Summary
In humans, pre-existing anti-HIV-1 neutralizing antibodies (nAbs) have not been associated with decreased HIV-1 acquisition. Here, we evaluate antibody-dependent cellular cytotoxicity (ADCC) present in pre-transmission infant and maternal plasma and breast milk (BM) against the contemporaneous maternal HIV-1 variants. HIV-1-exposed uninfected compared with HIV-1-exposed infected infants have higher ADCC and a combination of ADCC and nAb responses against their corresponding mother’s strains. ADCC does not correlate with nAbs, suggesting they are independent activities. The infected infants with high ADCC compared with low ADCC, but not those with higher ADCC plus nAbs, have lower morbidity up to 1 year after birth. A higher IgA to IgG ratio, observed in BM supernatants and in a higher proportion of the infected compared with the uninfected infants, associates with lower ADCC. Against the exposure strains, ADCC, more than nAbs, associates with both lower mother-to-child transmission and decreased post-infection infant morbidity.
Keywords: HIV-1, antibody-dependent cellular cytotoxicity, neutralization, mother-to-child transmission, envelope glycoprotein, IgA, breast milk, breastfeeding, antiretroviral, nutrition
Graphical abstract
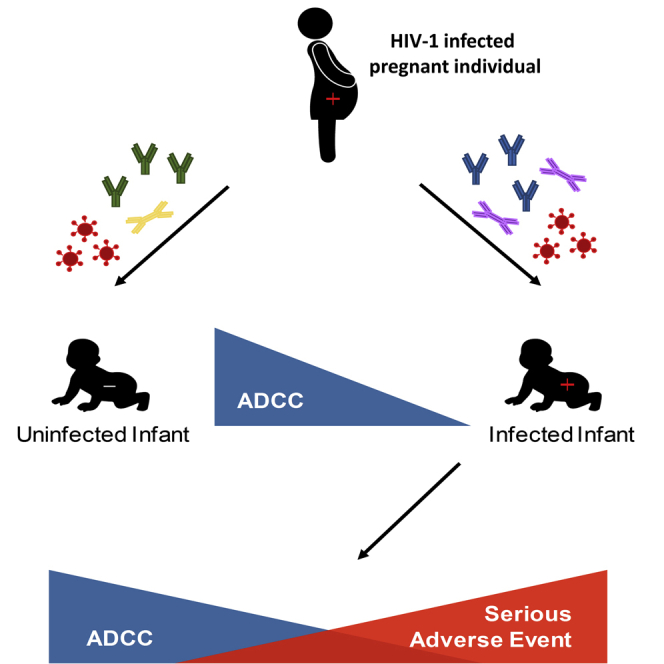
Highlights
Infants with higher ADCC against their mother’s strains acquire HIV less frequently
Infected infants with higher pre-transmission ADCC responses have better outcomes
ADCC activity does not correlate with neutralizing antibody responses
High IgA levels associate with lower ADCC activity
Thomas et al. show that higher pre-existing ADCC responses against exposure strains associate with less likelihood of HIV-1 mother-to-child transmission and lower morbidity in infected infants.
Introduction
It is imperative to identify immune factors that can decrease HIV-1 transmission in humans. The recent finding that passive infusion of large quantities of a broadly neutralizing antibody (bnAb) demonstrated no significant decrease in subsequent HIV-1 acquisition highlights this need.1 Examining mother-to-child transmission (MTCT) cohorts can be useful, because infants acquire HIV-1 at a lower frequency than may be expected, especially considering the long duration of viral exposure in utero and during breastfeeding. This risk of HIV-1 MTCT has been primarily associated with higher maternal plasma viral load and lower absolute CD4 counts.2 In the absence of antiretroviral treatment (ART), transmission risk during the breastfeeding period is approximately 10%–20% depending on duration, suggesting natural immune mechanisms may protect against acquisition.3 Infants passively acquire systemic and mucosal antibodies during gestation and breastfeeding, respectively,4,5 suggesting humoral immunity may protect against HIV-1 acquisition. However, studies from our group and others have shown that pre-existing broad and potent neutralizing antibody (nAb) responses do not associate with a lower risk of HIV-1 acquisition in highly exposed infants,6,7 although some investigations have suggested otherwise.8,9 In this study, we investigated the impact of antibody-dependent cellular cytotoxicity (ADCC) on HIV-1 MTCT.
ADCC is induced when the Fab region of an antibody binds to the HIV-1 envelope glycoprotein (Env) presented on the surface of infected cells. The Fc portion of the bound antibody can then interact with Fc receptors (FcRs) on various immune cells, such as FcγRIIIa (CD16), on natural killer (NK) cells.10 This Fc-FcR bridge induces the killing of the infected cell. ADCC was previously associated with the modest protection observed in the RV144 HIV-1 vaccine trial.11 HIV-specific ADCC activity present in infected mother’s breast milk (BM) supernatants was associated with lower MTCT via breastfeeding.12 Furthermore, passively transferred ADCC activity in HIV-infected infants was associated with improved infant survival.13,14 However, the role of ADCC in preventing transmission and in providing a therapeutic benefit remains controversial, primarily because animal models have often failed to corroborate the findings from human cohorts. Furthermore, emerging evidence suggests that the importance of antibody effector functions is likely situation specific and influenced by the route of transmission, targeted epitope, and Fc and Fab properties.15,16 However, no prior investigations have examined ADCC against the viruses circulating in infected mothers. Assessing responses that exist before transmission against maternal variants is most analogous to understanding how pre-existing antibodies may prevent infection from exposure strains and improve outcomes.
Here, we investigated ADCC present in maternal and infant plasma, as well as breast milk, against strains circulating in the chronically infected mother using an infection-based ADCC killing assay.17 We found that ADCC was higher in infants who remained uninfected compared with infants who acquired infection. Furthermore, higher ADCC was associated with lower infected infant morbidity and mortality up to 1 year after birth. Our observations suggest that eliciting ADCC against the exposure strains may provide both protection against transmission and therapeutic benefit in settings in which infected infants cannot get ART.
Results
Infant ADCC responses are associated with lower transmission
We examined ADCC responses in plasma and breast milk samples from mother-infant pairs in the control arm of the Breastfeeding, Antiretroviral, and Nutrition (BAN) study.18 In these individuals, ART was given for a maximum of 7 days, and HIV-1 transmission occurred at least 2 weeks after birth. We examined responses in the closest available sample collected before the first infant HIV-1 PCR-positive result to assess antibodies existing before the transmission event. Every transmitting mother (TM)-infant pair was matched to two dyads with no documented transmission based on maternal age, virus level, and absolute CD4 count. In addition, samples used in our study came from a time point just before transmission when infants acquired infection, and a similar time point was used in the matched uninfected infants. We examined ADCC against virus stocks incorporating HIV-1 Envs isolated from 16 TMs and 26 non-transmitting mothers (NTMs). Demographics were not significantly different among the mothers and infants in these two groups (Table S1). Similarly, the isolation method and the number of isolated maternal Envs were not different between the groups (Table S2). The Env sequences present in the individual maternal virus stocks were not examined. However, previous studies from our group and others suggest that the yeast homologous recombination methodology used here incorporates a large number of diverse variants.19,20
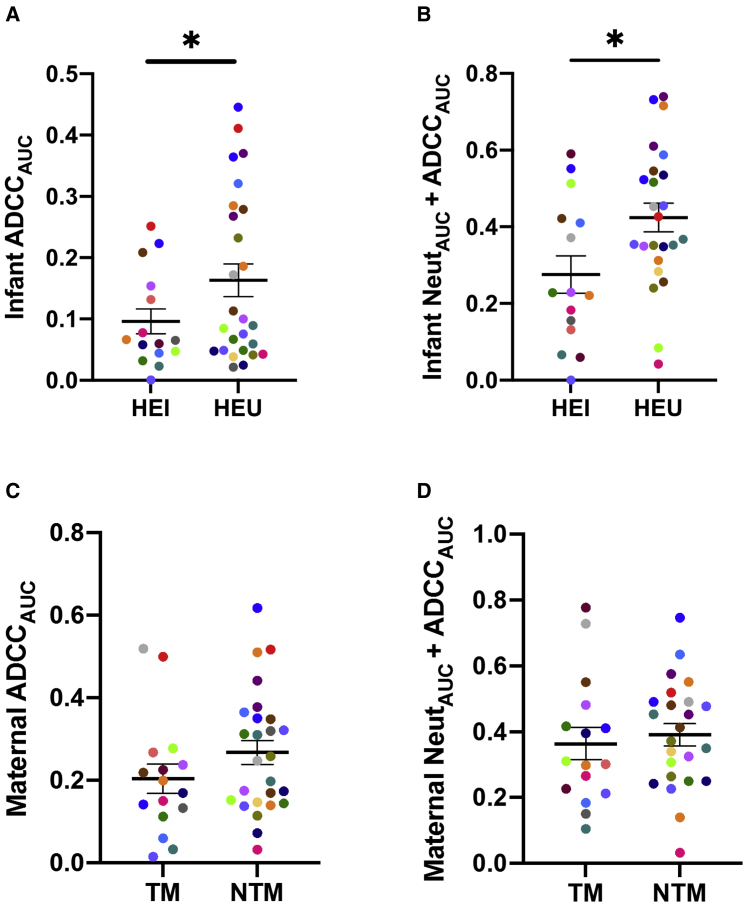
ADCC was estimated using a previously validated infected-cell-killing assay.17 A reporter cell line with trans-activator of transcription (Tat)-responsive luciferase expression was infected with virus stocks incorporating maternal Envs. NK cell line-mediated decrease in luciferase expression in the presence compared with the absence of serially diluted plasma was used to estimate ADCC. ADCC activity was compared using an area under the killing curve (AUC) estimate with a range from 0, signifying no ADCC, to 1, representing 100% ADCC at all dilutions.21 HIV-exposed uninfected (HEU) infants (mean 0.16, range 0.02–0.45) compared with HIV-exposed infected (HEI) infants (mean 0.10, range 0.00–0.25) had higher ADCC AUC (ADCCAUC) against the cells infected with their corresponding mother’s variants (p = 0.05; Figure 1A; Table S2). Multivariable logistic regression analysis demonstrated a trend that the odds of infection was around 60% lower with a 0.1 unit increase in pre-existing infant ADCCAUC (odds ratio [OR] 0.41, 95% confidence interval [CI] 0.16–1.07, p = 0.07) after accounting for maternal plasma virus level, absolute CD4 count, and duration between birth and sample collection.
Figure 1.
Infant and maternal ADCC and neutralization plus ADCC responses
HIV-exposed infected (HEI) and HIV-exposed uninfected (HEU) infants (A and B) and transmitting (TM) and non-transmitting (NTM) mothers (C and D) ADCCAUC (A and C) and NeutAUC + ADCCAUC (B and D) are shown in relation to transmission outcome. Colors signify matched pairs. Bars show mean and standard error. Group comparisons were done using Welch’s t test and/or multivariable logistic regression. ∗p ≤ 0.05 with one of these statistical tests. All values are means from a minimum of 2 replicates.
We also hypothesized that combined neutralization and ADCC responses contribute to protection against transmission. We previously measured infant and maternal plasma neutralization AUC (NeutAUC) against the same maternal virus strains, and we observed no significant differences based on transmission outcomes.6 A novel measure (NeutAUC + ADCCAUC) was significantly higher in HEU infants (mean 0.42, range 0.04–0.74) compared with HEI infants (mean 0.28, range 0.00–0.59, p = 0.02; Figure 1C). This measurement summed the AUC values from the neutralization assay (mean 0.14, range 0.00–0.33) and the ADCC assay (mean 0.24, range 0.02–0.60). In all instances, we tested the activity present in the same highest plasma dilution and the same series of plasma dilutions. No normalization was involved in either measure, and summed measurements could range from 0 to 2. Multivariate logistic regression analysis demonstrated that the odds of infection was around 40% lower with a 0.1 unit increase in pre-existing infant NeutAUC + ADCCAUC (OR 0.64, 95% CI 0.42–0.97, p = 0.04; Figure 1B) after accounting for maternal characteristics.
NTMs compared with TMs also had higher ADCCAUC (Figure 1C) and NeutAUC + ADCCAUC (Figure 1D). However, this was not significantly different in magnitude or in predicting the odds of transmission. In contrast to a previous study,12 TMs’ and NTMs’ ADCCAUC values from breast milk supernatant (Figure S1A) and from breast milk isolated immunoglobulin G (IgG) (Figure S1B) were not different in magnitude and did not associate with odds of transmission. Furthermore, the magnitude of the breast milk IgG or breast milk supernatant ADCC did not associate with transmission when the analysis was restricted to the women with high plasma virus levels (greater than 4.6 log10 copies per milliliter), as in the previous study.12 Similar to our previous reports,6,22 AUC estimates were directly correlated to the percentage decrease observed at the highest tested plasma/antibody dilution (p < 0.0001, ρ > 0.91 in all cases). In aggregate, higher ADCC and a combination of ADCC plus nAb responses in the exposed individual against the exposure strains associate with lower HIV-1 acquisition.
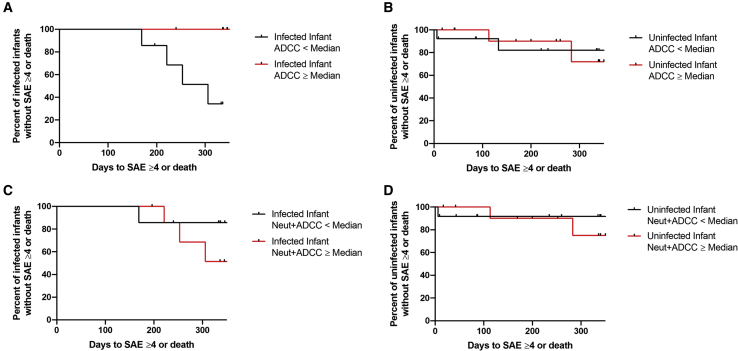
High infant ADCC responses in infected infants are associated with reduced morbidity and mortality up to one year after birth
ADCC activity may also affect the outcomes of infants who eventually become infected.13,14 As part of a pre-specified analysis, HEI and HEU infants were divided into 2 groups, those with less than and those with greater than or equal to median ADCC activity, similar to the analysis from our group and others.6,13 In the absence of ART, a serious adverse event (SAE) ≥ grade 4 or death occurred in 5 and 6 of the 15 HEI and 26 HEU infants, respectively, included in our analysis (Table S3). The time to this event occurred earlier among the HEI infants with low compared with high ADCC (log rank hazard ratio 9.2, 95% CI 1.4–62.0, p = 0.01; Figure 2A). Probability of incurring a SAE ≥ grade 4 or death was not different among the HEU infants who had low compared with high ADCC (Figure 2B). The probability of a SAE ≥ grade 4 or death was not significantly different among the HEI infants (Figure 2C) and HEU infants (Figure 2D) who had high compared with low NeutAUC + ADCCAUC. Thus, ADCC responses, but not a combination of ADCC and nAb responses, against the exposure strains associate with lower morbidity up to 1 year after birth in the infected infants.
Figure 2.
Morbidity associated with pre-existing humoral responses
Kaplan-Meier curves estimating time (days) to a grade 4 or greater serious adverse event (SAE) or death for HEI infants (A and C) and HEU infants (B and D) with ADCCAUC (A and B) and NeutAUC + ADCCAUC (C and D) greater than or equal to the cohort median (red) or less than the cohort median (black). Tick marks denote right censoring.
ADCC activity does not correlate with neutralization responses but is possibly affected by levels of IgA
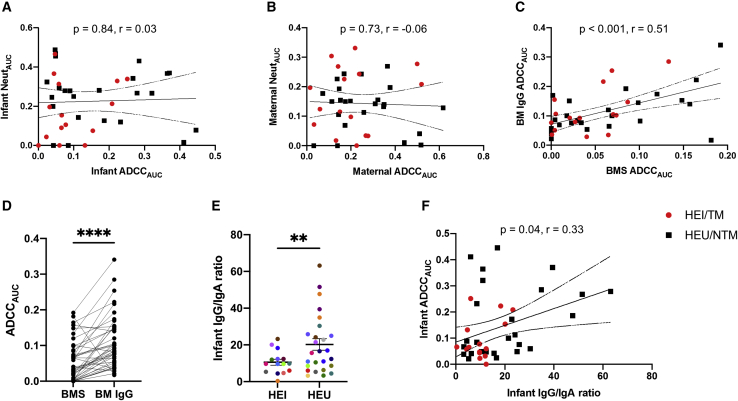
Autologous ADCC and neutralization responses were not correlated in infant plasma (p = 0.70, ρ = 0.06; Figure 3A) or maternal plasma (p = 0.97, ρ = −0.001; Figure 3B). Total IgG trended higher in HEI compared with HEU infant plasma (p = 0.06; Figure S2A). There was no difference in total IgG levels in TM and NTM plasma (p = 0.72; Figure S2B) or breast milk (p = 0.78; Figure S2C). Furthermore, ADCC activity in infant plasma IgG (p = 0.82, ρ = 0.04; Figure S2D), maternal plasma IgG (p = 0.56, ρ = −0.09; Figure S2E), or breast milk IgG (p = 0.88, ρ = −0.02; Figure S2F) did not correlate with total IgG magnitude in these samples. Thus, neutralization and ADCC against maternal strains are independent activities, and high ADCC does not merely reflect higher immunoglobulin levels.
Figure 3.
Plasma and breast milk ADCC relative to neutralization and immunoglobulin isotypes
(A and B) ADCCAUC correlation with NeutAUC among infants (A) and mothers (B).
(C and D) ADCC from breast milk (BM)-isolated IgG relative to breast milk supernatant (BMS).
(E and F) IgG to IgA ratio among infants (E), and IgG to IgA ratio among infants relative to infant ADCCAUC (F).
The red and black dots indicate TMs/HEI and NTMs/HEU, respectively, and colors in (E) signify matched pairs. In (E), bars show mean and standard error. Correlations were assessed using Spearman’s statistic. Lines indicate linear regression fit with a 95% confidence interval. Group comparisons were done using matched pairs Wilcoxon rank-sum (D) or Welch’s t test (E). ∗∗p ≤ 0.01, ∗∗∗∗p ≤ 0.0001. All values represent mean values from a minimum of 2 replicates.
ADCC from breast milk supernatant and breast milk IgG was highly correlated (p < 0.001, ρ = 0.51; Figure 3C). Interestingly, breast milk IgG consistently yielded higher ADCC relative to breast milk supernatant (p < 0.0001; Figure 3D). In contrast, we previously demonstrated that ADCC was not significantly different in plasma compared with IgG isolated from plasma.17 Breast milk supernatant compared with plasma contains high levels of immunoglobulin A (IgA),23 which may inhibit IgG-mediated ADCC. The HEU infants who had higher ADCC as a group (Figure 1) also had significantly higher IgG/IgA ratios compared with HEI infants (p = 0.01; Figure 3E). Furthermore, the infant IgG/IgA ratio demonstrated a modest significant correlation with infant ADCCAUC (p = 0.04, ρ = 0.33; Figure 3F). NTMs compared with TMs also had a non-significant higher IgG to IgA ratio in plasma (Figure S3A), but not in breast milk (Figure S3B). Collectively, these results support the idea that high IgA levels may interfere with IgG-mediated ADCC.11,24,25
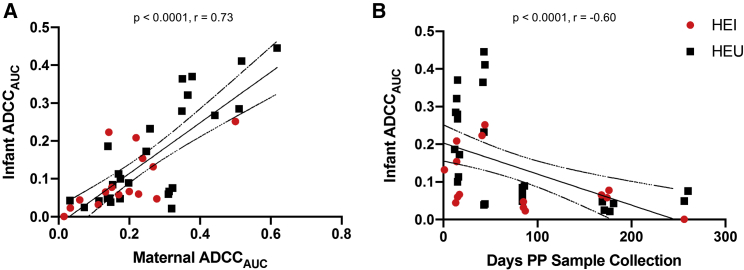
Infants acquire ADCC-inducing antibodies from their mothers and are not affected by the mother’s duration of disease
Infants passively acquire maternal antibodies throughout gestation, and these maternal antibodies wane over the course of 6 to 12 months.26 Maternal and infant ADCC responses were highly correlated (p < 0.0001, ρ = 0.73; Figure 4A), and infant responses negatively correlated with the days postpartum to sample collection (p < 0.0001, ρ = −0.60; Figure 4B). These results suggest that the ADCC responses are from the passive transfer of antibodies from the mother, rather than de novo production in the child.
Figure 4.
Infant and maternal ADCCAUC and duration after birth
Correlation between infant and maternal ADCCAUC (A) and infant ADCCAUC relative to days after birth (B). The red and black dots indicate TMs/HEI and NTMs/HEU, respectively. Correlations were assessed using Spearman’s statistic. Lines indicate linear regression fit with a 95% confidence interval.
In general, nAb responses are higher among individuals with high plasma virus levels and lower CD4 counts.27 In our cohort, maternal ADCC was not associated with maternal viral load (p = 0.83, ρ = −0.03; Figure S4A) or CD4 count (p = 0.31, ρ = 0.16; Figure S4C). Similarly, infant ADCC was not associated with maternal viral load (p = 0.57, ρ = 0.09; Figure S4B) or CD4 count (p = 0.48, ρ = 0.11; Figure S4D). These results imply that the duration of HIV-1 disease, as estimated by virus levels and number of CD4 cells, does not affect ADCC capacity in the mother or the infant.
Discussion
Efforts to develop an effective HIV-1 vaccine have aimed at eliciting antibodies that can prevent infection. Passive transfusion investigations in animal models have clearly demonstrated that nAbs, especially bnAbs, prevent infection against cell-free virus challenge.28 However, recent results from clinical trials and prior MTCT cohorts have not confirmed these findings, probably because there are significant differences between animal challenge models and common modes of HIV-1 acquisition.1,29 Identifying the characteristics of the pre-existing immune response that can limit acquisition in humans remains vital for future vaccine efforts.
In many respects, MTCT cohorts are an ideal model to examine this question, because infants passively acquire maternal antibodies before and while they are consistently exposed to the virus. Some previous MTCT studies have suggested that HIV-1-specific ADCC responses and various other antibody functionalities may limit virus acquisition.10,12,13,30,31 Differences in the assay methodology, as well as the number and source of the samples examined, have likely contributed to the conflicting results. In this study, we found that pre-existing infant ADCC responses are associated with both decreased transmission and lower HIV-1 infected infant morbidity. In contrast to all previous studies, we examined ADCC responses against variants encountered by naive infants. This is important because recent clinical trials of a passively infused bnAb showed that neutralization susceptibility of the infecting viruses is important for preventing transmission.1 Our observations potentially suggest that when neutralization is insufficient to block transmission, pre-existing ADCC responses may help prevent virus acquisition. Our results support the notion that enhancing ADCC responses against the variants present in the transmitting individual would decrease transmission. Furthermore, we estimated ADCC using an assay that quantified the elimination of infected targets only, not bystander cells.17,32 Infection-based assays that quantify actual infected cell killing have been deemed to have fewer artifacts compared with other methods that use gp120-coated targets or surrogate markers for effector cell activity.16,33 Furthermore, our methodology for generating replication-competent viruses incorporating the Envs of interest recapitulates the major strains present in a sample from a chronically infected individual.34 In general, Env expressed on the surface of infected cells is the most important, if not the only, target for ADCC.35 Thus, our studies are directly relevant for understanding how pre-existing antibodies in exposed individuals may prevent virus acquisition and subsequent morbidity.
Animal studies have clearly demonstrated that nAbs prevent transmission more efficiently in the presence of ADCC and other antibody-mediated functionalities.36, 37, 38, 39 This work, along with our previous study,6 demonstrates that ADCC alone and in conjunction with nAbs, but not nAbs alone, correlates with protection against acquisition in humans. ADCC, with and without nAbs, may protect against infection because the infected mother’s BM contains both cell-free virus and infected cells. Exposed infants with high ADCC may eliminate some of their mother’s cells with infectious virus that they ingest during breastfeeding. However, pre-existing neutralization response alone may not protect against transmission, because nAbs drive viral escape in the infected mothers.40, 41, 42, 43, 44 Still, in humans, the modifications conferring neutralization resistance may impart susceptibility to pre-existing antibodies in other ways. For instance, one previous study suggested that neutralization pressure renders viruses ADCC susceptible.45 Animal models may yield conflicting results because in general, the challenge stocks only contain cell-free virus that have demonstrated neutralization susceptibility to the passively infused antibody.
Along with previous studies from our group and others,6,13,14 we demonstrate that ADCC alone and not nAbs, with or without ADCC, associates with decreased morbidity in infected infants. In infected infants, high ADCC activity may eliminate some cells that become infected after virus acquisition. This may lower virus levels and preserve the nascent immune system. Both animal studies and an observed positive association between cervicovaginal ADCC levels and genital HIV-1 RNA loads support this potential mechanism.46, 47, 48 Beyond ADCC, nAbs may not further modulate the subsequent disease course because of the presence of mostly neutralization-resistant viruses. Similar association was not observed in the uninfected infants, presumably because they do have HIV-1 infected cells. However, to date, this possible mechanism has not been directly tested by quantifying the number of infected cells harboring infectious virus.
The observed correlations do not merely reflect differences in immunoglobulin levels but most likely result from as-yet-undefined antibody properties. Of note, we did not measure HIV-1-directed immunoglobulin levels because we were interested in responses against maternal exposure variants, not unrelated strains captured by consensus proteins. Therefore, it is possible that the magnitude of HIV-specific total IgG varies among groups and may contribute to the observed outcome differences. Similar to previous studies,43,49 our observations suggest that the selective transfer of specific antibodies from the mother to the infant, such as IgG and more so than IgA, is important. Furthermore, the significant difference in IgG/IgA ratios between HEI and HEU infants and in ADCC from breast milk IgG compared with supernatant supports previous arguments that IgA may interfere with ADCC.11,24,25 One previous study suggests that IgA binds the same epitopes as IgG at a higher affinity but does not induce ADCC, proposing a mechanism for IgA’s negative effect of ADCC.25 Future efforts should be aimed at both investigating the mechanisms for these associations and characterizing the antibodies that impart ADCC and potentially other effector activity. Our data highlight the benefits of ADCC and support designing vaccines that can enhance ADCC in highly exposed at-risk individuals.
Limitations of the study
This study demonstrates that higher pre-existing ADCC activity in exposed infants against the strains circulating in the corresponding infected mother is associated with lower risk of HIV-1 acquisition and lower morbidity and mortality up to 1 year after birth in infected babies. These associations are based on examination of 42 mother-infant dyads; virus stocks could not be generated from all 63 mothers in the original study. Examination of other cohorts would provide both confirmation and generalizability. We also did not characterize the viral strains isolated from the mothers; thus, differences among the exposure variants from TMs compared with NTMs may influence transmission outcomes. Our results further suggest that IgA inhibits IgG-mediated ADCC. However, this conclusion was based on examining the total concentrations, rather than the HIV-1-specific concentrations, of the different immunoglobulin isotypes.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| HIVIG | NIH AIDS Reagent Program | Cat#3957, Lot#130230 |
| Bacterial and virus strains | ||
| pNL4-3 | HIV Reagent Program | ARP-114 |
| Biological samples | ||
| Breastfeeding, Antiretroviral, and Nutrition (BAN) Study (ClincialTrials.gov no. NCT00164736) | Centers for Disease Control and Prevention | ClincialTrials.gov no. NCT00164736 |
| Critical commercial assays | ||
| Bio-Plex Pro Human Isotyping Assays Kit | Bio-Rad | Cat#171A3100M |
| Melon Gel IgG Spin Purification Kit | ThermoFisher | 45206 |
| Experimental models: Cell lines | ||
| CD16+KHYG-1 | Dr. David Evans | N/A |
| MT4-CCR5-Luc | Dr. Manish Sagar | N/A |
| Software and algorithms | ||
| R version 4.0.3 | https://www.npackd.org/p/r/4.0.3 | N/A |
| Prism (Version 8.0) | https://www.graphpad.com | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Manish Sagar (msagar@bu.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Antibodies and cell cultures
HIVIG was acquired from the NIH AIDS Reagent Program (Cat #3957, Lot #130230). Melon Gel IgG Spin Purification Kit (ThermoFisher, 45206) was used to isolate IgG from HIV-infected breast milk supernatant per manufacturer’s protocol. IgG isolation resulted in a 10-fold dilution relative to the breast milk supernatant, which was accounted for when setting up assays by adding ten times the amount of diluted IgG. MT4-CCR5-Luc cells were maintained at a density of 1 × 106 cells/ml in RPMI 1640 (Invitrogen) containing 10% FBS (Invitrogen), 25 mM HEPES (Invitrogen), 2mM L-glutamine (Invitrogen), 100 U/ml penicillin (Invitrogen), and 100 μg/ml streptomycin (Invitrogen). The NK cell line, CD16+KHYG-1, was generously provided by Dr. David Evans. Cells were maintained at a density of 5 × 105 cells/ml in RMPI 1640 (Invitrogen) containing 10% FBS (Invitrogen), 25 mM HEPES (Invitrogen), 2mM L-glutamine (Invitrogen), 0.1 mg/ml Primocin (InvivoGen), 1 μg/ml Cyclosporine A (CsA) (Sigma), and 10 U/ml interleukin-2 (IL-2) (NIH AIDS Reagent Program). Human epithelial kidney HEK293T cells and TZM-bl cells were acquired from the NIH AIDS Reagent Program. Cells were cultured in Dulbecco’s modified Eagle medium (DMEM) containing 10% FBS (Invitrogen), 2mM L-glutamine (Invitrogen), 100 U/ml penicillin (Invitrogen), and 100 μg/ml streptomycin (Invitrogen).
Human subjects and samples
Plasma and breast milk samples were acquired from the control arm of the Breastfeeding, Antiretroviral, and Nutrition (BAN) Study (ClincialTrials.gov no. NCT00164736). Each mother-infant pair involved in this study was treated peripartum with a single-dose oral Nevirapine followed by Zidovudine-Lamivudine (ZDV-3TC) for 7 days postpartum. For enrollment in the trial, infants were required to have a negative-DNA PCR at birth and 14 days postpartum to rule out intrauterine and intrapartum transmission, respectively. Mother-infant pairs were followed for 48 weeks with sample collection occurring every two weeks. An infant was deemed to have acquired HIV-1 through breast milk when HIV-1 RNA was detected and a previous sample was negative. All infant and maternal samples used in this study are from a time-point prior to documented HIV-1 acquisition in the baby. Every infant that acquired infection and their corresponding transmitting mother were then matched to two mother-infant pairs where transmission did not occur. Matching took into consideration maternal plasma viral load, maternal CD4+ T cell count, and days postpartum to sample collection.
Method details
Envelope isolation, amplification, and replication-competent virus stocks
Envelopes were isolated from the contemporaneous maternal plasma sample that was also assessed for humoral activity. HIV-1 RNA was isolated from 140 μL of maternal plasma using the QIAamp viral RNA Mini kit (QIAGEN, 52904), following manufacturer’s protocol. Reverse transcription was used to generated cDNA from the using either the CHAVI protocol or bulk PCR method50 (Table S3). Amplified products were purified using ExoSap IT (Affymetrix, 78201) prior to sequence analysis. Sequencing was used to confirm Envs were HIV-1 subtype C, commonly circulating in Malawi, and rule out contamination with common lab strains. Isolated Env amplicons were incorporated into an subtype C transmitted / founder (T/F) infectious molecular clone, pZM247Fv251 through a previously described yeast gap repair homologous recombination protocol34,52. Plasmids rescued from yeast were used to transform in Top10 electrocompetent E. coli cells. Bacterial plasmids were rescued using Qiaprep Miniprep kits (QIAGEN, 27106). 293T cells were transfected with 1-3 μg/μL of the Env containing plasmid, 1 μg/μL of helper plasmid, along with 94 μL DMEM, and 9 μL polyethylenimine (PEI)34. Transfected cells were cultured at 37°C for 48 h. Supernatants were filtered through a 0.45-μm-pore to remove cellular debris before storing at −80°C. 293T virus was passaged in CD4+ T cells for a maximum of 7 days. Supernatant were filtered through a 0.45-μm-pore upon harvest, and stored at −80°C. Viral titers were determined on TZM-bl cells in the presence of 10 μg/ml DEAE-dextran.
ADCC assay
All maternal and infant plasma samples, and breast milk supernatant, were heat inactivated for 1 h at 56°C. All ADCC assays were performed in duplicate or triplicate a minimum of 2 independent times using MT4-CCR5-Luc cells and CD16+KHYG-1 cells, as previously described17. Briefly, MT4-CCR5-Luc cells were infected with viruses incorporating maternal Envs by spinoculating at 1200 X g for 90 min before resting cells for 30 min at 37°C. Cells were then washed in PBS before incubating for approximately 72 h at 37°C. After incubating, infection was checked and cells were ready for use if the relative light units (RLU) was at least 10-fold over background. Approximately 1 × 105 infected cells were incubated with 6 2-fold serial dilutions of maternal plasma, infant plasma, and breast milk supernatant or isolated IgG starting with 1:50 dilution, for 20 min at 37°C in a 96 well plate (Corning, 3610). After incubation, 5 X 105 CD16+KHYG-1 cells were added to each well. After 24 h, luciferase levels were determined using Bright-Glo (Promega E2650). Differences between RLU in the presence or absence of plasma, breast milk supernatant, or IgG were used to determine %ADCC. Background RLU in uninfected MT4-CCR5-Luc cells and CD16+KHYG-1 cells were subtracted from all wells. Serial dilution curves were used to calculate area under the curve (AUC). The killing capacity of the CD16+KHYG-1 cells was assessed each time for every independent ADCC assay by assessing the ability of pooled immunoglobulin (HIVIG) to mediate ADCC against HIV-1 NL43-infected cells.
Human Isotyping Assay
All maternal plasma, infant plasma, and breast milk supernatant was tested using the Bio-Rad Bio-Plex Pro Human Isotyping Assays Kit (#171A3100M) to quantify IgG1, IgG2, IgG3, IgG4, IgA, and IgM per manufacturer’s protocol. Samples were tested at a 1:40,000 dilution and data was acquired on the MAGPIX through access from the Boston University Analytical Core. Total IgG was determined from summing quantities of IgG1, IgG2, IgG3, and IgG4.
Quantification and statistical analysis
All normally and not normally distributed unpaired comparisons used an unpaired t test with Welch’s correction and Mann-Whitney U test respectively. All correlations were assessed using Spearman statistic. Multivariate logistic regression analysis was conducted with the transmission status as the dependent variable. Predictors included ADCCAUC (categorical group) or ADCCAUC + NeutAUC, maternal plasma virus level, absolute CD4 count, and days from birth to sample collection. Conditional logistic regression analysis was also conducted to examine transmission relative to ADCCAUC or ADCCAUC + NeutAUC accounting for the matched mother infant pairs. The conditional logistic regression yielded similar results as multivariable logistic regression after accounting for baseline demographics. The conditional regression analysis, however, did not account for all individuals because in some instances there was no matching data for a transmitting or non-transmitting mother-infant pair. For brevity, conditional logistic regression analysis results were not included. Clinical adverse events were graded by the BAN study investigators prior to our sample evaluations and according to toxicity tables from the Division of AIDS at the National Institute of Allergy and Infectious Diseases (NIAID). For the Kaplan Meier event curves and log-rank (Mantel-Cox) analysis, ADCCAUC were dichotomized as high (ADCCAUC ≥ cohort median) versus low (ADCCAUC < cohort median). This analysis was stratified by HIV status of the infant. Cox proportional hazard models were not used because of the limited number of grade 4 or greater SAE in the cohort. Statistical analysis was done using R version 4.0.3 and GraphPad Prism (Version 8.0). All p values are based on two-sided test.
Additional resources
Ethics statement
The BAN Study was approved by the Malawi National Health Science Research Committee, the institutional review boards at the University of the North Carolina, the U.S. Centers for Disease Control and Prevention, and Boston University. All women provided written informed consent for themselves as well as on behalf of their infants.
Acknowledgments
We thank the participants of the BAN study, without whom none of this work would be possible. This study was supported by NIH grants R21-AI137119, K24-AI145661, and P30-AI042853. A.S.T. was supported by NIH grant T32-5T32AI00730928. The funders had no role in the study design, data collection, and interpretation or the decision to submit this work for publication. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author contributions
M.S. and A.S.T. conceived and designed the study. A.S.T., Y.M., and J.E.I. conducted the experiments. A.E. and A.P.K. provided clinical samples and data. M.S., A.S.T., W.J., and L.F.W. analyzed the data and conducted the statistical analyses. M.S. and A.S.T. wrote the manuscript with editorial assistance from the co-authors.
Declaration of interests
The authors declare no competing interests.
Published: October 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2021.100412.
Supplemental information
Data and code availability
All data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Corey L., Gilbert P.B., Juraska M., Montefiori D.C., Morris L., Karuna S.T., Edupuganti S., Mgodi N.M., deCamp A.C., Rudnicki E., HVTN 704/HPTN 085 and HVTN 703/HPTN 081 Study Teams Two Randomized Trials of Neutralizing Antibodies to Prevent HIV-1 Acquisition. N. Engl. J. Med. 2021;384:1003–1014. doi: 10.1056/NEJMoa2031738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andiman W., Bryson Y., de Martino M., Fowler M., Harris D., Hutto C., Korber B., Kovacs A., Landesman S., Lindsay M., International Perinatal HIV Group The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N. Engl. J. Med. 1999;340:977–987. doi: 10.1056/NEJM199904013401301. [DOI] [PubMed] [Google Scholar]
- 3.Aldrovandi G.M., Kuhn L. What infants and breasts can teach us about natural protection from HIV infection. J. Infect. Dis. 2010;202(Suppl 3):S366–S370. doi: 10.1086/655972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasetti M.F., Ackerman M.E., Hoen A.G., Alter G., Tsang J.S., Marchant A. Maternal determinants of infant immunity: Implications for effective immunization and maternal-child health. Vaccine. 2020;38:4491–4494. doi: 10.1016/j.vaccine.2020.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fouda G.G., Yates N.L., Pollara J., Shen X., Overman G.R., Mahlokozera T., Wilks A.B., Kang H.H., Salazar-Gonzalez J.F., Salazar M.G., Center for HIV/AIDS Vaccine Immunology HIV-specific functional antibody responses in breast milk mirror those in plasma and are primarily mediated by IgG antibodies. J. Virol. 2011;85:9555–9567. doi: 10.1128/JVI.05174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghulam-Smith M., Olson A., White L.F., Chasela C.S., Ellington S.R., Kourtis A.P., Jamieson D.J., Tegha G., van der Horst C.M., Sagar M. Maternal but not infant anti-HIV-1 neutralizing antibody response associates with enhanced transmission and infant morbidity. MBio. 2017;8:1–19. doi: 10.1128/mBio.01373-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch J.B., Nduati R., Blish C.A., Richardson B.A., Mabuka J.M., Jalalian-Lechak Z., John-Stewart G., Overbaugh J. The breadth and potency of passively acquired human immunodeficiency virus type 1-specific neutralizing antibodies do not correlate with the risk of infant infection. J. Virol. 2011;85:5252–5261. doi: 10.1128/JVI.02216-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarlatti G., Leitner T., Hodara V., Halapi E., Rossi P., Albert J., Fenyö E.M. Neutralizing antibodies and viral characteristics in mother-to-child transmission of HIV-1. AIDS. 1993;7(Suppl 2):S45–S48. doi: 10.1097/00002030-199311002-00010. [DOI] [PubMed] [Google Scholar]
- 9.Martinez D.R., Vandergrift N., Douglas A.O., McGuire E., Bainbridge J., Nicely N.I., Montefiori D.C., Tomaras G.D., Fouda G.G., Permar S.R. Maternal Binding and Neutralizing IgG Responses Targeting the C-Terminal Region of the V3 Loop Are Predictive of Reduced Peripartum HIV-1 Transmission Risk. J. Virol. 2017;91:1–16. doi: 10.1128/JVI.02422-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boesch A.W., Brown E.P., Ackerman M.E. The role of Fc receptors in HIV prevention and therapy. Immunol. Rev. 2015;268:296–310. doi: 10.1111/imr.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haynes B.F., Gilbert P.B., McElrath M.J., Zolla-Pazner S., Tomaras G.D., Alam S.M., Evans D.T., Montefiori D.C., Karnasuta C., Sutthent R. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabuka J., Nduati R., Odem-Davis K., Peterson D., Overbaugh J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog. 2012;8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milligan C., Richardson B.A., John-Stewart G., Nduati R., Overbaugh J. Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality. Cell Host Microbe. 2015;17:500–506. doi: 10.1016/j.chom.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaffe Z.A., Naiman N.E., Slyker J., Wines B.D., Richardson B.A., Hogarth P.M., Bosire R., Farquhar C., Ngacha D.M., Nduati R. Improved HIV-positive infant survival is correlated with high levels of HIV-specific ADCC activity in multiple cohorts. Cell Rep. Med. 2021;2:100254. doi: 10.1016/j.xcrm.2021.100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su B., Dispinseri S., Iannone V., Zhang T., Wu H., Carapito R., Bahram S., Scarlatti G., Moog C. Update on Fc-Mediated Antibody Functions Against HIV-1 Beyond Neutralization. Front. Immunol. 2019;10:2968. doi: 10.3389/fimmu.2019.02968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forthal D.N., Finzi A. Antibody-dependent cellular cytotoxicity in HIV infection. AIDS. 2018;32:2439–2451. doi: 10.1097/QAD.0000000000002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas A.S., Ghulam-Smith M., Olson A., Coote C., Gonzales O., Sagar M. A new cell line for assessing HIV-1 antibody dependent cellular cytotoxicity against a broad range of variants. J. Immunol. Methods. 2020;480:112766. doi: 10.1016/j.jim.2020.112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chasela C.S., Hudgens M.G., Jamieson D.J., Kayira D., Hosseinipour M.C., Kourtis A.P., Martinson F., Tegha G., Knight R.J., Ahmed Y.I., BAN Study Group Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N. Engl. J. Med. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudley D.M., Gao Y., Nelson K.N., Henry K.R., Nankya I., Gibson R.M., Arts E.J. A novel yeast-based recombination method to clone and propagate diverse HIV-1 isolates. Biotechniques. 2009;46:458–467. doi: 10.2144/000113119. [DOI] [PubMed] [Google Scholar]
- 20.Etemad B., Ghulam-Smith M., Gonzalez O., White L.F., Sagar M. Single genome amplification and standard bulk PCR yield HIV-1 envelope products with similar genotypic and phenotypic characteristics. J. Virol. Methods. 2015;214:46–53. doi: 10.1016/j.jviromet.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X., Gilbert P.B., Hioe C.E., Zolla-Pazner S., Self S.G. Statistical approaches to analyzing HIV-1 neutralizing antibody assay data. Stat. Biopharm. Res. 2012;4:1–13. doi: 10.1080/19466315.2011.633860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Registre L., Moreau Y., Ataca S.T., Pulukuri S., Henrich T.J., Lin N., Sagar M. JVI; 2019. HIV-1 co-receptor usage and variable loop contact impacts V3 loop bnAb susceptibility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson K.M., Nazar A.M. Breastfeeding, the immune response, and long-term health. J. Am. Osteopath. Assoc. 2006;106:203–207. [PubMed] [Google Scholar]
- 24.Ruiz M.J., Ghiglione Y., Falivene J., Laufer N., Holgado M.P., Socías M.E., Cahn P., Sued O., Giavedoni L., Salomón H. Env-Specific IgA from Viremic HIV-Infected Subjects Compromises Antibody-Dependent Cellular Cytotoxicity. J. Virol. 2016;90:670–681. doi: 10.1128/JVI.02363-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomaras G.D., Ferrari G., Shen X., Alam S.M., Liao H.X., Pollara J., Bonsignori M., Moody M.A., Fong Y., Chen X. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc. Natl. Acad. Sci. USA. 2013;110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front. Immunol. 2014;5:446. doi: 10.3389/fimmu.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rusert P., Kouyos R.D., Kadelka C., Ebner H., Schanz M., Huber M., Braun D.L., Hozé N., Scherrer A., Magnus C., Swiss HIV Cohort Study Determinants of HIV-1 broadly neutralizing antibody induction. Nat. Med. 2016;22:1260–1267. doi: 10.1038/nm.4187. [DOI] [PubMed] [Google Scholar]
- 28.Julg B., Barouch D. Broadly neutralizing antibodies for HIV-1 prevention and therapy. Semin. Immunol. 2021;51:101475. doi: 10.1016/j.smim.2021.101475. [DOI] [PubMed] [Google Scholar]
- 29.Thomas A.S., Ghulam-Smith M., Sagar M. Neutralization and beyond: Antibodies and HIV-1 acquisition. Curr. Top. Virol. 2018;15:73–86. [PMC free article] [PubMed] [Google Scholar]
- 30.Naiman N.E., Slyker J., Richardson B.A., John-Stewart G., Nduati R., Overbaugh J.M. Antibody-dependent cellular cytotoxicity targeting CD4-inducible epitopes predicts mortality in HIV-infected infants. EBioMedicine. 2019;47:257–268. doi: 10.1016/j.ebiom.2019.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hompe E.D., Jacobson D.L., Eudailey J.A., Butler K., Edwards W., Pollara J., Brummel S.S., Fouda G.G., Chinula L., Kamanga M. Maternal Humoral Immune Responses Do Not Predict Postnatal HIV-1 Transmission Risk in Antiretroviral-Treated Mothers from the IMPAACT PROMISE Study. MSphere. 2019;4:1–14. doi: 10.1128/mSphere.00716-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alpert M.D., Heyer L.N., Williams D.E.J., Harvey J.D., Greenough T., Allhorn M., Evans D.T. A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1- or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays. J. Virol. 2012;86:12039–12052. doi: 10.1128/JVI.01650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richard J., Prévost J., Baxter A.E., von Bredow B., Ding S., Medjahed H., Delgado G.G., Brassard N., Stürzel C.M., Kirchhoff F. Uninfected Bystander Cells Impact the Measurement of HIV-Specific Antibody-Dependent Cellular Cytotoxicity Responses. MBio. 2018;9 doi: 10.1128/mBio.00358-18. e00358-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatziandreou N., Arauz A.B., Freitas I., Nyein P.H., Fenton G., Mehta S.H., Kirk G.D., Sagar M. Sensitivity changes over the course of infection increases the likelihood of resistance against fusion but not CCR5 receptor blockers. AIDS Res. Hum. Retroviruses. 2012;28:1584–1593. doi: 10.1089/aid.2011.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koup R.A., Sullivan J.L., Levine P.H., Brewster F., Mahr A., Mazzara G., McKenzie S., Panicali D. Antigenic specificity of antibody-dependent cell-mediated cytotoxicity directed against human immunodeficiency virus in antibody-positive sera. J. Virol. 1989;63:584–590. doi: 10.1128/jvi.63.2.584-590.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hessell A.J., Hangartner L., Hunter M., Havenith C.E.G., Beurskens F.J., Bakker J.M., Lanigan C.M.S., Landucci G., Forthal D.N., Parren P.W.H.I. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 37.Bournazos S., Klein F., Pietzsch J., Seaman M.S., Nussenzweig M.C., Ravetch J.V. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halper-Stromberg A., Lu C.L., Klein F., Horwitz J.A., Bournazos S., Nogueira L., Eisenreich T.R., Liu C., Gazumyan A., Schaefer U. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158:989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu C.-L., Murakowski D.K., Bournazos S., Schoofs T., Sarkar D., Halper-Stromberg A., Horwitz J.A., Nogueira L., Golijanin J., Gazumyan A. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016;352:1001–1004. doi: 10.1126/science.aaf1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickover R., Garratty E., Yusim K., Miller C., Korber B., Bryson Y. Role of maternal autologous neutralizing antibody in selective perinatal transmission of human immunodeficiency virus type 1 escape variants. J. Virol. 2006;80:6525–6533. doi: 10.1128/JVI.02658-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonsignori M., Liao H.X., Gao F., Williams W.B., Alam S.M., Montefiori D.C., Haynes B.F. Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunol. Rev. 2017;275:145–160. doi: 10.1111/imr.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar A., Smith C.E.P., Giorgi E.E., Eudailey J., Martinez D.R., Yusim K., Douglas A.O., Stamper L., Labranche C.C., Montefiori D.C. Infant T/F HIV-1 Viruses from Peripartum Transmission are Neutralization Resistant to Paired Maternal Plasma. PLoS Pathog. 2018;14:192. doi: 10.1371/journal.ppat.1006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez D.R., Tu J.J., Kumar A., Mangold J.F., Mangan R.J., Goswami R., Giorgi E.E., Chen J., Mengual M., Douglas A.O. Maternal broadly neutralizing antibodies can select for neutralization-resistant, infant-transmitted/founder HIV variants. MBio. 2020;11:1–22. doi: 10.1128/mBio.00176-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X., Parast A.B., Richardson B.A., Nduati R., John-Stewart G., Mbori-Ngacha D., Rainwater S.M.J., Overbaugh J. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J. Virol. 2006;80:835–844. doi: 10.1128/JVI.80.2.835-844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mielke D., Bandawe G., Pollara J., Abrahams M.R., Nyanhete T., Moore P.L., Thebus R., Yates N.L., Kappes J.C., Ochsenbauer C. Antibody-Dependent Cellular Cytotoxicity (ADCC)-Mediating Antibodies Constrain Neutralizing Antibody Escape Pathway. Front. Immunol. 2019;10:2875. doi: 10.3389/fimmu.2019.02875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nag P., Kim J., Sapiega V., Landay A.L., Bremer J.W., Mestecky J., Reichelderfer P., Kovacs A., Cohn J., Weiser B., Baum L.L. Women with cervicovaginal antibody-dependent cell-mediated cytotoxicity have lower genital HIV-1 RNA loads. J. Infect. Dis. 2004;190:1970–1978. doi: 10.1086/425582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Himes J.E., Goswami R., Mangan R.J., Kumar A., Jeffries T.L., Jr., Eudailey J.A., Heimsath H., Nguyen Q.N., Pollara J., LaBranche C. Polyclonal HIV envelope-specific breast milk antibodies limit founder SHIV acquisition and cell-associated virus loads in infant rhesus monkeys. Mucosal Immunol. 2018;11:1716–1726. doi: 10.1038/s41385-018-0067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ng C.T., Jaworski J.P., Jayaraman P., Sutton W.F., Delio P., Kuller L., Anderson D., Landucci G., Richardson B.A., Burton D.R. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat. Med. 2010;16:1117–1119. doi: 10.1038/nm.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jennewein M.F., Goldfarb I., Dolatshahi S., Cosgrove C., Noelette F.J., Krykbaeva M., Das J., Sarkar A., Gorman M.J., Fischinger S. Fc Glycan-Mediated Regulation of Placental Antibody Transfer. Cell. 2019;178:202–215.e14. doi: 10.1016/j.cell.2019.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salazar M., Salazar-Gonzalez J.F., McPherson D. Molecular Biology and Sequencing Core, University of Alabama at Birmingham; 2007. Whole Genome Amplification of HIV-1 From a Single RNA Template. Standard Operating Procedure CHAVI-MBSC-2.https://www.uab.edu/medicine/cfar/images/chavi-mbsc-21.pdf [Google Scholar]
- 51.Salazar-Gonzalez J.F., Salazar M.G., Keele B.F., Learn G.H., Giorgi E.E., Li H., Decker J.M., Wang S., Baalwa J., Kraus M.H. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 2009;206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Etemad B., Fellows A., Kwambana B., Kamat A., Feng Y., Lee S., Sagar M. Human immunodeficiency virus type 1 V1-to-V5 envelope variants from the chronic phase of infection use CCR5 and fuse more efficiently than those from early after infection. J. Virol. 2009;83:9694–9708. doi: 10.1128/JVI.00925-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.