Abstract
Abstract
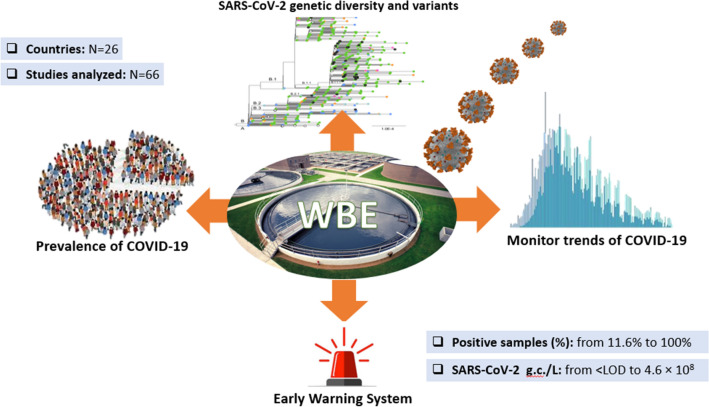
The outbreak of coronavirus infectious disease-2019 (COVID-19), caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has rapidly spread throughout the world. Several studies have shown that detecting SARS-CoV-2 in untreated wastewater can be a useful tool to identify new outbreaks, establish outbreak trends, and assess the prevalence of infections. On 06 May 2021, over a year into the pandemic, we conducted a scoping review aiming to summarize research data on SARS-CoV-2 in sewage. Papers dealing with raw sewage collected at wastewater treatment plants, sewer networks, septic tanks, and sludge treatment facilities were included in this review. We also reviewed studies on sewage collected in community settings such as private or municipal hospitals, healthcare facilities, nursing homes, dormitories, campuses, airports, aircraft, and cruise ships. The literature search was conducted using the electronic databases PubMed, EMBASE, and Web Science Core Collection. This comprehensive research yielded 1090 results, 66 of which met the inclusion criteria and are discussed in this review. Studies from 26 countries worldwide have investigated the occurrence of SARS-CoV-2 in sewage of different origin. The percentage of positive samples in sewage ranged from 11.6 to 100%, with viral concentrations ranging from ˂LOD to 4.6 × 108 genome copies/L. This review outlines the evidence currently available on wastewater surveillance: (i) as an early warning system capable of predicting COVID-19 outbreaks days or weeks before clinical cases; (ii) as a tool capable of establishing trends in current outbreaks; (iii) estimating the prevalence of infections; and (iv) studying SARS-CoV-2 genetic diversity. In conclusion, as a cost-effective, rapid, and reliable source of information on the spread of SARS-CoV-2 and its variants in the population, wastewater surveillance can enhance genomic and epidemiological surveillance with independent and complementary data to inform public health decision-making during the ongoing pandemic.
Graphic Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12560-021-09498-6.
Keywords: COVID-19, SARS-CoV-2, Wastewater, Surveillance, Sewage
Introduction
Coronavirus disease-2019 (COVID-19) emerged in China in December 2019 and has since become a global pandemic, with over 180.000.000 confirmed cases globally and 3.900.000 deaths as of July 03, 2021 (WHO, 2021). The true number of cases is likely to have been substantially greater than reported, since mild or asymptomatic infections have often been overlooked. On March 11, 2020, “deeply concerned by the alarming levels of spread and severity, and by the alarming levels of inaction”, WHO declared COVID-19 a global pandemic (WHO, 2020). Currently, the epidemiological scenario varies among countries, depending on their epidemic phase and mitigation measures. A growing number of SARS-CoV-2 variant sequences have been detected since the beginning of the pandemic, some of which are considered of global concern for possible increased transmissibility, virulence, or ability to evade host immune response (WHO, 2021).
The clinical manifestations of COVID-19 range from asymptomatic or mild to life-threatening disease. Symptoms are mainly respiratory. COVID-19 pneumonia can lead to severe respiratory distress requiring mechanical ventilation, multiple organ failure, and death (Ruan et al., 2020; Wu & McGoogan, 2020). Nevertheless, gastrointestinal infections have been reported, with symptoms such as diarrhea, nausea, vomiting, and abdominal pain (Alberca et al. 2021a).
Significant concentrations of SARS-CoV-2 RNA have been detected in the feces of infected individuals, both asymptomatic and symptomatic, even after recovery from respiratory symptoms (Alberca et al., 2021, Dergram et al., 2021; Joukar et al., 2021; Nishiura et al., 2020, Pedersen et al., 2021; Petrillo et al., 2021; Treibel et al., 2020, Wölfel et al., 2020; Wu et al., 2021; Zhang et al., 2021), with concentrations up to 107 copies per gram of feces, depending on the course of infection (Guo et al., 2021; Saawarn & Hait, 2021). This has raised concerns about the possibility of fecal–oral transmission of the virus, although the main infection routes are currently believed to be by respiratory droplets, airborne transmission, and direct or indirect contact (Greenhalgh et al., 2021; Lodder & de Roda Husman, 2020; van Doorn et al., 2020).
Given that SARS-CoV-2 RNA is detectable in feces, testing for it in sewersheds enables the monitoring of disease burden in the community. Thus far, SARS-CoV-2 RNA has been found in raw sewage and primary sludge worldwide, as well as in treated wastewater and river water (Amahmid et al., 2021; Foladori et al., 2020; Mohapatra et al., 2021). Contrary to clinical reporting systems, wastewater surveillance has the advantage of covering not only symptomatic individuals who had been tested, but also asymptomatic and symptomatic cases who had not been tested. It is, thus, an excellent tool to complement the clinical surveillance of populations under the threat of COVID-19.
This scoping review provides an overview of the studies published on SARS-CoV-2 wastewater analysis over a year into the pandemic. Its aim is to outline the current evidence regarding the potential of wastewater surveillance: (i) as an early warning system to identify early signs of outbreaks; (ii) to monitor trends in ongoing outbreaks (presence/absence, stagnation/increase/decrease in the number of cases); (iii) to estimate the prevalence of infections; and (iv) to study SARS-CoV-2 genetic diversity and variants in the community.
Methods
An electronic literature search was conducted on 06 May 2021, using the electronic databases PubMed, EMBASE, and Web Science Core Collection, with no restrictions on publication date or language. The search strategy included terms related to SARS-CoV-2 and the environmental matrix of interest (Supplementary Table S1).
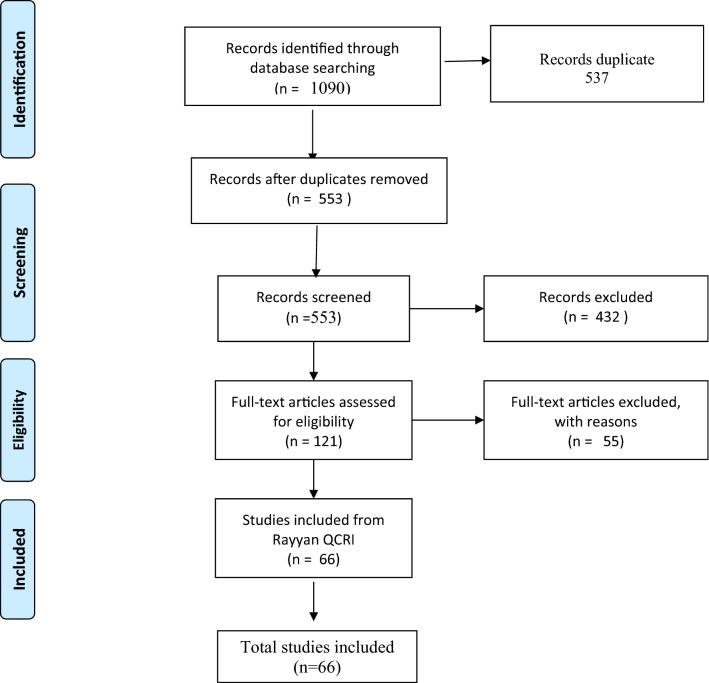
A total of 1090 articles were retrieved. After the removal of duplicates (n = 537), the remaining 553 articles were manually screened using the Rayyan review platform (Ouzzani et al., 2016), and assessed for eligibility by two independent reviewers (GLR and GBF). All articles dealing with (i) early warning to identify signs of outbreaks; (ii) trends in ongoing outbreaks (presence/absence, stagnation/increase/decrease in the number of cases); (iii) estimating the prevalence of infections; (iv) studying SARS-CoV-2 genetic diversity and variants in the community, were included in the study. Preprint articles were not included in this review. Based on these inclusion criteria, 432 records were excluded, leaving 121 articles. These were subjected to full-text screening, resulting in the exclusion of an additional 55 papers, which were either (i) unrelated to the detection of SARS-CoV-2 in sewage or to wastewater surveillance (e.g., articles dealing with the standardization of methods or disinfection procedures) (ii) reviews including data already retrieved directly from research articles.
Ultimately, 66 articles were included in the review. A flow chart illustrating the steps of paper selection can be found in Fig. 1.
Fig. 1.
PRISMA flow diagram
Results
The presence of SARS CoV-2 in sewage of different origin has been reported in a total of 26 countries, spanning practically all the continent (Europe, Americas, Asia, Oceania, and Africa). Sixty-six studies, conducted since the beginning of the pandemic, are reviewed and classified here, according to the type of sewage, into three different categories: raw sewage (n = 63), sludge (n = 7) and septic tank (n = 1). Five studies covered more than one sewage type (Chakraborty et al., 2021, D’Aoust et al. 2021b, Graham et al., 2021, Li et al., 2021a, Petala et al., 2021).
Details of the 66 studies (country, category, origin of sewage) are shown in Table 1 and in supplementary Table 2. For each study, information regarding country, sample type, collection time, number of Wastewater Treatment Plants (WWTPs) involved, percentages of positive samples and relative concentrations were retrieved (Table 1). The vast majority of the studies focused on samples collected in WWTPs (n = 57), followed by hospitals (n = 8), aircraft (n = 2), dormitories (n = 2), a sewer network (n = 1), a nursing home (n = 1), a COVID-19 isolation center (n = 1), a cruise ship (n = 1), a campus (n = 1) and a private residence (n = 1). The number of sewage treatment plants included in each study ranged from 2 to 33. The percentage of SARS-CoV-2 positive samples ranged from 11.6 to 100%, and the concentrations of SARS-CoV-2 in wastewater ranged from ˂LOD to 4.6 × 108 genome copies (g.c.)/L (see Table 1).
Table 1.
Characteristics of included studies
| Author, Year | Country | Sampling time | Sample type | Specific locations | No of sampling points | % of positive samples | Viral concentration | Use of wastewater surveillance | Main findings | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Agrawal, (2021a) | Germany | Apr/2020-Aug/2020 | Raw sewage | WTP | 2 | Not reported | 2 × 103 to 3 × 106 (g.c./L) | A, B |
SARS-CoV-2 RNA increase preceded cases by two weeks Corresponding increase in the viral load in sewage and incidence |
| 2 | Agrawal, (2021b) | Germany | Dec/2020 | Raw sewage | WTP | 3 | Not reported | Not reported | D | Mutations found in wastewater samples not identified in clinical settings in the investigated area |
| 3 | Ahmed, (2021a) | Australia | Feb/2020-May/2020 | Raw sewage | WTP | 3 | 21/63 (33%) | 1.35 × 103 to 1.19 × 105 (g.c./L) | A, B |
SARS-CoV-2 RNA detected up to three weeks before the first clinical case was reported there Decline of SARS-CoV-2 RNA correlates with the decline of the first epidemic wave No correlation of viral loads in wastewater with daily cases |
| 4 | Ahmed, (2021b) | Bangladesh | Jul/2020-Aug/2020 | Septic tank samples | COVID-19 isolation center | 3 | 21/63 (33%) | Up to 10 × 107 (g.c./L) | B |
First report of SARS-CoV-2 RNA in wastewaters in Bangladesh in the vicinity of COVID-19 isolation Center Distance in few meters from the excretion point had no significant influence on SARS-CoV-2 Ct-value |
| 5 | Ahmed, (2020a) | Australia | Apr/2020 | Raw sewage | aircraft, cruise ship | Not reported | 8/16 (50%) | < LOD to 5.9 × 103 (g.c./L) | B | SARS-CoV-2 RNA detected in wastewater from aircrafts and cruise ships, suggesting possible use of these samples for screening and contact tracing |
| 6 | Ahmed, (2020b) | Australia | Mar/2020-Apr/2020 | Raw sewage | WTP | 3 | 2/9 (22%) | 19 to 1.2 × 102 (g.c./L) | C |
First report of SARS-CoV-2 RNA in wastewaters in Australia Number of infected individuals in the catchment estimated via Monte Carlo simulation in agreement with clinical observations |
| 7 | Albastaki, (2021) | United Arab Emirates | Apr/2020-Jul/2020 | Raw sewage | WTP, aircraft | 9 | 6/27 (22%) | Not reported | B |
First report of SARS-CoV-2 RNA in wastewaters in the United Arab Emirates Viral load (reported using Ct values) in wastewaters decreased in correspondance with the decrease of COVID-19 cases 13.6% of wastewater samples from aircrafts were positive |
| 8 | Arora, (2020) | India | May/2020-Jun/2020 | Raw sewage | WTP, hospital |
6 WTP, 2 Hospital |
5/12 (42%), 1/5 (20%) |
Not reported | B |
First report of SARS-CoV-2 RNA in wastewaters in India Viral loads correlated with the increased number of COVID-19 positive patients in the same areas |
| 9 | Baldovin et al. (2021) | Italy | Apr/2020-May/2020 | Raw sewage | WTP | 4 | 4/9 (44%) | 6.3 × 104 to 7.9 × 104 (g.c./L) | C |
Report of SARS-CoV-2 RNA in wastewaters in Italy Hospitalization data suggested a WBE detection power of about 1 COVID-19 case per 531 inhabitants |
| 10 | Bertrand, (2021) | France | Apr/2020-May/2020 | Raw sewage | WTP | 1 | 4/12 (33%) | 2.1 × 107 to 1.6 × 107 (g.c./L) | B | Decrease of SARS-CoV-2 RNA concentration in wastewaters observed during lockdown, correlating with the decrease of COVID-19 cases in the area |
| 11 | Betancourt, (2021) | USA | Aug/2020-Nov/2020 | Raw sewage | campus | 1 | 6/14 (43%) | 1.0 × 104 to 1.06 × 106 (g.c./L) | A |
Sewage surveillance was used to monitor students at their return in the fall and identified positive individuals in a dorm (both symptomatic and asymptomatic) Sewage surveillance provided early warnings of infections in 13 dorms during the fall semester |
| 12 | Carrillo-Reyes, (2021) | Me × ico | Apr/2020-Jul/2020 | Raw sewage | WTP | 2 | 8/22 (36%) | 2.1 × 104 to 1.4 × 108 (g.c./L) | B | SARS-CoV-2 RNA in the influent of WTPs showed a significant correlation with the cumulative COVID-19 cases in the city |
| 13 | Chakraborty, (2021) | India | Sep/2020 | Raw sewage, Sludge samples | hospital | 9 | 12/17 (71%) |
STPs (range 9.66 × 104 to 1.99 × 105 (g.c./L); SPSs (range 1.41 × 104 to 9.96 × 104 g.c./L); Hospital (1.19 × 104 to 9.89 × 104 g.c./L) |
C | The estimated number of infected individuals calculated based on wastewater data was in line with the number of active COVID-19 cases in the catchment areas |
| 14 | Chavarria-Miró, (2021) | Spain | Dec/2019-Jul/2020 | Raw sewage | WTP | 2 | Not reported | Not reported | A, C |
SARS-CoV-2 RNA was detected in sewage 41 days before the reporting of the first COVID-19 case Wastewater surveillance anticipated the onset of the second epidemic wave The estimation of total active shedders from SARS-CoV-2 RNA in wastewaters pointed toward a high proportion of asymptomatic individuals and an infection prevalence of 2.0–6.5% SARS-CoV-2 detection in wastewater was estimated possible with an infection prevalence of around 0.12% and 0.09% of the total population |
| 15 | Colosi, (2021) | USA | Jul/2020 | Raw sewage | WTP; dormitorie; hospital; private residence | 1 each |
2/3 (67%); 15/29 (52%); 11/11 (100%); 0/1 (0%) |
Not reported | A |
Correspondence of SARS-CoV-2 RNA detection in wastewaters from hospital and college dormitories with presence/absence of COVID-19 cases detected via clinical testing SARS-CoV-2 RNA detection in presence of a small number of asymptomatic cases Poor correlation between PCR Ct values and number of new cases |
| 16 | Crits-Christoph, (2021) | USA | May/2020-Jul/2020 | Raw sewage | WTP | 4 | 7/22 (32%) | 1.0 × 103 to 1.0 × 106 (g.c./L) | D |
NGS of SARS-CoV-2 from sewage collected in the San Francisco Bay found sequences corresponding to genomes detected in clinical specimens from the same area Variants not found in clinical samples were found in wastewaters, providing evidence for the introduction of viral lineages |
| 17 | D’Aoust, (2021b) | Canada | Apr/2020-Jun/2020 | Raw sewage, Sludge samples | WTP | 2 |
(PCS): 4/ 5 (93%); (PGS): 5/6 (82%) |
(PCS) 1.7 × 103 to 3.8 × 105 (g.c./L) | A |
SARS-CoV-2 RNA detected in primary clarified sludge when < 1% positivity was recorded in clinical testing SARS-CoV-2 RNA concentration in wastewater increased 48 h prior to a reported increase in positive cases SARS-CoV-2 RNA concentration in wastewater increased approx 96 h prior to a reported increase in community hospitalizations |
| 18 | D'Aoust, (2021a) | Canada | Jun/2020-Aug/2020 | Sludge samples | WTP | 1 | Not reported | 1.0 × 104 to 3.01 × 104 copies/copies PMMoV (*normalized with PMMoV) | B |
SARS-CoV-2 RNA in primary clarified sludge showed a significant correlation with epidemiological data: daily cases, active cases and percent positive (strongest correlations observed with the number of active cases) Pepper mild mottle virus (PMMoV) normalization of RNA showed the strongest correlation to epidemiological metrics |
| 19 | Davó, (2021) | Spain | Oct/2020-Dec/2020 | Raw sewage | nursing home | 5 | 29/300 (9.6%) | 2.2 × 103 to 4.1 × 108 (g.c./L) | A, B |
Detection of SARS-CoV-2 RNA in sewage 5 to 19 days before the identification of cases (residents or staff) in a nursing home SARS-CoV-2 RNA in wastewater increased exponentially during the outbreak SARS-CoV-2 RNA was not detected anymore in wastewaters after the end of the outbreak |
| 20 | Fongaro, (2021) | Brazil | Oct/2019-Mar/2020 | Raw sewage | WTP | 1 | Not reported | 3.1 × 105 to 4.8 × 106 (g.c./L) | A |
Detection of SARS-CoV-2 RNA in wastewaters 56 days before (> 90 in the case of Brazil) the report of COVID-19 cases in the Americas Viral loads constant until early March 2020, followed by an increase coinciding with the onset of COVID-19 cases in the region |
| 21 | Gerrity, (2021) | USA | Mar/2020-May/2020 | Raw sewage | WTP | 2 | 23/36 (64%) | 104 to 106 (g.c./L) | B |
SARS-CoV-2 RNA concentration in wastewaters in two sewersheds (normalized using PMMoV) correlated with public health data in an early phase of the pandemic Wastewater surveillance might be a lagging indicator for declining infection rates, possibly due to prolonged viral shedding |
| 22 | Gibas, (2021) | USA | Sep/2020-Nov/2020 | Raw sewage | dormitories | 19 bldgs | 45/332 (13.5%) | Not reported | A |
Identification by wastewater surveillance of asymptomatic COVID-19 cases undetected by the campus monitoring program Detection power of one single asymptomatic individual in dorms with a resident populations of 150–200 |
| 23 | Gonçalves, (2021) | Slovenia | Jun/2020 | Raw sewage | hospital | 1 | 10/15 (67%) | Not reported | A, B |
First report of SARS-CoV-2 RNA in wastewaters in Slovenia Detection of SARS-CoV-2 RNA in hospital untreated wastewater in presence of only one hospitalized COVID patient |
| 24 | Gonzalez, (2020) | USA | Mar/2020 | Raw sewage | WTP | 9 | 98/198 (49.5%) | 102 to 105 (g.c./L) | B | Increasing (phase reopenings) and decreasing trends (lockdown phase) of SARS-CoV-2 RNA over a 21-week period, correlating with outbreak clinical data |
| 25 | Graham, (2020) | USA | Mar/2020-Jul/2020 | Raw sewage, Sludge samples | WTP | 2 |
Influents 5/12 (41.6%); Solids 7/12 (58.3%) |
350 and 3100 mL/g (*ratio N1-N2 in solids and influents) | B |
SARS-CoV-2 RNA concentrations in wastewater settled solids showed a significant correlation with COVID-19 clinically confirmed cases in the initial phase of the pandemic Testing wastewater solids may be more sensitive than testing influent Normalization by PMMoV did not substantially change correlation results with new COVID-19 cases |
| 26 | Hasan, (2021) | United Arab Emirates | May/2020-Jun/2020 | Raw sewage | WTP | 11 WTP, 38 locations | 11/11 (100%); 33/45 (73%) |
(WTP) 7.5 × 102 to 3.4 × 104;(g.c./L) (Other Locations) 2.86 × 102 to 2.9 × 104 (g.c./L) |
B, C |
First report quantifying SARS-CoV-2 RNA in wastewaters in the United Arab Emirates Decrease of viral loads in wastewater correlated with the reduction of COVID-19 cases The number of infected individuals was estimated using Monte Carlo simulation (approx 1.2 × 104) One region had higher estimates despite the lower viral loads when compared to another region, indicating that proper representation of the data is crucial in environmental surveillance of SARS-CoV-2 |
| 27 | Hata, (2020) | Japan | Mar/2020-May/2020 | Raw sewage | WTP | 5 | 21/45 (47%) | 1.0 × 101 to 3.5 × 104 (g.c./L) | A |
SARS-CoV-2 RNA was detectable before the number of cases reached < 1.0 per 100,000 people SARS-CoV-2 RNA detection frequency remained high even after cases stopped increasing, possibly due to detection of virus from discharged or undiagnosed individuals |
| 28 | Hemalatha et al. (2021) | India | Jul/2020-Aug/2020 | Raw sewage | WTP | - | 12/12 (100%) | 6.6 × 102 to 2.4 × 104 (g.c./L) | C | Based on wastewater data, the infected and actively shedding population in the area under observation was estimated to be between 30.000 and 3 million |
| 29 | Hokajärvi, (2021) | Finland | Apr/2020-May/2020 | Raw sewage | WTP | 1 | 1/2 (50%) | Not reported | B |
First report of SARS-CoV-2 RNA in wastewaters in Finland Confirmed COVID-19 cases were reported in the municipalities of the sewerage network area |
| 30 | Hong et al. (2021) | Saudi Arabia | Apr/2020 | Raw sewage | hospital | 2 | 43/57 (75%) | Not reported | C | Analysis on septic tanks and biological activated sludge tanks located onsite of a hospital showed that a range of 253 – 409 positive cases out of 10,000 persons are required for SARS-CoV-2 RNA detection in wastewater |
| 31 | Izquierdo-Lara, (2021) | The Netherlands and Belgium | Apr/2020-Jul/2020 | Raw sewage | WTP | 20 | 20/55 (36%) | Not reported | D | NGS of SARS-CoV-2 in sewage found clades (19A, 20A, and 20B) clustering with clinical samples from the same region |
| 32 | Johnson, (2021) | South Africa | Jun/2020 | Raw sewage | WTP | 5 | 5/5 (100%) | 4.6 × 106 to 454 × 106 (g.c./L) | B | Viral load in wastewater samples from two WTPs differed among each other and were in line with the number of COVID-19 cases in the catchment areas |
| 33 | Karthikeyan, (2021) | USA | Jul/2020-Oct/2020 | Raw sewage | WTP | 1 | 24/24 (100%) | 2.01 × 104 (g.c./L) | A |
Peaks of SARS-CoV-2 RNA in wastewaters were followed by peaks in clinically confirmed cases Using a prediction model cases could be anticipated by 3 weeks |
| 34 | Kitamura et al. (2021) | Japan | Jun/2020-Aug/2020 | Raw sewage |
WTP, manhole |
2, 1 |
18/32 (56%) | 1.6 × 102 to 1.3 × 104 (g.c./L) | B |
Significant correlation between COVID-19 cases and SARS-CoV-2 RNA concentration in wastewaters was detected during the second epidemic wave in areas with a high prevalence of the disease A significant correlation was found between the SARS-CoV-2 RNA concentration in wastewater and the number of COVID-19 cases with respect to the onset date |
| 35 | Kumar, (2021) | India | Aug/2020-Sep/2020 | Raw sewage | WTP | 4 | 40/43 (93%) | up to 1.2 × 103 (g.c./L) | A |
SARS-CoV-2 RNA concentration in sewage was higher in September compared to August 2020, corresponding to a ~ 2.2-fold rise in the number of confirmed cases The increase of RNA concentration was detected 1–2 weeks before the in increase of confirmed cases |
| 36 | Kumar, (2020) | India | May/2020 | Raw sewage | WTP | 1 | 2/2 (100%) | 5.6 × 10 to 3.5 × 102 (g.c./L) | B |
First report of SARS-CoV-2 RNA in wastewaters in India Increase in SARS-CoV-2 RNA correlating with active COVID-19 patients |
| 37 | La Rosa, (2020) | Italy | Feb/2020-Apr/2020 | Raw sewage | WTP | 3 | 6/12 (50%) | Not reported | A |
First report of SARS-CoV-2 RNA in wastewaters in Italy SARS-CoV-2 RNA detected few days after the first notified autochthonous case, when the total number of reported COVID-19 cases was very low |
| 38 | La Rosa, (2021a) | Italy | Oct/2019-Feb/2020 | Raw sewage | WTP | 5 | 15/40 (38%) | up to 5.6 × 104 (g.c./L) | A | SARS-COV-2 RNA detected in Northen Italy mid-December 2019, two months before the first notified autochthonous case |
| 39 | La Rosa, (2021b) | Italy | Sep/2020-Feb/2021 | Raw sewage | WTP | 5 | 23/48 (48%) | 1.6 × 103 to 3.0 × 104 (g.c./L) | D | Mutations characteristic of Variants Of Concern (alfa and gamma) and of lineage 20E.EU1were detected in sewage samples |
| 40 | Li, (2021a) | USA | Aug/2020-Oct/2020 | Raw sewage, Sludge samples | WTP | 2 | Not reported |
Liquid fraction: 103.0–105.1 g.c./L, 101.2- 104.5 g.c./L, and 102.0–104.5 (N1, N2 ed E assay) Solid Fraction: 104.1–105.5 g.c./g, 101.5–106.0 g.c./g, and 101.4–106.2 g.c./g (N1, N2 ed E assay |
B |
Downward trend of SARS-CoV-2 RNA in wastewater samples in correspondance to the decrease of new COVID-19 cases Significant daily fluctuation of SARS-CoV-2 RNA in wastewater were detected (fine-scale temporal dynamics of SARS-CoV-2) |
| 41 | Martin, (2020) | England | Mar/2020-Apr/2020 | Raw sewage | WTP | 1 | 3/11 (27%) | 3.1 × 103 to 6.0 × 105 (g.c./L) | D |
Sequencing of different regions of SARS-CoV-2 demonstrated changes in variant predominance SARS-CoV-2 sequences in sewage closely resembled those from clinical samples |
| 42 | Medema, (2020) | The Netherlands | Feb/2020-Mar/2020 | Raw sewage | WTP | 7 | 20/30 (66%) | 2.6 × 103 to 2.2 × 106 (g.c./L) | A, B |
No SARS-CoV-2 RNA detection 3 weeks before the first Dutch case was reported In one urban center, SARS-CoV-2 RNA detection in sewage 6 days before the first cases were reported Viral load increase correlated significantly with the increase in COVID-19 prevalence |
| 43 | Miyani, (2020) | USA | Apr/2020-May/2020 | Raw sewage | WTP | 1 | 54/54 (100%) | 104 to 105 (g.c./L) | B |
-SARS-COV-2 RNA was detected in 100% of untreated wastewater samples collected Michigan between April 8, 2020, and May 26, 2020 -Not an attempt to make predictions or statistical associations with clinical data was performed |
| 44 | Mlejnkova, (2020) | Czech Republic | Apr/2020-Jun/2020 | Raw sewage | WTP | 33 | 13/112 (12%) | Not reported | B | SARS-CoV-2 RNA was detected in wastewater samples at a lower than expected frequency (approx 12%), considering prevalence of COVID-19 cases in the areas (between 24 and 561 cases per 100,000 inhabitants) |
| 45 | Nasseri, (2021) | Iran | Apr/2020 | Raw sewage | WTP | 3 | 12/12 (100%) | Not reported | B | SARS-CoV-2 RNA detected in wastewater in 3 cities of Iran |
| 46 | Nemudryi, (2020) | USA | Mar/2020-Jun/2020 | Raw sewage | WTP | 1 | 13/17 (77%) | 2.2 × 101 to 6.1 × 103 (g.c./L) | D | A nearly complete SARS-CoV-2 genome sequence from a wastewater sample collected in USA on Jun 2020 allowed to infer viral ancestry by phylogenetic analysis |
| 47 | Peccia, (2020) | USA | Mar/2020-Jun/2020 | Sludge samples | WTP | 1 | 73/75 (97%) | 1.7 × 106 to 4.6 × 108 (g.c./L) | A |
Throughout a 10-week study, viral loads tracked the rise and fall of cases and of COVID-19 hospital admissions SARS-CoV-2 RNA in sludge showed an increase in March that was not observed in clinical testing or hospital admissions data; the sludge results led the number of positive tests by date of specimen collection by 0–2 days, the percentage of positive tests by date of specimen collection by 0–2 days, hospital admissions by 1–4 days, and the number of positive tests by report date by 6–8 days |
| 48 | Petala et al. (2021) | Greece | Apr/2020-May/2020 | Raw sewage, Sludge samples | WTP | 1 | 16/29 (55%) | 1.6 × 106 to 3.2 × 106 (g.c./L) | B | Viral loads in sewage samples showed a declining trend up to undetectable levels in line with the very low number of infections and hospital admissions in area under observation |
| 49 | Prado, (2020) | Brazil | Apr/2020 | Raw sewage | WTP, hospital, sewers network | 12 | 5/12 (42%) | Not reported | A |
SARS-CoV-2 RNA detected prevalently in samples from areas with a higher number of reported COVID-19 cases SARS-CoV-2 RNA was also detected in one sample from an area not yet reached by the outbreak |
| 50 | Prado, (2021) | Brazil | Apr/2020-Aug/2020 | Raw sewage | WTP | 2 | 188/223 (84.3%) | 6.3 × 105 to 5.0 × 106 (g.c./L) | A, D |
SARS-CoV-2 RNA was detected in sewage in a community where no COVID-19 cases had been reported Sequencing of SARS-CoV-2 in sewage showed three strains sharing the same nucleotide mutations (clade G, B.1.1.33), which were also observed in clinical strains circulating in the same area during the study period |
| 51 | Randazzo, (2020a) | Spain | Feb/2020-Apr/2020 | Raw sewage | WTP | 3 | 12/15 (83%) | 1.65 × 105 to 9.77 × 105 (g.c./L) | A |
SARS-CoV-2 RNA was consistently detected in wastewater samples taken in late February 2020, when COVID-19 cases in that region were only incipient RT-qPCR signal in wastewaters increased and reached a plateau faster than reported cases Strong indication that SARS-CoV-2 was undergoing community transmission earlier than previously believed |
| 52 | Randazzo, (2020b) | Spain | Mar/2020-Apr/2020 | Raw sewage | WTP | 6 | 35/42 (84%) | 2.5 × 105 (g.c./L) | A | SARS-CoV-2 RNA was detected in wastewater samples in low prevalence municipalities, 12–16 days before COVID-19 cases were reported |
| 53 | Rimoldi, (2020) | Italy | Apr/2020 | Raw sewage | WTP | 3 | 4/8 (50%) | Not reported | D | A SARS-CoV-2 genome from a sewage sample collected in Northern Italy in April 2020 was similar to a clinical sampe from the same region |
| 54 | Saguti, (2021) | Sweden | Feb/2020-Jul/2020 | Raw sewage | WTP | 5 | 18/21 (86%) | 7.9 × 103 to 1.8 × 106 (g.c./L) | A |
Analysis of multiple WTPs in one city displayed differences in the local incidence of SARS-CoV-2, thus enabling the detection of local outbreaks SARS-CoV-2 RNA peaks in wastewater preceded the peaks of COVID-19 hospitalized patients by 3–4 weeks |
| 55 | Saththasivam, (2021) | Qatar | Jun/2020-Aug/2020 | Raw sewage | WTP | 5 | 43/43 (100%) | 7.8 × 103 to 5.4 × 105 (g.c./L) | B, C |
First report of SARS-CoV-2 RNA in wastewaters in Qatar The trend of PCR Ct values in wastewater samples mirrored the number of new daily positive cases The number of infected subjects was estimated by mathematical model using viral concentrations of wastewater samples; the estimated number was significantly higher than the officially reported ceses |
| 56 | Sharma et al. (2021) | India | May/2020 | Raw sewage | WTP | 6 | 12/20 (60%) | Not reported | B |
SARS-CoV-2 RNA detected in sewage samples from six different sites Viral RNA detected in samples collected in May 2020, when the number of COVID-19 cases was in rapid increase |
| 57 | Sherchan, (2020) | USA | Jan/2020-Apr/2020 | Raw sewage | WTP | 2 | 2/7 (29%) | 3.1 × 103 to 7.5 × 103 (g.c./L) | B | First report of SARS-CoV-2 RNA in wastewater in NorthAmerica, including the USA |
| 58 | Tanhaei, (2021) | Iran | Jun/2020-Jul/2020 | Raw sewage | WTP | 4 | 1/1 (100%) | Not reported | B | First report of SARS-CoV-2 RNA in wastewaters in Teheran (Iran) |
| 59 | Trottier, (2020) | France | May/2020-Jul/2020 | Raw sewage | WTP | 1 | 7/7 (100%) | > 103 to 8 × 104 (g.c./L) | A | SARS-CoV-2 RNA loads in wastewater increased 2–3 weeks before the surge of new COVID-19 patients |
| 60 | Wang, (2020) | China | Feb/2020 | Raw sewage | hospital | 1 | 3/3 (100%) | Not reported | B | Detection of SARS-CoV-2 RNA in sewage samples from the inlets of preprocessing disinfection pool in a Chinese hospital |
| 61 | Weidhaas, (2021) | USA | Apr/2020-May/2020 | Raw sewage | WTP | 10 | 77/126 (61%) |
66 ± 154 × 103 (g.c./L)(rural sewersheds or lower COVID-19 caseloads) to 390 ± 489 × 103 (g.c./L)( (urban centers with higher COVID-19 caseloads) |
B, C |
Urban sewersheds serving > 100,000 individuals and tourist communities had higher SARS-CoV-2 detection frequencies than facilities serving medium-sized and rural communities Outbreaks of COVID-19 in two communities positively correlated with an increase of SARS-CoV-2 RNA in wastewater, while a decline in COVID-19 cases preceded the decline in RNA The estimated number of SARS-CoV-2 shedders in each sewershed areas linearly correlated with the cumulative diagnosed COVID-19 cases |
| 62 | Westhaus, (2021) | Germany | Apr/2020 | Raw sewage | WTP | 9 | 9/9 (100%) | 3.0 × 103 to 2.0 × 104 (g.c./L) | B |
First report of SARS-CoV-2 RNA in wastewaters in Germany Viral loads correlated with the cumulative and the acute COVID-19 cases reported in the catchment areas |
| 63 | Wilder, (2021) | USA | Apr/2020-Jun/2020 | Raw sewage | WTP | 28 | 111/169 (66%) | Avg: 2.16 × 104 (g.c./L) | A, C |
Higher SARS-CoV-2 RNA concentration in wastewaters were significantly associated to positive COVID-19 tests reported one week later wastewater sampling crAssphage co-detection displays potential to improve interpretations of wastewater surveillance data SARS-CoV-2 RNA was quantifiable in some wastewater service areas where daily positives tests were less than 1 per 10,000 people or when weekly positive test rates within a sewershed were as low as 1.7% |
| 64 | Wu, (2020) | USA | Jan/2020-Mar/2020 | Raw sewage | WTP | 2 | 12/12 (100%) | 5.7 × 104 to 3.0 × 105 (g.c./L) | B, C |
SARS-CoV-2 RNA concentration showed an increase between March and mid-April, followed by a declining trend Viral loads were significantly higher than expected based on clinically confirmed cases in the area The number of positive cases estimated from wastewater viral titers is orders of magnitude greater than the number of confirmed clinical cases |
| 65 | Wurtzer, (2020) | France | Mar/2020-Apr/2020 | Raw sewage | WTP | 3 | 25/25 (100%) | 5 × 104 to 3 × 106 (g.c./L) | A, B |
SARS-CoV-2 genome could be detected early in the pandemic and before the epidemic grew massively (around 8 March) The concentration of viral RNA in raw sewage was approx. 104 g.c./L in samples collected at the beginning of March, when less than 10 hospitalized COVID-19 cases were reported in the catchment areas (and only 635 in the whole country) The increase of SARS-CoV-2 concentration in raw wastewater accurately followed the increase of human COVID-19 cases A marked decrease of SARS-CoV-2 concentration in raw wastewater was observed concomitantly with the reduction of new COVID-19 cases, 29 days in the lockdown |
| 66 | Zhou et al., (2021) | China | Mar/2020 | Raw sewage | WTP, hospital | 10 | 3/10 (30%) | Not reported | B |
SARS-CoV-2 RNA was found in the liquid waste of COVID-19 healthcare facility system in Wuhan Viral RNA was also detected in the urban sewerage network |
A: early warning; B: occurrence and trends of infection (and correlation with epidemiological measures); C: estimation of COVID-19 prevalence (and of the power of wastewater surveillance to detect SARS-CoV-2); D: SARS-CoV-2 genetic diversity and variants
WTP wastewater treatment plant, STP Sewage treatment Plants, SPS Sewage pomping Station, Avg average, NGS next-generation sequencing, PGS, post grid solid, PCS, primary clarified sludge
The main findings (summarized in Table S2) are presented here according to the following main areas:
-
i
Wastewater surveillance as an early warning system;
-
ii
Wastewater surveillance to assess infection occurrence and trends, and its correlation with epidemiological measures;
-
iii
Wastewater surveillance to estimate the prevalence of COVID-19 and its power to detect SARS-CoV-2 in a sewershed;
-
iv
Wastewater surveillance to investigate SARS-CoV-2 genetic diversity and variants.
Some of the studies are relevant to more than one of these categories.
Wastewater Surveillance as an Early Warning System
Twenty-five studies reported either the detection of SARS-CoV-2 in the environment before the identification of clinical cases, or a rise in SARS-CoV-2 concentrations in the environment before these trends became visible in the numbers of cases or hospitalizations (Agrawal et al., 2021a; Ahmed et al., 2021a; Betancourt et al., 2021; Chavarria-Miró et al., 2021; Colosi et al., 2021; D'Aoust et al., 2021b; Davó et al., 2021; Fongaro et al., 2021; Gibas et al., 2021; Gonçalves et al., 2021; Hata et al., 2021; Karthikeyan et al., 2021; Kumar et al., 2021; La Rosa et al., 2020, 2021a; Medema et al., 2020; Peccia et al., 2020; Prado et al., 2020, 2021; Randazzo et al., 2020a, 2020b; Saguti et al., 2021; Trottier et al., 2020; Wilder et al., 2021; Wurtzer et al., 2020).
-
i.
Occurrence of SARS-CoV-2 RNA in wastewater where no COVID-19 cases had been reported before.
Two studies — one from Italy and one from Brazil — reported on the occurrence of SARS-CoV-2 as early as December 2019. Both studies used archival samples. The former found SARS-CoV-2 in sewage two months before the first autochthonous case was reported in Italy (La Rosa et al., 2021a), and the latter, more than 90 days ahead of the reports of COVID-19 cases in Brazil (Fongaro et al., 2021). Five other studies reported the occurrence of SARS-CoV-2 in wastewater prior to confirmed cases, 6 to 41 days ahead: in Amersfoort, the Netherlands (Medema et al., 2020), in Brazil, in the state of Rio de Janeiro (Prado et al., 2020, 2021), in Connecticut, USA (Peccia et al., 2020), in Spain, in low prevalence municipalities (Randazzo et al., 2020b), and in the metropolitan area of Barcelona (Chavarria-Miró et al., 2021), and in Brisbane, Australia (Ahmed et al., 2021a).
-
ii.
Occurrence of SARS-CoV-2 RNA in wastewater at the very beginning of the epidemic, when. COVID-19 cases were only incipient, or in low prevalence periods
Five studies detected SARS-CoV-2 RNA in wastewater or primary clarified sludge when the number of officially confirmed cases was still very low: in Milan, Italy, in February 2020 at a time when only 29 cases had been officially reported in the province (La Rosa et al., 2020), in France, in March and April 2020, when only 635 cases were reported in the whole country (Wurtzer et al., 2020), in the region of Valencia, Spain, in late February 2020, when COVID-19 cases were only incipient (Randazzo et al., 2020a), in Ottawa, Canada, SARS-CoV-2 was detected in sewage during the summer of 2020, when clinical testing recorded daily percent positivity below 1% (D'Aoust et al., 2021a). Finally, in The Netherlands, virus RNA was detected in sewage when the COVID-19 prevalence was still low (Medema et al., 2020).
-
iii.
Increment of SARS-CoV-2 RNA in the wastewater before rise in clinical COVID-19 cases.
Seven studies reported viral concentrations increasing days or weeks ahead of positive test results: two days before in the City of Ottawa, Canada (D'Aoust et al., 2021b), one/two weeks ahead in Connecticut, US (Peccia et al., 2020), in New York (Wilder et al., 2021), in India (Kumar et al., 2021), and in Frankfurt, Germany (Agrawal et al., 2021a), and roughly 2–3 weeks in the Montpellier area in France (Trottier et al., 2020). Finally, in San Diego, California, a model showed that environmental surveillance data could predict one-week COVID-19 cases with excellent accuracy, and 3-week cases with fair accuracy (Karthikeyan et al., 2021).
-
iv.
Increment of SARS-CoV-2 RNA in the wastewater before rise in local hospital admissions.
Three studies documented an increase in viral concentrations days or weeks ahead of local hospital admissions or increase in hospitalization: 1–4 days ahead in Connecticut, USA (Peccia et al., 2020), four days in the City of Ottawa, Canada (D'Aoust et al., 2021a) and three to four weeks in the city of Gothenburg and surrounding municipalities in Sweden (Saguti et al., 2021). In France, SARS-CoV-2 genome could be detected in wastewater samples during the months of March and April 2020, at a time when less than 10 hospitalized COVID-19 cases were reported in the catchment areas of the studied WWTPs (Wurtzer et al., 2020).
-
v.
Early warning system in congregate living settings.
All the studies described above were based on the analysis of raw sewage or sludge samples collected at WWTPs, and thus representing large communities. Other papers have addressed the usefulness of wastewater surveillance as an early warning system in congregate living settings like university dormitories, college, nursing homes, and hospitals.
In the USA, the results of wastewater samples collected from college dormitory complexes were highly consistent with known presence or absence of COVID-19 cases detected by clinical testing (Colosi et al., 2021). In a University campus in Arizona, the detection of virus RNA in wastewater led to selected clinical testing, resulting in the identification and isolation of infected individuals (both symptomatic and asymptomatic); subsequently, positive wastewater samples provided early warning of the presence of infections in a number of dorms, averting potential disease transmission (Betancourt et al., 2021). In another American university campus in North Carolina sewage surveillance allowed the identification of asymptomatic COVID-19 cases not detected by clinical monitoring programs, including in-house contact tracing, symptomatic testing, scheduled testing of student athletes, and daily symptom reporting (Gibas et al., 2021).
Wastewater surveillance applied to nursing homes in Valencia, Spain, detected SARS-CoV-2 infection cases. Moreover, the presence of SARS-CoV-2 RNA in sewage preceded the identification of cases among residents and staff or outbreak declaration, with lag times ranging from 5 to 19 days (Davó et al., 2021). In Slovenia, detection of SARS-CoV-2 RNA in hospital wastewater from an area with low COVID-19 prevalence was reported at a time in which only one patient was hospitalized (Gonçalves et al., 2021).
Wastewater Surveillance to Assess Infection Occurrence and Trends, and its Correlation with Epidemiological Measures
-
i.
Assessing SARS-COV-2 occurrence and trends in large communities
Thirty-five studies reported the importance of SARS-CoV-2 detection in the environment as a tool to monitor the spread of the virus in the community (Agrawal et al., 2021a, Ahmed et al., 2021a, Ahmed et al., 2021b, Ahmed et al., 2020a, Albastaki et al., 2021, Arora et al., 2020, Bertrand et al., 2021, Carrillo-Reyes et al., 2021, D’Aoust et al. 2021a, Davó et al., 2021, Gerrity et al., 2021, Gonçalves et al., 2021, Gonzalez et al., 2020, Graham et al., 2021, Hasan et al., 2021, Hokajarvi et al., 2021, Johnson et al., 2021, Kitamura et al., 2021, Kumar et al., 2020, Li et al., 2021a, Medema et al., 2020, Miyani et al., 2020, Mlejnkova et al., 2020, Nasseri et al., 2021, Petala et al., 2021, Saththasivam et al., 2021, Sharma et al., 2021, Sherchan et al., 2020, Tanhaei et al., 2021, Wang et al., 2020, Weidhaas et al., 2021, Westhaus 2021, Wu et al., 2020, Wurtzer et al., 2020, Zhou et al., 2021). Some limited their analyses to the presence or absence of the virus in wastewater without attempting to link wastewater data to epidemiological findings. The majority of studies, however, found that SARS-CoV-2 concentration reflected the circulation of the virus in the population and tracked the rise and fall of cases seen in SARS-CoV-2 clinical test results and local COVID-19 hospital admissions.
An increase in SARS-CoV-2 concentrations in sewage correlated significantly with the increase in reported COVID-19 cases or hospital admission in different studies from a number of countries: The Netherland (Medema et al., 2020), Utah (Weidhaas et al., 2021), Southern Nevada (Gerrity et al., 2021), Germany (Westhaus 2021; Agrawal et al., 2021a), Dubai (Albastaki et al., 2021), India (Arora et al., 2020; Kumar et al., 2020; Sharma et al., 2021), Mexico (Carrillo-Reyes et al., 2021), Japan (Kitamura et al., 2021).
Other studies found that a decrease in SARS-CoV-2 concentrations provided indirect evidence for a reduction in virus transmission, often in response to precautionary measures implemented by the government, including a lockdown, in the following countries: France (Bertrand et al., 2021; Wurtzer et al., 2020), Greece (Petala et al., 2021), Qatar (Saththasivam et al., 2021), Canada (city of Gatineau) (D’Aoust et al. 2021b), United Arab Emirates (Hasan et al., 2021), Hawaii (Li et al., 2021a, 2021b), and Czech Republic (Mlejnkova et al., 2020), Australia (Ahmed et al., 2021a).
Studies performed over long periods of time observed both increasing and decreasing trends, mirroring outbreak trends. In Virginia, surveillance showed an increase prior to lockdown measures, a fall and a plateau before reopenings, and a significant rise starting following reopenings (Gonzalez et al., 2020); in California, SARS-CoV-2 RNA in wastewater settled solids correlated positively and significantly with COVID-19 clinically confirmed case counts, across both rising and falling periods of the epidemiological curve (Graham et al., 2021).
While in general SARS-CoV-2 concentration reflected the circulation of the virus in the population, lack of correlation was also found. For example in Massachusetts observed viral titers in sewage were significantly higher than expected based on clinically confirmed cases (Wu et al., 2020).
Some studied found that while the increase in case counts may occur concurrently or precede the increase in SARS-CoV-2 RNA in wastewater, the decline in SARS-CoV-2 RNA in wastewater may lag the decline in case counts (Gerrity et al., 2021; Hata et al., 2021; Weidhaas et al., 2021).
One study performed in South Africa monitored different WWTPs located in different areas in a single collection date, comparing quantitative data with the number of positive COVID-19 cases detected in the areas at the time and found that SARS-CoV-2 virus titers were in line with the number of positive COVID-19 cases reported in the catchment areas (Johnson et al., 2021).
Finally, a few studies reported only on the first detection of SARS-CoV-2 in wastewater in a certain country, without examining correlations with clinical data. These studies were performed in the following countries: Bangladesh (Ahmed et al., 2021b); Finland (Hokajarvi et al., 2021), Michigan (Miyani et al., 2020), and Tehran (Nasseri et al., 2021; Tanhaei et al., 2021).
-
ii.
SARS-CoV-2 occurrence and trends in congregate living settings
Five studies demonstrated the usefulness of wastewater surveillance to study COVID-19 trends in congregate living settings, including nursing home facilities, hospitals, and large transport vessels.
One study found that RNA levels in wastewater samples collected at nursing home facilities surged exponentially over the course of outbreaks; disappearance of SARS-CoV-2 RNA from sewers, on the other hand, was associated with the control of outbreaks or with the absence of new documented cases following the implementation of adequate measures (Davó et al., 2021).
Detecting SARS-CoV-2 RNA in samples from both aircraft and cruise ship wastewater indicating that surveillance of wastewater from large transport vessels with their own sanitation systems has potential as a complementary source of data to perform clinical testing and contact tracing among disembarking passengers (Ahmed et al., 2020a).
Three studies investigated occurrence and concentrations of SARS-CoV-2 RNA in hospital sewage: in a Chinese hospital in February 2020, when a total of 33 laboratory-confirmed COVID-19 patients were hospitalized in the isolation wards (Wang et al. 2020a), in another medical facilities in China, in early March 2020 (Zhou et al., 2021), and in hospital wastewater from a low COVID-19 disease prevalence area in Slovenia at a time when only one patient was hospitalized (Gonçalves et al., 2021).
Wastewater Surveillance to Estimate the Prevalence of COVID-19 and its Power to Detect SARS-CoV-2 in a Sewershed
Given the correlations found between wastewater surveillance and clinical data, eleven studies have addressed SARS-CoV-2 detection in the environment as a useful tool to estimate the prevalence of SARS-CoV-2 in the community or to study the power of environmental surveillance to detect SARS-CoV-2 in a sewershed (i.e., the minimal number of shedding individuals needed for environmental samples to yield a positive result) (Ahmed et al., 2020b, Baldovin et al., 2021, Chakraborty et al., 2021, Chavarria-Miró et al., 2021, Hasan et al., 2021, Hemalatha et al., 2021, Hong et al., 2021, Saththasivam et al., 2021, Weidhaas et al., 2021, Wilder et al., 2021, Wu et al., 2020).
In Utah, USA, the estimated number of COVID-19 cases in sewersheds (ten WWTPs covering 1.26 M people), was found to be linearly correlated with the cumulative diagnosed COVID-19 cases in a sewershed (Weidhaas et al., 2021). The estimated wastewater SARS-CoV-2 concentration compared to case counts was 0.78:1, suggesting that the estimated sum of SARS-CoV-2 shedders is less than the sum of confirmed cases during the study period, possibly due to decay of the RNA signal in wastewater, or inefficiencies in the sample processing method, or to the fact not all COVID-19 individuals shed the virus in feces and the length and duration of shedding vary between individuals (Weidhaas et al., 2021).
On the other hand, the estimated number of infected individuals in the population, declining from 542,313 ± 51,159 to 31,181 ± 3081 over the course of the sampling period, was significantly higher than the officially reported numbers in Qatar, possibly due to the fact that sewage surveillance captures not only diagnosed, symptomatic cases, but undiagnosed, asymptomatic and paucisymptomatic cases (Saththasivam et al., 2021). Likewise, findings obtained in Massachusetts, USA showed that the number of positive cases estimated from wastewater viral titers collected at a large treatment facility (rough prevalence of 0.1% to 5% depending on the estimated average of viral genomes per ml in stool) were orders of magnitude greater than the number of confirmed clinical cases (0.026% confirmed for the state of Massachusetts) (Wu et al., 2020). The authors highlight that this discrepancy could arise from a number of factors, and additional data on viral shedding in stool over the clinical course of the disease in COVID-19 patients may be required to better interpret these findings.
In Spain, too, estimation of the total number of active shedders from SARS-CoV-2 RNA concentrations in wastewater, suggested a high proportion of asymptomatic infected individuals. The study estimated the prevalence of infection in the population to be 2.0–6.5%. Proportions of around 0.12% and 0.09% of the total population were determined to be required for successful detection of SARS-CoV-2 (Chavarria-Miró et al., 2021).
Wilder and co-workers found that SARS-CoV-2 RNA in wastewater was quantifiable in some service areas with daily positive test rates of less than 1 per 10,000 people, or when weekly positive test rates within a sewershed were as low as 1.7% (Wilder et al., 2021).
The Monte Carlo simulation was employed to estimate the number of infected individuals in a catchment area in Australia. The simulation estimated a median number of infections ranging from 1,090 on 27/3/2020 to 171 on 1/4/2020 in the catchment basin (median prevalence of 0.096% over the six-day surveillance). Estimates were in reasonable agreement with clinical observations (Ahmed et al., 2021a, 2021b).
An Italian study tested a WWTP serving the city of Padua, in northern Italy, and its hospital district, including a dedicated COVID-19 hospital, and estimated the detection power of wastewater surveillance using hospitalization data to be about 1 COVID-19 case per 500 inhabitants (Baldovin et al., 2021).
The number of infected people in different catchment areas was calculated in southern India post lockdown, in September 2020, using the estimated RNA (in gc/L) in wastewater samples, and was in line with the number of actual active COVID-19 cases in the catchment community (3983 vs 3418) (Chakraborty et al., 2021). In this same country, another study conducted from July 2020 toAugust 2020 estimated the spread of SARS-CoV-2 in the city of Hyderabad (nearly 10 million people), indicating that the number of infected people might be anywhere between thirty thousand and three million during the study period (Hemalatha et al., 2021); the possible number of infected people was calculated for three different shedding rates within the range (105, 106, and 107 copies/mL feces), owing to the uncertainty and difference in the number of viral particles excreted by infected individuals.
SARS-CoV-2 in wastewater samples collected from five WWTPs in two prefectures in Japan was more likely to be detected when COVID-19 was prevalent in the catchment area (> 10 confirmed cases per 100,000 people), but it was detectable in wastewater even before the number of cases reached 1 per 100,000 people (Hata et al., 2021).
Finally, Hong and co-workers explored the minimal number of positive cases in a community necessary to detect the virus in wastewater by analyzing wastewaters from a septic tank and a biological activated sludge tank located onsite at a hospital. They found that between 253–409 positive cases per 10,000 people are required for SARS-CoV-2 RNA to be detected in wastewater (Hong et al., 2021).
Wastewater surveillance to investigate SARS-CoV-2 diversity and variants in a community
Eight studies have reported the importance of sequencing environmental SARS-CoV-2 as a tool to determine strains circulating in the community and to study SARS-CoV-2 diversity (Agrawal et al., 2021b; Crits-Christoph et al., 2021; Izquierdo-Lara et al., 2021; La Rosa et al., 2021b; Martin et al., 2020; Nemudryi et al., 2020; Prado et al., 2021; Rimoldi et al., 2020).
The first “full-genome” sequence of SARS-CoV-2 from sewage, assembled using Ion Torrent PGM sequencer, with a mapping-based approach, was performed on a sample collected from a WWTP in northern Italy in April 2020. The phylogenetic analysis revealed that the sequenced strain was closely related to a SARS-CoV-2 strain isolated in the same region in March 2020, and to the commonest strains in Europe (Rimoldi et al., 2020).
In Germany, SARS-CoV-2 RNA from wastewater samples collected in December 2020 were sequenced. The analysis revealed 75 mutations, most of which had been previously reported in clinical samples only outside of Frankfurt, indicating that the sequencing of SARS-CoV-2 RNA in wastewater can provide insights into emerging variants in a city (Agrawal et al., 2021b).
Similar findings were reported by sequencing complete and near-complete SARS-CoV-2 consensus genomes from sewage collected in the San Francisco Bay in July 2020; the major consensus genotypes detected in the sewage were identical to clinical genomes from the region. Additional variants not found in clinical samples, were also identified in wastewaters (Crits-Christoph et al., 2021).
Martin and co-workers demonstrated changes in SARS-CoV-2 variant predominance in sewage collected in March/April 2020, using a nested RT-PCR approach targeting five different regions of the viral genome, concluding that viral RNA sequences found in sewage closely resemble those from clinical samples (Martin et al., 2020).
Genomic surveillance of strains detected in sewage in April/August 2020 in Brazil identified three complete consensus files, obtain by the assembled reads, showing the same nucleotide mutations, all belonging to clade G, B.1.1.33. These mutations had been observed in strains circulating in Rio de Janeiro during the period of the study (Prado et al., 2021).
Nemudryi and co-workers were able to obtain a nearly complete SARS-CoV-2 genome sequence from a wastewater sample collected in the USA in June 2020. Eleven single-nucleotide variants were detected in the assembled genome, which distinguished the wastewater SARS-CoV-2 sequence from the Wuhan-Hu-1/2019 reference sequence (Nemudryi et al., 2020).
Izquierdo-Lara and co-workers used next-generation sequencing of sewage samples to evaluate the diversity of SARS-CoV-2 in the Netherlands and Belgium between April and July 2020, finding the most prevalent clades (19A, 20A, and 20B), and clustering sewage samples with clinical samples from the same region. Several novel mutations in the SARS-CoV-2 genome were also detected (Izquierdo-Lara et al., 2021).
More recently, mutations characteristic of variants of concern (VOCs; Alpha and Gamma variant) and of other variants (20E (EU1)) were found in sewage samples collected in Italy between January and February 2021, using a long nested RT-PCR assay to detect key spike protein mutations distinctive of the major known circulating SARS-CoV-2 variants (La Rosa et al., 2021b).
Discussion
Environmental virologists have studied pathogens in sewage for decades. In 1946, Dr. Melnick, was the first to point out that the presence or absence of poliovirus in sewage can give not only information on the risk of waterborne transmission, but also epidemiological information (Melnick, 1947). Today, the WHO recognizes the importance of environmental surveillance for poliovirus. It recommends clinical surveillance as a gold standard, but underlines that environmental surveillance can give valuable supplementary information, particularly in urban populations where acute flaccid paralysis surveillance is absent or questionable, persistent virus circulation is suspected, or frequent virus re-introduction is perceived (World Health 2003). Indeed, the discovery of polio transmission in Israel in 2013 through sewage monitoring– the first re-emergence in that country since 1988 – provided evidence that sewage monitoring could be used to reveal possible silent transmission globally. Over the last decades, environmental surveillance has been successfully used to study the circulation of a number of other enteric viruses, such as norovirus, adenovirus, hepatitis A and E viruses, and others (La Rosa & Muscillo, 2013; Sinclair et al., 2008). Moreover, the usefulness of environmental surveillance for viral pathogens has been demonstrated for non-enteric viruses as well – viruses like papillomavirus and polyomavirus (Hamza & Hamza, 2018; La Rosa et al., 2013).
The analysis of wastewater to monitor the emergence and spread of infectious disease at a population level has received renewed attention in light of the current COVID-19 pandemic.
After the first detection of SARS-CoV-2 in wastewaters by Medema and co-workers in the Netherlands (Medema et al., 2020), a number of research groups have started working on monitoring SARS-CoV-2 in sewage. So far, National Wastewater Surveillance Systems have been implemented worldwide in response to the COVID-19 pandemic, in order to help public health officials to better understand the extent of SARS-CoV-2 infections in communities. Examples of European countries with an active WBE Surveillance system and related websites can be found in supplementary Table 3.
For further information on applicability of SARS-CoV-2 sewage surveillance for supporting public health decisions and actions, see also recent reviews (Amereh et al., 2021; Lundy et al., 2021; McClary-Gutierrez et al., 2021).
The aim of this review was to summarize the state of the art of sewage surveillance applied to SARS-CoV-2, focusing on the main findings in terms of its potential contribution to clinical COVID-19 surveillance and to efforts to control the pandemic.
Studies reviewed here reported the presence of SARS CoV-2 in sewage of different origin in 26 countries, covering practically every continent.
Twenty-five studies have reported the detection of SARS-CoV-2 in the environment before the emergence of clinical cases, or a rise of SARS-CoV-2 concentrations in the environment before these same trends became evident in the number of clinical cases or hospitalizations, illustrating the potential of WBE as an early warning system. Some of these studies documented the detection of SARS-CoV-2 RNA in sewage as early as December 2019 (Fongaro et al., 2021; La Rosa et al., 2021a) or in any case before the official notification of cases in the investigated areas (Ahmed 2021a; Chavarria-Miró et al., 2021; Medema et al., 2020; Prado et al., 2020; Randazzo et al., 2020b). Other studies found SARS-CoV-2 RNA in wastewater when COVID-19 cases in that region were incipient and only a limited number of clinical cases had been documented or reported (La Rosa et al., 2020; Randazzo et al., 2020a; Wurtzer et al., 2020). In one study, viral RNA was detectable even before the number of cases reached < 1.0 per 100,000 people (Hata et al., 2021).
What is more, different authors demonstrated increases in SARS-CoV-2 viral loads in wastewater anticipating the onset of COVID-19 reported cases or hospitalizations by days or even weeks illustrating the ability of wastewater surveillance to anticipate the onset of subsequent waves (Agrawal et al., 2021a; D'Aoust et al., 2021a; Karthikeyan et al., 2021; Kumar et al., 2021; Peccia et al., 2020; Saguti et al., 2021; Trottier et al., 2020; Wilder et al., 2021).
Some studies have also demonstrated the usefulness of sewage monitoring to provide early warning of infections in buildings, such as universities, dormitories, hospitals or nursing homes (Betancourt et al., 2021; Colosi et al., 2021; Davó et al., 2021; Gibas et al., 2021; Gonçalves et al., 2021), documenting the subsequent interventions taken following the alarm. Near-source tracking in sewers serving particular buildings has emerged as an interesting non-invasive tool that, when combined with subsequent targeted population screening, may enable rapid identification and control of facility outbreaks (Hassard et al., 2021). In general, the early detection of SARS-CoV-2 RNA in wastewater can identify hotspots and sound the alarm in the event of imminent danger, allowing public health officials to coordinate and implement interventions to control the spread of infections.
Thirty-four studies monitored the occurrence and trends of COVID-19. Some of these studies also correlated SARS-CoV-2 concentrations with epidemiological metrics, finding positive correlations between viral loads in sewage and COVID-19 cases (daily cases, active cases and percent positive) (Agrawal et al., 2021a, Albastaki et al., 2021, Arora et al., 2020, Bertrand et al., 2021, Carrillo-Reyes et al., 2021, D’Aoust et al. 2021b, Gerrity et al., 2021, Gonzalez et al., 2020, Graham et al., 2021, Hasan et al., 2021, Kitamura et al., 2021, Li et al., 2021a, Medema et al., 2020, Peccia et al., 2020, Petala et al., 2021, Saththasivam et al., 2021, Weidhaas et al., 2021, Westhaus 2021, Wurtzer et al., 2020). Moreover, the disappearance of SARS-CoV-2 RNA from sewage was associated with absence of new documented cases following the implementation of adequate preventive measures (Davó et al., 2021). Interestingly, some of the studies found that while the increase in case counts tends to occur concurrently or precede the increase in SARS-CoV-2 RNA in wastewater, the decline in SARS-CoV-2 RNA in wastewater may lag the decline in case counts, possibly due to prolonged viral shedding (Gerrity et al., 2021; Weidhaas et al., 2021).
At any rate, all these studies demonstrate that the quantitative monitoring of SARS-CoV-2 in raw sewage is a relevant indicator of the evolution of viral circulation in the population linked to a given sewage network.
About 20% of the studies included in the present review attempted to estimate the prevalence of COVID-19 in a community (residents of a WWTP’s catchment area) using SARS-CoV-2 concentration data, the WWTP's average daily influent flow rate, the size of the population served and correction factors (Ahmed et al., 2020b, Baldovin et al., 2021, Chakraborty et al., 2021, Chavarria-Miró et al., 2021, Hasan et al., 2021, Hemalatha et al., 2021, Hong et al., 2021, Saththasivam et al., 2021, Weidhaas et al., 2021, Westhaus 2021, Wilder et al., 2021, Wu et al., 2020). The results of these studies are very variable, and remain rough estimates. The estimated number of SARS-CoV-2 shedders was found to be linearly correlated with the cumulative number of diagnosed COVID-19 cases in the sewershed of one study, but was significantly higher than the officially reported numbers in another. SARS-CoV-2 RNA in wastewater was quantifiable in some service areas with less than 1 per 10,000 people testing positive daily (Wilder et al., 2021). Current limitations and future prospects for WBE as an approach for the estimation of disease burden are described in a recent review (Bhattacharya et al., 2021). Li et al., 2021a, 2021b reviewed uncertainties in assessing SARS-CoV-2 prevalence by wastewater-based epidemiology (Li et al., 2021b). They divided the estimation process into different steps involving virus shedding, in-sewer transportation, sampling and storage, analysis of SARS-CoV-2 RNA concentrations, and back-estimation, and summarized the uncertainties associated with each step (Li et al., 2021b). They concluded that considerable uncertainties arise due to the methodology and the complexity of various processes involved. The EU Commission Recommendation of 17.3.2021 on a common approach to establish a systematic surveillance of SARS-CoV-2 and its variants in wastewaters (EUROPEAN COMMISSION, 2021), also underlines the fact that “wastewater surveillance is a tool to observe trends and not an absolute means to draw conclusions about the prevalence of COVID-19 in the population.”
The Recommendation also addresses the potential of environmental surveillance to study SARS-CoV-2 variants worldwide. New virus variants are evolving and spreading across the world. Some variants are more transmissible, or have a higher propensity to cause severe disease, and therefore constitute a threat to the response against the virus. Alpha, Beta, Gamma, and Delta (B.1.1.7, B.1.351, P.1, and B.1.617.2 pango lineages, respectively, recently relabelled by the WHO) are the so-called variants of concern for which clear evidence is available indicating a significant impact on transmissibility, severity and/or immunity that is likely to affect the epidemiological situation (European Centre for Disease Prevention and Control 2021). On the other hand, for the so-called variants of interest (VOI), the evidence is still preliminary or uncertain. It is therefore of utmost importance to use all available means to detect variants as soon as possible in order to provide timely and suitable responses. Surveillance of SARS-CoV-2 variants in wastewaters can provide a cost-effective, rapid, and reliable source of information in this respect. The vast majority of papers included in the present review looked at the occurrence and quantity of SARS-CoV-2 in wastewaters, but a few studies also sequenced environmental SARS-CoV-2 in order to determine the strains circulating in the community and to study SARS-CoV-2 genetic diversity (Agrawal et al., 2021b; Crits-Christoph et al., 2021; Izquierdo-Lara et al., 2021; La Rosa et al., 2021b; Martin et al., 2020; Nemudryi et al., 2020; Prado et al., 2021; Rimoldi et al., 2020). Some of these studies provided insights into emerging variants in the areas investigated, seeing that sequences yet to be identified in clinical samples, were found in wastewaters. Also, SARS-CoV-2 VOCs have been detected in sewage (pango lineage B.1.1.7 and P.1), suggesting that tracking SARS-CoV-2 variants and their abundance in sewersheds could provide an early warning system for the emergence or spread of more infectious or virulent strains in the community. More recently, SARS-CoV-2 VOCs and VOIs were identified in wastewater samples matching those found in clinical isolates from the same time periods (Ai et al., 2021; Avgeris et al., 2021; Carcereny et al., 2021; Gregory et al., 2021; Heijnen et al., 2021; La Rosa et al., 2021c; Lee et al., 2021; Mishra et al., 2021; Rios et al., 2021; Swift et al., 2021; Wurtz et al., 2021; Yaniv et al., 2021).
Notably, some of the studies reviewed here demonstrated that the data on SARS-CoV-2 environmental monitoring were effectively used to guide public health decisions (Ahmed 2020b, Betancourt et al., 2021; Chavarria-Miró et al., 2021; Gibas et al., 2021; Prado et al., 2021; Saguti et al., 2021). For example, sewer monitoring of SARS-CoV-2 in Spain enabled the identification of specific COVID-19 hot spots and the rapid adoption of appropriate infection control measures, such as mass testing (Chavarria-Miró et al., 2021). In Sweden, an analysis of wastewater from different parts of the city of Gothenburg showing differences in local incidence of SARS-CoV-2 proved useful in the adoption of public health measures such as tracking to mitigate the spread, and in the planning of hospital bed availability and care needs (Saguti et al., 2021). In Brazil, environmental surveillance provided helped identify regions with unreported cases of the disease, mainly in socially vulnerable communities, and define regionalized coping strategies in priority areas and plan the distribution of tests and other resources (Prado et al., 2021). Based on sewage findings, an active surveillance was launched to search for individuals showing COVID-19-related symptoms, infected individuals were identified through testing and measures to control the spread of the disease were implemented. Similarly, Ahmed and co-workers showed that wastewater surveillance in large transport vessels with their own sanitation systems (aircraft and cruise ships) has potential as a complementary source of data to prioritize clinical testing and contact tracing among disembarking passengers (Ahmed et al., 2020b). Implementing building-level SARS-CoV-2 wastewater surveillance on a university campus enabled the identification of asymptomatic COVID-19 cases that were not detected by other components of the campus monitoring program (including in-house contact tracing, symptomatic testing, scheduled testing of student athletes, and daily symptom reporting). These findings were delivered to decision makers, who imposed lockdown and testing measures to control the spread of infection (Gibas et al., 2021).The University of Arizona utilized sewage surveillance paired with clinical testing as a surveillance tool to monitor the community for SARS-CoV-2 in near real time, as students re-entered campus in the fall, and identified one symptomatic and two asymptomatic individuals in a dorm, which was critical to COVID-19 containment (Betancourt et al., 2021).
In conclusion, SARS-CoV-2 was detected in wastewater before the first reported clinical cases in many countries, suggesting that monitoring of SARS-CoV-2 in wastewater could serve as an early warning system to identify signs of outbreaks and potentially prevent them in both large and small communities. Monitoring trends in viral concentrations in sewage make it possible to follow the spread and dynamics of the disease, thus allowing the implementation of timely response measures for the containment of outbreaks and wastewater-based epidemiology seems a promising approach for estimating population-wide COVID-19 prevalence. To date, however, uncertainties limit the reliability of this approach. Finally, tracking SARS-CoV-2 variants and their abundance in sewersheds could provide an early warning system for the emergence and/or spread of more infectious or virulent strains in the community.
Supplementary Information
Below is the link to the electronic supplementary material.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agrawal S, Orschler L, Lackner S. Long-term monitoring of SARS-CoV-2 RNA in wastewater of the Frankfurt metropolitan area in Southern Germany. Scientific Reports. 2021;11(1):5372. doi: 10.1038/s41598-021-84914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S, Orschler L, Lackner S. Metatranscriptomic Analysis Reveals SARS-CoV-2 mutations in wastewater of the frankfurt metropolitan area in Southern Germany. Microbiol Resour Announc. 2021 doi: 10.1128/MRA.00280-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W, Tscharke B, Bertsch PM, Bibby K, Bivins A, Choi P, Clarke L, Dwyer J, Edson J, Nguyen TMH, O'Brien JW, Simpson SL, Sherman P, Thomas KV, Verhagen R, Zaugg J, Mueller JF. SARS-CoV-2 RNA monitoring in wastewater as a potential early warning system for COVID-19 transmission in the community: A temporal case study. The Science of the Total Environment. 2021;761:144216. doi: 10.1016/j.scitotenv.2020.144216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed F, Islam MA, Kumar M, Hossain M, Bhattacharya P, Islam MT, Hossen F, Hossain MS, Islam MS, Uddin MM, Islam MN, Bahadur NM, Didar-Ul-Alam M, Reza HM, Jakariya M. First detection of SARS-CoV-2 genetic material in the vicinity of COVID-19 isolation Centre in Bangladesh: Variation along the sewer network. The Science of the Total Environment. 2021;776:145724. doi: 10.1016/j.scitotenv.2021.145724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W, Bertsch PM, Angel N, Bibby K, Bivins A, Dierens L, Edson J, Ehret J, Gyawali P, Hamilton KA, Hosegood I, Hugenholtz P, Jiang G, Kitajima M, Sichani HT, Shi J, Shimko KM, Simpson SL, Smith W, Symonds EM, Mueller JF. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: a surveillance tool for assessing the presence of COVID-19 infected travellers. Journal of Travel Medicine. 2020;27(5):116. doi: 10.1093/jtm/taaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W, Angel N, Edson J, Bibby K, Bivins A, O'Brien JW, Choi PM, Kitajima M, Simpson SL, Li J, Tscharke B, Verhagen R, Smith W, Zaugg J, Dierens L, Hugenholtz P, Thomas KV, Mueller JF. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. The Science of the Total Environment. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai Y, Davis A, Jones D, Lemeshow S, Tu H, He F, Ru P, Pan X, Bohrerova Z, Lee J. Wastewater SARS-CoV-2 monitoring as a community-level COVID-19 trend tracker and variants in Ohio. United States. Sci Total Environ. 2021;801:149757. doi: 10.1016/j.scitotenv.2021.149757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albastaki A, Naji M, Lootah R, Almeheiri R, Almulla H, Almarri I, Alreyami A, Aden A, Alghafri R. First confirmed detection of SARS-COV-2 in untreated municipal and aircraft wastewater in Dubai, UAE: The use of wastewater based epidemiology as an early warning tool to monitor the prevalence of COVID-19. Science of the Total Environment. 2021;760:143350. doi: 10.1016/j.scitotenv.2020.143350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberca GGF, Solis-Castro RL, Solis-Castro ME, Alberca RW. Coronavirus disease-2019 and the intestinal tract: An overview. World Journal Gastroenterol. 2021;27(13):1255–1266. doi: 10.3748/wjg.v27.i13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amahmid O, El Guamri Y, Rakibi Y, Ouizat S, Yazidi M, Razoki B, Kaid Rassou K, Asmama S, Bouhoum K, Belghyti D. Occurrence of SARS-CoV-2 in excreta, sewage, and environment: epidemiological significance and potential risks. International Journal of Environmental Health Research. 2021 doi: 10.1080/09603123.2021.1901865. [DOI] [PubMed] [Google Scholar]
- Amereh F, Negahban-Azar M, Isazadeh S, Dabiri H, Masihi N, Jahangiri-Rad M, Rafiee M. Sewage systems surveillance for SARS-CoV-2: identification of knowledge gaps, emerging threats, and future research needs. Pathogens. 2021;10(8):946. doi: 10.3390/pathogens10080946.PMID:34451410;PMCID:PMC8402176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S, Nag A, Sethi J, Rajvanshi J, Saxena S, Shrivastava SK, Gupta AB. Sewage surveillance for the presence of SARS-CoV-2 genome as a useful wastewater based epidemiology (WBE) tracking tool in India. Water Science and Technology : A Journal of the International Association on Water Pollution Research. 2020;82(12):2823–2836. doi: 10.2166/wst.2020.540. [DOI] [PubMed] [Google Scholar]
- Avgeris M, Adamopoulos PG, Galani A, Xagorari M, Gourgiotis D, Trougakos IP, Voulgaris N, Dimopoulos MA, Thomaidis NS, Scorilas A. Novel Nested-Seq Approach for SARS-CoV-2 Real-time epidemiology and in-depth mutational profiling in wastewater. International Journal of Molecular Sciences. 2021;22(16):8498. doi: 10.3390/ijms22168498.PMID:34445204;PMCID:PMC8395163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldovin T, Amoruso I, Fonzo M, Buja A, Baldo V, Cocchio S, Bertoncello C. SARS-CoV-2 RNA detection and persistence in wastewater samples: An experimental network for COVID-19 environmental surveillance in Padua, Veneto Region (NE Italy) The Science of the Total Environment. 2021;760:143329. doi: 10.1016/j.scitotenv.2020.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand, I., Challant, J., Jeulin, H., Hartard, C., Mathieu, L., Lopez, S., Scientific Interest Group Obépine, Schvoerer, E., Courtois, S., & Gantzer, C Epidemiological surveillance of SARS-CoV-2 by genome quantification in wastewater applied to a city in the northeast of France: Comparison of ultrafiltration- and protein precipitation-based methods. International Journal of Hygiene and Environmental Health. 2021;233:113692. doi: 10.1016/j.ijheh.2021.113692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt WQ, Schmitz BW, Innes GK, Prasek SM, Pogreba Brown KM, Stark ER, Foster AR, Sprissler RS, Harris DT, Sherchan SP, Gerba CP, Pepper IL. COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention. The Science of the Total Environment. 2021;779:146408. doi: 10.1016/j.scitotenv.2021.146408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P, Kumar M, Islam MT, Haque R, Chakraborty S, Ahmad A, Niazi NK, Cetecioglu Z, Nilsson D, Ijumulana J, van der Voorn T, Jakariya M, Hossain M, Ahmed F, Rahman M, Akter N, Johnston D, Ahmed KM. Prevalence of SARS-CoV-2 in communities through wastewater surveillance-a potential approach for estimation of disease burden. Current Pollution Reports. 2021 doi: 10.1007/s40726-021-00178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcereny A, Martínez-Velázquez A, Bosch A, Allende A, Truchado P, Cascales J, Romalde JL, Lois M, Polo D, Sánchez G, Pérez-Cataluña A, Díaz-Reolid A, Antón A, Gregori J, Garcia-Cehic D, Quer J, Palau M, Ruano CG, Pintó RM, Guix S. Monitoring Emergence of the SARS-CoV-2 B1.1.7 Variant through the Spanish National SARS-CoV-2 Wastewater Surveillance System (VATar COVID-19) Environmental Science and Technology. 2021 doi: 10.1021/acs.est.1c03589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Reyes J, Barragán-Trinidad M, Buitrón G. Surveillance of SARS-CoV-2 in sewage and wastewater treatment plants in Mexico. Journal of Water Process Engineering. 2021;40:101815. doi: 10.1016/j.jwpe.2020.101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P, Pasupuleti M, Jai Shankar MR, Bharat GK, Krishnasamy S, Dasgupta SC, Sarkar SK, Jones KC. First surveillance of SARS-CoV-2 and organic tracers in community wastewater during post lockdown in Chennai, South India: Methods, occurrence and concurrence. Science of the Total Environment. 2021;778:146252. doi: 10.1016/j.scitotenv.2021.146252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria-Miró G, Anfruns-Estrada E, Martínez-Velázquez A, Vázquez-Portero M, Guix S, Paraira M, Galofré B, Sánchez G, Pintó RM, Bosch A. Time evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wastewater during the first pandemic wave of COVID-19 in the Metropolitan Area of Barcelona. Spain. Applied and Environmental Microbiology. 2021;87(7):e02750–e2820. doi: 10.1128/AEM.02750-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosi LM, Barry KE, Kotay SM, Porter MD, Poulter MD, Ratliff C, Simmons W, Steinberg LI, Wilson DD, Morse R, Zmick P, Mathers AJ. Development of wastewater pooled surveillance of severe acute respiratory Syndrome Coronavirus 2 (SARS-CoV-2) from Congregate Living Settings. Applied and Environmental Microbiology. 2021;87(13):e0043321. doi: 10.1128/AEM.00433-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crits-Christoph A, Kantor RS, Olm MR, Whitney ON, Al-Shayeb B, Lou YC, Flamholz A, Kennedy LC, Greenwald H, Hinkle A, Hetzel J, Spitzer S, Koble J, Tan A, Hyde F, Schroth G, Kuersten S, Banfield JF, Nelson KL. Genome Sequencing of Sewage Detects Regionally Prevalent SARS-CoV-2 Variants. Mbio. 2021;12(1):2703–2720. doi: 10.1128/mBio.02703-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aoust PM, Graber TE, Mercier E, Montpetit D, Alexandrov I, Neault N, Baig AT, Mayne J, Zhang X, Alain T, Servos MR, Srikanthan N, MacKenzie M, Figeys D, Manuel D, Jüni P, MacKenzie AE, Delatolla R. Catching a resurgence: Increase in SARS-CoV-2 viral RNA identified in wastewater 48 h before COVID-19 clinical tests and 96 h before hospitalizations. The Science of the Total Environment. 2021;770:145319. doi: 10.1016/j.scitotenv.2021.145319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aoust PM, Mercier E, Montpetit D, Jia JJ, Alexandrov I, Neault N, Baig AT, Mayne J, Zhang X, Alain T, Langlois MA, Servos MR, MacKenzie M, Figeys D, MacKenzie AE, Graber TE, Delatolla R. Quantitative analysis of SARS-CoV-2 RNA from wastewater solids in communities with low COVID-19 incidence and prevalence. Water Research. 2021;188:116560. doi: 10.1016/j.watres.2020.116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davó L, Seguí R, Botija P, Beltrán MJ, Albert E, Torres I, López-Fernández PÁ, Ortí R, Maestre JF, Sánchez G, Navarro D. Early detection of SARS-CoV-2 infection cases or outbreaks at nursing homes by targeted wastewater tracking. Clinical Microbiology and Infection : the Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2021;27(7):1061–1063. doi: 10.1016/j.cmi.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergham J, Delerce J, Bedotto M, La Scola B, Moal V. Isolation of Viable SARS-CoV-2 virus from feces of an immunocompromised patient suggesting a possible fecal mode of transmission. Journal of Clinical Medicine. 2021;10(12):2696. doi: 10.3390/jcm10122696.PMID:34207314;PMCID:PMC8235306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (2021). SARS-CoV-2 variants of concern as of 24 June 2021.
- European Commission (2021) Commission RECOMMENDATION of 17.3.2021 on a common approach to establish a systematic surveillance of SARS-CoV-2 and its variants in wastewaters in the EU.
- Foladori P, Cutrupi F, Segata N, Manara S, Pinto F, Malpei F, Bruni L, La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: What do we know? A review. The Science of the Total Environment. 2020;743:140444. doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fongaro G, Stoco PH, Souza D, Grisard EC, Magri ME, Rogovski P, Schörner MA, Barazzetti FH, Christoff AP, de Oliveira L, Bazzo ML, Wagner G, Hernández M, Rodríguez-Lázaro D. The presence of SARS-CoV-2 RNA in human sewage in Santa Catarina, Brazil, November 2019. The Science of the Total Environment. 2021;778:146198. doi: 10.1016/j.scitotenv.2021.146198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity D, Papp K, Stoker M, Sims A, Frehner W. Early-pandemic wastewater surveillance of SARS-CoV-2 in Southern Nevada: Methodology, occurrence, and incidence/prevalence considerations. Water Research X. 2021 doi: 10.1016/j.wroa.2020.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibas C, Lambirth K, Mittal N, Juel M, Barua VB, Roppolo Brazell L, Hinton K, Lontai J, Stark N, Young I, Quach C, Russ M, Kauer J, Nicolosi B, Chen D, Akella S, Tang W, Schlueter J, Munir M. Implementing building-level SARS-CoV-2 wastewater surveillance on a university campus. The Science of the Total Environment. 2021;782:146749. doi: 10.1016/j.scitotenv.2021.146749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves J, Koritnik T, Mioč V, Trkov M, Bolješič M, Berginc N, Prosenc K, Kotar T, Paragi M. Detection of SARS-CoV-2 RNA in hospital wastewater from a low COVID-19 disease prevalence area. The Science of the Total Environment. 2021;755(Pt 2):143226. doi: 10.1016/j.scitotenv.2020.143226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Curtis K, Bivins A, Bibby K, Weir MH, Yetka K, Thompson H, Keeling D, Mitchell J, Gonzalez D. COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology. Water Research. 2020;186:116296. doi: 10.1016/j.watres.2020.116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KE, Loeb SK, Wolfe MK, Catoe D, Sinnott-Armstrong N, Kim S, Yamahara KM, Sassoubre LM, Mendoza Grijalva LM, Roldan-Hernandez L, Langenfeld K, Wigginton KR, Boehm AB. SARS-CoV-2 RNA in Wastewater Settled Solids Is Associated with COVID-19 Cases in a Large Urban Sewershed. Environmental Science & Technology. 2021;55(1):488–498. doi: 10.1021/acs.est.0c06191. [DOI] [PubMed] [Google Scholar]
- Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet (london, England) 2021;397(10285):1603–1605. doi: 10.1016/S0140-6736(21)00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory DA, Wieberg CG, Wenzel J, Lin CH, Johnson MC. Monitoring SARS-CoV-2 populations in wastewater by amplicon sequencing and using the Novel Program SAM Refiner. Viruses. 2021;13(8):1647. doi: 10.3390/v13081647.PMID:34452511;PMCID:PMC8402658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Tao W, Flavell RA, Zhu S. Potential intestinal infection and faecal-oral transmission of SARS-CoV-2. Nature Reviews. Gastroenterology & Hepatology. 2021;18(4):269–283. doi: 10.1038/s41575-021-00416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza H, Hamza IA. Oncogenic papillomavirus and polyomavirus in urban sewage in Egypt. The Science of the Total Environment. 2018;610–611:1413–1420. doi: 10.1016/j.scitotenv.2017.08.218. [DOI] [PubMed] [Google Scholar]
- Hasan SW, Ibrahim Y, Daou M, Kannout H, Jan N, Lopes A, Alsafar H, Yousef AF. Detection and quantification of SARS-CoV-2 RNA in wastewater and treated effluents: Surveillance of COVID-19 epidemic in the United Arab Emirates. Science of the Total Environment. 2021;764:1429. doi: 10.1016/j.scitotenv.2020.142929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassard F, Lundy L, Singer AC, Grimsley J, Di Cesare M. Innovation in wastewater near-source tracking for rapid identification of COVID-19 in schools. The Lancet. Microbe. 2021;2(1):e4–e5. doi: 10.1016/S2666-5247(20)30193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A, Hara-Yamamura H, Meuchi Y, Imai S, Honda R. Detection of SARS-CoV-2 in wastewater in Japan during a COVID-19 outbreak. The Science of the Total Environment. 2021;758:143578. doi: 10.1016/j.scitotenv.2020.143578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen L, Elsinga G, de Graaf M, Molenkamp R, Koopmans M, Medema G. Droplet digital RT-PCR to detect SARS-CoV-2 signature mutations of variants of concern in wastewater. The Science of the Total Environment. 2021;799:149456. doi: 10.1016/j.scitotenv.2021.149456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemalatha M, Kiran U, Kuncha SK, Kopperi H, Gokulan CG, Mohan SV, Mishra RK. Surveillance of SARS-CoV-2 spread using wastewater-based epidemiology: Comprehensive study. The Science of the Total Environment. 2021;768:144704. doi: 10.1016/j.scitotenv.2020.144704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokajärvi AM, Rytkönen A, Tiwari A, Kauppinen A, Oikarinen S, Lehto KM, Kankaanpää A, Gunnar T, Al-Hello H, Blomqvist S, Miettinen IT, Savolainen-Kopra C, Pitkänen T. The detection and stability of the SARS-CoV-2 RNA biomarkers in wastewater influent in Helsinki, Finland. The Science of the Total Environment. 2021;770:145274. doi: 10.1016/j.scitotenv.2021.145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, P. Y., Rachmadi, A. T., Mantilla-Calderon, D., Alkahtani, M., Bashawri, Y. M., Al Qarni, H., O'Reilly, K. M., & Zhou, J. (2021). Estimating the minimum number of SARS-CoV-2 infected cases needed to detect viral RNA in wastewater: To what extent of the outbreak can surveillance of wastewater tell us? Environmental Research, 195, 110748. 10.1016/j.envres.2021.110748 [DOI] [PMC free article] [PubMed]
- Izquierdo-Lara R, Elsinga G, Heijnen L, Munnink B, Schapendonk C, Nieuwenhuijse D, Kon M, Lu L, Aarestrup FM, Lycett S, Medema G, Koopmans M, de Graaf M. Monitoring SARS-CoV-2 Circulation and diversity through community wastewater sequencing, the Netherlands and Belgium. Emerging Infectious Diseases. 2021;27(5):1405–1415. doi: 10.3201/eid2705.204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Muller C, Ghoor S, Louw J, Archer E, Surujlal-Naicker S, Berkowitz N, Volschenk M, Bröcker L, Wolfaardt G, Van der Walt M, Mutshembele AM, Malema S, Gelderblom HC, Muhdluli M, Gray G, Mathee A, Street R. Qualitative and quantitative detection of SARS-CoV-2 RNA in untreated wastewater in Western Cape Province. South Africa. South African Medical Journal. 2021;111(3):198–202. doi: 10.7196/SAMJ.2021.v111i3.15154. [DOI] [PubMed] [Google Scholar]
- Joukar F, Yaghubi Kalurazi T, Khoshsorour M, Taramian S, Mahfoozi L, Balou HA, Jafarinezhad A, Pourkazemi A, Hesni E, Asgharnezhad M, Shenagari M, Jahanzad I, Naghipour M, Maroufizadeh S, Mansour-Ghanaei F. Persistence of SARS-CoV-2 RNA in the nasopharyngeal, blood, urine, and stool samples of patients with COVID-19: A hospital-based longitudinal study. Virol J. 2021;18(1):134. doi: 10.1186/s12985-021-01599-9.PMID:34210325;PMCID:PMC8248752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan S, Ronquillo N, Belda-Ferre P, Alvarado D, Javidi T, Longhurst CA, Knight R. High-Throughput Wastewater SARS-CoV-2 detection enables forecasting of community infection dynamics in San Diego County. mSYstems. 2021;6(2):45–21. doi: 10.1128/mSystems.00045-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura, K., Sadamasu, K., Muramatsu, M., & Yoshida, H. (2021). Efficient detection of SARS-CoV-2 RNA in the solid fraction of wastewater. Science of The Total Environment, 763, 144587. 10.1016/j.scitotenv.2020.144587 [DOI] [PMC free article] [PubMed]
- Kumar M, Joshi M, Patel AK, Joshi CG. Unravelling the early warning capability of wastewater surveillance for COVID-19: A temporal study on SARS-CoV-2 RNA detection and need for the escalation. Environmental Research. 2021;196:110946. doi: 10.1016/j.envres.2021.110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Patel AK, Shah AV, Raval J, Rajpara N, Joshi M, Joshi CG. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. The Science of the Total Environment. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G, Iaconelli M, Mancini P, Bonanno Ferraro G, Veneri C, Bonadonna L, Lucentini L, Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. The Science of the Total Environment. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G, Mancini P, Bonanno Ferraro G, Veneri C, Iaconelli M, Bonadonna L, Lucentini L, Suffredini E. SARS-CoV-2 has been circulating in northern Italy since December 2019: Evidence from environmental monitoring. The Science of the Total Environment. 2021;750:141711. doi: 10.1016/j.scitotenv.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G, Mancini P, Bonanno Ferraro G, Veneri C, Iaconelli M, Lucentini L, Bonadonna L, Brusaferro S, Brandtner D, Fasanella A, Pace L, Parisi A, Galante D, Suffredini E. Rapid screening for SARS-CoV-2 variants of concern in clinical and environmental samples using nested RT-PCR assays targeting key mutations of the spike protein. Water Research. 2021;197:117104. doi: 10.1016/j.watres.2021.117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G, Brandtner D, Mancini P, Veneri C, Bonanno Ferraro G, Bonadonna L, Lucentini L, Suffredini E. Key SARS-CoV-2 mutations of Alpha, Gamma and Eta variants detected in urban wastewaters in Italy by long-read amplicon sequencing based on nanopore technology. Water. 2021;13(18):2503. doi: 10.3390/w13182503. [DOI] [Google Scholar]
- La Rosa G, Fratini M, Accardi L, D'Oro G, Della Libera S, Muscillo M, Di Bonito P. Mucosal and cutaneous human papillomaviruses detected in raw sewages. PLoS ONE. 2013;8(1):e52391. doi: 10.1371/journal.pone.0052391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa, G. and Muscillo, M. (2013) Viruses in Food and Water. Cook, N. (ed), pp. 97–125, Woodhead Publishing.
- Lee, Wei Lin; Imakaev, Maxim; Armas, Federica; McElroy, Kyle A.; Gu, Xiaoqiong; Duvallet, Claire; Chandra, Franciscus; Chen, Hongjie; Leifels, Mats; Mendola, Samuel; Floyd-O’Sullivan, Róisín; Powell, Morgan M.; Wilson, Shane T.; Berge, Karl L. J.; Lim, Claire Y. J.; Wu, Fuqing; Xiao, Amy; Moniz, Katya H; Ghaeli, Newsha; Matus, Mariana; Thompson, Janelle; Alm, Eric J (2021) Quantitative SARS-CoV-2 Alpha Variant B117 Tracking in Wastewater by Allele-Specific RT-qPCR. Environmental Science & Technology Letters 8(8): 675–682 Doi: 10.1021/acs.estlett.1c00375
- Li B, Di DYW, Saingam P, Jeon MK, Yan T. Fine-Scale Temporal Dynamics of SARS-CoV-2 RNA Abundance in Wastewater during A COVID-19 Lockdown. Water Research. 2021;197:117093. doi: 10.1016/j.watres.2021.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang S, Shi J, Luby SP, Jiang G. Uncertainties in estimating SARS-CoV-2 prevalence by wastewater-based epidemiology. Chemical Engineering Journal. 2021;415:129039. doi: 10.1016/j.cej.2021.129039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W, de Roda Husman AM. SARS-CoV-2 in wastewater: Potential health risk, but also data source. Lancet Gastroenterol Hepatol. 2020;5(6):533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy L, Fatta-Kassinos D, Slobodnik J, Karaolia P, Cirka L, Kreuzinger N, Castiglioni S, Bijlsma L, Dulio V, Deviller G, Lai FY, Alygizakis N, Barneo M, Baz-Lomba JA, Béen F, Cíchová M, Conde-Pérez K, Covaci A, Donner E, Ficek A, Hassard F, Hedström A, Hernandez F, Janská V, Jellison K, Hofman J, Hill K, Hong PY, Kasprzyk-Hordern B, Kolarević S, Krahulec J, Lambropoulou D, de Llanos R, Mackuľak T, Martinez-García L, Martínez F, Medema G, Micsinai A, Myrmel M, Nasser M, Niederstätter H, Nozal L, Oberacher H, Očenášková V, Ogorzaly L, Papadopoulos D, Peinado B, Pitkänen T, Poza M, Rumbo-Feal S, Sánchez MB, Székely AJ, Soltysova A, Thomaidis NS, Vallejo J, van Nuijs A, Ware V, Viklander M. Making Waves: Collaboration in the time of SARS-CoV-2 - rapid development of an international co-operation and wastewater surveillance database to support public health decision-making. Water Research. 2021;1(199):117167. doi: 10.1016/j.watres.2021.117167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Klapsa D, Wilton T, Zambon M, Bentley E, Bujaki E, Fritzsche M, Mate R, Majumdar M. Tracking SARS-CoV-2 in Sewage: Evidence of Changes in Virus Variant Predominance during COVID-19 Pandemic. Viruses. 2020;12(10):1144. doi: 10.3390/v12101144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClary-Gutierrez JS, Mattioli MC, Marcenac P, Silverman AI, Boehm AB, Bibby K, Balliet M, de Los Reyes FL. SARS-CoV-2 Wastewater Surveillance for Public Health Action. Emerging Infectious Diseases. 2021;27(9):1–8. doi: 10.3201/eid2709.210753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G, Heijnen L, Elsinga G, Italiaander R, Brouwer A. Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in The Netherlands. Environmental Science & Technology Letters. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Melnick JL. Poliomyelitis virus in urban sewage in epidemic and in nonepidemic times. American Journal of Hygiene. 1947;45(2):240–253. doi: 10.1093/oxfordjournals.aje.a119132. [DOI] [PubMed] [Google Scholar]
- Mishra, S., Mindermann, S., Sharma, M., Whittaker, C., Mellan, T. A., Wilton, T., Klapsa, D., Mate, R., Fritzsche, M., Zambon, M., Ahuja, J., Howes, A., Miscouridou, X., Nason, G. P., Ratmann, O., Semenova, E., Leech, G., Sandkühler, J. F., Rogers-Smith, C., Vollmer, M., COVID-19 Genomics UK (COG-UK) Consortium Changing composition of SARS-CoV-2 lineages and rise of Delta variant in England. EClinicalMedicine. 2021;39:101064. doi: 10.1016/j.eclinm.2021.101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyani B, Fonoll X, Norton J, Mehrotra A, Xagoraraki I. SARS-CoV-2 in Detroit Wastewater. Journal of Environmental Engineering. 2020;146(11):06020004. doi: 10.1061/(ASCE)EE.1943-7870.0001830. [DOI] [Google Scholar]
- Mlejnkova H, Sovova K, Vasickova P, Ocenaskova V, Jasikova L, Juranova E. Preliminary Study of Sars-Cov-2 Occurrence in Wastewater in the Czech Republic. International Journal of Environmental Research and Public Health. 2020;17(15):5508. doi: 10.3390/ijerph17155508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S, Menon NG, Mohapatra G, Pisharody L, Pattnaik A, Menon NG, Bhukya PL, Srivastava M, Singh M, Barman MK, Gin KY, Mukherji S. The novel SARS-CoV-2 pandemic: Possible environmental transmission, detection, persistence and fate during wastewater and water treatment. The Science of the Total Environment. 2021;765:142746. doi: 10.1016/j.scitotenv.2020.142746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri S, Yavarian J, Baghani AN, Azad TM, Nejati A, Nabizadeh R, Hadi M, Jandaghi N, Vakili B, Vaghefi S, Baghban M, Yousefi S, Nazmara S, Alimohammadi M. The presence of SARS-CoV-2 in raw and treated wastewater in 3 cities of Iran: Tehran, Qom and Anzali during coronavirus disease 2019 (COVID-19) outbreak. Journal of Environmental Health Science & Engineering. 2021;19(1):1–12. doi: 10.1007/s40201-021-00629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A, Nemudraia A, Wiegand T, Surya K, Buyukyoruk M, Cicha C, Vanderwood KK, Wilkinson R, Wiedenheft B. Temporal Detection and Phylogenetic Assessment of SARS-CoV-2 in Municipal Wastewater. Cell Reports. Medicine. 2020;1(6):100098. doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H, Linton NM, Akhmetzhanov AR. Serial interval of novel coronavirus (COVID-19) infections. International Journal of Infectious Diseases : IJID : Official Publication of the International Society for Infectious Diseases. 2020;93:284–286. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Systematic Reviews. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J, Zulli A, Brackney DE, Grubaugh ND, Kaplan EH, Casanovas-Massana A, Ko AI, Malik AA, Wang D, Wang M, Warren JL, Weinberger DM, Arnold W, Omer SB. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nature Biotechnology. 2020;38(10):1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen RM, Tornby DS, Bang LL, Madsen LW, Skov MN, Jensen TG, Johansen IS, Andersen TE. Rectally shed SARS-CoV-2 lacks infectivity: Time to rethink faecal-oral transmission? Nature Reviews. Gastroenterology & Hepatology. 2021;18(9):669. doi: 10.1038/s41575-021-00501-w.PMID:34312524;PMCID:PMC8311632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petala M, Dafou D, Kostoglou M, Karapantsios T, Kanata E, Chatziefstathiou A, Sakaveli F, Kotoulas K, Arsenakis M, Roilides E, Sklaviadis T, Metallidis S, Papa A, Stylianidis E, Papadopoulos A, Papaioannou N. A physicochemical model for rationalizing SARS-CoV-2 concentration in sewage Case study: The city of Thessaloniki in Greece. The Science of the Total Environment. 2021;755(1):142855. doi: 10.1016/j.scitotenv.2020.142855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrillo M, Brogna C, Cristoni S, Querci M, Piazza O, Van den Eede G. Increase of SARS-CoV-2 RNA load in faecal samples prompts for rethinking of SARS-CoV-2 biology and COVID-19 epidemiology. F100Res. 2021;10:370. doi: 10.12688/f1000research.52540.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T, Fumian TM, Mannarino CF, Resende PC, Motta FC, Eppinghaus A. Wastewater-based epidemiology as a useful tool to track SARS-CoV-2 and support public health policies at municipal level in Brazil. Water Research. 2021;191:116810. doi: 10.1016/j.watres.2021.116810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T, Fumian TM, Mannarino CF, Maranhão AG, Siqueira MM, Miagostovich MP. Preliminary results of SARS-CoV-2 detection in sewerage system in Niterói municipality, Rio de Janeiro. Brazil. Memorias Do Instituto Oswaldo Cruz. 2020;115:e200196. doi: 10.1590/0074-02760200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W, Cuevas-Ferrando E, Sanjuán R, Domingo-Calap P, Sánchez G. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. International Journal of Hygiene and Environmental Health. 2020;230:113621. doi: 10.1016/j.ijheh.2020.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W, Truchado P, Cuevas-Ferrando E, Simón P, Allende A, Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Research. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi SG, Stefani F, Gigantiello A, Polesello S, Comandatore F, Mileto D, Maresca M, Longobardi C, Mancon A, Romeri F, Pagani C, Cappelli F, Roscioli C, Moja L, Gismondo MR, Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. The Science of the Total Environment. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios G, Lacoux C, Leclercq V, Diamant A, Lebrigand K, Lazuka A, Soyeux E, Lacroix S, Fassy J, Couesnon A, Thiery R, Mari B, Pradier C, Waldmann R, Barbry P. Monitoring SARS-CoV-2 variants alterations in Nice neighborhoods by wastewater nanopore sequencing. The Lancet regional health: Europe; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair RG, Choi CY, Riley MR, Gerba CP. Pathogen surveillance through monitoring of sewer systems. Advances in Applied Microbiology. 2008;65:249–269. doi: 10.1016/S0065-2164(08)00609-6.PMID:19026868;PMCID:PMC7112011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. China. Intensive Care Medicine. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saawarn B, Hait S. Occurrence, fate and removal of SARS-CoV-2 in wastewater: Current knowledge and future perspectives. Journal of Environmental Chemical Engineering. 2021;9(1):104870. doi: 10.1016/j.jece.2020.104870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saguti F, Magnil E, Enache L, Churqui MP, Johansson A, Lumley D, Davidsson F, Dotevall L, Mattsson A, Trybala E, Lagging M, Lindh M, Gisslén M, Brezicka T, Nyström K, Norder H. Surveillance of wastewater revealed peaks of SARS-CoV-2 preceding those of hospitalized patients with COVID-19. Water Research. 2021;189:116620. doi: 10.1016/j.watres.2020.116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saththasivam J, El-Malah SS, Gomez TA, Jabbar KA, Remanan R, Krishnankutty AK, Ogunbiyi O, Rasool K, Ashhab S, Rashkeev S, Bensaad M, Ahmed AA, Mohamoud YA, Malek JA, Abu Raddad LJ, Jeremijenko A, Abu Halaweh HA, Lawler J, Mahmoud KA. COVID-19 (SARS-CoV-2) outbreak monitoring using wastewater-based epidemiology in Qatar. The Science of the Total Environment. 2021;774:145608. doi: 10.1016/j.scitotenv.2021.145608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma DK, Nalavade UP, Kalgutkar K, Gupta N, Deshpande JM. SARS-CoV-2 detection in sewage samples: Standardization of method & preliminary observations. The Indian Journal of Medical Research. 2021;153(1 & 2):159–165. doi: 10.4103/ijmr.IJMR_3541_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan SP, Shahin S, Ward LM, Tandukar S, Aw TG, Schmitz B, Ahmed W, Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: A study in Louisiana, USA. The Science of the Total Environment. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift CL, Isanovic M, Correa Velez KE, Norman RS. Community-level SARS-CoV-2 sequence diversity revealed by wastewater sampling. Science of the Total Environment. 2021;801:149691. doi: 10.1016/j.scitotenv.2021.149691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanhaei, M., Mohebbi, S. R., Hosseini, S. M., Rafieepoor, M., Kazemian, S., Ghaemi, A., Shamloei, S., Mirjalali, H., Asadzadeh Aghdaei, H., & Zali, M. R. (2021) The first detection of SARS-CoV-2 RNA in the wastewater of Tehran, Iran. Environmental Science and Pollution Research International, 10.1007/s11356-021-13393-9. [DOI] [PMC free article] [PubMed]
- Treibel TA, Manisty C, Burton M, McKnight Á, Lambourne J, Augusto JB, Couto-Parada X, Cutino-Moguel T, Noursadeghi M, Moon JC. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet (london, England) 2020;395(10237):1608–1610. doi: 10.1016/S0140-6736(20)31100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier J, Darques R, Ait Mouheb N, Partiot E, Bakhache W, Deffieu MS, Gaudin R. Post-lockdown detection of SARS-CoV-2 RNA in the wastewater of Montpellier, France. One Health (amsterdam, Netherlands) 2020;10:100157. doi: 10.1016/j.onehlt.2020.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn AS, Meijer B, Frampton C, Barclay ML, de Boer N. Systematic review with meta-analysis: SARS-CoV-2 stool testing and the potential for faecal-oral transmission. Alimentary Pharmacology & Therapeutics. 2020;52(8):1276–1288. doi: 10.1111/apt.16036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Feng H, Zhang S, Ni Z, Ni L, Chen Y, Zhuo L, Zhong Z, Qu T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. International Journal of Infectious Diseases : IJID : Official Publication of the International Society for Infectious Diseases. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas J, Aanderud ZT, Roper DK, VanDerslice J, Gaddis EB, Ostermiller J, Hoffman K, Jamal R, Heck P, Zhang Y, Torgersen K, Laan JV, LaCross N. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. The Science of the Total Environment. 2021;775:145790. doi: 10.1016/j.scitotenv.2021.145790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhaus S, Weber FA, Schiwy S, Linnemann V, Brinkmann M, Widera M, Greve C, Janke A, Hollert H, Wintgens T, Ciesek S. Detection of SARS-CoV-2 in raw and treated wastewater in Germany - Suitability for COVID-19 surveillance and potential transmission risks. The Science of the Total Environment. 2021;751:141750. doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2020).WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020.
- WHO (2021). WHO announces simple, easy-to-say labels for SARS-CoV-2 Variants of Interest and Concern.
- Wilder ML, Middleton F, Larsen DA, Du Q, Fenty A, Zeng T, Insaf T, Kilaru P, Collins M, Kmush B, Green HC. Co-quantification of crAssphage increases confidence in wastewater-based epidemiology for SARS-CoV-2 in low prevalence areas. Water Research X. 2021;11:100100. doi: 10.1016/j.wroa.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- World Health . Guidelines for environmental surveillance of poliovirus circulation. World Health Organization; 2003. [Google Scholar]
- Wu F, Zhang J, Xiao A, Gu X, Lee WL, Armas F, Kauffman K, Hanage W, Matus M, Ghaeli N, Endo N, Duvallet C, Poyet M, Moniz K, Washburne AD, Erickson TB, Chai PR, Thompson J, Alm EJ. SARS-CoV-2 Titers in Wastewater Are Higher than Expected from Clinically Confirmed Cases. mSystems. 2020;5(4):00614. doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wu Y, Cheng X, Jiang G, Tang H, Ming S, Tang L, Lu J, Guo C, Shan H, Huang X. Altered oral and gut microbiota and its association with SARS-CoV-2 viral load in COVID-19 patients during hospitalization. NPJ Biofilms Microbiomes. 2021;7(1):61. doi: 10.1038/s41522-021-00232-5.PMID:34294722;PMCID:PMC8298611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz N, Revol O, Jardot P, Giraud-Gatineau A, Houhamdi L, Soumagnac C, Annessi A, Lacoste A, Colson P, Aherfi S, Scola B. Monitoring the Circulation of SARS-CoV-2 Variants by Genomic Analysis of Wastewater in Marseille, South-East France. Pathogens. 2021;10(8):1042. doi: 10.3390/pathogens10081042.PMID:34451505;PMCID:PMC8401729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S, Marechal V, Mouchel JM, Maday Y, Teyssou R, Richard E, Almayrac JL, Moulin L. Evaluation of lockdown effect on SARS-CoV-2 dynamics through viral genome quantification in waste water, Greater Paris, France, 5 March to 23. Euro Surveillance. 2020;25(50):2000776. doi: 10.2807/1560-7917.ES.2020.25.50.2000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Cen, M., Hu, M., Du, L., Hu, W., Kim, J. J., & Dai, N. (2021). Prevalence and Persistent Shedding of Fecal SARS-CoV-2 RNA in Patients With COVID-19 Infection: A Systematic Review and Meta-analysis. Clinical and translational gastroenterology, 12(4): 00343. 10.14309/ctg.0000000000000343. [DOI] [PMC free article] [PubMed]
- Yaniv K, Ozer E, Shagan M, Lakkakula S, Plotkin N, Bhandarkar NS, Kushmaro A. Direct RT-qPCR assay for SARS-CoV-2 variants of concern (Alpha, B117 and Beta, B1351) detection and quantification in wastewater. Environmental. 2021 doi: 10.1016/j.envres.2021.111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JB, Kong WH, Wang S, Long YB, Dong LH, He ZY, Liu MQ. Detection of SARS-CoV-2 RNA in Medical Wastewater in Wuhan During the COVID-19 Outbreak. Virologica Sinica. 2021 doi: 10.1007/s12250-021-00373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.