatosis, Lorlatinib, Magnetic resonance imaging
INTRODUCTION
Anaplastic lymphoma kinase (ALK) gene rearrangement occurs in a small group of patients with non-small cell lung cancer (NSCLC) [1]. Recently, treatment with tyrosine kinase inhibitors (TKIs) significantly improved the management of ALK-rearranged NSCLC, and a new generation of ALK-TKIs has been continuously introduced [1]. Lorlatinib is a third generation of ALK/ROS1TKI and intracranial response is rapid and effective [1].
Leptomeningeal metastasis occurs in 3% to 5% of patients with advanced NCSLC [2]. It is a serious complication of advanced disease, and the prognosis of patients with leptomeningeal metastasis is very poor [2].
We report a patient with severe neurological deterioration due to leptomeningeal metastases involving both brain and spinal cord, managed rapidly and successfully via lorlatinib therapy.
CASE REPORT
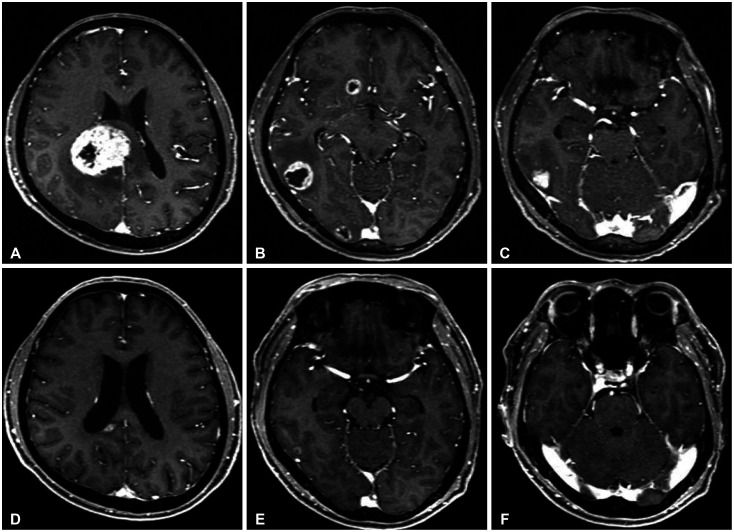
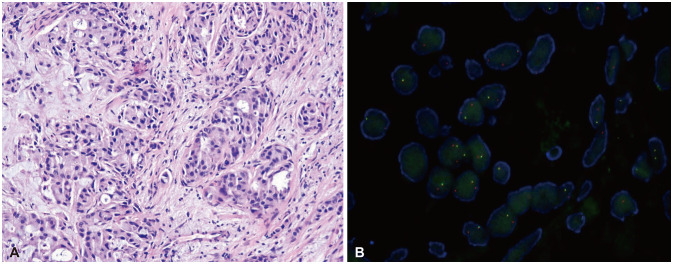
A 48-year-old male patient was diagnosed with stage IV NSCLC. The F-18 FDG PET scan showed pulmonary and pleural lesions with high 18F-FDG-uptake and gadolinium (Gd)-enhanced brain MRI showed numerous synchronous brain metastases. (Fig. 1A-C). Histopathological diagnosis of the lung mass revealed a well-differentiated adenocarcinoma with bronchoscopic biopsy (Fig. 2A). Additional molecular analysis revealed an ALK rearrangement (80%) confirmed via fluorescent in situ hybridization (Fig. 2B) and immunohistochemistry.
Fig. 1. Gadolinium-enhanced MRIs show severe brain metastases (A-C), which were well controlled (D-F) after two months of alectinib therapy and whole brain radiation therapy.
Fig. 2. The cancer cells show infiltrative atypical glands with mucinous differentiation (H&E, ×100) (A), and anaplastic lymphoma kinase fluorescence in situ hybridization shows many isolated red signals and spilt signals (×200) (B).
Initial management entailed pericardiostomy due to pericardial effusion and metastasis. Whole brain radiation therapy was also performed using a dose of 3,000 cGy in 10 fractions. Alectinib was started with a dose of 1,200 mg/day. After 3 months, the brain MRI showed dramatically improved previous metastases (Fig. 1D-F), and chest CT revealed improved primary cancer. Alectinib therapy was continued for one year and both primary cancer and brain metastases were stable during the treatment. However, after one year, locally developed brain recurrence was detected via serial brain MRI and the patient underwent gamma knife radiosurgery (GKRS). At that time, primary cancer showed recurrence, prompting radiation therapy. The systemic treatment was switched to doxorubicin/cisplatin (AP) chemotherapy from alectinib regimen.
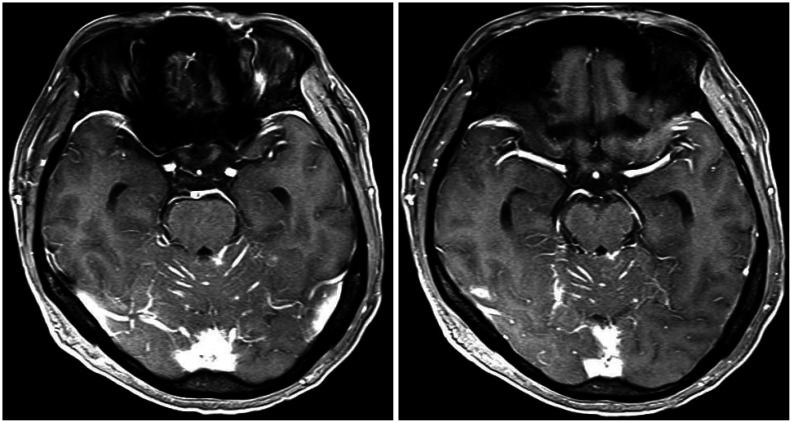
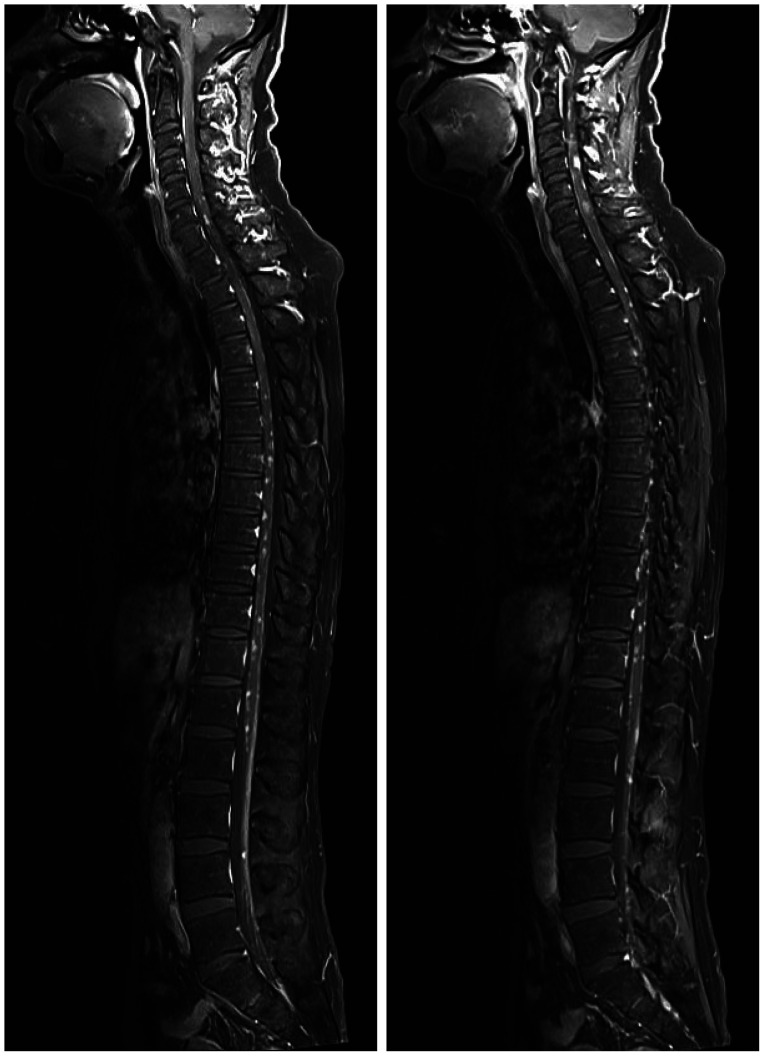
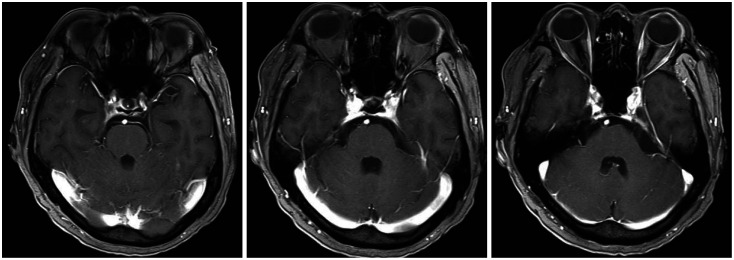
Three months after the GKRS, the patient visited the emergency room due to severe headache and drowsiness. He also presented with paraparesis grade I and severe, radiating pain to both legs. Brain MRI revealed newly developed leptomeningeal metastasis, especially in the infratentorial lesion (Fig. 3), although the brain metastases managed by previous GKRS were all under control. The spine MRIs showed multiple intramedullary and leptomeningeal metastases along the whole spinal axis (Fig. 4). Considering both the progression of the primary cancer in the chest CT and the newly developed brain and spinal metastasis, lorlatinib was initiated with a dose of 100 mg/day and radiation therapy (RT) for whole spine started with a dose of 3,000 cGy by 15 fractions. The medication was administered via Levin tube for two weeks and continued orally later. The spine RT was stopped after 5 fractions because of the patients’ drowsiness and re-started after substantial recovery of patients’ mental state and paraparesis two weeks after the start of lorlatinib medication.
Fig. 3. Gadolinium-enhanced brain MRIs show newly developed leptomeningeal enhancement along both cerebellar folia.
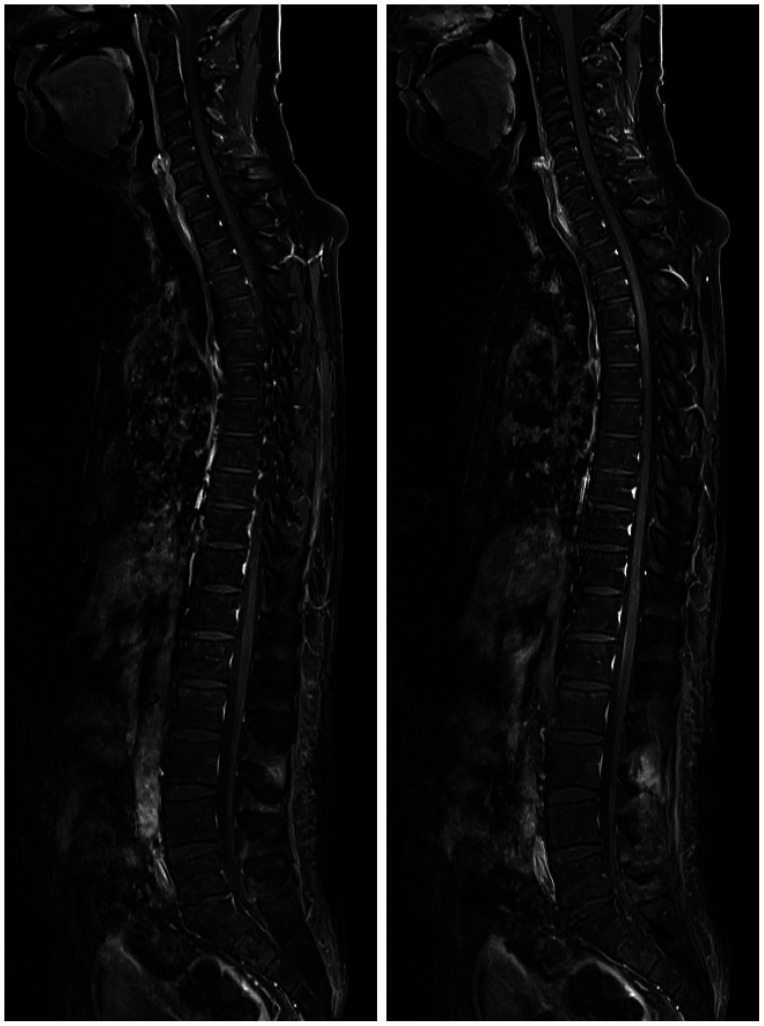
Fig. 4. The spine MRIs show multiple intramedullary and leptomenigeal metastases along the whole spinal axis.
Two months after the start of lorlatinib and spinal RT, the patient visited the outpatient department. Paraparesis was alleviated to almost normal condition and the patient walked without any assistive devices. No symptoms of severe headache or leg pains were detected, although they were very severe before lorlatinib therapy. The brain MRI showed improvement in leptomeningeal metastasis (Fig. 5), and chest CT showed regression of the primary cancer. One month later, the spine MRI revealed a dramatic improvement in spinal leptomeningeal metastases (Fig. 6). The patient was regularly monitored with continuing lorlatinib medication for 5 months without any systemic complication or neurological abnormality. However, the patient showed primary lung cancer recurrence after 5 months of lorlatinib treatment. Although the lorlatinib treatment was switched to platinum-based classical systemic treatment, the patient died of pulmonary ailments.
Fig. 5. The brain MRIs show improvement in leptomeningeal metastasis two months after lorlatinib treatment.
Fig. 6. The spine MRIs show improvement in leptomeningeal metastasis along the whole spinal axis three months after lorlatinib treatment.
DISCUSSION
ALK gene rearrangement is observed in a small group of patients with NSCLC, with the proportion varying from 3% to 13% [1]. Over the last few years, small molecule TKIs have shown significant benefit in the management of ALK-rearranged NSCLC [1], and a new generation of ALK-TKIs has been continuously introduced.
Lorlatinib is a third-generation ALK/ROS1TKI indicated for patients who are refractory to both crizotinib, which is a first-generation ALK-TKI, and ceritinib, a second-generation ALK-TKI [1,3]. Previous studies investigating lorlatinib for ALK-positive NSCLC patients reported an intracranial overall response rate of 60% (95% CI, 49% to 70%). Interestingly, the intracranial responses were rapid and the median time to the first tumor response was 1–4 months. Lorlatinib is even reported to be effective clinically regardless of the number of previous TKI treatments [1,3,4,5].
Leptomeningeal metastasis occurs in 3% to 5% of patients with advanced NCSLC [2,6]. It is a serious complication associated with advanced disease. The treatments are intended to improve both neurological symptoms and quality of life as well as prolong survival [2]. The prognosis of patients with leptomeningeal metastasis is poor. However, the median survival has improved from 1–3 months to 3–11 months due to novel systemic treatments [2,6,7,8]. For the management of brain metastasis, molecular targeted therapies and immunotherapy have been introduced and recently considered as promising treatments although classical chemotherapy and radiation therapy have also been used [2]. However, their role in leptomeningeal metastasis is not clearly known [2].Therapeutic efficacy in leptomeningeal metastasis is determined by therapeutic drug concentration in the cerebrospinal fluid (CSF) at the same doses administered systemically [2].
Lorlatinib is a highly potent macrocystic inhibitor and a poor P-glycoprotein substrate, with excellent passive permeability. Therefore, it has a high CSF penetration of 31% to 96%, and the ratio of CSF concentration to half-maximal inhibitory concentrations exceeds 90% [2].
For leptomeningeal metastasis, RT has been mostly used in combination with continuous systemic treatment. However, a second RT is seldom repeated for patients with newly developed leptomeningeal metastasis. Currently, intrathecal or intraventricular chemotherapy with methotrexate, cytarabine and thiotepa might be considered as a salvage treatment. However, the treatment result was not always satisfactory, and complications such as aseptic meningitis and chemotherapy-related central nervous system (CNS) toxicity should be considered [2]. Even in intraventricular chemotherapy, a surgical intervention via device insertion is needed and device-related complications have been reported [2].
In the present case, the patient’s neurological status was very poor. He presented with deep drowsiness and stupor along with severe paraparesis with motor grade I, and complained of severe headache and radiating pain to both legs. Even worse, he had undergone brain RT one year ago. Intratheral therapy could not be considered either because of his low Karnofsky performance score (<70). Based on the molecular study of ALK rearrangement of the patient’s primary cancer and the previous treatment history of ALK-TKI, lorlatinib was administered at the dose of 100 mg/day via Levin tube for one week in the neurological intensive care unit. Interestingly, the beginning of neurological improvement occurred within two weeks. Gafer et al. [1] reported rapid regression of neurological symptoms in two patients with brain metastastasis from ALK+ lung cancer, who were treated with lorlatinib. The two patients manifested a dramatic regression of brain metastases in MRI after one week and one month, respectively, following treatment with lorlatinib at doses of 100 mg/day. Hochmair et al. [9] also reported a patient with intrathecal metastasis from ALK-positive lung cancer, who showed dramatic remission of intrathecal spinal metastases following loralatinib therapy with the same dose of 100 mg/day. They found neurological improvement and radiological remission in spine MRI at two weeks and 4 months, respectively. Our patient also manifested rapid symptoms involving both brain and spinal cord in two weeks. Despite the addition of spine RT, we thought that lorlatinib therapy was effective against brain and spinal leptomeningeal metastases. Lorlatinib is also known to have a relatively higher CSF penetration of 31%–96% and effective CSF concentration [2,10], which might be one of the reasons for its effectiveness against leptomeningeal metastases of the CNS in the present case.
In conclusion, lorlatinib was effective for patients with CNS leptomeningeal metastases from ALK-positive lung cancer. The agent is easy to administer orally even via Levin tube. The effect was rapid and the neurological recovery was satisfactory.
Footnotes
- Conceptualization: In-Young Kim.
- Data curation: Min-Gwan Sun.
- Formal analysis: In-Young Kim.
- Supervision: Young-Jin Kim, Tae-Young Jung, Kyung-Sub Moon, Shin Jung, In-Je Oh, Young-Cheol Kim, Yoo-Duk Choi.
- Writing—original draft: Min-Gwan Sun.
- Writing—review & editing: In-Young Kim.
Conflicts of Interest: The author has no potential conflicts of interest.
Funding Statement: None
Ethics Statement
This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital with a waiver of informed consent (CNUHH-2021-175).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
- 1.Gafer H, de Waard Q, Compter A, van den Heuvel M. Rapid regression of neurological symptoms in patients with metastasised ALK+ lung cancer who are treated with lorlatinib: a report of two cases. BMJ Case Rep. 2019;12:e227299. doi: 10.1136/bcr-2018-227299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19:e43–e55. doi: 10.1016/S1470-2045(17)30689-7. [DOI] [PubMed] [Google Scholar]
- 3.Akamine T, Toyokawa G, Tagawa T, Seto T. Spotlight on lorlatinib and its potential in the treatment of NSCLC: the evidence to date. Onco Targets Ther. 2018;11:5093–5101. doi: 10.2147/OTT.S165511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw AT, Bauer TM, Felip E, et al. Clinical activity and safety of PF-06463922 from a dose escalation study in patients with advanced ALK+ or ROS1+ NSCLC. J Clin Oncol. 2015;33:8018 [Google Scholar]
- 5.Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 6.Remon J, Le Rhun E, Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: a continuing challenge in the personalized treatment era. Cancer Treat Rev. 2017;53:128–137. doi: 10.1016/j.ctrv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Le Rhun E, Galanis E. Leptomeningeal metastases of solid cancer. Curr Opin Neurol. 2016;29:797–805. doi: 10.1097/WCO.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 8.Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11:1962–1969. doi: 10.1016/j.jtho.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Hochmair MJ, Schwab S, Prosch H. Complete remission of intrathecal metastases with lorlatinib therapy in a heavily pretreated ALK-positive lung cancer patient. Anticancer Drugs. 2017;28:928–930. doi: 10.1097/CAD.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 10.Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57:4720–4744. doi: 10.1021/jm500261q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.