Key Points
Question
Is clinically indicated replacement of peripheral intravenous catheters (PVCs) compared with routine replacement every 96 hours associated with increased risk of peripheral intravenous catheter bloodstream infections (PVC-BSIs)?
Findings
In this cohort study of 412 631 PVCs, there was a significantly increased incidence rate ratio of PVC-BSIs after switching from routine replacement to clinically indicated replacement of PVCs. After a routine replacement strategy was reestablished, a reduction of PVC-BSI incidence was observed.
Meaning
These findings suggest that clinically indicated replacement of PVCs is associated with an increased risk of PVC-BSIs compared with routine replacement.
Abstract
Importance
Peripheral intravenous catheters (PVCs) are the most frequently used indwelling devices in hospitals worldwide. Peripheral intravenous catheter bloodstream infections (PVC-BSIs) are rare, but severe and preventable, adverse events.
Objective
To investigate the incidence of PVC-BSIs after changing the policy of routine PVC replacement every 96 hours to clinically indicated replacement.
Design, Setting, and Participants
This institution-wide, observational cohort study evaluated all patients hospitalized at a large university-affiliated hospital with 10 sites in Western Switzerland with a PVC insertion between January 1, 2016, and February 29, 2020.
Exposures
Peripheral intravenous catheters were routinely replaced every 96 hours until March 31, 2018 (baseline period). Between April 1, 2018, and October 15, 2019, PVCs were replaced if clinically indicated (intervention period). From October 16, 2019, PVCs were again routinely replaced every 96 hours (reversion period).
Main Outcomes and Measures
The PVC-BSI rates and PVC-BSI incidence rate ratios (IRRs) during each period.
Results
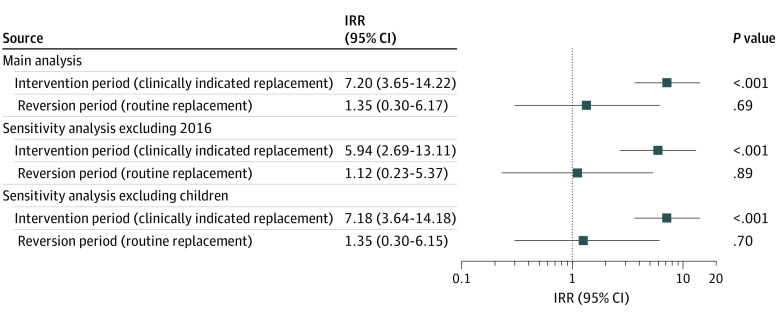
A total of 412 631 PVCs with documented catheter duration were included (164 331 patients; median [interquartile range] patient age, 51 [33-72] years; 88 928 [54.1%] female): 241 432 PVCs at baseline, 130 779 at intervention, and 40 420 at reversion. Eleven PVC-BSIs were observed during the baseline period, 46 during the intervention, and 4 during the reversion period. Although the monthly number of PVC-days remained stable during all study periods, the number of monthly inserted PVCs decreased during the intervention period. The number of PVCs still in place more than 4 or more than 7 days was higher during the intervention period compared with the baseline and reversion periods. A significantly increased IRR of PVC-BSIs was observed for the intervention period (IRR, 7.20; 95% CI, 3.65-14.22; P < .001) compared with baseline, whereas during the reversion period there was no significant increase (IRR, 1.35; 95% CI, 0.30 6.17; P = .69).
Conclusions and Relevance
The results of this cohort study using a large, prospective surveillance database suggest that replacement of PVCs only when clinically indicated may be associated with an increased risk of PVC-BSI compared with routine replacement. Even if PVC-associated BSI is a rare event, the use of PVCs in most patients makes this outcome relevant.
This cohort study examines the association between the incidence of peripheral intravenous catheter bloodstream infections and a change in hospital policy from routine catheter replacement to clinically indicated catheter replacement.
Introduction
Peripheral intravenous catheter (PVC) insertion is a frequent procedure in hospitalized patients. A global audit across 13 countries found that almost 60% of inpatients had at least 1 PVC in place.1 The estimated number of PVCs used across Europe or other regions of the world is unknown, although estimates from global device sales have been reported to be approximately 1.2 billion.2,3 In Switzerland, a mean of 48.6% (range, 47.7%-49.5%) of patients in acute care have a PVC in place every single day.4 Several complications of PVCs have been described in the literature, such as phlebitis, hematoma, extravasation, and bruising.5 The total burden of PVC-associated bloodstream infections (PVC-BSIs) is underestimated and probably represents an important proportion of nosocomial BSIs,2 as reported by a recent systematic review.6
Whether to replace catheters routinely or when clinically indicated was categorized as an unresolved question by the US Centers for Disease Control and Prevention; thus, no formal prevention recommendation was issued.7 A recent systematic review and meta-analysis8 of 7412 catheters did not find a difference in the incidence of catheter-related BSI (CRBSI) between the clinically indicated and the routine replacement groups, but only 3 PVC-BSI episodes were observed across the eligible studies. The objective of this study was to investigate the association between PVC-BSI incidence and hospital policy changes of discontinuing routine PVC replacement every 96 hours in favor of clinically indicated replacement, using a large, prospectively collected database.
Methods
Setting, Patients, and Catheters
This observational study using prospectively collected data was performed at the University of Geneva Hospitals, the largest tertiary care center in Switzerland, with 10 sites, 2008 beds, and approximately 60 000 admissions per year. All hospitalized patients between January 1, 2016, and February 29, 2020, with at least 1 PVC in place were included. We performed a retrospective analysis of prospectively collected data. The prospective surveillance was conducted as part of the routine quality improvement activities of our infection control program; thus, institutional review board approval (University of Geneva Hospitals) was not required. All data were deidentified. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.9
For more than 25 years, the University of Geneva Hospitals system has performed a hospital-wide prospective surveillance of all health care–associated BSIs, including PVC-BSIs. For each hospital-associated BSI episode, data on source of infection and clinical and microbiological characteristics are routinely collected by the infection control team. In brief, each new positive blood culture result (even without definite microbiological results; ie, prospectively) is routinely communicated from the central microbiology laboratory to the Infection Prevention and Control (IPC) team members during working days (ie, each potentially health care–associated positive blood culture result is assigned to a member of the IPC team), who prospectively follow up and investigate sources of health care–associated episodes. Criteria for investigating episodes were (1) occurrence more than 48 hours after hospital admission or previous hospitalization in the last 10 days, (2) surgery in the last 30 (nonimplant surgery) or 90 (implant surgery) days, (3) occurrence in neonatal units, (4) positive blood culture results in patient obstetric wards, and (5) positive blood culture results in hemodialysis and onco-hematologic outpatients. Data on BSI management and treatment and comorbidities at the time of diagnosis were not routinely collected. Peripheral intravenous catheter–related or PVC-associated episodes were routinely validated by 1 of 4 experts (N.B., M.-N.C., V.S., and W.Z.).
Data Sources and Variables Routinely Collected
Individual-level data on PVC (ward, insertion site, and dwell-time) and patient-level data (age and sex) were extracted from the electronic health record. All PVC-BSI data were collected from the hospital-wide database of prospective BSI surveillance.
Definitions
The primary outcome, PVC-BSI, was defined as a BSI that occurred from day of insertion until 48 hours after catheter removal and positive culture result with the same microorganism of a quantitative PVC tip culture of 103 colony-forming units (CFU) per mL or greater (or semiquantitative central venous catheter culture >15 CFU/mL) or positive superficial culture with the same microorganism from pus from insertion site (ie, catheter related), according to the European Centre for Disease Prevention and Control.10 Of note, catheter tip cultures were not routinely performed at the University of Geneva Hospitals. Alternatively, a BSI was associated with a catheter if occurring from day of insertion until 48 hours after catheter removal, the resolution of symptoms in 48 hours after catheter removal and the absence of any other infectious focus (ie, catheter associated). Common skin contaminants were considered relevant only if the patient had at least 1 sign or symptom (fever [temperature >38 °C], chills, or hypotension) of infection and 2 positive blood culture results from 2 separate blood samples within 48 hours. Common skin contaminants included coagulase-negative staphylococci, Bacillus species, Propionibacterium species, Corynebacterium species, or Micrococcus species.
Intervention and Infection Control Procedures
Hospital policy required routine replacement of PVCs every 96 hours until March 31, 2018 (baseline period). In April 2018, this policy was changed to replacing PVCs when clinically indicated only (intervention period). After October 15, 2019, the policy was changed back to routine replacement after interim analysis of the incidence of PVC-BSI (reversion period).
Infection control practices for PVC insertion and maintenance care did not change during the study periods: (1) the choice of the insertion site was left at the discretion of the nurse or physician, (2) only alcohol-based 2% chlorhexidine-gluconate was available for skin antisepsis on catheter insertion and dressing change, and (3) and semipermeable transparent dressings were used consistently and were changed when clinically indicated. Soiled, leaking, or wet dressings were required to be immediately replaced. The insertion site was required to be inspected daily for clinical signs, indicating malfunctioning or signs of phlebitis or local infection.
The choice of catheter type (eg, PVC or central venous catheter) was at the discretion of the physicians caring for each patient. In our institution, we do not have standardized algorithms or recommendations for catheter type selection. Hand hygiene adherence did not change during the intervention period (eFigure 1 in the Supplement).
Statistical Analysis
The statistical plan included 7 steps. First, we performed group comparisons between the different periods using the χ2 and Kruskal-Wallis tests for categorical and continuous variables, as appropriate, using catheters or patients as units of analysis. Second, monthly aggregated data on PVCs and PVC-days were graphically summarized from January 2016 to February 2020 (total number of PVC-days per month, number of PVCs inserted per month, number of PVC-days in PVC in situ >4 days, number of PVCs in situ >4 days, number of PVC-days in PVCs in situ >7 days, and number of PVCs in situ >7 days). Third, incidence rate ratios (IRRs) were calculated for the intervention and reversion periods, with baseline period as a reference, using segmented Poisson regression models on aggregated monthly data with catheter-days as an offset. Poisson goodness of fit was tested using the Pearson χ2 test. Overdispersion was investigated using the likelihood ratio test with subsequent fitting of a negative binomial model, where appropriate. Three sensitivity analyses were performed excluding (1) catheters inserted during the year 2016 (because few PVC-BSIs occurred during that year), (2) children (<18 years of age), or (3) PVCs inserted in the intensive care unit. Fourth, the incremental risk of PVC-BSI between the baseline and intervention period was analyzed adjusting for available covariates (sex, age, and insertion site) using a logistic regression model for rare events (Firth method) at the PVC level. Fifth, we evaluated secular trends using a Poisson regression adjusting for the effect of the intervention (as above) as well as time (in months) since start of the study. We evaluated whether there was interaction between time and the intervention. We plotted the predicted values of the model as well as the predictions under the counterfactual scenario (ie, as if the intervention was never implemented). Sixth, we conducted an interrupted time-series analysis of monthly rates of PVCs per 10 000 catheter-days for all 3 periods (baseline, intervention, and reversion) using Prais-Winsten regression (eMethods in the Supplement). Seventh, we evaluated differences in the distribution of microorganisms among PVC-BSI episodes between the routine replacement periods (baseline and reversion period) and the clinically indicated replacement period (intervention period) using the Fisher exact test. Information on missing data and censoring is summarized in the eMethods and eTable 1 in the Supplement. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc); R software, version 4.0.4 (R Foundation for Statistical Computing); RStudio, version 1.4.1717 (R Foundation for Statistical Computing); and Stata, version 14 (StataCorp LLC); and in particular the itsa command. A 2-sided P < .05 was considered statistically significant.
Results
Patients and Catheters
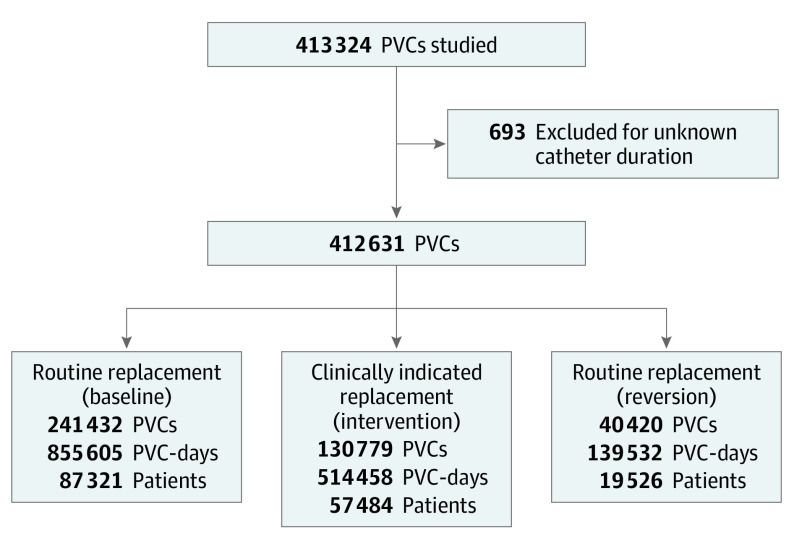
A total of 412 631 PVCs were identified in the electronic medical records and included for analysis (164 331 patients; median [interquartile range] patient age, 51 [33-72] years; 88 928 [54.1%] female): 241 432 PVCs were included in the baseline period, 130 779 in the intervention period, and 40 420 in the reversion period (Figure 1). Characteristics of patients and catheters are described as numbers (percentages) for qualitative variables and/or medians (interquartile ranges [IQRs]) for quantitative variables and are given in the Table.
Figure 1. Study Flowchart.
PVC indicates peripheral intravenous catheter.
Table. Characteristics of the Study Population by Study Perioda.
| Characteristic | Baseline | Intervention | Reversion | P value |
|---|---|---|---|---|
| Sexb | ||||
| Female | 47 114 (54.0) | 31 259 (54.4) | 10 555 (54.1) | .28 |
| Male | 40 207 (46.0) | 26 225 (45.6) | 8971 (45.9) | |
| Age, median (IQR)b | 51 (33-71) | 52 (33-72) | 55 (35-74) | <.001 |
| ICU admission | 7120 (2.9) | 2782 (2.1) | 732 (1.8) | <.001 |
| No. of catheters per patient, median (IQR)c | 1 (1-2) | 1 (1-2) | 1 (1-2) | <.001 |
| Dwell time, d | ||||
| >4 | 26 372 (10.9) | 26 656 (20.4) | 5170 (12.8) | <.001 |
| >7 | 5745 (2.4) | 10656 (8.1) | 947 (2.3) | <.001 |
| Insertion site | ||||
| Forearm | 130 877 (54.2) | 50 584 (38.7) | 15 276 (37.8) | <.001 |
| Arm | 6930 (2.9) | 2105 (1.6) | 675 (1.7) | |
| Elbow | 12 247 (5.1) | 21 508 (16.4) | 7530 (18.6) | |
| Hand | 69 615 (28.8) | 30 930 (23.7) | 9141 (22.6) | |
| Other | 6018 (2.5) | 2636 (2.0) | 771 (1.9) | |
| Wrist | 15 745 (6.5) | 23 016 (17.6) | 7027 (17.4) | |
| Operator | ||||
| Out-of-hospital | 18 909 (7.8) | 10 573 (8.1) | 2786 (6.9) | <.001 |
| In-hospital | 222 523 (92.2) | 120 206 (91.9) | 37 634 (93.1) | |
| PVC-BSI | 11 (<0.1) | 46 (<0.1) | 4 (<0.1) | <.001 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; PVC-BSI, peripheral intravenous catheter bloodstream infection.
Data are presented as number (percentage) of cases unless otherwise indicated.
For these 2 variables, patient data were used.
Data for patient per hospitalization were used (mean [SD], 1.8 [1.8] for the baseline period, 1.6 [1.5] for the intervention period, and 1.7 [1.5] for the reversion period).
During the intervention period, 20.4% of the PVCs were in place for more than 4 days, whereas 10.9% of PVCs were in place for more than 4 days in the baseline and 12.8% were in place for more than 4 days in the reversion periods (P < .001). The PVC insertion site changed significantly during the study periods, with PVCs inserted more frequently on the forearm (54.2%) and on the hand (28.8%) during the baseline period (P < .001). During the baseline period, PVCs were inserted less frequently on the wrist (6.5%) and elbow (5.1%). We observed 11 PVC-BSIs in the baseline period, 46 in the intervention period, and 4 in the reversion period.
Graphical Description
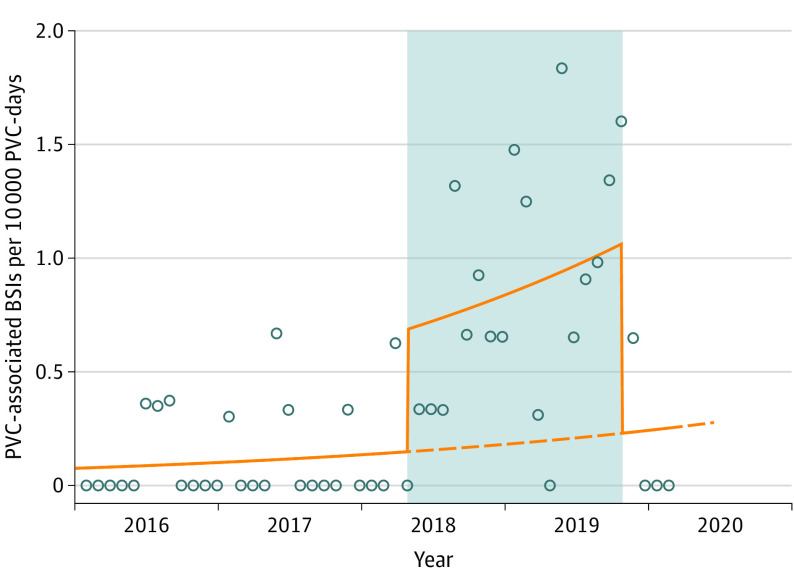
Using graphical description, we observed that the number of PVC-days per month remained stable across the study periods, whereas the number of PVCs per month decreased during the intervention period (eFigure 2 in the Supplement). The monthly numbers and percentages of PVCs in place for more than 4 days or more than 7 days were higher during the intervention period compared with the baseline and reversion periods (Figure 2). Similarly, among PVCs that were in place more than 4 days or more than 7 days, the total monthly number of PVC-days was higher during the intervention period compared with the baseline and reversion periods (Figure 2). The incidence rate of PVC-BSI during the intervention period was 0.9 per 10 000 catheter-days compared with 0.13 per 10 000 catheter-days during the baseline period (Figure 3; eFigure 3 in the Supplement).
Figure 2. Number of Peripheral Intravenous Catheter (PVC)–Days and PVCs Stratified by Catheter Duration During the 3 Study Periods.
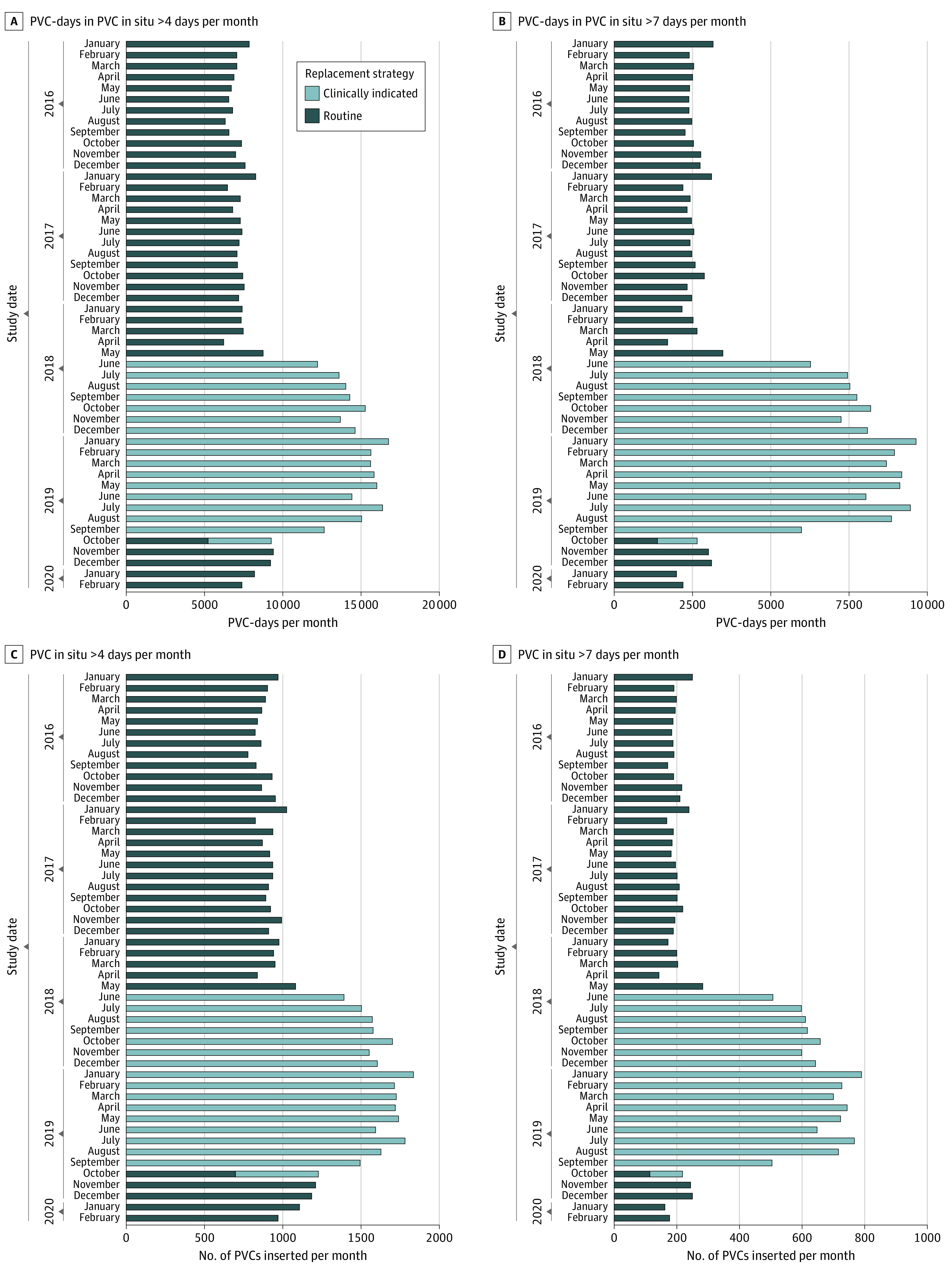
Figure 3. Monthly Incidence of Peripheral Venous Catheter (PVC)–Associated Bloodstream Infections (BSIs) During the 3 Study Periods.
The PVCs were routinely replaced every 96 hours until March 31, 2018 (baseline period). Between April 1, 2018, and October 15, 2019, the PVCs were replaced only if clinically indicated (intervention period, highlighted in gray). From October 16, 2019, the PVCs were again routinely replaced every 96 hours (reversion period). Circles indicate the monthly incidence density of PIVC-associated BSIs per 10 000 catheter-days; the solid orange line indicates the plot of the values (of PVC-associated BSIs per 10 000 catheter-days) predicted by the Poisson regression model; the dashed orange line indicates the predicted trend under counterfactual scenario (ie, if the intervention had never been implemented).
Incidence Rate Ratios
The risk of PVC-BSI in the intervention period was significantly higher (IRR, 7.20; 95% CI, 3.65-14.22; P < .001) compared with the baseline period. Conversely, the risk in the reversion period was not significantly different (IRR, 1.35; 95% CI, 0.30-6.17; P = .69) (Figure 4) compared with the baseline period. After excluding PVC data from 2016, we observed similar results for the intervention (IRR, 5.94; 95% CI, 2.69-13.11; P < .001) and reversion (IRR, 1.12; 95% CI, 0.23-5.37; P = .89) periods. After excluding PVCs inserted in children, we observed similar results for the intervention (IRR, 7.18; 95% CI, 3.64-14.18; P < .001) and reversion (IRR, 1.35; 95% CI, 0.30-6.15; P = .70) periods. After excluding PVCs inserted in the intensive care unit, we observed similar results for the intervention (IRR, 6.81; 95% CI, 3.53-13.13; P < .001) and reversion period (IRR, 1.26; 95% CI, 0.28-5.68; P = .76). Using individual catheter data and adjusting for age, sex, and insertion site, we observed an increased risk for the intervention period (adjusted odds ratio, 6.36; 95% CI, 3.60-11.23; P < .001) (eTable 2 in the Supplement). When adjusting for the time trend, the IRR for the intervention was slightly lower but still statistically significant (IRR, 4.63; 95% CI, 2.16-9.91; P < .001), and no independent trend was found for study months (IRR, 1.02; 95% CI, 0.99-1.06; P = .12) (Figure 3). The interrupted time-series analysis of monthly rates of PVCs per 10 000 catheter-days for all 3 periods (baseline, intervention, and reversion) using Prais-Winsten regression confirmed our results (eResults, eTable 3, and eFigure 4 in the Supplement).
Figure 4. Incidence Rate Ratios (IRRs) of Peripheral Venous Catheter–Associated Bloodstream Infections During the Intervention and Reversion Periods.
The baseline period (routine replacement) served as the reference.
Peripheral Intravenous Catheter Bloodstream Infections
Only 12 PVC-BSIs were observed in the first 4 days of catheter placement but 49 were observed thereafter, with a peak of 18 PVC-BSIs (29.0%) on day 5 of catheter placement (eFigure 5 in the Supplement). Characteristics of patients with PVC-BSI are given in eTable 4 in the Supplement. During the intervention period, the median dwell time in patients with PVC-BSIs (per patient) was 7 days (IQR, 5-12 days). In PVCs with more than 7 catheter-days (n = 17 330), we observed 18 PVC-BSIs (0.1%), whereas in catheters with 7 or fewer PVC-days (n = 395 240), we observed 43 PVC-BSIs (0.01%, P < .001).
We identified 15 microorganisms for the PVC-BSIs in the routine replacement periods (baseline and reversion periods) and 46 for the intervention period. During routine replacement, 9 (60.0%) were coagulase-negative staphylococci, whereas during clinically indicated replacement, more Staphylococcus aureus episodes (n = 10 [21.7%]) were observed (eTable 5 in the Supplement).
Discussion
To our knowledge, this cohort study includes the largest prospective surveillance data set ever analyzed on PVC-BSI risk after change in catheter replacement policy. We found that, after introducing a clinically indicated replacement strategy for PVC, the incidence of PVC-BSI increased substantially and returned to baseline when the policy returned to routine replacement. Our results were confirmed by different sensitivity analyses.
A recent Cochrane systematic review and meta-analysis8 of 7412 participants found that routine replacement probably reduces infiltration of fluid into surrounding tissues; moreover, rates of catheter failure caused by blockage were probably lower in the routine replacement group compared with the clinically indicated group. However, clinically indicated removal may have reduced device-related costs, and no clear difference in the incidence of thrombophlebitis between clinically indicated and routine replacement was observed.8 The authors found similar incidences of CRBSI (1 of 3590 patients in the clinically indicated replacement group vs 2 of 3733 patients in the routine replacement group). In light of these results, the authors suggested changing to a policy whereby catheters are replaced only if there is a clinical indication. Of interest, a more recent randomized clinical trial (RCT)11 of 1319 patients found similar results, but no catheter-associated BSIs were observed in the 2 groups. In general, RCTs have usually not specifically targeted PVC-BSI as a primary outcome because of its rare occurrence. In 5 of 7 RCTs, CRBSIs were not observed.11,12,13,14,15 Two trials reported 2 suspected BSIs16 and 1 CRBSI.17 Despite large numbers at first glance, all RCTs were underpowered for detecting differences in PVC-BSIs. Thus, large prospective cohort studies are required to complement the weak evidence generated by RCTs.
It is debatable whether an incidence increase to approximately 1 PVC-BSI per 10 000 catheter-days justifies routine replacement of PVCs. Indeed, PVC-BSI is the rarest of the many complications of vascular access, and we found that routine replacement is associated with a higher number of used catheters per hospital stay. Repeated insertion is associated with patient discomfort and decreased venous capital for patients, whereas for health care workers, it is associated with increased risks of needlestick injuries and is time consuming for vascular access teams. However, longer dwell time may also cause phlebitis, by far the most common complication of PVC use. Catheter-related infections are associated with increased mortality and length of stay,18 and a concerning increase of true pathogens (ie, other than common skin contaminants) was observed in the current study’s cohort19 during the intervention period. Not surprisingly, PVC-BSIs caused by S aureus in particular are significantly associated with mortality.20
Strengths and Limitations
This study has strengths, including the large number of PVCs observed in a real-world setting in an institution with a long-standing prospective BSI surveillance program. We noticed that several PVCs were not routinely replaced in our cohort even if recommended (ie, baseline and reversion periods). Most PVC-BSIs occurred in PVCs with long dwell times. After further relaxing of infection prevention measures and subsequent increase of PVCs associated with long dwell times, a significant increase of PVC-BSI was detected, which may explain the significant risk increase. Peripheral intravenous catheter bloodstream infections decreased after returning to routine replacement after 4 days. This cutoff was selected based on the available evidence base. However, our data suggest that the best cutoff for PVC replacement aimed at reducing PVC-BSI may be longer but remains to be further elucidated.
Our study has several limitations. First, although we used a large data set with high-quality prospective observational data, PVC replacement strategies were not randomized. However, to our knowledge, no large RCT with PVC-BSI as the primary outcome is currently planned, and even if it were, it may not reflect real-life conditions. It is conceivable that patients included in RCTs would be more carefully monitored and subsequently may be less prone to developing subsequent catheter infections. Second, insertion sites have changed over time (Table), which may influence our results. However, no differences were found in insertion sites between the intervention and reversion periods, which works against this hypothesis. Third, the reversion period was short (4.5 months of observation, until February 29, 2020). Initially, this period was intended to be longer. However, because of the COVID-19 pandemic, the hospital’s case mix and clinical practices changed markedly; thus, patients hospitalized during the pandemic may have been at increased risk of hospital-acquired infections.21,22 Fourth, data on monitoring of PVC cultures were not available; thus, we cannot exclude that more PVC tip cultures were performed during the intervention period. However, PVC tip cultures were performed rarely and only because of suggested infectious complications; PVC diagnosed by catheter tip cultures represented only 13% of all PVC-BSI episodes. Moreover, institutional recommendations regarding catheter tip cultures did not change during the study period.
Conclusions
In this study, clinically indicated replacement of PVCs was associated with an increased risk of PVC-BSI compared with routine replacement, which only could have been identified by analyzing a sufficiently large, prospective surveillance database. Even if PVC-associated BSI is a rare event, the use of PVCs in most patients makes this outcome relevant.
eFigure 1. Hand Hygiene Compliance During the Study Periods
eFigure 2. Number Of Peripheral Venous Catheters and Catheter-Days During the Study (January 2016-February 2020)
eFigure 3. Peripherally Inserted Catheter–Associated Bloodstream Infections During the Study Period (January 2016-February 2020).
eFigure 4. Interrupted Time-Series Analysis
eFigure 5. Peripherally Inserted Catheter–Associated Bloodstream Infections by Dwell Time.
eMethods. Interrupted Time-Series Analysis and Missing Data
eTable 1. Included and Excluded Peripheral Venous Catheters
eTable 2. Logistic Regression Model Adjusting for Sex, Age and Insertion Site
eTable 3. Interrupted Time-Series Analysis
eTable 4. Description of Patients With Peripherally Inserted Catheter–Associated Bloodstream Infections During the Three Different Study Periods.
eTable 5. Microbiological Etiology of Peripherally Inserted Catheter–Associated Bloodstream Infection, Stratified by Routine and Clinically Indicated Replacement Periods
eResults. Interrupted Time-Series Analysis
References
- 1.Alexandrou E, Ray-Barruel G, Carr PJ, et al. ; OMG Study Group . Use of short peripheral intravenous catheters: characteristics, management, and outcomes worldwide. J Hosp Med. 2018;13(5). doi: 10.12788/jhm.3039 [DOI] [PubMed] [Google Scholar]
- 2.Zingg W, Pittet D. Peripheral venous catheters: an under-evaluated problem. Int J Antimicrob Agents. 2009;34(suppl 4):S38–S42. doi: 10.1016/S0924-8579(09)70565-5 [DOI] [PubMed] [Google Scholar]
- 3.PR Newswire. Global peripheral I.V. catheter market 2014–2018. Accessed August 22, 2021. http://www.prnewswire.com/news-releases/globalperipheral-iv-catheter-market-2014–2018-257019061.html
- 4.Zingg W, Metsini A, Balmelli C, et al. National point prevalence survey on healthcare-associated infections in acute care hospitals, Switzerland, 2017. Eur Surveill. 2019;24(32):1800603. doi: 10.2807/1560-7917.ES.2019.24.32.1800603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beecham GB, Tackling G. Peripheral Line Placement. StatPearls; 2020. [PubMed] [Google Scholar]
- 6.Mermel LA. Short-term peripheral venous catheter-related bloodstream infections: a systematic review. Clin Infect Dis. 2017;65(10):1757-1762. doi: 10.1093/cid/cix562 [DOI] [PubMed] [Google Scholar]
- 7.US Centers for Disease Control and Prevention. CDC Guidelines for the Prevention of Intravascular Catheter-Related Infections. Updated 2017. Accessed July 1, 2021. https://www.cdc.gov/infectioncontrol/guidelines/bsi/recommendations.html
- 8.Webster J, Osborne S, Rickard CM, Marsh N. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2019;1:CD007798. doi: 10.1002/14651858.CD007798.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806-808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Centre for Disease Prevention and Control. Surveillance of healthcare-associated infections and prevention indicators in European intensive care units. 2017. Accessed July 1, 2021.https://www.ecdc.europa.eu/sites/default/files/documents/HAI-Net-ICU-protocol-v2.2_0.pdf
- 11.Vendramim P, Avelar AFM, Rickard CM, Pedreira MDLG. The RESPECT trial-replacement of peripheral intravenous catheters according to clinical reasons or every 96 hours: a randomized, controlled, non-inferiority trial. Int J Nurs Stud. 2020;107:103504. doi: 10.1016/j.ijnurstu.2019.103504 [DOI] [PubMed] [Google Scholar]
- 12.Rickard CM, McCann D, Munnings J, McGrail MR. Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: a randomised controlled trial. BMC Med. 2010;8:53. doi: 10.1186/1741-7015-8-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Donk P, Rickard CM, McGrail MR, Doolan G. Routine replacement versus clinical monitoring of peripheral intravenous catheters in a regional hospital in the home program: a randomized controlled trial. Infect Control Hosp Epidemiol. 2009;30(9):915-917. doi: 10.1086/599776 [DOI] [PubMed] [Google Scholar]
- 14.Xu L, Hu Y, Huang X, Fu J, Zhang J. Clinically indicated replacement versus routine replacement of peripheral venous catheters in adults: a nonblinded, cluster-randomized trial in China. Int J Nurs Pract. 2017;23(6). doi: 10.1111/ijn.12595 [DOI] [PubMed] [Google Scholar]
- 15.Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a Research base for Intravenous Peripheral cannula re-sites (DRIP trial): a randomised controlled trial of hospital in-patients. Int J Nurs Stud. 2007;44(5):664-671. doi: 10.1016/j.ijnurstu.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 16.Webster J, Clarke S, Paterson D, et al. Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial. BMJ. 2008;337:a339. doi: 10.1136/bmj.a339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rickard CM, Webster J, Wallis MC, et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380(9847):1066-1074. doi: 10.1016/S0140-6736(12)61082-4 [DOI] [PubMed] [Google Scholar]
- 18.Olaechea PM, Palomar M, Álvarez-Lerma F, Otal JJ, Insausti J, López-Pueyo MJ; ENVIN-HELICS Group . Morbidity and mortality associated with primary and catheter-related bloodstream infections in critically ill patients. Rev Esp Quimioter. 2013;26(1):21-29. [PubMed] [Google Scholar]
- 19.Heilmann C, Ziebuhr W, Becker K. Are coagulase-negative staphylococci virulent? Clin Microbiol Infect. 2019;25(9):1071-1080. doi: 10.1016/j.cmi.2018.11.012 [DOI] [PubMed] [Google Scholar]
- 20.Pujol M, Hornero A, Saballs M, et al. Clinical epidemiology and outcomes of peripheral venous catheter-related bloodstream infections at a university-affiliated hospital. J Hosp Infect. 2007;67(1):22-29. doi: 10.1016/j.jhin.2007.06.017 [DOI] [PubMed] [Google Scholar]
- 21.Buetti N, Ruckly S, de Montmollin E, et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med. 2021;47(2):180-187. doi: 10.1007/s00134-021-06346-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Zhang Y, Wu J, et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microbes Infect. 2020;9(1):1958-1964. doi: 10.1080/22221751.2020.1812437 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Hand Hygiene Compliance During the Study Periods
eFigure 2. Number Of Peripheral Venous Catheters and Catheter-Days During the Study (January 2016-February 2020)
eFigure 3. Peripherally Inserted Catheter–Associated Bloodstream Infections During the Study Period (January 2016-February 2020).
eFigure 4. Interrupted Time-Series Analysis
eFigure 5. Peripherally Inserted Catheter–Associated Bloodstream Infections by Dwell Time.
eMethods. Interrupted Time-Series Analysis and Missing Data
eTable 1. Included and Excluded Peripheral Venous Catheters
eTable 2. Logistic Regression Model Adjusting for Sex, Age and Insertion Site
eTable 3. Interrupted Time-Series Analysis
eTable 4. Description of Patients With Peripherally Inserted Catheter–Associated Bloodstream Infections During the Three Different Study Periods.
eTable 5. Microbiological Etiology of Peripherally Inserted Catheter–Associated Bloodstream Infection, Stratified by Routine and Clinically Indicated Replacement Periods
eResults. Interrupted Time-Series Analysis